Abstract
Airway microbial dysbiosis is associated with subsequent bronchopulmonary dysplasia (BPD) development in very preterm infants. However, the relationship of airway microbiome in normal pulmonary development has not been defined. To better understand the role of the airway microbiome, we compared normal and abnormal alveolar and pulmonary vascular development in mice with or without a microbiome. We hypothesized that the lungs of germ-free (GF) mice would have an exaggerated phenotypic response to hyperoxia compared with non–germ-free (NGF) mice. With the use of a novel gnotobiotic hyperoxia chamber, GF and NGF mice were exposed to either normoxia or hyperoxia. Alveolar morphometry, pulmonary mechanics, echocardiograms, inflammatory markers, and measures of pulmonary hypertension were studied. GF and NGF mice in normoxia showed no difference, whereas GF mice in hyperoxia showed protected lung structure and mechanics and decreased markers of inflammation compared with NGF mice. We speculate that an increase in abundance of pathogenic bacteria in NGF mice may play a role in BPD pathogenesis by regulating the proinflammatory signaling and neutrophilic inflammation in lungs. Manipulation of the airway microbiome may be a potential therapeutic intervention in BPD and other lung diseases.
Keywords: bronchopulmonary dysplasia, germ free, gnotobiotic, hyperoxia, lung microbiome
INTRODUCTION
Extremely premature infants are born with very immature lungs, which are highly susceptible to multiple forms of injury. Injury to developing lungs may affect normal alveolar and vascular development, potentially resulting in development of bronchopulmonary dysplasia (BPD) (1, 5, 14). The etiology of BPD is multifactorial, with oxygen toxicity and inflammation central to its pathogenesis (3, 8, 9, 12, 29–32). The pathogenic pathways for BPD are not well defined and include alterations in coordination of gene regulation and cell-to-cell interactions as well as cellular interactions with extracellular matrix (19). Recent data indicate that human airway and lungs harbor a commensal microbiota, which induce activation of an inflammatory response in various lung diseases (21, 27, 28). We have established that the neonatal airway harbors a distinct microbiome even at birth in both preterm and term infants (17). We also found that the airways of preterm infants with BPD are marked by distinct dysbiosis with relative increased abundance of phylum Gammaproteobacteria and decreased Firmicutes, especially genus Lactobacillus (17). Multiple other studies have reported associations between airway microbial dysbiosis and BPD or severity of BPD (18, 20, 35). Despite these studies demonstrating associations between microbial dysbiosis and BPD, mechanisms by which airway microbial dysbiosis contribute to BPD are unclear. We have recently established the possible role of airway microbiome in altering airway metabolome, thus modifying the risk of BPD (15). We also identified airway metabolomic changes with differential enrichment of metabolites involved in fatty acid activation and androgen and estrogen biosynthesis in BPD-predisposed infants compared with BPD-resistant infants (15).
To define the pathogenic importance of the airway microbiome, analyzing an environment devoid of microbiome is essential. Hence, to understand the role of airway microbiota in normal alveolar and vascular development in neonatal mice, we created a novel germ-free (GF) hyperoxia-exposed newborn mouse model and hypothesized that the GF mice would demonstrate an exaggerated oxygen-induced lung injury.
METHODS
Animal studies.
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and were consistent with the Public Health Service policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, 2002). Non–germ-free (NGF) Swiss Webster animals were bred in Volker Hall animal facility at the University of Alabama Birmingham. GF Swiss Webster animals were bred and obtained from University of Alabama Birmingham Gnotobiotic core facility. Hyperoxia exposure for GF animals was conducted in a gnotobiotic core facility in a germ-free environment. We used Swiss Webster animals because of their excellent reproductive performance. No strain-specific phenotypic differences were seen between Swiss Webster and C57BL6 animals in our preliminary hyperoxia experiments. Based on our previous results of hyperoxia-induced alveolar simplification in mice, to detect a 25% increase (2 SD) in the radial alveolar count (RAC), we needed 6 animals per group to provide 80% power with an α of 0.05. Hence, all experiments had a minimum of 6–8 mice per group, in approximately equal male-to-female ratio, per condition.
Germ-free hyperoxia system.
To execute hyperoxia experiments on GF animals, we designed and constructed a novel germ-free hyperoxia chamber at our gnotobiotic core facility at University of Alabama at Birmingham. GF animals were bred in a germ-free environment, and the experiment was conducted in this novel hyperoxia chamber until the day animals were euthanized.
Murine postnatal hyperoxia-induced BPD model.
Newborn GF and NGF dams and their pups were exposed to either room air (21% O2; control group) or hyperoxia (85% O2) from postnatal day (PN) 3–14 (period of maximal alveolar development) in a sealed Plexiglas chamber with continuous oxygen monitoring (13). Breeding and experiments were done for NGF animals in the regular room air environment animal facility in identical fashion. Surrogate dams were switched every 24 h from hyperoxia to normoxia to avoid maternal oxygen toxicity for both GF and NGF animals. Daily animal maintenance was carried out, with exposure of the animals to room air for < 10 min/day. A standard mouse pellet diet and water were provided ad libitum as per the regular animal facility protocol for the NGF animals, and an autoclave extruded diet was provided ad libitum as per the gnotobiotic facility protocol for GF animals.
Pulmonary function testing.
At the end of hyperoxia or air exposure, a subset of mouse pups at PN14 were anesthetized with isoflurane, and the trachea was cannulated using a 24-gauge Angiocath and fixed with a ligature of 3-0 silk. Pulmonary mechanics were evaluated using the flexiVent apparatus (SCIREQ, Montreal, QC, Canada) equipped with a module 1 as previously described (22).
Echocardiogram.
Transthoracic echocardiography was performed using a Visual Sonics Vevo 770 Imaging System (Toronto, ON, Canada). B-mode image sequences and pulsed-wave Doppler was recorded in the parasternal long axis with the angle of the probe aligned with the direction of the flow in the pulmonary valve outflow tract. We measured 1) pulmonary artery flow wave acceleration time (PAT), which is the time to peak from the onset of wave acceleration; 2) pulmonary artery flow wave total ejection time (PET), which is the time from onset to end of systolic flow; and 3) the diameter of the pulmonary artery immediately distal to the pulmonary artery valve. We calculated the estimated right ventricle systolic pressure based on the PAT-to-PET ratio using a validated equation. All measurements were averaged on three cardiac cycles (34).
Animal harvesting.
After lung mechanics and/or echocardiogram, the pups were euthanized. The pups were then randomly assigned for either lung histology, bronchoalveolar lavage fluid (BALF) collection, or lung homogenates for protein collection. The chest was opened; the lungs were inflation fixed via the trachea with 10% formalin at 20 cm H2O. BALF was conducted by instilling 0.3 mL of PBS intratracheally at least twice via tracheostomy. The sample was stored for protein analysis at −80°C. There were some time points when not all samples were obtained because of technical difficulties.
Analysis of alveolar morphometry.
Five-micrometer sections were stained with hematoxylin and eosin for pulmonary morphometry as previously described (11, 26). The software package MetaMorph v. 6.2r4 (Universal Imaging) interfaced with a Nikon Eclipse microscope equipped with a high-resolution charge-coupled device camera was used for image analysis. Evaluation of alveolar lung development was done using RAC (10) and mean linear intercept (MLI) (7). Alveolar number counts were not conducted as a part of morphometric analysis.
Analysis of myeloperoxidase by ELISA.
With the use of DuoSet ELISA (R&D Systems), natural and recombinant mouse myeloperoxidase (MPO) was measured in BALF cellular pellets. BALF was centrifuged at 10,000 g for 5 min and the pellets were resuspended in 200 μL 1 × PBS and further diluted 1:10. Plates were coated overnight to capture antibody. Sample (100 μL/well) was added and further treated with detection antibody, followed by the streptavidin-horseradish peroxidase (HRP) method. Optical density was measured at 450 nm.
Analysis of cytokines.
Custom-designed (MCYTOMAG-70K-08, Mouse Cytokine MAGNETIC Kit4, Millipore Sigma) total cytokines Milliplex Mouse Cytokine Magnetic kits were used to measure a panel of inflammatory cytokines [including Eotaxin, interferon γ (IFN γ), interleukin-1 β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), macrophage inhibitory protein-1α (MIP-1α), macrophage inhibitory protein-1β (MIP-1β), vascular endothelial growth factor (VEGF), and tumor necrosis factor α (TNF-α)]. Manufacturer directions were followed and median fluorescent intensity (MFI) data using a 5-parameter logistic method was used for calculating cytokine/chemokine concentrations in BALF samples (16). There were some time points when not all samples were analyzed because of technical difficulties.
Pulmonary vascularization.
Assessment of pulmonary microvasculature was done by immunohistochemical staining for endomucin using a monoclonal primary antibody (MAB2624; EMD Millipore, Burlington, MA) at 1:100 × 1 h, with a rat HRP-DAB secondary kit (CTS017; R&D systems). For quantification, six random high-power (×400) fields from three mice per group were evaluated and analyzed using the image analysis software (MetaMorph v.6.2). Thresholds for positive antibody staining compared with nonimmune serum controls were defined. Positive pixels were expressed as percentages of total tissue area, excluding air spaces, as previously described (6, 24).
Estimation of right ventricular hypertrophy.
The right ventricle (RV)-to-left ventricle (LV) free wall thickness ratio was used to estimate right ventricular hypertrophy. Measurement of the RV and LV free wall thickness was done just inferior to the mitral leaflet attachment, as described previously (2, 4, 23). This measurement is more accurate than the conventional Fulton index of RV/(LV + Septum) weight ratio in newborn mice, as the Fulton index is technically challenging in extremely small newborn mouse hearts.
Statistical analysis.
Data are expressed as means ± SE. Two-way ANOVA was used for all multigroup analyses. Multiple-comparison testing (Student–Newman–Keuls) was performed if statistical significance (P < 0.05) was noted by ANOVA. GraphPad Prism version 8.0 (GraphPad Software, La Jolla, CA, https://www.graphpad.com/) was used for all data analysis. A limitation of post hoc tests analysis is that most require equal group sizes and the ones that do not are not suitable for either some of the comparisons or a reasonable α error size.
RESULTS
GF and NGF mice have similar lung development and function in room air.
Our first objective was to define the lung development in GF animals in comparison to NGF mice under normal conditions. We found GF mice in normoxia had alveolar development and septation (RAC) not statistically different compared with NGF mice in normoxia (RAC and MLI: P > 0.1 at all time points; Fig. 1). We also found no difference in lung mechanics (lung resistance: P = 0.77; Fig. 2A and compliance: P = 0.50; Fig. 2B), signs of pulmonary hypertension (echocardiogram: P = 0.56; Fig. 3, A and B and RV/LV free wall thickness ratio: P = 0.99; Fig. 3C), pulmonary vascularization [endomucin-stained area (%) (PN7: P = 0.96; Fig. 4), and markers and modulators of inflammation (MPO and cytokines, P > 0.05; Fig. 5) between GF and NGF animals in normoxia.
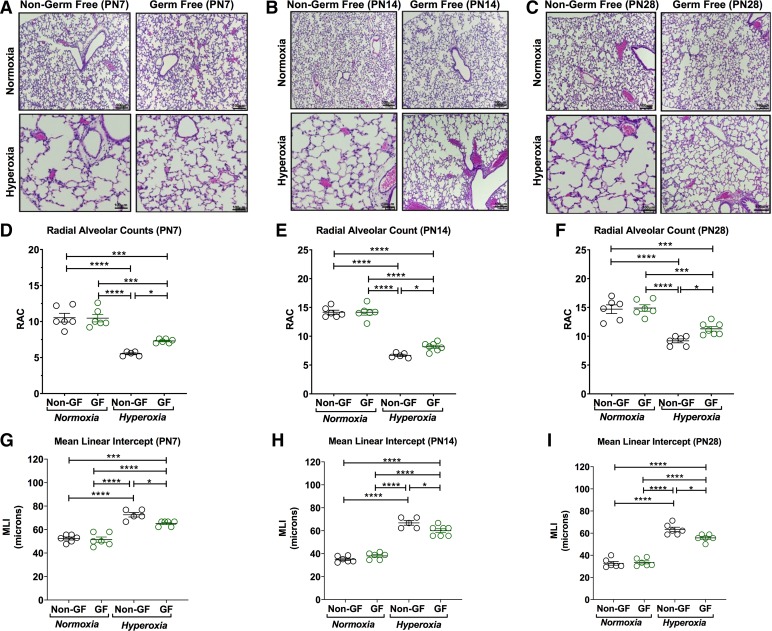
Fig. 1.
Germ-free (GF) animals have decreased lung injury in hyperoxia condition. A–C: representative photomicrographs of hematoxylin-eosin-stained sections of lungs from Swiss Webster [non–germ-free (NGF) and GF] mouse pups [A: postnatal age 7 (PN7); B: postnatal age 14 (PN14); and C: postnatal age 28 (PN28)] in normoxia (21% FiO2) or hyperoxia (85% FiO2) exposure from PN3–PN14 (magnification, ×100). Hyperoxia exposure was associated with marked alveolar hypoplasia in both NGF and GF mice at all postnatal ages compared with their respective normoxia group. D–F: radial alveolar counts (RACs) of both NGF and GF hyperoxia-treated pups were significantly lower than their respective normoxia groups at PN7, 14, and 28. However, GF hyperoxia-exposed pups have decreased alveolar simplification and alveolar hypoplasia compared with NGF hyperoxia group at PN7 (A), PN14 (B), and PN28 (C). The RACs of GF hyperoxia-treated groups were increased compared with NGF hyperoxia-exposed pups at PN7 (D), PN14 (E), and PN26 (F), indicating better alveorization in the GF hyperoxia group compared with the NGF hyperoxia group. G–I: alveolar size was comparatively larger in the NGF hyperoxia group compared with the GF hyperoxia group. No difference in RACs or mean linear intercepts (MLIs) was seen between NGF and GF normoxia groups. Data are means ± SE; n = 5–7 animals per group. *P < 0.05, ***P < 0.001, ****P < 0.0001.
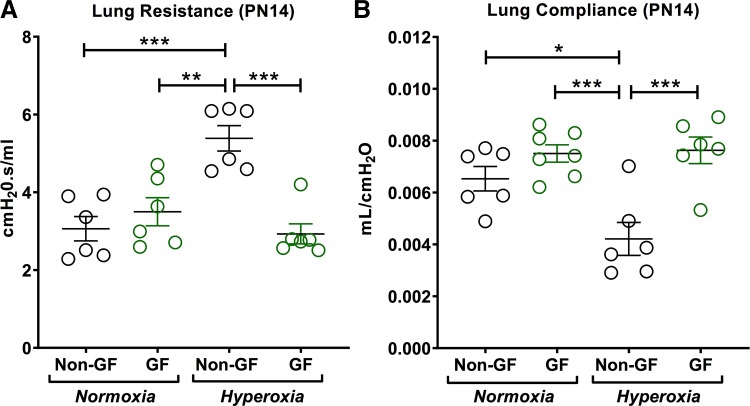
Fig. 2.
Germ-free (GF) animals have better lung mechanics than non–germ-free (NGF) animals in hyperoxia. A: total lung resistance was similar between NGF and GF normoxia pups. Hyperoxia significantly increased lung resistance in NGF pups compared with normoxia NGF pups. However, lung resistance was not increased in hyperoxia GF pups compared with GF normoxia pups. GF hyperoxia pups had decreased lung resistance compared with NGF hyperoxia pups. B: lung compliance was decreased in the hyperoxia NGF group; however, GF pups did not show reduction in lung compliance after hyperoxia exposure. GF hyperoxia pups had increased lung compliance compared with NGF hyperoxia pups. Data are means ± SE; n = 5–7 animals per group. *P < 0.05, **P < 0.01, ***P < 0.001. PN14, postnatal day 14.
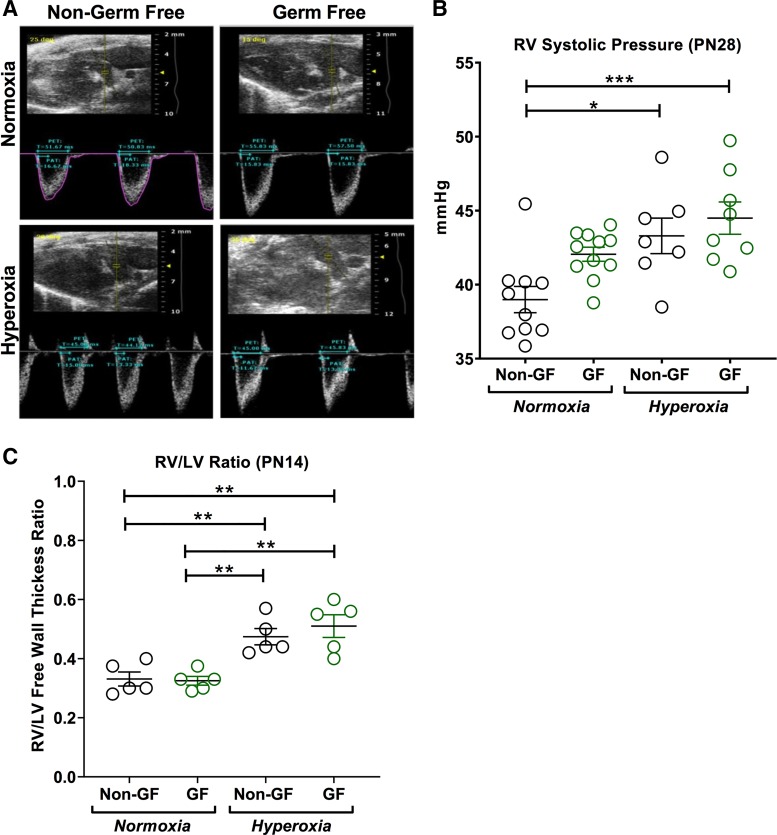
Fig. 3.
Neonatal hyperoxia increases right ventricular systolic pressure in both germ-free (GF) and non–germ-free (NGF) mice. A: representative echocardiogram of Swiss Webster NGF and GF mice at postnatal day (PN) 28 following normoxia and hyperoxia exposure. B: echocardiographic assessment of right ventricle (RV) systolic pressure at PN28 mice exposed to normoxia and hyperoxia. RV systolic pressure was increased in hyperoxia NGF mice compared with normoxia NGF mice. No differences in RV systolic pressure were seen between normoxia and hyperoxia GF mice. Also, no differences in RV systolic pressure were noted between NGF and GF mice in both normoxia and hyperoxia. C: right ventricle-to-left ventricle free wall thickness ratio (RV/LV) was measured at PN14 for right ventricular hypertrophy. Postnatal hyperoxia increased RV/LV in both NGF and GF mice compared with their respective normoxia group. No difference in RV/LV was seen between NGF and GF in either nomoxia or hyperoxia. Data are means ± SE; n = 5–10 animals per group. *P < 0.05, **P < 0.01, ***P < 0.001.
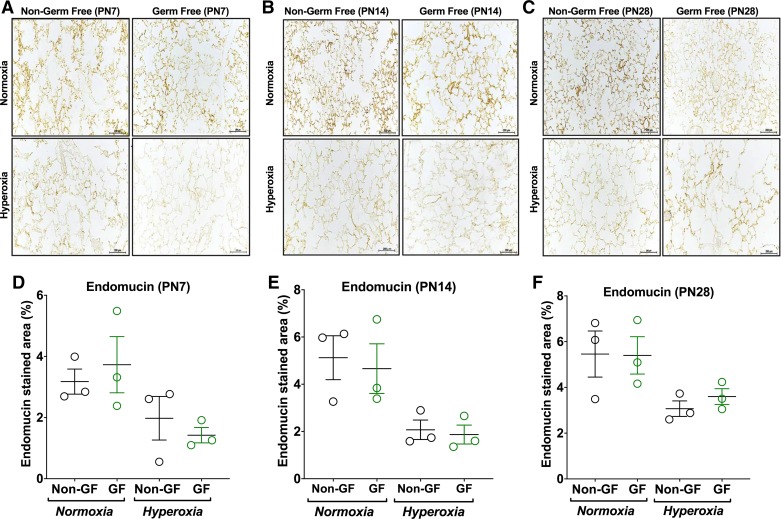
Fig. 4.
No difference in microvascular density is noticed between germ-free (GF) and non–germ-free (NGF) animals. A–C: representative photomicrographs of endomucin-stained sections of lungs from Swiss Webster (NGF and GF) mouse pups [postnatal age 7 (PN7; A), postnatal age 14 (PN14; B), and postnatal age 28 (PN28; C)] in normoxia (21% FiO2) or hyperoxia (85% FiO2) exposure from PN3–PN14 (magnification, ×200). D–F: hyperoxia exposure resulted in sparse microvasculature in both NGF and GF mice at all postnatal ages compared with their respective normoxia group on analysis using endomucin stained area (%). No difference was seen between NGF and GF groups in microvascular density in either normoxia or hyperoxia conditions. Data are means ± SE; n = 3 animals per group.
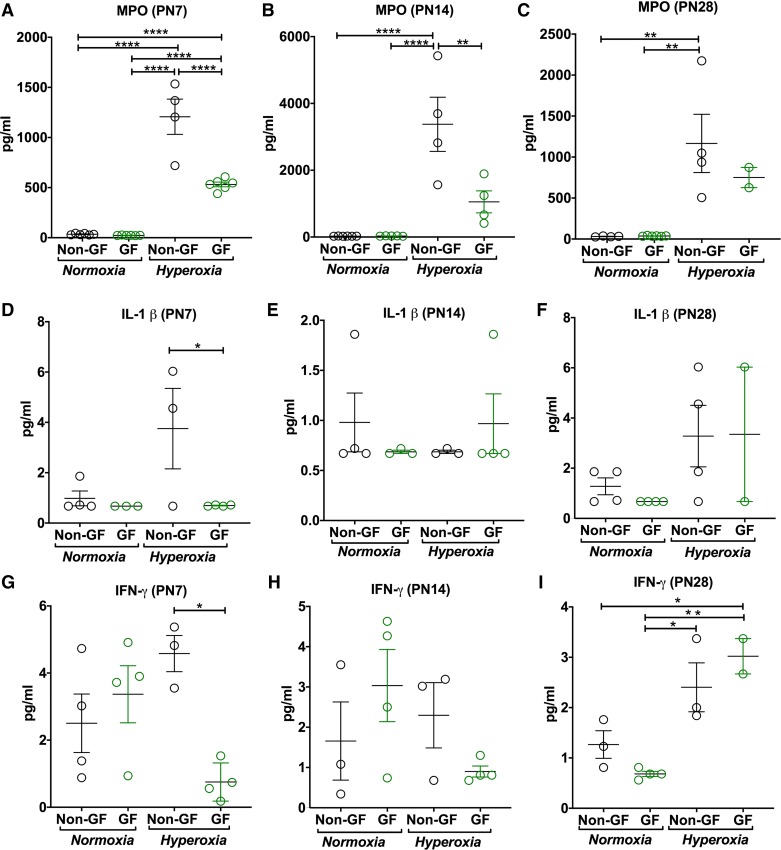
Fig. 5.
Germ-free (GF) animals mount decreased inflammation in hyperoxia condition. A–C: myeloperoxidase (MPO) activity, as a measure of neutrophil activity, measured in bronchoalveolar lavage fluid after normoxia or hyperoxia exposure. Hyperoxia-induced increments in MPO activity are less dramatic in GF mice at both postnatal day 7 (PN7) and PN14 compared with NGF mice. This difference was not seen at PN28. D–I: inflammatory cytokine levels using multiplex assays showed decreased interleukin-1β (IL-1β; D) and interferon γ (IFN-γ; G) in GF hyperoxia mice at PN7 compared with NGF hyperoxia group; however, results are more scattered. Data are means ± SE; n = 2–5 animals per group. *P < 0.05, **P < 0.01, ****P < 0.0001.
GF mice have decreased hyperoxia-induced lung injury compared with NGF mice.
Hyperoxia inhibited alveolar development in both GF and NGF animals, as noted by enlarged alveolar spaces with diminished septation. Both hyperoxia-exposed GF and NGF mice had reduced RAC and increased MLI at 7, 14, and 28 days of age (P < 0.05, Fig. 1, D–I). However, hyperoxia-exposed GF mice had better alveolarization, as demonstrated by increased RAC (PN7: P = 0.043, PN14: P = 0.04, and PN28: P = 0.047; Fig. 1, D–F) and decreased MLI (PN7: P = 0.027, PN14: P = 0.027, and PN28: P = 0.016; Fig. 1, G–I), as compared with hyperoxia-exposed NGF mice. This difference in RAC and MLI between GF and NGF in the hyperoxia group was also noted at different time points at PN7, PN14, and PN28 (Fig. 1, D–I).
GF animals have better lung function in hyperoxia.
Neonatal hyperoxia in NGF mice increased total lung resistance and decreased total lung compliance compared with air-exposed NGF mice. However, hyperoxia exposure in neonatal GF mice did not affect lung function. Also, compared with NGF hyperoxia-exposed mice, GF hyperoxia-exposed mice at PN14 showed significantly lower total lung resistance (P = 0.0001; Fig. 2A) and better total lung compliance (P = 0.0005; Fig. 2B).
GF and NGF mice show increased right ventricular systolic pressure and RV/LV ratio in hyperoxia.
Echocardiographic assessment of RV systolic pressure at PN28 showed increased RV systolic pressure in hyperoxia-exposed NGF compared with air-exposed NGF mice. In GF mice at PN28, this increase in RV systolic pressure after hyperoxia was not statistically significant (P = 0.21; Fig. 3B). However, GF mice in both groups had higher RV systolic pressures at baseline. Also, no significant differences in RV systolic pressure were seen between hyperoxia-exposed GF compared with hyperoxia-exposed NGF mice (P = 0.81; Fig. 3, A and B).
Hyperoxia increased the relative thickness of the RV free wall compared with the LV free wall in both NGF (P = 0.01) and GF (P = 0.001) mice at PN14 (Fig. 3C). No significant difference was seen in RV/LV free wall thickness ratio between GF and NGF in both normoxia (P = 0.99) and hyperoxia (P = 0.79) (Fig. 3C).
GF and NGF mice show similar pulmonary vascularization in both normoxia and hyperoxia.
No significant difference in pulmonary microvasculature using endomucin-stained area (%) were noted between GF and NGF mice in either normoxia or hyperoxia (P > 0.05; Fig. 4, D–F). However, both GF and NGF mice in hyperoxia had decreased pulmonary vascular density consistent with reduced capillary density compared with their respective air-exposed mice at all postnatal ages (P < 0.05; Fig. 4, D–F).
GF mice have decreased inflammation in hyperoxia compared with NGF mice.
Hyperoxia exposure resulted in increased MPO activity in BALF in both NGF and GF mice compared with their respective normoxia controls at both PN7 (P < 0.0001; Fig. 4D), PN14 (P < 0.0001; Fig. 4E), and PN28 (P = 0.004; Fig. 4F). However, GF mice exposed to hyperoxia had reduced MPO activity compared with NGF mice after hyperoxia exposure at PN7 (P < 0.0001; Fig. 4D) and PN14 (P = 0.004; Fig. 4E). No difference in MPO activity was seen in either NGF or GF normoxia groups (P < 0.004; Fig. 5, D–F).
At the same time points, inflammatory cytokine protein expression levels in BALF by multiplex assays showed decreased IFN-γ (P = 0.02; Fig. 5G) and IL-1β (P = 0.04; Fig. 5D) in BALF of GF hyperoxia mice compared with NGF mice in hyperoxia at PN7. No difference was seen in monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1, α and β (MIP-1α, MIP-1β), or tumor necrosis factor-α (TNF-α) (P > 0.05).
DISCUSSION
This is the first report of the impact of the absence of a respiratory microbiome on normal and abnormal lung development in a mouse model. This study indicates that neonatal hyperoxia exposure in mice with a microbiome results in worse alveolar hypoplasia and a greater impairment of lung mechanics, as compared with GF mice that are devoid of a respiratory microbiome. Decreased markers of pulmonary inflammation (MPO, IFN-γ, and IL-1β) were also seen in GF animals compared with NGF animals on hyperoxia exposure. Overall, neonatal GF mice have partial protection from hyperoxia-induced pulmonary changes compared with NGF mice.
The role of airway microbiome in BPD has been the subject of many recent studies. In the past decade, several human studies have reported an association between airway microbial dysbiosis (17, 18, 25, 33, 35) and neonatal chronic lung disease. Despite these associative studies, the direction of causality between airway injury during development and respiratory colonization with microorganisms still remains unsettled. Also, mechanistic studies have not yet determined the exact causal implication of dysbiosis in BPD. Our first aim was to answer the first question regarding the role of airway microbiome on overall lung development by using germ-free animals and a novel hyperoxia chamber modified for gnotobiotic animal work.
We found that GF mice in normoxia have similar lung structure and function compared with NGF mice. We also found that pulmonary vascularization in GF mice in normoxia is not different from that of NGF mice in normoxia. Additionally, markers of inflammation were also not different between the two normoxic group. These findings suggest that in the absence of additional external noxious stimuli, germ-free animals have similar alveolar and vascular development compared with NGF animals. Interestingly, and contrary to our initial hypothesis, we found that GF animals were less susceptible to hyperoxic injury compared with NGF animals. We speculate that the presence of predisposing pathogenic microbiota in NGF and humanized mice may accentuate the proinflammatory cascade in hyperoxia, thus leading to a worse phenotype as compared with GF animals. In the absence of these pathogenic microbiota, GF animals may not mount the inflammatory response and hence may have relative phenotypic protection. This finding is consistent with our previous human neonatal airway microbiome study, where we found increased neutrophilic activity (MPO) and a dysbiotic airway microbiome with proteobacterial preponderance in severe BPD patients (17).
A major strength of our study is the comprehensive evaluation of lung structure and mechanics using multiple parameters in addition to echocardiography, cytokines, and MPO in a newborn GF and NGF mouse model. A novel and first of its kind gnotobiotic hyperoxia facility was established for this study, which facilitated the continuous germ-free conditions in the lungs, even during the postnatal period. The limitations of the traditional newborn mouse hyperoxia model includes the lack of antenatal impacts (e.g., associated with chorioamnionitis, fetal growth restriction) and ventilator-induced lung injury postnatally. Nevertheless, this still remains the most widely used animal model of BPD. In conclusion, GF mice are relatively protected from the severe phenotypic changes resulting from hyperoxia exposure, as compared with their NGF counterparts.
These findings further highlight the role of the pulmonary microbiome in modulating the pathogenesis of BPD. This study lays the foundation in the complex process of evaluating microbiome effects on lung development and injury. Further studies using this background may indicate underlying mechanisms of microbiome-modulated hyperoxia-induced lung injury inform potential new therapeutic strategies for various lung diseases.
GRANTS
This work was supported in part by American Heart Association Grant 17SDG32720009 (C. V. Lal) and NIH National Heart, Lung, and Blood Institute Grant K08 HL141652 (C. V. Lal).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.V.L. conceived and designed research; K.D., T.J., and C.V.L. performed experiments; K.D., T.J., and C.V.L. analyzed data; K.D., A.E.F., G.R., G.A.P., X.X., T.J., J.E.B., A.G., N.A., and C.V.L. interpreted results of experiments; K.D., T.J., and C.V.L. prepared figures; K.D. and C.V.L. drafted manuscript; K.D., G.R., J.E.B., A.G., N.A., and C.V.L. edited and revised manuscript; K.D., A.E.F., G.R., G.A.P., X.X., T.J., J.E.B., A.G., N.A., and C.V.L. approved final version of manuscript.
REFERENCES
- 1.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Bronchopulmonary dysplasia: a review. Arch Gynecol Obstet 288: 325–333, 2013. doi: 10.1007/s00404-013-2753-8. [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005. doi: 10.1203/01.PDR.0000159512.55862.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Morty RE. Searching for better animal models of BPD: a perspective. Am J Physiol Lung Cell Mol Physiol 311: L924–L927, 2016. doi: 10.1152/ajplung.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008. doi: 10.1152/ajplung.00534.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari A, Bhandari V. Pathogenesis, pathology and pathophysiology of pulmonary sequelae of bronchopulmonary dysplasia in premature infants. Front Biosci 8: e370–e380, 2003. doi: 10.2741/1060. [DOI] [PubMed] [Google Scholar]
- 6.Brey EM, Lalani Z, Johnston C, Wong M, McIntire LV, Duke PJ, Patrick CW Jr. Automated selection of DAB-labeled tissue for immunohistochemical quantification. J Histochem Cytochem 51: 575–584, 2003. doi: 10.1177/002215540305100503. [DOI] [PubMed] [Google Scholar]
- 7.Caretta G, Piontelli E. Isolation of keratinophilic fungi from soil in Pavia, Italy. Sabouraudia 13: 33–37, 1975. doi: 10.1080/00362177585190061. [DOI] [PubMed] [Google Scholar]
- 8.Chess PR, D’Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 30: 171–178, 2006. doi: 10.1053/j.semperi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Coalson JJ, Winter V, deLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med 152: 640–646, 1995. doi: 10.1164/ajrccm.152.2.7633720. [DOI] [PubMed] [Google Scholar]
- 10.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1—postnatal lung growth. Thorax 37: 572–579, 1982. doi: 10.1136/thx.37.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiCarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol Lung Cell Mol Physiol 269: L690–L697, 1995. doi: 10.1152/ajplung.1995.269.5.L690. [DOI] [PubMed] [Google Scholar]
- 12.Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718, 1994. [PubMed] [Google Scholar]
- 13.James ML, Ross AC, Nicola T, Steele C, Ambalavanan N. VARA attenuates hyperoxia-induced impaired alveolar development and lung function in newborn mice. Am J Physiol Lung Cell Mol Physiol 304: L803–L812, 2013. doi: 10.1152/ajplung.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jónsson B, Rylander M, Faxelius G. Ureaplasma urealyticum, erythromycin and respiratory morbidity in high-risk preterm neonates. Acta Paediatr 87: 1079–1084, 1998. doi: 10.1111/j.1651-2227.1998.tb01418.x. [DOI] [PubMed] [Google Scholar]
- 15.Lal CV, Kandasamy J, Dolma K, Ramani M, Kumar R, Wilson L, Aghai Z, Barnes S, Blalock JE, Gaggar A, Bhandari V, Ambalavanan N. Early airway microbial metagenomic and metabolomic signatures are associated with development of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 315: L810–L815, 2018. doi: 10.1152/ajplung.00085.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal CV, Olave N, Travers C, Rezonzew G, Dolma K, Simpson A, Halloran B, Aghai Z, Das P, Sharma N, Xu X, Genschmer K, Russell D, Szul T, Yi N, Blalock JE, Gaggar A, Bhandari V, Ambalavanan N. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 3: e93994, 2018. doi: 10.1172/jci.insight.93994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B, Carlo WA, Keeley J, Rezonzew G, Kumar R, Morrow C, Bhandari V, Ambalavanan N. The airway microbiome at birth. Sci Rep 6: 31023, 2016. doi: 10.1038/srep31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res 76: 294–301, 2014. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 19.Mižíková I, Morty RE. The extracellular matrix in bronchopulmonary dysplasia: target and source. Front Med (Lausanne) 2: 91, 2015. doi: 10.3389/fmed.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourani PM, Harris JK, Sontag MK, Robertson CE, Abman SH. Molecular identification of bacteria in tracheal aspirate fluid from mechanically ventilated preterm infants. PLoS One 6: e25959, 2011. doi: 10.1371/journal.pone.0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhlebach MS, Hatch JE, Einarsson GG, McGrath SJ, Gilipin DF, Lavelle G, Mirkovic B, Murray MA, McNally P, Gotman N, Davis Thomas S, Wolfgang MC, Gilligan PH, McElvaney NG, Elborn JS, Boucher RC, Tunney MM. Anaerobic bacteria cultured from cystic fibrosis airways correlate to milder disease: a multisite study. Eur Respir J 52: 1800242, 2018. doi: 10.1183/13993003.00242-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol 301: L125–L134, 2011. doi: 10.1152/ajplung.00074.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olave N, Lal CV, Halloran B, Bhandari V, Ambalavanan N. Iloprost attenuates hyperoxia-mediated impairment of lung development in newborn mice. Am J Physiol Lung Cell Mol Physiol 315: L535–L544, 2018. doi: 10.1152/ajplung.00125.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. doi: 10.1152/ajplung.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne MS, Goss KC, Connett GJ, Kollamparambil T, Legg JP, Thwaites R, Ashton M, Puddy V, Peacock JL, Bruce KD. Molecular microbiological characterization of preterm neonates at risk of bronchopulmonary dysplasia. Pediatr Res 67: 412–418, 2010. doi: 10.1203/PDR.0b013e3181d026c3. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol Heart Circ Physiol 236: H818–H827, 1979. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 27.Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Bruce KD. characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16s ribosomal DNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol 42: 5176–5183, 2004. doi: 10.1128/JCM.42.11.5176-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers GB, Zain NM, Bruce KD, Burr LD, Chen AC, Rivett DW, McGuckin MA, Serisier DJ. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 11: 496–503, 2014. doi: 10.1513/AnnalsATS.201310-335OC. [DOI] [PubMed] [Google Scholar]
- 29.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol 34: 174–190, 2008. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 30.Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology 95: 353–361, 2009. doi: 10.1159/000209301. [DOI] [PubMed] [Google Scholar]
- 31.Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 79: 205–209, 2001. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 32.Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol 26, Suppl 1: S57–S62, 2006. doi: 10.1038/sj.jp.7211476. [DOI] [PubMed] [Google Scholar]
- 33.Stressmann FA, Connett GJ, Goss K, Kollamparambil TG, Patel N, Payne MS, Puddy V, Legg J, Bruce KD, Rogers GB. The use of culture-independent tools to characterize bacteria in endo-tracheal aspirates from pre-term infants at risk of bronchopulmonary dysplasia. J Perinat Med 38: 333–337, 2010. doi: 10.1515/jpm.2010.026. [DOI] [PubMed] [Google Scholar]
- 34.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, Halpern EF, Bloch KD, Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging 3: 157–163, 2010. doi: 10.1161/CIRCIMAGING.109.887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner BD, Sontag MK, Harris JK, Miller JI, Morrow L, Robertson CE, Stephens M, Poindexter BB, Abman SH, Mourani PM. Airway microbial community turnover differs by BPD severity in ventilated preterm infants. PLoS One 12: e0170120, 2017. doi: 10.1371/journal.pone.0170120. [DOI] [PMC free article] [PubMed] [Google Scholar]