Abstract
Asthma, a common disorder associated with airway inflammation and hyperresponsiveness, remains a significant clinical burden in need of novel therapeutic strategies. Patients are increasingly seeking complementary and alternative medicine approaches to control their symptoms, including the use of natural products. Ginger, a natural product that we previously demonstrated acutely relaxes airway smooth muscle (ASM), has long been reported to possess anti-inflammatory properties, although a precise mechanistic understanding is lacking. In these studies, we demonstrate that chronic administration of whole ginger extract or 6-shogaol, a bioactive component of ginger, mitigates in vivo house dust mite antigen-mediated lung inflammation in mice. We further show that this decrease in inflammation is associated with reduced in vivo airway responsiveness. Utilizing in vitro studies, we demonstrate that 6-shogaol augments cAMP concentrations in CD4 cells, consistent with phosphodiesterase inhibition, and limits the induction of nuclear factor-κB signaling and the production of proinflammatory cytokines in activated CD4 cells. Sustained elevations in cAMP concentration are well known to inhibit effector T cell function. Interestingly, regulatory T cells (Tregs) utilize cAMP as a mediator of their immunosuppressive effects, and we demonstrate here that 6-shogaol augments the Treg polarization of naïve CD4 cells in vitro. Taken together with previous reports, these studies suggest that ginger and 6-shogaol have the potential to combat asthma via two mechanisms: acute ASM relaxation and chronic inhibition of inflammation.
Keywords: cAMP, flexiVent, house dust mite antigen, phosphodiesterase, regulatory T cell
INTRODUCTION
Asthma is a chronic disease characterized by airway hyperresponsiveness and inflammation that affects almost 1 in 10 adults (3). Traditionally, short-acting and long-acting β-agonists and inhaled corticosteroids have been the mainstays of asthma therapy. More recently, biologic therapeutics have come into wider use. However, in the United States nearly half of all asthmatics report their symptoms are poorly controlled and experience at least one asthma attack a year (20). This indicates a persistent need for improved therapeutic approaches.
Many patients, including asthmatics, increasingly seek complementary health approaches to improve the management of their disease. Data from the National Health Interview Survey, conducted by the Centers for Disease Control and Prevention (CDC), indicate that 4 of 10 adults utilize complementary and alternative medicine (CAM) approaches each year, with nonvitamin, nonmineral, and natural products being the most commonly used therapy (6). We previously reported that the natural product ginger (Zingiber officinale) and several of its biologically active components (6-shogaol, 6-gingerol, and 8-gingerol) acutely relax airway smooth muscle (ASM) in a number of experimental paradigms (31). We also demonstrated that low concentrations of these active components potentiated β-agonist-mediated human ASM relaxation, potentially by augmenting intracellular cyclic adenosine monophosphate (cAMP) concentrations, as all three compounds inhibited phosphodiesterase 4D (PDE4D) activity in purified enzyme assays (32). Of the three biologically active components tested in our previous work, 6-shogaol was the most potent in augmenting β-agonist-mediated ASM relaxation and inhibiting PDE4 function.
Ginger and its components have long been reported to have anti-inflammatory properties with the potential to combat a host of conditions, including obesity, diabetes, and arthritis (4, 34). However, a precise mechanistic understanding of these anti-inflammatory effects is lacking. In light of our previous finding demonstrating that 6-shogaol inhibits PDE function in cell-free preparations, it is interesting to note that the PDE inhibitor roflumilast has demonstrated anti-inflammatory effects in asthma clinical trials (7, 13).
In the present study, we establish that chronic in vivo administration of whole ginger extract or 6-shogaol mitigates allergic lung inflammation and airway hyperresponsiveness in the murine house dust mite (HDM) antigen model of asthma. We further demonstrate that 6-shogaol augments cAMP concentrations in CD4 cells, consistent with PDE inhibition, and limits nuclear factor-κB (NF-κB) induction and proinflammatory cytokine release. Finally, we demonstrate that 6-shogaol augments regulatory T cell (Treg) polarization in vitro. Given our previous results indicating that ginger or 6-shogaol directly relaxes ASM, these compounds show promise in their ability to treat asthma via two mechanisms: direct ASM relaxation and inhibition of airway inflammation.
MATERIALS AND METHODS
Animals.
All animal studies were approved by the Columbia University Institutional Animal Care and Use Committee. Eight- to twelve-week-old C57/Bl6 mice of both sexes (Jackson Laboratories, Bar Harbor, ME) were used for all experiments.
Effect of ginger or 6-shogaol on in vivo house dust mite antigen-induced murine lung inflammation.
To induce allergic lung inflammation by HDM-sensitization, mice were briefly anesthetized with isoflurane (Baxter, Deerfield, IL) and exposed to intranasal HDM antigen (30 µg dissolved in 25 µL PBS; Greer Laboratories, Lenoir, NC) or PBS alone (nonsensitized controls) for 10 days. Throughout this period, the mice also received oral whole ginger extract (Ginger Extract, Pure Encapsulations, Sudbury, MA), intraperitoneal 6-shogaol (Dalton Chemical Laboratories, Toronto, ON), or the respective vehicle. The ginger extract experiments included three cohorts of mice: 1) intranasal PBS + vehicle (oral gavage 2% hydroxypropyl methylcellulose and 2.5% polyethylene glycol); 2) intranasal HDM + vehicle; and 3) intranasal HDM + oral gavage ginger extract 40 mg/kg twice daily. Similarly, intraperitoneal 6-shogaol experiments included 3 cohorts of mice: 1) intranasal PBS + vehicle (50 uL ip, DMSO; twice daily); 2) intranasal HDM + vehicle; 3) intranasal HDM + 6-shogaol (6.6 mM in 50 uL ip, DMSO; twice daily). After 10 days, bronchoalveolar lavages (BAL) were performed on euthanized mice (overdose of Fatal-Plus, 100 mg/kg ip; Vortech Pharmaceuticals, Dearborn, MI) by slowly infusing 1 mL of PBS into the lungs with a syringe (via an 18-gauge cannula inserted in the trachea through a tracheostomy) and then withdrawing the solution. This was repeated once more, and the 2 samples were combined for each mouse and centrifuged at 500 g for 10 min at room temperature. The cell pellet was resuspended in 200 µL PBS and a 5-µL aliquot was mixed with Trypan blue for BAL total cell counts using a hemocytometer. Differentials were performed by fluorescence-activated cell sorting (FACS) analyses of cell surface marker and forward-scatter (FSC)/side-scatter (SSC) characteristics using an LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ) and FSC Express software (De Novo Software, Glendale, CA). Cells were stained with anti-CD45 (30-F11), -CD3 (145-2C11), -CD11c (HL3), and -SiglecF (E50-2440) antibodies for 1 h at room temperature (1:100 antibody concentration; 1 × 106 cells in 100 µL of FACS buffer; BD Biosciences). Debris and doublets were gated out of analyses. BAL cells, as previously described, were defined as follows: T lymphocytes: CD45+, CD3+, SSClow; eosinophils: CD45+, CD3−, CD11c−, SiglecF+, SSChigh; macrophage: CD45+, CD3−, CD11c+ (21).
Lungs were also collected for histologic analysis and comparison of lung homogenate IL-4 concentrations. Briefly, one lung from each mouse was exposed to 10% formalin overnight, subsequently dehydrated through a graded ethanol series, paraffin-embedded, sectioned, dewaxed, and stained with hematoxylin and eosin. The remaining lung from each mouse was immediately placed into ice-cold digest buffer (1% Nonidet P-40; in mM: 10 Tris pH 7.5, 50 NaCl, 30 Na4P2O7, 50 m NaF, 1 Na3VO4) containing protease inhibitor cocktail (cOmplete Mini, Roche Diagnostics, Indianapolis, IN) and homogenized with a rotor stator homogenizer (top speed for 20 s on ice; IKA Works, Wilmington, NC). The samples were then centrifuged (12,000 g, 4°C for 10 min), and the supernatants were collected and stored at −80°C for subsequent total protein (Pierce BCA Protein Assay, ThermoFisher Scientific, Waltham, MA) and IL-4 concentration analyses (enzyme-linked immunosorbent assay, ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer instructions. IL-4 concentrations were normalized to total protein concentration for each sample.
Effect of 6-shogaol on house dust mite antigen-induced in vivo murine airway hyperresponsiveness.
Mice underwent HDM-sensitization for 3 wk while receiving 6-shogaol (6.6 mM in 50 uL ip, DMSO, twice daily) or vehicle (50 uL DMSO ip twice daily), as described above. Mice receiving 3 wk of intranasal PBS served as nonsensitized controls. In vivo airway responsiveness to inhaled methacholine was assessed by forced oscillation technique using a FlexiVent (SciReq, Montreal, QC, Canada), as previously described (36). Briefly, the mice were anesthetized (pentobarbital 50 mg/kg ip), paralyzed (succinylcholine 10 mg/kg ip), and ventilated using an 18-gauge cannula via a tracheostomy (tidal volume 10 mg/kg, 150 breaths/min, 3 cmH2O positive end expiratory pressure). Airway resistances were measured during a graded, nebulized methacholine challenge (50% duty cycle, 0–50 mg/mL; Sigma, St. Louis, MO). Airway resistance [resistance of respiratory system (Rrs)] was compared between groups using two-way ANOVA with matched comparisons and Bonferonni posttests to compare resistances at each methacholine concentration. A nonsensitized cohort (3 wk of intranasal PBS without HDM) was included to demonstrate the effect of HDM sensitization on resistance. This cohort was drawn from previous studies in compliance with IACUC regulations on the reduction of animal usage.
Effect of 6-shogaol on in vitro CD4 cell cAMP concentration, NF-κB induction, cytokine production, and polarization.
Primary splenocytes were obtained by mincing spleens from euthanized mice and gently crushing the fragments through a cell strainer. Red blood cells were lysed from the resultant suspension, the splenocytes were centrifuged at 300 g and resuspended in PBS. Primary naïve CD4 cells were isolated from the splenocytes by negative selection (MagniSort Mouse CD4 T cell Enrichment Kit, ThermoFisher Scientific). The CD4 cells (2 × 106 cells in 1 mL of HBSS) were then exposed to 10–25 µM 6-shogaol or vehicle (0.2% DMSO) with and without 0.5 µM prostaglandin E2 (PGE2; R&D Systems) for 30 min. The cells were then homogenized, and cAMP concentrations were assayed by ELISA according to the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY). cAMP concentrations were normalized to total protein concentration.
To assess the effect of 6-shogaol on the induction of NF-κB signaling, primary murine CD4 cells were isolated as described above and rested in culture for 2 h in serum-free RPMI-1640 media (ThermoFisher Scientific). The cells were then exposed to 10 µM of 6-shogaol or vehicle for 5 min before a 15-min exposure to anti-CD3/CD28-coated beads (Dynabeads Mouse T-Activator CD3/CD28, ThermoFisher Scientific; 1:1 bead-to-cell ratio) or vehicle. Whole cell protein lysates were then obtained from 2 × 106 cells and total and phosphorylated Ser536 p65 was assayed by ELISA (NF-κB p65 (pS536) + Total NF-κB p65 SimpleStep ELISA Kit, Abcam, Cambridge, UK).
In separate experiments, CD4 cells were cultured in the presence of anti-CD3/CD28-coated beads and 5 ng/mL TGF-β (R&D Systems), 10 µM 6-shogaol, 100 µM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich), TGF-β + 6-shogaol, TGF-β + IBMX, or vehicle (0.2% DMSO). Cells were cultured at 1 × 106 cells/mL in RPMI-1640 media supplemented with 10% fetal bovine serum, 1× antibiotics/antimycotics (ThermoFisher Scientific), and 50 µM β-mercaptoethanol (Sigma-Aldrich) at 37°C in the presence of 5% CO2. Media supernatants were sampled at 24, 72, and 96 h for cytokine concentration analyses using a mouse Th1/Th2/Th17 Cytometric Bead Array kit (BD Bioscience). At 4 and 7 days, cells were analyzed by flow cytometry for Treg polarization (CD4+CD25+FOXP3+) (29). Briefly, 1 × 106 cells were collected from culture, centrifuged at 300 g, washed with PBS, and stained for surface expression of CD4 (RM4-5, BD Bioscience) and CD25 (PC61.5, ThermoFisher Scientific) for 1 h at room temperature (1:100 antibody concentration; 1 × 106 cells in 100 µL of FACS buffer). The cells were then stained with a fixable viability dye according to the manufacturer’s instructions (ThermoFisher Scientific). The cells were subsequently fixed and permeabilized (FOXP3/Transcription Factor Staining Buffer Kit, ThermoFisher Scientific) and stained for intracellular FOXP3 (1:100 antibody concentration; 1 × 106 cells in 100 µL; FJK-16s, ThermoFisher Scientific). Analyses were performed using an LSRII flow cytometer and FSC Express software. Debris, non-singlet cells, and dead cells were gated out of analyses. Tregs were defined as CD4+CD25+FOXP3+ cells (3).
Statistics.
Data were analyzed using one- or two-way ANOVA with matched comparisons and the Bonferroni posttest analyses as appropriate using Prism 4 software (GraphPad, La Jolla, CA). Statistical significance was established at P < 0.05.
RESULTS
Chronic administration of whole ginger extract or 6-shogaol inhibits in vivo house dust mite antigen-induced lung inflammation in mice.
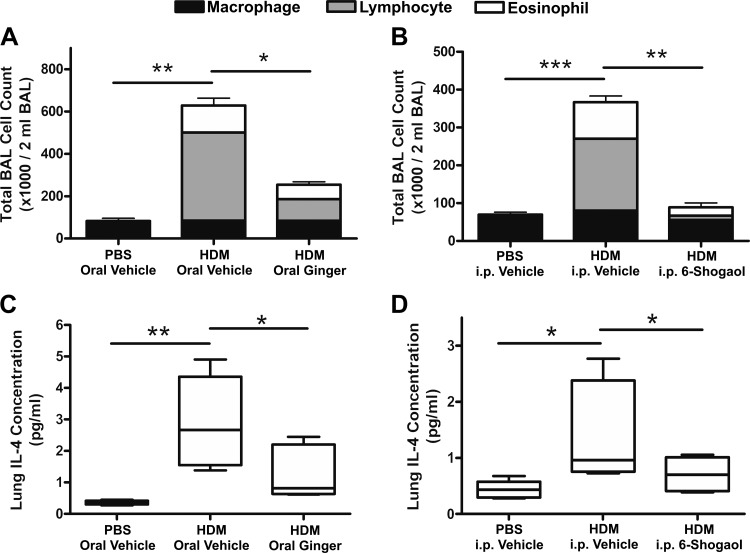
Ten days of intranasal HDM administration led to allergic lung inflammation in mice, characterized by peribronchial and perivascular leukocyte infiltration (Fig. 1). HDM sensitization led to significant increases in BAL total cell counts, primarily consisting of eosinophils and lymphocytes. Coadministration of oral whole ginger extract (Fig. 2A) or intraperitoneal 6-shogaol (Fig. 2B) along with intranasal HDM significantly decreased BAL cell counts compared with mice receiving HDM and the respective vehicles (n = 5, ANOVA with Bonferonni posttest analyses, P < 0.05 and P < 0.01, respectively). Consistent with this, oral whole ginger extract (Fig. 2C) or intraperitoneal 6-shogaol (Fig. 2D) also decreased lung concentration of IL-4 (n = 5, ANOVA with Bonferonni posttest analyses, P < 0.05), a key mediator of type 2 helper T cell (Th2)-driven inflammation, by 59 and 51% respectively, compared with vehicle controls in HDM-sensitized mice.
Fig. 1.
Hematoxylin and eosin-stained sections of mouse lungs. Insets: ×2 magnification of peribronchial regions. Ten days of intranasal house dust mite (HDM) exposure led to peribronchial and perivascular leukocyte infiltration (middle) compared with intranasal phosphate buffered saline (PBS) vehicle controls (left). Coadministration of either oral whole ginger extract (A, right; 40 mg/kg twice daily) or intraperitoneal (ip) 6-shogaol (B, right; 50 µL of 6.6 mM solution twice daily) attenuated the HDM-mediated inflammation. Scale bar, 500 µm.
Fig. 2.
Bronchoalveolar lavage (BAL) cell counts and lung IL-4 concentrations. Ten days of intranasal house dust mite (HDM) exposure led to a significant increase in total BAL cell counts in oral and intraperitoneal (ip) vehicle-treated control animals (A and B, respectively) compared with corresponding intranasal phosphate-buffered saline (PBS) vehicle controls. These increases, which were composed primarily by elevations in eosinophil and lymphocyte numbers, were significantly inhibited by coadministration of oral whole ginger extract (A; 40 mg/kg twice daily) or intraperitoneal 6-shogaol (B; 50 µL ip of 6.6 mM solution twice daily). Oral whole ginger extract and intraperitoneal 6-shogaol also significantly inhibited HDM-mediated increases in lung homogenate IL-4 concentration (C and D, respectively). Data in A and B are means ± SE, n = 5 mice, ANOVA with Bonferonni posttest comparisons. *P < 0.05, **P < 0.01, ***P < 0.001.
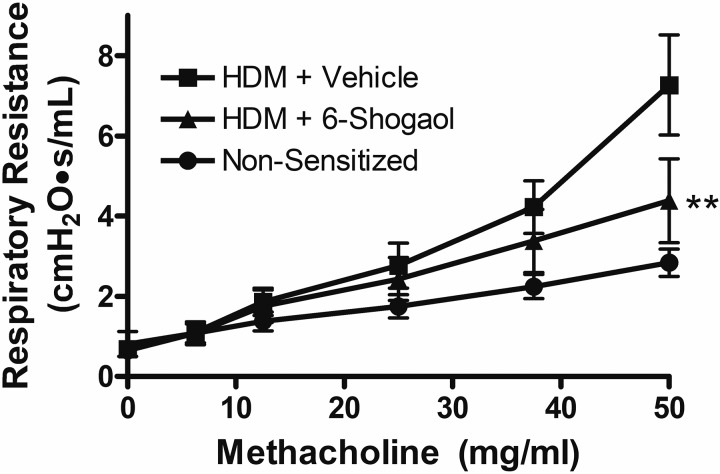
6-Shogaol inhibits house dust mite antigen-induced in vivo airway hyperresponsiveness in mice.
Consistent with decreased Th2-driven lung inflammation, mice receiving intraperitoneal 6-shogaol during a 3-wk HDM-sensitization protocol had significantly decreased in vivo airway responsiveness (Rrs; resistance in response to a graded, inhaled methacholine challenge) compared with mice receiving HDM plus vehicle as determined by forced oscillation technique (Fig. 3; n = 7, two-way ANOVA with matched comparisons and Bonferonni posttests, P < 0.01 comparing HDM-sensitized 6-shogaol vs. vehicle groups at 50 mg/mL methacholine). Given that 6-shogaol was administered chronically (not during or immediately before airway resistance testing), this difference is likely the result of decreased lung and airway inflammation (and the resultant decrease in airway smooth muscle sensitivity to methacholine) rather than an acute relaxant effect of 6-shogaol on ASM.
Fig. 3.
Mouse in vivo respiratory resistance measured by forced oscillation technique. Invasive airway resistance testing (flexiVent) was performed in anesthetized, paralyzed mice after 3 wk of intranasal house dust mite (HDM) exposure and coadministration of intraperitoneal 6-shogaol or vehicle (DMSO). Mice receiving 3 wk of intranasal PBS served as a nonsensitized control. Chronic 6-shogaol treatment significantly diminished the increase in respiratory resistance that occurred in response to a graded, inhaled methacholine challenge. Data are means ± SE; n = 7 mice, two-way ANOVA with Bonferonni posttests. **P < 0.01 comparing HDM + vehicle to HDM + 6-shogaol at 50 mg/mL methacholine.
6-Shogaol augments murine CD4 cell cAMP concentration in vitro.
In light of our report demonstrating that 6-shogaol inhibits phosphodiesterase (PDE) activity in cell-free assays (32), we sought to determine the effect of 6-shogaol on CD4 cell cyclic adenosine monophosphate (cAMP) concentrations in vitro. 6-Shogaol (10 and 25 µM) significantly and dose-dependently augmented cAMP concentrations in CD4 cells exposed to prostaglandin E2 (PGE2), a Gαs-coupled receptor agonist that induces adenylyl cyclase activity/cAMP production in CD4 cells, at 30 min compared with vehicle control (Fig. 4; n = 5 mice, ANOVA with matched comparisons and Bonferonni posttest analyses, P < 0.01 and P < 0.001 respectively). 6-Shogaol without PGE2 did not significantly increase basal cAMP concentrations compared with vehicle, consistent with inhibition of PDE-mediated cAMP degradation.
Fig. 4.
In vitro murine CD4 cell intracellular cAMP concentration. cAMP concentrations were measured by ELISA in 2 × 106 CD4 cells that were homogenized after 30 min of drug exposure in vitro. Cotreatment of CD4 cells exposed to prostaglandin E2 (PGE2; Gαs-coupled receptor agonist) with 10–25 µM 6-shogaol (6-S) led to dose-dependent increases in cAMP concentrations over cells treated with PGE2 alone. 6-Shogaol alone did not increase cAMP above DMSO (0.2%) vehicle control. These results are consistent with 6-shogaol-mediated inhibition of phosphodiesterase activity. n = 5 mice, ANOVA with matched comparisons and Bonferonni posttest analyses. **P < 0.01, ***P < 0.001.
6-Shogaol alters murine CD4 cell cytokine production in vitro.
Given that cAMP is a known direct, negative regulator of effector T (Teff) cell function (5) and a mediator of Treg-associated suppression of Teff function (28), we sought to determine the effect of 6-shogaol on naïve T cell cytokine production during T cell receptor (TCR) activation. Naïve primary murine CD4 cells were stimulated with anti-CD3/CD28-coated beads in vitro. The addition of 10 µM 6-shogaol to the culture media led to a significant reduction in media supernatant concentrations of Teff-associated cytokines (IL-2, TNF-α, and IFN-γ) from 24 –96 h compared with vehicle control (Fig. 5; n = 3 mice, ANOVA with matched comparisons and Bonferonni posttest analyses). Interestingly, 6-shogaol did not decrease IL-10, a cytokine associated with Treg function. The effect of 6-shogaol on cytokine production was similar to that of 5 ng/mL TGF-β, a known driver of Treg differentiation in vitro and in vivo (8). Neither 6-shogaol nor TGF-β decreased CD4 cell viability compared with vehicle control (data not shown).
Fig. 5.
Murine CD4 cells in vitro cytokine production. CD4 cells were culture for 96 h under T cell receptor stimulating conditions (CD3 and CD28 activation) in the presence of 10 µM 6-shogaol (6-S), 5 ng/mL TGF-β, both 6-shogaol and TGF-β, or vehicle (0.1% DMSO). Media supernatant cytokine concentrations were measured at 24, 72, and 96 h. 6-Shogaol significantly inhibited the production of several proinflammatory cytokines (IL-2, TNF-α, and IFN-γ) at multiple time points compared with vehicle control. However, IL-10 production was not inhibited. A similar pattern was seen with exposure to TGF-β, a known driver of regulatory T cell polarization. Data are means ± SE; n = 3 mice, ANOVA with matched comparisons and Bonferonni posttest analyses. *P < 0.05, **P < 0.01.
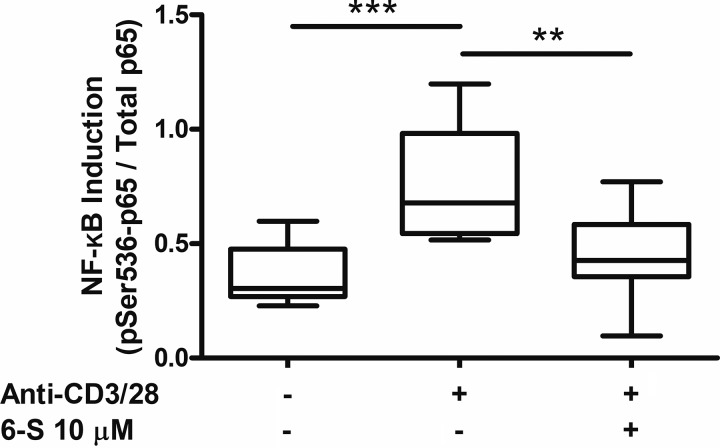
6-Shogaol limits CD3/CD28-mediated induction of NF-κB signaling in murine CD4 cells in vitro.
The NF-κB signaling pathway is a key mediator of CD4 cell activation and proinflammatory cytokine production. In murine CD4 cells stimulated with anti-CD3/CD28-coated beads in vitro, 10 µM 6-shogaol significantly inhibited the induction of NF-κB signaling, measured as phosphorylated-Ser536 p65/total p65, by 78% (Fig. 6; P < 0.01, n = 9, ANOVA with matched comparisons and Bonferonni posttest analyses). This is consistent with its effects on CD4 cell cytokine production.
Fig. 6.
In vitro murine CD4 cell NF-κB induction. Exposure of CD4 cells to T cell receptor-stimulating conditions (anti-CD3/CD28-coated beads: anti-CD3/28) led to a significant increase in NF-κB signaling, quantified by ELISA as phosphorylated Ser536 p65 (pSer536-p65) to total p65 ratio, at 15 min. 6-Shogaol (6-S) 10 µM significantly inhibited this effect. n = 9 mice, ANOVA with matched comparisons and Bonferonni posttest analyses. **P < 0.01, ***P < 0.001.
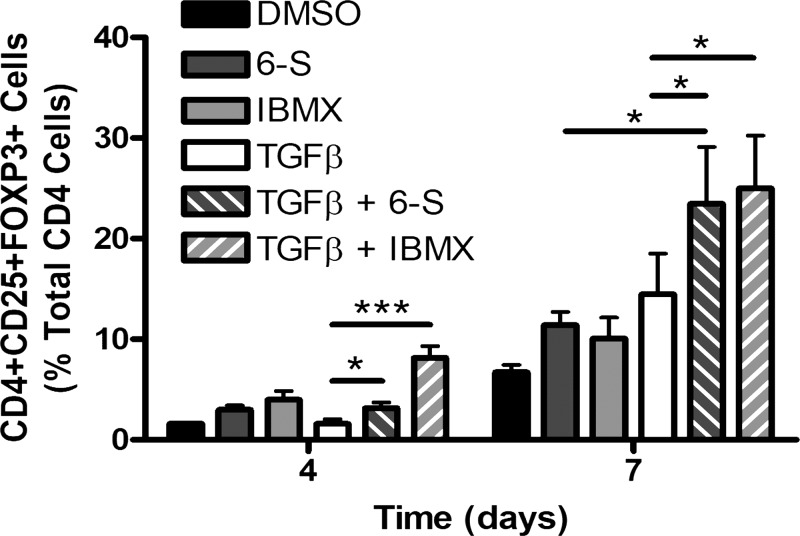
6-Shogaol augments murine Treg polarization in vitro.
At 7 days in culture, 10 µM 6-shogaol augmented TGF-β-mediated polarization of naïve CD4 cells to a Treg phenotype, defined as CD4+CD25+FOXP3+ cells, by 162% (Fig. 7; ANOVA with matched comparisons and Bonferonni posttest analyses, P < 0.05 compared with TGF-β alone, n = 4). This effect was mimicked by 100 µM IBMX, a known PDE inhibitor. Neither 6-shogaol nor IBMX decreased CD4 cell viability compared with vehicle control (data not shown).
Fig. 7.
In vitro murine regulatory T cell polarization. Naïve CD4 cells were cultured for 7 days under T cell receptor stimulating conditions (CD3 and CD28 activation) in the presence of 10 µM 6-shogaol (6-S), 100 µM IBMX, 5 ng/mL TGF-β, both 6-shogaol and TGF-β, both IBMX and TGF-β, or vehicle (0.1% DMSO). Coexposure of the cells to 6-shogaol and TGF-β led to a significant increase in regulatory T cell polarization at 4 and 7 days (defined as percentage of CD+CD25+FOXP3+ cells) compared with TGF-β alone. IBMX, a phosphodiesterase inhibitor, mimicked this effect. At 7 days, coexposure of cells to 6-shogaol and TGF-β also led to a significant increase in regulatory T cell (Treg) polarization compared with 6-shogaol alone. This is consistent with 6-shogaol-mediated augmentation of Treg polarization by TGF-β, a known driver of Treg polarization. Data are means ± SE; n = 4 mice, ANOVA with matched comparisons and Bonferonni posttest analyses. *P < 0.05, ***P < 0.001.
DISCUSSION
In this paper, we demonstrate that chronic administration of oral whole ginger extract or intraperitoneal 6-shogaol, a bioactive component of ginger, reduces allergic lung inflammation in the murine HDM model of asthma. Chronic 6-shogaol treatment also reduces in vivo airway hyperresponsiveness in reaction to an inhaled methacholine challenge in HDM-sensitized mice. Utilizing in vitro studies, we also demonstrate that 6-shogaol augments cAMP concentrations in CD4 cells exposed to PGE2, a Gαs-coupled receptor agonist. Furthermore, we show that 6-shogaol inhibits proinflammatory cytokine production in activated CD4 cells and augments Treg polarization in vitro.
Although we have previously shown that ginger or 6-shogaol directly and acutely relaxes ASM (31, 32), the decreased airway responsiveness we demonstrate in the present study likely results from the inhibition of chronic HDM-induced, Th2-driven lung inflammation as characterized by a reduction in BAL lymphocyte and eosinophil counts and lung IL-4 concentration. It is well established that chronic elevation of Th2-associated cytokines IL-13 and IL-4 leads to the development and maintenance of airway hyperresponsiveness in asthma models via multiple mechanisms, including effects on immune cells, airway nerves, airway epithelium, and directly on ASM (15, 26, 27). Considering that 6-shogaol was administered chronically in the current study and not during or immediately before airway resistance testing, the decreased responsiveness to inhaled methacholine observed in the present study is likely attributable to a chronic anti-inflammatory effect and not an acute relaxant effect on ASM. Interestingly, ASM is known to play an immunomodulatory role in asthmatic inflammation as well (35). The current studies do not address the potential of chronic whole ginger or 6-shogaol administration to alter these functions in particular. Thus, the present findings, coupled with our previous studies (31, 32), demonstrate a dual beneficial effect on ASM responses; an acute relaxation of ex vivo precontracted ASM by 6-shogaol and a chronic anti-inflammatory effect of 6-shogaol on in vivo ASM methacholine responsiveness.
This is the first report demonstrating a beneficial effect of ginger or 6-shogaol in an allergic lung inflammation model. Furthermore, we propose a novel mechanism for 6-shogaol’s anti-inflammatory effect: PDE inhibition. We previously demonstrated 6-shogaol inhibits PDE activity in cell-free assays (32). Studies here demonstrate that 6-shogaol augments intracellular cAMP concentrations in CD4 cells exposed to PGE2, a generally anti-inflammatory mediator that activates adenylyl cyclase activity/cAMP production in CD4 cells via activation of its Gαs-coupled receptors. However, 6-shogaol does not increase basal cAMP concentrations in the absence of PGE2, consistent with inhibition of PDE, the enzymes chiefly responsible for cAMP degradation.
Coligation of the TCR and the coreceptor CD28 leads to an increase in lymphocyte cAMP concentration that facilitates antigen detection (10). However, this elevation is transient (<10 min) as TCR activation also recruits PDE to lipid rafts where it degrades cAMP (1). Inhibition of PDE4 function, and the resultant sustained elevation in intracellular cAMP concentrations, suppresses conventional T cell function largely via protein kinase A (PKA)-dependent processes (5). Regulatory T cells (Tregs), on the other hand, have a relatively high intracellular cAMP concentration and inhibit immune responses primarily by increasing cAMP concentrations in Teffs and dendritic cells. This is thought to occur by one of two mechanisms (or potentially both). The first is the direct transfer of cAMP from Tregs into target cells through gap junctions. The second is the release of cAMP by Tregs, which is then converted to adenosine by ectonucleases on the cell surfaces. This adenosine then activates its Gαs-coupled receptors on target cells, leading to increased adenylyl cyclase activity and cAMP concentrations (18, 28). It is interesting to note that the clinically utilized PDE4 inhibitor roflumilast has demonstrated anti-inflammatory effects in human asthma (13).
In this study, we demonstrate that 6-shogaol suppresses induction of NF-κB signaling and proinflammatory cytokine (IL-2, TNF-α, IFN-γ) release from activated CD4 cells in vitro. Interestingly, IL-10 production is not inhibited by 6-shogaol. This cytokine profile is similar to that seen with exposure to TGF-β, a known driver of Treg polarization in vitro and peripheral Treg differentiation in vivo (8). IL-10, which is produced by a number of cell types including Tregs, has complex actions but generally functions to maintain homeostasis during inflammatory processes by limiting excessive immune responses (24). This led us to hypothesize that 6-shogaol was promoting Treg polarization of naïve CD4 cells. In fact, we demonstrate that 6-shogaol augments TGF-β-mediated Treg polarization in vitro, consistent with this hypothesis. Although the studies presented here do not provide conclusive evidence that this effect is PDE/cAMP-mediated, IBMX, a known PDE inhibitor, mimics 6-shogaol in augmenting Treg polarization. Furthermore, previous reports demonstrate that PGE2, a Gαs-coupled receptor agonist, induces FOXP3 expression and suppressive function in CD4+CD25+ and CD4+CD25- lymphocytes. This effect was mirrored by forskolin, a pharmacologic activator of adenylyl cyclase, and cholera toxin, an activator of the Gαs subunit of G proteins (30). These findings are all consistent with a cAMP-mediated effect on Treg polarization.
Several clinical trials have reported beneficial effects of ginger in conditions with significant inflammatory pathology. These include osteoarthritis (12, 25), primary dysmenorrhea (2), type 2 diabetes mellitus and metabolic syndrome (34, 37), and hypertension (17). There is also preliminary clinical evidence (9) and significant preclinical evidence that ginger and its components, particularly 6-shogaol, may have therapeutic effects in cancers as a result of antiproliferative, antioxidant, anti-invasive, and/or anti-inflammatory properties (11). Most previous reports on the mechanisms of ginger’s anti-inflammatory effects come from in vitro studies. Focusing on immune cells, 6-gingerol, another bioactive component of ginger, was shown to inhibit NF-κB induction and proinflammation cytokine production in lipopolysaccharide (LPS)-stimulated peritoneal macrophages (33). Another report in macrophage demonstrated that 6-gingerol decreased inducible nitric oxide synthase (iNOS) and TNF-α expression through suppression of NF-κB activation and protein kinase C (PKC)-α translocation (19). In LPS-stimulated cultures, 6-shogaol was shown to inhibit microglia activation by inhibiting proinflammatory cytokine production as well as cyclooxygenase (COX)-2 expression and NF-κB activity (16). Consistent with these reports, we demonstrate here that 6-shogaol limits induction of NF-κB signaling in stimulated CD4 cells as well. The mechanism by which 6-shogaol affects NF-κB activity is not known for certain. However, it is interesting to note that there is an extensive literature demonstrating that cAMP regulates NF-κB activity in multiple cell types (14). For example, Navarro et al. demonstrated that rolipram, a PDE4 inhibitor, decreased NF-κB activation in activated Jurkat cells and primary human T cells. This effect was mimicked by the addition of exogenous dibutyryl-cyclic AMP (dBcAMP), a cell permeable cAMP analog (22). Similarly, Neumann et al. (23) showed that forskolin inhibited formation of nuclear NF-κB complexes in murine T cells, likely by PKA-mediated stabilization of IκBα, a cytosolic inhibitor of κB factors.
This is the first demonstration of ginger- or 6-shogaol-mediated mitigation of lung inflammation and airway hyperresponsiveness in the murine HDM model of asthma. 6-Shogaol increases CD4 cell cAMP concentrations, inhibits the induction of NF-κB signaling and proinflammatory cytokine release, and augments Treg polarization in vitro. Experiments presented here suggest that these effects are the result of PDE inhibition. Given their anti-inflammatory effects and previously demonstrated ability to directly relax ASM, ginger and 6-shogaol are promising asthma therapeutics that target two key pathologic components of asthma: acute airway smooth muscle contraction and lung inflammation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHBLI) Grant HL140102 (G. T. Yocum); NHBLI Grant HL132203 (J. Danielsson); NHBLI Grant HL143052 (M. Mikami); NHBLI Grant HL122340 (C. W. Emala); and National Center for Complementary and Integrative Health (NCCIH) Grant AT009989 (C. W. Emala). G. T. Yocum and J. Danielsson are Louis V. Gerstner Jr. Scholars. J. J. Hwang is a Howard Hughes Medical Institute Medical Research Fellow. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under award S10RR027050.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.T.Y., J.J.H., and C.W.E. conceived and designed research; G.T.Y., J.J.H., M.M., J.D., A.S.K., and C.W.E. performed experiments; G.T.Y., J.J.H., and C.W.E. analyzed data; G.T.Y., J.J.H., M.M., J.D., A.S.K., and C.W.E. interpreted results of experiments; G.T.Y. prepared figures; G.T.Y. drafted manuscript; G.T.Y., J.J.H., M.M., J.D., A.S.K., and C.W.E. edited and revised manuscript; G.T.Y., J.J.H., M.M., J.D., A.S.K., and C.W.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Atrium Innovations (Pure Encapsulations) for supplying ginger extract.
REFERENCES
- 1.Abrahamsen H, Baillie G, Ngai J, Vang T, Nika K, Ruppelt A, Mustelin T, Zaccolo M, Houslay M, Taskén K. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR signaling J Immunol 173: 4847–4858, 2004. doi: 10.4049/jimmunol.173.8.4847. [DOI] [PubMed] [Google Scholar]
- 2.Adib Rad H, Basirat Z, Bakouei F, Moghadamnia AA, Khafri S, Farhadi Kotenaei Z, Nikpour M, Kazemi S. Effect of ginger and novafen on menstrual pain: a cross-over trial. Taiwan J Obstet Gynecol 57: 806–809, 2018. doi: 10.1016/j.tjog.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Fryar CD. Current Asthma Prevalence by Weight Status Among Adults: United States, 2001-2014 NCHS Data Brief 239: 1–8, 2016. [PubMed] [Google Scholar]
- 4.Al-Nahain A, Jahan R, Rahmatullah M. Zingiber officinale: a potential plant against rheumatoid arthritis. Arthritis (Egypt) 2014: 159089, 2014. doi: 10.1155/2014/159089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arumugham VB, Baldari CT. cAMP: a multifaceted modulator of immune synapse assembly and T cell activation. J Leukoc Biol 101: 1301–1316, 2017. doi: 10.1189/jlb.2RU1116-474R. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1–23, 2008. [PubMed] [Google Scholar]
- 7.Bateman ED, Goehring UM, Richard F, Watz H. Roflumilast combined with montelukast versus montelukast alone as add-on treatment in patients with moderate-to-severe asthma. J Allergy Clin Immunol 138: 142–149.e8, 2016. doi: 10.1016/j.jaci.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198: 1875–1886, 2003. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citronberg J, Bostick R, Ahearn T, Turgeon DK, Ruffin MT, Djuric Z, Sen A, Brenner DE, Zick SM. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: results from a pilot, randomized, and controlled trial. Cancer Prev Res (Phila) 6: 271–281, 2013. doi: 10.1158/1940-6207.CAPR-12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conche C, Boulla G, Trautmann A, Randriamampita C. T cell adhesion primes antigen receptor-induced calcium responses through a transient rise in adenosine 3′,5′-cyclic monophosphate. Immunity 30: 33–43, 2009. doi: 10.1016/j.immuni.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 11.de Lima RM, Dos Reis AC, de Menezes AP, Santos JV, Filho JW, Ferreira JR, de Alencar MV, da Mata AM, Khan IN, Islam A, Uddin SJ, Ali ES, Islam MT, Tripathi S, Mishra SK, Mubarak MS, Melo-Cavalcante AA. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: a comprehensive review. Phytother Res 32: 1885–1907, 2018. doi: 10.1002/ptr.6134. [DOI] [PubMed] [Google Scholar]
- 12.Drozdov VN, Kim VA, Tkachenko EV, Varvanina GG. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J Altern Complement Med 18: 583–588, 2012. doi: 10.1089/acm.2011.0202. [DOI] [PubMed] [Google Scholar]
- 13.Gauvreau GM, Boulet LP, Schmid-Wirlitsch C, Côté J, Duong M, Killian KJ, Milot J, Deschesnes F, Strinich T, Watson RM, Bredenbröker D, O’Byrne PM. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir Res 12: 140, 2011. doi: 10.1186/1465-9921-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlo S, Kooijman R, Beck IM, Kolmus K, Spooren A, Haegeman G. Cyclic AMP: a selective modulator of NF-κB action. Cell Mol Life Sci 68: 3823–3841, 2011. doi: 10.1007/s00018-011-0757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma Science 282: 2261–2263, 1998. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, Kim SY. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology 63: 211–223, 2012. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Hasani H, Arab A, Hadi A, Pourmasoumi M, Ghavami A, Miraghajani M. Does ginger supplementation lower blood pressure? A systematic review and meta-analysis of clinical trials. Phytother Res 33: 1639–1647, 2019. doi: 10.1002/ptr.6362. [DOI] [PubMed] [Google Scholar]
- 18.Klein M, Bopp T. Cyclic AMP represents a crucial component of Treg cell-mediated immune regulation. Front Immunol 7: 315, 2016. doi: 10.3389/fimmu.2016.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun 382: 134–139, 2009. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- 20.Mazurek JM, Syamlal G. Prevalence of asthma, asthma attacks, and emergency department visits for asthma among working adults—National Health Interview Survey, 2011–2016 MMWR Morb Mortal Wkly Rep 67: 377–386, 2018. doi: 10.15585/mmwr.mm6713a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49: 503–510, 2013. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro J, Punzón C, Jiménez JL, Fernández-Cruz E, Pizarro A, Fresno M, Muñoz-Fernández MA. Inhibition of phosphodiesterase type IV suppresses human immunodeficiency virus type 1 replication and cytokine production in primary T cells: involvement of NF-κB and NFAT J Virol 72: 4712–4720, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann M, Grieshammer T, Chuvpilo S, Kneitz B, Lohoff M, Schimpl A, Franza BR Jr, Serfling E. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A EMBO J 14: 1991–2004, 1995. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 50: 871–891, 2019. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Paramdeep G. Efficacy and tolerability of ginger (Zingiber officinale) in patients of osteoarthritis of knee Indian J Physiol Pharmacol 57: 177–183, 2013. [PubMed] [Google Scholar]
- 26.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J Allergy Clin Immunol 118: 410–419, 2006. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med 208: 853–867, 2011. doi: 10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rueda CM, Jackson CM, Chougnet CA. Regulatory T-cell-mediated suppression of conventional T-cells and dendritic cells by different cAMP intracellular pathways. Front Immunol 7: 216, 2016. doi: 10.3389/fimmu.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol 707: 3–17, 2011. doi: 10.1007/978-1-61737-979-6_1. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res 65: 5211–5220, 2005. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 31.Townsend EA, Siviski ME, Zhang Y, Xu C, Hoonjan B, Emala CW. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am J Respir Cell Mol Biol 48: 157–163, 2013. doi: 10.1165/rcmb.2012-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townsend EA, Zhang Y, Xu C, Wakita R, Emala CW. Active components of ginger potentiate β-agonist-induced relaxation of airway smooth muscle by modulating cytoskeletal regulatory proteins. Am J Respir Cell Mol Biol 50: 115–124, 2014. doi: 10.1165/rcmb.2013-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi S, Maier KG, Bruch D, Kittur DS. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Surg Res 138: 209–213, 2007. doi: 10.1016/j.jss.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci 1398: 83–98, 2017. doi: 10.1111/nyas.13375. [DOI] [PubMed] [Google Scholar]
- 35.Xia YC, Redhu NS, Moir LM, Koziol-White C, Ammit AJ, Al-Alwan L, Camoretti-Mercado B, Clifford RL. Pro-inflammatory and immunomodulatory functions of airway smooth muscle: emerging concepts. Pulm Pharmacol Ther 26: 64–74, 2013. doi: 10.1016/j.pupt.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Yocum GT, Turner DL, Danielsson J, Barajas MB, Zhang Y, Xu D, Harrison NL, Homanics GE, Farber DL, Emala CW. GABAA receptor α4-subunit knockout enhances lung inflammation and airway reactivity in a murine asthma model. Am J Physiol Lung Cell Mol Physiol 313: L406–L415, 2017. doi: 10.1152/ajplung.00107.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Chen H, Song Z, Wang X, Sun Z. Effects of ginger (Zingiber officinale Roscoe) on Type 2 diabetes mellitus and components of the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2018: 5692962, 2018. doi: 10.1155/2018/5692962. [DOI] [PMC free article] [PubMed] [Google Scholar]