Abstract
The nongenomic mechanisms by which glucocorticoids modulate β2 agonist-induced-bronchodilation remain elusive. Our studies aimed to elucidate mechanisms mediating the beneficial effects of glucocorticoids on agonist-induced bronchodilation. Utilizing human precision-cut lung slices (hPCLS), we measured bronchodilation to formoterol, prostaglandin E2 (PGE2), cholera toxin (CTX), or forskolin in the presence and absence of budesonide. Using cultured human airway smooth muscle (HASM), intracellular cAMP was measured in live cells following exposure to formoterol, PGE2, or forskolin in the presence or absence of budesonide. We showed that simultaneous budesonide administration amplified formoterol-induced bronchodilation and attenuated agonist-induced phosphorylation of myosin light chain, a necessary signaling event mediating force generation. In parallel studies, cAMP levels were augmented by simultaneous exposure of HASM cells to formoterol and budesonide. Budesonide, fluticasone, and prednisone alone rapidly increased cAMP levels, but steroids alone had little effect on bronchodilation in hPCLS. Bronchodilation induced by PGE2, CTX, or forskolin was also augmented by simultaneous exposure to budesonide in hPCLS. Furthermore, HASM cells expressed membrane-bound glucocorticoid receptors that failed to translocate with glucocorticoid stimulation and that potentially mediated the rapid effects of steroids on β2 agonist-induced bronchodilation. Knockdown of glucocorticoid receptor-α had little effect on budesonide-induced and steroid-dependent augmentation of formoterol-induced cAMP generation in HASM. Collectively, these studies suggest that glucocorticoids amplify cAMP-dependent bronchodilation by directly increasing cAMP levels. These studies identify a molecular mechanism by which the combination of glucocorticoids and β2 agonists may augment bronchodilation in diseases such as asthma or chronic obstructive pulmonary disease.
Keywords: asthma, bronchodilation, chronic obstructive pulmonary disease, corticosteroids, smooth muscle shortening
INTRODUCTION
Combination therapy including an inhaled glucocorticoid and a long-acting β2 agonist represents a cornerstone in the management of airway diseases, including asthma and chronic obstructive pulmonary disease. Conceptually, combined use of an anti-inflammatory agent with a bronchodilator improves medication adherence compared with using each drug separately (6, 19, 26). Use of an inhaled corticosteroid (ICS) with a long-acting β2 agonist (LABA) as rescue and maintenance therapy appears more effective than using an ICS or SABA alone or using an ICS/LABA for maintenance (2, 3, 12, 14, 15, 21, 22). Evidence now suggests that, apart from enhanced adherence, combination therapy augments efficacy of either drug alone (18). We posit that this effect is mediated by a rapid nongenomic effect of glucocorticoids. To date, the molecular mechanisms by which nongenomic effects of glucocorticoids modulate bronchodilation remain unknown (18).
Glucocorticoids primarily mediate their effects by activating the glucocorticoid receptor (GR). In this canonical signaling, the GR resides in the cytoplasm in its inactive state and, upon ligand activation, translocates to the cell nucleus to interact with glucocorticoid response elements (GREs) and produce genomic effects altering protein expression. Recent evidence has emerged to suggest that glucocorticoids manifest rapid nongenomic actions on several signaling processes (18). These noncanonical, nongenomic effects of glucocorticoids appear to involve nonspecific interactions with the cell membrane and/or specific interactions with cytosolic GRs (cGRs) or membrane-bound GRs (mGRs). The rapid nongenomic effects appear to, at least in part, be mediated through a putative mGR (18).
Several studies have reported an interaction of mGR with other membrane receptors, particularly G protein-coupled receptors (GPCR) (27). Involvement of mGR and GPCR-dependent mechanisms in the rapid effect (~1 min) of corticosterone on N-methyl-d-aspartate-evoked currents in hippocampal neurons was demonstrated (27), suggesting that mGR may couple with multiple G proteins, including Gαs and Gαq/11. Other studies suggest that mGR directly activates downstream intracellular signaling pathways. Corticosterone acted via mGR to rapidly elicit PKC-dependent activation of ERK1/2 MAPK pathway in PC12 cells (20). Interestingly, proteomic analysis of CCRF-CEM cells identified 128 proteins that were differentially regulated by activation of mGR using BSA-conjugated cortisol briefly (5 and 15 min) (25). These actions were unique to mGR, as no activation of cGR target genes (e.g., GILZ) were observed. Signal pathway analysis now provides evidence that mGR is involved in numerous pathways that are also regulated by glucocorticoids through cGR, suggesting that mGRs trigger early priming events, ultimately facilitating slower genomic activation by glucocorticoids (25).
Our study identifies a molecular mechanism by which glucocorticoids acutely amplify β2 agonist-induced bronchodilation. By rapidly stimulating cAMP production, glucocorticoids augment the primary second messenger signal of β2 agonists and other bronchodilators. Ultimately, identification of plasma membrane components through which glucocorticoids impact cAMP signaling and bronchodilation may provide novel therapeutic targets for airway diseases, an area that has seen little innovation in the past 45 years.
METHODS
Reagents.
Reagents were purchased from the following vendors: formoterol, forskolin, carbachol, and TNFα (Sigma Aldrich, St. Louis, MO); budesonide (AstraZeneca); cell culture media and components (ThermoFisher, Waltham, MA); fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA); GR antibody (Santa Cruz, Dallas, TX); β-actin antibody (Sigma Aldrich); phosphorylated GRα (Cell Signaling Technology, Danvers, MA); rabbit and mouse secondary antibodies for immunoblotting (LI-COR, Lincoln, NE); GRα-targeted siRNA (Ambion, Austin, TX); nontargeting siRNA (Dharmacon, Lafayette, CO); HiPerFect transfection reagent (Qiagen, Germantown, MD); and single analyte ELISA kits for IL-6, RANTES, and IL-8 (R&D Systems, Minneapolis, MN).
Human precision-cut lung slice generation and bronchodilation assays.
Human precision-cut lung slices (hPCLS) were prepared as previously described (11). Briefly, whole human lungs from donors without asthma were dissected and inflated using 2% (wt/vol) low melting point agarose. Once the agarose set, the lobe was sectioned, and cores of 8-mm diameter were made. Cores containing small airways, by visual inspection, were sliced at a thickness of 350 μm (Precisionary Instruments VF300 Vibratome, Greenville, NC) and collected in wells containing supplemented Ham’s F-12 medium. To study bronchodilation, small airways contained within hPCLS were contracted with carbachol (10−5 M), and then bronchodilated to formoterol (10−12–10−6 M), budesonide (10−12–10−5 M), forskolin (10−12–10−5 M), PGE2 (10−10–10−5 M), or cholera toxin (0.01–100 μg/mL) ± budesonide (10−5 M, simultaneous administration). Human lung tissue samples were commercially obtained from anonymous donors (National Disease Research Interchange, Philadelphia, PA or the International Institute for the Advancement of Medicine, Edison, NJ), and are therefore exempt from Institutional Review Board approval. Although these samples have demographic information, there is no information linking the subject’s identification to the tissue. Integrated area under the curve (AUC; % dilation/bronchodilator dose) was calculated from the dose-response curves and was plotted along with maximal bronchodilation achieved over the entire dose-response curve.
Human airway smooth muscle cells.
Human airway smooth muscle (HASM) cells were derived from tracheas obtained from nonasthmatic donor lungs that hPCLS were also derived from. HASM cell culture was performed as described previously (16). Briefly, the cells were cultured in Ham’s F-12 medium supplemented with 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 2.5 mg/mL amphotericin B, and this medium was replaced every 72 h. HASM cells in subculture during passages 1–5 were used because these cells retain expression of native contractile proteins, as demonstrated by immunocytochemical staining for smooth muscle actin and myosin (16).
cAMP assays.
For kinetic measurement of cAMP production in live cells, subconfluent HASM cells were plated in black-walled, clear-flat-bottom, 96-well plates with HASM media, BacMam virus expressing the green cAMP difference detector in situ (cADDis) cAMP sensor (Montana Molecular, Bozeman, MT), and 1 μM trichostatin-A (Sigma Aldrich, St. Louis, MO) per well and grown overnight. Media was aspirated and replaced with PBS without calcium or magnesium, and then the plate was covered and incubated at room temperature. Cell fluorescence was read from the plate bottom using excitation/emission wavelengths of 494 nm and 522 nm, respectively, using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). A kinetic read (5 min) on unstimulated cells was performed to determine variability in each well’s fluorescence (≤ 5%). Cells were stimulated with agonist and fluorescence changes were read at 30-s intervals for 30 min.
Cell-surface biotinylation.
Cell-surface protein biotinylation was carried out according to manufacturer’s protocols (Pierce Biotechnology, Rockford, IL). Cells were grown to 90%–95% confluence, incubated with a biotin solution, then scraped and lysed. Lysates were incubated with NeutrAvidin beads, and then bound proteins were eluted off. Eluates were assessed for glucocorticoid receptor using epidermal growth factor receptor (EGFR; Cell Signaling Technology, Danvers, MA) as a positive control for cell-surface biotinylation. Remaining cell lysates were examined for expression of GAPDH (Millipore, Burlington, MA) and cyclooxygenase IV (Cell Signaling Technology, Danvers, MA) as measures of intracellular/cytosolic proteins.
Immunohistochemistry.
HASM cells were grown in chamber slides until confluent, then serum starved for 18 h. Cells were then fixed with 1% paraformaldehyde, washed, then blocked with 1% BSA-PBS solution containing 10% FcR block (Miltenyi Biotec, Auburn, CA). Cells were stained with a GR antibody (rabbit, Santa Cruz Biotechnology, Dallas, TX) in 1% BSA/PBS solution overnight. The slides were washed, stained with biotin-coated donkey anti-rabbit antibody (Jackson Immunolabs, Bar Harbor, ME) in 1% BSA-PBS, washed, and then incubated with a streptavidin-Alexa Fluor 488-conjugated antibody (Jackson Immunolabs). The slides were washed and then the cells were permeabilized with 0.01% Triton X-100 and stained with DAPI. Slides were coverslipped and imaged.
Immunoblotting.
HASM cells were treated with carbachol (25 μM Cch, 10 min) and then with formoterol (100 pM, 5 min) ± simultaneous budesonide stimulation (1 μM, 5 min). Cells were then treated with 500 μM perchloric acid, plates were scraped, and cells were pelleted. Pellets were solubilized in RIPA and sonicated before being subjected to SDS-PAGE and transferred to nitrocellulose membranes, as previously described (1), and then assessed for phosphorylation of myosin light chain (MLC) and total MLC. Total GRα and phospho-GRα were assessed in cell lysates following siRNA transfection of HASM with nontargeting and GRα-targeted siRNA.
Single analyte ELISAs.
HASM transfected with nontargeting or GRα-targeted siRNA were treated with budesonide (100 nM, 1 h) before stimulation with TNFα (10 ng/mL, 24 h). Single analyte ELISAs were utilized to assess release of IL-6, RANTES, and IL-8 into the media. Each condition represents duplicate samples from a single donor, each run in triplicate.
Statistical analyses.
Standard curves for cAMP generation were fitted, and unknown values were extrapolated using GraphPad Prism 6.0h (GraphPad Software Inc., San Diego, CA). Data are presented as the mean ± SE. Statistical comparisons (t tests and one-way analysis of variance) were performed, and graphics were generated using GraphPad Prism 6.0h (GraphPad Software Inc.). Unpaired nonparametric analyses were used for hPCLS data that was not normally distributed to compare conditions. Paired parametric analysis was used for HASM experiments (ELISA, Western blots, and cAMP generation).
RESULTS
Budesonide enhances formoterol-induced bronchodilation.
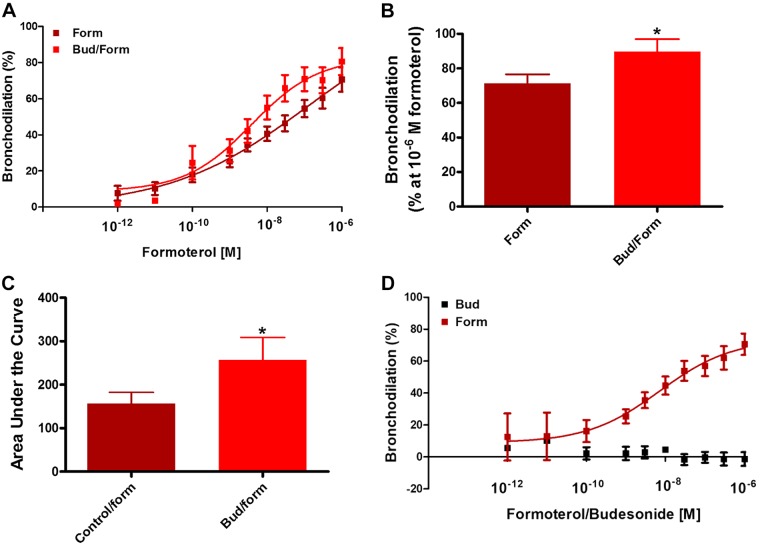
To examine whether a glucocorticoid and a β2 agonist can additively promote bronchodilation, human small airways in hPCLS were preconstricted to carbachol and then dilated to formoterol in the absence and presence of budesonide, with the budesonide being added simultaneously with the formoterol. Budesonide treatment augmented formoterol-induced bronchodilation (Fig. 1), increasing maximal levels of bronchodilation. Similarly, the integrated bronchodilator response, as represented by area under the curve (AUC), significantly increased. Budesonide alone had little effect on luminal diameter dilation despite being administered at similar concentrations as formoterol (data not shown). These data show that simultaneous administration of budesonide augments β2 agonist-induced dilation of human small airways.
Fig. 1.
Acute budesonide stimulation augments formoterol-induced bronchodilation but does not induce bronchodilation alone. A–C: simultaneous stimulation of human precision-cut lung slices (hPCLS) with budesonide (Bud) (10−5 M) and formoterol (form) (10−12 to 10−6 M) (A) augments maximal bronchodilation (formoterol vs. budesonide at 10−6 M formoterol, 71.4 ± 5% vs. 89.7 ± 7.3%) (B) and increases the integrated area under the curve (C) vs. formoterol alone (formoterol vs. formoterol + budesonide, 156.8 ± 25.9 vs. 257.1 ± 51.6). Data represent n = 15–25 donors. D: budesonide alone does not induce bronchodilation in hPCLS. Data represent 2 (budesonide alone) donors and 15 (formoterol) donors, 6–36 slices/condition. *P < 0.05 compared with form or control/form.
Budesonide amplifies PGE2-, cholera toxin-, and forskolin-mediated bronchodilation.
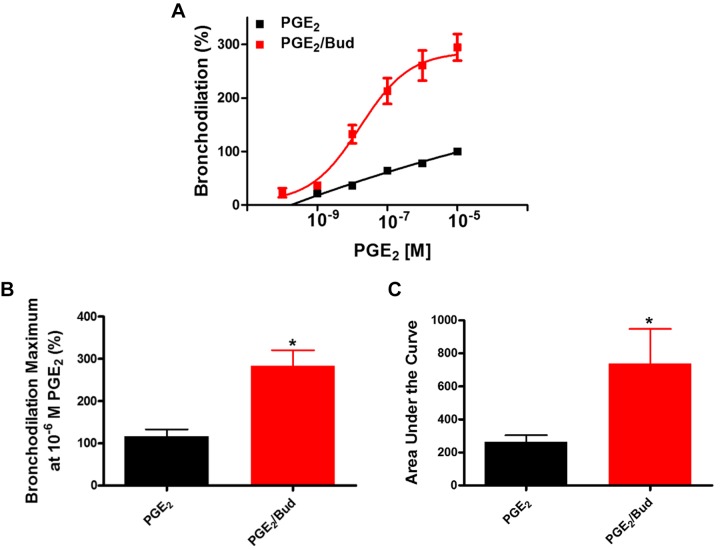
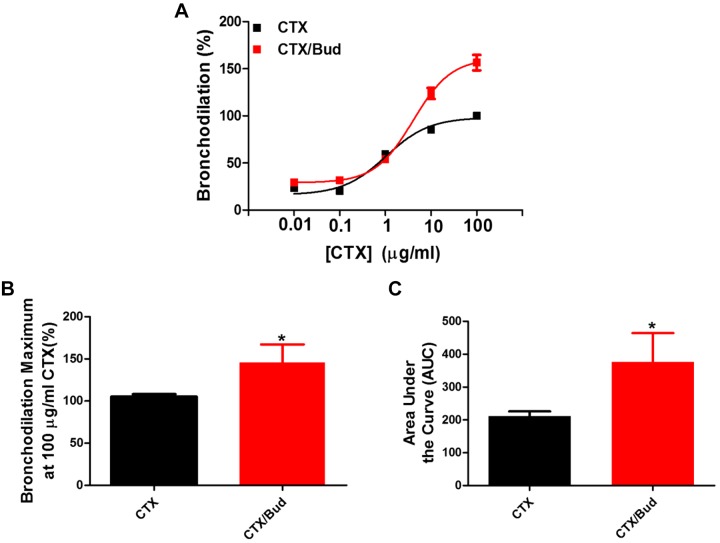
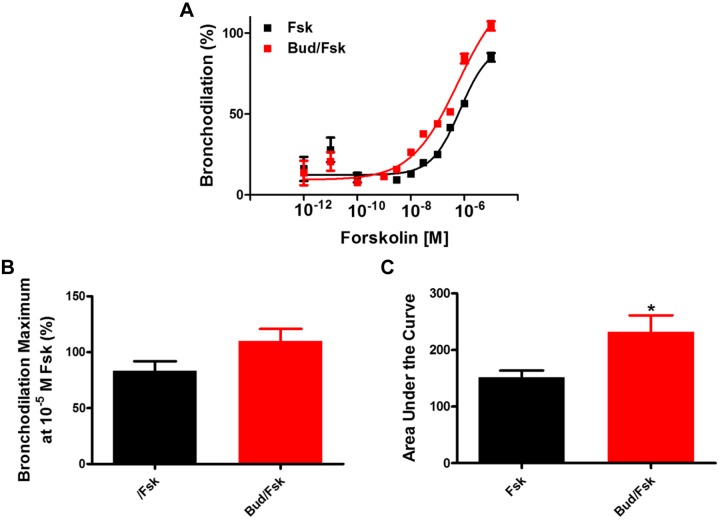
Given that budesonide augments airway dilation to a β2 agonist, we next examined whether budesonide enhances dilation mediated by activation of other GPCRs coupled to Gαs or via direct activation of Gαs or adenylyl cyclase (AC). We showed that simultaneous administration of budesonide enhances PGE2-induced bronchodilation of airways (Fig. 2), with increases in maximal bronchodilation and AUC to PGE2. To assess whether the effect of budesonide was due to activation of Gαs, a G protein shared between the β2-adrenergic (β2AR) and prostanoid E 2/4 (EP2/4) receptors, we utilized cholera toxin (CTX) to induce bronchodilation in hPCLS. Simultaneous administration of budesonide with CTX induced greater maximal bronchodilation overall responses to CTX compared with CTX alone (Fig. 3). To assess whether budesonide enhanced direct adenylyl cyclase (AC)-induced bronchodilation, hPCLS were exposed to forskolin (FSK) in the presence or absence of budesonide. Simultaneous budesonide administration enhanced FSK-induced bronchodilation (Fig. 4), significantly increasing the AUC to FSK compared with control.
Fig. 2.
Simultaneous budesonide treatment augments PGE2-induced bronchodilation of human small airways. Budesonide (Bud; 10 μM) was given simultaneously with PGE2 (10−10 to 10−5 M) and bronchodilation was assessed. Concentration-response curves (A) are represented as % dilation for the combination compared with PGE2-induced dilation alone. Maximal bronchodilation at 10−5 M PGE2 (B, PGE2 vs. PGE2 + budesonide at a maximum of 10−5 M PGE2, 116.6 ± 16.6% vs. 283.3 ± 37.3%) and integrated area under the curve (C, PGE2 vs. PGE2 + budesonide, 264.5 ± 40 vs. 736.2 ± 209.6) of PGE2-induced bronchodilation were significantly increased. Data represent n = 3 donors. *P < 0.05 compared with PGE2 stimulation alone.
Fig. 3.
Budesonide significantly augments cholera toxin-induced bronchodilation of human small airways. Budesonide (10 μM) was given simultaneously with cholera toxin (CTX, 0.01–100 μg/mL) and bronchodilation was assessed. Concentration-response curves (A) were normalized to CTX stimulation alone set to 100%. Maximum bronchodilation at 100 μg/mL (B, CTX vs. CTX + budesonide at a maximum of 100 μg/mL CTX, 105.5 ± 3.9% vs. 128.4 ± 17.5%) and area under the curve (AUC) (C, CTX vs. CTX + budesonide, 200.8 ± 13.8 vs. 361.5 ± 112.6) were significantly increased with budesonide stimulation. Data are representative of n = 5 donors, 11–13 slices/condition. *P < 0.05 compared with CTX stimulation alone.
Fig. 4.
Budesonide (Bud) augments forskolin-induced bronchodilation of human small airways. Budesonide (10 μM) was given simultaneously with forskolin (Fsk; 10−12 to 10−5 M) and bronchodilation was assessed. Concentration-response curves to Fsk (A) were plotted. Maximal bronchodilation at 10−5 M Fsk (B, Fsk vs. Fsk + budesonide at maximum of 10−5 M Fsk, 83.7 ± 8.5% vs. 110.1 ± 10.6%) and area under the curve (C, FSK vs. FSK + budesonide, 151.9 ± 11.8 vs. 232.3 ± 29.1) were significantly increased in the presence of budesonide. Data are representative of n = 5 donors. *P < 0.05 compared with control/Fsk.
Budesonide enhances formoterol-stimulated cAMP production.
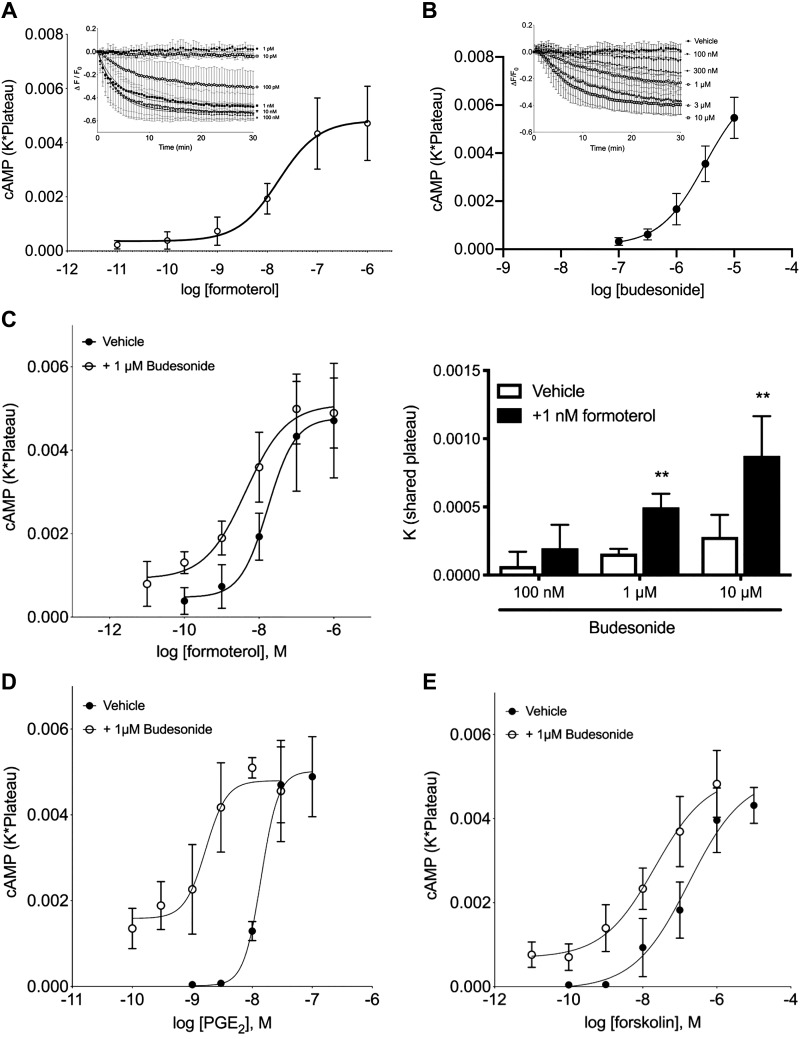
To dissect the molecular pathways by which budesonide augments bronchodilation of human airway smooth muscle (HASM), HASM were infected with a recombinant BacMam expressing a fluorescent cAMP sensor, cADDis, and cAMP levels were measured after exposure to varying concentrations of formoterol. The cADDis sensor decreases fluorescence upon binding cAMP, providing real-time assessment of intracellular cAMP levels without inclusion of phosphodiesterase (PDE) inhibitors. Formoterol decreased cADDis fluorescence within minutes that typically stabilized within 15–20 min (Fig. 5A, inset). To account for the rate and maximal levels of cAMP production produced by formoterol, the product of the decay rate (K) and the level at steady state (plateau) were plotted for each drug concentration. Using this analysis, formoterol increased cAMP levels (Fig. 5A). In parallel, formoterol-stimulated cAMP was measured in HASM treated with vehicle or 10−6 M budesonide given simultaneously. Budesonide shifted the formoterol concentration-response curve 3.9-fold leftward (Fig. 5B). Additionally, budesonide at 1 or 10 μM augmented formoterol-induced cAMP production, but that effect was not realized at 100 nM budesonide. The effect of budesonide on formoterol-stimulated cAMP production in HASM cells therefore mimicked the effect of budesonide on agonist-induced bronchodilation observed in hPCLS.
Fig. 5.
Budesonide alone induces cAMP production in human airway smooth muscle (HASM) cells and enhances formoterol, PGE2, and forskolin (Fsk)-induced cAMP production. HASM cells were incubated with recombinant BacMam virus expressing the cAMP difference detector in situ (cADDis) cAMP sensor for 24 h. After establishing baseline, fluorescence decay was monitored for 30 min after the addition of the drug. A: cADDis sensor fluorescent decay curves elicited by various concentrations of formoterol were fit by one-phase decay nonlinear regression analysis (inset; 10−12–10−6 M formoterol, log EC50 of −7.78 ± 0.185). The rate (K) was multiplied by the steady-state change in fluorescence (plateau) for each concentration of formoterol. Each point represents the mean ± SE of n = 5. B: budesonide alone (1 or 10 μM) elicits cAMP production in cells and is compared with forskolin (10 μM) stimulation. Each point represents the mean ± SE of n = 4–6 cell lines, and lines represent the fit by one-phase decay nonlinear regression analysis. *P < 0.05, **P < 0.01 of each time point compared with vehicle using multiple t tests and the Holm–Sidak method for correction of multiple comparisons. C: formoterol concentration-response curves in the presence of vehicle or 1 μM budesonide. Each point represents the mean ± SE of n = 5 cell lines (log EC50 formoterol vs. formoterol + budesonide, −7.76 ± 0.205 vs. −8.36 ± 0.189, P = 0.021). D: PGE2 concentration-response curves in the presence of vehicle or 1 μM budesonide. Each point represents the mean ± SE of n = 4 cell lines (log EC50 PGE2 vs. PGE2 + budesonide, −7.85 ± 0.062 vs. −8.78 ± 0.129, P = 0.046). E: forskolin concentration-response curves in the presence of vehicle or 1 μM budesonide. Each point represents the mean ± SE of n = 7 cell lines (log EC50 FSK vs. FSK + budesonide, −6.75 ± 0.168 vs. −7.67 ± 0.190, P = 0.005).
Budesonide enhances PGE2- and forskolin-stimulated cAMP production.
To determine whether budesonide also enhanced cAMP signaling stimulated by PGE2, PGE2-stimulated cAMP production was detected by cADDis in a concentration-dependent manner (Fig. 5C). Inclusion of budesonide (1 μM) shifted the PGE2 curve leftward 8.4-fold. We next examined cAMP responses to forskolin (FSK), finding that FSK-stimulated cAMP production was shifted leftward 8.2-fold by inclusion of 1 μM budesonide compared with vehicle (Fig. 5D). These data are consistent with the notion that budesonide enhances cAMP production initiated by multiple GPCRs in different signaling compartments in HASM cells and that budesonide-mediated augmentation of these responses is receptor independent.
Budesonide, fluticasone, and prednisone enhance directly stimulated cAMP production.
Because budesonide enhanced agonist-induced cAMP signaling, we investigated whether budesonide alone stimulated cAMP. Significant decreases in cADDis fluorescence was observed within seconds of addition of 1 or 10 μM budesonide (data not shown). Ten μM budesonide stimulated cAMP levels that were statistically different than vehicle within 2 min, while 1 μM induced significant changes within 5 min. The higher concentration of budesonide stimulated changes in cADDis fluorescence that were equivalent to maximal concentrations of forskolin (10 μM) or formoterol (not shown). However, cADDis is a readily saturated sensor, so high levels of cAMP may not be distinguishable (9). We also examined cAMP production by other corticosteroids, finding that fluticasone and prednisone (data not shown) stimulated cAMP production. Fluticasone was equi-effective as budesonide, although greater interexperimental variability was observed when prednisone was less efficacious and less potent.
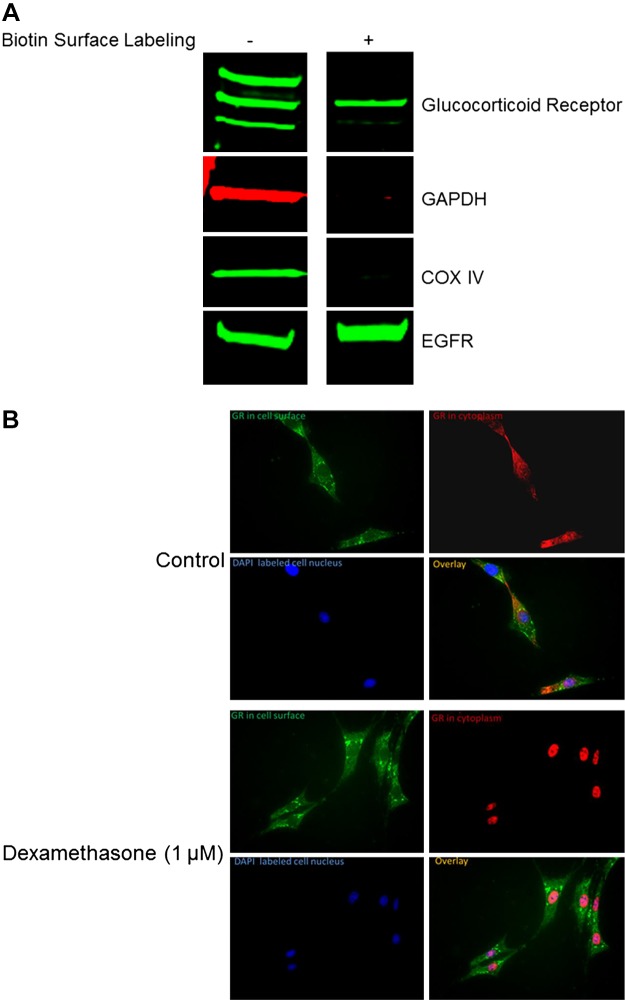
A membrane-bound form of GR is present in HASM cells.
Since budesonide effects on cAMP generation and bronchodilation were rapid, we posited that budesonide activates a membrane-associated receptor. Other laboratories have identified an mGR that can evoke immediate steroid effects in other cell types (13, 23, 24). Using cell-surface biotin labeling and immunohistochemistry (Fig. 6), we showed that HASM express mGRs that fail to translocate to the nucleus from the membrane despite stimulation with a glucocorticoid (dexamethasone). As expected, cytosolic GR translocated to the nucleus as shown in Fig. 6.
Fig. 6.
Membrane-bound glucocorticoid receptors exist in human airway smooth muscle (HASM). A: HASM from lung donors without disease was biotinylated, and purification of a membrane fraction determined the existence of a membrane-bound form of the glucocorticoid receptor. Presence of the epidermal growth factor receptor (EGFR) and absence of GAPDH; cytochrome c oxidase (COX) IV (cytosolic proteins) were used as controls for purity of membrane protein isolation. B: HASM cells were fixed with acetone following stimulation for 30 min with dexamethasone (1 μM). Fixed cells were then probed for glucocorticoid receptor and DAPI. Data are representative of three separate donors without asthma for both A and B.
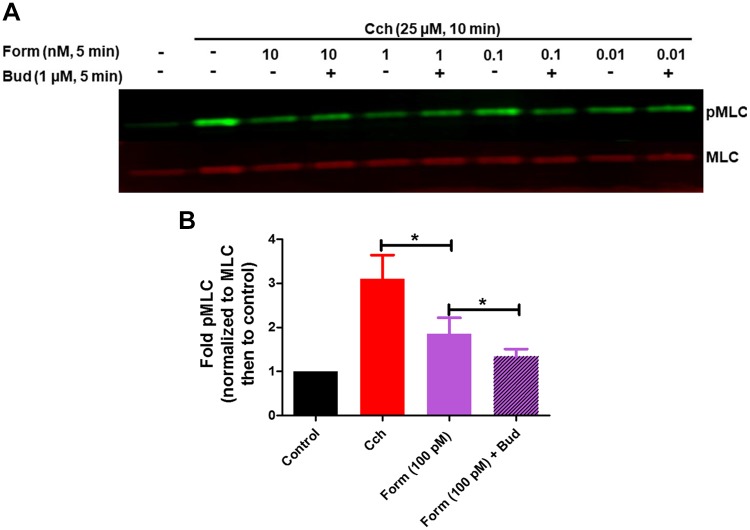
Budesonide augments formoterol-induced dephosphorylation of myosin light chain.
Contractile agonists activate GPCRs that evoke HASM shortening by promoting phosphorylation of myosin light chain (pMLC). To determine whether formoterol in the absence and presence of budesonide modulates agonist-induced excitation-contraction signaling, we examined attenuation of carbachol-induced pMLC by formoterol ± budesonide. Formoterol alone (10 pM – 10 nM) induced significant reversal of carbachol-induced pMLC, and simultaneous administration of budesonide with formoterol (100 pM) further augments formoterol-induced pMLC dephosphorylation (Fig. 7). These data suggest that glucocorticoids can also augment formoterol effects on procontractile pathways in HASM cells.
Fig. 7.
Budesonide augments formoterol-mediated attenuation of carbachol (Cch)-induced phosphorylation of myosin light chain. Human airway smooth muscle (HASM) cells were treated with Cch (25 μM, 15 min), then treated simultaneously with formoterol (Form; 10 pM–10 nM, 5 min) and budesonide (Bud; 1 μM, 5 min). Phosphorylation of myosin light chain was assessed by immunoblot, using total myosin light chain as a loading control (A). Data are expressed as fold compared with control (B) and are representative of five separate HASM donors (*P < 0.05).
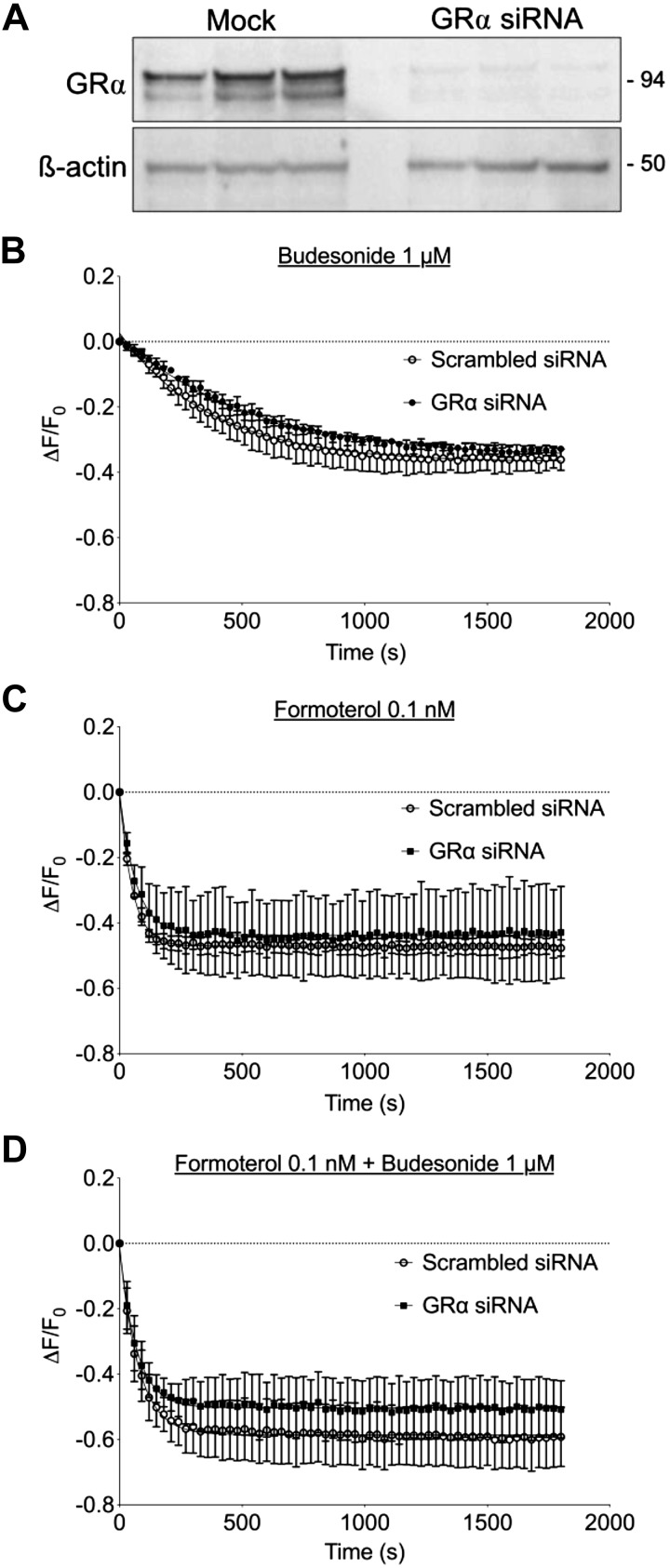
GRα knockdown in HASM cells has little effect on cAMP generation in response to budesonide or budesonide + formoterol.
To more directly assess the role of the GR in mediating rapid cAMP responses to glucocorticoids, we used siRNA to knock down GRα expression in HASM. Transfection of validated siRNA sequences into HASM led to a nearly complete loss of GRα immunoreactivity (detected as a doublet of ~94 kDa) compared with cells transfected with a scrambled siRNA (Fig. 8A). GRα mRNA expression in control HASM was readily detected by quantitative RT-PCR but was undetectable in cells transfected with GRα siRNA (not shown). We then assessed cAMP levels in HASM following siRNA transfection. Forskolin-stimulated cAMP responses were unaltered by GRα knockdown, implying that loss of GRα did not alter AC expression or function (data not shown). Budesonide (1 μM) or formoterol (0.1 nM) each elicited equivalent cAMP responses in both GRα knockdown and control cells (Fig. 8, B and C). cAMP responses to simultaneous addition of budesonide and formoterol were greater than either agent alone but were similar in both control and GRα-knockdown conditions (Fig. 8D). Knockdown of GRα was confirmed by immunoblot and showed reversal of budesonide attenuation of TNFα-induced inflammatory mediator release (data not shown). These results suggest that GRα expression may not be required for rapid, nongenomic signaling by glucocorticoid.
Fig. 8.
Knockdown of glucocorticoid receptor-α (GRα) had little effect on budesonide or budesonide + formoterol-induced cAMP production in human airway smooth muscle (HASM). HASM cells were transfected with siRNA specific for GRα or scrambled control (mock) for 72 h. A: lysates were collected and analyzed by immunoblotting using antibodies specific for GRα or β-actin. Each lane shows one of 3 separate experiments on individual cell lines. B, C and D: cAMP difference detector in situ sensor was expressed in HASM with a recombinant BacMam virus, and then cAMP responses to budesonide (1 μM, B), formoterol (0.1 nM, C), or both budesonide and formoterol (1 µM and 0.1 nM, respectively, D) were measured in HASM that was transfected with nontargeting siRNA or siRNA specific for GRα. Each point represents the mean ± SE of n = 4–5 cell lines.
DISCUSSION
In airway smooth muscle, contractile agonists stimulate Ca2+-dependent signaling, evoking cell shortening that is regulated in part through increased [Ca2+]i transients, inhibition of sarcoplasmic reticulum Ca2+-ATPase (SERCA), and pMLC, inducing actin-myosin cross-bridge cycling and force generation. Agents that mobilize cAMP and activate protein kinase A (PKA) protect against or reverse agonist-induced bronchoconstriction. In epithelial cells and neurons, glucocorticoids modulate signaling pathways that promote relaxation of HASM. Dexamethasone stimulation of bronchial cells attenuated [Ca2+]i currents, which was reversed by SERCA inhibitors, PKA, and activated adenylyl cyclase (AC) within minutes (24). Further, corticosterone treatment reversed ATP-induced [Ca2+]i transients in mouse HT4 neuroblastoma cells (7). In primary HASM cells, budesonide stimulation alone increased cAMP levels (data not shown). Interestingly, we show that simultaneous budesonide and formoterol significantly reduced carbachol-induced pMLC (Fig. 7), but there was little effect of budesonide alone. Others noted existence of an mGR that may mediate nongenomic, rapid effects of glucocorticoids. We demonstrate presence of mGR in HASM (Fig. 6) that does not translocate upon glucocorticoid stimulation. Our work suggests that expression of GRα is not necessary to elicit cAMP production in response to budesonide or budesonide + formoterol (Fig. 8). There is a possibility that residual GR protein, whose expression was not attenuated by siRNA transfection, may mediate the effect, but the existence of a modified GR that may not be subject to knockdown of the protein may mediate the effects of steroids on cAMP production/augmentation of bronchodilation. Overall, these data suggest that, despite the effect of glucocorticoids on attenuation of Ca2+-dependent pathways, glucocorticoids alone had little effect on agonist-induced bronchoconstriction of small airways (data not shown) in hPCLS but rather amplified β2 agonist-induced bronchodilation.
Although glucocorticoids alone induced cAMP production (data not shown), these steroids had little effect on bronchodilation of small airways in hPCLS (data not shown). Robust and sensitive assessment of cAMP pools is achieved using the cADDis reporter (9). Although the assay shows increases in cAMP production in response to budesonide alone, this increase in cAMP levels may be insufficient for promoting bronchodilation due to an inadequate magnitude or localization of the cAMP signal, despite utilizing the same concentrations of budesonide as formoterol in the hPCLS bronchodilation assays (10−12–10−5 M, data not shown). Glucocorticoids may therefore be increasing cAMP levels via direct activation of Gαs, activation of an unidentified GPCR or inhibition of phosphodiesterases. Others suggest that increases in intracellular cAMP elicited by glucocorticoids may be due to inhibition of ABCC4, an ATP binding cassette transporter that pumps cAMP into the extracellular space. In differentiated airway epithelial cells, dexamethasone, but not budesonide, augmented activity of GRE-luciferase when given with forskolin treatment. Additionally, blockade of ABCC4 potentiated activity of the GRE-luciferase in the presence of either dexamethasone or budesonide treatment (8). Unlike airway epithelial cells, β2 agonist or forskolin treatment had little effect on extracellular levels of cAMP in HASM (data not shown). However, we reported that ABCC4 is expressed in HASM cells, and its expression is augmented by budesonide treatment (10). In the aforementioned studies, glucocorticoid treatment was for 6–12 h, conceivably, the mechanisms modulating rapid glucocorticoid stimulation may differ. Future studies will address whether nongenomic effects of glucocorticoids are mediated by activation of ABCC4 proteins.
Others noted acute glucocorticoid stimulation activates signaling pathway components in non-muscle cells that are associated with bronchodilatory responses in smooth muscle. In bronchial epithelial cells, dexamethasone inhibited agonist-induced [Ca2+]i, with the inhibition sensitive to AC and PKA inhibition (24). Others show a nongenomic glucocorticoid effect that was PKA-dependent in a neural cell line (7). Accordingly, to assess mechanisms by which steroid may be augmenting agonist-induced bronchodilation, we examined the effect of glucocorticoids on 1) selectivity of responses we observed to the β2AR, 2) direct activation of Gαs, and 3) whether the response observed was receptor dependent. We found that PGE2-, CTX-, and FSK-stimulated bronchodilation of small airways (Figs. 2, 3, and 4, respectively) was augmented by coadministration of budesonide. Similarly, PGE2-, and FSK-induced cAMP production in HASM (Fig. 5, C and D) was augmented by budesonide coadministration. Despite the cADDis characterizing budesonide’s effects on CTX-induced cAMP production, CTX induced such saturating levels of cAMP production in HASM cells that we were unable to detect a synergism above CTX stimulation alone (data not shown).
Interestingly, synergism between glucocorticoids and β2 agonists has been examined previously. A recent study noted that beclomethasone augmented formoterol-induced reversal of precontracted airways (4) to about ~30% maximally (5). Although there was synergism between the two therapeutics, the beclomethasone dose was given overnight, with effects likely genomic. This is in direct contrast to what our studies show, where despite budesonide inducing cAMP production in HASM, it alone was unable to induce bronchodilation even at a concentration of 100 μM. Additionally, the 2016 study (5) utilized nonselective inhibitors of PKA and Gαs to establish roles for those proteins in beclomethasone-induced bronchodilation in lung slices. Our studies have the advantage of observing cAMP production in live primary HASM in real time, a system in which we can genetically manipulate components of signaling pathways to more rigorously ascertain the necessity and sufficiency specific molecules in the responses we observe.
Our studies identify mechanisms by which budesonide, a potent glucocorticoid, rapidly activates cAMP production and augments agonist-mediated bronchodilation. This is the first direct demonstration that glucocorticoids stimulate cAMP production in airway smooth muscle. These mechanisms may explain, in part, how combination therapy of β2 agonists and ICS offers greater efficacy in comparison to either drug alone. Interestingly, these beneficial effects occur separate and distinct from any anti-inflammatory effects (18). Combination therapy represents a cornerstone for maintenance therapy in asthma and chronic obstructive pulmonary disease. Evidence also suggests that this combination can serve as an anti-inflammatory reliever therapy (when added to maintenance) that decreases exacerbation rates, improves asthma symptoms, and enhances lung function compared with higher doses of inhaled corticosteroids (ICS) alone (14, 21, 22). Our data identify molecular mechanisms by which steroids and long-acting β2 agonists together improve bronchodilation.
GRANTS
These studies were funded by NIH Grants P01 HL11447 and UL1 TR003017 (R. A. Panettieri, Jr., C. Koziol-White, B. Deeney, S. Orfanos, G. Cao, and V. Parikh), R01 HL111541 (O. Tliba), and R01 GM107094 (R. S. Ostrom, T. B. Johnstone, and M. L. Corpuz) and by AstraZeneca (I. Dainty and R. A. Panettieri, Jr.).
DISCLOSURES
R. A. Panettieri, Jr., received funding to support scientific research from AstraZeneca. I. Dainty is employed by AstraZeneca. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.K.-W., T.B.J., G.C., S.O., V.P., O.T., R.S.O., I.D., and R.A.P. conceived and designed research; C.K.-W., T.B.J., M.L.C., G.C., S.O., V.P., B.D., and R.S.O. performed experiments; C.K.-W., T.B.J., M.L.C., G.C., S.O., V.P., B.D., and R.S.O. analyzed data; C.K.-W., T.B.J., M.L.C., G.C., S.O., V.P., B.D., R.S.O., and I.D. interpreted results of experiments; C.K.-W., T.B.J., G.C., S.O., V.P., B.D., and R.S.O. prepared figures; C.K.-W., O.T., R.S.O., and R.A.P. drafted manuscript; C.K.-W., T.B.J., M.L.C., G.C., S.O., V.P., B.D., O.T., R.S.O., I.D., and R.A.P. edited and revised manuscript; C.K.-W., T.B.J., M.L.C., G.C., S.O., V.P., B.D., O.T., R.S.O., I.D., and R.A.P. approved final version of manuscript.
REFERENCES
- 1.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, Laviolette M, Panettieri RA Jr, Druey KM. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun 6: 6763, 2015. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Siwek-Posluszna A, FitzGerald JM. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med 378: 1877–1887, 2018. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 3.Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Harrison T, Houghton C, Oldfield K, Papi A, Pavord ID, Williams M, Weatherall M; Novel START Study Team . Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 380: 2020–2030, 2019. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 4.Calzetta L, Matera MG, Facciolo F, Cazzola M, Rogliani P. Beclomethasone dipropionate and formoterol fumarate synergistically interact in hyperresponsive medium bronchi and small airways. Respir Res 19: 65, 2018. doi: 10.1186/s12931-018-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cazzola M, Calzetta L, Rogliani P, Puxeddu E, Facciolo F, Matera MG. Interaction between corticosteroids and muscarinic antagonists in human airways. Pulm Pharmacol Ther 36: 1–9, 2016. doi: 10.1016/j.pupt.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 344: 219–224, 1994. doi: 10.1016/S0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- 7.Han JZ, Lin W, Chen YZ. Inhibition of ATP-induced calcium influx in HT4 cells by glucocorticoids: involvement of protein kinase A. Acta Pharmacol Sin 26: 199–204, 2005. doi: 10.1111/j.1745-7254.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 8.Huff RD, Rider CF, Yan D, Newton R, Giembycz MA, Carlsten C, Hirota JA. Inhibition of ABCC4 potentiates combination beta agonist and glucocorticoid responses in human airway epithelial cells. J Allergy Clin Immunol 141: 1127–1130.e5, 2018. doi: 10.1016/j.jaci.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone TB, Smith KH, Koziol-White CJ, Li F, Kazarian AG, Corpuz ML, Shumyatcher M, Ehlert FJ, Himes BE, Panettieri RA Jr, Ostrom RS. PDE8 is expressed in human airway smooth muscle and selectively regulates cAMP signaling by β2-adrenergic receptors and adenylyl cyclase 6. Am J Respir Cell Mol Biol 58: 530–541, 2018. doi: 10.1165/rcmb.2017-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan M, Koziol-White C, Shumyatcher M, Johnson M, Jester W, Panettieri RA Jr, Himes BE. Airway smooth muscle-specific transcriptomic signatures of glucocorticoid exposure. Am J Respir Cell Mol Biol 61: 110–120, 2019. doi: 10.1165/rcmb.2018-0385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, Andrews A, Himes BE, Damoiseaux RD, Liggett SB, Di Carlo D, Kurten RC, Panettieri RA Jr. Inhibition of PI3K promotes dilation of human small airways in a rho kinase-dependent manner. Br J Pharmacol 173: 2726–2738, 2016. doi: 10.1111/bph.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract 61: 725–736, 2007. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meurs H, Santing RE, Remie R, van der Mark TW, Westerhof FJ, Zuidhof AB, Bos IS, Zaagsma J. A guinea pig model of acute and chronic asthma using permanently instrumented and unrestrained animals. Nat Protoc 1: 840–847, 2006. doi: 10.1038/nprot.2006.144. [DOI] [PubMed] [Google Scholar]
- 14.O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, Ekström T, Bateman ED. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 171: 129–136, 2005. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 15.O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Ivanov S, Reddel HK. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med 378: 1865–1876, 2018. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 16.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 256: C329–C335, 1989. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 18.Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci 40: 38–49, 2019. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, Ullman A; Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group . Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 337: 1405–1411, 1997. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 20.Qiu J, Wang P, Jing Q, Zhang W, Li X, Zhong Y, Sun G, Pei G, Chen Y. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem Biophys Res Commun 287: 1017–1024, 2001. doi: 10.1006/bbrc.2001.5691. [DOI] [PubMed] [Google Scholar]
- 21.Rabe KF, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, Lier PA, Jorup C. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest 129: 246–256, 2006. doi: 10.1378/chest.129.2.246. [DOI] [PubMed] [Google Scholar]
- 22.Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, Boulet LP, Naya IP, Hultquist C. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin 20: 1403–1418, 2004. doi: 10.1185/030079904X2051. [DOI] [PubMed] [Google Scholar]
- 23.Sun HW, Miao CY, Liu L, Zhou J, Su DF, Wang YX, Jiang CL. Rapid inhibitory effect of glucocorticoids on airway smooth muscle contractions in guinea pigs. Steroids 71: 154–159, 2006. doi: 10.1016/j.steroids.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Urbach V, Walsh DE, Mainprice B, Bousquet J, Harvey BJ. Rapid non-genomic inhibition of ATP-induced Cl- secretion by dexamethasone in human bronchial epithelium. J Physiol 545: 869–878, 2002. doi: 10.1113/jphysiol.2002.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernocchi S, Battello N, Schmitz S, Revets D, Billing AM, Turner JD, Muller CP. Membrane glucocorticoid receptor activation induces proteomic changes aligning with classical glucocorticoid effects. Mol Cell Proteomics 12: 1764–1779, 2013. doi: 10.1074/mcp.M112.022947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 153: 1481–1488, 1996. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Sheng H, Qi J, Ma B, Sun J, Li S, Ni X. Glucocorticoid acts on a putative G protein-coupled receptor to rapidly regulate the activity of NMDA receptors in hippocampal neurons. Am J Physiol Endocrinol Metab 302: E747–E758, 2012. doi: 10.1152/ajpendo.00302.2011. [DOI] [PubMed] [Google Scholar]