Abstract
Protein arginine methyltransferase 1 (PRMT1), which primarily causes asymmetric arginine methylation of histone and nonhistone proteins, has been found to activate gene expression and mediate multiple pathological processes. Its role in renal fibrosis, however, remains unclear. In the present study, we observed that PRMT1 and its specific epigenetic marker, asymmetric di-methylated histone 4 arginine 3 (H4R3Me2a), were highly expressed in cultured renal interstitial fibroblasts. Treatment of PRMT1 with AMI-1, a selective inhibitor of PRMT1, or silencing PRMT1 with siRNA inhibited serum-induced and transforming growth factor (TGF)-β1-induced expression of α-smooth muscle actin (α-SMA) and collagen type I, two hallmarks of renal fibroblast activation, in a dose-dependent and time-dependent manner. In a murine model of renal fibrosis induced by unilateral ureteral obstruction, PRMT1 expression and H4R3Me2a were also upregulated, which was coincident with increased expression of α-SMA, collagen type I, and fibronectin. Administration of AMI-1 reduced PRMT1 and H4R3Me2a expression, attenuated extracellular matrix protein deposition, and inhibited renal fibroblast activation and proliferation. Moreover, AMI-1 treatment inhibited Smad3 phosphorylation and TGF-β receptor I expression but prevented Smad7 downregulation both in the kidney after unilateral ureteral obstruction injury and in cultured renal interstitial fibroblasts exposed to TGF-β1. Collectively, these results demonstrate that PRMT1 may mediate renal fibroblast activation and renal fibrosis development through activation of the TGF-β/Smad3 signaling pathway. They also suggest that PRMT1 inhibition may be a potential therapeutic approach for the treatment of fibrotic kidney disease.
Keywords: α-smooth muscle actin, protein arginine methyltransferase 1, renal fibrosis, renal interstitial fibroblasts, transforming growth factor-β1
INTRODUCTION
Renal fibrosis is a common pathological change in the progression of chronic kidney disease (CKD) to the end stage of renal disease. During this process, renal interstitial fibroblasts differentiate into myofibroblasts, which express α-smooth muscle actin (α-SMA). Myofibroblasts produce a large amount of extracellular matrix (ECM) proteins, including collagen type I and fibronectin, which are deposited in the renal interstitium, leading to renal fibrosis (28, 38). Thus, identification of signaling pathways and genes that regulate activation of renal interstitial fibroblasts and accumulation of ECM proteins is critical for developing novel therapeutic treatments of renal fibrosis.
Mounting evidence demonstrates the crucial role of growth factors and cytokines such as transforming growth factor (TGF)-β1 in the activation of renal fibroblasts and production of excessive ECM proteins (16, 26). TGF-β1 exerts its biological responses through activation of heteromeric complexes of TGF-β type I and type II receptors (TGF-βRI and TGF-βRII, respectively). Once the receptors are activated, Smad3 protein is phosphorylated by TGF-βRI. Activated Smad3 protein forms a complex with Smad4 and then translocates to the nucleus to drive transcription of numerous target genes associated with fibrogenesis (17, 23). Smad3 protein also forms a complex with chromatin remodeling proteins that is required for transcriptional activation (33). The TGF-β/Smad3 signaling can be antagonized by Smad7, an inhibitory Smad (17, 23), through a mechanism involved in inhibiting Smad3 phosphorylation and enhancing the degradation of TGF-βRI (14, 24). In addition, the duration and intensity of signals transmitted to Smad3 protein are controlled by posttranslational modifications of various signaling components (33).
Many types of posttranslational modification have been identified in the regulation of signaling mediators. The role of acetylation, phosphorylation, and ubiquitination has been well studied in the context of TGF-β signaling; the importance of other types of protein modifications, such as methylation, has also been recognized recently. Protein methylation can occur through modification of lysine or arginine residues that are regulated by lysine and arginine methyltransferases, respectively. Our recent studies have demonstrated that EZH2, a lysine methyltransferase, is highly expressed in the kidney injured by unilateral urethral obstruction and in cultured renal interstitial fibroblasts exposed to TGF-β1. Blockade of EZH2 inhibits Smad3 phosphorylation by preserving Smad7 expression (37). SET9 lysine methyltransferase has also been reported to facilitate nuclear import of Smad3 and enhance Smad3 transcriptional activity (21). Interestingly, recent studies have shown that protein arginine methyltransferase 1 (PRMT1) can also induce methylation of Smad6 (10, 32) and Smad7 (12) as well as TGF-β1-induced epithelial-mesenchymal transition (EMT) of Smad7 (12); it is unclear whether PRMT1 is involved in the regulation of TGF-β/Smad3 signaling and renal fibroblast activation.
PRMT1 belongs to a family of arginine methyltransferases and is a predominant PRMT member in mammalian cells. It can catalyze methylation of the arginine 3 residue of histone H4, an epigenetic active marker (30, 32). Increasing evidence indicates that PRMT1 plays a crucial role in various biological processes and is linked to multiple pathological responses. For example, overexpression of PRMT1 was observed in several cancers (34) and is associated with pulmonary diseases (36), cardiovascular diseases (2), diabetes (11), and renal diseases (20). Furthermore, increased PRMT1 expression was found in lung myofibroblasts and alveolar type II cells in idiopathic pulmonary fibrosis and has been identified in fibrotic lesions of bleomycin-injured lungs (18, 35). This suggests that PRMT1 may contribute to the development of pulmonary fibrosis. However, the role of PRMT1 in renal fibrosis remains poorly understood.
In the present study, we conducted in vitro and in vivo experiments to examine the PRMT1 expression in renal interstitial fibroblasts and fibrotic kidneys, and we evaluated the effect of pharmacological PRMT1 abrogation on the activation of renal fibroblasts and development of renal fibrosis. Our results demonstrate that abundant PRMT1 is expressed in myofibroblasts and obstructed kidneys and that pharmacological inhibition of PRMT1 suppresses dedifferentiation of fibroblasts to myofibroblasts and development of renal fibrosis through a mechanism involved in the suppression of Smad3 activation.
MATERIALS AND METHODS
Antibodies and chemicals.
Asymmetric di-methylated histone 4 arginine 3 (H4R3me2a), PRMT1, fibronectin, collagen type Ι, Smad3, and phosphorylated (p-)Smad3 antibodies as well as AMI-1 were purchased from Abcam (Cambridge, MA). GAPDH antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). TGF-β1 was purchased from Cell Signaling Technology (Danvers, MA). The siRNA specifically for rat PRMT1 was purchased from RiboBio (Guangzhou, China). α-SMA antibody and all other chemicals were purchased from Sigma-Aldrich.
Animals and experimental design.
C57BL/6 male mice, weighing 20–22 g, were purchased from Shanghai Super-B&K Laboratory Animal Corporation in China and used in this study. To establish a model of unilateral ureteral obstruction (UUO), an incision along the midline of the abdominal cavity was made, and the left ureter was then isolated and ligated with 4-0 silk. The contralateral kidney of mice that underwent the same surgery without ligation of the ureter was used as a sham control. AMI-1 (10 mg/kg) in 50 μL DMSO was injected intraperitoneally daily in UUO mice for 7 days. The same dose of DMSO was administered in sham and UUO alone groups. At the end of experiment, animals were euthanized, and the kidneys were collected for histological examination and protein analysis. All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Tongji University Animal Welfare Committee.
Cell culture, inhibitor treatment, siRNA transfection, and proliferation measurement.
Rat renal interstitial fibroblasts (NRK-49F) were cultured in DMEM with F-12 media supplemented with 0.5% penicillin and streptomycin and 5% FBS in an atmosphere of 95% air and 5% CO2 at a temperature of 37°C. Various doses of AMI-1 were added directly to the subconfluent NRK-49F cells, which were subsequently cultured for the periods indicated in the figures. For the treatment with TGF-β1, serum-starved NRK-49F cells were placed in DMEM containing 0.5% serum for 24 h and then subjected to 5 ng/mL TGF-β1 for an additional 24 h with or without AMI-1. For siRNA transfection, siRNA oligonucleotides targeted specifically to PRMT1 (200 pmol) were transfected into NRK-49F cells by Lipofectamine 2000. As a control, 200 pmol of scrambled control siRNA were also transfected to NRK-49F cells in separate dishes. After transfection, cells were plated and cultured for 36 h in DMEM-F-12 with 5% FBS before cell lysates were prepared for immunoblot analysis. Cell proliferation was assessed by the Cell Counting Kit-8 assay according to the procedure provided by the manufacturer (Abcam). Cultured cells were also photographed, and cell numbers were counted under phase-contrast microscopy.
Immunoblot analysis.
After treatment, cells were harvested into a mixture of cell lysis solution (Biotech Well) with a protease inhibitor cocktail (Biotech Well). The samples of kidneys were homogenized, and cell lyses were subjected to immunoblot analysis. Proteins (10–25 μg) were intercepted by PVDF membranes after being segregated by SDS-PAGE. PVDF membranes were incubated with primary antibody at 4°C overnight and subsequently washed four times with Tris-buffered saline with Tween 20. PVDF membranes were then soaked in a 5% nonfat milk blend with proper horseradish peroxidase-marked secondary antibodies at room temperature for 1 h. The bound antibodies were examined by chemiluminescence scanning.
Histology, immunohistochemical staining, and immunofluorescencent staining.
The kidneys were fixed in 4% paraformaldehyde and then embedded in paraffin blocks for histological evaluation. Tissue sections (3 μm in thickness) were stained with periodic acid-Schiff reagent by standard protocol. Immunofluorescent staining was performed according to the procedures described in our previous study (18). The following primary antibodies were used: rabbit polyclonal anti-collagen type I antibody (Abcam), rabbit polyclonal anti-PRMT1 antibody (Abcam), rabbit polyclonal anti-fibronectin antibody (Abcam), and mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody (Arigobio, Hsinchu, Taiwan). Primary antibodies were incubated overnight at 4°C followed by staining with fluorescent conjugated secondary antibody (Santa Cruz Biotechnology). Nuclei were costained with 4′,6-diamidino-2-phenylindole. A Zeiss 710 Duo microscope was used to take the images. Positive staining areas were assessed by Image Pro-Plus software.
Statistical analysis.
Data are presented as means ± SD (n = 6). Each experiment was undertaken at least three times, and the statistics were subjected to one-way ANOVA. Tukey’s test was used to compare multiple means, and a t test was used to analyze the differences. P > 0.05, P < 0.05, P < 0.01, P < 0.001, and P < 0.001 were considered not significant, significant, very significant, highly significant, and extremely significant, respectively.
RESULTS
Inhibition of PRMT1 with AMI-1 reduces serum-induced activation of cultured renal interstitial fibroblasts in a time-dependent and dose-dependent manner.
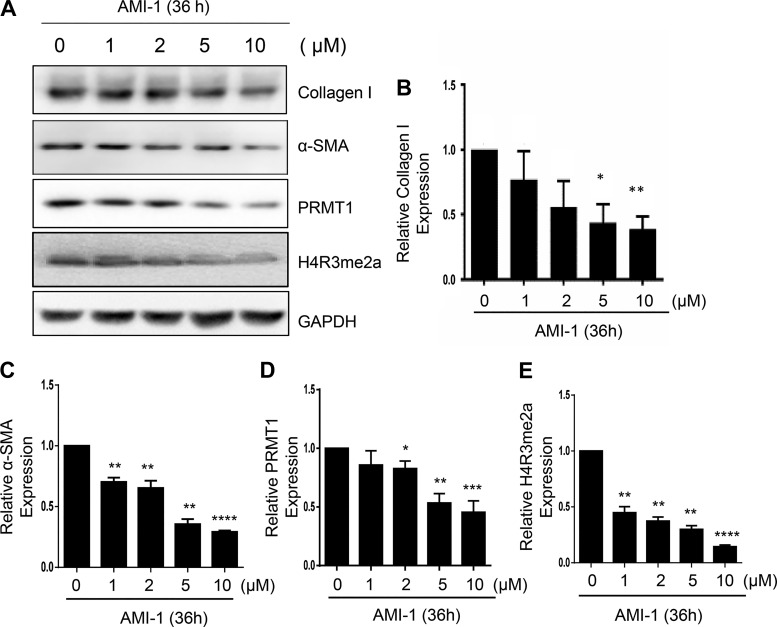
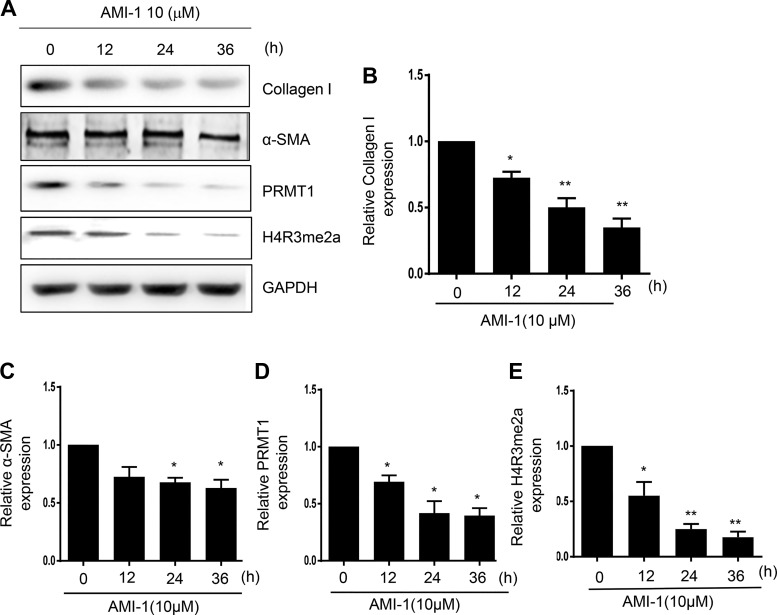
The development of renal fibrosis is characterized by transformation of renal interstitial fibroblasts to myofibroblasts as indicated by the expression of the mesenchymal marker α-SMA and production of ECM proteins such as collagen type Ι (7). To determine whether PRMT1 plays a role in the activation of renal fibroblasts, cultured rat renal interstitial fibroblasts (NRK-49F) with 5% FBS were exposed to various concentrations of AMI-1, a selective inhibitor for PRMT1, for 36 h. Immunoblot analysis of cell lysates indicated that α-SMA and collagen type Ι were expressed in NRK-49F cells and reduced in the presence of AMI-1 at 2 and 5 μM, and the maximum reduction occurred at 10 μM (Fig. 1, A–C). Similarly, AMI-1 treatment inhibited the expression of α-SMA in a time-dependent manner, with the maximum inhibition occuring when cells were treated with AMI-1 for 36 h (Fig. 2, A–C). To ensure the efficacy of AMI-1, we also examined the effect of AMI-1 on the expression of PRMT1 and H4R3me2a, a methylation marker of PRMT1. As shown in Fig. 1, A, D, and E, and Fig. 2, A, D, and E, PRMT1 and H4R3me2a were upregulated in serum-cultured NRK-49F cells, and AMI-1 treatment reduced their expression in a dose- and time-dependent manner. These data illustrate that PRMT1 activity is required for the differentiation of renal interstitial fibroblasts into myofibroblasts and proliferation.
Fig. 1.
Inhibition of protein arginine methyltransferase 1 (PRMT1) with AMI-1 reduces serum-induced activation of cultured renal interstitial fibroblasts in a dose-dependent manner. Normally cultured NRK-49F cells were treated with AMI-1 (0–10 μM) for 36 h. Cell lysates were then prepared and subjected to immunoblot analysis with antibodies against α-smooth muscle actin (α-SMA), collagen type I, PRMT1, asymmetric di-methylated histone 4 arginine 3 (H4R3me2a), or GAPDH (A). Collagen type I (B), α-SMA (C), PRMT1 (D), or H4R3me2a (E) were quantified by densitometry and normalized with GAPDH. Values are means ± SD of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.001 vs. controls.
Fig. 2.
Inhibition of protein arginine methyltransferase 1 (PRMT1) with AMI-1 reduces serum-induced activation of cultured renal interstitial fibroblasts in a time-dependent manner. Normally cultured NRK-49F cells were treated with AMI-1 (10 μM) for 0–36 h. Cell lysates were then prepared and subjected to immunoblot analysis with antibodies against collagen type I, α-smooth muscle actin (α-SMA), fibronectin, PRMT1, asymmetric di-methylated histone 4 arginine 3 (H4R3me2a), or GAPDH (A). Collagen type I (B), α-SMA (C), PRMT1 (D), or H4R3me2a (E) were quantified by densitometry and normalized with GAPDH. Values are means ± SD of at least three independent experiments. *P < 0.05 and **P < 0.01 vs. controls.
AMI-1 inhibits TGF-β1-induced activation of renal interstitial fibroblasts in culture.
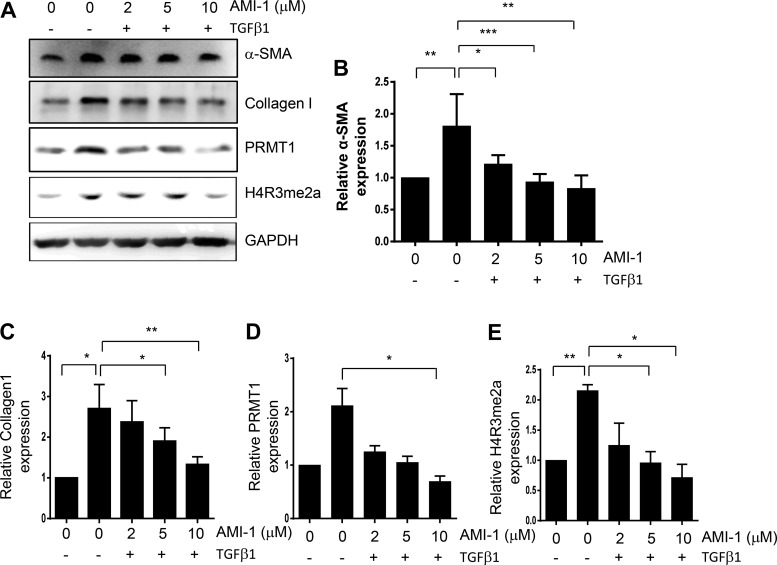
TGF-β1 is a potent cytokine involved in the activation of renal fibroblasts and renal fibrogenesis (16, 26). Thus, we further investigated the effect of PRMT1 inhibition on TGF-β1-induced activation of renal interstitial fibroblasts as well as expression of PRMT1 and H4R3me2a in cultured NRK-49F cells. Figure 3, A–C, shows that basal levels of α-SMA and collagen type I were detected in starved NRK-49F cells, whereas TGF-β1 treatment markedly enhanced their expression, indicating strengthened activation of renal fibroblasts by TGF-β1. AMI-1 treatment suppressed expressions of both α-SMA and collagen type I. Exposure to TGF-β1 also increased PRMT1 and H4R3me2a expression, which was suppressed by AMI-1 as well (Fig. 3, A, D, and E). These data demonstrated that PRMT1 activity is necessary for TGF-β1-induced activation of renal fibroblasts.
Fig. 3.
Inhibition of protein arginine methyltransferase 1 (PRMT1) with AMI-1 reduces transforming growth factor (TGF)-β1-induced activation of cultured renal interstitial fibroblasts in a dose-dependent manner. Serum-starved NRK-49F cells were pretreated with AMI-1 (0–10 μM) for 1 h and then exposed to TGF-β1 (5 ng/mL) for an additional 24 h. Cell lysates were prepared and subjected to immunoblot analysis with antibodies against α-smooth muscle actin (α-SMA), collagen type I, fibronectin, PRMT1, asymmetric di-methylated histone 4 arginine 3 (H4R3me2a), or GAPDH (A). α-SMA (B), collagen type I (C), PRMT1 (D), or H4R3me2a (E) were quantified by densitometry and normalized with GAPDH. Values are means ± SD of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. controls or other samples as indicated.
siRNA-mediated silencing of PRMT1 inhibits activation of cultured renal interstitial fibroblasts.
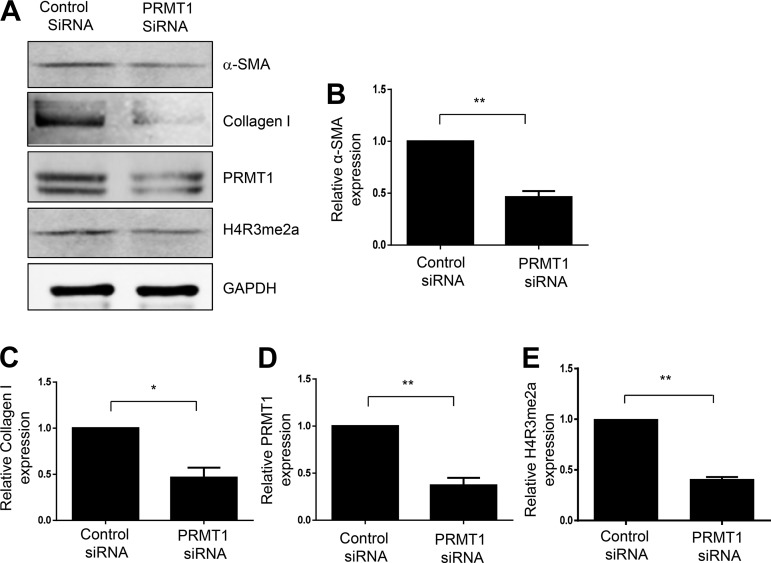
To validate the role of PRMT1 in mediating activation of renal interstitial fibroblasts, we examined the effect of siRNA specifically targeting PRMT1 on serum-induced activation of renal interstitial fibroblasts. Figure 4, A and D, shows that transfection with PRMT1 siRNA led to a 74% reduction of PRMT1 in cultured NRK-49F cells. Inhibition of PRMT1 activity by its siRNA was reflected in reduced expression of H4R3me2a (Fig. 4, A and E). In parallel with reduced expression and activity of PRMT1, transfection by PRMT1 siRNA largely inhibited expression of α-SMA and collagen type I induced by 5% FBS (Fig. 4, A–C). These data, together with the inhibitory effect of AMI-1 on the activation of renal interstitial fibroblasts, provide strong evidence for PRMT1 involvement in the activation of renal interstitial fibroblasts.
Fig. 4.
Knockdown of protein arginine methyltransferase 1 (PRMT1) by siRNA reduces activation of cultured renal interstitial fibroblasts. Serum-starved NRK-49F cells were transfected with siRNA specifically targeting PRMT1 or scrambled siRNA and then incubated in 5% FBS for an additional 36 h. Cell lysates were prepared for immunoblot analysis with antibodies against α-smooth muscle actin (α-SMA), collagen type I, PRMT1, asymmetric di-methylated histone 4 arginine 3 (H4R3me2a), or GAPDH (A). Expression levels of α-SMA (B), collagen type I (C), PRMT1 (D), or H4R3me2a (E) were quantified by densitometry and normalized with tubulin or GAPDH. Values are means ± SD of at least three independent experiments. *P < 0.05 and **P < 0.01 vs. siRNA controls.
Inhibition of PRMT1 by AMI-1 attenuates development of renal fibrosis in a murine model of obstructive nephropathy.
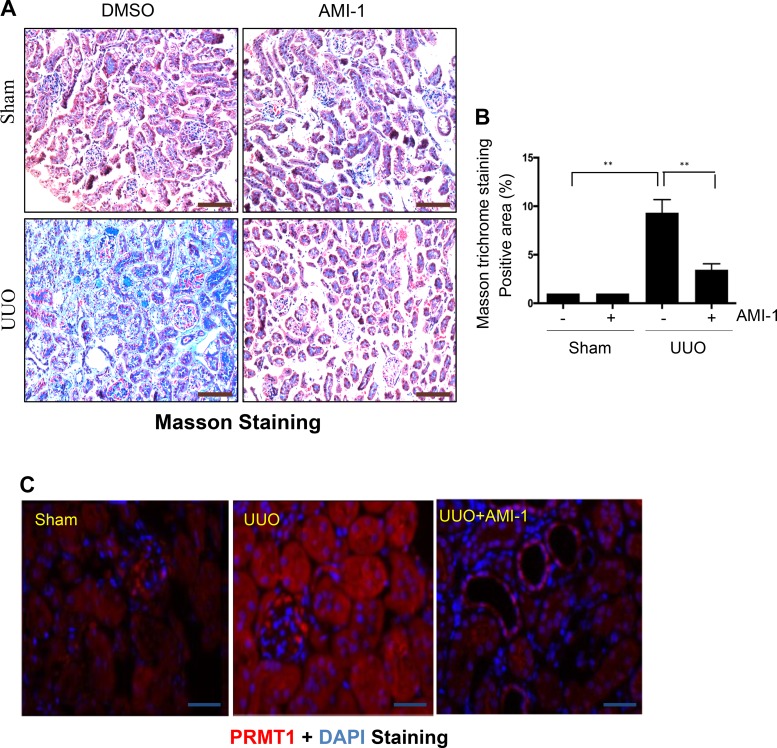
Given the importance of renal interstitial fibroblast activation in the development and progression of renal fibrosis, we further examined the effect of PRMT1 inhibition on renal fibrosis induced by UUO in mice. UUO injury induced deposition of collagen fibrils in the kidney at 7 days, as demonstrated by Masson trichrome staining; administration of AMI-1 significantly inhibited this response (Fig. 5, A and B). Immunofluorescent staining showed a weak signal of PRMT1 expression in the sham kidney, whereas UUO injury caused a dramatic increase of PRMT1 expression in the kidney. PRMT1 was clearly observed in the cytoplasm and nuclei of both renal tubular and interstitial cells (Fig. 5C). These results suggest that PRMT1 may be implicated in the pathological process of renal fibrosis by acting in both renal epithelial cells and renal interstitial fibroblasts. Notably, despite the fact that daily administration of AMI at 20 mg/kg for 7 or 14 days reduced body weight of mice compared with control mice that received vehicle (DMSO) alone, application of the AMI inhibitor at 10 mg/kg for 7 days did not affect either body weight and the weight of various main organs (heart, liver, spleen, lung, brain, and kidney without ureteral ligation) in mice compared with control mice (Tables 1 and 2), suggesting that at the dose used for inhibition of renal fibrosis, AMI-1 is not toxic.
Fig. 5.
A: administration of AMI-1 attenuates renal fibrosis in obstructed kidneys. Photomicrographs illustrate Masson trichrome staining of kidney tissue (magnification: ×200). B: the Masson trichrome-positive tubulointerstitial area relative to the whole area from 10 random cortical fields was analyzed. Data are presented as means ± SD; n = 6. **P < 0.01 vs. the sham control group or other groups as indicated. C: photomicrographs illustrating protein arginine methyltransferase 1 (PRMT1) expression in the kidney. Scale bar = 100 μm. UUO, unilateral ureteral obstruction.
Table 1.
Mouse body weight after daily administration of different doses of AMI-1 for 7 and 14 days
| AMI-1 | Sham, g | Sham + AMI-1, g |
|---|---|---|
| 7 days | ||
| 5 mg/kg | 24.8 ± 1.7 | 25.4 ± 1.6 |
| 10 mg/kg | 25.2 ± 1.4 | 24.9 ± 1.8 |
| 20 mg/kg | 25.3 ± 1.3 | 20.7 ± 0.7* |
| 14 days | ||
| 5 mg/kg | 26.2 ± 0.7 | 26.3 ± 0.9 |
| 10 mg/kg | 25.8 ± 1.6 | 24.5 ± 1.1 |
| 20 mg/kg | 26.1 ± 1.2 | 22.9 ± 1.4* |
Values are means ± SD.
P < 0.05 vs. the sham alone group.
Table 2.
Mouse organ weight after daily administration of AMI-1 at 10 mg/kg for 7 days
| Tissue | Sham, mg | Sham + AMI-1, mg |
|---|---|---|
| Heart | 122.15 ± 12.9 | 120.4 ± 10.3 |
| Liver | 898.41 ± 47.7 | 948.7 ± 92.7 |
| Spleen | 78.01 ± 11.1 | 83.0 ± 10.4 |
| Lung | 120.13 ± 8.3 | 101.6 ± 9.8 |
| Right kidney | 103.87 ± 9.4 | 113.2 ± 11.5 |
| Brain | 438.7 ± 20.6 | 429.3 ± 22.6 |
Values are means ± SD.
Administration of AMI-1 reduces accumulation of ECM proteins and activation of renal interstitial fibroblasts in the kidney after UUO injury.
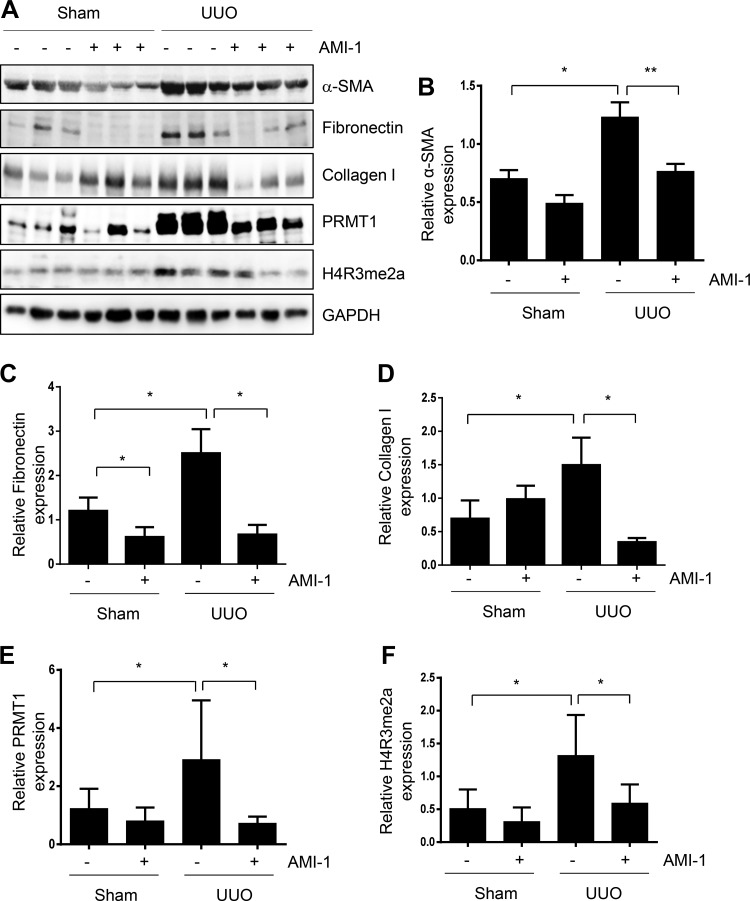
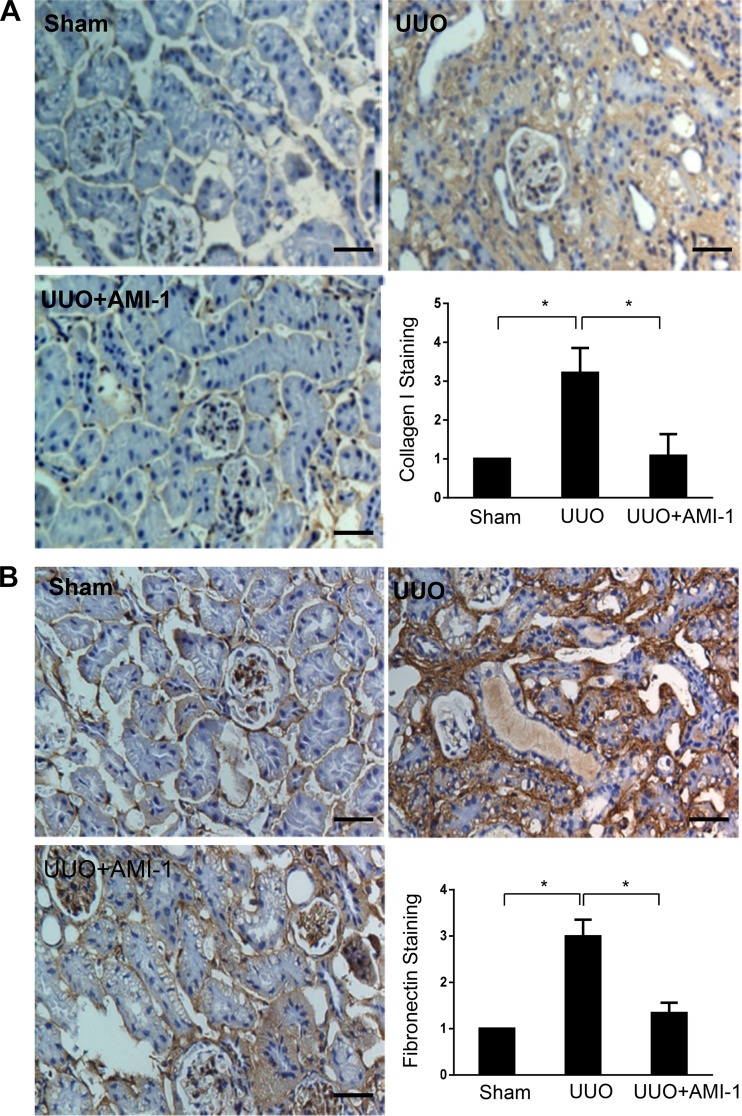
Activation of renal interstitial fibroblasts and accumulation of ECM are regarded as key events in the progression of renal dysfunction and of CKD to the end stage of renal disease (15). To investigate the effect of AMI-1 treatment on renal fibrogenesis, the whole kidney was collected at day 7 after ureteral ligation, and cell lysates were subjected to immunoblot analysis. Compared with sham kidneys, UUO kidneys demonstrated an increase in α-SMA, fibronectin, and collagen. Administration of AMI-1 reduced α-SMA, fibronectin, and collagen type I expression (Fig. 6, A–D). In line with those observations, AMI-1 was also effective in suppressing UUO-induced expression of collagen type I and fibronectin, as demonstrated by immunochemical staining (Fig. 7, A and B). As expected, UUO injury increased expression of PRMT1 and H4R3me2a, whereas AMI-1 reduced their expression (Fig. 6, A, E, and F). Notably, in contrast to collagen type I inhibition, AMI-1 increased expression of collagen type I in sham kidneys. The mechanism by which AMI-1 treatment resulted in the upregulation of collagen type I in sham kidneys remains unclear; this may be related to the distinct expression and functions of PRMT1 isoforms in diverse cell types within the kidney under physiological and pathological conditions. This issue is interesting and will be addressed in our future work. Overall, our data demonstrated that inhibition of PRMT1 expression and its activity by AMI-1 alleviates renal fibrosis after UUO injury. Additionally, AMI-1 treatment promotes collagen type I expression in the kidney of mice that received sham surgery.
Fig. 6.
Administration of AMI-1 inhibits expression of α-smooth muscle actin (α-SMA) and extracellular matrix proteins in obstructed kidneys. Kidney tissue lysates were subjected to immunoblot analysis with antibodies against α-SMA, collagen type I, fibronectin, protein arginine methyltransferase 1 (PRMT1), asymmetric di-methylated histone 4 arginine 3 (H4R3me2a), or GAPDH (A). α-SMA (B), fibronectin (C), collagen type I (D),PRMT1 (E), or H4R3me2a (F) were quantified by densitometry and normalized with GAPDH. Values are means ± SD of at least three independent experiments. *P < 0.05 and **P < 0.01 vs. the sham control group or other groups as indicated. UUO, unilateral ureteral obstruction.
Fig. 7.
Administration of AMI-1 inhibits expression of collagen type I and fibronectin in obstructed kidneys. Photomicrographs illustrate collagen type I (A) and fibronectin (B) staining of kidney tissue (magnification: ×200). The positive staining area of collagen type I or fibronectin relative to the whole area from 10 random cortical fields was analyzed. Data are presented as means ± SD (n = 6). *P < 0.05 vs. the sham control group and other groups as indicated. Scale bar = 100 μm. UUO, unilateral ureteral obstruction.
Blockade of PRMT1 inhibits phosphorylation of Smad3 in the UUO-injured kidney and cultured renal interstitial fibroblasts.
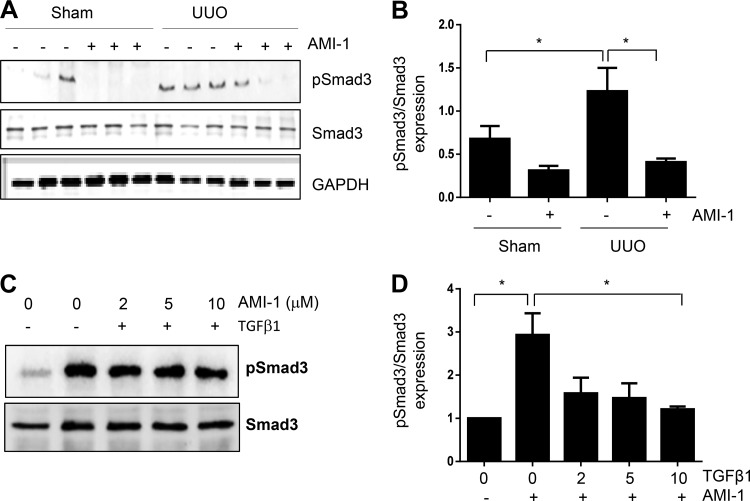
The TGF-β/Smad3 signaling pathway is well known for its role in driving expression of multiple fibrotic genes, including collagen type I (16, 26). PRMT1 has been reported to mediate arginine methylation of several nonhistone proteins, including Smurf2, a member of the E3 ligase family that acts as a negative regulator of TGF-β signaling by targeting Smads (3). We thus examined the effect of PRMT1 inhibition on the phosphorylation (activation) of Smad3 in the UUO kidney and cultured renal interstitial fibroblasts. As shown in Fig. 8, A and B, phosphorylation levels of Smad3 were increased in the obstructed kidney compared with p-Smad3 levels in sham kidneys and reduced in the kidneys of animals treated with AMI-1. Of note, AMI-1 treatment did not alter the expression of total Smad3. Similarly, AMI-1 dose-dependently inhibited TGF-β1-stimulated phosphorylation of Smad3 in cultured renal interstitial fibroblasts (Fig. 8, C and D). Therefore, it appears that PRMT1 activity is coupled with TGF-β/Smad3 signaling and mediates renal fibroblast activation and renal fibrosis.
Fig. 8.
AMI-1 inhibits phosphorylation of Smad3 in obstructed kidneys and cultured rat interstitial fibroblasts. Kidney tissue (A) or cell lysates (C) were subjected to immunoblot analysis with antibodies against phosphorylated (p)Smad3, Smad3, or GAPDH. pSmad3 was quantified by densitometry and normalized with Smad3 (B and D). Values are means ± SD of at least three independent experiments. *P < 0.05 vs. the sham control group and other groups as indicated. TGF-β1, transforming growth factor β1; UUO, unilateral ureteral obstruction.
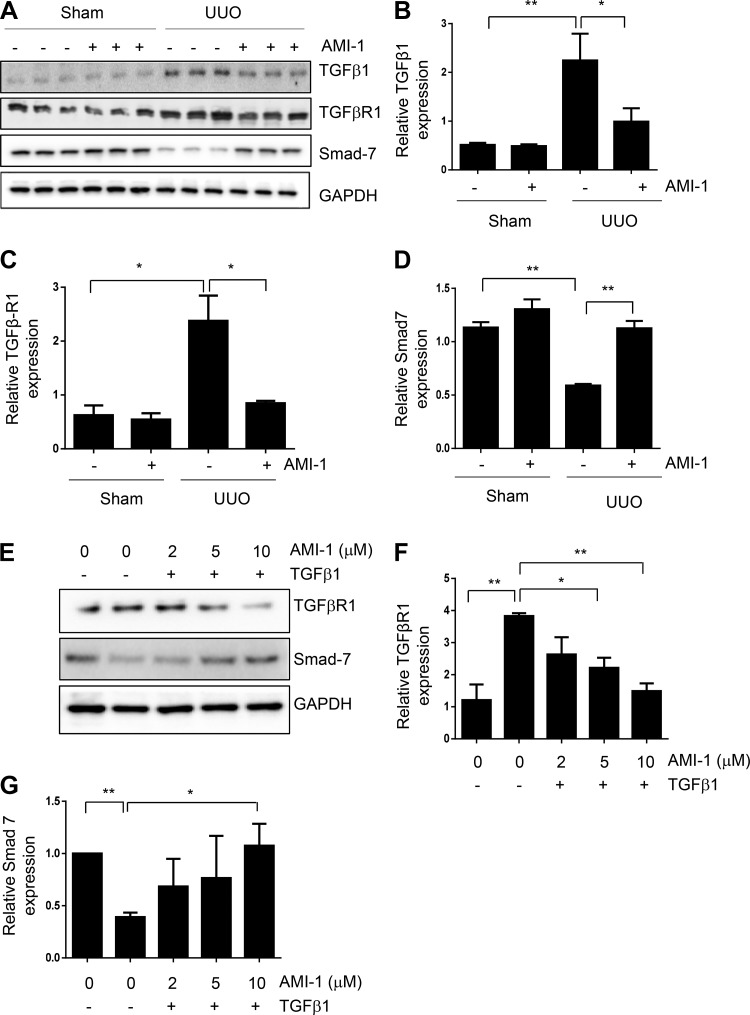
Blockade of PRMT1 inhibits expression of TGF-β1 and TGF-βRI and preserves expression of Smad7 in the kidney after UUO injury.
Given that Smad3 activation is initiated by interaction of TGF-β1 with TGF-βRI and is inhibited by Smad7 (14, 24), we further examined the effect of PRMT1 inhibition on expression of these molecules. As shown in Fig. 9, A–D, UUO injury to the kidney resulted in increased expression of TGF-β1 and TGF-βRI and decreased expression of Smad7, effects that were reversed by treatment with AMI-1. To verify the effect of AMI-1 on the expression of TGF-βRI and Smad7 in renal fibroblasts, we treated serum-starved NRK-49F cells with TGF-β1 in the absence or presence of AMI-1 and then analyzed expression levels of TGF-βRI and Smad7. Figure 9, E–G, shows that AMI-1 dose-dependently inhibited TGF-βR1 expression and restored Smad7 expression. Collectively, the above data illustrated that PRMT1 is critically involved in the activation of TGF-β/Smad3 signaling, possibly via a mechanism involved in the downregulation of Smad7.
Fig. 9.
AMI-1 inhibits transforming growth factor (TGF)-β1 and TGF-β receptor type I (TGFβRI) expression and prevents Smad7 downregulation in obstructed kidneys and suppresses TGFβRI expression and preserves Smad7 expression in cultured renal fibroblasts. Kidney tissue (A–D) and NRK-49F cells (E–G) were collected after various treatments as indicated in materials and methods. The prepared tissue or cell lysates were subjected to immunoblot analysis with antibodies against TGF-β1 (A), TGF-βRI (A and E), or Smad7 (A and E). TGF-β1 (B), TGF-βRI (C and F), or Smad7 (D and G) were quantified by densitometry and normalized with GAPDH. Values are means ± SD of at least three independent experiments. *P < 0.05 and **P < 0.01 vs. the sham control group or other groups as indicated. UUO, unilateral ureteral obstruction.
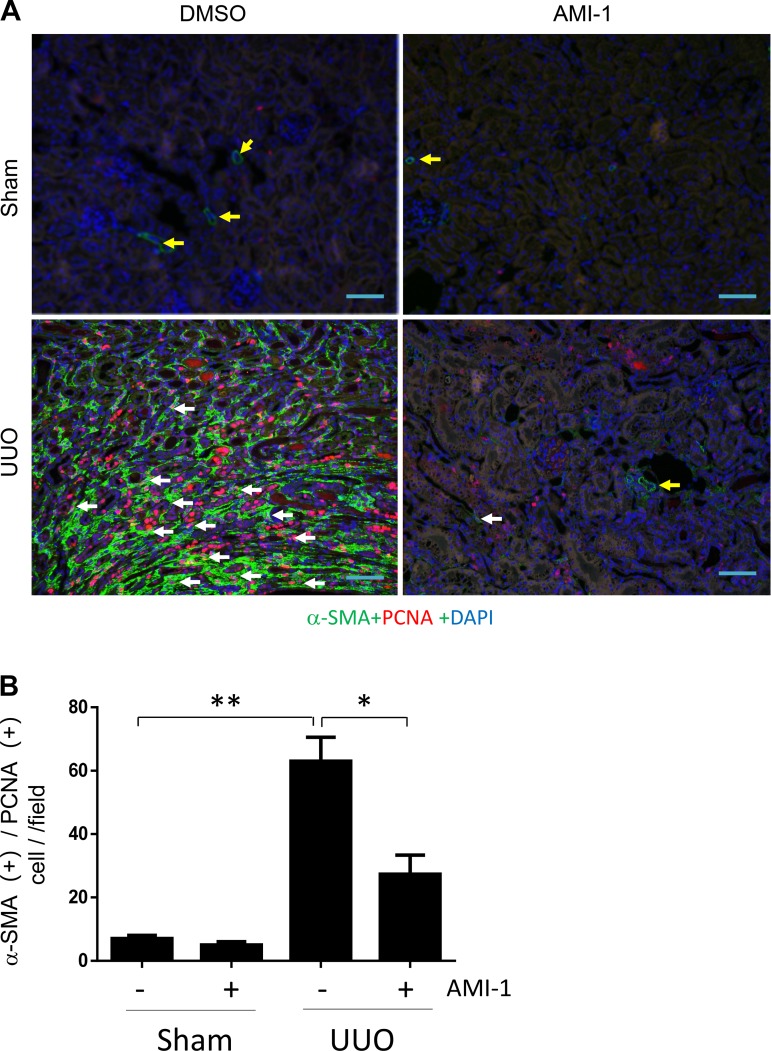
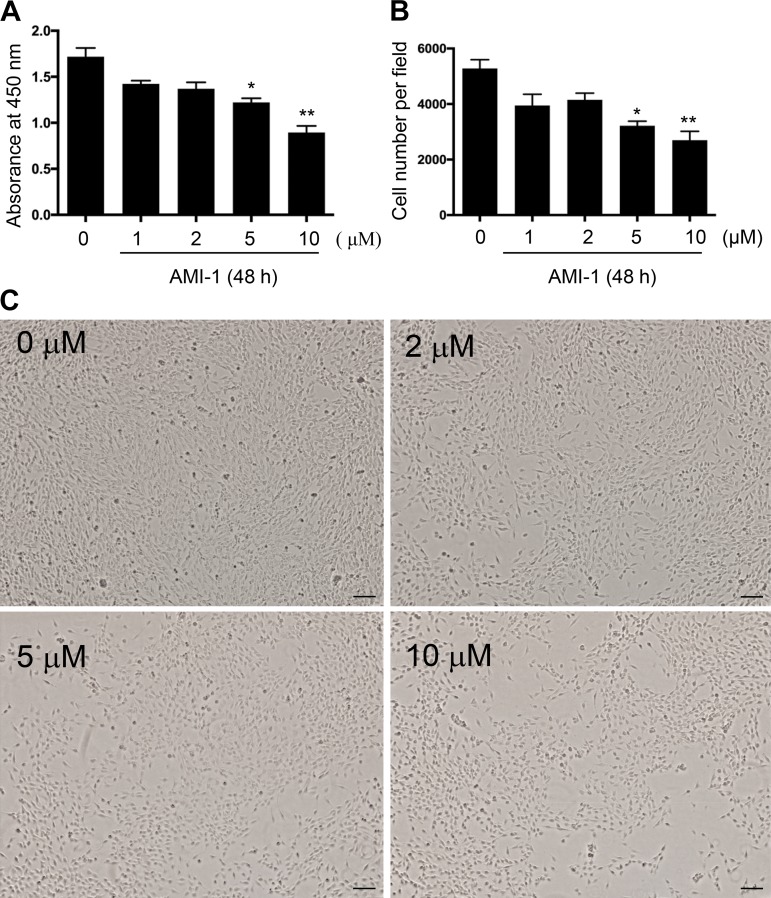
Blockade of PRMT1 inhibits proliferation of renal interstitial fibroblasts in vivo and in vitro.
Proliferation of renal interstitial fibroblasts is another profibrotic response after injury to the kidney (27). To understand the role of PRMT1 in this process, we determined the effect of AMI-1 on renal fibroblast proliferation in the kidney with UUO injury and in NRK49-F cells stimulated with serum. As shown in Fig. 10, A and B, UUO-injured kidneys displayed a larger number of myofibroblasts, as indicated by PCNA and α-SMA double positive staining compared with sham kidneys, whereas AMI-1 treatment resulted in a significant decrease in the number of myofibroblasts. To confirm this observation, we also examined the effect of AMI-1 on the proliferation of cultured NRK-49F cells; treatment with AMI-1 dose and time dependently inhibited serum-induced proliferation of this cell type, as determined by either Cell Counting Kit-8 assay or cell counting (Fig. 11, A and B). Dose-dependent inhibition of cell proliferation by AMI-1 was also observed under phase-contrast microscopy, with the maximum effect at 10 μM. The concentrations of AMI-1 used in these experiments neither changed cell morphology nor caused their detachment (Fig. 11C), suggesting that AMI-1-conferred decreased number of cells is not due to cell killing. Therefore, these data provide evidence that PRMT1 is required for the proliferation of renal interstitial fibroblasts in vivo and in vitro.
Fig. 10.
AMI-1 inhibits renal fibroblast proliferation in the kidneys after unilateral ureteral obstruction (UUO) injury. A: after UUO injury, collected kidney tissue was used for costaining with antibodies to α-smooth muscle actin (α-SMA) and proliferating cell nuclear antigen (PCNA) (magnification: ×200). B: interstitial cells with positive staining for both α-SMA and PCNA were counted in 10 high-power fields and expressed as means ± SD. *P < 0.05 and **P < 0.01 vs. the control group or other groups as indicated. Scale bar = 200 μm. Yellow arrows indicate small blood arteries; white arrows indicate proliferating myofibroblasts.
Fig. 11.
AMI-1 inhibits the proliferation of cultured rat interstitial fibroblasts stimulated with serum. Approximately 30% confluent NRK-49F cells were cultured in 5% FBS for 48 h in the absence or presence of AMI-1 (0–10 μM). Cell proliferation was assessed by the Cell Counting Kit-8 assay (A) and cell counting (B). Values are means ± SD of at least three independent experiments. *P < 0.05 and **P < 0.01 vs. control groups. C: phase-contrast photographs (×40) showing the effect of AMI-1 (0–10 μM) on cell proliferation. Scale bar = 500 μm.
DISCUSSION
In the present study, we demonstrated that 1) PRMT1 is highly expressed in renal interstitial fibroblasts and kidneys undergoing fibrotic changes, 2) pharmacological inhibition of PRMT1 with AMI-1 or siRNA-mediated PRMT1 silencing leads to decreased activation of renal interstitial fibroblasts in vitro, 3) AMI-1 administration attenuates the development of renal fibrosis in a murine model of UUO-induced renal fibrosis, and 4) AMI-1 is effective in suppressing Smad3 phosphorylation and TGF-βRI expression and in preventing Smad7 downregulation in cultured renal interstitial fibroblasts and fibrotic kidneys. These data suggest that PRMT1 may mediate renal fibroblast activation and renal fibrogenesis through a mechanism involved in the activation of TGF-β/Smad3 signaling.
PRMT1 is expressed ubiquitously during early embryogenesis (13). During embryonic development, it is mainly located peripherally and in the central nervous system (19). Although PRMT1 is also abundantly expressed in the developing kidney, its level and methyltransferase activity quickly decline after birth (31). This may explain why only a small amount of PRMT1 was detected in the kidneys of mice at 8 wk of age, as indicated in our animal experiments. However, we observed that expression levels of PRMT1 were increased in the kidney after UUO injury. Immunofluorescent staining indicated that PRMT1 was primarily located in renal tubules, interstitial cells, and some cells in the glomerulus. Currently, the mechanism by which UUO injury stimulates expression of PRMT1 remains unclear, but it may be related to oxidative stress present in the kidney after ureteral ligation. This hypothesis is supported by observations that PRMT1 mRNA or protein expression is increased under oxidative stress, a condition linked to a variety of disease states (25), and that administration of antioxidant agents suppressed expression of PRMT1 in diabetic retinopathy (4). In addition, we found that serum and TGF-β1 stimulated PRMT1 expression in renal interstitial fibroblasts, suggesting that expression of PRMT1 is subjected to regulation by profibrotic growth factors/cytokines. Additional studies are required to elucidate the signaling mechanism by which those factors promote PRMT1 expression.
The functional role of PRMT family members is related to their location in cells and tissues (9). Although most functional studies have focused on the nuclear functions of PRMT1, a small amount of PRMT1 is also found in the cytoplasm and associated with the membrane (9). This unique feature of PRMT1 allows it to function as a regulator in the cellular cytoplasm and membrane. In this context, it has been reported that PRMT1 can induce arginine methylation of some nonhistone proteins, such as Smad6, in the cytosol (29). Our study also suggests that this enzyme is required for the activation of TGF-β/Smad3 signaling since blockade of PRMT1 suppresses Smad3 phosphorylation (see below). Currently, how PRMT1 is coupled to the TGF-β signaling pathway and how that leads to Smad3 activation remain unclear. There are at least two possibilities: PRMT1 may directly induce methylation of Smad3 at certain arginine residues and then regulate its activity; alternatively, PRMT1 may regulate activation of TGF-β/Smad3 signaling by altering the activity of inhibitory Smads (Smad6 and Smad7). In support of the later hypothesis, Inamitsu et al. (10), using mass spectrometric analysis, demonstrated that PRMT1 can interact with and methylate Smad6; Xu et al. (32) reported that methylated Smad6 is dissociated from type I bone morphogenetic protein receptors, leading to activation of effector Smad1 and Smad5 through phosphorylation. In this context, methylated Smad7 is ubiquitinated and degraded by E3 ligase (5). Smad7 degradation can lead to a loss of its inhibitory effect on Smad3 and TGF-βR1, thereby increasing Smad3 activity. A recent report has shown that PRMT1 can induce Smad7 methylation and promote EMT (12), whereas our present study indicates that PRMT1 inhibition could preserve Smad7 expression and inhibited Smad3 phosphorylation and TGF-βR1 expression in both UUO injured kidneys and TGF-β1-stimulated renal fibroblasts in culture. Thus, it is likely that PRMT1-mediated Smad7 ubiquitination is also required for its downregulation and subsequent activation of the TGF-β/Smad3 signaling pathway. In addition, it is possible that PRMT1 may promote activation of this signaling pathway by increasing expression and release of TGF-β1. This hypothesis is supported by our immunoblot results showing that blockade of PRMT1 reduced expression of TGF-β1 in the kidney after ureteral obstruction. We will test all these hypotheses in our future studies.
PRMT1 may also regulate renal fibrogenesis by triggering EMT. It has been previously reported that PRMT1-mediated arginine 3 residue of histone H4 methylation at the ZEB1 promoter can activate its transcription and subsequently induce EMT by repressing transcription of E-cadherin in breast cancer cells (6). During the development of renal fibrosis, renal epithelial cells undergo a partial EMT and promote production and release of profibrotic growth factors/cytokines, including TGF-β1. Since Twist1 is the substrate of PRMT1 (1) and activation of Twist is required for the development of EMT in renal tubular cells (8), it is possible that PRMT1 may also be implicated in renal fibrosis through the induction of partial EMT by activation of Twist1. This hypothesis merits further investigation.
Increasing evidence indicates that overexpression of PRMT1 is associated with several chronic conditions, including cardiovascular disease, renal disease, diabetes, and pulmonary fibrosis (22, 34). In line with these observations, our present study revealed that PRMT1 is highly expressed in cultured renal fibroblasts and the fibrotic kidney and that inhibition of PRMT1 by AMI-1 was effective in suppressing renal fibroblast activation, proliferation, and fibrosis development. This suggests the importance of PRMT1 in mediating renal fibrogenesis. Since renal fibrosis is a key pathological process in progression of CKD resulting from many causes, PRMT1 could be a novel therapeutic target for the treatment of fibrotic kidney disease.
In summary, we demonstrate that UUO-elicited upregulation of PRMT1 contributes to the development of renal interstitial fibrosis by promoting activation and proliferation of renal interstitial fibroblasts and through activation of TGF-β/Smad3 signaling in the kidney. Therefore, pharmaceutical inhibition of PRMT1 may hold a therapeutic potential for CKD.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 81670623 and 81830021 (to S. Zhuang), Branch Grant 2018YFA0108802 of National Key R&D Program of China (to S. Zhuang), and National Institute of Diabetes and Digestive and Kidney Diseases Grant 1-R01-DK-113256-01A1 (to S. Zhuang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Z. and S.Z. conceived and designed research; Y.Z. and C.Y. performed experiments; Y.Z. and C.Y. analyzed data; Y.Z., C.Y., and S.Z. interpreted results of experiments; Y.Z. prepared figures; Y.Z. and S.Z. drafted manuscript; S.Z. edited and revised manuscript; C.Y. and S.Z. approved final version of manuscript.
REFERENCES
- 1.Avasarala S, Van Scoyk M, Karuppusamy Rathinam MK, Zerayesus S, Zhao X, Zhang W, Pergande MR, Borgia JA, DeGregori J, Port JD, Winn RA, Bikkavilli RK. PRMT1 is a novel regulator of epithelial-mesenchymal-transition in non-small cell lung cancer. J Biol Chem 290: 13479–13489, 2015. doi: 10.1074/jbc.M114.636050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouras G, Deftereos S, Tousoulis D, Giannopoulos G, Chatzis G, Tsounis D, Cleman MW, Stefanadis C. Asymmetric dimethylarginine (ADMA): a promising biomarker for cardiovascular disease? Curr Top Med Chem 13: 180–200, 2013. doi: 10.2174/1568026611313020007. [DOI] [PubMed] [Google Scholar]
- 3.Cha B, Park Y, Hwang BN, Kim SY, Jho EH. Protein arginine methyltransferase 1 methylates Smurf2. Mol Cells 38: 723–728, 2015. doi: 10.14348/molcells.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Xu X, Sheng M, Zhang X, Gu Q, Zheng Z. PRMT-1 and DDAHs-induced ADMA upregulation is involved in ROS- and RAS-mediated diabetic retinopathy. Exp Eye Res 89: 1028–1034, 2009. doi: 10.1016/j.exer.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Elkouris M, Kontaki H, Stavropoulos A, Antonoglou A, Nikolaou KC, Samiotaki M, Szantai E, Saviolaki D, Brown PJ, Sideras P, Panayotou G, Talianidis I. SET9-mediated regulation of TGF-β signaling links protein methylation to pulmonary fibrosis. Cell Reports 15: 2733–2744, 2016. doi: 10.1016/j.celrep.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Zhao Y, Zhang J, Lu Y, Liu X, Geng P, Huang B, Zhang Y, Lu J. The dual function of PRMT1 in modulating epithelial-mesenchymal transition and cellular senescence in breast cancer cells through regulation of ZEB1. Sci Rep 6: 19874, 2016. doi: 10.1038/srep19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grande MT, López-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328, 2009. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- 8.Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, Rowe RG, Weiss SJ, López-Novoa JM, Nieto MA. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21: 989–997, 2015. [Erratum in Nat Med 22: 217, 2016.] doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann F, Fackelmayer FO. Nucleo-cytoplasmic shuttling of protein arginine methyltransferase 1 (PRMT1) requires enzymatic activity. Genes Cells 14: 309–317, 2009. doi: 10.1111/j.1365-2443.2008.01266.x. [DOI] [PubMed] [Google Scholar]
- 10.Inamitsu M, Itoh S, Hellman U, Ten Dijke P, Kato M. Methylation of Smad6 by protein arginine N-methyltransferase 1. FEBS Lett 580: 6603–6611, 2006. doi: 10.1016/j.febslet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki H. Impaired PRMT1 activity in the liver and pancreas of type 2 diabetic Goto-Kakizaki rats. Life Sci 85: 161–166, 2009. doi: 10.1016/j.lfs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Katsuno Y, Qin J, Oses-Prieto J, Wang H, Jackson-Weaver O, Zhang T, Lamouille S, Wu J, Burlingame A, Xu J, Derynck R. Arginine methylation of SMAD7 by PRMT1 in TGF-β-induced epithelial-mesenchymal transition and epithelial stem-cell generation. J Biol Chem 293: 13059–13072, 2018. doi: 10.1074/jbc.RA118.002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause CD, Yang ZH, Kim YS, Lee JH, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol Ther 113: 50–87, 2007. doi: 10.1016/j.pharmthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol 32: 236–243, 2012. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 16.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediators Inflamm 2016: 8319283, 2016. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12: 325–338, 2016. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 18.Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawlak MR, Scherer CA, Chen J, Roshon MJ, Ruley HE. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol Cell Biol 20: 4859–4869, 2000. doi: 10.1128/MCB.20.13.4859-4869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwedhelm E, Böger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 7: 275–285, 2011. doi: 10.1038/nrneph.2011.31. [DOI] [PubMed] [Google Scholar]
- 21.Shuttleworth VG, Gaughan L, Nawafa L, Mooney CA, Cobb SL, Sheerin NS, Logan IR. The methyltransferase SET9 regulates TGFB1 activation of renal fibroblasts via interaction with SMAD3. J Cell Sci 131: jcs207761, 2018. doi: 10.1242/jcs.207761. [DOI] [PubMed] [Google Scholar]
- 22.Sun Q, Liu L, Roth M, Tian J, He Q, Zhong B, Bao R, Lan X, Jiang C, Sun J, Yang X, Lu S. PRMT1 upregulated by epithelial proinflammatory cytokines participates in COX2 expression in fibroblasts and chronic antigen-induced pulmonary inflammation. J Immunol 195: 298–306, 2015. doi: 10.4049/jimmunol.1402465. [DOI] [PubMed] [Google Scholar]
- 23.Sureshbabu A, Muhsin SA, Choi ME. TGF-β signaling in the kidney: profibrotic and protective effects. Am J Physiol Renal Physiol 310: F596–F606, 2016. doi: 10.1152/ajprenal.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y, Liao F, Wu G, Chang D, Yang Y, Dong X, Zhang Z, Zhang Y, Wu G. Ubiquitination and regulation of Smad7 in the TGF-β1/Smad signaling of aristolochic acid nephropathy. Toxicol Mech Methods 25: 645–652, 2015. doi: 10.3109/15376516.2015.1061082. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289: H2649–H2656, 2005. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 26.Vega G, Alarcón S, San Martín R. The cellular and signalling alterations conducted by TGF-β contributing to renal fibrosis. Cytokine 88: 115–125, 2016. doi: 10.1016/j.cyto.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Zhuang S. Src family kinases in chronic kidney disease. Am J Physiol Renal Physiol 313: F721–F728, 2017. doi: 10.1152/ajprenal.00141.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, Mundade R, Lange KC, Lu T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle 13: 32–41, 2014. doi: 10.4161/cc.27353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesche J, Kühn S, Kessler BM, Salton M, Wolf A. Protein arginine methylation: a prominent modification and its demethylation. Cell Mol Life Sci 74: 3305–3315, 2017. doi: 10.1007/s00018-017-2515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wysocka J, Allis CD, Coonrod S. Histone arginine methylation and its dynamic regulation. Front Biosci 11: 344–355, 2006. doi: 10.2741/1802. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Wang AH, Oses-Prieto J, Makhijani K, Katsuno Y, Pei M, Yan L, Zheng YG, Burlingame A, Brückner K, Derynck R. Arginine methylation initiates BMP-induced Smad signaling. Mol Cell 51: 5–19, 2013. doi: 10.1016/j.molcel.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu P, Lin X, Feng XH. Posttranslational regulation of Smads. Cold Spring Harb Perspect Biol 8: a022087, 2016. doi: 10.1101/cshperspect.a022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer 13: 37–50, 2013. doi: 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
- 35.Zakrzewicz D, Zakrzewicz A, Didiasova M, Korencak M, Kosanovic D, Schermuly RT, Markart P, Wygrecka M. Elevated protein arginine methyltransferase 1 expression regulates fibroblast motility in pulmonary fibrosis. Biochim Biophys Acta 1852: 2678–2688, 2015. doi: 10.1016/j.bbadis.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Zakrzewicz D, Zakrzewicz A, Preissner KT, Markart P, Wygrecka M. Protein arginine methyltransferases (PRMTs): promising targets for the treatment of pulmonary disorders. Int J Mol Sci 13: 12383–12400, 2012. doi: 10.3390/ijms131012383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Zang X, Ponnusamy M, Masucci MV, Tolbert E, Gong R, Zhao TC, Liu N, Bayliss G, Dworkin LD, Zhuang S. Enhancer of zeste homolog 2 inhibition attenuates renal fibrosis by maintaining Smad7 and phosphatase and tensin homolog expression. J Am Soc Nephrol 27: 2092–2108, 2016. doi: 10.1681/ASN.2015040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuang S, Liu N. EGFR signaling in renal fibrosis. Kidney Int Suppl (2011) 4: 70–74, 2014. doi: 10.1038/kisup.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]