The transforming growth factor-β (TGF-β) superfamily member, myostatin (MSTN) or growth differentiation factor 8 (GDF-8), is well known for its role in regulating skeletal muscle growth. Notably, a “double muscle” phenotype has been observed in several breeds of cattle whereby muscle mass is increased by ~20%. An 11-base pair deletion in the Mstn coding region on chromosome 2 was discovered to be the causative mutation underlying this profound muscle overgrowth phenotype in cattle (7). Since then, studies in mice have confirmed that MSTN is indeed a strong negative regulator of skeletal muscle growth across species (8). The growth-restrictive effects of MSTN are not limited to skeletal muscle, as subsequent research demonstrated that cardiac muscle mass was significantly elevated in MSTN knockout mice in response to hypertrophic stimulus (9). Others have reported that MSTN affects not only cardiomyocyte growth but also cardiac fibrosis, as MSTN overexpression in the heart caused increased cardiac interstitial fibrosis and compromised cardiac function in aged mice (1). However, these studies are not without controversy as genetic loss of MSTN neither resulted in ventricular hypertrophy nor attenuated cardiac fibrosis in the dystrophin deficient mdx mouse (5). Although there is some indication that MSTN has profibrotic and antihypertrophic effects in the heart, the pathological state, as well as the expression level of MSTN itself, at least in part, likely accounts for conflicting reports. In this issue of the American Journal of Physiology-Heart and Circulatory Physiology, Castillero et al. (3) aim to clarify some controversies in the field by directly testing the hypothesis that inhibition of the MSTN receptor attenuates adverse cardiac remodeling and fibrosis in a mouse model of heart failure (HF).
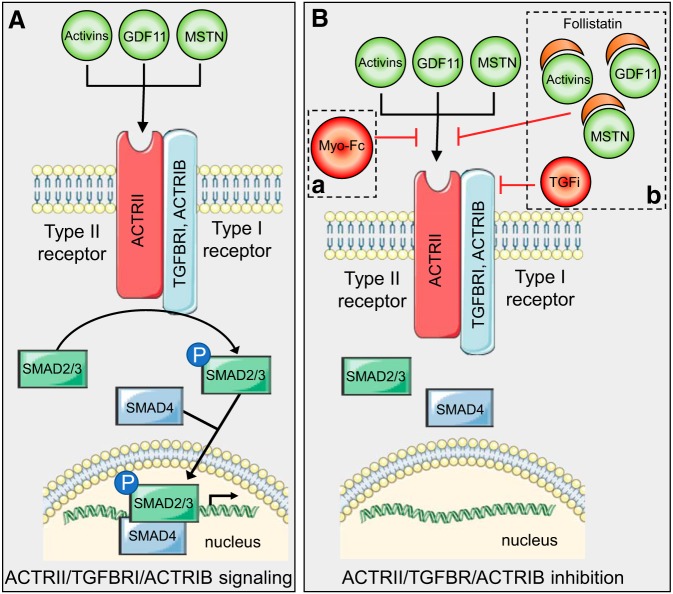
MSTN binds to the activin type II receptor (ACTRII) which couples with TGF-β receptor I (TGFBRI/ALK-4) or activin receptor type-1β (ACTR1B/ALK-5). This recruitment of type I receptor kinases initiates a signaling cascade via the transcription factors SMAD2/3, which regulates expression of myogenesis genes. Along with MSTN, GDF-11, and activin A/B also bind to and activate the ACTRII receptor (Fig. 1A). In humans, MSTN protein expression is elevated in patients with dilated cardiomyopathy, as well as patients with ischemic cardiomyopathy (6). Similar expression patterns have been observed in mice, where circulating and cardiac MSTN expression is increased shortly after myocardial infarction (MI) (4). Interestingly, inhibition of the MSTN receptor, ACTRII, in aged mice and mice with nonischemic HF resulted in improved cardiac function (11). Collectively, these data demonstrate that MSTN protein is elevated in the heart following injury and suggest that MSTN/ACTRII signaling contributes to adverse cardiac outcomes following injury. However, the direct role of ACTRII signaling in the post-cardiac injury response has not been comprehensively described, likely because of complex signaling of the TGF-β superfamily whereby several ligand-receptor interactions with overlapping signaling cascades exist.
Fig. 1.
A: schematic representation of canonical activin, growth differentiation factor 11 (GDF11), and myostatin (MSTN) signaling through the activin type II receptor/transforming growth factor-β (TGF-β) receptor I (ACTRII/TGFBRI) or ACTRII/activin receptor type-1β (ACTRII/ACTR1B) receptor. B: strategy implemented by Castillero et al. (3) to inhibit activin type II receptor signaling. Animals were administred either Myo-Fc (a, dashed square) or follistatin along with TGFBRI inhibitor (TGFi; b, dashed square).
In the current study, Castillero et al. (3) implemented two pharmacological depletion models to block ACTRII signaling and test the role of ACTRII signaling in cardiac outcomes following MI in mice. The first strategy aimed to block ACTRII signaling was by weekly administration of Myo-Fc, which is a decoy form of MSTN (Fig. 1B,a, dashed box). Myo-Fc competitively inhibits endogenous MSTN, GDF-11, and activins from binding to ACTRII (2). The second pharmacological strategy implemented was a combination of follistatin (Fol) and a TGFBRI inhibitor (TGFi, GW788388), which were administered daily (Fig. 1B,b, dashed box). Fol binds MSTN, GDF-11, and activins, thereby preventing these ligands from binding ACTRII (8). TGFi inhibits activation of the type I receptor to prevent TGFBRI-dependent phosphorylation of SMAD2/3. TGFi inhibits not only TGFBRI but also several other TGF receptors that redundantly phosphorylate SMAD2/3; therefore, TGFi administration efficiently depletes SMAD2/3 phosphorylation (10). These inhibitor treatments were administered to 8-wk-old- male mice after surgical left anterior descending ligation, resulting in MI or sham operation, and were also used for in vitro studies in AC16 cardiomyocyte-like cells.
The authors report that inhibiting ACTRII/TGFBR signaling through Myo-Fc or Fol+TGFi administration improved fractional shortening and attenuated fibrosis in mice following MI. Both pharmacological treatments showed similar and impressive improvement in percent fractional shortening and fibrosis, convincingly demonstrating that inhibiting ACTRII signaling could improve post-MI outcomes and prevent pathological cardiac remodeling. Considering the well-known role of MSTN signaling on preventing muscle hypertrophy, the authors investigated cardiomyocyte cell size and heart weights. In sham-operated mice, Myo-Fc and Fol+TGFi treatments both significantly increased cardiomyocyte cross-sectional area. However, neither treatment exacerbated cardiac hypertrophy after MI or worsened cardiac function. The authors attribute these findings to the anabolic properties of Myo-Fc in a nondisease setting. Furthermore, Myo-Fc treatment improved exercise tolerance following MI and also in sham-operated animals, which could be due to cardiac phenotypes or possibly effects on skeletal muscle as gastrocnemius muscle weight was significantly elevated following Myo-Fc treatment. Collectively, the authors establish a significant role for ACTRII signaling in both normal cardiac homeostasis and after cardiac injury.
Castillero et al. (3) further delve into the signaling mechanisms downstream of ACTRII/TGFBR using both mouse HF and in vitro models. Following MI in vivo, ACTRII/TGFBR inhibition prevented unfolded protein response (UPR) signaling, as well as normalized the activation of SMAD2/3, and p38. Previously reported in the literature and shown here, UPR, P-SMAD2/3, and P-p38 signaling all increase after MI and are hallmark features of HF. Taken together, these findings demonstrate that inhibiting ACTRII/TGFBR after MI can decrease cardiac dysregulation and apoptotic signaling mechanisms.
Inhibiting ACTRII/TGFBR with Myo-Fc and Fol+TGFi also promoted hypertrophic cardiac signaling as indicated by increased Akt phosphorylation (P-Akt) in vivo. With the use of AC16 cardiomyocyte-like cells, in vitro studies confirmed that P-Akt is increased in response to Myo-Fc and Fol+TGFi, and abundance of P-Akt is inversely correlated with P-SMAD2/3 and P-p38 regulation in cardiomyocytes under stress. These results further support a link between ACTRII signaling and Akt activation. The authors also provide ample evidence that ACTRII/TGFBR inhibition decreases not only fibrosis itself but also fibrotic markers connective tissue growth factor, fibronectin, collagen type I-α1 chain, α-smooth muscle actin, and matrix metallopeptidase 12. This supports the idea that ACTRII signaling can aggravate cardiac fibrosis in an injury setting.
Castillero et al. (3) provide compelling evidence that blocking ACTRII signaling could provide a potential therapeutic benefit in the heart following MI. The current findings that both Myo-Fc and Fol+TGFi attenuate fibrosis and improve fractional shortening after MI warrant additional studies aimed at determining cell types responding to pharmacological treatments in vivo. Furthermore, the comprehensive downstream signaling modulated by ACTRII inhibition, not only in the heart but in off-target tissues as well, has not been fully elucidated and also warrants further investigation. Additional studies designed to investigate the long-term effects of ACTRII inhibition will be critical for understanding the full therapeutic potential of ACTRII inhibition in patients with HF. In summary, Castillero et al. provide the cardiovascular community with novel and convincing insight into ACTRII signaling in cardiac remodeling and fibrosis following MI. We expect that future studies will confirm that downregulating SMAD2/3 through ACTRII inhibitors has potential clinical importance for improving pathological cardiac remodeling.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants R01-HL-141159 (to C. C. O’Meara) and T32-HL-007852 (to S. J. Paddock) and the Cardiovascular Center and Research and Educational Program Fund at the Medical College of Wisconsin (to C. C. O’Meara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.P. and C.C.O. prepared figures and drafted, edited, revised, and approved final manuscript.
REFERENCES
- 1.Biesemann N, Mendler L, Kostin S, Wietelmann A, Borchardt T, Braun T. Myostatin induces interstitial fibrosis in the heart via TAK1 and p38. Cell Tissue Res 361: 779–787, 2015. doi: 10.1007/s00441-015-2139-2. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19: 543–549, 2005. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 3.Castillero E, Akashi H, Najjar M, Ji R, Brandstetter LM, Wang C, Liao X, Zhang X, Sperry A, Gailes M, Guaman K, Recht A, Schlosberg I, Sweeney HL, Ali ZA, Homma S, Colombo PC, Ferrari G, Schulze PC, George I. Activin type II receptor ligand signaling inhibition after experimental ischemic heart failure attenuates cardiac remodeling and prevents fibrosis. Am J Physiol Heart Circ Physiol 318: TBA, 2019. doi: 10.1152/ajpheart.00302.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillero E, Akashi H, Wang C, Najjar M, Ji R, Kennel PJ, Sweeney HL, Schulze PC, George I. Cardiac myostatin upregulation occurs immediately after myocardial ischemia and is involved in skeletal muscle activation of atrophy. Biochem Biophys Res Commun 457: 106–111, 2015. doi: 10.1016/j.bbrc.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord 17: 290–296, 2007. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail 12: 444–453, 2010. doi: 10.1093/eurjhf/hfq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 17: 71–74, 1997. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306–9311, 2001. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99: 15–24, 2006. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen M, Thorikay M, Deckers M, van Dinther M, Grygielko ET, Gellibert F, de Gouville AC, Huet S, ten Dijke P, Laping NJ. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int 73: 705–715, 2008. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 11.Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, Platt C, Damilano F, Lindman BR, Elmariah S, Biersmith M, Lee SJ, Seidman CE, Seidman JG, Gerszten RE, Lach-Trifilieff E, Glass DJ, Rosenzweig A. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med 11: 11, 2019. doi: 10.1126/scitranslmed.aau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]