Abstract
As we go about our daily routines we are continuously bombarded with environmental feedback that requires appraisal and response. Sleep loss can compromise the efficiency by which these cognitive processes function. Operationally, poor performance caused by insufficient sleep translates to increased health and safety risks in settings where attention and timely and/or accurate decisions to respond are critical (e.g., at work, on the road, etc.). Current rodent tasks that assess altered cognition after sleep deprivation (SD) do not accurately model the continuous multisensory feedback that informs goal-oriented behavior in humans. Herein, we describe the vibration actuating search task (VAST), which consists of a vibrating open field with pseudo-randomly selected entrance and target destination points. To successfully complete a trial, mice use feedback from rotary motor-induced floor vibrations to navigate from the entrance point to the target destination. Sets of 20 trials were conducted on 3 consecutive days, and before testing on the third day control mice were undisturbed while other mice were sleep deprived for 10 h. On the first 2 days mice learned the task with high success rates. Alternatively, VAST performance was compromised following SD as measured by increased failures in task completion, time to target, time spent immobile, and decreased speed as compared with undisturbed mice. The VAST enables the analysis of continuous feedback via multiple sensory modalities in mice and is applicable to a variety of operational settings.
NEW & NOTEWORTHY The vibration actuating search task (VAST) is a novel performance assay that uses continuous auditory and haptic feedback to motivate and direct search behaviors in mice. The VAST is rapidly acquired by mice and performance is disrupted by sleep deprivation. The VAST has practical application in occupational settings. The cognitive aspects of the sensorimotor integration in the VAST may prove useful for rodent models of neurodegenerative disease.
Keywords: goal-oriented behavior, multisensory feedback, neurocognitive task, rodent model, sleep
INTRODUCTION
Human studies have shown that sleep deprivation (SD) can cause deficits in multiple aspects of cognition, including attention, memory, and executive function (Jackson and Van Dongen 2011; Pilcher and Huffcutt 1996). Though specific aspects of cognition are easily dissected and studied in humans, the effects of SD on underlying processes remain poorly understood (Jackson et al. 2013). Furthermore, SD-related performance deficits can lead to roadway and workplace accidents. From an operational perspective there are many interventions to combat compromised work and travel safety issues that result from SD-induced impairments in sustained attention. For example, long-haul drivers often implement self-initiated strategies such as blasting cold air, turning up the radio, consuming stimulants, or stopping for naps (May and Baldwin 2009). Larger-scale technologies have also become available in recent years, including alarmed warning systems that detect lane departures, eye closures, or head nodding; similarly, rumble strips provide both haptic and auditory feedback (Dinges et al. 2005; Hartley et al. 2000; Perrillo 1998). However, it is difficult to assess the efficacy of SD-related interventions without an understanding of the neurobiological circuitry that is taxed during SD.
To investigate the neurobiological basis of SD-induced cognitive performance deficits, relevant tasks must be designed and applied to animal models that can replicate the effects of human studies. Rodent tasks designed to probe the effects of SD on object recognition (Palchykova et al. 2006), spatial memory (Alhaider et al. 2010; Alvarenga et al. 2008; Hagewoud et al. 2010), and sustained attention (Christie et al. 2008; Córdova et al. 2006; Oonk et al. 2015; van Enkhuizen et al. 2014; Walker et al. 2011) have been developed, but the extent to which they reflect human behavior is variable and often not directly comparable to human tasks. Moreover, there is a dearth of work on the role of sleep in sensory integration (Clawson et al. 2018; Walker et al. 2011), although sleep is clearly crucial in sensorimotor integration during development (Del Rio-Bermudez et al. 2015).
It is particularly important to quantify and model the effects of sleep deprivation on sustained attention as this aspect of cognition is critically impacted by and reliably pronounced in performance impairments induced by fatigue (Lim and Dinges 2008). The increasing reliance on haptic and auditory feedback in warning systems creates the need for an animal model of attention that focuses on the same sensory modalities. Furthermore, such tasks need to be similarly sensitive to the effects of sleep deprivation to ensure the validity and improve the effectiveness of countermeasures for compromised attention.
With the vibration actuating search task (VAST), we have created a novel task that demonstrates that mice are capable of navigating to an unmarked target destination using gradient auditory/haptic feedback based on the rodent’s proximity to the target destination. Much like the children’s “hot-and-cold” game the frequency of the maze’s vibration is programmed to be dependent on the location of the mouse relative to the randomized target destination.
METHODS
Animals.
C57BL/6J mice were bred in-house. Breeding pairs were procured from Jackson Laboratories (Sacramento, CA). Male mice aged 8–12 wk at the start of testing (n = 21) were housed three to a cage at 23 ± 1°C, on a 12:12-h light-dark cycle with access to food and water ad libitum. To acclimate the mice to the experimenter and the behavioral paradigm, each animal was handled daily using an escalating handling protocol (see Handling protocol). All behavioral tasks (including handling) were performed within zeitgeber time (ZT) 10–12.65 under red light (25–30 lux). All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee.
Apparatus.
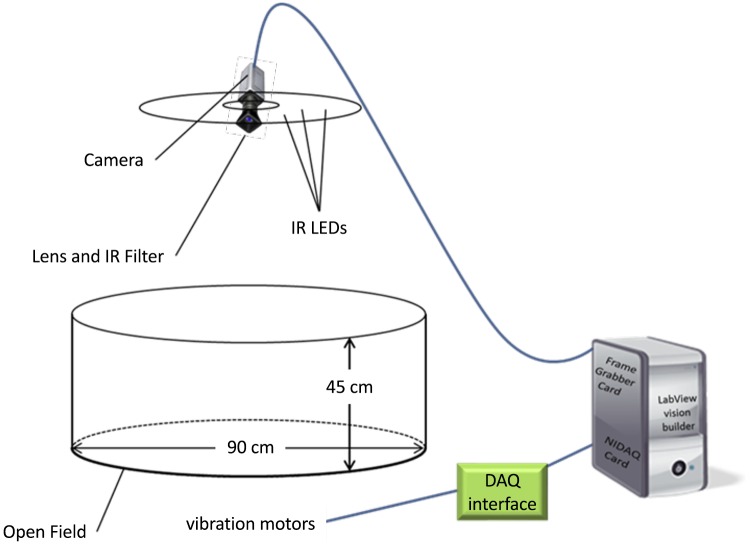
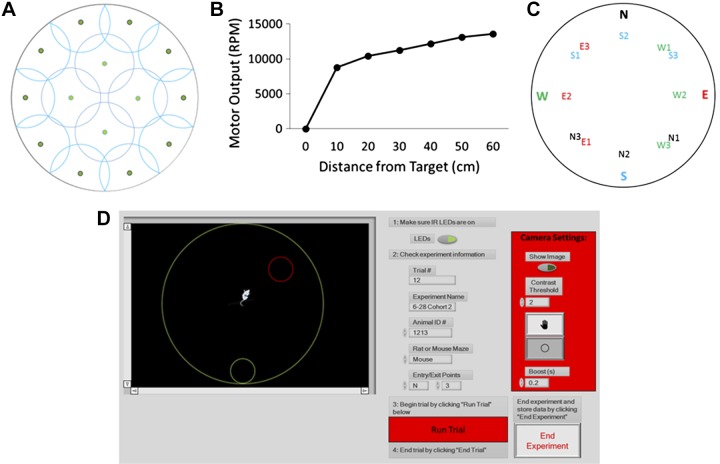
The maze was constructed using a round polycarbonate open field (90-cm diameter) encircled by a 45-cm-tall acrylic wall (Fig. 1). The maze was illuminated from above by infrared (IR) LEDs that were distributed on a circular chandelier and mounted above the open field. To minimize image noise from IR light reflections the base and wall of the open field were painted flat black. Fourteen small vibration motors (Precision Microdrives, London, UK; no. 312-401) were mounted on the underside of the base and spaced out based on circle packing within the maze diameter (Fig. 2A). These motors worked in tandem to create vibratory feedback based on the mouse’s distance from the exit point (Fig. 2B). Animal tracking, vibration frequency, and raw data output were controlled by a custom-built LabVIEW program (National Instruments, Austin, TX).
Fig. 1.
Schematic of vibration actuating search task (VAST) apparatus. The maze consists of a round open field, painted flat black. The maze was illuminated from above using infrared (IR) LEDs and recorded from above. Frames were analyzed by a custom LabVIEW program to determine how far the mouse was from the exit point/target location and adjust the vibration motor output accordingly. NIDAQ, National Instruments Data Acquisition Card.
Fig. 2.
A circle packing algorithm was used to determine the optimal placements of motors (green dots) on the underside of the open field (A). Change in vibration motor output as a function of mouse distance from the target location (B). Maze exit points were randomized via a Latin square design between 12 possibilities (N1, N2, N3, E1, E2, etc.). Each exit point was equidistant from its corresponding entrance point (designated north, east, west, or south; C). The graphical user interface prompted experimenters to turn on LEDs and to input trial information (trial name, date, animal name, species, and entrance point) as well as start and end each trial. The experimental field boundaries, region of interest, and the contrast threshold settings were also controlled by the interface (D).
Animal tracking.
To track mouse movement, a Manta G-125 monochrome camera equipped with an IR filter lens was mounted in the center of the chandelier and connected to a computer running the custom LabVIEW program. Information on the animal’s position was collected each time a frame was processed (30 fps). During initial setup, the experimenter identified a region of interest (ROI) to mask the peripheral image and restrict the camera view to the open field of the apparatus. This ROI information was used in all subsequent trials. A threshold was then applied to the ROI to distinguish the animal from the open field under IR lighting. Since the open field was darker, the reflection of the animal was the brightest part of the frame. This new image was then processed by taking the “center of mass” (average X,Y coordinate values) of the brightest pixels. The center of mass was the geometric center of the animal’s body as seen in the masked image and assigned as pixel coordinates (Fig. 2D). These coordinates rendered the raw position of the animal. The raw information was filtered using a moving average of the last six raw locations. The output of the filter was regarded as the actual position.
If the actual position of the mouse changed more than 6 pixels between frames, then the mouse was logged as mobile. This value was chosen to exclude changes in the actual position that were not the result of ambulation, such as a change in posture or noise in the image. If there was less than a 6-pixel change, the mouse was logged as immobile. While the mouse was ambulatory, a cumulative sum was logged to reflect distance traveled. All mouse position coordinates were given in pixels and converted to distance in centimeters by a ratio of the ROI diameter (measured in pixels) to the diameter of the open field (90 cm). These data were then saved to an Excel spreadsheet.
The data set consisted of [X,Y] coordinate pairs that represent the centroid of the animals’ location across each trial recorded at a 10-Hz sampling rate. To mitigate position tracking errors within the data set, outlier coordinate pairs were identified and replaced with a linear interpolation of neighboring coordinates. Coordinate pairs were identified as outliers if they were outside the boundary of the apparatus platform, or the Euclidean distance between it and the next coordinate pair was zero, or the Euclidean distance between it and the next coordinate pair exceeded 0.22 m.
Handling protocol.
A mouse handling protocol was designed to incrementally increase contact with the experimenter and apparatus. This protocol was adapted from the handling procedures described in Fridgeirsdottir et al. (2014).
Day 1: Mice were ear tagged early in the day. Later in the day, cages were moved to a ventilated table in the home room. Nesting material was removed to encourage exploration and the experimenter put a gloved hand in the cage, allowing the mice to explore at will for 5 min.
Day 2: Nesting material was removed, and mice were lifted by the tail to be placed on the experimenter’s gloved hand. Mice were then moved into an empty cage and back in this manner.
Day 3: Procedures from day 2 were repeated. However, mice climbed into the experimenter’s hand without encouragement. Mice were kept on the experimenter’s arm for 30 s before being returned to their home cage.
Day 4: Procedures from day 3 were repeated with the period on the experimenter’s arm extended to 90 s.
Day 5: Procedures from day 4 were repeated. However, mice were moved to the empty cage and back twice.
Day 6: Procedures from day 5 were repeated with the period on the experimenter’s arm being extended to 120 s.
Days 7–14: Procedures from day 6 were repeated. However, mice were moved to the empty cage and back thrice. Mice were handled on subsequent days until the entire procedure was completed within 10 min.
Days 12–14: During the last 3 days of handling, mice began the handling sessions in the open field of the VAST maze in the absence of vibration for 5 min to allow for habituation to the open field under red light.
Behavioral training.
Each testing session involved 20 trials and sessions were repeated over 3 consecutive days. Mice began each trial by being placed facing the outer wall of the field at one of four predetermined entry locations, each equidistant from the center of the maze (Fig. 2C). Once the mice were positioned and the experimenter’s hand withdrawn from view the motors began vibrating at a frequency consistent with the mouse’s position from the target point (Fig. 2B). The vibrations diminished as the mouse approached the target, stopping when the mouse entered the target circle. The presentation of the exit and entry points in each trial was pseudo-randomized using a Latin Square design. In each trial mice were given 2 min to reach the target. If the target was not reached during the allotted time the mouse was considered to have failed the trial and was removed from the maze. Three cage mates were tested per session under dim red overhead lights to restrict visual cues. Within a given session each cage mate was subjected to a trial in the same order so that all three mice completed trial 1 before any cage mate began trial 2. The maze was disinfected with 70% ethanol between each trial. A Froot Loop (Kellogg’s, Battle Creek, MI) was given to the mice as a reward once they completed a 20-trial session.
Experimental design.
All mice underwent 2 sessions of training (of 20 trials each) on 2 consecutive days to demonstrate task performance (n =18). Before the third session, mice were either sleep deprived for 10 h (ZT 0–10; n = 8; session 3-SD) or left to sleep undisturbed (n = 10; session 3-CONT).
The gentle handling SD method was implemented by the trained researchers, who were replaced hourly. In brief, when a mouse became motionless for several seconds a soft artist’s paintbrush was used to tap the cage or disturb the bedding around the animal to keep it awake. When necessary the back of the mouse was also gently stroked with the brush as previously reported (Davis et al. 2015, 2016, 2017).
Statistics.
Exclusion criteria were established to eliminate rodents that responded poorly to the vibratory stimulus (i.e., mice which displayed an average speed of 0.3 m/s or more throughout session 1) or were overly aggressive. Of five excluded mice, four failed over 50% of trials in session 1; this left 18 mice for statistical analysis.
For sessions 1 and 2 mice from both conditions were combined (n = 18) and two-way repeated measures ANOVAs with factors of SESSION and TRIAL were conducted. For session 3 three-way mixed repeated-measures ANOVAs with between factors of CONDITION (session 3-CONT versus session 3-SD) and within factors of SESSION and TRIAL were performed on single trials using SPSS v.17.0. Outcome measures included the success rate, total distance required to reach the target (path distance), total time to find the target destination (time to target), distance/time (speed), and time immobile during each trial (time immobile). To demonstrate SD-induced performance instability, the coefficients of variability for success rate, path distance, time to target, speed, and time immobile for session 2 were combined and compared with session 3-SD values using a paired t test. A Huynh–Feldt adjustment was used to account for sphericity violations of repeated measures ANOVAs, with unadjusted degrees of freedom being reported for clarity. When appropriate, Student’s t tests were used for pairwise comparisons. Alpha levels for statistical significance were set to P < 0.05.
RESULTS
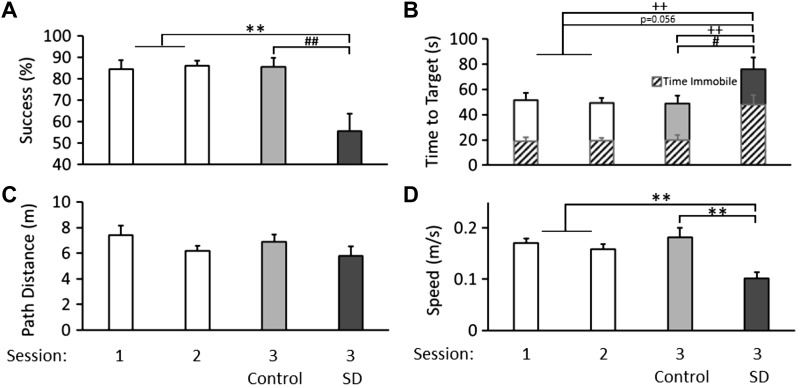
Mice were able to learn the task rapidly as evidenced by average success rates of 84 and 86% on sessions 1 and 2, respectively (Fig. 3A; CONDITION × SESSION: F2,32 = 7.59, P = 0.002). Following session 3-SD success rates declined to 56% which was 30% below the session 3-CONT (CONDITION: F1,16 = 10.33, P = 0.005) and session 2 success rates (SESSION: F1,7 = 12.32, P = 0.010), indicating performance deficits in navigating to the target destination before the 2-min trial expiration. Time to target decreased across sessions, indicating task acquisition (Fig. 3B; SESSION: F2,32 = 3.90, P = 0.030). However, following session 3-SD time to target increased by 26.4 s compared with session 3-CONT (CONDITION: F1,16 = 5.25, P = 0.036), but the 27.1-s increase compared with session 2 times did not reach statistical significance (SESSION: F1,7 = 5.23, P = 0.056). Mice showed progressive decreases in path distance across trials demonstrating within-session performance improvement (Fig. 3C; TRIAL: F3,51 = 6.70, P = 0.002). The average decrease in path distance from sessions 1 to 2 was 1.23 m, but this trend was not statistically significant (SESSION: F1,17 = 3.33, P = 0.086). Remarkably, the reduction in path distance from session 2 was not disrupted by SD, which may indicate that SD did not impact mouse orientation or task requirements, although session 3-SD mice sustained attentional deficits, impairing their goal-oriented ambulation. Furthermore, mouse speed decreased across the three sessions, suggesting that mouse movements may become more calculated with increased exposure to the VAST. It is not clear whether the 0.057 m/s decrease in speed from session 2 to 3-SD (F1,7 = 18.41, P = 0.004) was a continuation of task acquisition or whether decreased speed was the result of compromised performance due to SD (Fig. 3D; CONDITION × SESSION: F2,32 = 7.58, P = 0.002). The amount of time immobile was stable across sessions 1 and 2 (Fig. 3B). However, session 3-SD mice increased immobility by 28 s compared with session 3-CONT mice (CONDITION: F1,16 = 10.09, P = 0.006) and compared with their session 2 performance (F1,7 = 14.06, P = 0.007). The changes in time immobile coupled with the decrease in trial speed following SD is indicative of poor task persistence. Proportionally, time immobile constituted 37 and 39% of the total time to target in sessions 1 and 2, respectively, whereas time immobile following session 3-SD dramatically increased to 63% of the total time to target. The changes between session 3-CONT and session 3-SD mice also yielded performance instability as indicated by differences in the size of the error bars seen in (Fig. 3). To further illustrate this point, we computed the coefficient of variability for each of the five outcome measures in the session 3-SD mice and compared those values to their session 2 performance and confirmed increased variability incurred by SD (P = 0.045).
Fig. 3.
Means and standard errors for VAST performance outcomes. The VAST is acquired quickly, as shown by success rates over three sessions. Performance is compromised in session 3-SD after 10 h of sleep deprivation (SD). Results for sessions 1 and 2 (n = 18) are shown in white, and results for session 3 are shown in light gray (n = 10) and dark gray after 10 h of SD (n = 8; A). The height of the bar demonstrates the average time elapsed before the mouse arrives at the target location or is removed from the trial (trials timeout after 120 s). The solid portion of each bar demonstrates the average time spent moving around the open field during each session. The patterned segments demonstrate the average time spent immobile while in the open field. Overall, mice spent an increased percentage of time immobile while in the open field after 10 h of SD (B). The distance traveled within a trial decreased over the three sessions of VAST testing (C). Mouse speed within a trial decreases with SD (**P < 0.01 for differences between session 3-SD (n = 8) and sessions 1 and 2 (n = 18); #P < 0.05, ##P < 0.01 for differences between session 3-SD (n = 8) and session 3-CONT (n = 10); ++P < 0.01 for time immobile; D).
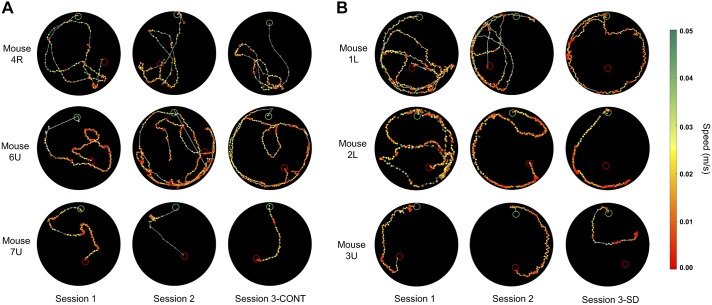
Representative path traces from trials occurring later in each session on 3 consecutive days from mice 4-R, 6-U, and 7-U with undisrupted sleep are provided as a demonstration of the changes manifest in Fig. 3 in session 3-CONT cohorts (Fig. 4A). In these trials, time immobile was low across sessions, as was total trial time. Path distance and speed remained consistent across sessions. Matched trials are provided from session 3-SD mice 1-L, 2-L, and 3-U to demonstrate compromised VAST performance after 10 h of SD (Fig. 4B). In these trials, the time immobile increased across sessions, which contributed to an increase in total trial time and a decrease in average speed. Together, these changes result in what appears to be more deliberate and directed movement toward the target destination on session 2 and session 3-CONT, and ultimately manifest with trial success. Whereas in session 3-SD, time to target, time immobile, and trials ending in failure increased.
Fig. 4.
Sets of path traces from six representative mice during a single late session trial on 3 consecutive days with session 3-CONT preceded by undisrupted sleep (A; mouse 4-R, trial 17; 6-U, trial 19; and 7-U, trial 18) and session 3-SD preceded by 10 h of sleep deprivation (B; mouse 1-L, trial 17; 2-L, trial 18; and 3-U, trial 19). The mouse’s path is portrayed on the open field by color-coded points that depict speed per the green-red color spectrum legend (right). The green and red open circles represent the entry point and target destinations, respectively.
DISCUSSION
The current findings demonstrate that mice can execute a spatial navigation task that is incentivized by graded haptic positional feedback. Specifically, the VAST uses exposure to vibrations to motivate performance. Our task further improves on the face and construct validity of sustained attention tasks by assessing contiguous spans of behavior rather than momentarily piqued attention. The validity of this task is further enhanced by the introduction of sensory stimuli and behavioral parameters that more closely model real world human behavior and standard human tasks of sustained attention, including the five-choice continuous performance task or the psychomotor vigilance task (PVT; Dinges and Powell 1985; van Enkhuizen et al. 2014). Additionally, the VAST appears to accurately model the fast acquisition of the human PVT, which is a rarity in rodent cognitive tasks as many tasks engage operant behaviors that can take weeks to train. Unlike the VAST, operant paradigms are motivated by consumed reward and often coupled with food or water deprivation (Córdova et al. 2006; Oonk et al. 2015; Walker et al. 2011; van Enkhuizen et al. 2014). Thus, the VAST confers the additional benefit of not being limited by satiation, as occurs with instrumental and operant conditioning.
From a hedonic perspective, the VAST likely motivates performance by eliciting aversive maneuvering, away from the field floor vibrations and toward the target destination. This reduces the floor vibration frequency until the mouse reaches the target and floor vibrations cease. The use of this type of tactile stimulus to both drive and direct behavior is novel. During each session in our study, mice were exposed to vibrations (with >80% in the high-frequency range of 150–240 Hz) for an average of 18.0 ± 1.6 min/day during ≤ 2 min bouts (maximum trial length) with an interval of 4–5 min between each exposure. No overt mouse behaviors were indicative of health concerns during or after VAST testing. It is important to note that low-frequency environmental vibrations have been reported to adversely affect mouse breeding colonies, heart rate, and blood pressure (Diercks et al. 2010; Farah et al. 2006; Norton et al. 2011). In contrast, high-frequency vibrations have demonstrated beneficial physiological outcomes including increased bone density, lower triglyceride levels, and decreased adipogenesis (Rubin et al. 2007; Xie et al. 2008). In addition to vibration, auditory cues from the motors could potentially motivate behavior in the VAST and aid in VAST acquisition. However, when we detached the motors from the VAST floor, decoupling the vibrations from the noise of the motors, mice (n = 3) exhibited chance performance with 31.7 ± 7.9% success rate, 102.8 ± 3.9 s time to target, 9.19 ± 0.55 m path distance, and 65.9 ± 5.6 s time immobile. Thus, auditory cues from the motors alone are not sufficient to drive task performance; instead these data reflect general non-goal-oriented exploration.
The haptic feedback of the VAST also has practical application, as it mimics the tactile vibration of rumble strips found on the sides of highways, which alert drivers when they veer from their lanes. One study examining the efficacy of rumble strips reports that drivers brake earlier and more often when approaching intersections with rumble strips than those without, even when sleep deprived (Harder and Bloomfield 2005). This operational feature of driver performance directly parallels the time immobile metric of the VAST. Driver fatigue is a multidimensional construct that can often be difficult to parse and counteract. Fatigue can be sleep-related or task-related (May and Baldwin 2009), and the VAST queries both subcategories. The accurate detection or interpretation of haptic feedback cues in the VAST is compromised by sleep loss resulting in increased time immobile and decreased speed during the task. Task-related performance is measured directly by success rate and time to target. Harder and Bloomfield (2005) reiterate the human finding that SD increases variability in steering late at night. Similarly, SD-induced increases in variability may explain the decreased success rates observed in session 3-SD. This is indicated by the differences in coefficient of variability and the larger error bars in the session 3-SD VAST parameters (Fig. 3). This is reflective of the state instability that follows SD, as manifest by increased lapses in the laboratory setting (Doran et al. 2001). Humans exhibit individual performance differences in their susceptibility to the effects of SD. The VAST post-SD performance reflects individual mouse differences in performance. For example, two of the eight mice were not adversely affected by sleep loss: one mouse showed session 3-SD performance outcomes similar to their session 2 performance, while another mouse slightly improved on success rates and time to target after SD. Thus, mice demonstrate individual differences in their resilience to sleep loss, as is observed in human populations (Doran et al. 2001; Van Dongen 2012).
It is not readily obvious whether the post-SD performance decrements reported herein are reflective of cognitive dysfunction, motor fatigue, altered sensory detection, or a combination of these sensorimotor integration components. It is doubtful that motor fatigue is a primary cause of the observed deficits as C57BL/6 mice given access to a running wheel will voluntarily travel 4,000–10,000 m/day (Roemers et al. 2018). In the VAST mice traveled 135.1 ± 9.6 m/day on average, a fraction of their physical capabilities. It is apparent that VAST performance is susceptible to SD as evidenced by decreases in success rates and speed, in addition to increases in both time to target and time immobile. Another consideration germane to the observed SD effects is the possibility that they are blunted by underlying circadian-driven performance enhancements at dark onset (toward the end of the session). Increased vigilance at the onset of the subjective waking phase of humans and rodents typically is accompanied by augmented cognitive task performance (Bougard et al. 2016; Gritton et al. 2012; Kline et al. 2010; Takahashi et al. 2013).
The VAST was first conceptualized as a task that could be easily learned, while remaining complex enough to engage SD-sensitive cortical structures. While it is counterintuitive that path distance is stable following SD, it suggests that there is a component of the VAST that is retained and uncompromised by sleep loss. Still the effects of SD on VAST performance are robust in that almost half of the post-SD trials ended in failure. Poor success rates coupled with the increase in time immobile during session 3-SD evidence the detrimental effects of SD on VAST performance. The present findings can be interpreted in several ways. One possibility is that SD generates “microsleeps” that compromise task performance by impeding goal-oriented behavior, thereby increasing reaction time (Li et al. 2014; Rechtschaffen and Bergmann 2002). Microsleeps are temporary intrusions of sleep during periods of prolonged wakefulness that may last for as little as a fraction of a second. During these periods, electroencephalographic markers and behavioral characteristics of sleep manifest in organisms that may otherwise appear awake (Durmer and Dinges 2005; Veasey et al. 2004). Another possible explanation of the observed performance deficits is that exposure to the VAST after SD increases anxiety levels and leads to increased freezing behavior. Sleep deprivation-induced anxiety effects in mice are reported with the elevated plus maze (Silva et al. 2004). Along these lines, a few mice defecated in the first few VAST trials of session 1 (a sign of anxiety), but this was not observed during session 3 trials even when trials followed SD. Moreover, the analysis of time immobile showed that sleep-deprived mice in the VAST would frequently stop and engage in subtle nonambulatory movements (e.g., turn their head, sniff, and groom, although these behaviors were not recorded) during the task, as opposed to the hallmark freezing behavior of fear conditioning. The SD-induced performance decrements could also stem from other sources including loss of motivation, decreased attention, and/or decreased sensitivity to the stimulus. For instance, SD has been shown to alter sensory feedback thresholds in humans and rodents. While strong evidence exists for increased pain sensitivity following SD (Alexandre et al. 2017; Onen et al. 2001; Roehrs et al. 2006), evidence in other sensory modalities is sparse. In humans the detection of auditory stimuli is less affected by SD than visual stimuli (Jung et al. 2011) and in rats extended SD elevates hearing thresholds (Jung et al. 2018). SD may also impair proprioception, olfaction, and even certain tastes (Furchtgott and Willingham 1956; Gomez et al. 2008; Killgore and McBride 2006). It is difficult to exclude changes in sensory threshold as an explanation of the VAST SD results. However, if disrupted sensory feedback was entirely responsible for the SD-compromised performance, an increase in path distance would be expected, which is not the case. Overall, none of the explanations above are mutually exclusive of one another and it is possible that each could contribute to time immobile over the course of a trial. More studies will be needed to determine the relevance of the proposed explanations.
In summary, the VAST is a rapidly learned mouse task that models real-time multimodal sensory feedback to guide search responses. The applications of instantaneous sensory feedback extend beyond the VAST as a potential alternative to foot shock in fear conditioning or swimming in the Morris water maze. VAST vibrations could also be paired with graded visual stimuli to assess cognitive flexibility in a stimulus reversal paradigm. The fast acquisition rates are an advantage of the VAST with respect to other mouse behavioral paradigms that measure performance, especially operant conditioning. Moreover, the use of vibration to motivate mice does not limit performance to presatiety behaviors. The integrated information processing component of the VAST with its moment-to-moment perception of auditory and tactile sensory inputs, detection of ambulatory motor response, and real-time situational appraisal may be useful in rodent studies of neurodegenerative diseases and determining the efficacy of their treatments. Finally, the VAST parallels important aspects of human attentional tasks and their responses to sleep loss and provides a novel assay that can be further investigated via electrophysiological and molecular constituents of insufficient sleep.
GRANTS
This work was funded by a Washington State University Faculty Seed Grant and NIH grant NS085605 to C. J. Davis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.N.B. and C.J.D. conceived and designed research; P.N.B., T.N., and D.O.H. performed experiments; P.B., J.N.K., D.O.H., and C.J.D. analyzed data; P.B., T.N., and C.J.D. interpreted results of experiments; P.N.B. and C.J.D. prepared figures; P.N.B. and C.J.D. drafted manuscript; P.N.B., J.N.K., T.N., D.O.H., and C.J.D. edited and revised manuscript; P.N.B., J.N.K., T.N., D.O.H., and C.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The mouse VAST apparatus was constructed as part of a senior project in the Washington State University Department of Electrical Engineering and Computer Sciences. The team included Nathan W. Calkins, Matthew D. Bryan, Daniel O. Harvey, and Bedelu A. Dender.
REFERENCES
- Alexandre C, Latremoliere A, Ferreira A, Miracca G, Yamamoto M, Scammell TE, Woolf CJ. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat Med 23: 768–774, 2017. doi: 10.1038/nm.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep 33: 437–444, 2010. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarenga TA, Patti CL, Andersen ML, Silva RH, Calzavara MB, Lopez GB, Frussa-Filho R, Tufik S. Paradoxical sleep deprivation impairs acquisition, consolidation, and retrieval of a discriminative avoidance task in rats. Neurobiol Learn Mem 90: 624–632, 2008. doi: 10.1016/j.nlm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Bougard C, Moussay S, Espié S, Davenne D. The effects of sleep deprivation and time of day on cognitive performance. Biol Rhythm Res 47: 401–415, 2016. doi: 10.1080/09291016.2015.1129696. [DOI] [Google Scholar]
- Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res 17: 376–384, 2008. doi: 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson BC, Durkin J, Suresh AK, Pickup EJ, Broussard CG, Aton SJ. Sleep promotes, and sleep loss inhibits, selective changes in firing rate, response properties and functional connectivity of primary visual cortex neurons. Front Syst Neurosci 12: 40, 2018. doi: 10.3389/fnsys.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdova CA, Said BO, McCarley RW, Baxter MG, Chiba AA, Strecker RE. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep 29: 69–76, 2006. [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Dunbrasky D, Oonk M, Taishi P, Opp MR, Krueger JM. The neuron-specific interleukin-1 receptor accessory protein is required for homeostatic sleep and sleep responses to influenza viral challenge in mice. Brain Behav Immun 47: 35–43, 2015. doi: 10.1016/j.bbi.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Taishi P, Honn KA, Koberstein JN, Krueger JM. P2X7 receptors in body temperature, locomotor activity, and brain mRNA and lncRNA responses to sleep deprivation. Am J Physiol Regul Integr Comp Physiol 311: R1004–R1012, 2016. doi: 10.1152/ajpregu.00167.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Zielinski MR, Dunbrasky D, Taishi P, Dinarello CA, Krueger JM. Interleukin 37 expression in mice alters sleep responses to inflammatory agents and influenza virus infection. Neurobiol Sleep Circadian Rhythms 3: 1–9, 2017. doi: 10.1016/j.nbscr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Bermudez C, Sokoloff G, Blumberg MS. Sensorimotor processing in the newborn rat red nucleus during active sleep. J Neurosci 35: 8322–8332, 2015. doi: 10.1523/JNEUROSCI.0564-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercks AK, Schwab A, Rittgen W, Kruspel A, Heuss E, Schenkel J. Environmental influences on the production of pre-implantation embryos. Theriogenology 73: 1238–1243, 2010. doi: 10.1016/j.theriogenology.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Maislin G, Brewster RM, Krueger GP, Carroll RJ. Pilot test of fatigue management technologies. Transp Res Rec 1922: 175–182, 2005. doi: 10.1177/0361198105192200122. [DOI] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput 17: 652–655, 1985. doi: 10.3758/BF03200977. [DOI] [Google Scholar]
- Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol 139: 253–267, 2001. [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol 25: 117–129, 2005. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Farah VM, Joaquim LF, Morris M. Stress cardiovascular/autonomic interactions in mice. Physiol Behav 89: 569–575, 2006. doi: 10.1016/j.physbeh.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Fridgeirsdottir GA, Hillered L, Clausen F. Escalated handling of young C57BL/6 mice results in altered Morris water maze performance. Ups J Med Sci 119: 1–9, 2014. doi: 10.3109/03009734.2013.847511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchtgott E, Willingham WW. The effect of sleep-deprivation upon the thresholds of taste. Am J Psychol 69: 111–112, 1956. doi: 10.2307/1418125. [DOI] [PubMed] [Google Scholar]
- Gomez S, Patel M, Berg S, Magnusson M, Johansson R, Fransson PA. Effects of proprioceptive vibratory stimulation on body movement at 24 and 36h of sleep deprivation. Clin Neurophysiol 119: 617–625, 2008. doi: 10.1016/j.clinph.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Gritton HJ, Kantorowski A, Sarter M, Lee TM. Bidirectional interactions between circadian entrainment and cognitive performance. Learn Mem 19: 126–141, 2012. doi: 10.1101/lm.023499.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res 19: 280–288, 2010. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- Harder JA, Bloomfield JR. The Effects of In-Lane Rumble Strips on the Stopping Behavior of Sleep-Deprived Drivers. Final Technical Report Minnesota Local Road Research Board, 2005. http://hdl.handle.net/11299/835. [Google Scholar]
- Hartley L, Horberry T, Mabbott N, Krueger GP. Review of Fatigue Detection and Prediction Technologies. Melbourne, Australia: National Road Transport Commission, 2000. [Google Scholar]
- Jackson ML, Gunzelmann G, Whitney P, Hinson JM, Belenky G, Rabat A, Van Dongen HP. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev 17: 215–225, 2013. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ML, Van Dongen HPA. Cognitive effects of sleepiness. In: Sleepiness: Causes, Consequences and Treatment, edited by Thorpy MJ, Billiard M. Cambridge, UK: Cambridge University Press, 2011, p. 72–81. [Google Scholar]
- Jung CM, Ronda JM, Czeisler CA, Wright KP Jr. Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J Sleep Res 20: 348–355, 2011. doi: 10.1111/j.1365-2869.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Kim M, Lee SJ, Lee E, Lee SA, Lee JD, Choi JH, Kim BG. Effect of sleep deprivation on hearing levels in rats. Int J Pediatr Otorhinolaryngol 112: 169–175, 2018. doi: 10.1016/j.ijporl.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Killgore WD, McBride SA. Odor identification accuracy declines following 24 h of sleep deprivation. J Sleep Res 15: 111–116, 2006. doi: 10.1111/j.1365-2869.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- Kline CE, Durstine JL, Davis JM, Moore TA, Devlin TM, Youngstedt SD. Circadian rhythms of psychomotor vigilance, mood, and sleepiness in the ultra-short sleep/wake protocol. Chronobiol Int 27: 161–180, 2010. doi: 10.3109/07420521003648604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Panossian LA, Zhang J, Zhu Y, Zhan G, Chou YT, Fenik P, Bhatnagar S, Piel DA, Beck SG, Veasey S. Effects of chronic sleep fragmentation on wake-active neurons and the hypercapnic arousal response. Sleep (Basel) 37: 51–64, 2014. doi: 10.5665/sleep.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 1129: 305–322, 2008. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- May JF, Baldwin CL. Driver fatigue: the importance of identifying causal factors of fatigue when considering detection and countermeasure technologies. Transp Res, Part F Traffic Psychol Behav 12: 218–224, 2009. doi: 10.1016/j.trf.2008.11.005. [DOI] [Google Scholar]
- Norton JN, Kinard WL, Reynolds RP. Comparative vibration levels perceived among species in a laboratory animal facility. J Am Assoc Lab Anim Sci 50: 653–659, 2011. [PMC free article] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res 10: 35–42, 2001. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Oonk M, Davis CJ, Krueger JM, Wisor JP, Van Dongen HP. Sleep deprivation and time-on-task performance decrement in the rat psychomotor vigilance task. Sleep (Basel) 38: 445–451, 2015. doi: 10.5665/sleep.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Meerlo P, Dürr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem 85: 263–271, 2006. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Perrillo K. The Effectiveness and Use of Continuous Shoulder Rumble Strips. Albany, NY: Federal Highway Administration, 1998. [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep 19: 318–326, 1996. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep 25: 18–24, 2002. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep 29: 145–151, 2006. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- Roemers P, Mazzola PN, De Deyn PP, Bossers WJ, van Heuvelen MJG, van der Zee EA. Burrowing as a novel voluntary strength training method for mice: a comparison of various voluntary strength or resistance exercise methods. J Neurosci Methods 300: 112–126, 2018. doi: 10.1016/j.jneumeth.2017.05.027. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA 104: 17879–17884, 2007. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RH, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Niigaki ST, Abílio VC, Tufik S, Frussa-Filho R. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology (Berl) 176: 115–122, 2004. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Sawa K, Okada T. The diurnal variation of performance of the novel location recognition task in male rats. Behav Brain Res 256: 488–493, 2013. doi: 10.1016/j.bbr.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP. Connecting the dots: from trait vulnerability during total sleep deprivation to individual differences in cumulative impairment during sustained sleep restriction. Sleep 35: 1031–1033, 2012. [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Acheson D, Risbrough V, Drummond S, Geyer MA, Young JW. Sleep deprivation impairs performance in the 5-choice continuous performance test: similarities between humans and mice. Behav Brain Res 261: 40–48, 2014. doi: 10.1016/j.bbr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Yeou-Jey H, Thayer P, Fenik P. Murine Multiple Sleep Latency Test: phenotyping sleep propensity in mice. Sleep 27: 388–393, 2004. doi: 10.1093/sleep/27.3.388. [DOI] [PubMed] [Google Scholar]
- Walker JL, Walker BM, Fuentes FM, Rector DM. Rat psychomotor vigilance task with fast response times using a conditioned lick behavior. Behav Brain Res 216: 229–237, 2011. doi: 10.1016/j.bbr.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol (1985) 104: 1056–1062, 2008. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]