Abstract
Inwardly rectifying K+ (Kir) channels are expressed in multiple organs and cell types and play critical roles in cellular function. Most notably, Kir channels are major determinants of the resting membrane potential and K+ homeostasis. The renal outer medullary K+ channel (Kir1.1) was the first renal Kir channel identified and cloned in the kidney over two decades ago. Since then, several additional members, including classical and ATP-regulated Kir family classes, have been identified to be expressed in the kidney and to contribute to renal ion transport. Although the ATP-regulated Kir channel class remains the most well known due to severe pathological phenotypes associated with their mutations, progress is being made in defining the properties, localization, and physiological functions of other renal Kir channels, including those localized to the basolateral epithelium. This review is primarily focused on the current knowledge of the expression and localization of renal Kir channels but will also briefly describe their proposed functions in the kidney.
Keywords: KCNJ10 (Kir4.1), KCNJ16 (Kir5.1), inwardly rectifying K+ channels, potassium homeostasis, renal outer medullary K+ channel
INTRODUCTION
Inwardly rectifying K+ (Kir) channels are encoded by 16 known KCNJ genes and are named for their “anomalous” nonlinear current-voltage relationship resulting from voltage-dependent channel blockade by intracellular cations (primarily Mg2+ or polyamines). Structurally, Kir channel subunits contain two transmembrane spanning domains, an ion selectivity filter, and intracellular carboxy- and amino-terminal domains, and they assemble as tetramers to form functional K+ channels containing a single pore (18). Kir channel expression and gating are regulated by a wide variety of intracellular and extracellular factors. Most notably, Mg2+ regulates gating by voltage-dependent intracellular channel blockade, and activation by phosphatidylinositol 4,5-bisphosphate binding is required for all Kir channels (17, 21). Kir channels can be blocked by extracellular Ba2+ and Cs+, in addition to small-molecule inhibitors being developed for specific channels (3, 62, 63). Modulation by other factors, including extracellular K+ concentration, intracellular H+, ATP, phosphorylation by PKA and PKC, and protein-protein interactions, is distinct to each Kir subfamily. Kir proteins are divided into seven subfamilies (Kir1–Kir7) and can be functionally and evolutionally categorized into four groups: ATP-regulated (transport channels), classical, G protein-gated, and ATP-sensitive Kir channels (16, 18, 37, 52).
Since their discovery, Kir channels have been found to be expressed in many cell and tissue types where they contribute to diverse cellular and organismal functions, including maintenance of the resting membrane potential, modulation of cellular excitability, and regulation of whole body electrolyte homeostasis. Several Kir channels are expressed in the kidney and are collectively integral to its function; thus, Kir channelopathies often involve severe renal impairments. Figure 1 summarizes current knowledge regarding the expression of well-characterized, as well as less-described, Kir channels and their reported localization along the nephron. Most of the well-characterized Kir channels expressed in the plasma membrane of renal epithelial cells are weakly rectifying and functionally classified as transport channels. Regardless of where on the nephron they are localized, the activity of these channels can be directly coupled to active transport (Na+- K+-ATPase or Na+-K+-2Cl− cotransporter), which enables their major contribution to Na+ and K+ homeostasis. Despite numerous reports of renal Kir channel expression in the literature, very few have been thoroughly investigated and supported with sufficient evidence. The study of these ion channels is complex and requires a multidisciplinary approach, which includes confirmation and localization of mRNA and/or protein expression, evaluation of physiological roles using genetic models and/or pharmacological targeting, and, most importantly, functional characterization using electrophysiological techniques. For initial channel characterization, in vitro electrophysiology studies in heterologous expression systems may be useful. However, ex vivo patch-clamp analysis is critical for proper functional evaluation of Kir channel properties in the kidney. Using a novel vibrodissociation approach to isolate renal tubules, we were able to report ex vivo basolateral Kir channel activity (presumably Kir4.1/Kir5.1) for the first time not only in rodents but in human kidneys as well (22). By this standard, only Kir1.1, Kir4.1, and Kir4.1/Kir5.1 channels can be considered appropriately characterized and validated in the kidney thus far. Other Kir channels require further investigation to firmly establish their expression and functional properties.
Fig. 1.
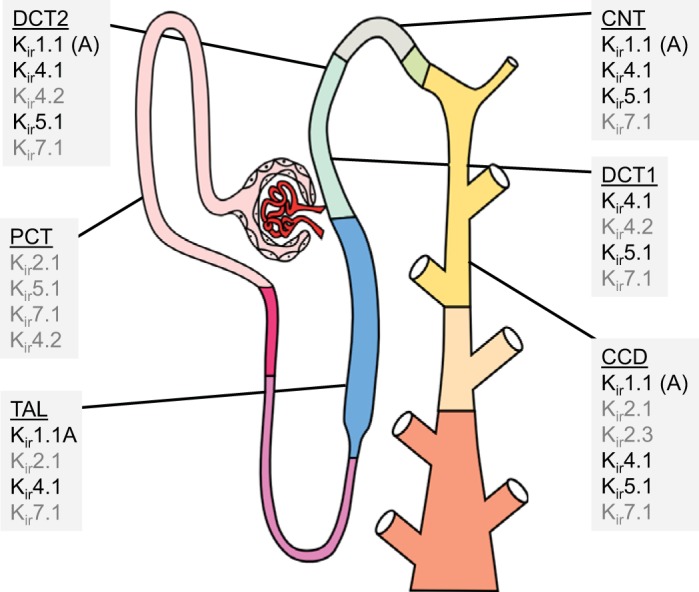
Inwardly rectifying K+ (Kir) channels expressed along the nephron. Shown is a summary of Kir subunits reported to be expressed in the proximal convoluted tubule (PCT), thick ascending limb (TAL), connecting tubule (CNT), distal convoluted tubule (DCT), and cortical collecting duct (CCD). Kir subunits noted in black have been validated using several models and techniques to assess mRNA, protein localization, and/or channel function. Gray indicates Kir subunits that have been reported but their expression and activity have not been thoroughly validated. All Kir subunits expressed basolaterally except for Kir1.1 [renal outer medullary K+ (ROMK) channel], which is expressed on the apical membrane (denoted by “A”).
Kir1.1 (RENAL OUTER MEDULLARY K+ CHANNEL)
The first inward rectifier family member to be identified in the kidney, Kir1.1, is encoded by KCNJ1 and is more commonly referred to as the renal outer medullary K+ channel (ROMK) (19). ROMK can be classified as an ATP-regulated or K+ transport channel with weak inward rectification. ROMK expression has been localized to the apical membrane of the thick ascending limb (TAL) of the loop of Henle, the distal convoluted tubule (DCT), the connecting tubule (CNT), and the cortical collecting duct (CCD) (24, 34). ROMK surface expression in the DCT, which is part of the aldosterone sensitive distal nephron (ASDN), has been shown to be regulated by extracellular K+ concentration and aldosterone signaling (57). Three alternatively spliced ROMK isoforms [ROMK1 (Kir1.1a), ROMK2 (Kir1.1b), and ROMK3 (Kir1.1c)] differ in sequence at the amino terminus, but all yield functional channels with high open probabilities and essentially identical biophysical properties (6, 58). Despite no detectable differences in function, the isoforms are distinct in their localization along the nephron. In the distal nephron, ROMK1 and ROMK3 are expressed specifically in principal cells and are largely responsible for K+ secretion and the maintenance of overall K+ homeostasis. In the TAL, ROMK2 is the primary determinant of the apical membrane conductance and drives Na+ transport through Na+-K+-2Cl− cotransporter activity, thereby contributing to Na+ reabsorption and urine concentration. Loss of function mutations in KCNJ1 causes Bartter syndrome, a severe condition that includes renal salt wasting, hypokalemia, and hypotension (7).
Kir4.1 AND Kir5.1
Kir4.1 (encoded by KCNJ10), a weak inward rectifier and ATP-regulated Kir channel, is exclusively localized to the basolateral membrane of epithelial cells in the TAL, DCT, CNT, and CCD (64). In the kidney, Kir4.1 contributes to both homotetrameric as well as heterotetrameric channels by assembling with Kir5.1 subunits (encoded by KCNJ16). The Kir5.1 subunit (a classical group of Kir protein) forms heterotetameric channels with other ATP-regulated or classical subunits. Presumably, Kir5.1 is coexpressed with Kir4.1 and forms the heteromeric Kir4.1/Kir5.1 basolateral channel in the ADSN. Compared with Kir4.1 homomeric channels, Kir4.1/Kir5.1 channels have higher single-channel conductance, greater pH sensitivity, and increased membrane expression (27, 56). Kir4.1/Kir5.1 channels have been determined to have a key role in setting the basolateral membrane potential and conductance. Ion transport through these channels is coupled to the activity of Na+-K+ ATPase allowing for K+ recycling across the basolateral membrane. Therefore, Kir4.1/Kir5.1 channels are significant determinants of the transepithelial voltage and provide the driving force controlling Na+ reabsorption and K+ secretion and thereby “fine tune” whole body electrolyte homeostasis, regulate blood volume, and ultimately contribute to long-term blood pressure control (37, 38). Kir4.1/Kir5.1 channels are sensitive to physiological pH changes, and we have shown that the expression of the Kir5.1 subunit is required for maintaining pH homeostasis (43). Furthermore, our recent studies have revealed a critical role of Kir5.1 in the control of the renin-angiotensin-aldosterone system (32). Unlike the Kir4.1 homomer, Kir4.1/Kir5.1 channel activity is regulated by extracellular K+ as well as intracellular Na+ (41, 45). Consistent with this, these channels have been shown to act as K+ sensors in the distal nephron (10) and to regulate the expression and/or activity of apical Na+ transport through epithelial Na+ channels and the NaCl cotransporter (20, 49, 65). The contribution of with no lysine (WNK)4 and Ste20-related proline/alanine-rich kinase (SPAK)/oxidative stress response 1 (OSR1) kinases to this mechanism has also been reported (48, 55). The WNK/SPAK/OSR1 pathway was found to be associated with other Kir channels as well, including Kir1.1, Kir2.1, and Kir2.3 (54, 61).
As key regulators of renal salt handling, Kir4.1/Kir5.1 channels may mediate blood pressure responses to dietary salt intake, and dysfunction in these channels has been proposed to contribute to the pathogenesis of salt-sensitive hypertension (36, 38). Loss-of-function mutations in KCNJ10 results in a number of neurological and renal pathologies in humans known as EAST/SeSAME syndrome (5, 44, 47). The prominent renal phenotype associated with this syndrome, including salt-wasting tubulopathy, hypokalemia, and metabolic alkalosis, has been proposed to be largely due to impaired function or mislocalization of the heteromeric Kir4.1/5.1 channel (46, 53, 60).
Kir5.1 has also been reported to be expressed in the proximal tubule (PCT) (56) and suggested as a negative regulator of the Kir2.1 channel due to their apparent colocalization on the basolateral membrane and evidence that heteromeric coassembly of Kir2.1 and Kir5.1 results in an electrically silent channel (12). However, this requires validation as expression of Kir5.1 in the PCT has not been firmly established.
LESS-STUDIED RENAL Kir CHANNELS
Kir7.1
A more recently identified ATP-regulated transport Kir channel, Kir7.1 (KCNJ13), has been shown by immunohistochemistry, Western blot, and/or RT-PCR to be expressed on the basolateral membrane of the PCT, TAL, DCT, CNT, and CCD segments (11, 35). However, it is evident that more work needs to be done to characterize Kir7.1 expression in the kidney, as reports of its localization are inconsistent and sometimes contradictory, which is likely due to limitations of antibody-based techniques and the inherent disconnect between mRNA abundance and protein expression. A recent study used CRISPR/Cas9 to create a Kir7.1 knockin mouse with a hemagglutinin epitope tagging the protein. In contrast to other reports, they found Kir7.1 expression to be limited to the inner medullary collecting ducts and absent from the cortex (9). Renal Kir7.1 channels are likely to be homotetramers, as no heteromeric Kir channels containing Kir7.1 have been identified. Compared with the rest of the Kir channel family, Kir7.1 has distinct properties resulting from an amino acid difference in the pore region (position 125): where other Kir channels have an arginine residue, Kir7.1 has a methionine residue (13, 25). As a result, Kir7.1 has a much lower affinity for Ba2+ and channel conductance is neither dependent on voltage nor extracellular K+ concentration (13). Regulation of Kir7.1 by PKA and PKC was found to be similar to other Kir channels (66). Functional studies of Kir7.1 have thus far been limited to in vitro heterologous expression systems, which may provide a foundation for future ex vivo experiments required. In addition, the physiological function of Kir7.1 in the kidney remains poorly understood. Some evidence suggests that Kir7.1 may play an important role in renal K+ excretion during development (50). It has also been proposed that Kir7.1 may be involved in K+ recycling and transport because it is expressed basolaterally like the Kir4.1/Kir5.1 channel. Knockout mouse and rat models of Kir7.1 are currently being developed and will reveal much more about the physiological function of these channels. Although Kir7.1 is expressed in numerous cell types, identified Kir7.1 channelopathies are primarily associated with retinal defects and congenital blindness and do not display a clear renal phenotype (26).
Kir4.2
Like Kir4.1, Kir4.2 (KCNJ15) is an ATP-regulated transport channel also proposed to be expressed on the basolateral side of DCT epithelia (31). However, a recent study (4) using RT-PCR on microdissected tubule segments showed its localization to be exclusive to the PCT. Kir4.2 has been found to be more pH sensitive than Kir4.1 with pKa values of 7.1 and 6.0, respectively (39, 40). Functional properties of Kir4.2 have been studied in vitro using heterologous expression systems. Like Kir4.1, Kir4.2 can form both a homomeric channel and a functional heterotetramer with Kir5.1. The heteromerization of Kir4.2 with Kir5.1 converts Kir4.2 from a strong to a weak rectifier, which renders it sensitive to intracellular pH (28, 39). Although Kir4.2 and Kir5.1 are coexpressed in the DCT, the presence of a Kir4.2/Kir5.1 channel has been proposed but not validated in the kidney (31). Like ROMK and Kir4.1, Kir4.2 channels are regulated by extracellular K+ content. However, extracellular K+ does not alter the surface expression of Kir4.2 but rather accelerates the transition from a deactivated channel state to an activated state (14). A recently developed Kcnj15 knockout mouse revealed that renal Kir4.2 channels play a role in acid-base homeostasis distinct from Kir4.1. The genetic deletion of Kir4.2 caused metabolic acidosis with intracellular alkalization and membrane depolarization in PCT cells (4). Although specific channelopathies of renal Kir4.2 have not been identified clinically thus far, KCNJ15 has been found to be downregulated in renal cell carcinoma and it has been suggested that it may have a role as a tumor suppressor and a potential therapeutic target (30).
Kir2.1 and Kir2.3
Kir2.1, encoded by the KCNJ2 gene, is a classical Kir channel and strong inward rectifier most known for its prominent role in the brain and heart. Although much less is known about its role in the kidney, Kir2.1 mRNA has been shown in the glomerulus, PCT, TAL, and CCD (12). However, functional studies to validate the expression of Kir2.1 and the role of strong rectification in renal cells are lacking. In the kidney, Kir2.1 has also been found in the afferent and efferent arterioles, and it has been shown to be a major determinant of the resting membrane potential in juxtaglomerular cells (8, 15). Genetic variations in KCNJ2 are present in ~90% of patients with inherited Andersen’s syndrome, which is characterized by hypokalemic periodic paralysis and includes some renal impairment (42).
Kir2.3 (encoded by the KCNJ4 gene) is also a classical Kir channel with strong inward rectification. It is expressed basolaterally in CCD cells, where it may contribute to driving K+ transport across the epithelium for its secretion through ROMK (59). Basolateral localization of this channel is likely due to the presence of a specific 15-amino acid basolateral sorting signal on the carboxy terminal (29). Kir2.3 has a high open probability and was previously proposed as the dominant channel responsible for basolateral K+ conductance in the CCD (33); however, more recent evidence implicates the Kir4.1/Kir5.1 channel for this role.
CONCLUSIONS
Kir channels are integral to overall renal physiology and play a key role in maintaining K+ homeostasis, modulating Na+ transport, and regulating blood pressure. Renal epithelial cells exclusively express subunits belonging to only two Kir channel classes (ATP regulated and classical), which function to transport K+ across their basolateral and apical membranes. In humans, hereditary Kir channel mutations, or Kir channelopathies, often include renal deficits with dysregulated Na+ and K+ homeostasis. In addition, growing evidence implicates renal Kir channel dysfunction in the pathophysiology of more common complex diseases, such as hypertension. The contribution of renal Kir channels to complex disease states may be particularly relevant in pathologies involving dysregulated Mg2+, such as diabetes (1), because gating by Mg2+ is universal to all Kir channels. Collectively, these channels’ known and hypothesized involvement in pathological conditions has warranted pharmacological studies targeting specific Kir channels, which has been historically problematic. Multiple nonspecific medications, such as fluoxetine (Kir4.1), amitriptyline (Kir4.1/Kir5.1), and diphenhydramine (Kir2.3), were found to modulate Kir channel activity as an off-target effect (3). Progress is currently being made in the development of specific small-molecule Kir channel modulators, which is sure to accelerate progress in the study of these channels. Drugs targeting Kir1.1 (VU591) (2), Kir4.1 (VU0134992) (23), and Kir7.1 (ML418) (51) have already been made available. More work is needed to precisely define and validate localization of specific Kir channel expression and their distribution along the nephron. Furthermore, greater focus on in vivo pharmacological assessment and ex vivo functional characterization will be required to fully appreciate the role of Kir channels in renal physiology and evaluate their utility as therapeutic targets.
GRANTS
This work was was partially supported by National Institutes of Health Grants R35-HL-135749 and P01-HL-116264 (to A. Staruschenko), R56-DK-121750 (to O. Palygin), F31-DK-122647 (to A. Manis), and R01-HL-122358 (to M. Hodges), American Heart Association Grant 16EIA26720006 (to A. Staruschenko), and Department of Veteran Affairs Grant I01 BX004024 (to A. Staruschenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.D.M. and O.P. prepared figures; A.D.M. and O.P. drafted manuscript; A.D.M., M.R.H., A.S., and O.P. edited and revised manuscript; A.D.M., M.R.H., A.S., and O.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize if additional relevant publications were not directly or fully discussed due to space limitation of this review.
REFERENCES
- 1.Barbagallo M, Dominguez LJ. Magnesium and type 2 diabetes. World J Diabetes 6: 1152–1157, 2015. doi: 10.4239/wjd.v6.i10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhave G, Chauder BA, Liu W, Dawson ES, Kadakia R, Nguyen TT, Lewis LM, Meiler J, Weaver CD, Satlin LM, Lindsley CW, Denton JS. Development of a selective small-molecule inhibitor of Kir1.1, the renal outer medullary potassium channel. Mol Pharmacol 79: 42–50, 2011. doi: 10.1124/mol.110.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhave G, Lonergan D, Chauder BA, Denton JS. Small-molecule modulators of inward rectifier K+ channels: recent advances and future possibilities. Future Med Chem 2: 757–774, 2010. doi: 10.4155/fmc.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bignon Y, Pinelli L, Frachon N, Lahuna O, Figueres L, Houillier P, Lourdel S, Teulon J, Paulais M. Defective bicarbonate reabsorption in Kir4.2 potassium channel deficient mice impairs acid-base balance and ammonia excretion. Kidney Int S0085-2538(19)31032-4, 2019. doi: 10.1016/j.kint.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1132–F1140, 1995. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CJ, Sung CC, Huang CL, Lin SH. Inward-rectifying potassium channelopathies: new insights into disorders of sodium and potassium homeostasis. Pediatr Nephrol 30: 373–383, 2015. doi: 10.1007/s00467-014-2764-0. [DOI] [PubMed] [Google Scholar]
- 8.Chilton L, Loutzenhiser K, Morales E, Breaks J, Kargacin GJ, Loutzenhiser R. Inward rectifier K(+) currents and Kir2.1 expression in renal afferent and efferent arterioles. J Am Soc Nephrol 19: 69–76, 2008. doi: 10.1681/ASN.2007010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornejo I, Villanueva S, Burgos J, López-Cayuqueo KI, Chambrey R, Julio-Kalajzić F, Buelvas N, Niemeyer MI, Figueiras-Fierro D, Brown PD, Sepúlveda FV, Cid LP. Tissue distribution of Kir7.1 inwardly rectifying K+ channel probed in a knock-in mouse expressing a haemagglutinin-tagged protein. Front Physiol 9: 428, 2018. doi: 10.3389/fphys.2018.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang CL, Ellison DH, Wang WH. Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017. doi: 10.1681/ASN.2016090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derst C, Hirsch JR, Preisig-Müller R, Wischmeyer E, Karschin A, Döring F, Thomzig A, Veh RW, Schlatter E, Kummer W, Daut J. Cellular localization of the potassium channel Kir7.1 in guinea pig and human kidney. Kidney Int 59: 2197–2205, 2001. doi: 10.1046/j.1523-1755.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- 12.Derst C, Karschin C, Wischmeyer E, Hirsch JR, Preisig-Müller R, Rajan S, Engel H, Grzeschik K, Daut J, Karschin A. Genetic and functional linkage of Kir5.1 and Kir2.1 channel subunits. FEBS Lett 491: 305–311, 2001. doi: 10.1016/S0014-5793(01)02202-5. [DOI] [PubMed] [Google Scholar]
- 13.Döring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J Neurosci 18: 8625–8636, 1998. doi: 10.1523/JNEUROSCI.18-21-08625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edvinsson JM, Shah AJ, Palmer LG. Potassium-dependent activation of Kir4.2 K+ channels. J Physiol 589: 5949–5963, 2011. doi: 10.1113/jphysiol.2011.220731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishman MC. Membrane potential of juxtaglomerular cells. Nature 260: 542–544, 1976. doi: 10.1038/260542a0. [DOI] [PubMed] [Google Scholar]
- 16.González C, Baez-Nieto D, Valencia I, Oyarzún I, Rojas P, Naranjo D, Latorre R. K(+) channels: function-structural overview. Compr Physiol 2: 2087–2149, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477: 495–498, 2011. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 19.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 20.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA. Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 100: 321–356, 2020. doi: 10.1152/physrev.00044.2018. [DOI] [PubMed] [Google Scholar]
- 21.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 391: 803–806, 1998. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 22.Isaeva E, Fedoriuk M, Bohovyk R, Klemens CA, Khedr S, Golosova D, Levchenko V, El-Meanawy A, Palygin O, Staruschenko A. Vibrodissociation method for isolation of defined nephron segments from human and rodent kidneys. Am J Physiol Renal Physiol 317: F1398–F1403, 2019. doi: 10.1152/ajprenal.00448.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharade SV, Kurata H, Bender AM, Blobaum AL, Figueroa EE, Duran A, Kramer M, Days E, Vinson P, Flores D, Satlin LM, Meiler J, Weaver CD, Lindsley CW, Hopkins CR, Denton JS. Discovery, characterization, and effects on renal fluid and electrolyte excretion of the Kir4.1 potassium channel pore blocker, VU0134992. Mol Pharmacol 94: 926–937, 2018. doi: 10.1124/mol.118.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohda Y, Ding W, Phan E, Housini I, Wang J, Star RA, Huang CL. Localization of the ROMK potassium channel to the apical membrane of distal nephron in rat kidney. Kidney Int 54: 1214–1223, 1998. doi: 10.1046/j.1523-1755.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 25.Krapivinsky G, Medina I, Eng L, Krapivinsky L, Yang Y, Clapham DE. A novel inward rectifier K+ channel with unique pore properties. Neuron 20: 995–1005, 1998. doi: 10.1016/S0896-6273(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 26.Kumar M, Pattnaik BR. Focus on Kir7.1: physiology and channelopathy. Channels (Austin) 8: 488–495, 2014. doi: 10.4161/19336950.2014.959809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- 28.Lam HD, Lemay AM, Briggs MM, Yung M, Hill CE. Modulation of Kir4.2 rectification properties and pHi-sensitive run-down by association with Kir5.1. Biochim Biophys Acta 1758: 1837–1845, 2006. doi: 10.1016/j.bbamem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Le Maout S, Welling PA, Brejon M, Olsen O, Merot J. Basolateral membrane expression of a K+ channel, Kir 2.3, is directed by a cytoplasmic COOH-terminal domain. Proc Natl Acad Sci USA 98: 10475–10480, 2001. doi: 10.1073/pnas.181481098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Wang H, Ni B, Zhang J, Li S, Huang Y, Cai Y, Mei H, Li Z. Loss of KCNJ15 expression promotes malignant phenotypes and correlates with poor prognosis in renal carcinoma. Cancer Manag Res 11: 1211–1220, 2019. doi: 10.2147/CMAR.S184368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manis AD, Palygin O, Khedr S, Levchenko V, Hodges MR, Staruschenko A. Relationship between renin-angiotensin-aldosterone system and renal Kir5.1 channels. Clin Sci (Lond) 133: 2449–2461, 2019. doi: 10.1042/CS20190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millar ID, Taylor HC, Cooper GJ, Kibble JD, Robson L. A Kir2.3-like K+ conductance in mouse cortical collecting duct principal cells. J Membr Biol 211: 173–184, 2006. doi: 10.1007/s00232-006-0036-z. [DOI] [PubMed] [Google Scholar]
- 34.Nüsing RM, Pantalone F, Gröne HJ, Seyberth HW, Wegmann M. Expression of the potassium channel ROMK in adult and fetal human kidney. Histochem Cell Biol 123: 553–559, 2005. doi: 10.1007/s00418-004-0742-5. [DOI] [PubMed] [Google Scholar]
- 35.Ookata K, Tojo A, Suzuki Y, Nakamura N, Kimura K, Wilcox CS, Hirose S. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Nephrol 11: 1987–1994, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: e92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palygin O, Pochynyuk O, Staruschenko A. Distal tubule basolateral potassium channels: cellular and molecular mechanisms of regulation. Curr Opin Nephrol Hypertens 27: 373–378, 2018. doi: 10.1097/MNH.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palygin O, Pochynyuk O, Staruschenko A. Role and mechanisms of regulation of the basolateral Kir 4.1/Kir 5.1K+ channels in the distal tubules. Acta Physiol (Oxf) 219: 260–273, 2017. doi: 10.1111/apha.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson WL, Dourado M, Schreiber M, Salkoff L, Nichols CG. Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver. J Physiol 514: 639–653, 1999. doi: 10.1111/j.1469-7793.1999.639ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pessia M, Imbrici P, D’Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol 532: 359–367, 2001. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J 15: 2980–2987, 1996. doi: 10.1002/j.1460-2075.1996.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plaster NM, Tawil R, Tristani-Firouzi M, Canún S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptácek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell 105: 511–519, 2001. doi: 10.1016/S0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 43.Puissant MM, Muere C, Levchenko V, Manis AD, Martino P, Forster HV, Palygin O, Staruschenko A, Hodges MR. Genetic mutation of Kcnj16 identifies Kir5.1-containing channels as key regulators of acute and chronic pH homeostasis. FASEB J 33: 5067–5075, 2019. doi: 10.1096/fj.201802257R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben-Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA 107: 14490–14495, 2010. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenhouse-Dantsker A, Sui JL, Zhao Q, Rusinova R, Rodríguez-Menchaca AA, Zhang Z, Logothetis DE. A sodium-mediated structural switch that controls the sensitivity of Kir channels to PtdIns(4,5)P(2). Nat Chem Biol 4: 624–631, 2008. doi: 10.1038/nchembio.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10). J Biol Chem 285: 36040–36048, 2010. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su XT, Ellison DH, Wang WH. Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K+ excretion. Am J Physiol Renal Physiol 316: F582–F586, 2019. doi: 10.1152/ajprenal.00412.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH. Disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol 310: F985–F993, 2016. doi: 10.1152/ajprenal.00584.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki Y, Yasuoka Y, Shimohama T, Nishikitani M, Nakamura N, Hirose S, Kawahara K. Expression of the K+ channel Kir7.1 in the developing rat kidney: role in K+ excretion. Kidney Int 63: 969–975, 2003. doi: 10.1046/j.1523-1755.2003.00806.x. [DOI] [PubMed] [Google Scholar]
- 51.Swale DR, Kurata H, Kharade SV, Sheehan J, Raphemot R, Voigtritter KR, Figueroa EE, Meiler J, Blobaum AL, Lindsley CW, Hopkins CR, Denton JS. ML418: the first selective, sub-micromolar pore blocker of Kir7.1 potassium channels. ACS Chem Neurosci 7: 1013–1023, 2016. doi: 10.1021/acschemneuro.6b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka-Kunishima M, Ishida Y, Takahashi K, Honda M, Oonuma T. Ancient intron insertion sites and palindromic genomic duplication evolutionally shapes an elementally functioning membrane protein family. BMC Evol Biol 7: 143, 2007. doi: 10.1186/1471-2148-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanemoto M, Abe T, Uchida S, Kawahara K. Mislocalization of K+ channels causes the renal salt wasting in EAST/SeSAME syndrome. FEBS Lett 588: 899–905, 2014. doi: 10.1016/j.febslet.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Taylor CA IV, An SW, Kankanamalage SG, Stippec S, Earnest S, Trivedi AT, Yang JZ, Mirzaei H, Huang CL, Cobb MH. OSR1 regulates a subset of inward rectifier potassium channels via a binding motif variant. Proc Natl Acad Sci USA 115: 3840–3845, 2018. doi: 10.1073/pnas.1802339115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomson MN, Cuevas CA, Bewarder TM, Dittmayer C, Miller LN, Si J, Cornelius RJ, Su XT, Yang CL, McCormick JA, Hadchouel J, Ellison DH, Bachmann S, Mutig K. WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia. Am J Physiol Renal Physiol 318: F216–F228, 2020. doi: 10.1152/ajprenal.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tucker SJ, Imbrici P, Salvatore L, D’Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem 275: 16404–16407, 2000. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- 57.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol Renal Fluid Electrolyte Physiol 275: F239–F245, 1998. doi: 10.1152/ajprenal.1998.275.2.F239. [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annu Rev Physiol 59: 413–436, 1997. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 59.Welling PA. Primary structure and functional expression of a cortical collecting duct Kir channel. Am J Physiol Renal Fluid Electrolyte Physiol 273: F825–F836, 1997. doi: 10.1152/ajprenal.1997.273.5.F825. [DOI] [PubMed] [Google Scholar]
- 60.Williams DM, Lopes CM, Rosenhouse-Dantsker A, Connelly HL, Matavel A, O-Uchi J, McBeath E, Gray DA. Molecular basis of decreased Kir4.1 function in SeSAME/EAST syndrome. J Am Soc Nephrol 21: 2117–2129, 2010. doi: 10.1681/ASN.2009121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu P, Gao ZX, Su XT, Ellison DH, Hadchouel J, Teulon J, Wang WH. Role of WNK4 and kidney-specific WNK1 in mediating the effect of high dietary K+ intake on ROMK channel in the distal convoluted tubule. Am J Physiol Renal Physiol 315: F223–F230, 2018. doi: 10.1152/ajprenal.00050.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaika O, Palygin O, Tomilin V, Mamenko M, Staruschenko A, Pochynyuk O. Insulin and IGF-1 activate Kir4.1/5.1 channels in cortical collecting duct principal cells to control basolateral membrane voltage. Am J Physiol Renal Physiol 310: F311–F321, 2016. doi: 10.1152/ajprenal.00436.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaika OL, Mamenko M, Palygin O, Boukelmoune N, Staruschenko A, Pochynyuk O. Direct inhibition of basolateral Kir4.1/5.1 and Kir4.1 channels in the cortical collecting duct by dopamine. Am J Physiol Renal Physiol 305: F1277–F1287, 2013. doi: 10.1152/ajprenal.00363.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang C, Wang L, Su XT, Lin DH, Wang WH. KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol 308: F1288–F1296, 2015. doi: 10.1152/ajprenal.00687.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111: 11864–11869, 2014. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W, Zitron E, Bloehs R, Müller-Krebs S, Scholz E, Zeier M, Katus H, Karle C, Schwenger V. Dual regulation of renal Kir7.1 potassium channels by protein kinase A and protein kinase C. Biochem Biophys Res Commun 377: 981–986, 2008. doi: 10.1016/j.bbrc.2008.10.110. [DOI] [PubMed] [Google Scholar]


