Abstract
Reactive oxygen species (ROS), mitochondrial dysfunction, and excessive vasoconstriction are important contributors to chronic hypoxia (CH)-induced neonatal pulmonary hypertension. On the basis of evidence that PKCβ and mitochondrial oxidative stress are involved in several cardiovascular and metabolic disorders, we hypothesized that PKCβ and mitochondrial ROS (mitoROS) signaling contribute to enhanced pulmonary vasoconstriction in neonatal rats exposed to CH. To test this hypothesis, we examined effects of the PKCβ inhibitor LY-333,531, the ROS scavenger 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL), and the mitochondrial antioxidants mitoquinone mesylate (MitoQ) and (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO) on vasoconstrictor responses in saline-perfused lungs (in situ) or pressurized pulmonary arteries from 2-wk-old control and CH (12-day exposure, 0.5 atm) rats. Lungs from CH rats exhibited greater basal tone and vasoconstrictor sensitivity to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U-46619). LY-333,531 and TEMPOL attenuated these effects of CH, while having no effect in lungs from control animals. Basal tone was similarly elevated in isolated pulmonary arteries from neonatal CH rats compared with control rats, which was inhibited by both LY-333,531 and mitochondria-targeted antioxidants. Additional experiments assessing mitoROS generation with the mitochondria-targeted ROS indicator MitoSOX revealed that a PKCβ-mitochondrial oxidant signaling pathway can be pharmacologically stimulated by the PKC activator phorbol 12-myristate 13-acetate in primary cultures of pulmonary artery smooth muscle cells (PASMCs) from control neonates. Finally, we found that neonatal CH increased mitochondrially localized PKCβ in pulmonary arteries as assessed by Western blotting of subcellular fractions. We conclude that PKCβ activation leads to mitoROS production in PASMCs from neonatal rats. Furthermore, this signaling axis may account for enhanced pulmonary vasoconstrictor sensitivity following CH exposure.
NEW & NOTEWORTHY This research demonstrates a novel contribution of PKCβ and mitochondrial reactive oxygen species signaling to increased pulmonary vasoconstrictor reactivity in chronically hypoxic neonates. The results provide a potential mechanism by which chronic hypoxia increases both basal and agonist-induced pulmonary arterial smooth muscle tone, which may contribute to neonatal pulmonary hypertension.
Keywords: mitochondria, oxidative stress, PKCβ, pulmonary hypertension, vascular disease
INTRODUCTION
Chronic hypoxia (CH) contributes to the pathogenesis of neonatal pulmonary hypertension (pHTN) (1, 28) by elevating postnatal pulmonary vascular resistance (PVR) through both vasoconstriction and arterial smooth muscle proliferation (5, 9, 26). Vasoconstrictor responses to CH are multifaceted, including acute hypoxic pulmonary vasoconstriction (26), increased myogenic tone (57), and enhanced reactivity to vasoconstrictor stimuli (15, 26). Oxidative stress, increased vascular smooth muscle (VSM) Ca2+ sensitivity, and elevated intracellular Ca2+ appear to be common mediators of the vasoconstrictor component of various forms of pHTN in both adults and neonates (3, 26, 29, 30). However, the enzymatic sources of reactive oxygen species (ROS) that mediate oxidant signaling and the underlying mechanisms of vasoconstriction in this setting are not well understood.
The mitochondrial electron transport chain has emerged as a major source of ROS in the pulmonary circulation, mediating both vasoconstrictor responses to acute hypoxia (64) and aberrant cellular signaling leading to oxidative damage and cell death in ischemia-reperfusion injury (36). Mitochondrial ROS (mitoROS) are additionally elevated in the pulmonary vasculature of fetal lambs with pHTN caused by prenatal ligation of the ductus arteriosus (3, 20), suggesting a potential contribution of mitochondrial oxidative stress to the development of neonatal pHTN. MitoROS production can be increased by a variety of physiological stimuli, including activation of PKCβ, a classical PKC isoform that contributes to several cardiovascular and metabolic disorders through a primary mechanism of mitochondrial dysfunction and apoptosis (62). Interestingly, PKCβ has been implicated in mediating augmented pulmonary vasoconstrictor reactivity in an adult rodent model of sleep apnea (intermittent hypoxia)-induced pHTN (55). On the basis of these observations, we hypothesized that a PKCβ-mitoROS signaling axis contributes to enhanced pulmonary vasoconstriction in neonatal rats exposed to CH.
To test our hypothesis, we used an isolated (in situ) perfused lung preparation to evaluate the contribution of ROS and PKCβ to basal and agonist-induced pulmonary vasoconstriction in lungs from normoxic and CH neonatal (2 wk old) rats. Additional experiments examined the contribution of PKCβ and mitoROS to basal tone development in isolated pressurized pulmonary arteries from neonates in both groups and assessed effects of CH on pulmonary arterial PKCβ expression and subcellular localization by Western blotting. Finally, we examined the signaling relationship between PKCβ and mitoROS with pulmonary arterial smooth muscle cells (PASMCs) collected from neonatal rats. Our findings demonstrate a novel effect of neonatal CH to augment both basal tone and agonist-induced pulmonary arterial constriction through PKCβ-mitoROS signaling.
METHODS
Animals and CH Exposure Protocol
Animal protocols used for this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center (Albuquerque, NM).
Neonatal rats.
Timed-pregnant Sprague-Dawley (Harlan Industries) rats arrived 1 wk before their delivery date to allow for acclimation. Birth of the litter occurred under ambient barometric pressure (~630 mmHg for Albuquerque, NM). On day 2 of life, litters selected for CH exposure were housed in a hypobaric hypoxic chamber with barometric pressure maintained at 380 ± 5 mmHg (0.5 atm) for 12 days. Age-matched control litters were housed in similar conditions under ambient barometric pressure. Litters in either group were housed with their birthing dam. We have previously reported that this CH exposure protocol results in elevated right ventricular systolic pressure and hypertrophy, indicative of pHTN (54).
Adult rats.
To evaluate whether PKCβ-mediated pulmonary vasoconstriction following CH is developmentally regulated, we performed additional studies using adult male Sprague-Dawley (Harlan Industries) rats (200–250 g body wt). Animals in the CH group were housed in a hypobaric chamber with barometric pressure maintained at 380 ± 5 mmHg for 4 wk. Age-matched control animals were housed for an equal duration at ambient barometric pressure. This protocol for CH exposure results in pHTN in adult rats (48).
For all animal groups exposed to CH, the hypobaric chamber was opened twice per week to provide animals with food, water, and clean bedding. Animals were housed on a 12:12-h light-dark cycle.
Isolated (in Situ) Perfused Neonatal Rat Lung Preparation
This protocol has been previously described by our group (14, 54). Briefly, neonatal rats (postnatal day 14) were anesthetized with pentobarbital sodium (200 mg/kg ip). The animal was placed on a heating block with temperature maintained at 37°C. The trachea was cannulated and connected to a mouse ventilator (Hugo Sachs Electronik-Harvard Apparatus) with a tidal volume of 5 mL/kg body mass at a rate of 100 breaths/min with a warmed and humidified gas mixture (21% O2-6% CO2-balance N2). End-expiratory pressure was maintained at 3 cmH2O. After removal of the frontal rib cage, heparin (5 U; 50 μL) was injected into the right ventricle and the pulmonary artery was cannulated with custom-fitted PE-90 tubing (Becton Dickinson). The preparation was immediately perfused with physiological saline solution (PSS) containing (in mM) 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose with 4% (wt/vol) bovine serum albumin (BSA) (all reagents from Sigma) with a peristaltic pump (Ismatec) at an initial rate of 0.1 mL/min. After the onset of perfusion, the left ventricle was cannulated with custom-fitted PE-160 tubing. Perfusion rate (Q) was gradually increased to a maximum flow of 15 mL·min−1·kg body mass. Perfusion was nonrecirculating until the perfusate exiting the lung was nearly free of blood. Recirculation was then initiated and maintained for the duration of the experiment. Total recirculating volume was ~3.5 mL. All cannulas were secured in place with braided 4-0 silk suture. Lungs were kept in zone 3 conditions with venous pressure maintained at 3 mmHg and airway pressure at 1 mmHg. Pulmonary arterial (Pa) and venous (Pv) pressures were simultaneously recorded with P-75 pressure transducers (Hugo Sachs Electronik-Harvard Apparatus) connected to the pulmonary arterial and venous lines. Data were recorded and processed with data acquisition software and hardware (AT-CODAS; Dataq Instruments). Total PVR (Rt) was calculated as (Pa − Pv)/Q.
Assessment of Segmental Vascular Resistances
A double occlusion method was used to obtain segmental vascular resistances as previously reported (14, 49). In short, lungs were isolated as above and both inflow and outlet lines were simultaneously occluded, allowing arterial and venous pressures to rapidly equilibrate to microvascular capillary pressure (Pc). This estimation of Pc agrees with values of Pc assessed by isogravimetric methods (60). Pulmonary arterial resistance (Ra) was calculated with the formula (Pa − Pc)/Q, and pulmonary venous resistance (Rv) was calculated with (Pc − Pv)/Q.
Basal Tone and Agonist-Induced Vasoconstriction in Isolated (in Situ) Perfused Rat Lung
To assess the contribution of ROS and PKCβ to augmented pulmonary vasoconstriction following CH, isolated (in situ) lungs from neonatal rats were exposed either to vehicle or the ROS scavenger 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL) (47) (1 mM final concentration; Sigma), the general PKC inhibitor Ro 31-8220 (55) (5 μM; Enzo), or the selective PKCβ inhibitor LY-333,531 (55, 59) (10 nM; AdipoGen). Previous observations from our group demonstrate that neonatal CH enhances basal pulmonary arterial tone and augments vasoconstrictor sensitivity to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U-46619) through mechanisms independent of changes in reactivity to endogenous nitric oxide (NO) (54). Therefore, all experiments were performed in the presence of Nω-nitro-l-arginine (l-NNA, 300 μM; Sigma) (51) to eliminate complicating effects of endogenous NO production on the observed responses. Lungs from neonates in both groups were allowed to equilibrate with PSS + 4% BSA, after which a baseline double occlusion was performed. The contribution of active tone to baseline PVR was assessed by administration of the exogenous NO donor 1,3-propanediamine, N-{4-[1-(3-aminopropyl)-2-hydroxy-2-nitro-sohydrazino]butyl} (spermine NONOate) (14, 49) (100 μM; Cayman Chemical) to dilate the pulmonary vasculature. A double occlusion was performed after stabilization of the response to NONOate. Basal tone is expressed as the change in resistance to spermine NONOate.
The effect of ROS and PKCβ to enhance vasoconstrictor reactivity after CH was determined by performing cumulative concentration-response curves to the thromboxane analog U-46619 (56) (Cayman Chemical) with and without ROS scavenging and PKCβ inhibition. Double occlusions were performed to assess Pc under baseline conditions and after development of a stable pressor response to each concentration of U-46619. Vasoconstrictor responses were calculated as changes in resistance to U-46619 from baseline.
Basal Tone in Isolated Pulmonary Arteries
To evaluate the contribution of PKCβ and mitoROS to enhanced basal tone development in neonates exposed to CH, small pulmonary arteries were isolated and cannulated from adult and neonatal animals in both groups, as previously described (43, 55). Pulmonary arteries [~150- to 200-μm inner diameter (ID)] from the left lung were dissected free from surrounding parenchyma and cannulated on a glass microcannula secured in a vessel superperfusion chamber (CH-1; Living Systems Instrumentation). All vessels were pressurized to 12 mmHg, and vessel ID was measured with bright-field videomicroscopy. Cannulated, pressurized vessels were superfused with recirculating heated (37°C) PSS bubbled with a 6% CO2-10% O2-balance N2 gas mixture. After a 20-min equilibration period, l-NNA (300 μM) was added to the superfusate, leading to an increase in vasomotor tone. Experiments were allowed to progress for a minimum of 20 min or until the vasoconstrictor response stabilized. After stabilization of basal tone, vessels were superfused with nonrecirculating Ca2+-free PSS that contained (in mM) 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 5.5 glucose, and 3 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA; Sigma) (7, 12) for 30 min. After a 30-min Ca2+-free PSS washout, recirculation was initiated and ionomycin [Ca2+ ionophore (2, 44, 56)] (3 μM; Calbiochem) was added to maximally dilate the vessel. Basal tone is expressed as percentage of maximally dilated, Ca2+-free diameter with the formula [(Ca2+-free ID − constricted ID)/Ca2+-free ID] × 100.
In isolated vessel studies conducted in the presence of inhibitors of either PKCβ or mitoROS, the inhibitor was present throughout the experiment, i.e. during the vessel dissection, cannulation, equilibration, and l-NNA exposure. Basal tone studies were performed in the presence and absence of the selective PKCβ inhibitor LY-333,531 (10 nM), the mitochondrial antioxidants (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO) (16) (200 μM; Sigma) and mitoquinone mesylate (MitoQ) (17) (1 μM; gift from MitoQ Ltd.), or vehicle.
Transient Culture of Pulmonary Arterial Smooth Muscle Cells and Fluorescence Detection of Mitochondrial ROS
PKC-dependent increases in levels of mitochondrial ROS (mitoROS) were assessed in transiently cultured primary pulmonary arterial smooth muscle cells (PASMCs). The protocol to collect and transiently culture primary PASMCs has been previously described by our group (46). Briefly, control neonates were anesthetized with pentobarbital sodium (200 mg/kg ip), and the heart and lungs were exposed via midline thoracotomy. Left and right lungs were removed and placed in sterile 20 mM Ca2+ Hanks’ balanced salt solution (HBSS) plus 1% (vol/vol) penicillin-streptomycin (pen/strep; Lonza). Intrapulmonary arteries (~2nd–5th order) were dissected from surrounding lung parenchyma. To isolate PASMCs, intrapulmonary arteries from both lungs were enzymatically digested in 20 mM Ca2+ HBSS containing papain (9.5 U/mL), type 1 collagenase (1,750 U/mL), and dithiothreitol (1 mM) at 37°C for 15 min. After enzymatic digestion, collected pulmonary arteries were added to a 70-μm cell strainer and rinsed with 10 mL of Ca2+-free HBSS. Pulmonary arteries were then added to a fresh Eppendorf tube containing Ca2+-free HBSS, and PASMCs were dispersed with a Pasteur pipette. The cell suspension was then placed on gelatin-coated 25-mm glass coverslips and cultured in Ham’s F-12 medium (Life Technologies) supplemented with 5% fetal bovine serum (FBS; Thermo Fisher) and 1% pen/strep for 48 h at 37°C.
Cultured PASMCs from control neonates were serum starved for 24 h in Ham’s F-12 medium plus 1% FBS and 1% pen/strep before experimentation. Cells were then treated with PMA (10 μM; Calbiochem) to stimulate PKC-dependent mitoROS production. After a 30-min exposure to PMA, mitochondrial was detected with the mitochondria-targeted indicator MitoSOX (5 μM; ThermoFisher) (32). Depending on the experimental protocol, cells were pretreated with scavengers of cellular TEMPOL (1 mM) or Tiron (10 mM). A selective scavenger of mitochondrial was also used [(2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO, 200 μM). Finally, LY-333,531 (10 nM) was used to selectively inhibit PKCβ. Cells were pretreated with ROS scavengers or LY-333,531 for 20 min at 37°C and were present for the duration of MitoSOX treatment. After the pretreatment period, PASMCs were exposed to MitoSOX for 30 min before being fixed with 4% paraformaldehyde. Coverslips containing MitoSOX-labeled PASMCs were then added to a slide with Fluoro-Gel (Electron Microscopy Sciences) and imaged by confocal microscopy.
Fluorescence images were acquired with a ×63 80% glycerol-immersion objective on a confocal microscope (Leica TCS SP5; 1.3 numerical aperture). MitoSOX was excited with a DPSS 561 laser, and the emission was processed through an Acousto-Optical Beam Splitter (AOBS; Leica) crystal set to an emission bandwidth of 570–620 nm. Images were obtained without an optical zoom (x–y pixel dimensions of 0.49 μm × 0.49 μm); all images were obtained with a 512 × 512 frame and frame averaging of 2 scans. Scan speed was set to 400 Hz. Laser power was 60% for the DPSS 561 laser. z Stacks were obtained at z steps of 0.8 μm. Detector gain and offset setting were held constant for all acquired z stacks at a gain setting of 985 and an offset of −12 for the photomultiplier tube set to collect bandwidth of 570–620 nm.
Quantification of fluorescence was performed with SlideBook 6 (3i: Intelligent Imaging Innovations). MitoSOX fluorescence intensity is represented as an average fluorescence intensity with an inclusive threshold of 10,000–65,535 (maximum for 16-bit images). Each z stack was thresholded to select for positive-stained areas with fluorescence intensity values above background (cells not treated with MitoSOX). Data are reported as means ± SE, and n represents the number of individual animals that yielded cells for experimentation. Each individual n is an average of two technical replicates of cells from the same animal.
Western Blotting for PKCβ
The effect of CH on pulmonary arterial PKCβ protein expression was evaluated via Western blot as previously reported (49). Isolated pulmonary arteries (~2nd–5th order) were collected from neonatal pups, snap frozen in liquid N2, and stored at −80°C until ready for blotting. On the day of the experiment, frozen pulmonary arteries were homogenized in a 1-mL Tenbroeck tissue grinder (Wheaton) with 150 μL of ice-cold homogenization buffer [in mM: 10 Tris·HCl (pH 7.4), 250 sucrose, 10 EDTA] containing both a protease inhibitor cocktail (Sigma catalog no. P8340) and a phosphatase inhibitor cocktail (Sigma catalog no. P0044). Homogenates were spun at 700 g for 10 min in a desktop centrifuge (Beckman) at 4°C to pellet debris. Protein concentration of the supernatant was assessed via a Qubit 4 Fluorometer (Thermo Fisher). Samples (15 μg protein/lane) were resolved by SDS-PAGE with 12% acrylamide along with molecular weight standards (Bio-Rad). The separated proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad) and blocked for 1 h at room temperature with 5% nonfat milk (Carnation) and 0.1% Tween 20 (Bio-Rad) in a Tris-buffered saline solution (TTBS) containing 10 mM Tris·HCl and 50 mM NaCl (pH 7.5). Blots were then incubated overnight at 4°C with slight agitation in a TTBS solution containing 5% nonfat milk and a rabbit polyclonal primary antibody against PKCβ (1:1,000; Proteintech, catalog no. 12919-1-AP, lot no. 00019327). This antibody has been previously validated (69). To control for protein loading, all membranes were probed with a rabbit polyclonal antibody to β-actin (1:14,000; Abcam, catalog no. ab8227, lot no. GR186254-1) in TTBS + 5% nonfat milk for 1 h at room temperature. The blots were then probed with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:3,000; Bio-Rad, catalog no. 172-1019, lot no. 64026773) in TTBS + 5% nonfat milk for 1 h at room temperature to achieve immunochemical labeling. Chemiluminescence labeling was performed according to manufacturer instructions (ECL substrate; Thermo Scientific). Protein bands were detected via exposure of a chemiluminescence-sensitive film to the membrane, and band density was quantified with ImageJ (NIH).
An additional mechanism by which PKCβ could stimulate enhanced mitoROS production is through translocation to the mitochondria. To test this possibility, we collected pulmonary arteries from both control and CH neonates and snap froze them in liquid N2 until ready for use. We followed the Western blotting protocol outlined in the previous section with the following differences. After the initial centrifugation (Eppendorf Centrifuge 5415R) of the arterial homogenates at 700 g for 10 min at 4°C to clear tissue debris, we spun the collected supernatant at 10,000 g for 1 h at 4°C to separate the mitochondria-enriched (pellet) and cytosolic/microsomal (supernatant) fractions. The pellet was resuspended in 20 μL of homogenization buffer plus protease and phosphatase inhibitors. Fifteen micrograms of the fractionated samples was then separated with the SDS-PAGE protocol described above. To verify mitochondrial isolation, blots containing fractionated homogenates were probed with a mouse monoclonal primary antibody against a subunit of cytochrome-c oxidase (1:10,000; Thermo Fisher, catalog no. A21348, lot no. 1890863) for 1 h at room temperature, followed by 1 h with HRP-conjugated goat anti-mouse secondary antibody (1:3,000; Bio-Rad, catalog no. 172-1011, lot no. 350002594). Data are expressed as the ratio of PKCβ band density (Image J, NIH) in the mitochondria-enriched fraction to that in the cytoplasmic/microsomal fraction.
Calculations and Statistics
All data are expressed as ± SE. Values of n represent numbers of animals. A t-test, one-way analysis of variance (ANOVA), or two-way ANOVA was used to make comparisons between groups. If differences were detected by ANOVA, individual groups were further evaluated with a Student-Newman-Keuls post hoc comparison. Data expressed as percentages were arcsin-transformed before statistical comparison to allow for parametric analysis. P values of <0.05 were considered significant.
RESULTS
Consistent with our previous findings (54), body weight of CH pups was significantly lower than that of age-matched control pups (control: 28.1 ± 0.7 g, CH 17.6 ± 0.2 g; P < 0.05).
ROS Mediate Augmented Basal Pulmonary Arterial Tone Following Neonatal CH
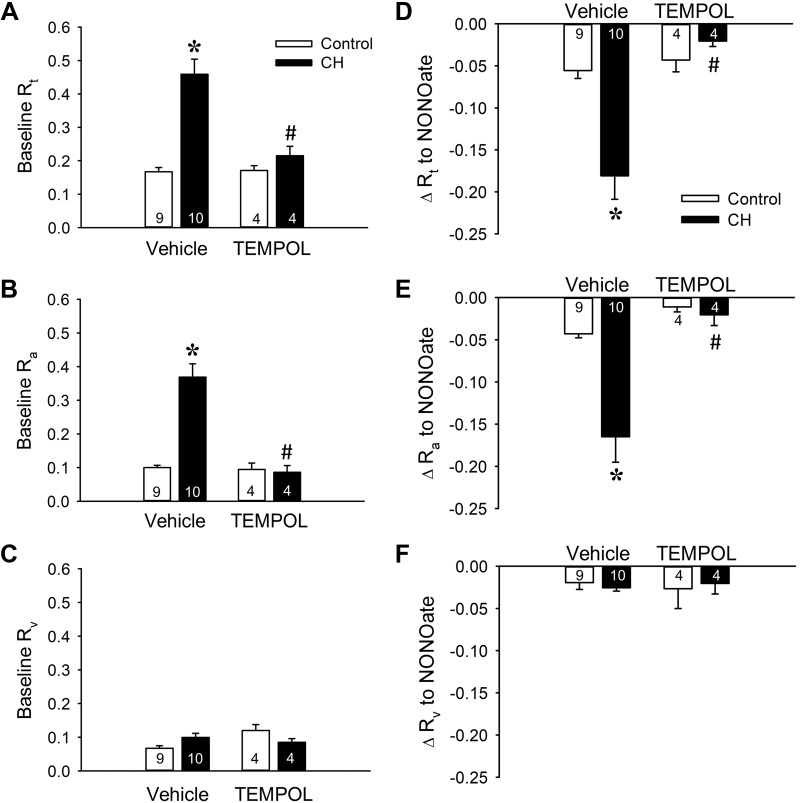
The contribution of to CH-induced increases in basal PVR in neonatal rats was evaluated with the superoxide dismutase (SOD) mimetic TEMPOL in an (in situ) saline-perfused lung preparation. Consistent with a prior study from our group (54), lungs from neonates exposed to CH demonstrated greater baseline total (Rt) and arterial (Ra) resistances than control lungs, with no difference in venous resistance (Rv) between groups (Fig. 1, A–C), indicating that CH-dependent increases in Ra account for elevations in Rt. Interestingly, the greater baseline Rt and Ra in the CH group were diminished to the levels of control rats by the SOD mimetic TEMPOL (Fig. 1, A and B), whereas TEMPOL was without effect on Rv (Fig. 1C). Furthermore, total and segmental resistances were unaltered by scavenging in lungs from control rats. These results indicate that the CH-induced elevation in baseline PVR in this model is due entirely to a ROS-mediated increase in Ra.
Fig. 1.
Chronic hypoxia (CH) increases baseline pulmonary vascular resistance and basal tone through reactive oxygen species signaling. A–C: total (Rt; A), arterial (Ra; B), and venous (Rv; C) baseline vascular resistance (mmHg·mL−1·kg·min) in lungs (in situ) from control and CH neonatal rats in the presence or absence of the superoxide dismutase mimetic 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL, 1 mM). D–F: the contribution of basal tone to total (D), arterial (E), and venous (F) resistance is expressed as the change in resistance (ΔR) to 1,3-propanediamine, N-{4-[1-(3-aminopropyl)-2-hydroxy-2-nitro-sohydrazino]butyl} (spermine NONOate, 100 μM) in lungs (in situ) from control and CH neonates. All experiments were conducted in the presence of Nω-nitro-l-arginine (300 μM). Values are means ± SE; n = 4–10 rats/group (indicated in bars). *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA followed by Student-Newman-Keuls post hoc comparison.
To specifically evaluate the contribution of basal tone to CH-dependent elevations in PVR, we assessed effects of the NO donor spermine NONOate to vasodilate lungs from both control and CH neonates. Absolute changes in Rt and Ra to spermine NONOate were greater in CH compared with control lungs (Fig. 1, D and E). In contrast, effects of exogenous NO on baseline Rv were minimal in both groups, and unaffected by exposure to CH, demonstrating that the venous segment exhibits little to no basal tone (Fig. 1F). Therefore, greater basal Rt after CH appears to be mediated by enhanced pulmonary arterial tone.
Consistent with effects of TEMPOL to normalize baseline resistances between groups (Fig. 1, A–C), pretreatment with TEMPOL nearly abolished total and arterial vasodilatory responses to spermine NONOate after CH (Fig. 1, D and E), while having no effect on the venous segment (Fig. 1F). This finding suggests that augmented baseline Rt and Ra observed after neonatal CH exposure (Fig. 1, A and B) are due entirely to ROS-dependent vasoconstriction. Additionally, we observed a modest degree of basal tone in control animals that was also largely arterial (Fig. 1, D–F). However, this level of tone was not significantly altered by scavenging of with TEMPOL (Fig. 1, D–F). In summary, these findings suggest that greater baseline Ra after CH is mediated by a ROS-dependent vasoconstrictor mechanism rather than structural hemodynamic consequences of vascular remodeling.
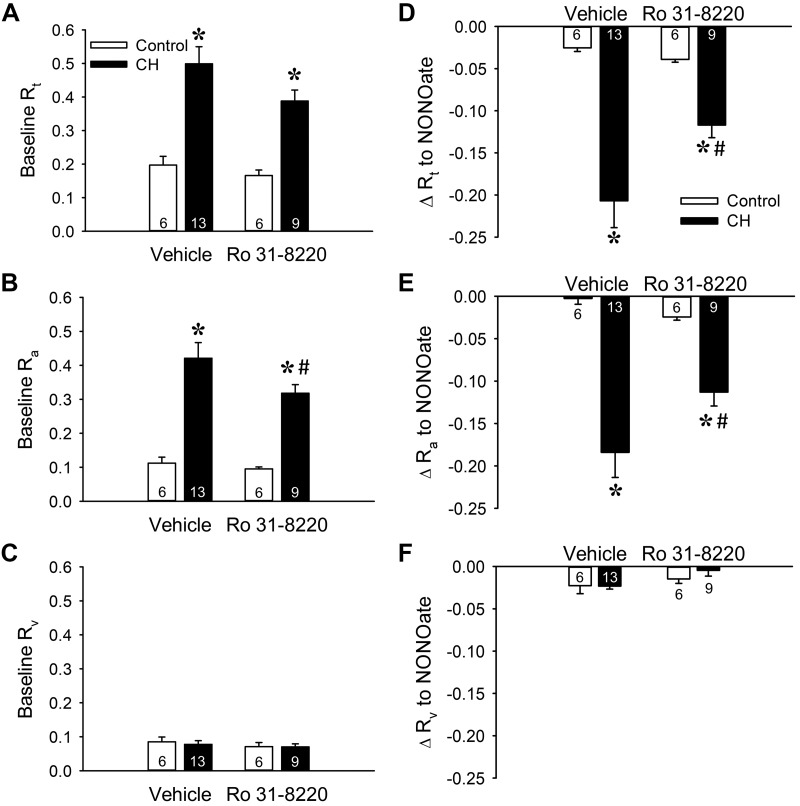
PKCβ Facilitates Enhanced Basal Pulmonary Arterial Tone Following CH in Neonates
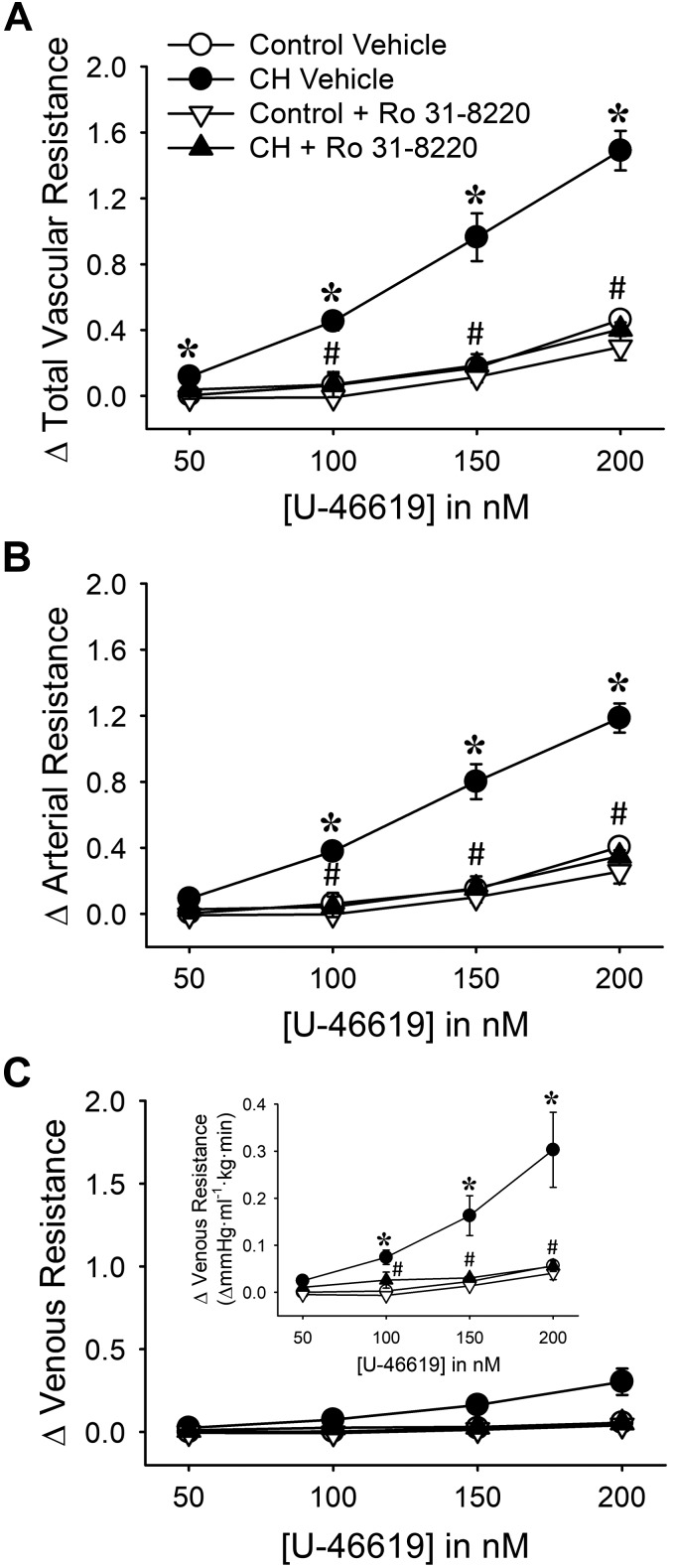
We initially examined the contribution of PKC to CH-induced increases in baseline PVR in neonatal rat lungs with the broad-spectrum PKC inhibitor Ro 31-8220. PKC inhibition tended to reduce both baseline Rt and Ra selectively in lungs from CH neonates, although significance was achieved only for Ra (Fig. 2, A and B). In contrast, Ro 31-8220 was without effect on Rv in lungs from either group (Fig. 2C), demonstrating that arterial PKC signaling contributes to increased PVR following CH exposure.
Fig. 2.
PKC contributes to augmented baseline pulmonary vascular resistance and basal tone in lungs from neonatal rats exposed to chronic hypoxia (CH). A–C: total (Rt; A), arterial (Ra; B), and venous (Rv; C) baseline vascular resistance (mmHg·mL−1·kg·min) in lungs from control and CH neonatal rats in the presence or absence of the PKC inhibitor Ro 31-8220 (10 μM). D–F: the contribution of basal tone to total (D), arterial (E), and venous (F) resistance is expressed as the change in resistance (ΔR) to 1,3-propanediamine, N-{4-[1-(3-aminopropyl)-2-hydroxy-2-nitro-sohydrazino]butyl} (spermine NONOate, 100 μM) in lungs (in situ) from control and CH neonates. Experiments were conducted in the presence of Nω-nitro-l-arginine (300 μM). Values are means ± SE; n = 6–13 rats/group (indicated in bars). *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA followed by Student-Newman-Keuls post hoc comparison.
We next examined the contribution of PKC to elevated basal pulmonary vascular tone after CH by assessing vasodilatory responses to spermine NONOate in the presence of Ro 31-8220. Consistent with effects of PKC inhibition on baseline resistances, Ro 31-8820 attenuated responses to spermine NONOate in lungs from CH rats, suggesting that PKC contributes to basal pulmonary vascular tone after CH (Fig. 2D). This effect of PKC inhibition was solely dependent on a reduction in basal arterial tone (Fig. 2, E and F). In contrast, PKC inhibition did not affect basal tone in lungs from control neonates (Fig. 2).
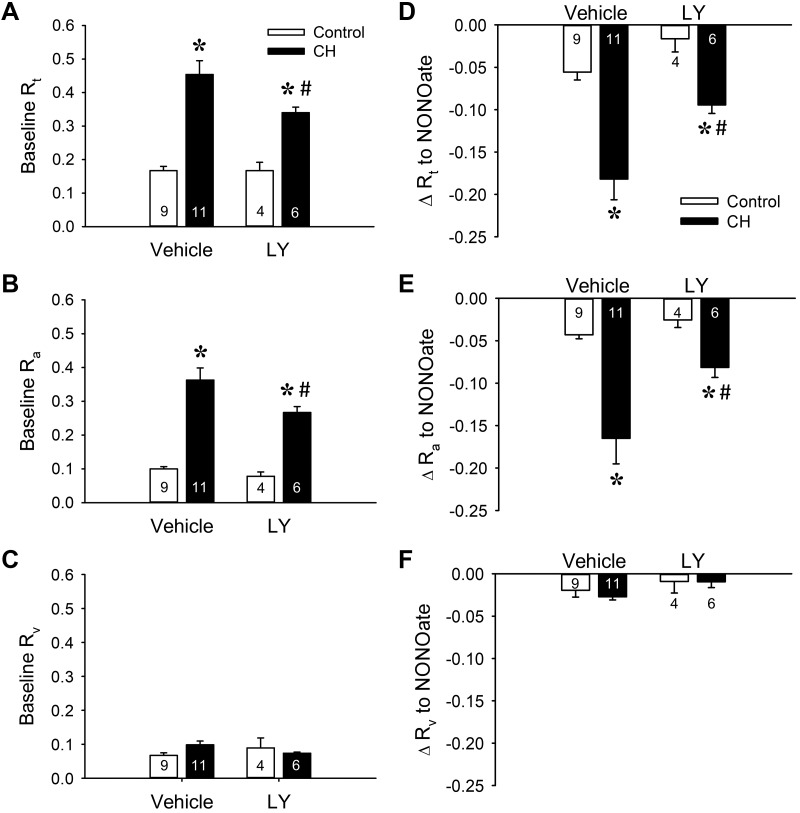
PKCβ is a PKC isoform that has been implicated as an upstream mediator of mitochondrial dysfunction and resultant oxidative stress in several disease states, including ischemia-reperfusion injury (62) and obesity (38). We therefore examined the contribution of PKCβ to greater basal pulmonary vascular tone after CH by repeating the above experiments in the presence of the PKCβ inhibitor LY-333,531. Similar to effects of Ro 31-8220, selective PKCβ inhibition significantly decreased baseline PVR and in neonates exposed to CH (Fig. 3A), a response that was entirely dependent on a reduction in Ra (Fig. 3, B and C) and pulmonary arterial tone (Fig. 3, E and F). Additionally, PKCβ inhibition was without effect on basal tone in lungs from control neonates (Fig. 3). Collectively, these findings suggest that both PKCβ and ROS contribute to enhanced basal pulmonary vascular tone in neonates with CH-induced pHTN by selectively promoting pulmonary arterial constriction.
Fig. 3.
Chronic hypoxia (CH) increases baseline pulmonary vascular resistance and basal tone through PKCβ signaling. A–C: total (Rt; A), arterial (Ra; B), and venous (Rv: C) baseline vascular resistance (mmHg·mL−1·kg·min) in lungs (in situ) from control and CH neonatal rats in the presence or absence of the PKCβ inhibitor LY-333,531 (LY, 10 nM). D–F: the contribution of basal tone to total (D), arterial (E), and venous (F) resistance is expressed as the change in resistance (ΔR) to 1,3-propanediamine, N-{4-[1-(3-aminopropyl)-2-hydroxy-2-nitro-sohydrazino]butyl} (spermine NONOate, 100 μM) in lungs from control and CH neonates. Experiments were conducted in the presence of Nω-nitro-l-arginine (300 μM). Values are means ± SE; n = 4–11 rats/group (indicated in bars). *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA followed by Student-Newman-Keuls post hoc comparison.
ROS and PKCβ Mediate Enhanced Vasoconstrictor Sensitivity to U-46619 After CH Exposure in Neonatal Rats
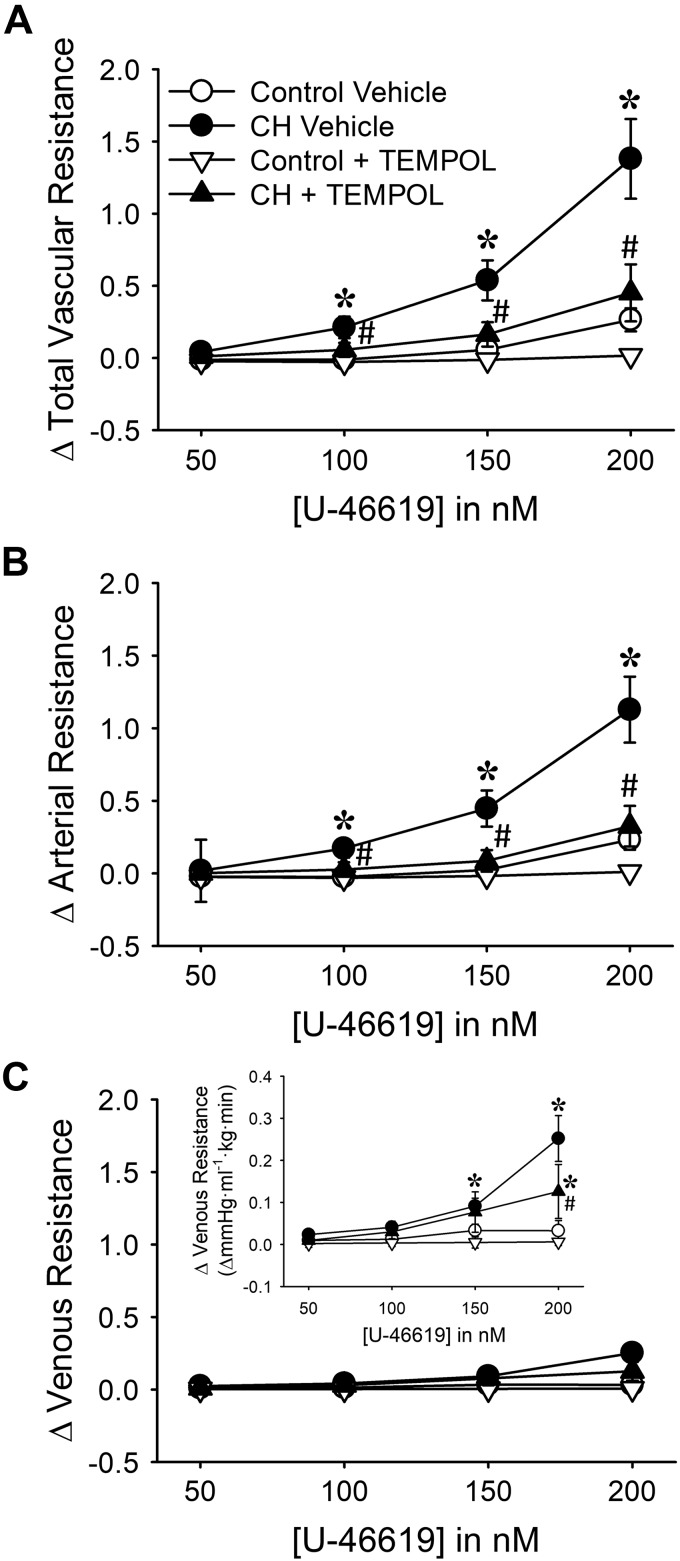
Consistent with effects of CH to increase basal pulmonary arterial tone, vasoconstrictor responses to the thromboxane mimetic U-46619 were significantly augmented by CH (Fig. 4). This effect of CH was primarily a function of increased arterial reactivity to U-46619 (Fig. 4B), although venous constriction to U-46619 was also augmented by CH exposure (Fig. 4C). TEMPOL attenuated total, arterial, and venous responses to U-46619 in lungs from CH rats (Fig. 4). Although TEMPOL also tended to reduce vascular reactivity to U-46619 in control lungs, this effect did not achieve statistical significance (Fig. 4).
Fig. 4.
Reactive oxygen species (ROS) mediate enhanced vasoconstrictor sensitivity in lungs from chronic hypoxia (CH) neonates: changes in total (A), arterial (B), and venous (C) resistance (mmHg·mL−1·kg·min) to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U-46619) in lungs from control and CH neonates. Inset in C uses a smaller y-axis scale to allow better visualization of differences between groups. Experiments were conducted in the presence of the ROS scavenger 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL, 1 mM) or vehicle. All experiments were conducted in the presence of Nω-nitro-l-arginine (300 μM). Values are means ± SE; n = 6 rats for control vehicle, n = 5 rats for CH vehicle, n = 6 rats for control + TEMPOL, n = 4 rats for CH + TEMPOL. *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA at each U-46619 concentration ([U-46619]) with a Student-Newman-Keuls post hoc comparison.
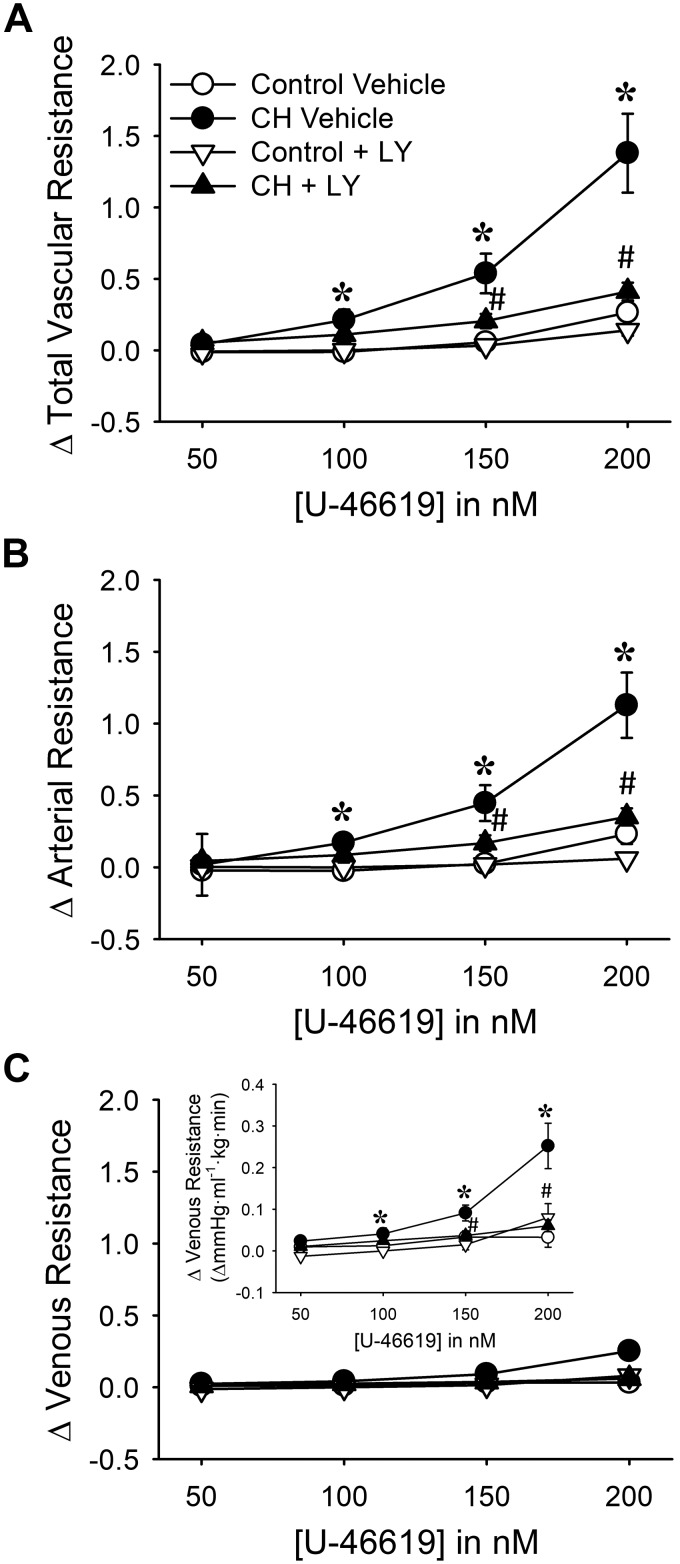
Similar to effects of TEMPOL, both Ro 31-8220 and LY-333,531 reduced CH-dependent increases in U-46619-induced vasoconstriction to control levels (Figs. 5 and 6). However, neither general PKC inhibition nor selective PKCβ inhibition altered pulmonary vasoreactivity to U-46619 in lungs from control neonates (Figs. 5 and 6). Together, these findings suggest that both and PKCβ are required for elevated pulmonary vasoconstrictor sensitivity to U-46619 after neonatal CH.
Fig. 5.
Greater vasoconstrictor sensitivity in lungs from chronic hypoxia (CH) vs. control neonates is PKC dependent: changes in total (A), arterial (B), and venous (C) resistance (mmHg·mL−1·kg·min) to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U-46619) in lungs from control and CH neonates. Inset in C uses a smaller y-axis scale to allow better visualization of differences between groups. Experiments were conducted in the continued presence of Nω-nitro-l-arginine (300 μM) with or without the PKC inhibitor Ro 31-8220 (10 μM). Values are means ± SE; n = 5 rats for control vehicle, n = 6 rats for CH vehicle, n = 5 rats for control + Ro 31-8220, n = 4 rats for CH + Ro 31-8220. *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA at each U-46619 concentration ([U-46619]) with a Student-Newman-Keuls post hoc test.
Fig. 6.
PKCβ mediates greater vasoconstrictor sensitivity in lungs from chronic hypoxia (CH) neonates: changes in total (A), arterial (B), and venous (C) resistance (mmHg·mL−1·kg·min) to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U-46619) in lungs from control and CH neonates. Inset in C uses a smaller y-axis scale to allow better visualization of differences between groups. Experiments were conducted in the continued presence of Nω-nitro-l-arginine (300 μM) with or without the PKCβ inhibitor LY-333,531 (LY, 10 nM). Values are means ± SE; n = 6 rats for control vehicle, n = 5 rats for CH vehicle, n = 5 rats for control + LY, n = 5 rats for CH + LY. *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA at each U-46619 concentration ([U-46619]) with a Student-Newman-Keuls post hoc test.
PKCβ and MitoROS Mediate CH-Induced Basal Tone in Isolated Pulmonary Arteries from Neonatal Rats
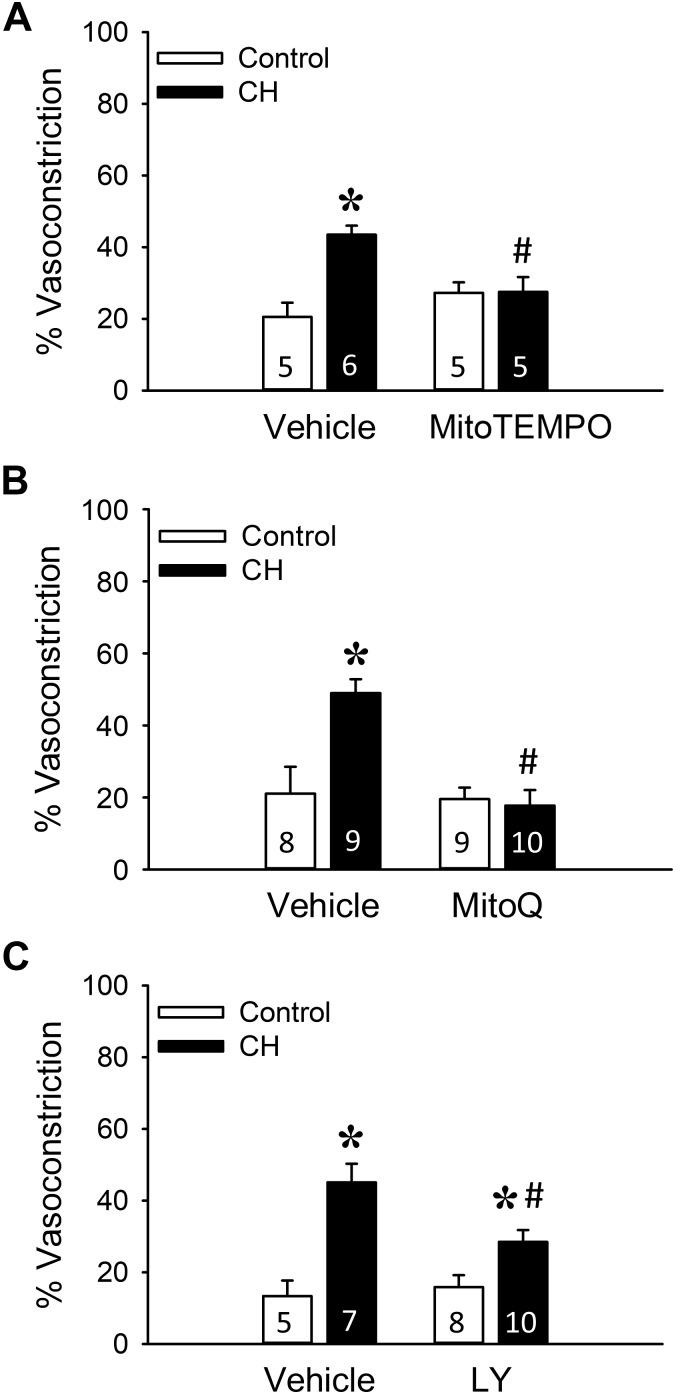
Given the importance of mitoROS in PKCβ signaling (4, 20), we directly evaluated the contribution of these mediators to basal arterial constriction in isolated, pressurized (∼150- to 200-μm ID) pulmonary arteries from control and CH neonates. Arteries from CH neonates exhibited greater basal tone compared with control vessels (Fig. 7), in agreement with perfused lung studies (Figs. 1–3). This response to CH was abolished by pretreatment with the mitochondrial antioxidants MitoTEMPO and MitoQ (Fig. 7, A and B). In contrast, there was no significant effect of either MitoTEMPO or MitoQ to alter tone in vessels from control neonates (Fig. 7, A and B).
Fig. 7.
PKCβ and mitochondrial reactive oxygen species facilitate enhanced basal tone in isolated pulmonary arteries from chronic hypoxia (CH) neonates. Experiments were conducted in the presence of the mitochondria-targeted scavenger (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO, 200 μM; A), the coenzyme Q10 analog mitoquinone mesylate (MitoQ, 1 μM; B), the PKCβ inhibitor LY-333,531 (LY, 10 nM; C), or vehicle. Basal tone (% vasoconstriction) is expressed as % maximally dilated (Ca2+ free) inner diameter. All experiments were conducted in the presence of Nω-nitro-l-arginine (300 μM). Values are means ± SE; n = 5–10 rats/group (indicated in bars). *P < 0.05 vs. control, #P < 0.05 vs. vehicle, analyzed by 2-way ANOVA followed by Student-Newman-Keuls post hoc test.
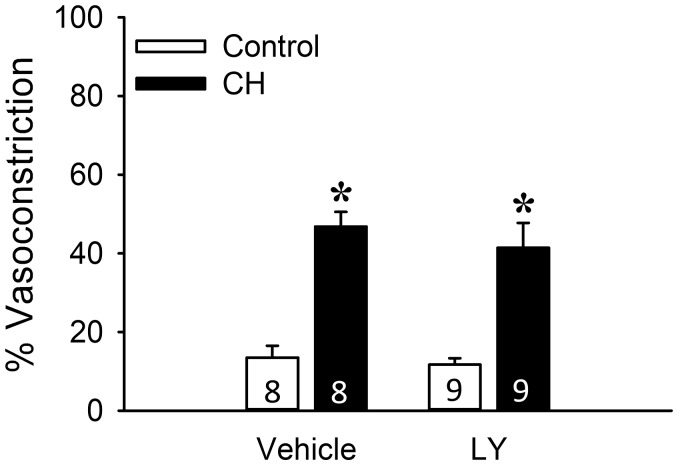
Consistent with effects of LY-333,531 to limit CH-dependent increases in basal tone in the (in situ) lung preparation, PKCβ inhibition also reduced basal tone development in isolated, pressurized pulmonary arteries from CH neonates (Fig. 7C) but not control neonates. However, in contrast to effects of PKCβ inhibition in arteries from neonatal rats, LY-333,531 was without effect on basal levels of constriction in isolated pulmonary arteries from adult CH or control rats (Fig. 8). These findings further support the specificity of the inhibitor and suggest that PKCβ-dependent basal tone development following CH is lost in adult rats. In summary, these results support a major contribution of both PKCβ and mitoROS to enhanced pulmonary arterial tone following neonatal CH.
Fig. 8.
PKCβ does not contribute to elevated basal tone in isolated pulmonary arteries from chronic hypoxia (CH) adult rats. Experiments were conducted in the presence of the PKCβ inhibitor LY-333,531 (LY, 10 nM) or vehicle. Basal tone (% vasoconstriction) is expressed as % maximally dilated (Ca2+ free) inner diameter. All experiments were conducted in the presence of Nω-nitro-l-arginine (300 μM). Values are means ± SE; n = 8 or 9 rats/group (indicated in bars). *P < 0.05 vs. control, analyzed by 2-way ANOVA followed by Student-Newman-Keuls post hoc test.
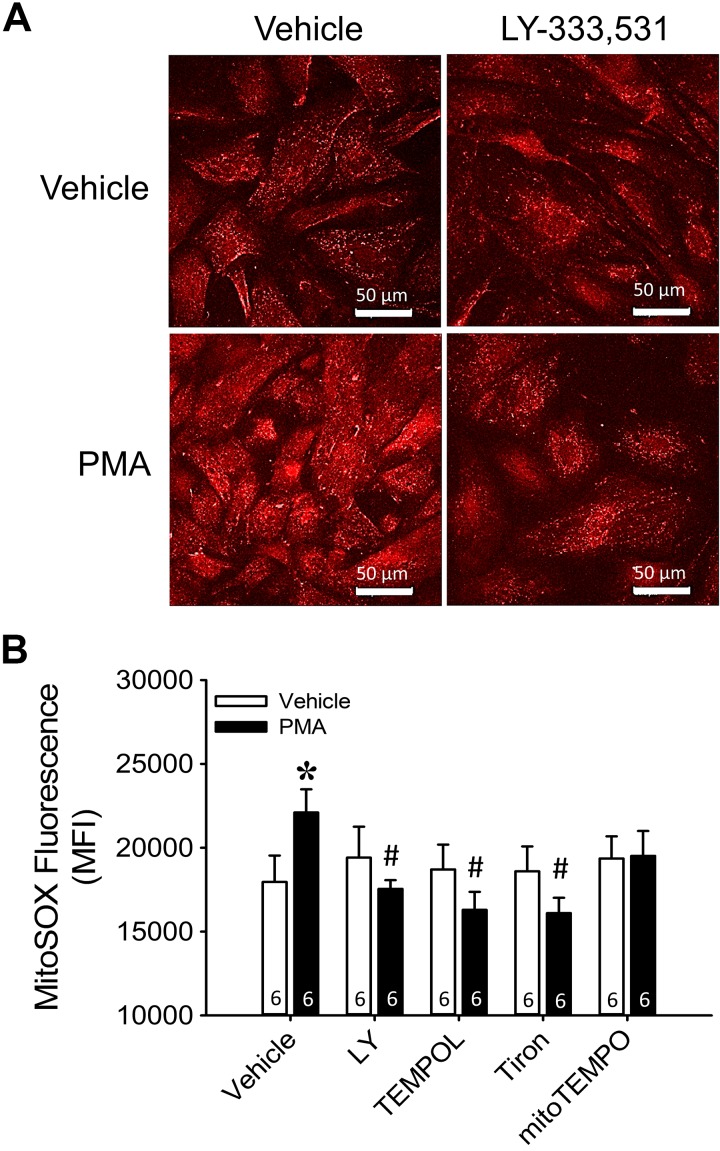
PMA Stimulates PKCβ-Dependent mitoROS Production in Isolated PASMCs from Control Neonates
Although PKCβ and mitoROS contribute to basal tone development after CH exposure, the signaling relationship between these mediators is unclear. Therefore, we next sought to determine whether an intact PKCβ-mitoROS signaling mechanism is present in control PASMCs that can be stimulated pharmacologically to allow improved mechanistic analysis of this pathway. In primary cultures of PASMCs collected from arteries of control neonates, the PKC activator PMA stimulated mitochondrial production as assessed with the mitochondria-targeted fluorescent indicator MitoSOX (Fig. 9). Furthermore, PMA-induced mitoROS production was prevented by either PKCβ inhibition with LY-333,531 or scavenging with TEMPOL or Tiron. MitoTEMPO similarly prevented PMA-mediated increases in mitoROS generation, although MitoSOX fluorescence in the presence of PMA was not significantly different between MitoTEMPO and vehicle treatments (P = 0.055). These inhibitors were without effect on MitoSOX fluorescence in the absence of PMA treatment. These findings suggest that PKCβ stimulation promotes mitochondrial production, a signaling pathway that may contribute to enhanced pulmonary arterial tone after exposure to CH in neonates.
Fig. 9.
PKCβ stimulation leads to mitochondrial reactive oxygen species production in pulmonary arterial smooth muscle cells (PASMCs) from control neonates. A: representative photomicrographs of MitoSOX fluorescence from transiently cultured PASMCs collected from pulmonary arteries of control neonates. B: summary data of MitoSOX mean fluorescence intensity (MFI) under vehicle conditions or in response to the PKC agonist PMA (10 μM) in the presence of each treatment condition: PKCβ inhibition with LY-333,531 (LY, 10 nM), oxidant scavenging with the superoxide dismutase mimetics 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL, 1 mM) and Tiron (10 mM), and the mitochondria-targeted scavenger (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO, 200 μM). Values are means ± SE; n = 6 rats/group (indicated in bars). *P < 0.05 vs. vehicle, #P < 0.05 vs. PMA vehicle, analyzed by 2-way ANOVA followed by Student-Newman-Keuls post hoc test.
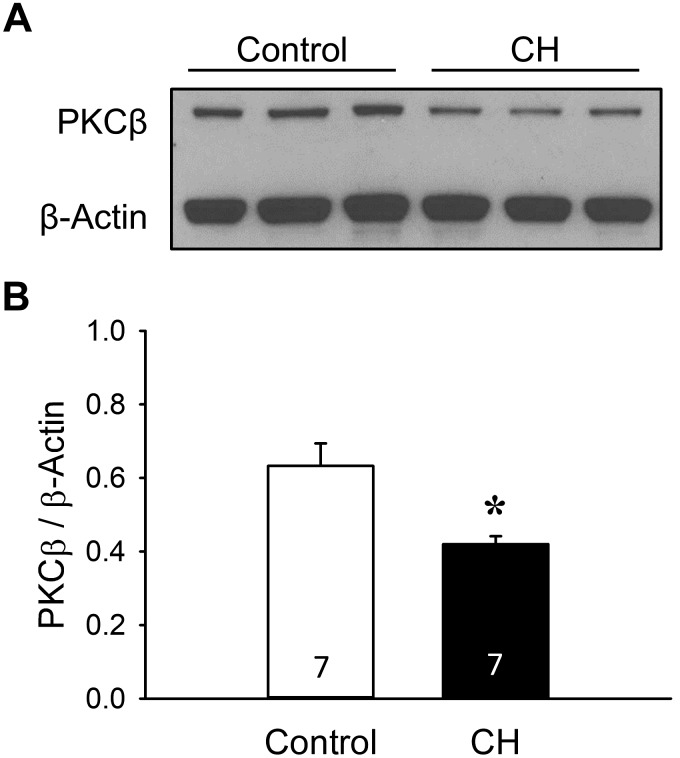
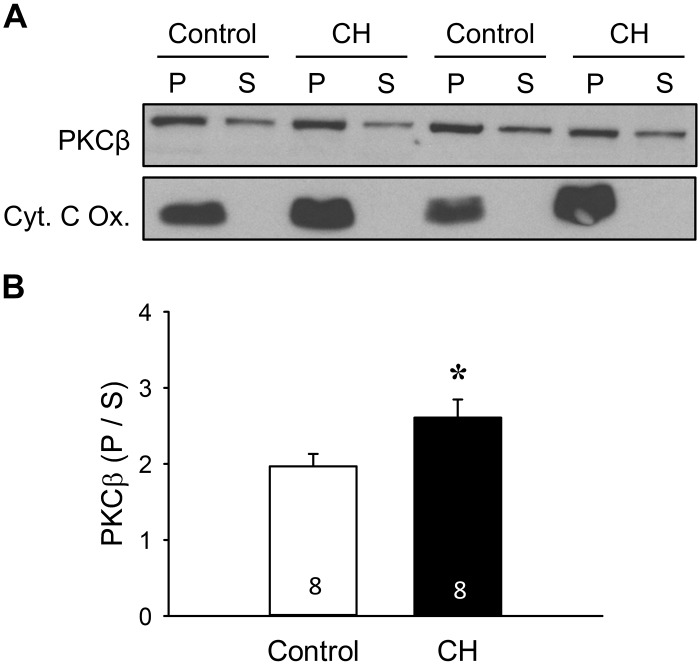
CH Limits PKCβ Protein Expression but Increases Mitochondrial Localization of PKCβ in Pulmonary Arteries from Neonatal Rats
Expression of PKCβ in pulmonary arterial homogenates from each group was confirmed by Western blot analysis (Fig. 10). Surprisingly, we found that PKCβ levels were lower in pulmonary artery homogenates from neonates exposed to CH compared with control neonates (Fig. 10). To address whether enhanced pulmonary arterial tone after CH is associated with increased localization of PKCβ to mitochondria, we assessed the proportion of PKCβ in mitochondrial and extramitochondrial fractions of pulmonary arterial homogenates from each group of rats by Western blotting. Although PKCβ was predominantly localized to mitochondria in pulmonary arteries from both control and CH neonates (Fig. 11A), CH significantly increased the proportion of PKCβ in the mitochondrial fraction (Fig. 11B). Mitochondrial isolation was confirmed by expression of cytochrome-c oxidase. There were no significant differences in protein loading between groups/fractions as assessed by densitometric analysis of Coomassie-stained blots [band density (arbitrary units): control pellet 80.6 ± 3.9, CH pellet 80.3 ± 2.9, control supernatant 74.7 ± 3.9, CH supernatant ± 74.0 ± 2.4]. These findings suggest that PKCβ-mediated increases in pulmonary arterial tone following CH are independent of altered protein expression but are associated with increased mitochondrial localization of PKCβ.
Fig. 10.
Pulmonary arterial PKCβ protein expression is lower in chronic hypoxia (CH) compared with control neonates. A: representative Western blot for PKCβ. B: summary data for PKCβ levels in pulmonary arterial homogenates from control and CH neonatal rats. PKCβ bands were normalized to those of β-actin. Values are means ± SE; n = 7 rats/group (indicated in bars). *P < 0.05 vs. control, analyzed by unpaired t-test.
Fig. 11.
Mitochondrially localized PKCβ is greater in intrapulmonary arteries from neonatal chronic hypoxia (CH) vs. control rats. A: representative Western blot for PKCβ and cytochrome-c oxidase (Cyt. C Ox.) in mitochondria-enriched pellet (P) and extramitochondrial supernatant (S) fractions. B: summary data for the P-to-S PKCβ ratio in fractionated pulmonary arterial homogenates collected from control and CH neonatal rats. Values are means ± SE; n = 8 rats/group (indicated in bars). *P < 0.05 vs. control, analyzed by unpaired t-test.
DISCUSSION
The present study examined the contribution of PKCβ and mitoROS signaling to enhanced pulmonary vasoconstriction in neonatal rats with CH-induced pHTN. The major findings from this study are that 1) neonatal CH increases basal and agonist-induced pulmonary arterial constriction that relies on and PKCβ; 2) the contribution of PKCβ to CH-induced basal pulmonary arterial tone appears to be developmentally regulated, being important in neonates but not adults; 3) PKCβ stimulation increases mitoROS production in neonatal PASMCs; and 4) neonatal CH decreases pulmonary arterial PKCβ protein expression but increases mitochondrial localization of PKCβ. Taken together, these data demonstrate an effect of CH in neonatal rats to augment pulmonary arterial constriction in a PKCβ- and mitoROS-dependent manner. These findings may provide a mechanistic basis for the vasoconstrictor component of CH-induced neonatal pHTN.
CH impacts the developing lung circulation and contributes to neonatal pHTN through several mechanisms involving elevated blood viscosity (52), altered blood vessel structure (39) and number (35), and vasoconstriction (37). We have previously reported that this vasoconstrictor response to CH includes both increased basal pulmonary arterial tone and augmented U-46619-induced vasoconstrictor sensitivity in isolated, perfused lungs from neonatal rats (54). The present study expands on these findings by demonstrating a primary role for ROS in these responses. Consistent with these observations is evidence that CH exposure in newborn piglets unmasks an effect of acetylcholine to constrict isolated pulmonary arteries (23). Furthermore, isolated pulmonary arteries from newborn piglets with CH-induced pHTN exhibit vasoconstriction in response to ROS generated by exogenous xanthine oxidase (22). This vasoconstrictor response was dependent on thromboxane synthesis, consistent with evidence from this same laboratory that chronic thromboxane inhibition reduces CH-induced pHTN in these animals (25). Consequently, it is conceivable that endogenous thromboxanes contribute to the elevated basal pulmonary arterial tone observed in the present study. ROS production has additionally been implicated in impaired endothelium-dependent vasodilation through a reduction in NO bioavailability in pulmonary hypertensive neonatal rats and lambs (20, 29) and attenuated vasorelaxation to exogenous NO in fetal lambs with pHTN resulting from ligation of the ductus arteriosus (10, 19).
Neonatal pHTN is associated with increased pulmonary levels of ROS (19) from a variety of sources, including NADPH oxidase (27), xanthine oxidase (29), and uncoupled endothelial nitric oxide synthase (34). However, mitochondria represent a major source of ROS in the pulmonary circulation, and neonatal lambs with pHTN display elevated mitoROS production (4). In agreement with these observations, our present findings demonstrate a novel role for mitoROS in mediating elevated basal tone in pressurized small pulmonary arteries after neonatal exposure to CH. There are several potential mechanisms whereby lung mitoROS levels may be enhanced in neonatal pHTN. For example, cyclic stretch stimulates mitochondrial production in cultured PASMCs from newborn lambs (65), a stimulus that may be accentuated in neonates with elevated pulmonary arterial pulse pressures. Furthermore, mitoROS levels are increased in endothelial cells from pulmonary hypertensive lambs because of a reduction in SOD2 protein expression and activity (4). However, given the importance of PKCβ in mediating mitoROS production and cellular oxidative stress in disease states such as multiple sclerosis and cardiac ischemia-reperfusion injury (38, 62), an alternative possibility is that elevated mitoROS production in neonatal CH rats results from increased PKCβ activity.
PKC signaling is an important mediator of basal vascular tone in isolated lungs from piglets with CH-induced pHTN (6). Furthermore, PKCβ contributes to enhanced vasoconstrictor sensitivity in isolated pulmonary arteries from adult rats with intermittent hypoxia-induced pHTN (55). Findings from the present study using both isolated (in situ) perfused lungs and isolated pulmonary arteries further demonstrate that selective PKCβ inhibition limits enhanced basal tone and vasoconstrictor sensitivity observed in neonatal rats exposed to CH. Moreover, this effect of PKCβ inhibition was entirely dependent on a reduction in arterial resistance, suggesting that CH exposure selectively promotes PKCβ-dependent pulmonary arterial constriction.
Consistent with evidence that mitoROS generation is an important downstream consequence of PKCβ activation (38, 62), we observed that selective PKCβ inhibition prevented PMA-induced increases in MitoSOX fluorescence in isolated PASMCs from neonatal rats. Although the mechanisms by which PKCβ stimulates pulmonary mitoROS are unknown, previous studies have implicated the redox enzyme p66shc in mediating adipocyte dysfunction in obesity, as well as oxidative stress, apoptosis, and remote organ injury induced by intestinal ischemia-reperfusion (38, 62). An additional potential mechanism whereby PKCβ promotes the generation of mitoROS involves activation of mitochondrial ATP-sensitive K+ (mitoKATP) channels (45) and electron transport chain uncoupling, which can facilitate mitoROS production (21). Further studies are therefore needed to determine the potential contribution of p66shc or mitoKATP channels to enhanced vasoconstriction following neonatal exposure to CH.
Interestingly, although we found that scavenging eliminated basal tone in both isolated lungs and pulmonary arteries from CH neonates, PKCβ inhibition was only partially effective. Although it is possible that the concentration of LY-333,531 employed does not provide maximal inhibition of PKCβ, arguing against this possibility is evidence from the isolated lung studies that PKCβ inhibition abolished effects of CH to augment vasoconstrictor responsiveness to U-46619 in lungs, similar to effects of TEMPOL. These findings suggest that mechanisms other than PKCβ signaling promote mitochondrial production and resultant basal tone, whereas PKCβ plays a primary role in mediating ROS-dependent vasoconstriction to U-46619.
Mitochondrial may increase VSM contractility by altering Ca2+ signaling and/or by increasing myofilament Ca2+ sensitivity. Supporting a potential role for elevated Ca2+ in this response are studies demonstrating that mitoROS contribute to acute hypoxia-dependent increases in intracellular Ca2+ concentration ([Ca2+]i) and subsequent VSM contraction in PASMCs from adult mice (63). Additionally, treatment of mouse and human fibroblasts with the electron transport chain complex 1 inhibitor rotenone reduced mitoROS production and limited cellular attachment and membrane spreading (68), processes driven by actin polymerization (68). Actin polymerization may promote myofilament Ca2+ sensitization by enhancing transmission force to the extracellular matrix (58) and by increasing the assembly of contractile units between actin and myosin (58). ROS-induced actin polymerization has additionally been implicated in mediating increased basal and agonist-induced pulmonary vasoconstriction following CH in adult rats (66). However, whether a similar mechanism contributes to altered pulmonary vasoreactivity following neonatal CH remains to be determined.
We further observed an interesting dichotomy in the contribution of PKCβ to enhanced basal pulmonary arterial tone between CH neonates and adults. Specifically, whereas PKCβ contributed to basal tone in neonates, no such effect was observed in arteries from CH adult rats. This finding is consistent with prior work from our group showing that enhanced vasoconstrictor reactivity to endothelin-1 (31) and KCl (11) after CH in adult rats is unaltered by general PKC inhibition. Rather, these responses are mediated by Rho kinase-induced Ca2+ sensitization (12, 31). Interestingly, PKC appears to be an important regulator of arterial constriction in pulmonary normotensive neonatal piglets (6). Additionally, chronic PKC inhibition with chelerythrine limits CH-induced pHTN in neonatal piglets (6), suggesting that PKC contributes to pulmonary arterial tone in otherwise healthy neonates and PKC-dependent pulmonary vasoconstriction is an important mediator of CH-induced neonatal pHTN. It is further possible that neonatal CH exposure attenuates the fall in pulmonary vascular resistance after birth, resulting in a persistent vasoconstriction of the fetal state. Whether PKCβ and mitoROS contribute to fetal pulmonary vasoconstriction represents an interesting avenue of future investigation.
Although the mechanism by which CH mediates PKCβ activation to augment basal and agonist-induced vasoconstriction is unknown, it is possible that a more oxidized cellular environment or increases in [Ca2+]i are contributing factors. Indeed, PKCβ is a classical PKC isoform that can be stimulated by diacylglycerol (41) and increases in [Ca2+]i (41). Additionally, oxidative stress has previously been shown to promote PKCβ signaling (38, 62) through protein sulfenylation (33). Therefore, elevated cellular ROS levels observed in neonatal pHTN may directly promote activity of PKCβ. In addition to Ca2+ and the cellular redox environment contributing to activity of PKCβ, protein expression and subcellular localization are important mediators of its activity (40). Although PKCβ protein expression in pulmonary artery homogenates from CH neonates was lower relative to control neonates, mitochondrial localization of PKCβ was elevated after CH. Future studies are therefore needed to evaluate the contribution of [Ca2+]i, oxidative stress, and/or PKCβ mitochondrial localization to PKCβ-induced mitoROS production and vasoconstriction in CH-induced neonatal pHTN.
Enhanced mitoROS-dependent vasoconstriction after neonatal CH may result from an inherent change in VSM phenotype that imparts increased sensitivity to membrane stretch or receptor activation. Alternatively, CH may alter endothelial function to mediate this response, possibly by increasing production of endothelial ROS or endothelin-1 that in turn leads to VSM PKCβ activation (50). Therefore, a goal of future studies is to address the potential role of endothelial dysfunction in increased VSM mitoROS generation and vasoconstriction following neonatal CH.
Consistent with previous work from our laboratory (54), neonatal rats exposed to CH exhibited a lower body weight compared with their normoxic counterparts. Interestingly, investigations using other neonatal animal models of CH-induced pHTN have demonstrated that CH limits growth (8, 18, 24) and human infants born at high altitude weigh less than those born at sea level (42). Although we did not measure the body weight of the dams used in the present study, it is likely that CH also led to a reduction in their body weight, similar to that which occurs in adult male rats (13). Therefore, we cannot exclude the possibility that such effects of CH were associated with limitations in milk supply to the pups and, if so, whether such effects contributed to the observed increases in pulmonary vasoreactivity. Additionally, neonatal CH exposure is associated with elevated levels of corticosteroids and reduced levels of growth hormone and insulin-like growth factor 1 (67), which may contribute to CH-induced growth restriction. Neonatal CH exposure has also been associated with brain injury (53) and impaired lung development (61), both severe developmental deficits that, together with growth restriction, highlight the complexity of neonatal CH exposure and underscore the importance of studies to investigate underlying mechanisms of disease in neonates exposed to CH.
In conclusion, these studies demonstrate a previously undescribed role for PKCβ and mitoROS to mediate enhanced pulmonary vasoconstriction following neonatal CH exposure, a response associated with greater mitochondrial localization of PKCβ. This effect of CH was not observed in adult rats, suggesting that the pathophysiological mechanisms contributing to the vasoconstrictor component of pHTN may be developmentally regulated. Additionally, we found that PKCβ signals proximal to mitoROS generation in neonatal PASMCs. These results improve our understanding of cellular pathways contributing to CH-induced pulmonary arterial constriction in the neonate, a response important to the development of neonatal pHTN (37, 57). Understanding of how CH activates PKCβ, the mechanistic link between PKCβ activation and mitoROS production, and the role of these mediators in the development of neonatal pHTN represent important avenues of future investigation, which may ultimately reveal novel therapeutic interventions that target components of this signaling axis in infants with pHTN.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant F31-HL-131334 (to J. R. Sheak) and NIH Grants T32-HL-007736, R01-HL-132883, R01-HL-088192, and K12-GM-088021 and American Heart Association Grant 16GRNT27700010 (to T. C. Resta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.S. and T.C.R. conceived and designed research; J.R.S., S.Y., L.W.-C., and R.A. performed experiments; J.R.S., S.Y., L.W.-C., and R.A. analyzed data; J.R.S., S.Y., L.W.-C., R.A., B.R.W., N.L.J., and T.C.R. interpreted results of experiments; J.R.S., S.Y., L.W.-C., and T.C.R. prepared figures; J.R.S. drafted manuscript; J.R.S., S.Y., L.W.-C., R.A., B.R.W., N.L.J., and T.C.R. edited and revised manuscript; J.R.S., S.Y., L.W.-C., R.A., B.R.W., N.L.J., and T.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Minerva Murphy (University of New Mexico) for technical support and to MitoQ Ltd. for the generous donation of MitoQ.
REFERENCES
- 1.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132: 2037–2099, 2015. [Erratum in Circulation 133: e368, 2016.] doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 2.Abramov AY, Duchen MR. Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium 33: 101–112, 2003. doi: 10.1016/S0143-4160(02)00203-8. [DOI] [PubMed] [Google Scholar]
- 3.Afolayan AJ, Eis A, Alexander M, Michalkiewicz T, Teng RJ, Lakshminrusimha S, Konduri GG. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L40–L49, 2016. doi: 10.1152/ajplung.00392.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012. doi: 10.1152/ajplung.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen SW, Chatfield BA, Koppenhafer SA, Schaffer MS, Wolfe RR, Abman SH. Circulating immunoreactive endothelin-1 in children with pulmonary hypertension. Association with acute hypoxic pulmonary vasoreactivity. Am Rev Respir Dis 148: 519–522, 1993. doi: 10.1164/ajrccm/148.2.519. [DOI] [PubMed] [Google Scholar]
- 6.Berkenbosch JW, Baribeau J, Ferretti E, Perreault T. Role of protein kinase C and phosphatases in the pulmonary vasculature of neonatal piglets. Crit Care Med 29: 1229–1233, 2001. doi: 10.1097/00003246-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol Cell Physiol 242: C404–C408, 1982. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- 8.Bierer R, Nitta CH, Friedman J, Codianni S, de Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Gonzalez Bosc LV. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol 301: L872–L880, 2011. doi: 10.1152/ajplung.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blood AB, Terry MH, Merritt TA, Papamatheakis DG, Blood Q, Ross JM, Power GG, Longo LD, Wilson SM. Effect of chronic perinatal hypoxia on the role of rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol 304: R136–R146, 2013. doi: 10.1152/ajpregu.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 11.Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010. doi: 10.1152/ajplung.00276.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 13.Chicoine LG, Avitia JW, Deen C, Nelin LD, Earley S, Walker BR. Developmental differences in pulmonary eNOS expression in response to chronic hypoxia in the rat. J Appl Physiol (1985) 93: 311–318, 2002. doi: 10.1152/japplphysiol.01083.2001. [DOI] [PubMed] [Google Scholar]
- 14.Chicoine LG, Paffett ML, Girton MR, Metropoulus MJ, Joshi MS, Bauer JA, Nelin LD, Resta TC, Walker BR. Maturational changes in the regulation of pulmonary vascular tone by nitric oxide in neonatal rats. Am J Physiol Lung Cell Mol Physiol 293: L1261–L1270, 2007. doi: 10.1152/ajplung.00235.2006. [DOI] [PubMed] [Google Scholar]
- 15.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009. doi: 10.1152/ajplung.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9: 1825–1836, 2007. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 18.Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol 43: 20–28, 2008. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- 19.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 174: 272–281, 2010. doi: 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci 14: 1197–1218, 2009. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fike CD, Aschner JL, Slaughter JC, Kaplowitz MR, Zhang Y, Pfister SL. Pulmonary arterial responses to reactive oxygen species are altered in newborn piglets with chronic hypoxia-induced pulmonary hypertension. Pediatr Res 70: 136–141, 2011. doi: 10.1203/PDR.0b013e3182207ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol 286: L1244–L1254, 2004. doi: 10.1152/ajplung.00345.2003. [DOI] [PubMed] [Google Scholar]
- 24.Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol (1985) 77: 2853–2862, 1994. doi: 10.1152/jappl.1994.77.6.2853. [DOI] [PubMed] [Google Scholar]
- 25.Fike CD, Zhang Y, Kaplowitz MR. Thromboxane inhibition reduces an early stage of chronic hypoxia-induced pulmonary hypertension in piglets. J Appl Physiol (1985) 99: 670–676, 2005. doi: 10.1152/japplphysiol.01337.2004. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Raj JU. Hypoxic pulmonary hypertension of the newborn. Compr Physiol 1: 61–79, 2011. doi: 10.1002/cphy.c090015. [DOI] [PubMed] [Google Scholar]
- 27.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 28.Haworth SG, Hislop AA. Lung development-the effects of chronic hypoxia. Semin Neonatol 8: 1–8, 2003. doi: 10.1016/S1084-2756(02)00195-1. [DOI] [PubMed] [Google Scholar]
- 29.Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 30.Jernigan NL, Resta TC. Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation 21: 259–271, 2014. doi: 10.1111/micc.12096. [DOI] [PubMed] [Google Scholar]
- 31.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes—the ultimate approach for intra- and extracellular superoxide detection. Biochim Biophys Acta 1840: 739–744, 2014. doi: 10.1016/j.bbagen.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem 275: 24136–24145, 2000. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 34.Lakshminrusimha S, Wiseman D, Black SM, Russell JA, Gugino SF, Oishi P, Steinhorn RH, Fineman JR. The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 293: H1491–H1497, 2007. doi: 10.1152/ajpheart.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562, 2002. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 36.Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta 1813: 1382–1394, 2011. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol 294: L205–L213, 2008. doi: 10.1152/ajplung.00234.2007. [DOI] [PubMed] [Google Scholar]
- 38.Mehta NK, Mehta KD. Protein kinase C-beta: an emerging connection between nutrient excess and obesity. Biochim Biophys Acta 1841: 1491–1497, 2014. doi: 10.1016/j.bbalip.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Meyrick B, Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol 101: 527–542, 1980. [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson CP, Willets JM, Davies NW, Challiss RA, Standen NB. Visualizing the temporal effects of vasoconstrictors on PKC translocation and Ca2+ signaling in single resistance arterial smooth muscle cells. Am J Physiol Cell Physiol 295: C1590–C1601, 2008. doi: 10.1152/ajpcell.00365.2008. [DOI] [PubMed] [Google Scholar]
- 41.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem 270: 28495–28498, 1995. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 42.Niermeyer S, Andrade-M MP, Vargas E, Moore LG. Neonatal oxygenation, pulmonary hypertension, and evolutionary adaptation to high altitude (2013 Grover Conference series). Pulm Circ 5: 48–62, 2015. doi: 10.1086/679719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal 18: 1777–1788, 2013. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norton CE, Jernigan NL, Kanagy NL, Walker BR, Resta TC. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: regulation by reactive oxygen species. J Appl Physiol (1985) 111: 980–988, 2011. doi: 10.1152/japplphysiol.01286.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol 286: H468–H476, 2004. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 46.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L419–L430, 2014. doi: 10.1152/ajplung.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramiro-Diaz JM, Nitta CH, Maston LD, Codianni S, Giermakowska W, Resta TC, Gonzalez Bosc LV. NFAT is required for spontaneous pulmonary hypertension in superoxide dismutase 1 knockout mice. Am J Physiol Lung Cell Mol Physiol 304: L613–L625, 2013. doi: 10.1152/ajplung.00408.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol Heart Circ Physiol 276: H699–H708, 1999. doi: 10.1152/ajpheart.1999.276.2.H699. [DOI] [PubMed] [Google Scholar]
- 49.Resta TC, Walker BR. Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am J Physiol Heart Circ Physiol 270: H888–H896, 1996. doi: 10.1152/ajpheart.1996.270.3.H888. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfield BA, Abman SH. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr 123: 109–114, 1993. doi: 10.1016/S0022-3476(05)81552-5. [DOI] [PubMed] [Google Scholar]
- 51.Russ RD, Walker BR. Maintained endothelium-dependent pulmonary vasodilation following chronic hypoxia in the rat. J Appl Physiol (1985) 74: 339–344, 1993. doi: 10.1152/jappl.1993.74.1.339. [DOI] [PubMed] [Google Scholar]
- 52.Ruth V, Widness JA, Clemons G, Raivio KO. Postnatal changes in serum immunoreactive erythropoietin in relation to hypoxia before and after birth. J Pediatr 116: 950–954, 1990. doi: 10.1016/S0022-3476(05)80659-6. [DOI] [PubMed] [Google Scholar]
- 53.Sathyanesan A, Kundu S, Abbah J, Gallo V. Neonatal brain injury causes cerebellar learning deficits and Purkinje cell dysfunction. Nat Commun 9: 3235, 2018. doi: 10.1038/s41467-018-05656-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheak JR, Weise-Cross L, deKay RJ, Walker BR, Jernigan NL, Resta TC. Enhanced NO-dependent pulmonary vasodilation limits increased vasoconstrictor sensitivity in neonatal chronic hypoxia. Am J Physiol Heart Circ Physiol 313: H828–H838, 2017. doi: 10.1152/ajpheart.00123.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snow JB, Gonzalez Bosc LV, Kanagy NL, Walker BR, Resta TC. Role for PKCβ in enhanced endothelin-1-induced pulmonary vasoconstrictor reactivity following intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 301: L745–L754, 2011. doi: 10.1152/ajplung.00020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol (1985) 104: 110–118, 2008. doi: 10.1152/japplphysiol.00698.2005. [DOI] [PubMed] [Google Scholar]
- 57.Storme L, Parker TA, Kinsella JP, Rairigh RL, Abman SH. Chronic hypertension impairs flow-induced vasodilation and augments the myogenic response in fetal lung. Am J Physiol Lung Cell Mol Physiol 282: L56–L66, 2002. doi: 10.1152/ajplung.2002.282.1.L56. [DOI] [PubMed] [Google Scholar]
- 58.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 279: 51722–51728, 2004. doi: 10.1074/jbc.M408351200. [DOI] [PubMed] [Google Scholar]
- 59.Tang S, Xiao V, Wei L, Whiteside CI, Kotra LP. Protein kinase C isozymes and their selectivity towards ruboxistaurin. Proteins 72: 447–460, 2008. doi: 10.1002/prot.21942. [DOI] [PubMed] [Google Scholar]
- 60.Townsley MI, Korthuis RJ, Rippe B, Parker JC, Taylor AE. Validation of double vascular occlusion method for Pc,i in lung and skeletal muscle. J Appl Physiol (1985) 61: 127–132, 1986. doi: 10.1152/jappl.1986.61.1.127. [DOI] [PubMed] [Google Scholar]
- 61.Truog WE, Xu D, Ekekezie II, Mabry S, Rezaiekhaligh M, Svojanovsky S, Soares MJ. Chronic hypoxia and rat lung development: analysis by morphometry and directed microarray. Pediatr Res 64: 56–62, 2008. doi: 10.1203/PDR.0b013e31817289f2. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, Chen Z, Zhang F, Jing H, Xu W, Ning S, Li Z, Liu K, Yao J, Tian X. Blockade of PKCβ protects against remote organ injury induced by intestinal ischemia and reperfusion via a p66shc-mediated mitochondrial apoptotic pathway. Apoptosis 19: 1342–1353, 2014. doi: 10.1007/s10495-014-1008-x. [DOI] [PubMed] [Google Scholar]
- 63.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med 42: 642–653, 2007. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wedgwood S, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L196–L203, 2015. doi: 10.1152/ajplung.00097.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weise-Cross L, Sands MA, Sheak JR, Broughton BR, Snow JB, Gonzalez Bosc LV, Jernigan NL, Walker BR, Resta TC. Actin polymerization contributes to enhanced pulmonary vasoconstrictor reactivity after chronic hypoxia. Am J Physiol Heart Circ Physiol 314: H1011–H1021, 2018. doi: 10.1152/ajpheart.00664.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zayour D, Azar ST, Azar N, Nasser M, Obeid M, Mroueh S, Dbaibo GS, Bitar FF. Endocrine changes in a rat model of chronic hypoxia mimicking cyanotic heart disease. Endocr Res 29: 191–200, 2003. doi: 10.1081/ERC-120022301. [DOI] [PubMed] [Google Scholar]
- 68.Zeller KS, Riaz A, Sarve H, Li J, Tengholm A, Johansson S. The role of mechanical force and ROS in integrin-dependent signals. PLoS One 8: e64897, 2013. doi: 10.1371/journal.pone.0064897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Zhan Y, Zhang D, Dai B, Ma W, Qi J, Liu R, He L. Eupolyphaga sinensis walker displays inhibition on hepatocellular carcinoma through regulating cell growth and metastasis signaling. Sci Rep 4: 5518, 2014. doi: 10.1038/srep05518. [DOI] [PMC free article] [PubMed] [Google Scholar]