Abstract
The application of blood flow restriction (BFR) during resistance exercise is increasingly recognized for its ability to improve rehabilitation and for its effectiveness in increasing muscle hypertrophy and strength among healthy populations. However, direct comparison of the skeletal muscle adaptations to low-load resistance exercise (LL-RE) and low-load BFR resistance exercise (LL-BFR) performed to task failure is lacking. Using a within-subject design, we examined whole muscle group and skeletal muscle adaptations to 6 wk of LL-RE and LL-BFR training to repetition failure. Muscle strength and size outcomes were similar for both types of training, despite ~33% lower total exercise volume (load × repetition) with LL-BFR than LL-RE (28,544 ± 1,771 vs. 18,949 ± 1,541 kg, P = 0.004). After training, only LL-BFR improved the average power output throughout the midportion of a voluntary muscle endurance task. Specifically, LL-BFR training sustained an 18% greater power output from baseline and resulted in a greater change from baseline than LL-RE (19 ± 3 vs. 3 ± 4 W, P = 0.008). This improvement occurred despite histological analysis revealing similar increases in capillary content of type I muscle fibers following LL-RE and LL-BFR training, which was primarily driven by increased capillary contacts (4.53 ± 0.23 before training vs. 5.33 ± 0.27 and 5.17 ± 0.25 after LL-RE and LL-BFR, respectively, both P < 0.05). Moreover, maximally supported mitochondrial respiratory capacity increased only in the LL-RE leg by 30% from baseline (P = 0.006). Overall, low-load resistance training increased indexes of muscle oxidative capacity and strength, which were not further augmented with the application of BFR. However, performance on a muscle endurance test was improved following BFR training.
Keywords: BFR resistance exercise, capillary, high-resolution respirometry, low-load, mitochondria, repetition failure
INTRODUCTION
Traditional resistance training is well established as an exercise modality to increase muscle strength and hypertrophy. The use of blood flow restriction (BFR) during training has garnered substantial interest among athletes, clinicians, and sports scientists as an effective method to augment training adaptations when low-intensity resistance exercise is used (24, 53). BFR training typically involves the proximal application of a tourniquet above the contracting muscle, which reduces arterial inflow and prevents venous outflow during low-load [<40% 1-repetition maximum (1-RM)] resistance exercise (LL-RE) (40). Recent reports using this technique demonstrate similar increases in muscle size, but not strength, compared with free blood flow, high-load resistance exercise (>65% 1-RM) (30). Low-load BFR exercise (LL-BFR) has been proposed to increase the metabolic stimulus within the exercising muscle, leading to augmentation of muscle protein synthesis (15, 16, 20, 38, 52), alterations in gene expression (8, 12, 29, 32), and changes in muscle fiber size, damage, and repair (7, 36, 37, 56, 58), which are greater than and/or similar to volume-matched LL-RE or high-load controls, respectively. In general, the previous literature has indicated that BFR positively influences skeletal muscle remodeling to a greater extent when LL-RE is volume-matched to LL-BFR; however, an increase in muscle fiber recruitment and/or fatigue is expected to occur earlier with the application of BFR during resistance exercise than in the LL-RE control condition (27, 57). Since performing resistance exercise to task failure (irrespective of load) appears to be an important stimulus for muscle adaptations in the free blood flow condition when equal exercise sets are performed (23, 49), volume-matched studies may attenuate potential training-induced skeletal muscle remodeling. Moreover, the role that blood flow manipulation may play in muscle adaptations is difficult to discern by comparing LL-BFR with high-load resistance exercise.
A limited amount of work has investigated the muscle adaptations of BFR training performed to task failure using high- or low-load controls. Similar improvements in the number of repetitions performed during a muscle endurance test (MET) have been demonstrated following LL-RE and LL-BFR training to task failure (10, 54). Likewise, after 6 wk of upper-body LL-BFR and LL-RE training using a within-subject design (11), similar increases in muscle strength and size occur despite different exercise volumes (load × repetitions), leading to muscle fatigue. Three other studies have directly compared LL-BFR with high-load protocols and found similar outcomes in muscle fiber cross-sectional area (CSA), mitochondrial protein content and function, and subcellular protein synthesis between both groups (9, 19, 52). However, in the absence of a group performing LL-RE to task failure, the training adaptations at the skeletal muscle level that occur as a direct result of BFR remain unknown.
The mechanism by which BFR influences training adaptations remains poorly understood, although recent reviews (45, 50) and investigations (12, 25) have suggested that the reduced arterial blood flow (i.e., increasing tissue hypoxia) and increased reactive oxygen species (ROS) production could be mechanisms that facilitate adaptations related to enhancement of skeletal muscle oxidative capacity. Interestingly, we have shown a decrease in mitochondrial ROS emission following a single bout of LL-BFR compared with LL-RE when both are performed to task failure (43). Recent evidence (34, 41, 44) suggests that ROS-mediated signaling may contribute to a greater muscle oxidative phenotype following aerobic exercise training; however, it is unknown whether this acute reduction in ROS following LL-BFR has physiological consequences related to skeletal muscle oxidative capacity adaptations compared with LL-RE. Therefore, the aims of this study were to examine 1) muscle strength and endurance performance, 2) skeletal muscle microvascular and morphological properties, and 3) mitochondrial protein content and function after 6 wk of lower-body training with LL-RE or LL-BFR performed to task failure. It was hypothesized that both types of training would increase all outcomes relative to baseline, and we explored whether the application of BFR differentially altered indexes of skeletal muscle oxidative capacity (i.e., microvascular and mitochondrial properties).
METHODS
Subjects.
Ten healthy, young men {mean [95% confidence interval (CI)]: 24 (22, 27) yr, 1.77 (175, 179) m, 78 (72, 85) kg} who previously participated in an investigation of a single bout of LL-RE and LL-BFR (43) consented to perform 6 wk of training. Before enrollment, participants completed the Physical Activity Readiness Questionnaire (PAR-Q+), were informed of the risks involved, and provided written informed consent. Inclusion criteria consisted of the absence of major injuries in the past 6 mo, the absence of musculoskeletal disease, no previous lower-body resistance training experience (≥1 day/wk) for ≥6 mo, and abstinence from a periodization program for ≥1 mo before study commencement. The study was reviewed and approved by the University of Guelph research ethics board (REB no. 17-12-005) in accordance with the Declaration of Helsinki.
Study design.
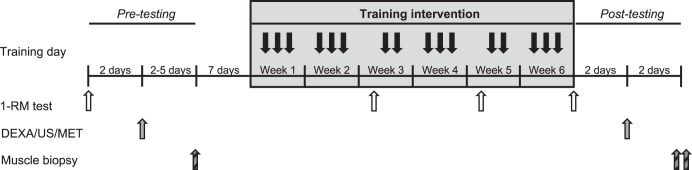
We employed a within-subject unilateral model to mitigate the role of interindividual variability in skeletal muscle adaptations, as previously discussed (31). A schematic representation of the study design is provided in Fig. 1. Each leg was randomly assigned, with matching for dominance, to perform LL-RE and LL-BFR three times per week for 6 wk. Strength testing was performed at baseline, at the start of weeks 3 and 5, and following 6 wk of training. Muscle biopsies were taken before commencement of the 6 wk of training and after all postintervention testing (i.e., 5 days following the last training intervention). Immediately following each training session, participants consumed ~25 g of whey protein (~2.5 g leucine/serving; Canadian Protein, Windsor, ON, Canada) to standardize postexercise nutrition. Participants were instructed to avoid other lower-body aerobic or resistance exercises throughout the study. Every participant completed all training sessions. During data acquisition and analysis, investigators were not blinded to experimental conditions. Participants abstained from tobacco/caffeine for ≥12 h and alcohol for ≥24 h before all testing sessions.
Fig. 1.
Schematic representation of the study design. DEXA, dual-energy X-ray absorptiometry; US, ultrasound; MET, muscle endurance test; RM, repetition maximum.
Resistance training protocol.
With each leg, participants performed three sets of single-leg squats in a robotically resisted Smith machine (1080 Quantum, 1080 Motion, Lidingö, Sweden) at a load corresponding to 30% 1-RM to task failure, with rest periods set at 100 s, for 6 wk. The use of this robotically resisted machine (1080 Quantum) is described in detail elsewhere (59). Biweekly 1-RM testing on each leg was considered a training day that week. Training order was randomized, such that half of the participants performed LL-RE followed by LL-BFR each day, and vice versa. Participants rested for 5–10 min between training bouts on each leg. Repetitions were performed at a 3.5-s duty cycle (1.75-s eccentric and 1.75-s concentric contraction, with pace set to an audible signal), and if either leg performed >30 repetitions in set 1, the load was increased by 2 kg for the subsequent training day on that leg only. An 11-cm-wide tourniquet was placed as high as possible (above biopsy sites) on the upper thigh of the LL-BFR leg, and the pressure was set corresponding to 60–70% of the lowest effective occlusive pressure (LOP) in the seated position. The LOP is defined as the lowest pressure required to occlude arterial blood flow and was determined using the personalized tourniquet system (Delfi, Vancouver, BC, Canada). The tourniquet remained inflated until completion of set 3 during LL-BFR. LOP was determined on each training day, and no differences were observed in pressure applied between the first and the last training day (153 ± 7 and 156 ± 6 mmHg, P = 0.5).
Muscle biopsies.
After baseline testing, a single resting muscle biopsy was obtained from the vastus lateralis. Baseline samples were randomized, such that 50% of the biopsies were from the nondominant and dominant legs, respectively. To reduce participant strain, we obtained only a single resting biopsy under the assumption of minimal biochemical/histological differences between legs, as previously discussed (31). After the 6 wk of training, a muscle biopsy was obtained from each leg within 5 days of the last training session. Biopsies were performed under local anesthesia (2% lidocaine with epinephrine) using the Bergström technique. Samples were freed from blood and/or connective tissue, and a section of each sample was quickly placed into ice-cold preservation buffer [BIOPS; in mM: 50 MES, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 dithiothreitol, 20 taurine, 5.77 ATP, 15 PCr, and 6.56 MgCl2·H2O (pH 7.1)] for high-resolution respirometry or embedded in optimal cutting temperature (OCT) medium in isopentane cooled by liquid nitrogen for future immunohistochemical analysis. The remainder of each sample was flash-frozen in liquid nitrogen. Subjects were asked to refrain from lower-body exercise for ≥48 h and food for 12 h before each sampling.
Strength testing.
The 1-RM testing was performed using the robotically resisted Smith machine according to the methodology described previously (43). Pretesting was undertaken with participants refraining from lower-body exercise for ≥72 h before, while, during, and after the 6 wk of training was done with at least 24 h of rest.
Body composition and ultrasound for quadriceps muscle thickness.
Participants were fasted, with no food or liquid for 12 h, and whole body densitometry was performed by dual-energy X-ray absorptiometry (DEXA) using the Discovery DEXA system (Christie InnoMed, Mississauga, ON, Canada) calibrated to a manufacturer-provided phantom spine. Only the fat-free lean mass (bone mineral content subtracted) from each thigh was analyzed from the midneck to below the distal portion of the femur (Hologic Software, Hologic, Bedford, MA) due to only controlling for the lower-body training.
Muscle thickness was measured by ultrasound and used as an index of whole muscle hypertrophy, which is strongly correlated to muscle CSA determined by magnetic resonance imaging (14). After the DEXA scan, the vastus lateralis and rectus femoris muscles were landmarked to ensure consistency of measurements at each time point. The vastus lateralis site was located at 50% of the distance between the greater trochanter and lateral epicondyle of the femur; the rectus femoris was located at 50% of the distance between the anterior superior iliac spine and superior aspect of the patella. The 50% distance for each muscle was recorded and replicated pre- and postintervention, with the results expressed as an average of two separate images acquired from each muscle. Participants rested supine for 10 min before commencement of measurements. Images were acquired using B-mode ultrasound with a linear-array probe (8L-RS Transducer, GE Healthcare, Chicago, IL) with minimal pressure applied to the probe and analyzed immediately postacquisition from the superficial to the deep aponeurosis (Vivid I Software, GE Healthcare). Reliability of these measurements for the sonographer was assessed over 3 consecutive days on preserved human cadavers at the University of Guelph human anatomy laboratory, and the measurements were found to have a coefficient of variation of 1.7% within the same day and 4.8% between days. Measurements were taken distal to the site of the tourniquet due to the potential attenuation of muscle adaptations caused by compression (26) in the LL-BFR leg.
Muscle endurance test.
A custom-built leg-extension dynamometer (1080 Quantum, 1080 Motion), which has been shown to be reliable for measuring human power output (59), was used during a maximal-effort muscle endurance task. Briefly, the “model A” configuration was used, and anatomical positioning was performed according to the methods of Whinton et al. (59), such that the distal shin pad was ~2 cm above the lateral malleolus and the knee joint angle was ~90° knee flexion. A similar protocol was used to determine single-leg 1-RM leg extension for each leg on the same day following baseline 1-RM squats. Because a single measurement (such as number of repetitions performed) may not provide sufficient resolution to determine differences between legs to repeatedly maintain power outputs across multiple muscle contractions, we opted to perform a test fixed for the number of repetitions (30 repetitions) and maximal concentric velocity of 180°/s, resulting in a duration of ~60 s. Participants were familiarized with the protocol and allowed two to three practice repetitions. After ~5 min of rest, they were instructed to perform 30 maximal concentric effort repetitions at 30% of the 1-RM. Subjects were instructed to extend the leg with maximal speed and force to full extension, followed by a controlled eccentric return to ~90° knee flexion. Feedback was provided to ensure a full stop between repetitions, and strong verbal encouragement was given throughout the test. Half of the participants performed the test with the LL-RE leg first with a 10- to 15-min rest followed by the LL-BFR leg, and vice versa. The load, time of day, and anatomical positions were recorded and replicated pre- and posttraining. The data represent the mean concentric power output for repetitions 1–30. Power output was expressed in both absolute and relative terms to account for individual differences. Relative power output was calculated by dividing each repetition by the highest power output obtained from a single repetition of each trial and expressed as a percentage by multiplying each value by 100.
Mitochondrial respiration measurements.
Muscle samples in ice-cold BIOPS were trimmed of nonmuscle tissue, separated with fine-tipped forceps under a microscope, incubated with saponin (40 µg/µL) for 30 min, and then washed in mitochondrial respiration buffer [MiR05; 0.5 mM EGTA, 3 mM MgCl2·6H2O, 60 mM potassium lactobionate, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, 20 mM taurine, and 1 g/L fatty acid-free BSA (pH 7.1)] for 15 min. Respiration experiments were performed using high-resolution respirometry (Oxygraph-2K, Oroboros Instruments, Innsbruck, Austria), as previously described (34, 43). Briefly, ADP was titrated in the presence of 5 mM pyruvate and 1 mM malate. After ADP titrations, 10 mM glutamate and 10 mM succinate were sequentially added to determine maximal complex I- and complex I/II-linked respiration (i.e., maximally supported respiration). Cytochrome c (10 µM) was added at the end of all experiments to confirm mitochondrial membrane integrity by a <10% increase in respiration, which resulted in exclusion of four individual fiber bundles (total 78) from this investigation. Respiratory control ratios (RCR) were determined (state 3/state 4 respiration) to demonstrate the mitochondrial coupling. After completion of experiments, fiber bundles were recovered and freeze-dried to normalize all data to fiber weight. Because of the potential high variability during high-resolution respirometry experiments (4), the same investigator performed all experiments, the same stock reagents were used, and two to four fiber bundles were used at each time point/condition averaged as a single value. The average within-day coefficient of variation for each stage with saturating ADP was 11.7–12.8%. ADP titrations were analyzed using Michaelis-Menten kinetics with a constraint of 100 in Prism 8 (GraphPad, La Jolla, CA).
Western blot analysis.
Freeze-dried muscle fibers were digested in a lysis buffer containing 10% glycerol, 5% β-mercaptoethanol, and 2.3% SDS in 62.5 mM Tris·HCl and 0.01% bromophenol blue for 1 h at 65°C with gentle shaking (2). Samples were vortexed briefly every 15 min to improve digestion, and 5 µL of digested lysate were loaded onto SDS-polyacrylamide gels for Western blot protein quantification. Proteins were separated by electrophoresis at 150 V for 1 h and transferred at 100 V for 1 h to polyvinylidene difluoride membranes. After the membranes were blocked in 5–7.5% skim milk or BSA, commercially available antibodies were used to detect cytochrome c oxidase complex IV (COXIV, 1:30,000 dilution; catalog no. A21347, Invitrogen), oxidative phosphorylation complex cocktail (OXPHOS, 1:500 dilution; catalog no. ab110413, MitoSciences), voltage-dependent anion-selective channel protein (VDAC, 1:1,000 dilution; catalog no. ab14734, Abcam), mitochondrial creatine kinase (MiCK, 1:1,000 dilution; catalog no. ab131188, Abcam), SOD2 (1:5,000 dilution; catalog no. ab13533, Abcam), and pyruvate dehydrogenase E1 α-subunit (PDHE1α, 1:1,000 dilution; catalog no. 459400, Invitrogen). Appropriate washes and secondary antibodies [mouse anti-rabbit (catalog no. sc-2357, Santa Cruz Biotechnology) and goat anti-mouse (catalog no. 155-035-003, Jackson ImmunoResearch), diluted 1:1,000–1:5,000] were applied. All samples for each protein were transferred on the same membrane to limit variability and quantified via chemiluminescence using a FluorChem HD imaging system (Alpha Innotech, Santa Clara, CA). Equal loading across groups was confirmed using Ponceau staining for each membrane.
Immunohistochemical preparation.
Muscle samples embedded in OCT medium were cut into 10-μm-thick sections with a cryostat (Thermo Fisher Scientific, Mississauga, ON, Canada) maintained at −20°C. To reduce experimental variation, samples from each condition were placed on the same slide. Muscle fiber type was determined as previously described (3) with slight modifications. Slides were incubated with primary antibodies against myosin heavy chain (MHC) I (BA-F8; 1:20 dilution), MHC IIa (SC-71; 1:200 dilution), and MHC IIx (6H1; 1:40 dilution) for 1 h. Similarly, serial sections were utilized for determination of muscle fiber capillary supply (51). Briefly, sections were incubated overnight with primary antibodies specific for endothelium [collagen IV (M3F7), 1:50 dilution] and sarcolemma [dystrophin (MANDYS1), 1:100 dilution]. After overnight incubation and appropriate washes/secondary antibody incubation, slides were counterstained for cell nuclei using DAPI (30 μM) for 5 min and then washed three times for 5 min each with 1× PBS. Before imaging, sections were mounted with ProLong antifade reagent (Life Technologies, Burlington, ON, Canada), and all images were acquired 6–24 h thereafter. All primary antibodies were purchased from the Developmental Studies Hybridoma Bank (University of Iowa), and secondary antibodies were purchased from Invitrogen (Burlington, ON, Canada).
Sections were visualized with an Axio Observer Z1 microscope (Carl Zeiss, Jena, Germany), and individual images were taken across the entire cross section and then assembled into a composite image using Zen software. The entire section for each sample was analyzed completely. Because of the lack of type IIX fibers in most participants, we classified fibers as type I or II only. Type I/IIA hybrid fibers were excluded from analysis because of their low abundance, and any peripheral, longitudinal/oblong, and/or damaged fibers were not analyzed. We observed no differences in fiber number [81 (95% CI: 58, 105) at baseline (Pre), 86 (95% CI: 57, 116) after LL-RE, 70 (95% CI: 54, 87) after LL-BFR, P = 0.6] or type [47 (95% CI: 30, 64) Pre, 48 (95% CI: 32, 64) after LL-RE, 38 (95% CI: 25, 52) after LL-BFR for type I (P = 0.5); 34 (95% CI: 22, 47) Pre, 38 (95% CI: 22, 54) after LL-RE, 32 (95% CI: 24, 40) after LL-BFR for type II (P = 0.8)].
Immunohistochemical analysis.
Muscle fiber CSA and perimeter were calculated manually by tracing around each fiber using ImageJ (National Institutes of Health, Bethesda, MD). For analysis of capillary supply, several properties were examined: capillary contacts (number of capillaries/fiber), sharing factor (number of fibers sharing each capillary), individual capillary-to-fiber ratio, and capillary-to-fiber perimeter (CFPE) index (number of capillaries/1,000-μm perimeter), as described previously (22). Myonuclei were quantified by clear positive staining to be within the sarcolemma, and the myonuclear domain was determined by dividing the individual fiber CSA by the number of myonuclei per fiber.
One sample was not acquired for a participant at the end of the study due to tissue constraints and was excluded from analysis. This was similarly true for baseline samples in two other participants. However, at baseline an additional sample from the same two participants was acquired 2 h following an acute bout of exercise on the same day (43) and was used for analysis. Statistical analysis on morphology did not reveal changes with (n = 9) or without (n = 7) these samples; thus they were included in the final data. Myonuclei analysis was performed without these samples (n = 7) due to the potential of satellite cell infiltration following an acute bout of exercise (56).
Statistical analysis.
All data were analyzed in SPSS version 25 (IBM, Armonk, NY), with alpha set a priori at P < 0.05, and data were visually inspected for normality using Q-Q plots. Repeated-measures ANOVA (2-group × 2-time) were performed on exercise parameters: exercise volume and indexes for muscle hypertrophy (muscle thickness and thigh lean mass). Strength (1-RM) and repetitions per set were analyzed using a 2-group × 4-time and 2-group × 3-set repeated-measures ANOVA, respectively. A 2-group × 2-time × 30-repetition repeated-measures ANOVA was also used to analyze absolute and relative power outputs from the MET as an entire data set. To aid in practical interpretation, absolute and relative power outputs are further expressed as changes from baseline and were analyzed in tertiles (i.e., repetitions 1–10, 11–20, and 21–30) by repeated-measures ANOVA (2-group × 10-repetition). Analysis in tertiles was used to account for potential early increases in power output (resulting from increases in strength) and to remove the influence of the expected decrease in power output near the completion of the test. All skeletal muscle results were analyzed using repeated-measures ANOVA. Where a significant interaction occurred, post hoc tests were performed to compare baseline values with LL-RE and LL-BFR. One-tailed tests were used for comparison with baseline, because previous resistance training interventions showed either no change or increases for all measurements compared with baseline. Two-tailed tests were performed to directly compare LL-RE and LL-BFR between each other. Post hoc tests were corrected for multiple comparisons using Bonferroni’s correction factor. Data in text and tables are expressed as mean and 95% CI. Where possible, figures show individual data and group mean. For clarity, the MET data are presented as means ± SE.
RESULTS
Exercise volume and muscle strength.
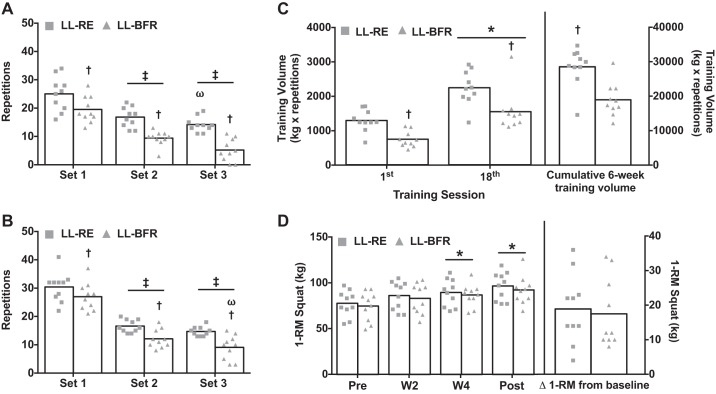
Similar 1-RM values were observed at baseline between legs [77 (95% CI: 67, 87) and 74 (95% CI: 63, 86) kg for LL-RE and LL-BFR, respectively, P = 0.2], although the training load (i.e., 30% 1-RM) was slightly greater for the LL-RE leg [23.4 (95% CI: 20.1, 26.8) vs. 22 (95% CI: 18.5, 25.5) kg, P = 0.03]. In the first training session, both legs performed more repetitions in set 1 than in sets 2 and 3; however, across all sets, the LL-RE leg performed more repetitions than the LL-BFR leg [25 (95% CI: 21, 29) vs. 20 (95% CI: 16, 23) in set 1, 17 (95% CI: 14, 19) vs. 9 (95% CI: 8, 11) in set 2, and 14 (95% CI: 12, 16) vs. 5 (95% CI: 2, 8) in set 3, all P < 0.05; Fig. 2A]. By the last training session, similar to the first training session, both legs performed more repetitions in set 1 than in sets 2 and 3, with the LL-RE leg performing more repetitions than the LL-BFR leg [30 (95% CI: 27, 34) vs. 27 (95% CI: 24, 31) in set 1, 17 (95% CI: 15, 18) vs. 12 (95% CI: 10, 14) in set 2, and 15 (95% CI: 14, 16) vs. 9 (95% CI: 6, 12) in set 3, all P < 0.05; Fig. 2B]. Additionally, the LL-RE leg lifted a greater exercise load than the LL-BFR leg by the last training session [36 (95% CI: 31, 41) vs. 32 (95% CI: 27, 37) kg, P = 0.004].
Fig. 2.
Exercise training volume and muscle strength changes following 6 wk of low-load resistance exercise (LL-RE) or low-load blood flow restriction resistance exercise (LL-BFR) training. A: repetitions to failure for each set of the first training session. B: repetitions for each set of the last training session. C: daily training volume for the first and last (18th) training sessions. Cumulative exercise training volume is shown at right. D: dynamic muscle strength before (Pre) and after 2 wk (W2), 4 wk (W4), and 6 wk (Post) of training. Absolute change in each group is shown at right. RM, repetition maximum. ‡P < 0.05 vs. set 1. ωP < 0.05 vs. set 2. †P < 0.01 vs. LL-RE at similar time points. *P < 0.01 vs. Pre. Symbols represent individual values, and bars represent group means (n = 10/group).
As a result of the differences in load and/or repetitions performed, total exercise volume (load × repetitions) during the first and last training sessions was less during LL-BFR than LL-RE; however, both legs increased exercise training volume by nearly twofold by the final training session (both P < 0.01; Fig. 2C). As a result, cumulative 6-wk training volume was ~33% (P < 0.0001) lower for the LL-BFR leg compared with the LL-RE leg (Fig. 2C). Despite large differences in exercise volume, both legs increased their 1-RM [96 (95% CI: 85, 107) and 92 (95% CI: 81, 103) kg for LL-RE and LL-BFR, respectively, both P = 0.001], with no differences between legs following the 6 wk of training (Fig. 2D).
Indexes of whole muscle hypertrophy.
After both protocols, an effect of time (P = 0.043) was observed for vastus lateralis muscle thickness, which increased from baseline in the LL-RE and LL-BFR legs (4.9% and 7.4%, respectively), whereas this effect was not observed for rectus femoris muscle thickness or thigh lean mass in either leg (Table 1).
Table 1.
Thigh lean mass and quadriceps muscle thickness before and after 6 wk of LL-RE and LL-BFR training in young men
|
P Value |
|||||
|---|---|---|---|---|---|
| Pre | Post | Time | Group | Time × group | |
| Thigh lean mass, kg | |||||
| LL-RE LL-BFR |
7.19 (6.56, 7.81) 7.16 (6.50, 7.81) |
7.23 (6.60, 7.86) 7.24 (6.55, 7.93) |
0.6 | 0.9 | 0.14 |
| Muscle thickness, cm | |||||
| Rectus femoris | |||||
| LL-RE LL-BFR |
2.56 (2.36, 2.76) 2.59 (2.41, 2.77) |
2.58 (2.41, 2.75) 2.70 (2.51, 2.88) |
0.13 | 0.4 | 0.09 |
| Vastus lateralis | |||||
| LL-RE LL-BFR |
2.80 (2.52, 3.08) 2.72 (2.59, 2.86) |
2.92 (2.70, 3.13) 2.92 (2.74, 3.10) |
0.043 | 0.7 | 0.2 |
Values are means (95% confidence interval); n = 10/group. Thigh lean mass was acquired using dual-energy X-ray absorptiometry, and muscle thickness was acquired by ultrasound at 50% of muscle length. LL-RE, low-load resistance exercise; LL-BFR, low-load blood flow restriction resistance exercise.
Muscle endurance test.
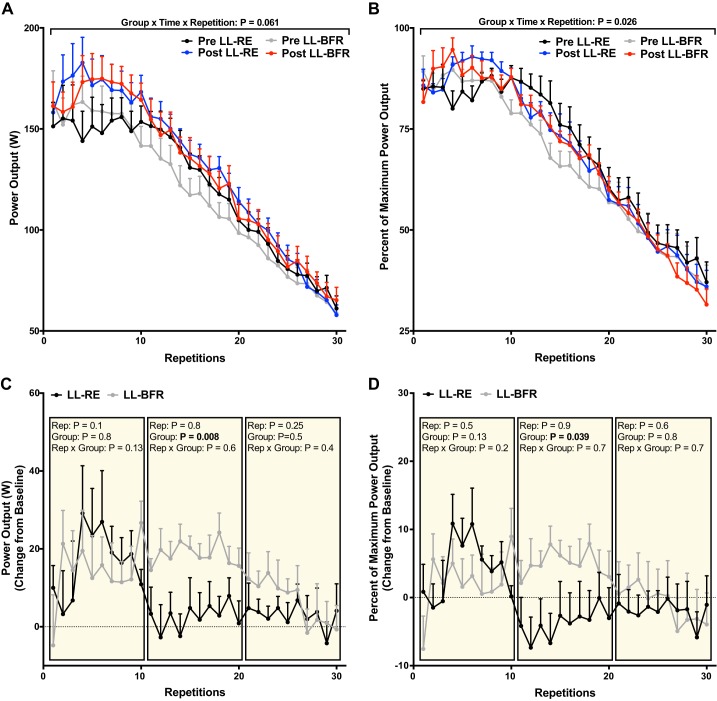
Power output during all 30 repetitions before and after training, expressed in absolute and relative terms, is presented in Fig. 3, A and B. An expected effect of time (P = 0.001) was observed for the average power output across all 30 repetitions [119 (95% CI: 105, 134) and 128 (95% CI: 113, 144) W before and after, respectively, for LL-RE and 116 (95% CI: 99, 134) vs. 130 (95% CI: 116, 145) W before and after, respectively, for LL-BFR], with a nonsignificant (P = 0.11) increase from baseline in maximum power output for a single repetition [177 (95% CI: 144, 209) and 189 (95% CI: 163, 215) W before and after, respectively, for LL-RE and 183 (95% CI: 146, 219) and 192 (95% CI: 168, 217) W before and after, respectively, for LL-BFR]. Absolute power outputs trended toward an interaction (group × time × repetition) in absolute terms (P = 0.061), and when data were expressed as a relative percentage of highest power output obtained from a single repetition to control for interindividual variation, a significant interaction occurred (P = 0.026).
Fig. 3.
Concentric power output during a muscle endurance test before (Pre) and after (Post) 6 wk of low-load resistance exercise (LL-RE) or low-load blood flow restriction resistance exercise (LL-BFR) training. A: absolute power output for each repetition. B: relative power output expressed as percentage of the highest power output of a single repetition. C and D: change from baseline for absolute and relative power output, respectively, which was analyzed in tertiles. Rep, repetition. Values are means ± SE (n = 10/group).
Both legs similarly improved the average power output during the first 10 repetitions following training [152 (95% CI: 129, 178) and 168 (95% CI: 146,190) W before and after LL-RE, respectively; 157 (95% CI: 130, 183) and 171 (95% CI: 145, 196) W before and after LL-BFR, respectively, P = 0.027]. Interestingly, only the LL-BFR leg had an ∼18% increase in power output compared with baseline values throughout the middle of the test (repetitions 11–20), which was greater than in the LL-RE leg [3 (95% CI: −6, 11) and 19 (95% CI: 13, 24) W for LL-RE and LL-BFR, respectively, P = 0.008; Fig. 3C]. A similar pattern was observed when the data were analyzed as a relative percentage, such that the LL-BFR leg maintained a greater change from baseline than the LL-RE leg across repetitions 11–20 [−4 (95% CI: −12, 4) vs. 5 (95% CI: −0.6, 11)%, P = 0.039; Fig. 3D].
Muscle fiber characteristics.
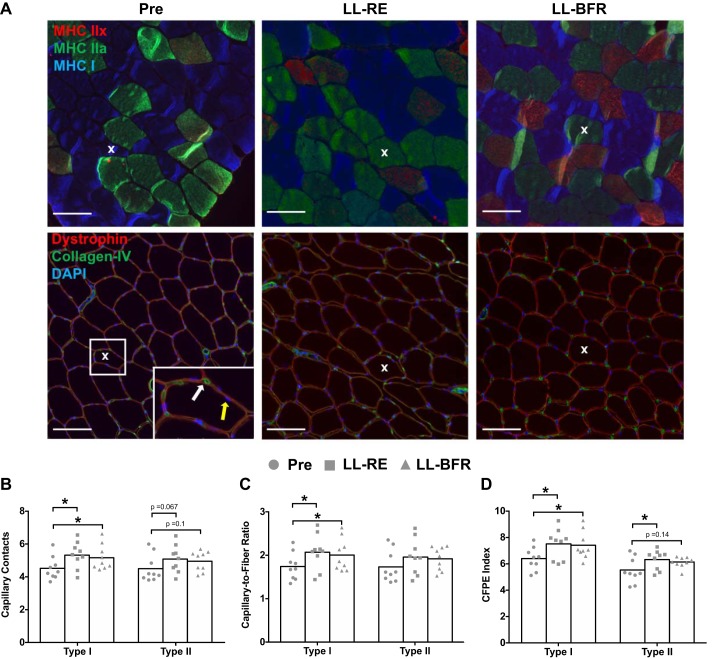
Fiber type distribution, CSA, perimeter, sharing factor, myonuclei per fiber, and myonuclear domain did not significantly differ following the 6 wk of training for either leg, irrespective of fiber type (Table 2). On the other hand, both LL-RE and LL-BFR effectively increased the capillary contacts with type I fibers by ~18% (P = 0.018) and ~14% (P = 0.006), respectively, an effect that was trending toward an increase for type II fibers relative to baseline for both LL-RE (P = 0.067) and LL-BFR (P = 0.1; Fig. 4B). The individual capillary-to-fiber ratio for type I fibers increased in both legs relative to baseline values [1.76 (95% CI: 1.53, 2.00) at baseline vs. 2.09 (95% CI: 1.79, 2.40) for LL-RE and 2.01 (95% CI: 1.73, 2.3) for LL-BFR, both P < 0.05; Fig. 4C]. Repeated-measures ANOVA revealed only a trend for differences in capillary-to-fiber ratio for type II fibers following training [1.74 (95% CI: 1.46, 2.02) at baseline vs. 1.96 (95% CI: 1.66, 2.28) and 1.93 (95% CI: 1.72, 2.14) after LL-RE and LL-BFR, respectively, P = 0.08]. Finally, the CFPE index increased for type I fibers in both legs [6.38 (95% CI: 5.72. 7.05) at baseline vs. 7.49 (95% CI: 6.60, 8.38) and 7.39 (95% CI: 6.58, 8.20) after LL-RE and LL-BFR, respectively, both P < 0.05] and only in the LL-RE leg for type II fibers [5.51 (95% CI: 4.76, 6.25) vs. 6.30 (95% CI: 5.73, 6.88) after LL-RE, P = 0.048; Fig. 4D].
Table 2.
Muscle fiber characteristics and structural properties
| Pre | LL-RE | LL-BFR | P Value | |
|---|---|---|---|---|
| Fiber type, % | ||||
| Type I | 57 (45, 69) | 57 (50, 64) | 52 (43, 62) | 0.6 |
| Type II | 43 (31, 55) | 43 (36, 50) | 47 (38, 57) | 0.6 |
| Fiber CSA, µm2 | ||||
| Type I | 4,393 (3,659, 5,127) | 5,088 (4,192, 5,985) | 4,838 (4,034, 5,642) | 0.116 |
| Type II | 5,770 (4,762, 6,778) | 6,291 (4,984, 7,599) | 6,280 (5,086, 7,475) | 0.5 |
| Fiber perimeter, µm | ||||
| Type I | 261 (238, 284) | 281 (254, 306) | 273 (250, 296) | 0.14 |
| Type II | 299 (272, 327) | 309 (277, 342) | 313 (281, 343) | 0.5 |
| Sharing factor | ||||
| Type I | 2.70 (2.64, 2.78) | 2.67 (2.55, 2,79) | 2.69 (2.62, 2.77) | 0.7 |
| Type II | 2.73 (2.64, 2.82) | 2.69 (2.58, 2.81) | 2.68 (2.62, 2.73) | 0.8 |
| Myonuclei | ||||
| Type I | 2.17 (1.89, 2.45) | 2.33 (1.83, 2.82) | 2.17 (1.83, 2.50) | 0.7 |
| Type II | 2.80 (2.59, 2.99) | 2.83 (2.20, 3.47) | 2.73 (2.33, 3.13) | 0.9 |
| Myonuclear domain, µm2 | ||||
| Type I | 2,369 (1,813, 2,924) | 2,671 (2,293, 3,049) | 2,701 (2,085, 3,317) | 0.4 |
| Type II | 2,594 (1,977, 3,210) | 2,668 (2,158, 3,177) | 2,947 (2,220, 3,673) | 0.6 |
Values are means (95% confidence interval); n = 9/group, except for myonuclei (n = 7/group). LL-RE, low-load resistance exercise; LL-BFR, low-load blood flow restriction resistance exercise; CSA, cross-sectional area.
Fig. 4.
Skeletal muscle microvascular properties before (Pre) and after 6 wk of low-load resistance exercise (LL-RE) or low-load blood flow restriction resistance exercise (LL-BFR) training. A: representative images for immunohistochemical staining of serial cross sections. Top: type I [myosin heavy chain (MHC) I, blue] and type II (MHC IIa, green; MHC IIx, red) muscle fibers. Bottom: muscle fiber boarder (dystrophin; red), capillary (collagen IV, green), and nuclei (DAPI, blue). White and yellow arrows represent a capillary and a myonucleus, respectively. X depicts the same fiber within each condition for both sets of images. Scale bars = 100 μm. B–D: capillary contacts, capillary-to-fiber ratio, and capillary-to-fiber perimeter exchange (CFPE) index. *P < 0.05 vs. Pre. Symbols represent individual values, and bars represent group means (n = 9/group).
Mitochondrial content and function.
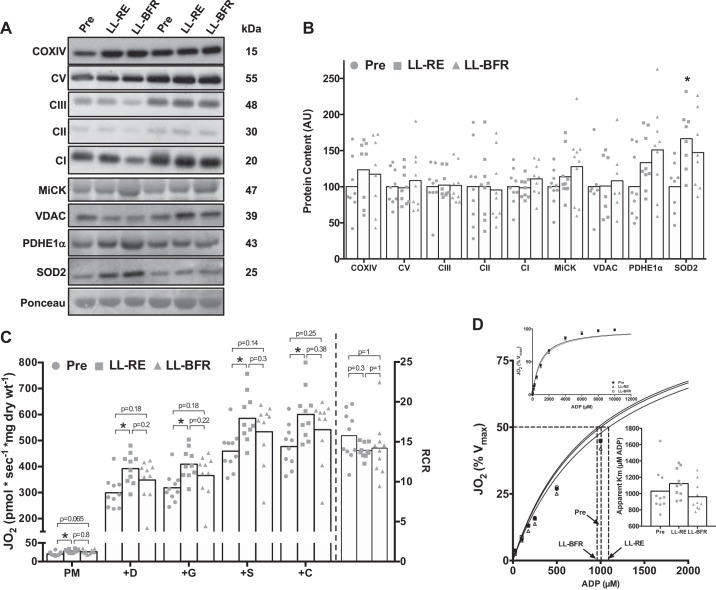
Irrespective of training modality, protein content of electron transport chain subunits [complexes V, III, II, I, and IV (COX)] and mitochondrial membrane transporters (VDAC and MiCK) was not different following 6 wk of training, although there was an increase in the antioxidant protein SOD2 in the LL-RE leg (P = 0.02; Fig. 5, A and B). Moreover, despite the absence of change in protein content for electron transport chain subunits or mitochondrial membrane transporters following training, initial repeated-measures ANOVAs illustrated significant differences at each respiration stage (all P < 0.05; Fig. 5C). Post hoc analysis revealed that LL-RE increased respiration at each stage from baseline (all P < 0.01), an effect that was not observed following LL-BFR training. We also observed no differences in mitochondrial ADP sensitivity following 6 wk of training in either leg [apparent Km = 1,030 (95% CI: 842, 1,217), 1,122 (95% CI: 999, 1,245), and 960 (95% CI: 835, 1,085) μM at baseline, after LL-RE, and after LL-BFR, respectively, P = 0.2; Fig. 5D].
Fig. 5.
Mitochondria-located protein expression and respiratory capacity in permeabilized muscle fibers before (Pre) and after 6 wk of low-load resistance exercise (LL-RE) or low-load blood flow restriction resistance exercise (LL-BFR) training. A: representative Western blot images for each protein for 2 participants. COXIV, cytochrome c oxidase complex IV; CI–CIII and CV, complexes I–III and V; MiCK, mitochondrial creatine kinase; VDAC, voltage-dependent anion-selective channel protein; PDHE1α, pyruvate dehydrogenase E1 α-subunit. B: quantification of Western blots for proteins located within mitochondria. AU, arbitrary units. C: high-resolution respirometry illustrating maximal complex I/II-linked respiration. D: Michaelis-Menten kinetic curves and apparent Km for ADP. Jo2, O2 consumption; PM, pyruvate + malate; +D, PM + ADP; +G, PMD + glutamate; +S, PMDG + succinate; +C, PMDGS + cytochrome c; RCR, respiratory control ratio. *P < 0.01 vs. Pre. Symbols represent individual values, and bars represent group means [n = 7–10/group (Western blot data) and n = 10/group (respirometry data)].
DISCUSSION
In the present study we demonstrate that when LL-RE and LL-BFR were performed to task failure during a 6-wk training program, outcomes in muscle strength and size were similar, despite accumulation of ~33% lower training volume in the LL-BFR than the LL-RE leg. Interestingly, sustained power output was greater during a muscle endurance task following LL-BFR training, which could not be explained by changes in muscle fiber size, microvascular supply, or mitochondrial respiration capacity. These findings provide novel insight into the muscle adaptations following low-load resistance training, with or without BFR, and may have implications for the prescription of these training modalities to healthy or clinical populations.
Exercise volume and muscle strength and size.
We observed an ∼25% increase in 1-RM following the 6 wk of training in both LL-BFR and LL-RE conditions, a finding that has been corroborated previously (11). However, in both groups, only vastus lateralis muscle thickness demonstrated a measurable increase, whereas nonsignificant changes occurred in other indexes of muscle hypertrophy (lean thigh mass, rectus femoris muscle thickness, and muscle fiber CSA) or factors that have been proposed to influence hypertrophy (e.g., number of myonuclei or myonuclear domain). We included several different estimates of muscle size due to the variety of methods used to assess muscle hypertrophy, each with its limitations and/or variability, as recently highlighted (21). The failure to reach a statistically significant change for the indexes that demonstrated trends (e.g., type I muscle fiber CSA) could be explained in part by the study being insufficiently powered to detect relatively small-to-moderate effect sizes (Cohen’s d) for changes in type I muscle fiber CSA from baseline (d = 0.65 and 0.45 for LL-RE and LL-BFR, respectively). Such effect sizes may be due to our studied population (i.e., resistance-trained individuals) and exercise sets/week during the study (48). As such, a longer duration of training (e.g., 8–12 wk) and/or more exercise sets (e.g., 4–6 sets) may lead to appreciable increases in these variables, and future research examining the optimal frequency, intensity, and duration of programs is warranted for this population. In total, we did not detect differences in strength or estimates of muscle size between training interventions when performed to task failure, despite different total exercise volume accumulated over the 6-wk study. These findings further support a more prominent role of within-subject intrinsic factors as opposed to extrinsic factors (e.g., exercise volume) in influencing adaptations such as muscle strength or hypertrophy when similar exercise sets are performed with low loads with or without BFR.
Skeletal muscle microvascular properties.
Previous work has shown that, compared with volume-matched LL-RE, a single-bout of LL-BFR leads to a superior increase in genes associated with angiogenesis (12). It is important to note, however, that acute signaling responses and chronic adaptations in humans are not always aligned. We were interested in determining if LL-BFR would induce greater muscle capillarity when exercise was not work-matched but, rather, performed to task failure, as we believe resistance training to volitional fatigue is an important stimulus to elicit muscle remodeling when equal numbers of exercise sets are performed. The present study did demonstrate type I muscle fiber angiogenesis following low-load resistance training, regardless of the intentional BFR, a finding that was primarily driven by an increase in the number of capillaries in contact with each individual fiber as opposed to the number of fibers supplied by a single capillary (i.e., no change in the sharing factor). Although microvascular expansion was not observed in type II muscle fibers, which were trending toward significance, previous work has shown that both fiber types respond positively to high- and low-load resistance training (23), and in agreement with this, we did not find evidence that BFR or LL-RE training greatly influenced the capillary response in a fiber-type-specific manner. Whether the mechanisms and temporal responses leading to microvascular expansion are similar between the two modalities remains to be determined.
Mitochondrial function and content after LL-RE and LL-BFR.
As our previous investigation demonstrated an acute reduction in mitochondrial-derived ROS emission following LL-BFR, but not LL-RE (43), we explored if these differences chronically influenced mitochondrial function and protein content. Muscle mitochondrial respiratory capacity increased ∼30% from baseline following LL-RE training; this response did not reach significance following LL-BFR training, although no statistical differences existed between LL-RE and LL-BFR. Previous work has demonstrated a 40% increase in maximally linked complex I/II respiration after 6 wk of LL-BFR (19). The discrepancy in the magnitude of change between our findings (nonsignificant 18% change) and those of Groennebaek et al. (19) (significant 40% change) following LL-BFR may be due in part to the participant population: untrained participants who had not participated in resistance- or aerobic-type exercise 6 mo prior the commencement of the study were used by Groennebaek et al., whereas our cohort was resistance-trained and had only refrained from a periodized resistance training program for ≥1 mo. As such, the exercise stimulus (e.g., LL-BFR) used by Groennebaek et al. may have been more robust, leading to greater intramuscular signaling events (6) and subsequent remodeling (60), which results in a greater effect size. However, the lack of a volume-matched LL-RE group to LL-BFR and/or midpoint measurements in the present study or the study of Groennebaek et al. (19) prevents a clear explanation.
Despite an increased respiratory capacity in the LL-RE leg, there was no increase in any markers of mitochondrial protein content, a finding that has been demonstrated previously in young individuals following resistance training (19, 42, 46). This is interesting given previous evidence that mitochondrial protein fractional synthesis rates increase following resistance training (19) and acutely after a novel bout of resistance exercise, an effect that is blunted in the trained state (60). The brief, intermittent nature of resistance exercise seems insufficient to increase mitochondrial content after 6 wk of training in young, resistance-trained individuals, with or without BFR. Our findings suggest intrinsic changes within mitochondria, such as posttranslational modifications of proteins (55) or alterations in supercomplex abundance (18), resulting in a greater respiratory capacity, although the underlying mechanisms remain to be determined. In opposition to our findings, a recent study using isolated mitochondria reported no change in respiratory capacity following 12 wk of high-load resistance training in young individuals (47), but the methodological differences of using isolated mitochondria must be recognized (28), and this could help explain our differential results. In summary, future work using gold-standard approaches is required to directly examine the influence of training experience, exercise volume, and task failure during resistance training with and without BFR and their influence on mitochondrial function and content.
Muscle endurance test.
We provide evidence that LL-BFR training results in greater sustained, voluntary power output during a MET. While the mechanisms of muscle fatigue are multifactorial and highly dependent on the mode, intensity, and duration (1, 13, 17), in the present study the improvement in endurance following LL-BFR cannot be explained by alterations in muscle strength, size, microvasculature supply, or respiratory capacity. Although currently speculative, given the nature of our protocol (~60 s), the improved voluntary muscle power output following LL-BFR training may be related to an improved anaerobic maintenance of muscle ATP and/or preservation of proper ion gradients, both of which would allow repeated excitation-contraction coupling of muscle fibers and sustained force output (35, 39). Recent literature has demonstrated greater exercise performance accompanied by a reduction in K+ efflux from exercising muscle following intermittent BFR cycling for 6 wk, which was attributed in part to an increase in Na+-K+-ATPase isoforms (5). Therefore, it is possible that chronic LL-BFR training results in similar adaptations, leading to sustained power outputs during intense exercise, although this remains an unexplored avenue of research. Despite this knowledge gap, our data suggest that the fatigue resistance following BFR training was likely not a result of improved muscle aerobic capacity.
Limitations.
Methodological limitations within this study are worth mentioning. First, our analyses were not performed in a blinded manner and, thus, could be subjected to potential bias in our measures. Second, after each training session, the participants consumed a source of protein to better model nutritional approaches outside a laboratory setting. It is unknown whether the nutritional intervention could have influenced our finding; however, it is unlikely that this would alter our overall interpretation, given the within-subject design. Third, a single muscle biopsy sample was collected from one leg at baseline, as opposed to each leg being sampled independently, to avoid undo participant discomfort. While it is possible that the legs differed at the myocellular level at baseline, our previous work suggests limited differences in select markers (i.e., mitochondrial respiratory capacity) between legs; thus we believe this approach to be appropriate. Fourth, the use of indirect measures (protein content) to estimate changes in mitochondrial content may not parallel actual changes in density following a training intervention, as recently demonstrated (33); thus, caution is warranted when inferring our findings of an increased respiratory capacity without changes in protein content. Fifth, the MET was performed at a relatively high intensity for ~60 s. Since skeletal muscle relies on a balance of anaerobic and aerobic means for ATP provision at different exercise intensities, it remains unclear if differences in performance between LL-RE and LL-BFR training would occur following a lower-intensity muscle endurance task of a longer duration.
Perspectives and Significance
We evaluated the effect of resistance exercise with and without BFR to repetition failure for 6 wk in young men by examining a combination of whole muscle group performance and skeletal muscle cellular properties. The present investigation provides evidence of potentially different functional muscle adaptations following training with LL-BFR, in particular with muscle endurance after only 6 wk of training, that cannot be explained by changes in muscle strength, size, and skeletal muscle mitochondrial or microvascular adaptations. These findings may be of significant interest for athletes in terms of sports performance or injury rehabilitation. However, future studies are required to fully elucidate the roles of blood flow manipulation, exercise volume, and task failure in mediating resistance training adaptation. Specifically, the inclusion of both volume- and failure-matched LL-RE groups is required to further our understanding of BFR during resistance exercise.
GRANTS
This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant 03974 (J. F. Burr), Canadian Foundation for Innovation Grant 35460 (J. F. Burr), and Ontario Ministry of Research, Innovation, and Science Grant 460597 (J. F. Burr).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.P., H.L.P., G.J.F.H., G.P.H., and J.F.B. conceived and designed research; C.P., H.L.P., and F.K. performed experiments; C.P. and H.L.P. analyzed data; C.P., H.L.P., F.K., G.J.F.H., J.Q., G.P.H., and J.F.B. interpreted results of experiments; C.P. prepared figures; C.P. and J.F.B. drafted manuscript; C.P., H.L.P., F.K., G.J.F.H., J.Q., G.P.H., and J.F.B. edited and revised manuscript; C.P., H.L.P., F.K., G.J.H., J.Q., G.P.H., and J.F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects who volunteered to participate in the study for their effort throughout training. We also thank Dr. Erin Weersink for performing the muscle biopsies.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.Barbeau P-A, Miotto PM, Holloway GP. Mitochondrial-derived reactive oxygen species influence ADP sensitivity, but not CPT-I substrate sensitivity. Biochem J 475: 2997–3008, 2018. doi: 10.1042/BCJ20180419. [DOI] [PubMed] [Google Scholar]
- 3.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardinale DA, Gejl KD, Ørtenblad N, Ekblom B, Blomstrand E, Larsen FJ. Reliability of maximal mitochondrial oxidative phosphorylation in permeabilized fibers from the vastus lateralis employing high-resolution respirometry. Physiol Rep 6: e13611, 2018. doi: 10.14814/phy2.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiansen D, Eibye KH, Rasmussen V, Voldbye HM, Thomassen M, Nyberg M, Gunnarsson TGP, Skovgaard C, Lindskrog MS, Bishop DJ, Hostrup M, Bangsbo J. Cycling with blood flow restriction improves performance and muscle K+ regulation and alters the effect of anti-oxidant infusion in humans. J Physiol 597: 2421–2444, 2019. doi: 10.1113/JP277657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20: 190–192, 2006. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- 7.Cumming KT, Paulsen G, Wernbom M, Ugelstad I, Raastad T. Acute response and subcellular movement of HSP27, αB-crystallin and HSP70 in human skeletal muscle after blood-flow-restricted low-load resistance exercise. Acta Physiol (Oxf) 211: 634–646, 2014. doi: 10.1111/apha.12305. [DOI] [PubMed] [Google Scholar]
- 8.Drummond MJ, Fujita S, Takashi A, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008. [Erratum in Med Sci Sports Exerc 40: 1191, 2008.] doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellefsen S, Hammarström D, Strand TA, Zacharoff E, Whist JE, Rauk I, Nygaard H, Vegge G, Hanestadhaugen M, Wernbom M, Cumming KT, Rønning R, Raastad T, Rønnestad BR. Blood flow-restricted strength training displays high functional and biological efficacy in women: a within-subject comparison with high-load strength training. Am J Physiol Regul Integr Comp Physiol 309: R767–R779, 2015. doi: 10.1152/ajpregu.00497.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahs CA, Loenneke JP, Thiebaud RS, Rossow LM, Kim D, Abe T, Beck TW, Feeback DL, Bemben DA, Bemben MG. Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct Imaging 35: 167–176, 2015. doi: 10.1111/cpf.12141. [DOI] [PubMed] [Google Scholar]
- 11.Farup J, de Paoli F, Bjerg K, Riis S, Ringgard S, Vissing K. Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sports 25: 754–763, 2015. doi: 10.1111/sms.12396. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson RA, Hunt JEA, Lewis MP, Martin NRW, Player DJ, Stangier C, Taylor CW, Turner MC. The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur J Sport Sci 18: 397–406, 2018. doi: 10.1080/17461391.2017.1422281. [DOI] [PubMed] [Google Scholar]
- 13.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Franchi MV, Longo S, Mallinson J, Quinlan JI, Taylor T, Greenhaff PL, Narici MV. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand J Med Sci Sports 28: 846–853, 2018. doi: 10.1111/sms.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985) 108: 1199–1209, 2010. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985) 103: 903–910, 2007. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 17.Green HJ. Mechanisms of muscle fatigue in intense exercise. J Sports Sci 15: 247–256, 1997. doi: 10.1080/026404197367254. [DOI] [PubMed] [Google Scholar]
- 18.Greggio C, Jha P, Kulkarni SS, Lagarrigue S, Broskey NT, Boutant M, Wang X, Conde Alonso S, Ofori E, Auwerx J, Cantó C, Amati F. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 25: 301–311, 2017. doi: 10.1016/j.cmet.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Groennebaek T, Jespersen NR, Jakobsgaard JE, Sieljacks P, Wang J, Rindom E, Musci RV, Bøtker HE, Hamilton KL, Miller BF, de Paoli FV, Vissing K. Skeletal muscle mitochondrial protein synthesis and respiration increase with low-load blood flow restricted as well as high-load resistance training. Front Physiol 9: 1796, 2018. doi: 10.3389/fphys.2018.01796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 306: E1198–E1204, 2014. doi: 10.1152/ajpendo.00600.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haun CT, Vann CG, Roberts BM, Vigotsky AD, Schoenfeld BJ, Roberts MD. A critical evaluation of the biological construct skeletal muscle hypertrophy: size matters but so does the measurement. Front Physiol 10: 247, 2019. doi: 10.3389/fphys.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hepple RT, Mackinnon SLM, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on V̇o2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 82: 1305–1310, 1997. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- 23.Holloway TM, Morton RW, Oikawa SY, McKellar S, Baker SK, Phillips SM. Microvascular adaptations to resistance training are independent of load in resistance-trained young men. Am J Physiol Regul Integr Comp Physiol 315: R267–R273, 2018. doi: 10.1152/ajpregu.00118.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med 51: 1003–1011, 2017. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 25.Hunt JEA, Galea D, Tufft G, Bunce D, Ferguson RA. Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J Appl Physiol (1985) 115: 403–411, 2013. doi: 10.1152/japplphysiol.00040.2013. [DOI] [PubMed] [Google Scholar]
- 26.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports 21: e231–e241, 2011. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 27.Krustrup P, Söderlund K, Relu MU, Ferguson RA, Bangsbo J. Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: effect of thigh occlusion. Scand J Med Sci Sports 19: 576–584, 2009. doi: 10.1111/j.1600-0838.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 29.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc 44: 2077–2083, 2012. doi: 10.1249/MSS.0b013e3182625928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lixandrão ME, Ugrinowitsch C, Berton R, Vechin FC, Conceição MS, Damas F, Libardi CA, Roschel H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med 48: 361–378, 2018. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 31.MacInnis MJ, McGlory C, Gibala MJ, Phillips SM. Investigating human skeletal muscle physiology with unilateral exercise models: when one limb is more powerful than two. Appl Physiol Nutr Metab 42: 563–570, 2017. doi: 10.1139/apnm-2016-0645. [DOI] [PubMed] [Google Scholar]
- 32.Manini TM, Vincent KR, Leeuwenburgh CL, Lees HA, Kavazis AN, Borst SE, Clark BC. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol (Oxf) 201: 255–263, 2011. doi: 10.1111/j.1748-1716.2010.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meinild Lundby A-K, Jacobs RA, Gehrig S, de Leur J, Hauser M, Bonne TC, Flück D, Dandanell S, Kirk N, Kaech A, Ziegler U, Larsen S, Lundby C. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol (Oxf), 222: 2018. doi: 10.1111/apha.12905. [DOI] [PubMed] [Google Scholar]
- 34.Miotto PM, Holloway GP. Exercise-induced reductions in mitochondrial ADP sensitivity contribute to the induction of gene expression and mitochondrial biogenesis through enhanced mitochondrial H2O2 emission. Mitochondrion 46: 116–122, 2019. doi: 10.1016/j.mito.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, Bangsbo J. Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflugers Arch 448: 452–456, 2004. doi: 10.1007/s00424-004-1257-6. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen JL, Aagaard P, Bech RD, Nygaard T, Hvid LG, Wernbom M, Suetta C, Frandsen U. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol 590: 4351–4361, 2012. doi: 10.1113/jphysiol.2012.237008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen JL, Frandsen U, Prokhorova T, Bech RD, Nygaard T, Suetta C, Aagaard P. Delayed effect of blood flow–restricted resistance training on rapid force capacity. Med Sci Sports Exerc 49: 1157–1167, 2017. doi: 10.1249/MSS.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 38.Nyakayiru J, Fuchs CJ, Trommelen J, Smeets JSJ, Senden JM, Gijsen AP, Zorenc AH, van Loon LJC, Verdijk LB. Blood flow restriction only increases myofibrillar protein synthesis with exercise. Med Sci Sports Exerc 51: 1137–1145, 2019. doi: 10.1249/MSS.0000000000001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overgaard K, Nielsen OB, Clausen T. Effects of reduced electrochemical Na+ gradient on contractility in skeletal muscle: role of the Na+-K+ pump. Pflugers Arch 434: 457–465, 1997. doi: 10.1007/s004240050421. [DOI] [PubMed] [Google Scholar]
- 40.Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, Abe T, Nielsen JL, Libardi CA, Laurentino G, Neto GR, Brandner C, Martin-Hernandez J, Loenneke J. Blood flow restriction exercise position stand: considerations of methodology, application, and safety. Front Physiol 10: 533, 2019. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J Physiol 592: 1887–1901, 2014. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301: R1078–R1087, 2011. doi: 10.1152/ajpregu.00285.2011. [DOI] [PubMed] [Google Scholar]
- 43.Petrick HL, Pignanelli C, Barbeau PA, Churchward-Venne TA, Dennis KMJH, van Loon LJC, Burr JF, Goossens GH, Holloway GP. Blood flow restricted resistance exercise and reductions in oxygen tension attenuate mitochondrial H2O2 emission rates in human skeletal muscle. J Physiol 597: 3985–3997, 2019. doi: 10.1113/JP277765. [DOI] [PubMed] [Google Scholar]
- 44.Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevičius G, Paužas H, Mekideche A, Kayser B, Martinez-Redondo V, Ruas JL, Bruton J, Truffert A, Lanner JT, Skurvydas A, Westerblad H. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci USA 112: 15492–15497, 2015. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope ZK, Willardson JM, Schoenfeld BJ. Exercise and blood flow restriction. J Strength Cond Res 27: 2914–2926, 2013. doi: 10.1519/JSC.0b013e3182874721. [DOI] [PubMed] [Google Scholar]
- 46.Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47: 1922–1931, 2015. doi: 10.1249/MSS.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoenfeld BJ, Contreras B, Krieger J, Grgic J, Delcastillo K, Belliard R, Alto A. Resistance training volume enhances muscle hypertrophy but not strength in trained men. Med Sci Sports Exerc 51: 94–103, 2019. doi: 10.1249/MSS.0000000000001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res 31: 3508–3523, 2017. doi: 10.1519/JSC.0000000000002200. [DOI] [PubMed] [Google Scholar]
- 50.Scott BR, Slattery KM, Sculley DV, Dascombe BJ. Hypoxia and resistance exercise: a comparison of localized and systemic methods. Sports Med 44: 1037–1054, 2014. doi: 10.1007/s40279-014-0177-7. [DOI] [PubMed] [Google Scholar]
- 51.Scribbans TD, Edgett BA, Vorobej K, Mitchell AS, Joanisse SD, Matusiak JBL, Parise G, Quadrilatero J, Gurd BJ. Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLoS One 9: e98119, 2014. doi: 10.1371/journal.pone.0098119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sieljacks P, Wang J, Groennebaek T, Rindom E, Jakobsgaard JE, Herskind J, Gravholt A, Møller AB, Musci RV, de Paoli FV, Hamilton KL, Miller BF, Vissing K. Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in healthy males. Front Physiol 10: 649, 2019. doi: 10.3389/fphys.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slysz J, Stultz J, Burr JF. The efficacy of blood flow restricted exercise: a systematic review & meta-analysis. J Sci Med Sport 19: 669–675, 2016. doi: 10.1016/j.jsams.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Sousa J, Neto GR, Santos HH, Araújo JP, Silva HG, Cirilo-Sousa MS. Effects of strength training with blood flow restriction on torque, muscle activation and local muscular endurance in healthy subjects. Biol Sport 34: 83–90, 2017. doi: 10.5114/biolsport.2017.63738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stram AR, Payne RM. Post-translational modifications in mitochondria: protein signaling in the powerhouse. Cell Mol Life Sci 73: 4063–4073, 2016. doi: 10.1007/s00018-016-2280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wernbom M, Apro W, Paulsen G, Nilsen TS, Blomstrand E, Raastad T. Acute low-load resistance exercise with and without blood flow restriction increased protein signalling and number of satellite cells in human skeletal muscle. Eur J Appl Physiol 113: 2953–2965, 2013. doi: 10.1007/s00421-013-2733-5. [DOI] [PubMed] [Google Scholar]
- 57.Wernbom M, Järrebring R, Andreasson MA, Augustsson J. Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J Strength Cond Res 23: 2389–2395, 2009. doi: 10.1519/JSC.0b013e3181bc1c2a. [DOI] [PubMed] [Google Scholar]
- 58.Wernbom M, Paulsen G, Nilsen TS, Hisdal J, Raastad T. Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol 112: 2051–2063, 2012. doi: 10.1007/s00421-011-2172-0. [DOI] [PubMed] [Google Scholar]
- 59.Whinton AK, Thompson KMA, Power GA, Burr JF. Testing a novel isokinetic dynamometer constructed using a 1080 Quantum. PLoS One 13: e0201179, 2018. doi: 10.1371/journal.pone.0201179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]