Abstract
Whether SLC4A11 transports ammonia and its potential mode of ammonia transport (, NH3, or NH3-2H+ transport have been proposed) are controversial. In the absence of ammonia, whether SLC4A11 mediates significant conductive H+(OH−) transport is also controversial. The present study was performed to determine the mechanism of human SLC4A11 ammonia transport and whether the transporter mediates conductive H+(OH−) transport in the absence of ammonia. We quantitated H+ flux by monitoring changes in intracellular pH (pHi) and measured whole cell currents in patch-clamp studies of HEK293 cells expressing the transporter in the absence and presence of NH4Cl. Our results demonstrate that SLC4A11 mediated conductive H+(OH−) transport that was stimulated by raising the extracellular pH (pHe). Ammonia-induced HEK293 whole cell currents were also stimulated by an increase in pHe. In studies using increasing NH4Cl concentrations with equal extracellular and intracellular concentrations, the shift in the reversal potential (Erev) due to the addition of ammonia was compatible with NH3-H+ transport competing with H+(OH−) rather than NH3-nH+ (n ≥ 2) transport. The increase in equivalent H+(OH−) flux observed in the presence of a transcellular H+ gradient was also compatible with SLC4A11-mediated NH3-H+ flux. The NH3 versus Erev data fit a theoretical model suggesting that NH3-H+ and H+(OH−) competitively interact with the transporter. Studies of mutant SLC4A11 constructs in the putative SLC4A11 ion coordination site showed that both H+(OH−) transport and ammonia-induced whole cell currents were blocked suggesting that the H+(OH−) and NH3-H+ transport processes share common features involving the SLC4A11 transport mechanism.
Keywords: ammonia, patch clamp, proton, SLC4A11, transport
INTRODUCTION
SLC4A11 belongs to the SLC4 gene family of and transporters (42). The amino acid sequence of SLC4A11 is least homologous to other SLC4 transporters, and its function has remained controversial. It was originally thought that SLC4A11 functions as an electrogenic sodium borate cotransporter [Na+(n)-B(OH−)4−] or that it mediates cation (Na+ or H+) permeation (41). It was subsequently reported that SLC4A11 mediates Na+-OH− cotransport (equivalent to Na+/H+ exchange) (20, 38), transport (38), water flux (50, 54), H+(OH−) transport (22, 23, 37, 41), NH3-2H+ cotransport (ammonia-driven H+ transport) (23, 30, 61), and NH3 transport (33). SLC4A11 does not transport or unlike other SLC4 transporters (20, 33, 38).
SLC4A11 is expressed in multiple tissues including testis, thyroid gland, inner ear, mammary glands, esophagus, cerebellum, kidney, salivary glands, pancreas, spleen, liver, cornea, and trachea (8, 34, 41, 43). Naturally occurring mutations in SLC4A11 cause corneal disorders, including all cases of autosomal recessive congenital hereditary endothelial dystrophy (CHED) (2, 21, 51, 55), Harboyan syndrome (CHED with sensorineural hearing loss) (11, 49), and some cases of Fuchs’ endothelial corneal dystrophy (44, 50, 56). Patients with CHED have diffuse corneal edema shortly after birth with a diffusely edematous corneal stroma, and there is thickening of Descemet’s membrane and abnormal corneal endothelial morphology (12). In mice the loss of the Slc4a11 gene causes corneal endothelial dystrophy recapitulating the human disease (17), auditory sensorineural abnormalities (34), and renal ion and water transport abnormalities (15, 17).
The corneal endothelium utilizes glutamine to produce ATP for Na+-K+-ATPase activity to support physiological pump function (60). High levels of glutaminolysis lead to the production of ammonia as a byproduct. Slc4a11−/− mice have disrupted expression of enzymes involved in glutamine metabolism with a predicted impairment in pump function and elevated levels of nitrotyrosine staining, a general marker of ammonia toxicity (60). Similar changes in glutaminolysis enzyme expression were detected in a conditionally immortal Slc4a11−/− mouse corneal endothelial cell line (62). Given the potential importance of glutamine metabolism resulting in the generation of ammonia, there is a need for a cellular ammonia transport process to prevent its accumulation with resultant ammonia toxicity.
The role of SLC4A11 in mediating ammonia transport and the underlying transport mechanism is controversial. Ogando et al. (38) first suggested that human SLC4A11 expressed in PS120 cells transports . Zhang et al. (61) reported that the transporter mediates NH3-2H+ cotransport in PS120 cells. Loganathan et al. (33) found that SLC4A11 expressed in oocytes transports NH3 rather than NH3-2H+. Myers et al. (37) concluded that mouse Slc4a11 does not transport ammonia per se. In the absence of ammonia, whether SLC4A11 mediates conductive H+(OH−) transport is also controversial. Kao et al. (22, 23) and Myers et al. (37) have provided evidence for SLC4A11-mediated conductive H+(OH−) transport whereas others have been unable to detect significant conductive H+(OH−) transport (33, 61). These discrepant results prompted our re-examination of the transport properties of SLC4A11, specifically whether it mediates H+(OH−) transport and the mechanism of ammonia transport. Our results indicate that SLC4A11 mediates conductive H+(OH−) transport in the absence of ammonia, and in the presence of ammonia our findings are compatible with an NH3-H+ mode of transport. Furthermore, we have generated a theoretical model characterizing H+(OH−) and NH3-H+ competition for transport through SLC4A11.
METHODS
HEK293 Cell Culture and Transfection
The cells were grown in DMEM supplemented with 10% FBS, l-glutamine (200 mg/L), and 1% penicillin-streptomycin at 37°C in a 5% CO2 atmosphere and passaged onto 60-mm dishes (sulpho-NHS-SS-biotin studies) or onto PEI-coated coverslips (functional studies). The cells were transiently transfected using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's protocol except that the transfection solution was removed after a 2-h exposure and the cells were studied 24 h posttransfection. The cells were transfected with empty pTT vector (mock transfected) or various human SLC4A11-C constructs. Enhanced green fluorescent protein (eGFP)-coupled constructs were used to identify expressing cells in the patch-clamp experiments.
Measurement of Intracellular pH and H+ Flux
Following transient transfection with the various constructs, intracellular pH (pHi) was measured in cells grown on coated coverslips after 24 h. The coverslips were placed in a custom-designed chamber on the stage of a microscope fluorometer (28) and loaded with the fluorescent pHi probe BCECF using esterified BCECF-AM (Life Technologies) at room temperature for ∼20 min. The composition of the solution used for dye loading was (in mM) 140 KCl, 5 tetramethylammonium chloride (TMACl), and 5 HEPES. In each experiment, the fluorescence excitation ratio (500 nm/440 nm; 530-nm emission) obtained from ∼200 cells was averaged. The bathing solutions continuously perfused the coverslips at 2 ml/min (37°C). At the end of each experiment the intracellular fluorescence excitation ratio was calibrated with 5 μM valinomycin (Sigma-Aldrich) and 26 μM nigericin (Sigma-Aldrich) to equilibrate pHi, extracellular pH (pHe), and K+ (45). With the use of addition/removal protocols, the intrinsic cell buffer capacity (βi) was measured in HEPES-buffered solutions over a range of intracellular H+ (Hin+) values and calculated as Δ[]/Δ[Hin+]. In the initial 10–15 s following a bath solution switch, the rate of change of pHi (dpHi/dt) was measured and converted to the rate of change of [Hin+] (d[Hin+]/dt). H+ flux (mM/s) was calculated as βi × (d[Hin+]/dt). In NH4Cl-containing solutions, the ammonia buffer capacity βNH4 was calculated as 2.303 []i and added to βi to calculate the equivalent H+ flux. The flux of H+ versus OH− flux cannot be distinguished thermodynamically and is referred to in the text as SLC4A11-mediated H+(OH−) flux for simplicity. The solutions used were nominally -free, and since HEK293 cells have endogenous Na+-coupled transport activity (40) to prevent the generation of from ambient CO2, the solutions were bubbled continuously with 100% O2.
H+(OH−) Flux Following an Increase in pHe
Transient H+(OH−) flux was induced following an increase in bath pH from 7.4 to 8.0 followed by a return to pH 7.4 under Na+-free conditions. The cells were initially bathed in a solution containing (in mM) 140 KCl, 5 TMACl, and 5 HEPES, pH 7.4, and after pHi stabilized, the external solution pH was acutely increased from pH 7.4 to 8.0. H+(OH−) flux was measured following the acute increase in external pH to 8.0 and following the return to pH 7.4.
Equivalent H+(OH−) Flux Following an Increase in pHe in the Presence of NH4Cl
Transient H+ flux was induced following an increase in bath pH from 7.4 to 8.0 followed by a return to pH 7.4 under Na+-free conditions in the presence of 5 mM NH4Cl. In these experiments the NH3 concentration was kept constant at 0.153 mM at both pH values. The concentration decreased from 4.85 to 1.22 mM at pH 8.0. The cells were initially bathed in solution that contained (in mM) 140 KCl, 5 NH4Cl, and 5 HEPES, pH 7.4, and after pHi stabilized, the external pH was acutely increased from pH 7.4 to 8.0 with a solution that contained (in mM) 140 KCl, 3.63 TMACl, and 1.37 NH4Cl, and 5 HEPES, pH 8.0. Equivalent H+(OH−) flux was measured following the acute increase in external pH to 8.0 and following the return to pH 7.4.
Patch-Clamp Measurements of Electrogenic H+(OH−) and Ammonia-Induced Whole Cell Currents
The cells were replated (1:10 dilution) onto coated 35-mm tissue culture inserts (Bioptechs) 24 h following transfection. The tissue culture inserts were put onto a chamber on the stage of a fluorescent microscope (Axioskop 2 FS plus; Carl Zeiss). Cells with moderate eGFP fluorescence were chosen 2–3 h later for electrophysiological recording using a MultiClamp 700B patch amplifier (Molecular Devices). Borosilicate glass patch pipettes were prepared with a tip resistance 4–6.5 MΩ (tip diameter of 1–1.5 μm). A microagar salt bridge containing 2 M KCl was built into the electrode holder to ensure stable electrode potentials during recording (47). Using whole cell voltage clamp, steady-state currents were measured with a holding potential of −15 mV and a series of voltage pulses (400-ms) at 10-mV increments (−70 to +40 mV). Using the pClamp software (Clampex 10, Molecular Devices), the signal was low-pass filtered at 400 Hz and sampled at 2 kHz. Junction potentials calculated with Clampex 10 that were generated from different compositions of patch pipette and bath solutions were corrected for at all applied potentials. With the auto-whole cell capacitance and series resistance compensation, whole cell capacitance and series resistance were determined. Typically, the series resistance was compensated 80% (both correction and prediction). The cells were continuously perfused at a flow rate of ∼2 ml/min (22–24°C). Whole cell currents were measured using the solutions in the protocols described below.
H+(OH−) currents at different pHe values.
The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 1 CaCl2, 10 tetraethylammonium chloride (TEA-Cl), 10 EGTA, and 100 HEPES, pH 7.4. For this and subsequent protocols, 100 mM HEPES were used to minimize any patch pipette pH changes due to pHe changes or changes in extracellular NH3; the bath solutions contained (in mM) 140 TMACl, 1.5 CaCl2, 10 CsCl, and 10 HEPES. The bath pH was varied from 7.0 to 8.0. In this and subsequent protocols, cesium hydroxide (55 mM) was added to the patch pipette solution to pH the solution to 7.4. The final measured patch pipette osmolality was ~265 mosmol/kgH2O with a bath osmolality of ~295 mosmol/kgH2O.
Ammonia-induced currents at different pHe values at constant concentration and varying NH3 concentrations.
The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 2 NH4Cl (0.023 NH3; 1.977 ), 1 CaCl2, 8 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution pH 7.0 contained (in mM) 138.014 TMACl, 1.986 NH4Cl (0.009 NH3; 1.977 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES; pH 7.4 contained (in mM) 138 TMACl, 2 NH4Cl (0.023 NH3; 1.977 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES; pH 7.6 contained (in mM) 137.987 TMACl, 2.013 NH4Cl (0.036 NH3; 1.977 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES; pH 7.8 contained (in mM) 137.966 TMACl, 2.034 NH4Cl (0.057 NH3; 1.977 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES; and pH 8.0 contained (in mM) 137.932 TMACl, 2.068 NH4Cl (0.091 NH3; 1.977 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES.
Ammonia-induced currents following an increase in bath pH from 7.4 to pH 8.0 with an concentration gradient at pH 8.0 and no NH3 concentration gradient.
Protocols a–e were utilized. Protocol a used patch pipette and bath pH 7.4: NH3 = 0.006 mM; = 0.494 mM; bath 8.0: NH3 = 0.006 mM; = 0.124 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 0.5 NH4Cl, 1 CaCl2, 9.5 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 139.5 TMACl, 0.5 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 139.87 TMACl, 0.13 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol b used patch pipette and bath pH 7.4: NH3 = 0.011 mM; = 0.989 mM; bath 8.0: NH3 = 0.011 mM; = 0.249 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 1.0 NH4Cl, 1 CaCl2, 9.0 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 139.0 TMACl, 1.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 139.74 TMACl, 0.26 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol c used patch pipette and bath pH 7.4: NH3 = 0.023 mM; = 1.977 mM; bath 8.0: NH3 = 0.023 mM; = 0.497 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 2 NH4Cl, 1 CaCl2, 8 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 138 TMACl, 2.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 139.48 TMACl, 0.52 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol d used patch pipette and bath pH 7.4: NH3 = 0.034 mM; = 2.966 mM; bath 8.0: NH3 = 0.034 mM; = 0.746 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 3 NH4Cl, 1 CaCl2, 7 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 137 TMACl, 3.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 139.22 TMACl, 0.78 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol e used patch pipette and bath pH 7.4: NH3 = 0.057 mM; = 4.943 mM; bath 8.0: NH3 = 0.057 mM; =1.243 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 5 NH4Cl, 1 CaCl2, 5 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 135 TMACl, 5.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 138.7 TMACl, 1.3 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0.
Ammonia-induced currents following an increase in bath pH from 7.4 to pH 8.0 with an NH3 concentration gradient at pH 8.0 and no concentration gradient.
Protocols a-f were utilized. Protocol a used patch pipette and bath pH 7.4: NH3 = 0.006 mM; = 0.494 mM; bath 8.0: NH3 = 0.023 mM; = 0.494 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 0.5 NH4Cl, 1 CaCl2, 9.0 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 139.5 TMACl, 0.5 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 139.483 TMACl, 0.517 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol b used patch pipette and bath pH 7.4: NH3 = 0.011 mM; = 0.989 mM; bath 8.0: NH3 = 0.045 mM; 0.989 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 1.0 NH4Cl, 1 CaCl2, 9.0 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 139.0 TMACl, 1.0 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 138.966 TMACl, 1.034 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol c used patch pipette and bath pH 7.4: NH3 = 0.023 mM; = 1.977 mM; bath 8.0: NH3 = 0.091 mM; = 1.977 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 2 NH4Cl, 1 CaCl2, 8 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 138 TMACl, 2.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 137.932 TMACl, 2.068 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol d used patch pipette and bath pH 7.4: NH3 = 0.034 mM; = 2.966 mM; bath 8.0: NH3 = 0.136 mM; = 2.966 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 3 NH4Cl, 1 CaCl2, 7 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 137 TMACl, 3.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 136.898 TMACl, 3.102 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol e used patch pipette and bath pH 7.4: NH3 = 0.057 mM; = 4.943 mM; bath 8.0: NH3 = 0.227 mM; = 4.943 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 5 NH4Cl, 1 CaCl2, 5 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 135 TMACl, 5.0 NH4Cl,1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 134.83 TMACl, 5.17 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0. Protocol f used patch pipette and bath pH 7.4: NH3 = 0.114 mM; = 9.886 mM; bath 8.0: NH3 = 0.454 mM; = 9.886 mM. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 10 NH4Cl, 1 CaCl2, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution contained (in mM) 130 TMACl, 10 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 7.4; 129.66 TMACl, 10.34 NH4Cl, 1.5 CaCl2, 10 CsCl, and 10 HEPES, pH 8.0.
Mutant H+(OH−) currents and ammonia-induced currents at pH 8.0 with an NH3 concentration gradient at pH 8.0 and no concentration gradient.
In the absence of ammonia, H+(OH−) currents were measured at external pH 7.4 and 8.0. The patch pipette solution contained (in mM) 45 cesium methanesulfonate, 1 CaCl2, 10 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solutions contained (in mM) 140 TMACl, 1.5 CaCl2, 10 CsCl, and 10 HEPES. In the presence of ammonia, the patch pipette solution contained (in mM) 45 cesium methanesulfonate, 0.500 mM NH4Cl (0.006 NH3; 0.494 ), 1 CaCl2, 10 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution pH 7.4 contained (in mM) 139.5 TMACl, 0.500 NH4Cl (0.006 NH3; 0.494 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES; and at pH 8.0 contained (in mM) 140 TMACl, 0.517 NH4Cl (0.023 NH3; 0.494 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES.
Patch-Clamp Experiments Monitoring of pHi
EYFP is a pH-sensitive fluorophore with a pK of 7.1 (32). pHi was monitored with EYFP in patch-clamped cells expressing a pcDNA3-EYFP-SLC4A11 construct. Fluorescent images of the cell expressing EYFP were acquired using a Mightex camera (CXE-B013-U; Mightexsystems) every 10 s, and the cell fluorescence was quantitated and subtracted from the background fluorescence. pHi was monitored while the bath pH was changed from pH 7.4 to 8.0. The following solutions were used: the patch pipette solution contained (in mM) 45 cesium methanesulfonate, 1 CaCl2, 10 TEA-Cl, 10 EGTA, and 100 HEPES, pH 7.4; the bath solutions contained (in mM) 140 TMACl, 1.5 CaCl2, 10 CsCl, and 10 HEPES. In separate experiments, pHi was monitored while the extracellular NH3 concentration was increased from 0.114 to 0.454 mM at the same time the bath pH was increased from pH 7.4 to 8.0. The solutions used were as follows: the patch pipette solution contained (in mM) 45 cesium methanesulfonate, 10 NH4Cl (0.114 NH3; 9.886 ), 1 CaCl2, 10 EGTA, and 100 HEPES, pH 7.4; the bath solution pH 7.4: contained (in mM) 130 TMACl, 10 NH4Cl (0.114 NH3; 9.886 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES; and the bath solution pH 8.0 contained (in mM) 129.66 TMACl, 10.34 NH4Cl (0.454 NH3; 9.886 ), 1.5 CaCl2, 10 CsCl, and 10 HEPES.
Sulfo-NHS-SS-Biotin Plasma Membrane Labeling
Plasma membrane proteins were labeled with sulfo-NHS-SS-biotin and using streptavidin-agarose resin were pulled down according to the following protocol: 24 h after transfection, the cells were washed repeatedly with ice-cold PBS (pH 8.0) and then incubated at pH 8.0 (4°C for 30 min) with 1.1 mM sulfo-NHS-SS-biotin (Thermo Fisher Scientific). The reaction was stopped with 50 mM Tris buffer (containing 140 mM NaCl, pH 8.0, 4°C). The cells were then collected, washed with PBS, and lysed on ice in a buffer containing 150 mM NaCl, 1% (vol/vol) Igepal (Sigma-Aldrich), 0.5% sodium deoxycholate (Thermo Fisher Scientific), 5 mM EDTA (Sigma-Aldrich), and 10 mM Tris·HCl, pH 7.5, with protease inhibitors (Roche Life Sciences). Following the pelleting of insoluble material [10-min centrifugation (20,000 g at 4°C)], the supernatant which contained >90% of the plasma membrane protein fraction was collected and incubated for 4 h (4°C) with 50 μl of streptavidin-agarose resin (Thermo Fisher Scientific) on a rotating shaker. Following brief centrifugation to pellet the resin and washing with the lysis buffer, bound proteins were eluted at 60°C for 5 min with 2× SDS buffer containing 2% β-mercaptoethanol (EMD Millipore, Billerica, MA). A previously characterized rabbit polyclonal antibody (1:3,000 dilution) against a carboxy terminus epitope in human SLC4A11 was used to probe immunoblots of plasma membrane pulled down proteins and whole cell lysates (23, 34).
Modeling SLC4A11 Ion Coordination Site
A homology model of the outward facing state of SLC4A11 was built with Modeller 9.18 (58), using the available crystal structure of human AE1 (4) and cryoEM structure of human NBCe1-A (19) as templates. The multiple sequence alignment of SLC4A11 and the two templates (AE1 and NBCe1) was done in advance with ClustalW (53). Five hundred three-dimensional (3D) models of SLC4A11 were then generated with the automodel algorithm implemented in Modeller 9.18, and their overall quality was assessed with DOPE and GA341 scores (36, 48). The best 10 models were analyzed for further quality assessment by manual comparison of several key structural elements identified previously from functional mutagenesis in the two templates. One final model, reproducing successfully the position and orientation of the functionally critical residues in the templates was selected. The SLC4a11 ion coordination site figure was prepared with PyMol (46).
Statistics
Student’s t test was used to compare group means. One-way ANOVA and Dunnett’s t test were used to compare multiple study group means with a control group. The results are depicted as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
H+(OH−) Flux Following an Increase in pHe in the Absence of Ammonia
pHi transients were monitored following an increase in pHe from 7.4 to 8.0 and return to pH 7.4. As shown in representative experiments in Fig. 1, the changes in pHi induced by the bath pH changes were significantly greater in SLC4A11-expressing cells. The flux of H+(OH−) (pH 7.4 to 8.0) was 0.011 ± 0.002 mM/s (n = 8) in mock-transfected cells versus 0.053 ± 0.004 (n = 19) (P < 0.001) in SLC4A11-expressing cells and the flux of H+(OH−) (pH 8.0 to 7.4) was 0.009 ± 0.002 mM/s (n = 8) in mock-transfected cells versus 0.062 ± 0.005 mM/s (n = 19) (P < 0.001) in SLC4A11-expressing cells.
Fig. 1.
Changes in intracellular pH (pHi) induced by extracellular pH (pHe) changes (7.4–8.0–7.4). Transient H+(OH−) flux was induced following an increase in bath pH from 7.4 to 8.0 followed by a return to pH 7.4. A: mock-transfected cells. B: SLC4A11-transfected cells.
Whole Cell H+ Currents at Various pHe Values
Whole cell currents were measured at pHe values between 7.0 and 8.0 with the patch pipette at pH 7.4 in mock-transfected and SLC4A11-expressing cells. The whole cell currents are depicted in Fig. 2, A–C. Following mock subtraction, the current was measured at +40 mV and the conductance was measured at 0 mV at each pH value. The results were as follows: pH 7.0 (n = 6): 5.25 ± 0.588 pA; 0.292 ± 0.038 nS; pH 7.4 (n = 6): 5.36 ± 1.71 pA (n = 6); 0.304 ± 0.046 nS; pH 7.6 (n = 5): 32.1 ± 3.46 pA; 0.820 ± 0.066 nS; pH 7.8 (n = 7): 58.0 ± 3.56 pA; 1.04 ± 0.084 nS; pH 8.0 (n = 3): 92.7 ± 5.45 pA; 1.41 ± 0.078 nS. The reversal potential (Erev) was measured at each pH value following mock subtraction. Figure 2D depicts a plot of Erev versus pHi-pHo showing that the data fall on the predicted line representing the following equation: E = 58 × (pHi-pHo) demonstrating that the whole cell currents represent H+(OH−) currents through the transporter. In separate experiments, whole cell currents measured at the same H+ gradient and opposite direction from pH 7.4 are compared (Fig. 3); pH 7.15 versus 8.0 (H+ gradient ± 30 nM); pH 7.19 versus pH 7.8 (H+ gradient ± 24.2 nM); pH 7.26 versus pH 7.6 (H+ gradient ± 14.9 nM); pH 7.30 versus pH 7.52 (H+ gradient ± 10.1 nM). The currents measured following background subtraction at +40 mV at each pH value and conductance at 0 mV were as follows: pH 7.4: 5.36 ± 1.71 pA; 0.304 ± 0.049 nS; pH 7.15: 6.00 ± 1.14 pA; 0.330 ± 0.059 nS (n = 3) and pH 8.0: 94.1 ± 13.0 pA (n = 3, P < 0.01 versus pH 7.15); 1.43 ± 0.156 nS (n = 3, P < 0.05 versus pH 7.15); pH 7.19: 1.38 ± 0.275 pA; 0.382 ± 0.080 nS (n = 4) and pH 7.8: 64.3 ± 9.63 pA (n = 4, P < 0.005 versus pH 7.19); 1.24 ± 0.176 nS (n = 4, P < 0.05 versus pH 7.19); pH 7.26: 4.15 ± 1.96 pA; 0.337 ± 0.062 nS (n = 3) and pH 7.6: 34.1 ± 3.56 pA (n = 5, P < 0.05 versus pH 7.26); 0.779 ± 0.074 nS (n = 5, P < 0.05 versus pH 7.26); pH 7.30: 5.25 ± 1.21 pA; 0.365 ± 0.071 nS (n = 4) and pH 7.52: 22.6 ± 3.45 pA (n = 4, P < 0.05 versus pH 7.30); 0.662 ± 0.096 nS (n = 4, P < 0.05 versus pH 7.30). These findings show that raising the pHe per se stimulates SLC4A11-mediated H+(OH−) transport.
Fig. 2.
Whole cell currents were measured at various extracellular pH (pHe) values with an intracellular pH (pHi) of 7.4 and current-voltage data are depicted. The bath pH was varied from 7.0 to 8.0. A: mock-transfected cells. B: SLC4A11-transfected cells. C: subtraction of the mock data from the SLC4A11 data. D: reversal potential (Erev) measured at various pHe values with an intracellular pH (pHi) of 7.4.
Fig. 3.
Whole cell currents were measured at various extracellular pH (pHe) values that differed from pH 7.4 with the same H+ gradient and opposite direction are compared and current-voltage data are depicted. A, D, G, and J: mock-transfected cells. B, E, H, and K: SLC4A11-transfected cells. C, F, I, and L: subtraction of the mock data from the SLC4A11 data.
Equivalent H+(OH−) Flux Following an Increase in pHe From 7.4 to pH 8.0 at Constant NH3 Concentration With a Decrease in the Concentration at pH 8.0
Following an increase in pHe from 7.4 to 8.0 and return to pH 7.4 in the presence of ammonia pHi transients were monitored (Fig. 4). In these experiments the extracellular NH3 concentration was 0.153 mM in both the pH 7.4 and 8.0 solutions and the concentration was decreased from 4.85 mM at pH 7.4 to 1.22 mM at pH 8.0 creating a transcellular and H+ gradient. As shown in representative experiments in Fig. 4, in mock-transfected cells equivalent H+(OH−) flux was greater in the presence of ammonia following generation of a transcellular H+ gradient: pH 7.4 to 8.0: 0.019 ± 0.003 mM/s (n = 7) versus 0.011 ± 0.002 mM/s (n = 8) in controls (P < 0.05) and pH 8.0 to 7.4: −0.025 ± 0.003 (n = 7) versus −0.009 ± 0.002 mM/s (n = 8) (P < 0.001) in controls. In SLC4A11-expressing cells the equivalent H+(OH−) flux was significantly greater in the presence of ammonia following generation of a transcellular H+ gradient; pH 7.4 to 8.0: 0.144 ± 0.009 mM/s (n = 8) versus 0.053 ± 0.004 mM/s (n = 19) in controls (P < 0.001) and pH 8.0 to 7.4: −0.112 ± 0.005 mM/s (n = 8) versus −0.062 ± 0.005 mM/s (n = 19) in controls (P < 0.001). The data are compatible with NH3 coupled H+ transport (or transport) contributing with H+(OH−) flux to the total equivalent H+(OH−) flux in SLC4A11-expressing cells in the presence of ammonia following an increase and decrease in pHe.
Fig. 4.
Changes in intracellular pH (pHi) induced by extracellular pH (pHe) changes (7.4–8.0–7.4) in the presence of 5 mM NH4Cl at a constant NH3 concentration of 0.153 mM with a decrease in the concentration from 4.85 to 1.22 mM at pH 8.0. A: mock-transfected cells. B: SLC4A11-transfected cells. C: summary of the pH 7.4–8.0 flux data in mock and SLC4A11-transfected cells. D: summary of the pH 8.0–7.4 flux data in mock and SLC4A11-transfected cells. *P < 0.05; #P < 0.001, presence versus absence of 5 mM NH4Cl in the same cell type (Student’s t test).
Whole Cell Currents in the Presence of a Constant Concentration and Varying Extracellular NH3 Concentrations as a Function of pHe
In the presence of 2 mM NH4Cl, whole cell currents were measured at pHe values between 7.0 and 8.0 with the patch pipette at 7.4 in mock-transfected and SLC4A11-expressing cells. The concentration was kept constant, and there was no patch pipette to bath gradient. The NH3 patch pipette to bath gradient varied with the pH and was equal and opposite to the H+ gradient at each pH value. The ammonia-induced whole cell currents are summarized in Fig. 5, A–C. In the absence and presence of ammonia, the SLC4A11-mediated whole cell currents were subtracted from the mock induced currents. The results at +40 mV and conductances measured at 0 mV in the absence of ammonia are shown above (Whole Cell H+ Currents at Various pHe Values and Fig. 2C). The results in the presence of ammonia were: pH 7.0: 8.45 ± 1.13 pA (n = 5), P < 0.05 vs. control, and 0.529 ± 0.030 nS (n = 5), P < 0.001 vs. control; pH 7.4: 18.7 ± 2.83 pA (n = 4), P < 0.001 vs. control, and 0.452 ± 0.020 nS (n = 4), P < 0.05 vs. control; pH 7.6: 47.6 ± 4.76 pA (n = 4), P < 0.05 vs. control, and 1.12 ± 0.104 nS (n = 4), P < 0.05 vs. control; pH 7.8: 89.7 ± 8.30 pA (n = 5), P < 0.01 vs. control, and 1.72 ± 0.180 nS (n = 5), P < 0.01 vs. control; and pH 8.0: 129.5 ± 7.77 pA (n = 4), P < 0.05 vs. control, and 2.08 ± 0.126 nS (n = 4), P < 0.01 vs. control. Figure 5, D and E, shows the comparison with the data obtained in the absence of ammonia. The results demonstrate that the ammonia-induced whole cell currents are stimulated by increasing the pHe.
Fig. 5.
Whole cell currents were measured at various extracellular pH (pHe) values with an intracellular pH (pHi) of 7.4 in the presence of 2 mM NH4Cl, and current-voltage data are depicted. The concentration was kept constant and there was no patch pipette to bath gradient. The NH3 patch pipette to bath gradient varied with the pH and was equal and opposite to the H+ gradient at each pH value. A: mock-transfected cells. B: SLC4A11-transfected cells. C: subtraction of the mock data from the SLC4A11 data. D: summary of the subtraction (SLC4A11 minus mock) data of the effect of ammonia on whole cell currents (+40 mV) measured at various pHe values. E: summary of the subtraction (SLC4A11 minus mock) data of the effect of ammonia on conductance (0 mV) measured at various pHe values. *P < 0.05; +P < 0.01; #P < 0.001, presence versus absence of 2 mM NH4Cl at each specific pH (Student t test).
Ammonia-Induced Currents Following an Increase in pHe From 7.4 to pH 8.0 With an Concentration Gradient at pH 8.0 and No NH3 Concentration Gradient
Whole cell currents were measured in mock-transfected and SLC4A11-expressing cells following an increase in bath pH from 7.4 to 8.0 in solutions containing various NH3 and concentrations without a patch pipette to bath NH3 concentration gradient in the presence of patch pipette to bath 4:1 gradient at pH 8.0. The Erev value was measured in each solution. The mock-transfected data were subtracted from the SLC4A11 data. Figure 6A shows a plot of Erev versus the extracellular NH3 concentration. The theoretical Erev based on the H+ gradient of 4:1 (intracellular to extracellular) is −34.8, which approximates the Erev measured in the absence of ammonia (−30.4 mV). As shown in Fig. 6A, the Erev measured at each NH3 concentration remained unchanged in the ammonia containing solutions. The latter is what is theoretically expected for an NH3-nH+ (n ≥ 1) or -nH+ (n ≥ 0) transporter (theoretical Erev also −34.8 in the ammonia-containing solutions).
Fig. 6.
A: Whole cell currents were measured in mock-transfected and SLC4A11-expressing cells following an increase in bath pH from 7.4 to 8.0 in solutions containing various NH3 and concentrations in the presence of patch pipette to bath 4:1 gradient (pH 8.0) without a patch pipette to bath NH3 concentration gradient. Depicted is a plot of reversal potential (Erev) versus the extracellular NH3 concentration. B: following an increase in bath from 7.4 to 8.0 whole cell currents were measured in mock-transfected and SLC4A11-expressing cells in solutions containing various NH3 and concentrations in the presence of patch pipette to bath 1:4 NH3 gradient (pH 8.0) that was equal and opposite to the H+ gradient without a patch pipette to bath concentration gradient. Depicted is a plot of Erev versus the extracellular NH3 concentration.
Ammonia-Induced Currents Following an Increase in pHe from 7.4 to pH 8.0 with an NH3 Concentration Gradient at pH 8.0 and no Concentration Gradient
Following an increase in bath from 7.4 to 8.0, whole cell currents were measured in mock-transfected and SLC4A11-expressing cells in solutions containing various NH3 and concentrations without a patch pipette to bath concentration gradient in the presence of patch pipette to bath 1:4 NH3 gradient that was equal and opposite to the H+ gradient. In each solution protocol, the Erev was measured. The mock-transfected data were subtracted from the SLC4A11 data. A plot of Erev versus the extracellular NH3 concentration is shown in Fig. 6B. With an H+ gradient of 4:1 (intracellular to extracellular), the theoretical Erev is −34.8, which approximates the Erev measured in the absence of ammonia (−30.4 mV). In the presence of ammonia, the Erev expected from NH3-H+ transport is zero given equal and opposite H+ and NH3 gradients. As shown in Fig. 6B as the NH3 concentration increased, the measured Erev shifted closer to zero reaching −9.6 mV at a patch pipette and bath concentration of 9.89 mM and a patch pipette and bath NH3 concentration of 0.114 and 0.454 mM, respectively. The data are compatible with NH3-H+ transport (or transport) competing with H+(OH−) for transport on SLC4A11 with increasing NH3 concentration. The data are not compatible with NH3-2H+-mediated SLC4A11 transport since Erev at the higher ammonia concentrations should have approached −17.6 mV (the Erev expected from an NH3-2H+ transport mode). Other transport modes such as NH3-3H+ (expected Erev −23.4) or -H+ (expected Erev −17.6 mV) are also excluded.
Monitoring pHi in the Patch-Clamp Experiments Using EYFP
pHi was monitored in patch-clamped cells following a change in pHe from 7.4 to 8.0 (pHi 7.4) and following a change to pH 8.0 in the presence ammonia with 4:1 bath to patch pipette NH3 gradient (patch pipette and bath NH3 concentration of 0.114 mM and 0.454 mM respectively). Figure 7 depicts representative experiments where the pHi remained unchanged following an increase in pHe to 8.0 in the absence (n = 7) and presence (n = 6) of 4:1 NH3 gradient. These findings indicate that changes in pHi were not a factor affecting the data.
Fig. 7.
Intracellular pH (pHi) was monitored using EYFP in patch-clamped cells following a change in pHe from 7.4 to 8.0 (pHi 7.4) and following a change to pH 8.0 in the presence of ammonia with a patch pipette and bath NH3 concentration of 0.114 and 0.454, mM, respectively (4:1 bath to patch pipette NH3 gradient). Representative experiments are depicted following an increase in pHe to 8.0 and in the absence and presence of 4:1 NH3 gradient.
Effect of SLC4A11 Mutations on H+(OH−) Currents and Ammonia-Induced Currents
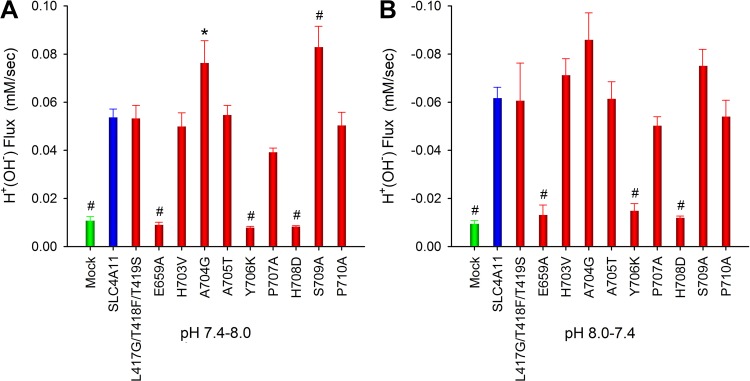
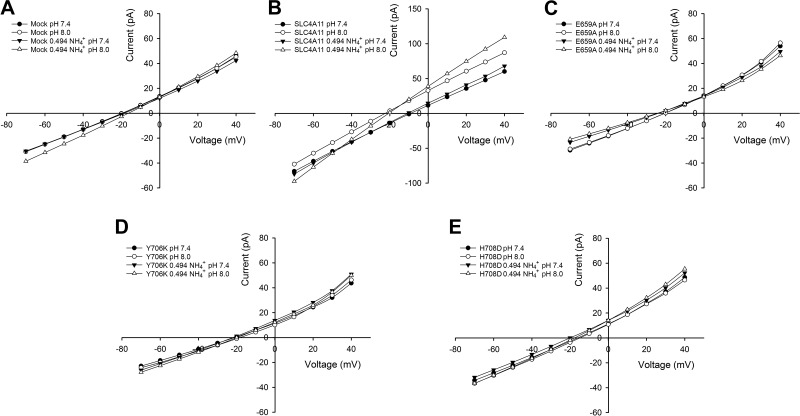
On the basis of homology with NBCe1-A and AE1, the predicted SLC4A11 ion coordination site includes residues adjacent to or within TM3, TM8, and TM10. We mutated residues in these locations as shown in Fig. 8 and measured H+(OH−) flux following an increase in bath pH from 7.4 to 8.0 and from pH 8.0 to 7.4. These results are shown in Fig. 8. We identified three residues E659, Y706, and H708 within or adjacent to TMs 8 and 10 that when mutated significantly decreased H+(OH−) flux. H+(OH−) currents and ammonia-induced currents were also measured in these mutant SLC4A11-expressing cells to determine whether H+(OH−) and NH3-H+ interact (and compete) with the same SLC4A11 residues (Fig. 9). In SLC4A11-expressing cells whole cell currents were measured following an increase in bath pH from 7.4 to 8.0. In separate experiments whole cell currents were measured following an increase in pHe from 7.4 to 8.0 in the presence of ammonia with the bath concentration 0.494 mM at pH 7.4 and 8.0 while the bath NH3 concentration increased from 0.006 mM at pH 7.4 to 0.023 mM at pH 8.0 (patch pipette to bath NH3 gradient equal and opposite to the H+ gradient). As shown in Fig. 9, in all mutants there was a decrease in both H+(OH−) currents and ammonia-induced currents suggesting that these transport processes share common features involving the SLC4A11 transport mechanism. Figure 10 shows the results of sulpho-NHS-SS-biotin experiments showing the plasma membrane expression of these transporters in addition to P707A, which had a modest decrease in H+(OH−) flux (Fig. 8). The involved residues are depicted in the SLC4A11 predicted ion coordination site (Fig. 11).
Fig. 8.
Based on homology with NBCe1-A and AE1, specific residues adjacent to or within TM3, TM8, and TM10 were predicted to be in the SLC4A11 ion coordination site. Residues in these locations were mutated and extracellular pH (pHe) was changed from 7.4 to 8.0–7.4 while intracellular pH (pHi) was monitored to calculate the H+(OH−) flux. A: flux (pH 7.4–8.0). B: flux (pH 8.0–7.4). E659, Y706, and H708 when mutated significantly decreased H+(OH−) flux. *P < 0.05; #P < 0.001, comparison with wild-type SLC4A11 (one-way ANOVA and Dunnett’s t test).
Fig. 9.
Residues near the putative ion coordination site were mutated and H+(OH−) currents and ammonia-induced currents were measured to determine whether H+(OH−) and NH3-H+ interact (and compete) with the same SLC4A11 residues. Whole cell currents were measured in mutant SLC4A11-expressing cells following an increase in bath pH from 7.4 to 8.0 in the absence of ammonia and in the presence of 0.5 mM NH4Cl solutions, and current-voltage data are depicted. The bath concentration was 0.494 mM at pH 7.4 and 8.0 while the bath NH3 concentration increased from 0.006 mM at pH 7.4 to 0.023 mM at pH 8.0 (1:4 patch pipette to bath NH3 gradient equal and opposite to the H+ gradient).
Fig. 10.
Sulpho-NHS-SS-biotin experiments showing the plasma membrane expression of the mutant SLC4A11 transporters. A: representative experiment showing immunoblot analysis of cell surface expression of wild-type and mutant SLC4A11 proteins. B: densitometric analysis of the ratio of SLC4A11 proteins to lysate intensity (n = 5).
Fig. 11.
Predicted SLC4A11 ion coordination site: A homology model of the outward facing state of SLC4A11 was built with Modeller 9.18, using the human AE1 crystal structure (4) and human NBCe1 cryo-EM structure (19) as templates. Multiple sequence alignment of SLC4A11 and the two templates (AE1 and NBCe1) was done in advance with ClustalW (53). Five hundred three-dimentional models of SLC4A11 were then generated with the automodel algorithm implemented in Modeller 9.18, and their overall quality was assessed with DOPE and GA341 scores (36, 48). The best 10 models were analyzed for further quality assessment and one final model, reproducing successfully the position and orientation of the functionally critical residues in the templates, was chosen.
A Model for SLC4A11 Ammonia Transport: NH3-H+ and H+ Competitively Interact with the Transporter
For a model where the transport of NH3-H+ and H+ occur independently and since NH3-H+ and H+ are not coupled, the GHK equation (3) is applicable. For the Erev calculation, if SLC4A11 transports NH3-H+ and H+ independently in parallel, we have:
| (1) |
where PNH3 is the affinity of NH3-H+ and PH is affinity of H+. If we assume that NH3-H+ and H+ interact competitively with the transporter:
| (2) |
where ANH3-H is the affinity of NH3-H+ and AH is the affinity of H+. We modeled the data from the protocol in Fig. 6B with Eq. 2 where ammonia-induced currents were measured following an increase in pHe from 7.4 to pH 8.0 at various NH4Cl concentrations without an concentration gradient at pH 7.4 and 8.0. We fitted the NH3 versus Erev data with Eq. 2 (nonlinear least squares method). Figure 12 shows the best fit to the data with Erev as a function of NH3 concentration which gives an estimate of ANH3-H+/(ANH3 + AH+) = 0.99995. The model described by Eq. 2 is consistent with the NH3 versus Erev data.1 In addition, applying the model to the data in Fig. 5C, the predicted Erev values at each pH were as follows: pH 7.0, +18.1 mV; pH 7.4, 0 mV; pH 7.6, −7.45 mV; pH 7.8, −13.6 mV; and pH 8.0, −18.4 mV. These values closely approximate the measured Erev values: pH 7.0, +15.3 mV; pH 7.4, 0.248 mV; pH 7.6, −7.78 mV; pH 7.8, −12.3 mV; and pH 8.0, −20.0 mV (Fig. 13).
Fig. 12.
Model (NH3-H+ versus H+) of the data in Fig. 6B depicting the NH3 versus reversal potential (Erev) data using a nonlinear least squares method (Eq. 2) where ammonia-induced currents were measured following an increase in extracellular pH (pHe) from 7.4 to pH 8.0 at various NH4Cl concentrations without a change in concentration at pH 8.0. The data were fitted with Eq. 2 and depicted is the best fit to the data with Erev as a function of NH3 concentration. The model described by Eq. 2 is consistent with the NH3 versus Erev data.
Fig. 13.
The NH3-H+ versus H+ model of the data in Fig. 6B was used to predict the pH-dependent reversal potential (Erev) values in Fig. 5C. Plotted are the measured Erev values, which closely approximate the predicted Erev values.
DISCUSSION
In the present study H+(OH−) flux was quantitated by monitoring changes in pHi and measuring whole cell currents in HEK293 cells expressing the transporter. Stimulation of whole cell currents by ammonia was measured under various conditions to determine the mode of transport. In the absence of ammonia, SLC4A11-mediated H+(OH−) transport that was stimulated by raising the pHe. An increase in pHe was also found to stimulate ammonia-induced whole cell currents. These effects are likely mediated by the deprotonization of key residues in the transporter involved in the ion transport mechanism. In experiments using varying NH4Cl concentrations with equal extracellular and intracellular concentrations and opposing H+ and NH3 gradients (NH3-H+ Erev = 0), the Erev shifted from −30.4 mV (absence of ammonia) to less negative values (−9.6 mV) compatible with NH3-H+ transport [rather than NH3-nH+ (n ≥ 2) transport], where NH3-H+ competes with H+(OH−) for transport. In separate experiments, in the presence of ammonia and an acute transcellular H+ gradient there was an increase in the measured equivalent H+(OH−) flux as expected if NH3-H+ flux was contributing to the observed pH changes. A theoretical model was created and used to fit the NH3 versus Erev data, which showed that NH3-H+ and H+(OH−) competitively interact with the transporter with an affinity ratio ANH3-H+/(ANH3-H+ + AH+) of 0.99995. Both H+(OH−) transport and ammonia-induced whole cell currents were decreased in several mutant SLC4A11 constructs involving the putative ion coordination site suggesting that H+(OH−) and NH3-H+ transport processes share common features involving the SLC4A11 transport mechanism.
Ogando et al. (38) first reported that when PS120 fibroblasts expressing SLC4A11 were exposed to NH4Cl, after the initial alkalinization phase due to cellular NH3 entry, the acidification phase due to entry was greater than mock-transfected cells. There was also a greater degree of acidification after NH4Cl removal consistent with greater influx. Similar findings were reported by Zhang et al. (61) who noted that NH3-H+ transport would give similar results. In contrast, Loganathan et al. (33) reported that the alkalinization phase was greater in SLC4A11-expressing HEK293 cells that was attributed to SLC4A11-mediated NH3 flux per se. Li et al. (30) also reported a greater alkalinization phase.
Zhang et al. (61) first reported NH4Cl-induced changes in whole cell currents in PS120 fibroblasts expressing SLC4A11. Based on Erev shifts in response to changes in external NH3 and pH, it was suggested that SLC4A11 mediates NH3-2H+ cotransport. In studies where the pH was changed to generate varying NH3 and concentrations, the authors concluded that NH3-2H+ rather than was the transport mode because NH3 varied proportionally with the change in whole cell current. In addition, K+, which shares transport processes with , is not transported by SLC4A11 (16, 61). In keeping with these findings our data are compatible with NH3-H+ cotransport. It should be noted that our data do not distinguish NH3-H+ from as the transported species or provide information as whether interconversion of these species within SLC4A11 occurs during the transport cycle as in the AmtB channel (25). However, in our model of the data, the affinity ratio ANH4+/(ANH4+ + AH+) is close to zero suggesting that NH3-H+ is being transported.
Loganathan et al. (33) studied SLC4A11-mediated ammonia transport in Xenopus oocytes. Exposure of control oocytes to NH4Cl led to an intracellular acidification due to the preferential influx of rather than NH3. In SLC4A11-expressing oocytes, pHi increased suggesting that NH3 was the species being transported. The whole cell currents induced by ammonia exposure were attributed to an alkalinization-dependent opening of endogenous channels rather than NH3-2H+ cotransport. Experiments measuring pHi in HEK 293 cells following NH4Cl addition were also compatible with SLC4A11-mediated NH3 transport per se. SLC4A11-mediated conductive H+(OH−) transport in the absence of ammonia was also not detected in this study. Why these results differ from the present study is unclear.
Myers et al. (37) studied mouse Slc4a11 expressed in Xenopus oocytes. The authors concluded that the currents observed in the presence of ammonia do not involve Slc4a11-mediated NH3 or transport or an endogenous channel. Mouse Slc4a11 functioned as an H+(OH−) transporter that was stimulated by an increase in pHe or pHi. Several mechanisms for the increased currents observed were hypothesized including the following: 1) cell depolarization due to influx, which would result in increased Slc4a11-mediated H+(OH−) flux; 2) the increase in pHi due to enhanced H+(OH−) flux would have an independent effect on stimulating the transporter; and 3) pHi may have been increased due to cellular NH3 influx thereby stimulating the transporter.
Changes in patch pipette pH due to pHe changes or changes in extracellular NH3 were not a factor affecting the data. The patch pipette solution contained 100 mM HEPES to minimize any patch pipette solution pH changes. As shown in Fig. 7 using EYFP to monitor changes in pHi in the patch-clamp experiments, following pHe changes (experiments in Fig. 2), there were no changes in pHi detected. In addition, increasing the extracellular NH3 concentration and pHe (experiments in Fig. 5) did not change pHi. In experiments where the pHe was changed (Fig. 2), the measured Erev approximated the value predicted from the transcellular H+ gradient (bath to patch pipette). In the data in Fig. 6A, the Erev remained unchanged approximating the theoretical value of −34.8 in all NH4Cl containing solutions indicating that the H+ gradient between the patch pipette and bath remained at ~4:1 in all solutions. Ammonia-induced changes in membrane potential were also not a likely factor affecting the data. Voltage clamping the cells prevented ammonia-induced changes in cell membrane potential from independently driving H+(OH−) flux. In experiments where the cells were not voltage clamped (data in Fig. 4), a decrease in bath and increase in bath pH in this protocol could potentially have increased the cell membrane potential (more negative), but this would have decreased equivalent H+(OH−) flux in contrast to the increase that was detected.
Our data demonstrate that in the absence of ammonia, SLC4A11 functions as an H+(OH−) conductive pathway. These findings complement previous studies measuring changes in pHi and electrophysiological studies on mouse Slc4a11 (37). Previous studies by Zhang et al. (61) and Loganathan et al. (33) in Xenopus oocytes with measurements at −60- and −40-mV holding potential, respectively, concluded that SLC4A11-does not mediate significant conductive H+(OH−) transport. Our data demonstrate that conductive SLC4A11-mediated conductive H+(OH−) transport is more easily detected at membrane voltages that are less negative. Our findings are in agreement with Myers et al. (37) who concluded that mouse Slc4a11 mediates conductive H+(OH−) transport. Because of the activity of mouse Slc4a11 was independently stimulated by an in increase in pHe and pHi, it was suggested that OH− is the most likely the transported species. Whether SLC4A11 transports H+ versus OH− is difficult to address given that 1) the transport of each species affects pH identically; 2) H+ and OH− transcellular concentration gradients are equal and opposite in direction; and 3) they have the same Nerst potentials. Of interest, there is evidence for OH− transport in (C6F2)2Hg planar phospholipid membranes and freshwater algae Chara spp. Channels (1, 35).
Based on homology with the ion coordination sites in the SLC4 transporters NBCe1-A (19) and AE1 (4), we examined residues adjacent to or within TM3, TM8, and TM10 that could be involved in SLC4A11 ion coordination. Our results show that mutants E659A (TM8), Y706D (TM10), and H708K (TM10) (SLC4A11-C numbering) impair H+(OH−) flux and block both conductive H+(OH−) transport and ammonia-stimulated whole cell currents. E659A and H708D (SLC4A11-C numbering) are known mutations causing CHED (24, 27). We hypothesize that these residues are involved in ion coordination in the transporter and that H+(OH−) and NH3-H+ transport processes share common features involving the SLC4A11 transport mechanism. The presence of E659 in the putative ion coordination site suggests that H+ rather than OH− is the transported species. These findings complement our competitive binding model (Eq. 2) depicting competitive interaction of H+(OH−) and NH3-H+ with the transporter. NBCe1-A and AE1 likely undergo conformational changes from outward facing to inward facing conformations during the transport cycle. By analogy, given its sequence homology, it might be predicted that SLC4A11 is a transporter that undergoes similar conformational changes. It is also possible however that SLC4A11 functions as an H+(OH−) and NH3-H+ channel. H+ conduction in proteins is thought to occur either by a reversible protonation-deprotonation of specific residues or via a Grotthuss-type (water-hopping) mechanism (10). Interestingly, it has been suggested that a variant of the Grotthuss mechanism involves putative H+ hopping in channels that are occupied with ammonia appearing macroscopically as an increase in NH3-H+ (or ) permeability (52).
In addition to mediating ammonia and H+(OH−) transport, SLC4A11 transports water (54). Aquaporin proteins such AQP8 and AtTIP2;1 also transport ammonia and water via a mechanism involving uptake and deprotonation (26). Aquaporins typically efficiently exclude protons from the pore via electrostatic interactions rather than proton wire interruptions (9, 13). Rat AQP1 mutated in the ar/R constriction region develops ammonia stimulated proton transport and a proton permeability (5). Artificial water channels can transport protons at a high rate (14, 31). The transport of water by SLC4A11 is analogous to the transport of water by other membrane transport proteins such as SGLT1, NIS, MCT1, NKCC1, and LeuT (29, 59, 63) that are thought to develop transient water-conducting (channel-like) states during the conformational changes that provide alternating access to substrates. Whether water permeates SLC4A11 through these transient water-conducting states or SLC4A11 functions as a water channel like aquaporin is currently unknown. In addition, whether there are structural overlapping features common to its water, ammonia, and H+(OH−) transport pathways is unknown. Of the known SLC4A11 mutations, the R125H CHED-causing mutation blocks all three modes of transport suggesting structurally (or structural interaction) shared features between these pathways (23).
In conclusion, our results suggest that SLC4A11 mediates conductive H+(OH−) transport and in the presence of ammonia the transporter also functions in the NH3-H+ transport mode. An important question to be addressed is the role of SLC4A11-mediated transport in vivo. In corneal endothelial cells, SLC4A11 has been reported to be expressed on the basolateral membrane (54) and more recently in the inner mitochondrial membrane (39). To calculate the electrochemical gradient across the transporter, whereas the stromal pH is estimated to be 7.39–7.54 (depending on whether the eye is open or closed) (6) with a pHi of ~7.37 (7), the stromal and intracellular ammonia concentrations have not been determined. There is also uncertainty regarding the Vm of endothelial cells (18, 57) making it difficult to predict whether the transporter acts as a cellular H+ and ammonia loader versus an H+ and ammonia efflux pathway. It is hypothesized that in mitochondria SLC4A11 acts as an H+ influx pathway resulting in mitochondrial uncoupling and depolarization (39). Further studies are needed to address the measurements of corneal stromal, endothelial, and mitochondrial ammonia concentrations in vivo to predict the direction of SLC4A11 transport.
GRANTS
I. Kurtz is supported by funds from the NIH (National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-077162), the Allan Smidt Charitable Fund, the Ralph Block Family foundation, and the Factor Family Foundation. Work in laboratory of S. Noskov was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC Grant RGPIN-315019). H. Zhekova was supported by the Alberta Innovates Health Solution Post-Doctoral Fellowship. Molecular dynamics simulations were performed on the Canada Foundation for Innovation-supported GladOS cluster at the University of Calgary and on the West-Grid/Compute Canada clusters under a Research Allocation Award (to S. Noskov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.M.S. and I.K. conceived and designed research; L.K., R.A., N.A., and D.N. performed experiments; L.K., X.M.S., and I.K. analyzed data; L.K., X.M.S., and I.K. interpreted results of experiments; L.K. and H.Z. prepared figures; H.Z. and I.K. drafted manuscript; L.K., S.N., A.P., and I.K. edited and revised manuscript; L.K., R.A., X.M.S., N.A., D.N., H.Z., S.N., A.P., and I.K. approved final version of manuscript.
Footnotes
As to another possible alternative model, transport competing with H+, fitting this model to our data in Fig. 6B did obtain a good fit. However, the affinity ratio ANH4+/(ANH4+ + AH+) from the fitting of the data was 8.18E-6. This suggests that in the experiment shown in Fig. 6B, the transport of contributes little compared to H+ to the current and reversal potential. The affinity ratio for NH3- H+ transport competing with H+ is high (0.99995), whereas the affinity ratio for is close to zero. This modeling analysis suggests that although both models, NH3+-H+ competing with H+ and competing with H+, fit the data in Fig. 6B, the fitting parameter affinity ratio favors the model of NH3-H+competing with H+.
REFERENCES
- 1.Al Khazaaly S, Beilby MJ. Zinc ions block H+/OH− channels in Chara australis. Plant Cell Environ 35: 1380–1392, 2012. doi: 10.1111/j.1365-3040.2012.02496.x. [DOI] [PubMed] [Google Scholar]
- 2.Aldave AJ, Yellore VS, Bourla N, Momi RS, Khan MA, Salem AK, Rayner SA, Glasgow BJ, Kurtz I. Autosomal recessive CHED associated with novel compound heterozygous mutations in SLC4A11. Cornea 26: 896–900, 2007. doi: 10.1097/ICO.0b013e318074bb01. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez O, Latorre R. The enduring legacy of the “constant-field equation” in membrane ion transport. J Gen Physiol 149: 911–920, 2017. doi: 10.1085/jgp.201711839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, Abe Y, Hino T, Ikeda-Suno C, Kuma H, Kang D, Murata T, Hamakubo T, Cameron AD, Kobayashi T, Hamasaki N, Iwata S. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350: 680–684, 2015. doi: 10.1126/science.aaa4335. [DOI] [PubMed] [Google Scholar]
- 5.Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 103: 269–274, 2006. doi: 10.1073/pnas.0507225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonanno JA, Polse KA. Measurement of in vivo human corneal stromal pH: open and closed eyes. Invest Ophthalmol Vis Sci 28: 522–530, 1987. [PubMed] [Google Scholar]
- 7.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. II. Na+: cotransport and Cl−/ exchange. Invest Ophthalmol Vis Sci 33: 3068–3079, 1992. [PubMed] [Google Scholar]
- 8.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293: R2136–R2146, 2007. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- 9.de Groot BL, Grubmüller H. The dynamics and energetics of water permeation and proton exclusion in aquaporins. Curr Opin Struct Biol 15: 176–183, 2005. doi: 10.1016/j.sbi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 10.DeCoursey TE. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the HV family. Physiol Rev 93: 599–652, 2013. doi: 10.1152/physrev.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet 44: 322–326, 2007. doi: 10.1136/jmg.2006.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlers N, Módis L, Møller-Pedersen T. A morphological and functional study of congenital hereditary endothelial dystrophy. Acta Ophthalmol Scand 76: 314–318, 1998. doi: 10.1034/j.1600-0420.1998.760312.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg B. Why can’t protons move through water channels? Biophys J 85: 3427–3428, 2003. doi: 10.1016/S0006-3495(03)74763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopinadhan K, Hu S, Esfandiar A, Lozada-Hidalgo M, Wang FC, Yang Q, Tyurnina AV, Keerthi A, Radha B, Geim AK. Complete steric exclusion of ions and proton transport through confined monolayer water. Science 363: 145–148, 2019. doi: 10.1126/science.aau6771. [DOI] [PubMed] [Google Scholar]
- 15.Gröger N, Fröhlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem 285: 14467–14474, 2010. doi: 10.1074/jbc.M109.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall JA, Yan D. The molecular basis of K+ exclusion by the Escherichia coli ammonium channel AmtB. J Biol Chem 288: 14080–14086, 2013. doi: 10.1074/jbc.M113.457952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SB, Ang HP, Poh R, Chaurasia SS, Peh G, Liu J, Tan DT, Vithana EN, Mehta JS. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest Ophthalmol Vis Sci 54: 6179–6189, 2013. doi: 10.1167/iovs.13-12089. [DOI] [PubMed] [Google Scholar]
- 18.Hodson S, Wigham C. A near-zero membrane potential in transporting corneal endothelial cells of rabbit. J Physiol 412: 365–374, 1989. doi: 10.1113/jphysiol.1989.sp017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh KW, Jiang J, Abuladze N, Tsirulnikov K, Kao L, Shao X, Newman D, Azimov R, Pushkin A, Zhou ZH, Kurtz I. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nat Commun 9: 900, 2018. doi: 10.1038/s41467-018-03271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54: 4330–4340, 2013. doi: 10.1167/iovs.13-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao X, Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Gangopadhyay N, Hejtmancik JF, Kannabiran C. Autosomal recessive corneal endothelial dystrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet 44: 64–68, 2007. doi: 10.1136/jmg.2006.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao L, Azimov R, Abuladze N, Newman D, Kurtz I. Human SLC4A11-C functions as a DIDS-stimulatable H+(OH−) permeation pathway: partial correction of R109H mutant transport. Am J Physiol Cell Physiol 308: C176–C188, 2015. doi: 10.1152/ajpcell.00271.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao L, Azimov R, Shao XM, Frausto RF, Abuladze N, Newman D, Aldave AJ, Kurtz I. Multifunctional ion transport properties of human SLC4A11: comparison of the SLC4A11-B and SLC4A11-C variants. Am J Physiol Cell Physiol 311: C820–C830, 2016. doi: 10.1152/ajpcell.00233.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaul H, Suman M, Khan Z, Ullah MI, Ashfaq UA, Idrees S. Missense mutation in SLC4A11 in two Pakistani families affected with congenital hereditary endothelial dystrophy (CHED2). Clin Exp Optom 99: 73–77, 2016. doi: 10.1111/cxo.12276. [DOI] [PubMed] [Google Scholar]
- 25.Khademi S, O’Connell J 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science 305: 1587–1594, 2004. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 26.Kirscht A, Kaptan SS, Bienert GP, Chaumont F, Nissen P, de Groot BL, Kjellbom P, Gourdon P, Johanson U. Crystal structure of an ammonia-permeable aquaporin. PLoS Biol 14: e1002411, 2016. doi: 10.1371/journal.pbio.1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodaganur SG, Kapoor S, Veerappa AM, Tontanahal SJ, Sarda A, Yathish S, Prakash DR, Kumar A. Mutation analysis of the SLC4A11 gene in Indian families with congenital hereditary endothelial dystrophy 2 and a review of the literature. Mol Vis 19: 1694–1706, 2013. [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz I. Apical Na+/H+ antiporter and glycolysis-dependent H+-ATPase regulate intracellular pH in the rabbit S3 proximal tubule. J Clin Invest 80: 928–935, 1987. doi: 10.1172/JCI113184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Shaikh SA, Enkavi G, Wen PC, Huang Z, Tajkhorshid E. Transient formation of water-conducting states in membrane transporters. Proc Natl Acad Sci USA 110: 7696–7701, 2013. doi: 10.1073/pnas.1218986110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Hundal KS, Chen X, Choi M, Ogando DG, Obukhov AG, Bonanno JA. R125H, W240S, C386R, and V507I SLC4A11 mutations associated with corneal endothelial dystrophy affect the transporter function but not trafficking in PS120 cells. Exp Eye Res 180: 86–91, 2019. doi: 10.1016/j.exer.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Licsandru E, Kocsis I, Shen YX, Murail S, Legrand YM, van der Lee A, Tsai D, Baaden M, Kumar M, Barboiu M. Salt-excluding artificial water channels exhibiting enhanced dipolar water and proton translocation. J Am Chem Soc 138: 5403–5409, 2016. doi: 10.1021/jacs.6b01811. [DOI] [PubMed] [Google Scholar]
- 32.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci USA 95: 6803–6808, 1998. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loganathan SK, Schneider HP, Morgan PE, Deitmer JW, Casey JR. Functional assessment of SLC4A11, an integral membrane protein mutated in corneal dystrophies. Am J Physiol Cell Physiol 311: C735–C748, 2016. doi: 10.1152/ajpcell.00078.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem 284: 26882–26896, 2009. doi: 10.1074/jbc.M109.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas WJ. Alkaline band formation in Chara corallina: due to OH efflux or H influx? Plant Physiol 63: 248–254, 1979. doi: 10.1104/pp.63.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melo F, Sánchez R, Sali A. Statistical potentials for fold assessment. Protein Sci 11: 430–448, 2002. doi: 10.1002/pro.110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers EJ, Marshall A, Jennings ML, Parker MD. Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH− conductance pathway that is stimulated by rises in intracellular and extracellular pH. Am J Physiol Cell Physiol 311: C945–C959, 2016. doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na+ permeable pHi regulator. Am J Physiol Cell Physiol 305: C716–C727, 2013. doi: 10.1152/ajpcell.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogando DG, Choi M, Shyam R, Li S, Bonanno JA. Ammonia sensitive SLC4A11 mitochondrial uncoupling reduces glutamine induced oxidative stress. Redox Biol 26: 101260, 2019. doi: 10.1016/j.redox.2019.101260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlowski A, Vargas LA, Aiello EA, Álvarez BV. Elevated carbon dioxide upregulates NBCn1 Na+/ cotransporter in human embryonic kidney cells. Am J Physiol Renal Physiol 305: F1765–F1774, 2013. doi: 10.1152/ajprenal.00096.2013. [DOI] [PubMed] [Google Scholar]
- 41.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun 282: 1103–1109, 2001. doi: 10.1006/bbrc.2001.4692. [DOI] [PubMed] [Google Scholar]
- 44.Riazuddin SA, Vithana EN, Seet LF, Liu Y, Al-Saif A, Koh LW, Heng YM, Aung T, Meadows DN, Eghrari AO, Gottsch JD, Katsanis N. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophy. Hum Mutat 31: 1261–1268, 2010. doi: 10.1002/humu.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436: 424–427, 2005. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 46.Schrödinger, Inc The PyMOL Molecular Graphics System, version 1.8 Mannheim, Germany: Schrödinger, 2015. [Google Scholar]
- 47.Shao XM, Feldman JL. Micro-agar salt bridge in patch-clamp electrode holder stabilizes electrode potentials. J Neurosci Methods 159: 108–115, 2007. doi: 10.1016/j.jneumeth.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci 15: 2507–2524, 2006. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siddiqui S, Zenteno JC, Rice A, Chacón-Camacho O, Naylor SG, Rivera-de la Parra D, Spokes DM, James N, Toomes C, Inglehearn CF, Ali M. Congenital hereditary endothelial dystrophy caused by SLC4A11 mutations progresses to Harboyan syndrome. Cornea 33: 247–251, 2014. doi: 10.1097/ICO.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soumittra N, Loganathan SK, Madhavan D, Ramprasad VL, Arokiasamy T, Sumathi S, Karthiyayini T, Rachapalli SR, Kumaramanickavel G, Casey JR, Rajagopal R. Biosynthetic and functional defects in newly identified SLC4A11 mutants and absence of COL8A2 mutations in Fuchs endothelial corneal dystrophy. J Hum Genet 59: 444–453, 2014. doi: 10.1038/jhg.2014.55. [DOI] [PubMed] [Google Scholar]
- 51.Sultana A, Garg P, Ramamurthy B, Vemuganti GK, Kannabiran C. Mutational spectrum of the SLC4A11 gene in autosomal recessive congenital hereditary endothelial dystrophy. Mol Vis 13: 1327–1332, 2007. [PubMed] [Google Scholar]
- 52.Takeda K, Barry PH, Gage PW. Effects of ammonium ions on endplate channels. J Gen Physiol 75: 589–613, 1980. doi: 10.1085/jgp.75.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680, 1994. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet 22: 4579–4590, 2013. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38: 755–757, 2006. doi: 10.1038/ng1824. [DOI] [PubMed] [Google Scholar]
- 56.Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet 17: 656–666, 2008. doi: 10.1093/hmg/ddm337. [DOI] [PubMed] [Google Scholar]
- 57.Watsky MA, Rae JL. Resting voltage measurements of the rabbit corneal endothelium using patch-current clamp techniques. Invest Ophthalmol Vis Sci 32: 106–111, 1991. [PubMed] [Google Scholar]
- 58.Webb B, Sali A. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics 54: 5.6.1–5.6.37, 2016. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeuthen T, MacAulay N. Transport of water against its concentration gradient: fact or fiction? WIREs Membr Transp Signal 1: 373–381, 2012. doi: 10.1002/wmts.54. [DOI] [Google Scholar]
- 60.Zhang W, Li H, Ogando DG, Li S, Feng M, Price FW Jr, Tennessen JM, Bonanno JA. Glutaminolysis is essential for energy production and ion transport in human corneal endothelium. EBioMedicine 16: 292–301, 2017. doi: 10.1016/j.ebiom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W, Ogando DG, Bonanno JA, Obukhov AG. Human SLC4A11 Is a novel NH3/H+ co-transporter. J Biol Chem 290: 16894–16905, 2015. doi: 10.1074/jbc.M114.627455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W, Ogando DG, Kim ET, Choi MJ, Li H, Tenessen JM, Bonanno JA. Conditionally immortal Slc4a11−/− mouse corneal endothelial cell line recapitulates disrupted glutaminolysis seen in Slc4a11−/− mouse model. Invest Ophthalmol Vis Sci 58: 3723–3731, 2017. doi: 10.1167/iovs.17-21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C, Noskov SY. The role of local hydration and hydrogen-bonding dynamics in ion and solute release from ion-coupled secondary transporters. Biochemistry 50: 1848–1856, 2011. doi: 10.1021/bi101454f. [DOI] [PubMed] [Google Scholar]