Abstract
The introduction of duplex Doppler ultrasound almost half a century ago signified a revolutionary advance in the ability to assess limb blood flow in humans. It is now widely used to assess blood flow under a variety of experimental conditions to study skeletal muscle resistance vessel function. Despite its pervasive adoption, there is substantial variability between studies in relation to experimental protocols, procedures for data analysis, and interpretation of findings. This guideline results from a collegial discussion among physiologists and pharmacologists, with the goal of providing general as well as specific recommendations regarding the conduct of human studies involving Doppler ultrasound-based measures of resistance vessel function in skeletal muscle. Indeed, the focus is on methods used to assess resistance vessel function and not upstream conduit artery function (i.e., macrovasculature), which has been expertly reviewed elsewhere. In particular, we address topics related to experimental design, data collection, and signal processing as well as review common procedures used to assess resistance vessel function, including postocclusive reactive hyperemia, passive limb movement, acute single limb exercise, and pharmacological interventions.
Keywords: blood flow, endothelial function, vascular function
INTRODUCTION
Cardiovascular disease is a leading cause of morbidity and mortality worldwide (228). In the United States alone, approximately 600,000 deaths (25%) each year are attributed to cardiovascular disease (17). The burden associated with cardiovascular disease is projected to sharply escalate in the Western world because of the changing age structure of the population, in addition to the growing prevalence of physical inactivity, obesity, and diabetes (84, 180, 286, 300). In many clinical syndromes linked to underlying cardiovascular disease, overt changes in “cardiovascular control” are observed, which may encompass disease-related changes in neural, metabolic, and myogenic control of skeletal muscle resistance vessels. Notably, in various metabolic diseases such as diabetes, impaired vasomotor reactivity of the resistance arteries, including feed arteries and arterioles, typically manifests before vascular dysfunction or disease can be detected in larger, more proximal arteries. In fact, assessment of resistance vessel function may have the potential to provide prognostic information beyond the predictive value of traditional risk factors (4, 119, 262). Furthermore, given that skeletal muscle resistance vessel function is positively associated with exercise tolerance and fitness (1, 54, 138, 173), blood pressure control (147, 199, 200), glucose homeostasis (12, 146), and lower cardiovascular risk (4, 109, 119, 172), improved vasomotor function in skeletal muscle resistance arteries (defined broadly as the ability to vasoconstrict or vasodilate appropriately) is a marker of overall health status.
A landmark in technological development for assessment of skeletal muscle blood flow and downstream resistance vessel function in humans was the introduction of duplex Doppler ultrasound (9). This methodology was, along with the cold saline thermodilution technique, instrumental to the initial recordings of limb blood flow during rhythmic muscle contraction (291) and to the subsequent seminal work undertaken at the Copenhagen Muscle Research Center and led by Prof. Bengt Saltin (135, 212–214, 216, 217, 232). Ever since then, Doppler ultrasound has been widely used to assess blood flow under a variety of experimental conditions [e.g., reactive hyperemia, passive limb movement (PLM), single limb exercise, pharmacological interventions] designed to interrogate aspects of skeletal muscle resistance vessel function (Fig. 1). Accordingly, the purpose of this report is to provide some recommendations regarding the conduct of human studies involving Doppler ultrasound-based assessments of resistance vessel function in skeletal muscle. Indeed, we focus on the methods used to assess resistance vessel function rather than upstream conduit artery function (i.e., macrovasculature), which has been expertly reviewed elsewhere (96, 262). This commissioned compendium reflects the discussion among several independent research groups that routinely employ these methodologies for vascular studies in humans. For comparisons of Doppler ultrasound with other technologies to assess blood flow, the reader is referred to recent reviews (27, 83).
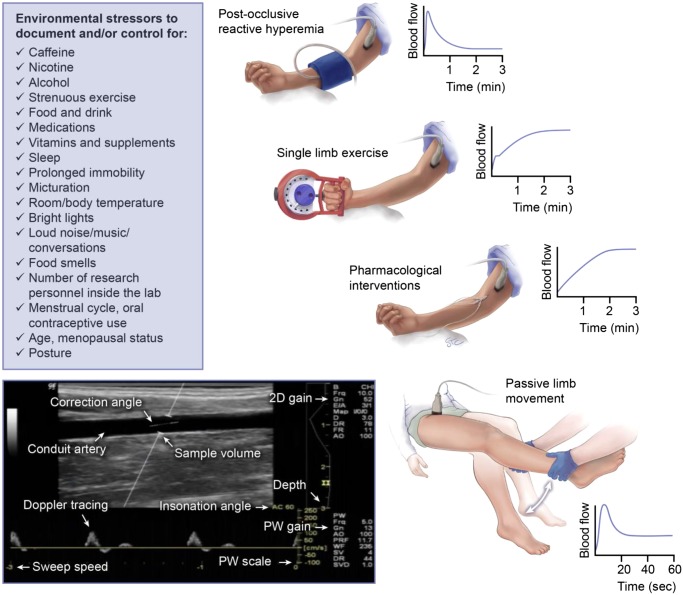
Fig. 1.
Schematic illustrating common Doppler ultrasound-based procedures to assess resistance vessel function in humans and list of environmental stressors that should be taken into consideration during such studies. As noted in the text, these procedures can be applied to the upper and lower limbs and can also be overlaid. For example, drug infusions can occur in the resting limb but also during exercise or passive limb movement. Likewise, cuff occlusion can also be coupled with exercise to produce a “maximal” hyperemic response. The profile of typical blood flow responses is also illustrated; however, these are largely dependent on the precise stimulus (e.g., intensity of exercise, drug type, and dose, etc.), as well as the subject population studied.
EXPERIMENTAL DESIGN
Subject-Specific Considerations
When appropriate experimental controls are in place, Doppler ultrasound-based determinations of limb blood flow to assess resistance vessel function can be reliably compared between study populations and/or within the same individuals before and after perturbation or over time (213, 247). To ensure measures of resistance vessel function are both accurate and repeatable, human research participants should avoid a number of potential environmental stressors for a period of time preceding assessment (Fig. 1). At the minimum, research participants should refrain from caffeine, nicotine, alcohol, and strenuous exercise for 12–24 h (26, 183, 261, 284). In addition, food and drink (other than sips of water) should be avoided for at least 4 h before the initiation of measurements. In many cases, subjects have to be fasted for these measures. Indeed, in some studies, overnight fasting is required; this may be the case for insulin clamp studies or studies in clinical populations (i.e., insulin resistance), where a 4-h fast may not be sufficient time to confidently assure that glucose/insulin levels are low and/or at steady-state. Furthermore, blood flow distribution is impacted during the postprandial state. A high-fat meal, for example, not only alters blood flow distribution but can also exert detrimental effects on vascular function (130, 198). With this, another option may be to feed a standardized low-fat, low-glycemic index meal to participants, for example, during day-long experiments with multiple testing sessions where it is not feasible to have subjects continuously fasted.
Medication considerations.
When conducting research with human volunteers, it is standard practice to inquire about past and current medical history to determine whether individuals meet eligibility criteria. In such instances, medications known to impact the cardiovascular system should be documented. Such medications include but are not limited to nonsteroidal anti-inflammatory medications (118, 240), certain vitamins and/or supplements (66, 129, 233), antianxiety and antidepressants (101, 206), central acting sympatholytics (86), β-blockers (143, 171), ACE inhibitors (171), angiotensin II receptor blockers, statins, and other lipid-lowering agents (170, 287, 307). Adequate experimental control may consist of withholding certain medications/vitamins before participation. For example, “as needed” medications (i.e., ibuprofen taken PRN) or subject-elected supplements/vitamins can be withheld if not prescribed and the subject is willing. From a clinical standpoint, a typical washout period for medication is five half-lives, which typically falls within a 24–48-h time window. The potential impact of medication on vascular outcomes is well illustrated by two recently published studies in patients with heart failure with a reduced ejection fraction (HFrEF). Using an identical knee extension exercise paradigm, Barett-O’Keefe et al. (14) identified a 20–35% reduction in exercise-induced muscle blood flow in patients with HFrEF compared with controls, whereas Espoito et al. (67) failed to identify any disease-related reduction in limb blood flow. One potential explanation for the divergent results from these two studies using almost identical methodologies may be the choice to withhold β-blockers for 48 h before the experimental day in the latter study. This example highlights the importance of carefully considering background pharmacotherapy in highly medicated patient groups. That said, it is important to mention that in some clinical populations, individuals may be unable to refrain from certain medications, and it may be unsafe to do so; in these instances, studies should be conducted a standard period of time following medication intake (262). If the medication cannot be withheld, and it interacts with main study outcomes, the individual may need to be excluded from the study. For example, amlodipine (angioselective calcium channel blocker) is a medication that would impact the results of a vascular study, and also should not be withheld if prescribed. If the medication was withheld, elimination half-life is ∼40 h, so five half-lives would be ∼200 h (and could be even longer in older adults). It could be argued that in some experiments the impact of these medications (taken at the appropriate time of day and dose) is actually of interest and ecologically valid. Indeed, in the real world, a patient is the individual plus their medications. Accordingly, whether drugs are sanctioned or allowed ultimately depends on the research question, the experimental design, and the population studied. These decisions may also depend upon physician recommendation, the Institutional Review Board, and/or the local ethics committee.
Study timing.
Study visits should be scheduled for the same time of day, after a normal night’s sleep, during wakefulness. There is evidence to support diurnal variation in vascular function (68, 133, 134, 197, 243) as well as an effect of sleep (5, 25). Indeed, it is advised that investigators collect information regarding sleep patterns, as a short-night sleep duration has recently been associated with depressed endothelial-dependent vasodilator function due to diminished nitric oxide (NO) bioavailability (5). Furthermore, subjects should be kept awake during testing to avoid acute effects of sleep on main outcome variables. Prolonged inactivity/bed rest is also associated with reduced vascular function (265). In this regard, protocols lasting more than 2 h should consider the potential impact of prolonged immobility on experimental measures and how such factors may impact results in later trials. Time-controlled experiments may be necessary to account for the effect of prolonged sedentary time on outcome variables (219, 285). Alternatively, and depending on the experimental model (e.g., single-limb exercise), the contralateral limb may be used as internal control, an approach that significantly strengthens the experimental design (16, 117, 174, 220, 254, 263, 266, 269, 292, 293).
Before instrumentation for even relatively short protocols, subjects should be strongly encouraged to void their bladder, clearly explaining that they will not be able to get up to use the restroom once the study starts. For prolonged studies, a urinal should be available, or other plan should be in place for what will be done if an individual needs to use the restroom, given the significant changes in cardiovascular homeostasis that can occur with increasing bladder distension (72). During testing, the study room should be quiet, temperature controlled, dimly lit, and void of distractions, including but not limited to conversations, loud music, and people coming in and out of the laboratory. Likewise, to reduce subject burden, the number of research personnel should be limited to those needed to execute the study protocols and measurements. For reasons related to both biological safety and a controlled environment, food and food smells (e.g., coffee) should also be avoided.
All studies conducted in premenopausal women of childbearing age should be menstrual cycle controlled, meaning women are studied during the same hormone phase of the menstrual cycle (92). The majority of researchers choose to study participants during the early follicular phase of the menstrual cycle (days 1–5) and/or placebo phase of oral hormonal contraceptive use because circulating female sex hormone concentrations (estrogen, progesterone) are at their lowest. However, depending on the question at hand, it may be more appropriate to study women during the luteal phase of the menstrual cycle and/or active pill phase of oral hormonal contraceptive use. In reality, the optimal design may be to conduct measurements in both high- and low-estrogen phases. Under these circumstances, measurements of sex hormones would be important. We refer readers to a recent review on this topic (251). Along these lines, when studying older individuals, it is important to document and/or control for menopausal status as well as the potential use of hormone replacement therapy. Age at onset of menopause should also be considered. We now understand that there are a number of vascular control mechanisms that differ between men and women as well as between women using oral contraceptive pills and hormone replacement therapy, women with natural menstrual cycles, and women after menopause (7, 155, 160, 272). Thus, controlling for menstrual phase, pill phase, and menopausal status is an important aspect of study design for women.
Attempting to Isolate Skeletal Muscle Blood Flow
Doppler-based assessments are made in the conduit arteries, and thus changes in flow (i.e., blood velocity) are largely reflective of changes in tone of the downstream resistance vessels, particularly if changes in conduit artery diameter do not occur. With this, researchers need to consider the potential impact of all distal resistance arteries. For example, at rest and under thermoneutral conditions, ∼30% of forearm blood flow is directed to the skin (42), indicating that a nontrivial portion of forearm blood flow resides in the cutaneous circulation (250). Notably, under thermal stress, 75–80% of forearm blood flow is directed to the skin circulation (132, 196); therefore, cutaneous vascular flow becomes a primary determinant of whole limb blood flow. Accordingly, when the intent is to study skeletal muscle blood flow, it is of paramount importance to limit extraneous flow to the skin. By maintaining an ambient temperature of the study room of ∼21–22°C (to keep core temperature stable and exposed limb in direct contact with cool air), variability in blood flow to the cutaneous circulation can be reduced. In such instances, it is also important to limit body coverings such as long-sleeved shirts, pants, and/or heavy blankets. The use of blankets has the potential to also limit visibility of the extremities, which can be problematic when attempting to control for limb movement. Directing a fan toward the limb of study has also been used to limit extraneous flow to the skin (47, 221). Nevertheless, it is important for other cardiovascular measurements (e.g., heart rate) that the subject is not cold.
In addition, inflation of a wrist (during forearm measurements) or ankle (during leg measurements) blood pressure cuff to suprasystolic pressures can be used to limit flow to the hand/foot, which is composed primarily of skin (47, 75, 221). Historically, this approach has been applied to study differences in vasomotor control between acral and non-acral skin. Of note is that this approach has the undesirable effect of creating tissue ischemia, and therefore, it may not be appropriate for prolonged periods of data collection. There is also the aspect of subject discomfort that requires consideration. The usefulness and appropriateness of wrist occlusion varies with the conditions and techniques (i.e., Doppler ultrasound versus venous occlusion plethysmography) under which forearm blood flow is being assessed. For example, in the case of postocclusion reactive hyperemia, because the entire limb circulation is affected, a wrist cuff is required for accurate measurement of forearm blood flow via venous occlusion plethysmography. This is because the strain gauge around the forearm does not provide assessment of the hand, yet the hand microcirculation is also dilated because of occlusion. In contrast, Doppler ultrasound measures conduit artery inflow to the entire limb, and thus, in the absence of a wrist cuff, dilation of the hand circulation remains appropriately captured as part of the total hyperemic response. In the setting of forearm exercise, an occlusion cuff around the wrist would lead to metaboreflex activation of the muscles in the hand and consequently alter blood flow responses as assessed via Doppler ultrasound. With venous occlusion plethysmography, inflating the wrist cuff for the brief period of flow measurement when exercise is temporarily stopped would be appropriate.
To determine whether skin blood flow is adequately controlled, one can observe the pattern of resting blood flow across the cardiac cycle. At rest, there is often systolic but not diastolic flow, and flow remains relatively stable. When there is periodic diastolic flow, this is an indication of rhythmic increases and decreases in skin blood flow. These oscillations should be identified. Typically, extending the resting period (i.e., waiting a bit) until diastolic flow dissipates is adequate. Another option is to apply local (e.g., forearm) cooling until stability and zero diastolic flow is observed. For review of the oscillatory flow due to rhythmic opening and closing of arteriovenous anastomoses in human skin and how local cooling eliminates this and leads to stable, low flow through the forearm conduit artery, see the review by Walløe (290). It is most often blood flow within the hand that creates concern, and an ice pack on the hand can speed up the process of eliminating skin flow. When systolic-only flow is achieved, one can assume that the majority of skin blood flow is eliminated and the ice pack should be removed. If this technique is employed, it is important to recognize the potential impact of an ice pack on the hand on other cardiovascular variables (e.g., blood pressure) as well as the effect of such cooling on sympathetic nervous system activity (e.g., cold pressor test), which must be assessed and avoided. However, it should be noted that cold pressor test is also a pain response (148) and is much more extreme than the local cooling we are describing here. Although muscle sympathetic nerve activity can be increased if sufficient whole body cooling is evoked (87), this increase requires considerable reduction in skin temperature before it manifests. Participants experiencing the room temperatures recommended in this review are not experiencing the sensation of being cold, as they are fully clothed and comfortable. However, the exposed limb is experiencing direct air contact, and thus the local cooling effect of such an environment on the arteriovenous anastomoses is the goal of the recommended room temperature. Importantly, currently there is no approach to exclusively isolate muscle blood flow from other tissues in the limb (e.g., cutaneous, adipose, bone) using ultrasound technology, and thus the obtained data should be interpreted with this understanding.
Model Selection
A number of past studies have focused on blood flow to a single limb (arm or leg) and extrapolated these findings to all skeletal muscle vascular beds to determine systemic vascular function. However, we now know there are clear limb differences in vascular control mechanisms (i.e., arm versus leg) (184, 191, 209, 223), and thus any conclusions drawn are likely specific to the vascular bed studied. In addition, resistance vessel function in dominant/trained limbs may differ from nondominant/untrained limbs (93, 144, 185, 256, 301). In these instances, it is important to document and control such factors between subjects and visits as much as is possible.
Regardless of known limb differences, the forearm is often studied for a number of reasons, including 1) isolation of intrinsic mechanisms without influence of central hemodynamics (e.g., cardiac output) (63, 90, 91), although some would argue that one-legged knee extension exercise also poses minimal cardiopulmonary stress, particularly at submaximal work rates (8, 176–178, 190, 231); 2) significance of the forearm to activities of daily living (e.g., carrying groceries, opening jars); and 3) relatively easy access to venous and arterial vessels (192). It should be noted that the initial studies using forearm plethysmography were done by clinical pharmacologists. In the early era of cardiovascular drug development, they needed an in vivo human model to translate preclinical/animal work, but one where the consequences of potential side effects were limited to one limb and did not potentially inflict serious events.
The vascular bed studied will dictate subject posture during testing. The majority of studies in the forearm are conducted with the subject in a supine position, whereas studies in the leg may be conducted in the supine, seated, semirecumbent, or prone position. Variations in posture have the potential to alter systemic vascular control mechanisms, including differences in venous pooling and venous return, baroreceptor stretch, and hydrostatic pressure (24, 50, 260, 278). For example, when going from lying to sitting, sympathetic nerve activity directed to the skeletal muscle vasculature can double, thus significantly impacting peripheral vascular tone (24, 127). Therefore, posture (and/or limb height relative to heart-level) should be controlled for, reported in manuscripts, and considered in data interpretation.
DATA COLLECTION AND SIGNAL PROCESSING
There are a number of technical aspects of Doppler ultrasound that require significant training to ensure mastery of the technique. It is only through consistent handling of the ultrasound probe and a clear understanding of software settings that one can be assured the method is accurate, valid, and reliable, with low day-to-day variability (9, 81–83, 96, 153, 213, 291, 295).
Doppler Technology
When the use of Doppler technology was first introduced for assessment of blood velocity (53, 95), data were captured, stored and analyzed via non-imaging systems. One limitation of non-imaging devices is the inability to measure arterial diameter which, as highlighted below, is an essential component of the calculation of blood flow. To control for this, blood velocity collected via non-imaging Doppler devices can be combined with diameter measurements from a separate ultrasound imaging system; however, this approach introduces potential variability in the probe recording sites and does not allow for precise time alignment between the velocity and diameter, which can be critical for accurate analysis of blood flow. In this regard, it should be mentioned that while earlier studies were limited by equipment without capabilities for simultaneous B-mode imaging and Doppler velocity measurements, most of the current Doppler ultrasound systems possess the duplex modality thus allowing for continuous and simultaneous assessment of diameter and blood velocity (9, 81, 82, 88, 89, 112, 299).
Using duplex Doppler ultrasound, ultrasound waves of a given frequency (typically 4 to 12 MHz when using a linear array ultrasound probe) are reflected by red blood cells (erythrocytes) traveling through the conduit vessel of interest on their way to downstream resistance vessels. When these sound waves are reflected by moving erythrocytes, there is a shift in the Doppler ultrasound frequency that is sensed by the receiver. The difference in the transmitted and received ultrasound frequency is used to calculate erythrocyte velocity (27, 82, 83, 212). To limit error within the estimate of blood flow, an insonation angle (the angle at which the ultrasound beam meets moving erythrocytes) of 60° or less to the axis of the vessel is required (212). This can be achieved through the Doppler software interface and by manually tilting the probe.
Because commercial ultrasound systems are primarily designed to provide high-resolution images (B-mode), the majority of devices, including newer systems, emit a beam with a thin, finite width (21). Unfortunately, most of these thin-width beams are narrower than a typical brachial or femoral artery and do not insonate the entire cross-sectional area of the vessel. Thus, the ultrasound system may not measure velocity near the vessel lamina, which is theoretically lower (in accordance with the principles of laminar flow). As a result, when the average velocity is calculated, the slower velocities are not included in the calculation, leading to an overestimation of mean blood velocity by ≤33% (21). It should be clarified that thin-width beam is a newer development, so it is quite likely to play a role in current research. The reader is directed to the review by Buck et al. (21) for details on how to assess the beam width in one’s own system. Validation studies that compared Doppler ultrasound to blood flow determined by thermodilution in humans relied on earlier generations of ultrasound that likely did not have thin-beam technology (213, 303), and therefore, these early validation studies cannot be generalized to current systems (21).
Although this “thin-beam” error exists, our point herein is twofold. First, this source of error is largely underreported, and we want investigators to know of and acknowledge the issue. Second, if beam and vessel width are known, this overestimation can be corrected and thin-beam error minimized using a published equation (21, 70). By reporting depth and diameter of the blood vessel, as well as specifications of the ultrasound probe, the impact of thin-beam ultrasound on study results can be examined and controlled (21). Another approach is to create a calibration between the velocity voltage signal and actual measured flow through Tygon tubing of various diameters. With knowledge the actual tubing diameter and flow, true mean velocity can be calculated, and Doppler voltage can be calibrated accordingly.
Regarding signal processing, there are a number of available technologies currently in use by groups around the world. We will name just a few of them here, which include commercially available technologies such as the Multigon spectral analyzer (221), DAT (112), and FlowWave (41) as well as custom-made, image/video capture systems (88, 89, 299). Such approaches may be used to analyze the Doppler shift frequency and subsequently determine mean blood velocity from the weighted mean of the spectrum of Doppler shift frequencies. The pros/cons to each of these approaches are too numerous to address in this review and will likely become obsolete as new technologies are developed. Nevertheless, the preferred approach is one that relies upon digital analysis of the graphical user interface and can be simultaneously recorded and aligned with other physiological parameters for beat-to-beat analysis (50, 73, 74). An alternative approach that allows for analog velocity signal output and/or RCA phono jacks (allowing for the conversion of Doppler audio signals to real-time velocity signals) is also available (112). The issue of transferring signals from commercial/clinical ultrasound devices to a data acquisition program is not trivial, and an understanding of how the signal is being processed is of high importance.
Technical Settings
In addition to insonation angle, depth, diameter, and specifications of the ultrasound probe, there are a number of other technical settings that must be recorded and kept consistent between participants and/or visits to ensure accuracy and repeatability of measurements. First, the position of the ultrasound probe on the skin should be marked to ensure that measurements are occurring at the same location throughout the study. Some groups use a probe “holder” to securely fix/clamp the probe, mark the position on the skin with a permanent marker, take a picture, and/or identify anatomic landmarks within the B-mode image. For studies requiring multiple visits, investigators can provide the subject with a permanent marker to remark the spot of interest in between visits. Alternatively, henna tattoos (i.e., dye drawn on the skin that may last a couple of weeks) can be used.
Regarding anatomic landmarks, the probe should be positioned ≥2 to 3 cm proximal to vessel bifurcations and/or branches on a relatively straight segment of vessel to avoid potential vortices (83); this is not the same as noise from turbulence at the vessel wall, which can be rejected with the use of a low-velocity filter. Second, following optimization of the image, ultrasound settings such as 2D gain, PW gain, sample volume, and sweep speed should not be altered within a given experiment and should be reproduced between study visits. Furthermore, the preferred approach by several laboratories is to use a sample volume that encompasses the entire width of the vessel (192). However, this is somewhat controversial. As noted above, one of the issues with insonation sample volume is that, although the thickness of the volume can be adjusted to encompass the entire vessel, in most cases the width of the insonation field is unknown (21). In this way, some groups derive the peak envelope from the center of the vessel and then use standardized equations to calculate mean flow (69). Use of a large sample volume that spans the entire vessel also increases the risk of spurious velocity measurements during experimental paradigms where significant limb movement is expected, such as high-intensity handgrip or knee extensor exercise. Regardless of approach, standardization of such measures can be easily accomplished by documenting this information in a laboratory notebook or by taking a screenshot. A summary of settings that should be optimized and kept constant can be found in Fig. 1.
Estimation of Blood Flow
The Doppler ultrasound velocity signal, when combined with brachial artery or common femoral artery diameter, provides an estimate of blood flow to the entire limb. Blood velocity is the time- and space-averaged, angle-corrected, and amplitude-weighted mean blood velocity (81, 83, 213). The gold-standard approach includes steady-state mean blood velocity recordings collected in duplex mode (2D and Doppler) during simultaneous 2D vessel visualization and audiovisual blood velocity feedback (83). Vessel diameter measurements are obtained from a perpendicular B-mode (2D mode) image, using care to ensure a clear image of the borders between lumen and intima of the artery, and are used to calculate vessel cross-sectional area [π × (artery diameter ÷ 2)2]. Blood flow is then calculated as the product of mean blood velocity (cm/s) and artery cross-sectional area (cm2) and is multipled by 60 s/min to convert from microliters per second and achieve units of microliters per minute. This calculation, as noted, requires accurate measures of vessel diameter (82).
To ensure accuracy in the measurement of conduit vessel diameter, some groups record diameter images continuously (e.g., multiple frames per second using custom-designed or commercially available wall-tracking software), whereas others get a “snapshot” of vessel diameter at various times during the measurement period. There are data to suggest whether diameter does/does not change with various physiological stressors, depending on the cohort studied, limb of interest, and location within the artetial tree where measurements are made (202). For example, the large majority of studies using knee extension exercise have failed to identify any appreciable change in femoral artery diameter, even at very high exercise intensities (213, 215, 304), although there are exceptions (85, 201). However, conduit vessel diameter has been known to increase in the more distal portions of the leg (e.g., superficial femoral, popliteal arteries) that are commonly used for lower-limb, flow-mediated vasodilation testing (265). Furthermore, brachial artery vasodilation is often observed at moderate- and high-intensity handgrip exercise (245, 306), so much so that this experimental model has been adopted to assess endothelium-dependent vasodilation to exercise-induced “sustained shear” (273). Undoubtedly, the recommended practice is to measure diameter and velocity simultaneously at high temporal resolution (e.g., 30 Hz) using an edge detection and wall tracking software. If this is not possible and diameter is measured at a single time point using calipers, it is advised that all measures are conducted 1) during the same time of the cardiac cycle; 2) by the same investigator, ideally blinded to the condition; and 3) in triplicate (at minimum) and the values averaged. Regardless, before selecting the region of interest, it is good practice to play back the video or images to identify the arterial segment that is consistently the most clear and free of movement artifact.
When assessments of blood flow (mL/min) are combined with measurements of arterial blood pressure, limb vascular conductance (flow/pressure gradient) and/or resistance (pressure gradient/flow) can be calculated. Vascular conductance is typically calculated as limb blood flow (mL/min) divided by mean arterial blood pressure (mmHg) and multiplied by 100 to achieve units of mL·min−1·100 mmHg−1. The multiplication by 100 is arbitrary, allowing for more direct comparison between flow and conductance values (i.e., similar decimal places). Importantly, this determination of regional conductance/resistance is distinct from the more traditional calculation of systemic vascular resistance, a clinical metric that is mean blood pressure divided by cardiac output.
The combination of flow and pressure signals (e.g., conductance, resistance) is helpful for developing a deeper understanding of downstream vascular tone. More specifically, an increase in conductance may be assumed to signify peripheral vasodilation (an increase in flow when normalized for driving pressure), whereas an increase in resistance may signify vasoconstriction. Mathematically speaking, there is a reciprocal relationship between resistance and conductance. Although neither resistance nor conductance are perfect, changes in conductance are thought to provide a better index of the regional vasomotor response than changes in resistance (195). This is because when perfusion pressure remains constant and changes in blood flow occur (similar to what is observed with the majority of maneuvers described herein), calculated changes in resistance are not added algebraically, whereas changes in conductance yield consistent results (i.e., conductance is linearly related to flow, whereas resistance is curvilinear) (151). In this way, flow is proportional to 1/resistance and is directly proportional to conductance. On the other hand, blood pressure is proportional to resistance, but to 1/conductance. It is the direct versus inverse proportional effect that encourages the use of the index proportional to the variable that is changing (i.e., conductance and flow, as well as resistance and pressure). In this situation, vasoconstriction will induce a large decrease in conductance with little increase in resistance (151, 195). To summarize a relatively complicated, contentious, and often confused point 1) conductance is the preferred assessment when flow is the primary variable of interest, and it is expected that changes in flow will be substantially greater than changes in pressure (i.e., dynamic exercise); and 2) resistance is preferred when pressure is the primary variable of interest, and it is expected that pressure will change more than flow.
Some options for blood pressure recording include continuous methodologies (e.g., finger photoplethysmography, arterial catheter connected to a pressure transducer) or “snapshot” approaches (e.g., brachial artery cuff with sphygmomanometer). However, continuous methodologies are preferred given that the “snapshot” approach provides a single systolic and diastolic pressure across a time frame of oscillating blood pressures and associated limb blood flows, potentially resulting in over- or underestimation of dynamic responses. To ensure accurate measures when performing experiments within the forearm circulation with a subject in the supine position, blood pressure devices should be kept at heart level (or corrected for this factor), and finger photoplethysmography technologies should be calibrated to an upper arm sphygmomanometer when assessing mean arterial pressure. On the contrary, in leg-based studies that involve placement of indwelling arterial and venous catheters, it is commonplace to keep the pressure transducer at the level of blood flow assessment (i.e., femoral artery) to obtain accurate leg blood pressure values that allow for calculation of perfusion pressure across the limb. This level of accuracy is required to obtain a true measure of leg vascular conductance. It is important to acknowledge that these limb-specific examples are less about the limb and more about limb position and the need to quantify the effective pressure gradient for flow. However, if blood pressure is the main variable of interest, then the transducer should be placed at heart level.
As alluded to above, depending on the nature of the investigation, the limb may be in a position that varies relative to heart level. The question then arises as to what represents the effective perfusion pressure gradient for blood flow (since vascular conductance is calculated using an estimate of this effective perfusion pressure). A key factor that needs to be considered when estimating effective perfusion pressure is the hydrostatic contribution to local limb arterial and venous blood pressure. Because of hydrostatic contributions, blood pressure on both the venous and arterial side increases in proportion to the distance below the heart that the vascular bed of interest is positioned. Because, at rest, there is a continuous column of blood on the arterial and venous side, this does not elevate the pressure gradient from arterial to venous circulation. In that instance, the use of heart level arterial pressure and the assumption of a central venous pressure close to zero (if the person is in an upright position) are appropriate. For these reasons, it is common for research groups to use mean arterial blood pressure to calculate vascular conductance.
In contrast, at rest in a limb positioned above heart level, there is a loss of hydrostatic pressure on the arterial side but not on the venous side, because the venous circulation volume is “unstressed” volume from just above central vein level onward (unstressed volume is volume in the veins that does not create pressure). Therefore, the further above the heart, the lower the arterial to venous pressure gradient becomes in proportion to the height of the hydrostatic column on the arterial side. This is the case regardless of whether the person is supine or upright. Thus, with increasing limb height above heart level, the effective perfusion pressure used to calculate vascular conductance decreases.
If vascular conductance in exercising muscle is the variable of interest, quantification of the effective perfusion pressure gradient is based on the following. First, contraction empties the veins of the muscle so that upon relaxation the local venous pressure is zero. Second, the hydrostatic contribution locally contributes to the local arterial pressure. Therefore, the local effective perfusion pressure gradient for flow would be the local arterial blood pressure. In contrast, above heart level there has been no change in the arterial to venous pressure gradient as a result of contraction because venous pressure was essentially zero already. We know that the local exercising muscle arterial pressure represents the effective perfusion pressure because of the findings of Walker et al. (288). These investigators had participants perform rhythmic forearm exercise at a moderate intensity. During steady-state exercise, the forearm was moved from above to below heart level during one of the contractions. Upon release of the contraction, flow was immediately higher than during the preceding relaxation that took place above heart level. The reverse occurred when moving the forearm back above heart level. In summary, accounting for effective perfusion pressure differences as described above is critical if one is interested in comparing vascular conductance between limb positions. Most important is the consistency of measurements and being aware of these issues.
COMMON PROCEDURES
Postocclusive Reactive Hyperemia
Protocol used during the flow-mediated dilation test.
One of the most common Doppler ultrasound-based methodologies for noninvasive assessment of vascular function in humans is brachial artery flow-mediated dilation (FMD), which was introduced by Celermajer et al. (35) in 1992. This technique involves a transient (5 min) suprasystolic (200–250 mmHg) forearm occlusion to generate a reactive hyperemic-induced shear stress stimulus that consequently elicits dilation of the brachial artery, which is detectable via ultrasound. In contrast to the forearm, reactive hyperemia in lower limbs (e.g., popliteal or femoral artery) often requires a cuff occlusion of ≥250 mmHg (71).
FMD is endothelium dependent (207), and as such it reflects conduit artery endothelial function (102, 224, 262, 267). Importantly, the magnitude of reactive hyperemia following transient limb ischemia is assessed via Doppler ultrasound and serves as an index of resistance vessel function. The reactive hyperemic response should not be confused with FMD. Although interrelated, the measure of reactive hyperemia reflects the magnitude of dilation in downstream resistance arteries. Conversely, FMD is a measure of dilation of the upstream conduit artery in response to the hyperemic shear stress stimulus. More information regarding the measure of conduit artery FMD can be found in previous reviews (102, 224, 262, 267).
Continuous and simultaneous monitoring of arterial diameter (B-mode) and blood velocity (Doppler mode) is the preferred approach to appropriately capture reactive hyperemia (210). Data can be expressed as a peak change in blood flow or velocity, typically occurring approximately within the first 10 s from cessation of occlusion, or as blood flow or velocity area under the curve (AUC) across an extended postocclusion period (e.g., 60 s to 2 min) (210). It remains unknown what metric is most reflective of resistance vessel function. Accordingly, at this time it is recommended that data are presented in multiple ways (i.e., absolute blood flow and velocity, peak, and AUC) to be comprehensive and allow for comparisons with other studies. It is also important that placement and size of the pneumatic cuff, as well as the duration and magnitude of occlusion, are standardized across subjects and time points because the degree and extent of muscle ischemia is a dominant determinant of the hyperemic response. If researchers are interested in simultaneously also obtaining FMD data conforming to current guidelines (102, 224, 262, 267), it is recommended that the cuff is positioned distal to the site of imaging (Fig. 1). If not, then placing the cuff on the upper arm will provide a more robust hyperemia.
Of significance is that Mitchell et al. (172) were the first to provide evidence that cardiovascular risk factors are more closely related to reactive hyperemia than to FMD itself, a finding confirmed by a subsequent study (205). The importance of studying the function of small resistance arteries is further supported by additional evidence collectively suggesting reactive hyperemia as 1) an independent predictor (i.e., beyond other risk factors) of adverse cardiovascular events and a measure that can discriminate subjects with increased cardiovascular disease risk (4, 109, 119, 271) and 2) a measure that is responsive to changes in physical activity levels (99, 219, 285). The degree to which reactive hyperemia is endothelium dependent has not been experimentally resolved; however, it is prudent to assume that, unlike FMD in conduit arteries, reactive hyperemia after a 5-min cuff occlusion is not an endothelial-specific test. In this regard, reactive hyperemia should be considered a general vascular metric resulting from an agglutination of factors, namely the local production, release, and diffusion of a myriad of vasodilator substances (e.g., metabolites) during muscle ischemia combined with the responsiveness of the underlying resistance arteries. Accordingly, changes in reactive hyperemia can be attributed to both alterations in the magnitude/diversity of stimuli and the phenotype of the responding vascular cells. Notwithstanding the potential predictive value of reactive hyperemia, it should be noted that this vascular response is minimally mediated by NO (49, 61, 179, 257). In fact, using venous occlusion plethysmography to assess forearm blood flow, work by Crecelius et al. (49) demonstrated that, in young healthy subjects, activation of inwardly rectifying potassium (KIR) channels is the primary determinant of peak reactive hyperemia, whereas activation of both KIR channels and Na+/K+-ATPase contributes to almost all of the total AUC reactive hyperemic response. The extent to which these vasodilator pathways are blunted in patient populations remains to be deciphered. However, on the basis of these findings and work relating reactive hyperemia with cardiovascular risk factors and outcomes, it is conceivable to conclude that vascular health extends beyond NO-dependent vasodilator function (49). Future studies are needed to elucidate whether the determinants of reactive hyperemia are the same in the upper and lower extremities as well as to further establish its prognostic value in large cohort studies. Likewise, further consideration for quantification of the metabolic rate of the ischemic limb (i.e., the ischemic stimulus) is warranted. Indeed, recent work suggests that the ischemic stimulus to vasodilate varies widely across individuals and that the level of reactive hyperemia is often coupled to the magnitude of tissue desaturation (225, 226).
Protocol used to achieve peak (or “maximal”) reactive hyperemia.
There is a long history of the assessment of peak reactive hyperemic blood flow response as an index of the overall structural capacity of a resistance vessel bed in humans (40, 77, 181, 203, 258). The general principle, introduced by Folkow and colleagues (76, 77) in the 1950s in the context of assessment of the mechanisms associated with primary hypertension, is that by dilating the resistance vessels maximally, it is possible to indirectly gauge their structure. Folkow proposed that dilator capacity might ultimately be limited by increased arterial wall thickness and that peak dilator capacity is therefore linked to hyperresponsiveness of resistance vessels to all forms of vasoactive stimulation. Conway (40) established that peak reactive hyperemia elicited by prolonged cuff inflation could not be modulated by concurrent intra-arterial vasodilator or vasoconstrictor drug infusions, whereas Takeshita and Mark (258) established that 10 min of ischemia elicited a peak reactive hyperemic response that could not be modified by sympathetic nervous system activation.
These pioneering early studies were completed using forearm strain gauge plethysmography. In more recent years, Naylor et al. (181) and Tinken and colleagues (269, 270) applied these concepts using Doppler ultrasound assessments and identified that forearm occlusion (5 min), coupled with handgrip exercise (3 min; 1 contraction every 3 s using a 3-kg load), elicits a maximal blood flow response that is not exceeded by either a longer period of ischemia or the combination of ischemia and vasodilator drug administration. As a consequence, this ischemic exercise stimulus has been used in multiple studies as an index of structural conduit and resistance vessel remodeling (268, 269). Indeed, hyperemia following ischemic exercise is considered a measure of skeletal muscle blood flow capacity (149, 181, 229) that is augmented with exercise training (90, 91, 149, 166, 249, 252, 253, 255). Increases in skeletal muscle blood flow capacity can result from increases in microvascular density (i.e., increased number and/or size of microvessels) (149, 150) as well as enlargement of the larger upstream arterioles (i.e., those that lie proximal to the interstitial level) and feed arteries, where the majority of the resistance to flow resides (241). Changes in the wall-to-lumen ratio, as proposed by Folkow and colleagues (76, 77) in the 1950s, can also occur (19, 59, 94, 229, 230, 264). Furthermore, the idea that blood flow capacity also relies on functional vascular adaptations (i.e., changes in the phenotype of endothelial and smooth muscle cells) cannot be dismissed (149, 150). In conclusion, maximal reactive hyperemia in the arm can be achieved with ischemic forearm exercise, and blood flow changes in this response are determined largely by structural vascular adaptations in skeletal muscle.
Passive Limb Movement
Passive limb movement (PLM) is the manipulation of a limb (e.g., leg) without voluntary effort or muscle contraction. At the onset of PLM, a robust and transient increase in blood flow occurs. This hyperemia is evoked by mechanical deformation/stretch of the skeletal muscle, leading to a cascade of vasodilatory events in the resistance vessels (80, 175, 216, 279). Unlike reactive hyperemia observed following cuff occlusion, the PLM-induced response occurs without tissue ischemia. Compared with active exercise, which will be discussed in detail below, the hyperemia during PLM occurs with negligible increase in skeletal muscle activation and metabolism (111, 274). During volitional exercise, the increase in oxygen demand caused by elevated skeletal muscle metabolism dominates the regulation of blood flow. Because of the number of “moving parts” during active exercise, interrogation of vasodilatory pathways is difficult. PLM and/or single voluntary muscle contractions may be used as reductionist models to better understand the regulation of blood flow in response to mechanical factors (such as stretch or shear stress) without an increase in metabolism and oxygen demand (302). Remarkably, the time courses of hyperemia in response to single voluntary contraction and PLM are quite similar. Moreover, as will be discussed later, the control of hyperemia between the two models appears to be similar; therefore, insight gained from PLM-based measures may inform the regulation of blood flow during single voluntary contractions and vice versa (34). Moreover, a growing body of evidence supports the notion that PLM may be a potentially clinically relevant approach to assess vascular function (80).
The first evidence of a PLM-induced hyperemic response was reported by Rådegran and Saltin (216). In preparation for active exercise, PLM was performed in the leg, and a 3.3-fold increase in blood flow through the common femoral artery was observed. Following this observation, Wray et al. (302) used PLM to elucidate mechanisms of blood flow regulation and reported a transient and significant increase in blood flow during PLM and concomitant cardioacceleration. Through a series of studies, the role of central and peripheral responses to PLM was described, and it became clear that PLM results in a central hemodynamic response (increase in heart rate and cardiac output) that involves afferent feedback from the moved limb (98, 169, 274, 277). Based on these studies, it was concluded that central hemodynamic response to PLM is linked to the peripheral response; however, the magnitude of the central response does not dictate the peripheral response. This is an important distinction when considering the relevance of PLM to assess resistance vessel function.
Role of nitric oxide.
Accumulating evidence supports an increase in hyperemia during PLM, and now the mechanisms regulating the vasodilatory response have been elucidated. With the use of intra-arterial administration of the nitric oxide (NO) synthase inhibitor l-NG-monomethyl arginine (l-NMMA; see Pharmacological Interventions for more detail regarding this technique), it has been reported that ∼80% of the hyperemic response to PLM is NO dependent in young healthy men (175, 277). Subsequent studies in healthy older adults confirmed that the age-related reduction in vasodilation to PLM is largely due to diminished NO bioavailability (98, 276). Together, these findings suggest that the hyperemic response to PLM, being largely NO mediated, may provide a useful and novel approach to assess endothelial function of the skeletal muscle resistance arteries. Comparisons of PLM with more established methods of assessing endothelial function (i.e., FMD and infusion of acetylcholine) support this notion. With respect to brachial artery FMD, Rossman et al. (227) reported significant correlations between FMD and PLM in young (r = 0.77) and older (r = 0.45) subjects. Similar relationships between popliteal FMD and PLM have been reported (r = 0.68) (289). These studies related functional measures between conduit arteries and resistance vessels. Notably, within the same vascular bed, Mortensen et al. (175) reported an even stronger relationship between the increase in leg blood flow in response to acetylcholine and the PLM-induced hyperemic response (r = 0.84).
PLM, despite being a relatively novel approach, is capable of identifying vascular dysfunction that accompanies aging, disease, and inactivity. In patients with overt disease, including heart failure, peripheral artery disease, chronic obstructive pulmonary disease, and systemic sclerosis, the PLM response is diminished by ≤90% (39, 115, 175, 289, 298). Importantly, PLM is also highly responsive to acute disease conditions such as sepsis, where systemic inflammation evokes a critical vascular component, leading to impaired vasodilatory capacity (as evidenced by diminished PLM) (182). Physical activity and cardiorespiratory fitness also modulate the PLM response such that more active and fit individuals possess a greater PLM response than their sedentary counterparts (97). It should be noted that the NO contribution to disease and inactivity-related reductions in the PLM response are inferred from direct NO synthase inhibition studies performed in healthy adults, as similar invasive studies have yet to be performed in patient groups. In addition, there are still no prospective prognostic data on large cohorts, which is required to determine the clinical relevance of this test.
Technical considerations.
Detailed step-by-step instructions for preparing, performing, and interpreting PLM have been presented by Gifford and Richardson (80) (see reference for detailed instructions). The following section will highlight several critical factors to consider when performing PLM. The performance of the PLM test is rather simple; however, as with any human-based physiological assessment, reproducibility and validity are highly dependent upon participant preparation and the careful attention to detail by the investigators. Duplex Doppler ultrasound is required for arterial imaging and simultaneous acquisition of second-by-second or beat-by-beat (i.e., cardiac cycle) blood velocity. PLM can be performed in either the supine or upright seated positions, with the upright seated position being preferred as the hyperemic response, and contribution of NO is augmented in this position (97, 278).
Standard precautions for the use of Doppler ultrasound must be followed (see above). Familiarization trials should be performed to ensure that the subject can remain passive during the movement and the common femoral artery can be properly imaged. Once the subject has assumed the seated position with their legs extended and supported, a 15-min period of rest and instrumentation is required. This time frame is important to ensure stable baseline hemodynamics. Upon hemodynamic stabilization, baseline Doppler data are collected for ≥60 s. During the transition from baseline to PLM, it is it important to minimize unwanted movement and to ensure there is no insensible muscle activation using simultaneous electromyography. This is best achieved by fully supporting the subject’s legs at chair level with a table that can be easily moved. The investigator performing the PLM then manually supports the leg to be moved and slides the table away, allowing for complete range of motion. PLM is performed across a 90° range of motion (starting at a knee extension of 180°) for ≥1 min and at a rate of 1 Hz. Following data acquisition, the hyperemic response can be determined and analyzed according to peak response, change from baseline, and/or total hyperemic response (i.e., AUC). As mentioned above, it is recommended that data are presented in multiple ways to allow for comparisons with other studies.
Single-Limb Exercise
The use of Doppler ultrasound for assessment of peripheral artery blood flow during exercise was first used in the 1980s (291, 295). Unlike other techniques that have been used for the assessment of blood flow during exercise (venous occlusion plethysmography and indicator dilution methods), Doppler ultrasound is unique in that it can sample blood velocity continuously (i.e., beat-by-beat or second-by-second) (213). Additionally, it has the capability to detect rapid changes in the pulsatile nature of the blood velocity profile and provide a comprehensive view of skeletal muscle blood flow during each duty cycle (a muscular contraction and relaxation period) during exercise.
As discussed earlier in this review, the assessment of arterial blood flow via duplex Doppler ultrasound is calculated as the product of the cross-sectional area of the artery and the mean velocity in which blood moves through that artery. Changes in arterial blood velocity during exercise reflect the vasomotor tone of the downstream circulation within the contracting skeletal muscle and ultimately provide insight to resistance vessel function in humans. While understanding that the mechanisms involved in the complex integration and regulation of skeletal muscle blood flow during exercise are important and may be of interest to the reader, this area has previously been covered in detail elsewhere (136). Instead, our goal for this section is to outline key experimental considerations when using Doppler ultrasound for the assessment and interpretation of skeletal muscle blood flow responses to exercise.
To examine skeletal muscle blood flow measurements during what is classically viewed as exercise, measurements would ideally be made during whole body exercise engaging large muscle groups such as those involving locomotion or cycling. However, because maintaining a recommended angle of insonation (≤60°) is critical for obtaining accurate and reproducible blood flow assessments, the use of Doppler ultrasound during whole body exercise is not always a feasible option. In this regard, Doppler ultrasound during maximal exercise is technically challenging and requires a highly skilled operator and significant training. To avoid insonation failures that would inherently occur with upright, whole body exercise, models incorporating brachial and femoral artery blood flow assessments during submaximal forearm handgrip or lower-limb knee extensor exercise, respectively, have been developed and are commonly used (2, 3, 247).
Forearm versus leg models.
The forearm model typically involves subjects performing rhythmic handgrip exercise on either a hand dynamometer or custom-built handgrip device involving a weighted pulley system while in a supine position. Despite some differences across studies in the design of the forearm exercise protocol, the contraction frequency commonly employed for rhythmic dynamic exercise tends to be ∼20–60 contractions/min, with workloads ranging from a light to a moderate intensity [∼10–40% of maximum voluntary contraction (MVC)]. In such instances, target force output can be shown in real time to guide participant contraction force and duration. This approach can help ensure sufficient force development with each duty cycle, and tension over time can be quantified. In such models, brachial artery blood velocity and diameter are measured proximal to the elbow and catheter insertion site (for studies involving intra-arterial drug infusions, discussed in detail below).
In the one-legged knee extension exercise model, the exercise is confined to the quadriceps muscle group. This is achieved by having subjects “kick” the lower part of the leg through an ∼90–170° range of motion during extension at a constant cadence (typically 40–60 kicks/min) against a resistance, whereas the flexion portion of the exercise is a passive movement (2, 201, 222). Subjects are placed in either a seated semirecumbent position or supine position for the exercise protocol. The measurement of blood velocity in the femoral artery feeding the active thigh muscles is commonly made distal to the inguinal ligament, but above the bifurcation to the branches of the superficial and deep femoral arteries. These models are ideal for accurate blood flow assessment during exercise, as they allow the limb and artery to remain in a fixed position, increase the stability of the Doppler probe position, and ultimately help to minimize motion artifact and insonation angle variability. Additionally, the use of a single-limb exercise model (when paired with intra-arterial drug infusions) also permits the study of the local vasoregulatory mechanisms in contracting human skeletal muscle, with minimal interference from the cardiovascular reflexes that are engaged during whole body exercise (29, 110, 232).
Although both the forearm handgrip and one-legged knee extension exercise models have been instrumental in identifying mechanisms involved in the local regulation of skeletal muscle blood flow during exercise, it is important to consider potential limb-specific differences in terms of vascular responsiveness to exercise and the interpretation of any age- and disease-related reported reductions in muscle blood flow. In this context, evidence suggests the vasodilator responsiveness differs between the arms and legs of humans. That is, in response to physiological vasodilator stimuli (e.g., limb ischemia) as well as pharmacological stimuli (e.g., intra-arterial infusions of both endothelium-dependent and independent vasodilators), the arms exhibit greater vascular reactivity than the legs (184, 187). Conversely, the legs demonstrate greater sympathetically mediated vasoconstrictor responsiveness than the arms (191, 204). To date, direct comparisons of forearm and leg blood flow and vasodilator responses to exercise, particularly in the same cohort, are scarce. This is likely attributable to several confounding variables at play when trying to compare responses to exercise between limbs.
First, the forearm contains less muscle mass than the quadriceps, and therefore, hypothetically, the capacity to increase flow in response to exercise may be lower. One way to help circumvent the influence of this confounding factor is to compare responses normalized for muscle mass. Using this approach, Donato et al. (60) examined forearm and leg blood flow responses normalized to muscle mass to a range of relative handgrip and one-leg knee extension workloads, respectively, in the same cohort of young and older adults. Although the primary purpose of their study was to determine whether aging has differing effects on blood flow in the arm and leg, close inspection of the data clearly indicate that the normalized blood flow response to exercise is greater in the leg compared with the forearm in both the young and older groups. Similarly, leg blood flow responses (normalized to quadriceps muscle mass) to exercise appear to be greater than blood flow responses in the forearm of the same cohort of young obese adults (154). Interestingly, more recent work using the knee extension exercise modality in young healthy male subjects failed to identify a relationship between quadriceps muscle mass and leg blood flow across a wide range of exercise intensities, challenging the notion that muscle mass is a determinant of the hyperemic response during exercise (79).
Second, the bipedal nature of humans exposes the upper and lower limbs to differing chronic homeostatic challenges and uses. In this context, the legs represent a large vascular bed that is exposed to greater hydrostatic forces and used regularly for locomotion, whereas the arms are not (223). These inherent limb differences likely impact skeletal muscle metabolism and, therefore, the blood flow responses to exercise.
Finally, it is important to consider the type of muscle contraction being performed when considering possible differences in the blood flow responses between the forearm and the leg. The type of muscle contraction performed in most exercise studies involving the forearm and leg differ slightly. The single-leg knee extensor model employed usually consists of contraction of the quadriceps during the concentric portion of the duty cycle, followed by a passive relaxation during return to the 90° leg flexion position (e.g., no resistance during the eccentric portion) (2, 121, 201, 222). In contrast, the weight is constant during the contraction and relaxation phases of the forearm contractions (no passive relaxation) in studies using a pulley system ergometer (58, 154, 239), although this is not the case for the “static intermittent” exercise performed with a handgrip dynamometer. Therefore, it is conceivable that differences in the blood flow and vasodilator responses to exercise between limbs are confounded to some degree by the type of muscle contraction performed. Additionally, the method for determining submaximal exercise workloads tends to differ for the forearm handgrip and one-legged knee extensor exercise models. That is, submaximal workloads for the one-legged knee extensor studies are often based on a work rate maximum derived from an incremental maximal exercise test. Conversely, the submaximal workloads commonly used in forearm studies are based on a percentage of an isometric MVC, such that comparing the blood flow responses between limbs at the same percent of work rate maximum (leg) versus MVC (forearm) may not truly represent the same relative workloads. Taken together, these notable differences have made it difficult to make direct comparisons in the blood flow response to exercise between limbs of humans. However, some groups have used the same incremental maximal test approach to identify workloads in the forearm (18, 234), which makes relative workload comparisons between forearm and leg possible.
Exercise intensities and timing of the response.
Regardless of the limb being studied, when designing an exercise experiment with blood flow assessments, it is important to consider absolute and relative exercise intensities. Given the well-established relationship between metabolic demand and blood flow/oxygen delivery, it is often advantageous to incorporate at least two relative and two absolute exercise intensities, an approach that is especially important when comparing groups with marked differences in exercise capacity or muscle strength (young versus old or control versus disease). If one considers that oxygen demand dictates blood flow, then absolute workload would be used for comparison. In such instances, the parallel assessment of arterial oxygen content and oxygen uptake of the limb would strengthen blood flow measurements during exercise. This is particularly the case when comparing young and older adults or men and women, where differences in arterial oxygen content can be expected.
One of the main advantages of using Doppler ultrasound in the assessment of peripheral blood flow in humans is that it allows for continuous sampling of blood velocity and can detect rapid changes in blood flow during the transition from rest to exercise and/or the transition between workloads. To date, the majority of studies that have assessed blood flow during exercise in humans have commonly done so under steady-state conditions, that is, quantifying the absolute amount or magnitude of change in flow (from rest) for a given workload once the flow has likely plateaued. Depending on the intensity of exercise and assuming the workload is kept constant throughout, this usually occurs within the first few minutes of exercise, with the majority of studies waiting 2 to 3 min before data collection to allow establishment of a hemodynamic steady state. Thereafter, steady-state exercising muscle blood flow is usually derived from the blood velocity data, and arterial diameter measurements are collected continuously during the last 30–60 s of a given exercise workload and/or bout (33, 142, 154). The assessment of steady-state responses has allowed for the interrogation of whether blood flow to the contracting muscle is impaired in patient populations known to have diminished exercise capacity. Along these lines, Doppler ultrasound-assessed steady-state exercise blood flow has been reported to be attenuated with age in both forearm and single-knee extensor models (105), although this dogma has been challenged in recent years. Furthermore, the impairments in steady-state blood flow appear to be even greater in the presence of disease states such as heart failure (14, 15), chronic obstructive pulmonary disease (125), and peripheral artery disease (103, 145).
Although examining the blood flow response under steady-state conditions has proven to be extremely useful in detecting age and disease-related alterations in vascular control mechanisms, the dynamic response of muscle blood flow in the transition from rest is often overlooked. At the onset of exercise there is a rapid (within 1 to 2 s) and substantial increase in blood flow. The mechanisms responsible for the immediate rise in flow during the first few seconds of voluntary exercise likely involve the skeletal muscle pump and some combination of vasodilating substances. The extensive list of possible mechanisms has been covered previously in other reviews (38, 136, 282). Using a single voluntary muscle contraction model in humans elicits an immediate and robust rise in limb blood flow and vascular conductance in both the forearm (20, 28, 34, 43, 46, 139, 280) and leg (50, 120, 122, 123). The blood flow and vasodilator response, commonly termed rapid onset vasodilation, tend to peak at approximately three to six cardiac cycles after completion of the single muscle contraction, and these responses are graded with contraction intensity (20, 43, 139, 280). This is accomplished by having a subject perform a single brief (∼1 s) muscle contraction and relaxation using either the forearm handgrip or single knee extensor model and then tracking the blood flow and vasodilator response in the active limb for 30–45 cardiac cycle postcontraction. This approach allows for the assessment of the immediate (first cardiac cycle postcontraction), peak, and total (expressed as AUC) blood flow and vasodilator response to a single voluntary muscle contraction. Reporting each of these measures is important to allow comparisons across studies. An important benefit of examining the blood flow and vasodilator response to a single-muscle contraction is that it allows assessment of the local mechanisms underlying exercise hyperemia without the confounding effects of subsequent contractions. Importantly, the immediate vasodilation following the first muscle contraction is thought to serve a critical role in helping the transition and timing between the onset of exercise and steady-state exercise conditions (235, 248).
The dynamic nature of the blood flow response across an exercise transient (from rest to steady-state exercise) can also be assessed using Doppler ultrasound coupled with either the forearm handgrip or one-legged knee extension models. During rhythmic dynamic submaximal exercise, skeletal muscle blood flow shows an exponential rise over time that typically presents in either a monophasic or biphasic pattern (32, 121, 124, 161, 280), although a triphasic response can be observed with heavier exercise intensities (234). With a biphasic response commonly observed during moderate-intensity forearm or leg exercise, there is an immediate and significant rise in flow that plateaus within ∼5–7 s, followed by a second, slower phase beginning at ∼20 s and progressing until steady state is achieved (234, 244, 282). Using an exponential model allows for the quantification of various dynamic response characteristics of exercising muscle blood flow, including 1) the time delay of the response from the onset of exercise, 2) a time constant (τ) that represents the rate at which flow increases in a given phase, and 3) the amplitude of the response for an individual phase or overall total response (281). It could be argued that if the kinetic response is the primary response of interest, multiple transitions should be performed, similar to what is done with V̇o2 kinetics. Typically, four transitions are collected and then ensemble averaged. Also, exercise intensity domain (determined by gas exchange threshold) should be accounted for, as this will influence the development or lack thereof of a slow component (208). To adequately identify specific phases and model the blood flow response across the entire exercise transient, it is imperative to maintain a recommended angle of insonation and to minimize any motion artifact. Additionally, averaging the blood velocity over a complete contraction/relaxation cycle helps to minimize variability in the blood flow across and between cardiac cycles, which is impacted by the mechanical impedance of muscle contraction to arterial inflow (281). Indeed, using an averaging over the contraction/relaxation cycle approach to assess the blood flow and vasodilator kinetic response to forearm exercise is reproducible (32).
Pharmacological Interventions
Pharmacological interventions have been integral in advancing our understanding of vascular control mechanisms in humans. For example, the use of intra-arterial acetylcholine and/or sodium nitroprusside infusions (mentioned above) has enhanced our understanding of endothelium-dependent and -independent vasodilation in humans as well as the important contribution of NO in vascular control in both health and disease (162, 193, 194, 217). Of note is that a large amount of the information contained within this section is pertinent to other approaches beyond Doppler ultrasound (e.g., venous occlusion plethysmography). Indeed, regardless of the technique to assess limb blood flow, a number of key considerations are needed when employing pharmacological agents.
When designing studies using pharmacological interventions in humans, findings from preclinical work can inform experimental design; however, there are a number of additional important considerations. The first step in drug and dose selection is often literature review to ascertain previous use of the drug and model in humans. Many physiological studies have used pharmacological approaches successfully in the past, allowing the researcher to capitalize on prior knowledge for study design (see Tables 1 and 2 for recent examples).
Table 1.
Example of drugs used in regional studies
| Drug | Drug Class | Ref. No. |
|---|---|---|
| Acetylcholine | Vasodilator: cholinergic | 64 |
| Adenosine | Vasodilator: adenosine | 165 |
| Aminophylline | Adenosine antagonist | 165 |
| Angiotensin II* | Angiotensin II | 16 |
| Ascorbic acid | Vitamin C | 275 |
| Atropine | Cholinergic antagonist | 20 |
| Barium chloride (BaCl2)* | KIR channels | 45 |
| BHT-933* | α2-Adrenergic agonist | 128 |
| BQ123* | ETA receptor antagonist | 13 |
| BQ788* | ETB receptor antagonist | 55 |
| Celecoxib† | Cyclooxygenase-2 inhibitor | 64 |
| Clonidine | α2-Adrenergic agonist | 167 |
| Dexmedetomidine | α2-Adrenergic agonist | 168 |
| Dipyridamole | Adenosine transporter antagonist | 164 |
| Endothelin-1* | Vasoconstrictor: endothelin-1 | 188 |
| Glibencamide* | ATP-sensitive potassium (KATP) channel antagonist | 238 |
| Ibuprofen† | Cyclooxygenase inhibitor | 11 |
| Indomethacin† | Cyclooxygenase inhibitor | 259 |
| Isoproterenol | β1- and β2-Adrenergic agonist | 104 |
| Ketorolac | Cyclooxygenase inhibitor | 30 |
| Milrinone | Phosphodiesterase III inhibitor | 65 |
| NG-monomethyl-l-arginine acetate* | Nitric oxide synthase inhibitor | 186 |
| Nitroglycerin | Vasodilator: nitric oxide | 218 |
| Norepinephrine | α- and β1-Adrenergic agonist | 36 |
| Ouabain octahydrate* | Na+/K+-ATPase | 45 |
| Phentolamine | α1- and α2-Adrenergic antagonist | 297 |
| Phenylephrine | α1-Adrenergic agonist | 168 |
| Prazosin* | α1-Adrenergic antagonist | 167 |
| Propranolol | β1- and β2-Adrenergic antagonist | 297 |
| Prostacyclin | Vasodilator: prostacyclin | 186 |
| Sildenafil† | Phosphodiesterase V inhibitor | 137 |
| Sodium Nitroprusside | Vasodilator: nitric oxide | 218 |
| Tyramine* | Vasoconstrictor: endogenous norepinephrine | 31 |
| Yohimbine* | α1-Adrenergic antagonist | 56 |
Historical (not Food and Drug Administration approved, limited availability, or no longer manufactured) or
orally administered and vascular study was preformed regionally.
Table 2.
Example of drugs used in systemic studies
| Drug | Class | Ref. No. |
|---|---|---|
| Dopamine | α- and β-Dopamine agonist | 131 |
| Dexmedetomidine | α2-Adrenergic agonist | 296 |
| Epinephrine | α- and β-Adrenergic agonist | 62 |
| Glycopyrrolate | Cholinergic antagonist | 296 |
| Insulin | Hormone-hypoglycemic | 10 |
| Phentolamine | α1- and α2-Adrenergic antagonist | 237 |
| Phenylephrine† | Vasoconstrictor: α1-agonist | 159 |
| Phenylephrine‡ | Vasoconstrictor: α1-agonist | 37 |
| Propranolol | β1- and β2-Adrenergic antagonist | 158 |
| Sodium nitroprusside† | Vasodilator: nitric oxide | 159 |
| Sodium nitroprusside‡ | Vasodilator: nitric oxide | 37 |
| Terbutaline | β2-Adrenergic agonist | 113 |
| Trimethaphan* | Ganglionic blocker | 6 |
Historical (not Food and Drug Administration approved, limited availability, or no longer manufactured);
infusion titration, or
modified Oxford technique.
Model selection.
When preparing a study protocol, model selection is an important consideration. Both dosage and pharmacokinetics (absorption, distribution, metabolism, and excretion) will differ greatly between regional (e.g., forearm, leg) and systemic physiological studies. The model system will also determine dosing. For example, because both regional (e.g., forearm size) and systemic (e.g., body size) volumes will differ between groups and individuals, doses are typically indexed for limb volume (e.g., µg·dL forearm volume−1·min−1) and/or body size (e.g., µg·kg−1·min−1).
As noted above, many physiological studies using Doppler ultrasound often employ a regional/forearm model, although the leg/quadriceps have been extensively interrogated by several research groups (15, 126, 189, 304, 305). In either model system, this method requires placement of an arterial catheter under local anesthesia and aseptic conditions by a trained individual (i.e., physician) for drug administration and direct blood pressure measurement via the indwelling catheter connected to a pressure transducer. Catheters are typically placed using the Seldinger technique (242) in a retrograde direction. The pharmaceutical agent is then administered directly into the vasculature of interrogation (i.e., forearm or leg circulation) to create a local effect, under free-flowing conditions (90, 91). Of note is that, depending on the catheter placement, the ultrasound probe can be placed proximal or distal to the site of drug infusion. Some laboratories scan distal to the end of the catheter, an approach that allows visualization of the catheter in the artery. In such instances, care should be taken to 1) select an imaging site that is not in the direct line of the catheter or where the catheter movement is minimal (catheter movement may impact the blood velocity profile assessed by Doppler) or 2) scan far enough away from catheter that it is not in the field of view.
An alternative and less common approach to intra-arterial infusions is a regional intravenous technique known as a Bier-block (51, 52, 100, 152, 246). Briefly, a catheter is placed intravenously in the antecubital fossa of the arm for regional administration of a pharmacological agent. The forearm vasculature is then drained by elevating the arm above heart level, followed by sequential exsanguination of forearm blood volume via compression. Shortly thereafter, the pharmacological agent is infused intravenously in the occluded arm via the catheter, allowing the drug to diffuse into the forearm tissue. Circulatory arrest is maintained for 20 min, after which time the cuff is deflated and the subject rests for an additional 15–60 min before measurement. Importantly, groups have conducted control studies on separate study days and have shown no effect of the Bier-block procedure itself (52). To ensure the forearm and Doppler probe return to the same position following the exsanguination procedure, it is important to fix the position and posture of the arm while also marking the recording site on the skin (51). It is important to note that 1) this technique can be applied to studies using a limited number of pharmacological agents and 2) the drugs and doses are not interchangeable with those administered intra-arterially.
Dosing scheme and solution.
The next pharmacological consideration is whether a single dose and/or dose-response curve needs to be developed. In some instances, augmentation or blockade at a single, continuous dose is sufficient; this is the standard approach when using the Bier-block technique. Conversely, other agents (i.e., acetylcholine) may be administered intra-arterially via a dose-response curve. Benefits of a dose-response curve include the ability to compare responses across a range of receptor stimuli, determination of a “maximal” local dose (i.e., a plateau in the sigmoidal dose response curve, such that increasing doses do not elicit an additional change in blood flow; see Ref. 303), and determination of half-maximal effective concentration (EC50). One can also assess the slope of the response to detect physiological differences that may be more nuanced. Often, three to five ascending doses are sufficient to produce a drug dose-response curve. Depending on the agent, a loading dose may be required before initiating experimental trials. A loading dose is followed by a continuous maintenance dose (211). This type of dosing scheme (loading/continuous maintenance) may be necessary when using pharmacological agents with a long half-life when an immediate, full effect is desired, which is often the case with receptor antagonist drugs. An alternative option would be to administer the maintenance dose for a longer period of time until concentrations/effect reach steady-state. A final note on dosing is that when drugs are infused in the dilated state (e.g., exercise and/or a “high flow control,” described in more detail below), the dose needs to be adjusted to the flow environment so that it is not inadvertently diluted (57, 106, 108, 283).
Once the dose is determined, consideration should be given to the final solution to be delivered. First, the compatibility of the drug in the desired diluent must be ensured and appropriate experimental controls put in place. In many instances, normal saline (0.9% NaCl) is appropriate; however, some drugs (e.g., prostacyclin) require an alternative diluent to be stable. If an alternative diluent is used, this diluent should be used as the experimental control given that some diluents alone can exhibit effects on the peripheral vasculature (i.e., glycine- or ethanol-containing solutions). Second, concentration of the solution should be predetermined to ensure the final volume administered is appropriate. For example, intra-arterial drug infusion rates should be within the 2 to 4 mL/min range in forearm studies to reduce the potential for noise from the infusion catheter that has the potential to interfere with the Doppler signal (83). Therefore, infusing a final concentration that is too dilute will result in excess fluid administration, whereas a final concentration that is too concentrated may lead to very slow infusion rates and potential errors if dead space of the catheter/tubing is not accurately accounted for (20). For example, if the calculated dead space for a femoral catheter plus tubing and stopcock is 0.9 mL and the pump rate for the drug is 1 mL/min, it will take almost 1 min for the drug to reach the circulation. For simplicity, the total volume of dead space within the tubing and catheter may be established before the experimental day, and tubing may be “primed” with the study drug to minimize run-in time and increase confidence that the drug is getting into the limb when slow infusion rates are required.
Regarding dosing times, a good rule of thumb is to expect five half-lives for a drug to be at a steady-state concentration when the drug is administered systemically. However, regional administration differs greatly from systemic administration, and drug steady state is often reached faster within a regional compartment compared with systemic administration. For example, intra-arterial administration of the α1-adrenergic agonist phenylephrine locally into the forearm circulation elicits a maximal reduction in limb blood flow within 45 s; in contrast, 30 min of continuous infusion is needed to establish a similar level of vasoconstriction in response to endothelin-1 (304). Regardless of the initial regional administration, the systemic half-life of the drug needs to be a consideration. All pharmacological agents are expected to leave the local circulation (i.e., forearm, leg) and enter the systemic circulation. Systemic spillover of a vasoactive drug is typically minimized during arm-based studies where small doses are required. In contrast, in leg-based studies, the potential for systemic spillover is augmented because of the larger limb mass and higher drug doses required to elicit a vasoactive effect. Irrespective of the limb being studied, central hemodynamics (i.e., heart rate and mean arterial blood pressure) should be measured continuously to document any potential systemic effects, which could confound the interpretation of the studies. With this, the potential for systemic accumulation (i.e., spillover) must be considered. For example, pharmaceutical agents with long half-lives (e.g., milrinone) will accumulate systemically following redistribution from the forearm due to their dependence on renal and/or hepatic clearance. Therefore, dosage and/or infusion time should be reduced as needed to limit systemic effects. This is highly important with use of a Bier-block, where removal of the tourniquet could result in sudden release of the drug into the systemic circulation. With this, it is important to recognize that although hemodynamics (e.g., forearm blood flow) may return to baseline before five half-lives have elapsed, there is still the drug in the body, which may elicit an effect.
Safety and other considerations.
With the design of any intervention in humans, a major decision point is safety of the selected model. When proposing a new agent that has not been studied previously, it is essential to consult a physician and/or pharmacist familiar with the pharmacology and safe administration of the desired drug before finalizing a research protocol. For example, although hexamethonium has been used systemically (23) and in research safely in regional studies (114), administration via inhalation may produce fatal results (236). Additionally, intravenous promethazine is commonly used clinically as an anti-emetic; however, inappropriate intra-arterial administration in a regional forearm model may result in loss of limb (78). Last, barium chloride and ouabain have been used extensively in recent years in the forearm circulation (44, 48, 108, 211); however, these drugs should not be given in the leg because of concerns for systemic spillover and potentially serious consequences on K+ channels in the heart. Identifying potential allergic reactions and/or interactions with existing pathology and pharmacological interventions is also essential to be considered before a subject is enrolled in these types of studies. For example, an aspirin allergy would exclude individuals for studies, including oral indomethacin, and asthmatics would be excluded from studies using systemic trimethaphan. Finally, additional consideration and consultation is required when designing drug infusion studies in patient groups to ensure the intended study drug is not contraindicated and to consider the potential interaction and/or duplicity with the patient’s existing pharmacotherapy.
With the design of a human physiological study using pharmacological agents, regulatory requirements are also key considerations. First and foremost, the selected agent must be approved by the FDA and produced under good manufacturing practices (GMP). There are a number of compounds used historically that no longer meet the requirement of GMP and/or are no longer manufactured, which precludes their use (see Table 1). Thus, it is highly recommended that the availability of a compound is carefully explored before study, especially in the context of GMP. Even if produced under GMP, the FDA mandates that an Investigational New Drug (IND) application be filed. An IND application is required for drug studies in humans using agents that are not FDA approved as well as agents that are approved but will be used outside their FDA-approved terms (116). Simply, if it is not a currently approved drug by the FDA used according FDA-approved guidelines, an IND is required, even if the compound is of pharmaceutical quality. In such cases, the research team should inquire with the FDA regarding an IND exemption, and the FDA will determine whether the proposed agent/protocol is exempt or not. An IND exemption may be granted if all exemption criteria are met (for more information, please see the FDA website). Whether exempt or not, in addition to the other required criteria, special attention should be given to ensure that the route of administration, dose, and patient population, as well as other factors, do not significantly increase risk or decrease acceptability of risk associated with the use of the study drug.
In contrast to regional administration (i.e., local forearm infusions), a systemic model design is selected when total body hemodynamic effects are desired and/or when drugs require hepatic activation (i.e., many pro-drugs). One large pharmacological advantage of systemic usage is that typical dosing and pharmacokinetics in normal therapeutic use are reasonably well defined. With this, pharmacokinetics is important in systemic study design. When used intravenously, the bioavailability of systemically administered drugs is 100%. However, if a medication is administered orally, the bioavailability will be dependent upon absorption and/or hepatic extraction (i.e., first-pass metabolism), which has the potential to reduce the available amount of drug considerably. Measurement periods will next need to consider distribution time; this careful consideration is necessary to ensure that data are collected within the window of peak plasma concentration of the study drug. For example, a centrally acting α2-agonist (e.g., dexmedetomidine) given systemically to block central sympathetic outflow will have a greater time to onset of effect than a directly acting vascular agent (e.g., sodium nitroprusside) due to the distribution time into the central nervous system.
As with any factor of study design, study population needs to be carefully considered. For example, drug half-life is dependent on clearance, and this information is readily available in the literature for FDA-approved medications. However, most pharmacokinetic studies are performed in small sample sizes and in healthy male populations. Studies in different populations (e.g., aging, renal insufficiency, etc.) will have differing volumes of distribution and clearances and, therefore, altered drug half-lives when compared with the heathy male population. Accordingly, certain populations may require an adjustment in dose. For example, in anticipation that older adults will exhibit attenuated adrenergic receptor responsiveness, some groups elect to use different doses in young versus older adults (221). When pharmacological agents are dosed relative to limb size (mL·dL−1 forearm volume ·min−1), it may be appropriate to also present flow and conductance values relative to limb size (i.e., mL·min−1·dL−1 forearm volume/100 mmHg); this is especially true when comparing across groups of individuals of varying sizes (156, 157). Finally, it should be acknowledged that in the older plethysmography studies of drug infusion (e.g., l-NMMA, acetylcholine, and sodium nitroprusside), it was typical to include a dose-response curve to an agent independent of the pathway in question (i.e., NO) to prove that any abnormality was specific to that pathway and not generalized in nature. For example, a study focused on endothelin-1-mediated vasoconstriction should also consider including dose-response curves to other agonists (e.g., phenylephrine) to determine whether alterations in endothelin-1-induced vasoconstriction are indeed specific to changes in endothelin-1 signaling. These experimental controls should be included when possible.
Although pharmacological interventions can be a powerful experimental tool, there are limitations of this approach. Many experimental considerations have been addressed above; however, there are a number of nuances to this approach that cannot be adequately summarized in a few paragraphs. To this end, it is paramount that investigators not formally trained in pharmacology seek collaboration with individuals experienced in such approaches to ensure both sound experimental design and subject safety.
ANALYSIS AND REPORTING OF FINDINGS
It is critical that one considers how data will be reported early in study design. Depending on the research question of interest, blood flow and/or vascular conductance measures may be reported as an average over time (mean), a peak response, a steady-state response, or an integrated AUC. Typically, a quiet resting period of a minimum of 10 to 20 min is required before hemodynamics become stable following instrumentation and/or an intervention period (i.e., washout). Following this period of time, baseline hemodynamic data are often reported as an average over 30 s to 2 min to be confident that subjects have achieved steady state and to limit the influence of beat-by-beat variability. This is particularly relevant if the subject has arrythmias.
As noted above, a benefit of the use of Doppler ultrasound in human physiological research is the ability to assess blood flow continuously. In instances when responses to an intervention may be transient (such as in response to a single muscle contraction, passive movement of the limb, or short-acting pharmacological agent), data collected at 30 Hz can be analyzed beat by beat (20) or in short, time-dependent (i.e., 3-s) bins (107). In such instances, the peak change or integrated AUC is often identified and reported.
After the assessment of resting baseline hemodynamics, most researchers are interested in the dynamic response to an experimental perturbation (i.e., the vasodilator or vasoconstrictor response to an intervention). A common issue encountered in studies where baseline vascular tone may differ across groups and/or conditions is the proper quantification of the blood flow responses (106). As such, it is preferred to quantify and present data as both an absolute (Δ) and relative (%) change in blood flow (or conductance) from baseline within a given condition (163, 221, 294).
This issue of data presentation has received much attention particularly as it relates to the study of functional sympatholysis and the combined study design of both exercise and intra-arterial pharmacological interventions (141, 283), due in large part to the capacity for large increases in skeletal muscle blood flow in the exercising limb. Mathematically, percent changes become smaller as the starting value increases; for example, a 50% reduction in resting leg blood flow (≈250 ml/min) may reflect an absolute change of 125 ml/min, whereas this same change in absolute blood flow during moderate-intensity knee extensor exercise, where leg blood flow may increase 10-fold (≈2,500 ml/min), would be <1%. Thus, it is critical to evaluate blood flow in both absolute units, as well as absolute change and percent change, to properly interpret the physiological significance of responses. However, quantification of data as an absolute (Δ) versus a relative change (%) from baseline to steady-state exercise can lead to discrepancies in the interpretation of results (106). To address this issue, a vasodilating agent may be administered to match the anticipated hemodynamic response to exercise (i.e., a “high-flow control”) before studying drug administration (58, 140). Alternately, post hoc calculation of the magnitude of change in blood flow or vascular conductance, expressed as a percentage, can be determined from rest to exercise (58). Indeed, it is the belief of many that the relative (%) change in vascular conductance (from rest to exercise or pre/post-drug infusion) is the most appropriate index of vasoconstrictor responses (22, 283). In a review on the topic, Buckwalter and Clifford (22) demonstrate that, despite differences in baseline blood flow, a relative (%) change in vascular conductance reflects a similar relative reduction in blood vessel radius (i.e., vasoconstriction). Although the relative (%) change in blood flow and/or conductance is commonly viewed as the most appropriate index for assessing vasomotor responses, one must ultimately consider the nature of the experimental question being addressed. For example, under conditions of altered arterial oxygen content (i.e., hypoxia), assessing the absolute change in blood flow and vascular conductance needed to help “compensate” and keep oxygen delivery to the active muscles relatively constant is likely more appropriate (33).
FINAL REMARKS
The use of Doppler ultrasound in human cardiovascular studies has rapidly escalated over recent years. The widespread adoption of this technology to assess limb blood flow is largely driven by the increased recognition that resistance vessel function is an important determinant of cardiovascular health that may also provide prognostic information. Another advantage is that from the same technique one can typically derive information about both conduit and resistance vessel function. However, the prevalent use of Doppler ultrasound to assess resistance vessel function has also exposed the marked variability between studies and laboratories with respect to experimental approaches, procedures, and analysis, revealing a clear need for guidelines regarding the conduct of human studies involving this methodology.
The National Institutes of Health and other research funding agencies strive to exemplify and promote the highest level of scientific integrity and enhance reproducibility through rigor and transparency. In support of this, in 2015, the editors of AJP-Heart and Circulatory Physiology began working with experts in various fields to develop landmark guidelines in cardiovascular research. As part of this initiative, the present commissioned article is the reflection of collegial discussions among physiologists and pharmacologists from several independent research groups with expertise in Doppler ultrasound-based measures of resistance vessel function in humans. Here, we portray a collection of considerations and recommendations on topics related to experimental design, data acquisition, and signal processing in addition to delivering an overview of the common procedures used to assess resistance vessel function via Doppler ultrasound. We expect that this expert consensus report will serve as a useful resource for both trainees and more established investigators engaged in human cardiovascular research.
GRANTS
J. K. Limberg is supported by National Institutes of Health (NIH) Grant R00-HL-130339; D. P. Casey is supported by American Diabetes Association Grant 1-16-ICTS-015; J. D. Trinity is supported by NIH Grant R01-HL-142603 and Veterans Affairs (VA) Merit Award I01CX001999; D. W. Wray is supported by NIH Grant R01-HL-118313 and VA Merit Award I01RX001311; M. E. Tschakovsky is supported by Natural Sciences and Engineering Research Council of Canada Discovery Grant RGPIN/05078-2016 and Research Tools and Instruments Grant EQPEQ0407690-11, as well as infrastructure grants from the Canadian Foundation for Innovation and the Ontario Innovation Trust; D. J. Green is supported by National Health and Medical Research Council Principal Research Fellow Grant 1080914; Y. Hellsten is supported by The Independent Research Fund Denmark-Medical Sciences, The Lundbeck Foundation, and The Danish Ministry of Culture Foundation for Sport Science; P. J. Fadel is supported by NIH Grant R01-HL127071; M. J. Joyner is supported by NIH Grant R35-HL-139854; and J. Padilla is supported by NIH Grant R01-HL137769.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., M.J.J., and J.P. conceived and designed research; J.P. prepared figures; J.K.L., D.P.C., J.T., W.T.N., and J.P. drafted manuscript; J.K.L., D.P.C., J.T., W.T.N., D.W.W., M.E.T., D.J.G., Y.H., P.J.F., M.J.J., and J.P. edited and revised manuscript; J.K.L., D.P.C., J.T., W.T.N., D.W.W., M.E.T., D.J.G., Y.H., P.J.F., M.J.J., and J.P. approved final version of manuscript.
REFERENCES
- 1.Alomari MA, Solomito A, Reyes R, Khalil SM, Wood RH, Welsch MA. Measurements of vascular function using strain-gauge plethysmography: technical considerations, standardization, and physiological findings. Am J Physiol Heart Circ Physiol 286: H99–H107, 2004. doi: 10.1152/ajpheart.00529.2003. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59: 1647–1653, 1985. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation 123: 163–169, 2011. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 5.Bain AR, Weil BR, Diehl KJ, Greiner JJ, Stauffer BL, DeSouza CA. Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Atherosclerosis 265: 41–46, 2017. doi: 10.1016/j.atherosclerosis.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Baker SE, Limberg JK, Dillon GA, Curry TB, Joyner MJ, Nicholson WT. Aging Alters the Relative Contributions of the Sympathetic and Parasympathetic Nervous System to Blood Pressure Control in Women. Hypertension 72: 1236–1242, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangsbo J, Kjær M, Hellsten Y. Bengt Saltin (1935-2014). J Physiol 592: 5149–5151, 2014. doi: 10.1113/jphysiol.2014.285411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber FE, Baker DW, Nation AW, Strandness DE Jr, Reid JM. Ultrasonic duplex echo-Doppler scanner. IEEE Trans Biomed Eng 21: 109–113, 1974. doi: 10.1109/TBME.1974.324295. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa TC, Kaur J, Holwerda SW, Young CN, Curry TB, Thyfault JP, Joyner MJ, Limberg JK, Fadel PJ. Insulin increases ventilation during euglycemia in humans. Am J Physiol Regul Integr Comp Physiol 315: R84–R89, 2018. doi: 10.1152/ajpregu.00039.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes JN, Charkoudian N, Matzek LJ, Johnson CP, Joyner MJ, Curry TB. Acute cyclooxygenase inhibition does not alter muscle sympathetic nerve activity or forearm vasodilator responsiveness in lean and obese adults. Physiol Rep 2: e12079, 2014. doi: 10.14814/phy2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol 271: E1067–E1072, 1996. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. doi: 10.1152/ajpheart.00603.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MA, Nativi-Nicolau J, Stehlik J, Richardson RS, Wray DW. Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol 307: H1512–H1520, 2014. doi: 10.1152/ajpheart.00527.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett-O’Keefe Z, Lee JF, Ives SJ, Trinity JD, Witman MAH, Rossman MJ, Groot HJ, Sorensen JR, Morgan DE, Nelson AD, Stehlik J, Richardson RS, Wray DW. α-Adrenergic receptor regulation of skeletal muscle blood flow during exercise in heart failure patients with reduced ejection fraction. Am J Physiol Regul Integr Comp Physiol 316: R512–R524, 2019. doi: 10.1152/ajpregu.00345.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett-O’Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE, Sander M, Richardson RS, Wray DW. Angiotensin II potentiates α-adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124: 413–422, 2013. doi: 10.1042/CS20120424. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum in: Circulation 135: e646, 2017.] doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentley RF, Kellawan JM, Moynes JS, Poitras VJ, Walsh JJ, Tschakovsky ME. Individual susceptibility to hypoperfusion and reductions in exercise performance when perfusion pressure is reduced: evidence for vasodilator phenotypes. J Appl Physiol (1985) 117: 392–405, 2014. doi: 10.1152/japplphysiol.01155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol (1985) 112: 1653–1658, 2012. doi: 10.1152/japplphysiol.01489.2011. [DOI] [PubMed] [Google Scholar]
- 20.Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol (1985) 85: 2249–2254, 1998. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- 21.Buck TM, Sieck DC, Halliwill JR. Thin-beam ultrasound overestimation of blood flow: how wide is your beam? J Appl Physiol (1985) 116: 1096–1104, 2014. doi: 10.1152/japplphysiol.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Burch RR. The effects of intravenous hexamethonium on venous pressure of normotensive and hypertensive patients with and without congestive heart failure. Circulation 11: 271–279, 1955. doi: 10.1161/01.CIR.11.2.271. [DOI] [PubMed] [Google Scholar]
- 24.Burke D, Sundlöf G, Wallin G. Postural effects on muscle nerve sympathetic activity in man. J Physiol 272: 399–414, 1977. doi: 10.1113/jphysiol.1977.sp012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S, Somers VK. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J Am Heart Assoc 3: e001143, 2014. doi: 10.1161/JAHA.114.001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter JR, Stream SF, Durocher JJ, Larson RA. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am J Physiol Endocrinol Metab 300: E771–E778, 2011. doi: 10.1152/ajpendo.00674.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey DP, Curry TB, Joyner MJ. Measuring muscle blood flow: a key link between systemic and regional metabolism. Curr Opin Clin Nutr Metab Care 11: 580–586, 2008. doi: 10.1097/MCO.0b013e32830b5b34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casey DP, Joyner MJ. Influence of α-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol (1985) 113: 1201–1212, 2012. doi: 10.1152/japplphysiol.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol (1985) 111: 1527–1538, 2011. doi: 10.1152/japplphysiol.00895.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey DP, Joyner MJ. Prostaglandins do not contribute to the nitric oxide-mediated compensatory vasodilation in hypoperfused exercising muscle. Am J Physiol Heart Circ Physiol 301: H261–H268, 2011. doi: 10.1152/ajpheart.00222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey DP, Joyner MJ, Claus PL, Curry TB. Vasoconstrictor responsiveness during hyperbaric hyperoxia in contracting human muscle. J Appl Physiol (1985) 114: 217–224, 2013. doi: 10.1152/japplphysiol.01197.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey DP, Ranadive SM, Joyner MJ. Aging is associated with altered vasodilator kinetics in dynamically contracting muscle: role of nitric oxide. J Appl Physiol (1985) 119: 232–241, 2015. doi: 10.1152/japplphysiol.00787.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey DP, Shepherd JR, Joyner MJ. Sex and vasodilator responses to hypoxia at rest and during exercise. J Appl Physiol (1985) 116: 927–936, 2014. doi: 10.1152/japplphysiol.00409.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol (1985) 115: 446–455, 2013. doi: 10.1152/japplphysiol.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 36.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572: 821–827, 2006. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 287: H1658–H1662, 2004. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- 38.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol (1985) 97: 393–403, 2004. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 39.Clifton HL, Machin DR, Groot HJ, Frech TM, Donato AJ, Richardson RS, Wray DW. Attenuated nitric oxide bioavailability in systemic sclerosis: Evidence from the novel assessment of passive leg movement. Exp Physiol 103: 1412–1424, 2018. doi: 10.1113/EP086991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conway J. A vascular abnormality in hypertension. A study of blood flow in the forearm. Circulation 27: 520–529, 1963. doi: 10.1161/01.CIR.27.4.520. [DOI] [PubMed] [Google Scholar]
- 41.Coolbaugh CL, Bush EC, Caskey CF, Damon BM, Towse TF. FloWave.US: validated, open-source, and flexible software for ultrasound blood flow analysis. J Appl Physiol (1985) 121: 849–857, 2016. doi: 10.1152/japplphysiol.00819.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol 128: 258–267, 1955. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a Brief Contraction of Forearm Muscles on Forearm Blood Flow. J Appl Physiol 19: 142–146, 1964. doi: 10.1152/jappl.1964.19.1.142. [DOI] [PubMed] [Google Scholar]
- 44.Crecelius AR, Kirby BS, Hearon CM Jr, Luckasen GJ, Larson DG, Dinenno FA. Contracting human skeletal muscle maintains the ability to blunt α1 -adrenergic vasoconstriction during KIR channel and Na(+)/K(+) -ATPase inhibition. J Physiol 593: 2735–2751, 2015. doi: 10.1113/JP270461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol 590: 5349–5359, 2012. doi: 10.1113/jphysiol.2012.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol 301: H1302–H1310, 2011. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol 307: H782–H791, 2014. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 113: 1023–1032, 2013. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Credeur DP, Holwerda SW, Restaino RM, King PM, Crutcher KL, Laughlin MH, Padilla J, Fadel PJ. Characterizing rapid-onset vasodilation to single muscle contractions in the human leg. J Appl Physiol (1985) 118: 455–464, 2015. doi: 10.1152/japplphysiol.00785.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui J, Leuenberger UA, Blaha C, Yoder J, Gao Z, Sinoway LI. Local adenosine receptor blockade accentuates the sympathetic responses to fatiguing exercise. Am J Physiol Heart Circ Physiol 298: H2130–H2137, 2010. doi: 10.1152/ajpheart.00083.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui J, Moradkhan R, Mascarenhas V, Momen A, Sinoway LI. Cyclooxygenase inhibition attenuates sympathetic responses to muscle stretch in humans. Am J Physiol Heart Circ Physiol 294: H2693–H2700, 2008. doi: 10.1152/ajpheart.91505.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis JG, Richards KL, Greene D. A sample volume tracking unit for pulsed Doppler echocardiography. IEEE Trans Biomed Eng 26: 285–288, 1979. doi: 10.1109/TBME.1979.326404. [DOI] [PubMed] [Google Scholar]
- 54.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 55.Diehl KJ, Stauffer BL, Dow CA, Bammert TD, Brunjes DL, Greiner JJ, DeSouza CA. Chronic Nebivolol Treatment Suppresses Endothelin-1-Mediated Vasoconstrictor Tone in Adults With Elevated Blood Pressure. Hypertension 67: 1196–1204, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol 540: 1103–1110, 2002. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol 534: 287–295, 2001. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 61.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001. doi: 10.1042/cs1010629. [DOI] [PubMed] [Google Scholar]
- 62.Du S, Joyner MJ, Curry TB, Eisenach JH, Johnson CP, Schrage WG, Jensen MD. Effect of β2-adrenergic receptor polymorphisms on epinephrine and exercise-stimulated lipolysis in humans. Physiol Rep 2: e12017, 2014. doi: 10.14814/phy2.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol 488: 259–265, 1995. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eisenach JH, Gullixson LR, Allen AR, Kost SL, Nicholson WT. Cyclo-oxygenase-2 inhibition and endothelium-dependent vasodilation in younger vs. older healthy adults. Br J Clin Pharmacol 78: 815–823, 2014. doi: 10.1111/bcp.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elvebak RL, Eisenach JH, Joyner MJ, Nicholson WT. The function of vascular smooth muscle phosphodiesterase III is preserved in healthy human aging. Clin Transl Sci 3: 239–242, 2010. doi: 10.1111/j.1752-8062.2010.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esposito F, Wagner PD, Richardson RS. Incremental large and small muscle mass exercise in patients with heart failure: evidence of preserved peripheral haemodynamics and metabolism. Acta Physiol (Oxf) 213: 688–699, 2015. doi: 10.1111/apha.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Etsuda H, Takase B, Uehata A, Kusano H, Hamabe A, Kuhara R, Akima T, Matsushima Y, Arakawa K, Satomura K, Kurita A, Ohsuzu F. Morning attenuation of endothelium-dependent, flow-mediated dilation in healthy young men: possible connection to morning peak of cardiac events? Clin Cardiol 22: 417–421, 1999. doi: 10.1002/clc.4960220610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans DH. On the measurement of the mean velocity of blood flow over the cardiac cycle using Doppler ultrasound. Ultrasound Med Biol 11: 735–741, 1985. doi: 10.1016/0301-5629(85)90107-3. [DOI] [PubMed] [Google Scholar]
- 70.Evans DH. Some aspects of the relationship between instantaneous volumetric blood flow and continuous wave Doppler ultrasound recordings—I. The effect of ultrasonic beam width on the output of maximum, mean and RMS frequency processors. Ultrasound Med Biol 8: 605–609, 1982. doi: 10.1016/0301-5629(82)90116-8. [DOI] [PubMed] [Google Scholar]
- 71.Fadel PJ, Stromstad M, Wray DW, Smith SA, Raven PB, Secher NH. New insights into differential baroreflex control of heart rate in humans. Am J Physiol Heart Circ Physiol 284: H735–H743, 2003. doi: 10.1152/ajpheart.00246.2002. [DOI] [PubMed] [Google Scholar]
- 72.Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension 14: 511–517, 1989. doi: 10.1161/01.HYP.14.5.511. [DOI] [PubMed] [Google Scholar]
- 73.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol 305: H867–H874, 2013. doi: 10.1152/ajpheart.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fairfax ST, Padilla J, Vianna LC, Holwerda SW, Davis MJ, Fadel PJ. Myogenic responses occur on a beat-to-beat basis in the resting human limb. Am J Physiol Heart Circ Physiol 308: H59–H67, 2015. doi: 10.1152/ajpheart.00609.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Folkow B. The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med Suppl 4: 3s–22s, 1978. doi: 10.1042/cs055003s. [DOI] [PubMed] [Google Scholar]
- 77.Folkow B, Griyby G, Thulesius O. Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand 44: 255–272, 1958. doi: 10.1111/j.1748-1716.1958.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 78.Foret AL, Bozeman AP, Floyd WE III. Necrosis caused by intra-arterial injection of promethazine: case report. J Hand Surg Am 34: 919–923, 2009. doi: 10.1016/j.jhsa.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 79.Garten RS, Groot HJ, Rossman MJ, Gifford JR, Richardson RS. The role of muscle mass in exercise-induced hyperemia. J Appl Physiol (1985) 116: 1204–1209, 2014. doi: 10.1152/japplphysiol.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gifford JR, Richardson RS. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gill RW. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound Med Biol 11: 625–641, 1985. doi: 10.1016/0301-5629(85)90035-3. [DOI] [PubMed] [Google Scholar]
- 82.Gill RW. Pulsed Doppler with B-mode imaging for quantitative blood flow measurement. Ultrasound Med Biol 5: 223–235, 1979. doi: 10.1016/0301-5629(79)90014-0. [DOI] [PubMed] [Google Scholar]
- 83.Gliemann L, Mortensen SP, Hellsten Y. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol 118: 1081–1094, 2018. doi: 10.1007/s00421-018-3880-5. [DOI] [PubMed] [Google Scholar]
- 84.Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep 21: 21, 2019. doi: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 85.Gonzales JU, Miedlar JA, Parker BA, Proctor DN. Relation of femoral diameter, shear rate, and dilatory response to knee extensor exercise. Med Sci Sports Exerc 42: 1870–1875, 2010. doi: 10.1249/MSS.0b013e3181dd1c99. [DOI] [PubMed] [Google Scholar]
- 86.Gourdin M, Dubois P, Mullier F, Chatelain B, Dogné JM, Marchandise B, Jamart J, De Kock M. The effect of clonidine, an alpha-2 adrenergic receptor agonist, on inflammatory response and postischemic endothelium function during early reperfusion in healthy volunteers. J Cardiovasc Pharmacol 60: 553–560, 2012. doi: 10.1097/FJC.0b013e31827303fa. [DOI] [PubMed] [Google Scholar]
- 87.Greaney JL, Kenney WL, Alexander LM. Sympathetic function during whole body cooling is altered in hypertensive adults. J Appl Physiol (1985) 123: 1617–1624, 2017. doi: 10.1152/japplphysiol.00613.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O’Driscoll G. Effect of lower limb exercise on forearm vascular function: contribution of nitric oxide. Am J Physiol Heart Circ Physiol 283: H899–H907, 2002. doi: 10.1152/ajpheart.00049.2002. [DOI] [PubMed] [Google Scholar]
- 89.Green D, Cheetham C, Reed C, Dembo L, O’Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol (1985) 93: 361–368, 2002. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- 90.Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR. Modification of forearm resistance vessels by exercise training in young men. J Appl Physiol (1985) 77: 1829–1833, 1994. doi: 10.1152/jappl.1994.77.4.1829. [DOI] [PubMed] [Google Scholar]
- 91.Green DJ, Fowler DT, O’Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol (1985) 81: 943–948, 1996. doi: 10.1152/jappl.1996.81.2.943. [DOI] [PubMed] [Google Scholar]
- 92.Green DJ, Hopkins ND, Jones H, Thijssen DH, Eijsvogels TM, Yeap BB. Sex differences in vascular endothelial function and health in humans: impacts of exercise. Exp Physiol 101: 230–242, 2016. doi: 10.1113/EP085367. [DOI] [PubMed] [Google Scholar]
- 93.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Green DJ, Spence A, Rowley N, Thijssen DH, Naylor LH. Vascular adaptation in athletes: is there an ‘athlete’s artery’? Exp Physiol 97: 295–304, 2012. doi: 10.1113/expphysiol.2011.058826. [DOI] [PubMed] [Google Scholar]
- 95.Greene ER, Richards KL, Nelson C, Davis J. Variable sample volume dimension in pulse Doppler echocardiography. Biomed Sci Instrum 15: 91–100, 1979. [PubMed] [Google Scholar]
- 96.Greyling A, van Mil AC, Zock PL, Green DJ, Ghiadoni L, Thijssen DH; TIFN International Working Group on Flow Mediated Dilation . Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis 248: 196–202, 2016. doi: 10.1016/j.atherosclerosis.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 97.Groot HJ, Rossman MJ, Garten RS, Wang E, Hoff J, Helgerud J, Richardson RS. The Effect of Physical Activity on Passive Leg Movement-Induced Vasodilation with Age. Med Sci Sports Exerc 48: 1548–1557, 2016. doi: 10.1249/MSS.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928, 2015. doi: 10.1113/JP270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 27: 2650–2656, 2007. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 98: 584–596, 1996. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hantsoo L, Czarkowski KA, Child J, Howes C, Epperson CN. Selective serotonin reuptake inhibitors and endothelial function in women. J Womens Health (Larchmt) 23: 613–618, 2014. doi: 10.1089/jwh.2013.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hart CR, Layec G, Trinity JD, Le Fur Y, Gifford JR, Clifton HL, Richardson RS. Oxygen availability and skeletal muscle oxidative capacity in patients with peripheral artery disease: implications from in vivo and in vitro assessments. Am J Physiol Heart Circ Physiol 315: H897–H909, 2018. doi: 10.1152/ajpheart.00641.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harvey RE, Barnes JN, Charkoudian N, Curry TB, Eisenach JH, Hart EC, Joyner MJ. Forearm vasodilator responses to a β-adrenergic receptor agonist in premenopausal and postmenopausal women. Physiol Rep 2: e12032, 2014. doi: 10.14814/phy2.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594: 2261–2273, 2016. doi: 10.1113/JP270593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hearon CM Jr, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Endothelium-dependent vasodilatory signalling modulates α1 -adrenergic vasoconstriction in contracting skeletal muscle of humans. J Physiol 594: 7435–7453, 2016. doi: 10.1113/JP272829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hearon CM Jr, Richards JC, Racine ML, Luckasen GJ, Larson DG, Dinenno FA. Amplification of endothelium-dependent vasodilatation in contracting human skeletal muscle: role of KIR channels. J Physiol 597: 1321–1335, 2019. doi: 10.1113/JP276998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hearon CM Jr, Richards JC, Racine ML, Luckasen GJ, Larson DG, Joyner MJ, Dinenno FA. Sympatholytic effect of intravascular ATP is independent of nitric oxide, prostaglandins, Na+/K+ -ATPase and KIR channels in humans. J Physiol 595: 5175–5190, 2017. doi: 10.1113/JP274532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 110.Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol 590: 6297–6305, 2012. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- 112.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hesse C, Schroeder DR, Nicholson WT, Hart EC, Curry TB, Penheiter AR, Turner ST, Joyner MJ, Eisenach JH. beta2-Adrenoceptor gene variation and systemic vasodilatation during ganglionic blockade. J Physiol 588: 2669–2678, 2010. doi: 10.1113/jphysiol.2010.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hodge RL, Whelan RF. Effect of hexamethonium on the vascular response to noradrenaline in man. Br J Pharmacol Chemother 18: 331–336, 1962. doi: 10.1111/j.1476-5381.1962.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Høier B, Rufener N, Bojsen-Møller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol 588: 3833–3845, 2010. doi: 10.1113/jphysiol.2010.190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holbein ME. Understanding FDA regulatory requirements for investigational new drug applications for sponsor-investigators. J Investig Med 57: 688–694, 2009. doi: 10.2310/JIM.0b013e3181afdb26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holder SM, Dawson EA, Brislane Á, Hisdal J, Green DJ, Thijssen DHJ. Fluctuation in shear rate, with unaltered mean shear rate, improves brachial artery flow-mediated dilation in healthy, young men. J Appl Physiol (1985) 126: 1687–1693, 2019. doi: 10.1152/japplphysiol.00009.2019. [DOI] [PubMed] [Google Scholar]
- 118.Holowatz LA, Kenney WL. Chronic low-dose aspirin therapy attenuates reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol (1985) 106: 500–505, 2009. doi: 10.1152/japplphysiol.91215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hughes WE, Kruse NT, Casey DP. Sympathetic nervous system activation reduces contraction-induced rapid vasodilation in the leg of humans independent of age. J Appl Physiol (1985) 123: 106–115, 2017. doi: 10.1152/japplphysiol.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hughes WE, Kruse NT, Ueda K, Casey DP. Habitual exercise training in older adults offsets the age-related prolongation in leg vasodilator kinetics during single-limb lower body exercise. J Appl Physiol (1985) 125: 746–754, 2018. doi: 10.1152/japplphysiol.00235.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hughes WE, Ueda K, Casey DP. Chronic endurance exercise training offsets the age-related attenuation in contraction-induced rapid vasodilation. J Appl Physiol (1985) 120: 1335–1342, 2016. doi: 10.1152/japplphysiol.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hughes WE, Ueda K, Treichler DP, Casey DP. Rapid onset vasodilation with single muscle contractions in the leg: influence of age. Physiol Rep 3: e12516, 2015. doi: 10.14814/phy2.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hughson RL, Tschakovsky ME. Cardiovascular dynamics at the onset of exercise. Med Sci Sports Exerc 31: 1005–1010, 1999. doi: 10.1097/00005768-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 125.Iepsen UW, Munch GW, Rugbjerg M, Ryrsø CK, Secher NH, Hellsten Y, Lange P, Pedersen BK, Thaning P, Mortensen SP. Leg blood flow is impaired during small muscle mass exercise in patients with COPD. J Appl Physiol (1985) 123: 624–631, 2017. doi: 10.1152/japplphysiol.00178.2017. [DOI] [PubMed] [Google Scholar]
- 126.Iepsen UW, Munch GW, Ryrsø CK, Secher NH, Lange P, Thaning P, Pedersen BK, Mortensen SP. Muscle α-adrenergic responsiveness during exercise and ATP-induced vasodilation in chronic obstructive pulmonary disease patients. Am J Physiol Heart Circ Physiol 314: H180–H187, 2018. doi: 10.1152/ajpheart.00398.2017. [DOI] [PubMed] [Google Scholar]
- 127.Incognito AV, Samora M, Cartafina RA, Guimarães GMN, Daher M, Millar PJ, Vianna LC. Pharmacological assessment of the arterial baroreflex in a young healthy obese male with extremely low baseline muscle sympathetic nerve activity. Clin Auton Res 28: 593–595, 2018. doi: 10.1007/s10286-018-0559-2. [DOI] [PubMed] [Google Scholar]
- 128.Ives SJ, Fadel PJ, Brothers RM, Sander M, Wray DW. Exploring the vascular smooth muscle receptor landscape in vivo: ultrasound Doppler versus near-infrared spectroscopy assessments. Am J Physiol Heart Circ Physiol 306: H771–H776, 2014. doi: 10.1152/ajpheart.00782.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol (1985) 103: 1715–1721, 2007. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 130.Johnson BD, Padilla J, Harris RA, Wallace JP. Vascular consequences of a high-fat meal in physically active and inactive adults. Appl Physiol Nutr Metab 36: 368–375, 2011. doi: 10.1139/h11-028. [DOI] [PubMed] [Google Scholar]
- 131.Johnson BD, Peinado AB, Ranadive SM, Curry TB, Joyner MJ. Effects of intravenous low-dose dopamine infusion on glucose regulation during prolonged aerobic exercise. Am J Physiol Regul Integr Comp Physiol 314: R49–R57, 2018. doi: 10.1152/ajpregu.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol 39: 920–924, 1975. doi: 10.1152/jappl.1975.39.6.920. [DOI] [PubMed] [Google Scholar]
- 133.Jones H, Green DJ, George K, Atkinson G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol 298: R427–R432, 2010. doi: 10.1152/ajpregu.00442.2009. [DOI] [PubMed] [Google Scholar]
- 134.Jones H, Lewis NC, Thompson A, Marrin K, Green DJ, Atkinson G. Diurnal variation in vascular function: role of sleep. Chronobiol Int 29: 271–277, 2012. doi: 10.3109/07420528.2012.654554. [DOI] [PubMed] [Google Scholar]
- 135.Joyner M, Kjaer M, Larsen PO. The Copenhagen Muscle Research Centre (CMRC) 1994-2004. Scand J Med Sci Sports 25, Suppl 4: 22–28, 2015. doi: 10.1111/sms.12598. [DOI] [PubMed] [Google Scholar]
- 136.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kellawan JM, Limberg JK, Scruggs ZM, Nicholson WT, Schrage WG, Joyner MJ, Curry TB. Phosphodiesterase-5 inhibition preserves exercise-onset vasodilator kinetics when NOS activity is reduced. J Appl Physiol (1985) 124: 276–282, 2018. doi: 10.1152/japplphysiol.00483.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kingwell BA, Tran B, Cameron JD, Jennings GL, Dart AM. Enhanced vasodilation to acetylcholine in athletes is associated with lower plasma cholesterol. Am J Physiol 270: H2008–H2013, 1996. doi: 10.1152/ajpheart.1996.270.6.H2008. [DOI] [PubMed] [Google Scholar]
- 139.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional α-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. doi: 10.1113/jphysiol.2010.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. doi: 10.1016/S0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 144.Koch DW, Newcomer SC, Proctor DN. Blood flow to exercising limbs varies with age, gender, and training status. Can J Appl Physiol 30: 554–575, 2005. doi: 10.1139/h05-141. [DOI] [PubMed] [Google Scholar]
- 145.Kruse NT, Ueda K, Hughes WE, Casey DP. Eight weeks of nitrate supplementation improves blood flow and reduces the exaggerated pressor response during forearm exercise in peripheral artery disease. Am J Physiol Heart Circ Physiol 315: H101–H108, 2018. doi: 10.1152/ajpheart.00015.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 147.Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol 22: 316–320, 2007. doi: 10.1097/HCO.0b013e3281ca710d. [DOI] [PubMed] [Google Scholar]
- 148.Larson RA, Carter JR. Total sleep deprivation and pain perception during cold noxious stimuli in humans. Scand J Pain 13: 12–16, 2016. doi: 10.1016/j.sjpain.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 150.Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol 59, Suppl 7: 71–88, 2008. [PMC free article] [PubMed] [Google Scholar]
- 151.Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvasc Res 37: 230–236, 1989. doi: 10.1016/0026-2862(89)90040-X. [DOI] [PubMed] [Google Scholar]
- 152.Lee F, Shoemaker JK, McQuillan PM, Kunselman AR, Smith MB, Yang QX, Smith H, Gray K, Sinoway LI. Effects of forearm bier block with bretylium on the hemodynamic and metabolic responses to handgrip. Am J Physiol Heart Circ Physiol 279: H586–H593, 2000. doi: 10.1152/ajpheart.2000.279.2.H586. [DOI] [PubMed] [Google Scholar]
- 153.Lewis P, Psaila JV, Davies WT, McCarty K, Woodcock JP. Measurement of volume flow in the human common femoral artery using a duplex ultrasound system. Ultrasound Med Biol 12: 777–784, 1986. doi: 10.1016/0301-5629(86)90075-X. [DOI] [PubMed] [Google Scholar]
- 154.Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol (1985) 108: 349–355, 2010. doi: 10.1152/japplphysiol.00551.2009. [DOI] [PubMed] [Google Scholar]
- 155.Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol (1985) 109: 1360–1368, 2010. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Limberg JK, Harrell JW, Johansson RE, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Microvascular function in younger adults with obesity and metabolic syndrome: role of oxidative stress. Am J Physiol Heart Circ Physiol 305: H1230–H1237, 2013. doi: 10.1152/ajpheart.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Limberg JK, Johansson RE, Peltonen GL, Harrell JW, Kellawan JM, Eldridge MW, Sebranek JJ, Schrage WG. β-Adrenergic-mediated vasodilation in young men and women: cyclooxygenase restrains nitric oxide synthase. Am J Physiol Heart Circ Physiol 310: H756–H764, 2016. doi: 10.1152/ajpheart.00886.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Limberg JK, Malterer KR, Matzek LJ, Levine JA, Charkoudian N, Miles JM, Joyner MJ, Curry TB. Resting sympathetic activity is associated with the sympathetically mediated component of energy expenditure following a meal. Physiol Rep 5: e13389, 2017. doi: 10.14814/phy2.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Limberg JK, Ott EP, Holbein WW, Baker SE, Curry TB, Nicholson WT, Joyner MJ, Shoemaker JK. Pharmacological assessment of the contribution of the arterial baroreflex to sympathetic discharge patterns in healthy humans. J Neurophysiol 119: 2166–2175, 2018. doi: 10.1152/jn.00935.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Limberg JK, Peltonen GL, Johansson RE, Harrell JW, Kellawan JM, Eldridge MW, Sebranek JJ, Walker BJ, Schrage WG. Greater Beta-Adrenergic Receptor Mediated Vasodilation in Women Using Oral Contraceptives. Front Physiol 7: 215, 2016. doi: 10.3389/fphys.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol (1985) 85: 1622–1628, 1998. doi: 10.1152/jappl.1998.85.5.1622. [DOI] [PubMed] [Google Scholar]
- 162.Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001. doi: 10.1016/S0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 163.Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF, Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol 589: 1979–1990, 2011. doi: 10.1113/jphysiol.2011.205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martin EA, Nicholson WT, Curry TB, Eisenach JH, Charkoudian N, Joyner MJ. Adenosine transporter antagonism in humans augments vasodilator responsiveness to adenosine, but not exercise, in both adenosine responders and non-responders. J Physiol 579: 237–245, 2007. doi: 10.1113/jphysiol.2006.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol (1985) 101: 1678–1684, 2006. doi: 10.1152/japplphysiol.00546.2006. [DOI] [PubMed] [Google Scholar]
- 166.Martin WH III, Kohrt WM, Malley MT, Korte E, Stoltz S. Exercise training enhances leg vasodilatory capacity of 65-yr-old men and women. J Appl Physiol (1985) 69: 1804–1809, 1990. doi: 10.1152/jappl.1990.69.5.1804. [DOI] [PubMed] [Google Scholar]
- 167.Masuki S, Dinenno FA, Joyner MJ, Eisenach JH. Selective alpha2-adrenergic properties of dexmedetomidine over clonidine in the human forearm. J Appl Physiol (1985) 99: 587–592, 2005. doi: 10.1152/japplphysiol.00147.2005. [DOI] [PubMed] [Google Scholar]
- 168.Masuki S, Eisenach JH, Dinenno FA, Joyner MJ. Reduced forearm alpha1-adrenergic vasoconstriction is associated with enhanced heart rate fluctuations in humans. J Appl Physiol (1985) 100: 792–799, 2006. doi: 10.1152/japplphysiol.00586.2005. [DOI] [PubMed] [Google Scholar]
- 169.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mercuro G, Zoncu S, Saiu F, Sarais C, Rosano GM. Effect of atorvastatin on endothelium-dependent vasodilation in postmenopausal women with average serum cholesterol levels. Am J Cardiol 90: 747–750, 2002. doi: 10.1016/S0002-9149(02)02602-4. [DOI] [PubMed] [Google Scholar]
- 171.Millgård J, Lind L. Divergent effects of different antihypertensive drugs on endothelium-dependent vasodilation in the human forearm. J Cardiovasc Pharmacol 32: 406–412, 1998. doi: 10.1097/00005344-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 172.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004. [Erratum in: Hypertension 45: e9, 2005.] doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 173.Montero D, Walther G, Diaz-Cañestro C, Pyke KE, Padilla J. Microvascular Dilator Function in Athletes: A Systematic Review and Meta-analysis. Med Sci Sports Exerc 47: 1485–1494, 2015. doi: 10.1249/MSS.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 174.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mortensen SP, González-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol (1985) 107: 1757–1762, 2009. doi: 10.1152/japplphysiol.00638.2009. [DOI] [PubMed] [Google Scholar]
- 178.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- 179.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. doi: 10.1161/01.RES.88.2.145. [DOI] [PubMed] [Google Scholar]
- 180.Mutie PM, Giordano GN, Franks PW. Lifestyle precision medicine: the next generation in type 2 diabetes prevention? BMC Med 15: 171, 2017. doi: 10.1186/s12916-017-0938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naylor LH, Weisbrod CJ, O’Driscoll G, Green DJ. Measuring peripheral resistance and conduit arterial structure in humans using Doppler ultrasound. J Appl Physiol (1985) 98: 2311–2315, 2005. doi: 10.1152/japplphysiol.01047.2004. [DOI] [PubMed] [Google Scholar]
- 182.Nelson AD, Rossman MJ, Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS, Richardson RS. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study. J Appl Physiol (1985) 120: 991–999, 2016. doi: 10.1152/japplphysiol.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, Schmid RW, Maurer G, Stefenelli T. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J Am Coll Cardiol 39: 251–256, 2002. doi: 10.1016/S0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- 184.Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 556: 1001–1011, 2004. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- 186.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nishiyama SK, Wray DW, Richardson RS. Sex and limb-specific ischemic reperfusion and vascular reactivity. Am J Physiol Heart Circ Physiol 295: H1100–H1108, 2008. doi: 10.1152/ajpheart.00318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nishiyama SK, Zhao J, Wray DW, Richardson RS. Vascular function and endothelin-1: tipping the balance between vasodilation and vasoconstriction. J Appl Physiol (1985) 122: 354–360, 2017. doi: 10.1152/japplphysiol.00772.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nyberg M, Christensen PM, Mortensen SP, Hellsten Y, Bangsbo J. Infusion of ATP increases leg oxygen delivery but not oxygen uptake in the initial phase of intense knee-extensor exercise in humans. Exp Physiol 99: 1399–1408, 2014. doi: 10.1113/expphysiol.2014.081141. [DOI] [PubMed] [Google Scholar]
- 190.Nyberg M, Mortensen SP, Saltin B, Hellsten Y, Bangsbo J. Low blood flow at onset of moderate-intensity exercise does not limit muscle oxygen uptake. Am J Physiol Regul Integr Comp Physiol 298: R843–R848, 2010. doi: 10.1152/ajpregu.00730.2009. [DOI] [PubMed] [Google Scholar]
- 191.Nyberg M, Piil P, Kiehn OT, Maagaard C, Jørgensen TS, Egelund J, Isakson BE, Nielsen MS, Gliemann L, Hellsten Y. Probenecid Inhibits α-Adrenergic Receptor-Mediated Vasoconstriction in the Human Leg Vasculature. Hypertension 71: 151–159, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nyberg SK, Berg OK, Helgerud J, Wang E. Reliability of forearm oxygen uptake during handgrip exercise: assessment by ultrasonography and venous blood gas. Physiol Rep 6: e13696, 2018. doi: 10.14814/phy2.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.O’Driscoll G, Green D, Maiorana A, Stanton K, Colreavy F, Taylor R. Improvement in endothelial function by angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 33: 1506–1511, 1999. doi: 10.1016/S0735-1097(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 194.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 95: 1126–1131, 1997. doi: 10.1161/01.CIR.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 195.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol 260: H632–H637, 1991. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- 196.Ooue A, Ichinose TK, Inoue Y, Nishiyasu T, Koga S, Kondo N. Changes in blood flow in conduit artery and veins of the upper arm during leg exercise in humans. Eur J Appl Physiol 103: 367–373, 2008. doi: 10.1007/s00421-008-0706-x. [DOI] [PubMed] [Google Scholar]
- 197.Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 109: 2507–2510, 2004. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- 198.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. Eur J Appl Physiol 98: 256–262, 2006. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 199.Padilla J, Jenkins NT, Laughlin MH, Fadel PJ. Blood pressure regulation VIII: resistance vessel tone and implications for a pro-atherogenic conduit artery endothelial cell phenotype. Eur J Appl Physiol 114: 531–544, 2014. doi: 10.1007/s00421-013-2684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 201.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol (1985) 103: 1583–1591, 2007. doi: 10.1152/japplphysiol.00662.2007. [DOI] [PubMed] [Google Scholar]
- 202.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol (1985) 104: 655–664, 2008. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- 203.Patterson GC, Whelan RF. Reactive hyperaemia in the human forearm. Clin Sci 14: 197–211, 1955. [PubMed] [Google Scholar]
- 204.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol (1985) 92: 2105–2113, 2002. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- 205.Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F, Anderson TJ. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. Am J Cardiol 103: 1610–1615, 2009. doi: 10.1016/j.amjcard.2009.01.376. [DOI] [PubMed] [Google Scholar]
- 206.Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther 86: 527–532, 2009. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 207.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986. doi: 10.1161/01.HYP.8.1.37. [DOI] [PubMed] [Google Scholar]
- 208.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol 2: 933–996, 2012. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- 209.Proctor DN, Newcomer SC. Is there a difference in vascular reactivity of the arms and legs? Med Sci Sports Exerc 38: 1819–1828, 2006. doi: 10.1249/01.mss.0000230340.79247.52. [DOI] [PubMed] [Google Scholar]
- 210.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol (1985) 102: 1510–1519, 2007. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 211.Racine ML, Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. Inhibition of Na+/K+ -ATPase and KIR channels abolishes hypoxic hyperaemia in resting but not contracting skeletal muscle of humans. J Physiol 596: 3371–3389, 2018. doi: 10.1113/JP275913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rådegran G. Limb and skeletal muscle blood flow measurements at rest and during exercise in human subjects. Proc Nutr Soc 58: 887–898, 1999. doi: 10.1017/S0029665199001196. [DOI] [PubMed] [Google Scholar]
- 213.Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol (1985) 83: 1383–1388, 1997. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- 214.Rådegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 171: 177–185, 2001. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- 215.Rådegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol 278: H162–H167, 2000. doi: 10.1152/ajpheart.2000.278.1.H162. [DOI] [PubMed] [Google Scholar]
- 216.Rådegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol 274: H314–H322, 1998. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- 217.Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol 276: H1951–H1960, 1999. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- 218.Ranadive SM, Eugene AR, Dillon G, Nicholson WT, Joyner MJ. Comparison of the vasodilatory effects of sodium nitroprusside vs. nitroglycerin. J Appl Physiol (1985) 123: 402–406, 2017. doi: 10.1152/japplphysiol.00167.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Richards JC, Crecelius AR, Larson DG, Luckasen GJ, Dinenno FA. Impaired peripheral vasodilation during graded systemic hypoxia in healthy older adults: role of the sympathoadrenal system. Am J Physiol Heart Circ Physiol 312: H832–H841, 2017. doi: 10.1152/ajpheart.00794.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 75: 1911–1916, 1993. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- 223.Richardson RS, Secher NH, Tschakovsky ME, Proctor DN, Wray DW. Metabolic and vascular limb differences affected by exercise, gender, age, and disease. Med Sci Sports Exerc 38: 1792–1796, 2006. doi: 10.1249/01.mss.0000229568.17284.ab. [DOI] [PubMed] [Google Scholar]
- 224.Rodriguez-Miguelez P, Seigler N, Harris RA. Ultrasound assessment of endothelial function: a technical guideline of the flow-mediated dilation test. J Vis Exp (110): 2016. doi: 10.3791/54011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rosenberry R, Munson M, Chung S, Samuel TJ, Patik J, Tucker WJ, Haykowsky MJ, Nelson MD. Age-related microvascular dysfunction: novel insight from near-infrared spectroscopy. Exp Physiol 103: 190–200, 2018. doi: 10.1113/EP086639. [DOI] [PubMed] [Google Scholar]
- 226.Rosenberry R, Trojacek D, Chung S, Cipher DJ, Nelson MD. Interindividual differences in the ischemic stimulus and other technical considerations when assessing reactive hyperemia. Am J Physiol Regul Integr Comp Physiol 317: R530–R538, 2019. doi: 10.1152/ajpregu.00157.2019. [DOI] [PubMed] [Google Scholar]
- 227.Rossman MJ, Groot HJ, Garten RS, Witman MA, Richardson RS. Vascular function assessed by passive leg movement and flow-mediated dilation: initial evidence of construct validity. Am J Physiol Heart Circ Physiol 311: H1277–H1286, 2016. doi: 10.1152/ajpheart.00421.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 372: 1333–1341, 2015. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rowley NJ, Dawson EA, Birk GK, Cable NT, George K, Whyte G, Thijssen DH, Green DJ. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol (1985) 110: 1190–1195, 2011. doi: 10.1152/japplphysiol.01371.2010. [DOI] [PubMed] [Google Scholar]
- 230.Rowley NJ, Dawson EA, Hopman MT, George KP, Whyte GP, Thijssen DH, Green DJ. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc 44: 844–849, 2012. doi: 10.1249/MSS.0b013e31823f6887. [DOI] [PubMed] [Google Scholar]
- 231.Saltin B, Mortensen SP. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol 590: 6269–6275, 2012. doi: 10.1113/jphysiol.2012.241026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- 233.Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 9: 187–208, 2017. doi: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Saunders NR, Pyke KE, Tschakovsky ME. Dynamic response characteristics of local muscle blood flow regulatory mechanisms in human forearm exercise. J Appl Physiol (1985) 98: 1286–1296, 2005. doi: 10.1152/japplphysiol.01118.2004. [DOI] [PubMed] [Google Scholar]
- 235.Saunders NR, Tschakovsky ME. Evidence for a rapid vasodilatory contribution to immediate hyperemia in rest-to-mild and mild-to-moderate forearm exercise transitions in humans. J Appl Physiol (1985) 97: 1143–1151, 2004. doi: 10.1152/japplphysiol.01284.2003. [DOI] [PubMed] [Google Scholar]
- 236.Savulescu J, Spriggs M. The hexamethonium asthma study and the death of a normal volunteer in research. J Med Ethics 28: 3–4, 2002. doi: 10.1136/jme.28.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schmitt JA, Joyner MJ, Charkoudian N, Wallin BG, Hart EC. Sex differences in alpha-adrenergic support of blood pressure. Clin Auton Res 20: 271–275, 2010. doi: 10.1007/s10286-010-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schrage WG, Dietz NM, Joyner MJ. Effects of combined inhibition of ATP-sensitive potassium channels, nitric oxide, and prostaglandins on hyperemia during moderate exercise. J Appl Physiol (1985) 100: 1506–1512, 2006. doi: 10.1152/japplphysiol.01639.2005. [DOI] [PubMed] [Google Scholar]
- 239.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol (1985) 109: 768–777, 2010. doi: 10.1152/japplphysiol.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Segal SS, Duling BR. Communication between feed arteries and microvessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circ Res 59: 283–290, 1986. doi: 10.1161/01.RES.59.3.283. [DOI] [PubMed] [Google Scholar]
- 242.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol 39: 368–376, 1953. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 243.Shaw JA, Chin-Dusting JP, Kingwell BA, Dart AM. Diurnal variation in endothelium-dependent vasodilatation is not apparent in coronary artery disease. Circulation 103: 806–812, 2001. doi: 10.1161/01.CIR.103.6.806. [DOI] [PubMed] [Google Scholar]
- 244.Shoemaker JK, Hughson RL. Adaptation of blood flow during the rest to work transition in humans. Med Sci Sports Exerc 31: 1019–1026, 1999. doi: 10.1097/00005768-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 245.Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovasc Res 35: 125–131, 1997. doi: 10.1016/S0008-6363(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 246.Shoemaker JK, McQuillan PM, Sinoway LI. Upright posture reduces forearm blood flow early in exercise. Am J Physiol 276: R1434–R1442, 1999. [DOI] [PubMed] [Google Scholar]
- 247.Shoemaker JK, Pozeg ZI, Hughson RL. Forearm blood flow by Doppler ultrasound during test and exercise: tests of day-to-day repeatability. Med Sci Sports Exerc 28: 1144–1149, 1996. doi: 10.1097/00005768-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 248.Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol 76: 418–427, 1998. doi: 10.1139/y98-033. [DOI] [PubMed] [Google Scholar]
- 249.Silber D, McLaughlin D, Sinoway L. Leg exercise conditioning increases peak forearm blood flow. J Appl Physiol (1985) 71: 1568–1573, 1991. doi: 10.1152/jappl.1991.71.4.1568. [DOI] [PubMed] [Google Scholar]
- 250.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol (1985) 110: 389–397, 2011. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sims ST, Heather AK. Myths and Methodologies: Reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol 103: 1309–1317, 2018. doi: 10.1113/EP086797. [DOI] [PubMed] [Google Scholar]
- 252.Sinoway LI, Musch TI, Minotti JR, Zelis R. Enhanced maximal metabolic vasodilatation in the dominant forearms of tennis players. J Appl Physiol (1985) 61: 673–678, 1986. doi: 10.1152/jappl.1986.61.2.673. [DOI] [PubMed] [Google Scholar]
- 253.Sinoway LI, Shenberger J, Wilson J, McLaughlin D, Musch T, Zelis R. A 30-day forearm work protocol increases maximal forearm blood flow. J Appl Physiol (1985) 62: 1063–1067, 1987. doi: 10.1152/jappl.1987.62.3.1063. [DOI] [PubMed] [Google Scholar]
- 254.Sjøberg KA, Frøsig C, Kjøbsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA, McConell GK. Exercise Increases Human Skeletal Muscle Insulin Sensitivity via Coordinated Increases in Microvascular Perfusion and Molecular Signaling. Diabetes 66: 1501–1510, 2017. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 255.Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol (1985) 62: 606–610, 1987. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- 256.Soares RN, George MA, Proctor DN, Murias JM. Differences in vascular function between trained and untrained limbs assessed by near-infrared spectroscopy. Eur J Appl Physiol 118: 2241–2248, 2018. doi: 10.1007/s00421-018-3955-3. [DOI] [PubMed] [Google Scholar]
- 257.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90: 2285–2290, 1994. doi: 10.1161/01.CIR.90.5.2285. [DOI] [PubMed] [Google Scholar]
- 258.Takeshita A, Mark AL. Decreased vasodilator capacity of forearm resistance vessels in borderline hypertension. Hypertension 2: 610–616, 1980. doi: 10.1161/01.HYP.2.5.610. [DOI] [PubMed] [Google Scholar]
- 259.Taylor JL, Hines CN, Nicholson WT, Joyner MJ, Barnes JN. The effect of ageing and indomethacin on forearm reactive hyperaemia in healthy adults. Exp Physiol 99: 859–867, 2014. doi: 10.1113/expphysiol.2014.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Teixeira AL, Daher M, Souza MC, Ramos PS, Fisher JP, Vianna LC. Sympathetically mediated cardiac responses to isolated muscle metaboreflex activation following exercise are modulated by body position in humans. Am J Physiol Heart Circ Physiol 314: H593–H602, 2018. [DOI] [PubMed] [Google Scholar]
- 261.Tesselaar E, Nezirevic Dernroth D, Farnebo S. Acute effects of coffee on skin blood flow and microvascular function. Microvasc Res 114: 58–64, 2017. doi: 10.1016/j.mvr.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 262.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 264.Thijssen DH, Dawson EA, van den Munckhof IC, Birk GK, Timothy Cable N, Green DJ. Local and systemic effects of leg cycling training on arterial wall thickness in healthy humans. Atherosclerosis 229: 282–286, 2013. [Erratum in: Atherosclerosis 232: 259, 2014.] doi: 10.1016/j.atherosclerosis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 265.Thijssen DH, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol (1985) 111: 244–250, 2011. doi: 10.1152/japplphysiol.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Thijssen DH, Schreuder TH, Newcomer SW, Laughlin MH, Hopman MT, Green DJ. Impact of 2-Weeks Continuous Increase in Retrograde Shear Stress on Brachial Artery Vasomotor Function in Young and Older Men. J Am Heart Assoc 4: e001968, 2015. doi: 10.1161/JAHA.115.001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 268.Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008. doi: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 271.Title LM, Lonn E, Charbonneau F, Fung M, Mather KJ, Verma S, Anderson TJ. Relationship between brachial artery flow-mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Vasc Med 13: 263–270, 2008. doi: 10.1177/1358863X08095154. [DOI] [PubMed] [Google Scholar]
- 272.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol 292: H2874–H2880, 2007. doi: 10.1152/ajpheart.00762.2006. [DOI] [PubMed] [Google Scholar]
- 273.Tremblay JC, Pyke KE. Flow-mediated dilation stimulated by sustained increases in shear stress: a useful tool for assessing endothelial function in humans? Am J Physiol Heart Circ Physiol 314: H508–H520, 2018. doi: 10.1152/ajpheart.00534.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Trinity JD, Broxterman RM, Richardson RS. Regulation of exercise blood flow: Role of free radicals. Free Radic Biol Med 98: 90–102, 2016. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe A, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O’Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011. doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Trinity JD, Richardson RS. Physiological impact and clinical relevance of passive exercise/movement. 49: 1365–1381, 2019. doi: 10.1007/s40279-019-01146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol (1985) 96: 639–644, 2004. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- 281.Tschakovsky ME, Saunders NR, Webb KA, O’Donnell DE. Muscle blood-flow dynamics at exercise onset: do the limbs differ? Med Sci Sports Exerc 38: 1811–1818, 2006. doi: 10.1249/01.mss.0000230341.86870.4f. [DOI] [PubMed] [Google Scholar]
- 282.Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol (1985) 97: 739–747, 2004. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- 283.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Umemura T, Ueda K, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Jitsuiki D, Soga J, Goto C, Chayama K, Yoshizumi M, Higashi Y. Effects of acute administration of caffeine on vascular function. Am J Cardiol 98: 1538–1541, 2006. doi: 10.1016/j.amjcard.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 285.Vranish JR, Young BE, Stephens BY, Kaur J, Padilla J, Fadel PJ. Brief periods of inactivity reduce leg microvascular, but not macrovascular, function in healthy young men. Exp Physiol 103: 1425–1434, 2018. doi: 10.1113/EP086918. [DOI] [PubMed] [Google Scholar]
- 286.Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, Kaur A, Friedemann Smith C, Wilkins E, Rayner M, Roberts N, Scarborough P. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 5: e002495, 2016. doi: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 60: 1517–1523, 2012. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Walker KL, Saunders NR, Jensen D, Kuk JL, Wong SL, Pyke KE, Dwyer EM, Tschakovsky ME. Do vasoregulatory mechanisms in exercising human muscle compensate for changes in arterial perfusion pressure? Am J Physiol Heart Circ Physiol 293: H2928–H2936, 2007. doi: 10.1152/ajpheart.00576.2007. [DOI] [PubMed] [Google Scholar]
- 289.Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y, Askew CD. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 246: 98–105, 2016. doi: 10.1016/j.atherosclerosis.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 290.Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature (Austin) 3: 92–103, 2016. doi: 10.1080/23328940.2015.1088502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Walløe L, Wesche J. Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol 405: 257–273, 1988. doi: 10.1113/jphysiol.1988.sp017332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Walsh LK, Ghiarone T, Olver TD, Medina-Hernandez A, Edwards JC, Thorne PK, Emter CA, Lindner JR, Manrique-Acevedo C, Martinez-Lemus LA, Padilla J. Increased endothelial shear stress improves insulin-stimulated vasodilatation in skeletal muscle. J Physiol 597: 57–69, 2019. doi: 10.1113/JP277050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Walsh LK, Restaino RM, Martinez-Lemus LA, Padilla J. Prolonged leg bending impairs endothelial function in the popliteal artery. Physiol Rep 5: e13478, 2017. doi: 10.14814/phy2.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wesche J. The time course and magnitude of blood flow changes in the human quadriceps muscles following isometric contraction. J Physiol 377: 445–462, 1986. doi: 10.1113/jphysiol.1986.sp016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wilkins BW, Hesse C, Sviggum HP, Nicholson WT, Moyer TP, Joyner MJ, Eisenach JH. Alternative to ganglionic blockade with anticholinergic and alpha-2 receptor agents. Clin Auton Res 17: 77–84, 2007. doi: 10.1007/s10286-006-0387-7. [DOI] [PubMed] [Google Scholar]
- 297.Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178: 232–238, 2015. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol (1985) 91: 929–937, 2001. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 300.Wou C, Unwin N, Huang Y, Roglic G. Implications of the growing burden of diabetes for premature cardiovascular disease mortality and the attainment of the Sustainable Development Goal target 3.4. Cardiovasc Diagn Ther 9: 140–149, 2019. doi: 10.21037/cdt.2018.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wray DW, Donato AJ, Nishiyama SK, Richardson RS. Acute sympathetic vasoconstriction at rest and during dynamic exercise in cyclists and sedentary humans. J Appl Physiol (1985) 102: 704–712, 2007. doi: 10.1152/japplphysiol.00984.2006. [DOI] [PubMed] [Google Scholar]
- 302.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555: 545–563, 2004. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007. doi: 10.1152/ajpheart.00867.2007. [DOI] [PubMed] [Google Scholar]
- 305.Wray DW, Nishiyama SK, Richardson RS. Role of alpha1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009. doi: 10.1152/ajpheart.01016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Young BE, Holwerda SW, Vranish JR, Keller DM, Fadel PJ. Sympathetic Transduction in Type 2 Diabetes Mellitus. Hypertension 74: 201–207, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]