Abstract
Compensatory endocytosis (CE) is one of the primary mechanisms through which cells maintain their surface area after exocytosis. Considering that in eggs massive exocytosis of cortical granules takes place after fertilization, the aim of this work was to evaluate the occurrence of CE following cortical exocytosis in mouse eggs. For this purpose, we developed a pulse-chase assay to detect cortical granule membrane internalization. Results showed internalized labeling in SrCl2-activated and fertilized eggs when chasing at 37°C, but not at a non-permissive temperature (4°C). The use of kinase and calcineurin inhibitors led us to conclude that this internal labeling corresponded to CE. Further experiments showed that CE in mouse eggs is dependent on actin dynamics and dynamin activity, and could be associated with a transient exposure of phosphatidylserine. Finally, CE was impaired in A23187 ionophore-activated eggs, highlighting once again the mechanistic differences between activation methods. Altogether, these results demonstrate for the first time that egg activation triggers CE in mouse eggs after exocytosis of cortical granules, probably as a plasma membrane homeostasis mechanism.
Keywords: compensatory endocytosis, egg activation, phosphatidylserine, fertilization, cortical granules
Introduction
Calcium-regulated exocytosis is a feature common to a variety of cell types. It involves the fusion of secretory vesicles with the plasma membrane resulting in an increase in the plasma membrane surface. As a consequence, exocytosis should be coupled to an endocytic process known as compensatory endocytosis (CE) that retrieves excess surface membrane into endosomes allowing the plasma membrane to recover its integrity and constituting one of the primary mechanisms through which cells maintain their surface area (Covian-Nares, Smith, & Vogel, 2008; Engisch & Nowycky, 1998). In professional secretory cells such as neurons and neuroendocrine cells, the dynamics and mechanisms involved in these processes have been extensively studied but are still debated. Evidence for exo-endocytosis coupling came from morphological and functional studies in neuroendocrine cells showing that granule membranes are maintained together as “microdomains” after exocytosis and are subsequently recaptured without intermixing with the plasma membrane (Houy et al., 2013). Among other factors, phospholipid scrambling in the plasma membrane has been proposed as a signal to trigger CE. In particular, it has been shown that surface exposure of phosphatidylserine (PS) in chromaffin cells, although not involved in granule exocytosis, is required for the subsequent membrane CE (Ory et al., 2013).
In eggs, exocytic vesicles known as cortical granules (CG) are Golgi apparatus–derived membrane bound organelles that accumulate during oogenesis and form a uniform layer in the cortex after egg maturation to metaphase II stage. Fertilization triggers in eggs long-lasting Ca2+ oscillations that initiate a series of changes collectively known as “egg activation”. One of the first events occurring during egg activation is cortical granule exocytosis (CGE), which is important for the prevention of polyspermy. CGE is different from most other regulated vesicle secretion processes because CG are not renewed after their fusion with the plasma membrane (M. Liu, 2011). Nonetheless, several studies indicate that CE occurs in sea urchin and Xenopus eggs to restore cell surface homeostasis (Bement, Benink, Mandato, & Swelstad, 2000; Whalley, Terasaki, Cho, & Vogel, 1995). Moreover, membrane capacitance studies performed in hamster eggs suggest that an endocytic phenomenon may also occur in mammalian eggs (Kline & Stewart-Savage, 1994). In view of this, in the present work we have evaluated and characterized the occurrence of compensatory endocytosis after CGE in mouse eggs. In addition, we show that CE could be associated to the transient and calcium-dependent exposure of PS that occurs after egg activation (Curia et al., 2013).
Materials and Methods
Ethics statement
Animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of IBYME (024/2016) and of the University of Massachusetts (OPAS 119-1580). Experiments were performed in strict accordance with the Guide for Care and Use of Laboratory Animals approved by the National Institutes of Health (NIH).
Animals and reagents
Hybrid C57BL/6xBALB/cF1 or CD1 young adult (30-60 days) female or adult (60-120 days) male mice used in this study were maintained at 23°C with a 12 h L:12 h D cycle.
All reagents and chemicals were of molecular biology grade, and were purchased from Sigma-Aldrich Chemicals (St Louis, MO, USA) or Invitrogen (Carlsbad, CA, USA), unless otherwise specified.
Egg collection
Female mice were superovulated by an injection (i.p.) of equine chorionic gonadotropin (eCG; 5 UI), followed by the administration (i.p.) of human chorionic gonadotropin (hCG; 5 UI) 48 h later. Eggs were collected from the oviducts of superovulated animals 12-13 h after hCG administration, and maintained in Whitten’s-HEPES-PVA or Tyrode’s Lactate HEPES (TL-HEPES) medium supplemented with 5% heat-treated fetal calf serum (FCS). Cumulus cells were removed by incubating the cumulus-oocyte complexes for 5 min in 0.3 mg/ml hyaluronidase (type IV). Zonae pellucidae were dissolved by treating the eggs with acid Tyrode’s solution (pH 2.5) for 10-20 sec.
Calcium measurement
Intracellular Ca2+ concentration ([Ca2+]i) imaging was performed as previously described (Zhang, Yoon, Parys, & Fissore, 2015). Briefly, zona-free eggs were loaded with 1.25 μM Fura-2 AM supplemented with 0.02% pluronic acid for 20 min. Eggs were thoroughly washed in Ca2+/Mg2+-free FCS-free TL-HEPES and attached to glass-bottom dishes (MatTeck Corp, Ashland, MA, USA). Fura-2 fluorescence was excited with 340 nm and 380 nm wavelengths every 20 sec and emitted light was collected at wavelengths above 510 nm with a Nikon Diaphot microscope fitted for fluorescence measurements. After 10 min, SrCl2 was added to a final concentration of 10 mM and Ca2+/Sr2+ oscillations were recorded for at least 1 h.
Cortical exudate staining
Metaphase II-arrested (MII) zona-free eggs were cultured in the presence of 10mM SrCl2 at 37°C in Ca2+/Mg2+-free Chatot, Ziomek and Bavister (CZB) medium for different time-points, washed and incubated for additional 30 min at 4°C in the presence of 5 μg/ml DyLight 649-conjugated Lens culinaris agglutinin (LCA, Vector Laboratories, Burlingame, CA, USA) in complete CZB medium. Cells were then fixed for 45 min in 3.7% w/v paraformaldehyde, mounted and analyzed with a Nikon A1 Spectral Confocal microscope. The amount of cortical exudate in each egg was determined by measuring the intensity of fluorescent labeling in the membrane using FIJI (Schindelin et al., 2012).
LCA internalization assays (pulse-chase experiments)
For LCA internalization studies, zona-free eggs were treated with 10 mM SrCl2 for 15 min or 10 μM A23187 Ca2+ ionophore for 5 min in Ca2+/Mg2+-free Whittens-HEPES-PVA medium. To synchronize with SrCl2-activated eggs, ionophore-treated eggs were incubated in complete medium for additional 10 min. Pulse with LCA was performed as described in Cortical exudate staining. Following the pulse, eggs were washed rapidly and incubated at 37°C or 4°C for 30 min to allow LCA internalization (chase). Cells were then fixed and mounted, and Z-stack confocal images were taken from each egg (1 μm step size, at least 15 images per cell) using a Nikon Eclipse E800 confocal microscope. Images were analyzed using FIJI and the number of fluorescent dots per confocal plane was determined.
When performing pulse-chase experiments in the presence of 25 nM Staurosporine (Abcam, Cambridge, MA, USA) or 1μM Cyclosporin A (Abcam), inhibitors were added 15 min before stimulus and kept during stimulus, pulse and chase. To determine possible effects on cortical exocytosis, some eggs were fixed and mounted after pulse, and the amount of cortical exudate was determined as mentioned before. For experiments with actin disruptors (10 μM Latrunculin A, Calbiochem, San Diego, CA, USA; 10 μM Cytochalasin D), Jasplakinolide (0.5 μM), filipin (5 μM), PitStop 2 (15 μM) and dynasore (80 μM), drugs were added during pulse and chase, and images were taken with a Nikon A1 Spectral Confocal microscope or an Olympus IX83 Spinning Disk microscope. In all cases, inhibitors were diluted from a solution stock in dimethyl sulfoxide (DMSO). The same volume of DMSO was added to control incubation medium.
Actin staining
For actin microfilament staining, eggs were fixed, washed in 0.4% w/v bovine serum albumin (BSA) 0.1 M glycine in PBS (PBS-BSA4) and permeabilized 15 min with 0.1% v/v Triton X-100 in PBS-BSA4. After washing, eggs were incubated 30 min with Acti-stain™ 488 (100 nM, Cytoskeleton, Denver, CO, USA), washed again, stained with Hoechst 33342, mounted and analyzed with a Nikon A1 Spectral Confocal microscope.
In vitro fertilization
Mouse in vitro fertilization was performed as previously described (Curia et al., 2013). Briefly, epididymal mouse sperm were recovered in 300 μl of capacitation medium supplemented with 0.3% w/v BSA under paraffin oil (Ewe, Sanitas SA, Buenos Aires, Argentina). Aliquots of the suspension were added to 300 μl of fresh medium to give a final concentration of 1-3 x 106 cells/ml and incubated for 90 min under paraffin oil at 37°C in an atmosphere of 5% v/v CO2 in air. Zona-free eggs were inseminated with capacitated sperm (final concentration: 0.5 x 104 cells/ml) and gametes were co-incubated for 15 min at 37°C in an atmosphere of 5% v/v CO2 in air. Eggs were then washed and used for pulse-chase assays (as described in LCA internalization assays).
PS and CG co-localization studies
After 1 h incubation with 10 mM SrCl2 or activation with 10 μM A23187, zona-free eggs were exposed to FITC-conjugated Annexin V (FITC-ANX5, 1:25, BD Biosciences, San Diego, CA, USA) for 1 h to detect PS externalization. For the detection of CG exudate, 5 μg/ml LCA was added during the last 15 min. Eggs were then fixed, washed, mounted with Vectashield containing 10 μg/ml Hoechst 33342 and analyzed with a Nikon A1 Spectral Confocal microscope. Co-localization tests were performed with FIJI.
Statistical Analysis
Values are presented as mean ± SEM from at least 3 independent experiments. Statistical analyses were performed by using GraphPad Prism Software (San Diego, CA, USA). Statistical significance of the data was analyzed with Kruskal-Wallis non-parametric test with Dunn’s post-test for time curve of cortical exocytosis, Student’s t-test for cortical exocytosis measurement and the internalized fluorescent dots in pulse-chase assays and one-way ANOVA for pulse-chase experiments in fertilized eggs. In pulse-chase assays with inhibitors, relative internalized fluorescent labeling was considered statistically different to control whenever the 95% confidence interval did not include 1. In all cases, differences were considered significant at p<0.05.
Results
As a first step towards the study and characterization of CE in mouse eggs, we established a detection system to analyze the reincorporation of the cortical granule membrane into the cytoplasm. For this purpose, we took advantage of the ability of LCA (Lens culinaris agglutinin) to tag exocytosed CG material in activated eggs (Raz, Skutelsky, Amihai, Hammel, & Shalgi, 1998). In this way, we could incubate living cells with labeled LCA and follow the labeling fate after exocytosis. First, we determined the minimum time of incubation with the parthenogenetic activating agent SrCl2 required to detect LCA binding to the egg surface. Quantification of fluorescent labeling on eggs exposed to 10 mM SrCl2 at different-time points showed that labeling for LCA was detectable on the egg surface after 15 min of incubation with SrCl2, and that it increased up to a maximum after 30 min of incubation (Fig. 1A, B). Coincident with this, most eggs incubated with SrCl2 presented the first Ca2+/Sr2+ peak during the first 15 min (Fig. 1C). For the above reasons, and to avoid the possibility of missing early endocytic events, 15 min of incubation with SrCl2 was chosen for subsequent experiments. Endocytosis was then evaluated by pulse-chase experiments and confocal microscopy, measuring the internalization of the fluorescent LCA bound to the cell surface after exocytosis. Fluorescent dots were observed in the egg cytoplasm when chase was performed at 37°C, but not at 4°C, a non-permissive temperature used as control (Figure 2A, Suppl. Video 1 and 2). Further quantification analysis indicated that the number of dots per confocal plane at 37°C was significantly different from that at 4°C (Figure 2B), supporting the occurrence of endocytosis of previously exocytosed CG material. Considering that CE in sea urchin eggs is inhibited by non-specific kinase and calcineurin inhibitors (Covian-Nares et al., 2008), we evaluated the effect of 25 nM Staurosporine (Stp) and 1 μM Cyclosporin A (CsA) on mouse egg endocytosis. Whereas the total amount of fluorescence on the plasma membrane in activated eggs after pulse was similar among treatments (Figure 2C), indicative of a similar CGE rate, the relative number of fluorescent dots within the cytoplasm was significantly lower in inhibitor-treated eggs compared to controls (Figure 2D).
Figure 1. Time curve of cortical exocytosis.
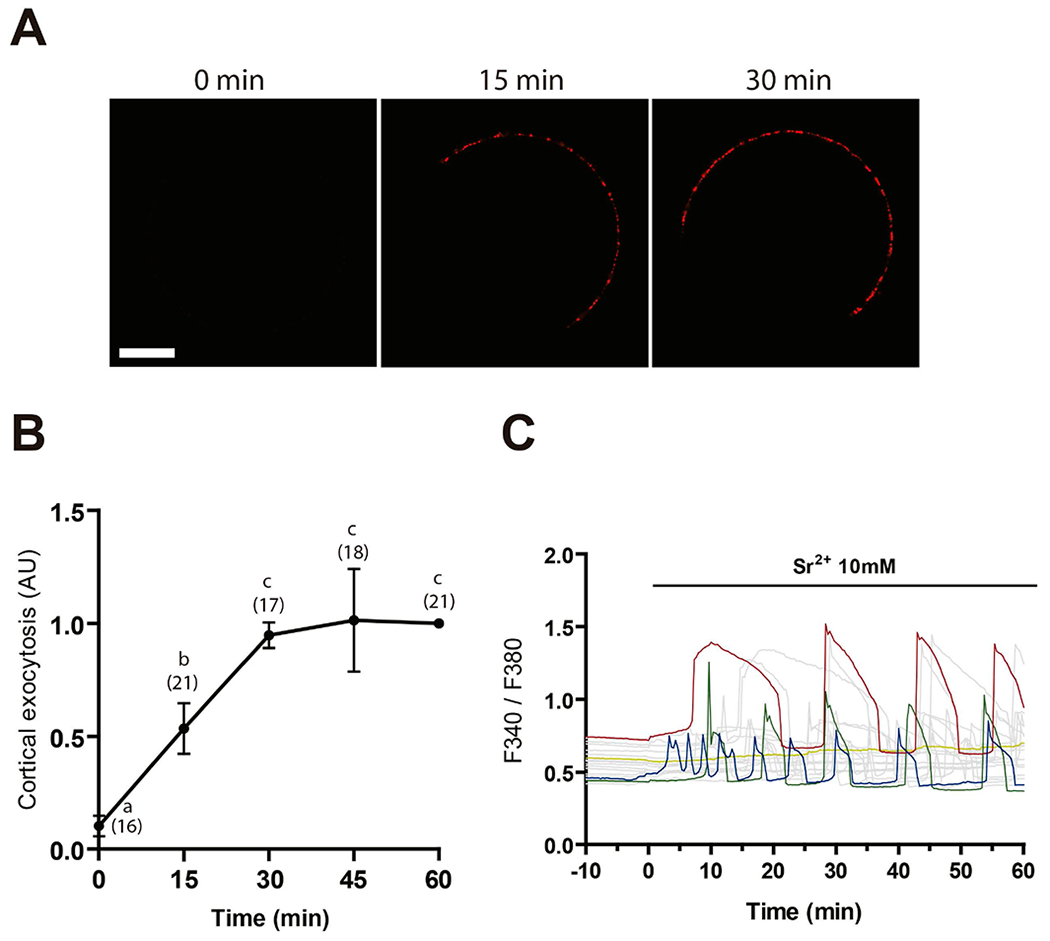
(A) Zona-free eggs were incubated with 10 mM SrCl2 in Ca2+/Mg2+-free medium for different time-points, and cortical exudate was detected with DyLight 649-LCA. Bar = 20 μm. (B) Fluorescent labeling in the plasma membrane was quantified and relativized to intensity after 60 min stimulus with SrCl2. The total number of evaluated eggs for each group is shown in brackets. Points with different letters are significantly different (p<0.05). (C) Representative Ca2+/Sr2+ responses induced by 10 mM SrCl2.
Figure 2. Compensatory endocytosis occurs in SrCl2-activated and fertilized eggs after cortical exocytosis.
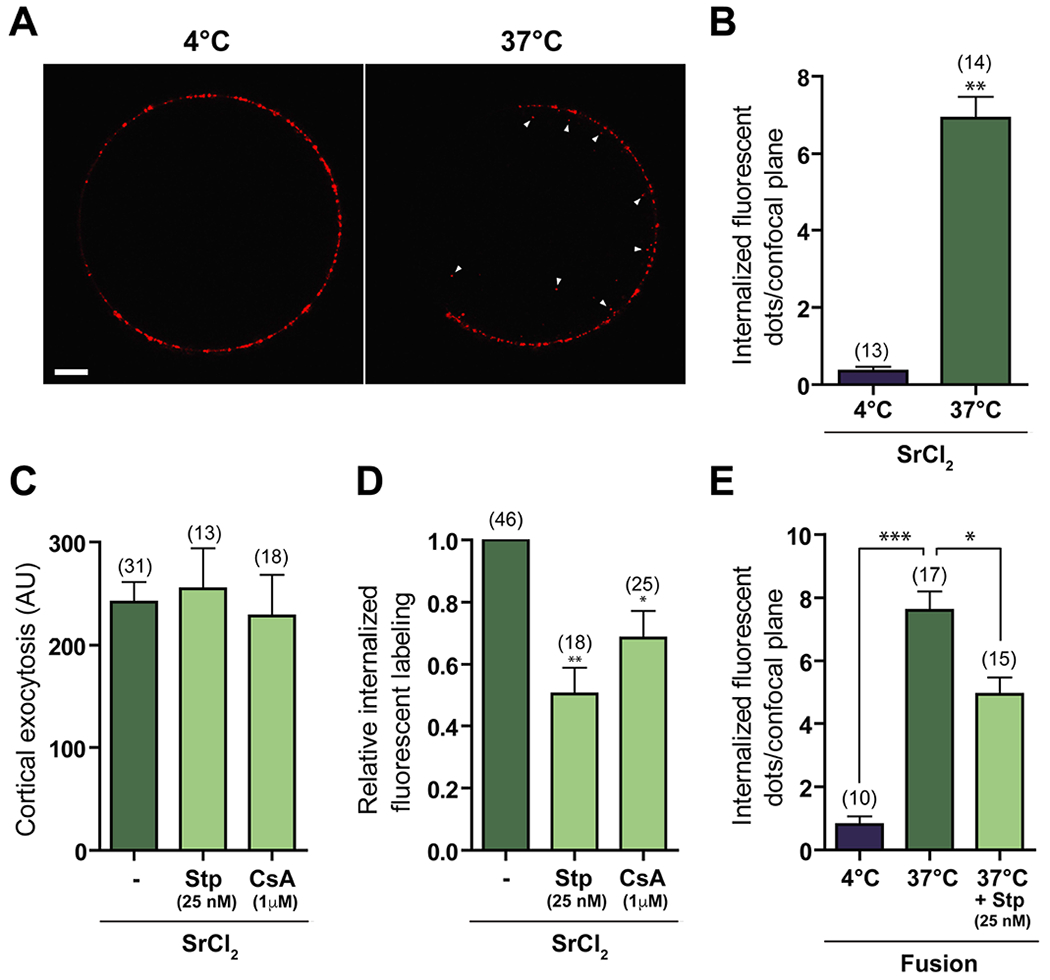
(A) Zona-free eggs were incubated for 15 min with 10 mM SrCl2 in Ca2+/Mg2+-free medium and stained with LCA for 30 min at 4°C (pulse), and chase was performed for 30 min at 4°C or 37°C in LCA-free medium. Note the internalized fluorescent dots after 37°C-chase (right, arrowheads), and their absence after 4°C-chase (left). Bar = 10 μm. AU: Arbitrary Units. (B) Quantification of total internalized fluorescent dots per confocal plane in SrCl2-treated eggs after 4°C- or 37°C-chase. (C, D) Pulse-chase experiments were performed in the presence of 25 nM Stp or 1 μM CsA. Cortical exocytosis was measured as the fluorescent intensity in the membrane after pulse (C) and the number of fluorescent dots per confocal plane after chase relative to control (-, DMSO) was determined (D). (E) Zona-free eggs were inseminated with capacitated mouse spermatozoa. After 15 min of co-incubation, eggs were thoroughly washed, stained with LCA at 4°C and chase at 4°C or 37°C was performed. Some eggs were pre-incubated and treated during the whole experiment with 25 nM Stp. The number of internalized fluorescent dots per confocal plane was determined. The total number of evaluated eggs for each group is shown in brackets. *p<0.05, **p<0.01.
Although parthenogenetic activation with SrCl2 resembles the activation induced by the fertilizing sperm (Kline & Kline, 1992), we evaluated whether CE also occurs during fertilization. For this purpose, the number of fluorescent dots within the egg was quantified in recently fertilized zona-free eggs after pulse-chase experiments. When chase was performed at 37°C, a significantly higher number of dots per confocal plane was observed compared to controls (chase at 4°C) (Figure 2E). Moreover, the presence of Stp significantly decreased this parameter (Figure 2E), supporting that CE also occurs in fertilized eggs. It is interesting to note that the number of fluorescent dots per confocal plane was similar in fertilized and SrCl2-activated eggs (compare Figures 2E and 2B).
After establishing the occurrence of CE in SrCl2-activated and fertilized eggs, we next characterized this endocytic process. Considering the cytoskeleton modifications that take place in the egg after fertilization/activation (Sun & Schatten, 2006), we then investigated whether actin filament dynamics are involved in CE. For this purpose, pulse-chase experiments were repeated in SrCl2-activated eggs in the presence of cytoskeleton disruptors such as Cytochalasin D (CytD, 10 μM) and Latrunculin A (LatA, 10 μM) or Jasplakinolide (Jas, 0.5 μM) which stabilizes microfilaments. In all cases, the drugs were added right after treatment with SrCl2 to avoid possible interference with CGE. The effectiveness of the disruptors was confirmed by both a decreased F-actin labeling with phalloidin and a disruption in meiotic spindle rotation and polar body extrusion (Suppl. Figure S1). The presence of cytoskeletal drugs caused a remarkable decrease in the relative amount of cytoplasmic labeling for LCA (Figure 3A) indicating that CE in eggs is strongly dependent on cytoskeleton dynamics.
Figure 3. Compensatory endocytosis in activated eggs requires actin cytoskeleton dynamics and dynamin.
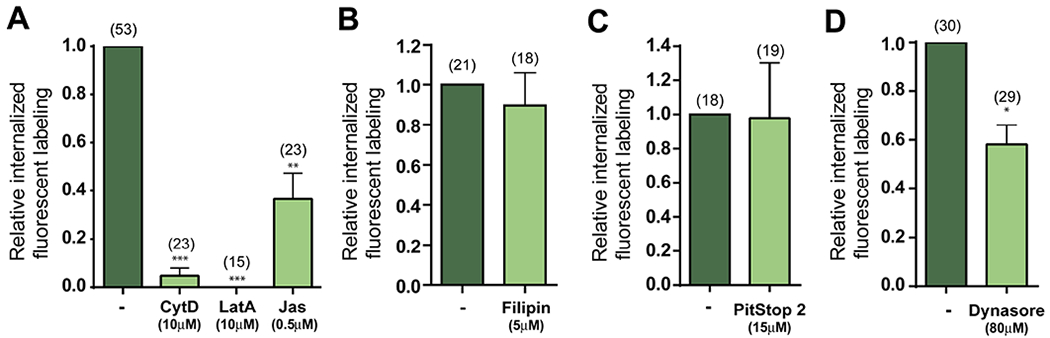
Quantification of internalized fluorescent labeling after pulse-chase experiments in the presence of 10 μM CytD,10 μM LatA or 0.5 μM Jas (A), 5 μM filipin (B), 15 μM PitStop 2 (C) or 80 μM dynasore (D). The number of internalized fluorescent dots was relativized to control (-, DMSO). Inhibitors were added during pulse to prevent possible deleterious effects on cortical exocytosis. The total number of evaluated eggs for each group is shown in brackets. *p<0.05, **p<0.01.
To get further insights into the endocytic pathways involved in LCA internalization, we next evaluated the effect of inhibitors of different modulators of endocytosis during our pulse-chase assays. We first examined the effect of the addition of 5 μM filipin, a cholesterol binding compound that disrupts lipid rafts and inhibits caveolae-mediated endocytosis. Results showed no differences in the relative number of LCA-labeled dots in filipin-treated eggs compared to controls (Figure 3B). Although different forms of endocytosis occur through clathrin-mediated mechanisms (Milosevic, 2018), PitStop 2 (15 μM), a compound that blocks clathrin N-terminal domain, did not decrease internalized LCA-labeling compared to controls (Figure 3C). Finally, the GTPase dynamin has been proposed to be involved in the fission of retrieved vesicles from the plasma membrane (Holroyd, Lang, Wenzel, De Camilli, & Jahn, 2002). Consistent with this possibility, the addition of 80 μM dynasore, an inhibitor of the GTPase activity of dynamin, produced a significant decrease in the internalization of LCA in activated eggs compared to controls (Figure 3D).
The calcium ionophore A23187 is commonly used for inducing [Ca2+]i increases and egg activation. However, the number of Ca2+ rises, its source and the [Ca2+]i achieved in the egg differ from those produced by SrCl2 (Carvacho, Lee, Fissore, & Clapham, 2013; Vincent, Cheek, & Johnson, 1992; Wakai, Zhang, Vangheluwe, & Fissore, 2013). In view of this, we evaluated the occurrence of CE in ionophore-activated eggs. The total fluorescence on the egg membrane after LCA pulse corresponding to cortical exudate was similar to that obtained in SrCl2-activated eggs (Figure 4A). However, the number of internalized dots per plane was significantly lower in A23187-activated eggs (Figure 4B), supporting the notion that the method of egg activation is important for CE, probably due to how the increase in [Ca2+]i is achieved. In addition, the observed difference resembles the low ability of ionophore-activated eggs to expose phosphatidylserine (PS), a phenomenon observed in both fertilized and SrCl2-activated eggs (Curia et al., 2013). In this regard, when ionophore-activated eggs were stained with FITC-ANX5 and Dylight 649-labeled LCA for detection of exposed PS and CG exudate, respectively, we observed a limited amount of FITC-ANX5 fluorescence on the cell surface despite normal levels of LCA labeling (Figure 5A). Interestingly, when these studies were performed with SrCl2-activated eggs in which CE is not impaired, we detected co-localization of both labels (Pearson correlation coefficient: +0.736) (Figure 5A, B) suggesting that PS exposure could be associated with CE rather than with CGE.
Figure 4. Compensatory endocytosis is impaired in A23187-activated eggs.
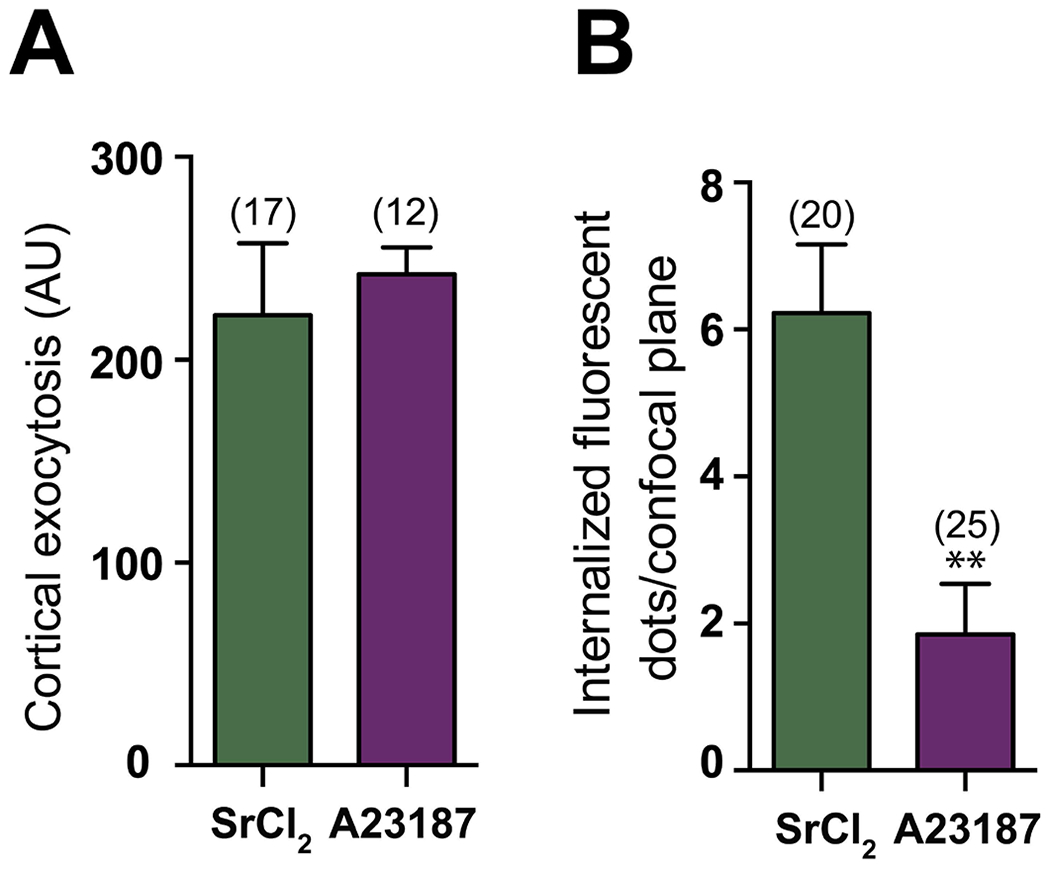
Zona-free eggs were incubated with 10 μM Ca2+ ionophore A23187 in Ca2+/Mg2+-free medium for 5 min, thoroughly washed, incubated for additional 10 min in complete medium, and pulse-chase experiments were performed. Cortical exocytosis was measured as the fluorescent intensity in the membrane after pulse (A) and the relative number of fluorescent dots per confocal plane after chase was determined (B). AU: Arbitrary Units. The total number of evaluated eggs for each group is shown in brackets. *p<0.05, **p<0.01, ***p<0.001.
Figure 5. Co-localization of cortical exudate and PS exposure.
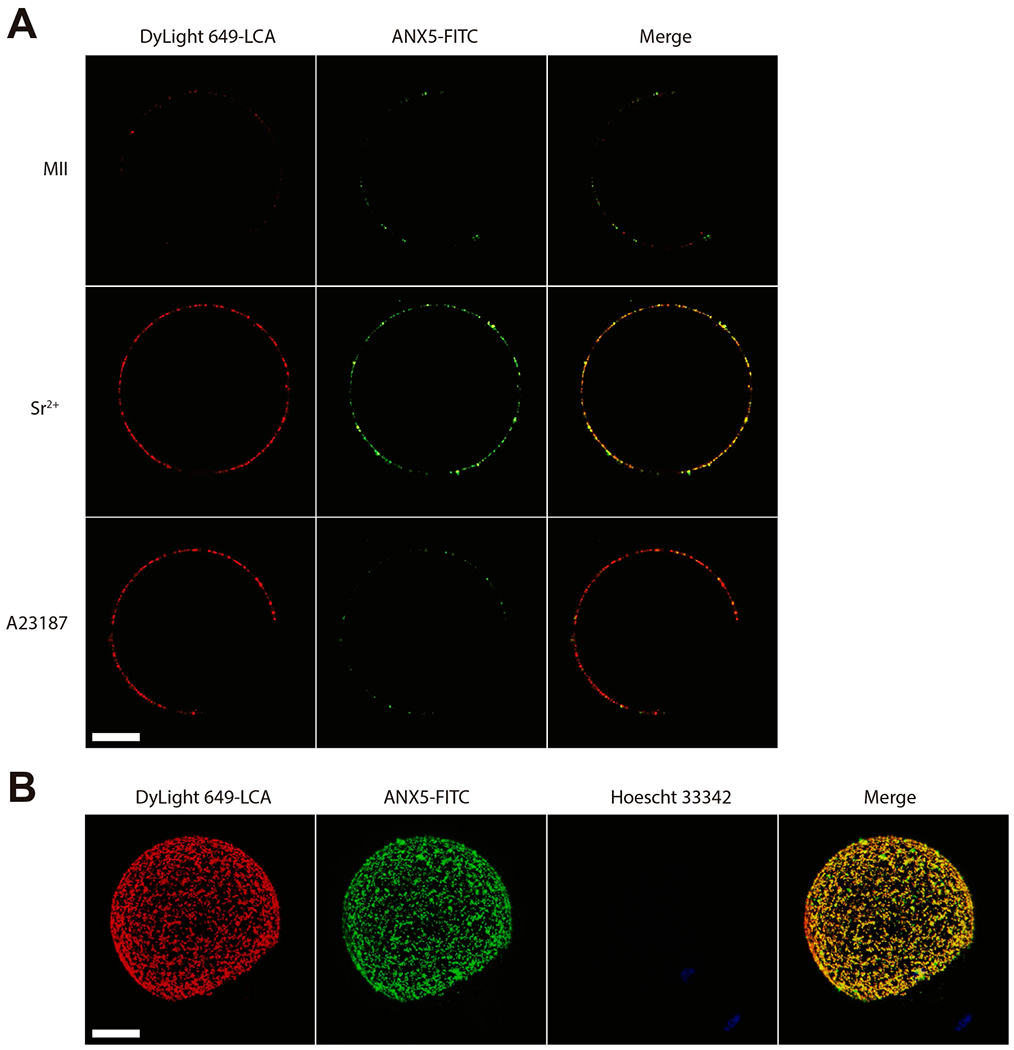
(A) MII eggs, SrCl2-activated or A23187-activated eggs were exposed to FITC-ANX5 (green) for 1 h, and LCA (red) was added during the last 15 min of incubation. Eggs were fixed and different confocal planes were analyzed with a confocal microscope. For SrCl2 or A23187-activated eggs, only those exhibiting a second polar body were studied. Note that LCA labeling is absent in the region where polar body extrusion occurred as it is cortical granule-free. Results are representative of 3 independent experiments in each of which 5–15 eggs/group were evaluated. (B) 3D reconstruction of a SrCl2-activated egg stained with both probes. DNA was stained with Hoechst 33342. Bar = 20 μm.
Discussion
Although much has been studied about the mechanisms involved in secretory vesicle fusion, less attention has been paid towards understanding how cells maintain a homeostatic membrane balance after this secretory event. Previous evidence in sea urchin showed that CE is constrained to sites of exocytosis during egg activation (Smith et al., 2000). Whereas membrane capacitance experiments suggested the occurrence of endocytosis after CGE in hamster eggs (Kline & Stewart-Savage, 1994), it was not demonstrated it occurred by CE in mammalian eggs. The experimental design used in the present work allowing the assessment of the internalization of exocytosed CG material led us to show, for the first time, the occurrence of CE in mouse eggs following CGE induced by both sperm entry and parthenogenetic activation with SrCl2.
In both sea urchin and Xenopus eggs (Bement et al., 2000; Whalley et al., 1995), as well as in other cellular systems (Gundelfinger, Kessels, & Qualmann, 2003), CE occurs rapidly after exocytosis. In contrast, in mouse CGE, which is more protracted, it was necessary to closely time the period of observation to avoid the loss of detection of possible early endocytic events. We, therefore, chose the shortest time of stimulation with SrCl2 that produced a significant increase of the cortical exudate in the membrane (15 min). After a pulse with LCA and a chase at 37˚C, internalization of the fluorescent probe was detected in the egg cytoplasm that was not observed after chase at a non-permissive temperature (Tomoda, Kishimoto, & Lee, 1989). This result indicated not only the existence of an endocytic phenomenon in the egg but also that the technique used was adequate to evaluate endocytosis under our experimental conditions.
The use of calmodulin and calcineurin inhibitors both proposed to mediate CE in different cellular systems (Chiang et al., 2014; Covian-Nares et al., 2008; Igarashi & Watanabe, 2007; Wu et al., 2014) led us to conclude that the endocytic process that takes place after CGE corresponded to CE. We observed that eggs exposed to the calcineurin inhibitor CsA showed fewer numbers of fluorescent dots within the cytoplasm, confirming the participation of calcineurin in this process. Stp, a kinase inhibitor that modulates CE in other cellular systems, also inhibited fluorescence internalization reinforcing the occurrence of CE in mouse eggs. Although the precise mechanism involved in these pharmacological studies cannot be deduced due to the broad effect of Stp, at least one of its targets, PKC, plays a key role in the endocytosis of KATP channels in somatic cells (Han et al., 2018) as well as in egg activation (Halet, 2004). It is interesting to note that none of the two inhibitors completely prevented LCA internalization. This could be attributed to the fact that the Ca2+ elevation necessary for exo-endocytic processes could be also acting through other mediators not affected by these inhibitors. In this regard, synaptotagmin, a major Ca2+ sensor for synaptic vesicle exocytosis expressed in maturing mouse eggs (Zhu et al., 2012) has been postulated as a Ca2+ downstream link between exo- and endocytosis in other systems (Maritzen, Podufall, & Haucke, 2010).
Several cellular elements have been proposed to compartmentalize the plasma membrane and, thereby, organize the sites of exo- and endocytosis. Among them, membrane lipid domains, i.e. lipid rafts/caveolae, and the underlying cytoskeleton are the most attractive candidates for such function (Gundelfinger et al., 2003). In addition, the relevance of the correct assembly of lipid rafts in mouse eggs for post-fertilization events has been reported (Buschiazzo et al., 2013). Our results using filipin as an inhibitor of caveolae-mediated endocytosis (Rothberg et al., 1992; Rothberg, Ying, Kamen, & Anderson, 1990; Schnitzer, Oh, Pinney, & Allard, 1994) suggest that the lipid rafts/caveolae domains might not be relevant for CE in eggs. On the other hand, the profound inhibition of fluorescence internalization observed in the presence of actin cytoskeleton modulators suggests that the remodeling of the actin cytoskeleton that occurs after fertilization is critical for CE in eggs. In systems where exocytosis occurs through total fusion of the exocytosed vesicles, clathrin-mediated endocytosis takes place adjacent to the active zone of exocytosis (Gundelfinger et al., 2003; Milosevic, 2018). Clathrin has been detected in immature and mature mouse oocytes playing roles in GPR3-mediated endocytosis or meiotic spindle assembly, respectively (Lowther, Nikolaev, & Mehlmann, 2011; Lu et al., 2017). In our case, clathrin inhibition did not produce a reduction in the internalization of LCA-labeled vesicles, suggesting that CE would be independent of the action of this protein. In different types of endocytosis, the GTPase dynamin is responsible for the release of the invaginated vesicles of the plasma membrane through the formation of ring-shaped structures (Holroyd et al., 2002; Meunier & Gutiérrez, 2016). Also, dynamin would be able to promote actin assembly around fused vesicles for subsequent invagination (Lee & De Camilli, 2002; Merrifield, Feldman, Wan, & Almers, 2002). Our experiments with dynasore showed that dynamin participates in endocytic events in eggs, consistent with results in other cellular systems including immature mouse oocytes (Lowther et al., 2011). It has been further described that dynamin must be dephosphorylated by calcineurin to fulfill its action during endocytosis (J. P. Liu, Sim, & Robinson, 1994). Therefore, the inhibition of LCA internalization observed following addition of CsA could be in part attributed to an impaired dephosphorylation of dynamin. Overall, these experiments indicate that CE in mouse eggs is dependent on actin dynamics and dynamin activity, and seems to be independent of clathrin and lipid rafts. Our results are, therefore, consistent with an endocytic mechanism known as “kiss-and-coat” that has been proposed to operate in oocytes of different non-mammalian species and characterized by the coating of the vesicles with F-actin (Sokac & Bement, 2006). In particular, in Xenopus oocytes, G-actin forms filaments around GCs fused to the membrane (Sokac, Co, Taunton, & Bement, 2003). This coating would not only stabilize the secreted vesicle during exocytosis but could be responsible for its later retrieval by CE (Sokac, Schietroma, Gundersen, & Bement, 2006). While there is no evidence regarding a “kiss-and-coat” mechanism in mammalian eggs, the finding that treatment with actin disruptors such as CytD and LatA almost completely prevented LCA internalization supports the importance of actin filaments in mouse egg endocytic events.
Research on several cell types supports a role for the endocytic machinery in facilitating the retrieval of previously exocytosed material from the plasma membrane (Gundelfinger et al., 2003; Maritzen et al., 2010). In eggs, membrane retrieval appears not to extend to the total amount of newly exocytosed CG membranes as fluorescent labeling for CG material persisted on the egg surface up to 6 h after activation (data not shown). In this regard, we cannot discard the possibility that the extent of CE is underestimated due to subcortical localization of some endocytosed vesicles not detectable with the confocal system used in our study. Nevertheless, our findings are consistent with the view that CGE in eggs is a unique event compared to exocytosis in neurons and neuroendocrine cells where several rounds of exocytosis depend on complete recycling of secretory vesicle components from the plasma membrane (Houy et al., 2013). CE could, therefore, be involved in the achievement of plasma membrane homeostasis in eggs, although the contribution of other mechanisms induced in activated eggs, as the release of exosomes into the perivitelline space (Bianchi, Doe, Goulding, & Wright, 2014; Miyado et al., 2008), cannot be ruled out.
CE is observed not only in SrCl2-activated eggs, but also in in vitro fertilized eggs supporting its physiological significance. However, the functional consequences of this endocytic process are unknown. Although it cannot be discounted that CE in eggs represents an evolutionary remnant mechanism from that occurring in specialized secretory cells with several rounds of exo-endocytosis, it has been clearly established that membrane recycling is crucial for plasma membrane area adaptation to variations in cell surface area and membrane homeostasis (Lecuit & Pilot, 2003; Rowghanian & Campàs, 2017). Additionally, both membrane recycling and the resulting plasma membrane dynamics may affect the distribution and movements of transmembrane proteins, which could be essential for various cellular processes (Asnacios & Hamant, 2012; Goehring & Grill, 2013). How these effects on membrane dynamics could be relevant for a correct early embryo development is still unknown and requires further investigation.
Our results show that, although exocytosis occurred similarly in SrCl2 and ionophore-activated eggs, lower levels of CE were observed in ionophore-activated ones. Even though both SrCl2 and calcium ionophore A23187 have proven to be very effective in activating mouse eggs, the mechanisms involved in the intracellular calcium increase are quite different: while SrCl2 provokes long duration repetitive Ca2+/Sr2+ rises mainly through influx via TRPV3 membrane channel (Carvacho et al., 2013; Kline & Kline, 1992), A23187 in a Ca2+-free medium induces a single transient Ca2+ increase coming mostly from internal stores such as the ER (Vincent et al., 1992). Therefore, the difference in CE observed in our study between these treatments could be attributed to the differences in the cortical Ca2+ concentrations producing local changes in membrane-associated processes. In this regard, the relevance of external Ca2+ entry for activation has been described in mammals (Bernhardt et al., 2018) and Drosophila (Hu & Wolfner, 2019). In addition, and despite accumulating evidence for the close coupling of exo- and endocytosis in different cellular systems, our results with ionophore-activated eggs show that these two phenomena can be dissociated. This dissociation is accompanied by a reduction in PS externalization in the egg plasma membrane after activation. Similar results were obtained in chromaffin cells in which the expression of a constitutively inactive form of PLSCR1 scramblase inhibited both PS exposure and CE but not exocytosis (Ory et al., 2013). Moreover, considering our hypothesis that CE is associated to PS exposure, it is relevant to mention that Jas was able to inhibit PS exposure after activation in mouse eggs (Curia et al., 2013). Therefore, the inhibition of CE observed with Jas could be attributed, at least in part, to the lower levels of PS exposure under this condition. The possible association between CE and PS exposure was evaluated by microinjecting mutant Plscr1 into mouse eggs activated with SrCl2 (kindly provided by Dr Gasman, University of Strasbourg, France); however, PS exposure was not impaired in these eggs (data not shown) likely due to compensation by other scramblases (Rysavy et al., 2014). Additional studies are necessary to check the occurrence of this mechanism in eggs. In spite of this, results obtained in other cellular systems supporting the involvement of different lipid species in CE (Gundelfinger et al., 2003) favor the idea that PS exposure may contribute to exo-endocytic coupling in eggs. PS could serve as a molecular anchor for proteins containing the PS-binding domain C2 (Fairn & Grinstein, 2008; Yeung et al., 2009) or positively charged molecules, thereby regulating the recruitment of different factors involved in downstream events required for the exo-endocytic process (i.e. synaptotagmin, Rab, Rac1, MARCKS) (Pérez-Lara et al., 2016; Rysavy et al., 2014). Moreover, the suggested role of PS on the acquisition of membrane curvature necessary for vesicle formation further supports its participation in this process (Rysavy et al., 2014). Therefore, externalization of PS that specifically occurs in exocytotic spots could be regulating the subsequent recapture of CG membrane.
In conclusion, our studies show that mouse eggs display CE after GCE probably as a plasma membrane homeostasis mechanism. Nevertheless, future studies should address the precise function(s) of this process in mammalian eggs and embryos as well as the identification of the intermediate molecular steps underlying this process. On the other hand, our results showing that fertilized eggs exhibited PS exposure and CE, whereas ionophore-activated eggs did not (Curia et al., 2013 and this study) reinforce the notion that different activation procedures induce different downstream mechanisms, providing a point of caution regarding the widespread use of untested methods of artificial oocyte activation in clinical practice.
Supplementary Material
Supplementary Figure S1. Impairment of polar body extrusion and actin disruption with LatA treatment. MII or SrCl2-activated eggs were treated with 10 μM LatA or DMSO (control) during pulse-chase experiments, fixed, and stained with Hoechst 33342 to visualize DNA (A). Some of them were permeabilized, and stained with Acti-stain™ 488 to analyze actin distribution (B). Results are representative of 3 independent experiments in each of which 5–10 eggs/group were evaluated. Bar = 20 μm.
Supplementary Video 1. Compensatory endocytosis in SrCl2-activated eggs. Z-stack images of SrCl2-activated eggs after chase at 4°C collected at 1 μm step size. Note the absence of internalized fluorescent dots.
Supplementary Video 2. Compensatory endocytosis in SrCl2-activated eggs. Z-stack images of SrCl2-activated eggs after chase at 37°C collected at 1 μm step size. Note the presence of internalized fluorescent dots.
Acknowledgements
The authors would like to thank Goli Ardestani, M.Sc. (UMass) and Dr. Pablo Pomata (IBYME-CONICET) for technical assistance, the Light Microscopy Facility (UMass, Amherst), and all the members of our laboratory for their helpful comments.
Funding: This study was supported by the National Agency for Scientific and Technological Promotion Grants (Grants No. PICT 2016-1057 to DJC, and PICT 2015-0471 to PSC), the National Institutes of Health (Grant No. RO1 HD092499 to RAF), Fundación René Baron, and a Fulbright Scholarship to MDGE.
Footnotes
Data availability statement: The data that support the findings of this study are available from the corresponding author, DJC, upon request.
Conflict of interest
We declare that we have no conflicts of interest regarding this study.
References
- Asnacios A, & Hamant O (2012). The mechanics behind cell polarity. Trends in Cell Biology, 22(11), 584–91. 10.1016/j.tcb.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Bement WM, Benink H, Mandato CA, & Swelstad BB (2000). Evidence for direct membrane retrieval following cortical granule exocytosis in Xenopus oocytes and eggs. The Journal of Experimental Zoology, 286(7), 767–75. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10797329 [DOI] [PubMed] [Google Scholar]
- Bernhardt ML, Stein P, Carvacho I, Krapp C, Ardestani G, Mehregan A, … Williams CJ (2018). TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization. Proceedings of the National Academy of Sciences of the United States of America, 115(44), E10370–E10378. 10.1073/pnas.1810422115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Doe B, Goulding D, & Wright GJ (2014). Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature, 508(7497), 483–487. 10.1038/nature13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschiazzo J, Ialy-Radio C, Auer J, Wolf J-P, Serres C, Lefèvre B, & Ziyyat A (2013). Cholesterol Depletion Disorganizes Oocyte Membrane Rafts Altering Mouse Fertilization. PLoS ONE, 8(4), e62919 10.1371/journal.pone.0062919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho I, Lee HC, Fissore RA, & Clapham DE (2013). TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Reports, 5(5), 1375–86. 10.1016/j.celrep.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H-C, Shin W, Zhao W-D, Hamid E, Sheng J, Baydyuk M, … Wu L-G (2014). Post-fusion structural changes and their roles in exocytosis and endocytosis of dense-core vesicles. Nature Communications, 5(1), 3356 10.1038/ncomms4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covian-Nares JF, Smith RM, & Vogel SS (2008). Two independent forms of endocytosis maintain embryonic cell surface homeostasis during early development. Developmental Biology, 316(1), 135–148. 10.1016/j.ydbio.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia CA, Ernesto JI, Stein P, Busso D, Richard M, Cuasnicu PS, … Cohen DJ (2013). Fertilization induces a transient exposure of phosphatidylserine in mouse eggs. PLoS One, 8(8), e71995 10.1371/journal.pone.0071995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, & Nowycky MC (1998). Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. The Journal of Physiology, 506 ( Pt 3), 591–608. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9503324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairn GD, & Grinstein S (2008). Cell biology. A one-sided signal. Science (New York, N.Y.), 320(5875), 458–60. 10.1126/science.1158173 [DOI] [PubMed] [Google Scholar]
- Goehring NW, & Grill SW (2013). Cell polarity: mechanochemical patterning. Trends in Cell Biology, 23(2), 72–80. 10.1016/j.tcb.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Gundelfinger ED, Kessels MM, & Qualmann B (2003). Temporal and spatial coordination of exocytosis and endocytosis. Nature Reviews Molecular Cell Biology, 4(2), 127–139. 10.1038/nrm1016 [DOI] [PubMed] [Google Scholar]
- Halet G (2004). PKC signaling at fertilization in mammalian eggs. Biochimica et Biophysica Acta, 1742(1–3), 185–9. 10.1016/j.bbamcr.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Han Y-E, Chun JN, Kwon MJ, Ji Y-S, Jeong M-H, Kim H-H, … Ho W-K (2018). Endocytosis of K ATP Channels Drives Glucose-Stimulated Excitation of Pancreatic β Cells. Cell Reports, 22(2), 471–481. 10.1016/j.celrep.2017.12.049 [DOI] [PubMed] [Google Scholar]
- Holroyd P, Lang T, Wenzel D, De Camilli P, & Jahn R (2002). Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proceedings of the National Academy of Sciences of the United States of America, 99(26), 16806–11. 10.1073/pnas.222677399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houy S, Croisé P, Gubar O, Chasserot-Golaz S, Tryoen-Tóth P, Bailly Y, … Gasman S (2013). Exocytosis and Endocytosis in Neuroendocrine Cells: Inseparable Membranes! Frontiers in Endocrinology, 4, 135 10.3389/fendo.2013.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, & Wolfner MF (2019). The Drosophila Trpm channel mediates calcium influx during egg activation. Proceedings of the National Academy of Sciences of the United States of America, 201906967 10.1073/pnas.1906967116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, & Watanabe M (2007). Roles of calmodulin and calmodulin-binding proteins in synaptic vesicle recycling during regulated exocytosis at submicromolar Ca2+ concentrations. Neuroscience Research, 58(3), 226–233. 10.1016/j.neures.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Kline D, & Kline JT (1992). Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Developmental Biology, 149(1), 80–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1728596 [DOI] [PubMed] [Google Scholar]
- Kline D, & Stewart-Savage J (1994). The Timing of Cortical Granule Fusion, Content Dispersal, and Endocytosis during Fertilization of the Hamster Egg: An Electrophysiological and Histochemical Study. Developmental Biology, 162(1), 277–287. 10.1006/dbio.1994.1085 [DOI] [PubMed] [Google Scholar]
- Lecuit T, & Pilot F (2003). Developmental control of cell morphogenesis: a focus on membrane growth. Nature Cell Biology, 5(2), 103–8. 10.1038/ncb0203-103 [DOI] [PubMed] [Google Scholar]
- Lee E, & De Camilli P (2002). Dynamin at actin tails. Proceedings of the National Academy of Sciences, 99(1), 161–166. 10.1073/pnas.012607799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Sim AT, & Robinson PJ (1994). Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science (New York, N.Y.), 265(5174), 970–3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8052858 [DOI] [PubMed] [Google Scholar]
- Liu M (2011). The biology and dynamics of mammalian cortical granules. Reproductive Biology and Endocrinology, 9(1), 149 10.1186/1477-7827-9-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther KM, Nikolaev VO, & Mehlmann LM (2011). Endocytosis in the mouse oocyte and its contribution to cAMP signaling during meiotic arrest. Reproduction, 141(6), 737–747. 10.1530/REP-10-0461 [DOI] [PubMed] [Google Scholar]
- Lu A, Zhou C-J, Wang D-H, Han Z, Kong X-W, Ma Y-Z, … Liang C-G (2017). Cytoskeleton-associated protein 5 and clathrin heavy chain binding regulates spindle assembly in mouse oocytes. Oncotarget, 8(11), 17491–17503. 10.18632/oncotarget.15097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritzen T, Podufall J, & Haucke V (2010). Stonins-Specialized Adaptors for Synaptic Vesicle Recycling and Beyond? Traffic, 11(1), 8–15. 10.1111/j.1600-0854.2009.00971.x [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Feldman ME, Wan L, & Almers W (2002). Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nature Cell Biology, 4(9), 691–698. 10.1038/ncb837 [DOI] [PubMed] [Google Scholar]
- Meunier FA, & Gutiérrez LM (2016). Captivating New Roles of F-Actin Cortex in Exocytosis and Bulk Endocytosis in Neurosecretory Cells. Trends in Neurosciences, 39(9), 605–613. 10.1016/j.tins.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Milosevic I (2018). Revisiting the Role of Clathrin-Mediated Endoytosis in Synaptic Vesicle Recycling. Frontiers in Cellular Neuroscience, 12, 27 10.3389/fncel.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, … Umezawa A (2008). The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proceedings of the National Academy of Sciences, 105(35), 12921–12926. 10.1073/pnas.0710608105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory S, Ceridono M, Momboisse F, Houy S, Chasserot-Golaz S, Heintz D, … Gasman S (2013). Phospholipid scramblase-1-induced lipid reorganization regulates compensatory endocytosis in neuroendocrine cells. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(8), 3545–56. 10.1523/JNEUROSCI.3654-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Lara Á, Thapa A, Nyenhuis SB, Nyenhuis DA, Halder P, Tietzel M, … Jahn R (2016). PtdInsP2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. eLife, 5 10.7554/eLife.15886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz T, Skutelsky E, Amihai D, Hammel I, & Shalgi R (1998). Mechanisms leading to cortical reaction in the mammalian egg. Molecular Reproduction and Development, 51(3), 295–303. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, & Anderson RG (1992). Caveolin, a protein component of caveolae membrane coats. Cell, 68(4), 673–82. 10.1016/0092-8674(92)90143-z [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, & Anderson RG (1990). Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. The Journal of Cell Biology, 111(6 Pt 2), 2931–8. 10.1083/jcb.111.6.2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowghanian P, & Campàs O (2017). Non-equilibrium Membrane Homeostasis in Expanding Cellular Domains. Biophysical Journal, 113(1), 132–137. 10.1016/j.bpj.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysavy NM, Shimoda LMN, Dixon AM, Speck M, Stokes AJ, Turner H, & Umemoto EY (2014). Beyond apoptosis: the mechanism and function of phosphatidylserine asymmetry in the membrane of activating mast cells. Bioarchitecture, 4(4–5), 127–37. 10.1080/19490992.2014.995516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, … Cardona A (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods, 9(7), 676–82. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, & Allard J (1994). Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. The Journal of Cell Biology, 127(5), 1217–32. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7525606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, Kaczmarek LK, & Vogel SS (2000). Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. The Journal of Cell Biology, 148(4), 755–67. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10684256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac AM, & Bement WM (2006). Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Molecular Biology of the Cell, 17(4), 1495–502. 10.1091/mbc.e05-10-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac AM, Co C, Taunton J, & Bement W (2003). Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nature Cell Biology, 5(8), 727–732. 10.1038/ncb1025 [DOI] [PubMed] [Google Scholar]
- Sokac AM, Schietroma C, Gundersen CB, & Bement WM (2006). Myosin-1c Couples Assembling Actin to Membranes to Drive Compensatory Endocytosis. Developmental Cell, 11(5), 629–640. 10.1016/j.devcel.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q-Y, & Schatten H (2006). Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction, 131(2), 193–205. 10.1530/rep.1.00847 [DOI] [PubMed] [Google Scholar]
- Tomoda H, Kishimoto Y, & Lee YC (1989). Temperature effect on endocytosis and exocytosis by rabbit alveolar macrophages. The Journal of Biological Chemistry, 264(26), 15445–50. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2768271 [PubMed] [Google Scholar]
- Vincent C, Cheek TR, & Johnson MH (1992). Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. Journal of Cell Science, 103 ( Pt 2, 389–96. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1478942 [DOI] [PubMed] [Google Scholar]
- Wakai T, Zhang N, Vangheluwe P, & Fissore RA (2013). Regulation of endoplasmic reticulum Ca 2+ oscillations in mammalian eggs. Journal of Cell Science, 126(24), 5714–5724. 10.1242/jcs.136549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley T, Terasaki M, Cho MS, & Vogel SS (1995). Direct membrane retrieval into large vesicles after exocytosis in sea urchin eggs. The Journal of Cell Biology, 131(5), 1183–92. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8522582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-S, Zhang Z, Zhao W-D, Wang D, Luo F, & Wu L-G (2014). Calcineurin Is Universally Involved in Vesicle Endocytosis at Neuronal and Nonneuronal Secretory Cells. Cell Reports, 7(4), 982–988. 10.1016/j.celrep.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Heit B, Dubuisson J-F, Fairn GD, Chiu B, Inman R, … Grinstein S (2009). Contribution of phosphatidylserine to membrane surface charge and protein targeting during phagosome maturation. The Journal of Cell Biology, 185(5), 917–28. 10.1083/jcb.200903020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Yoon SY, Parys JB, & Fissore RA (2015). Effect of M-phase kinase phosphorylations on type 1 inositol 1,4,5-trisphosphate receptor-mediated Ca2+ responses in mouse eggs. Cell Calcium, 58(5), 476–88. 10.1016/j.ceca.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X-L, Qi S-T, Liu J, Chen L, Zhang C, Yang S-W, … Sun Q-Y (2012). Synaptotagmin1 is required for spindle stability and metaphase-to-anaphase transition in mouse oocytes. Cell Cycle, 11(4), 818–826. 10.4161/cc.11.4.19329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Impairment of polar body extrusion and actin disruption with LatA treatment. MII or SrCl2-activated eggs were treated with 10 μM LatA or DMSO (control) during pulse-chase experiments, fixed, and stained with Hoechst 33342 to visualize DNA (A). Some of them were permeabilized, and stained with Acti-stain™ 488 to analyze actin distribution (B). Results are representative of 3 independent experiments in each of which 5–10 eggs/group were evaluated. Bar = 20 μm.
Supplementary Video 1. Compensatory endocytosis in SrCl2-activated eggs. Z-stack images of SrCl2-activated eggs after chase at 4°C collected at 1 μm step size. Note the absence of internalized fluorescent dots.
Supplementary Video 2. Compensatory endocytosis in SrCl2-activated eggs. Z-stack images of SrCl2-activated eggs after chase at 37°C collected at 1 μm step size. Note the presence of internalized fluorescent dots.


