Abstract
Myostatin (MSTN) is a transforming growth factor (TGF)-β superfamily member that acts as a negative regulator of muscle growth and may play a role in cardiac remodeling. We hypothesized that inhibition of activin type II receptors (ACTRII) to reduce MSTN signaling would reduce pathological cardiac remodeling in experimental heart failure (HF). C57BL/6J mice underwent left anterior descending coronary artery ligation under anesthesia to induce myocardial infarction (MI) or no ligation (sham). MI and sham animals were each randomly divided into groups (n ≥ 10 mice/group) receiving an ACTRII or ACTRII/TGFβ receptor-signaling inhibiting strategy: 1) myo-Fc group (weekly 10 mg/kg Myo-Fc) or 2) Fol + TGFi group (daily 12 µg/kg follistatin plus 2 mg/kg TGFβ receptor inhibitor), versus controls. ACTRII/TGFBR signaling inhibition preserved cardiac function by echocardiography and prevented an increase in brain natriuretic peptide (BNP). ACTRII/TGFBR inhibition resulted in increased phosphorylation (P) of Akt and decreased P-p38 mitogen-activated protein kinase (MAPK) in MI mice. In vitro, Akt contributed to P-SMAD2,3, P-p38, and BNP regulation in cardiomyocytes. ACTRII/TGFBR inhibition increased sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) levels and decreased unfolded protein response (UPR) markers in MI mice. ACTRII/TGFBR inhibition was associated with a decrease in cardiac fibrosis and fibrosis markers, connective tissue growth factor (CTGF), type I collagen, fibronectin, α-smooth muscle actin, and matrix metalloproteinase (MMP)-12 in MI mice. MSTN exerted a direct regulation on the UPR marker eukaryotic translation initiation factor-2α (eIf2α) in cardiomyocytes. Our study suggests that ACTRII ligand inhibition has beneficial effects on cardiac signaling and fibrosis after ischemic HF.
NEW & NOTEWORTHY Activin type II receptor ligand inhibition resulted in preserved cardiac function, a decrease in cardiac fibrosis, improved SERCA2a levels, and a prevention of the unfolded protein response in mice with myocardial infarction.
Keywords: cardiac fibrosis, cardiac remodeling, heart failure, myocardial infarction, myostatin
INTRODUCTION
Myostatin (MSTN), also known as growth differentiation factor-8 (GDF8), is a signaling protein from the transforming growth factor (TGF)-β superfamily that acts as one of the few known inhibitors of muscle growth. In cardiomyocytes, MSTN is induced both after mechanical stretch and following ischemic injury (33, 34). Whether cardiac MSTN upregulation in heart failure (HF) is pathological or compensatory is still under debate. Previous studies in MSTN-knockout (KO) mice have reported unchanged cardiac function (12, 27) accompanied by either no effect on cardiomyocyte size or cardiac hypertrophy (12) or slightly smaller hearts in MSTN-KO mice (27). Other studies have reported reduced ejection fraction and eccentric hypertrophy in constitutive MSTN-KO mice (20, 31), whereas constitutive overexpression of MSTN in the heart (16) or in the heart and skeletal muscle (2, 30) reduced cardiac mass without effects on cardiac systolic function. Another study has affirmed that genetic inactivation of MSTN in adult mice is enough to cause cardiac hypertrophy and HF (3), suggesting that MSTN activation can be a physiological compensatory effect to curb pathological hypertrophy in HF. Some studies have suggested that MSTN blockade-mediated cardiac hypertrophy resembles eccentric physiological hypertrophy found in athletes (20, 31), making it clinically desirable in the setting of severe ventricular dysfunction. Cardiac MSTN may also be involved in cardiomyocyte fibrosis. The influence of MSTN in cardiac fibrosis is controversial, as different studies have found either a lack of effect (12) or an involvement of MSTN in cardiac interstitial fibrosis in older mice (4). MSTN signaling is exerted downstream from activin type II receptors (ACTRII); after binding ACTRIIA/B, ACTRII couples with a type 1 receptor kinase (ACTRIB or TGFBRI) and activates signaling via SMAD2,3 transcription factors. Growth differentiation factor (GDF)-11 and activins are other ACTRII ligands whose role has been studied in the heart. GDF11 was notably reported to inhibit age-related cardiac hypertrophy (24), although those results have been challenged (36). Increased activin A levels have been reported to predict LV remodeling in ST-elevation myocardial infarction (MI) (23). TGFBRI and SMAD2,3 mediate not only ACTRII-dependent but also TGFBRII-dependent signaling, and therefore, they can be active in the absence of ACTRII stimulation.
Consequences of pharmacological inhibition of MSTN and ACTRII ligands have been reported primarily in studies analyzing the effects on skeletal muscle, in healthy KO model studies without physiological evidence of HF, or in isolated cell studies. A recent study has shown improved cardiac function following ACTRII inhibition in aged mice and mice with nonischemic heart failure (32). To our knowledge, this is the first study to date analyzing the effect of inhibiting MSTN or ACTRII ligands after established ischemic HF in an animal model or humans. We previously reported increased cardiac MSTN in patients with dilated cardiomyopathy; MSTN activity was associated with compensatory myocyte hypertrophy in human HF (14), and support with mechanical devices led to significant changes in MSTN and insulin-like growth factor-1 (IGF-1). Moreover, we have reported an early increase in cardiac and circulating MSTN in a mouse model of MI (9). In the same study, we found a lack of increase in circulating GDF11 and activin A after MI. Given the complexity of HF and its effect on multiple organ systems, it is critical to evaluate MSTN response in the appropriate clinical setting of HF or after cardiac injury, in which MSTN may mediate a role in myocardial homeostasis or repair. In the present work, we seek to study the effect of ACTRII inhibition in a well-established small-animal HF model. We employed two different pharmacological strategies aimed to reduce ACTRII-dependent signaling: 1) a decoy MSTN (Myo-Fc) to prevent ACTRII ligands (MSTN, GDF11, and activins) from binding the receptor and 2) a combination of the endogenous antagonist follistatin (Fol) to prevent MSTN, GDF11, and activins from binding to ACTRIIs (22) together with a TGFBRI inhibitor to reduce TGFBRI-dependent SMAD2,3 activation. We analyzed whether ACTRII signaling inhibition could prevent cardiac remodeling in experimental HF and analyzed the cellular mechanisms mediating the effect of ACTRII inhibition in cardiomyocytes. We hypothesized that inhibition of ACTRII ligands after ischemia may preserve cardiomyocyte geometry, improve remodeling and hypertrophic signaling, and reduce myocardial fibrosis.
MATERIALS AND METHODS
Study groups and study design.
This study was approved by the Institutional Animal Care and Use Committee of Columbia University, which conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 85-23, Revised 1996). Eight-week-old male C57BL/6 mice (Jackson, Bar Harbor, ME) were randomly assigned to either surgery or sham surgery. Under ketamine-xylazine (100 mg/kg/10 mg/kg ip) anesthesia, mice underwent surgical ligation of the left anterior descending coronary artery (LAD) through a left thoracotomy incision, causing MI. Sham-operated animals underwent the same procedure without ligation of the coronary artery. Two weeks after surgery, MI was confirmed by echocardiography, and MI mice without cardiac dysfunction [fractional shortening (FS) > 35] or with severely decreased function (FS < 10) were excluded from the experiment. Sham (n = 30) and MI mice (n = 34) were each randomly divided into three groups (n ≥ 10 mice/group) receiving a ACTRII ligand inhibiting treatment: 1) Myo-Fc group receiving weekly intraperitoneal 10 mg/kg doses of Myo-Fc (Pfizer, Cambridge, MA), 2) Fol + TGFi group receiving daily 12 µg/kg doses of intraperitoneal Fol (Biovision, Milpipas, CA) plus daily 2 mg/kg doses of oral TGFBRI inhibitor (GW788388; Tocris), or 3) corresponding vehicle for each drug. The Myo-Fc compound has been described previously (7). A MSTN propeptide is fused to a murine IgG-Fc region to increase its stability. The MSTN propeptide-Fc fusion inhibits binding of MSTN, GDF11, and activin B to receptor ACTRIIB with an IC50 of 1 nM. GW788388 inhibits both TGFBRI and TGFBRII (29). Doses were chosen based on previous literature (7, 18, 29), and decreased phosphorylated SMAD2,3 (P-SMAD2,3)/SMAD2,3 in tissue was the chosen end point to confirm effective ACTRII/TGFBRI signaling inhibition. After 8 wk of treatment/vehicle, echocardiography was repeated, running endurance tests were performed, and mice were euthanized. Heart and gastrocnemius muscles were collected and stored at −80 C until analysis. A portion of the heart was stored in formalin for histology analysis.
Echocardiography.
Cardiac function was assessed by echocardiography under isoflurane anesthesia (inhaled, 1–5%) 2 wk post-MI and before euthanasia to assess successful MI induction and the possible effect of the treatment on myocardial function and remodeling with a 30-MHz high-frequency ultrasound transducer (Visualsonics Vevo770, Toronto, ON, Canada), as previously described (37).
Running endurance testing.
Mice were run on a treadmill (Columbus Instruments, Columbus, OH) until exhaustion, as previously described (37). Results were expressed in a Kaplan Meier survival curve.
Histology.
Standard histology was performed in heart samples (n = 6/group) for myocyte size (hematoxylin and eosin) and fibrosis (Masson’s trichrome). For myocyte size, 10 pictures were taken of each slide, and 10 myocytes were measured in each picture using ImageJ. For fibrosis assessment, 10 pictures from the MI-remote areas of each slide were scored as follows: 0, no fibrosis; 1, traces of fibrosis; 2, apparent fibrosis; 3, extended fibrosis; 4, massive fibrosis. Apoptosis was assessed in slides from the same specimens by chromogenic TUNEL staining of DNA fragmentation (no. ab206386; Abcam, Cambridge, MA).
Cell culture.
To study whether Akt mediated the effect of ACTRII inhibition in cardiomyocyte signaling, and to confirm the direct effect of MSTN on markers of calcium handling and unfolded protein response, we employed in vitro studies using the human adult female left ventricular cardiomyocyte-like cell line AC16 (Sigma, St. Louis, MO). Culture and characterization of AC16 was performed as recently described (10) by confirming expression levels of cardiac markers [brain natriuretic peptide (BNP), cardiac actin] and the absence of a fibroblast marker (FSP1) and a skeletal muscle marker (MyoD) in comparison with fresh human septal cardiomyocytes. The cell division capacity of AC16 cells is absent in primary human cardiomyocytes, making AC16 a useful tool for in vitro studies; levels of all the cardiac markers studied were comparable in AC16 and primary human cardiomyocytes (10). We tested the effect of 24-h treatment of several molecular components of heart failure stress in MSTN upregulation as follows: H2O2 (100 μM), tumor necrosis factor-α (TNFα; 10ng/ml), LPS (50 μg/ml), angiotensin II (ANG II; 10 μM), dexamethasone (10 μM), and 10% of pooled serum from mice 10 wk after LAD ligation, as previously described (9). AC16 cells were transfected with MSTN siRNA, Akt siRNA, or nontargeting (scrambled) siRNA (Dharmacon, Lafayette, CO) with the transfection reagent Lipofectamine RNAiMAX (Invitrogen, Grand Island, NY). The siRNA constructs were added to the culture medium at a concentration of 165 nM in combination with lipofectamine. After 4 h, the medium was changed to fresh DMEM containing 2% FBS and incubated for 48 h. Cells were incubated with MI serum in the presence or absence of either 0.2 μg/ml Myo-Fc or a combination on 1 μM of GW-788388 and 10 ng/ml Fol in 0.01% DMSO. To test the role of the ACTRII/TGFBRI inhibiting treatments on fibroblasts, we performed studies on Normal Human Ventricular Cardiac Fibroblasts (Lonza, Walkersville, MD). Fibroblasts (Fb) were grown on poly-l-lysine-coated plates and used between passages 3 and 5. Fbs were incubated with profibrotic compounds (ANG II, 10 μM; or TGFβ1, 10ng/ml) or 10% MI serum in the presence or absence of 0.2 μg/ml Myo-Fc or 1μM of GW-788388 plus 10 ng/ml Fol in 0.01% DMSO.
Protein analysis.
Protein extraction and analysis of heart and gastrocnemius (n ≥ 8/group) and AC16 cell isolates was performed by Western blot analysis, as recently described (9). The following primary antibodies were used at a 1:1,000 dilution: anti-MSTN (no. ab71808; Abcam), anti-SMAD2,3 (no. sc-133098; Santa Cruz Biotechnology, Santa Cruz, CA), anti-P-SMAD2,3 (no. sc-11769-R; Santa Cruz Biotechnology), anti-IGF-1 (no. ab9572; Abcam), anti-Akt (no. 9272; Cell Signaling Technology, Danvers, MA), anti-P-Akt (no. 9271, Cell Signaling Technology), anti-p38 (no. 9212; Cell Signaling Technology), anti-P-p38 (no. 9211; Cell Signaling Technology), anti-sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a; no. A010-2, Badrilla, Leeds, UK), anti-eukaryotic translation initiation factor-2α (eIF2α; no. 9722; Cell Signaling Technology), anti-P-eIF2α (no. 9721; Cell Signaling Technology), anti-X-box binding protein (XBP1; no. ab37152; Abcam), and anti-matrix metalloproteinase-12 (MMP12; no. ab128030; Abcam). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-365062, Santa Cruz Biotechnology) was used as a loading control at a 1:5,000 dilution. Analysis of circulating interleukin-6 (IL-6) as a marker of systemic inflammation was performed by ELISA (no. ab100712; Abcam). Representative full blots to show antibody specificity are provided in Supplemental Fig. S1; Supplemental Material for this article is available at https://doi.org/10.6084/m9.figshare.11204690).
Real-time PCR.
Real-time PCR analysis of mRNA levels of MSTN, GDF11, inhibin A (to reflect activin A), BNP, atrial natriuretic peptide (ANP), IGF-1, unfolded protein response genes [binding immunoglobulin protein (BiP), activating transcription factor 4 (ATF4), and C/EBP homologous protein (CHOP)], fibrosis-related genes [connective tissue growth factor (CTGF), type I collagen (Col1a1), fibronectin, and α-smooth muscle actin (α-SMA)], and inflammation-related genes [TNFα and early growth response gene-2 (EGR2)] was performed in cardiac tissue (n = 5/group), as recently described (9). To analyze the effects of the treatments and gene silencing on MSTN, Akt and targets of MSTN in AC16 cells, we analyzed the mRNA levels of MSTN, Akt, and BNP. Expression of fibrosis-related genes was tested in cultures of human cardiac fibroblasts treated with ACTRII inhibitors. The corresponding sequences were as follows: mouse MSTN forward, 5′-tggccatgatcttgctgtaa-3′; mouse MSTN reverse, 5′-ccttgacttctaaaaagggattca-3′; mouse GDF11 forward, 5′-acagacctggctgtcacctc-3′; mouse GDF11 reverse, 5′-tcgaagctccatgaaaggat-3′; mouse INHB forward, 5′-gatcatcagctttgcagagaca-3′; mouse INHB reverse, 5-tgccttcattagagacgaagaa-3′; mouse BNP forward, 5′-gtcagtcgtttgggctgtaac-3′; mouse BNP reverse, 5′-agacccaggcagagtcagaa-3′; mouse ANP forward, 5′-acctgctagaccacctggag-3′; mouse ANP reverse, 5′-ccttggctgttatcttcggtaccgg-3′; mouse IGF1 forward, 5′-agcagccttccaactcaattat-3′; mouse IGF1 reverse, 5′-tgaagacgacatgatgtgtatctttat-3′; mouse BiP forward, 5′-ctgaggcgtatttgggaaag-3′; mouse BiP reverse, 5′-tcatgacattcagtccagcaa-3′; mouse ATF4 forward, 5′-atgatggcttggccagtg-3′; mouse ATF4 reverse, 5′-ccattttctccaacatccaatc-3′; mouse CHOP forward, 5′-gcgacagagccagaataaca-3′; mouse CHOP reverse, 5′-atgactgcacgtggaccag-3′; mouse CTGF forward, 5′-tgacctggaggaaaacattaaga-3′; mouse CTGF reverse, 5′-agccctgtatgtcttcacactg-3′; mouse Col1a1 forward, 5′-atgttcagctttgtggacct-3′; mouse Col1a1 reverse 5′-gcagctgacttcagggatgt-3′; mouse fibronectin forward, 5′-cagaagagtgagcccctgat-3′; mouse fibronectin reverse, 5-attggggtgtggaagggta-3′; mouse α-SMA (Acta2) forward, 5′-acatagctggagcagcgtct-3′; mouse α-SMA reverse, 5′-taacccttcagcgttcagc-3′; mouse TNFα forward, 5′-tcttctcattcctgcttgtgg-3′; mouse TNFα reverse, 5′-ggtctgggccatagaactga-3′; mouse EGR2 forward, 5′-ctacccggtggaagacctc-3; EGR2 reverse, 5′-aatgttgatcatgccatctcc-3′; human MSTN forward, 5′-tggtcatgatcttgctgtaacc-3′; human MSTN reverse, 5′-cttgacctctaaaaacggattca-3′; human Akt forward, 5′-ggctattgtgaaggagggttg-3′; human Akt reverse, 5′-tccttgtagccaatgaaggtg-3′; human BNP forward, 5′- cgcaaaatggtcctctacac-3′; human BNP reverse, 5′- ccgtggaaattttgtgctc-3′; human CTGF forward, 5-ctcctgcaggctagagaagc-3; human CTGF reverse, 5′-gatgcactttttgcccttctt-3′; human Col1a1 forward, 5′-gggattccctggacctaaag-3′; human Col1a reverse, 5′-ggaacacctcgctctcca-3′; human fibronectin forward, 5′-gggagaataagctgtaccatcg-3′; human fibronectin reverse, 5′-tccattaccaagacacacacact-3′; human α-SMA forward, 5′-cctatccccgggactaagac-3′; human α-SMA reverse, 5′-aggcagtgctgtcctcttct-3′.
Data analysis.
Results are reported as means ± SE. Statistical analysis was performed by ANOVA, followed by Tukey’s post hoc test, or t-test (MSTN silencing experiments), as appropriate. For all analyses, P values were two-sided, and a P < 0.05 was considered significant. Endurance running test was analyzed by log-rank test. All data were analyzed using SPSS 22 (SPSS, Chicago, IL).
RESULTS
Inhibition of ACTRII/TGFBR signaling after MI is associated with preserved cardiac hypertrophy and increased skeletal muscle mass.
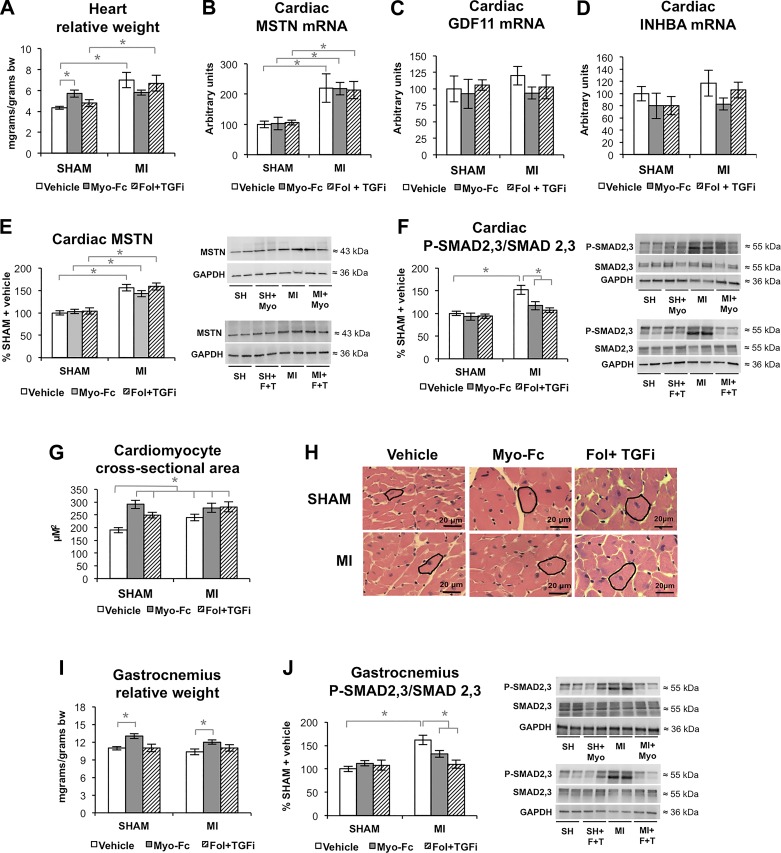
Neither MI nor ACTRII/TGFBR-inhibiting treatments resulted in changes in total body weight (average 29 ± 0.4 g body wt), adipose tissue mass, or mortality among groups. LAD ligation resulted in an increase in cardiac relative mass in mice treated with vehicle (Fig. 1A) in comparison with sham-operated mice (P < 0.05). Treatment with Myo-Fc or a combination of Fol and a TGFβ receptor inhibitor (Fol + TGFi) did not prevent or worsen increased cardiac relative mass in MI mice; however, sham mice treated with Myo-Fc had heavier hearts than sham mice treated with vehicle, suggesting anabolic properties of this compound in a nondisease setting. Real-time PCR showed an increase in cardiac MSTN mRNA in MI mice (Fig. 1B), whereas neither GDF11 (Fig. 1C) or inhibin βA-subunit (INHBA; Fig. 1D) were increased, suggesting that inhibition of ACTRII signaling in the heart after MI corresponds mainly to inhibition of MSTN signaling. MSTN propeptide protein levels were likewise increased in the heart of MI mice (Fig. 1E). As expected, the treatments did not change the expression or protein levels of MSTN. Figure 1F shows that Myo-Fc and Fol + TGFi decreased ACTRII/TGFBR cardiac signaling, attenuating the activation (phosphorylation) of SMAD2,3 in MI mice. Both treated and untreated MI mice showed cardiomyocyte hypertrophy (Figs. 1G and 1H). The anabolic effects of ACTRII/TGFBR inhibition were also manifested in sham mice, which showed enlarged cardiomyocytes comparable with MI mice. Figure 1I shows the effect of ACTRII/TGFBR inhibition on the relative mass of the gastrocnemius muscle of mice. Although we did not find an effect of MI or Fol + TGFi treatment on gastrocnemius mass, treatment with Myo-Fc increased gastrocnemius relative mass in both sham and MI mice (P < 0.05). Like in the heart, gastrocnemius P-SMAD2,3/SMAD2,3 increase in MI mice was prevented by Myo-Fc and Fol-TGFi (Fig. 1J).
Fig. 1.
Inhibition of activin type II receptor (ACTRII)/TGFβ receptor (TGFBR) signaling after myocardial infarction (MI) is associated with preserved cardiac hypertrophy and increased skeletal muscle mass. A: heart relative weight [body weight (bw)] in sham and mice with MI treated with vehicle, Myo-Fc, or a combination of follistatin and a TGFβ receptor inhibitor (Fol + TGFi); n ≥ 10 mice/group. B–D: cardiac mRNA levels of myostatin (MSTN; B), growth differentiation factor-11 (GDF11; C), and inhibin A (D); inhibin βA-subunit (INHBA), n = 5 mice/group. E and F: cardiac MSTN (E) and total and phosphorylated (P) SMAD2,3 ratio (F) and representative blots; n ≥ 8 mice/group. G and H: cardiomyocyte cross-sectional area (G) and representative images (H); n = 6 mice/group. I: gastrocnemius muscle relative weight; n ≥ 10 mice/group. J: gastrocnemius total and P-SMAD2,3 ratio and representative blots; n = 8 mice/group. *P < 0.05 by 2-way ANOVA.
Inhibition of ACTRII/TGFBR signaling is associated with preserved cardiac function and improved exercise tolerance.
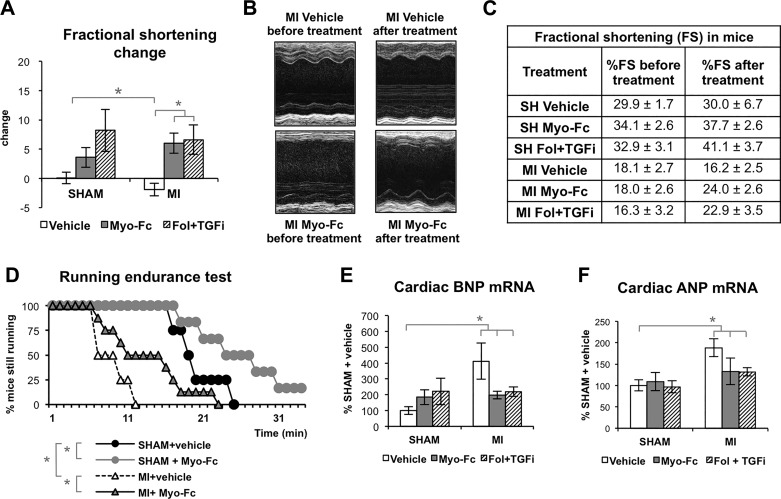
Figure 2, A–C, shows the effect of 8 wk of ACTRII/TGFBR inhibiting treatments (from 2 to 10 wk after surgery) on fractional shortening (FS). Cardiac function remained stable in sham mice and continued to worsen in MI mice treated with vehicle. Treatment of MI mice with either Myo-Fc or Fol + TGFi resulted in a significantly improved cardiac function, as measured by change in FS (P < 0.05; Fig. 2A) and improved absolute FS (Fig. 2C). Sham mice treated with ACTRII/TGFBR inhibitors showed normal cardiac function similar to untreated sham mice (Fig. 2, A and C). Additional echocardiographic parameters are shown in Supplemental Fig. S2). The preserved cardiac function by the Myo-Fc compound was also evident in the running endurance test (Fig. 2D). Whereas MI mice treated with vehicle endured running for significantly shorter times than sham mice, Myo-Fc treatment significantly increased running time in MI as well as in sham mice. We measured expression of BNP and ANP as markers of cardiac stress (Fig. 2, E and F). Correlation between MSTN and BNP has been reported previously in human HF (15). As expected, MI mice showed significantly increased BNP gene expression (412% sham mice) and ANP (188% sham). Treatment with either Myo-Fc or Fol + TGFi resulted in lower BNP (199, 220% sham mice), and moderately reduced ANP levels in MI mice, suggesting reduced cardiac stress in response to ACTRII/TGFBR signaling inhibition.
Fig. 2.
Inhibition of activin type II receptor (ACTRII)/TGFβ receptor (TGFBR) signaling is associated with preserved cardiac function and exercise tolerance. A: change in fractional shortening (FS) from 2 wk after LAD surgery to the end of treatment/vehicle (10 wk after surgery) in sham and mice with myocardial infarction (MI) treated with vehicle, Myo-Fc, or a combination of follistatin and a TGFβ receptor inhibitor (Fol + TGFi); n ≥ 10 mice/group. B and C: representative echocardiography M-mode images (B) and table showing absolute FS in sham and MI mice before and after treatment (C). D: running time (min) in running endurance test at increasing speed at the end of treatment/vehicle (10 wk after surgery) in sham and mice with myocardial infarction (MI) treated with vehicle or Myo-Fc; n ≥ 10 mice/group. E and F: cardiac mRNA levels of BNP (E) and ANP (F); n = 5 mice/group. *P < 0.05 by 2-way ANOVA (A, E, and F) or log rank test (D).
Inhibition of ACTRII/TGFBR signaling promotes hypertrophic cardiac signaling in MI mice.
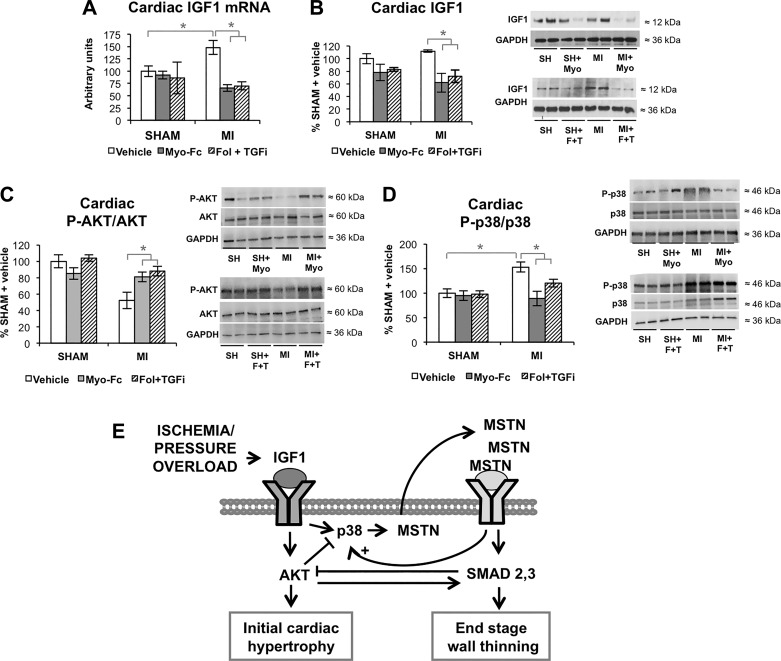
Figure 3 shows the changes in atrophic/hypertrophic cardiac signaling associated with ACTRII/TGFBR inhibition. Myo-Fc and Fol + TGFi significantly decreased pro-hypertrophic IGF-1 mRNA (Fig. 3A) and protein levels (Fig. 3B) in MI mice (P < 0.05). Phosphorylation (activation) of Akt, which is the main effector of IGF1 signaling in growth and hypertrophy, was decreased in MI mice, but treatment with the ACTRII/TGFBR inhibitors resulted in a normalization of Akt activation (P < 0.05; Fig. 3C). Increased P-p38 in MI mice was significantly prevented by Myo-Fc and Fol + TGFi treatment (both P < 0.05; Fig. 3D). Figure 3E summarizes IGF1/MSTN signaling following cardiac stress. IGF-1 signaling is exerted preferentially thorough Akt in physiological conditions, and p38 signaling gets activated under stress conditions like stretch (34). Signaling by MAP kinase (MAPK) p38 can be activated by both MSTN and IGF-1 (34) and also mediates MSTN activation downstream from IGF-1 (34). The relative ratio of Akt pathway versus p38 may determine whether the end ventricular phenotype is hypertrophy or dilation.
Fig. 3.
Inhibition of activin type II receptor (ACTRII)/TGFβ receptor (TGFBR) promotes hypertrophic cardiac signaling. A: cardiac mRNA levels of insulin-like growth factor-1 (IGF-1) in sham and mice with myocardial infarction (MI) mice treated with vehicle, Myo-Fc, or a combination of follistatin and a TGFβ receptor inhibitor (Fol + TGFi); n = 5 mice/group. B–D: cardiac protein levels of IGF-1 (B), total and phosphorylated (P) Akt (C), and total and phosphorylated MAPK p38 (D); n ≥ 8 mice/group. *P < 0.05 by 2-way ANOVA. E: schematic representation of IGF-1/myostatin (MSTN) regulation.
AKT modulation contributes to cellular effects of ACTRII/TGFBR signaling inhibition.
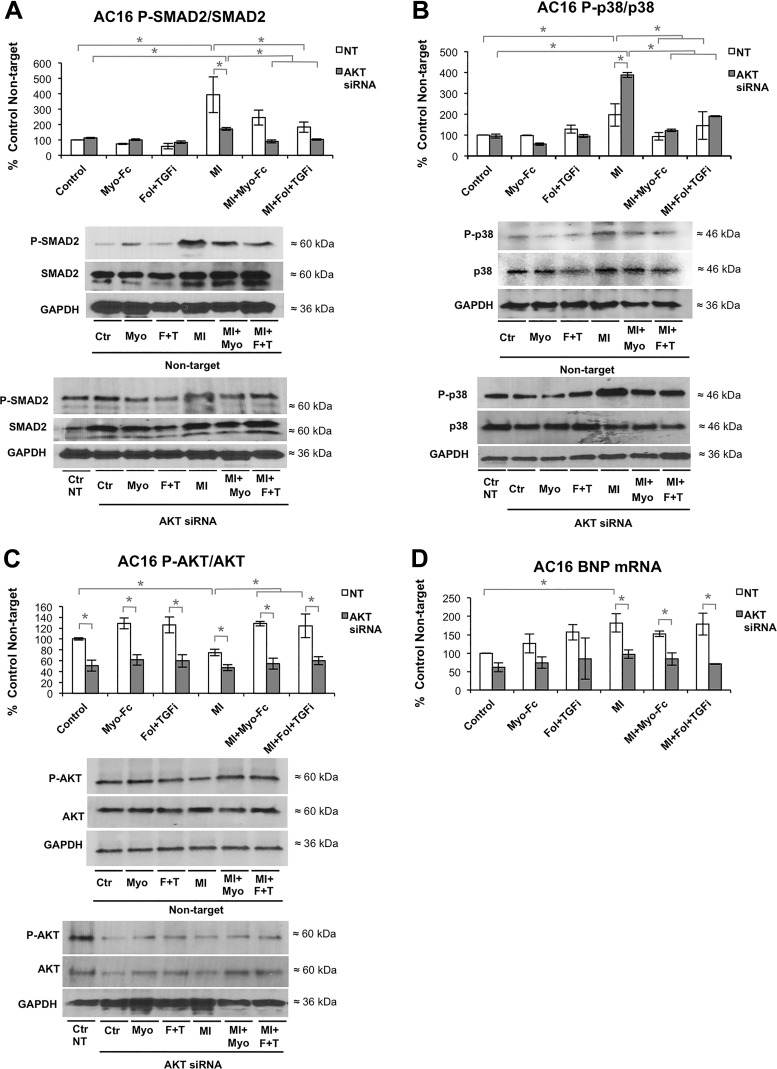
We hypothesized that the normalization of P-Akt in response to ACTRII/TGFBR inhibition may partially mediate the effect of the treatments preserving hypertrophy and preventing cardiac dysfunction. To test this hypothesis, we employed in vitro culture of AC16 cardiomyocyte-like cells. To achieve upregulation of MSTN signaling, we treated cells as previously described (9) with pooled sera from mice 10 wk after LAD ligation. Treatment of AC16 cells with MI sera increased MSTN expression to a similar degree as other in vitro models of cardiac stress like TNFα, LPS, and ANG II (Supplemental Fig. S3A). PCR analysis confirmed the downregulation of Akt [39% expression compared with non-targeting (NT) siRNA] achieved by Akt siRNA (Supplemental material Fig. S3B). The in vitro effects of post-MI serum and the ACTRII/TGFBR inhibitors were as follows: 1) MI serum resulted in an increase in P-SMAD2/SMAD2 and P-p38/p38 (Fig. 4, A and B) and a decrease in P-Akt/Akt (Fig. 4C) and 2) treatment with Myo-Fc or Fol + TGFi attenuated the effect of post-MI serum in cells transfected with NT siRNA (P < 0.05; Fig. 4, A–C). MI serum-induced P-SMAD2 increase was lower in cells transfected with Akt siRNA vs. NT (Fig. 4A), whereas P-p38/p38 increase was higher in cells transfected with Akt siRNA vs. NT (Fig. 4B). Myo-Fc- and Fol + TGFi-mediated reduction in P-SMAD2 and P-p38 was preserved in cells transfected with Akt siRNA (Fig. 4, A and B). BNP gene expression was increased in cells treated with MI sera (Fig. 4D). Myo-Fc or Fol + TGFi did not prevent BNP expression increase in vitro, whereas Akt silencing decreased BNP mRNA in all MI sera-treated groups (P < 0.05; Fig. 4D). These results indicate that Akt is involved in P-SMAD2, P-p38, and BNP regulation in cardiomyocytes under stress and may partially mediate changes in BNP levels in response to ACTRII/TGFBR inhibition.
Fig. 4.
Akt modulation contributes to cellular effects of activin type II receptor (ACTRII)/TGFβ receptor (TGFBR) signaling inhibition. Protein levels of total and phosphorylated (P) SMAD2 (A), total and phosphorylated MAPK p38 (B), and total and phosphorylated Akt (C) and gene expression levels of brain natriuretic peptide (BNP; D) in AC16 cultured cardiomyocytes transfected with nontargeting (NT) or Akt siRNA, followed by 24-h treatment with sham (control) or 10-wk post-myocardial infarction (MI) mouse serum, in the presence or absence of 0.2 μg/ml Myo-Fc or a combination of follistatin (10 ng/ml) and a TGFβ receptor inhibitor (1 μM) (Fol + TGFi). Results are expressed as %NT control; n = 3/condition. *P < 0.05 by 2-way ANOVA.
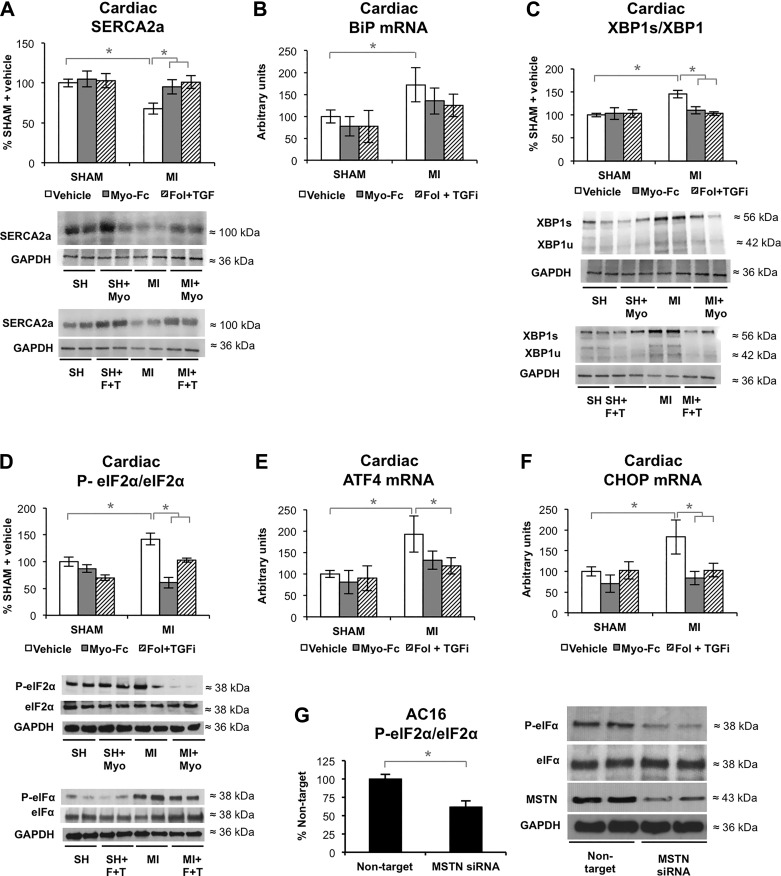
ACTRII/TGFBR signaling inhibition may improve calcium cycling and prevents unfolded protein response after experimental MI.
Ca2+ cycling by decreased SERCA2a is one of the hallmarks of HF (10). Treatment with Myo-Fc and Fol + TGFi significantly prevented SERCA2a decrease in MI mice (Fig. 5A). Impaired Ca2+ cycling can lead to the activation of endoplasmic reticulum stress and subsequent unfolded protein response (UPR) (10) and apoptosis. The expression of the master UPR switch BiP was increased in MI mice and unchanged by the treatments in sham and MI mice (Fig. 5B). BiP activates three different UPR pathways: serine/threonine-protein kinase/endoribonuclease (IRE1), activating-transcription factor 6 (ATF6), and eukaryotic translation initiation factor-2α kinase 3 (PERK). IRE1 and ATF6 signaling branches converge by activating XBP1 and its target genes. Activated PERK phosphorylates eIF2α, which attenuates translation initiation and protein synthesis, and activates apoptosis via ATF4 and CHOP. MI mice (Figs. 5, C–F) had increased XBP1 (spliced/total XBP1 ratio), eIF2α (phosphorylation), and expression of eIF2α targets ATF4 and CHOP. Myo-Fc and Fol + TGFi prevented the increase in XBP1, eIF2α, ATF4, and CHOP. Despite the effect seen in CHOP expression, apoptosis (DNA fragmentation staining), which was limited to the LV dilation area in MI mice, was not visibly decreased by Myo-Fc or Fol + TGFi treatments (Supplemental Fig. S4A). To investigate whether MSTN exerts a direct regulation of SERCA2a, XBP1, and eIF2α in cardiomyocytes, we tested the effect of MSTN silencing on AC16 cells. MSTN silencing by siRNA reduced MSTN signaling to a similar degree as MSTN inhibitors, as shown in Supplemental Fig. S3, B–E. Silencing of MSTN (52% knockdown) on AC16 cells did not affect SERCA2a or XBP1 activation (Supplemental Fig. S4, B and C). MSTN knockdown significantly decreased eIF2α phosphorylation (Fig. 5G), suggesting a direct effect of MSTN on the UPR by interfering with eIF2α activation.
Fig. 5.
Inhibition of activin type II receptor (ACTRII)/TGFβ receptor (TGFBR) may improve calcium cycling and prevents unfolded protein response (UPR) after experimental myocardial infarction (MI). A: cardiac protein levels of increased sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) and representative blots of sham and MI mice treated with vehicle, Myo-Fc, or a combination of follistatin and a TGFβ receptor inhibitor (Fol + TGFi); n ≥ 8 mice/group. B: cardiac gene expression levels of binding immunoglobulin protein (BiP); n = 5 mice/group. C and D: spliced/total ratio of X-box binding protein (XBP1; C) and total and phosphorylated eukaryotic translation initiation factor-2α (eIf2α) and representative blots (D), n ≥ 8 mice/group. E and F: cardiac gene expression levels of activating transcription factor 4 (ATF4; E) and C/EBP homologous protein (CHOP; F); n = 5 mice/group. G: total and phosphorylated eIF2α and representative blots of AC16 cultured cardiomyocytes transfected with nontargeting or MSTN siRNA. Results are expressed as %nontarget; n = 4/condition. *P < 0.05 by 2-way ANOVA (A–F) or t-test (G).
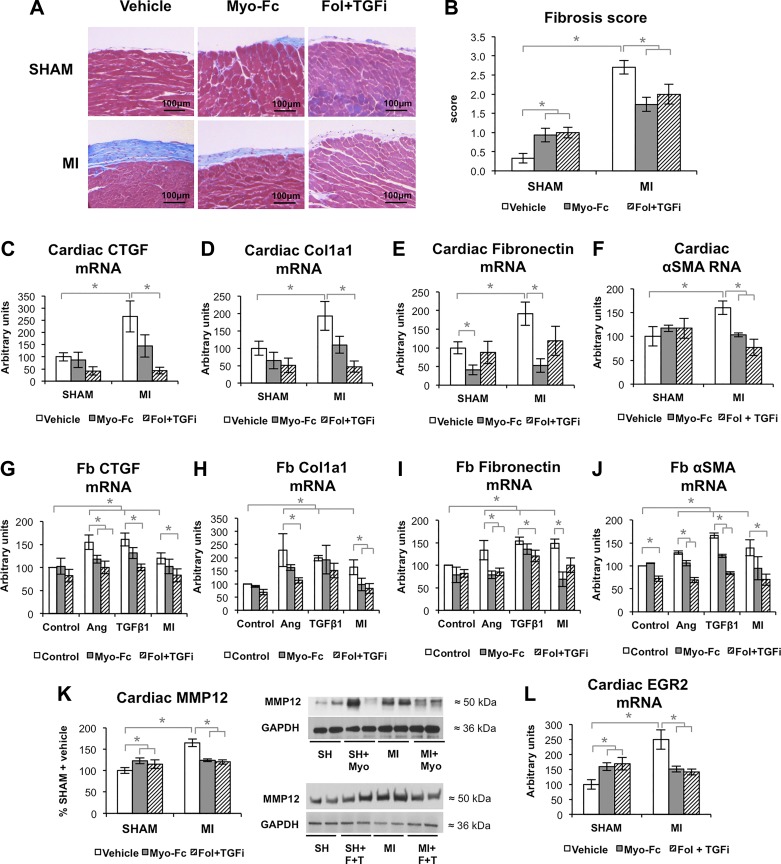
ACTRII/TGFBR signaling inhibition prevents fibrosis after experimental MI.
MI mice had increased interstitial and epicardial fibrosis in the infarction-remote area (Figs. 5A and 6B and Supplemental Fig. S5) as well as perivascular fibrosis (Supplemental Fig. S6). Treatment with the ACTRII/TGFBR inhibitors significantly decreased the degree of fibrosis in MI mice; however, both Myo-Fc and Fol + TGFi induced fibrosis in sham-operated mice. PCR analysis showed significantly increased CTGF, Col1a1, fibronectin, and α-SMA mRNA levels in MI mice (Fig. 6, C–F). The combination of Fol and a TGFBR inhibitor resulted in significantly decreased CTGF, Col1a1, and α-SMA mRNA levels in MI mice, whereas Myo-Fc treatment significantly decreased fibronectin levels and α-SMA and in MI mice. To test the antifibrotic properties of Myo-Fc and Fol + TGFi in cardiac fibroblasts, we tested whether they could prevent the pro-fibrotic phenotype induced by MI serum, TGFβ1, and ANG II. In vitro results (Fig. 6, G–J) confirmed the in vivo findings, with Fol + TGFi preventing CTGF increase as well as ANG II- and MI-induced increase in Col1a1, and Myo-Fc preventing ANG II- and MI serum-induced increase in fibronectin mRNA. α-SMA increase in response to ANG II, TGFβ1, and MI serum was prevented by Fol + TGFi, whereas Myo-Fc partially decreased ANG II and TGFβ1 effect increased α-SMA. We assessed the cardiac levels of several MMPs and tissue inhibitors of MMPs (TIMPs), which are involved in the maturation phase of the fibrotic scar. We found that MMP12 (Fig. 6K) was significantly lower in treated MI animals versus vehicle (both P < 0.05) and increased in sham mice. To test a possible role of inflammation and cardiac macrophages in MMP12 levels, we tested the expression of TNFα as a marker of pro-inflammatory macrophages and EGR2 as a marker of alternative, anti-inflammatory, M2-like macrophages (19). TNFα expression was not increased in the heart or the skeletal muscle or MI mice (Supplemental Fig. S7, A and B), and neither were the circulating levels of IL6 (Supplemental Fig. S7C). Conversely, EGR2 expression levels followed a pattern similar to MMP12 and fibrosis, suggesting a possible link between M2-like macrophages, MMP12, and fibrosis in response to MI and ACTRII/TGFBR inhibition.
Fig. 6.
Inhibition of activin type II receptor (ACTRII)/TGFβ receptor (TGFBR) signaling prevents fibrosis after experimental myocardial infarction (MI). A and B: representative images of epicardial fibrosis (A) and fibrosis score (B) of sham and mice with MI treated with vehicle, Myo-Fc, or a combination of follistatin and a TGFβ receptor inhibitor (Fol + TGFi); n = 6 mice/group. C–F: cardiac mRNA levels of connective tissue growth factor (CTGF; C), type I collagen (Col1a1; D), fibronectin (E), and α-smooth muscle actin (α-SMA; F); n = 5 mice/group. mRNA levels of CTGF (G), Col1a1 (H), fibronectin (I) and α-SMA (J) in cultures of human ventricular cardiac fibroblasts (Fb) treated for 24 h with sham (control) or 10 wk post-MI mouse serum (MI), angiotensin II (ANG II; 10 μM) or TGFβ1 (10 ng/ml) in the presence or absence of 0.2 μg/ml Myo-Fc or a combination of follistatin (10 ng/ml) and a TGFβ receptor inhibitor (1 μM; Fol + TGFi). K: cardiac protein levels of matrix metalloproteinase 12 (MMP12) and representative blots, n ≥ 8 mice/group. L: cardiac early growth response gene-2 (EGR2) mRNA; n = 5 mice/group. *P < 0.05 by 2-way ANOVA.
DISCUSSION
In this study, we report that ACTRII/TGFBR signaling blockade after MI can prevent pathological cardiac remodeling, potentially preserve cardiac function, and reduce fibrosis. We propose Akt signaling, MSTN-mediated SMAD2,3 blockade, fibrosis reduction, and UPR as possible mechanisms mediating the cardiac effects of ACTRII/TGFBR inhibition after MI. The major findings in our study are as follows: 1) ACTRII/TGFBR inhibition improved FS by echocardiography in mice with established HF; 2) ACTRII/TGFBR inhibition was associated with cardiac hypertrophy in sham mice, but it did not worsen cardiac function in sham animals and did not exacerbate cardiomyocyte hypertrophy after MI; 3) treatment with ACTRII/TGFBR inhibitors normalized cardiac P-SMAD2,3 and P-p38 in MI as well as prevented P-Akt downregulation; 4) Akt partially mediated the effect of ACTRII/TGFBR inhibitors in cardiomyocyte-like cells under stress; 5) ACTRII/TGFBR inhibition was associated with increased SERCA2a levels and a prevention of UPR in MI mice; 6) ACTRII/TGFβ inhibition was associated with a fibroblast-driven decrease in cardiac fibrosis, including fibroblast activation in MI mice; and 7) MSTN exerted a direct regulation on the activation of UPR marker eIF2α in cardiomyocyte-like cells. Inhibition of TGFR by GW788388 was associated only with a stronger effect than inhibition of ACTRII alone on markers of fibrosis (CTGF, Col1a1, and αSMA). It is important to note that ACTRII/TGFBR inhibition in sham mice was associated with two potential undesirable effects, cardiomyocyte hypertrophy and mild increase in fibrosis, although cardiac function was not negatively affected, and sham mice treated with Myo-Fc performed significantly longer time in the running test. The lack of upregulation of GDF11 and INHBA mRNA in the heart of MI mice suggests that the effect of the treatments can be attributed to the inhibition of increased MSTN signaling after MI. Studies by us (9) and others (16) have suggested that circulating MSTN, likely from cardiac origin, may activate SMAD2,3 signaling and protein degradation in the skeletal muscle after MI and in HF. As expected, ACTRII/TGFBR inhibition increased skeletal muscle mass and normalized SMAD2,3 activation in our study.
The positive results that we found in cardiac function and remodeling in MI mice contrast in some regard with the study by Biesemann et al. (4), who described that blockade of cardiac MSTN in adult mice is enough to cause HF. It has been suggested that synergistic toxicity of MSTN knockdown together with the high dose of tamoxifen used in the study may have mediated the development of HF in mice (26). In the study by Biesemann et al. (4), cardiomyocyte-specific deletion of MSTN increased lethality after 10 days and caused atrial and ventricular dilation and impaired LV function, as well as fibrosis. The role of TGFβ signaling on cardiac fibrosis is well established, and MSTN may mediate fibrosis in skeletal muscle (25). We measured fibrosis after ACTRII/TGFBR inhibition and, not unexpectedly, found that fibrosis, CTGF, fibronectin, Col1a1, and α-SMA decreased along with reductions in SMAD2,3 signaling and MMP12 levels; limitation of fibrosis after MI can serve as an important component to preserve ventricular function. The parallel increase in MMP12 protein levels and fibrosis in sham mice treated with ACTRII/TGFBR inhibitors suggested a possible involvement of macrophages, which are an important source of MMP12. Moreover, MSTN inhibition is associated with macrophage infiltration in the skeletal muscle (35). Inflammation, as reflected by IL-6 and TNFα, was not increased in MI mice and may be more characteristic of earlier time points in post-MI remodeling. Anti-inflammatory, M2-like macrophages are involved in cardiac fibrosis (28). The expression of the M2 marker EGR2 followed the same trend among groups as fibrosis and MMP12, suggesting that a macrophage subpopulation may be involved in the observed effect in sham mice treated with ACTRII/TGFBR inhibitors.
The effect of ACTRII/TGFBR inhibition on cardiac remodeling may be partially mediated by IGF-1/Akt-dependent signaling. Treatment with Myo-Fc or Fol + TGFi was associated with decreased cardiac IGF-1; however, the activation of the IGF-1 main effector, Akt, was increased in MI mice treated with the inhibitors. The observed restoration of P-Akt levels by ACTRII/TGFBR inhibitors is consistent with several studies that have reported an inhibitory effect of MSTN on Akt activation (5, 17, 27). Our in vitro data support the hypothesis that Akt partially mediates the observed effect of ACTRII/TGFBR inhibitors in cardiac remodeling. The decreased BNP expression levels in MI mice treated with inhibitors may be mediated by restored Akt. Although under Akt silencing MI serum still resulted in an upregulation of P-SMAD2,3, this increase was lower than in the NT siRNA-treated cells. A role of Akt decreasing SMADs has previously been reported in different cell populations, although the cross-talk between SMADs and Akt is complex (13).
Under stress conditions like stretch, p38 mediates IGF1-dependent cardiac MSTN activation (34). Conversely, MSTN signaling can activate p38, which may in turn contribute to increased MSTN levels via feedback regulation (4). As expected, ACTRII/TGFBR inhibition resulted in a decrease in p38 phosphorylation. Overall, our results indicate a pro-hypertrophic profile in MI mice treated with MSTN inhibitors 10 wk after MI induction. An adequate balance between pro-hypertrophic and anti-hypertrophic signaling may be necessary to preserve cardiac function and avoid LV dilation, particularly after MI. An initial, limited upregulation of MSTN immediately after MI may be beneficial to help restrict hypertrophic signaling, reduce myocardial oxygen demand and thereby lessen ischemic injury, and improve cardiac contractility, whereas extended activation of MSTN conversely may lead to late dilation and fibrosis (8, 14). We hypothesize that MSTN blockade after the onset of cardiac hypertrophic stress response signaling and the formation of the initial fibrotic scar may be the most advantageous strategy to prevent end-stage cardiac wall thinning.
Restoration of adequate Ca2+ cycling and attenuated UPR with ACTRII/TGFBR inhibition may play a role in the improved contractility seen in treated animals. Impaired SERCA2a function is considered central to Ca2+ deregulation in HF, and the improvement in SERCA2a found may reflect an overall improvement in cellular processes, as suggested by the lack of a direct effect of MSTN on SERCA2a levels in cardiomyocyte-like cells. Elevated SERCA2a levels have been described in MSTN-KO mice (21) and following ACTRII inhibition in aged mice and mice with nonischemic heart failure (32). It has been previously reported that endoplasmic reticulum stress can activate MSTN (6); our results suggest that, in turn, MSTN may activate eIF2α in cardiomyocytes.
Despite our best efforts, this study has several limitations. Besides the pathways we propose (IGF-1-related signaling, Ca2+ cycling and UPR), other signaling mechanisms may be involved in the effect of ACTRII/TGFBR inhibition treatments. Specifically, the relationship between ACTRII ligands and inflammatory cells in cardiac repair, which is likely complex due to the multiple cell populations involved in post-MI remodeling, will need to be explored in follow-up studies. Logistic reasons prevented us for performing the running test in the Fol + TGFi groups. Hemodynamics were not assessed, and it is possible that changes in whole body hemodynamics indirectly affect cardiac behavior. Cardiac function as measured by fractional shortening alone is imprecise in small animals, and interpretation of these data requires caution; however, support of cardiac signaling showing positive benefits and exercise tolerance/endurance testing is highly suggestive that Myo-Fc treated animals had improved cardiac performance. Finally, this study was performed only in male mice, whereas AC16 cells are female. Possible sex-specific effects of the treatments cannot be excluded.
Our observations are important from a clinical standpoint regarding cardiac remodeling and cachexia management, as they support the hypothesis that SMAD2,3 downregulation by ACTRII inhibitors has the potential of improving cardiac homeostasis as well as preserving muscle mass, which could result in an improved quality of life for HF patients. The prevalence of cardiac cachexia has been estimated to be between 8 and 42%, depending on the diagnosis criteria, and its presence dramatically negatively affects survival, with a hazard ratio for death of 3.73 (1). The cardiac remodeling effects of ACTRII/TGFBR inhibition observed in sham and MI mice make it important to identify the specific patients and stages of HF disease that could benefit from this pharmacological strategy. A timely blockade of ACTRII after MI, at a time when pro-hypertrophic signaling has remitted, could be beneficial to prevent worsening wall thinning or aid in myocardial recovery programs. A second population that may benefit are HF patients supported with LV assisted devices (LVAD); these patients have increased MSTN levels and are at risk of myocardial atrophy with prolonged durations of LVAD support (1, 11).
Conclusions.
To conclude, the current study showed that ACTRII/TGFBR signaling inhibition after myocardial infarction has beneficial effects preserving cardiac function and signaling and preventing fibrosis, which may attenuate the progression to end-stage HF.
GRANTS
This work was supported in part by an American Association of Thoracic Surgeons Graham Research Award 2016-2018 (to I. Geroge), a 2017 Irving Institute/Clinical Trials Office Pilot Award (to E. Castillero), and National Heart, Lung, and Blood Institute Grants R01-122805 and R01-131872 (to G. Ferrari).
DISCLOSURES
The MSTN inhibitor Myo-Fc was kindly provided by Pfizer. P. C. Colombo is recipient of a research grant from Abbott, also serving as a consultant (with no honoraria) for the same company.
AUTHOR CONTRIBUTIONS
E.C., H.L.S., and I.G. conceived and designed research; E.C., H.A., M.N., R.J., L.M.B., X.L., X.Z., A.S., M.G., K.G., A.R., and I.S. performed experiments; E.C., H.A., M.N., R.J., and X.L. analyzed data; E.C., C.W., X.L., P.C.C., G.F., P.C.S., and I.G. interpreted results of experiments; E.C. prepared figures; E.C. drafted manuscript; E.C., H.A., M.N., R.J., C.W., X.Z., H.L.S., Z.A.A., S.H., P.C.C., G.F., P.C.S., and I.G. edited and revised manuscript; I.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the kind generosity of Pfizer and Dr. Peter Bialek, Dr. Jane Owens, and Dr. Michael St. Andre for support of this project.
REFERENCES
- 1.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349: 1050–1053, 1997. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 2.Artaza JN, Reisz-Porszasz S, Dow JS, Kloner RA, Tsao J, Bhasin S, Gonzalez-Cadavid NF. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol 194: 63–76, 2007. doi: 10.1677/JOE-07-0072. [DOI] [PubMed] [Google Scholar]
- 3.Biesemann N, Mendler L, Wietelmann A, Hermann S, Schäfers M, Krüger M, Boettger T, Borchardt T, Braun T. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circ Res 115: 296–310, 2014. doi: 10.1161/CIRCRESAHA.115.304185. [DOI] [PubMed] [Google Scholar]
- 4.Biesemann N, Mendler L, Kostin S, Wietelmann A, Borchardt T, Braun T. Myostatin induces interstitial fibrosis in the heart via TAK1 and p38. Cell Tissue Res 361: 779–787, 2015. doi: 10.1007/s00441-015-2139-2. [DOI] [PubMed] [Google Scholar]
- 5.Bish LT, Morine KJ, Sleeper MM, Sweeney HL. Myostatin is upregulated following stress in an Erk-dependent manner and negatively regulates cardiomyocyte growth in culture and in a mouse model. PLoS One 5: e10230, 2010. doi: 10.1371/journal.pone.0010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420: 418–421, 2002. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19: 543–549, 2005. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart A, Auger-Messier M, Molkentin JD, Heineke J. Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting. Am J Physiol Heart Circ Physiol 300: H1973–H1982, 2011. doi: 10.1152/ajpheart.00200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillero E, Akashi H, Wang C, Najjar M, Ji R, Kennel PJ, Sweeney HL, Schulze PC, George I. Cardiac myostatin upregulation occurs immediately after myocardial ischemia and is involved in skeletal muscle activation of atrophy. Biochem Biophys Res Commun 457: 106–111, 2015. doi: 10.1016/j.bbrc.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillero E, Akashi H, Pendrak K, Yerebakan H, Najjar M, Wang C, Naka Y, Mancini D, Sweeney HL, D’Armiento J, Ali ZA, Schulze PC, George I. Attenuation of the unfolded protein response and endoplasmic reticulum stress after mechanical unloading in dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 309: H459–H470, 2015. doi: 10.1152/ajpheart.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillero E, Ali ZA, Akashi H, Giangreco N, Wang C, Stöhr EJ, Ji R, Zhang X, Kheysin N, Park JS, Hegde S, Patel S, Stein S, Cuenca C, Leung D, Homma S, Tatonetti NP, Topkara VK, Takeda K, Colombo PC, Naka Y, Sweeney HL, Schulze PC, George I. Structural and functional cardiac profile after prolonged duration of mechanical unloading: potential implications for myocardial recovery. Am J Physiol Heart Circ Physiol 315: H1463–H1476, 2018. doi: 10.1152/ajpheart.00187.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord 17: 290–296, 2007. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res 69: 15–25, 2006. doi: 10.1016/j.cardiores.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail 12: 444–453, 2010. doi: 10.1093/eurjhf/hfq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF. Increased plasma myostatin in heart failure. Eur J Heart Fail 13: 734–736, 2011. doi: 10.1093/eurjhf/hfr024. [DOI] [PubMed] [Google Scholar]
- 16.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 121: 419–425, 2010. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitachi K, Nakatani M, Tsuchida K. Myostatin signaling regulates Akt activity via the regulation of miR-486 expression. Int J Biochem Cell Biol 47: 93–103, 2014. doi: 10.1016/j.biocel.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Hosoyama T, Yamanouchi K, Nishihara M. Role of serum myostatin during the lactation period. J Reprod Dev 52: 469–478, 2006. doi: 10.1262/jrd.18009. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado JD, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 macrophages. PLoS One 10: e0145342, 2015. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson MF, Luong D, Vang DD, Garikipati DK, Stanton JB, Nelson OL, Rodgers BD. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J Endocrinol 213: 263–275, 2012. doi: 10.1530/JOE-11-0455. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MF, Li N, Rodgers BD. Myostatin regulates tissue potency and cardiac calcium-handling proteins. Endocrinology 155: 1771–1785, 2014. doi: 10.1210/en.2013-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306–9311, 2001. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JF, Hsu SY, Teng MS, Wu S, Hsieh CA, Jang SJ, Liu CJ, Huang HL, Ko YL. Activin A predicts left ventricular remodeling and mortality in patients with st-elevation myocardial infarction. Acta Cardiol Sin 32: 420–427, 2016. doi: 10.6515/acs20150415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153: 828–839, 2013. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 118: 3531–3541, 2005. doi: 10.1242/jcs.02482. [DOI] [PubMed] [Google Scholar]
- 26.McLean BA, Oudit GY. Letter by McLean and Oudit regarding article, “myostatin regulates energy homeostasis in the heart and prevents heart failure”. Circ Res 116: e51–e52, 2015. doi: 10.1161/CIRCRESAHA.115.306124. [DOI] [PubMed] [Google Scholar]
- 27.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99: 15–24, 2006. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Rourke SA, Dunne A, Monaghan MG. The Role of Macrophages in the Infarcted Myocardium: Orchestrators of ECM Remodeling. Front Cardiovasc Med 6: 101, 2019. doi: 10.3389/fcvm.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen M, Thorikay M, Deckers M, van Dinther M, Grygielko ET, Gellibert F, de Gouville AC, Huet S, ten Dijke P, Laping NJ. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int 73: 705–715, 2008. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 30.Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab 285: E876–E888, 2003. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers BD, Interlichia JP, Garikipati DK, Mamidi R, Chandra M, Nelson OL, Murry CE, Santana LF. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J Physiol 587: 4873–4886, 2009. doi: 10.1113/jphysiol.2009.172544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, Platt C, Damilano F, Lindman BR, Elmariah S, Biersmith M, Lee SJ, Seidman CE, Seidman JG, Gerszten RE, Lach-Trifilieff E, Glass DJ, Rosenzweig A. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med 11: eaau8680, 2019. doi: 10.1126/scitranslmed.aau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol 180: 1–9, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 68: 405–414, 2005. doi: 10.1016/j.cardiores.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, Kambadur R, Sharma M. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther 15: 1463–1470, 2007. doi: 10.1038/sj.mt.6300182. [DOI] [PubMed] [Google Scholar]
- 36.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res 117: 926–932, 2015. doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zizola C, Kennel PJ, Akashi H, Ji R, Castillero E, George I, Homma S, Schulze PC. Activation of PPARδ signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction. Am J Physiol Heart Circ Physiol 308: H1078–H1085, 2015. doi: 10.1152/ajpheart.00679.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]