Abstract
Insulin resistance and right ventricular (RV) dysfunction are associated with lipotoxicity in heritable forms of pulmonary arterial hypertension (PAH), commonly due to mutations in bone morphogenetic protein receptor type 2 (BMPR2). How BMPR2 dysfunction in cardiomyocytes alters glucose metabolism and the response of these cells to insulin are unknown. We hypothesized that BMPR2 mutation in cardiomyocytes alters glucose-supported mitochondrial respiration and impairs cellular responses to insulin, including glucose and lipid uptake. We performed metabolic assays, immunofluorescence and Western analysis, RNA profiling, and radioactive isotope uptake studies in H9c2 cardiomyocyte cell lines with and without patient-derived BMPR2 mutations (mutant cells), with and without insulin. Unlike control cells, BMPR2 mutant cardiomyocytes have reduced metabolic plasticity as indicated by reduced mitochondrial respiration with increased mitochondrial superoxide production. These mutant cells show enhanced baseline phosphorylation of insulin-signaling protein as indicated by increased Akt, AMPK, and acetyl-CoA carboxylase phosphorylation that may negatively influence fatty acid oxidation and enhance lipid uptake, and are insulin insensitive. Furthermore, mutant cells demonstrate an increase in milk fat globule-EGF factor-8 protein (MFGE8), which influences the insulin-signaling pathway by phosphorylating AktSer473 via phosphatidylinositol 3-kinase and mammalian target of rapamycin. In conclusion, BMPR2 mutant cardiomyocytes have reduced metabolic plasticity and fail to respond to glucose. These cells have enhanced baseline insulin-signaling pattern favoring insulin resistance with failure to augment this pattern in response to insulin. BMPR2 mutation possibly blunts glucose uptake and enhances lipid uptake in these cardiomyocytes. The MFGE8-driven signaling pathway may suggest a new mechanism underlying RV lipotoxicity in PAH.
Keywords: BMPR2 mutation, H9c2 cultured cardiomyocytes, insulin signaling protein intermediates, MFGE8, pulmonary arterial hypertension, right ventricular dysfunction and lipotoxicity
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a fatal disease characterized by right ventricular (RV) dysfunction, failure, and ultimately death. In PAH, clinical outcomes are poor, with RV failure often within 2–3 years after diagnosis (14). We and others have published that patients with heritable PAH, commonly due to bone morphogenetic protein receptor type 2 (BMPR2) mutations, have disproportionate RV failure, manifested by an earlier age at presentation, reduced RV compensation, and impaired cardiomyocyte fatty acid metabolism (3, 33).
In heritable (HPAH) and idiopathic PAH, RV dysfunction and failure are associated with RV lipid accumulation (13), which is a result of impaired fatty acid metabolism, mitochondrial dysfunction, and increased lipid uptake via altered localization of CD36, the major fatty acid transporter protein (13, 35). Insulin resistance, common in PAH, is associated with disproportional RV dysfunction and is closely linked to lipid accumulation (40, 43); hyperglycemia and dyslipidemia play a significant role in the etiology of the disease (9, 27, 36). The specific impact of insulin on glucose and lipid metabolism in RV cardiomyocytes with BMPR2 mutation is not known.
We hypothesized that BMPR2 mutation in cardiomyocytes alters metabolic plasticity and glucose uptake in the presence of glucose as a substrate and impairs responses to insulin. We demonstrate that BMPR2 mutant cardiomyocytes show reduced mitochondrial oxygen consumption (independent of available substrate) associated with increased superoxide production, and enhanced phosphorylation of Akt, AMPK, and acetyl-CoA carboxylase (ACC) proteins unaffected by insulin. In BMPR2 mutant cardiomyocytes, glucose transporter protein 4 (Glut4) protein expression is unchanged while CD36 protein expression is increased. Furthermore, these cardiomyocytes have variable glucose uptake and are insensitive to insulin but express an exaggerated palmitate uptake and are partially responsive to insulin. Finally, we identified a novel pathway involving milk fat globule-EGF factor-8 protein (MFGE8), which influences phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) proteins potentially mediating lipid uptake and metabolism.
METHODS
H9c2 cell culture and insulin stimulation.
H9c2 (ATCC CRL-1446; see Refs. 1 and 2), a rat (Rattus norvegicus) myoblast adherent cell line obtained from heart/myocardium tissue, is a suitable transfection host. The sex of the host was not documented. These H9c2 cells were stably transfected with mutant BMPR2 plasmids [mutant 1 (M1), BMPR2 gene with a 2579-2580delT resulting in a frame shift at amino acid 859 resulting in 10 missense amino acids and a stop and mutant 2 (M2), BMPR2 gene with a C993T mutation resulting in R332X], or empty vector (control) was used (35). G418 was used for the selection of positive clones. H9c2 cells from passage 8 to 12 were used for experiments. H9c2 cardiomyocytes were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 4.5 g/L d-glucose, 2 mM glutamine, 1 mM pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin in humidified air (5% CO2) at 37°C. Differentiation into cardiac myocytes was induced by transferring H9c2 myoblasts to differentiation medium, comprised of DMEM, 1% FBS, 2 mM glutamine, 1 mM pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin. Cells grown in differentiation medium for 48 h were used for experiments (22). For insulin stimulation, H9c2 in differentiation media were stimulated with 100 ng/mL insulin (Sigma-Aldrich, St. Louis, MO) for 5, 30, or 60 min. These cells exhibit the classical adult cardiomyocyte phenotype (35). All of the H9c2 clones expressed cardiac-specific markers (Acta1; Acta2; desmin; troponin T2, cardiac type; tropomyosin 1α) and demonstrated immunoreactivity for cardiac troponin T, markers of cardiomyocytes.
Measurement of H9c2 mitochondrial bioenergetics using the Seahorse XFe96 extracellular flux analyzer.
An XFe96 analyzer (Seahorse Biosciences, North Billerica, MA) was used to measure bioenergetic function in intact H9c2 cells in real time (7). Briefly, H9c2 were seeded at a density of 20,000 cells/well in Seahorse Bioscience XFe96 cell culture plates in 100 μL of cardiomyocyte differentiation media. The plates were incubated in a 37°C humidified incubator with 5% CO2 for 48 h to reach 70–80% confluency. Measurements of extracellular flux were made in unbuffered media. For these experiments, the media was changed 1 h before the start of the extracellular flux assay to unbuffered (pH 7.4) Seahorse base media containing 2 mM GlutaMax, 1 mM sodium pyruvate, and 4.5 g/L d-glucose (Complete media) or DMEM containing 1 g/L glucose (low glucose), DMEM containing 4.5 g/L glucose (high glucose), or DMEM containing 1 mM sodium pyruvate and incubated at 37°C without CO2. Normalization to the total number of nuclei per well following the experiment was used to control for variation in cell number. On completion of the assay, the cells were stained with Coomassie blue, and cell nuclei were counted using a cell counter. Oxygen consumption rate (OCR) data are expressed as pmol/min.
MitoSOX staining for superoxide production in H9c2 cells.
H9c2 cells were plated in four-well chamber slides (catalog no.: 354104; Falcon) and grown until they reached 70% confluency and differentiated. Following stimulation with insulin (100 ng/mL; Sigma-Aldrich), 1 mL of 5 μM MitoSOX reagent (M36008; Molecular Probes, Eugene, OR) was applied. Cells were incubated for 10 min at 37°C, protected from light, and washed with warm HBSS-Ca-Mg buffer. The slides were mounted using Vectashield mounting media containing DAPI (Vector Laboratories, Burlingame, CA) for confocal microscopy.
Western blotting protocol for H9c2 cells.
H9c2 cells were homogenized in RIPA buffer (PBS, 1% Ipegal, 0.5% sodium deoxycholate, 0.1% SDS) with proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). Protein concentration was determined by Bradford assay (Pierce, Rockford, IL) and stored at –70°C until use. Primary antibodies used for Western blot included CD36 [3313; Cell Signaling Technologies (CST), Danvers, MA], Glut4 (ab354; Abcam, Cambridge, MA), phospho-Akt (Thr308, 9275; CST), phospho-Akt (Ser473, 4060s; CST), Akt (4685; CST), phospho-AMPK (Thr172, 2535; CST), phospho-AMPK (Ser485, 4185; CST), AMPK (2532; CST), phospho-ACC (Ser79, 3661; CST), phospho-ACC1 (Ser79)/phospho-ACC (Ser221, CG1001; Cell Applications, San Diego, CA), PI3K p85 (4292; CST), MFGE8 (AF2805; R&D Systems, Minneapolis, MN), rapamycin-insensitive companion of mTOR (Rictor, 2140; CST), and β-actin (ab8227; Abcam). Donkey anti-rabbit (711-035-152; Jackson ImmunoResearch Laboratories, West Grove, PA), donkey anti-mouse (715-035-150; Jackson ImmunoResearch Laboratories), and donkey anti-goat (705-035-147; Jackson ImmunoResearch Laboratories) were used as secondary antibodies.
RNA sequencing for H9c2 cells.
For RNA sequencing (RNA-Seq) analysis, for each control and mutant cells, the cells were plated in triplicates and combined together for RNA-Seq analysis. The two controls represent control H9c2 cells collected from two consecutive passages. In case of mutant cells, the cells collected from two consecutive passages for M1 and M2 cells were combined and compared for analysis with the control cells.
RNA-Seq for H9c2 cells was performed using Illumina TruSeq stranded cDNA library prep, with sequencing on an Illumina HiSeq 2500 with 51-bp single-end reads. All analyses were carried out using the Amazon EC2, ubuntu HVM r3.2x large instance with 8 cores, 61 GB RAM, and 160 GB SSD storage. The FastQC v0.11.3 tool was applied for a basic analysis of generated sequence. For the six samples, 29.5–37.5 million reads, 51 bp in length, with GC content of 49% were generated. High-quality sequence was obtained as evidenced by Phred score between 30 and 40 for all nucleotide positions for the majority of reads. Using Trimmomatic v0.33, low-quality bases were trimmed from reads. Leading and trailing bases with Phred 33 quality score below 3 or ambiguous “N” were trimmed. Using a four-base sliding window, bases with quality score below 10 were removed. After trimming, only reads that had a minimum length of 25 bp were retained for further analyses. The trimmed reads of each sample were aligned to the rat genome, using STAR v 2.4.0. Reference genome and transcriptome annotation belonged to rat assembly build Rnor_5.0 provided by Ensembl, downloaded from illumina iGenomes on April 23, 2015. Star alignment run options were chosen such that unannotated noncanonical junctions were removed, and rare junctions were filtered. The Python module HTSeq-Count v0.6.1p1was used to count aligned reads at the gene level in the intersection nonempty mode, using the same Ensembl gtf used at the alignment step. Count tables generated using HTSeq were read in using package DESeq2 from Rv3.1.3/Bioconductor v 3.0. Counts were normalized by library size and estimation of dispersion, following a negative binomial model. The Wald test was used for statistical inference of difference between the mutant samples from control. There were over 26,000 genes in the counts table. After filtering for genes with zero counts and flagging counts as outliers, based on a diagnostic measure of Cook’s distance, 12,378 genes remained for differential expression testing for each of the two contrasts; 362 genes were deemed significant in the kinase domain mutant vs. control contrast and 253 in the tail domain mutant vs. control contrast, based on an adjusted P value <0.05, after multiple testing correction. Comparative annotation enrichment for the two gene lists obtained was performed using ToppCluster. This tool uses hypergeometric distribution as the statistical model to obtain the probability of multiple random occurrences of an annotation term in a gene list. A Bonferroni correction is applied to the P values.
Animal studies.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Vanderbilt University School of Medicine. We used a mouse model of mutant Bmpr2 expression (1): the Rosa26-rtTA2 Å~TetO7-Bmpr2R899X FVB/N mice previously described (15, 41), called BMPR2R899X for brevity (39), in which mutant BMPR2 is universally expressed. Expression of transgene occurs only after initiation of doxycycline. Transgene-negative mice were used as littermate controls and were administered doxycycline as well. BMPR2R899X mice were fed a high-fat chow (60% lard, Western diet) with 1 g/kg doxycycline beginning at 6 wk of age. Western diet has been shown to increase penetrance of PH in this model (40). This diet was continued for 6 wk, at which time mice were euthanized and tissue was harvested.
Gene expression analysis in H9c2 cells and mouse RV by PCR.
To determine the expression of mutant BMPR2 in the H9c2 clones stably transfected with plasmid containing mutated BMPR2 gene and to confirm the expression of genes identified by RNA sequencing, we performed real-time PCR analysis using the primer sets described in Table 1. The expression of these genes was also confirmed in RV tissue obtained from the mouse model of RV dysfunction with BMPR2 mutation by real-time PCR using the primer set described in Table 1. RNA from H9c2 clones was isolated using RNeasy kits (Qiagen, Valencia, CA) and converted to cDNA using a high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturers’ protocols. Gene expression was assessed using SYGR gene expression assay rat probe sets. The expression of the genes is calculated as 2ΔΔT, and HPRT is the housekeeping gene.
Table 1.
Primers used for RT-PCR for H9c2 cardiomyocytes and mouse RV
| Primer Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| Human Bmpr2 | TGC TTT GGC ATC AGT CTC TG | ATC AAG AGA GGG TTC GGA TG |
| Rat Bmpr2 | GGC GAA AAG ATC AAG AGA CG | CAC TGC CAT TGT TGT TGA CC |
| Rat Hprt | GCA GAC TTT GCT TTC CTT GG | TCC AAC ACT TCG AGA GGT CC |
| Rat Htra1 | GGCTCCGGAACTCCGATATG | ATCCCAATCACCTCGCCATC |
| Rat Flnc | TCATCACCATCCGCTTTGGG | CACTTCCGCAGGCTCCTC |
| Rat Ctgf | AGACACATTTGGCCCTGACC | CATTGGTAACCCGGGTGGAG |
| Rat Mfge8 | TGCTTCCACGATGCCAAATG | TCTCCATCCAGGTAGGAGGTG |
| Rat Tgfb3 | GCCTCAGTCTTTGGGATCTGG | ACCACCAGAGCCCTTTGTAAG |
| Rat Myo5b | CCAGAGGTGCACAGAGATCG | CCTCGTGTACCGGCTGTAG |
| Rat IL6st | GCAGGACTGAGCTCCCTATG | CACGCTGGTAACTCCCTACC |
| Rat Pdia3 | CTCCCATCTCGGTTCTCTGG | TCAGTTCCAACACGTCTGAGG |
| Rat Igfbp5 | GAGTCATCCCTGCACCTGAG | TCCTTTGCGGTCACAGTTGG |
| Rat Igfbp4 | GGTGTATGCACGGAGCTGTC | GAAGCTGTTGTTGGGATGCTC |
| Rat Timp2 | AGGACCTGACAAGGACATCG | TCCGCCTTCCCTGCAATTAG |
| Rat Col6a2 | GCAACATGACGCTGTTCTCTG | TGTGCACTGGGCCACATAGAG |
| Mouse Htra1 | CAGTCACCACTGGGATCGTC | ACCTCGCCATCCAGGTTTAC |
| Mouse Flnc | CAGATGCGCCAGCCCTATG | GGATTCCAGAGGCTCCACAG |
| Mouse Ctgf | CCTAGCTGCCTACCGACTG | GGTAACTCGGGTGGAGATGC |
| Mouse Mfge8 | GTGAAACCGGTTGTTCTACACAG | GCTGCAAGCCCATGAAACC |
| Mouse Tgfb3 | AGCGCTACATAGGTGGCAAG | GACCCAAGTTGGACTCTCTCC |
| Mouse Il6st | CTCTGGGTGGAATGGACACC | GGAGGCGTCCTCTCAAGTG |
| Mouse Pdia3 | GGGCTCATGCTAGTCGAGTTC | GGCAGTGCAATCCACCTTTG |
| Mouse Igfbp5 | GCGAGCAAACCAAGATAGAGAG | TTCACAGCCTCAGCCTTCAG |
| Mouse Igfbp4 | TACATTGATGCACGGGCAAG | GCTCTCATCCTTGTCAGAGGTC |
| Mouse Timp2 | GTGCAAGATCACTCGCTGTC | CGCGCAAGAACCATCACTTC |
RV, right ventricular; BMPR2, bone morphogenic protein receptor 2; Hprt, hypoxanthine-guanine phosphoribosyltransferase; Htra1, HtrA serine peptidase 1; Flnc, filamin-C; Ctgf, connective tissue growth factor; Mfge8, milk fat globule-EGF factor 8 protein; Tgfb3, transforming growth factor-β3; Myo5b, myosin VB; IL6st, interleukin-6 signal transducer; PDia3, protein disulfide isomerase family A member 3; Igfbp5, insulin-like growth factor-binding protein 5; Igfbp4, insulin-like growth factor-binding protein 4; Timp2, tissue inhibitor of metalloproteinases 2; Col6a2, collagen type VI α2-chain.
Immunofluorescence staining for CD36 and Glut4 in H9c2 cells.
For localization of CD36 and Glut4, H9c2 cells were plated in four-well chamber slides (catalog no.: 354104; Falcon) and grown until they reached 70% confluency and differentiated. Following stimulation with insulin (100 ng/mL; Sigma-Aldrich), H9c2 cells were fixed with 4% paraformaldehyde for 15 min at 37°C and permeabilized (permeabilization buffer: 20 mM HEPES, pH 7.4, E8 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2) for 10 min at room temperature, followed by incubation with primary [CD36 (3313; CST), Glut4 (ab354; Abcam)] antibodies overnight at 4°C. The next day, cells were incubated with Alexa Fluor 594-labeled secondary antibody (Sigma Aldrich). The slides were mounted using Vectashield mounting media containing DAPI (Vector Laboratories, Burlingame, CA) for confocal microscopy. Semiquantitative analysis of immunofluorescence staining was done using NIS-Elements AR 4.11.00 64-bit software. All mages were collected at the same time under the same conditions and were subjected to the same exposure time for images to be taken. The immunofluorescence intensity was calculated by dividing sum immunofluorescence intensity measured per slice by the number of nuclei in that slice.
Immunohistochemical staining for pAktSer473, pAMPKThr172, and pACCSer79 in human RV tissue.
Immunolocalization was performed on archival paraffin-embedded human RV tissue obtained from controls (n = 4–7; Cooperative Human Tissue Network) and PAH patients (n = 6–8; Vanderbilt University Medical Center). The study protocol was approved by the Institutional Review Board of Vanderbilt University Medical Center (IRB 9401). RV sections were deparaffinized, rehydrated, and blocked with 5% normal goat serum, followed by overnight incubation with primary antibody [pAktSer473, no. 4060 (CST, Danvers, MA); pAMPKThr172, no. 2535 (CST); pACCSer79, no. 3661 (CST)] at 4°C. The sections were then incubated with biotinylated secondary antibody followed by incubation with HRP-conjugated streptavidin. Diaminobenzidine was used as a substrate for HRP. The sections were dehydrated and mounted in Cytoseal XYL (Richard-Allan Scientific, Kalamazoo, MI) for light microscopic examination. All images were collected at the same time under the same conditions and were subjected to the same exposure time for images to be taken. The area of positive immunostaining per section (total of 5 random sections per RV section) was calculated using Image J software.
Radioactive [14C]palmitate and 2-deoxy-[3H]glucose uptake by H9c2 cardiomyocytes.
Palmitate or glucose uptake by H9c2 cardiomyocytes with and without mutant BMPR2 was quantified using the methods described by Stuck et al. (32) and Ha and Pak (10). Briefly, control, M1, and M2 cells were plated (2 × 104 cells/well) in six-well plates in two sets of triplicates. After differentiation into cardiac myocytes, the cells were incubated with or without insulin (100 ng/mL) along with 0.75 μCi/mL of [14C]palmitate or 1 μCi/mL of 2-deoxy-[3H]glucose for 60 min at 37°C and terminated by rapid washing with 1 mL of ice-cold PBS. Cells were disrupted with 1 mL of lysis buffer and scraping, and cell-associated radioactivity was determined by scintillation counting.
Transfection in H9c2 cells using MFGE8 and control siRNA.
MFGE8 siRNA and siRNA control were purchased from Biocompare (San Diego, CA). MFGE8 siRNA and siRNA control were transfected using Lipofectamine 2000 Transfection Reagent (ThermoFisher Scientific). For each well of a six-well plate, 1 µL of MFGE8 siRNA or siRNA control was added to 250 mL Opti-MEM medium. After 5 min, siRNA mixture was mixed with Lipofectamine 2000 mixture. After 20 min, the mixture was added to the cells in six-well plates. After transfection (4 h), the cells were fed with culture medium and incubated for 24 h before conducting the radioactive palmitate experiment.
Statistical analysis.
Statistical analyses for continuous variables were carried out using either one-way ANOVA or unpaired two-tailed t tests (GraphPad Prism Software, La Jolla, CA). Data are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
BMPR2 mutation reduces the rate of mitochondrial oxygen consumption, increases superoxide production, and impairs glucose responsiveness of cultured cardiomyocytes.
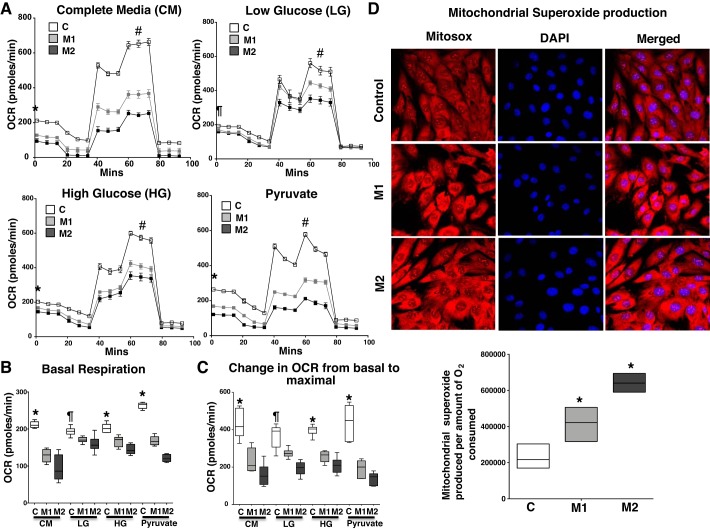
In cardiomyocytes, while lipid is the primary energy source, glucose is an important secondary source of energy production, and the ability to change fuel source dependent on substrate availability and concentration, termed as metabolic plasticity, is a key feature of normal cardiomyocytes. We sought to determine mitochondrial respiration in mutant and control cells in response to glucose concentration and its glycolytic metabolite pyruvate. Using the Seahorse XFe96 Extracellular Flux Analyzer, we assayed mitochondrial oxygen consumption rate (OCR) in the presence of two glucose concentrations [low glucose (1 g/L) and high glucose (4.5 g/L)], sodium pyruvate [a substrate that directly enters the tricarboxylic acid (TCA) cycle bypassing glycolysis], and complete media. As shown in Fig. 1A, with complete media, high glucose, or pyruvate as a substrate, basal mitochondrial OCR was significantly reduced (P < 0.001) in mutant cells (M1 and M2) compared with controls. Also, with low glucose as a substrate, the mitochondrial basal OCR was reduced (P < 0.01) only in M2 cells compared with controls. However, irrespective of the glucose concentration or pyruvate addition, the maximal mitochondrial OCR and change in OCR from basal to maximal respiration was significantly reduced (P < 0.001) in mutant cells compared with controls (Fig. 1, A–C), suggesting that mutant cells are impaired in utilizing glucose and its metabolites as substrates.
Fig. 1.
Bone morphogenetic protein receptor type 2 (BMPR2) mutation reduces the rate of mitochondrial oxygen consumption, increases superoxide production, and impairs glucose responsiveness in cardiomyocytes. A: oxygen consumption rate (OCR) measured over time following the addition of inhibitors of electron transport chain in mutant and control cells grown in complete media (CM, 10 mM/mL glucose, 1 mM sodium pyruvate, and 2 mM glutamine) low glucose (1 mM/mL glucose, LG), high glucose (10 mM/mL glucose, HG), and pyruvate (1 mM sodium pyruvate). B and C: bar graph representing reduction in basal (B) and change in OCR (C) from basal to maximal in mutant cells compared with controls. Open squares, control (n = 6); gray squares, mutant 1 (M1, n = 6); black squares, mutant 2 (M2, n = 6). *P < 0.001 comparing OCR of M1 and M2 with control (C). #P < 0.001, maximal OCR of M1 and M2 vs. control. ¶P < 0.01 comparing basal OCR of M2 with control. D: mitochondrial superoxide production is detected using MitoSOX. Bar graphs represent fluorescence mitochondrial superoxide production/amount of O2 consumed in control and mutant cells. *P < 0.05 comparing control with M1 and M2. Open squares, control; gray squares, M1; black squares, M2. Also see Supplemental Fig. S1. Statistical test used was one-way ANOVA (nonparametric).
BMPR2 mutation has been shown to increase oxidative stress in the pulmonary vasculature in humans and animal models of PAH (18), which relates to altered mitochondrial oxygen consumption (25). We investigated mitochondrial superoxide production in mutant and control cardiomyocytes using MitoSOX. As shown in Fig. 1D, although Mitosox fluorescence intensity in mutant cells was not significantly increased (Supplemental Fig. S1; Supplemental Material is available at https://doi.org/10.6084/m9.figshare.10137767), the mitochondrial superoxide production per amount of O2 consumed was significantly increased in M1 (P < 0.05) and M2 (P < 0.002) cells compared with controls, suggesting that, although mutant cells are consuming less O2, a significantly larger proportion is used for mitochondrial superoxide production. Thus, in cultured cardiomyocytes, BMPR2 mutation-mediated reduced metabolic plasticity is associated with increased mitochondrial superoxide production.
BMPR2 mutation in cardiomyocytes predominately enhances the expression of proteins influencing insulin sensitivity and mainly blunts their response to insulin.
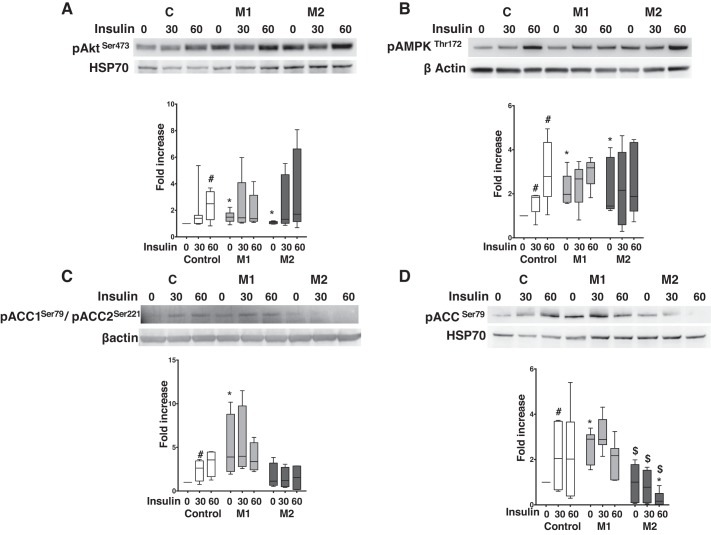
We next sought to determine BMPR2-mediated changes in proteins related to insulin signaling. In the insulin-signaling pathway, phosphorylation of Akt and its targets is indicative of insulin sensitivity (2). In cultured cardiomyocytes, at baseline, pAktSer473 protein expression was significantly higher (P < 0.05) in mutant cells compared with controls. Insulin significantly increased (P < 0.05) pAktSer473 protein expression at 60 min in controls but showed no significant change in mutant cells (Fig. 2A). pAktThr308 protein expression at baseline was similar in control and mutant cells, which significantly increased (P < 0.05) within 30 min of insulin stimulation in controls but not in mutant cells (Supplemental Fig. S2). Thus, in cultured cardiomyocytes, BMPR2 mutation primarily increases phosphorylation of AktSer473, which fails to respond to insulin.
Fig. 2.
Bone morphogenetic protein receptor type 2 (BMPR2) mutation in cardiomyocytes predominately enhances the expression of proteins influencing insulin sensitivity and blunts their response to insulin. A: Western blot gels and bar graph for phosphorylated (p) AktSer473 in mutant and control at baseline and following insulin stimulation. B: Western blot gels and bar graph for p-AMPKThr172 in mutant and control at baseline and following insulin stimulation. C: Western blot gels and bar graph for p-acetyl-CoA carboxylase (ACC)Ser79/pACCSer221 in mutant and control at baseline and following insulin stimulation. D: Western blot gels and bar graph for pACCSer79 in mutant and control at baseline and following insulin stimulation. Open bars, control (n = 3–5); light gray bars, mutant 1 (M1, n = 3–5); dark gray bars, mutant 2 (M2, n = 3–5). P < 0.05, protein expression in mutant cells (M1 or M2) vs. controls (*), following insulin stimulation (#), and M1 vs. M2 cells ($). Also see Supplemental Fig. S2. Statistical test used was t test.
Phosphorylated Akt regulates AMPK activity (37). AMPK is a fuel-sensing enzyme that enhances insulin sensitivity in various tissues, including heart (8, 12). In cardiomyocytes, at baseline, pAMPKThr172 protein expression was significantly higher (P < 0.05) in mutant cells compared with controls (Fig. 2B). Insulin significantly increased (P < 0.05) pAMPKThre172 protein expression in control cells in 30 min, which remained unchanged in mutant cells (Fig. 2B). Thus, similar to Akt phosphorylation, mutant cells have enhanced baseline AMPK phosphorylation and fail to respond to insulin, suggesting a lack of insulin sensitivity.
AMPK is the main regulator of ACC, the rate-limiting enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA (26), a key step in fatty acid metabolism. AMPK can phosphorylate ACC1 at Ser79 and ACC2 at Ser219 (because of the NH2-terminal extension). ACC2 phosphorylation is involved in regulation of fatty acid oxidation, whereas ACC1 phosphorylation promotes fatty acid synthesis (11). In cardiomyocytes (using an antibody that can recognize both phosphorylation sites on ACC) at baseline, pACC1Ser79/pACC2Ser221 protein expression was significantly increased (P < 0.05) in M1 cells but not in M2 cells compared with controls. Insulin significantly increased (P < 0.05) pACC1Ser79/pACC2Ser221 protein expression in control cells, which remained unchanged in mutant cells (Fig. 2C). Using the antibody that recognizes phosphorylation of ACC at serine 79, at baseline, pACCSer79 protein expression was significantly increased (P < 0.01) only in M1 cells compared with controls and M2 cells (Fig. 2D). Insulin significantly increased (P < 0.05) pACCSer79 protein expression in control cells at 30 min but showed a variable response in mutant cells with no significant change in pACCSer79 protein expression in M1 cells but significant reduction (P < 0.05) in M2 cells (Fig. 2D). Thus, BMPR2 mutant cardiomyocytes predominantly phosphorylating ACC1 and not ACC2 suggest a pro-fatty acid synthesis state at baseline, and BMPR2 mutation-specific insulin-dependent reduction in ACC1 phosphorylation, also suggest influencing fatty acid synthesis.
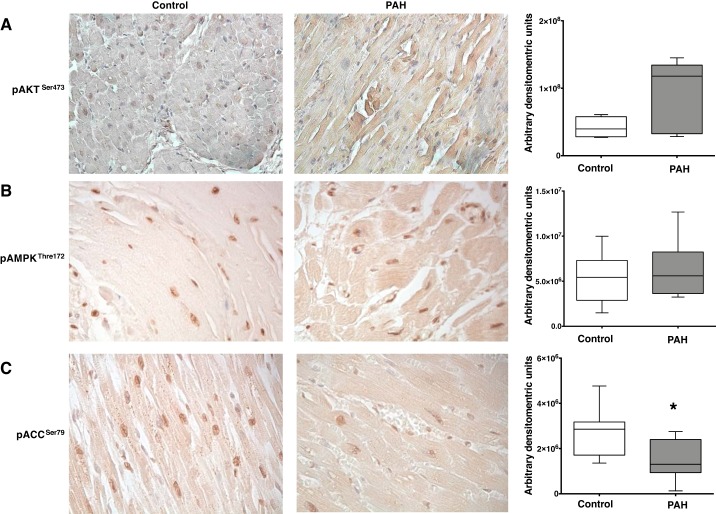
We confirmed the expression of these protein intermediates of the insulin-signaling pathway in human control and PAH RV tissue. In PAH, we observed a trend toward an increase (P = 0.08) in the expression of pAktSer473 compared with controls (Fig. 3A). Although there was no change in the expression of pAMPKThr172 (Fig. 3B) in PAH compared with controls, there was significant decrease in the expression of pACCSer79 (P < 0.05, Fig. 3C), suggesting that pAMPKThr172 activity required for dephosphorylation of ACC could be inhibited.
Fig. 3.
Immunohistochemical analysis of phosphorylated (p)-AktSer473, p-AMPKThr172, and p-acetyl-CoA carboxylase (ACC)Ser79 in human right ventricular (RV) tissue from control and pulmonary arterial hypertension (PAH) patients. A: pAktSer473 staining the RV tissue is in brown and nuclei were counterstained with hematoxylin. Magnification ×400. Bar graphs represent semiquantitative analysis of brown staining using Fiji software [control (n = 4), PAH (n = 6)]. B: pAMPKThr172 staining the RV tissue is in brown and nuclei were counterstained with hematoxylin. Magnification ×400. Bar graphs represent semiquantitative analysis of brown staining using Fiji software [control (n = 7), PAH (n = 8)]. C: pACCSer79 staining the RV tissue is in brown and nuclei were counterstained with hematoxylin. Magnification ×400. Bar graphs represent semiquantitative analysis of brown staining using Fiji software [control (n = 7), PAH (n = 8)]. *P < 0.05. Statistical test used was t test.
Response of BMPR2 mutant cardiomyocytes to glucose and insulin is variable and mutation specific.
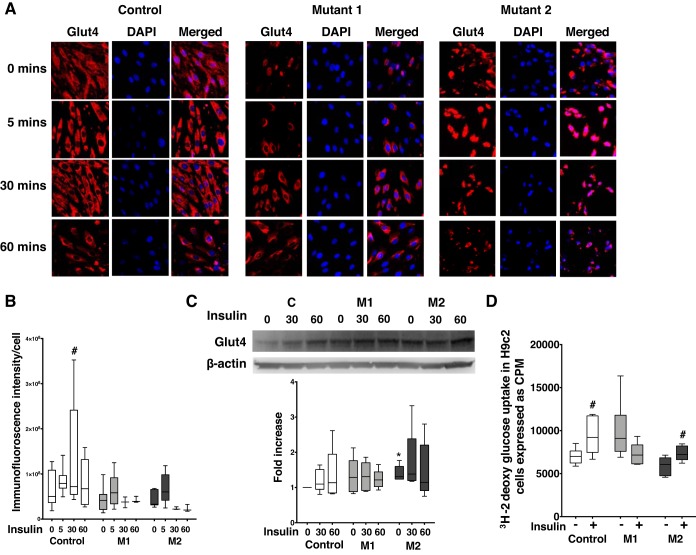
Loss of myocardial metabolic flexibility and impaired insulin sensitivity contributes to left ventricular dysfunction (16) and is characterized by impaired trans-sarcolemmal glucose transport via glucose transporter protein 4 (Glut4) (21). We investigated the effect of insulin on Glut4 protein expression in mutant and control cells. In cardiomyocytes, at baseline, Glut4 protein was ubiquitously present in cytoplasm and perinuclear space in control cells, whereas it was prominently perinuclear in mutant cells with intense immunostaining in M2 cells (Fig. 4A). Insulin significantly increased (P < 0.05) Glut4 immunofluorescence intensity (Fig. 4B) in control cells at 30 min; however, it remained unchanged and appeared to remain perinuclear in mutant cells (Fig. 4A). Moreover, total Glut4 protein expression in control and M1 cells was similar but increased in M2 (P < 0.05) at baseline and remained unchanged with insulin stimulation (Fig. 4C). To demonstrate the functional effect of BMPR2 mutation on insulin-mediated glucose uptake in cardiomyocytes, we performed glucose uptake assay in control and mutant cells using 2-deoxy-[3H]glucose after 1 h of insulin stimulation. At baseline, glucose uptake in mutant cells was influenced by the type of BMPR2 mutation because there was a trend toward increased glucose uptake (P = 0.07) in M1 cells and decreased glucose uptake (P = 0.07) in M2 cells (Fig. 4D) compared with controls. Insulin stimulation led to significant uptake of glucose (P < 0.05) in control cells while mutant cells, yet again, demonstrated a variable response to insulin, wherein M1 cells indicated a trend toward reduction (P = 0.07) in glucose uptake and M2 cells showed significant increase (P < 0.03) in glucose uptake. Overall, although glucose uptake in response to insulin increased in M2 cells, it was lower compared with control cells. These findings indicate that BMPR2 mutant cardiomyocytes have impaired insulin-mediated glucose transport responses, which is influenced by the type of BMPR2 mutation.
Fig. 4.
Response of bone morphogenetic protein receptor type 2 (BMPR2) mutant cardiomyocytes to glucose and insulin is variable and mutation-specific. A: immunofluorescence staining of glucose transporter protein 4 (Glut4, in red) and nucleus is stained blue in control, mutant 1 (M1), and mutant 2 (M2) cells. Magnification ×400. B: semiquantitative analysis of Glut4 immunofluorescence intensity in control and mutant cells. C: Western blot gels and bar graphs representing Glut4 protein in mutant and control at baseline and following insulin stimulation (n = 5). D: 2-deoxy-[3H]glucose uptake in control and mutant cells following insulin stimulation for 60 min (n = 6). *P < 0.05, baseline Glut4 protein expression in M2 vs. control (C). #P < 0.05, 2-deoxy-[3H]glucose uptake in control and mutant cells following insulin stimulation for 60 min. Statistical test used was t test.
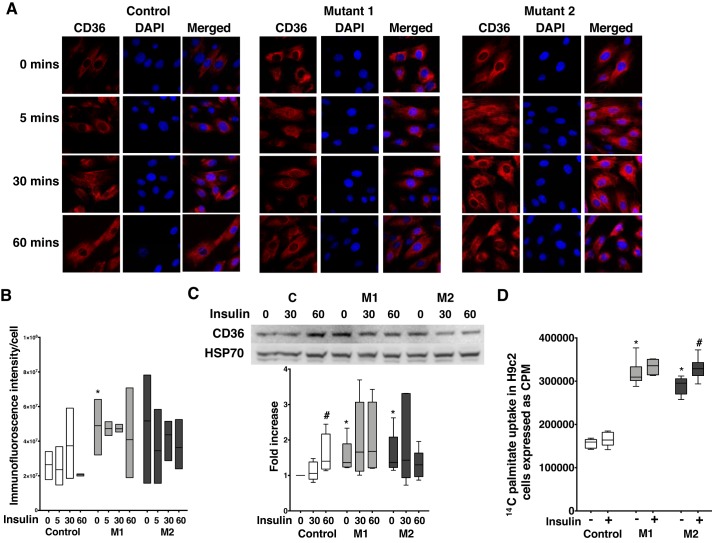
BMPR2 mutation increases CD36 protein expression and palmitate uptake, and insulin-stimulated palmitate uptake is dependent on the type of BMPR2 mutation.
Lipid accumulation in RV tissue and BMPR2 mutant cardiomyocytes is well established (4, 13, 35), and insulin is known to promote long-chain fatty acid uptake through cellular distribution of CD36 in the left ventricle (19). We investigated the effect of insulin on CD36 protein expression and lipid uptake in BMPR2 mutant cardiomyocytes. In cardiomyocytes, at baseline, CD36 protein expression was localized to the cytoplasm in control cells and was both cytoplasmic and perinuclear in mutant cells (Fig. 5A), with significant increase (P < 0.05) in immunofluorescence intensity in M1 cells (Fig. 5B), which remained unchanged with response to insulin in control and mutant cells. At baseline, total CD36 protein expression was significantly higher (P < 0.05) in mutant cells compared with controls (Fig. 5C), which was significantly increased (P < 0.05) at 60 min in control cells in response to insulin but remained unaffected in mutant cells. To determine the functional role of this observed increase in CD36 in fatty acid uptake with insulin stimulation in mutant BMPR2 cardiomyocytes, we performed radioactive palmitate uptake assay in control and mutant cells using [14C]palmitate. At baseline, there was significant increase in uptake of radioactive palmitate in mutant cells (P < 0.0001) compared with controls (Fig. 5D). Insulin did not increase palmitate uptake in control cells but showed a modest but significant increase (P < 0.05) in M2 cells. Thus, BMPR2 mutation in cardiomyocytes increases CD36 protein expression and palmitate uptake likely via CD36, with a further insulin-stimulated increase dependent on the type of BMPR2 mutation.
Fig. 5.
Bone morphogenetic protein receptor type 2 (BMPR2) mutation increases CD36 protein expression and palmitate uptake, and insulin-stimulated palmitate uptake is dependent on the type of BMPR2 mutation. A: immunofluorescence staining of CD36 (in red) and nucleus (in blue) in control, mutant 1 (M1), and mutant 2 (M2) cells. Magnification ×400. B: semiquantitative analysis of CD36 immunofluorescence intensity in control and mutant cells (*P < 0.05). C: Western blot gels and bar graphs representing CD36 protein in mutant and control at baseline and following insulin stimulation (n = 6). P < 0.05, protein expression in mutant cells vs. controls (*) and following insulin stimulation (#). D: [14C]palmitate uptake in control and mutant cells following insulin stimulation for 60 min (n = 6). *P < 0.0001, fold increase in palmitate uptake in mutant cells compared with control. #P < 0.05, fold increase in palmitate uptake in mutant cells following 60 min of insulin stimulation. Statistical test used was t test.
BMPR2 mutation increases MFGE8, milk fat globule-EGF-factor 8, gene expression in cultured cardiomyocytes.
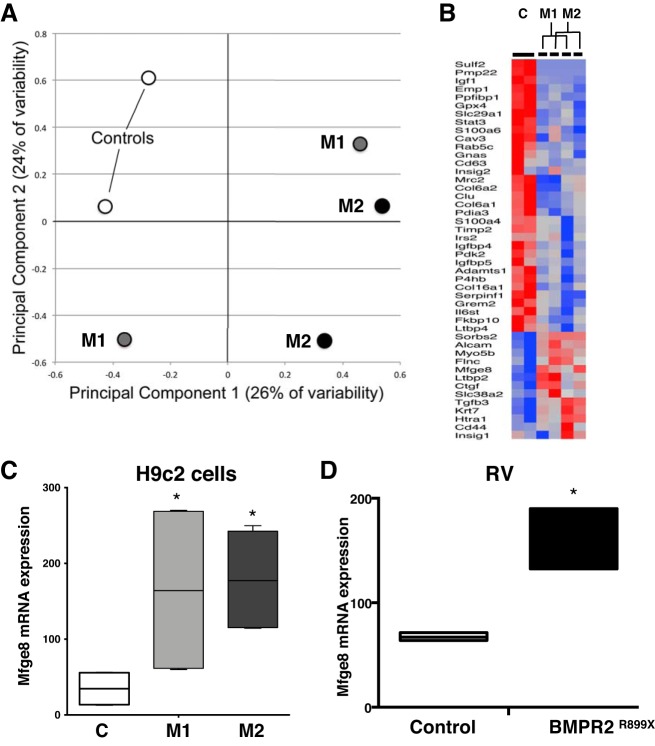
We next sought to determine whether genes related to insulin signaling underlie these functional changes in BMPR2 cardiomyocytes and discover new metabolic mechanisms differentiating mutant BMPR2 cardiomyocytes from control cells by RNA-Seq. Using principal component analysis, we found that mutant cells are distinctly different from controls and 253, and 362 unique sets of genes were differentially expressed (P < 0.05) by M1 and M2 cells compared with controls, respectively (Fig. 6A). These genes were involved in regulation of various metabolic pathways, including sensitivity to insulin, extracellular matrix organization, cytoskeletal development, and cell-cell communications. From the cluster of genes involved in insulin sensitivity (Fig. 6B), by quantitative RT-PCR, we analyzed 12 genes (6 upregulated: Htra1, Flnc, Ctgf, Mfge8, Tgfb3, and Myo5b and 6 downregulated: Il6st, Pdia3, Igfbp5, Igfbp4, Timp2, and Col6a2) that were significantly (P < 0.05) altered in mutant cells compared with controls. Ten out of 12 selected genes were expressed in mutant and control cells, of which HTRA1, CTGF, and MFGE8 were significantly increased and IL6ST and IGFBP4 significantly decreased (Fig. 6C and Supplemental Fig. S3, A and B). However, when the expression of these genes was confirmed in RV tissue from control and BMPR2 mutant mice, only Mfge8 gene expression was significantly increased in mutant RV compared with control RV (Fig. 6D and Supplemental Fig. S3, C and D).
Fig. 6.
Bone morphogenetic protein receptor type 2 (BMPR2) mutation increases milk fat globule-EGF factor 8 (MFGE8) gene expression in cultured cardiomyocytes. A: principal component analysis for control (open circles) and mutant (black circles) cells. B: heat map of genes involved in insulin sensitivity. C: MFGE8 mRNA expression in control and mutant cells. Open bars, control; dark gray bars, mutant 1 (M1); light gray bars, mutant 2 (M2). *P < 0.05, MFGE8 gene expression in M1 and M2 vs. control (C). D: MFGE8 mRNA expression in right ventricular (RV) tissue from BMPR2R899X mice compared with control mice. Open bars, control; black bars, mutant and Rosa26R899X. Also see Supplemental Fig. S3. Statistical test used was t test. *P < 0.05, MFGE8 gene expression in BMPR2 mutant mouse RV vs. control mouse RV.
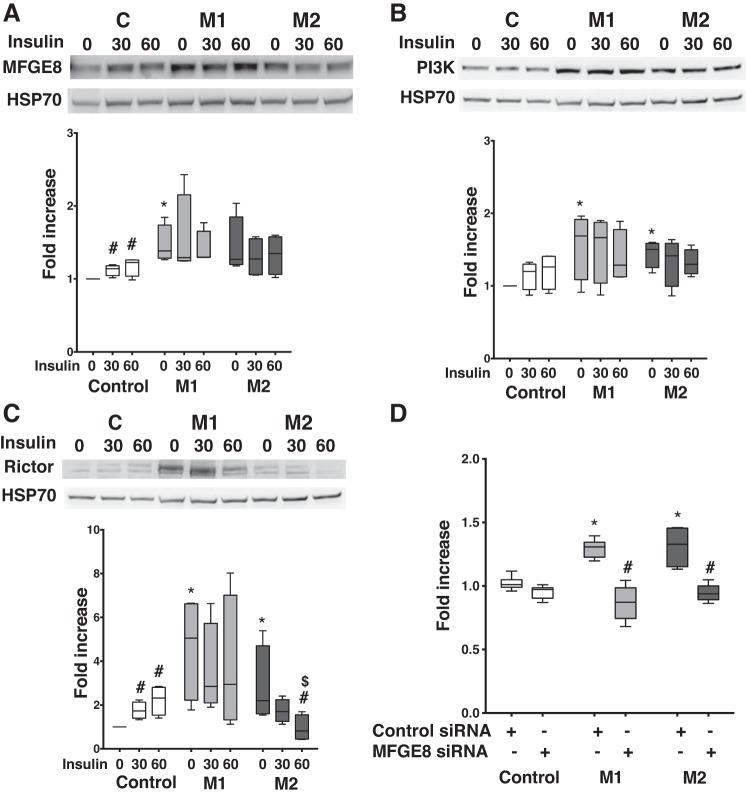
Increase in MFGE8 protein expression in cultured cardiomyocytes with BMPR2 mutation influences PI3K and mTOR proteins involved in insulin signaling.
Originally identified as part of the milk fat globule membrane, Mfge8 is a multifunctional glycoprotein that promotes fatty acid uptake by translocating CD36 to the plasma membrane in a PI3K-dependent mechanism (17). Mfge8 is increased in human obesity and in mice fed high-fat diet (17) and thus may have relevance to the PAH RV. We investigated the expression of MFGE8 in response to insulin and its effect of insulin-signaling intermediates in mutant and control cells. At baseline, compared with controls, there was a significant increase in MFGE8 protein expression in M1 cells (P < 0.05), and it trended higher in M2 (P = 0.07). In control cells, insulin significantly increased (P < 0.05) MFGE8 protein expression within 30 min but not in mutant cells (Fig. 7A). We then assessed the expression of PI3K and Rictor (one of the protein subunits of mTOR complex 2; see Ref. 17) at baseline and following insulin stimulation. At baseline, PI3K-1 protein expression was significantly higher (P < 0.05) in mutant cells compared with controls, and insulin did not affect PI3K-1 protein expression in control or mutant cells (Fig. 7B). At baseline, Rictor protein expression was also significantly higher (P < 0.05) in mutant cells compared with controls (Fig. 7C). In controls, insulin significantly increased (P < 0.05) Rictor protein expression within 30 min and had a variable effect in mutant cells [in M1 cells, there was no change in the levels of Rictor protein, but, in M2 cells, Rictor protein levels were significantly decreased (P < 0.05)]. Thus, in cultured BMPR2 mutant cardiomyocytes, MFGE8 is increased but unresponsive to insulin and may coordinate Akt phosphorylation at Ser473 via both the PI3K and mTOR pathway, which may play a role in increasing fatty acid uptake. Insulin negatively influenced the mTOR pathway, depending on the type of BMPR2 mutation, but not the PI3K pathway.
Fig. 7.
Increase in milk fat globule-EGF factor 8 (MFGE8) protein expression in cultured cardiomyocytes with bone morphogenetic protein receptor type 2 (BMPR2) mutation influences phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) proteins involved in insulin signaling. A: MFGE8 protein expression in control and mutant cells at baseline and following insulin stimulation (n = 4). B: Western blot gels and bar graph representing PDK1 protein expression in control and mutant cells at baseline and following insulin stimulation (n = 4). P < 0.05, protein expression in mutant cells vs. controls (*) and following insulin stimulation (#). C: Western blot gels and bar graph representing rapamycin-insensitive companion of mTOR (Rictor) protein expression in control and mutant cells at baseline and following insulin stimulation (n = 4). P < 0.05, protein expression in mutant cells vs. controls (*), following insulin stimulation (#), and in mutant 1 (M1) vs. mutant 2 (M2) cells ($). Statistical test used was t test. D: [14C]palmitate uptake in control and mutant cells for 60 min (n = 6). *P < 0.01, fold increase in palmitate uptake in mutant cells compared with control. #P < 0.001, fold decrease in palmitate uptake in mutant cells compared with control. Statistical test used was t test.
MFGE8 is shown to influence lipid uptake via insulin-signaling intermediates (17). We next conducted experiments using MFGE8 siRNA to demonstrate the effect of MFGE8 on lipid uptake in H9c2 with mutant BMPR2. In mutant H9c2 cells, as expected, there was >25% increase (P < 0.01) in lipid uptake compared with controls (Fig. 7D). Transfection with MFGE8 siRNA demonstrated ~50% reduction in MFGE8 gene expression in H9c2 cells (both in control and mutant cells; Supplemental Fig. S4A) and ~20% reduction in protein expression in mutants only (Supplemental Fig. S5, A and B). This led to 35 and 28% reduction (P < 0.001) in radioactive palmitate uptake in M1 and M2 cells, respectively, indicating that, in mutant H9c2 cells, MFGE8 may be involved in increasing lipid uptake and thus influence increased lipid accumulation in these cells (35).
DISCUSSION
In this series of experiments, we explored the effect of BMPR2 mutation in cultured cardiomyocytes on mitochondrial respiration in response to glucose and its metabolites, insulin sensitivity, and on glucose and lipid uptake. Our findings indicate that 1) BMPR2 mutation reduces mitochondrial respiration in cultured cardiomyocytes, and these cells fail to respond to glucose and its metabolites, 2) the proteins influencing insulin sensitivity are augmented as a result of BMPR2 mutation in cardiomyocytes and generally unresponsive to insulin, 3) cultured mutant cardiomyocytes consistently demonstrated increased palmitate uptake that is partially responsive to insulin stimulation while glucose uptake is not enhanced at baseline and is similarly modestly responsive to insulin, and 4) MFGE8 protein is increased in BMPR2 mutant cardiomyocytes and may influence PI3K and mTOR proteins involved in lipid biosynthesis and insulin signaling. Taken together, our data suggests an altered metabolic state in BMPR2 mutant cardiomyocytes characterized by impaired metabolic plasticity, impaired insulin signaling, and enhanced fat uptake and biosynthesis.
Although under normal physiological conditions cardiomyocytes demonstrate metabolic plasticity in response to available substrate (28), the PAH RV has mitochondrial-metabolic abnormalities due to both impaired fatty acid and glucose metabolism (1, 31); specifically, we have shown that BMPR2 mutant cardiomyocytes have reduced mitochondrial oxidation in the presence of fat as a substrate, and mitochondrial volume is not reduced in BMPR2 mutant cardiomyocytes (35). Here, using cultured BMPR2 mutant cardiomyocytes, we show that, irrespective of glucose concentration or availability of substrate that bypasses the glycolysis pathway, mitochondrial respiration is impaired. This suggests that the increase in myocardial glucose uptake in PAH patients (24) may not adequately compensate for impaired mitochondrial β-oxidation in PAH RV and that the PAH RV lacks normal capacity to augment glucose use and therefore cannot be most energetically maximally efficient regardless of glucose availability.
BMPR2 mutations have been shown to activate p38MAPK signaling, which accounts for proliferation and inhibition of apoptosis in pulmonary vasculature (6). Our data show that, in cultured cardiomyocytes, intracellular insulin-signaling intermediates, which include pAktSer473, are also activated by BMPR2 mutation, leading to impaired insulin sensitivity that fails to respond to insulin. Furthermore, site-specific ACC phosphorylation, which can influence lipid metabolism or lipid synthesis (11), indicates that BMPR2 mutant cardiomyocytes have enhanced fatty acid synthesis. Furthermore, our data on Glut4 and CD36 indicate that, in BMPR2 mutant cardiomyocytes, although Glut4 protein levels are unchanged, involvement of Glut4 protein in glucose uptake as seen in PAH RV (23, 24) may be influenced by the type of BMPR2 mutation. It is also possible that other glucose transporters, for example, Glut1, shown to have increased expression in PAH (30), may be involved. BMPR2 mutant cardiomyocytes also appear to have impaired insulin responses measured by protein phosphorylation and functional glucose uptake. Increase in palmitate uptake in BMPR2 mutant cardiomyocytes at baseline is similar to the increase in fatty acid uptake previously seen in PAH RV (23), and insulin-dependent enhanced lipid uptake seen in some BMPR2 mutant cardiomyocytes may further promote lipid deposition and lipotoxicity. Taken together, these data suggest that BMPR2 mutant cardiomyocytes have impaired insulin sensitivity and enhanced lipid uptake and, perhaps, biosynthesis.
In cultured BMPR2 mutant cardiomyocytes, using an unbiased approach, we identified MFGE8 as a new protein of interest that may be involved in modulating insulin sensitivity. In humans, Mfge8 gene is located in the region linked with susceptibility to obesity (29), and circulating concentrations of MFGE8 are elevated in diabetes (5, 42). MFGE8 increases fatty acid uptake by translocating CD36 to the plasma membrane via the PI3K-Akt insulin-signaling pathway, thus regulating the absorption and storage of dietary fats and leading to obesity and insulin resistance (17). Our initial studies in cultured cardiomyocytes with mutant BMPR2 indicate that MFGE8 protein may play a role by increasing fatty acid uptake via both the PI3K and mTOR pathway. This is similar to results of Khalifeh-Soltani et al. (17) and may be further investigated. In addition, the mTOR pathway (depending on the type of BMPR2 mutation), but not the PI3K pathway, is negatively influenced by insulin in BMPR2 mutant cardiomyocytes.
BMPR2 mutant cardiomyocytes can be used to model the PAH RV with BMPR2 mutation or suppression, and prior findings in these cells, despite their limitations, had been translated faithfully into animal models and human disease, suggesting a high degree of relevance to the PAH RV (4, 13, 35). We have used two of the many BMPR2 mutations described in the literature in our experiments: 1) frame shift mutation in BMPR2 gene at amino acid 859 results in 10 missense amino acids with a stop codon, which leads to deletion of the cytoplasmic tail of BMPR2 protein (M1 cells), and 2) a point mutation in the kinase domain of BMPR2 gene with a C993T mutation resulting in R332X substitution (M2 cells). In past studies, we have demonstrated pleiotropic effects of these BMPR2 mutations in cell culture and animals studies (20, 34, 38), which is consistent with the differences we observe with regard to insulin sensitivity and glucose and palmitate uptake in response to insulin in these cultured BMPR2 mutant cardiomyocytes. Differences in the cytoplasmic tail signaling may underlie some of our observed variability in cell culture and likely underlie the clinical heterogeneity across HPAH families.
Our data are not without limitations. In our [14C]palmitate experiments, we measured cellular radioactivity. It is possible that [14C]CO2 is released from cells after full β-oxidation, which would be unmeasured in our experiments. Here, we demonstrated increased cellular 14C in cells with BMPR2 mutation after culture with [14C]palmitate, and, in prior work (35), we have demonstrated reduced β-oxidation in cardiomyocytes with BMPR2 mutation. Thus we hypothesize that there would be reduced production of [14C]CO2 in cardiomyocytes with BMPR2 mutation but have not specifically measured 14C liberation from cells. Secondly, in this study, we limited our investigation to understanding the role of CD36 and Glut4 proteins in insulin signaling in BMPR2 mutant cardiomyocytes. The role of other fatty acid and glucose transporter proteins in insulin signaling requires further investigation in BMPR2 mutant cardiomyocytes.
In summary, our data show that BMPR2 mutation in cardiomyocytes is associated with reduced maximal respiration, impaired metabolic plasticity, especially with respect to glucose handling, enhanced expression of proteins influencing insulin sensitivity with blunted insulin response suggesting insulin insensitivity, and variable glucose but enhanced fatty acid uptake. On the basis of preliminary studies, our data point to a potential role for MFGE8 in mediating lipid uptake through Akt phosphorylation and suggest a new mechanism underlying right ventricular lipotoxicity in PAH. Finally, we aim to validate these findings in animal models of PAH with BMPR2 mutation and human tissues to strengthen the impact of these studies.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants P01 HL108800-01A1, 1 R01 HL122417-01A1, and K08 HL121174.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R.H., M.F., and M.H.T. conceived and designed research; X.C., S.Z., N.L.F., C.J., and M.H.T. performed experiments; A.R.H. and M.H.T. analyzed data; J.P.F. and M.H.T. interpreted results of experiments; M.H.T. prepared figures; A.R.H. and M.H.T. drafted manuscript; A.R.H., J.H.N., J.D.W., and M.H.T. edited and revised manuscript; A.R.H., J.P.F., X.C., S.Z., N.L.F., C.J., M.F., J.H.N., J.D.W., and M.H.T. approved final version of manuscript.
REFERENCES
- 1.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ 3: 144–152, 2013. doi: 10.4103/2045-8932.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6: a009191, 2014. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittain EL, Pugh ME, Wheeler LA, Robbins IM, Loyd JE, Newman JH, Larkin EK, Austin ED, Hemnes AR. Shorter survival in familial versus idiopathic pulmonary arterial hypertension is associated with hemodynamic markers of impaired right ventricular function. Pulm Circ 3: 589–598, 2013. doi: 10.1086/674326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brittain EL, Talati M, Fessel JP, Zhu H, Penner N, Calcutt MW, West JD, Funke M, Lewis GD, Gerszten RE, Hamid R, Pugh ME, Austin ED, Newman JH, Hemnes AR. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 133: 1936–1944, 2016. doi: 10.1161/CIRCULATIONAHA.115.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng M, Li BY, Li XL, Wang Q, Zhang JH, Jing XJ, Gao HQ. Correlation between serum lactadherin and pulse wave velocity and cardiovascular risk factors in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 95: 125–131, 2012. doi: 10.1016/j.diabres.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Dewachter L, Adnot S, Guignabert C, Tu L, Marcos E, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Eddahibi S. Bone morphogenetic protein signalling in heritable versus idiopathic pulmonary hypertension. Eur Respir J 34: 1100–1110, 2009. doi: 10.1183/09031936.00183008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dott W, Mistry P, Wright J, Cain K, Herbert KE. Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol 2: 224–233, 2014. doi: 10.1016/j.redox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291: E867–E877, 2006. doi: 10.1152/ajpendo.00207.2006. [DOI] [PubMed] [Google Scholar]
- 9.Grinnan D, Farr G, Fox A, Sweeney L. The role of hyperglycemia and insulin resistance in the development and progression of pulmonary arterial hypertension. J Diabetes Res 2016: 2481659, 2016. doi: 10.1155/2016/2481659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha H, Pak Y. Modulation of the caveolin-3 and Akt status in caveolae by insulin resistance in H9c2 cardiomyoblasts. Exp Mol Med 37: 169–178, 2005. doi: 10.1038/emm.2005.23. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30: 1064–1070, 2002. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 12.Hegarty BD, Turner N, Cooney GJ, Kraegen EW. Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxf) 196: 129–145, 2009. doi: 10.1111/j.1748-1716.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 13.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, Disalvo T, West J. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 189: 325–334, 2014. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jaïs X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA, West J. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 302: L474–L484, 2012. doi: 10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 5: 68, 2018. doi: 10.3389/fcvm.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalifeh-Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, Turner SM, Chawla A, Stahl A, Atabai K. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med 20: 175–183, 2014. doi: 10.1038/nm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane KL, Talati M, Austin E, Hemnes AR, Johnson JA, Fessel JP, Blackwell T, Mernaugh RL, Robinson L, Fike C, Roberts LJ II, West J. Oxidative injury is a common consequence of BMPR2 mutations. Pulm Circ 1: 72–83, 2011. doi: 10.4103/2045-8932.78107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51: 3113–3119, 2002. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 20.Majka S, Hagen M, Blackwell T, Harral J, Johnson JA, Gendron R, Paradis H, Crona D, Loyd JE, Nozik-Grayck E, Stenmark KR, West J. Physiologic and molecular consequences of endothelial Bmpr2 mutation. Respir Res 12: 84, 2011. doi: 10.1186/1465-9921-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montessuit C, Lerch R. Regulation and dysregulation of glucose transport in cardiomyocytes. Biochim Biophys Acta 1833: 848–856, 2013. doi: 10.1016/j.bbamcr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Nobuhara M, Saotome M, Watanabe T, Urushida T, Katoh H, Satoh H, Funaki M, Hayashi H. Mitochondrial dysfunction caused by saturated fatty acid loading induces myocardial insulin-resistance in differentiated H9c2 myocytes: a novel ex vivo myocardial insulin-resistance model. Exp Cell Res 319: 955–966, 2013. doi: 10.1016/j.yexcr.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Ohira H, deKemp R, Pena E, Davies RA, Stewart DJ, Chandy G, Contreras-Dominguez V, Dennie C, Mc Ardle B, Mc Klein R, Renaud JM, DaSilva JN, Pugliese C, Dunne R, Beanlands R, Mielniczuk LM. Shifts in myocardial fatty acid and glucose metabolism in pulmonary arterial hypertension: a potential mechanism for a maladaptive right ventricular response. Eur Heart J Cardiovasc Imaging 17: 1424–1431, 2016. doi: 10.1093/ehjci/jev136. [DOI] [PubMed] [Google Scholar]
- 24.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, Shirato K. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 45: 1849–1855, 2005. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 25.Pak O, Sommer N, Hoeres T, Bakr A, Waisbrod S, Sydykov A, Haag D, Esfandiary A, Kojonazarov B, Veit F, Fuchs B, Weisel FC, Hecker M, Schermuly RT, Grimminger F, Ghofrani HA, Seeger W, Weissmann N. Mitochondrial hyperpolarization in pulmonary vascular remodeling. Mitochondrial uncoupling protein deficiency as disease model. Am J Respir Cell Mol Biol 49: 358–367, 2013. doi: 10.1165/rcmb.2012-0361OC. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol (1985) 92: 2475–2482, 2002. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- 27.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 30: 904–911, 2011. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem Supplm 55: 1–11, 1994. doi: 10.1002/jcb.240550002. [DOI] [PubMed] [Google Scholar]
- 29.Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14: 529–644, 2006. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 30.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest 138: 1234–1239, 2010. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan J, Dasgupta A, Huston J, Chen KH, Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med (Berl) 93: 229–242, 2015. doi: 10.1007/s00109-015-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuck BJ, Lenski M, Böhm M, Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem 283: 32562–32569, 2008. doi: 10.1074/jbc.M801904200. [DOI] [PubMed] [Google Scholar]
- 33.Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, Montani D, Souza R, Simonneau G, Soubrier F, Humbert M. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med 177: 1377–1383, 2008. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 34.Talati M, West J, Zaynagetdinov R, Hong CC, Han W, Blackwell T, Robinson L, Blackwell TS, Lane K. BMP pathway regulation of and by macrophages. PLoS One 9: e94119, 2014. doi: 10.1371/journal.pone.0094119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talati MH, Brittain EL, Fessel JP, Penner N, Atkinson J, Funke M, Grueter C, Jerome WG, Freeman M, Newman JH, West J, Hemnes AR. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med 194: 719–728, 2016. doi: 10.1164/rccm.201507-1444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trammell AW, Talati M, Blackwell TR, Fortune NL, Niswender KD, Fessel JP, Newman JH, West JD, Hemnes AR. Pulmonary vascular effect of insulin in a rodent model of pulmonary arterial hypertension. Pulm Circ 7: 624–634, 2017. doi: 10.1086/689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valentine RJ, Coughlan KA, Ruderman NB, Saha AK. Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch Biochem Biophys 562: 62–69, 2014. doi: 10.1016/j.abb.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West J, Austin E, Fessel JP, Loyd J, Hamid R. Rescuing the BMPR2 signaling axis in pulmonary arterial hypertension. Drug Discov Today 19: 1241–1245, 2014. doi: 10.1016/j.drudis.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol 295: L744–L755, 2008. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J 41: 861–871, 2013. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasuda T, Tada Y, Tanabe N, Tatsumi K, West J. Rho-kinase inhibition alleviates pulmonary hypertension in transgenic mice expressing a dominant-negative type II bone morphogenetic protein receptor gene. Am J Physiol Lung Cell Mol Physiol 301: L667–L674, 2011. doi: 10.1152/ajplung.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu F, Li BY, Li XL, Cai Q, Zhang Z, Cheng M, Yin M, Wang JF, Zhang JH, Lu WD, Zhou RH, Gao HQ. Proteomic analysis of aorta and protective effects of grape seed procyanidin B2 in db/db mice reveal a critical role of milk fat globule epidermal growth factor-8 in diabetic arterial damage. PLoS One 7: e52541, 2012. doi: 10.1371/journal.pone.0052541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 33: 318–324, 2009. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]