Abstract
Rodent models are frequently employed in cardiovascular research, yet our understanding of pediatric cardiac physiology has largely been deduced from more simplified two-dimensional cell studies. Previous studies have shown that postnatal development includes an alteration in the expression of genes and proteins involved in cell coupling, ion channels, and intracellular calcium handling. Accordingly, we hypothesized that postnatal cell maturation is likely to lead to dynamic alterations in whole heart electrophysiology and calcium handling. To test this hypothesis, we employed multiparametric imaging and electrophysiological techniques to quantify developmental changes from neonate to adult. In vivo electrocardiograms were collected to assess changes in heart rate, variability, and atrioventricular conduction (Sprague-Dawley rats). Intact, whole hearts were transferred to a Langendorff-perfusion system for multiparametric imaging (voltage, calcium). Optical mapping was performed in conjunction with an electrophysiology study to assess cardiac dynamics throughout development. Postnatal age was associated with an increase in the heart rate (181 ± 34 vs. 429 ± 13 beats/min), faster atrioventricular conduction (94 ± 13 vs. 46 ± 3 ms), shortened action potentials (APD80: 113 ± 18 vs. 60 ± 17 ms), and decreased ventricular refractoriness (VERP: 157 ± 45 vs. 57 ± 14 ms; neonatal vs. adults, means ± SD, P < 0.05). Calcium handling matured with development, resulting in shortened calcium transient durations (168 ± 18 vs. 117 ± 14 ms) and decreased propensity for calcium transient alternans (160 ± 18- vs. 99 ± 11-ms cycle length threshold; neonatal vs. adults, mean ± SD, P < 0.05). Results of this study can serve as a comprehensive baseline for future studies focused on pediatric disease modeling and/or preclinical testing.
NEW & NOTEWORTHY This is the first study to assess cardiac electrophysiology and calcium handling throughout postnatal development, using both in vivo and whole heart models.
Keywords: calcium handling, cardiac maturation, electrophysiology, development, optical mapping
INTRODUCTION
Cardiovascular research studies focused on disease modeling or pharmacological safety assessment often employ small rodent animal models, as the rodent and human heart follow a similar sequence of structural cardiac development and electrophysiological patterning (27, 34, 54). In both species, the mammalian heart begins as a mesoderm-derived tube with slow electrical conduction, an underdeveloped sarcoplasmic reticulum, and limited contractile force (18, 54). In addition, in both species, the heart continues to develop postnatally, not reaching maturity in small rodents until weeks, or in humans until years, after birth (55). Indeed, age-dependent changes in voltage-gated potassium (17, 57, 58), calcium (56, 61), and sodium channel (6) current have been described in a variety of mammalian cell models. Additionally, immature mammalian cardiomyocytes have underdeveloped intercalated disks, which can lead to reduced intercellular coupling and slowed electrical conduction (55). Moreover, neonatal cardiomyocytes lack fully developed transverse tubules and sarcoplasmic reticulum (66), which influences calcium cycling (4, 13, 56) and contractile function (32, 41). Because of the extended period of postnatal maturation, the juvenile mammalian heart is uniquely dynamic and often considered a “moving target” (39). As such, there are considerable gaps in our understanding of normal cardiac physiology throughout the postnatal developmental period. These gaps in knowledge can have unintended consequences, particularly when pharmacological therapies, toxicological studies, or disease models are designed and tested using only adult animals.
The objective of this study was to evaluate age-dependent changes in cardiac electrophysiology and calcium handling throughout postnatal development. To date, cardiac developmental studies have largely been limited to rodent cell models (2). However, the results gleaned from these models may not fully translate to a whole heart with specialized anatomy, cell populations (atrial, nodal, and ventricular), spatial tissue heterogeneity, and a coordinated conduction system. Therefore, we used in vivo and ex vivo (whole heart) models to fully characterize temporal changes in rat cardiac electrophysiology and calcium handling. The described study used both an electrophysiology study (EP) and simultaneous, dual-optical mapping of transmembrane voltage and intracellular calcium using parameter sensitive dyes.
METHODS
Animal protocols were approved by the Institutional Animal Care and Use Committee at Children’s Research Institute and followed the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Experiments were performed using Sprague-Dawley rats from postnatal day (PND) 0 to adulthood (2–3 mo) from Taconic Biosciences (n = 146). Animals were housed in conventional acrylic rat cages in the Research Animal Facility, under standard environmental conditions (12-h:12-h light-dark cycle, 18–25°C, 30–70% humidity, free access to reverse osmosis water, corn cob bedding, and no. 2918 rodent chow, Envigo).
Gene expression analysis.
Age-dependent changes in gene and protein expression have previously been characterized (51). To confirm similar changes in cardiac maturation, total RNA was isolated from ventricular tissue using a RNeasy fibrous tissue kit (Qiagen). RNA was reverse transcribed using a SuperScript VILO cDNA kit (Thermo Scientific), and Taqman gene expression assays (Thermo Scientific) were used for quantitative real-time PCR (qPCR) analysis via a Quantstudio 7 platform (Applied Biosystems). Relative gene expression was assessed using the comparative CT method, or ΔΔCT, whereby ΔΔCT values were converted into ratios by 2−ΔΔCT and averaged across replicates. Data normalization was tested using two reference genes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein L4 (RPL4) (5). Neither reference gene exhibited differential expression in the qRT-PCR assay (data not shown), and as such, RPL4 was used for additional analysis. Fold change values are reported (average of technical replicates), with each assay including a minimum of three individual biological replicates. Data are presented as means ± SD. Statistical analysis was performed using two-way ANOVA, with a false discovery rate (0.1) to correct for multiple comparisons testing (GraphPad Prism). Significance was defined as P ≤ 0.05.
Based on previous cardiomyocyte maturation studies (44, 51, 65), genes of interest included myosin heavy chain 7 (MYH7), myosin heavy chain 6 (MYH6), gap junction protein-α1 (GJA1), desmoplakin (DSP), junctophilin 2 (JPH2), junction plakoglobin (JUP), n-cadherin (CDH2), caveolin-3 (CAV3), tight junction protein 1 (TJP1), SERCA Ca2+-ATPase (SERCA), calsequestrin 2 (CASQ2), ryanodine receptor 2 (RYR2), sodium-calcium exchanger 1 (NCX), voltage-dependent T-type calcium channel α-1G (CACNA1G), voltage-dependent L-type calcium channel subunit α-1C (CACNA1C), cardiac troponin I (TNNI3), tropomyosin 1 (TPM1), voltage-gated sodium channel subunit a5 (SCN5A), voltage-gated potassium channel subunit Kv4.2 (KCND2), voltage-gated potassium channel subunit Kv1.5 (KCNA5), voltage-gated potassium channel subunit Kv4.3 (KCND3), and cardiac inward rectifier potassium channel (KCNJ2).
Protein expression analysis.
Ventricular tissue was isolated and snap frozen, and protein was isolated using T-PER extraction reagent (Thermo Fisher) containing protease and phosphatase inhibitors (Halt Cocktail, Thermo Fisher). Protein concentration was determined via BCA assay (Thermo Fisher), with 10 μg total protein loaded into 4–20% Tris-Glycine gels (Bio-Rad) and transferred to PVDF membrane (Millipore Sigma). A total protein stain was used for protein expression normalization (Li-Cor). Primary antibodies included connexin-43 (1:2,000, Sigma-Aldrich no. C6219), N-cadherin (1:1,000, Abcam no. ab12221), GAPDH (1:1,000, Thermo Fisher no. MA5-15738), SERCA2A (1:500, Thermo Fisher no. MA3-919), and MYH6 (1:1,000, Proteintech no. 222811-AP); and secondary antibodies included anti-rabbit near-IR 680 (Li-Cor no. 925-68071) and anti-mouse near-IR 800 (Li-Cor no. 925-32210).
In vivo surface electrocardiogram recordings.
In vivo electrocardiograms (ECG) were collected from conscious animals using an ecgTUNNEL system (emka Technologies). The platform electrodes were coated with ultrasound gel before the animal was placed in the system. A clear half-tunnel was carefully positioned over the top of older animals (>PND 6), to limit movement, which can introduce noise in the signal. The animals were acclimated to the platform for 5 min, and biosignals were continuously acquired for 2 min using iox2 (emka Technologies). ECG signals were collected to analyze heart rate, atrial depolarization, atrioventricular conduction (PR interval), ventricular depolarization time (QRS width), and heart rate variability, including root means successive square difference (rMSSD) and standard deviation of the normal heart rate (SDNN). ECG parameters were computed in ecgAUTO (emka Technologies). In a subset of studies, an intraperitoneal injection of isoproterenol (5 mg/kg) was administered to assess cardiovascular response to β-adrenergic stimulation in unconscious animals. In the latter, a baseline was acquired (5 min), isoproterenol was administered intraperitoneally, and electrocardiograms were collected continuously thereafter (15–25 min). A 5-min period was averaged at the peak heart rate as an estimate of adrenergic response.
Isolated heart preparation and electrophysiology measurements.
Animals were anesthetized with 3% isoflurane; the heart was rapidly excised, and the aorta was cannulated. The heart was then transferred to a temperature-controlled (37°C) constant-pressure (70 mmHg) Langendorff-perfusion system (29). Excised hearts were perfused with Krebs-Henseleit buffer bubbled with 5% CO2-95% oxygen throughout the duration of the experiment, as previously described (~1 h) (24). Electrocardiograms were recorded throughout the duration of the experiment in a lead II configuration. ECG parameters were acquired in iox2 and analyzed in ecgAUTO.
An intact, whole heart EP study was performed by positioning a stimulus electrode on the right atria and a second electrode on the left ventricle’s epicardium, as previously described (24). A Bloom Classic electrophysiology stimulator (Fisher Medical) was set at a pacing current 1.5 times the minimum pacing threshold (1–2 mA) with a 1-ms monophasic pulse width. For each parameter, the pacing cycle length (PCL) was first decremented stepwise by 10 ms (250–50 ms) and then 1-ms intervals to pinpoint the PCL before loss of capture was observed. An S1-S1 pacing interval was applied to the right atria to determine the Wenckebach cycle length (WBCL), or shortest S1-S1 interval that resulted in 1:1 atrioventricular conduction. Similarly, an S1-S2 pacing interval was applied to the right atria to pinpoint the atrioventricular nodal refractory period (AVNERP). A S1-S2 pacing interval was applied to the left ventricle to find the shortest coupling interval that resulted in 1:1 ventricular depolarization, signifying the ventricular effective refractory period (VERP). In a subset of studies, cardiovascular response to β-adrenergic stimulation was assessed by delivering isoproterenol via syringe pump to achieve a final concentration of 10 μM. Cardiac EP parameters and coronary flow rate were compared before (baseline) and after isoproterenol application.
Optical mapping.
To reduce motion during imaging experiments, the heart was perfused with Krebs-Henseleit buffer supplemented with 10 μM (−/−) blebbistatin (Sigma-Aldrich) (14, 50). Epicardial imaging was performed by sequentially loading the heart with parameter-sensitive dyes. A calcium indicator dye (50 μg Rhod2) was added and allowed to stabilize for 10 min, followed by a potentiometric dye (62.1 μg RH237). The epicardium was illuminated with an LED light (525 nm, 1.4 mW/mm2, Thor Laboratories), fitted with an optical filter (535 ± 25 nm, Chroma Technologies). Fluorescence signals were acquired using an image splitting device (Optosplit II, Cairn Research, Ltd.) positioned in front of a sCMOS camera (Zyla 4.2Plus, Andor Technologies). The path splitter was configured with a dichroic mirror (660+ nm, Chroma Technologies) that passed RH237 emission and reflected Rhod-2 fluorescence. RH237 fluorescence was longpass filtered (ET710, Chroma Technologies), and Rhod-2 was bandpass filtered (ET585 ± 40 nm, Chroma Technologies) (23). A fixed focal length 17mm/F0.95 lens was attached to the image splitting device (Schneider no. 21–010456). MetaMorph (Molecular Devices, LLC) was used for optosplit image alignment and LED on/off triggering. Transmembrane voltage and calcium signals were acquired simultaneously at 800 frames/s. Dynamic ventricular pacing was applied, and images were acquired during each PCL (250–50 ms, S1-S1).
Signal processing.
Following image acquisition, signal processing and data analysis were performed using a custom MATLAB script, as previously described (23–25). A region of interest (ROI) was selected in identical locations on the split image of the heart for each raw image. The signals from each ROI (<2 × 2 mm) corresponding to transmembrane voltage and calcium transients, were spatially filtered (uniform kernel) and plotted against time. For myocardial tissue, a kernel size <3 × 3 mm is appropriate for spatial filtering in transient analysis, as previously described in detail by Mironov et. al. (35) and applied by Lee et. al (30). A peak detector algorithm was applied, and characteristics of each waveform were measured and averaged, including action potential duration at 30% (APD30) and 80% (APD80) repolarization, APD triangulation (APD80-APD30) (21), and calcium transient duration at 30% (CaD30) and 80% (CaD80) reuptake. Calcium transient alternans were defined as sequential calcium transient measurements that differed by >5%.
Statistical analysis.
Data are presented as means ± SD. Statistical analysis was performed using one or two-way ANOVA, with a false discovery rate (0.1) to correct for multiple comparisons testing (GraphPad Prism). Significance was defined as P ≤ 0.05. Optical signals were analyzed using custom algorithms (MATLAB). Optical signals were collected for 2 s per PCL, resulting in 8–25 signals per group.
RESULTS
Characterization of postnatal cardiac maturation and electrophysiology in vivo.
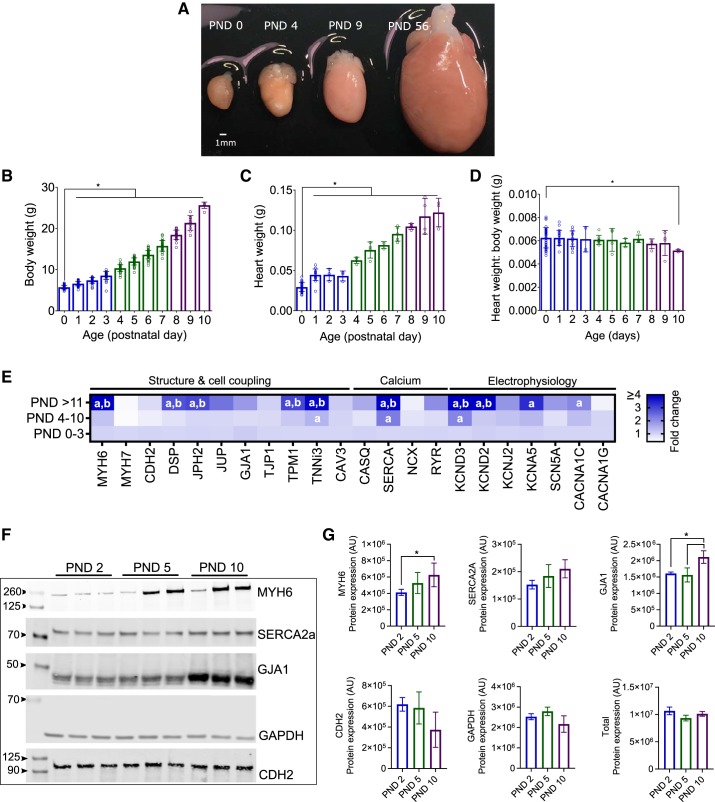
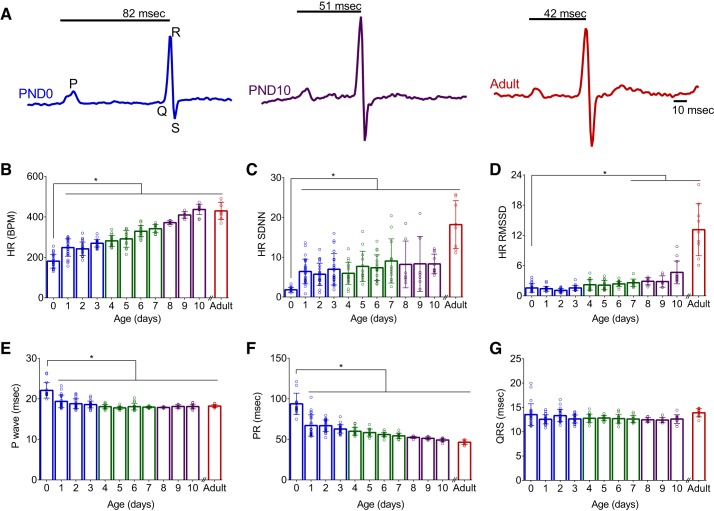
Age-dependent changes in gene and protein expression have previously been characterized (44, 51, 65). Therefore, we first aimed to confirm similar changes in rodent cardiac maturation by measuring ion channel, calcium handling, and cell structure gene expression. As the postnatal heart exhibits limited proliferative potential, cardiac development transitions from hyperplasia to hypertrophic growth shortly after birth (Fig. 1) (31). The functional and morphological changes during this progression occur in conjunction with alterations in electrical activity and calcium handling. In rodents, postnatal heart development is associated with a shift in myosin heavy chain expression (MYH7 to MYH6) (33). Compared with immature hearts (PND 0–3), we observed a 4.9-fold increase in MYH6 expression in older hearts (PND ≥11, P < 0.0001; Fig. 1E. In rodents, a shift in myosin heavy chain isoform expression is coupled to a change in heart rate, as MYH6 kinetics are three times faster than MYH7 (16). As cardiac development progressed, we observed a temporal change in MYH expression that corresponded with a linear increase in the in vivo resting heart rate from 181 ± 34 vs. 429 ± 13 beats/min (PND 0 vs. adult, P < 0.0001; Fig. 2B). No significant difference in resting heart rate was observed in animals older than PND 10. Autonomic regulation of heart rate increased postnatally (43) and was quantified via time-domain indices of heart rate variability. Compared with the earliest age (PND 0), standard deviation of heart rate (SDNN) increased by 357 and 901% in older animals (PND 10 and adult, respectively, P < 0.0001) and the root means successive square difference (rMSSD) increased by 197 and 737% in older animals (PND 10 and adult, respectively, P < 0.0001; Fig. 2, C and D).
Fig. 1.
Postnatal development and cardiac maturation A: isolated whole hearts with age denoted. PND, postnatal day. B–D: age-dependent increase in body weight (B), heart weight (C), and slight decrease in the heart weight to body weight ratio (D). *P < 0.05 compared with youngest age (PND 0). E: developmental time course corresponds to shift in myosin heavy chain gene expression and increased expression of key genes involved in intercellular coupling, cardiac electrophysiology, and calcium handling. Data are expressed as a fold change relative to the youngest age group (PND 0–3), normalized to ribosomal protein L4 (RPL4) housekeeping gene. Increased expression: darker shades of blue (>1-fold); decreased expression: white (<1-fold). aGene expression significantly different from PND 0–3 group. bSignificantly different from PND 4–10. F: representative immunoblots demonstrate an age-dependent increase in myosin heavy chain 6 (MYH6) and gap junction protein-α1 (GJA1) protein expression. G: average protein expression data, normalized to total protein staining. AU, arbitrary units. Significance was determined by one- or two-way ANOVA with 0.1 false discovery rate. Data are means ± SD; n ≥ 3 independent experiments. *P < 0.05.
Fig. 2.
Age-dependent alterations in in vivo electrocardiogram parameters. A: example of noninvasive electrocardiogram waveforms recorded from postnatal day 0 (PND 0), 10 (PND 10), and adult animal; PR interval time is denoted. B–F: postnatal development was associated with an age-dependent increase in heart rate (HR; B), heart rate variability (C and D), shortening of P-wave duration (E), and shortening of the PR interval (F). G: no significant difference in the QRS interval time was observed using noninvasive recordings. SDNN, standard deviation of the normal heart rate; RMSSD, root means successive square difference. *P < 0.05, statistically significant difference from earliest measured time point (PND 0). Data are means ± SD; n ≥ 7 animals per age.
Along with an age-dependent increase in heart rate, we observed a progressive shortening of in vivo electrocardiogram parameters during resting sinus rhythm. Noninvasive electrocardiogram recordings in a cohort of neonatal rats showed atrial conduction (P-wave duration) decreased from 22.1 ± 1.9 (PND 0) to 18.1 ± 0.6 ms (PND 10, P < 0.0001), and atrioventricular conduction time (PR interval) shortened from 93.8 ± 13.2 (PND 0) to 49.2 ± 2.1 ms (PND 10, P < 0.0001; Fig. 2, A, E, and F). An age-dependent trend in ventricular depolarization time (QRS interval) was not observed (Fig. 2G). Age-dependent shortening of atrial and atrioventricular conduction can be partly attributed to remodeling of cardiomyocyte size (49) and more defined intercellular connections (55), which have both been shown to enhance electrical propagation. Compared with immature hearts (PND 0–3), older hearts (PND ≥11) had an increased expression of cell coupling genes encoding desmosomal proteins (2.8-fold DSP, 3-fold JPH2), as well as an increase in L-type calcium channel (2.1-fold CACNA1C) and multiple potassium channels (5.7-fold KCND3, 6.9-fold KCND2, and 3.3-fold KCNA5; Fig. 1E).
Assessment of cardiac electrophysiology ex vivo.
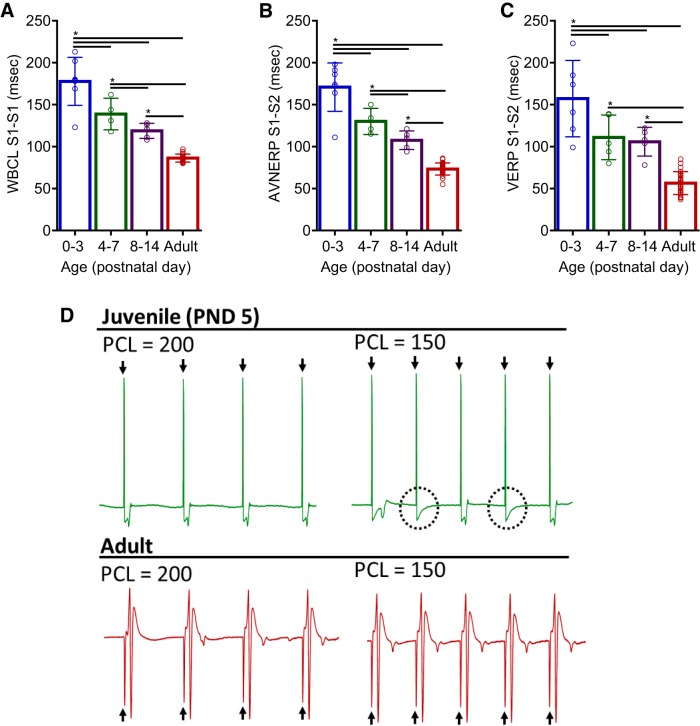
To examine the effects of postnatal maturation on cardiac electrophysiology in the absence of autonomic regulation, an EP study was performed on isolated Langendorff-perfused hearts (Fig. 3, A–D). In agreement with in vivo PR interval (ECG) measurements, immature hearts displayed slower atrioventricular conduction compared with adults. WBCL shortened progressively during development, from 178 ± 29 (PND 0–3) to 86 ± 5 ms in adult hearts, P < 0.0001 (Fig. 3A). In addition, an age-dependent shortening of AV node refractoriness (AVNERP) was observed, decreasing from 171 ± 29 (PND 0–3) to 73 ± 7 ms in adult hearts, (P < 0.0001; Fig. 3B). Both in vivo and ex vivo measurements indicate that atrioventricular conduction time shortens with cardiac development, in the presence or absence of autonomic influences. Additionally, an epicardial pacing protocol was performed to measure the ventricular effective refractory period (VERP), an indicator of repolarization. An age-dependent shortening of VERP was observed, decreasing from 157 ± 45 in younger hearts (PND 0–3) to 57 ± 14 ms in adult hearts (P < 0.0001; Fig. 3, C and D).
Fig. 3.
Cardiac electrophysiology assessment ex vivo. A–C: age-dependent shortening of Wenckebach cycle length (WBCL; A), as determined by S1-S1 atrial pacing, atrioventricular nodal effective refractory period atrioventricular nodal refractory period (AVNERP; B), as determined by S1-S2 atrial pacing, and ventricular effective refractory period (VERP; C), as determined by S1-S2 ventricular pacing. D: excised hearts were paced near the apex at 2 pacing cycle lengths (PCL = 200 and 150 ms) and electrocardiograms were recorded. Pacing spikes denoted with arrows. Juvenile heart displays a loss of capture (circled) at a shorter cycle length, compared with adult heart that shows ventricular response to each pacing spike, at both pacing frequencies. Data were binned into the following age groups: PND 0–3, PND 4–7, PND 8–14, and adult (2–3 mo). Data are means ± SD; n ≥ 5 animals per age. *P < 0.05.
Age-dependent alterations in cardiac action potential morphology.
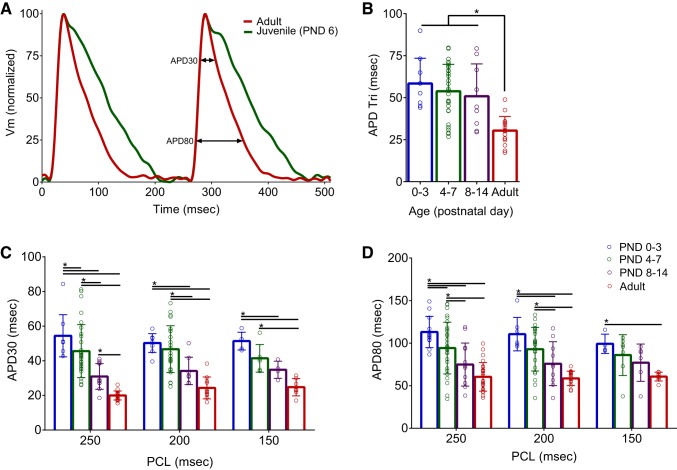
Results of our EP study indicate that juvenile hearts have increased ventricular refractoriness compared with adults. This is likely due to an increase in potassium channel expression in adult myocytes (compared with neonatal cells), which expedites repolarization time and shortens the action potential duration (APD) time (12, 57, 61). As previously noted, we observed an age-dependent increase in potassium channel gene expression during postnatal cardiac maturation. This included a 6.9-fold increase in KCND2 and 5.7-fold increase in KCND3 gene expression (PND 0–3 vs. >11 hearts), which contribute to the outward It0 (Fig. 1E). Importantly, It0 is responsible for the rapid repolarization and lack of a plateau phase in the adult rodent action potential (26, 58).
To evaluate action potential shape and duration in the whole heart, transmembrane voltage signals were recorded from the epicardial surface of isolated, Langendorff-perfused hearts. Optical signals acquired from juvenile hearts were binned into three age groups (PND 0–3, PND 4–7, and PND 8–14) and compared with adults. Prolonged APDs were consistently observed in younger hearts at multiple pacing frequencies. At 250-ms PCL, APD30 shortened from 54.4 ± 12 to 20 ± 2.6 ms and APD80 shortened from 113.2 ± 18.4 to 60.5 ± 16.8 ms (PND 0–3 vs. adult, respectively; P < 0.0001; Fig. 4). At faster pacing rates (150-ms PCL), APD30 and APD80 prolongation was observed in younger hearts, albeit the difference between intermediate age groups was modest. With increased ventricular refractoriness in younger hearts, loss of capture at PCLs <150 ms prevented APD measurements at faster pacing frequencies. Triangulation of action potential shape was also more pronounced in younger hearts (58.4 ± 15.1 ms, PND 0–3) compared with older hearts (30.4 ± 8.4 ms, adult; P < 0.0001; Fig. 4B). Increased potassium channel current can be mechanistically linked to shorter, less triangulated action potentials (17).
Fig. 4.
Age-dependent shortening of action potential duration time. A: transmembrane voltage (Vm) signals optically mapped from the epicardial surface of excised, intact hearts [250-ms pacing cycle lengths (PCL)]. B–D: prolonged action potential durations were observed in younger hearts at 30 and 80% repolarization (APD30 and APD80), which also displayed more triangulated action potentials. Data were binned into the following age groups: postnatal day (PND) 0–3, PND 4–7, PND 8–14, and adult (2–3 mo). APD Tri, triangulation APD80-APD30. Data are means ± SD; n ≥ 5 animals per age. *P < 0.05.
Age-dependent alterations in calcium handling and incidence of alternans.
Cardiomyocyte maturation includes the invagination of transverse tubules, formation of couplons, and synchronized calcium-induced calcium release (4, 66). To evaluate calcium handling in the whole heart, calcium transients were recorded from the epicardial surface of isolated Langendorff-perfused hearts. Rate adaptation of the calcium transient duration time was observed in all age groups. For example, in the PND 0–3 age group CaD80 decreased from 168 to 151 to 133 ms (PCLs = 250, 200, and 150 ms). However, immature hearts had consistently slower calcium handling at each pacing cycle. At slower pacing frequencies (250-ms PCL), an age-dependent shortening of CaD30 from 95 ± 16 to 57.2 ± 16.3 ms and CaD80 from 168.7 ± 17.7 to 117.9 ± 13.9 ms was observed (PND 0–3 vs. adult, P < 0.0001; Fig. 5, A–C). At faster pacing cycles (150 ms), CaD30 and CaD80 were longer in younger hearts, but differences between age groups were modest.
Fig. 5.
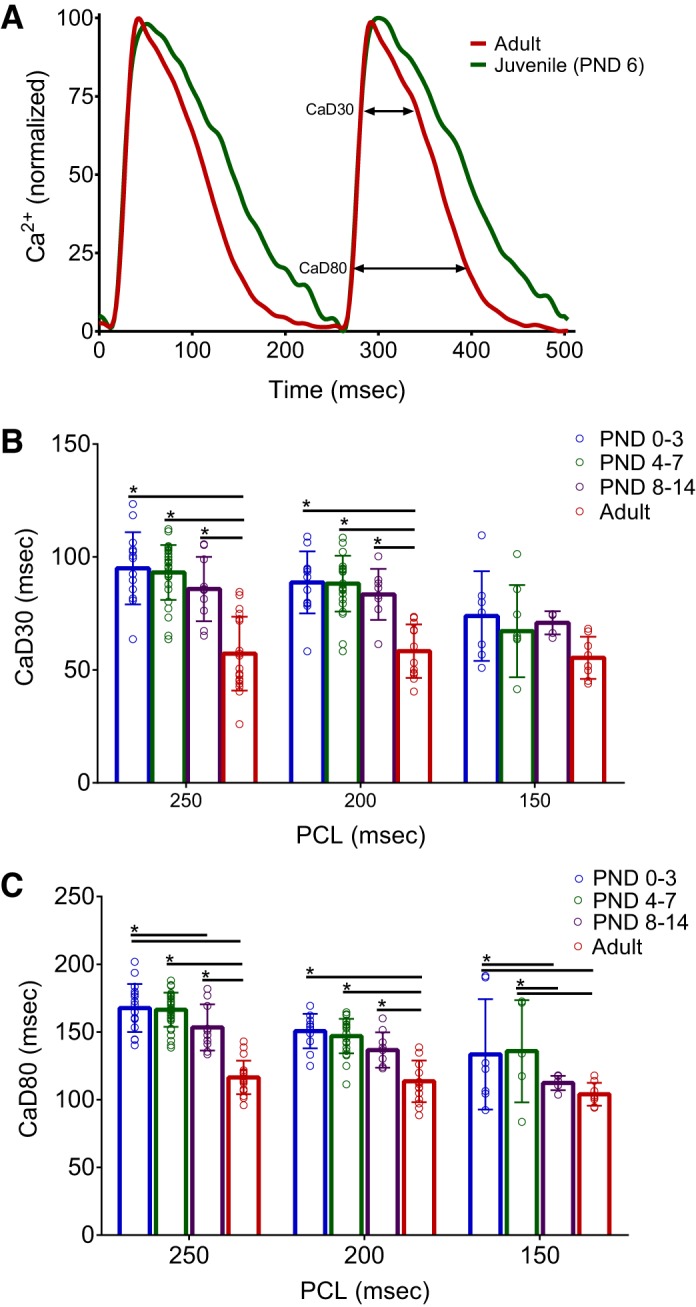
Age-dependent shortening of calcium handling. A: calcium transients recorded from the epicardial surface of excised, intact hearts [250-ms pacing cycle lengths (PCL)]. B and C: prolonged calcium transient durations were observed in younger hearts at 30 and 80% repolarization (CaD30 and CaD80). Data were binned into the following age groups: postnatal day (PND) 0–3, PND 4–7, PND 8–14, and adult (2–3 mo). Data are means ± SD; n ≥ 5 animals per age. *P < 0.05.
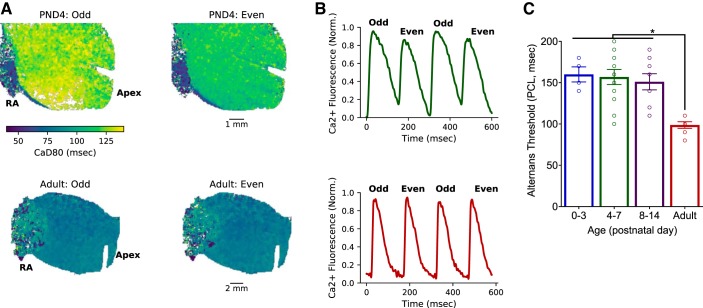
Faster calcium handling in adult hearts may be linked to an age-dependent increase in genes associated with calcium-induced calcium release (CACNA1C), calcium-reuptake into the sarcoplasmic reticulum (SERCA), and excitation-contraction coupling (TPM1 and TNNi3; Fig. 1E), whereas immature cardiomyocytes rely less on calcium-induced calcium release and more on sarcolemma calcium influx via the sodium-calcium exchanger (NCX) and T-type calcium channels (CACNA1G) (32). Disturbances in calcium handling have also been associated with an increased incidence of alternans or beat-to-beat alterations in calcium transient amplitude and kinetics (11, 42). A dynamic pacing protocol was implemented to pinpoint the alternans threshold or the longest pacing cycle length required to elicit calcium transient alternans. Immature hearts displayed an increased propensity for calcium transient alternans at slower pacing frequencies (160 ± 18.2 ms, PND 0–3) compared with adult hearts (99 ± 11.2 ms, P < 0.001; Fig. 6).
Fig. 6.
Increased incidence of calcium transient alternans in immature hearts. A: immature hearts (top) displayed an increased susceptibility to calcium transient alternans compared with adult hearts (bottom). Images show peak fluorescence at 150-ms pacing cycle lengths (PCL). RA, right atria. B: green and red traces represent 4 paced beats from the neonatal [postnatal day (PND) 4] and adult heart, respectively. C: the slowest PCL that resulted in alternating calcium transients (alternans threshold) was significantly slower in younger hearts compared with adults. Data were binned into the following age groups: PND 0–3, PND 4–7, PND 8–14, and adult (2–3 mo). Data are means ± SD; n ≥ 4 animals per age. *P < 0.05.
Age-related differences in isoproterenol response.
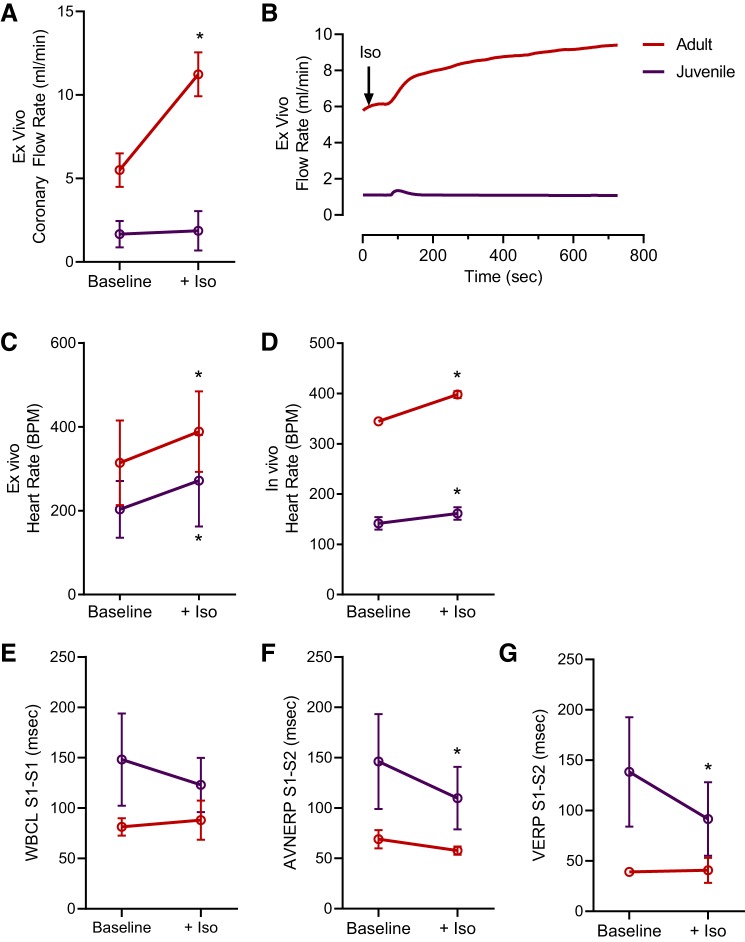
Maturation of the cardiovascular system can have a profound effect on the efficacy and potency of pharmacological agents. Previous studies have documented an age-dependent cardiovascular response to isoproterenol, a nonspecific β-adrenergic agonist, in both in vivo [e.g., canine, guinea pig (10, 63)] and isolated cardiomyocyte preparations [e.g., rat (28)]. Isoproterenol exerted a greater effect on the coronary vasculature in adult isolated whole hearts, increasing the coronary flow rate in adults by 104% compared with 12% in juvenile hearts (P < 0.0001; Fig. 7, A and B). Cardiac automaticity increased in both age groups after administration of isoproterenol, both in vivo and ex vivo (Fig. 7, C and D). However, EP parameters shortened in isolated juvenile hearts only, with a 25% decrease in AVNERP and 34% decrease in VERP compared with baseline measurements (P < 0.05; Fig. 7, E–G).
Fig. 7.
Age-related differences in isoproterenol response. A: isoproterenol (Iso) significantly increased the coronary flow rate in isolated adult hearts, compared with baseline, but not juvenile hearts. B: example traces of coronary flow rate across time. C and D: isoproterenol increased heart rate in vivo and ex vivo, in both age groups, relative to baseline. E–G: isoproterenol shortened cardiac electrophysiology parameters in isolated juvenile hearts but had minimal effects on adult cardiac electrophysiology. WBCL, Wenckebach cycle length; VERP, ventricular effective refractory period; AVNERP, atrioventricular nodal refractory period. Data are means ± SD; n ≥ 3 animals per age. *P < 0.05.
DISCUSSION
The neonatal heart continues to develop postpartum, not fully reaching maturity until ~21 days postnatal in rats (54) and >7 yr postnatal in humans (36, 55). In this study, we aimed to examine the relationship between postnatal cardiomyocyte maturation on cardiac electrophysiology and calcium handling using in vivo and ex vivo models. We show that the immature heart displays slowed atrioventricular conduction, prolonged action potential duration time, and longer ventricular effective refractory periods, compared with adult hearts. Age-dependent alterations in cardiac electrophysiology were associated with changes in genes encoding voltage-gated potassium channels and intercalated disk proteins that facilitate intercellular coupling. Calcium handling was also slowed in the immature heart, which coincided with less developed excitation-contraction coupling machinery and an increased propensity for calcium transient alternans. Finally, we demonstrate an age-dependent responsiveness to β-adrenergic stimulation: wherein adult hearts were more responsive to vasodilation of the vasculature and juvenile hearts to alterations in myocardial electrical conduction.
Postnatal changes in cardiomyocyte morphology.
Postnatal cardiac maturation includes the formation of intercellular connections between neighboring cardiomyocytes or intercalated disks. The cardiac intercalated disk includes the colocalization of adherens junctions, desmosomes, gap junctions, and sodium and potassium channels, which facilitates the rapid transmission of electrical activity, initiating contractile forces between neighboring myocytes (37, 48, 60). Indeed, the development of intercellular low-resistance pathways is vital to the heart’s ability to function as a highly coordinated syncytium. The spatial distribution of desmosomal, fascia adherens, and gap junction proteins shifts throughout postnatal development, from sporadically distributed to densely concentrated at the terminal ends of neighboring adult cardiomyocytes. In rodents, the intercalated disks are formed within the first ~20 days after birth but continue to develop well past maturity (1). Conversely in humans, the process is gradual with the colocalizing ion channels, adherens junctions, and gap junctions not being apparent until 6–7 yr after birth (40, 55).
In the presented study, we reported an increase in the relative mRNA abundance of key genes involved in intercellular connections and communication via the intercalated disks. From PND 0 to >11, older hearts had increased mRNA expression of desmosomal genes, including desmoplakin (DSP) and junctophilin 2 (JPH2). Notably, well-established intercalated disks have a direct influence on cell-cell communication and electrical conduction (22). Similarly, we observed a progressive shortening of ECG parameters (P wave, PR interval) with postnatal age that coincided with increased expression of intercalated disk genes. Notably, an age-dependent shortening of atrioventricular conduction and ventricular repolarization was observed both in vivo, as well as ex vivo, in the absence of autonomic influences.
Postnatal changes in excitation-contraction coupling.
The immature heart transitions from hyperplasia to hypertrophic growth shortly after birth (31, 32), and as the cardiomyocytes increase in size, transverse tubules begin to form and invaginate into the cell interior and the sarcoplasmic reticulum becomes more developed (52). This transition is characterized by major changes in calcium handling and the mechanism of excitation-contraction coupling. In the neonate, the functional role of the immature t-tubule system and sarcoplasmic reticulum is diminished, ceding calcium regulation to the sarcolemma. Excitation-contraction coupling is initially dependent on membrane depolarization, which directly controls calcium flux. Morphological changes facilitate the formation of dyads and couplons, wherein ryanodine receptors and L-type calcium channels are in close proximity (47). Concomitant with these organizational changes, cardiomyocytes become less dependent on the sarcolemma calcium influx and more reliant on sarcoplasmic reticulum-mediated calcium-induced calcium release (13, 19, 66). Ziman et al. (66) correlated the timing of t-tubule and couplon formation with improved excitation-contraction coupling in isolated cardiomyocytes aged 10–20 days. The authors showed that myocytes up to PND 10 lacked a t-tubule system, whereas the t-tubule system of cells isolated from PND 20 hearts were indistinguishable from adult myocytes.
In the presented study, we observed a similar developmental time course, with increased mRNA expression of the sarcoplasmic reticulum calcium ATPase and L-type calcium channel in older hearts (>11 days) compared with 0–3 days. Consequently, younger, immature hearts displayed prolonged calcium transient duration times and an increased propensity for calcium transient alternans. Importantly, calcium alternans can be associated with T-wave alternans and electrical instabilities (8, 9, 11). Our observations of slowed calcium handling in neonates is in agreement with previously published work by Escobar et al. (13), which showed that isolated neonatal cardiomyocytes were less sensitive to ryanodine receptor antagonism compared with older myocytes. The latter indicates minimal involvement of calcium-induced calcium release in the excitation-contraction coupling of immature hearts.
Postnatal changes in cardiac electrophysiology.
Postnatal cardiac maturation in both humans and rodents include an increase in cell size, formation of intercalated disks, invagination of t-tubules, and increased dependence on calcium-induced calcium release for excitation-contraction coupling. One of the inherent dissimilarities between species is an age-dependent increase in the heart rate of small rodents (20), which necessitates a progressively shorter action potential as rodent cardiomyocytes mature (12, 57, 61). In the presented study, we describe a linear increase in the heart rate and shortening of ECG parameters in older versus younger animals. This time course corresponds with a shift in myosin heavy chain expression to MYH6, which has kinetics that are three times faster than MYH7 (16). Although humans and rodents exhibit electrical restitution properties and an action potential that is rate dependent, the rodent action potential lacks a plateau phase due, in part, to differences in outward potassium current (17, 26). We observed an age-dependent increase in the expression of voltage-gated potassium channels: namely KCND2 and KCND3 that encode Kv4.2 and Kv4.3 and facilitate It0. Indeed, It0 is responsible for >50% of total outward potassium current and the very short APD that is characteristic of the adult mouse myocardium (59). In our study, action potentials showed rate dependency in all age groups, but immature hearts displayed longer action potential duration times and ventricular effective refractory periods.
Cardiac responsiveness to β-adrenergic stimulation.
Gaps in our understanding of juvenile cardiovascular physiology can have unintended consequences, particularly when pharmacological therapies or toxicological studies are designed and tested using only adult cardiac models: targeting ion channels and/or signaling pathways that may be underdeveloped in the immature myocardium. Indeed, a number of age-dependent cardiac responses to antiarrhythmics [e.g., dofetilide (38), sotalol (46)] and inotropes [e.g., dobutamine, isoproterenol (10)] have been reported. In our study, we highlighted age-dependent differences in cardiovascular responsiveness to isoproterenol, a nonspecific β-adrenergic agonist. Chruscinski et al. (7) showed that in a β2-knockout mouse model, the vasodilatory effect of isoproterenol was attenuated; however, in a double knockout of both β1 and β2, vasodilation was abolished. We noted a vasodilatory response to isoproterenol on the coronary vasculature of isolated, intact adult hearts, but this effect was minimal in juvenile animals. This suggests a reduction in endogenous adrenergic receptor functionality or expression in the neonatal coronary vasculature. Interestingly, an increase in automaticity was observed in both adults and juveniles, but EP parameters shortened significantly in isolated juvenile hearts. The magnitude of this response was in agreement with previously published studies, which suggest that myocardial β-adrenergic receptor sensitivity may be increased in noninnervated neonatal hearts (28, 64).
Limitations.
The scope of our study was limited to the age-dependent effects on cardiac electrophysiology and calcium handling using a rat model; therefore, potential differences with human physiology should be considered since prominent species-specific characteristics exist. For instance, an age-dependent increase in the heart rate of small rodents (20) is coupled to changes in excitation-contraction coupling that allow the rodent heart to adapt to faster beating rates. Accordingly, the rodent action potential (measured via intracellular microelectrode) becomes progressively shorter as cardiomyocytes mature (12, 57, 61). The neonatal human heart has minimal cardiac reserve, displaying a limited response to alterations in preload or afterload. Instead, studies suggest that the immature human heart is more reliant on changes in heart rate to increase cardiac output (3). The neonatal human heart is also less compliant with limited contractile reserve. To maintain contractile function, a high surface area-to-volume ratio allows immature cardiomyocytes to subsist on sarcolemma calcium flux versus calcium-induced calcium release (4). Humans also have a more pronounced force-frequency response compared with rodents, which develops at an early age (62). These notable mechanisms help maintain cardiac function, despite myocardial immaturity.
Conclusion.
Rodent models remain a valuable tool for understanding cardiac maturation, as an “ideal” human cardiac research model does not currently exist. Differentiated human embryonic stem cells and induced pluripotent stem cells hold promise, but methodologies to reproducibly mature these derived myocytes are still a work in progress (15, 44, 45, 53) and cell-based models cannot fully replicate a three-dimensional whole heart. Nevertheless, the mammalian heart continues to mature postnatally, and we demonstrate significant developmental changes in cardiac electrophysiology and calcium handling within the first few weeks after birth. We anticipate that these dynamic postnatal changes in cardiac physiology may contribute to age-dependent differences observed in disease modeling and/or preclinical safety testing.
GRANTS
This work was supported by the National Institutes of Health Grants R00-ES-023477 and R01-HL-139472 (to N. G. Posnack), Children’s Research Institute, and Children’s National Heart Institute. This publication was also supported by the Gloria and Steven Seelig family.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.S., M.B., and N.G.P. conceived and designed research; L.S., M.B., D.G., M. Reilly, M. Ramadan, D.M., T.P., C.M., A.C., and N.G.P. performed experiments; L.S., M.B., D.G., M. Reilly, M. Ramadan, D.M., T.P., C.M., A.C., R.J., and N.G.P. analyzed data; L.S., M.B., D.G., M. Reilly, M. Ramadan, D.M., C.M., R.J., and N.G.P. interpreted results of experiments; L.S., M.B., D.G., M. Reilly, and N.G.P. prepared figures; L.S. and N.G.P. drafted manuscript; L.S., M.B., D.G., and N.G.P. edited and revised manuscript; L.S., M.B., D.G., M. Reilly, M. Ramadan, D.M., T.P., C.M., A.C., R.J., and N.G.P. approved final version of manuscript.
REFERENCES
- 1.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80: 88–94, 1997. doi: 10.1161/01.RES.80.1.88. [DOI] [PubMed] [Google Scholar]
- 2.Artman M, Henry G, Coetzee WA. Cellular basis for age-related differences in cardiac excitation-contraction coupling. Prog Pediatr Cardiol 11: 185–194, 2000. doi: 10.1016/S1058-9813(00)00049-7. [DOI] [PubMed] [Google Scholar]
- 3.Baum VC, Palmisano BW. The immature heart and anesthesia. Anesthesiology 87: 1529–1548, 1997. doi: 10.1097/00000542-199712000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Brattelid T, Winer LH, Levy FO, Liestøl K, Sejersted OM, Andersson KB. Reference gene alternatives to Gapdh in rodent and human heart failure gene expression studies. BMC Mol Biol 11: 22, 2010. doi: 10.1186/1471-2199-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai B, Mu X, Gong D, Jiang S, Li J, Meng Q, Bai Y, Liu Y, Wang X, Tan X, Yang B, Lu Y. Difference of sodium currents between pediatric and adult human atrial myocytes: evidence for developmental changes of sodium channels. Int J Biol Sci 7: 708–714, 2011. doi: 10.7150/ijbs.7.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of β-adrenergic receptor subtypes in blood vessels of knockout mice lacking β(1)- or β(2)-adrenergic receptors. Mol Pharmacol 60: 955–962, 2001. doi: 10.1124/mol.60.5.955. [DOI] [PubMed] [Google Scholar]
- 8.Clusin WT. Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit Rev Clin Lab Sci 40: 337–375, 2003. doi: 10.1080/713609356. [DOI] [PubMed] [Google Scholar]
- 9.Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol 294: H1–H10, 2008. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll DJ, Gillette PC, Fukushige J, Lewis RM, Contant C, Hartley CJ, Entman ML, Schwartz A, Dunn F. Comparison of the cardiovascular action of isoproterenol, dopamine, and dobutamine in the neonatal and mature dog. Pediatr Cardiol 1: 307–314, 1980. doi: 10.1007/BF02336436. [DOI] [Google Scholar]
- 11.Edwards JN, Blatter LA. Cardiac alternans and intracellular calcium cycling. Clin Exp Pharmacol Physiol 41: 524–532, 2014. doi: 10.1111/1440-1681.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escande D, Loisance D, Planche C, Coraboeuf E. Age-related changes of action potential plateau shape in isolated human atrial fibers. Am J Physiol Heart Circ Physiol 249: H843–H850, 1985. doi: 10.1152/ajpheart.1985.249.4.H843. [DOI] [PubMed] [Google Scholar]
- 13.Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Pérez CG, Mejía-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol 286: H971–H978, 2004. doi: 10.1152/ajpheart.00308.2003. [DOI] [PubMed] [Google Scholar]
- 14.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 15.Feric NT, Radisic M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv Drug Deliv Rev 96: 110–134, 2016. doi: 10.1016/j.addr.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galler S, Puchert E, Gohlsch B, Schmid D, Pette D. Kinetic properties of cardiac myosin heavy chain isoforms in rat. Pflugers Arch 445: 218–223, 2002. doi: 10.1007/s00424-002-0934-6. [DOI] [PubMed] [Google Scholar]
- 17.Grandy SA, Trépanier-Boulay V, Fiset C. Postnatal development has a marked effect on ventricular repolarization in mice. Am J Physiol Heart Circ Physiol 293: H2168–H2177, 2007. doi: 10.1152/ajpheart.00521.2007. [DOI] [PubMed] [Google Scholar]
- 18.Günthel M, Barnett P, Christoffels VM. Development, proliferation, and growth of the mammalian heart. Mol Ther 26: 1599–1609, 2018. doi: 10.1016/j.ymthe.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaguchi S, Kawakami Y, Honda Y, Nemoto K, Sano A, Namekata I, Tanaka H. Developmental changes in excitation-contraction mechanisms of the mouse ventricular myocardium as revealed by functional and confocal imaging analyses. J Pharmacol Sci 123: 167–175, 2013. doi: 10.1254/jphs.13099FP. [DOI] [PubMed] [Google Scholar]
- 20.Heier CR, Hampton TG, Wang D, Didonato CJ. Development of electrocardiogram intervals during growth of FVB/N neonate mice. BMC Physiol 10: 16, 2010. doi: 10.1186/1472-6793-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation 103: 2004–2013, 2001. doi: 10.1161/01.CIR.103.15.2004. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh YC, Lin JC, Hung CY, Li CH, Lin SF, Yeh HI, Huang JL, Lo CP, Haugan K, Larsen BD, Wu TJ. Gap junction modifier rotigaptide decreases the susceptibility to ventricular arrhythmia by enhancing conduction velocity and suppressing discordant alternans during therapeutic hypothermia in isolated rabbit hearts. Heart Rhythm 13: 251–261, 2016. doi: 10.1016/j.hrthm.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Jaimes R 3rd, McCullough D, Siegel B, Swift L, Hiebert J, McInerney D, Posnack NG. Lights, camera, path splitter: a new approach for truly simultaneous dual optical mapping of the heart with a single camera. BMC Biomed Eng 1: 25, 2019. doi: 10.1186/s42490-019-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaimes R 3rd, McCullough D, Siegel B, Swift L, McInerney D, Hiebert J, Perez-Alday E, Trenor B, Sheng J, Saiz J, Tereshchenko L, Posnack N. Plasticizer interaction with the heart: chemicals used in plastic medical devices can interfere with cardiac electrophysiology. Circ Arrhythm Electrophysiol 12: e007294, 2019. doi: 10.1161/CIRCEP.119.007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaimes R 3rd, Walton RD, Pasdois P, Bernus O, Efimov IR, Kay MW. A technical review of optical mapping of intracellular calcium within myocardial tissue. Am J Physiol Heart Circ Physiol 310: H1388–H1401, 2016. doi: 10.1152/ajpheart.00665.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knollmann BC, Schober T, Petersen AO, Sirenko SG, Franz MR. Action potential characterization in intact mouse heart: steady-state cycle length dependence and electrical restitution. Am J Physiol Heart Circ Physiol 292: H614–H621, 2007. doi: 10.1152/ajpheart.01085.2005. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan A, Samtani R, Dhanantwari P, Lee E, Yamada S, Shiota K, Donofrio MT, Leatherbury L, Lo CW. A detailed comparison of mouse and human cardiac development. Pediatr Res 76: 500–507, 2014. doi: 10.1038/pr.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuznetsov V, Pak E, Robinson RB, Steinberg SF. β2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res 76: 40–52, 1995. doi: 10.1161/01.RES.76.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Langendorff O. Untersuchungen am uberlebenden Saugethierherzen [Investigations on the surviving mammalian heart]. Arch Gesante Physiol 61: 291–332, 1895. doi: 10.1007/BF01812150. [DOI] [Google Scholar]
- 30.Lee P, Calvo CJ, Alfonso-Almazán JM, Quintanilla JG, Chorro FJ, Yan P, Loew LM, Filgueiras-Rama D, Millet J. Low-cost optical mapping systems for panoramic imaging of complex arrhythmias and drug-action in translational heart models. Sci Rep 7: 43217, 2017. doi: 10.1038/srep43217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 28: 1737–1746, 1996. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 32.Louch WE, Koivumäki JT, Tavi P. Calcium signalling in developing cardiomyocytes: implications for model systems and disease. J Physiol 593: 1047–1063, 2015. doi: 10.1113/jphysiol.2014.274712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahdavi V, Lompre AM, Chambers AP, Nadal-Ginard B. Cardiac myosin heavy chain isozymic transitions during development and under pathological conditions are regulated at the level of mRNA availability. Eur Heart J 5, Suppl F: 181–191, 1984. doi: 10.1093/eurheartj/5.suppl_F.181. [DOI] [PubMed] [Google Scholar]
- 34.Marcela SG, Cristina RM, Angel PG, Manuel AM, Sofía DC, Patricia LR, Bladimir R-R, Concepción SG. Chronological and morphological study of heart development in the rat. Anat Rec (Hoboken) 295: 1267–1290, 2012. doi: 10.1002/ar.22508. [DOI] [PubMed] [Google Scholar]
- 35.Mironov SF, Vetter FJ, Pertsov AM. Fluorescence imaging of cardiac propagation: spectral properties and filtering of optical action potentials. Am J Physiol Heart Circ Physiol 291: H327–H335, 2006. doi: 10.1152/ajpheart.01003.2005. [DOI] [PubMed] [Google Scholar]
- 36.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kühn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA 110: 1446–1451, 2013. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noorman M, van der Heyden MA, van Veen TA, Cox MG, Hauer RN, de Bakker JM, van Rijen HV. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol 47: 23–31, 2009. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Obreztchikova MN, Sosunov EA, Plotnikov A, Anyukhovsky EP, Gainullin RZ, Danilo P Jr, Yeom ZH, Robinson RB, Rosen MR. Developmental changes in IKr and IKs contribute to age-related expression of dofetilide effects on repolarization and proarrhythmia. Cardiovasc Res 59: 339–350, 2003. doi: 10.1016/S0008-6363(03)00360-2. [DOI] [PubMed] [Google Scholar]
- 39.Pesco-Koplowitz L, Gintant G, Ward R, Heon D, Saulnier M, Heilbraun J. Drug-induced cardiac abnormalities in premature infants and neonates. Am Heart J 195: 14–38, 2018. doi: 10.1016/j.ahj.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation 90: 713–725, 1994. doi: 10.1161/01.CIR.90.2.713. [DOI] [PubMed] [Google Scholar]
- 41.Racca AW, Klaiman JM, Pioner JM, Cheng Y, Beck AE, Moussavi-Harami F, Bamshad MJ, Regnier M. Contractile properties of developing human fetal cardiac muscle. J Physiol 594: 437–452, 2016. doi: 10.1113/JP271290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramadan M, Sherman M, Jaimes R 3rd, Chaluvadi A, Swift L, Posnack NGNG. Disruption of neonatal cardiomyocyte physiology following exposure to bisphenol-a. Sci Rep 8: 7356, 2018. doi: 10.1038/s41598-018-25719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson RB. Autonomic receptor–effector coupling during post-natal development. Cardiovasc Res 31, Suppl 1: E68–E76, 1996. doi: 10.1016/S0008-6363(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 44.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556: 239–243, 2018. [Erratum in Nature 572: E16–E17, 2019.]. 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 134: 1557–1567, 2016. doi: 10.1161/CIRCULATIONAHA.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saul JP, Ross B, Schaffer MS, Beerman L, Melikian AP, Shi J, Williams J, Barbey JT, Jin J, Hinderling PH; Pediatric Sotalol Investigators . Pharmacokinetics and pharmacodynamics of sotalol in a pediatric population with supraventricular and ventricular tachyarrhythmia. Clin Pharmacol Ther 69: 145–157, 2001. doi: 10.1067/mcp.2001.113795. [DOI] [PubMed] [Google Scholar]
- 47.Scriven DR, Asghari P, Moore ED. Microarchitecture of the dyad. Cardiovasc Res 98: 169–176, 2013. doi: 10.1093/cvr/cvt025. [DOI] [PubMed] [Google Scholar]
- 48.Scuderi GJ, Butcher J. Naturally engineered maturation of cardiomyocytes. Front Cell Dev Biol 5: 50, 2017. doi: 10.3389/fcell.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res 86: 302–311, 2000. doi: 10.1161/01.RES.86.3.302. [DOI] [PubMed] [Google Scholar]
- 50.Swift LM, Asfour H, Posnack NG, Arutunyan A, Kay MW, Sarvazyan N. Properties of blebbistatin for cardiac optical mapping and other imaging applications. Pflugers Arch 464: 503–512, 2012. doi: 10.1007/s00424-012-1147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talman V, Teppo J, Pöhö P, Movahedi P, Vaikkinen A, Karhu ST, Trošt K, Suvitaival T, Heikkonen J, Pahikkala T, Kotiaho T, Kostiainen R, Varjosalo M, Ruskoaho H. Molecular atlas of postnatal mouse heart development. J Am Heart Assoc 7: e010378, 2018. doi: 10.1161/JAHA.118.010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka H, Sekine T, Nishimaru K, Shigenobu K. Role of sarcoplasmic reticulum in myocardial contraction of neonatal and adult mice. Comp Biochem Physiol A Mol Integr Physiol 120: 431–438, 1998. doi: 10.1016/S1095-6433(98)10043-0. [DOI] [PubMed] [Google Scholar]
- 53.Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, Levent E, Raad F, Zeidler S, Wingender E, Riegler J, Wang M, Gold JD, Kehat I, Wettwer E, Ravens U, Dierickx P, van Laake LW, Goumans MJ, Khadjeh S, Toischer K, Hasenfuss G, Couture LA, Unger A, Linke WA, Araki T, Neel B, Keller G, Gepstein L, Wu JC, Zimmermann W-H. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135: 1832–1847, 2017. doi: 10.1161/CIRCULATIONAHA.116.024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Weerd JH, Christoffels VM. The formation and function of the cardiac conduction system. Development 143: 197–210, 2016. doi: 10.1242/dev.124883. [DOI] [PubMed] [Google Scholar]
- 55.Vreeker A, van Stuijvenberg L, Hund TJ, Mohler PJ, Nikkels PG, van Veen TA. Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS One 9: e94722, 2014. doi: 10.1371/journal.pone.0094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner MB, Wang Y, Kumar R, Tipparaju SM, Joyner RW. Calcium transients in infant human atrial myocytes. Pediatr Res 57: 28–34, 2005. doi: 10.1203/01.PDR.0000148066.34743.10. [DOI] [PubMed] [Google Scholar]
- 57.Wahler GM, Dollinger SJ, Smith JM, Flemal KL. Time course of postnatal changes in rat heart action potential and in transient outward current is different. Am J Physiol Heart Circ Physiol 267: H1157–H1166, 1994. doi: 10.1152/ajpheart.1994.267.3.H1157. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Duff HJ. Developmental changes in transient outward current in mouse ventricle. Circ Res 81: 120–127, 1997. doi: 10.1161/01.RES.81.1.120. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res 79: 79–85, 1996. doi: 10.1161/01.RES.79.1.79. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, Lin JL, Wu KH, Wang D-Z, Reiter RS, Sinn HW, Lin CI, Lin CJ. Xin proteins and intercalated disc maturation, signaling and diseases. Front Biosci (Landmark Ed) 17: 2566–2593, 2012. doi: 10.2741/4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Xu H, Kumar R, Tipparaju SM, Wagner MB, Joyner RW. Differences in transient outward current properties between neonatal and adult human atrial myocytes. J Mol Cell Cardiol 35: 1083–1092, 2003. doi: 10.1016/S0022-2828(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 62.Wiegerinck RF, Cojoc A, Zeidenweber CM, Ding G, Shen M, Joyner RW, Fernandez JD, Kanter KR, Kirshbom PM, Kogon BE, Wagner MB. Force frequency relationship of the human ventricle increases during early postnatal development. Pediatr Res 65: 414–419, 2009. doi: 10.1203/PDR.0b013e318199093c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woulfe KC, Wilson CE, Nau S, Chau S, Phillips EK, Zang S, Tompkins C, Sucharov CC, Miyamoto SD, Stauffer BL. Acute isoproterenol leads to age-dependent arrhythmogenesis in guinea pigs. Am J Physiol Heart Circ Physiol 315: H1051–H1062, 2018. doi: 10.1152/ajpheart.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE 2001: re15, 2001. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 65.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 114: 511–523, 2014. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziman AP, Gómez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol 48: 379–386, 2010. doi: 10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]