Abstract
The excitability of resting motoneurons increases following spinal cord injury (SCI). The extent to which motoneuron excitability changes during voluntary muscle activity in humans with SCI, however, remains poorly understood. To address this question, we measured F waves by using supramaximal electrical stimulation of the ulnar nerve at the wrist and cervicomedullary motor-evoked potentials (CMEPs) by using high-current electrical stimulation over the cervicomedullary junction in the first dorsal interosseous muscle at rest and during 5 and 30% of maximal voluntary contraction into index finger abduction in individuals with chronic cervical incomplete SCI and aged-matched control participants. We found higher persistence (number of F waves present in each set) and amplitude of F waves at rest in SCI compared with control participants. With increasing levels of voluntary contraction, the amplitude, but not the persistence, of F waves increased in both groups but to a lesser extent in SCI compared with control participants. Similarly, the CMEP amplitude increased in both groups but to a lesser extent in SCI compared with controls. These results were also found at matched absolutely levels of electromyographic activity, suggesting that these changes were not related to decreases in voluntary motor output after SCI. F-wave and CMEP amplitudes were positively correlated across conditions in both groups. These results support the hypothesis that the responsiveness of the motoneuron pool during voluntary activity decreases following SCI, which could alter the generation and strength of voluntary muscle contractions.
NEW & NOTEWORTHY How the excitability of motoneurons changes during voluntary muscle activity in humans with spinal cord injury (SCI) remains poorly understood. We found that F-wave and cervicomedullary motor-evoked potential amplitude, outcomes reflecting motoneuronal excitability, increased during voluntary activity compared with rest in SCI participants but to a lesser extent that in controls. These results suggest that the responsiveness of motoneurons during voluntary activity decreases following SCI, which might affect functionally relevant plasticity after the injury.
Keywords: muscle activity, spinal circuits, spinal motoneurons, tetraplegia
INTRODUCTION
Evidence has shown that the excitability of resting motoneurons increases following spinal cord injury (SCI; D’Amico et al. 2014). In animals, weeks and months after SCI, persistent inward currents (PICs) reappear in motoneurons and contribute to the amplification of synaptic inputs (Binder 2002; Gorassini et al. 2004; Lee and Heckman 2000; Prather et al. 2001) and to the self-sustained excitatory drive that allows motoneurons to fire repetitively after brief synaptic excitation (Conway et al. 1988; Hounsgaard and Mintz 1988). Animals with chronic SCI show long-lasting reflexes, muscle spasms and hyperreflexia (Bennett et al. 1999, 2004; Li et al. 2004). Similarly, in humans with SCI, PICs facilitate the activation of resting motoneurons contributing to the generation of muscle spasms and self-sustained firing (Gorassini et al. 2004). Individuals with SCI show exaggerated tendon reflexes (Mailis and Ashby 1990), decreased inhibition in spinal neuronal circuits (Crone et al. 2003; Faist et al. 1994), and alterations in group Ia projections to motoneurons (Mailis and Ashby 1990) compared with control participants. However, the extent to which motoneuron excitability changes during voluntary muscle activity in humans with SCI compared with controls remains poorly understood.
A voluntary contraction increases “excitability” at the motoneuron pool. Inferences about changes in motoneuron excitability in humans can be made by examining the amplitude and persistence of F waves and the size of cervicomedullary motor-evoked potentials (CMEPs; (McNeil et al. 2013). F waves reflect the back firing of a small number of motoneurons activated antidromically after supramaximal electrical stimulation of a peripheral nerve (Eccles 1955). Despite the small number of motoneurons that produce F waves, likely high-threshold motoneurons (Espiritu et al. 2003; Khan et al. 2012), these neurons have shown to be sensitive to detect task-dependent adaptations during a voluntary behavior (Giesebrecht et al. 2011; Khan et al. 2012). CMEPs likely reflect changes in the efficacy of cortico-motoneuronal synapses or motoneuron excitability (Gandevia et al. 1999; Taylor and Gandevia 2004; Ugawa et al. 1991). CMEP size increases markedly during a voluntary contraction, without changing its latency, showing a large dependence on the excitability of the motoneuron pool due to transsynaptic stimulation of spinal tracts (Taylor et al. 2002; Ugawa et al. 1991).
Although little is known about changes in motoneuron excitability during voluntary muscle activity in humans with SCI, some studies allow us to make predictions. For example, long-interval intracortical inhibition, a measurement that reflects changes at a spinal level (McNeil et al. 2011), decreases in magnitude during voluntary activity in controls but not in humans with SCI not taking baclofen (Barry et al. 2013), suggesting reduced facilitation of spinal pathways during a voluntary behavior. Changes in motoneuron excitability may alter motoneuron recruitment and firing rate during voluntary contractions (Matthews 1997), and motor unit firing rates and firing rate modulation during voluntary muscle activity are reduced in SCI compared with control participants (Zijdewind and Thomas 2001). The distribution and efficiency of the synaptic inputs to the motoneuron pool also change after SCI (Calancie et al. 2002; Mailis and Ashby 1990), which could affect the motoneuronal output (Powers and Heckman 2017). We hypothesized that motoneuron excitability during voluntary muscle activity increases to a lesser extent in individuals with SCI compared with control participants.
MATERIALS AND METHODS
Participants.
Sixteen individuals with SCI (mean age: 46.1 ± 06 yr, 5 women; Table 1) and 18 age-matched controls (mean age: 38.7 ± 1 yr, P = 0.14, 6 women) participated in the study. All participants gave informed consent to experimental procedures, which were approved by the local ethics committee at the University of Miami. Participants with SCI had a chronic (≥1 yr), cervical injury (C2–C7), with an intact (score = 2) or impaired (score = 1), but not absent, innervation in dermatomes C6, C7, and C8 during light touch and pin prick stimulus using the American Spinal Injury Association Impairment Scale (AIS) sensory scores and residual hand motor function. One individual with SCI was categorized as AIS A (complete injury) due to the lack of sacral sparing (Marino et al. 2003) despite being able to elicit voluntary contractions with hand muscles, and the other 15 individuals were classified as incomplete AIS C and D (Table 1). Participants were able to exert maximal voluntary contraction (MVC; measured as the highest mean rectified electromyographic (EMG) activity found in 1 s during the MVC burst) into index finger abduction (controls = 0.84 ± 0.2 mV; SCI = 0.28 ± 0.1 mV; P < 0.001).
Table 1.
Spinal cord injury participants
| Subject | Age, yr | Sex | Injury Level | AIS | Etiology |
|---|---|---|---|---|---|
| 1 | 38 | M | C5 | C | T |
| 2 | 72 | M | C7 | A | T |
| 3 | 71 | M | C2 | C | T |
| 4 | 42 | M | C4 | D | T |
| 5 | 61 | M | C5 | D | T |
| 6 | 56 | M | C5 | C | T |
| 7 | 52 | W | C5 | D | T |
| 8 | 39 | M | C5 | C | T |
| 9 | 45 | W | C3 | C | T |
| 10 | 20 | M | C2 | C | T |
| 11 | 57 | W | C4 | C | T |
| 12 | 20 | W | C4 | C | T |
| 13 | 32 | M | C5 | C | T |
| 14 | 65 | M | C4 | D | T |
| 15 | 40 | M | C4 | C | T |
| 16 | 27 | W | C5 | C | T |
AIS, American Spinal Injury Association Impairment Scale; M, man; W, woman; C, cervical; T, traumatic.
EMG recordings.
EMG was recorded from the first dorsal interosseous (FDI) muscle of the right side in controls and from the less affected hand in individuals with SCI through surface electrodes secured to the skin over the belly of each muscle (Ag-AgCl, 10-mm diameter). The signals were amplified (×200), filtered (30–1,000 Hz), and sampled at 5 kHz for offline analysis (CED 1401 with Signal software, Cambridge Electronic Design).
Experimental setup.
All participants were seated in an armchair with both arms relaxed and flexed at the elbow by 90° with the forearm pronated and the wrist and forearm restrained by straps. At the start of the experiment, participants performed three brief MVCs (3–5 s) with the index finger into abduction, separated by 30 s. During index finger abduction, participants were instructed to press with the index finger against a custom lever into the abduction direction. Electrophysiological measurements (F waves and CMEPs, see detail methodology below) were tested at rest and during 5% (controls = 4.6 ± 1.2% of MVC; SCI = 5.1 ± 2.6% of MVC; P = 0.46) and 30% (controls = 25.7 ± 4.3% of MVC; SCI = 26.1 ± 4.9% of MVC; P = 0.81) of MVC into index finger abduction. F waves were recorded from the FDI muscle by ulnar nerve stimulation (Fig. 1A), and CMEPs were elicited by electrical stimulation at the cervicomedullary junction (Fig. 1B) at rest, and during 5% of MVC and 30% of MVC (Fig. 1C). Because SCI participants were weaker than control participants, additional experiments were conducted in controls matching the absolute EMG level exerted by SCI participants in all conditions (see below). Mean rectified EMG activity in the FDI muscle was shown online on a computer screen located in front of the participants by using Signal software to ensure that individuals were able to match EMG activity both muscles during all tasks.
Fig. 1.
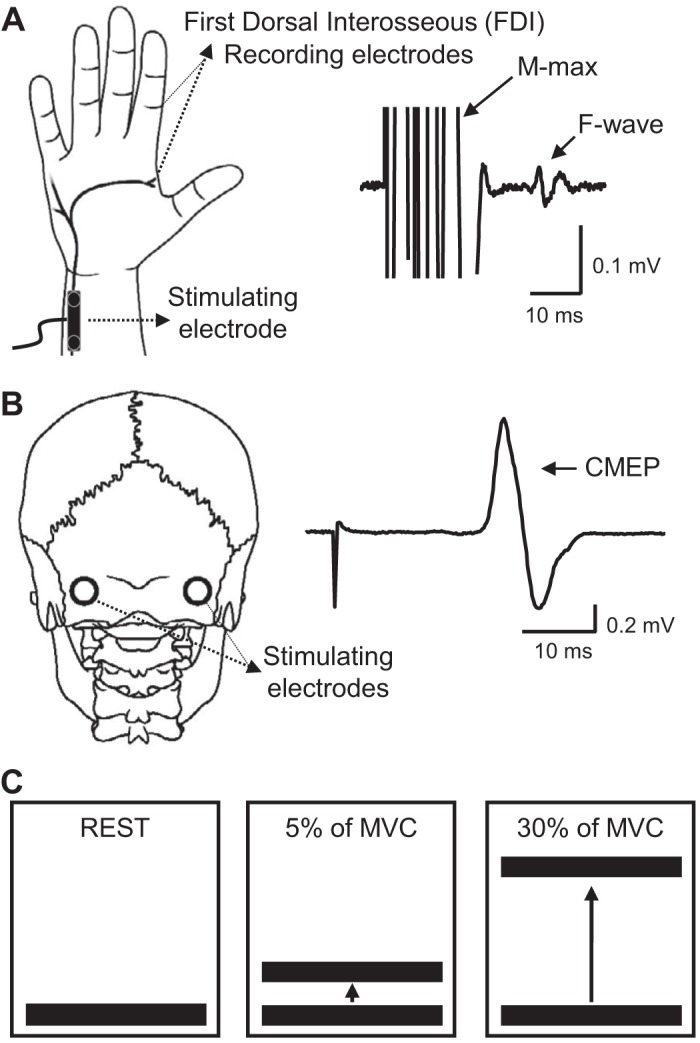
Experimental setup. A: schematic representation of the hand showing the ulnar nerve and F waves recorded from the first dorsal interosseous (FDI) muscle. B, left: schematic representation of the head showing the electrodes placed at the cervicomedullary junction. B, right: a raw trace showing a cervicomedullary motor-evoked potential (CMEP). C: cartoon showing the concept of the visual feedback. Individuals were tested at rest (left) and during 5% (middle) and 30% (right) of maximal voluntary contraction (MVC).
F waves.
F waves were examined by using supramaximal stimulus intensity to the ulnar nerve at the wrist (100-μs pulse duration; DS7A; Digitimer) with a monopolar bar electrode with the cathode positioned proximally (controls, n = 18; SCI, n = 16). The stimuli were delivered at 1 Hz at an intensity of 150% of the maximal motor response (M-max; controls = 69–128 mA; SCI = 82–195 mA). M-max values were reduced in SCI compared with control participants in the FDI muscle (controls = 22.1 ± 4.1 mV; SCI = 13.5 ± 4.1 mV; P < 0.001). For each trial, we quantified each F-wave peak-to-peak amplitude (expressed relative to the M-max) and F-wave persistence (the percentage of stimuli evoking a response; Bunday and Perez 2012). F-wave trials were filtered using a second-order Bessel high-pass filter (200 Hz) to “flatten the tail of the M wave” (Khan et al. 2012). An F wave was considered to be present if a response with a proper latency (minimum of 20 ms) had an amplitude ≥20 µV (Perez and Rothwell 2015). In an additional control experiment, we measured F waves in control participants (F-wave adjusted; n = 5) matching the absolute level of EMG during voluntary contraction exerted by the SCI group allowing us to assess motoneurons excitability at similar level of voluntary contraction between groups. We recorded 2 blocks of 60 trials, and then we put them together creating 1 block of 120 trials. The ratio of F wave amplitude to M-max was calculated for each trial and then averaged across all trials. The F-wave persistence was calculated in one block of 120 trials. During voluntary contraction, mean rectified EMG activity in the muscle tested was shown online to participants to ensure proper completion of the task with 1–2 min of rest in between trials.
CMEPs.
We stimulated the corticospinal tract using high-voltage electrical current (200-μs duration, Digitimer DS7AH) passed between two small gold-cup electrodes (GRASS Technologies, Astro Med, Warwick, RI) placed behind the mastoid process at the cervicomedullary level (controls, n = 11; SCI = 9). The stimulation intensity was adjusted in steps of ~50 mA to produce a response of ~3–5% of the M-max in the FDI muscle in 2 consecutive trials (controls = 3.7 ± 1.9% of M-max, 220–350 mA; SCI = 6.1 ± 3.9% of M-max, 310–450 mA; P = 0.13). We monitored the stimulation to ensure that it was below the intensity required to activate the peripheral nerve directly by increasing the intensity and observing a decrease in latency. A decrease in latency of ~2 ms indicates that the response reflects a mixture of pre- and postsynaptically activated motoneurons (Taylor and Gandevia 2004). In an additional control experiment, we measured CMEPs in control participants (CMEP adjusted; n = 7) matching the absolute level of EMG during voluntary contraction exerted by the SCI group allowing us to assess motoneurons excitability at similar level of voluntary contraction between groups. Five to 10 CMEPs were averaged on each condition at 4 s intervals.
Data analysis.
Normal distribution was tested by the Shapiro-Wilk's test and homogeneity of variances by the Mauchly’s test of sphericity. When sphericity could not be assumed, the Greenhouse- Geisser correction statistic was used. Repeated-measures ANOVA was performed to determine the effect of GROUP (controls and SCI) and CONDITION (rest and 5 and 30% of MVC) in the amplitude and persistence of F waves, CMEPs, and mean background EMG activity. Newman-Keuls post hoc analysis was used to test for significant comparisons. In addition, we calculated the slope of changes in F waves and CMEPs at increasing levels of MVC by using linear regression analysis, and independent t tests were used to determine significant differences between groups. Pearson correlation analysis was used as needed. Significance was set at P < 0.05. Group data are presented as the means ± SD in the text.
RESULTS
EMG.
Figure 2A illustrates examples of rectified EMG activity measured in the FDI muscle across conditions tested (rest and 5 and 30% of MVC) in a representative control and SCI participant. Note that EMG activity increased during increasing levels of voluntary contraction in both participants compared with rest. Repeated measures ANOVA showed no effect of GROUP [F(1,32) = 1.0, P = 0.3] but CONDITION [F(2,64) = 760.4, P < 0.001] and not in their interaction [F(2,64) = 0.08, P = 0.9] on mean rectified EMG activity normalized to the MVC. Post hoc analysis revealed that EMG activity increased at 30% (controls, P < 0.001; SCI, P < 0.001) and 5% (controls, P < 0.001; SCI, P < 0.001) of MVC compared with rest condition in both groups (Fig. 2B). EMG activity was also larger at 30% compared with 5% of MVC during index finger abduction in both groups (controls, P < 0.001; SCI, P < 0.001).
Fig. 2.
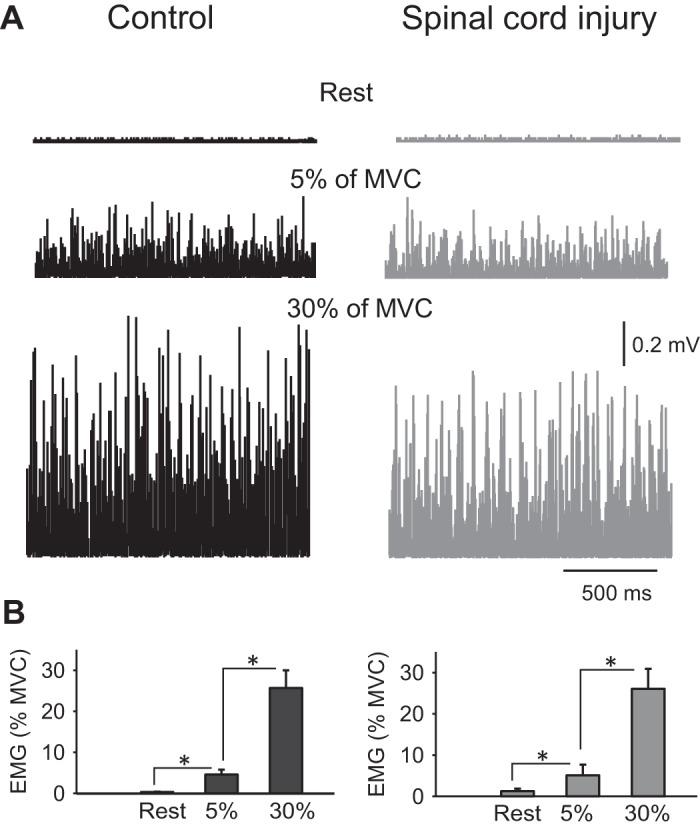
Electromyographic (EMG) activity. A: rectified EMG activity measured in the first dorsal interosseous (FDI) muscle in a representative control subject (left) and in an individual with cervical spinal cord injury (SCI; right) when the FDI muscle was at rest or performed 5 and 30% of maximal voluntary contraction (MVC). B: group data (controls, n = 18; SCI, n = 16) showing mean rectified EMG activity (expressed as a %MVC) at rest and during index finger abduction. Error bars indicate SD. *P < 0.05.
F waves.
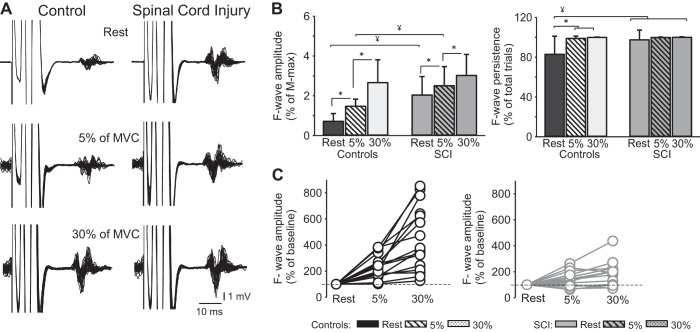
Figures 3A illustrates examples of F waves in the FDI muscle across conditions in two representative participants. Note that the amplitude of F waves at rest was larger in the SCI compared with the control participant. Also, note that F-wave amplitude increased during increasing levels of voluntary activity in both participants but to a lesser extent in the individual with SCI.
Fig. 3.
F-wave amplitude and persistence. A: motor response (M wave) and F-waves recorded from the first dorsal interosseous (FDI) muscles at REST and during 5% of maximal voluntary contraction (MVC) and 30% of MVC during index finger abduction in a representative control subject (left) and in an individual with cervical spinal cord injury (SCI: right). B: group data (controls, n = 18; SCI, n = 16) showing F-wave amplitude and persistence. The x-axis shows all conditions tested [F-wave mean amplitude (left) and F-wave persistence (right) at rest (black bars: controls; gray bars: SCI) and during 5% (dashed white bars: controls; dashed gray bars: SCI) and 30% (light gray bars: controls; dark gray bars: SCI) of MVC during index finger abduction]. The y-axis shows the F-wave amplitude [%maximal motor response (% of M-max)] and F-wave persistence (% of total trials). C: individual data showing F-wave amplitude in control (black circles) and SCI (gray circles) participants at rest and during 5% and 30% of MVC. Error bars indicate SD. *P < 0.05, for comparisons within participants. ¥P < 0.05, for comparisons between participants.
Repeated measures ANOVA showed an effect of GROUP [F(1,32) = 15.8, P = 0.003] and CONDITION [F(2,64) = 40.7, P < 0.001] and in their interaction [F(2,64) = 4.5, P = 0.01] on F-wave amplitude. Post hoc analysis showed that F-wave amplitude was larger at 30% (2.6 ± 1.1% of M-max; P < 0.001) and 5% (1.4 ± 0.3% of M-max; P < 0.001) of MVC compared with rest (0.7 ± 0.4% of M-max; P < 0.001) in control participants. F-wave amplitude was also larger at 30% compared with 5% of MVC (P < 0.001; Fig. 3B, left). Similarly, in SCI participants, F-wave amplitude was larger at 30% (3.0 ± 1.0% of M-max; P < 0.001) and 5% (2.5 ± 0.9% of M-max; P = 0.03) of MVC compared with rest (2.0 ± 0.9% of M-max) and at 30% compared with 5% (P < 0.001) of MVC. Post hoc analysis showed that F-wave amplitude was higher in SCI compared with controls at rest (P < 0.001) and 5% of MVC (P = 0.002), while no differences between the two groups were observed at 30% of MVC (P = 0.23).
Repeated measures ANOVA showed an effect of GROUP [F(1,32) = 7.3, P = 0.007] and CONDITION [F(2,64) = 13.7, P < 0.001] and in their interaction [F(2,64) = 8.2, P = 0.006] on F-wave persistence (% of total trials; Fig. 3B, right). Post hoc analysis showed that F-wave persistence increased in controls at 30% (99.9 ± 0.3% of total trials; P < 0.001) and 5% (98.8 ± 2.3% of total trials; P < 0.001) of MVC compared with rest (82.9 ± 18% of total trials). No differences in persistence were observed between 5 and 30% of MVC (P = 0.93). In SCI, F-wave persistence remained high across conditions (30% of MVC = 99.9 ± 0.002% of total trials; 5% of MVC = 99.8 ± 0.4% of total trials; rest = 97.5 ± 9.7% of total trials). F-wave persistence was higher in SCI compared with controls at rest (P < 0.001) but not at 5% (P = 0.7) and at 30% (P = 0.9) of MVC.
CMEPs.
Figure 4 illustrates an example of CMEPs in the FDI muscle across conditions in two representative participants. Note that the amplitude of CMEPs increased during increasing levels of voluntary activity in both participants but to a lesser extent in the individual with SCI.
Fig. 4.
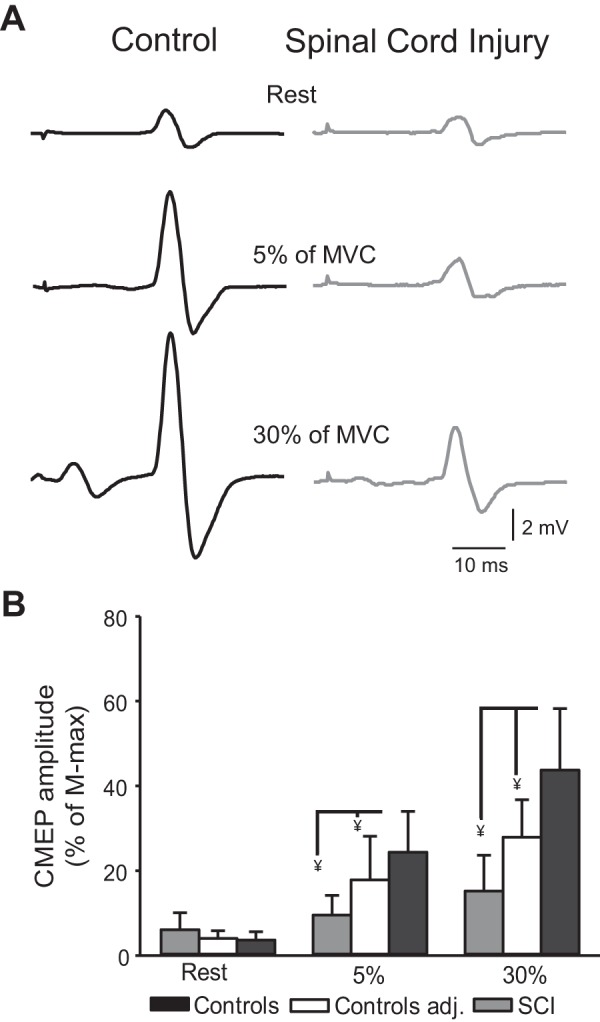
Cervicomedullary motor-evoked potentials (CMEPs). A: CMEPs recorded from the resting first dorsal interosseous (FDI) of a control subject (black) and an individual with cervical spinal cord injury (SCI; gray) at REST and during 5 and 30% of maximal voluntary contraction (MVC) into index finger abduction. B: group data showing CMEP amplitude [% of maximal motor response (M-max)] in controls (black bars), spinal cord injury (SCI; gray bars), and control participants who complete a control experiment (white bars) where they matched the same amount of absolute EMG level as the SCI group. The x-axis shows the conditions tested (REST and 5 and 30% of MVC during index finger abduction, and y-axis shows the size of the FDI CMEPs as a % of M-max). Error bars indicate SD. ¥P < 0.05, for comparisons between participants.
At rest, CMEP amplitude was similar across groups (controls = 3.6 ± 1.9% of M-max; SCI = 6.1 ± 3.9% of M-max; P = 0.13). Repeated measures ANOVA showed an effect of GROUP [F(1,18) = 19.3, P = 0.003] and CONDITION [F(2,36) = 77.2, P < 0.001] and in their interaction [F(2,36) = 30.8, P < 0.001] on CMEP amplitude (% of M-max). Post hoc analysis showed that in controls CMEP amplitude increased at 30% (43.7 ± 14.5% of M-max; P < 0.001) and 5% (24.3 ± 9.6% of M-max; P < 0.001) of MVC compared with rest (3.6 ± 1.9% of M-max). CMEPs were larger at 30% compared with 5% of MVC (P < 0.001). In SCI participants, CMEP amplitude increased at 30% (15 ± 8.4% of M-max, P = 0.007) and 5% (10.9 ± 4.4% of M-max, P = 0.04) of MVC compared with rest (6.1 ± 3.9% of M-max). CMEPs were also larger at 30% compared with 5% of MVC (P = 0.04). CMEP amplitude was higher in controls compared with SCI at 30% of MVC (P < 0.001) and at 5% of MVC (P = 0.001).
In an additional control experiment, where we matched EMG levels across groups, repeated measures ANOVA showed an effect of GROUP [F(1,14) = 4.7, P = 0.015] and CONDITION [F(2,28) = 48.4, P < 0.001] and in their interaction [F(2,28) = 10.4, P = 0.004] on CMEP amplitude (% of M-max). Post hoc analysis showed that CMEP amplitude in controls was higher at 30% (27.8 ± 8.8% of M-max) and 5% (17.8 ± 10.2% of M-max; P = 0.001) of MVC compared with rest (4.0 ± 1.8% of M-max P < 0.001). CMEP amplitude was also higher in controls compared with SCI at 30% (P = 0.01) and 5% (P = 0.02) of MVC, while no differences between groups were observed at rest (P = 0.15).
Slope of F waves and CMEPs.
Figure 5 illustrates the slope of F waves and CMEPs in the FDI muscle across conditions in control and SCI participants. The slope of F-wave amplitude across conditions was higher in controls (0.06 ± 0.03) compared with SCI (0.03 ± 0.02; P = 0.04) participants. We also compared the slope of participants with similar F-wave amplitude at rest (controls = 1.0% of M-max, n = 7; SCI = 1.4% of M-max, n = 6; P = 0.13). Here, we found that the slope was higher in controls compared with SCI participants (P = 0.03), supporting our previous results. Note that most controls and SCI participants showed an increase in the F waves at 5% (controls, 16/16, Fig. 3C, left; SCI, 12/17, Fig. 3C, right) and 30% (controls, 16/16, Fig. 3C, left; SCI, 14/17; Fig. 3C, right) of MVC. We also compared the slope of the F waves at similar levels of absolute EMG background across conditions (controls = 0.5 ± 0.09 mV, n = 5; SCI = 0.4 ± 0.13 mV, n = 5; P = 0.1). Here, we also observed that the slope of F waves was higher in controls compared with SCI participants (P = 0.03).
Fig. 5.
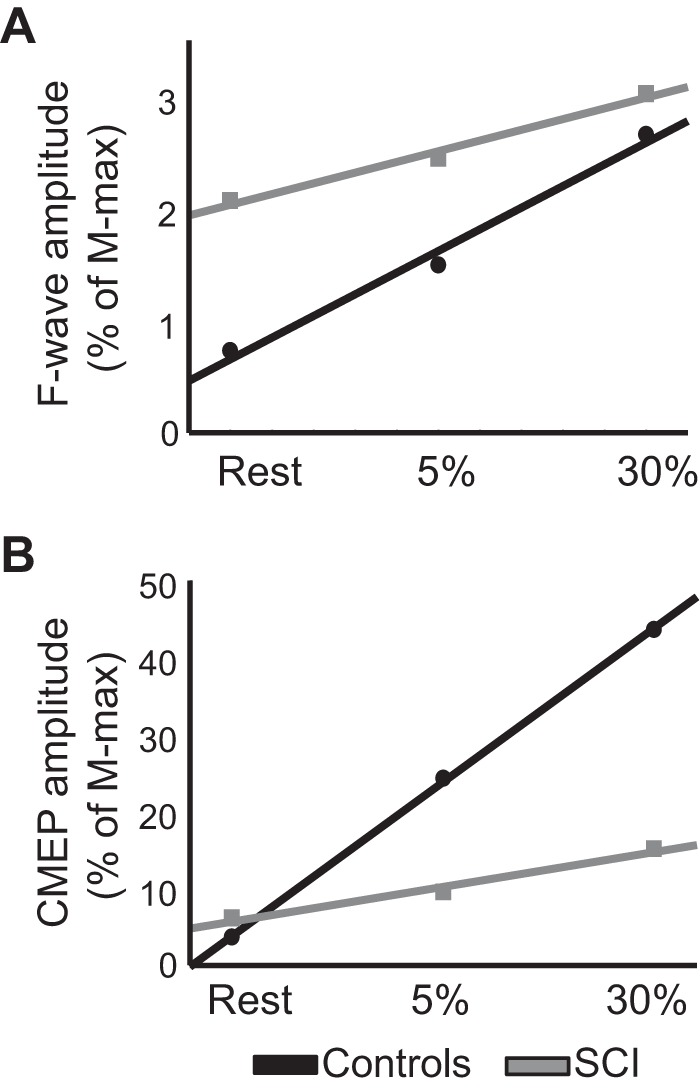
Slope of cervicomedullary motor-evoked potentials (CMEPs) and F waves. A and B: slope of F waves (A) and CMEPs (B) in controls (black) and spinal cord injury (SCI; gray) participants in all conditions tested. In A and B, the abscissa shows the conditions tested [REST and 5 and 30% of maximal voluntary contraction during index finger abduction] and the ordinate shows F-wave (A) and CMEPs (B) amplitudes as % of maximal motor response (M-max).
The slope of CMEPs was higher in controls (1.2 ± 0.3) compared with SCI (0.3 ± 0.2; P < 0.001). This was also observed in the additional control experiment where we matched EMG levels across groups. Here, we found that the slope of CMEPs was higher in controls (1.6 ± 0.2) compared with SCI (0.3 ± 0.2; P = 0.01) participants. We found a positive correlation between CMEPs and F-wave amplitude during all levels of voluntary contraction (controls: r = 0.72; P < 0.001; SCI: r = 0.54, P = 0.03).
DISCUSSION
In the present study we investigated whether a voluntary muscle contraction influences motoneuron excitability in humans with chronic cervical SCI to a similar extent as in control participants. To make inferences about changes in motoneuron excitability we tested F waves and CMEPs at rest and during different levels of voluntary muscle contraction. We found higher persistence and amplitude of F waves at rest in SCI compared with control participants. With increasing levels of voluntary contraction, the amplitude of F waves and CMEPs increased in controls and, to a lesser extent, in SCI participants. F-wave and CMEP amplitudes were positively correlated during all levels of voluntary contraction. These results suggest that the responsiveness of the motoneuron pool decreases during voluntary activity following SCI, which could alter the generation and strength of voluntary muscle contractions.
Changes in motoneuron excitability after SCI.
Consistent with previous results (Bunday et al. 2013; Bunday and Perez 2012), we found that the amplitude and persistence of F waves in the resting FDI muscle was higher in SCI than in control participants. Evidence showed that the presence of F waves in hand muscles changes according to the level of SCI (Thomas et al. 2017). In agreement, we found that F waves were present in all SCI participants in whom motoneurons for the FDI muscle were located below the injury level (Butler and Thomas 2003). Here, for the first time we measured F waves in humans with chronic incomplete SCI during voluntary muscle activity. We found that the amplitude of F waves increased during 5 and 30% of MVC compared with rest in SCI to a lesser extent than in control participants. In controls, our results are consistent with previous findings showing that the amplitude of the F waves increases with increasing levels of tonic voluntary activity (Suzuki et al. 1993). Even some reports showed that the size of F waves increases by imagining increasing levels of tonic voluntary activity (Bunno et al. 2014; Suzuki et al. 2014). It is possible that in humans with SCI the responsiveness of the motoneuron pool decreases during voluntary activity. This is consistent with evidence showing that other measurements reflecting changes at the spinal level, such as long-interval intracortical inhibition (McNeil et al. 2011), decrease in magnitude during voluntary activity in controls but not in humans with SCI not taking baclofen (Barry et al. 2013), suggesting reduced facilitation of spinal pathways during a voluntary behavior. Changes in sensory-evoked synaptic activation of motoneurons after SCI might have contributed to our findings. Humans with SCI showed prolonged postsynaptic potentials in motoneurons in response to brief afferent stimulation consistent with the prolonged excitatory postsynaptic potentials observed in chronically spinalized animals (Norton et al. 2008). The time course of excitatory postsynaptic potentials can be altered by changes in the intrinsic properties of the motoneuron, which can profoundly affect the input-output properties of motoneurons (Heckmann et al. 2005). A critical question is if F waves are sensitive to detect changes during voluntary activity. It is thought that F waves reflect the back firing of a small number of motoneurons activated antidromically after supramaximal electrical stimulation of a peripheral nerve (Eccles 1955). Changes in F-wave responses recorded during voluntary contraction can be difficult to interpret because collision between voluntary orthodromic and antidromic impulses will leave some motor axons free to transmit an H reflex to the muscle, although this is likely less of a problem during small levels of voluntary activity (Espiritu et al. 2003). Note that in our study we found a lesser facilitation of F-wave amplitudes not only at 30% but also at 5% of MVC in SCI compared with control participants. The same results were observed at matched absolute levels of EMG activity, suggesting that these changes were not related to decreases in voluntary motor output found in SCI participants. The same results were also observed at matched amplitude of resting normalized F waves across groups, suggesting that these changes were not related to increases in resting background motoneuron excitability found in SCI participants. Despite the small number of motoneurons that produce F waves, strong evidence showed that these neurons can reflect activity-dependent changes in excitability (Giesebrecht et al. 2011; Khan et al. 2012; Mazzocchio et al. 2008). Even during voluntary contraction of hand muscles with one side of the body, F waves measured in the contralateral resting homonymous muscle are sensitive to detect activity-dependent changes (Bunday et al. 2013). F-wave persistence was higher at rest in SCI compared with control participants, as previously shown (Bunday et al. 2013; Bunday and Perez 2012), but this outcome was less sensitive to detect changes during voluntary activity in both groups. In controls, increases were only present at 5% of MVC, and in SCI participants, F-wave persistence did not change between rest and voluntary activity. The most logical explanation is that the persistence is already high at rest and thus reaches a ceiling effect rapidly during voluntary muscle contraction. Indeed, our values for F-wave persistence at rest were ~80% of trials in controls and ~95% of trials in SCI participants, consistent with previous results (Espiritu et al. 2003). Even at rest, studies have reported no differences in F-wave persistence between SCI and control participants (Butler and Thomas 2003) consistent with the view that F-wave persistence is a less sensitive outcome.
The possibility that following SCI the responsiveness of motoneuron decreases during voluntary activity is supported by our CMEPs results. Evidence showed that CMEP size increases markedly during a voluntary contraction, without changing its latency, which shows a large dependence on the excitability of the motoneuron pool due to transsynaptic stimulation of spinal tracts (Taylor et al. 2002; Ugawa et al. 1995). We found that CMEP size increased during 5% and 30% of MVC compared with rest in all SCI participants but to a lesser extent than in control participants. This was also found at matched levels of absolute EMG activity across groups decreasing the possibility that muscle weakness present in SCI participants contributed to our findings. The distribution and efficiency of synaptic inputs to the motoneuron pool (Faist et al. 1994; Mailis and Ashby 1990) and the intrinsic properties of motoneurons (D’Amico et al. 2014) change after SCI. PICs reappear in motoneuron of animals (Binder 2002; Lee and Heckman 2000; Prather et al. 2001) and humans (Gorassini et al. 2004) with SCI contributing to amplify synaptic inputs and to the self-sustained firing of motoneurons. Thus one might expect that during voluntary activity motoneuron excitability could be even larger in SCI compared with control participants. However, PICs require long depolarization to fully activate (Li et al. 2004; Li and Bennett 2003; Moritz et al. 2007). The slow but regular spontaneous unit discharge that develops after chronic SCI is likely mediated by activation of a subthreshold, sodium-mediated PIC (Gorassini et al. 2004; Li et al. 2004). During small levels of voluntary activity, background firing motor unit activity may occur with partial calcium-mediated PIC activation (Elbasiouny et al. 2006). The background level of membrane depolarization of resting motoneurons increases following SCI (Elbasiouny et al. 2006; Thomas et al. 2017). Thus it is possible that during voluntary activity membrane depolarization of motoneurons is excessive and if the motoneuron is hyperpolarized, the antidromic spike fails to invade the soma resulting in no further increase in F-wave amplitude. This is supported by studies in rats with spinal cord transection showing that increases in motoneuron excitability might relate to a more depolarized resting membrane potential (Harvey et al. 2006). After SCI, during voluntary activity, synaptic noise to the motoneurons and the firing of the motor units and spike-to-spike variability increase (Gorassini et al. 2004), which might contribute to the excessive motoneuron depolarization after the injury. Axonal threshold changes during voluntary activity (Vagg et al. 1998) and the threshold for activating peripheral motor axons below the injury level also increase following SCI (Lin et al. 2007). However, it is unlikely that the reduced changes in motoneuron excitability observed in SCI participants were related to a reduction in the number of axons activated by the stimulation or increases in threshold during voluntary activity. We used supramaximal stimulation (150% of the intensity needed to evoke the M-max) throughout testing, and the size of the M-max remained constant across conditions. It is also unclear if depolarized membrane changes of a peripheral nerve after human SCI reflect motoneuron properties (Lin et al. 2007).
Functional considerations.
After SCI, a voluntary muscle contraction is controlled by a reduced number of motoneurons due to death (Grumbles and Thomas 2017) and affected by changes in the properties of motoneurons (D’Amico et al. 2014). Because changes in the distribution and properties of motoneurons influence strength and muscle activity, our results might have implications for voluntary motor behaviors in humans with SCI. We show that voluntary muscle activity enhances motoneurons excitability, as shown by higher F-wave amplitude and persistence and CMEP amplitude in humans with SCI, but to a lesser extent, than in control participants. Although both F waves and CMEPs are measurements commonly used to test motoneuron excitability (McNeil et al. 2013), variations in the amplitude of CMEPs likely reflect changes in the efficacy of cortico-motoneuronal synapses and/or motoneuron excitability (Gandevia et al. 1999; Taylor and Gandevia 2004; Ugawa et al. 1991). Given that changes in CMEPs and F waves were similar, our results strongly suggest that motoneuronal excitability during tonic voluntary activity increases to a lesser extent after SCI compared with control participants. Thus strategies targeting motoneurons or their inputs might represent an avenue to enhance motor recovery following SCI. This is consistent with studies showing that targeted spinal cord plasticity is beneficial for functional recovery in humans with SCI (Christiansen and Perez 2018; Taccola et al. 2018; Thompson and Wolpaw 2015). It might be important to consider changing the strength and distribution of synaptic inputs to motoneurons during voluntary activity (Bunday et al. 2018) to promote motoneuronal output and functionally relevant plasticity following SCI.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01-NS-090622-01 and R01 NS090622 (to M. A. Perez) and U.S. Department of Veterans Affairs Grant I01RX002474 (to M. A. Perez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.V. and M.A.P. conceived and designed research; R.V. performed experiments; R.V. and M.A.P. analyzed data; R.V. and M.A.P. interpreted results of experiments; R.V. and M.A.P. prepared figures; R.V. and M.A.P. drafted manuscript; R.V. and M.A.P. edited and revised manuscript; R.V. and M.A.P. approved final version of manuscript.
REFERENCES
- Barry MD, Bunday KL, Chen R, Perez MA. Selective effects of baclofen on use-dependent modulation of GABAB inhibition after tetraplegia. J Neurosci 33: 12898–12907, 2013. doi: 10.1523/JNEUROSCI.1552-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16: 69–84, 1999. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Binder MD. Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Brain Res Rev 40: 1–8, 2002. doi: 10.1016/S0165-0173(02)00183-2. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Oudega M, Perez MA. Aberrant crossed corticospinal facilitation in muscles distant from a spinal cord injury. PLoS One 8: e76747, 2013. doi: 10.1371/journal.pone.0076747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Perez MA. Impaired crossed facilitation of the corticospinal pathway after cervical spinal cord injury. J Neurophysiol 107: 2901–2911, 2012. doi: 10.1152/jn.00850.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Urbin MA, Perez MA. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul 11: 1083–1092, 2018. doi: 10.1016/j.brs.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Bunno Y, Yurugi Y, Onigata C, Suzuki T, Iwatsuki H. Influence of motor imagery of isometric opponens pollicis activity on the excitability of spinal motor neurons: a comparison using different muscle contraction strengths. J Phys Ther Sci 26: 1069–1073, 2014. doi: 10.1589/jpts.26.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Thomas CK. Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. J Appl Physiol (1985) 94: 567–575, 2003. doi: 10.1152/japplphysiol.01176.2001. [DOI] [PubMed] [Google Scholar]
- Calancie B, Molano MR, Broton JG. Interlimb reflexes and synaptic plasticity become evident months after human spinal cord injury. Brain 125: 1150–1161, 2002. doi: 10.1093/brain/awf114. [DOI] [PubMed] [Google Scholar]
- Christiansen L, Perez MA. Targeted-plasticity in the corticospinal tract after human spinal cord injury. Neurotherapeutics 15: 618–627, 2018. doi: 10.1007/s13311-018-0639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol 405: 369–384, 1988. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sørensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126: 495–507, 2003. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- D’Amico JM, Condliffe EG, Martins KJB, Bennett DJ, Gorassini MA. Corrigendum: Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci 8: 1–24, 2014. doi: 10.3389/fnint.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. The central action of antidromic impulses in motor nerve fibres. Pflugers Arch Gesamte Physiol Menschen Tiere 260: 385–415, 1955. doi: 10.1007/BF00363548. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol 570: 355–374, 2006. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiritu MG, Lin CS, Burke D. Motoneuron excitability and the F wave. Muscle Nerve 27: 720–727, 2003. doi: 10.1002/mus.10388. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain 117: 1449–1455, 1994. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol 521: 749–759, 1999. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesebrecht S, Martin PG, Gandevia SC, Taylor JL. Altered corticospinal transmission to the hand after maximum voluntary efforts. Muscle Nerve 43: 679–687, 2011. doi: 10.1002/mus.21938. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Grumbles RM, Thomas CK. Motoneuron death after human spinal cord injury. J Neurotrauma 34: 581–590, 2017. doi: 10.1089/neu.2015.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol 398: 591–603, 1988. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SI, Giesebrecht S, Gandevia SC, Taylor JL. Activity-dependent depression of the recurrent discharge of human motoneurones after maximal voluntary contractions. J Physiol 590: 4957–4969, 2012. doi: 10.1113/jphysiol.2012.235697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Lin CS, Macefield VG, Elam M, Wallin BG, Engel S, Kiernan MC. Axonal changes in spinal cord injured patients distal to the site of injury. Brain 130: 985–994, 2007. doi: 10.1093/brain/awl339. [DOI] [PubMed] [Google Scholar]
- Mailis A, Ashby P. Alterations in group Ia projections to motoneurons following spinal lesions in humans. J Neurophysiol 64: 637–647, 1990. doi: 10.1152/jn.1990.64.2.637. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM; ASIA Neurological Standards Committee 2002 . International standards for neurological classification of spinal cord injury. J Spinal Cord Med 26, Suppl 1: S50–S56, 2003. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Spindle and motoneuronal contributions to the phase advance of the human stretch reflex and the reduction of tremor. J Physiol 498: 249–275, 1997. doi: 10.1113/jphysiol.1997.sp021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R, Gelli F, Del Santo F, Popa T, Rossi A. Effects of posture-related changes in motor cortical output on central oscillatory activity of pathological origin in humans. Brain Res 1223: 65–72, 2008. doi: 10.1016/j.brainres.2008.05.024. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Butler JE, Taylor JL, Gandevia SC. Testing the excitability of human motoneurons. Front Hum Neurosci 7: 152, 2013. doi: 10.3389/fnhum.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res 209: 287–297, 2011. doi: 10.1007/s00221-011-2552-z. [DOI] [PubMed] [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol 98: 1042–1047, 2007. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008. doi: 10.1093/brain/awn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Rothwell JC. Distinct influence of hand posture on cortical activity during human grasping. J Neurosci 35: 4882–4889, 2015. doi: 10.1523/JNEUROSCI.4170-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Heckman CJ. Synaptic control of the shape of the motoneuron pool input-output function. J Neurophysiol 117: 1171–1184, 2017. doi: 10.1152/jn.00850.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol 85: 43–53, 2001. doi: 10.1152/jn.2001.85.1.43. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bunno Y, Onigata C, Tani M, Uragami S. Excitability of spinal neural function by motor imagery with isometric opponens pollicis activity: influence of vision during motor imagery. NeuroRehabilitation 34: 725–729, 2014. doi: 10.3233/NRE-141085. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fujiwara T, Takeda I. Excitability of the spinal motor neuron pool and f-waves during isometric ipsilateral and contralateral contraction. Physiother Theory Pract 9: 19–24, 1993. doi: 10.3109/09593989309036482. [DOI] [Google Scholar]
- Taccola G, Sayenko D, Gad P, Gerasimenko Y, Edgerton VR. And yet it moves: recovery of volitional control after spinal cord injury. Prog Neurobiol 160: 64–81, 2018. doi: 10.1016/j.pneurobio.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol (1985) 96: 1496–1503, 2004. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol 541: 949–958, 2002. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CK, Häger CK, Klein CS. Increases in human motoneuron excitability after cervical spinal cord injury depend on the level of injury. J Neurophysiol 117: 684–691, 2017. doi: 10.1152/jn.00676.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Targeted neuroplasticity for rehabilitation. In: Progress in Brain Research, edited by Dancause N, Nadeau S, Rossignol S. Amsterdam, The Netherlands: Elsevier, 2015, p. 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Genba-Shimizu K, Kanazawa I. Electrical stimulation of the human descending motor tracts at several levels. Can J Neurol Sci 22: 36–42, 1995. doi: 10.1017/S0317167100040476. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol 29: 418–427, 1991. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol 507: 919–925, 1998. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve 24: 952–962, 2001. doi: 10.1002/mus.1094. [DOI] [PubMed] [Google Scholar]