Abstract
The objective of this study was to interrogate the link between mitochondrial dysfunction and mitochondrial-specific autophagy in skeletal muscle. C57BL/6J mice were used to establish a time course of mitochondrial function and autophagy induction after fatigue (n = 12), eccentric contraction-induced injury (n = 20), or traumatic freeze injury (FI, n = 28); only FI resulted in a combination of mitochondrial dysfunction, i.e., decreased mitochondrial respiration, and autophagy induction. Moving forward, we tested the hypothesis that mitochondrial-specific autophagy is important for the timely recovery of mitochondrial function after FI. Following FI, there is a significant increase in several mitochondrial-specific autophagy-related protein contents including dynamin-related protein 1 (Drp1), BCL1 interacting protein (BNIP3), Pink1, and Parkin (~2-fold, P < 0.02). Also, mitochondrial-enriched fractions from FI muscles showed microtubule-associated protein light chain B1 (LC3)II colocalization suggesting autophagosome assembly around the damaged mitochondrial. Unc-51 like autophagy activating kinase (Ulk1) is considered necessary for mitochondrial-specific autophagy and herein we utilized a mouse model with Ulk1 deficiency in adult skeletal muscle (myogenin-Cre). While Ulk1 knockouts had contractile weakness compared with littermate controls (−27%, P < 0.02), the recovery of mitochondrial function was not different, and this may be due in part to a partial rescue of Ulk1 protein content within the regenerating muscle tissue of knockouts from differentiated satellite cells in which Ulk1 was not genetically altered via myogenin-Cre. Lastly, autophagy flux was significantly less in injured versus uninjured muscles (−26%, P < 0.02) despite the increase in autophagy-related protein content. This suggests autophagy flux is not upregulated to match increases in autophagy machinery after injury and represents a potential bottleneck in the clearance of damaged mitochondria by autophagy.
Keywords: mitophagy, muscle contractility, muscle regeneration, two-photon microscopy
INTRODUCTION
Mitochondria play an important role during muscle regeneration because they provide crucial energy for the repair of initial membrane disruption, the remodeling of damaged muscle fibers, and satellite cell proliferation and differentiation (14, 38). Mitochondria can be affected by muscle injury, as we and others have reported a decrease in mitochondrial content and a subsequent rise in mitochondrial biogenesis during muscle regeneration (7, 10, 25, 37). Our first motivation for this study was to define the extent to which fatigue, eccentric contraction-induced injury, and traumatic freeze injury cause mitochondrial dysfunction. Each of these muscle stressors has been implicated in mitochondrial stress without thorough assessment of mitochondrial oxygen consumption, i.e., respiration. Additionally, fatigue, eccentric contraction-induced injury, and freeze injury are reported to increase autophagy, a cellular response indicative of mitochondrial damage, which we have previously linked to the success of the regenerative process (7, 25).
We have previously highlighted that when portions of the mitochondria network are stressed or damaged there is an increase in proteins related to autophagy (7, 25). Autophagy is a cellular process that degrades dysfunctional organelles and proteins into their original amino acid and fatty acid components to be recycled in the cell. Autophagy is always basally active in skeletal muscle but can increase in response to stress stimuli such as exercise or caloric restriction (22, 42). Autophagy is initiated under such stressful conditions by phosphorylation of the autophagy-related protein Unc-51 like autophagy activating kinase (Ulk1) at serine 555 by AMPK (11). Ulk1-mediated autophagy has been reported by us and others to play an important role in exercise-induced metabolic adaptations and recovery of muscle strength following traumatic injury, and is considered to be necessary for mitochondrial-specific autophagy referred to as mitophagy (7, 11, 20, 35). The secondary objective of this study was to further define the link between mitochondrial stress and Ulk1-mediated autophagy, and to determine if Ulk1 was necessary for the functional recovery of mitochondria following muscle injury. Understanding the specific role of Ulk1-mediated autophagy after muscle fiber damage may be leveraged to develop targeted therapeutic modalities to address muscle regeneration in conditions such as aging and muscular dystrophy where deficits in muscle repair and autophagy have been reported (23, 27, 31).
We initially hypothesized that fatigue, eccentric contraction-induced injury, and traumatic freeze injury would cause a temporary loss in mitochondrial function. We also hypothesized that these muscle stressors would subsequently cause an increase in mitochondrial-specific autophagy (to degrade the dysfunctional mitochondria), and that Ulk1-mediated autophagy would therefore be necessary for the timely recovery of mitochondrial function following muscle fiber damage. Herein, we quickly determined that only traumatic freeze injury corresponds to a loss of mitochondrial function and we subsequently interrogated the link between this traumatic injury and the autophagy response.
METHODS
Ethical approval.
All animal protocols were approved by the University of Georgia Animal Care and Use Committee under the national guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care.
Animal models.
Male and female C57BL/6J mice aged 3–4 mo were bred in-house and housed 5 per cage in a temperature-controlled facility with a 12:12-h light/dark cycle. Muscle-specific Unc-51 like autophagy knockout mice (Ulk1 MKO) with myogenin-Cre and LoxP flanked Ulk1 and their myogenin-Cre negative littermates (LM) were used to test the necessity of Ulk1 for mitochondrial function and strength recovery after traumatic freeze injury. All mice had ad libitum access to food and water throughout the experiments.
Experimental design.
This study was originally designed to investigate the time course of mitochondrial dysfunction and subsequent autophagic response to fatigue, eccentric contraction-induced injury, and traumatic freeze injury as these muscle stressors have all been linked to mitochondrial damage. Early results strongly indicate a lack of mitochondrial dysfunction following fatigue and eccentric contraction-induced injury; therefore, only traumatic freeze injury was utilized on mouse cohorts 2 and 3 that further investigated the link between mitochondrial damage and autophagy.
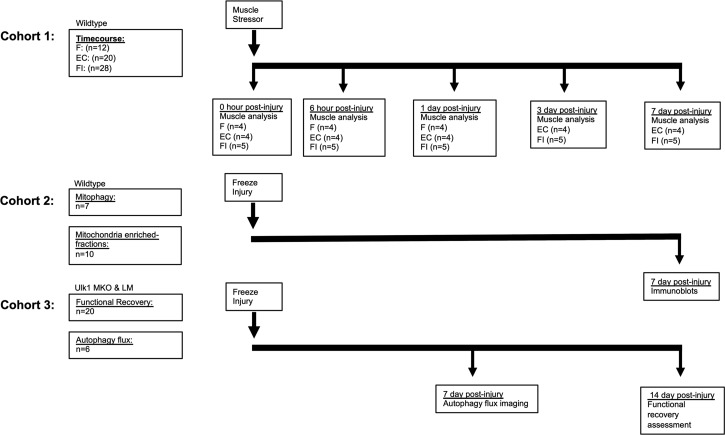
The first cohort of wild-type C57BL/6J mice were used to assess the time course of mitochondrial function and autophagy induction after various muscle stressors (Fig. 1). Briefly, mice were randomized into three groups: 1) a nondamaging metabolically fatiguing challenge (n = 12), 2) eccentric contraction-induced injury (n = 20) (5), and 3) traumatic freeze injury (n = 28). Tissues were analyzed for mitochondrial function, mitochondrial content, and autophagy induction immediately, 6 h, and 1 day post for all groups. No additional time points were assessed for the fatigue group because initial data indicated that there were no significant changes (Figs. 2 and 3). Additional time points, 3 and 7 days post, were assessed for the eccentric contraction-induced and freeze injury groups to characterize changes in mitochondrial function during the first week of recovery.
Fig. 1.
Study design. Diagram representing the 3 different cohorts of mice used in the study. Cohort 1 was used to determine the time course of mitochondrial dysfunction and autophagy induction after different muscle stressors (F, fatigue; EC, eccentric contraction; FI, freeze injury). Cohort 2 was used to determine the amount of mitochondrial-specific autophagy after traumatic freeze injury. Cohort 3 was used to determine the necessity of Unc-51 like autophagy activity kinase 1 (Ulk1)-mediated autophagy in the recovery of mitochondrial function and in regulating autophagy flux.
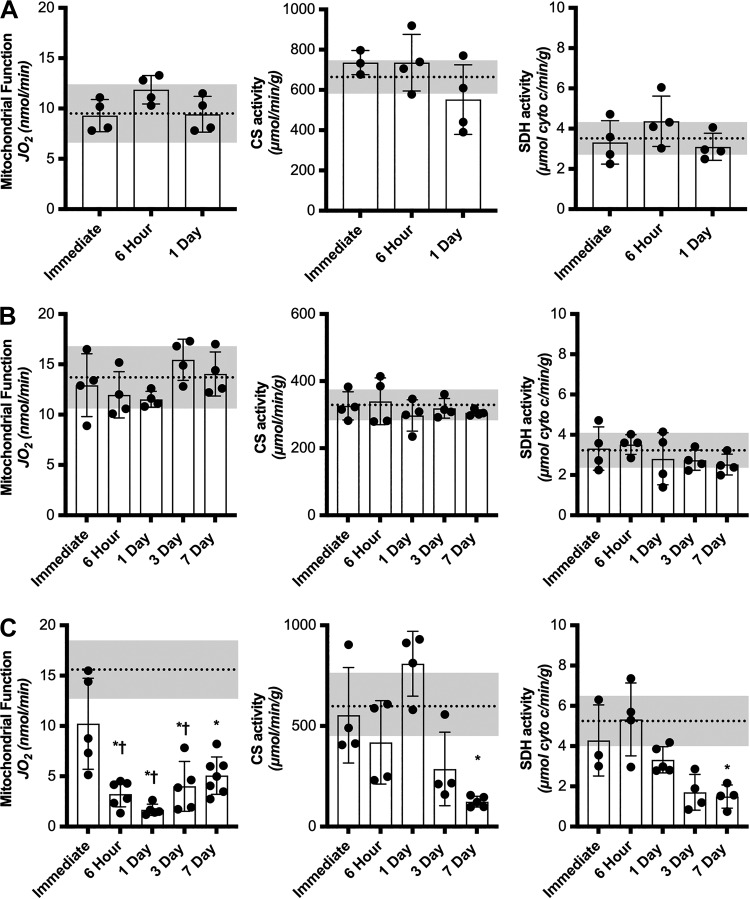
Fig. 2.
Time course of mitochondrial function and content changes after different muscle stressors. Oxygen consumption measurements of permeabilized tibialis anterior (TA) or extensor digitorum longus (EDL) muscle fibers and mitochondrial enzyme assays (citrate synthase, CS, and succinate dehydrogenase, SDH) immediately, 6 h, 1, 3, and 7 days after fatiguing protocol (n = 12) (A), eccentric contraction-induced injury (n = 20) (B), and traumatic freeze injury (n = 28) (C). Dashed line represents average contralateral control limb and shaded gray regions are ± SD of control limb. Stressed limb data are presented as means ± SD. Differences in mitochondrial function and content at different time points after muscle stress were analyzed by RM ANOVA with the repeated measures being the injured vs. uninjured contralateral control limb and the other factor being time. *Significantly different from uninjured. †Significantly different from immediate injured.
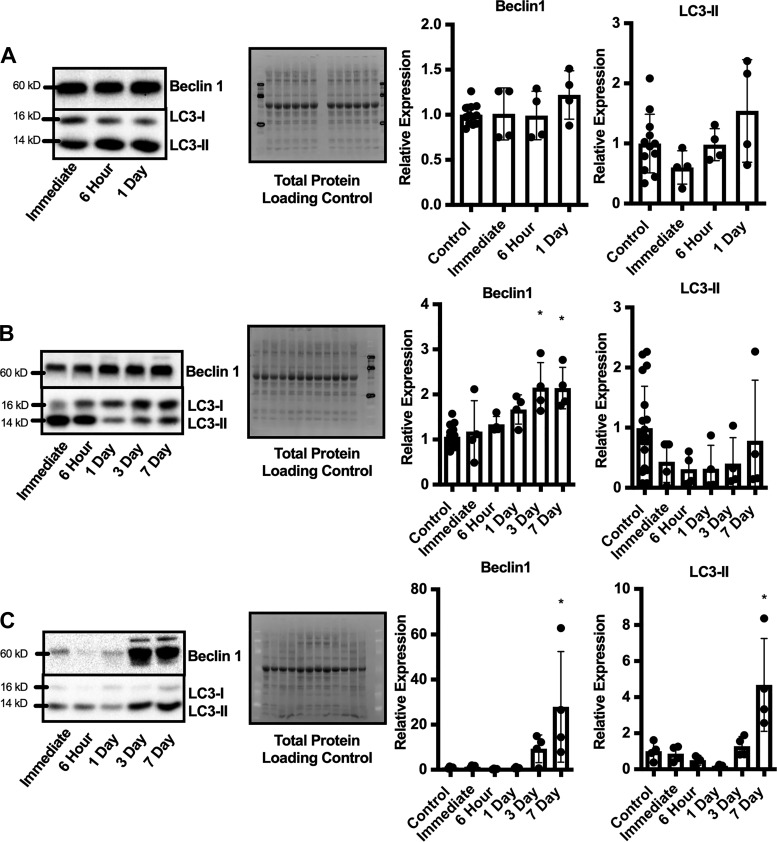
Fig. 3.
Time course of autophagy-related protein expression after different muscle stressors. Representative immunoblot and semiquantitative analysis of Beclin1 and microtubule-associated protein light chain B1 (LC3)II relative expression compared with control uninjured limb immediately, 6 h, 1, 3, and 7 days after fatiguing protocol (n = 12) (A), eccentric contraction-induced injury (n = 20) (B), and traumatic freeze injury (n = 28) (C). Blots were normalized to total protein as a loading control and are presented as relative expression to control uninjured limbs. Data are presented as means ± SD. Differences in protein expression at different time points after muscle stress were analyzed by RM ANOVA with the repeated measures being the injured vs. uninjured contralateral control limb and the other factor being time. *Significantly different from control limb.
A second cohort of wild-type C57BL/6J mice were used to analyze mitochondrial-specific autophagy following traumatic freeze injury based on results from the first cohort (Fig. 1). Unilateral freeze injuries were performed on all mice before randomization into two groups. The first group (n = 7) were used to probe mitochondrial-specific autophagy-related proteins at 7 days after injury via immunoblotting. The second group (n = 10) were used to determine the accumulation of autophagy-related proteins to the mitochondria relying on differential centrifugation and immunoblotting.
A third cohort of mice included Ulk1 MKO and LM mice to test the necessity of Ulk1 for autophagy flux (i.e., ongoing autophagy) and for recovery of mitochondrial function after injury (Fig. 1) (11, 16, 18). A first group of Ulk1 MKO and LM mice were electroporated with the green fluorescent protein (GFP)-microtubule-associated protein light chain B1 (LC3)-red fluorescent protein (RFP)-LC3ΔG obtained from Addgene (RRID: Addgene_84572) that allows fluorescent assessment of autophagy flux as a GFP portion is quenched during autophagy flux while the RFP portion serves as an external reference control. Mice were bilaterally transfected 3 wk before unilateral freeze injury (n = 6), and 7 days after injury, autophagy flux was assessed via GFP:RFP ratio captured through two-photon microscopy imaging. A second group of Ulk1 MKO and LM mice (n = 20) underwent unilateral freeze injuries and the recovery of peak-isometric dorsiflexion torque and mitochondrial function were determined at 14 days after injury. The selection of 14 days after injury was based on the results from cohort 1 showing that mitochondrial function was less than one-third of uninjured at 7 days after injury (Fig. 2) and on results from our previous studies indicating that 14 days is sufficient to observe differences in mitochondrial content with insufficient autophagy (7, 25).
Metabolic fatiguing protocol.
Mice were anesthetized using 1–2% isoflurane in oxygen, and left hindlimb was shaved and aseptically prepared. The foot was positioned into a foot-plate attached to the servomotor (model 129 300C-LR; Aurora Scientific, Aurora, ON, Canada) where the ankle joint was adjusted to a 90° angle and secured at the knee joint. Platinum-iridium (Pt-Ir) needle electrodes were inserted percutaneously on both sides of the peroneal nerve and the testing platform was maintained at 37°C throughout the optimization and muscle stressor protocols. Optimal muscle stimulation was achieved by finding peak-isometric torque of the ankle dorsiflexors [tibialis anterior (TA), extensor digitorum longus (EDL), extensor hallucis longus muscles] through increasing the current stimulating the peroneal nerve at a 200 Hz pulse frequency before executing the muscle stressor protocol. The fatiguing protocol consisted of 30 min of continuous 10 Hz stimulation which was modeled after muscle activation during a 30 min treadmill run (4).
Eccentric contraction-induced injury.
Mice were prepared, optimal muscle stimulation was verified following the methods listed above, and eccentric contraction-induced injury protocol was executed as previously described (5, 6). Briefly, for the eccentric contraction-induced injury, the foot was passively moved from the 0° position (perpendicular to the tibia) to 20° of dorsiflexion. The ankle dorsiflexor muscles were stimulated at 200 Hz for a 100-ms isometric contraction followed by an additional 50-ms stimulation while moving from 20° dorsiflexion to 20° plantarflexion at an angular velocity of 2,000°/s. Eccentric contractions were repeated every 10 s until a total of 100 electronically stimulated eccentric contractions were complete.
Freeze injury.
Freeze injury was performed as previously described (39). Before surgery, mice were anesthetized using isoflurane and given a local anesthetic injection of bupivacaine (5 mg/kg) (17). Afterwards, the left limb was aseptically prepared, a 1.5 cm incision was made over the TA muscle, and a steel probe cooled with dry ice was applied to the belly of the TA for 10 s. Upon completion of the freeze injury, the incision was closed with nylon suture and mice were administered meloxicam (2 mg/kg) for pain management immediately and again 12 h after surgery (39).
Oxygen consumption rates.
Mitochondrial function was assessed via oxygen consumption rates in dissected permeabilized muscle fiber bundles from both the stressed and the contralateral control limb using methods adapted from Kuznetsov et al. (19) and as we have previously described (33). To ensure that we were testing homogenously stressed muscle fibers, entire EDL muscles were permeabilized for the eccentric contraction-induced injury and fatiguing protocol and TA muscle fibers were dissected from the affected area for the freeze injury group. Oxygen consumption rates were made through the use of a Clark-type electrode (Hansetech) kept at a constant 25°C with constant stirring. State III respiration was accomplished by addition of glutamate (10 mM), malate (5 mM), succinate (10 mM), and ADP (5 mM). Oxygen consumption rates during State III respiration were normalized to tissue mass loaded into chamber.
Enzyme assays.
Both citrate synthase (CS) (17) and succinate dehydrogenase (SDH) enzyme assays were performed to quantify mitochondrial content in the stressed and contralateral control limbs after muscle fatigue, eccentric contraction-induced injury, and freeze injury. The portion of muscle remaining after fiber dissection for oxygen consumption rates was weighed and homogenized in 33 mM phosphate buffer (pH 7.4) at a muscle to buffer ratio of 1:40 using a glass tissue grinder. Citrate synthase activity was measured from the reduction of DTNB overtime as previously described (25). Succinate dehydrogenase activity was measured from the reduction of cytochrome c as previously described (13).
Immunoblotting.
The following autophagy-related proteins were analyzed using immunoblotting (1:1,000 dilution) to determine protein contents within whole muscle homogenates or enriched fractions, i.e., cytosolic and mitochondrial: Beclin1 (RRID:AB_1903911); LC3 B (RRID:AB_915950); dynamin-related protein DRP1 (D6C7) (RRID:AB_10950498); Pink1 (RRID:AB_11179069); Parkin (RRID:AB_10693040); BCL1 interacting protein BNIP3 (RRID:AB_2259284); CoxIV (RRID:AB_2085424); Ulk1 (RRID:AB_11178668). Protein was extracted from stressed and contralateral control muscles. Twenty-five micrograms of total protein was separated by SDS-PAGE, transferred onto a PVDF membrane, and immunoblotted as previously described (25). Immunoblots were normalized to total protein in lane and quantified using Bio-Rad Laboratories Image Laboratory software (Hercules, CA) (9, 36, 44).
Differential centrifugation.
Differential centrifugation was used to gain insight into mitochondrial-specific autophagy following muscle fiber damage utilizing an approach reported by Laker and colleagues (20) analyzing the amount of autophagy-related protein LC3 present in mitochondrial-enriched fractions. To obtain mitochondrial-enriched fractions and cytosolic fractions, differential centrifugation was performed on injured and contralateral uninjured TA muscles 14 days after injury as described (20). Briefly, muscles were homogenized in fractionation buffer (20 mM HEPES, 250 mM sucrose, 0.1 mM EDTA, plus protease and phosphatase) in a glass tissue homogenizer at a 1:20 tissue to buffer ratio. Homogenates were then spun at 800 g for 10 min at 4°C, supernatant was removed and then spun at 9,000 g for 10 min at 4°C. The supernatant was again removed and resuspended in an equal volume of 2× Laemmli buffer resulting in the cytosolic fraction. The remaining mitochondrial pellets were resuspended in fractionation buffer then spun at 11,000 g for 10 min at 4°C. Resulting mitochondrial-enriched pellets were resuspended in 20 μL of 2× Laemmli buffer resulting in the mitochondrial-enriched fraction. Both fractions were boiled for 5 min at 97°C, then frozen at −80°C until immunoblot analysis.
Autophagy flux plasmid transfection.
Autophagy flux was assessed utilizing the in vivo expression of a GFP-LC3-RFP-LC3ΔG plasmid imaged via two-photon microscopy. GFP-LC3-RFP-LC3ΔG plasmid (no. 84572, Addgene) was prepared by CsCl gradient centrifugation method from 1 liter inoculated LB media (30). In vivo electroporation of the TA muscle was performed as previously described (1). Briefly, 30 μg/μL of plasmid was injected into the TA. A BTX ECM 830 system equipped with 5 mm 2-needle arrays was used for electroporation. Three electrical pulses set at a voltage of 100 V and pulse length of 50 ms were used for transfection. After three pulses were completed the needles were reversed and three additional pulses were done with the same settings.
Two-photon scanning microscopy and image analysis.
We used our home-built microscope (34) for multiphoton microscopy of TA muscles. We used 940 nm excitation laser for excitation and GFP and 775 nm light for excitation of RFP (both 120 fs, linearly polarized). For GFP and RFP we used 509/22 nm and 585/40 nm (Semrock) filters, respectively. Bundles of muscle fibers were extracted, stabilized on a dissection gel, and placed under the microscope’s objective lens. Imaging was performed using a ×60 water immersion objective lens (Olympus LUMFLN 60x) directly on the tissue immersed in Krebs Ringer buffer. To process the images, we first denoised the images by 3D Gaussian blur filtering (standard deviation = 380 nm) and produced a binary mask from the RFP channel data using Otsu’s method. We then applied the same mask to both channels, and calculated the average fluorescence reading of the masked area in each channel, and the ratios of GFP to RFP for each stack.
Immunofluorescent staining for satellite cells.
Satellite cell dynamics were evaluated as previously described (41). Briefly, injured muscles from MKO mice (n = 6) and LM mice (n = 6) were isolated at 10 days postinjury and subjected to cryosectioning. Muscle sections were stained with primary antibody, Pax7 (1:5; DSHB at the University of Iowa) and Ki67 (1:1,000; RRID:AB_302459) overnight at 4°, followed with a secondary antibody stain at room temperature for 1 h. To evaluate satellite cell dynamics, muscle cross sections (at 3 representative levels) were used to enumerate the total number of satellite cells (Pax7+), proliferating satellite cells (Pax7+/Ki67+ and self-renewing satellite cells (Pax7+Ki67−).
Statistics.
Differences in mitochondrial function, mitochondrial content, and autophagy protein expression after different muscle stressors were analyzed by two-way repeated measures (RM) analysis of variance (ANOVA) with the repeated measures being the injured versus uninjured contralateral control limb and the other factor being time. Differential centrifugation immunoblots were analyzed by two-way RM ANOVA with the repeated measures being the injured versus uninjured contralateral control limb and the other factor being fraction (mitochondria-enriched or cytosolic). Ulk1 KO and LM comparisons were analyzed by two-way RM ANOVA with the repeated measures being the injured versus uninjured contralateral control limb and the other factor being genotype. All data were required to pass normality (Shapiro-Wilk) and equal variance tests (Brown-Forsythe F test) before proceeding with the two-way RM ANOVA. Significant interactions were tested with Tukey’s post hoc test using JMP statistical software (SAS, Cary, NC) to find differences between groups. Group main effects are reported where significant interactions were not observed. An α-level of 0.05 was used for all analyses and all values are means ± SD.
RESULTS
Time course of mitochondrial dysfunction and content after different muscle stressors.
Across all conditions of muscle stress, there was no effect of time on mitochondrial function or content in the contralateral control limb (P ≥ 0.55) therefore only the collective mean (dashed line) and standard deviations (gray region) are represented in each panel of Fig. 2. There was no difference in mitochondrial function or enzyme content between stressed and contralateral control limbs at any time point after the metabolic fatigue protocol (Main Effect: Limb, P ≥ 0.24, Fig. 2A). Similarly, there was no difference in mitochondrial function or content following the eccentric contraction-induced injury protocol (Main Effect: Limb, P ≥ 0.17, Fig. 2B). Mitochondrial function was significantly decreased 6 h after traumatic freeze injury (20% of uninjured), continued to decline to its lowest functional capacity 1 day after injury (12% of uninjured), and by 7 days after injury had recovered to ~34% of uninjured control limbs (Significant Interaction: P = 0.006, Fig. 2C). Mitochondrial content was not significantly different from contralateral control limbs until day 7 postinjury (Significant Interaction, P ≤ 0.021, Fig. 2C) suggesting a disproportionate loss of mitochondrial function early after freeze injury.
Time course of autophagy induction after different muscle stressors.
Immunoblots of Beclin1 and LC3II were analyzed to determine the time course of autophagy induction after different muscle stressors. There was no change in relative expression of Beclin1 or LC3II in the stressed limb compared with the control limb after the fatiguing protocol (P ≥ 0.728, Fig. 3A). Beclin1 expression increased 2-fold 3 days after the eccentric contraction-induced injury and remained elevated through 7 days postinjury (Significant Interaction, P = 0.014, Fig. 3B); however, no significant change was observed with LC3II (P = 0.9023). In contrast, traumatic freeze injury resulted in a robust autophagy induction evident by a 28-fold increase in Beclin1 expression and a 5-fold increase in LC3II at 7 days after injury (P ≤ 0.008, Fig. 3C).
Mitochondrial specific autophagy after traumatic freeze injury.
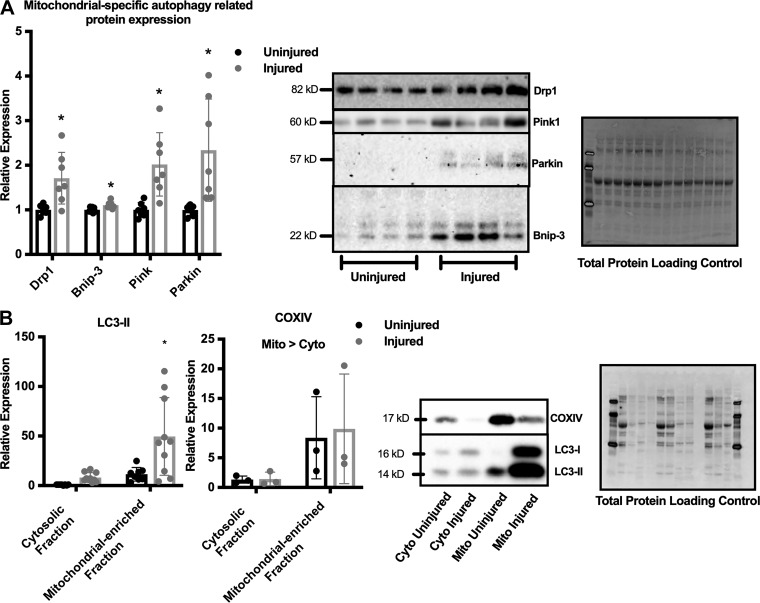
Because autophagy induction appeared to accompany mitochondrial dysfunction after freeze injury (Figs. 2 and 3), immunoblots of mitochondrial-specific autophagy-related proteins were analyzed. The mitochondrial fission-associated protein Drp1, the mitochondrial-specific autophagy machinery recruiting protein Bnip-3, and the damaged mitochondrial targeting proteins Pink1 and Parkin expression increased ~2-fold after injury (P ≤ 0.0138, Fig. 4A). Additionally, cytosolic fractions and mitochondrial-enriched fractions were subjected to immunoblot analysis of LC3II to further elucidate the extent of mitochondrial-specific autophagy. COXIV expression was increased 8-fold in the mitochondrial-enriched fractions compared with the cytosolic fractions (Fig. 4B) (COXIV Main Effect: Fraction, P = 0.049). Interestingly, LC3II expression was 37 times greater in the injured mitochondrial-enriched fractions compared with the injured cytosolic fractions, suggesting a large mitochondrial-specific autophagy response to traumatic freeze injury (Fig. 4B) (Significant Interaction, P = 0.017), in agreement with previous reports of accumulation of autophagy-related proteins at the mitochondria after physiological muscle stress (7, 20).
Fig. 4.
Mitochondrial-specific autophagy related protein expression and mitochondria localized autophagy response after freeze injury. A: semiquantitative analysis of dynamin-related protein (Drp1), BCL1 interacting protein 3 (Bnip-3), Pink, and Parkin protein expression 7 days after freeze injury and representative immunoblot images. (n = 7) Blots are normalized to total protein as a loading control. Differences in protein expression after injury were detected by RM ANOVA between uninjured and injured limbs. *Significantly different from uninjured limb. B: semiquantitative analysis of microtubule-associated protein light chain B1 (LC3)II expression and COXIV expression in cytosolic and mitochondrial-enriched fractions from mice 7 days after freeze injury followed by representative immunoblot images (n = 8). Blots are normalized to total protein as a loading control and presented as relative expression to uninjured cytosol fraction. Differences were analyzed by two-way RM ANOVA with the repeated measures being the injured vs. uninjured contralateral control limb and the other factor being fraction (mitochondria-enriched or cytosolic). *Significantly different from all other groups. Data are presented as means ± SD. DRP1; dynamin-related protein.
Recovery of strength, mitochondrial function, and mitochondrial content in Ulk1 MKO mice after traumatic freeze injury.
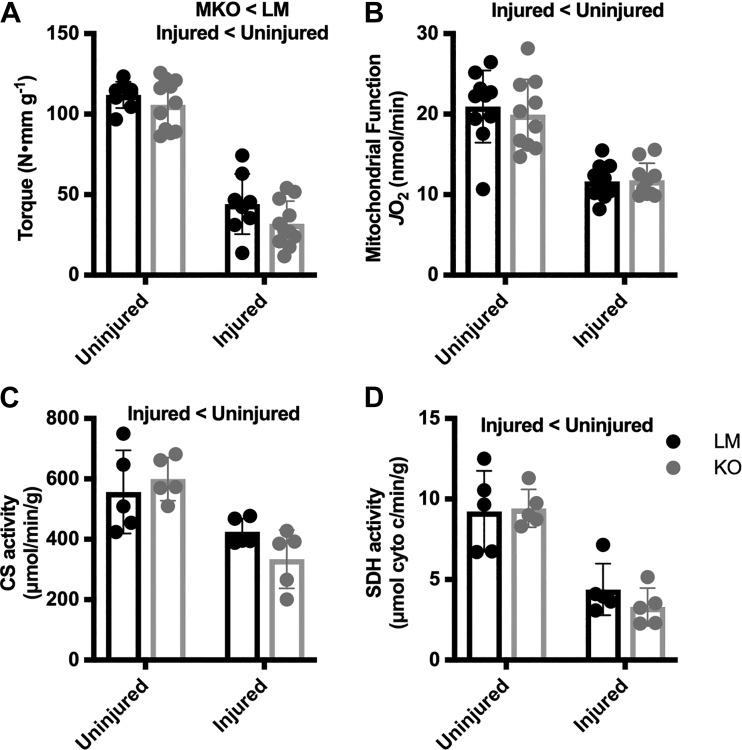
Mitochondrial-specific autophagy is mediated by the autophagy-related protein Ulk1 (7). We and others have investigated the role of Ulk1 following muscle stress and specifically mitochondrial stress (20, 25); however, whether Ulk1 is required for the recovery of mitochondrial function after injury has not been investigated. To ascertain the role of Ulk1-mediated autophagy in the recovery of mitochondrial function, we crossed myogenin-Cre mice with floxed-Ulk1 mice to generate muscle-specific Ulk1 knockout mice (MKO) (7). Prior to injury we observed no difference in max torque measurements between MKO and littermate (LM), genetically wild-type mice; however, 14 days after injury, MKO mice produced 27% less torque than the LM mice (Main Effect: Injury and Genotype, P ≤ 0.012, Fig. 5A). Mitochondrial function was decreased 43% in the injured limbs independent of genotype (Main Effect: Injury, P < 0.0001, Fig. 5B), and mitochondrial content was reduced by 34% and 58%, CS and SDH respectively, independent of genotype (Main Effect: Injury, P ≤ 0.0006, Fig. 5, C and D).
Fig. 5.
Muscle torque, mitochondrial function, and mitochondrial content before and after injury in Unc-51 like autophagy activity kinase 1 (Ulk1) muscle-specific knockout (MKO) mice. A: comparison of Ulk1 MKO (n = 10) and littermate (LM; n = 10) dorsiflexion muscle torque before (uninjured) and 14 days after freeze injury. B: oxygen consumption of permeabilized tibialis anterior (TA) muscle in injured and contralateral uninjured limbs 14 days after freeze injury. C and D: citrate synthase (CS) activity (C) and succinate dehydrogenase (SDH) activity (D) in TA muscles of LM (n = 5) and Ulk1 MKO (n = 5) mice in both injured and contralateral control limbs. Differences were analyzed by two-way RM ANOVA with the repeated measures being the injured vs. uninjured contralateral control limb and the other factor being genotype. Main effects are reported.
Autophagy-related protein induction in Ulk1 MKO mice after freeze injury.
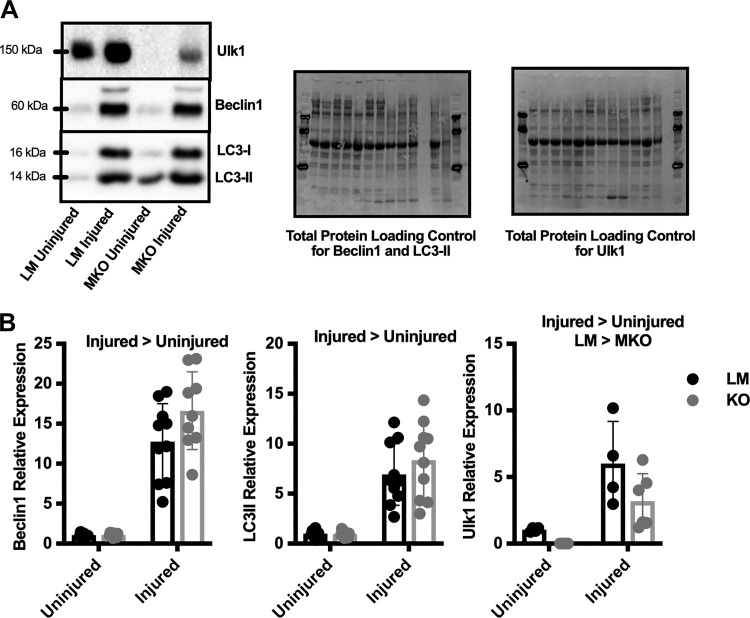
LC3II expression increased more than 7-fold in the injured limbs compared with the uninjured limb, independent of genotype (Main Effect: Injury, P ≤ 0.0001, Fig. 6B). Additionally, Beclin1 expression increased more than 14-fold with injury, independent of genotype (Main Effect: Injury, P ≤ 0.0001, Fig. 6B). Prior to injury, MKO mice had no Ulk1 expression as expected; however, after injury, both the LM and MKO mice had similar levels of Ulk1 protein content (Main Effect: Injury and Genotype, P ≤ 0.042, Fig. 6B).
Fig. 6.
Autophagy-related protein induction in Unc-51 like autophagy activity kinase 1 (Ulk1) muscle-specific knockout (MKO) mice after traumatic freeze injury. A and B: representative immunoblots (A) and semiquantitative analysis (B) of Beclin1, microtubule-associated protein light chain B1 (LC3)II, and Ulk1 protein expression in both injured and contralateral uninjured limbs of Ulk1 MKO (n = 10) and littermate (LM; n = 10) mice 14 days after freeze injury. Blots were normalized to total protein as a loading control and are presented as relative expression to LM uninjured limbs. Data are presented as individual values ± SD. Differences were analyzed by two-way RM ANOVA with the repeated measures being the injured vs. uninjured contralateral control limb and the other factor being genotype. Main effects are reported.
Reduced autophagy flux after injury in both LM and Ulk1 MKO mice.
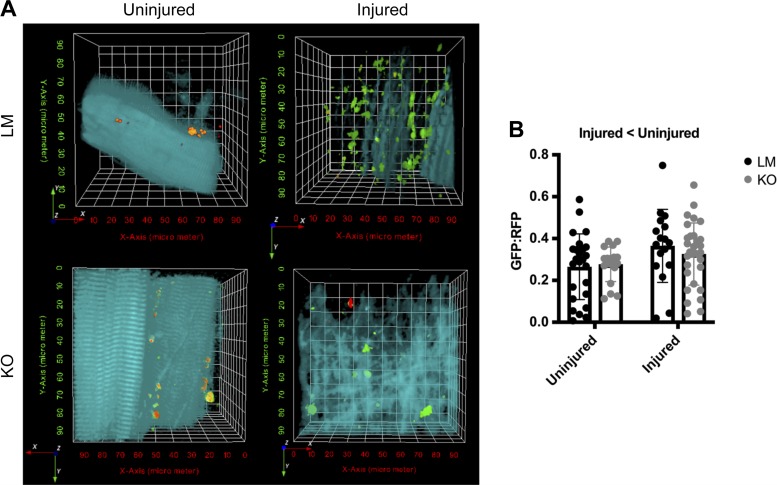
Static measurements of autophagy are a poor indicator of dynamic autophagy activity; therefore we measured autophagy flux using a GFP-LC3-RFP-LC3ΔG plasmid where the GFP portion is incorporated into the autophagosome and is consequently degraded when the autophagolysosome degrades. The RFP portion is cleaved and remains in the cytosol to serve as an internal control; therefore an increase in the GFP:RFP ratio signifies less autophagy flux. At 7 days after freeze injury, the GFP:RFP ratio increased 26% in the injured limb compared with the uninjured limb, independent of genotype (Main effect: Injury, P = 0.0189, Fig. 7). This is a striking feature as it suggests autophagy flux does not increase to meet the demands of increased number of autophagosomes (e.g., greater autophagy-related protein content, Figs. 2–3).
Fig. 7.
Autophagy flux is reduced in mice after traumatic freeze injury. A: representative two-photon microscopy images from tibialis anterior (TA) muscles of littermate (LM) and Unc-51 like autophagy activity kinase (ULK) muscle-specific knockout (MKO) mice transfected with green fluorescent protein (GFP)-microtubule-associated protein light chain B1 (LC3)-red fluorescent protein (RFP)-LC3ΔG plasmid. B: quantitative analysis of the GFP:RFP ratio which represents autophagosome turnover. Autophagy flux was greater in uninjured muscle as opposed to injured muscle. Data are presented as individual values ± SD. Differences were analyzed by two-way RM ANOVA with the repeated measures being the injured vs. uninjured contralateral control limb and the other factor being genotype. Main effects are reported.
Impaired satellite cell proliferation in Ulk1 MKO mice.
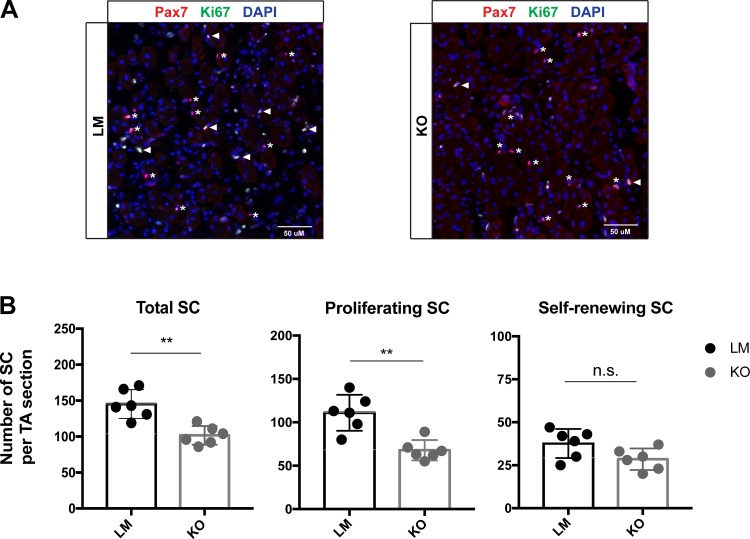
At the conclusion of our study we decided to explore satellite cell dynamics in Ulk1 KO mice because: 1) the strength deficit in the Ulk1 MKO mice, which is in agreement with our previous reports (17), suggests that Ulk1 knockout in myofibers impairs regenerative myogenesis; and 2) satellite cells are essential stem cells for regenerative myogenesis in skeletal muscle. Ten days postinjury there were a greater number of total and proliferating satellite cells in the freeze injured muscles of LM compared with Ulk1 MKO mice (P ≤ 0.016, Fig. 8), and no difference between mice in the number of self-renewing SC (P = 0.140). This result suggests that Ulk1 may influence satellite cell dynamics during muscle regeneration, even when Ulk1 deficiency is in the adult muscle fiber, not satellite cells.
Fig. 8.
Muscle-specific Unc-51 like autophagy activity kinase 1 (Ulk1) knockout impairs satellite cell (SC) proliferation after traumatic freeze injury. A: representative immunofluorescence imaging of muscle cross sections from littermate (LM; n = 6) and Ulk1 muscle-specific knockout (MKO; n = 6) mice at 10 days after injury. Arrowheads: Pax7+/Ki67+ proliferative satellite cells. Asterisks: Pax7+/Ki67- self-renewing satellite cells. B: numbers of total, proliferating, and self-renewing satellite cells per muscle cross section.
DISCUSSION
A primary goal for this study was to address several knowledge gaps in the field related to mitochondrial dysfunction after skeletal muscle stress, and the role of autophagy in mediating a response between the two. Mitochondria are appreciated as contributing to the regenerative potential, plasticity, and overall quality of skeletal muscle, and, therefore, investigating the muscle fiber-mitochondrial relationship may produce important targets for rehabilitation and disease prevention. However, there appear to be inconsistencies in the literature regarding what types of muscle stressors elicit mitochondrial dysfunction (20, 28, 29, 32), as well as the extent to which autophagy is necessary for the timely repair of mitochondrial dysfunction after muscle stress (7). Herein, we relied on oxygen consumption as the marker of mitochondrial function, enzyme activities of succinate dehydrogenase and citrate synthase as markers of mitochondrial content, and a muscle-specific Ulk1 knockout mouse to test the necessity of autophagy for the recovery of mitochondrial function and content.
The first knowledge gap we explored was the extent to which three often utilized muscle stressors (fatigue, eccentric contraction-induced injury, and traumatic injury) that produce a loss in muscle contractility (21, 39) and an autophagic response (3, 7, 25) will cause mitochondrial dysfunction, i.e., a decline in oxygen consumption. Reduced mitochondrial oxygen consumption was only observed after traumatic freeze injury and was not decreased until 6 h after injury (Fig. 2) in stark contrast to the immediate ~70% loss in force production (39). Fatiguing exercises and eccentric contraction-induced injury have been reported to cause mitochondrial dysfunction (20, 24) in contrast to our findings. A likely explanation for these conflicting findings may be the tools utilized to investigate mitochondrial dysfunction and/or damage. Specifically, immunohistological techniques like confocal microscopy used by Laker et al. (20) are useful for mechanistic investigations into localized mitochondrial events but do not necessarily reflect changes across the entire mitochondrial network; whereas oxygen consumption measurements test the functional capacity of the entire mitochondrial reticulum but do not capture more nuanced physiology such as fission and fusion events. It is irrefutable that functional, biochemical, and immunohistological approaches can provide valuable insight into mitochondrial physiology, yet when appropriate, future studies may consider the culminative advantage of combining more than one technique to avoid further inconsistencies in the literature.
We and others have previously reported that traumatic muscle injury results in a loss of mitochondrial content (7, 10, 25, 37), and herein we report that it also results in a loss of mitochondrial function (Fig. 2). A remaining question was the extent to which mitochondrial-specific autophagy, sometimes referred to as mitophagy, participates in the clearing of dysfunctional mitochondria after traumatic muscle injury. Laker et al. (20) demonstrated that mitochondrial-specific autophagy was critical for clearing damaged, i.e., ROS-producing mitochondria after muscle fatigue; therefore, it is logical to hypothesize that mitochondrial-specific autophagy may occur after a more severe muscle stressor such as traumatic muscle injury to clear away the dysfunctional mitochondria. To address this knowledge gap, we analyzed mitophagy-related proteins as well as autophagy-related proteins in mitochondrial-enriched fractions and cytosolic fractions. We found a 2-fold increase in mitophagy-related proteins and a robust accumulation of autophagy-related proteins localized to the mitochondria after freeze injury, suggesting that autophagy does participate in the clearance of dysfunctional mitochondrial after traumatic muscle injury (Fig. 4). This is the first study linking autophagy to mitochondrial dysfunction in injured skeletal muscle and understanding the process of clearing dysfunctional mitochondria after muscle injury may provide targets to facilitate muscle recovery.
To specifically test the extent to which mitochondrial-specific autophagy is important for muscle recovery after traumatic muscle injury we utilized a Ulk1 MKO mouse model. We and others have reported that Ulk1 may play an important role in both mitochondrial function and strength recovery after injury (7, 25). Our initial results suggested that Ulk1 is not required for the recovery of mitochondrial function after freeze injury (Fig. 5); however, there is a major consideration worth noting. Our Ulk1 MKO mouse model is a myogenin-Cre driven gene knockout, meaning Ulk1 is not expressed in adult muscle fibers, but is present in Pax7-expressing satellite cells. Quiescent satellite cells are activated upon injury, proliferate, differentiate, and ultimately provide new myonuclei for the regenerating fiber (43) leading to Ulk1 expression in the regenerated muscle fiber. Additionally, following traumatic muscle injury there are many other cell types that migrate into the injured territory to aid in muscle regeneration. These include inflammatory cells such as neutrophils and macrophages, fibro-adipogenic precursor cells (FAPs), fibroblasts, and endothelial cells all of which potentially express Ulk1 sufficient for autophagy induction (40). This premise is supported by our immunoblots showing Ulk1 protein content within the injured limbs of Ulk1 MKO mice (Fig. 6). This is a clear physiological limitation of this study and limits our ability to determine the necessity of Ulk1 for mitochondrial remodeling after traumatic injury. To circumvent this problem for future experiments, we are exploring the use of Pax7CreER mouse lines to effectively knockout Ulk1 in satellite cells and adult muscle fibers.
Previous muscle damage research, including our own, has failed to investigate the dynamic properties of autophagy after skeletal muscle injury. Specifically, our previous work (7, 25) and this current work (Fig. 3) show that the autophagy-related protein response scales to the magnitude of muscle damage after injury, but it remains unclear the extent to which autophagy flux matches the increases in autophagy machinery. Autophagy flux is described as the rate at which the entire process of autophagy occurs, meaning how quickly autophagosomes form around damaged content, fuse with lysosomes, and subsequently degrade (17). In contrast, an increase in autophagy machinery is a static measurement and is not always connected to an increase in autophagy flux. Static measurements of LC3II protein content are often used as proxies for autophagy flux but can actually indicate 1) an inhibition in lysosomal fusion to the autophagosome, 2) inhibition of autolysosome degradation, 3) an increase in the amount of autophagosomes (without an increase in flux), or 4) an increase in autophagy flux (17). Therefore, to assess changes in autophagy flux after injury, we used a GFP-LC3-RFP-LC3ΔG plasmid to assess autophagosome turnover through a GFP:RFP ratio. We found that autophagy flux was actually decreased in the injured limb compared with the uninjured (Fig. 7). This reduction in flux may represent a limiting factor, or bottleneck, in the ability of autophagy to quickly clear away damaged proteins and organelles, and it serves as a novel target to expedite the healing process in injured skeletal muscle. As mentioned above, we cannot make an interpretation on the role of Ulk1 in mediating autophagy flux due to the partial protein content rescue following injury (Fig. 6). However, it is intriguing to consider for future studies that the Ulk1 contribution from differentiating satellite cells may provide protection from changes in autophagy flux capacity when Ulk1 is deficient in the adult muscle fiber.
In this study, our finding that Ulk1 MKO impairs satellite cell proliferation (Fig. 8) raises an intriguing question: how does muscle fiber autophagy indirectly affect satellite cell dynamics? Satellite cells are essential stem cells for muscle regeneration and after traumatic injury (e.g., freeze injury), satellite cells exit quiescence and proliferate to form myoblasts (42). Myofiber-derived FGF2 and FGF6 are important mitogens for satellite cells during muscle regeneration (2, 8, 12, 15, 26). One possibility is that Ulk1-dependent autophagy may be pivotal for FGF2/6 expression and secretion in damaged myofibers. Alternatively, Ulk1-dependent autophagy may contribute to the degeneration of damaged myofibers by autophagy-induced cell death, which would be critical for setting the stage for satellite cell proliferation via timely recruitments of macrophages and FAPs. No matter what mechanism is involved, the observations in this study suggest an indirect positive influence of autophagy in myofibers on satellite cell proliferation, which may be therapeutically targeted in the future for improving muscle regeneration.
In conclusion, this work advances the field in three substantive ways. First, physiological muscle stressors that cause a decrease in muscle contractility and potentially result in mitochondrial stress do not always elicit a decline in mitochondrial function, as assessed via oxygen consumption. Second, despite an increase in autophagy machinery, autophagy flux is actually decreased following traumatic muscle injury, and this may represent a critical bottleneck to address with targeted therapies to enhance the recovery of muscle function. Third, autophagy appears to participate in the clearance of damaged mitochondria following traumatic injury in line with what has been reported following noninjurious muscle stressors (20). Therefore, future investigations into the role of autophagy following muscle stress associated with mitochondria should strongly consider an evaluation of mitochondrial function to complement a localized analysis of mitochondria stress and the use of a lysosomal inhibitor or plasmid to determine autophagy flux. Unfortunately, we were unable to fully determine the necessity of Ulk1 for timely recovery of mitochondrial function due to the limitation of the mouse model; however, we are intrigued by the strength deficits in the mice and potential crosstalk between Ulk1 in the adult muscle fiber and satellite cells during muscle regeneration.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1R01AR070178.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.N., W.M.S., K.F.T., L.J.M., and J.A.C. conceived and designed research; A.S.N., W.M.S., K.F.T., A.E.Q., A.B.F., G.H.M., A.Y., H.Y., and J.A.C. performed experiments; A.S.N., W.M.S., K.F.T., A.E.Q., A.B.F., G.H.M., A.Y., L.J.M., H.Y., and J.A.C. analyzed data; A.S.N., W.M.S., K.F.T., A.E.Q., A.B.F., G.H.M., A.Y., H.Y., and J.A.C. interpreted results of experiments; A.S.N., W.M.S., K.F.T., L.J.M., and J.A.C. prepared figures; A.S.N., W.M.S., H.Y., and J.A.C. drafted manuscript; A.S.N., W.M.S., K.F.T., A.E.Q., A.B.F., G.H.M., A.Y., L.J.M., H.Y., and J.A.C. edited and revised manuscript; A.S.N., W.M.S., A.E.Q., A.B.F., G.H.M., A.Y., L.J.M., H.Y., and J.A.C. approved final version of manuscript.
REFERENCES
- 1.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 16: 867–870, 1998. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JE, Mitchell CM, McGeachie JK, Grounds MD. The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res 216: 325–334, 1995. doi: 10.1006/excr.1995.1041. [DOI] [PubMed] [Google Scholar]
- 3.Ato S, Makanae Y, Kido K, Sase K, Yoshii N, Fujita S. The effect of different acute muscle contraction regimens on the expression of muscle proteolytic signaling proteins and genes. Physiol Rep 5: e13364, 2017. doi: 10.14814/phy2.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Call JA, Cochrane GD, Laker RC, Yan Z, Lowe DA. Exercise training improves plantar flexor muscle function in mdx mice. Med Sci Sports Exerc 44: 1671–1679, 2012. doi: 10.1249/MSS.0b013e31825703f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Call JA, Eckhoff MD, Baltgalvis KA, Warren GL, Lowe DA. Adaptive strength gains in dystrophic muscle exposed to repeated bouts of eccentric contraction. J Appl Physiol (1985) 111: 1768–1777, 2011. doi: 10.1152/japplphysiol.00942.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Call JA, Warren GL, Verma M, Lowe DA. Acute failure of action potential conduction in mdx muscle reveals new mechanism of contraction-induced force loss. J Physiol 591: 3765–3776, 2013. doi: 10.1113/jphysiol.2013.254656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Call JA, Wilson RJ, Laker RC, Zhang M, Kundu M, Yan Z. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. Am J Physiol Cell Physiol 312: C724–C732, 2017. doi: 10.1152/ajpcell.00348.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature 490: 355–360, 2012. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins MA, An J, Peller D, Bowser R. Total protein is an effective loading control for cerebrospinal fluid western blots. J Neurosci Methods 251: 72–82, 2015. doi: 10.1016/j.jneumeth.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duguez S, Féasson L, Denis C, Freyssenet D. Mitochondrial biogenesis during skeletal muscle regeneration. Am J Physiol Endocrinol Metab 282: E802–E809, 2002. doi: 10.1152/ajpendo.00343.2001. [DOI] [PubMed] [Google Scholar]
- 11.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461, 2011. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev 11: 2040–2051, 1997. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foltz SJ, Luan J, Call JA, Patel A, Peissig KB, Fortunato MJ, Beedle AM. Four-week rapamycin treatment improves muscular dystrophy in a fukutin-deficient mouse model of dystroglycanopathy. Skelet Muscle 6: 20, 2016. doi: 10.1186/s13395-016-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P. Autophagy maintains stemness by preventing senescence. Nature 529: 37–42, 2016. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 15.Kästner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem 48: 1079–1096, 2000. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algül H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso J et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222, 2016. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112: 1493–1502, 2008. doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 20.Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8: 548, 2017. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le G, Lowe DA, Kyba M. Freeze injury of the tibialis anterior muscle. Methods Mol Biol 1460: 33–41, 2016. doi: 10.1007/978-1-4939-3810-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27: 4184–4193, 2013. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 45: 2288–2301, 2013. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnar AM, Servais S, Guichardant M, Lagarde M, Macedo DV, Pereira-Da-Silva L, Sibille B, Favier R. Mitochondrial H2O2 production is reduced with acute and chronic eccentric exercise in rat skeletal muscle. Antioxid Redox Signal 8: 548–558, 2006. doi: 10.1089/ars.2006.8.548. [DOI] [PubMed] [Google Scholar]
- 25.Nichenko AS, Southern WM, Atuan M, Luan J, Peissig KB, Foltz SJ, Beedle AM, Warren GL, Call JA. Mitochondrial maintenance via autophagy contributes to functional skeletal muscle regeneration and remodeling. Am J Physiol Cell Physiol 311: C190–C200, 2016. doi: 10.1152/ajpcell.00066.2016. [DOI] [PubMed] [Google Scholar]
- 26.Olwin BB, Hauschka SD. Identification of the fibroblast growth factor receptor of Swiss 3T3 cells and mouse skeletal muscle myoblasts. Biochemistry 25: 3487–3492, 1986. doi: 10.1021/bi00360a001. [DOI] [PubMed] [Google Scholar]
- 27.Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S, Petrof BJ. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol 181: 583–592, 2012. doi: 10.1016/j.ajpath.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Rattray B, Caillaud C, Ruell PA, Thompson MW. Heat exposure does not alter eccentric exercise-induced increases in mitochondrial calcium and respiratory dysfunction. Eur J Appl Physiol 111: 2813–2821, 2011. doi: 10.1007/s00421-011-1913-4. [DOI] [PubMed] [Google Scholar]
- 29.Rattray B, Thompson M, Ruell P, Caillaud C. Specific training improves skeletal muscle mitochondrial calcium homeostasis after eccentric exercise. Eur J Appl Physiol 113: 427–436, 2013. doi: 10.1007/s00421-012-2446-1. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Russell DW. The inoue method for preparation and transformation of competent e. Coli: “ultra-competent” cells. CSH Protoc 2006: pdb.prot3944, 2006. doi: 10.1101/pdb.prot3944. [DOI] [PubMed] [Google Scholar]
- 31.Sandri M, Coletto L, Grumati P, Bonaldo P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J Cell Sci 126: 5325–5333, 2013. doi: 10.1242/jcs.114041. [DOI] [PubMed] [Google Scholar]
- 32.Silva LA, Bom KF, Tromm CB, Rosa GL, Mariano I, Pozzi BG, Tuon T, Stresck EL, Souza CT, Pinho RA. Effect of eccentric training on mitochondrial function and oxidative stress in the skeletal muscle of rats. Braz J Med Biol Res 46: 14–20, 2013. doi: 10.1590/1414-431X20121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southern WM, Nichenko AS, Shill DD, Spencer CC, Jenkins NT, McCully KK, Call JA. Skeletal muscle metabolic adaptations to endurance exercise training are attainable in mice with simvastatin treatment. PLoS One 12: e0172551, 2017. doi: 10.1371/journal.pone.0172551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tehrani KF, Latchoumane CV, Southern WM, Pendleton EG, Maslesa A, Karumbaiah L, Call JA, Mortensen LJ. Five-dimensional two-photon volumetric microscopy of in-vivo dynamic activities using liquid lens remote focusing. Biomed Opt Express 10: 3591–3604, 2019. doi: 10.1364/BOE.10.003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian W, Li W, Chen Y, Yan Z, Huang X, Zhuang H, Zhong W, Chen Y, Wu W, Lin C, Chen H, Hou X, Zhang L, Sui S, Zhao B, Hu Z, Li L, Feng D. Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS Lett 589: 1847–1854, 2015. doi: 10.1016/j.febslet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Vigelsø A, Dybboe R, Hansen CN, Dela F, Helge JW, Guadalupe Grau A. GAPDH and β-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J Appl Physiol (1985) 118: 386–394, 2015. doi: 10.1152/japplphysiol.00840.2014. [DOI] [PubMed] [Google Scholar]
- 37.Wagatsuma A, Kotake N, Yamada S. Muscle regeneration occurs to coincide with mitochondrial biogenesis. Mol Cell Biochem 349: 139–147, 2011. doi: 10.1007/s11010-010-0668-2. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Pickrell AM, Rossi SG, Pinto M, Dillon LM, Hida A, Rotundo RL, Moraes CT. Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool. Hum Mol Genet 22: 3976–3986, 2013. doi: 10.1093/hmg/ddt251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren GL, Summan M, Gao X, Chapman R, Hulderman T, Simeonova PP. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol 582: 825–841, 2007. doi: 10.1113/jphysiol.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wosczyna MN, Rando TA. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev Cell 46: 135–143, 2018. doi: 10.1016/j.devcel.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie L, Yin A, Nichenko AS, Beedle AM, Call JA, Yin H. Transient HIF2A inhibition promotes satellite cell proliferation and muscle regeneration. J Clin Invest 128: 2339–2355, 2018. doi: 10.1172/JCI96208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Licastro D, Cava E, Veronese N, Spelta F, Rizza W, Bertozzi B, Villareal DT, Hotamisligil GS, Holloszy JO, Fontana L. Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep 14: 422–428, 2016. doi: 10.1016/j.celrep.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 43.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeitler AF, Gerrer KH, Haas R, Jiménez-Soto LF. Optimized semi-quantitative blot analysis in infection assays using the Stain-Free technology. J Microbiol Methods 126: 38–41, 2016. doi: 10.1016/j.mimet.2016.04.016. [DOI] [PubMed] [Google Scholar]