Abstract
Strong evidence supports a major role for heterodimers of the type 1 taste receptor (T1R) family in the taste transduction of sugars (T1R2+T1R3) and amino acids (T1R1+T1R3), but there are also neural and behavioral data supporting T1R-independent mechanisms. Most neural evidence for alternate mechanisms comes from whole nerve recordings in mice with deletion of a single T1R family member, limiting conclusions about the functional significance and T1R independence of the remaining responses. To clarify these issues, we recorded single-unit taste responses from the nucleus of the solitary tract in T1R double-knockout (double-KO) mice lacking functional T1R1+T1R3 [KO1+3] or T1R2+T1R3 [KO2+3] receptors and their wild-type background strains [WT; C57BL/6J (B6), 129X1/SvJ (S129)]. In both double-KO strains, responses to sugars and a moderate concentration of an monosodium glutamate + amiloride + inosine 5′-monophosphate cocktail (0.1 M, i.e., umami) were profoundly depressed, whereas a panel of 0.6 M amino acids were mostly unaffected. Strikingly, in contrast to WT mice, no double-KO neurons responded selectively to sugars and umami, precluding segregation of this group of stimuli from those representing other taste qualities in a multidimensional scaling analysis. Nevertheless, residual sugar responses, mainly elicited by monosaccharides, persisted as small “sideband” responses in double-KOs. Thus other receptors may convey limited information about sugars to the central nervous system, but T1Rs appear critical for coding the distinct perceptual features of sugar and umami stimuli. The persistence of amino acid responses supports previous proposals of alternate receptors, but because these stimuli affected multiple neuron types, further investigations are necessary.
NEW & NOTEWORTHY The type 1 taste receptor (T1R) family is crucial for transducing sugars and amino acids, but there is evidence for T1R-independent mechanisms. In this study, single-unit recordings from the nucleus of the solitary tract in T1R double-knockout mice lacking T1R1+T1R3 or T1R2+T1R3 receptors revealed greatly reduced umami synergism and sugar responses. Nevertheless, residual sugar responses persisted, mainly elicited by monosaccharides and evident as “sidebands” in neurons activated more vigorously by other qualities.
Keywords: NST, sweet, T1R, umami
INTRODUCTION
The gustatory system is critical for distinguishing the chemical properties of substances before ingestion, a process that begins with specialized receptors in taste bud cells. Over a decade ago, a seminal discovery revealed that sugars and amino acids, cardinal constituents of carbohydrates and proteins, are sensed through the type 1 taste receptor family. This family, comprised of three class-C G protein-coupled receptors, includes T1R3, which is a common subunit that combines with T1R1 to form a heterodimer (T1R1+T1R3) for amino acid detection, and T1R2 (T1R2+T1R3) to detect sugars (Nelson et al. 2001, 2002). For both classes of nutrients, however, there is evidence for alternate receptor mechanisms.
Genetically rendering either subunit of the T1R2+T1R3 heterodimer nonfunctional severely disrupts sugar preference (Zukerman et al. 2009), detection (Treesukosol and Spector 2012), and concentration-dependent licking in brief-access tests that minimize postingestive influences (Nelson et al. 2001; Treesukosol et al. 2009, 2011a; Zhao et al. 2003) (but see Delay et al. 2006). Moreover, double-knockout mice lacking both T1R2 and T1R3 receptors (KO2+3) appear even more impaired and, regardless of concentration, display virtually no responsiveness to sugars in brief-access tests (Treesukosol et al. 2011a; Zhao et al. 2003). Nonetheless, extensive exposure to individual presentations of fructose and glucose that allows learning about the postingestive consequences of the different sugars reveals that KO2+3 mice display heightened licking of glucose over fructose, suggesting that there are T1R-independent mechanisms for detecting this stimulus class. Indeed, the T1Rs are not significantly involved in transducing other types of carbohydrates. In particular, KO2+3 mice display relatively normal behaviors toward Maltrin or Polycose, highly preferred oligosaccharide mixtures hypothesized to generate a distinct, nonsweet “polysaccharide” taste (Nissenbaum and Sclafani 1987; Smith and Spector 2017; Treesukosol and Spector 2012; Yee et al. 2011). Likewise, the cephalic phase response elicited by glucose is dependent on the ATP-sensitive K+ (KATP) channel not T1R3 (Glendinning et al. 2015, 2017).
Whole nerve recordings also support residual sensitivity to sugars in T1R KO mice in both the chorda tympani nerve (CT), which innervates the anterior tongue, and the glossopharyngeal nerve (GL), which supplies taste buds on the posterior tongue. In T1R3 null mice, the CT and GL exhibit some, albeit reduced, sugar responses (Damak et al. 2003; Zhao et al. 2003). Single-unit recordings from the nucleus of the solitary tract (NST) of T1R3 KO mice support the persistence of weak responses to sugars and reveal that across-neuron patterns change dramatically (Lemon and Margolskee 2009). Although whole nerve CT responses to both mono- and disaccharides were eliminated when both T1R2 and T1R3 were deleted (Zhao et al. 2003), responses to sugars in KO2+3 mice have not been evaluated in the GL nor the greater superficial petrosal (GSP) nerve, which innervates the palate. Because these neurophysiological data in double-KO mice are limited and not consistent with evidence for the remaining behavioral responses discussed above, more comprehensive studies exploring residual gustatory sensitivity throughout the oral cavity are necessary.
Evidence likewise suggests T1R-independent mechanisms for detecting amino acids. Amino acid ligands for T1R1+T1R3 include sodium and potassium salts of l-glutamate [monosodium glutamate (MSG) and monopotassium glutamate (MPG)] and in rodents, other amino acids. Glutamate responses exhibit synergism in the presence of ribonucleotides such as inosine 5′-monophosphate (IMP) (Zhang et al. 2008), and glutamate/ribonucleotide cocktails are considered prototypical “umami” stimuli. Concentration-dependent licking for mixtures of MSG + IMP is virtually eliminated in brief-access tests for mice lacking either (Zhao et al. 2003), or both T1R1 and T1R3 (KO1+3) (Blonde and Spector 2017; Zhao et al. 2003) but KO1+3 mice initiate a comparable number of trials, indicating unaltered appetitive motivation (Blonde and Spector 2017). Moreover, at higher concentrations, both KO1+3 (Blonde et al. 2018) and KO2+3 (Smith and Spector 2014) mice can discriminate a mixture of MSG and amiloride from water but only if IMP is present (Smith and Spector 2014). Previous studies suggest that the metabotropic glutamate receptors, mGLUR1 and mGLUR4, best characterized in the posterior tongue, may be responsible for this T1R-independent glutamate responsiveness (Chaudhari et al. 1996, 2000; Chaudhari and Roper 1998).
Ablation of T1R1, T1R3, or both subunits strongly depresses synergistic whole nerve CT responses to an MSG/IMP mixture (Damak et al. 2003; Zhao et al. 2003), but MSG and MPG still elicit relatively robust CT and GL responses (Damak et al. 2003; Kusuhara et al. 2013; Yasumatsu et al. 2012). Single-fiber recordings from the CT reveal key aspects of this remaining responsiveness in T1R3 KO mice (Yasumatsu et al. 2012), with some neurons responding to MPG but not KCl and a few of these fibers exhibiting a small amount of IMP enhancement. These results are consistent with a T1R-independent mechanism for detecting glutamate and the behavioral results showing that KO1+3 mice can detect higher concentrations of MSG + IMP mixed in amiloride (Blonde et al. 2018). However, questions remain since T1R1, which is expressed without T1R3 in some taste bud cells (Nelson et al. 2001), was still present. Moreover, without single-unit recordings, it is unclear whether residual GL responses arose from glutamate or the electrolyte components of MSG and MPG.
To more conclusively determine the degree to which amino acid and sugar responsiveness are T1R independent, the present experiment recorded NST responses arising from taste receptor subpopulations throughout the mouth using two strains of double-KO mice, KO1+3 and KO2+3. Stimuli of particular interest in light of the behavioral capacities of T1R KO mice included MSG with and without IMP, mono- and disaccharides, and Maltrin. By using single-unit recording, we were able to define chemosensitive response profiles and across-neuron patterns of activity to evaluate the potential of the remaining responses to support the behavioral capacities of T1R double-KO mice.
METHODS
Animals.
Subjects were 106 adult male (60) and female (46) mice weighing 18–35 g. Double-knockout mice were generated from male and female breeding pairs homozygous null for the Tas1r1, Tas1r2, or Tas1r3 gene [initially derived from S129 backcrossed with B6 mice and generously provided by Dr. Charles Zuker while at University of California, San Diego (now at Columbia University)]. After an additional backcross with B6 mice (Jackson Laboratory), homozygous-null mice for the Tas1r1 or Tas1r3 were paired to generate mice heterozygous for both Tas1r1 and Tas1r3. These heterozygous mice were used to generate mice heterozygous, homozygous null, and wild type for Tas1r1 and/or Tas1r3, which were in turn paired to generate mice homozygous-null for both Tas1r1 and Tas1r3. These double-KO mice were paired in a homozygous breeding strategy to generate the experimental double-KO mice. A similar breeding strategy was used to generate mice lacking both Tas1r2 and Tas1r3. According to single nucleotide polymorphism genome scanning analysis of known polymorphisms between B6 and S129 strains (completed by Jackson Laboratory), the double-KO mice have ~20–30% contribution from the S129 strain. Thus, for wild-type (WT) controls, we used the two strains, C57BL/6J (B6) and129X1/SvJ (S129), which comprised their genetic background (Jackson Laboratory, Bar Harbor, ME). Random WT and all double-KO mice were genotyped (Transnetyx, Inc., Cordova, TN) both before and after the experiment. Animals were housed in a vivarium that maintained a 12-h light/dark cycle and an ambient temperature of ~22.2 ± 0.6°C. Food and water were available ad libitum. All procedures were approved by Institutional Laboratory Animal Care and Use Committee at the Ohio State University.
Surgery.
Animals were deeply anesthetized with urethane (1 g/kg) followed by a maintenance dose of 0.25 g/kg as necessary. Urethane was supplemented with isoflurane for both induction (2%) and for maintenance (0.5–1%) during surgery, but in the majority of cases, isoflurane was discontinued during recording. Body temperature was monitored rectally and maintained at ∼37°C using a heating pad. To prevent tongue movements and reflexes during oral taste stimulation, the hypoglossal nerve was transected. To aid respiration each mouse was tracheotomized with PE60 (polyethylene tubing). Sutures were passed through the four corners of the lips and inserted shallowly in the lingual mucosa just anterior to foliates to extend the tongue and better view the oral cavity (Frank 1991; Halsell et al. 1993). Each mouse was secured in the stereotaxic instrument (custom-modified Kopf) equipped with nontraumatic earbars. A scalpel was used to make an incision (~1 cm) to open the scalp and the skull leveled between bregma and lambda. A hole (~4 mm diameter) was drilled through the skull just posterior to lambda to reach the NST for electrophysiological recordings.
The search for the taste-responsive NST typically commenced at 2.5 ± 0.6 mm posterior, 0.8 ± 0.4 mm lateral to lambda. We used tungsten microelectrodes (Frederic Haer Company or World Precision Instruments; impedance = ~1–3 mΩ). Electrodes were angled at 4° pointing rostrally and lowered through the overlying cerebellum and vestibular nucleus using a hydraulic microdrive (model no. 640; David Kopf Instruments) to reach the taste-responsive NST at 3.5–4.5 mm from the cerebellar surface. Neuronal activity was amplified and filtered (10,000×, 0.6–3 KHz), sampled at 10 kHz, and monitored using SPIKE 2-CED data acquisition system (model no. 140; Cambridge Electronic Design, Cambridge, UK). In selected instances, the recording site was marked by an electrolytic lesion (5–8 µA for 5–8 s) either at the site of recording, below (reticular formation), or above (vestibular nucleus) the NST on the same track. At the end of the experimental session, each animal was injected with a lethal dose of anesthesia (80 mg/kg ketamine and 100 mg/kg xylazine) and intracardially perfused using phosphate-buffered saline (PBS), and 4% paraformaldehyde (in 0.1 M phosphate buffer) containing 1.4% l-lysine acetate and 0.2% sodium metaperiodate (McLean and Nakane 1974). Subsequently, the brain was extracted, fixed overnight in 20% sucrose paraformaldehyde, and then blocked in the coronal plane and stored in sucrose-phosphate buffer before sectioning.
Tastants and stimulation.
Table 1 lists stimuli, concentrations, and the number of neurons sampled for each tastant. Chemicals were dissolved in artificial saliva (AS; Breza et al. 2010) and presented at room temperature (~23.5°C). We used representatives of the five standard gustatory qualities and other tastants. The choice of concentrations and additional stimuli was based on the behavioral abilities in T1R KO mice. Behavioral discrimination experiments (Blonde et al. 2018; Smith and Spector 2014) have demonstrated that a “umami” stimulus, a mixture of monosodium glutamate (MSG) with amiloride and inosine 5′-monophosphate (IMP), can be detected by KO1+3 mice at intense concentrations and by WT mice at lower intensities. However, eliminating IMP makes this stimulus difficult or impossible to detect regardless of genotype although MSG without amiloride can be discriminated readily based on the sodium component (Smith and Spector 2014). Thus we used umami, MSG with amiloride (MSGa), and MSG alone at an intense concentration of 0.6 M. In addition, two amino acids differentially affected by T1R deletion were included. Glycine detection is impaired but not abolished (Smith and Spector 2017) whereas l-lysine is relatively unaffected by T1R deletion (Smith and Spector 2014, 2017). Similarly, although preference and discrimination for sugars is markedly diminished in mice devoid of the T1R2 and/or T1R3 subunits, behavior toward maltodextrin solutions is relatively unaltered (Smith and Spector 2017; Treesukosol et al. 2009; Zukerman et al. 2009). Thus Maltrin 580 (a generous gift of Grain Processing Corporation, Muscatine, IA), a glucose oligomer mixture with an average chain length of ~6, was also tested [Maltrin 580 is similar to Polycose (Ross Laboratories), the stimulus used in the original explorations of this polysaccharide taste, but this product is no longer available]. Finally, based on the persistence of the cephalic phase response to glucose in T1R3 knockout mice (Glendinning et al. 2015) and the ability of KO2+3 mice to hedonically discriminate glucose from fructose after ingestive experience with these sugars (Schier and Spector 2016), we tested both glucose and fructose. We began by using these monosaccharides at the same concentration as sucrose (0.3 M) but early in the study switched to 1.0 M in an attempt to reveal more responses in double-KO mice with a concentration reported to be highly efficacious in eliciting the cephalic phase glucose response in T1R3 KO mice (Glendinning et al. 2015). Over 70% of neurons were tested with the higher monosaccharide concentration in each mouse strain. For simplicity, the two concentrations were combined for analysis after determining that conclusions were similar for either intensity. Likewise, in our initial experiments, we noted many neurons in the double-KO mice that responded to umami at 0.6 M and were concerned that these responses were due to the high concentration of the Na+ cation, even in the presence of amiloride. Thus we added 0.1 M umami and 0.6 M sodium to distinguish the adequate stimulus in these neurons.
Table 1.
Stimuli, concentrations, and the number of neurons sampled for each tastant
| Neurons (No.) |
||||||
|---|---|---|---|---|---|---|
| Stimulus/Group | Concentrations | Acronym | B6 | S129 | KO1+3 | KO2+3 |
| Group 1 (Probe) | ||||||
| Sucrose | 0.3 M | S | 47 | 46 | 45 | 56 |
| Umami* | 0.6 M MSG + 100 µM a + 2.5 mM IMP | U 0.6 | 47 | 46 | 45 | 56 |
| NaCl | 0.1 M | N | 47 | 46 | 45 | 56 |
| Citric acid | 0.01 M | I | 47 | 46 | 45 | 56 |
| Bitter mixture† | 2.7 mM Q + 0.01 mM CY | B | 47 | 46 | 45 | 56 |
| Maltrin | 16% | MAL | 47 | 46 | 45 | 56 |
| Group 2 | ||||||
| MSG | 0.6 M | MSG | 46 | 45 | 45 | 55 |
| MSG + amiloride | 0.6 M MSG + 100 µM a | MSGa | 46 | 44 | 43 | 55 |
| Glycine | 0.6 M | GLY | 47 | 45 | 45 | 56 |
| Lysine | 0.6 M | LYS | 45 | 44 | 45 | 55 |
| Glucose | 0.3 or 1.0 M | GLU | 47 | 46 | 44 | 56 |
| Fructose | 0.3 or 1.0 M | FRU | 45 | 46 | 43 | 56 |
| Group 3 | ||||||
| Umami | 0.1 M MSG + 100 µM a + 2.5 mM IMP | U 0.1 | 42 | 43 | 41 | 52 |
| NaCl | 0.6 M | N 0.6 | 41 | 41 | 42 | 54 |
| Group 4 | ||||||
| Artificial saliva‡ | AS | 38 | 35 | 40 | 46 | |
| NH4Cl | 0.3 M | NH4 | 29 | 34 | 34 | 43 |
Umami: monosodium glutamate (MSG) + amiloride (a) + inosine 5′-monophosphate (IMP).
Bitter mixture: quinine monohydrochloride (Q) + cycloheximide (CY).
Artificial saliva composition: potassium chloride (KCl), 0.022 M; sodium chloride (NaCl), 0.015 M; calcium chloride (CaCl2), 0.0003 M; magnesium chloride (MgCl2), 0.0006 M (Breza et al. 2010). KO, knockout.
Stimuli were delivered at a rate of 0.6 ml/s using a pressurized flow system controlled by CED software. Stimuli flowed through separate tubes to a 16:1 manifold (Warner Systems), connected to a single glass capillary (1.2-mm inner diameter) positioned to bathe all the oral taste receptor subpopulations [anterior tongue (AT), foliate (FOL), circumvallate papillae (CV), nasoincisor ducts (NID), and soft palate (SP)], which we confirmed visually though the operating microscope. The sequence of stimulation was as follows: 10 s of prerinse with AS followed by 10 s of stimulus delivery and 20 s of postrinse with AS. The interstimulus interval period was at least 60 s. Sometimes it was necessary to wait for a longer period to allow neural activity to return to baseline. For example, this occurred frequently when testing sodium-selective neurons with stimuli containing amiloride. When possible, we repeated stimulations. If the cell remained viable after testing chemosensitivity, the receptive field location was assessed as described in Geran and Travers (2013) and Geran and Travers (2006, 2009). Briefly, using the operating microscope, we used a small paintbrush or a precision applicator brush (S379; Parkell, Inc.) to apply a taste mixture to different receptor subpopulations. The location of the applied stimulus was evident due to reflections from the fluid. In some cases, it was possible to glean specific information about receptive fields and to specify the responsible cranial nerve branch(es). However, because of the very small size of the mouse mouth, it was often difficult to constrain stimulus spread in the posterior oral cavity. In these cases, receptive fields were simply classified as anterior (A-AT and/or NID), posterior (P-FOL, CV, and/or SP), or Mixed (M-Both A and P) receptive fields.
Protocol.
An obvious drop in the amplitude of spontaneous activity usually accompanied the transition from the overlying vestibular nucleus to the dorsal border of NST at which point the electrode was lowered more slowly while testing for taste-responsive activity. Stimulation with a taste mixture consisting of one representative of four of the five standard taste qualities (in mM: 300 sucrose, 10 citric acid, 100 NaCl, and 0.01 cycloheximide) along with individual compounds, including amino acids, monosaccharides, and Maltrin was used to probe for single taste-responsive neurons. Once a unit was isolated, testing with individual stimuli commenced. Stimuli were presented in varied order with some exceptions; we noted that for neurons preferentially activated by sodium, amiloride-containing stimuli often markedly reduced spontaneous activity and subsequent sodium-elicited responses for an extended period (2–3 min). Thus, in these cases, we delayed testing amiloride-containing stimuli until sodium salts were presented. Table 1 categorizes stimuli in four groups, based on the number of neurons tested with each: The “probe stimuli” (group 1) included representatives of the five standard taste qualities (using 0.6 M umami) and Maltrin; these were tested on all neurons. Group 2 consisted of MSG, MSG + amiloride, other amino acids, and the monosaccharides; these tastants were tested on all but one or two neurons. Group 3 stimuli, 0.1 M umami and 0.6 M NaCl, added after the beginning of the experiment, were tested on the majority, but fewer, cells. Group 4 stimuli were tested on the fewest neurons and included AS (to provide a “no taste” control) and NH4Cl. Stimuli in groups 1–3 were used for all the analyses described in the results, but the group 4 stimuli were only included for the across-neuron pattern analyses. Although tested on the fewest neurons, group 4 stimuli provided a useful extension for this analysis since the correlations between stimuli are most meaningful when comparing similarities and differences between many qualitatively similar and distinct compounds.
Neurophysiological data analysis.
Statistical analysis and quantification were performed using Excel (Versions 2013 and 2016) and Systat (Version 13.1). Net responses were the number of spikes during the 10-s stimulus delivery minus those during the prestimulus AS period. When stimuli were repeated, the mean of the trials was calculated. The means ± SD of the spontaneous and prerinse AS activity were also calculated across trials for each neuron. The response criteria were a net-evoked firing rate ≥1 Hz (i.e., at least 10 spikes for 10 s) and 2.5× the SD of the mean response to AS (Geran and Travers 2009; Nishijo et al. 1991). However, in three cases (WT, n = 1; double-KO, n = 2), we included neurons without responses to any tested stimulus. These neurons were determined to be in the NST, based on histology and/or simultaneously occurring background responses to taste stimuli. We included these cells because it was important to determine whether more neurons in the double-KO mice were unresponsive. The proportions of significant responses for each stimulus were determined across strains and compared using χ2-tests. Response magnitudes across stimuli and strains were evaluated using one-way and two-way ANOVA with repeated measures as appropriate. Because double-KO mice were somewhat older than the WT mice (see below), we used age as a covariate in the main ANOVA analyses as a precaution, but this covariate was never significant. Post hoc comparisons between strains were made were using Fisher’s least significant difference (LSD) tests or, when contrasts were between WT and double-KO strains, paired t tests. Due to the large number of stimuli compared, we were concerned about adopting an overly conservative approach and thus we present only the raw P values from the t tests. Significance was set at P ≤ 0.05 for all comparisons. Errors and error bars are means ± SE.
To identify similarities between response profiles we used hierarchical cluster analysis based on responses to representatives of the five standard taste qualities (the probe stimuli), Pearson’s correlations, and an average amalgamation schedule. Response profiles across an extended array of stimuli (groups 1–3) were visualized using multidimensional scaling. Across-neuron patterns of activity evoked by the various stimuli in WT and double-KO mice were quantified using Pearson’s r. Statistically significant differences in across-neuron correlations between WT and KO mice were made using Z tests. Breadth of tuning across the probe stimuli was quantified as the noise:signal ratio (Spector and Travers 2005) and using the entropy measure (Smith and Travers 1979). Sample sizes for the numbers of neurons and mice for each analysis are summarized in Table 2.
Table 2.
Summary of sample sizes for each analysis
| B6 | S129 | KO1+3 | KO2+3 | Total | Refer | Analyses | |
|---|---|---|---|---|---|---|---|
| Mice | 22 | 24 | 26 | 30 | 102 | Fig. 1 | ANOVAs: mean responses (except U 0.1 and N 0.6) |
| Neurons | 43 | 43 | 41 | 53 | 180 | ||
| Mice | 20 | 23 | 25 | 29 | 97 | Fig. 1 | ANOVA: U 0.1 |
| Neurons | 38 | 41 | 39 | 51 | 169 | ||
| Mice | 21 | 24 | 26 | 30 | 101 | Fig. 1 | ANOVA: N 0.6 |
| Neurons | 38 | 40 | 40 | 52 | 170 | ||
| Mice | 22 | 5 | 26 | 29 | 102 | Fig. 2 | Cluster analysis |
| Neurons | 44 | 44 | 38 | 49 | 175 | ||
| Mice | 22 | 25 | 29 | 30 | 106 | Text | ANOVAs: sex and age |
| Neurons | 47 | 46 | 45 | 56 | 194 | Text | Correlations: age and responses |
| Fig. 3 | MDS: neurons | ||||||
| Fig. 4 | Across-neuron correlations | ||||||
| Fig. 4 | MDS for stimuli (maximum N) | ||||||
| Mice | 9 | 7 | 16 | Fig. 5 | Various analyses for: sugar-responsive double-KO neurons | ||
| Neurons | 10 | 8 | 18 | ||||
| Mice | 22 | 24 | 25 | 29 | 100 | Fig. 6 | ANOVA: MSGa vs. U 0.6 |
| Neurons | 45 | 43 | 41 | 52 | 181 | ||
| Mice | 18 | 19 | 18 | 21 | 76 | Fig. 7 | χ2-test: receptive fields |
| Neurons | 32 | 28 | 28 | 37 | 125 | ||
| Mice | 15 | 16 | 14 | 23 | 68 | Fig. 8 | ANOVA: neuron locations |
| Neurons | 25 | 23 | 18 | 33 | 99 |
Numbers of mice and neurons are listed. KO, knockout; MDS, multidimensional scaling; MSGa, monosodium glutamate with amiloride; U, umami; N, NaCl.
Histology and immunohistochemistry.
Coronal sections of the medulla (40 µM) were sectioned into two series and processed immediately or stored at –20°C in cryoprotectant until processing. One series was stained with black gold for delineating myelinated fibers (~85% of mice; Schmued and Slikker 1999) or with Cresyl violet (Deitch and Moses 1957). Double immunohistochemistry for P2X2 and NeuN was performed on the second series as described in Breza and Travers (2016). Except as noted, all steps were at room temperature and the diluent and rinsing agent was PBS. Sections were rinsed before and after treatment with 1% sodium borohydride and 0.5% H2O2. Nonspecific binding was blocked in a cocktail of 0.3% Triton, 3% bovine serum albumin and 7.5% donkey serum before adding the primary antibodies {anti-P2X2, 1:10,000 and anti-NeuN, 1:1,000 [P2X2: rabbit polyclonal antibody against the intracellular COOH terminus of the P2X2 receptor, Alomone Labs, cat. no. APR003, Research Resource Identifier (RRID): AB_2040054, lots AN-05 and AN- 09; NeuN: mouse monoclonal antibody, Millipore MAB 377, RRID: 2298772, lots 2819579, 2919676, and 3018822]}. After incubation in the primary (48–72 h, 4°C), sections were rinsed then soaked in secondary antibody (biotinylated anti-rabbit IgG, 1:500, Jackson ImmunoResearch Laboratories, Inc., RRID: AB_2337965) in the same blocking solution for 1.5 h and then in an avidin-biotin mixture (Elite Kit, Vector, RRID: ABb_2336819) diluted in 0.1 M PB and 0.1% BSA. The chromagen reaction began in 0.05% 3, 3′-diaminobenzidine-HCl with 0.015% nickel ammonium sulfate, followed by the oxidation stage, initiated by addition of H2O2 to achieve a concentration of 0.0015%. This labeled P2X2 afferents dark brown to black. For labeling cell bodies with NeuN, we repeated the steps commencing with the secondary antibody incubation (biotinylated anti-mouse IgG, Jackson ImmunoResearch Laboratories, Inc., RRID: AB_2338570) but did the chromagen reaction without nickel to produce a light brown reaction product. Tissue sections were examined under dark field and bright-field optics, which facilitated demarcating the rostral (r)NST borders and centers of electrolytic lesions. For each mouse, the caudal and rostral borders of the rNST were determined. Locations of recorded neurons were calculated and plotted on a series of standard sections according to this calculation and/or the appearance of the most closely matching section. With lesions made at the recording site, a symbol was placed in the nucleus. If the lesion was made above or below the nucleus, we drew a line indicating the projected track. To maintain accuracy of reconstruction, tracks were only plotted if a dorsal or ventral lesion was ≤200 µM from the recording site or if there was evidence that the plane of section was parallel to the electrode track. Protocol notes indicating the depth of the neuron below the dorsal border of the NST provided the most reliable information for the z-axis.
RESULTS
Basic characteristics.
A total of 106 mice from four different strains were used to record from 194 NST taste-responsive single units. There were 93 neurons from WT [B6, n = 47 (19 females, 28 males); S129, n = 46 (15 females, 31 males)] and 101 neurons from T1R double-KO mice [KO1+3, n = 45 (19 females, 26 males); KO2+3, n = 56 (30 females, 26 males)]. Collapsed across strains, responses to the probe stimuli did not differ as a function of sex (ANOVA, stimulus, P < 0.0005, sex, P > 0.1, stimulus × sex, P > 0.1), and therefore, we combined male and female mice in subsequent analyses. Ages ranged from 2-17 mo with a similar range for WT (2–17 mo) and double-KO mice (3–17 mo). Nevertheless, due to their limited availability, on average, double-KO mice were 3–4 mo older than WT mice (P < 0.0005). This difference had minimal effects on responsiveness: correlations between age and responses to five standard tastants were low and insignificant, although there was a small negative correlation (r = −0.19) between 0.1 M NaCl and age for the double-KOs that approached significance (P = 0.052).
Responses to sugars and moderate concentrations of umami are profoundly depressed in T1R double-KO mice.
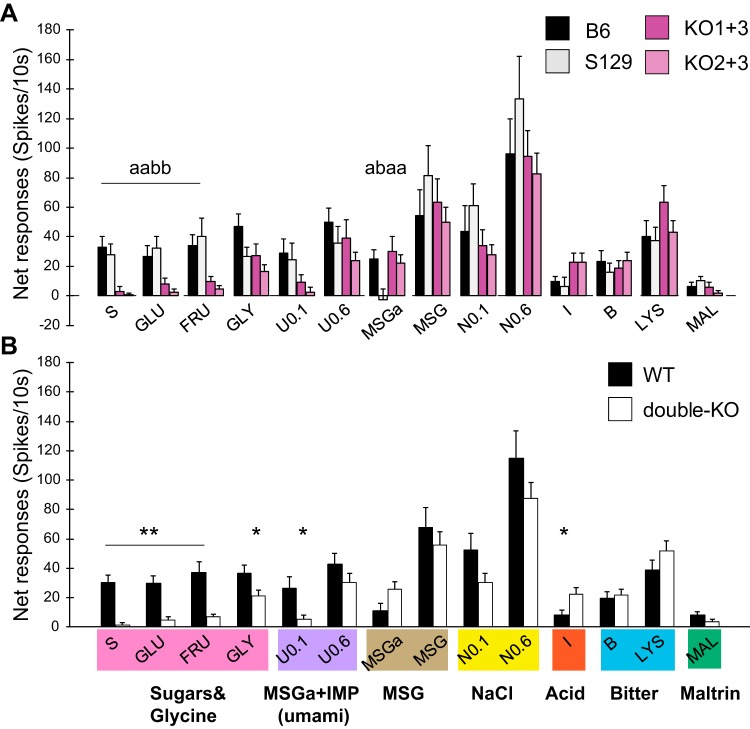
Figure 1A shows mean net responses for each WT and double-KO strain across stimuli. Responses to the sugars and 0.1 M umami were markedly smaller in the double-KO mice. ANOVAs using the four strains yielded no overall effect of strain (P > 0.1) but a significant effect of stimulus (P < 0.0005) and a stimulus × strain interaction (P = 0.001). Subsequent ANOVAs for individual stimuli were significant for sucrose, glucose, and fructose (all Ps < 0.005), and pairwise comparisons showed that responses to all sugars were significantly larger in either WT than either double-KO strain (all Ps ≤ 0.005, Fisher’s LSD). No other stimulus, including amino acids tested at 0.6 M, elicited significantly smaller responses in T1R double-KO mice when assessed across all four strains. Although not even approaching significance, it is worth noting that 0.6 M amino acids produced slightly larger responses in the KO1+3 than in the KO2+3 strain. Thus retention of the T1R1 subunit did not confer increased sensitivity to amino acids. Unexpectedly, the MSGa response was significantly smaller in S129 mice. This appeared due to a predominance of amiloride-sensitive versus insensitive sodium-responsive neurons in this strain. With this single exception, there were no differences between the two WT or double-KO strains, and therefore, to increase power and simplify analysis, we examined mean responses collapsed across WT and double-KO mice (Fig. 1B; ANOVA; strain, n.s.; stimulus, P < 0.0005; stimulus × strain, P < 0.0005). Across WT strains, the mean responses to glucose, fructose, sucrose, and 0.1 M umami were 5–20 times larger than in the double-KO mice (Ps < 0.0005 for sugars; P = 0.01 for umami, unadjusted t tests). The disparity in WT and double-KO responses to glycine was smaller but still significant (P = 0.03). Interestingly, the response to citric acid was larger in the double-KO mice (P = 0.01). Mean responses to Maltrin were small and did not vary significantly as a function of KO status although they were nominally smaller in the double-KO mice.
Fig. 1.
Taste responses of nucleus of the solitary tract neurons from type 1 taste receptor (T1R) double-knockout (double-KO) mice and their wild-type (WT) controls. Groups of stimuli are color coded below the x-axis in B roughly according to qualitative similarity. Note that many stimuli are qualitatively complex. A: means (±SE) net responses (spikes/10 s) across 4 strains: 2 WT (B6, n = 43; S129, n = 43) and 2 T1R-double-KO strains (KO1+3, n = 41; KO2+3, n = 53); separate ANOVAs were performed for 0.1 M umami (U0.1) and 0.1 M NaCl (N) due to 5–6 missing data points. Different letters indicate significant differences between strains for a given stimulus. B: same data set as in A but responses are pooled across WT (black filled bars) and double-KO strains (open bars). **P < 0.0001 and *P < 0.05 between WT and double-KO mice using unadjusted P values. Raw P values are P < 0.000001, 0.0001, and 0.0001 for sucrose (S), glucose (GLU), and fructose (FRU) and would withstand Bonferroni adjustment. Raw P values for 0.1 M umami (U0.1) and citric acid (I) were 0.01 and 0.01, respectively, and would not withstand this correction. B, bitter; MSGa, monosodium glutamate with amiloride; IMP, inosine 5′-monophosphate; GLY, glycine; LYS, lysine; MAL, Maltrin.
Some chemosensitive neuron groups are distinct for WT versus T1R double-KO mice.
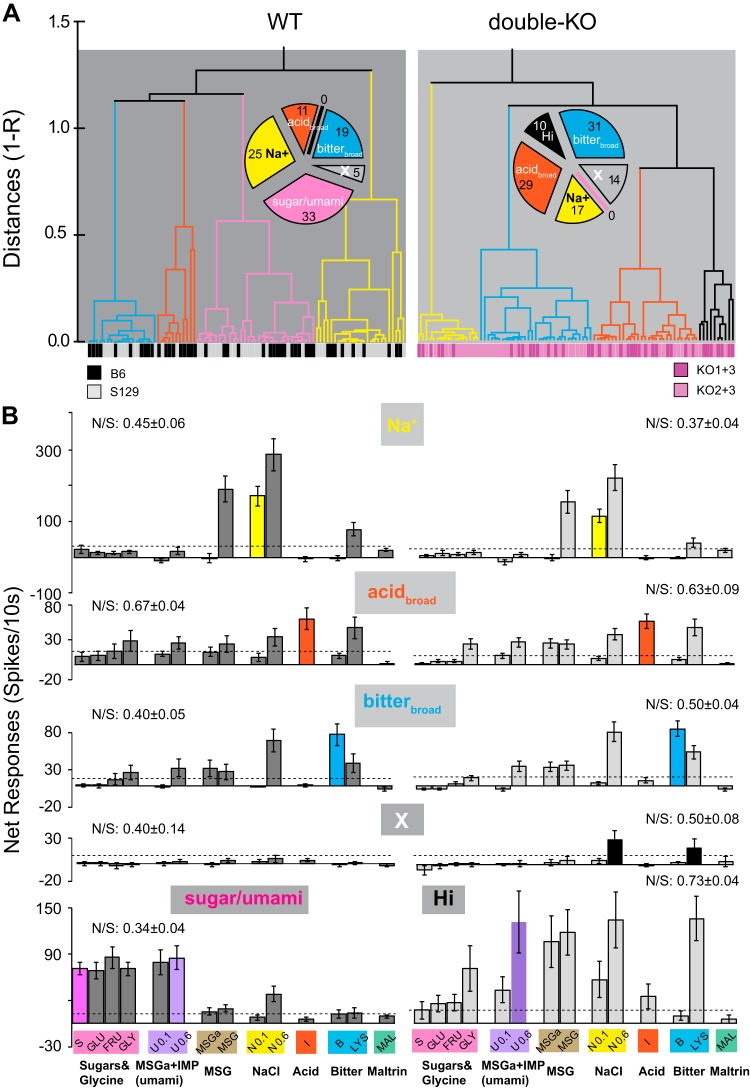
Cluster analysis identified repeating patterns of chemosensitivity in single neurons. We used the probe stimuli to generate clusters because they included representatives of the five standard qualities and were tested on all neurons. Before the analysis, 19 neurons (5/86 WT and 14/94 double-KO) that did not meet the response criterion for at least one probe stimulus were removed and assigned to a separate “X” category. Except in three cases (see methods), these cells responded to other chemicals in the array, often 0.6 M amino acids or 0.6 M NaCl.
For the remaining neurons, cluster analyses identified four groups each for WT and double-KO cells. The mean response profiles of three groups were almost identical for WT and T1R double-KO mice (Fig. 2). Sizable numbers of WT and double-KO neurons were selective for sodium (“Na+”). Notably, MSG responses in Na+ neurons were markedly smaller in the presence of the sodium epithelial channel (ENaC) blocker amiloride. The remaining two clusters were optimally responsive to citric acid (“acidbroad”) or the bitter mixture (“bitterbroad”), respectively. These neurons were narrowly tuned to the probe stimuli, but across the entire stimulus array, each type responded notably to lysine and other 0.6 M stimuli. On average, neither acidbroad, nor bitterbroad neurons were amiloride sensitive. Although response profiles in the Na+, acidbroad, and bitterbroad WT and double-KO groups were similar, their frequency varied. In WT mice, acidbroad neurons were the scarcest and Na+ the most frequent. In the double-KO mice, acidbroad and bitterbroad neurons were overrepresented (χ2-test, P < 0.02).
Fig. 2.
A: cluster trees for wild-type (WT; left) and double-knockout (double-KO; right). The distance metric on the y-axis is 1-Pearson’s r. Color bars at the bottom of the cluster trees specify individual mouse strains (WT: B6 or S129; double-KO: KO1+3 or KO2+3). The pie chart shows numbers of WT or double-KO neurons in each cluster, including neurons not responsive to any probe stimulus. These cells were precategorized as a separate group, “X”. B: mean response profiles across all 12 stimuli for each cluster for WT (left) and double-KO mice (right). Rows 1–4: clusters common to both groups of mice (Na+, acidbroad, bitterbroad, and X). Row 5: responses of the unique clusters (sugar/umami, WT; Hi, double-KO). Dotted horizontal lines in each graph denote the average response criterion. Note that y-axis scales are different for cluster types. The breadth of tuning across the 5 probe stimuli, quantified as the noise:signal (N/S) ratio (Spector and Travers 2005), appears above each graph. We compared the breadth of tuning of the WT and double-KO neurons in the 3 common clusters: Na+ neurons were broader in the WT mice (P < 0.03), but acidbroad and bitterbroad neurons were similar regardless of KO status (P > 0.1 for both). Similar conclusions were apparent with the entropy measure (Smith and Travers 1979). MSGa, monosodium glutamate with amiloride; IMP, inosine 5′-monophosphate.
Two additional groups were uniquely present in the WT or double-KO strains. In WT mice a large cluster, “sugar/umami,” responded optimally and similarly to the three sugars and glycine as well as the umami stimuli. These neurons exhibited relatively narrow tuning. The unique cluster in the double-KO mice (“Hi,” i.e., responsive to high concentrations) responded best and broadly to stimuli at the 0.6 M concentration. This cluster appeared defined based on an optimal response to 0.6 M umami. Importantly, whereas both WT sugar/umami cluster neurons and double-KO Hi neurons responded robustly to 0.6 M umami, WT sugar/umami neurons responded equivalently to 0.1 M umami and the sugars but double-KO Hi neurons responded minimally to these tastants (ANOVA across umami stimuli, glycine, and the sugars; sugar/umami neurons, P > 0.1, n = 33; Hi neurons, P < 0.0005, n = 10). Figure 3 shows a multidimensional scaling (MDS) plot of individual WT and double-KO neurons based on responses to all 12 stimuli and includes the X cells not used in the cluster analysis (n = 194). The MDS space supports the cluster analysis and suggests that incorporating more stimuli does not significantly alter neuron groupings. Moreover, the overlap of the WT and double-KO Na+, acidbroad, and bitterbroad clusters in the space stresses how closely these clusters resemble one another regardless of T1R status. Sugar/umami WT neurons form a coherent separate cluster.
Fig. 3.
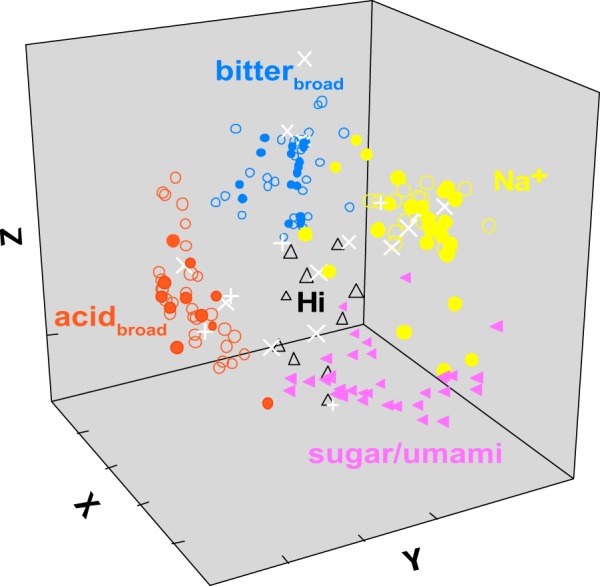
Multidimensional scaling (MDS) of all 194 wild-type (WT) and double-knockout (double-KO) neurons according to their pattern of responsiveness across 12 stimuli. Neurons are color coded to match the clusters identified in Fig. 2. Filled and unfilled shapes denote neurons from WT and double-KO mice, except for the “X” neurons, which are denoted by the white “+” symbol for the WT and “X” for double-KOs. The MDS analysis yielded a solution that agreed well with the cluster analysis. The MDS plot emphasizes the close correspondence between the WT and double-KO neurons in the Na+, bitterbroad, and acidbroad groups and the unique position of the sugar/umami cells. Although not as tightly clustered or segregated, the Hi neurons also occupy a unique location between the other groups. X neurons are dispersed throughout the space.
Although “Hi” double-KO neurons also occupied a defined location in the center of the other groups, they did not group as tightly and “X” neurons were widely dispersed. This suggests that both these groups had more idiosyncratic profiles. Because X neurons did not respond significantly to any probe stimulus and Hi neurons only to 0.6 M umami, both exhibited limited responsiveness to moderate concentrations of standard tastants. Notably, in double-KO mice, X and Hi cells together comprised 24% of the population but only 5% in the WT mice (χ2-test, P < 0.0005).
T1R deletion disrupts taste coding of sugars and umami stimuli.
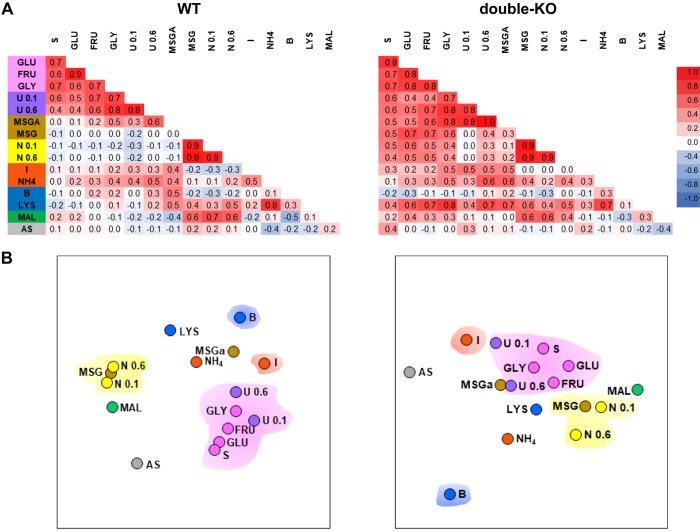
The much smaller responses elicited by the sugar and umami tastants and lack of sugar/umami neurons in T1R mice impaired representation of the sweet and umami qualities as defined by across-neuron patterns of activity. Figure 4 shows across-neuron correlations for WT and double-KO mice and the MDS spaces. Correlations among the sweet (sucrose, glucose, and glycine) and umami stimuli were virtually identical in the WT and double-KO mice (mean correlations = 0.65 ± 0.03 WT and 0.66 ± 0.03 double-KO, P > 0.1, Z test, n = 15), but correlations between sweet/umami and tastants associated with other qualities rose markedly in knockouts (mean correlations = 0.06 ± 0.03 WT and 0.37 ± 0.04 double-KO, P < 0.0005, n = 54, Z tests). For example, 0.1 M NaCl and the three sugars correlated slightly negatively (r = −0.1 for all) in WT mice but positively (r = 0.5–0.6) in the double-KOs. Interestingly, this was not true for every contrast between the sweet/umami and other categories. Notably, the cyloheximide + quinine bitter stimulus correlated poorly with sugars, glycine, and the moderate concentration of umami in both WT (r = −0.1–0.2) and double-KO (r = −0.3–0.1) mice. Nevertheless, the cumulative impact of the changes in across-neuron correlations produced an MDS representation (Fig. 4B) in the double-KOs characterized by less separation of the sweet and umami stimuli from other qualities.
Fig. 4.
A: across-neuron correlations (Pearson’s r) for wild-type (WT; left: B6 and S129) and double-knockout (double-KO) mice (right: KO13 and KO2+3) for all taste stimulus pairs. Individual WT and double-KO strains are combined because the main conclusions are the same as for the individual strains. Magnitudes of the correlations appear as a heat map (scale bar at right). The background color for the stimulus labels at left denote tastants with a common quality (some stimuli are complex, so this is an oversimplified categorization). Note that 2 additional stimuli, ammonium chloride (NH4) and artificial saliva (AS), were included in this analysis. B: 2-dimensional MDS plots (left: WT; right: double-KO) summarizing the relationships between stimuli as defined by the across-neuron correlations. Four color clouds encompass stimuli characterized as sweet or umami (pink), salty (yellow), sour (orange), or bitter (blue). In comparison with WT mice, the sweet/umami stimulus group in the double-KOs was poorly segregated from other qualities, especially salty and sour. Maltrin lies close to the salty stimuli in both groups of mice. MSGa, monosodium glutamate with amiloride; IMP, inosine 5′-monophosphate; GLY, glycine; S, sucrose; GLU, glucose; FRU, fructose; I, citric acid; B, bitter; U, umami; N, NaCl; LYS, lysine; MAL, Maltrin.
T1R-independent responses to sugars.
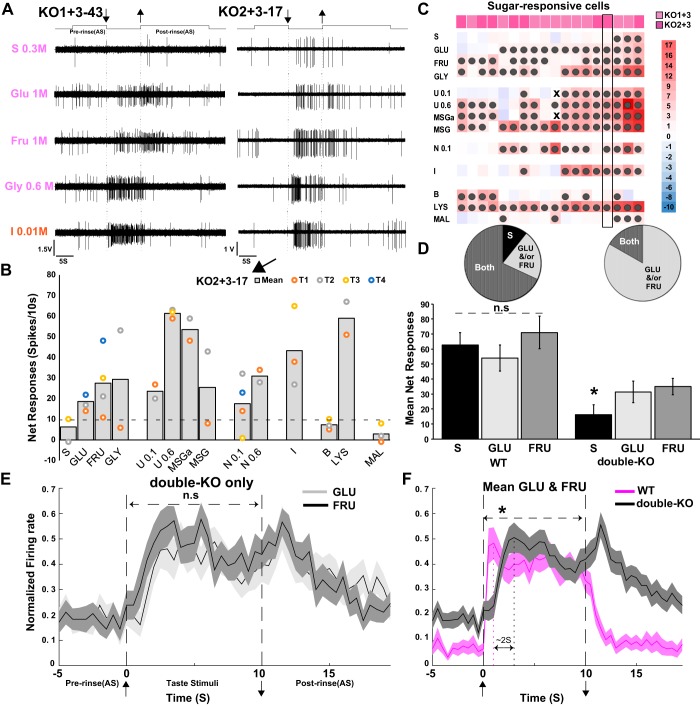
As described above, T1R deletion strongly reduced mean sugar responses. Nevertheless, sugar-elicited responses occurred in both KO1+3 (n = 10 neurons) and KO2+3 (n = 8 neurons) mice, although they were markedly less frequent and smaller than in WT controls. Overall, 18% (n = 18) of neurons from the double-KOs responded to at least one sugar, in comparison to 51% (n = 47) of the WT neurons (P < 0.0005, χ2-test).
Figure 5, A and B, shows examples of T1R-independent responses from each double-KO strain. These T1R-independent sugar responses comprised modest, often perithreshold responses in neurons optimally responsive to other taste qualities (Fig. 5C). Nevertheless, these responses were reliable. Whenever possible stimuli were repeated and when the mean response to a given sugar met the criterion, a majority of individual trials met criterion (2/2 sucrose, 10/13 glucose and 7/12 fructose responses). Interestingly, in double-KO mice, the monosaccharides glucose and fructose appeared more efficacious than sucrose (Fig. 5D). Thus among sugar-responsive neurons, only 17% of double-KO neurons responded to sucrose compared with 79% of WT neurons (χ2-test, P < 0.0005) whereas similar proportions of WT and double-KO neurons responded to glucose (70 vs. 78%, P > 0.1) or fructose (85 vs. 79%, P > 0.1). In addition, among sugar-responsive neurons, the mean response magnitude across the three sugars was comparable in WT neurons (ANOVA, P > 0.1, n = 47) but larger for the monosaccharides in double-KOs (ANOVA, P < 0.0005, post hoc t tests: glucose > sucrose, P = 0.001; fructose > sucrose, P < 0.0005, n = 18). Because the incidence and magnitude of glucose and fructose responses was similar in double-KO mice, but behavioral data suggest that double-KOs can discriminate them, we compared the mean time course of the responses to these two monosaccharides but they were very similar in the KOs (Fig. 5E) and WT mice (not shown). However, a comparison of double-KO and WT monosaccharide responses revealed that the double-KO responses were more sluggish, with both the onset and offset lagging those from WT mice by ~2 s (Fig. 5F, ANOVA, stimulus × strain, P > 0.1; strain × time, P < 0.0005, n = 65).
Fig. 5.
A: examples of type 1 taste receptor (T1R)-independent responses to sugars and glycine from double-knockout (double-KO) mice (left: KO1+3; right: KO2+3). Responses to citric acid are shown for comparison. Upward deflections of the line above the traces indicate artificial saliva flow. Arrows denote stimulus onset and offset. For both neurons, responses to monosaccharides are larger than to sucrose. B: mean net responses from the KO2+3 neuron shown in A (right) for the entire stimulus array. Individual trials are colored dots. Responses to glucose and fructose were much smaller than those to 0.6 M umami, monosodium glutamate with amiloride, and lysine. C: heat map of responses from all double-KO neurons that exhibited significant responses to at least 1 sugar (KO1+3, n = 10; KO2+3, n = 8). Scale at right shows response magnitudes as a multiple of the response criterion (a response that just meets criterion = 1). Dark-filled circles = significant responses. Small x's = missing data. The representative KO2+3 cell shown in A and B is highlighted with a black rectangular box. Although each of these neurons was sugar responsive, invariably, another quality was more effective. D: proportions and average net responses to the sugars compared between WT and double-KO mice only for cells responsive to at least 1 sugar (WT, n = 47; double-KO, n = 18). Among WT cells that were sugar responsive, sucrose and the monosaccharides activated mostly the same neurons (“Both” slice of the pie charts) and evoked responses of similar magnitudes. In contrast, in double-KO neurons that were sugar-responsive, glucose and fructose activated more cells than did sucrose and elicited larger mean responses. (*ANOVA, P < 0.0005, fructose vs. sucrose, P = 0.001; glucose vs. sucrose, P < 0.0005; fructose vs. glucose, P > 0.1). E: glucose and fructose responses in the double-KO mice only, averaged over successive 500 ms for the 18 sugar-responsive cells normalized to the peak 500-ms bin. The time courses of the responses to the 2 monosaccharides are similar. F: normalized time courses averaged across glucose and fructose for double-KO versus WT mice. Responses in the double-KO mice are more sluggish (*ANOVA: strain × time, P < 0.0005). MSGa, monosodium glutamate with amiloride; IMP, inosine 5′-monophosphate; GLY, glycine; S, sucrose; GLU, glucose; FRU, fructose; I, citric acid; B, bitter; U, umami; N, NaCl; n.s, not significant; LYS, lysine; MAL, maltrin.
MSGa vs. MSGa + IMP (umami).
The magnitudes of the mean responses elicited by 0.6 M umami were not different in WT and double-KO mice (see Fig. 1), but T1R KO strongly affected IMP enhancement of the MSGa response. In WT mice, 0.6 M umami elicited a mean response 3.9 times as great as MSGa (P < 0.0005). In KO mice, the response to umami was 1.2 times larger, slightly, albeit significantly different from MSGa (P = 0.02). Figure 6A shows responses of individual neurons to MSGa and umami. The much more striking enhancement of MSGa responses by IMP in the WT neurons is readily apparent but subtle enhancement also occurred in the double-KOs. Figure 6B quantifies the mean difference between pairs of responses for individual neurons. Across all WT cells, on average, umami evoked 32.8 ± 4.7 spikes/10 s more than did MSGa, compared with just 4.5 ± 0.9 spikes/10s in the double-KOs (P < 0.0005). The IMP-induced increase in the MSGa response varied by cluster.
Fig. 6.
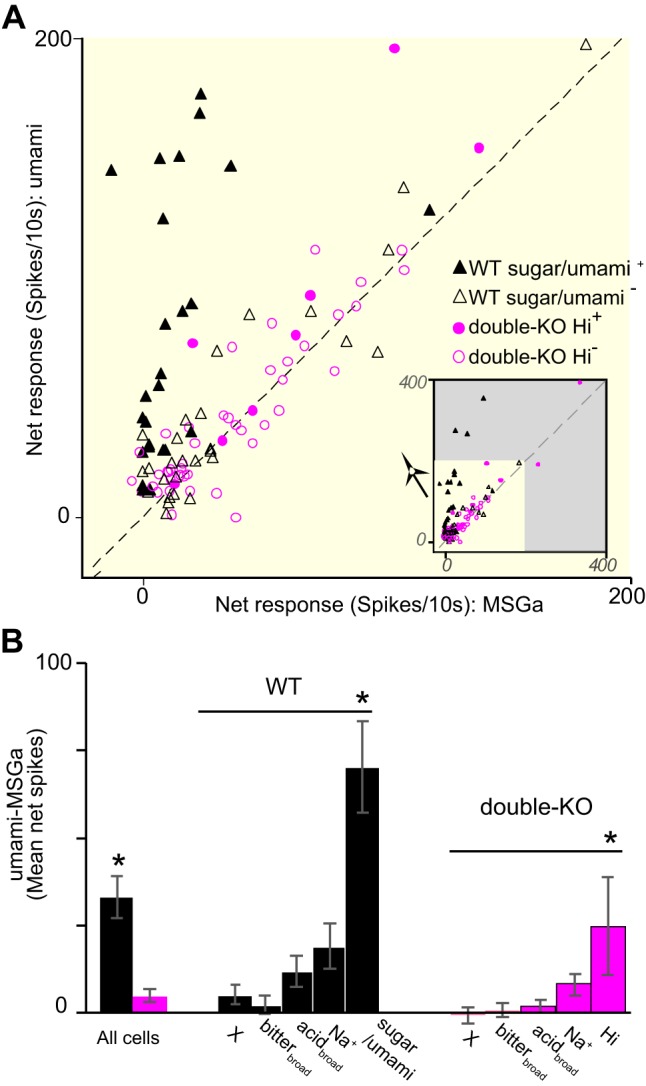
A: responses to monosodium glutamate with amiloride (MSGa) and MSGa + inosine 5′-monophosphate (IMP) (umami) for individual neurons. For clarity, only neurons responding significantly to at least 1 of these stimuli are included. The large plot magnifies the inset that shows all responses. The dotted line indicates equivalent responses for MSGa and umami. Wild-type (WT) responses to MSGa (black triangles) often showed a large enhancement with IMP, especially the sugar/umami neurons (filled triangles). Enhancement of double-knockout (double-KO) responses (pink circles) was very subtle though frequent for the smallest responses and more pronounced for a handful of cells, including a few from the Hi cluster (filled circles). B: mean difference between responses to umami and MSGa for all WT and double-KO neurons and for different clusters. *All cells, WT vs. double-KO, P < 0.0005, *WT, sugar/umami vs. other clusters, all P's < 0.03; *double-KO, *Hi vs. other clusters, all P's < 0.02).
As observed in previous peripheral and central studies (Ninomiya 1992; Tokita et al. 2012; Yasumatsu et al. 2012), the mean difference was much larger for sugar/umami WT neurons (filled triangles in Fig. 6A) than other WT clusters (ANOVA, P < 0.0005; post hoc Fisher’s LSD, all Ps < 0.03). In double-KO cells, an apparent effect of cluster was also observed, with responses in Hi cluster neurons (filled circles in Fig. 6A) showing the largest enhancement (ANOVA, P = 0.004, Fisher’s LSD, all Ps < 0.02). However, the degree of augmentation for Hi cluster neurons was variable and these cells were relatively few in number.
Maltrin responses are small and unaffected by T1R double-KO.
Maltrin 580 (Maltrin) is a maltodextrin composed of glucose oligomers with a nominal molecular weight of 1,000 and an average glucose chain length of ~6. This and similar stimuli, for example, Polycose, taste bland (though detectable) to humans (Lapis et al. 2014) but produce strong preferences and hedonic responsiveness in rodents (Sclafani and Clyne 1987). Such motivated behavior as well as taste detection thresholds are relatively unaltered by T1R deletion (Smith and Spector 2017; Treesukosol et al. 2009, 2011a; Treesukosol and Spector 2012; Zukerman et al. 2009). Consistent with the lack of a behavioral contribution of T1Rs, the incidence of Maltrin responses did not differ between double-KO and WT mice (9 vs. 13%, P > 0.1 χ2-test) and the average response magnitude was only marginally greater in the WTs (WT: 8.1 ± 1.8 and double-KO: 3.7 ± 1.7, P = 0.07). Despite this concurrence with regard to T1R involvement, the low incidence and weak responses to Maltrin (see Fig. 1) were at odds with the behavioral efficacy of this tastant. Maltrin was never an optimal stimulus for a neuron in either WT or double-KO mice. Instead, responses occurred as small “sidebands” in Na+ and sugar/umami clusters in the WT mice and the Na+ and Hi clusters in double-KO mice. Across-neuron correlations were higher between NaCl and Maltrin (r = 0.6 in double-KO and 0.7 in WT) than sucrose and Maltrin (r = 0.2 in double-KO and 0.4 in WT, see Fig. 4), indicative of a stronger relationship to sodium sensitivity.
Receptive fields and locations of taste neurons in T1R double-KO mice are similar to WT.
Figure 7 classifies receptive fields for neurons. In each mouse strain, we recorded from neurons with diverse receptive fields. For approximately one-half of the neurons, the receptive field was assessed precisely enough to determine whether the cell received input from a particular nerve branch. Cells with various combinations of inputs from the chorda tympani (CT), greater superficial (GSP), and glossopharyngeal (GL) were sampled from WT and double-KO mice. Across neurons that could be characterized for a given nerve, 57% responded to the anterior tongue, 54% to the nasoincisor ducts or soft palate, and 42% to the foliate and/or circumvallate papillae and thus received input from the CT, GSP, and GL, respectively (many received inputs from multiple nerves). Figure 7B shows that similar proportions of WT and double-KO neurons responded to the palate or posterior tongue although a higher proportion of double-KO (73%) than WT (42%) neurons were anterior tongue/CT-responsive (χ2-test, P = 0.001). In 64% of the cells, the receptive field could be characterized as being located in the anterior mouth, posterior mouth, or both regions, providing an opportunity to assess convergence (n = 125, Fig. 7C). These proportions were not significantly different for WT and double-KO mice (χ2-test, P > 0.1). There was likewise no difference for KO1+3 versus KO2+3 mice (χ2-test, P = 0.1). However, a greater fraction of neurons with anterior receptive fields were recorded from the S129 strain, whereas B6 neurons with posterior receptive fields were more dominant (χ2-test, P = 0.019).
Fig. 7.
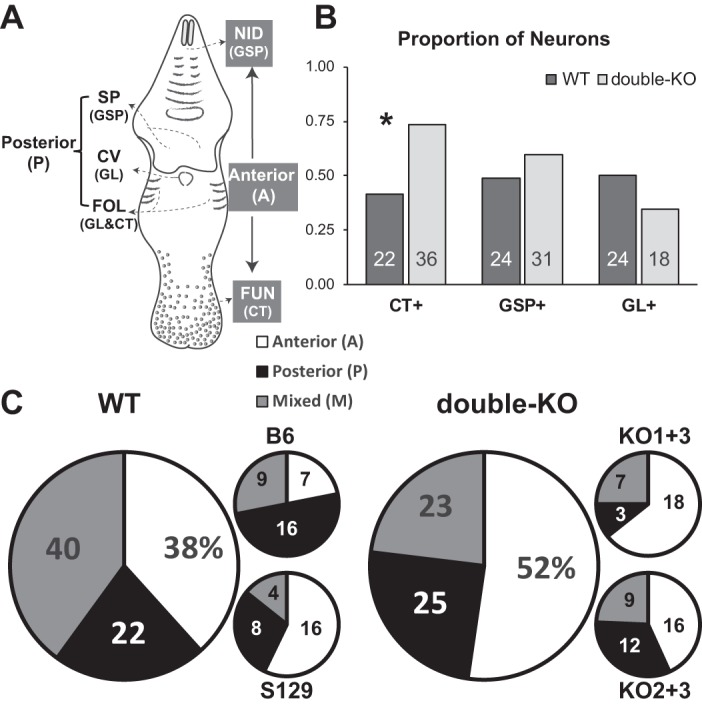
Receptive fields of recorded neurons. A: schematic of the mouse oral cavity depicting locations of the different taste receptor subpopulations and their innervation. Lingual taste buds include the fungiform papilla on the anterior tongue (FUN) innervated by the chorda tympani (CT) branch of the 7th nerve, and foliate (FOL), and circumvallate (CV) papillae on the posterior tongue, mostly innervated by the glossopharyngeal nerve (GL; the CT also innervates a few foliate taste buds). Taste buds occur in 2 regions of the palate, anteriorly in the nasoincisor ducts (NID) and posteriorly on the soft palate (SP); both palatal fields are innervated by the greater superficial petrosal (GSP) branch of the 7th nerve. B and C: proportions and numbers of neurons with receptive fields classified by innervation or oral cavity location (receptive fields were only determined for a subset of the recorded neurons). There was a higher proportion of double-KO neurons with CT inputs, as shown by *. B: the proportions (y-axis) and numbers (shown in bars) of neurons that could be classified as receiving or not receiving inputs from a given nerve. Note that proportions do not add up to “1.” C: pie charts showing the distribution of neurons with receptive fields in the anterior mouth (A) (FUN and/or NID), posterior (P) (FOL, SP, and/or CV), and mixed (M) (both A and P). Large pie charts represent pooled data for wild-type (WT) and double-knockouts (KOs) with percentages indicated. The smaller graphs break down the distribution for the individual strains, with numbers indicating the sample sizes.
Figure 8A shows examples of WT and double-KO neurons with lesions located at sites of recorded cells. Locations for 99/194 neurons for which recording sites could be reconstructed are summarized in Fig. 8B. Both WT and double-KO neurons were recorded from the caudal and rostral halves of the rNST. There was no difference in the mean locations of WT versus double-KO neurons quantified as a percentage of the distance from the posterior to the anterior limit of rNST (WT = 64 ± 3%; double-KO = 67 ± 2%, P > 0.1). Likewise, locations of WT and double-KO neurons did not vary as a function of depth (WT = 33 + 5 µm; double-KO = 41 + 5 µm, P > 0.1). Neurons with purely PO receptive fields were further posterior than those with mixed or AO receptive fields, regardless of KO status (ANOVA; RF, P = 0.003; KO status, P = 0.04; location × RF, P > 0.1). Similarly, when cluster types common to WT and double-KO neurons were considered, Na+ neurons were significantly further rostral, regardless of KO status (ANOVA; cluster, P = 0.001; KO status, P = 0.02; location × RF, P > 0.1).
Fig. 8.
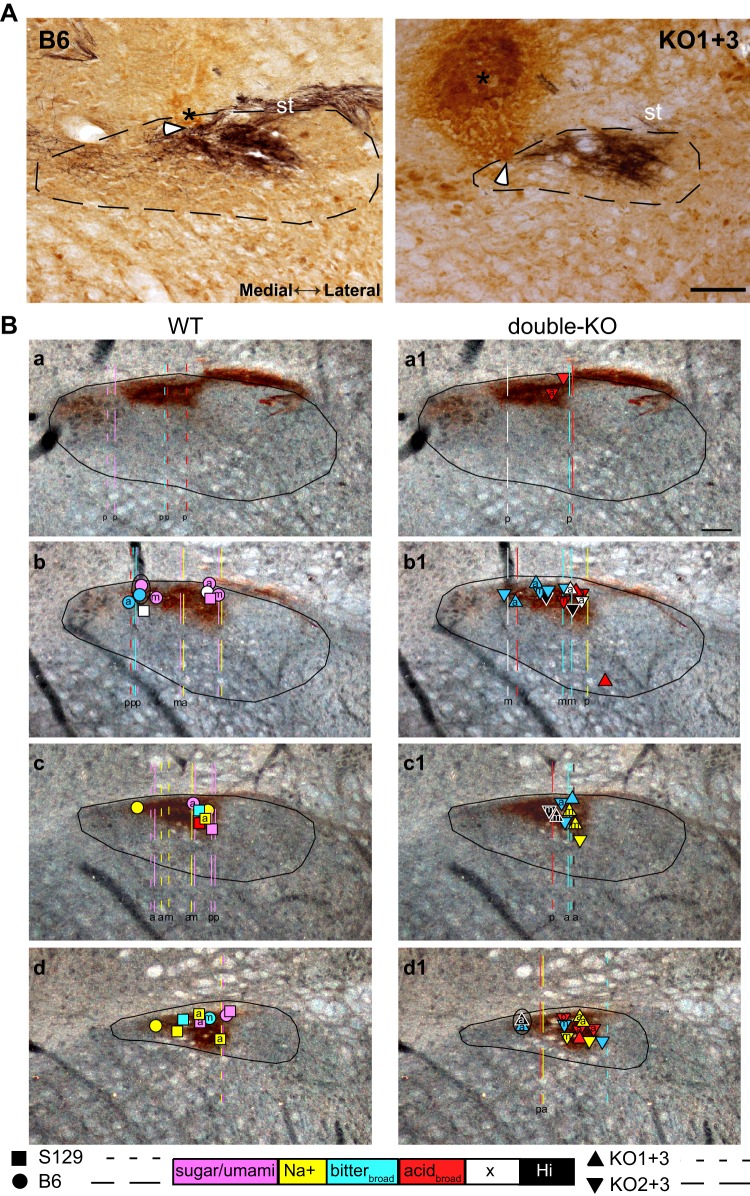
A: bright-field photomicrographs of P2X2 (black)/NeuN (brown)-stained sections showing examples of wild-type (WT; B6) and double-knockout (double-KO; KO1+3) cases with lesions made at the recording sites. In the P2X2/NeuN-stained tissue, lesions were apparent on the basis of brown nonspecific immunostaining, presumably due to the anti-mouse secondary antibody for NeuN. Note that, in these cases, cells were recorded within 15 µM deep to the electrophysiologically encountered dorsal border of the nucleus, as evident by the first appearance of gustatory activity. Thus the base of the lesions (white arrowheads), not the lesion centers (asterisks), must correspond with the recording sites. Two cells were recorded simultaneously in both instances. The section from the B6 recordings is further caudal. Dotted lines denote the approximate borders of the nucleus; st, solitary tract. Scale = 100 µm. B: darkfield photomicrographs of coronal sections of the rostral nucleus of the solitary tract (rNST) immunostained for P2X2 with superimposed symbols and lines indicating locations of the recorded neurons. Sections are arranged from caudal to rostral (a,a1–d,d1). P2X2 staining, indicating the location of gustatory afferents, which appear dark brown. Left: WT neurons. Right: type 1 taste receptor (T1R) double-KO neurons. Symbols depict cases where lesions were made at the site of recording; dashed lines show the estimated trajectory of tracks through the nucleus in cases where lesions were made dorsal or ventral to the nucleus. Symbols and tracks are color-coded based on chemosensitive neuron clusters (see Fig. 2). The letters inside the symbols or at the base of the tracks denote receptive fields [anterior (a), posterior (p), or mixed (m)]. Cells were usually inside or just outside the P2X2 field. WT and double-KO neurons were recorded in similar locations. Neurons with posterior receptive fields were significantly further posterior; Na+ neurons were anterior. Scale = 100 µm. Lateral is to the right and medial is to the left in A and B.
DISCUSSION
The present investigation revealed a major suppression of central responses to sugars and synergistic responses to MSGa + IMP in mice lacking T1R1+T1R2 or T1R2+T1R3, supporting previous evidence that the T1R family of receptors plays a crucial role in transducing taste stimuli referred to as sweet and savory (i.e., umami) by humans (Nelson et al. 2001, 2002). Effects were similar for the two strains of double-KO mice, stressing the importance of the T1R3 protein, a common subunit in both receptors, for conveying information about these taste qualities. Nevertheless, with both subunits of either the “sweet” or “umami” receptors ablated, a degree of responsiveness to sugars and amino acids remained, suggesting that other receptors also can convey limited information about these tastants.
Responsiveness to sugars.
Sucrose, glucose, and fructose each elicited robust responses in B6 and S129 WT mice. Response magnitudes decreased drastically in both double-KO strains. Thus no advantage was conferred in KO1+3 mice who retained T1R2, making it unlikely that this subunit acts as a low-affinity sugar receptor in vivo (Zhao et al. 2003). That both strains had similarly diminished sugar responses emphasizes the critical role of T1R3. Although most taste cells with T1Rs express either the T1R1+T1R3 or the T1R2+T1R3 heterodimer, some express T1R3 alone (Nelson et al. 2001) and heterologous expression of this subunit demonstrates its sufficiency for responding to high concentrations of sugars in vitro (Zhao et al. 2003). Behavioral tests in knockout mice lacking one subunit of the T1R2+T1R3 heterodimer reveal a very small but potential advantage in discriminating sugars from water for mice retaining T1R3 compared with those retaining T1R2 (Treesukosol and Spector 2012). It would be informative to know what central sugar responses remain in mice that retain just T1R3.
Despite the attenuation in sugar-elicited neural activity in T1R double-KOs, some responsiveness persisted. Interestingly, residual sugar responses were more frequent and robust for the monosaccharides than sucrose. In agreement with this finding, previous reports have observed less of an impact of T1R3 KO on CT responses to glucose relative to sucrose (Damak et al. 2003; Ohkuri et al. 2009). This differential susceptibility was also observed for inhibition of sweet-elicited responses by gurmarin (Ohkuri et al. 2009) and following genetic deletion of gustducin (Danilova et al. 2006) or TRPM5 (Damak et al. 2006), downstream signaling pathways for T1Rs (and T2Rs) (Zhang et al. 2003). Because taste cells express α-glucosidase enzymes, including sucrase-isomaltase, which hydrolyzes sucrose (Sukumaran et al. 2016), one might expect comparable responses to sucrose and the monosaccharides, at least in cases where equivalent concentrations are tested. However, in the present study, even when sucrose and the monosaccharides were tested at 0.3 M, sucrose usually elicited smaller responses in the double-KOs, suggesting that sucrose hydrolysis was not complete. In any case, the persistence of monosaccharide responses implies a T1R-independent mechanism. Previous studies suggest that the glucose transporters (GLUTs) in combination with KATP channels may be involved (Glendinning et al. 2017; Yee et al. 2011). Several GLUTs, SGLT1 and both components of the KATP channel, SUR1 and Kir6.1, are expressed, at least in posterior tongue taste buds. Indeed, immunohistochemical staining in T1R3-GFP mice demonstrated preferential expression of SGLT1, GLUT4, and SUR1 in T1R3-expressing taste bud cells, leading the authors to suggest that these transporters and channels comprise a second glucose-sensing pathway in cells that express the canonical “sweet” taste receptor. However, the specificity of these sensing mechanisms for glucose leaves open the question of how fructose is being transduced in the double-KOs. Clearly, more investigation into potential alternative sugar sensing mechanisms is warranted.
Despite observing a modest number of sugar responses in both T1R double-KO strains, in contrast to either WT strain, there was a striking lack of KO neurons that responded optimally to sugars. Rather, T1R-independent sugar-evoked activity occurred exclusively as “sideband” responses in neurons activated more robustly by other qualities. The loss of the sugar/umami cluster neurons gave rise to a markedly less distinct across-neuron pattern of activity generated by sugars (and umami) versus other qualities. Similarly, Yasumatsu et al. (2012) reported a lack of “sucrose/umami best” CT neurons in T1R3 KO mice. However, in that study some cells still responded optimally (although at a low rate) to sucrose and not umami. The lack of sugar/umami neurons in the present study is in agreement with Lemon and Margolskee (2009), who recorded from single NST neurons in T1R3 KO mice and likewise observed a dearth of cells optimally responsive to stimuli considered sweet and a less distinct representation of this quality in a multidimensional scaling space. We hypothesize that the absence of sweet/umami-best neurons, coupled with the low magnitude of the remaining sugar-evoked responses, implies that these T1R-independent responses would produce a weak sensation with a mixed and/or ill-defined quality.
Can such responses explain the residual behavioral capacities of T1R KO mice? In the case of the oral detection of the presence or absence of the stimulus at high concentrations as measured in operant conditioning-based discrimination tasks, it would seem plausible (Treesukosol and Spector 2012). Moreover, it might also explain why concentration-dependent licking to sugars can emerge in T1R KO mice after repeated brief-access testing seemingly as a result of the pairing of a potential orosensory cue with the reinforcing postingestive consequences of an entire session of licking (Treesukosol et al. 2009, 2011a, 2011b). The same logic applies to longer-term two-bottle preference tests (Damak et al. 2003; Nelson et al. 2001; Treesukosol et al. 2009; Treesukosol and Spector 2012; Zhao et al. 2003; Zukerman et al. 2009). Importantly, none of these behavioral abilities requires a qualitative discrimination per se; in other words, it is not necessary for the stimulus to taste “sweet,” only that the stimulus produce some discriminative cue that can be associated with reward. It seems possible that the residual sugar responses that we observed meet this criterion.
Other instances of preserved behavioral function are more difficult to explain. Recent studies show that, with extensive exposure to glucose and fructose under conditions that permit association of each stimulus with their postingestive consequences, both rats (Schier and Spector 2016) and mice (Schier et al. 2019) will display heightened licking of glucose relative to fructose in a brief access test. This hedonic sugar discrimination survives deletion of the T1R2+T1R3 heterodimer. Likewise, the cephalic-phase insulin response is triggered by contact of tastants with oral chemosensory receptors, appears specific for glucose, and is not dependent on the presence of T1R3 (Glendinning et al. 2017, 2018; Grill et al. 1984). Thus, together with the observation that sugars did not preferentially activate any NST neurons, the fact that glucose and fructose were equally effective, and even showed a similar time course, is at odds with the stimulus-specific nature of these T1R-independent learned and innate capabilities.
Responsiveness to amino acids.
Most of the amino acid stimuli we used were at a concentration of 0.6 M. Except for MSGa without IMP, both WT and T1R KO mice can discriminate these tastants from water, although some impairment for glycine is obvious (Blonde et al. 2018; Smith and Spector 2014). At this relatively strong intensity, MSGa with and without IMP, glycine, and lysine, each elicited responses in WT and T1R double-KO mice (Fig. 1). Response profiles for the different clusters provide clues to the adequate stimulus driving these responses (Fig. 2). Two 0.6 M amino acids, glycine and umami, preferentially activated sugar/umami cluster neurons in WT mice. In the presence of IMP, MSGa elicited responses in sugar/umami cells that rivaled those evoked by sugars, whereas MSG and MSGa elicited perithreshold responses. Glycine was similarly efficacious.
In addition to these selective effects in WT sugar/umami neurons, each 0.6 M amino acid activated neurons in other clusters. Lysine elicited significant responses in the WT and double-KO Na+, bitterbroad, and acidbroad clusters, with responses in acidbroad neurons nearly as large as citric acid. Likewise, although 0.6 M MSG with amiloride, with or without IMP, failed to drive Na+ cluster neurons, it elicited at or above-criterion activity in the acidbroad and bitterbroad groups, as did glycine. Moreover, each of these stimuli gave rise to robust responses in the unique double-KO cluster that we named “Hi” precisely because these cells responded robustly to all the 0.6 M stimuli. The observation that the 0.6 M amino acids activated multiple clusters implies that much of the evoked activity arose from receptors tuned to chemical properties other than those associated with the canonical chemical structure of amino acids. For example, the relatively low pH of lysine HCl (~5.8–6) probably contributed. Indeed, amino acids are qualitatively complex, eliciting multiple qualities in humans and generalizing poorly to any one taste quality in rats (Pritchard and Scott 1982; Schiffman et al. 1981).
Although 0.6 M amino acids did not exert very selective effects in T1R double-KO mice in the present study, previous data suggest the specific metabotropic receptors mGluR1 (San Gabriel et al. 2005, 2009) and/or mGluR4 (Chaudhari and Roper 1998; Chaudhari et al. 1996; Yasumatsu et al. 2012) play a role in transducing glutamate taste in rodents. It is possible that a component of the residual glutamate responses that we observed arose from mGluRs, but their characteristics were not consistent with this hypothesis. Thus amino acid responses in the double-KO mice did not arise preferentially from the posterior tongue, where mGluR receptors have been best characterized (Chaudhari et al. 1996), nor resemble the MPG-selective CT fibers observed by Yasumatsu and colleagues, which were preserved in T1R3 KO mice and suppressed by mGluR antagonists (Yasumatsu et al. 2012).
Although the present findings revealed minimal effects on average responses to 0.6 M amino acids, there were major consequences of T1R deletion on umami responses. In T1R KO mice, the mean response to 0.1 M MSGa + IMP was greatly suppressed as was umami synergism, and consequently, neurons with robust, selective responses to umami (and sugar) stimuli were lacking. These neural effects are consistent with the depression of concentration-dependent licking in brief-access tests and reduced capacity of T1R KO mice to discriminate umami tastants from water. At the same time, the retention of some umami responsiveness is consistent with the partial behavioral competence of T1R KO mice (Blonde and Spector 2017; Blonde et al. 2018; Smith and Spector 2014).
Despite this general correspondence between neural and behavioral amino acid responsiveness, puzzling aspects remain. Thus, when amiloride is used to suppress ENaC-dependent responses to the sodium component of MSG, neither WT nor T1R KO mice can discriminate MSG from water but when IMP is added, both can discriminate this stimulus, though the KOs require higher MSG intensities (Blonde et al. 2018; Smith and Spector 2014). This implies that some enhancement of the MSGa response by IMP occurs in T1R KO as well as WT mice. Consistent with previous peripheral (Nelson et al. 2002; Niki et al. 2011; Yasumatsu et al. 2012) and central (Nishijo et al. 1991; Tokita and Boughter 2012; Tokita et al. 2012) studies, we observed a nearly fourfold enhancement of the MSG response by IMP in WT neurons, mostly in WT sugar/umami cluster cells. Although there was also a statistically significant enhancement in the T1R double-KO mice, it was amounted to just a 20% increase. Whether this small augmentation can account for the behavioral enhancement of MSG discrimination by IMP in the T1R KO mice is uncertain. Ongoing studies in the laboratory are extending this finding over a larger range of behaviorally relevant concentrations to address this question. Similar to our results, Yasumatsu et al. (2012) observed that some enhancement of the MPG response by IMP remained in CT neurons in T1R3 KO mice but it occurred exclusively in neurons with selective responses to MPG. The neurons in which we observed this enhancement did not exhibit selective responses to glutamate.
Responsiveness to maltodetxtrin (what gives?).
Over three decades ago, Nissenbaum and Sclafani (1987) and Sclafani (2004) published an intriguing series of experiments demonstrating that rodents display great avidity for maltodextrin solutions and that the sensation produced by such compounds is distinct from that evoked by sucrose. The underlying basis for the behavioral potency and distinctiveness of such compounds, however, remains a mystery. The present findings demonstrated little to no effect of T1R1+T1R3 or T1R2+T1R3 KO on responses to Maltrin, a prototypical maltodextrin stimulus, in agreement with the relative lack of T1R gene deletion on behavioral (Treesukosol et al. 2009, 2011a; Zukerman et al. 2009) or CT responses to this or the similar compound, Polycose (Zukerman et al. 2009). These results are consistent with most single-unit studies employing polysaccharides, which show poor correlations between responses to tastants considered sweet and polysaccharides in both the CT nerve and NST of anesthetized rodents (Giza et al. 1991; Rehnberg et al. 1996; Travers and Norgren 1991). In the NST of the awake rat, however, Nakamura and Norgren (1993) demonstrated a high across-neuron correlation between Polycose and sugars, perhaps due to salivary amylase digestion to shorter-chain glucose polymers in the alert state (Rehnberg et al. 1996). Nevertheless, even in the awake rat neurophysiological responses to Polycose were small (Nakamura and Norgren 1993). In the present experiment, Maltrin responses exhibited the highest correlations with NaCl responses. These results concur with earlier experiments in rat, which demonstrated a closer relationship between Polycose and electrolytes than Polycose and sucrose (Giza et al. 1991; Travers and Norgren 1991). Interestingly, Rehnberg et al. (1996) demonstrated that amiloride suppressed hamster CT responses to Polycose, confirming that this stimulus can exert some effect via ENaC channels in anterior tongue taste buds, presumably due to trace amounts of sodium. Similar amounts of trace sodium are present in Maltrin (~2.4 mM for a 16% concentration of Maltrin; Maltrin QD 580 Product Sheet, Grain Processing Corporation). In sum, while succeeding in defining certain boundary conditions for maltodextrin responses, neurophysiological studies have so far failed to provide a compelling explanation for the behavioral potency and specificity of this class of compounds.
Consequences of T1R gene deletion on the organization of neural responses in the NST.
The knockout models we studied present an opportunity to assess the possibility of plasticity in the first-order gustatory relay resulting from long-term reduced afferent activity. Most sensory systems exhibit changes in central organization when deprived of such activity, especially when deprivation takes place during early life (Hubel and Wiesel 1962; Orczyk and Garraghty 2015). In the gustatory system, such alterations have been studied after dietary Na+ deprivation and following genetic deletion of functional ENaC channels in taste buds. These manipulations produce anatomical changes suggestive of central reorganization. Terminal fields of the primary afferent gustatory nerves are denser and fill a larger area of the nucleus, and there is more overlap between the CT, GSP, and GL (Skyberg et al. 2017; Sun et al. 2017). In the case of reduced signals arising from T1R receptors in KO1+3 and KO2+3 mice, however, gustatory responses occupied a similar region of rNST as in WT mice. Moreover, the proportions of cells receiving inputs from taste bud subpopulations in the anterior, posterior, or both regions of the mouth were similar, suggesting no increase in convergence. Likewise, assessing the breadth of chemosensitive tuning provides little evidence for increases in convergence in the neuron groups common to WT and double-KO mice. Indeed, Na+ neurons were slightly more broadly tuned in the WT mice, and the breadth of tuning of the acidbroad and bitterbroad groups in WT and double-KO mice was virtually identical. The apparent lack of large-scale changes in topography and convergence could suggest that, unlike the case with deprived sodium signaling (Skyberg et al. 2017; Sun et al. 2017), central reorganization does not occur after the removal of sweet and umami inputs. On the other hand, more subtle changes occurred, consistent with some degree of functional reorganization. Thus, in double-KO mice, a unique group of neurons emerged with response profiles characterized by very broad responsiveness to all 0.6 M amino acid stimuli. Moreover, mean responses to citric acid were significantly larger in the double-KO mice and T1R double-KO mice had higher proportions of bitterbroad and acidbroad cells. These results are similar to those in T1R3 KO mice, where more bitter-best neurons were observed (Lemon and Margolskee 2009). Enhanced responses to acids or bitter stimuli were not observed in recordings from the CT nerve in T1R2+3 knockout mice (Nelson et al. 2001; Treesukosol et al. 2009, 2011a; Zhao et al. 2003), suggesting that these changes occurred centrally. In addition to these hints supporting reorganization, at the same time, there was evidence that more NST neurons in the double-KO mice lacked sensory inputs altogether since the proportion of neurons not responsive any probe stimulus was nearly three times as great as that seen in WT. We tentatively conclude that removal of sensory signals arising from the T1R1+T1R3 or T1R2+T1R3 receptors leave one population of NST neurons without salient afferent inputs but that other neurons destined to receive T1R inputs become more responsive to other qualities.
Final remarks.
The current findings in T1R double-KO mice support previous observations of residual neural responses to sugars and amino acids in mice with deletion of a single T1R subunit. Both subunits of the T1R heterodimers were nonfunctional in the present study, making it highly likely that the residual responses are T1R independent. In the case of persistent responses to amino acids, receptors for mediating the perception of other taste qualities are likely to provide part of the explanation. Whether or not the mGluRs are also involved requires further investigation. T1R-independent sugar responses were more prominent for the monosaccharides, making it tempting to conclude that they arise from glucose transporters and/or the K+ATP channels in taste receptor cells. However, such a conclusion requires additional data, especially given that most of these mechanisms cannot be engaged by fructose. In any case, the residual responses that we observed should not obscure the major decrement in sugar and umami responses, the obliteration of sucrose/umami best neurons, and impaired neural representation of this class of stimuli evident in both KO1+3 and KO2+3 mice. These findings emphasize a primary role of the T1Rs in coding for sugar and umami taste stimuli.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grant DC-004574 (to A. C. Spector).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.D.B., A.C.S., and S.P.T. conceived and designed research; B.K. and G.D.B. performed experiments; B.K. and S.P.T. analyzed data; B.K., A.C.S., and S.P.T. interpreted results of experiments; B.K. and S.P.T. prepared figures; B.K. and S.P.T. drafted manuscript; B.K., G.D.B., A.C.S., and S.P.T. edited and revised manuscript; B.K., G.D.B., A.C.S., and S.P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Joseph B. Travers who made valuable comments on the initial draft. In addition, special thanks to Grace Houser, Cemaliye Semmedi, Andrew Harley, and Sanika Pinnanath for excellent technical assistance in immunochemistry.
REFERENCES
- Blonde GD, Spector AC. An examination of the role of l-glutamate and inosine 5′-monophosphate in hedonic taste-guided behavior by mice lacking the T1R1 + T1R3 receptor. Chem Senses 42: 393–404, 2017. doi: 10.1093/chemse/bjx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonde GD, Travers SP, Spector AC. Taste sensitivity to a mixture of monosodium glutamate and inosine 5′-monophosphate by mice lacking both subunits of the T1R1+T1R3 amino acid receptor. Am J Physiol Regul Integr Comp Physiol 314: R802–R810, 2018. doi: 10.1152/ajpregu.00352.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breza JM, Nikonov AA, Contreras RJ. Response latency to lingual taste stimulation distinguishes neuron types within the geniculate ganglion. J Neurophysiol 103: 1771–1784, 2010. doi: 10.1152/jn.00785.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breza JM, Travers SP. P2X2 receptor terminal field demarcates a “transition zone” for gustatory and mechanosensory processing in the mouse nucleus tractus solitarius. Chem Senses 41: 515–524, 2016. doi: 10.1093/chemse/bjw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3: 113–119, 2000. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. Molecular and physiological evidence for glutamate (umami) taste transduction via a G protein-coupled receptor. Ann N Y Acad Sci 855: 398–406, 1998. doi: 10.1111/j.1749-6632.1998.tb10598.x. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci 16: 3817–3826, 1996. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Danilova V, Damak S, Margolskee RF, Hellekant G. Taste responses to sweet stimuli in alpha-gustducin knockout and wild-type mice. Chem Senses 31: 573–580, 2006. doi: 10.1093/chemse/bjj062. [DOI] [PubMed] [Google Scholar]
- Deitch AD, Moses MJ. The Nissl substance of living and fixed spinal ganglion cells. II. An ultraviolet absorption study. J Biophys Biochem Cytol 3: 449–456, 1957. doi: 10.1083/jcb.3.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 31: 351–357, 2006. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol 65: 1452–1463, 1991. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- Geran L, Travers S. Temporal characteristics of gustatory responses in rat parabrachial neurons vary by stimulus and chemosensitive neuron type. PLoS One 8: e76828, 2013. doi: 10.1371/journal.pone.0076828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol 101: 1598–1612, 2009. doi: 10.1152/jn.91168.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza BK, Scott TR, Sclafani A, Antonucci RF. Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res 555: 1–9, 1991. doi: 10.1016/0006-8993(91)90852-M. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Frim YG, Hochman A, Lubitz GS, Basile AJ, Sclafani A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am J Physiol Regul Integr Comp Physiol 312: R597–R610, 2017. doi: 10.1152/ajpregu.00433.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Lubitz GS, Shelling S. Taste of glucose elicits cephalic-phase insulin release in mice. Physiol Behav 192: 200–205, 2018. doi: 10.1016/j.physbeh.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, Vasselli JR, Sclafani A. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am J Physiol Regul Integr Comp Physiol 309: R552–R560, 2015. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am J Physiol Regul Integr Comp Physiol 246: R88–R95, 1984. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers JB, Travers SP. Gustatory and tactile stimulation of the posterior tongue activate overlapping but distinctive regions within the nucleus of the solitary tract. Brain Res 632: 161–173, 1993. doi: 10.1016/0006-8993(93)91151-H. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol 160: 106–154, 1962. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol 591: 1967–1985, 2013. doi: 10.1113/jphysiol.2012.236604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapis TJ, Penner MH, Lim J. Evidence that humans can taste glucose polymers. Chem Senses 39: 737–747, 2014. doi: 10.1093/chemse/bju031. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101: 2459–2471, 2009. doi: 10.1152/jn.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: an extended stimulus array. J Neurophysiol 70: 879–891, 1993. doi: 10.1152/jn.1993.70.3.879. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. doi: 10.1016/S0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Niki M, Takai S, Kusuhara Y, Ninomiya Y, Yoshida R. Responses to apical and basolateral application of glutamate in mouse fungiform taste cells with action potentials. Cell Mol Neurobiol 31: 1033–1040, 2011. doi: 10.1007/s10571-011-9702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Kurenuma S, Nomura T, Uebayashi H, Kawamura H. Taste synergism between monosodium glutamate and 5′-ribonucleotide in mice. Comp Biochem Physiol A Comp Physiol 101: 97–102, 1992. doi: 10.1016/0300-9629(92)90634-3. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Norgren R. Parabrachial gustatory neural responses to monosodium glutamate ingested by awake rats. Physiol Behav 49: 965–971, 1991. doi: 10.1016/0031-9384(91)90209-7. [DOI] [PubMed] [Google Scholar]
- Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: a two-carbohydrate taste model. Neurosci Biobehav Rev 11: 187–196, 1987. doi: 10.1016/S0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol 296: R960–R971, 2009. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczyk JJ, Garraghty PE. Reconciling homeostatic and use-dependent plasticity in the context of somatosensory deprivation. Neural Plast 2015: 290819, 2015. doi: 10.1155/2015/290819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Scott TR. Amino acids as taste stimuli. II. Quality coding. Brain Res 253: 93–104, 1982. doi: 10.1016/0006-8993(82)90676-X. [DOI] [PubMed] [Google Scholar]
- Rehnberg BG, MacKinnon BI, Hettinger TP, Frank ME. Analysis of polysaccharide taste in hamsters: behavioral and neural studies. Physiol Behav 59: 505–516, 1996. doi: 10.1016/0031-9384(95)02092-6. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 90: 743S–746S, 2009. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chem Senses 30, Suppl 1: i25–i26, 2005. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- Schier LA, Inui-Yamamoto C, Blonde GD, Spector AC. T1R2+T1R3-independent chemosensory inputs contributing to behavioral discrimination of sugars in mice. Am J Physiol Regul Integr Comp Physiol 316: R448–R462, 2019. doi: 10.1152/ajpregu.00255.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Spector AC. Behavioral evidence for more than one taste signaling pathway for sugars in rats. J Neurosci 36: 113–124, 2016. doi: 10.1523/JNEUROSCI.3356-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Sennewald K, Gagnon J. Comparison of taste qualities and thresholds of D- and L-amino acids. Physiol Behav 27: 51–59, 1981. doi: 10.1016/0031-9384(81)90298-5. [DOI] [PubMed] [Google Scholar]
- Schmued L, Slikker W Jr. Black-gold: a simple, high-resolution histochemical label for normal and pathological myelin in brain tissue sections. Brain Res 837: 289–297, 1999. doi: 10.1016/S0006-8993(99)01624-8. [DOI] [PubMed] [Google Scholar]
- Sclafani A. The sixth taste? Appetite 43: 1–3, 2004. doi: 10.1016/j.appet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Clyne AE. Hedonic response of rats to polysaccharide and sugar solutions. Neurosci Biobehav Rev 11: 173–180, 1987. doi: 10.1016/S0149-7634(87)80023-4. [DOI] [PubMed] [Google Scholar]
- Skyberg R, Sun C, Hill DL. Maintenance of mouse gustatory terminal field organization is disrupted following selective removal of peripheral sodium salt taste activity at adulthood. J Neurosci 37: 7619–7630, 2017. doi: 10.1523/JNEUROSCI.3838-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Sens Flavor 4: 215–229, 1979. doi: 10.1093/chemse/4.3.215. [DOI] [Google Scholar]
- Smith KR, Spector AC. The importance of the presence of a 5′-ribonucleotide and the contribution of the T1R1 + T1R3 heterodimer and an additional low-affinity receptor in the taste detection of L-glutamate as assessed psychophysically. J Neurosci 34: 13234–13245, 2014. doi: 10.1523/JNEUROSCI.0417-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Spector AC. Detection of maltodextrin and its discrimination from sucrose are independent of the T1R2 + T1R3 heterodimer. Am J Physiol Regul Integr Comp Physiol 313: R450–R462, 2017. doi: 10.1152/ajpregu.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- Sukumaran SK, Yee KK, Iwata S, Kotha R, Quezada-Calvillo R, Nichols BL, Mohan S, Pinto BM, Shigemura N, Ninomiya Y, Margolskee RF. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc Natl Acad Sci USA 113: 6035–6040, 2016. doi: 10.1073/pnas.1520843113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Hummler E, Hill DL. Selective deletion of sodium salt taste during development leads to expanded terminal fields of gustatory nerves in the adult mouse nucleus of the solitary tract. J Neurosci 37: 660–672, 2017. doi: 10.1523/JNEUROSCI.2913-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Boughter JD Jr. Sweet-bitter and umami-bitter taste interactions in single parabrachial neurons in C57BL/6J mice. J Neurophysiol 108: 2179–2190, 2012. doi: 10.1152/jn.00465.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Yamamoto T, Boughter JD Jr. Gustatory neural responses to umami stimuli in the parabrachial nucleus of C57BL/6J mice. J Neurophysiol 107: 1545–1555, 2012. doi: 10.1152/jn.00799.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SP, Norgren R. Coding the sweet taste in the nucleus of the solitary tract: differential roles for anterior tongue and nasoincisor duct gustatory receptors in the rat. J Neurophysiol 65: 1372–1380, 1991. doi: 10.1152/jn.1991.65.6.1372. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855–R865, 2009. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci 31: 13527–13534, 2011a. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, Spector AC. The functional role of the T1R family of receptors in sweet taste and feeding. Physiol Behav 105: 14–26, 2011b. doi: 10.1016/j.physbeh.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol 303: R218–R235, 2012. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol 590: 1155–1170, 2012. doi: 10.1113/jphysiol.2011.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA 108: 5431–5436, 2011. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA 105: 20930–20934, 2008. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003. doi: 10.1016/S0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. doi: 10.1016/S0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866–R876, 2009. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]