Abstract
Complications associated with spinal cord injury (SCI) result from unregulated reflexes below the lesion level. Understanding neurotransmission distal to the SCI could improve quality of life by mitigating complications. The long-term impact of SCI on neurovascular transmission is poorly understood, but reduced sympathetic activity below the site of SCI enhances arterial neurotransmission (1). We studied sympathetic neurovascular transmission using a rat model of long-term paraplegia (T2–3) and tetraplegia (C6–7). Sixteen weeks after SCI, T2–3 and C6–7 rats had lower blood pressure (BP) than sham rats (103 ± 2 and 97 ± 4 vs. 117 ± 6 mmHg, P < 0.05). T2–3 rats had tachycardia (410 ± 6 beats/min), and C6–7 rats had bradycardia (299 ± 10 beats/min) compared with intact rats (321 ± 4 beats/min, P < 0.05). Purinergic excitatory junction potentials (EJPs) were measured in mesenteric arteries (MA) using microlectrodes, and norepinephrine (NE) release was measured using amperometry. NE release was similar in all groups, while EJP frequency-response curves from T2–3 and C6–7 rats were left-shifted vs. sham rats. EJPs in T2–3 and C6–7 rats showed facilitation followed by run-down during stimulation trains (10 Hz, 50 stimuli). MA reactivity to exogenous NE and ATP was similar in all rats. In T2–3 and C6–7 rats, NE content was increased in left cardiac ventricles compared with intact rats, but was not changed in MA, kidney, or spleen. Our data indicate that peripheral purinergic, but not adrenergic, neurotransmission increases following SCI via enhanced ATP release from periarterial nerves. Sympathetic BP support is reduced after SCI, but improving neurotransmitter release might maintain cardiovascular stability in individuals living with SCI.
NEW & NOTEWORTHY This study revealed increased purinergic, but not noradrenergic, neurotransmission to mesenteric arteries in rats with spinal cord injury (SCI). An increased releasable pool of ATP in periarterial sympathetic nerves may contribute to autonomic dysreflexia following SCI, suggesting that purinergic neurotransmission may be a therapeutic target for maintaining stable blood pressure in individuals living with SCI. The selective increase in ATP release suggests that ATP and norepinephrine may be stored in separate synaptic vesicles in periarterial sympathetic varicosities.
Keywords: excitatory junction potential, mesenteric arteries, purinergic neurotransmission, spinal cord injury, sympathetic neurotransmission
INTRODUCTION
When the spinal cord is injured, autonomic control becomes unbalanced as autonomic functions above the level of the injury remain under brain stem control whereas autonomic functions below the level of the injury are affected by diminished supraspinal input. The dysregulated reflexes are facilitated by structural neuroplastic changes in the spinal cord and peripheral tissues which lead to several complications associated with spinal cord injury (SCI) (3, 7, 19, 23, 24, 31, 32, 38, 40, 43, 51, 52, 61, 69). Accordingly, a better understanding of neuroeffector mechanisms below the level of the injury may improve the quality of life for individuals and families living with SCI.
SCI causes impaired mobility, chronic pain, spasticity, and autonomic dysfunction. These cardiovascular complications are most likely to occur in individuals with cervical (C6–7) or high thoracic injuries (T2–3) (25). Disruption of descending autonomic control and unregulated spinal reflexes play a major role in cardiovascular instability and cause autonomic dysreflexia, which is a life-threatening episode of severe hypertension caused by unregulated spinal reflexes. Although spinal neurons regenerate after SCI (29, 30), the restored synaptic inputs to sympathetic preganglionic neurons are altered (39) as preganglionic neurons may receive more inputs from spinal sympathetic interneurons (30, 60). Therefore, synapses in sympathetic ganglia and the neurovascular junction are also affected, causing enhanced neurogenic vascular contraction (1). It is known that that reduced sympathetic activity below the level of a SCI causes enhancement of neurovascular transmission to arteries (1). Accordingly, reflex activation of sympathetic neurons below the SCI causes vasoconstriction, increased vascular resistance, reduced blood flow, and hypertension.
The neuroeffector mechanisms responsible for enhanced neurogenic vasoconstriction are unknown. Sympathetic nerves supplying mesenteric arteries (MA) release norepinephrine (NE), ATP (or related purine), and neuropeptide Y (NPY). NE is the dominant vasoconstrictor transmitter released from periarterial sympathetic nerves (17, 35). ATP coreleased with NE contributes to arterial constriction although ATP may be stored in separate synaptic vesicles from other neurotransmitters (48). NPY is also released from periarterial sympathetic nerves during high-frequency stimulation. NPY sensitizes arterial smooth muscle to the constrictor effects of NE and directly causes arterial constriction (15, 55). However, previous studies using bladder distention to evoke a pressor response in rats with SCI showed that the pressor response was blocked by combined treatment with α- and β-adrenergic receptor antagonists while there was no change in circulating NPY (59). These data suggest that NPY does not contribute significantly to autonomic reflexes.
Here, we demonstrate differential alterations in purinergic and adrenergic sympathetic neurotransmission to MA from chronic T2–3 and C6–7 rats at 16 wk after SCI. Real-time nerve-evoked NE release from periarterial nerves was measured by continuous amperometry with carbon fiber electrodes (54). Excitatory junction potentials (EJPs) recorded from arterial smooth muscle cells were used as an indirect measure of neuronal ATP release (12). Understanding SCI-induced alterations in the function of the neurovascular junction may identify new therapeutic targets for maintaining stable cardiovascular function for individuals living with SCI.
MATERIALS AND METHODS
Animals and experimental procedures.
Experimental procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Michigan State University (AUF 10/17–179–00) and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. We used male Sprague-Dawley rats (Envigo, Indianapolis, IN) starting at 10 wk of age and continuing for 16–17 wk. Following the spinal cord or sham transection, rats were housed separately in standard rat cages (~13,350 cm3) in a dedicated animal room where ambient temperature was maintained at the thermoneutral zone for rats between 28 and 32°C. Entry into the animal room was restricted to the investigators, ensuring that the animals were not unnecessarily disturbed. Arterial pressure, heart rate, and activity counts were recorded using radiotelemeters in conscious animals for 20 s every minute, 24 h/day for 12 consecutive weeks. At the end of the 12-wk period, rats were deeply anesthetized with isoflurane and the heart was excised. The mesentery was surgically removed and kept in cold Krebs buffer before electrophysiology recordings.
Surgical procedures.
Two survival surgeries were performed on each animal. Before each surgery, animals received atropine (0.05 mg/kg). In addition, multimodal preemptive analgesia was achieved using a local infiltration of bupivacaine (diluted to 0.25%, 5 mg/kg) and lidocaine (diluted to 0.5%, 5 mg/kg) around the incision site (sc) and the administration of a nonsteroidal anti-inflammatory agent (carprofen; 10 mg/kg ip). Animals were anesthetized initially with 3–5% isoflurane and intubated. Subsequently, animals were supported by a ventilator delivering isoflurane at 1–3%. Carprofen and buprenorphine (0.1 mg/kg) were administered for 3 days postoperatively. In addition, the antibiotic cefazolin (10 mg/kg) was administered preoperatively and for 3 days postoperatively. All peri- and postoperative agents were purchased from Covetrus North America (formerly Henry Schein Animal Health, Columbus, OH).
First surgical procedure.
Using aseptic conditions and after anesthesia, a catheter from a telemetry device (PA-C40, Data Sciences International) was inserted into the left carotid artery and advanced into the descending aorta for recording arterial pressure, heart rate, and locomotor activity, and a second catheter was implanted into the intraperitoneal space (ip) for the infusion of fluids and drugs. The catheter was exteriorized on the dorsal aspect of the neck.
All animals remained on the feedback-based temperature control system and ventilator until recovery from the anesthesia. Once the animals regained consciousness, they were placed in a “rodent recovery cage” (Thermocare Intensive Care Unit, Paso Robles, CA). Animals were returned to the housing room when fully recovered from the anesthesia and gained the ability to maintain an upright body position and body temperature. At least 14 days were allowed for recovery. During the recovery period, the rats were provided with supplemental enrichment treats (Bio-Serv, Flemington, NJ), handled, and weighed.
Second surgical procedure.
This procedure has been described in previously published work (42, 44). Following recovery from the first surgery and after the rats returned to their presurgical weight, the second surgical procedure was performed. Rats were anesthetized as described earlier and subjected to a complete C6–7, T2–3, or sham spinal cord transection. Specifically, the spinous process and laminae of the sixth cervical vertebrae were removed exposing the sixth to seventh cervical spinal cord segment. The T2–3 spinal cord segment is readily seen without a laminectomy. The spinal cord was subsequently transected between C6–7 or T2–3 using a microknife (10316-14, Fine Science Tools) and Vannas spring scissors (15000-08, Fine Science Tools). Spinal cord transection between C6–7 eliminates supraspinal control of all sympathetic preganglionic neurons. Spinal cord transection between T2–3 preserves supraspinal control to some sympathetic preganglionic neurons. The extensiveness of the spinal cord transection was established by visually inspecting the transection site. Similar procedures were followed for the sham-transected rats, except the spinal cord was not transected.
Injury reproducibility is an important characteristic of experimental models of SCI because it limits the variability in outcome measures. Although spinal cord contusion injuries may be the most clinically relevant model since most injuries in humans occur in a similar manner, this model has a greater variability in outcome measures and spared descending systems compared with the complete spinal cord transection model. Therefore, to enhance injury reproducibility, we used the complete spinal cord transection model.
Acute postoperative care for spinal cord-injured animals.
Immediately following the spinal cord transection, animals present with flaccid paralysis and loss of sensation below the level of the lesion. This poses a major challenge to the C6–7- injured animal that requires 24-h assistance with basic needs including eating, drinking, and micturition. This specialized care for the C6–7-injured animals continues until the animals can eat and drink on their own as well as the return of reflexive micturition. Typically, this period lasts 7–10 days posttransection. The T2–3-injured animals do not require assistance eating or drinking; however, their bladders must be expressed every 4 h for ~7–10 days. All animals were also provided with novel, nutritionally complete, gel diets (Bio-Serv) and fruit to encourage eating. In addition, using a syringe, the C6–7-injured animals were hand fed liquid diets such as BOOST nutritional drink or diluted gel diets (Bio-Serv) several times a day. Once automatic voidance was observed, manual compression continued 4×/day to completely empty any residual urine that may risk infections.
Body temperature regulation is a major challenge for animals, and maintaining a thermoneutral environment was critical for recovery. Therefore, animals were housed in a dedicated animal room where the ambient temperature was maintained at the thermoneutral zone for rats (between 28 and 32°C).
Finally, injured animals were handled several times daily to passively move joints and massage muscles below the level of the lesion. These procedures prevented pressure sores and contractures. Sham-operated animals were also housed in a thermoneutral environment, handled daily, and received nutritional supplements.
Tissue preparation.
Sixteen weeks after spinal cord transection or sham transection, the rats were euthanized following isoflurane anesthesia by excising the heart. The mesentery was surgically removed and maintained in cold Krebs buffer. Tertiary branches of MA were carefully dissected, secured using 50-µm-diameter stainless steel pins in a recording chamber coated with Sylgard (Dow Chemical). The vessel surface was opened as a small window by careful removal of surrounded adipose tissue under a dissecting microscope to allow the electrode access to the vessel. Tissues were superfused with warm (35–36°C), oxygenated (95% O2-5% CO2) Krebs buffer (117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 11 mM dextrose). Nifedipine (1 µM) and prazosin (0.1 µM) were added to the Krebs buffer and applied throughout the experiment to inhibit vasoconstriction during electrical stimulation to maintain smooth muscle microelectrode position. Tissues were equilibrated for 30 min before beginning experiments.
Electrophysiological recordings.
Excitatory junction potential (EJP) recording is an indirect measurement of neuronal ATP release (49). Intracellular recordings from individual smooth muscle cells (SMCs) were obtained using sharp microelectrodes (80- to 140-MΩ tip resistance) prepared using a vertical pipette puller (PUL-100, WPI). The electrode was filled with 2 M KCl and connected to an Amplifier (IX2–700 dual intracellular preamp, Dagan Corp.) to record smooth muscle membrane potentials. Signals were filtered using a HumBug 60-Hz Noise Eliminator (Digitimer). Cell impalements were accepted if resting membrane potential quickly dropped below −50 mV. The actual resting membrane potential was determined after impalements were ended by subtracting the electrode tip potential from the measured membrane potential. The experiment began when the cell impalement and membrane potentials were stable for at least 30 s. The periarterial nerves were stimulated using a bipolar focal stimulating electrode containing two parallel Ag/AgCl electrodes connected to a constant current stimulus isolation unit (WPI), which was coupled to a pulse/train generator (Master 8, A.M.P.I., Jerusalem, Israel). The stimulating electrode was positioned parallel to the blood vessel by using a micromanipulator (Narishige Instruments, Amityville, NY). Signals were then sent to an oscilloscope (TBS1052B Digital Oscilloscope, Tektronix) and an analog/digital converter (Digidata1200, Molecular Devices, San Jose, CA) and recorded by Axoscope 10.0 software (Molecular Devices). Data were analyzed using Clampfit 10.7 software (Molecular Devices) and a computer.
Amperometric detection of NE release.
Amperometry is an electrochemical technique with high sensitivity that has been widely used to study synaptic vesicle exocytosis (18, 50, 65, 66) and neurotransmitter release (11, 53, 54, 70). The protocol for carbon-fiber electrode fabrication and amperometric detection of nerve-evoked NE release from MA were described in our previous work (11, 53, 54). Briefly, during the amperometric recording, a carbon-fiber electrode (700-µm tip length) was held at a fixed potential of +500 mV to oxidize NE. The electrode was gently pressed against the blood vessel using a micromanipulator (Narishige Instruments). Focal electrical stimulation (50 stimuli) was used to evoke neuronal NE release. NE release was quantified by a transfer of electrons after NE molecules were oxidized. A new carbon-fiber electrode was used daily. NE standard curves (0.001–1 µM) were obtained at the end of an experiment for each electrode. Oxidation currents were then converted to NE concentration. Furthermore, the rise slope of the peak current was used to describe neurotransmitter release (50). The data were acquired at sampling rate of 3 kHz. Oxidation currents were low-pass filtered at 10 Hz before data analysis.
Drug application.
Drugs were added to the Krebs buffer. Tissues were exposed to drugs for 7 min before testing the drug’s effect. It took approximately 1 min for the drug to reach the recording chamber after starting drug application.
Measurement of NE content by HPLC.
High-pressure liquid chromatography (HPLC; ESA Biosciences) was used to measure NE content in tissues using protocols described previously (28). Briefly, MA, and small pieces of heart, kidney and spleen, were weighed, homogenized in 0.1 M perchloric acid in 4 to 1 ratio and centrifuged at 15,000 g for 10 min. The supernatant was removed into a new tube and analyzed by HPLC. The HPLC electrochemical detector potential was set at −300 mV with a HR-80 reverse-phase column (Thermoscientific, Waltham, MA). Cat-A-Phase II (Thermo) was the mobile phase with a flow rate of 1.1 mL/min, the separation column was maintained at 35°C. Quantification was performed by comparing sample area measurements with a calibration curve. Standards were run every fifth sample to verify the identity of the peaks of interest on the chromatogram. The limit of detection was 0.01 ng/ml, and NE content was expressed as nanograms per gram tissue weight.
Vascular reactivity in pressurized MA in vitro.
MA (60-mmHg pressurized, ≈200-μm inner diameter) were mounted in a pressure myograph with Krebs solution, without intraluminal flow (67, 68). Changes in MA inner diameter (ID) were recorded using Diamtrak software (Adelaide, Australia). MA contractility was assessed using cumulative concentration-response curves for phenylephrine (PE; 0.001–10 μmol/l) and norepinephrine (NE; 0.001–10 μmol/l). Noncumulative concentration-response curves were constructed for ATP (0.001–3 mmol/l) to minimize P2X receptor desensitization. A 20-min wash period was followed after application of each ATP concentration.
Immunohistochemistry.
For localization of tyrosine hydroxylase (TH)- and vesicular nucleotide transporter (VNUT)-immunoreactive nerve fibers, second-order MA were dissected, and perivascular fat was removed. The MA were fixed in Zamboni fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) for 24 h at 4°C. After fixation, samples were stored in 70% ethanol until use. Samples were then placed in 0.1 M phosphate (pH 7.4) buffer for 5 min, then blocked in blocking buffer (0.1 M phosphate buffer, 0.2% Tx-100 with 4% donkey serum) for 1 h at room temperature. Immunostaining was performed with 1:500 rabbit anti-TH (BML-TZ1010–0050, Enzo Life Sciences, Farmingdale, NY) in blocking buffer overnight at 4°C. Tissues were washed the next day with 0.1 phosphate buffer (4 × 5 min each) and then stained with a 1:1,000 secondary donkey anti-rabbit Alexa 594 antibody (A21207,https://www.ncbi.nlm.nih.gov/nuccore/ThermoFisher Scientific) for 1 h at room temperature. Samples were then washed in 0.1 M phosphate buffer (4 × 5 min each) and then mounted on slides with mounting media containing DAPI (P36962,ThermoFisher Scientific,). Images were taken on an Olympus FluoView FV1000 confocal laser-scanning microscope. Five images were taken per tissue sample. The density of TH-containing nerves that supply MA was analyzed by counting the number of TH-containing nerve fibers crossing horizontal and vertical grids superimposed on blood vessel images (×40 objective magnification) by using Image J software.
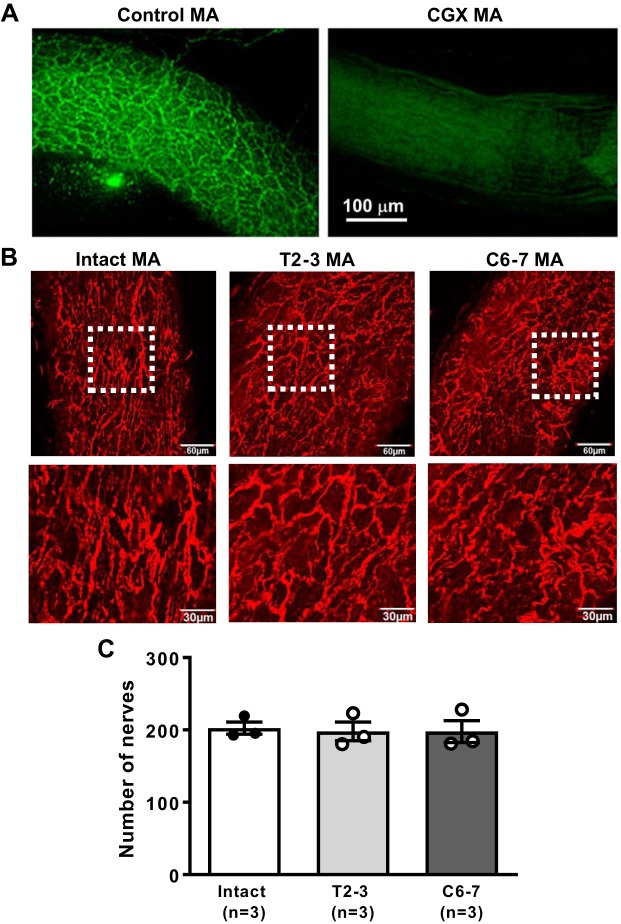
The specificity of the TH antibody for periarterial sympathetic nerves was confirmed by testing for TH immunoreactivity in MA from a control rat and from a rat that had undergone celiac ganglionectormy to remove the source of sympathetic nerve fibers supplying MA. Details of the celiac ganglionectomy procedure have been described previously (35).
Drugs.
Nifedipine, prazosin, pyridoxal phosphate-6-azo (benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS), NE, phenylephrine (PE), ATP, and tetrodotoxin (TTX) were obtained from Sigma-Aldrich. Nifedipine was dissolve in DMSO. Prazosin was dissolved in methanol. Others were dissolved in deionized water. Working solution of nifedipine and prazosin contain <0.01% DMSO and methanol, respectively. Final solutions were made in Krebs’ buffer on the day of the experiment.
Statistics.
Data are means ± SE, and n values are either the number of animals or the number of cells as indicated in each experiment. Data were analyzed using Student’s t-test, one-way ANOVA with Dunnett’s multiple comparison test or two-way ANOVA with Bonferroni’s multiple comparison test, as appropriate using Prism 6.0. Differences were considered significant when P < 0.05.
RESULTS
Blood pressure, heart rate, and body weight.
Resting blood pressure (BP), heart rate (HR), and body weight (BW) are shown in Table 1. Resting mean arterial pressures (MAP) of T2–3 and C6–7 rats were decreased compared with sham-operated rats (Table 1). C6–7 rats exhibited profound hypotension. T2–3 rats had higher HR than sham rats (Table 1). In contrast, C6–7 rats exhibited decreased HR compared with sham rats (Table 1). BW was lower in T2–3 and C6–7 rats compared with sham rats (Table 1).
Table 1.
BW, MAP, and HR in intact and SCI rats at 16 wk after SCI
| BW, g | MAP, mmHg | HR, beats/min | |
|---|---|---|---|
| Intact | 459 ± 18 | 117 ± 6 | 321 ± 4 |
| T2–3 transection | 380 ± 5* | 103 ± 2* | 410 ± 6* |
| C6–7 transection | 340 ± 12* | 97 ± 4* | 299 ± 10* |
Values are means ± SE and were analyzed using a 1-way ANOVA with Dunnett’s multiple comparison test. SCI, spinal cord injury; BW, body weight; MAP, mean arterial pressure; HR, heart rate.
P < 0.05 vs. intact.
Increased EJP amplitude recorded in mesenteric arteries from SCI rats.
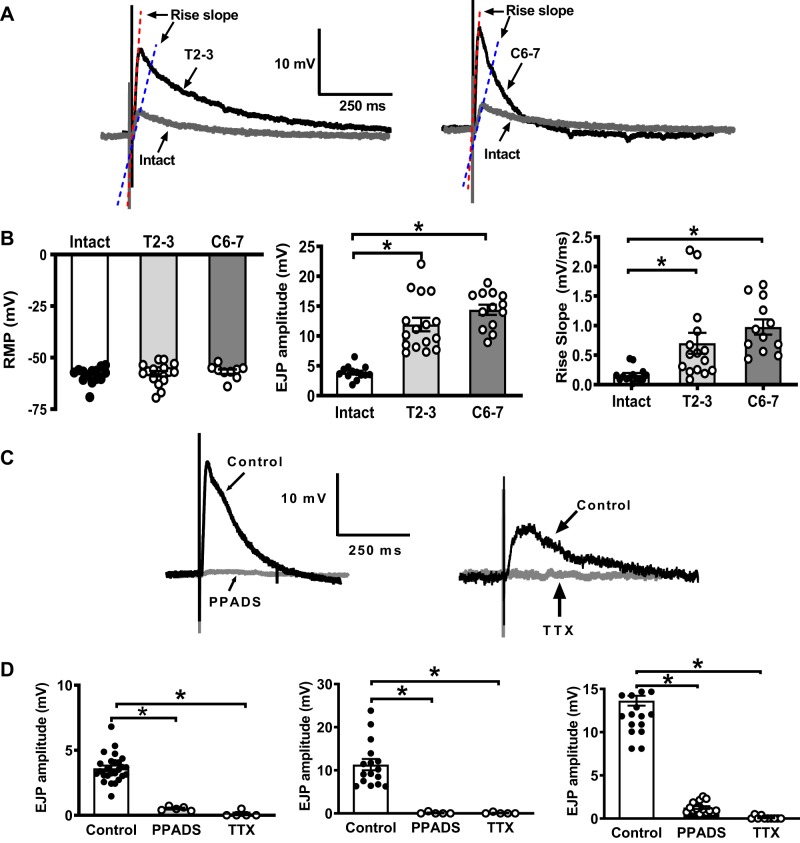
To assess purinergic neurotransmission in MA, EJPs were recorded from arterial SMCs as an indirect measurement of neuronal ATP release. Figure 1A shows representative recordings of EJPs from sham vs. T2–3 (left) and vs. C6–7 MA (right). The resting membrane potential (RMP) of vascular SMCs from all groups was similar (Fig. 1B, left). MA were electrically stimulated at 0.1 Hz by five stimuli. Mean amplitudes of EJP1-EJP5 and rise slope were analyzed. EJP amplitudes from T2–3 and C6–7 MA were greater than intact MA (11.9 ± 1.1, and 14.4 ± 0.8 vs. 3.9 ± 0.3 mV, respectively, P < 0.05; Fig. 1B, middle). Moreover, the EJP rise slope of T2–3 and C6–7 arteries was greater compared with sham intact arteries (0.7 ± 0.2, and 0.98 ± 0.13 vs. 0.16 ± 0.03 mV/ms, P < 0.05; Fig. 1B, right). EJPs from all groups were blocked by a P2X receptor antagonist, PPADS (10 µM), and a Na+ channel blocker, TTX (0.3 µM), indicating that EJPs were neuronally mediated (Fig. 1, C and D).
Fig. 1.
Electrophysiological properties of mesenteric artery (MA) smooth muscle cells (MA SMCs) from intact, T2–3, and C6–7 rats 16 wk after spinal cord injury (SCI). A: representative excitatory junction potentials (EJPs) recorded from MA from intact and T2–3 rats (left) and intact and C6–7 (right) rats. B: resting membrane potential (RMP) of MA SMCs from intact, T2–3, and C6–7 rats were similar (left, n = 15–17 cells from 4–6 animals/group). EJP amplitudes (middle; *P < 0.001, 1-way ANOVA and Tukey’s multiple comparison test (n = 13–16 cells from 4–6 animals/group) and rise slope (right; *P < 0.01, 1-way ANOVA and Tukey’s multiple comparison test, n = 12–15 cells from 4–6 animals/group) recorded from MA SMCs of T2–3 and C6–7 rats were significantly greater compared with MA from intact rats. C: representative recordings of EJPs blocked by pyridoxalphosphate-6-azophenyl-2,4-disulfonic acid tetrasodium salt (PPADS) and tetrodotoxin (TTX). D: mean EJP amplitudes recorded from MA from intact (left), T2–3 (middle), and C6–7 rats (right). EJPs in all groups were blocked by PPADS and TTX. *P < 0.0001 vs. control, 1-way ANOVA and Tukey’s multiple comparison test (n = 3–4 animals/group).
Increased EJP amplitude in SCI rats is due to prejunctional changes.
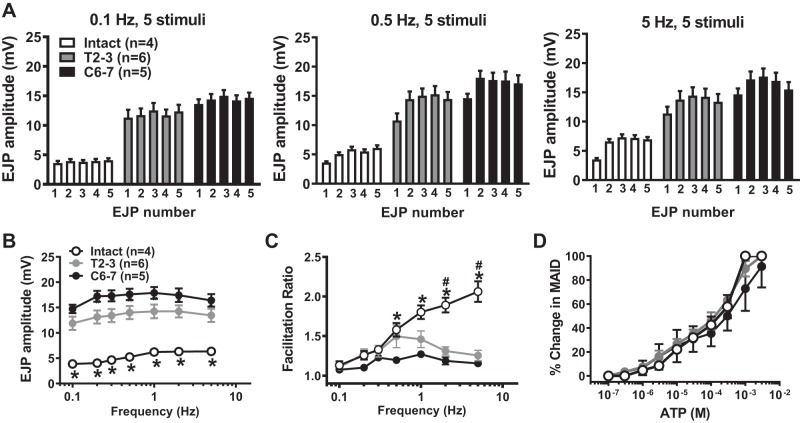
Frequency (0.1–5 Hz)-response curves evoked by five electrical stimuli were recorded from intact and SCI MA. EJP amplitude and degree of facilitation were measured and compared. EJP amplitudes were measured as the difference between the peak and the membrane potential at the time each stimulus was applied. The individual mean amplitudes of the first EJP to fifth EJP at 0.1-, 0.5-, and 5-Hz stimulation are shown in Fig. 2A, and frequency-response curves are shown in Fig. 2B. EJP amplitudes were increased in a frequency-dependent manner in intact, T2–3, and C6–7 MA (Fig. 2B). T2–3 and C6–7 MA exhibited an approximately threefold upward shift of frequency-EJP response curves compared with sham MA (P < 0.05, 2-way ANOVA, Fig. 2B).
Fig. 2.
Frequency-response curves for stimulation-evoked excitatory junction potentials (EJPs) recorded from MA from intact, T2–3, and C6–7 rats. A: mean amplitudes of the 1st to 5th EJP evoked by 0.1-, 0.5-, and 5-Hz trains of nerve stimulation. B: frequency-response curves for stimulation-evoked EJPs. *Intact EJPs are significantly different from T2–3 and C6–7 EJPs at each frequency (P < 0.008). C: frequency response curves for EJP facilitation. *Significantly different from C6–7 rats (P < 0.01). #Significantly different from T2–3 rats (P < 0.009). D: concentration-response curves (CRCs) for ATP-induced mesenteric artery (MA) contractions. There were no differences in CRCs between groups. Data were analyzed using a 2-way ANOVA and Tukey’s multiple comparison test.
EJP facilitation was measured next. Facilitation involves increased amplitude of consecutive EJPs that occurs during trains of nerve stimulation. Facilitation is caused by calcium accumulation in sympathetic varicosities with each electrical stimulus (62). The facilitation ratio was calculated by dividing the amplitude of the fifth EJP with the amplitude of the first EJP. A higher ratio indicates greater facilitation. No facilitation occurred at 0.1-Hz stimulation in any group (Fig. 2A, left, and C). EJP facilitation increased in a frequency-dependent manner in sham, but not T2–3 and C6–7 MA (Fig. 2C). The initial large EJP amplitude in T2–3 and C6–7 MA accounts for their flat facilitation curves. MA reactivity to exogenous ATP was tested in pressurized vessels. ATP concentration-response curves were similar in MA from all rats (Fig. 2D).
EJP rundown in MA from SCI rats.
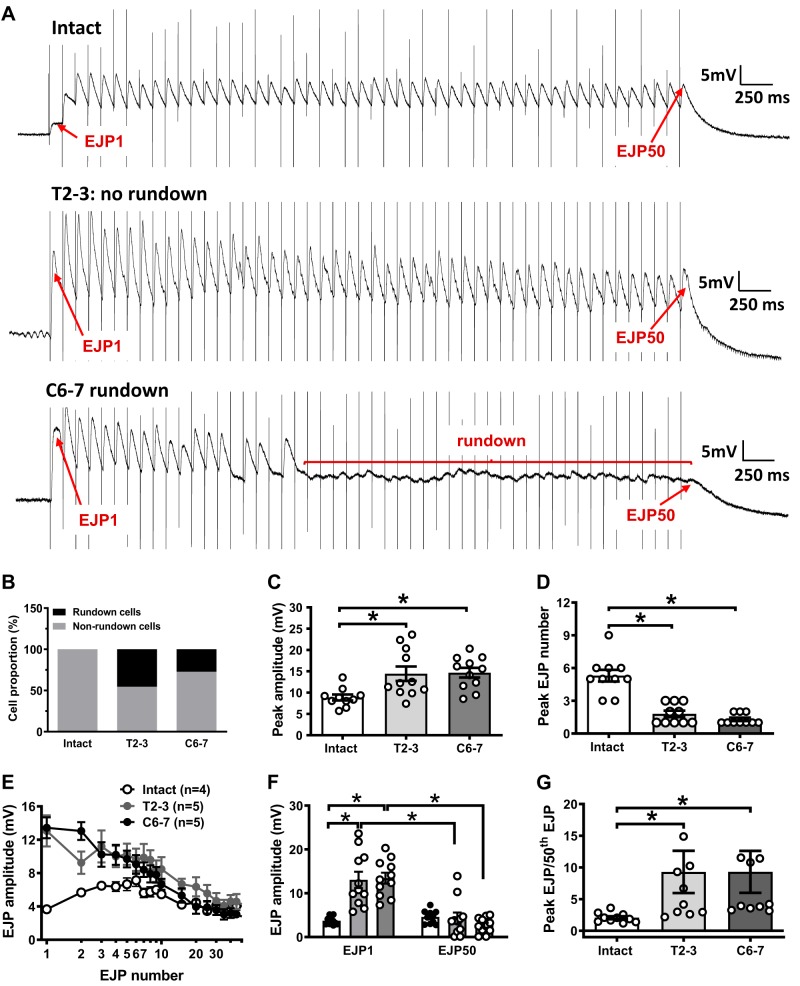
We further investigated whether the markedly increased ATP release in SCI rats caused by nerve stimulation would induce faster depletion of the releasable pool of ATP. Trains of 50 stimuli at 10 Hz were used to deplete neuronal ATP stores (12, 14, 37). Figure 3A shows representative traces of EJPs recorded from intact sham and SCI arteries. The amplitude of EJPs recorded from sham MA was maintained throughout the stimulation train. Following the initial EJP facilitation occurring during the first four to five stimuli, the peak EJP amplitude recorded from sham MA was maintained throughout the rest of the stimulus train (10 cells, Fig. 3B). Interestingly, EJP rundown occurred in some MA from T2–3 (5 of 11 cells, 45.5%), and C6–7 rats (3 of 11 cells, 27.3%; Fig. 3, A and B). Maximum EJP amplitude and the location of the peak during the stimulus train were also determined (Fig. 3, C and D). EJP peak amplitude was higher in MA from T2–3 and C6–7 rats compared with intact sham arteries (14.4 ± 1.7 and 14.7 ± 1.2 vs. 8.8 ± 0.7 mV, respectively, P < 0.05, 1-way ANOVA; Fig. 3C). Peak EJP amplitude also occurred earlier in T2–3 and C6–7 MA compared with MA from control rats (Fig. 3D, EJP number 1.8 ± 0.3, and 1.3 ± 0.2 vs. 5.3 ± 0.5, respectively; n = 10–11 cells for each group, P < 0.05, 1-way ANOVA).
Fig. 3.
Excitatory junction potential (EJP) rundown caused by trains of stimulation in mesenteric artery (MA) from intact, T2–3, and C6–7 rats. A: trains of stimulation (10 Hz, 50 stimuli) caused initial EJP facilitation followed by a decline to a stable level in MA from intact and T2–3 rats. EJPs recorded from C6–7 MA ran down completely after the 15th stimulus. B: proportion of EJP recordings that exhibited rundown in MA from intact (no rundown), T2–3, and C6–7 rats. C: peak EJP amplitude was greater in MA from T2–3 and C6–7 rats compared with intact rats. D: peak EJP amplitude occurred later in the stimulation train in intact MA compared with MA from T2–3 and C6–7 rats. E: mean data from experiments similar to those shown in A. F: mean amplitude of the 1st and 50th EPJ in MA from intact, T2–3, and C6–7 rats. G: the ratio of peak EJP amplitude to the 50th EJP amplitude was calculated. This ratio was compared between intact sham and spinal cord injury (SCI) rats. *P < 0.05, 1-way ANOVA followed by Tukey’s multiple comparison test.
Individual mean EJP amplitudes were analyzed for the first 10 EJPs in the train and then the 15th through the 50th EJP were measured and compared between sham and SCI rats. All amplitudes were measured using the differences between the baseline and peak for each stimulus. MA from T2–3 and C6–7 rats exhibited larger EJPs following stimuli early in the train compared with MA from intact rats. Peak facilitation occurred after the first stimulus, whereas peak facilitation in intact MA occurred between the third and sixth stimuli (Fig. 3A). Then, mean EJP amplitudes in MA from control and SCI rats decayed to the same level (Fig. 3A). Figure 3F illustrates differences between the 1st and 50th EJPs among intact sham and SCI rats. Stimulation of MA from T2–3 and C6–7 rats elicited a larger amplitude first EJP but failed to maintain EJP amplitude throughout the train, causing a reduction in the amplitude of the 50th EJP (Fig. 3F). The degree of rundown was also measured and compared between control and SCI MA. The magnitude of EJP rundown was analyzed by dividing the amplitude of the largest EJP (peak EJP) occurring early in the train by the amplitude of the 50th EJP (Fig. 3G). T2–3 and C6–7 MA had stronger EJP rundown compared with MA from sham-operated rats (9.3 ± 3.3 vs. 2.1 ± 0.3, P < 0.05, unpaired t test, and 9.3 ± 3.3 vs. 2.1 ± 0.3, P < 0.05, unpaired t test, respectively; Fig. 3G).
Spontaneous EJPs in MA of SCI rats.
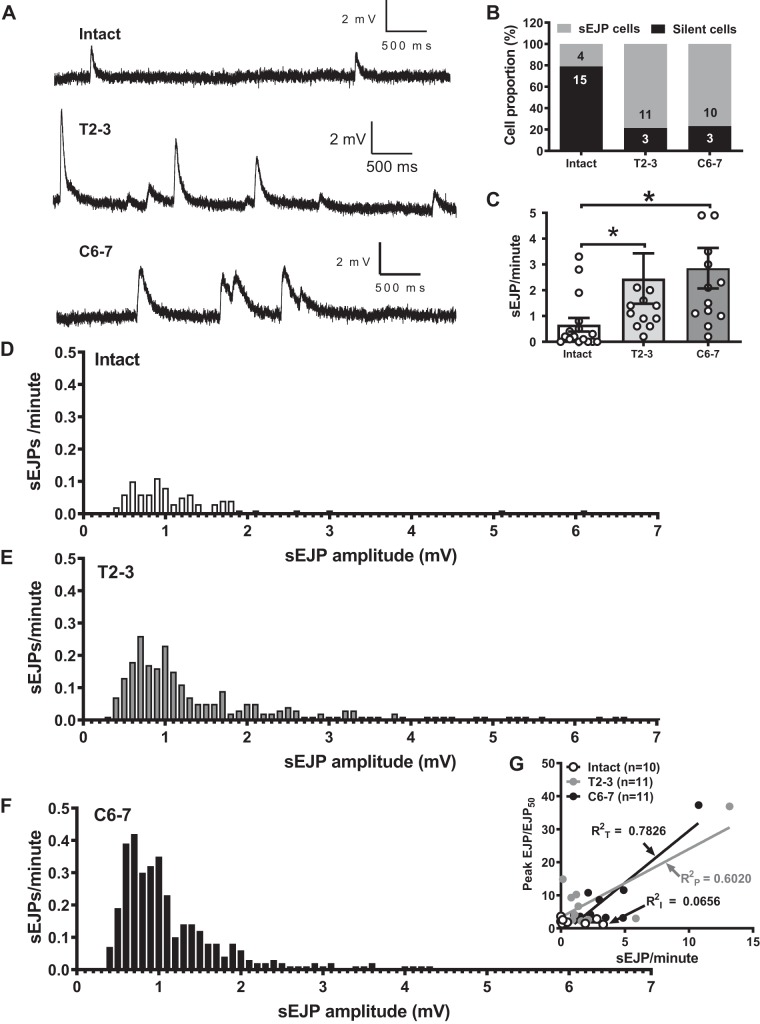
Spontaneous EJPs (sEJPs) were detected in some MA smooth muscle cells from intact and SCI rats but with different patterns (Fig. 4, A and B). sEJPs represent the quantal release of neurotransmitter in arteries. Some cells exhibited robust sEJPs, while most cells were silent in the absence of stimulation. The majority of T2–3 and C6–7 MA smooth muscle cells showed frequent sEJPs, while smooth muscle cells in MA from intact rats were mostly silent (Fig. 4B, 78.6 and 76.9 vs. 21.0%, respectively). Furthermore, sEJP frequency was calculated for individual cells by dividing the total number of sEJP events by the recording time. The sEJP frequency was higher in T2–3 and C6–7 rats compared with intact MA (2.5 ± 1 vs. 0.7 ± 0.3 events/min; P < 0.05, unpaired t test, and 2.8 ± 0.8 vs. 0.7 ± 0.3 events/min; P < 0.05, unpaired t test, respectively; Fig. 4C). Moreover, the distribution of sEJP amplitude was also analyzed (Fig. 4, D–F). Larger amplitude sEJPs were recorded from T2–3 and C6–7 rats compared with intact rats. These data suggest that the frequency of ATP release and/or the quantal content of individual packets of ATP are increased in periarterial sympathetic nerve varicosities of T2–3 and C6–7 rats.
Fig. 4.
Spontaneous EJPs (sEJPs). A: representative sEJP recordings from MA from intact, T2–3, and C6–7 rats. B: proportion of cells that exhibited sEJPs from all groups. T2–3 and C6–7 had higher frequency of sEJP events compare with MA from intact rats. C: frequency of sEJPs recorded from MA of intact, T2–3, and C6–7 rats. *P < 0.05 compared with intact, 1-way ANOVA followed by Tukey’s multiple comparison test. D–F: frequency distribution of sEJP amplitudes in MA from intact, T2–3, and C6–7 rats. sEJP were more frequent and larger in amplitude in MA from SCI rats. G: there is a positive correlation between sEJP frequency and rundown ratio in MA from T2–3 and C6–7 but not intact rats.
EJP rundown is related to the frequency of sEJP.
As EJP rundown and sEJPs occurred only in MA from T2–3 and C6–7 rats, we determined the correlation between the magnitude of rundown and sEJP frequency. There was a positive correlation between the sEJP rate and rundown in MA from T2–3 and C6–7 rats, but not intact rats (Fig. 4G), suggesting that the robust sEJPs in MA from T2–3 and C6–7 rats contribute to EJP rundown in these animals.
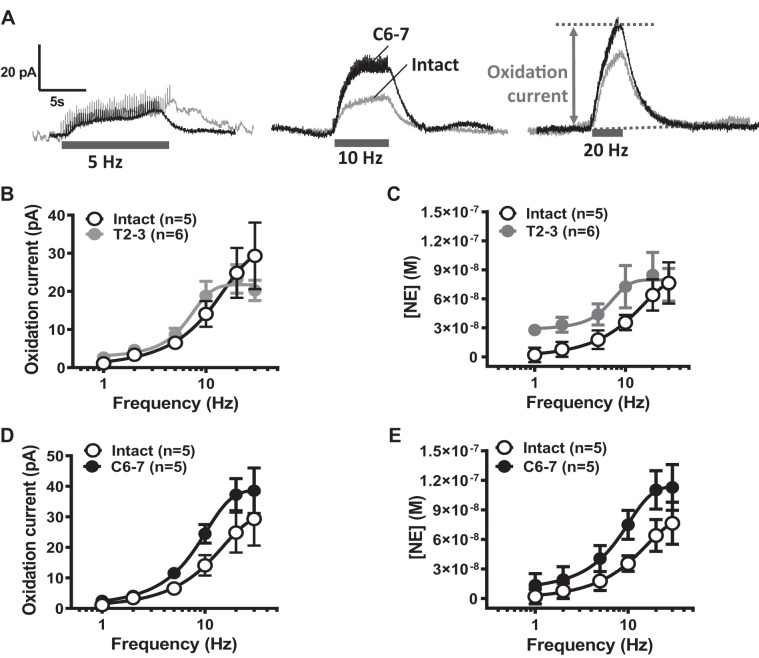
Effect of SCI on NE release from periarterial sympathetic nerves.
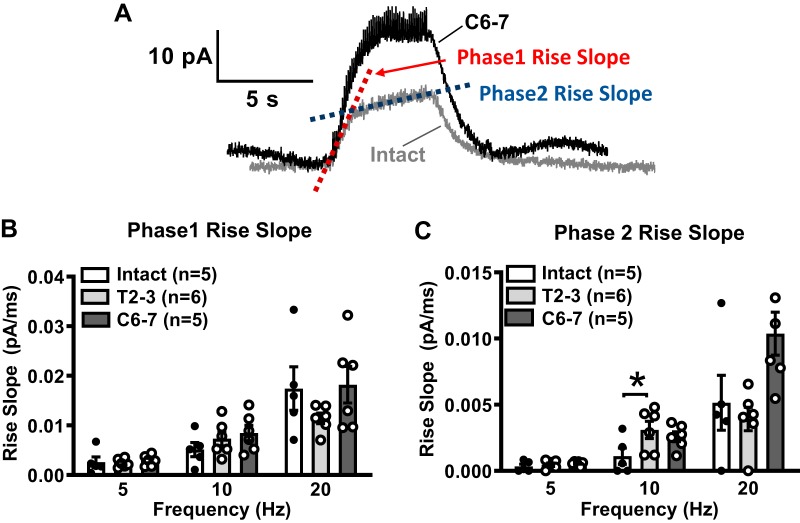
As purinergic neurotransmission increased in T2–3 and C6–7 MA, we next determined whether changes in noradrenergic neurotransmission also occurred in MA from SCI rats. Amperometry using carbon-fiber electrodes was used to detect real-time NE release in response to focal nerve stimulation (50 stimuli at 1–30 Hz). Representative traces of MA NE oxidation currents caused by stimulation trains at 5, 10, and 20 Hz from intact and C6–7 rats are shown in Fig. 5A. NE oxidation currents recorded from MA from intact and T2–3 rats (Fig. 5B) and C6–7 rats (Fig. 5D) were then converted to NE concentration (Fig. 5, C and E). This provided a quantitative measure of NE release from periarterial sympathetic nerves from intact vs. SCI rats. Neuronal NE release from intact and SCI MA increased in a frequency (1–30 Hz)-dependent manner (Fig. 5D). The stimulation frequency (Hz) producing half-maximum release (S50), maximum NE release (Emax; nmol), and Hill slope values are shown in Table 2. There were no significant differences in S50, Emax, or Hill slope between MA from SCI and intact MA (P > 0.05, 1-way ANOVA). Furthermore, the rise slope of NE oxidation currents was analyzed to determine whether there was an increased and faster neuronal NE release in SCI MA. The rise in the oxidation current occurred in two phases, where the first phase (phase 1) was faster than the later and slower phase 2 (Fig. 6A). Phase 1 and phase 2 rise slopes increased in a frequency-dependent manner in all groups (Fig. 6, B and C) There were no differences in the phase 1 rise slope at any frequency when data from intact, T2–3, and C6–7 rats were assessed (Fig. 6B). The rise slope of the phase 2 oxidation current was slower than phase 1, and there was a difference only between intact and T2–3 rise slopes at the 10-Hz stimulation frequency (Fig. 6C).
Fig. 5.
Effect of spinal cord injury (SCI) on norepinephrine (NE) release from periarterial sympathetic nerves. Electrical stimulation-evoked NE release was measured by amperometry using carbon-fiber electrodes (+500-mV oxidation potential). A: representative traces of stimulation-evoked NE oxidation current in MA from an intact and C6–7 rat. B: frequency (1–30 Hz)-response curves for stimulation-evoked NE oxidation currents recorded from MA from intact and T2–3 rats. C: conversion of the oxidation current to NE concentration by using exogenous NE standard curves. NE release was not significantly different between MA from intact and T2–3 rats. D: frequency (1–30 Hz)-response curves for stimulation-evoked NE oxidation currents recorded from MA from intact and C6–7 rats. E: conversion of oxidation current to NE concentration. There were no statistically significant differences between NE oxidation currents or NE release from sympathetic nerves supplying MA from intact and C6–7 rats (P > 0.05, 2-way ANOVA).
Table 2.
Statistical analysis of frequency-response curves for NE release from MA of intact, paraplegic, and tetraplegic rats
| n | S50 | Emax, nM | Hill Slope | |
|---|---|---|---|---|
| Intact | 5 | 10.2 ± 1.3 | 75.8 ± 21.8 | 0.12 ± 0.01 |
| T2–3 | 6 | 7.0 ± 0.7 | 80.5 ± 20.4 | 0.41 ± 0.17 |
| C6–7 | 5 | 8.4 ± 0.7 | 115.2 ± 22.5 | 0.16 ± 0.01 |
Values are means ± SE. NE, norepinephrine; MA, mesenteric arteries; S50, stimulation frequency (Hz) producing half-maximum release; Emax, maximum NE release (nmol); n = number of rats. There were no significant differences in S50, Emax, or Hill slope between MA from SCI and intact rats (P > 0.05, 1-way ANOVA).
Fig. 6.
Analysis of norepinephrine (NE) oxidation current rise slope. A: time course of rising phase of NE oxidation current includes two phases. Phase 1 and 2 were analyzed and compared across MA from intact, T2–3, and C6–7 rats. B: rise slopes of phase 1 of the NE oxidation current were not different at 5-, 10-, and 20-Hz stimulation. C: phase 2 rise slope was slower than the phase 1 rise slope for NE release from nerves supplying MA in all groups of rats. *P < 0.05 vs. intact at 10-Hz stimulation; n = number of rats.
Distribution of sympathetic nerves supplying MA.
TH, the rate-limiting enzyme for NE synthesis, was used as a marker for sympathetic nerves. The distribution of TH-containing nerve fibers was used to determine whether postganglionic sympathetic nerves degenerate following chronic SCI. TH staining revealed a dense network of nerve varicosities supplying MA (Fig. 7A). The average number of horizontal and vertical nerve crossings was similar in MA from intact, T2–3, and C6–7 rats (Fig. 7B; n = 3 rats/group).
Fig. 7.
Comparison of tyrosine hydroxylase (TH) immunoreactive nerves supplying mesenteric arteries (MA) of intact, T2–3, and C6–7 rats. A: immunohistochemical labeling of TH-positive sympathetic nerves supplying a MA in the small intestine. Left: TH labeling of a MA from a control rat shows a dense nerve plexus. Right: TH-positive nerves are absent from a MA from a rat 7 days after celiac ganglionectomy (CGX). Scale bar applies to both images. B: representative images showing the distribution of sympathetic nerve fibers on the adventitial surface of MA. C: nerve fiber grid crossing counts show that the nerve density was similar in MA from intact, T2–3, and C6–7 rats.
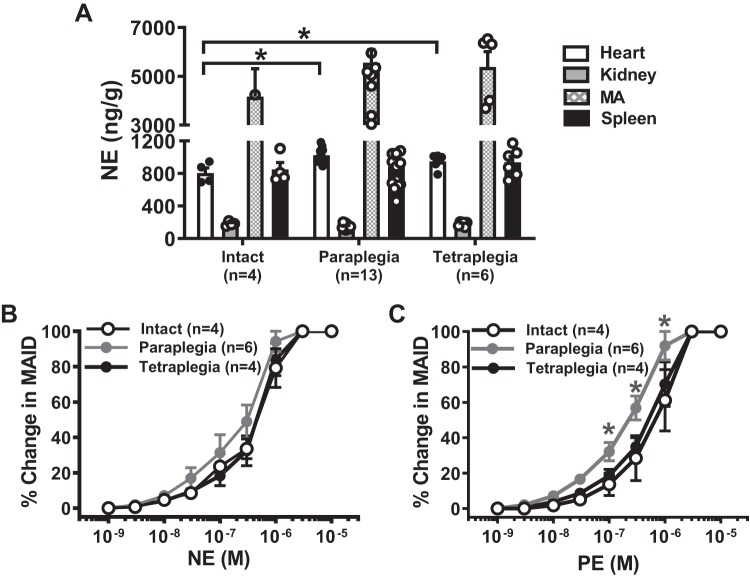
Measurement of NE content after chronic SCI.
NE content was measured from the heart, kidney, MA, and spleen by using HPLC. NE content in the heart from T2–3 and C6–7 animals was higher than in the intact sham (P < 0.05, 1-way ANOVA, Fig. 8A). There was no change in NE content in MA, kidney, and spleen from SCI animals (Fig. 8A).
Fig. 8.
Norepinephrine (NE) content in nerves supplying mesenteric arteries (MA), spleen, kidney, and heart of intact and spinal cord injury (SCI) rats. A: there was a small but significant increase in NE content in the heart of T2–3 and C6–7 compared with intact rats (*P < 0.05, 1-way ANOVA and Tukey’s post hoc test). B: there were no differences in NE concentration-response curves for MA constriction between intact, T2–3, and C6–7 rats. C: there was a slight but significant leftward shift in the phenylephrine (PE) concentration-response curve in MA from T2–3 rats (2-way ANOVA followed by Tukey’s post hoc test, *P < 0.05).
Vascular sensitivity to exogenous adrenergic vasoconstrictors.
Vascular reactivity to NE and phenylephrine (PE), an α1-adrenergic receptor agonist, were used to determine whether postjunctional receptor changes occur in SCI MA. NE concentration (0.001–10 µM)-response curves were similar in all groups (Fig. 8B). T2–3, but not C6–7, MA were more sensitive to PE compared with intact MA (Fig. 8C, P < 0.05, 2-way ANOVA).
DISCUSSION
Although tremendous progress has been made to understand the mechanisms responsible for blood pressure dysregulation after SCI, few studies have focused on postganglionic sympathetic neurotransmission to blood vessels, especially in the mesenteric circulation (1, 6, 7, 57). ATP and NE are cotransmitters released from postganglionic sympathetic varicosities at vascular neuroeffector junctions. The dual transmitter collaboration permits graded vasoconstriction that is determined by the number and frequency of sympathetic nerve action potentials. Single stimuli are sufficient to cause EJPs and brief arterial constriction while multiple action potentials release ATP and NE that collaborate to produce more sustained arterial constriction. This study focused on the long-term impact of SCI on the adrenergic and purinergic components of postganglionic neurovascular transmission. Amperometry allowed direct measurements of NE released from perivascular nerves in real time (36). EJP recordings allowed us to indirectly measure ATP released from periarterial nerves in response to nerve stimulation (9, 10). MA were used because they are densely innervated by postganglionic sympathetic nerves, and they are important resistance blood vessels where small changes in the vascular tone contribute to marked changes in BP (9). We studied the mechanisms contributing to increased sympathetic neurotransmission to MA at 4 mo after SCI, which is considerably longer than earlier studies (7, 30, 39, 46). Assessing postganglionic purinergic and adrenergic neurotransmission to MA provides an improved understanding of the mechanisms driving cardiovascular instability in individuals living with SCI. An improved understanding of neurovascular transmission may help in the development of new treatments that restore normal cardiovascular function in chronic SCI. We found that resting mean arterial pressures (MAP) of T2–3 and C6–7 rats were decreased compared with sham-operated rats, but C6–7 rats had more pronounced hypotension. Our T2–3 rats had increased HR compared with sham-operated rats, while C6–7 rats had decreased HR compared with sham rats. These differences can be attributed to the site of the SCI, Specifically, a wide range of spinal cord levels (C8–T6) project to the stellate ganglia (which provide over 90% of sympathetic supply to the heart), with a peak at the T2 level. High-thoracic spinal cord injury (i.e., T2–3 SCI) spares upper limb function and some (C8–T2) supraspinal control of sympathetic preganglionic neurons to the heart. In contrast, cervical SCI disconnects supraspinal control from all sympathetic preganglionic neurons. Thus sympathetic activity is reduced in C6–7 rats, whereas sympathetic output to the heart is elevated in T2–3 rats.
Differential cardiovascular changes between T2–3 and C6–7 rats.
Sixteen weeks after T2–3 and C6–7 SCI, rats exhibited prolonged hypotension that was more severe in C6–7 rats compared with T2–3 rats owing to complete cervical (C6–7) transection. These data indicate that the severity of hypotension is associated with the injury level. The cervical spinal cord transection was above the level of sympathetic supply to the heart; therefore, cardiac sympathetic efferent control mediated by reduced arterial baroreflex activation cannot modulate BP by increasing HR. Thus the C6–7 rats had hypotension with bradycardia. In contrast, sympathetic innervation of the heart remains mainly intact in T2–3 cord transection rats (10, 41, 44), enabling an increase in BP in response to reflex tachycardia (45). (4, 33) However, there are also limitations to the specificity of the spinal cord transections at C6–7 and T2–3 as these two injury sites are separated by only two vertebrae (T1 and C8). Accordingly, we cannot rule out the possibility that the C6–7 transection injured the T2–3 site either through a mechanical mechanism or through local bleeding and spread of inflammation and inflammatory cells. Similarly, the T2–3 transection could also injure the C6–7 site through similar mechanisms. Moreover, T2–3 and C6–7 rats exhibited significant weight loss (17% in T2–3 and 26% in C6–7 rats compared with control rats). This is partly related to long-term limited ability to perform physical activity contributing to loss of bone and muscle mass, which is also found in individuals living with SCI months after the injury (20, 21).
Altered purinergic neurotransmission.
ATP is released from postganglionic sympathetic nerves and binds to ligand gated P2X1 receptors on vascular SMC, causing cation influx into smooth muscle cells and resulting in a transient depolarization known as the EJP. Intracellular EJP recordings from arterial smooth muscle cells provide an indirect measure of neuronal ATP release from postganglionic sympathetic nerves (56). We recorded EJPs that were inhibited by TTX and PPADS in sham and SCI rats, confirming that EJPs are caused by ATP released from sympathetic nerves. In MA from C6–7 and T2–3 rats, EJP amplitude and rise slope were approximately threefold greater compared with MA from sham rats. McLachlan and Brock (46) also reported a twofold increase in EJP amplitude in rat tail arteries 8 wk after T7–8 spinal cord transection in female Wistar rats. Increased EJP amplitude and rise slope could be due to either increased prejunctional release of ATP or increased postjunctional responsiveness. We found no differences in exogenous ATP dose-response curves measured in MA from intact and SCI rats. Vascular smooth muscle resting membrane potentials were also similar in MA from intact and SCI rats, although nifedipine and prazosin that were added to the Krebs solution can alter the arterial smooth muscle membrane potential (26, 56). Therefore, it is likely that the increased EJP amplitude and rise slope in SCI rats are due to prejunctional changes in sympathetic nerve varicosities. Prejunctional changes in sympathetic nerves of SCI rats could include increased numbers of synaptic vesicles, increased ATP content in vesicles, upregulation of nerve terminal Ca2+ channels; impaired function of prejunctional adenosine A1 (58)- and/or α2-adrenergic autoreceptors (17), or a combination of each of these changes.
Facilitation of purinergic neurotransmission in long-term SCI rats.
Facilitation occurs throughout the nervous system during short bursts of synaptic activity. Synaptic facilitation is a successive increase in the amplitude of postsynaptic excitatory postsynaptic potentials (EPSPs) or EJPs due to an increase in transmitter release. We found that facilitation of EJPs increased in a frequency-dependent manner in MA from intact rats. This is consistent with previous findings that also found frequency-dependent facilitation of ATP release in the mouse vas deferens (5). Hardy and Brock (22) showed that Ca2+ entry into nerve terminals is crucial for EJP facilitation in guinea pig vas deferens. At high stimulation frequencies, Ca2+ enters the nerve terminal faster than it can be cleared and this contributes to increased neurotransmitter release. Interestingly, stimulation-frequency curves for MA from T2–3 and C6–7 rats were flat. However, we cannot conclude that EJP facilitation is impaired in SCI rats because T2–3 and C6–7 MA exhibit initially large EJP amplitudes during short trains of nerve stimulation, accounting for the flat facilitation ratio curves. Instead, we suggest that the first EJP in the stimulus train is already facilitated. There are two possible explanations for this interesting finding: 1) upregulation of the prejunctional N-type Ca2+ channels may be involved in the initial increased EJP amplitude, or 2) loss of supraspinal sympathetic outflow may lead to more ATP-containing vesicles mobilized to the active zone and ready to be released, causing augmentation of EJP amplitude in response to the first stimulus in a stimulus train. Investigating the contribution of N-type Ca2+ channels merits future consideration.
Depletion of the releasable pool of vesicular ATP in MA from SCI rats.
We tested the hypothesis that the loss of supraspinal control to sympathetic preganglionic neurons leads to a larger pool of ATP available for release during trains of stimulation and that a long train of stimulation can cause the rundown of ATP stores and a decline in EJP amplitude (12). Experiments using a high-frequency long train of nerve stimulation produced an initial large EJP in MA from SCI rats followed by rundown of EJP amplitude in MA. In contrast, in MA from control rats, the first EJP during a train of high-frequency stimulation was smaller than that in SCI rats, but subsequent stimuli produced facilitation. These data suggest that the readily releasable pool of ATP containing synaptic vessels is larger in sympathetic varicosities of SCI rats compared with control rats, but the pool is quickly depleted during a burst of action potentials. The amplitude of the EJP evoked by the 30th stimulus is similar in MA from all rats, suggesting that a steady-state pool of ATP-containing vesicles is equivalent in the three groups of rats. These data suggest that there is a shift in the distribution of synaptic vesicles to the readily releasable pool in sympathetic nerves supplying MA from SCI rats.
Spontaneous EJPs in MA of SCI rats.
Postganglionic sympathetic nerve varicosities release ATP onto smooth muscle cells producing sEJPs in the absence of electrical stimulation (8). sEJPs occurred more frequently in MA of T2–3 and C6–7 rats, which is consistent with a previous study (46). Brain and coworkers (5) suggested that only cells that are in close apposition with the sympathetic nerve varicosities containing numerous ATP vesicles are likely to exhibit sEJPs. The origin of sEJPs is due in part to spontaneous prejunctional Ca2+ transients (5). Frequent spontaneous ATP release can accelerate EJP rundown during high-frequency nerve stimulation.
EJP rundown is correlated with the frequency of sEJP in SCI rats.
Previous studies showed that EJP rundown is a result of the depletion of a releasable pool of ATP-containing synaptic vesicles (12). EJP rundown occurred in MA smooth muscle cells from T2–3 and C6–7 rats. Interestingly, sEJPs were found more frequently in MA of T2–3 and C6–7 rats compared with intact sham rats. Therefore, we determined the relationship between SCI and sEJPs and found a positive correlation between sEJP frequency and EJP rundown in T2–3 and C6–7 MA. Taken together, the depletion of neuronal ATP in chronic SCI rats is probably caused by two mechanisms: 1) spontaneous release of ATP from periarterial sympathetic nerve varicosities and 2) an increase in the size of the readily releasable pool of ATP-containing vesicles in active zones after chronic loss of supraspinal input. Alternatively, the rundown of EJP amplitude could be due to action potential failure in sympathetic axons during long trains of stimulation, as has been shown to occur in individuals with SCI (36).
Noradrenergic neurotransmission.
Previous studies using the α1-adrenergic receptor agonist methoxamine showed that downregulation of the NE transporter (NET) plays a role in the enhancement of sympathetic neurotransmission after SCI in several tissues (1, 7, 34). Although PE and methoxamine are α1-adrenergic receptor agonists, only PE is a substrate for NET (64). Downregulation of NET function leads to PE hypersensitivity but not methoxamine hypersensitivity in MAs from SCI rats. In the presence of desmethylimipramine, a NET blocker, the PE dose-response curves were similar between SCI and intact MA, indicating impaired NET function (7). We found a slight, but not significant, leftward shift in the stimulation/NE oxidation current-response curves that is consistent with downregulation of NET expression or function as shown previously in rat MA (7), although we cannot rule out the possibility that NE release mechanisms are also altered by SCI.
We also examined whether there are alterations in adrenergic signaling at the postjunctional site after 16 wk of SCI using a pressure myograph in the absence of nifedipine. T2–3, but not C6–7, MA are slightly more sensitive to the constrictor effects of PE. However, the sensitivity to NE of MA was unchanged for both T2–3 and C6–7 compared with intact sham MA. One explanation for this interesting finding is that PE is a selective α1-adrenergic receptor agonist, whereas NE activates both α- and β-adrenergic receptors. β-Adrenergic receptor activation attenuates NE-mediated contractions. The increased vascular reactivity to PE at 16 wk after SCI could be due to decreased PE clearance by NET as mentioned above. Unchanged adrenoceptors at the postjunctional site have been reported in many tissues, including the carotid artery, brachial artery, renal artery, and femoral artery at 8 wk after SCI (34). Al Dera et al. (2) showed increased neurogenic constriction of the tail arteries from SCI rats, and this was reduced by the L-type Ca2+ blocker nifedipine. The authors concluded that SCI caused an upregulation of L-type Ca2+ channels in arterial smooth muscle, and this could compensate for the loss of sympathetically mediated arterial constriction.
Brock and colleagues (10) found that MA from SCI rats were more sensitive to the constrictor effects of PE compared with MA from control rats. Contractile response to α,β-methylene ATP did not differ in MA from SCI and control rats. These data differ from the data we present here. We did find a small but statistically significant leftward shift in the PE concentration-response curve in MA from paraplegic (but not tetraplegic) rats, but there were no differences in NE concentration-response curves. The predominant effect of SCI on vascular neuromuscular transmission in our studies was prejunctional. The frequency-response curve for NE release (measured using amperometry) was left shifted in T2–3 and C6–7 rats, and ATP release (measured as EJP amplitude) was also increased in these rats. It is important to note that Brock and colleagues used female Wistar rats while we used male Sprague-Dawley rats. In addition, our studies were done 16 wk after SCI while Brock et al. did their studies 7.5 wk after SCI. Strain and time course differences could contribute to study differences in postjunctional sensitivity to neutrally released NE and exogenously applied PE.
Enhanced sympathetic neurovascular transmission is due to increased ATP release.
Previous studies demonstrated that ATP and NE are cotransmitters in sympathetic nerve varicosities (16, 47, 63). However, it is still unclear how NE and ATP are stored in nerve varicosities. ATP could be stored in separate vesicles in the same varicosities that release NE or ATP, and NE could be stored in the same vesicles. Finally, there could also be a mixed population of ATP/NE-containing vesicles, ATP-only vesicles, and NE-only vesicles.
In our study, SCI caused an increase in neuronal ATP release without significantly affecting NE release. This finding supports the idea that ATP and NE are stored in separate vesicles. ATP-and NE-containing vesicles may couple with different soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins required for exocytosis. Kalkhoran and coworkers (27) recently suggested the possibility that ATP and NE could be stored in distinct vesicles within the same varicosities, and their release required 1) different subtypes of synaptotagmin-1, a SNARE protein required for vesicle fusion, and 2) distinct subtypes of the voltage-gated Ca2+-channels. This can explain why the amplitude of stimulation evoked EJPs recorded from MA of SCI rats was much larger than EJPs recorded from of MA from intact rats across the range of stimulation frequencies. Different storage and release mechanisms could also explain why there is a stimulation frequency-dependent increase in the amplitude of NE oxidation current amplitudes. This may also suggest differences in the size of the vesicle pools, where the size of the releasable pool of ATP vesicles is smaller than the pool of NE-containing vesicles under normal physiological conditions. A detailed mechanistic study of how SCI contributes to selective upregulation of the ATP-containing vesicular pool merits future consideration.
Conclusions.
SCI is associated with several severe autonomic disturbances, including disruption of cardiovascular reflexes and BP control. Our data show that there is an enhancement of purinergic but not noradrenergic neurotransmission to MA in T2–3 and C6–7 rats. ATP acts at P2X1 purinergic receptors expressed by MA to cause rapidly developing but short-lived constrictions while NE caused slower developing but longer duration MA constriction. As purinergic signaling may predominate in MA of SCI rats, BP control maybe less stable, leading to hypotension. Orthostatic hypotension can become severe, and this reduces quality of life for individuals and families living with SCI.
GRANTS
This study was supported by National Institutes of Health Grants P01-HL-70687 and R01-HL-122223.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S., H.L.L., and S.E.D. conceived and designed research; S.S., H.X., R.F., H.L.L., and S.E.D. performed experiments; S.S., H.X., R.F., G.D.F., and J.J.G. analyzed data; S.S., H.X., R.F., G.D.F., and J.J.G. interpreted results of experiments; S.S., R.F., and J.J.G. prepared figures; S.S. drafted manuscript; S.S., H.X., G.D.F., S.E.D., and J.J.G. edited and revised manuscript; S.S., H.X., R.F., G.D.F., H.L.L., S.E.D., and J.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Robert Burnett for performing the HPLC measurements.
REFERENCES
- 1.Al Dera H, Brock JA. Changes in sympathetic neurovascular function following spinal cord injury. Auton Neurosci 209: 25–36, 2018. doi: 10.1016/j.autneu.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Al Dera H, Habgood MD, Furness JB, Brock JA. Prominent contribution of L-type Ca2+ channels to cutaneous neurovascular transmission that is revealed after spinal cord injury augments vasoconstriction. Am J Physiol Heart Circ Physiol 302: H752–H762, 2012. doi: 10.1152/ajpheart.00745.2011. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JM, Feng QP, Delaney GA, Teasell RW. Autonomic dysreflexia in tetraplegic patients: evidence for α-adrenoceptor hyper-responsiveness. Clin Auton Res 5: 267–270, 1995. doi: 10.1007/BF01818891. [DOI] [PubMed] [Google Scholar]
- 4.Besecker EM, Deiter GM, Pironi N, Cooper TK, Holmes GM. Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury. Am J Physiol Regul Integr Comp Physiol 312: R146–R156, 2017. doi: 10.1152/ajpregu.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brain KL, Jackson VM, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol 541: 849–862, 2002. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock JA, Cunnane TC. Neurotransmitter release mechanisms at the sympathetic neuroeffector junction. Exp Physiol 78: 591–614, 1993. doi: 10.1113/expphysiol.1993.sp003709. [DOI] [PubMed] [Google Scholar]
- 7.Brock JA, Yeoh M, McLachlan EM. Enhanced neurally evoked responses and inhibition of norepinephrine reuptake in rat mesenteric arteries after spinal transection. Am J Physiol Heart Circ Physiol 290: H398–H405, 2006. doi: 10.1152/ajpheart.00712.2005. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Holman ME. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol 155: 115–133, 1961. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen KL, Mulvany MJ. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res 30: 73–79, 1993. doi: 10.1159/000158978. [DOI] [PubMed] [Google Scholar]
- 10.Coote JH, Chauhan RA. The sympathetic innervation of the heart: important new insights. Auton Neurosci 199: 17–23, 2016. doi: 10.1016/j.autneu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Demel SL, Dong H, Swain GM, Wang X, Kreulen DL, Galligan JJ. Antioxidant treatment restores prejunctional regulation of purinergic transmission in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Neuroscience 168: 335–345, 2010. doi: 10.1016/j.neuroscience.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demel SL, Galligan JJ. Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension 52: 322–329, 2008. doi: 10.1161/HYPERTENSIONAHA.108.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008, 1997. doi: 10.1016/S0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 15.Donoso MV, Brown N, Carrasco C, Cortes V, Fournier A, Huidobro-Toro JP. Stimulation of the sympathetic perimesenteric arterial nerves releases neuropeptide Y potentiating the vasomotor activity of noradrenaline: involvement of neuropeptide Y-Y1 receptors. J Neurochem 69: 1048–1059, 1997. doi: 10.1046/j.1471-4159.1997.69031048.x. [DOI] [PubMed] [Google Scholar]
- 16.Donoso MV, Steiner M, Huidobro-Toro JP. BIBP 3226, suramin and prazosin identify neuropeptide Y, adenosine 5′-triphosphate and noradrenaline as sympathetic cotransmitters in the rat arterial mesenteric bed. J Pharmacol Exp Ther 282: 691–698, 1997. [PubMed] [Google Scholar]
- 17.Dunn WR, Brock JA, Hardy TA. Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br J Pharmacol 128: 174–180, 1999. doi: 10.1038/sj.bjp.0702760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fathali H, Cans A-S. Amperometry methods for monitoring vesicular quantal size and regulation of exocytosis release. Pflügers Arch 470: 125–134, 2018. doi: 10.1007/s00424-017-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001. doi: 10.1016/S0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 20.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 29: 489–500, 2006. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile— part I. J Spinal Cord Med 37: 693–702, 2014. doi: 10.1179/2045772314Y.0000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy TA, Brock JA. Effects of modulating Ca2+ entry and activating prejunctional receptors on facilitation of excitatory junction potentials in the guinea-pig vas deferens in vitro. Naunyn Schmiedebergs Arch Pharmacol 363: 515–525, 2001. doi: 10.1007/s002100000394. [DOI] [PubMed] [Google Scholar]
- 23.Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171: 153–169, 2001. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- 24.Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol 509: 382–399, 2008. doi: 10.1002/cne.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol 4: 1419–1453, 2014. doi: 10.1002/cphy.c130045. [DOI] [PubMed] [Google Scholar]
- 26.Jensen LJ, Salomonsson M, Jensen BL, Holstein-Rathlou NH. Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels. Br J Pharmacol 142: 709–718, 2004. doi: 10.1038/sj.bjp.0705841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mojard Kalkhoran S, Chow SH, Walia JS, Gershome C, Saraev N, Kim B, Poburko D. VNUT and VMAT2 segregate within sympathetic varicosities and localize near preferred Cav2 isoforms in the rat tail artery. Am J Physiol Heart Circ Physiol 316: H89–H105, 2019. doi: 10.1152/ajpheart.00560.2018. [DOI] [PubMed] [Google Scholar]
- 28.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 294: R1262–R1267, 2008. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 29.Krassioukov AV, Bunge RP, Pucket WR, Bygrave MA. The changes in human spinal sympathetic preganglionic neurons after spinal cord injury. Spinal Cord 37: 6–13, 1999. doi: 10.1038/sj.sc.3100718. [DOI] [PubMed] [Google Scholar]
- 30.Krassioukov AV, Weaver LC. Morphological changes in sympathetic preganglionic neurons after spinal cord injury in rats. Neuroscience 70: 211–225, 1996. doi: 10.1016/0306-4522(95)00294-S. [DOI] [PubMed] [Google Scholar]
- 31.Krassioukov AV, Weaver LC. Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin Exp Hypertens 17: 361–373, 1995. doi: 10.3109/10641969509087077. [DOI] [PubMed] [Google Scholar]
- 32.Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 19: 7405–7414, 1999. doi: 10.1523/JNEUROSCI.19-17-07405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird AS, Carrive P, Waite PM. Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J Physiol 577: 539–548, 2006. doi: 10.1113/jphysiol.2006.116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laird AS, Finch AM, Waite PM, Carrive P. Peripheral changes above and below injury level lead to prolonged vascular responses following high spinal cord injury. Am J Physiol Heart Circ Physiol 294: H785–H792, 2008. doi: 10.1152/ajpheart.01002.2007. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci 154: 66–73, 2010. doi: 10.1016/j.autneu.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Lin CS, Macefield VG, Elam M, Wallin BG, Engel S, Kiernan MC. Axonal changes in spinal cord injured patients distal to the site of injury. Brain 130: 985–994, 2007. doi: 10.1093/brain/awl339. [DOI] [PubMed] [Google Scholar]
- 37.Lin YQ, Graham K, Bennett MR. Depression of transmitter release at synapses in the rat superior cervical ganglion: the role of transmitter depletion. Auton Neurosci 88: 16–24, 2001. doi: 10.1016/S1566-0702(00)00287-3. [DOI] [PubMed] [Google Scholar]
- 38.Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol 435: 226–240, 2001. doi: 10.1002/cne.1204. [DOI] [PubMed] [Google Scholar]
- 39.Llewellyn-Smith IJ, Weaver LC, Keast JR. Effects of spinal cord injury on synaptic inputs to sympathetic preganglionic neurons. Prog Brain Res 152: 11–26, 2006. doi: 10.1016/S0079-6123(05)52001-6. [DOI] [PubMed] [Google Scholar]
- 40.Lujan HL, Chen Y, Dicarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus, and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009. doi: 10.1152/ajpheart.01286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007. doi: 10.1152/ajpheart.01019.2007. [DOI] [PubMed] [Google Scholar]
- 42.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol (1985) 113: 1332–1341, 2012. doi: 10.1152/japplphysiol.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lujan HL, Palani G, DiCarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol 299: R985–R995, 2010. doi: 10.1152/ajpregu.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lujan HL, Tonson A, Wiseman RW, DiCarlo SE. Chronic, complete cervical6-7 cord transection: distinct autonomic and cardiac deficits. J Appl Physiol (1985) 124: 1471–1482, 2018. doi: 10.1152/japplphysiol.01104.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathias CJ, Low DA. Autonomic disturbances in spinal cord injuries. In: Primer on the Autonomic Nervous System (3rd ed.), edited by Robertson D, Biaggioni I, Burnstock G, Low PA, and Paton JFR. San Diego, CA: Academic, 2012, chapt. 104, p. 505–509. [Google Scholar]
- 46.McLachlan EM, Brock JA. Adaptations of peripheral vasoconstrictor pathways after spinal cord injury. Prog Brain Res 152: 289–297, 2006. doi: 10.1016/S0079-6123(05)52019-3. [DOI] [PubMed] [Google Scholar]
- 47.Meldrum LA, Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol 92: 161–163, 1983. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- 48.Menéndez-Méndez A, Díaz-Hernández JI, Ortega F, Gualix J, Gómez-Villafuertes R, Miras-Portugal MT. Specific Temporal Distribution and Subcellular Localization of a Functional Vesicular Nucleotide Transporter (VNUT) in Cerebellar Granule Neurons. Front Pharmacol 8: 951, 2017. doi: 10.3389/fphar.2017.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyahara H, Suzuki H. Pre- and post-junctional effects of adenosine triphosphate on noradrenergic transmission in the rabbit ear artery. J Physiol 389: 423–440, 1987. doi: 10.1113/jphysiol.1987.sp016664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat Methods 2: 651–658, 2005. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- 51.Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol 184: 373–380, 2003. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Pan B, Kim EJ, Schramm LP. Increased close appositions between corticospinal tract axons and spinal sympathetic neurons after spinal cord injury in rats. J Neurotrauma 22: 1399–1410, 2005. doi: 10.1089/neu.2005.22.1399. [DOI] [PubMed] [Google Scholar]
- 53.Park J, Galligan JJ, Fink GD, Swain GM. Alterations in sympathetic neuroeffector transmission to mesenteric arteries but not veins in DOCA-salt hypertension. Auton Neurosci 152: 11–20, 2010. doi: 10.1016/j.autneu.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J, Galligan JJ, Fink GD, Swain GM. Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging. J Physiol 584: 819–834, 2007. doi: 10.1113/jphysiol.2007.134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ralevic V, Edvinsson L, Burnstock G. Inhibition of neuropeptide Y-induced augmentation of noradrenaline-induced vasoconstriction by D-myo-inositol 1,2,6-trisphosphate in the rat mesenteric arterial bed. Acta Physiol Scand 151: 309–317, 1994. doi: 10.1111/j.1748-1716.1994.tb09750.x. [DOI] [PubMed] [Google Scholar]
- 56.Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR. ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol 582: 745–754, 2007. doi: 10.1113/jphysiol.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rummery NM, Tripovic D, McLachlan EM, Brock JA. Sympathetic vasoconstriction is potentiated in arteries caudal but not rostral to a spinal cord transection in rats. J Neurotrauma 27: 2077–2089, 2010. doi: 10.1089/neu.2010.1468. [DOI] [PubMed] [Google Scholar]
- 58.Sangsiri S, Dong H, Swain GM, Galligan JJ, Xu H. Impaired function of prejunctional adenosine A1 receptors expressed by perivascular sympathetic nerves in DOCA-salt hypertensive rats. J Pharmacol Exp Ther 345: 32–40, 2013. doi: 10.1124/jpet.112.199612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santajuliana D, Zukowska-Grojec Z, Osborn JW. Contribution of alpha- and beta- adrenoceptors and neuropeptide-Y to autonomic dysreflexia. Clin Auton Res 5: 91–97, 1995. doi: 10.1007/BF01827469. [DOI] [PubMed] [Google Scholar]
- 60.Schramm LP. Spinal sympathetic interneurons: their identification and roles after spinal cord injury. Prog Brain Res 152: 27–37, 2006. doi: 10.1016/S0079-6123(05)52002-8. [DOI] [PubMed] [Google Scholar]
- 61.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 62.Smith AB, Cunnane TC. Omega-conotoxin GVIA-resistant neurotransmitter release in postganglionic sympathetic nerve terminals. Neuroscience 70: 817–824, 1996. doi: 10.1016/S0306-4522(96)83018-1. [DOI] [PubMed] [Google Scholar]
- 63.Sneddon P, Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol 106: 149–152, 1984. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- 64.Trendelenburg U, Maxwell RA, Pluchino S. Methoxamine as a tool to assess the importance of intraneuronal uptake of l-norepinephrine in the cat’s nictitating membrane. J Pharmacol Exp Ther 172: 91–99, 1970. [PubMed] [Google Scholar]
- 65.van Kempen GT, vanderLeest HT, van den Berg RJ, Eilers P, Westerink RH. Three distinct modes of exocytosis revealed by amperometry in neuroendocrine cells. Biophys J 100: 968–977, 2011. doi: 10.1016/j.bpj.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westerink RH. Exocytosis: using amperometry to study presynaptic mechanisms of neurotoxicity. Neurotoxicology 25: 461–470, 2004. doi: 10.1016/j.neuro.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Xu H, Bian X, Watts SW, Hlavacova A. Activation of vascular BK channel by tempol in DOCA-salt hypertensive rats. Hypertension 46: 1154–1162, 2005. doi: 10.1161/01.HYP.0000186278.50275.fa. [DOI] [PubMed] [Google Scholar]
- 68.Xu H, Fink GD, Galligan JJ. Increased sympathetic venoconstriction and reactivity to norepinephrine in mesenteric veins in anesthetized DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 293: H160–H168, 2007. doi: 10.1152/ajpheart.01414.2006. [DOI] [PubMed] [Google Scholar]
- 69.Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol 561: 583–596, 2004. doi: 10.1113/jphysiol.2004.074948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao H, Bian X, Galligan JJ, Swain GM. Electrochemical measurements of serotonin (5-HT) release from the guinea pig mucosa using continuous amperometry with a boron-doped diamond microelectrode. Diamond Related Materials 19: 182–185, 2010. doi: 10.1016/j.diamond.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]