Abstract
This review is based on the Carl Ludwig Distinguished Lecture, presented at the 2019 Experimental Biology Meeting in Orlando, FL, and provides a snapshot of >40 years of work done in collaboration with the late Gerard L. Gebber and colleagues to highlight the importance of considering the rhythmic properties of sympathetic nerve activity (SNA) and brain stem neurons when studying the neural control of autonomic regulation. After first providing some basic information about rhythms, I describe the patterns and potential functions of rhythmic activity recorded from sympathetic nerves under various physiological conditions. I review the evidence that these rhythms reflect the properties of central sympathetic neural networks that include neurons in the caudal medullary raphe, caudal ventrolateral medulla, caudal ventrolateral pons, medullary lateral tegmental field, rostral dorsolateral pons, and rostral ventrolateral medulla. The role of these brain stem areas in mediating steady-state and reflex-induced changes in SNA and blood pressure is discussed. Despite the common appearance of rhythms in SNA, these oscillatory characteristics are often ignored; instead, it is common to simply quantify changes in the amount of SNA to make conclusions about the function of the sympathetic nervous system in mediating responses to a variety of stimuli. This review summarizes work that highlights the need to include an assessment of the changes in the frequency components of SNA in evaluating the cardiovascular responses to various manipulations as well as in determining the role of different brain regions in the neural control of the cardiovascular system.
Keywords: brain stem sympathetic neurons, cardiac-related activity, central sympathetic network, correlation analysis, 10-Hz rhythm
INTRODUCTION
This review is based on the Carl Ludwig Distinguished Lecture that I had the honor of presenting at the 2019 Experimental Biology Meeting in Orlando, FL. The field of neural control of autonomic regulation owes much to the remarkable insight and research endeavors of Carl Ludwig, a German physician and physiologist, and his students in the 19th century (45, 53, 106). Much of the work done in this field, even in the past 50 years, has been predicated on the seminal discoveries of these insightful investigators. By using simultaneous recordings of intrathoracic pressure and arterial blood pressure (ABP), Ludwig introduced the concept of cardiorespiratory synchronization (45, 53). Ludwig and his Russian pupil Elie de Cyon introduced the concept of reflex regulation of ABP and heart rate by stimulating the vascular afferent nerve endings in the “depressor nerve,” i.e., the baroreceptor reflex (53). While working in Ludwig's laboratory in Leipzig, P. Owsjannikow and C. Dittmar made seminal discoveries about the brain regions responsible for setting the steady-state level of ABP (45, 106). By recording ABP while making serial transections of the brain, Owsjannikow determined that ABP was primarily controlled by the rostral two-thirds of the medulla and the caudal one-third of the pons; Dittmar made the first reference to a medullary “vasomotor center” within the rostral ventrolateral medulla (RVLM) at the level of the facial nucleus.
These discoveries of Ludwig and colleagues had impactful implications about the role of the sympathetic nervous system in the control of the circulation, although these early studies preceded by >50 years the use of methodology to record directly sympathetic nerve activity (SNA). Adrian et al. (2) published the first recordings of SNA; the bursts within cervical and abdominal SNA of anesthetized cats and rabbits were locked to the arterial pulse (AP; cardiac-related activity), and the amplitude of these bursts fluctuated on the time scale of the respiratory cycle (respiratory-related activity). About 36 years later, Karl-Erik Hagbarth inserted a needle into his ulnar nerve to record muscle SNA (MSNA) and reported that the pulse-synchronous bursts in MSNA are most prominent in the expiratory phase of the respiratory cycle (42, 67, 110). These findings of cardiac- and respiratory-related rhythmicity in SNA are evidence for the cardiorespiratory integration and baroreflex control, as first proposed by Ludwig and colleagues. In 1946, Alexander (3) not only confirmed the early results on the presumed origin of the neural basis for ABP control but also reported that SNA was also dependent on the integrity of the caudal one-third of the pons and rostral two-thirds of the medulla, as predicted by Ludwig’s students.
These early studies set the stage for my >40 years of work in collaboration with the late Gerard L. Gebber and colleagues in addressing the origins of rhythmic SNA. I entered the field at a fortunate time, as technologies such as chemical activation and inactivation of central neurons, neuroanatomical tract-tracing technology, and laboratory computers capable of assessing neural signals were being introduced. After first describing some basic information about biological rhythms, I describe the types of rhythms appearing in SNA, potential functions of rhythmic SNA, the evidence that these rhythms reflect the properties of anatomically distributed central autonomic neural networks, and data showing that one can make erroneous conclusions regarding the effects on SNA of some manipulations by measuring only the steady-state level of activity and overlooking its rhythmicity.
RHYTHMS AND REGULATION OF BIOLOGICAL PROCESSES
Periodic oscillations with cycle times ranging from fractions of seconds to years are ubiquitous among biological processes in small (e.g., unicellular eukaryotes) and large (e.g., humans) organisms; these rhythms are critical for the regulation of many physiological behaviors (60). Some rhythms are a reaction to an environmental stimulus: a common example is the circadian rhythm that regulates many biological processes, including the sleep-wake cycle, and even alterations in cardiovascular variables (102). Other rhythms arise from the bidirectional and highly branched connectivity of central neurons, allowing the activity in multiple brain regions to oscillate simultaneously (40). Rhythmicity offers advantages over randomly occurring events or stochastic patterns of activity (40, 99, 100, 102). For example, periodic regulation or entrainment synchronizes events to operate as a unit, such as when the pacemaker activity of the sinoatrial node ensures that the heart functions as a unit (111). Rhythmicity allows one to predict repetitive events and, thus, leads to more efficient activation of a behavior (40); this can be exemplified by the role of the circadian rhythm generator in preparing an organism for physiological actions that recur on a daily cycle (89).
Like so many physiological control systems, autonomic target organs, such as the heart and gastrointestinal tract, are characterized by their rhythmic activity. As mentioned above, cardiac- and respiratory-related discharges are commonly seen in recordings from sympathetic nerves. Sympathetic neural circuits are complex and capable of generating periodic activity patterns that range from ~0.04 to ≥10 Hz (e.g., diurnal variations), associated with the physiological or pathophysiological status of the animal, the behavioral state, the type of nerve being analyzed (innervation of the skin or muscle vasculature or the viscera), the age of the subject, and the species (19, 41–43, 45, 64, 68, 73, 80–83, 89).
The presence of periodic components in a signal can be quantified in the time domain by construction of an autocorrelogram or in the frequency domain by construction of a power-density spectrum (autospectrum). Autocorrelation analysis calculates a correlation coefficient between a signal and a copy of itself at lags of 0, ±1, ±2, ±3, etc., sampling intervals (34, 37, 52, 72). There are several algorithms for analyzing a signal in the frequency domain, but my laboratory has used fast-Fourier transform, which decomposes an analog signal into its frequency components by matching the signal to sine waves with a broad range of frequencies (34, 37, 72, 85).
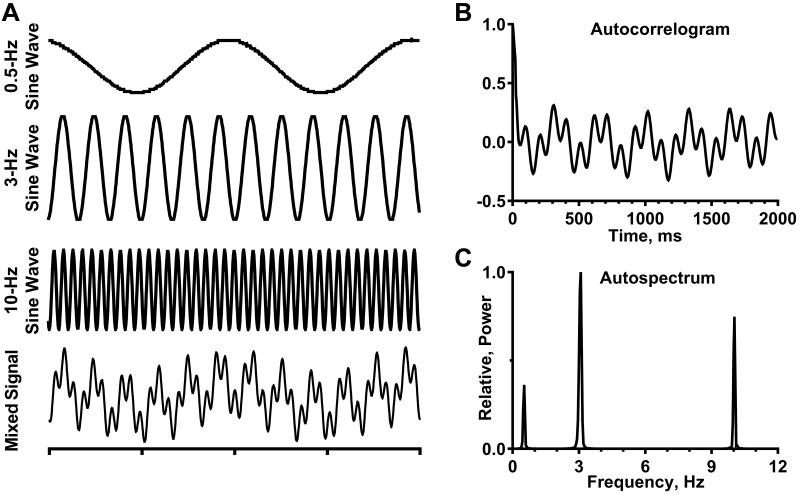
Figure 1 demonstrates that frequency-domain analysis is more suitable than time-domain analysis to reflect accurately the activity pattern of a signal like SNA, which has multiple frequency components. This point is illustrated by summing the outputs of three sine-wave generators (0.5-, 3-, and 10-Hz frequencies) to form a mixed signal (Fig. 1A). These three frequencies were selected, because they are comparable to the frequency components (respiratory-related, cardiac-related, and 10-Hz rhythms, respectively) identified in SNA of urethane-anesthetized, decerebrate-unanesthetized, or conscious cats (8, 19, 20, 24, 43, 44, 63, 82, 90). Autocorrelation analysis of the mixed signal (Fig. 1B) fails to show reliably the three distinct frequency components of this signal. In contrast, the autospectrum of the mixed signal (Fig. 1C) faithfully reproduces the frequencies of each of the three sine-wave generators that formed the mixed signal.
Fig. 1.
Comparison of time- and frequency-domain analyses to quantify the characteristics of a complex analog signal. A: outputs of 3 sine-wave generators are shown separately (0.5, 3.0, and 10 Hz), as well as in combination (mixed signal). Time scale = 1 s/division. B: autocorrelogram is the time-domain analysis of a 5-min recording of the mixed signal; 5-ms bin resolution. C: autospectrum is the frequency-domain analysis of the same 5-min recording of the mixed signal; it is based on 29 20-s windows with 50% overlap and frequency resolution of 0.04 Hz per bin. A Digidata 1322A digitizer (Axon Instruments, Union City, CA) was used to acquire data.
As described in detail below, my colleagues and I have used time- and frequency-domain analyses to characterize the properties of SNA and the activity of individual neurons deemed to be part of a brain stem sympathetic network. Importantly, these methods allow one to assess the relationship between SNA and the AP and phrenic nerve activity (PNA), between the activity of two or more sympathetic nerves, and, importantly, between SNA and the activity of individual brain stem neurons. In combination with techniques such as microinjection of agonists or antagonists of various receptors and antidromic activation, these approaches have proven to be indispensable for identifying the organization of central sympathetic networks.
QUANTIFYING THE RHYTHMS IN SNA
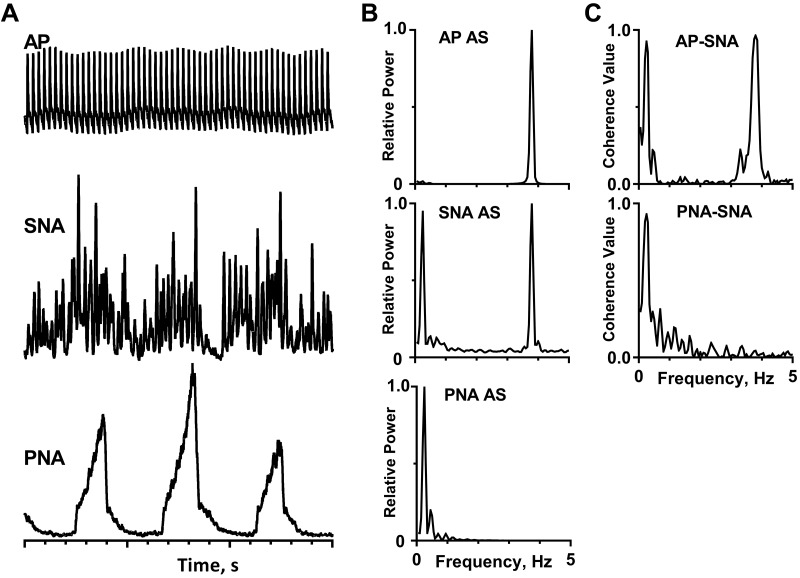
From the traces in Fig. 2A, it is easy to see that the amplitude of bursts of left inferior cardiac SNA fluctuates on the time scale of the respiratory cycle (as reflected by integrated PNA) in a barbiturate-anesthetized, paralyzed, and artificially ventilated cat. Figure 2B shows the SNA autospectrum; it has two marked peaks: one at 0.25 Hz, which is the same as the peak in the PNA autospectrum, and the other at 3.80 Hz, which coincides with the peak in the AP autospectrum.
Fig. 2.
Cardiac- and respiratory-related rhythms in inferior cardiac sympathetic nerve activity (SNA) of a barbiturate-anesthetized, paralyzed, and artificially ventilated cat. A: traces (top to bottom) show arterial pulse (AP), SNA, integrated phrenic nerve activity (PNA), and time base (1 s/division). The capacity-coupled preamplifier band-pass setting was 30–3,000 Hz (SNA) or 10–1,000 Hz (PNA). Signals were passed through a 50/60-Hz noise eliminator (Hum Bug, Quest Scientific, North Vancouver, BC, Canada) and a moving averager (model MA-821RSP, CWE, Ardmore, PA) with a 50-ms (SNA) or 100-ms (PNA) time constant. B: autospectra (AS) of these signals. C: coherence functions showing the relationship between pairs of these signals. Spectra are based on 35 20-s windows with 50% overlap and frequency resolution of 0.05 Hz per bin. A Digidata 1322A digitizer (Axon Instruments, Union City, CA) was used to acquire data.
In addition to identifying the periodicities in individual signals, spectral analyses can be used to quantify the strength of linear correlation (phase-locking) among the frequency components of two signals (34, 37, 57, 72). A coherence value of 1.0 denotes a perfect correlation, and a coherence value of 0 denotes the absence of a correlation. Figure 2B shows that the 0.25-Hz frequency component of SNA was strongly correlated to PNA (i.e., confirming that it is respiratory-related) and the 3.80-Hz frequency component of SNA was strongly correlated to the AP (i.e., confirming that it is cardiac-related).
There is compelling evidence that the cardiac- and respiratory-related rhythms in SNA reflect the influence of baroceptor afferents and a combination of lung inflation afferents and central respiratory neurons, respectively, on a central sympathetic network capable of generating oscillatory activity of various frequencies (6, 7, 9, 44, 54, 98, 109, 116). Cardiac-related SNA is typically replaced by irregular 2- to 6-Hz oscillations following bilateral section of the carotid sinus, aortic depressor, and vagus nerves (baroreceptor denervation) in barbiturate-anesthetized cats (11, 43, 54, 55, 109) and is often replaced by a 10-Hz rhythm after baroreceptor denervation in urethane-anesthetized, decerebrate-unanesthetized, or conscious cats (15, 24, 63, 90).
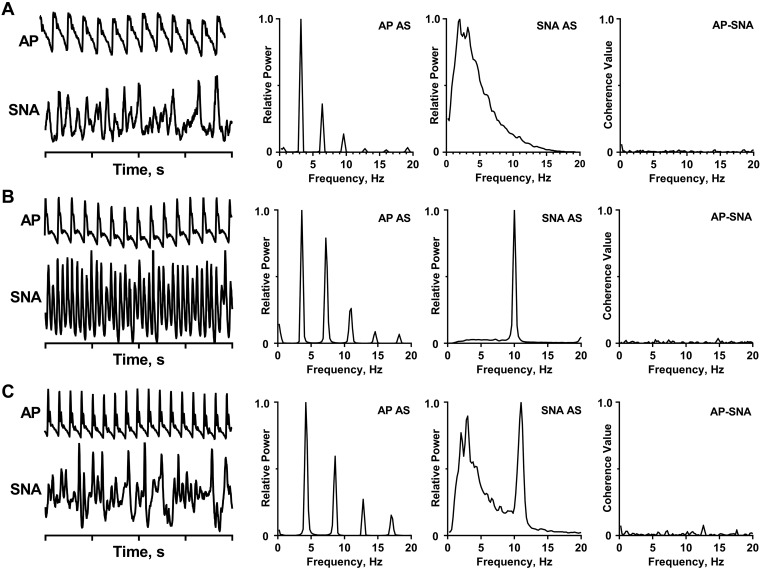
Figures 3 and 4 illustrate activity patterns recorded from sympathetic nerves of cats under different physiological conditions. Figure 3A shows an example of 2- to 6-Hz slow waves in SNA of a baroreceptor-denervated, barbiturate-anesthetized cat. The bursts of SNA vary in their duration and interburst intervals. SNA is not synchronized to the AP, as quantified by the absence of a peak at 3.2 Hz (the heart rate) in the AP-SNA coherence function (Fig. 3A, right). Figure 3, B and C, shows SNA recordings from two urethane-anesthetized, baroreceptor-denervated cats. In the first example, SNA has a prominent 10-Hz rhythm, with most of the activity distributed within a very narrow frequency band. In the second example, SNA is distributed over two frequency bands: most of the activity falls within the low-frequency (<6-Hz) band, and a second peak occurs near 10 Hz. The absence of a cardiac-related rhythm is verified by the coherence functions (Fig. 3, B and C, right). Figure 4 shows the coexistence of the cardiac-related and 10-Hz rhythms in SNA in a cat in which the carotid sinus nerve is intact but the aortic depressor and vagus nerves are cut (partial baroreceptor denervation).
Fig. 3.
Different frequency components in sympathetic nerve activity (SNA) from cats under different physiological conditions. A: recording of arterial pulse (AP) and SNA (preamplifier band pass = 30–3,000 Hz, moving average = 50-ms time constant) of a barbiturate-anesthetized, baroreceptor-denervated cat. B: recording of AP and SNA (preamplifier bandpass = 1–1,000 Hz) of a urethane-anesthetized, baroreceptor-denervated cat. C: recording of AP and SNA (preamplifier band pass = 1–1,000 Hz) of a urethane-anesthetized, baroreceptor-denervated cat. All spectra are based on 36 5-s windows with frequency resolution of 0.2 Hz. A Digidata 1322A digitizer (Axon Instruments, Union City, CA) was used to acquire data.
Fig. 4.
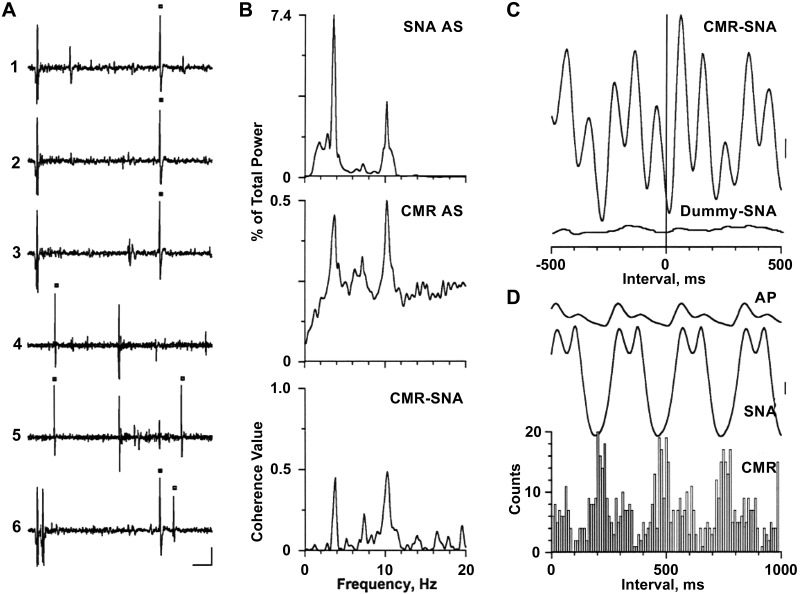
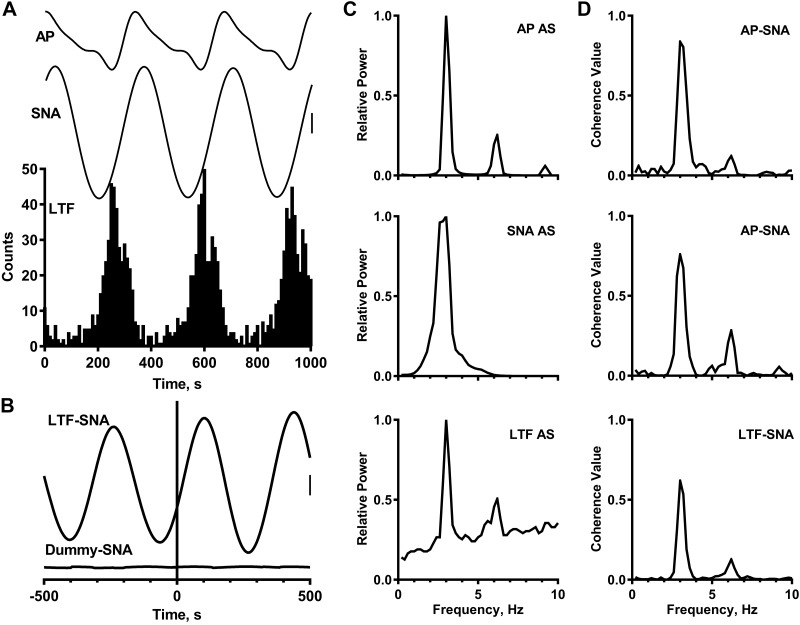
Spinal axonal trajectory for a caudal medullary raphe (CMR) neuron with activity correlated to both the cardiac-related and 10-Hz rhythms in left inferior cardiac sympathetic nerve activity (SNA). A1–A3: 3 consecutive antidromic responses to stimuli applied once every 1.2 s to a site in the first thoracic dorsolateral funiculus. A4 and A5: time-controlled collision test for antidromic activation. A6: estimation of axonal refractory period with paired stimuli. Black squares, Stimulus-induced or naturally occurring action potentials. Vertical calibration = 100 μV. B: traces (top to bottom) are autospectra (AS) of SNA and CMR neuronal activity and corresponding coherence function (CMR-SNA). Spectra are based on 32 5-s windows with frequency resolution of 0.2 Hz per bin. C: spike-triggered (top) and dummy-triggered (bottom) averages of SNA (415 trials each, 5-ms bin width). A series of randomly generated pulses with the same mean frequency as the neuronal spike train were used to construct the dummy average. D: arterial pulse (AP)-triggered analysis of SNA and CMR neuronal activity (289 trials, 5-ms bin width for averages, 10-ms bin width for histogram). Vertical calibrations = 50 and 80 μV in C and D, respectively. [Modified from Ref. 17]
FUNCTIONS OF RHYTHMS IN SNA
Despite the widespread existence of oscillatory behavior, the physiological relevance of some biological rhythms is not always readily apparent. Why is there a cardiac-related or 10-Hz rhythm in SNA if the vascular smooth muscle oscillates at frequencies <0.4 Hz? Barman and Gebber (19) showed that a spontaneous transition in SNA from a prominent cardiac-related rhythm to a dominant 10-Hz rhythm is accompanied by a marked increase in ABP. Similarly, manipulations that eliminate the 10-Hz rhythm (e.g., chemical inactivation or blockade of excitatory amino acid neurotransmission within various brain stem regions) induce a significant reduction in ABP (25, 27, 30, 115). Also, ABP is increased by perturbations (e.g., removal of the posterior vermis) that enhance the magnitude of the 10-Hz rhythm in SNA without a change in total power in SNA (21). Barman and Gebber (19) proposed that synchrony within the central sympathetic circuits responsible for the 10-Hz rhythm activates more preganglionic and postganglionic sympathetic neurons to induce an increase in ABP.
Several studies have linked the modulation of vascular smooth muscle contraction to various frequency components of naturally occurring or stimulus-induced SNA and concluded that high-frequency oscillations of renal SNA in rabbits contribute to setting the level of vasoconstrictor tone (71, 76). Malpas (80, 81) reviewed the evidence that stimulation of sympathetic nerves at different frequencies may lead to the release of different neurotransmitters; he suggested that different frequency patterns in SNA may not simply modulate the degree of vasoconstriction but may reflect distinct functional responses as a result of the release of specific neurotransmitters
By simultaneously recording the activity of two or more sympathetic nerves, it is apparent that there is a stronger correlation between the rhythmic activity than the asynchronous activity in pairs of sympathetic nerves (24, 58, 59, 115). This is the case even when a rhythmic component accounts for only a small component of the total power in SNA. This high coherence of rhythmic SNA in pairs of sympathetic nerves is evident when pairing postganglionic sympathetic nerves emanating from the same ganglion (e.g., inferior cardiac and vertebral nerves), when pairing a postganglionic sympathetic nerve that receives its input from the upper thoracic spinal cord with one that receives its input from the lower lumbar spinal cord (e.g., cardiac and lumbar nerves), and when pairing the activity of postganglionic nerves from ganglia on the left and right sides of the body. The finding that the rhythmic activity in two nerves is more strongly coherent than their asynchronous activity implies that rhythms help coordinate the activity of different elements of the sympathetic nervous system. Also, based on recordings from multiple postganglionic sympathetic neurons that project to the caudal ventral artery of the rat tail, Chang et al. (41) concluded that synchronous activity in a functionally defined population of neurons contributes to the ability of the central nervous system to formulate distinct patterns of sympathetic responses that are appropriate to support specific behaviors.
SEARCHING FOR BRAIN STEM NEURONS THAT COMPRISE A CENTRAL SYMPATHETIC NETWORK
Since the seminal work of Ludwig and his colleagues, scientists have strived to identify the brain regions that are critical for steady-state and reflex control of the cardiovascular system in health and disease. There is general agreement that vasomotor tone is dependent on brain stem neurons (3, 46, 47, 65, 66, 106), but the extent to which different brain stem regions contribute to this function remains a subject of investigation. Higher brain regions also contribute to changes in resting levels of ABP and heart rate, especially under conditions of stress, exercise, and other behaviors (32, 33, 46–48, 74, 75). Moreover, the brain stem is not unique to generating rhythmic SNA, as both the forebrain and spinal cord can generate rhythmicity in SNA (4, 74, 75, 84).
Prior to the 1980s, most of the studies aimed at identifying brain stem neurons responsible for setting the level of SNA and ABP relied on finding neurons that responded to electrical- or pressure-induced activation of baroreceptor nerves (36, 70, 79). A limitation of this approach is that baroreceptor activation can influence physiological processes such as respiration, muscle spindle activity, and cortical activity (6, 35, 101). When searching the medullary reticular formation, including the medullary lateral tegmental field (LTF), Biscoe and Sampson (36) were unsuccessful in locating neurons with pulse-synchronous activity. Gootman et al. (62) were the first to record successfully from a few medullary neurons (exact location not stated), the activity of which was correlated to the slow waves in splanchnic SNA of a baroreceptor-intact cat. In 1981, Gebber and Barman reported the results of a comprehensive investigation of neurons in the classic pressor area of the LTF; they used time-domain analyses to identify individual neurons with naturally occurring action potentials that were synchronized to the cardiac- and respiratory-related rhythms in SNA of barbiturate-anesthetized, baroreceptor-innervated cats (10) and to the 2- to 6-Hz slow waves in SNA of baroreceptor-denervated cats (55). Two years later, Barman and Gebber (11) were the first to identify RVLM neurons with activity correlated to SNA in cats with intact or bilaterally severed baroreceptor afferents. The failure of earlier attempts (36) to locate medullary neurons with pulse-synchronous activity in anesthetized cats likely reflects the fact that most of the brain stem neurons with sympathetic nerve-related activity do not fire in every cardiac cycle, requiring computer-aided approaches to detect this periodicity.
Figure 5 shows the types of time- and frequency-domain analyses that can be applied to characterize the interrelationships of the AP, SNA, and the activity of an individual neuron. The AP-triggered averages and histogram show the cardiac-related rhythms in SNA and LTF neuronal activity (Fig. 5A). The LTF neuronal spike-triggered average of SNA (Fig. 5B) shows that this LTF neuron was most prone to fire near the beginning of the cardiac-related burst recorded from the inferior cardiac postganglionic sympathetic nerve. Note the much-larger-amplitude deflections in the spike-triggered average than in the dummy-triggered average constructed by using a randomly generated train of pulses with the same mean frequency as the LTF neuronal spike train. Both the SNA autospectrum and the autospectrum of LTF neuronal activity (Fig. 5C) have a sharp peak at the frequency of the heart rate, and the coherence functions (Fig. 5D) confirm that both SNA and LTF neuronal activity are correlated to the AP and to each other at this frequency.
Fig. 5.
Time- and frequency-domain analyses of the relationship between arterial pulse (AP), left inferior cardiac sympathetic nerve activity (SNA), and medullary lateral tegmental field (LTF) neuronal activity in a baroreceptor-intact, urethane-anesthetized cat. A: AP-triggered analysis (500 trials) of AP, SNA, and LTF neuronal activity (5-ms bin width for averages, 10-ms bin width for histogram, 40-µV vertical calibration for SNA). B: LTF neuronal spike-triggered (top) and dummy-triggered (bottom) averages of SNA (400 trials each, 5-ms bin width, 20-µV vertical calibration). C: autospectra (AS) of AP and SNA and LTF neuronal activity (LTF-SNA). D: coherence functions relating pairs of these signals. Spectra are based on 36 5-s windows with frequency resolution of 0.2 Hz per bin. A Digidata 1322A digitizer (Axon Instruments, Union City, CA) was used to acquire data.
The classic approach of assessing how a neuron responds to baroreceptor activation (36, 38, 39, 70, 79) can be used in conjunction with time- and frequency-domain analyses to determine if a neuron is part of a sympathoexcitatory or sympathoinhibitory pathway. For this purpose, the firing rates of medullary neurons with cardiac-related activity were compared at baseline and during activation of the arterial baroreceptors (e.g., by a rapid rise in ABP produced by a brief partial obstruction of the abdominal aorta with inflation of a balloon-tipped Fogarty embolectomy catheter). Baroreceptor reflex activation can reduce SNA by inhibiting central sympathetic neurons that lead to activation of SNA or by activating central sympathetic neurons that function to suppress SNA. The firing rate of most of the LTF neurons with cardiac-related activity is reduced in parallel to SNA during a rise in ABP; thus these neurons are classified as sympathoexcitatory neurons; a smaller group of LTF neurons were classified as sympathoinhibitory neurons, because their firing rate was increased during activation of the baroreceptor reflex (13, 14, 56). These findings corroborate work showing that chemical activation (glutamate microinjection) of this area leads to either increases or decreases in ABP (50, 61, 83). Moreover, chemical inactivation (muscimol microinjection) or blockade of N-methyl-d-aspartate (NMDA) excitatory amino acid receptors in this region leads to significant reductions in ABP and SNA (23, 96), verifying its primary role as a sympathoexcitatory region. As reviewed by Dampney (46) and Guyenet (65, 66), the RVLM is known to be a major source of excitatory input to sympathetic preganglionic neurons in the intermediolateral nucleus (IML) of the thoracolumbar spinal cord. Chemical activation of the RVLM leads to an increase in ABP (47, 88, 103, 105). As expected, most of the RVLM neurons with activity correlated to SNA were identified as serving a sympathoexcitatory function, because their firing rate was suppressed during activation of the baroreceptor reflex (13, 17). Moreover, blockade of excitatory amino acid receptors in the RVLM leads to a significant decrease in SNA and ABP (23, 29).
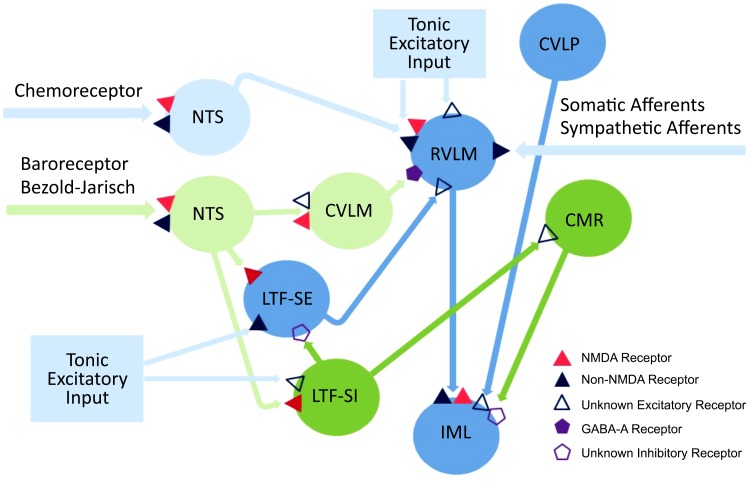
My colleagues and I have used several approaches to characterize the elements of central sympathetic circuits, including the neurons responsible for the cardiac-related and 10-Hz rhythmic components of SNA, those that transmit rhythmic activity to preganglionic sympathetic neurons in the IML, and those that mediate reflex-induced changes in SNA. These approaches included 1) using time- and frequency-domain correlation analyses to identify the location of neurons with activity correlated to the cardiac-related, but not the 10-Hz, component of SNA, those with activity correlated to the 10-Hz rhythm, but not to cardiac-related SNA, and those with activity correlated to both rhythms in SNA; 2) applying the technique of antidromic activation to identify spinal projections and intramedullary connections of presumed brain stem sympathetic neurons; and 3) microinjecting agonists or antagonists of putative central neurotransmitters (e.g., excitatory amino acids, GABA, serotonin, or catecholamines) into specific medullary and pontine areas to assess the impact of these manipulations on the level and pattern of basal SNA or reflex-induced changes in SNA. Most of our effort was focused on the following brain stem regions: caudal medullary raphe (CMR), caudal ventrolateral medulla (CVLM), caudal ventrolateral pons (CVLP), medullary LTF, rostral dorsolateral pons (RDLP), and RVLM (15–18, 20, 22, 23, 25–30, 87, 92, 93, 95–98). Table 1 and Fig. 6 summarize the major findings from these experiments. Figure 6 integrates information from anatomical and electrophysiological studies in both cats and rats, which sometimes differs (5, 12–14, 17, 18, 26, 27, 30, 38, 39, 46, 47, 51, 65, 66, 69, 78, 79, 86–88, 91, 93, 96, 98, 104, 107, 108, 114). Notably, there is a projection from the CVLM to RVLM that is a critical link within the baroreceptor reflex pathway in rats (104, 105, 108), but not in cats (93).
Table 1.
Characteristics of brain stem regions involved in control of steady-state and reflex-induced SNA
| CMR | CVLM | CVLP | LTF | RDLP | RVLM | |
|---|---|---|---|---|---|---|
| Pattern of neuronal activity related to SNA* | CR & 10 Hz (85%), 10 Hz (13%), CR (2%) | 10-Hz (90%), CR (10%) | CR & 10 Hz (54%), CR (23%), 10 Hz (23%) | CR (100%) | 10 Hz (50%), CR & 10 Hz (25%), CR (25%) | CR & 10 Hz (55%), 10 Hz (34%), CR (11%) |
| Function based on baroreceptor reflex activation | SI neurons (83%), SE neurons (16%) | SE neurons (75%), SI neurons (12%) | SE neurons (100%) | SE neurons (54%), SI neurons (28%) | SE neurons (70%), SI neurons (26%) | SE neurons (82%), SI neurons (5%) |
| Axonal trajectory to thoracic IML | CR & 10-Hz activity (99%) | None | CR & 10-Hz activity (92%) | None | None | CR & 10-Hz activity (91%); only 10-Hz activity (10%) |
| Intra-brain stem axonal trajectory | Project to and excite or inhibit CVLM neurons | Project to and excite CMR neurons; do not project to RVLM | Not tested | SE neurons project to and excite RVLM neurons; SI neurons project to and excite CMR neurons | Not tested | Project through LTF, emit collaterals |
| Response to muscimol microinjection† | SNA: ↓10-Hz power; ↑<6 Hz power; total power, ns; ↓MAP | SNA: ↓10-Hz power; ↑<6-Hz power; ↑total power, ns; MAP, ns | SNA: ↓10-Hz power; <6-Hz power, ns; ↓total power; ↓MAP | SNA: ↓10-Hz power; <6-Hz power, ns; total power, ns; ↓MAP | SNA: ↓10-Hz power; <6-Hz power, ns; total power, ns; ↓MAP | SNA: ↓10-Hz power; <6-Hz power, ns; ↓total power; ↓MAP |
| Response to blockade of NMDA receptors† | Not tested | SNA: ↓10-Hz power; total power, ns; MAP, ns | Not tested | SNA: ↓10-Hz power; <6-Hz power, ns; total power, ns; MAP, ns | Not tested | SNA: ↓10-Hz power; ↑<6-Hz power; ↓total power; ↓MAP |
| Response to blockade of non-NMDA receptors† | SNA: ↓10-Hz power; ↓total power; MAP, ns | SNA: ↓10-Hz power; ↑<6-Hz power; ↓total power; ↓MAP | SNA: ↓10-Hz power; ↑<6-Hz power; ↓total power; ↓MAP | |||
| Response to microinjection of 8-OHDPAT† | SNA: ↓10-Hz power; ↑<6-Hz power; total power, ns; ↓MAP | SNA: ↓10-Hz power; <6-Hz power, ns; ↓total power; ↓MAP | Not tested | Not tested | Not tested | SNA: ↓10-Hz power; <6-Hz power, ns; ↓total power; ↓MAP |
| Response to microinjection of clonidine† | SNA:10-Hz power, ns; <6-Hz power, ns; total power, ns; MAP, ns | SNA: ↓10-Hz power; <6-Hz power, ns; total power, ns; MAP, ns | SNA: ↓10-Hz power; ↓<6-Hz power; ↓total power; ↓MAP |
Data are from Refs. 13–18, 20, 22, 23, 25, 27–29, 87, 92–97, 115. ↓, Significantly decreased (P ≤ 0.05); ↑, significantly increased (P ≤ 0.05); ns, no significant change. SNA, sympathetic nerve activity; CR, cardiac-related; 8-OHDPAT, 8-hydroxy-2-(di-n-propylamino)tetralin; CMR, caudal medullary raphe; CVLM, caudal ventrolateral medulla; CVLP, caudal ventrolateral pons; IML, intermediolateral nucleus; MAP, mean arterial pressure; NMDA, N-methyl-d-aspartate; RDLP, rostral dorsolateral pons; RVLM, rostral ventrolateral medulla; SE, sympathoexcitatory; SI, sympathoinhibitory.
Percentages refer to percentage of those neurons with sympathetic nerve-related activity in cats in which both the cardiac-related and 10-Hz rhythms were present in SNA.
In baroreceptor-denervated cats with 10-Hz activity.
Fig. 6.
Central autonomic pathways based on a composite of work in feline and rodent models. The basis for the various connections and receptor types is described in the text. CMR, caudal medullary raphe; CVLM, caudal ventrolateral medulla; CVLP, caudal ventrolateral pons; LTF, lateral tegmental field; NMDA, N-methyl-d-aspartate; NTS, nucleus of the solitary tract; RVLM, rostral ventrolateral medulla; SE, sympathoexcitatory; SI, sympathoinhibitory. Data from numerous anatomical and electrophysiological studies demonstrate the connections shown here (5, 12–14, 17, 18, 26, 27, 30, 38, 39, 47, 51, 69, 78, 79, 86–88, 91, 93, 96, 98, 104, 107, 108, 114).
Do the Same Brain Stem Neurons Generate Both the Cardiac-Related and 10-Hz Sympathetic Rhythms?
By varying the level of ABP in a partially baroreceptor-innervated cat (carotid sinus nerve intact, aortic depressor and vagus nerves cut), one can easily change the balance between the cardiac-related and 10-Hz rhythms in SNA (17, 18, 22). At a mean ABP of >150 mmHg, the cardiac-related rhythm typically dominates; at a mean ABP of ~100 mmHg, the 10-Hz SNA often dominates. At intermediate levels of mean ABP, the two rhythms can coexist. The coexistence of cardiac-related and 10-Hz rhythms in SNA was of great value in the search for brain stem neurons responsible for the different rhythmic patterns in SNA. Figure 4 shows the results of this analysis for a CMR neuron recorded when both rhythms were evident in the SNA autospectrum (Fig. 4B, top); the autospectrum of the discharges of this CMR neuron also has peaks at the frequency of the heartbeat (3.8 Hz) and near 10 Hz (Fig. 4B, middle). AP-triggered analyses show the cardiac-related activity in both SNA and CMR neuronal activity (Fig. 4D). Importantly, coherence analysis (Fig. 4B, bottom) and spike-triggered averaging (Fig. 4C) show that CMR neuronal activity is correlated to both rhythms in SNA.
As summarized in Table 1, when this this approach was used, LTF was determined to be virtually devoid of neurons with activity correlated to the 10-Hz rhythm in SNA (16). In contrast, the activity of the majority of presumed sympathetic CVLM neurons was correlated to only the 10-Hz component of SNA (27); the activity of a few CVLM neurons was correlated to only the cardiac-related rhythm, but these neurons were likely interneurons in the baroreceptor reflex pathway, as they became quiescent when ABP fell below the level needed to entrain SNA to the cardiac cycle. The other areas surveyed (CMR, CVLP, RDLP, and RVLM) had a mixed population of neurons, including those with activity correlated to both rhythms in SNA, to only cardiac-related SNA, and to only the 10-Hz rhythm (17, 18, 22).
Use of Antidromic Activation to Map Axonal Trajectories of Brain Stem Neurons with Activity That Is Synchronized to Rhythms in SNA
My colleagues and I have used antidromic activation to search for spinal and intramedullary projections of medullary neurons with activity correlated to the rhythmic components of SNA (12, 16–18, 22, 27, 87). None of the LTF, CVLM, or RDLP neurons with activity correlated to SNA could be antidromically activated by electrical stimulation of the white matter of the first thoracic spinal segment (16, 22, 27). On the other hand, as shown in Table 1, the other three regions (CMR, RVLM, and CVLP) contain presumed sympathetic neurons with axons that project directly to the thoracolumbar IML (12, 17, 18, 87). These findings support results of anatomical studies showing brain stem projections to the IML (5, 86, 107, 114), as well as electrophysiological studies showing a spinal trajectory of presumed sympathetic neurons (38, 39, 51, 69, 78, 88, 91), in various species.
Since some brain stem regions have neurons with activity that is correlated to only the cardiac-related or to only the 10-Hz rhythm in SNA, these two rhythms must be generated by different pools of neurons. Also, since the activity of some brain stem neurons is synchronized to both rhythms, the outputs of the two rhythm generators must converge at a supraspinal level. Barman and Gebber (17, 18, 22) hypothesized that the outputs of the cardiac-related and 10-Hz rhythm generators converge on spinally projecting neurons. If so, the axons of all neurons with activity correlated to both sympathetic rhythms should project to the spinal cord (i.e., be antidromically activated by electrical stimulation of the upper thoracic spinal cord). On the other hand, if the outputs of the two sympathetic rhythm generators converge at a supraspinal site, one would expect to encounter medullary neurons with activity correlated to both rhythms that cannot be antidromically activated by thoracic spinal cord stimulation.
Figure 4A shows an example of antidromic activation of a CMR neuron with activity correlated to both the cardiac-related and 10-Hz rhythms in SNA. Antidromic activation was indicated by a constant-onset latency of activation in response to single shocks applied to the dorsolateral funiculus of the first thoracic spinal segment and collision of a stimulus-induced action potential with a naturally occurring action potential (77). In support of their hypothesis, Barman and Gebber (17, 18) showed that virtually all RVLM, CMR, and CVLP neurons with activity synchronized to both sympathetic rhythms projected to the thoracic spinal cord. On the other hand, none of the RDLP neurons with activity correlated to both the cardiac-related and 10-Hz rhythms were antidromically activated by thoracic spinal cord stimulation (22). This might mean that the RDLP is the site of convergence of the two sympathetic rhythm generators or that these RDLP neurons receive input from the CMR, CVLP, or RVLM. There is anatomical evidence for the latter (46, 49).
Besides searching for spinal trajectories of brain stem neurons with sympathetic nerve-related activity, my colleagues and I used the technique of antidromic mapping to identify interconnections of these neurons (Table 1). Figure 6 depicts some of the identified connections, and the potential physiological significance of these interactions is summarized here. The axons of LTF sympathoexcitatory neurons project to and likely terminate in the RVLM, and microstimulation of the LTF activates RVLM-spinal neurons (13). These data suggest that LTF neurons may be a source of excitatory drive to RVLM-spinal neurons. Similarly, the axons of LTF sympathoinhibitory neurons project to and likely terminate in the CMR, and microstimulation of the LTF activates CMR-spinal neurons, implying that LTF neurons may be a source of excitatory input to CMR-spinal neurons (14). Also, the axons of CVLM neurons with activity synchronized to the 10-Hz rhythm in SNA project to and likely terminate in the CMR, and some CMR neurons with activity correlated to the 10-Hz rhythm project to and likely terminate in the CVLM (27).
Which Brain Stem Regions Mediate Steady-State and Reflex-Induced Changes in SNA and ABP?
My colleagues and I have studied the effects of microinjection of excitatory amino acid receptor antagonists, the GABA agonist muscimol, and catecholamine or serotonin receptor agonists and antagonists into various brain stem regions on SNA and ABP. Table 1 and Fig. 6 summarize the results of numerous studies on the involvement of brain stem regions in setting the steady-state level and pattern of basal SNA and ABP and in mediating various autonomic reflexes.
As shown in Table 1, chemical inactivation (muscimol microinjection) of any one of the areas surveyed (CMR, CVLP, LTF, RDLP, or RVLM) in baroreceptor-denervated cats causes a significant decrease in the power in the 10-Hz band of SNA that is accompanied by a marked reduction in mean ABP (16, 22, 25, 27, 29, 115). This was even the case for the LTF, which does not contain neurons with activity synchronized to the 10-Hz sympathetic rhythm, implying that the LTF plays a permissive, but not a direct, role in the genesis of the 10-Hz rhythm (16). A significant reduction in mean ABP often occurred, even if the total power in SNA was unchanged, suggesting that the 10-Hz rhythm contributed to setting the resting level of ABP before chemical inactivation of the brain stem.
The microinjection of excitatory amino acid receptor antagonists in several brain stem regions also affected the 10-Hz rhythm in SNA of baroreceptor-denervated cats (Table 1). The 10-Hz rhythm was nearly abolished by the blockade of either NMDA or non-NMDA receptors in any one of the following brain stem regions: CVLM, LTF, or RVLM (23, 29). These data support the view that excitatory amino acid-mediated synaptic transmission in the LTF, RVLM, and CVLM contributes to the expression of the 10-Hz rhythm in SNA. The near abolition of this sympathetic rhythm by the blockade of these receptors in any one of these regions supports the hypothesis that the 10-Hz rhythm in SNA arises from an anatomically distributed network of brain stem neurons (19). The fact that excitatory amino acid-mediated synaptic transmission within the RVLM contributes to setting the level of SNA is counter to a theory that their activity is dependent on intrinsic pacemaker currents (65); whether these neurons also exhibit pacemaker activity under a physiological condition remains a topic of debate (1, 45).
Interference with catecholaminergic, cholinergic, and serotonergic central neurotransmission also influences the expression of the 10-Hz rhythm in SNA (92, 94, 95, 97). Microinjection of clonidine into the CVLM or RVLM, but not into the CMR, nearly eliminates the 10-Hz rhythm (97). This also occurs following intravenous administration of this α2-adrenoceptor agonist; intravenous administration of an α2-adrenoceptor antagonist enhances or induces the 10-Hz rhythm in SNA. These data support the hypothesis that central catecholaminergic neurons contribute to the expression of the 10-Hz rhythm. Central cholinergic neurons play a modulatory role in the control of the 10-Hz rhythm, as it can be induced by the intravenous administration of physostigmine, a cholinesterase inhibitor (94). This action is reversed by administration of the muscarinic receptor antagonist atropine; however, atropine did not interfere with the naturally occurring 10-Hz rhythm in SNA. Microinjection of the 5-HTIA receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin into the CMR, CVLM, or RVLM blocks or diminishes the 10-Hz rhythm in SNA, supporting the view that CMR serotonergic neurons, as well as serotonergic inputs to the RVLM and CVLM, contribute to the generation and/or transmission of the 10-Hz rhythm in SNA (92, 95).
My colleagues and I have also used the responses to microinjection of excitatory or inhibitory amino acid receptors into select brain stem regions to assess their role in mediating various reflex-induced changes in SNA. As shown in Fig. 6, these studies determined that NMDA receptors in the LTF play a prominent role in mediating the baroreceptor reflex in terms of both synchronizing SNA to the cardiac cycle and mediating the inhibition of SNA during a pressor response (93). This was later confirmed at the level of single neurons in the LTF by elimination of their cardiac-related activity by the iontophoresis of an NMDA receptor antagonist onto their cell bodies (28). In contrast to the well-documented role of the CVLM in mediating the baroreceptor reflex in rodents (104, 105, 108), blockade of neither NMDA nor non-NMDA receptors in a wide region of the CVLM (both rostral and caudal to the obex) disrupted the reflex; in fact, blockade of non-NMDA receptors enhanced the cardiac-related power in SNA (93). Microinjection of muscimol into the LTF showed that this region plays a critical role in mediating the effects of vagal lung inflation afferents on SNA as well as the cardiovascular, but not respiratory, effects of the Bezold-Jarisch reflex (29, 98). Microinjection of either muscimol or a non-NMDA receptor antagonist into the LTF also eliminates the sympathoexcitatory response to activation of arterial chemoreceptors, but not the sympathoexcitatory effects of electrical stimulation of vagal, trigeminal, or sciatic afferents (96). Blockade of non-NMDA receptors in the RVLM markedly attenuates the sympathoexcitatory responses induced by electrical stimulation of visceral afferent fibers in either the inferior cardiac or splanchnic nerve (26). Neither blockade of excitatory amino acid receptors nor chemical inactivation of the CVLM, LTF, or nucleus of the solitary tract alters these sympathosympathetic reflexes.
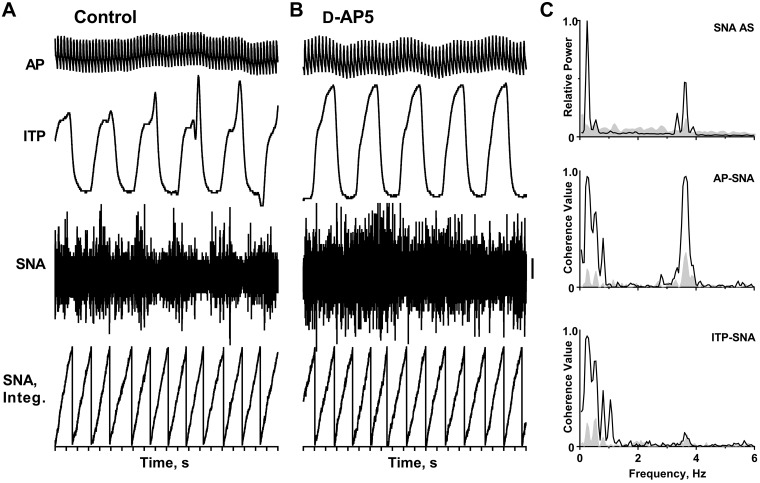
Does assessment of changes in rhythmic SNA provide more than a quantification of changes in the “amount” of activity in response to various manipulations? The data in Fig. 7 indicate that the answer is yes. These data show the changes in SNA following the bilateral microinjection of an NMDA receptor antagonist into the LTF; a recording of intratracheal pressure (ITP) was used as an index of respiration in this spontaneously breathing, urethane-anesthetized cat. An infusion of norepinephrine bitartrate (1–3 µg/min iv) was used to maintain mean blood pressure at ~110 mmHg throughout the experiment. There was a modest increase in heart rate (from 3.6 to 3.65 Hz); respiratory rate was unchanged at 0.25 Hz (15/min) in this experiment, as determined from AP and ITP autospectra (not shown). Based simply on assessment of the level of SNA by cumulative integration, as shown in Fig. 7, A and B, one would conclude that NMDA receptors in the LTF do not have a remarkable influence on SNA, since the average epoch duration is similar before and after bilateral NMDA receptor blockade in this region. However, frequency-domain analysis revealed that the microinjection of an NMDA receptor antagonist into the LTF nearly eliminated the respiratory- and cardiac-related rhythms in SNA. The revised conclusion from this analysis is that NMDA receptors in the LTF are needed for vagal lung inflation afferents and baroreceptor afferents to entrain slow waves in SNA to the respiratory and cardiac cycles, respectively. Similar data were reported in an earlier report (8). These data indicate that it is important to include an assessment of the frequency characteristics of SNA when studying the responses to various perturbations to the central or peripheral nervous system.
Fig. 7.
Effects of blockade of N-methyl-d-aspartate (NMDA) receptors in the medullary lateral tegmental field (LTF) on left inferior cardiac sympathetic nerve activity (SNA) of a spontaneously breathing, urethane-anesthetized cat. A and B: traces (top to bottom) show arterial pulse (AP), intratracheal pressure (ITP, as an index of respiration), SNA (preamplifier band pass = 30–3,000 Hz, 50-µV vertical calibration), and integrated SNA (cumulative integration) before (control) and after bilateral microinjection of d-2-amino-5-phosphonopentanoate (D-AP5). C: traces show autospectra (AS) of SNA and coherence functions relating SNA to the AP and ITP before (black line) and after (gray-shaded area) bilateral microinjection of D-AP5 into the LTF. Spectra are based on 35 20-s windows with 50% overlap and frequency resolution of 0.05 Hz per bin. They are shown on the same power scale before and after blockade of NMDA receptors. For spectral analysis, the sympathetic nerve recording was passed through a 50/60-Hz noise eliminator and further processed with a moving averager (50-ms time constant). A Digidata 1322A digitizer (Axon Instruments, Union City, CA) was used to acquire data.
Perspectives and Significance
At the start of this review, I mentioned that I entered the field of neural control of the cardiovascular system at a time when new technologies allowed researchers to better probe the brain to determine which areas are critical for maintaining or modulating SNA and ABP. Over the past nearly 50 years, autonomic neuroscientists have used primarily anesthetized animals to answer probing questions and to provide valuable information about the complexity of central autonomic networks. In the 21st century, there has been considerable interest in assessing the neural control of autonomic regulation in conscious animals in both health and disease (31, 32, 66, 81, 112, 113). Neural recordings of central sympathetic neurons in awake animals are needed to determine how central sympathetic circuits and SNA are modified by various behaviors (32). There is only one study reporting the responses of RVLM neurons with cardiac-related activity to an intervention (in this case, removal of vestibular inputs) in a conscious animal (31). The discovery of specific neuronal phenotypes of central neurons has promoted the use of optogenetic and pharmacogenetic technologies to define the influence of specific neuronal types in the control of SNA and ABP (66, 113). Advancements in telemetric methods for chronic recordings of SNA in conscious animals have enabled researchers to assess whether a change in SNA is temporally related to the development and/or progression of a cardiovascular disease (81). Wehrwein and Barman (112) reviewed several studies designed to determine if there is a correlation between the changes in SNA and ABP as hypertension progresses. There is a paucity of data to show explicitly that an increase in SNA precedes the development of hypertension. A factor not considered in these studies is the potential that the rise in ABP in these animal models of hypertension is caused by a change in the pattern (rhythmicity) of SNA. Perhaps in the future there will be a greater appreciation of the need to look not only for changes in the level of activity, but also for changes in the frequency characteristics of SNA.
Charkoudian and Wallin (42) refer to the sympathetic nervous system as the “ultimate integrator of cardiovascular function” and encourage researchers to interrogate ways by which the sympathetic nervous system integrates with brain pathways that regulate thermoregulation, exercise, and mental stress, which are often accompanied by changes in ABP. The time is ripe for researchers to take advantage of recent technological advancements and sophisticated data analysis methods to design hypothesis-driven, integrative studies using whole animals or human subjects that will answer probing questions about how changes in SNA (including changes in the pattern of SNA) influence an array of physiological and pathophysiological behaviors.
GRANTS
Studies from the author’s laboratory were funded by National Heart, Lung, and Blood Institute Grants HL-33266 (S. M. Barman) and HL-13187 (G. L. Gebber).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
S.M.B. prepared figures; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dr. Erica Wehrwein for reading a draft of this review and providing valuable comments.
This review is based on the Carl Ludwig Distinguished Lectureship of the American Physiological Society Neural Control and Autonomic Regulation Section presented at the 2019 Experimental Biology Meeting on 8 April 2019 in Orlando, FL.
REFERENCES
- 1.Accorsi-Mendonça D, da Silva MP, Souza GM, Lima-Silveira L, Karlen-Amarante M, Amorim MR, Almado CE, Moraes DJ, Machado BH. Pacemaking property of RVLM presympathetic neurons. Front Physiol 7: 424, 2016. doi: 10.3389/fphys.2016.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrian ED, Bronk DW, Phillips G. Discharges in mammalian sympathetic nerves. J Physiol 74: 115–133, 1932. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander RS. Tonic and reflex functions of medullary sympathetic cardiovascular centers. J Neurophysiol 9: 205–217, 1946. doi: 10.1152/jn.1946.9.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Ardell JL, Barman SM, Gebber GL. Sympathetic nerve discharge in chronic spinal cat. Am J Physiol Heart Circ Physiol 243: H463–H470, 1982. doi: 10.1152/ajpheart.1982.243.3.H463. [DOI] [PubMed] [Google Scholar]
- 5.Amendt K, Czachurski J, Dembowsky K, Seller H. Bulbospinal projections to the intermediolateral cell column: a neuroanatomical study. J Auton Nerv Syst 1: 103–117, 1979. doi: 10.1016/0165-1838(79)90009-2. [DOI] [PubMed] [Google Scholar]
- 6.Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir Physiol Neurobiol 174: 135–145, 2010. doi: 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainton CR, Richter DW, Seller H, Ballantyne D, Klein JP. Respiratory modulation of sympathetic activity. J Auton Nerv Syst 12: 77–90, 1985. doi: 10.1016/0165-1838(85)90041-4. [DOI] [PubMed] [Google Scholar]
- 8.Barman SM. What can we learn about neural control of the cardiovascular system by studying rhythms in sympathetic nerve activity? Int J Psychophysiol 103: 69–78, 2016. doi: 10.1016/j.ijpsycho.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barman SM, Gebber GL. Basis for synchronization of sympathetic and phrenic nerve discharges. Am J Physiol 231: 1601–1607, 1976. doi: 10.1152/ajplegacy.1976.231.5.1601. [DOI] [PubMed] [Google Scholar]
- 10.Barman SM, Gebber GL. Brain stem neuronal types with activity patterns related to sympathetic nerve discharge. Am J Physiol Regul Integr Comp Physiol 240: R335–R347, 1981. doi: 10.1152/ajpregu.1981.240.5.R335. [DOI] [PubMed] [Google Scholar]
- 11.Barman SM, Gebber GL. Sequence of activation of ventrolateral and dorsal medullary sympathetic neurons. Am J Physiol Regul Integr Comp Physiol 245: R438–R447, 1983. doi: 10.1152/ajpregu.1983.245.3.R438. [DOI] [PubMed] [Google Scholar]
- 12.Barman SM, Gebber GL. Axonal projection patterns of ventrolateral medullospinal sympathoexcitatory neurons. J Neurophysiol 53: 1551–1566, 1985. doi: 10.1152/jn.1985.53.6.1551. [DOI] [PubMed] [Google Scholar]
- 13.Barman SM, Gebber GL. Lateral tegmental field neurons of cat medulla: a source of basal activity of ventrolateral medullospinal sympathoexcitatory neurons. J Neurophysiol 57: 1410–1424, 1987. doi: 10.1152/jn.1987.57.5.1410. [DOI] [PubMed] [Google Scholar]
- 14.Barman SM, Gebber GL. Lateral tegmental field neurons of cat medulla: a source of basal activity of raphespinal sympathoinhibitory neurons. J Neurophysiol 61: 1011–1024, 1989. doi: 10.1152/jn.1989.61.5.1011. [DOI] [PubMed] [Google Scholar]
- 15.Barman SM, Gebber GL. Rostral ventrolateral medullary and caudal medullary raphe neurons with activity correlated to the 10-Hz rhythm in sympathetic nerve discharge. J Neurophysiol 68: 1535–1547, 1992. doi: 10.1152/jn.1992.68.5.1535. [DOI] [PubMed] [Google Scholar]
- 16.Barman SM, Gebber GL. Lateral tegmental field neurons play a permissive role in governing the 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol Regul Integr Comp Physiol 265: R1006–R1013, 1993. doi: 10.1152/ajpregu.1993.265.5.R1006. [DOI] [PubMed] [Google Scholar]
- 17.Barman SM, Gebber GL. Subgroups of rostral ventrolateral medullary and caudal medullary raphe neurons based on patterns of relationship to sympathetic nerve discharge and axonal projections. J Neurophysiol 77: 65–75, 1997. doi: 10.1152/jn.1997.77.1.65. [DOI] [PubMed] [Google Scholar]
- 18.Barman SM, Gebber GL. Classification of caudal ventrolateral pontine neurons with sympathetic nerve-related activity. J Neurophysiol 80: 2433–2445, 1998. doi: 10.1152/jn.1998.80.5.2433. [DOI] [PubMed] [Google Scholar]
- 19.Barman SM, Gebber GL. “Rapid” rhythmic discharges of sympathetic nerves: sources, mechanisms of generation, and physiological relevance. J Biol Rhythms 15: 365–379, 2000. doi: 10.1177/074873000129001468. [DOI] [PubMed] [Google Scholar]
- 20.Barman SM, Gebber GL. Role of ventrolateral medulla in generating the 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol Regul Integr Comp Physiol 293: R223–R233, 2007. doi: 10.1152/ajpregu.00085.2007. [DOI] [PubMed] [Google Scholar]
- 21.Barman SM, Gebber GL. The posterior vermis of the cerebellum selectively inhibits 10-Hz sympathetic nerve discharge in anesthetized cats. Am J Physiol Regul Integr Comp Physiol 297: R210–R217, 2009. doi: 10.1152/ajpregu.90989.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barman SM, Gebber GL, Kitchens H. Rostral dorsolateral pontine neurons with sympathetic nerve-related activity. Am J Physiol Heart Circ Physiol 276: H401–H412, 1999. doi: 10.1152/ajpheart.1999.276.2.H401. [DOI] [PubMed] [Google Scholar]
- 23.Barman SM, Gebber GL, Orer HS. Medullary lateral tegmental field: an important source of basal sympathetic nerve discharge in the cat. Am J Physiol Regul Integr Comp Physiol 278: R995–R1004, 2000. doi: 10.1152/ajpregu.2000.278.4.R995. [DOI] [PubMed] [Google Scholar]
- 24.Barman SM, Gebber GL, Zhong S. The 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol Regul Integr Comp Physiol 262: R1006–R1014, 1992. doi: 10.1152/ajpregu.1992.262.6.R1006. [DOI] [PubMed] [Google Scholar]
- 25.Barman SM, Kitchens HL, Leckow AB, Gebber GL. Pontine neurons are elements of the network responsible for the 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol Heart Circ Physiol 273: H1909–H1919, 1997. doi: 10.1152/ajpheart.1997.273.4.H1909. [DOI] [PubMed] [Google Scholar]
- 26.Barman SM, Orer HS. Rostral ventrolateral medullary but not medullary lateral tegmental field neurons mediate sympatho-sympathetic reflexes in cats. Am J Physiol Regul Integr Comp Physiol 299: R1269–R1278, 2010. doi: 10.1152/ajpregu.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barman SM, Orer HS, Gebber GL. Caudal ventrolateral medullary neurons are elements of the network responsible for the 10-Hz rhythm in sympathetic nerve discharge. J Neurophysiol 72: 106–120, 1994. doi: 10.1152/jn.1994.72.1.106. [DOI] [PubMed] [Google Scholar]
- 28.Barman SM, Orer HS, Gebber GL. Differential effects of an NMDA and a non-NMDA receptor antagonist on medullary lateral tegmental field neurons. Am J Physiol Regul Integr Comp Physiol 282: R100–R113, 2002. doi: 10.1152/ajpregu.2002.282.1.R100. [DOI] [PubMed] [Google Scholar]
- 29.Barman SM, Orer HS, Gebber GL. Role of medullary excitatory amino acid receptors in mediating the 10-Hz rhythm in sympathetic nerve discharge of cats. Brain Res 1049: 249–253, 2005. doi: 10.1016/j.brainres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Barman SM, Phillips SW, Gebber GL. Medullary lateral tegmental field mediates the cardiovascular but not respiratory component of the Bezold-Jarisch reflex in the cat. Am J Physiol Regul Integr Comp Physiol 289: R1693–R1702, 2005. doi: 10.1152/ajpregu.00406.2005. [DOI] [PubMed] [Google Scholar]
- 31.Barman SM, Sugiyama Y, Suzuki T, Cotter LA, DeStefino VJ, Reighard DA, Cass SP, Yates BJ. Rhythmic activity of neurons in the rostral ventrolateral medulla of conscious cats: effect of removal of vestibular inputs. Am J Physiol Regul Integr Comp Physiol 301: R937–R946, 2011. doi: 10.1152/ajpregu.00265.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barman SM, Yates BJ. Deciphering the neural control of sympathetic nerve activity: status report and directions for future research. Front Neurosci 11: 730, 2017. doi: 10.3389/fnins.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benarroch EE. Central Autonomic Network: Functional Organization and Clinical Correlations. Armonk, NY: Futura Publishing Company, 1997. [Google Scholar]
- 34.Bendat JS, Piersol AG. Random Data. Analysis and Measurement Procedures (2nd ed.). New York: Wiley, 1986. [Google Scholar]
- 35.Birznieks I, Boonstra TW, Macefield VG. Modulation of human muscle spindle discharge by arterial pulsations—functional effects and consequences. PLoS One 7: e35091, 2012. doi: 10.1371/journal.pone.0035091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biscoe TJ, Sampson SR. Responses of cells in the brain stem of the cat to stimulation of the sinus, glossopharyngeal, aortic and superior laryngeal nerves. J Physiol 209: 359–373, 1970. doi: 10.1113/jphysiol.1970.sp009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brazier MAB, Walter DO. Frequency and correlation analysis. In: Handbook of Electroencephalography and Clinical Neurophysiology, edited by Remond A. Amsterdam: Elsevier, 1973, vol. 5, part A. [Google Scholar]
- 38.Brown DL, Guyenet PG. Cardiovascular neurons of brain stem with projections to spinal cord. Am J Physiol Regul Integr Comp Physiol 247: R1009–R1016, 1984. doi: 10.1152/ajpregu.1984.247.6.R1009. [DOI] [PubMed] [Google Scholar]
- 39.Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985. doi: 10.1161/01.RES.56.3.359. [DOI] [PubMed] [Google Scholar]
- 40.Buzsáki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci 14: 345–367, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang HS, Staras K, Smith JE, Gilbey MP. Sympathetic neuronal oscillators are capable of dynamic synchronization. J Neurosci 19: 3183–3197, 1999. doi: 10.1523/JNEUROSCI.19-08-03183.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. doi: 10.1002/cphy.c130038. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MI, Gootman PM. Periodicities in efferent discharge of splanchnic nerve of the cat. Am J Physiol 218: 1092–1101, 1970. doi: 10.1152/ajplegacy.1970.218.4.1092. [DOI] [PubMed] [Google Scholar]
- 44.Cohen MI, Gootman PM, Feldman JL. Inhibition of sympathetic discharge by lung inflation. In: Arterial Baroreceptors and Hypertension, edited by Sleight P. New York: Oxford University Press, 1980, p. 161–167. [Google Scholar]
- 45.Coote JH. Landmarks in understanding the central nervous control of the cardiovascular system. Exp Physiol 92: 3–18, 2007. doi: 10.1113/expphysiol.2006.035378. [DOI] [PubMed] [Google Scholar]
- 46.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 47.Dampney RAL, Goodchild AK, Robertson LG, Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res 249: 223–235, 1982. doi: 10.1016/0006-8993(82)90056-7. [DOI] [PubMed] [Google Scholar]
- 48.Dampney RAL, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 49.Davern PJ. A role for the lateral parabrachial nucleus in cardiovascular function and fluid homeostasis. Front Physiol 5: 436, 2014. doi: 10.3389/fphys.2014.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dempesy CW, Richardson DE, Fontana CJ. Sympathetically-mediated cardiovascular responses induced by neurochemical microinjection in the brainstem lateral tegmental field of cat. Brain Res 704: 141–144, 1995. doi: 10.1016/0006-8993(95)01249-4. [DOI] [PubMed] [Google Scholar]
- 51.Deuchars SA, Morrison SF, Gilbey MP. Medullary-evoked EPSPs in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol 487: 453–463, 1995. doi: 10.1113/jphysiol.1995.sp020892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dutilleul P. Rhythms and autocorrelation analysis. Biol Rhythm Res 26: 173–193, 1995. doi: 10.1080/09291019509360334. [DOI] [Google Scholar]
- 53.Fye WB. Carl Ludwig and the Leipzig Physiological Institute: A factory of new knowledge. Circulation 74: 920–928, 1986. doi: 10.1161/01.CIR.74.5.920. [DOI] [PubMed] [Google Scholar]
- 54.Gebber GL. Basis for phase relations between baroreceptor and sympathetic nervous discharge. Am J Physiol 230: 263–270, 1976. doi: 10.1152/ajplegacy.1976.230.2.263. [DOI] [PubMed] [Google Scholar]
- 55.Gebber GL, Barman SM. Sympathetic-related activity of brain stem neurons in baroreceptor-denervated cats. Am J Physiol Regul Integr Comp Physiol 240: R348–R355, 1981. doi: 10.1152/ajpregu.1981.240.5.R348. [DOI] [PubMed] [Google Scholar]
- 56.Gebber GL, Barman SM. Lateral tegmental field neurons of cat medulla: a potential source of basal sympathetic nerve discharge. J Neurophysiol 54: 1498–1512, 1985. doi: 10.1152/jn.1985.54.6.1498. [DOI] [PubMed] [Google Scholar]
- 57.Gebber GL, Barman SM, Kocsis B. Coherence of medullary unit activity and sympathetic nerve discharge. Am J Physiol Regul Integr Comp Physiol 259: R561–R571, 1990. doi: 10.1152/ajpregu.1990.259.3.R561. [DOI] [PubMed] [Google Scholar]
- 58.Gebber GL, Zhong S, Barman SM. Synchronization of cardiac-related discharges of sympathetic nerves with inputs from widely separated spinal segments. Am J Physiol Regul Integr Comp Physiol 268: R1472–R1483, 1995. doi: 10.1152/ajpregu.1995.268.6.R1472. [DOI] [PubMed] [Google Scholar]
- 59.Gebber GL, Zhong S, Barman SM, Paitel Y, Orer HS. Differential relationships among the 10-Hz rhythmic discharges of sympathetic nerves with different targets. Am J Physiol Regul Integr Comp Physiol 267: R387–R399, 1994. doi: 10.1152/ajpregu.1994.267.2.R387. [DOI] [PubMed] [Google Scholar]
- 60.Goldbeter A. Biological rhythms: clocks for all times. Curr Biol 18: R751–R753, 2008. doi: 10.1016/j.cub.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 61.Goodchild AK, Dampney RAL. A vasopressor cell group in the rostral dorsomedial medulla of the rabbit. Brain Res 360: 24–32, 1985. doi: 10.1016/0006-8993(85)91216-8. [DOI] [PubMed] [Google Scholar]
- 62.Gootman PM, Cohen MI, Piercey MP, Wolotsky P. A search for medullary neurons with activity patterns similar to those in sympathetic nerves. Brain Res 87: 395–406, 1975. doi: 10.1016/0006-8993(75)90436-9. [DOI] [PubMed] [Google Scholar]
- 63.Green JH, Heffron PF. Studies upon the relationship between baroreceptor and sympathetic activity. Q J Exp Physiol Cogn Med Sci 53: 23–32, 1968. doi: 10.1113/expphysiol.1968.sp001942. [DOI] [PubMed] [Google Scholar]
- 64.Guild SJ, Barrett CJ, McBryde FD, Van Vliet BN, Head GA, Burke SL, Malpas SC. Quantifying sympathetic nerve activity: problems, pitfalls and the need for standardization. Exp Physiol 95: 41–50, 2010. doi: 10.1113/expphysiol.2008.046300. [DOI] [PubMed] [Google Scholar]
- 65.Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. Oxford: Oxford University Press, 1990, p. 145–167. [Google Scholar]
- 66.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 67.Hagbarth K-E, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968. doi: 10.1111/j.1365-201X.1968.tb10904.x. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto M, Kuwahara M, Tsubone H, Sugano S. Diurnal variation of autonomic nervous activity in the rat: investigation by power spectral analysis of heart rate variability. J Electrocardiol 32: 167–171, 1999. doi: 10.1016/S0022-0736(99)90095-X. [DOI] [PubMed] [Google Scholar]
- 69.Huangfu DH, Koshiya N, Guyenet PG. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Physiol Regul Integr Comp Physiol 261: R393–R402, 1991. doi: 10.1152/ajpregu.1991.261.2.R393. [DOI] [PubMed] [Google Scholar]
- 70.Humphrey DR. Neuronal activity in the medulla oblongata of the cat evoked by stimulation of the carotid sinus nerve. In: Baroreceptors and Hypertension, edited by Kedzi P. Oxford: Pergamon, 1967, p. 131–167. [Google Scholar]
- 71.Janssen BJ, Malpas SC, Burke SL, Head GA. Frequency-dependent modulation of renal blood flow by renal nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 273: R597–R608, 1997. doi: 10.1152/ajpregu.1997.273.2.R597. [DOI] [PubMed] [Google Scholar]
- 72.Jenkins GM, Watts DG. Spectral Analysis and Its Applications. San Francisco: Holden-Day, 1968. [Google Scholar]
- 73.Johnson CD, Gilbey MP. On the dominant rhythm in the discharges of single postganglionic sympathetic neurones innervating the rat tail artery. J Physiol 497: 241–259, 1996. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kenney MJ, Weiss ML, Mendes T, Wang Y, Fels RJ. Role of paraventricular nucleus in regulation of sympathetic nerve frequency components. Am J Physiol Heart Circ Physiol 284: H1710–H1720, 2003. doi: 10.1152/ajpheart.00673.2002. [DOI] [PubMed] [Google Scholar]
- 75.Kenney MJ, Weiss ML, Patel KP, Wang Y, Fels RJ. Paraventricular nucleus bicuculline alters frequency components of sympathetic nerve discharge bursts. Am J Physiol Heart Circ Physiol 281: H1233–H1241, 2001. doi: 10.1152/ajpheart.2001.281.3.H1233. [DOI] [PubMed] [Google Scholar]
- 76.Kishi E, Ootsuka Y, Rong W, Terui N. Functional significance of the 10 Hz rhythmic discharges in sympathetic nerves. Clin Exp Pharmacol Physiol 25: 464–467, 1998. doi: 10.1111/j.1440-1681.1998.tb02236.x. [DOI] [PubMed] [Google Scholar]
- 77.Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- 78.Lipski J, Kanjhan R, Kruszewska B, Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study “in vivo”. J Physiol 490: 729–744, 1996. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lipski J, McAllen RM, Spyer KM. The sinus nerve and baroreceptor input to the medulla of the cat. J Physiol 251: 61–78, 1975. doi: 10.1113/jphysiol.1975.sp011081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malpas SC. The rhythmicity of sympathetic nerve activity. Prog Neurobiol 56: 65–96, 1998. doi: 10.1016/S0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 81.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 82.Malpas SC, Ninomiya I. Fundamental rhythm of renal sympathetic nerve activity in anesthetized cats. J Auton Nerv Syst 37: 11–18, 1992. doi: 10.1016/0165-1838(92)90140-C. [DOI] [PubMed] [Google Scholar]
- 83.Marchenko V, Sapru HN. Cardiovascular responses to chemical stimulation of the lateral tegmental field and adjacent medullary reticular formation in the rat. Brain Res 977: 247–260, 2003. doi: 10.1016/S0006-8993(03)02719-7. [DOI] [PubMed] [Google Scholar]
- 84.Marina N, Taheri M, Gilbey MP. Generation of a physiological sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol 571: 441–450, 2006. doi: 10.1113/jphysiol.2005.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller WL, Sigvardt KA. Spectral analysis of oscillatory neural circuits. J Neurosci Methods 80: 113–128, 1998. doi: 10.1016/S0165-0270(97)00185-4. [DOI] [PubMed] [Google Scholar]
- 86.Miura M, Onai T, Takayama K. Projections of upper structure to the spinal cardioacceleratory center in cats: an HRP study using a new microinjection method. J Auton Nerv Syst 7: 119–139, 1983. doi: 10.1016/0165-1838(83)90041-3. [DOI] [PubMed] [Google Scholar]
- 87.Morrison SF, Gebber GL. Axonal branching patterns and funicular trajectories of raphespinal sympathoinhibitory neurons. J Neurophysiol 53: 759–772, 1985. doi: 10.1152/jn.1985.53.3.759. [DOI] [PubMed] [Google Scholar]
- 88.Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J Neurosci 8: 1286–1301, 1988. doi: 10.1523/JNEUROSCI.08-04-01286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Somers VK. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension 39: 168–172, 2002. doi: 10.1161/hy1201.097302. [DOI] [PubMed] [Google Scholar]
- 90.Ninomiya I, Akiyama T, Nishiura N. Mechanism of cardiac-related synchronized cardiac sympathetic nerve activity in awake cats. Am J Physiol Regul Integr Comp Physiol 259: R499–R506, 1990. doi: 10.1152/ajpregu.1990.259.3.R499. [DOI] [PubMed] [Google Scholar]
- 91.Ootsuka Y, Blessing WW. Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit. Am J Physiol Regul Integr Comp Physiol 288: R909–R918, 2005. doi: 10.1152/ajpregu.00564.2004. [DOI] [PubMed] [Google Scholar]
- 92.Orer HS, Barman SM, Gebber GL. Effects on sympathetic activity of 8-OHDPAT and clonidine in cat medullary lateral tegmental field. Am J Physiol Heart Circ Physiol 281: H613–H622, 2001. doi: 10.1152/ajpheart.2001.281.2.H613. [DOI] [PubMed] [Google Scholar]
- 93.Orer HS, Barman SM, Gebber GL, Sykes SM. Medullary lateral tegmental field: an important synaptic relay in the baroreceptor reflex pathway of the cat. Am J Physiol Regul Integr Comp Physiol 277: R1462–R1475, 1999. doi: 10.1152/ajpregu.1999.277.5.R1462. [DOI] [PubMed] [Google Scholar]
- 94.Orer HS, Barman SM, Zhong S, Gebber GL. A modulatory role of central cholinergic transmission in control of the 10-Hz rhythm in sympathetic nerve discharge. Brain Res 661: 283–288, 1994. doi: 10.1016/0006-8993(94)91205-X. [DOI] [PubMed] [Google Scholar]
- 95.Orer HS, Gebber GL, Barman SM. Role of serotonergic input to the ventrolateral medulla in expression of the 10-Hz sympathetic nerve rhythm. Am J Physiol Regul Integr Comp Physiol 294: R1435–R1444, 2008. doi: 10.1152/ajpregu.00012.2008. [DOI] [PubMed] [Google Scholar]
- 96.Orer HS, Gebber GL, Phillips SW, Barman SM. Role of the medullary lateral tegmental field in reflex-mediated sympathoexcitation in cats. Am J Physiol Regul Integr Comp Physiol 286: R451–R464, 2004. doi: 10.1152/ajpregu.00569.2003. [DOI] [PubMed] [Google Scholar]
- 97.Orer HS, Zhong S, Barman SM, Gebber GL. Central catecholaminergic neurons are involved in expression of the 10-Hz rhythm in SND. Am J Physiol Regul Integr Comp Physiol 270: R333–R341, 1996. doi: 10.1152/ajpregu.1996.270.2.R333. [DOI] [PubMed] [Google Scholar]
- 98.Phillips SW, Gebber GL, Barman SM. Medullary lateral tegmental field: control of respiratory rate and vagal lung inflation afferent influences on sympathetic nerve discharge. Am J Physiol Regul Integr Comp Physiol 288: R1396–R1410, 2005. doi: 10.1152/ajpregu.00632.2004. [DOI] [PubMed] [Google Scholar]
- 99.Pinsker HM, Ayers J. Neuronal oscillators. In: The Clinical Neurosciences, edited by Willis WD. New York: Churchill Livingstone, 1983, vol. 5, chapt. 9, p. 203–246. [Google Scholar]
- 100.Rapp PE. Why are so many biological systems periodic? Prog Neurobiol 29: 261–273, 1987. doi: 10.1016/0301-0082(87)90023-2. [DOI] [PubMed] [Google Scholar]
- 101.Rau H, Pauli P, Brody S, Elbert T, Birbaumer N. Baroreceptor stimulation alters cortical activity. Psychophysiology 30: 322–325, 1993. doi: 10.1111/j.1469-8986.1993.tb03359.x. [DOI] [PubMed] [Google Scholar]
- 102.Reinberg A, Ashkenazi I. Concepts in human biological rhythms. Dialogues Clin Neurosci 5: 327–342, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci 4: 474–494, 1984. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 105.Schreihofer AM, Sved AF. The ventrolateral medulla and sympathetic regulation of arterial pressure. In: Central Regulation of Autonomic Functions (2nd ed.), edited by Llewellyn-Smith IJ, Verberne AJM. New York: Oxford University Press, 2011, p. 78–97. [Google Scholar]
- 106.Seller H. Carl Ludwig and the localization of the medullary vasomotor center: old and new concepts of the generation of sympathetic tone. Pflugers Arch 432, Suppl: R94–R98, 1996. [PubMed] [Google Scholar]
- 107.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 491: 274–296, 1989. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 108.Sved AF, Ito S, Madden CJ. Baroreflex dependent and independent roles of the caudal ventrolateral medulla in cardiovascular regulation. Brain Res Bull 51: 129–133, 2000. doi: 10.1016/S0361-9230(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 109.Taylor DG, Gebber GL. Baroreceptor mechanisms controlling sympathetic nervous rhythms of central origin. Am J Physiol 228: 1002–1003, 1975. doi: 10.1152/ajplegacy.1975.228.4.1002. [DOI] [PubMed] [Google Scholar]
- 110.Vallbo ÅB, Hagbarth K-E, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 96: 1262–1269, 2004. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- 111.Vetulli HM, Elizari MV, Naccarelli GV, Gonzalez MD. Cardiac automaticity: basic concepts and clinical observations. J Interv Card Electrophysiol 52: 263–270, 2018. doi: 10.1007/s10840-018-0423-2. [DOI] [PubMed] [Google Scholar]
- 112.Wehrwein E, Barman SM. Highlights in basic autonomic neurosciences: Is an increase in sympathetic nerve activity involved in the development and maintenance of hypertension? Auton Neurosci 180: 1–4, 2014. doi: 10.1016/j.autneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 113.Wenker IC, Abe C, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Blood pressure regulation by the rostral ventrolateral medulla in conscious rats: effects of hypoxia, hypercapnia, baroreceptor denervation, and anesthesia. J Neurosci 37: 4565–4583, 2017. doi: 10.1523/JNEUROSCI.3922-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zagon A, Smith AD. Monosynaptic projections from the rostral ventrolateral medulla oblongata to identified sympathetic preganglionic neurons. Neuroscience 54: 729–743, 1993. doi: 10.1016/0306-4522(93)90243-9. [DOI] [PubMed] [Google Scholar]
- 115.Zhong S, Huang Z-S, Gebber GL, Barman SM. The 10-Hz sympathetic rhythm is dependent on raphe and rostral ventrolateral medullary neurons. Am J Physiol Regul Integr Comp Physiol 264: R857–R866, 1993. doi: 10.1152/ajpregu.1993.264.5.R857. [DOI] [PubMed] [Google Scholar]
- 116.Zhong S, Zhou S-Y, Gebber GL, Barman SM. Coupled oscillators account for the slow rhythms in sympathetic nerve discharge and phrenic nerve activity. Am J Physiol Regul Integr Comp Physiol 272: R1314–R1324, 1997. doi: 10.1152/ajpregu.1997.272.4.R1314. [DOI] [PubMed] [Google Scholar]