Abstract
Aerobic exercise capacity is critical to bodily health. As a model to investigate the mechanisms that determine health and disease, we employed low (LCR) and high (HCR) capacity running rat models selectively bred to concentrate the genes responsible for divergent aerobic running capacity. To investigate the skeletal muscle contribution to this innate difference in running capacity we employed an approach combining examination of the myofilament protein composition and contractile properties of the fast fiber extensor digitorum longus (EDL) and slow fiber soleus (SOL) muscles from LCR and HCR rats. Intact muscle force experiments demonstrate that SOL, but not EDL, muscles from LCR rats exhibit a three times greater decrease in fatigued force. To investigate the mechanism of this increased fatigability in the LCR SOL muscle, we determined the myofilament protein composition and functional properties. Force-Ca2+ measurements demonstrate decreased Ca2+ sensitivity of single skinned SOL muscle fibers from LCR compared with that of HCR rats. Segregating SOL fibers into fast and slow types demonstrates that the decreased Ca2+ sensitivity in LCR SOL results from a specific decrease in slow-type SOL fiber Ca2+ sensitivity such that it was similar to that of fast-type fibers. These results identify that the altered myofilament contractile properties of LCR SOL slow-type fibers result in a fast muscle type Ca2+ sensitivity and the LCR muscle phenotype. Overall our findings demonstrate alterations of the myofilament proteins could contribute to fatigability of the SOL muscle and the decreased innate aerobic running performance of LCR compared with HCR rats.
Keywords: contractile proteins, muscle fatigue, myosin isoforms, single fiber, troponin isoforms
INTRODUCTION
Large-scale clinical studies over the past two decades show low exercise capacity to be a stronger predictor of morbidity and mortality relative to other commonly reported risk factors including hypertension, type II diabetes, obesity, and smoking (25). Dysfunctional aerobic energy metabolism has also been implicated in essentially all age-related disease conditions including cardiac arrhythmias and sudden cardiac death (2). The mechanisms, however, responsible for this association are not completely understood. In 1996, Koch and Britton (23) initiated a large-scale artificial divergent selection for low [low capacity runners (LCR)] and high [high capacity runners (HCR)] treadmill running in rats as a model to investigate untrained aerobic exercise capacity. At generation 6, LCR and HCR aerobic treadmill running capacity differed by 171% (22). As selective breeding has continued, these LCR and HCR rat models have been demonstrated to diverge in a number of physiological characteristics. These characteristics include that LCR rats have been shown to have higher body weight, higher mean blood pressure, higher fasting insulin and impaired glucose tolerance, higher visceral adiposity, higher plasma triglycerides (34), and lower maximal oxygen consumption (V̇o2max) (35).
Significant to the lower aerobic capacity performance of LCR rats is an impairment in skeletal muscle metabolism, impaired skeletal muscle O2 utilization (15, 18, 19), lower mitochondrial density (10, 31), and altered skeletal muscle gene expression consistent with impaired mitochondrial function (29, 30). While mitochondria are key determinants of oxidative capacity, the skeletal muscle contractile properties of these aerobic capacity models have not been determined. Given the mechanical loads imposed by exercise, we hypothesized that the difference in running capacity between LCR and HCR involves selection for alterations in contractility and fatigue resistance of the skeletal muscles.
While the selective breeding process has continued, here we studied LCR and HCR rats at the 10th generation. To determine the skeletal muscle contribution to the difference in LCR and HCR intrinsic running capacity, ex vivo muscle studies were conducted on fast fiber extensor digitoriun longus (EDL) and slow fiber soleus (SOL) muscles. The EDL and SOL muscles were chosen to investigate as they represent contrasting types of muscle. The EDL is a fast glycolytic muscle while the SOL is a slow oxidative muscle. Results demonstrate that the decrease in contractile force following fatigue in SOL muscle from LCR rats is three times greater than muscles from HCR rats without a fatigue difference in EDL muscles. To further investigate the mechanism of increased LCR fatigability in the SOL muscle we determined the myofilament protein composition and segregated SOL single fibers into fast and slow fiber types. Segregated fiber functional measurements demonstrate that the Ca2+ sensitivity of LCR SOL slow fibers is decreased compared with HCR slow fibers such that LCR SOL slow fibers are similar to that of fast EDL fibers. Together these results identify the specific change in SOL slow fiber properties that contribute to the increased fatigability of LCR SOL muscle and decreased aerobic running performance of LCR compared with HCR rats.
MATERIALS AND METHODS
Animal models.
All procedures were carried out with approval by the Medical College of Ohio or Case Western Reserve University Institutional Animal Care and Use Committees and were conducted in accordance with the Guiding Principles in the Care and Use of Animals as approved by the National Institutes of Health (U.S. Public Health Service).
The development of the LCR (low capacity runners) and HCR (high capacity runners) through generation 6 has been previously described in detail (22, 23). Briefly, artificial, two-way, selective breeding was used to create low and high lines for untrained aerobic capacity as measured by endurance running. A founder population of 80 male and 88 female genetically heterogeneous rats (N:NIH stock) was the phenotype for running capacity with 13 of the lowest and 13 of the highest capacity rats from each gender randomly paired for mating (16). At each subsequent generation, 11-wk-old offspring were tested for treadmill running capacity and subjected to within-family selection from 13 mating pairs (17). Rats in this study were from generation 10 of selective breeding and were phenotyped for untrained aerobic running capacity at the Medical College of Ohio (now The University of Toledo College of Medicine and Life Sciences) before being sent to Case Western Reserve University for muscle phenotype evaluation including intact isolated muscle contractile and skinned fiber contractile characterization. All rats used were males and were 5–6 mo old at the time of aerobic running capacity phenotype evaluation. Intrinsic aerobic running capacity was determined as previously described (23). Briefly, rats were acclimated to treadmill running for 5 min at a slope of 15° over 5 consecutive days. On days 1 and 2 the treadmill speed was 5 m/min while on days 3–5 the treadmill speed was 10 m/min. The following week a maximal treadmill running test was conducted daily over a 5-day period. The best single day of performance was recorded as the animal’s intrinsic exercise capacity. Each day rats were tested at a constant slope of 15° and a starting speed of 10 m/min with the speed increased by 1 m/min every 2 min until the rats reached exhaustion. The longest distance run before exhaustion was considered as the animals running capacity. This amount of treadmill running is below that required to produce a measurable change in aerobic capacity (4, 13).
Intact muscle force measurement.
Following untrained aerobic running capacity phenotyping at the Medical College of Ohio, rats were shipped to Case Western University for intact muscle experiments that were performed on muscles from the same running capacity phenotyped rats at 12–16 mo of age as previously described (5, 9, 11, 26, 28). Briefly, following CO2 euthanasia the intact EDL (10–12 muscles from 6 LCR and 6 HCR rats) and soleus (SOL; 10–12 muscles from 6 LCR and 6 HCR rats) muscles were mounted between an isometric force transducer and the stationary post on a Radnoti (Monrovea, CA) system continually perfused with a modified Ringer solution [in mM: 142 NaCl, 4.0 KCl, 2.5 CaCl2, 2.0 MgCl2, 5.0 HEPES, and 10 glucose, pH 7.4 and continuously bubbled at room temperature (21°C) with 100% O2]. Isolated muscles were then treated with a fatigue protocol as previously described (5, 9, 11, 26, 28). Briefly, muscles were stimulated at a range of frequencies at the optimal muscle length of each muscle tested, until the frequency producing maximal tetanic force (Tmax) was found and then allowed to equilibrate for 20 min stimulated with a 500-ms electrical pulse-train (330 mA, ~70 Hz for EDL, ~40 Hz for SOL) administered with a periodicity of 1 min at the frequency that produced Tmax (1 tetanus/min with a 500-ms duration, thus a duty cycle of ~2%). Following 20-min equilibration, the muscles in the fatigue groups were subjected to a 5-min fatiguing protocol consisting of the same stimulatory pattern administered at a 1-s periodicity (duty cycle of 50%). Following the fatigue treatment, muscles were allowed to recover for 20 min at 20% duty cycle. Tmax for each muscle during the experimental protocol was normalized to the 100% value, i.e., the maximal tetanic contraction just before the fatigue treatment. Tmax was also normalized to the cross-sectional area of each muscle and is presented in millinewton per square millimeter (mN/mm2).
Skinned muscle force measurement.
Skinned muscle experiments were conducted on muscles from rats at 12–16 mo of age as previously described (8, 11, 20). Single EDL (7 fibers from LCR and HCR rats) and single SOL (8 fibers from LCR and HCR rats) fibers from freshly isolated muscles were dissected in relax solution [in mM: 1 Mg2+, 1 Mg-ATP, 15 phosphocreatine, 140 potassium methanesulfonate, and 50 imidazole; 170 ionic strength, 20 mM EGTA, pCa >8.5, pH 7.0 at room temperature (21°C)]. Following dissection, single fibers were exposed to relaxing solution containing 0.5% wt/wt Triton X-100 and protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM leupeptin, 1.0 mM benzamidine, and 10 µM aprotinin) for 30 min to permeabilize the sarcolemmal membrane and all subcellular organelles (7, 8). Single fibers were then mounted between an optoelectric force transducer (Scientific Instruments GmbH, Heidelberg, Germany) and a movable arm by wrapping the fibers three times around with small stainless steel wires. After mounting, muscle fibers were briefly exposed to pCa 6.0 to secure the mounting, relaxed in pCa 8.5 and stretched to optimal length (sarcomere length ~2.6 ± 0.1 µm determined with laser diffraction) (8), and the diameter of the fiber was measured. Single muscle fibers were then exposed to solutions of varying Ca concentrations (pCa 8.5 to 4.0) to determine the force versus pCa relationship. The composition of all solutions was calculated by using a computer program (Borland International, Scotts Valley, CA) employing the stability constants (14). At the end of each experiment, sarcomere length (SL) and the diameter of each fiber were measured again. Fibers where the SL changed by more than 0.1 µm during the force versus pCa study were discarded (<10% of all fibers). Each force versus pCa relationship was analyzed as described previously (7, 8, 11). Maximum calcium-activated force (Fmax; in mN/mm2) was recorded and normalized to the cross-sectional area of each fiber or to the maximal force produced by each fiber (100%, Fmax). A computer program (Sigma Plot 5.0 and Origin 6.0, Jandel Scientific) was used to fit the force versus pCa curve for each fiber to the Hill equation: % Fmax = 100[Ca2+]n/[(Ca50)n + [Ca2+]n]. Ca2+ sensitivity was evaluated from Ca50 (the Ca concentration producing half-maximal force). The steepness of the curve was evaluated from n, the Hill coefficient. After each parameter was obtained for individual fibers, an average force versus pCa was calculated.
Biochemical analyses of single muscle fibers.
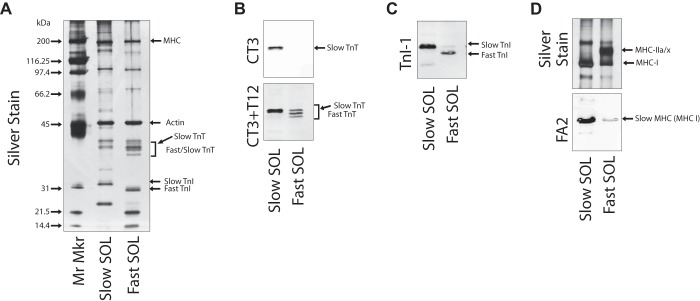
These experiments followed previously established protocols (11, 21, 27, 33, 36). Briefly, fibers from the skinned muscle contractility experiments were rapidly removed from their anchors, transferred to microtubes containing 10 µl of 1% SDS-buffer, and stored at −80°C for biochemical analyses. The total protein extract of these single muscle fibers was resolved by SDS-PAGE on 14% Laemmli gels with an acrylamide-to-bisacrylamide ratio of 180:1 and visualized by silver stain or transferred to nitrocellulose for Western blotting with the mAbs FA2 [type I myosin heavy chain (MHC)], CT3 [slow troponin (Tn) T], T12 (fast TnT), and TnI-1 (TnI) (20).
Statistics.
Data are presented as means ± SD. SigmaStat 3.0 software (SPSS, Chicago, IL) was used for all statistical analyses. The criterion for statistical significance was a P ≤ 0.05. For statistical analysis, either t test or one-way ANOVA (SigmaStat) followed by Tukey's post hoc test was used where appropriate.
RESULTS
Animal model.
The present study used the LCR and HCR rats developed by Koch and Britton (23) that had undergone 10 generations of aerobic capacity selection. At this generation, untrained LCR rats reached exhaustion 3.4 times faster and ran 7.5 times shorter in distance than HCR rats on an endurance treadmill running test (Table 1). This substantial difference in intrinsic, untrained aerobic treadmill running performance between HCR and LCR groups provides an ideal model system to investigate the effects of genetic selection for untrained aerobic performance on skeletal muscle function.
Table 1.
HCR and LCR rat running characteristics
| Body Weight, g | Running Time, min | Running Distance, m | |
|---|---|---|---|
| HCR | 253 ± 9 | 47.61 ± 1.04 | 1,021 ± 35 |
| LCR | 315 ± 12* | 10.89 ± 1.26* | 137 ± 20* |
Values are means ± SD; n = 12 animals. LCR, low capacity runners; HCR, high capacity runners.
P < 0.05 by t test.
Artificial selection for aerobic performance alters intact skeletal muscle function.
To investigate the effects of divergent aerobic selection on fast fiber type skeletal muscle performance, intact EDL muscles were isolated from the hindlimb of untrained LCR or HCR rats and subjected to a fatigue protocol to determine the muscles resistance to intermittent fatigue. At the end of the equilibration period, maximal developed tension in EDL muscles from LCR rats was 17% greater than muscles from HCR rats (Table 2). Following fatigue, however, there was no difference in the loss of contractile force, while upon recovery from fatigue LCR EDL muscle force was decreased compared with HCR muscles indicating a decreased recovery from fatigue (Fig. 1A).
Table 2.
Ex vivo EDL and SOL muscle performance
| Equilibration Force, mN/mm2 | Fatigue, % Equilibration Force | Recovery, % Equilibration Force | |
|---|---|---|---|
| EDL | |||
| HCR | 290 ± 43 | 7 ± 4 | 58 ± 4 |
| LCR | 350 ± 53* | 6 ± 4 | 47 ± 6* |
| SOL | |||
| HCR | 170 ± 28 | 79 ± 12 | 90 ± 10 |
| LCR | 210 ± 38 | 37 ± 10* | 82 ± 9 |
Values are means ± SD; n = 12 animals. EDL, extensor digitorum longus; SOL, soleus; LCR, low capacity runners; HCR, high capacity runners.
P < 0.05 vs. HCR for that muscle by t test.
Fig. 1.
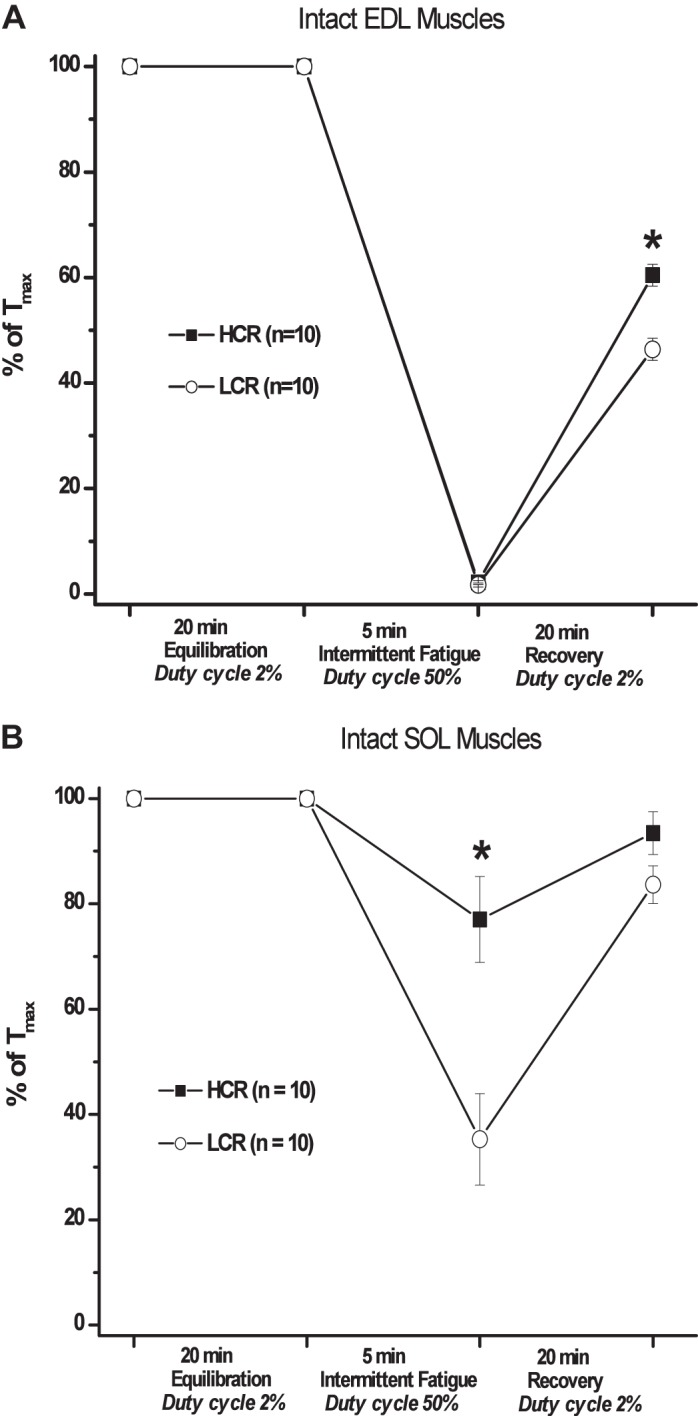
Intact ex vivo muscle force development. Force development of isolated muscles was determined before, following 5-min intermittent fatigue and after 20-min recovery and represented as a percentage of maximal tetanic force at the end of the equilibration period (% of Tmax). A: force of isolated extensor digitorum longus (EDL) muscles was similarly decreased in high capacity runner (HCR; black square) and low capacity runner (LCR; white circle) muscles following fatigue treatment and LCR force remained decreased following recovery compared with HCR. B: force of isolated soleus (SOL) LCR muscle was half that of HCR following fatigue but similar after recovery. *P < 0.05.
To investigate the effects of divergent aerobic selection on slow fiber type skeletal muscle performance, the resistance to fatigue of isolated LCR and HCR SOL muscles was determined using the same fatigue protocol. Before fatigue, the contractile function of SOL muscles from both LCR and HCR was not different (Table 2). Following fatigue, force development in LCR SOL muscles was half that of HCR muscles, while upon recovery from fatigue both LCR and HCR SOL muscles exhibited similar contractile force (Fig. 1B). These results demonstrate the artificial selection for aerobic performance, in the absence of training, altered the fatigue resistance of the slow-type SOL, but not the fast-type EDL, muscle and recovery from fatigue in the EDL but not SOL. Together these finding suggest a fiber type-specific alteration of genetically determined innate aerobic performance.
Increased LCR muscle fatigability results from alterations in the myofilament.
Altered intact skeletal muscle fatigue following the selection for aerobic performance could result from nonforce-producing cellular properties, such as altered metabolism and Ca2+ cycling, or from alterations of the properties intrinsic to myofilament force production itself. To determine the contribution of myofilament force production to muscle fatigue from LCR and HCR rats independent of other functions, we measured force development in single skinned fibers where Ca2+ and metabolic substrate concentrations were experimentally controlled. Nonfatigue maximal force development of skinned fast-type EDL fibers from LCR and HCR rats was not different (Table 3), and the Ca2+ sensitivity of force development in EDL fibers from LCR rats was minimally decreased compared with that of fibers from HCR rats (Fig. 2A). Similarly, maximal force development in SOL fibers from LCR and HCR rats was not different (Table 3); however, the Ca2+ sensitivity of SOL fibers from LCR rats was dramatically decreased compared with fibers from HCR rats (Fig. 2B). Average mechanical properties of EDL and SOL skinned fibers are presented in Table 3. See Supplemental Fig. S1 for ANOVA analysis comparing Fast SOL, Slow SOL, and EDL fiber mechanics (Supplemental Material is available at https://doi.org/10.35092/yhjc.11319998). These results demonstrate the selection for aerobic performance alters innate SOL myofilament Ca2+-sensitive force production.
Table 3.
Skinned fiber mechanical properties
| Fmax, mN/mm2 | EC50, μmol/L | Hill | n | |
|---|---|---|---|---|
| EDL | ||||
| HCR | 339 ± 39 | 1.50 ± 0.023 | 2.47 ± 0.40 | 7 |
| LCR | 379 ± 45 | 1.62 ± 0.040* | 2.62 ± 0.23 | 7 |
| SOL | ||||
| HCR | 261 ± 41 | 1.05 ± 0.001 | 1.97 ± 0.13 | 8 |
| LCR | 253 ± 33 | 1.49 ± 0.002* | 2.11 ± 0.23 | 8 |
| SOL | ||||
| HCR Fast | 372 ± 69 | 1.29 ± 0.020 | 2.15 ± 0.12 | 3 |
| HCR Slow | 199 ± 34 | 0.91 ± 0.050 | 1.78 ± 0.20 | 5 |
| LCR Fast | 352 ± 69 | 1.65 ± 0.004 | 2.32 ± 0.28 | 3 |
| LCR Slow | 194 ± 21 | 1.37 ± 0.002 | 1.89 ± 0.25 | 5 |
Values are means ± SD; n, number of fibers. LCR, low capacity runners; HCR, high capacity runners; Fmax, maximally developed tension normalized to cross-sectional area; EC50, Ca2+ concentration required to develop 50% maximal tension; Hill, slope of the tension-Ca2+ plot. See Supplemental Fig. S1 for ANOVA analysis comparing Fast soleus (SOL), Slow SOL, and extensor digitorum longus (EDL) fiber mechanics (https://doi.org/10.35092/yhjc.11319998).
P < 0.05 LCR vs. HCR for EDL and SOL muscles by t test.
Fig. 2.
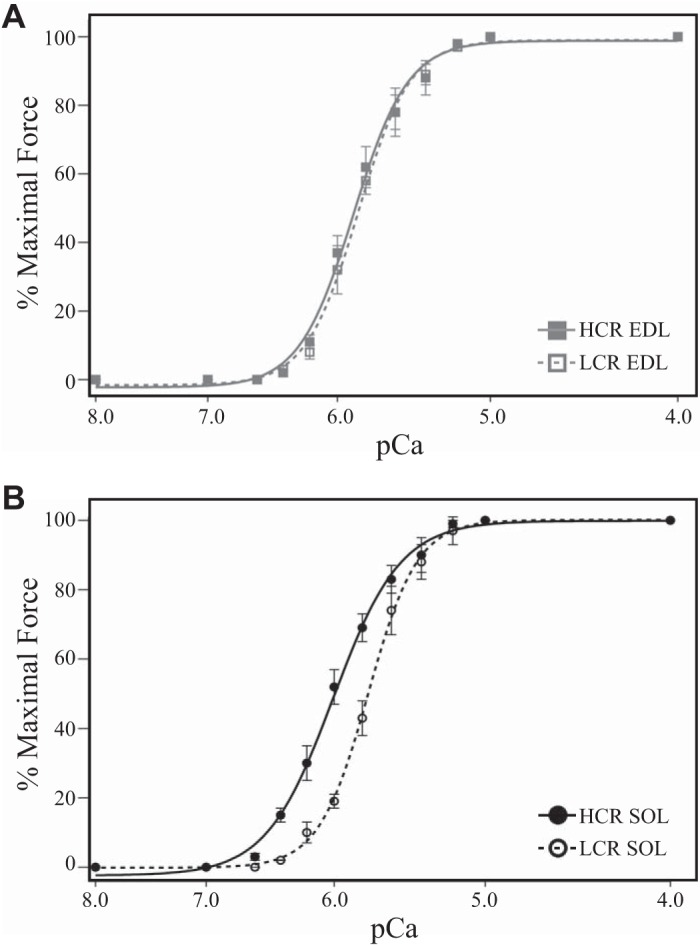
Skinned fiber force-pCa relationship. A: the Ca2+-sensitive force development of high capacity runner (HCR; gray square) and low capacity runner (LCR; white square) extensor digitorum longus (EDL) fibers was minimally different. B: the Ca2+-sensitive force production of LCR soleus (SOL; white circle) fibers was significantly decreased compared with that of HCR SOL (black circle) fibers.
To further explore the myofilament mechanism responsible for the LCR decrease in Ca2+ sensitivity of SOL muscles, we determined the myofilament protein composition of SOL muscles from LCR and HCR rats. Whole muscle homogenates from LCR and HCR rats SOL muscle express a mix of fast and slow troponin isoforms (Fig. 3A). To identify fast and slow fibers from SOL muscle for functional comparisons, all SOL single fibers used in determining the force-pCa relationship were collected and individually examined with silver-stained SDS-PAGE gel and Western blot to assess their myofilament protein isoform content. Single SOL fibers from both LCR and HCR rats demonstrated one of two distinct isoform expression patterns containing either slow TnT, slow TnI, and slow MHC-I (Slow SOL) or fast TnT and TnI with mixed amounts of slow MHC-I and fast MHC-IIa or MHC-IIx (Fast SOL) (Fig. 3, B–D). LCR and HCR rat SOL muscles both contained Fast SOL and Slow SOL type single fibers in similar amounts as previously demonstrated (10). Since the phenotype of type IIx and type IIa MHC is debated, SOL fibers were separated into Slow SOL and Fast SOL groups based on their troponin isoform content (6).
Fig. 3.
Soleus (SOL) fiber myofilament protein isoform expression. A: silver stain of representative single SOL fiber homogenates demonstrating two classes of SOL fibers that express either slow troponin (Tn)T, slow TnI, and slow myosin heavy chain (MHC)-I (Slow SOL) or fast TnT, fast TnI, and mixed slow MHC-I and fast MHC-IIa and MHC-IIx (Fast SOL). B–D: Western blots of representative single Slow SOL and Fast SOL fiber homogenates with mAb CT3 (slow skeletal muscle TnT specific) alone or in combination with mAb T12 (fast skeletal muscle TnT specific) (B), mAb TnI-1 (fast and slow skeletal muscle TnI) (C), and silver stain or FA2 (type I slow MHC specific) (D). Mr Mkr, molecular marker.
To investigate the effect of LCR and HCR aerobic selection on myofilament fiber type force development, we segregated SOL fibers by the troponin isoform expressed into LCR and HCR Fast SOL and Slow SOL single fibers. Consistent with the properties of the pooled fibers (Fig. 2) and their slow troponin isoform content (Fig. 3), HCR Slow SOL fiber Ca2+ sensitivity was increased compared with that of the HCR Fast SOL and HCR EDL fibers that express fast troponin isoforms (Fig. 4A). Contrary to its slow troponin isoform content, LCR Slow SOL fiber Ca2+ sensitivity was only minimally increased compared with that of LCR Fast SOL and LCR EDL fibers that express fast troponin isoforms (Fig. 4B). Unlike the HCR Slow SOL fibers, LCR Slow SOL fiber Ca2+ sensitivity was not different from that of the HCR EDL fast-type fibers (Fig. 4C). No differences were observed between LCR and HCR groups in the amount of maximal force developed. Average mechanical properties of the skinned SOL Fast and SOL Slow fibers are presented in Table 3. The finding that, contrary to its slow troponin isoform content, LCR Slow SOL fiber Ca2+ sensitivity is similar to that of the fast-type fibers (LCR EDL, HCR EDL, LCR Fast SOL, and HCR Fast SOL) while HCR Slow SOL fiber Ca2+ sensitivity was increased, demonstrates the loss of Ca2+ sensitivity in LCR Slow SOL fibers, and not LCR Fast SOL fibers, as contributing to the increased fatigability observed in the intact SOL muscle of LCR rats.
Fig. 4.
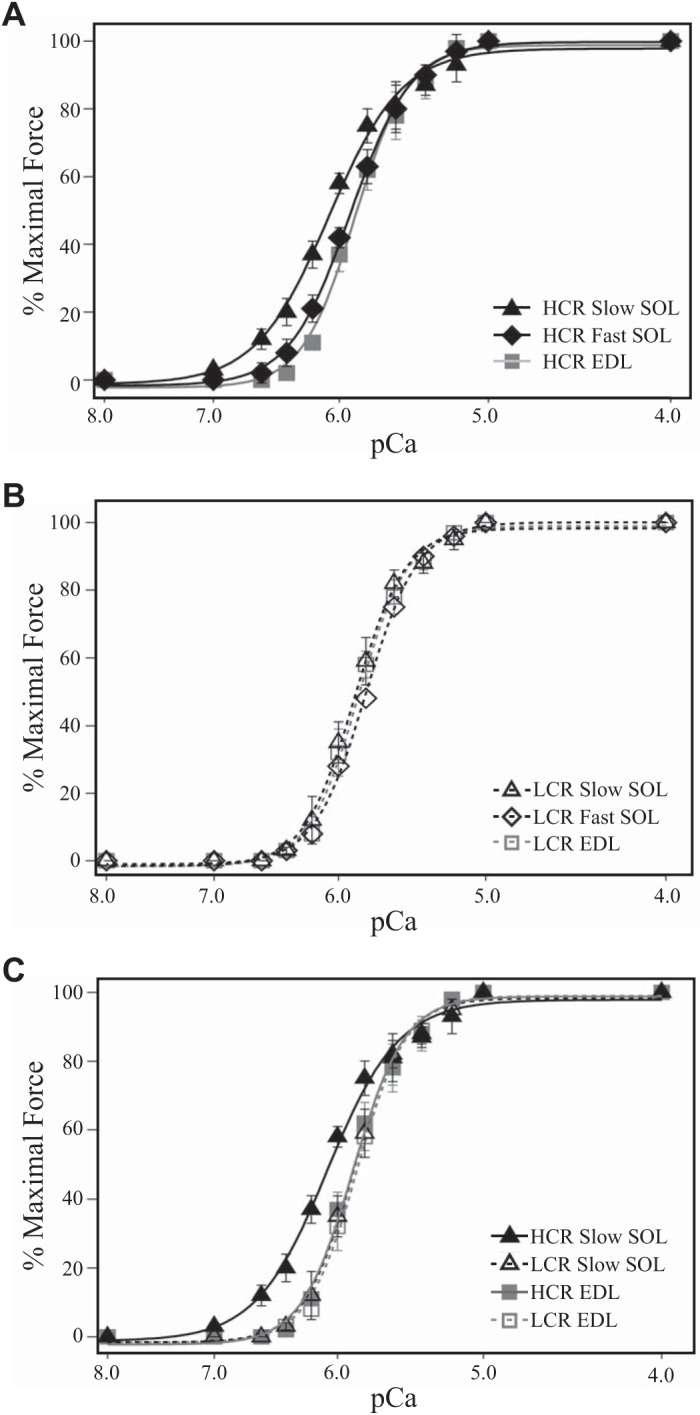
Skinned force-pCa relationship in fast and slow-type muscle fibers. A: Ca2+-sensitive force development of high capacity runner (HCR) Slow soleus (SOL) fibers (black triangle) was increased compared with both HCR Fast SOL (black diamond) and extensor digitorum longus (EDL; gray square) fibers. B: contrary to its slow troponin isoform content, the Ca2+-sensitive force development of low capacity runner (LCR) Slow SOL (white triangle) fibers was not different from LCR Fast SOL (white diamond) or EDL (white square) fibers. C: Ca2+-sensitive force development of HCR Slow SOL fibers was increased compared with that of LCR Slow SOL that was not different from EDL fibers.
DISCUSSION
To investigate the differences in intrinsic aerobic running capacity of rats selectively bred for low and high aerobic running capacity (23) we employed an approach combining examination of the myofilament protein composition and contractile properties of contrasting fast- and slow-type skeletal muscles from LCR and HCR rats. Major findings from this study demonstrate the following: 1) nonfatigue performance of intact SOL muscles from LCR and HCR rats was not different; 2) force development in intact SOL muscles from LCR rats was half that of HCR SOL muscles following fatigue while there was no difference in fatigue between LCR and HCR EDL muscles; 3) the Ca2+ sensitivity of skinned SOL fibers from LCR rats was decreased compared with HCR SOL fibers; and 4) the Ca2+ sensitivity of slow troponin expressing SOL fibers from the LCR rat was decreased such that they were no longer different from fast troponin expressing SOL or EDL fibers. Together these findings demonstrate the decreased Ca2+ sensitivity in LCR SOL slow troponin fibers as a contributor to the increased fatigability of SOL muscle and the decreased aerobic running capacity of LCR rats.
LCR selection increases intact muscle fatigability.
Aerobic capacity can be limited by whole body metabolic, pulmonary, and cardiovascular functions as well as factors intrinsic to the muscle itself. Trovinen et al. (33), previously demonstrated maximal torque development of the LCR rat triceps surae muscle complex was decreased following in vivo fatigue compared with that of the HCR rat. This finding of decreased LCR muscle torque supports altered muscle function, yet decreased torque in this model may also result from bodily contributors. The specific role of the muscle to LCR muscle fatigue is unknown.
To investigate if increased LCR rat fatigue results from mechanisms intrinsic to the muscle we evaluated isolated muscle performance, devoid of body influence. We demonstrate isolated LCR SOL muscle force development following fatigue is half that of HCR SOL muscle (Fig. 1B). This increased SOL fatigability was not caused by decreased innate force producing ability as LCR and HCR SOL intact muscles exhibited similar force in the absence of fatigue (Table 2). Furthermore, the LCR decrease in fatigue force was not observed in the LCR EDL muscle indicating a SOL muscle-specific effect (Fig. 1). These findings demonstrate that selected inheritable changes intrinsic to the SOL muscle are sufficient to decrease fatigue resistance and contribute to the decreased running capacity of LCR rats.
LCR selection alters myofilament function contraction.
The finding that isolated SOL muscle from LCR rats exhibits decreased force upon fatigue demonstrates a muscle level contribution to LCR decreased aerobic capacity. Previous studies have identified that LCR rat skeletal muscles exhibit impaired O2 utilization (15, 18, 19), lower mitochondrial density (10), and skeletal muscle gene expression consistent with impaired mitochondrial function (31). In addition to this altered muscle metabolism, decrease LCR SOL fatigue could also result from altered intrinsic muscle myofilament mediated force generation.
To determine the effects of selection for decreased aerobic capacity on the LCR myofilament function we investigated force development in skinned fiber preparations. In the skinned fiber preparation, treatment of the fiber with Triton X-100 (a nonionic detergent) removes all membranes (including sarcolemmal, sarcoplasmic reticulum, and mitochondrial) rendering the fiber devoid of Ca2+ and metabolic regulatory systems. Bathing the skinned fiber in solutions containing controlled Ca2+ concentration and metabolic substrates results in force development dependent solely on thin filament Ca2+ activation and myosin motor contractile properties. At saturating Ca2+ activation, skinned fiber maximal force production is largely dependent on the properties of myosin. Consistent with previous findings, we did not observe any difference in MHC isoform composition between LCR and HCR muscles (10). We further demonstrate that at saturating Ca2+ activation, maximal force development of neither EDL nor SOL was different between LCR and HCR skinned fibers (Table 3). The similar maximal force at saturating Ca2+ in LCR and HCR skinned fibers indicates that the innate myosin mediated force development is not different between LCR and HCR muscles.
Intact muscle force production is also dependent on Ca2+ activation of the thin filament. We observed only a minimal difference in Ca2+-sensitive force development between LCR and HCR EDL skinned fibers (Fig. 2A). Alternatively, the Ca2+-sensitive force development of skinned LCR SOL fibers was drastically lower than that of HCR SOL fibers (Fig. 2B). Unlike the EDL muscle, the SOL expresses both fast and slow troponin isoforms (6). Segregating the Ca2+-sensitive force development of LCR and HCR rat SOL single fibers based on their troponin isoform expression demonstrates LCR Fast SOL fiber Ca2+ sensitivity was similar to HCR Fast SOL fibers (Table 3). In contrast, LCR Slow SOL fiber Ca2+ sensitivity was decreased compared with HCR Slow SOL fibers. This decrease was such that LCR Slow SOL fiber Ca2+ sensitivity was not different from LCR Fast SOL, HCR Fast SOL, or EDL fibers (Fig. 4C and Table 3). The similar Ca2+ sensitivity of LCR SOL fibers containing slow troponin isoforms and SOL fibers containing fast troponin isoforms suggests LCR selection affected yet to be determined myofilament alterations such as decreasing myosin regulatory light chain (24) or myosin binding protein C expression and/or phosphorylation (1). While beyond the scope of this study, it will be important for future studies to investigate the myofilament modifications responsible for this decreased Ca2+ sensitivity. Overall, the results of our skinned fiber findings demonstrate LCR selection results in decreased SOL Ca2+-sensitive force development specifically in SOL fibers expressing slow troponin isoforms such that LCR Slow SOL fibers exhibit a fast fiber Ca2+ sensitivity. This myofilament change, specifically in the slow but not fast fiber environment, constitutes a selective modification in the LCR rat.
Decreased LCR Ca2+ sensitivity and fatigue.
LCR rats selectively breed for decreased running capacity run for less time and for shorter distances than HCR rats selected for increased running capacity (Table 1). In addition to previously identified alterations in LCR skeletal muscle metabolism, O2 utilization, and mitochondrial density (10, 15, 18, 19, 31), we now demonstrate LCR rat intact SOL muscle exhibits decreased force production following fatigue (Fig. 1B). LCR SOL skinned fiber Ca2+ sensitivity is decreased (Fig. 2 and 4C) indicating a myofilament mediated mechanism. The decrease in LCR SOL fiber Ca2+ sensitivity will result in decreased LCR SOL muscle force at the given intracellular Ca2+ level. Repetitive muscle usage induces fatigue as the result of decreased muscle performance and force production. Decreased force can result from a number of factors; however, within the muscle decreased force results from the accumulation of metabolic by-products and decreased intracellular Ca2+ (3, 12). Thus decreased LCR rat SOL Ca2+ sensitivity will result in faster and further depression of SOL force production during the fatigue-mediated decrease in intracellular Ca2+ compared with the HCR SOL. Ultimately, acceleration of LCR decreased SOL force will limit running ability. Support for LCR SOL decreased Ca2+ sensitivity as contributing to increase fatigability is provided by recent work from Tikunova et al. (32), who demonstrated decreasing SOL muscle Ca2+ sensitivity by a point mutation in troponin C resulted in decreased SOL muscle force production following fatigue. Overall, the findings from this study demonstrate that, in addition to alterations of LCR rat muscle metabolism, alteration of LCR rat slow-type fiber troponin isoforms and Ca2+ desensitization contribute to the increased fatigability of LCR SOL muscle.
Conclusions.
Artificial divergent selection for maximal aerobic running capacity in genetically heterogeneous rats provides a useful model to investigate the mechanistic determinants of untrained aerobic exercise capacity that is critical to health. While previous reports have identified impaired metabolism in LCR rats as a main linkage to decreased aerobic capacity, the contribution of skeletal muscle function to this decreased capacity is largely unexplored. Our findings identify that LCR rats also exhibit increased SOL fatigability and altered slow fiber function at the myofilament level that is not present in the fast-type EDL muscle. This myofilament change in the slow but not fast fiber environment, constitutes a selective modification in the LCR rat. In addition to depressed metabolism these results suggest that altered LCR SOL slow fiber Ca2+ sensitivity contributes to increased SOL muscle fatigue resulting in decreased treadmill running capacity of LCR rats.
GRANTS
This work was supported by grants from the United States Public Health Service, National Institutes of Health to T. M. Nosek and M. A. P. Brotto (National Heart, Lung, and Blood Institute Grant HL060304), J.-P. Jin (National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-048816), and B. J. Biesiadecki (National Heart, Lung, and Blood Institute Grant HL114940). M. A. Brotto is also supported by funding from the Hazel Jay Endowment. The LCR-HCR rat model system is currently funded by NIH Office of Research Infrastructure Programs Grant P40OD021331 (to L. G. Koch and S. L. Britton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.B. and J.-P.J. conceived and designed research; B.J.B., M.A.B., L.S.B., and L.G.K. performed experiments; B.J.B., M.A.B., L.S.B., L.G.K., T.M.N., and J.-P.J. analyzed data; B.J.B., M.A.B., L.S.B., L.G.K., S.L.B., T.M.N., and J.-P.J. interpreted results of experiments; B.J.B. and M.A.B. prepared figures; B.J.B., M.A.B., T.M.N., and J.-P.J. drafted manuscript; B.J.B., M.A.B., L.S.B., L.G.K., S.L.B., T.M.N., and J.-P.J. edited and revised manuscript; B.J.B., M.A.B., L.S.B., L.G.K., S.L.B., T.M.N., and J.-P.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The rat models for low and high exercise capacity are maintained as an international resource with support from the Department of Physiology and Pharmacology, The University of Toledo, College of Medicine, Toledo, OH. Contact L. G. Koch at Lauren.Koch2@UToledo.Edu or S. L. Britton at brittons@umich.edu for information on the rat models.
REFERENCES
- 1.Ackermann MA, Kerr JP, King B, Ward CW, Kontrogianni-Konstantopoulos A. The phosphorylation profile of myosin binding protein-C slow is dynamically regulated in slow-twitch muscles in health and disease. Sci Rep 5: 12637, 2015. [Erratum in Sci Rep 8: 46969, 2018]. doi: 10.1038/srep12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin KM, Cooke DA, Cheadle WG. Time course adaptations in cardiac and skeletal muscle to different running programs. J Appl Physiol 42: 267–272, 1977. doi: 10.1152/jappl.1977.42.2.267. [DOI] [PubMed] [Google Scholar]
- 5.Brotto MA, Andreatta-van Leyen S, Nosek CM, Brotto LS, Nosek TM. Hypoxia and fatigue-induced modification of function and proteins in intact and skinned murine diaphragm muscle. Pflugers Arch 440: 727–734, 2000. doi: 10.1007/s004240000327. [DOI] [PubMed] [Google Scholar]
- 6.Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM, Jin JP. Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am J Physiol Cell Physiol 290: C567–C576, 2006. doi: 10.1152/ajpcell.00422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brotto MA, Fogaça RT, Creazzo TL, Godt RE, Nosek TM. The effect of 2,3-butanedione 2-monoxime (BDM) on ventricular trabeculae from the avian heart. J Muscle Res Cell Motil 16: 1–10, 1995. doi: 10.1007/BF00125305. [DOI] [PubMed] [Google Scholar]
- 8.Brotto MA, Nosek TM. Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J Appl Physiol (1985) 81: 731–737, 1996. doi: 10.1152/jappl.1996.81.2.731. [DOI] [PubMed] [Google Scholar]
- 9.Brotto MA, Nosek TM, Kolbeck RC. Influence of ageing on the fatigability of isolated mouse skeletal muscles from mature and aged mice. Exp Physiol 87: 77–82, 2002. doi: 10.1113/eph8702224. [DOI] [PubMed] [Google Scholar]
- 10.Burniston JG, Kenyani J, Gray D, Guadagnin E, Jarman IH, Cobley JN, Cuthbertson DJ, Chen YW, Wastling JM, Lisboa PJ, Koch LG, Britton SL. Conditional independence mapping of DIGE data reveals PDIA3 protein species as key nodes associated with muscle aerobic capacity. J Proteomics 106: 230–245, 2014. doi: 10.1016/j.jprot.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Paula Brotto M, van Leyen SA, Brotto LS, Jin JP, Nosek CM, Nosek TM. Hypoxia/fatigue-induced degradation of troponin I and troponin C: new insights into physiologic muscle fatigue. Pflugers Arch 442: 738–744, 2001. doi: 10.1007/s004240100587. [DOI] [PubMed] [Google Scholar]
- 12.Debold EP. Decreased myofilament calcium sensitivity plays a significant role in muscle fatigue. Exerc Sport Sci Rev 44: 144–149, 2016. doi: 10.1249/JES.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 13.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol 53: 844–850, 1982. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- 14.Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279–297, 1982. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez NC, Howlett RA, Henderson KK, Koch LG, Britton SL, Wagner HE, Favret F, Wagner PD. Systemic oxygen transport in rats artificially selected for running endurance. Respir Physiol Neurobiol 151: 141–150, 2006. doi: 10.1016/j.resp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res 8: 477–479, 1984. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- 17.Horvat S, Bünger L, Falconer VM, Mackay P, Law A, Bulfield G, Keightley PD. Mapping of obesity QTLs in a cross between mouse lines divergently selected on fat content. Mamm Genome 11: 2–7, 2000. doi: 10.1007/s003350010002. [DOI] [PubMed] [Google Scholar]
- 18.Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. Selected contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J Appl Physiol (1985) 94: 1682–1688, 2003. doi: 10.1152/japplphysiol.00556.2002. [DOI] [PubMed] [Google Scholar]
- 19.Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, Koch LG, Wagner PD. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol (1985) 106: 1819–1825, 2009. doi: 10.1152/japplphysiol.00914.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin JP, Brotto MA, Hossain MM, Huang QQ, Brotto LS, Nosek TM, Morton DH, Crawford TO. Truncation by Glu180 nonsense mutation results in complete loss of slow skeletal muscle troponin T in a lethal nemaline myopathy. J Biol Chem 278: 26159–26165, 2003. doi: 10.1074/jbc.M303469200. [DOI] [PubMed] [Google Scholar]
- 21.Jin JP, Malik ML, Lin JJ. Monoclonal antibodies against cardiac myosin heavy chain. Hybridoma 9: 597–608, 1990. doi: 10.1089/hyb.1990.9.597. [DOI] [PubMed] [Google Scholar]
- 22.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Koch LG, Meredith TA, Fraker TD, Metting PJ, Britton SL. Heritability of treadmill running endurance in rats. Am J Physiol Regul Integr Comp Physiol 275: R1455–R1460, 1998. doi: 10.1152/ajpregu.1998.275.5.R1455. [DOI] [PubMed] [Google Scholar]
- 24.MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue–regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci 125: 2105–2114, 2012. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- 25.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 26.Nagaraj RY, Nosek CM, Brotto MA, Nishi M, Takeshima H, Nosek TM, Ma J. Increased susceptibility to fatigue of slow- and fast-twitch muscles from mice lacking the MG29 gene. Physiol Genomics 4: 43–49, 2000. doi: 10.1152/physiolgenomics.2000.4.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Nixon BR, Walton SD, Zhang B, Brundage EA, Little SC, Ziolo MT, Davis JP, Biesiadecki BJ. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol 72: 177–185, 2014. doi: 10.1016/j.yjmcc.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nosek TM, Brotto MA, Essig DA, Mestril R, Conover RC, Dillmann WH, Kolbeck RC. Functional properties of skeletal muscle from transgenic animals with upregulated heat shock protein 70. Physiol Genomics 4: 25–33, 2000. doi: 10.1152/physiolgenomics.2000.4.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Overmyer KA, Evans CR, Qi NR, Minogue CE, Carson JJ, Chermside-Scabbo CJ, Koch LG, Britton SL, Pagliarini DJ, Coon JJ, Burant CF. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab 21: 468–478, 2015. doi: 10.1016/j.cmet.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren YY, Koch LG, Britton SL, Qi NR, Treutelaar MK, Burant CF, Li JZ. Selection-, age-, and exercise-dependence of skeletal muscle gene expression patterns in a rat model of metabolic fitness. Physiol Genomics 48: 816–825, 2016. doi: 10.1152/physiolgenomics.00118.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson EJ, Stepto NK, Koch LG, Britton SL, Hawley JA. Divergent skeletal muscle respiratory capacities in rats artificially selected for high and low running ability: a role for Nor1? J Appl Physiol (1985) 113: 1403–1412, 2012. doi: 10.1152/japplphysiol.00788.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikunova S, Belevych N, Doan K, Reiser PJ. Desensitizing mouse cardiac troponin C to calcium converts slow muscle towards a fast muscle phenotype. J Physiol 596: 4651–4663, 2018. doi: 10.1113/JP276296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torvinen S, Silvennoinen M, Piitulainen H, Närväinen J, Tuunanen P, Gröhn O, Koch LG, Britton SL, Kainulainen H. Rats bred for low aerobic capacity become promptly fatigued and have slow metabolic recovery after stimulated, maximal muscle contractions. PLoS One 7: e48345, 2012. doi: 10.1371/journal.pone.0048345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 35.Wisloff U, Sanchez E.R., Brickman T., Koch L.G., Britton S.L.. Rat genetic models of aerobic capacity segregate for VO2max and heat shock protein hsp70i. In: Miami Nature Biotechnology Winter Symposia Miami, Ohio: 2003. [Google Scholar]
- 36.Yu ZB, Zhang LF, Jin JP. A proteolytic NH2-terminal truncation of cardiac troponin I that is up-regulated in simulated microgravity. J Biol Chem 276: 15753–15760, 2001. doi: 10.1074/jbc.M011048200. [DOI] [PubMed] [Google Scholar]