Historically, the lungs were considered sterile in healthy individuals, because standard bacterial culture methods failed to consistently detect microbial organisms peaceably inhabiting this niche (4). However, next-generation sequencing platforms have recently identified diverse communities of microbial organisms that reside in healthy human lungs (2). It is now generally accepted that, although at much lower density and diversity than communities in the human gut, a distinct microbiome does exist in the respiratory tract (3). Some studies further suggest the presence of a distinct lower airway microbiome with a composition that is distinct from the better characterized communities in the upper airway (4). Associations between alterations in airway microbiota and airway diseases are also becoming more apparent. It has been postulated that asthma and allergy represent interplay among consequences of abnormalities in microbial colonization and the composition of bronchial airway microbiota. Numerous studies have demonstrated that the composition of airway microbiota in asthmatic patients is different from that of healthy controls (1).
Bronchopulmonary dysplasia (BPD) is a multifactorial disease that results from the exposure of immature, preterm lungs to several noxious stimuli (8). Recent data from Lal et al. (5), our group (12), and others (9) indicate that human organs, specifically airways, harbor a commensal microbiota at the time of birth and possibly even in utero. Lal et al. (7) have previously reported that the airways of preterm infants with severe BPD are marked by distinct dysbiosis with relative increased abundance of Gammaproteobacteria and decreased Firmicutes, in particular the genus Lactobacillus (5). Multiple other studies have reported associations between airway microbial dysbiosis and BPD or severity of BPD (10). Exploring a variety of inputs connecting the airway microbiome to lung development, Lal and colleagues (6) have also shown that microbial dysbiosis may impact microRNA signaling in BPD. In subsequent microbial metagenomics analysis, Lal et al. (5) found that BPD-predisposed infants had elevated concentrations of metabolites involved in fatty acid activation, estrogen, and androgen biosynthesis, thus suggesting that the airway microbiome could alter lung development via alterations in metabolites and dysregulation of downstream signaling pathways. Taken together, these data suggest that the early airway microbiome modulates exosomal content of miRNA and induces changes in multiple metabolites. Despite these studies demonstrating association between development of BPD and microbial dysbiosis, the direction of causality between airway injury during development and respiratory colonization by organisms remained unsettled (8).
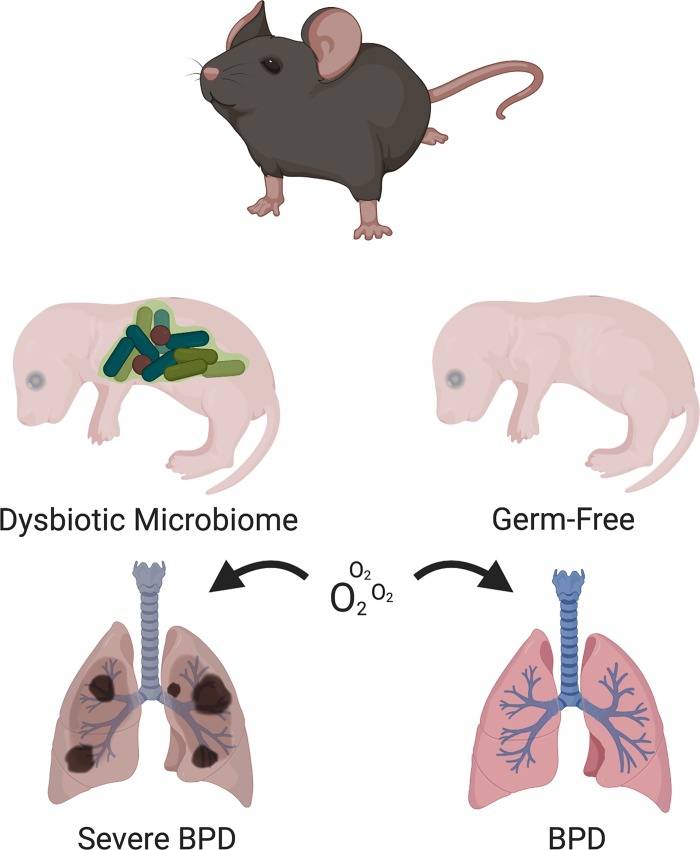
In this issue of American Journal of Physiology-Lung Cellular and Molecular Physiology, Lal and colleagues (2) developed a novel germ-free (GF) hyperoxia-exposed newborn mouse model to interrogate their hypothesis that GF mice, which are devoid of a microbiome, develop enhanced hyperoxia exposure-induced lung injury. In order to understand how the airway microbiome impacts airway development, analyzing an environment devoid of microbiome is an essential foundational step to define the pathogenic importance of the airway microbiome to the development of the mouse lung. This is the first report of the impact of the absence of a respiratory microbiome on normal and abnormal lung development in a mouse model. This study indicates that GF mice have similar lung development and function in room air to specific pathogen free (SPF) mice, which have an intact microbiome. However, contrary to their hypothesis, the authors found that neonatal hyperoxia exposure in mice with a microbiome resulted in worse alveolar hypoplasia and a greater impairment of lung mechanics as compared with GF mice. Decreased markers of pulmonary inflammation [myeloperoxidase (MPO), IFN-γ, and IL-1β] were also seen in GF animals as compared with SPF animals with hyperoxia exposure. Overall, the lung architecture of GF mice upon exposure to hyperoxia was relatively protected compared with SPF mice (Fig. 1). These findings are consistent with the group’s previous human neonatal airway microbiome study where they found increased granulocytic activity and a dysbiotic airway microbiome with a Proteobacterial preponderance in severe BPD patients (7). While some caution should be exhibited due to the intrinsic immune disruption in GF mice (11), these results make us wonder if the presence of pathogenic microbiota in SPF and humanized mice may potentiate the proinflammatory cascade in hyperoxia, thus leading to a worse phenotype as compared with GF animals. In the absence of a microbiome, GF animals may not mount a sufficient inflammatory response and therefore may exhibit phenotypic protection.
Fig. 1.
Germ-free mice are partially protected against hyperoxia exposure-induced lung injury. [Figure made in BioRender.]
A major strength of this study is the comprehensive evaluation of lung structure and mechanics using multiple parameters in addition to echocardiography, cytokines, and MPO in a newborn GF mouse model. The gnotobiotic hyperoxia facility developed for this study was a significant technical feat, which facilitated the maintaining continuous microbe-free conditions in the lungs during the postnatal period while providing a hyperoxic environment to induce BPD. A limitation of this study, and that of the traditional newborn mouse hyperoxia model in general, is the lack of antenatal confounder effects, such as chorioamnionitis, fetal growth restriction, and steroid use. Furthermore, evaluation of maternal and neonatal antibiotic exposure and the role of prenatal/maternal microbiome in postnatal lung development is lacking and would make excellent next steps.
This report sets the stage for future studies evaluating the complex processes involved in examining how the microbiome affects lung development and injury. To overcome the intrinsic immune disruption of GF mice (11), it will be important to build on this current study by using gnotobiotic mice, which have a defined microbiome amenable to systematic manipulation, to assess the therapeutic potential of altering the airway microbiome composition.
GRANTS
This work was supported in part by the Marshall Klaus Award from American Academy of Pediatrics and by the Fellow’s Basic Research Award from the Society for Pediatric Research to K. A. Willis. This work was also supported by grants from NIH to S. A. Cormier (R01AI090059, R01ES015050, and P42ES013648).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.W. prepared figure; K.A.W. drafted manuscript; K.A.W., J.F.P., S.A.C., and A.J.T. edited and revised manuscript; K.A.W., J.F.P., S.A.C., and A.J.T. approved final version of manuscript.
REFERENCES
- 1.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, Investigators C, Mohn WW, Turvey SE, Finlay BB. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7: 307ra152 307ra152, 2015. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 2.Dolma K, Freeman AE, Rezonzew G, Payne GA, Xu X, Jilling T, Blalock JE, Gaggar A, Ambalavanan N, Lal CV. Effects of hyperoxia on alveolar and pulmonary vascular development in germ-free mice. Am J Physiol Lung Cell Mol Physiol. In press. doi: 10.1152/ajplung.00316.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Lung Cell Mol Physiol 309: L1047–L1055, 2015. doi: 10.1152/ajplung.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiley JP, Caler EV. The lung microbiome. A new frontier in pulmonary medicine. Ann Am Thorac Soc 11, Suppl 1: S66–S70, 2014. doi: 10.1513/AnnalsATS.201308-285MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal CV, Kandasamy J, Dolma K, Ramani M, Kumar R, Wilson L, Aghai Z, Barnes S, Blalock JE, Gaggar A, Bhandari V, Ambalavanan N. Early airway microbial metagenomic and metabolomic signatures are associated with development of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 315: L810–L815, 2018. doi: 10.1152/ajplung.00085.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lal CV, Olave N, Travers C, Rezonzew G, Dolma K, Simpson A, Halloran B, Aghai Z, Das P, Sharma N, Xu X, Genschmer K, Russell D, Szul T, Yi N, Blalock JE, Gaggar A, Bhandari V, Ambalavanan N. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 3: e93994, 2018. doi: 10.1172/jci.insight.93994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B, Carlo WA, Keeley J, Rezonzew G, Kumar R, Morrow C, Bhandari V, Ambalavanan N. The Airway Microbiome at Birth. Sci Rep 6: 31023, 2016. doi: 10.1038/srep31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lignelli E, Palumbo F, Myti D, Morty RE. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol ajplung.00369.2019, 2019. doi: 10.1152/ajplung.00369.2019. [DOI] [PubMed] [Google Scholar]
- 9.Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res 76: 294–301, 2014. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 10.Pammi M, Lal C, Wagner BD, Mourani PM, Lohmann P, Luna R, Sisson A, Shivanna B, Hollister EB, Abman SH, Versalovic J, Connett GJ, Bhandari V, Ambalavanan N. Airway microbiome and development of bronchopulmonary dysplasia in preterm infants: a systematic review. J Pediatr 204: 126–133.e2, 2019. doi: 10.1016/j.jpeds.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Round JL, Palm NW. Causal effects of the microbiota on immune-mediated diseases. Sci Immunol 3: eaao1603, 2018. doi: 10.1126/sciimmunol.aao1603. [DOI] [PubMed] [Google Scholar]
- 12.Willis KA, Purvis JH, Myers ED, Aziz MM, Karabayir I, Gomes CK, Peters BM, Akbilgic O, Talati AJ, Pierre JF. Fungi form interkingdom microbial communities in the primordial human gut that develop with gestational age. FASEB J 33: 12825–12837, 2019. doi: 10.1096/fj.201901436RR. [DOI] [PMC free article] [PubMed] [Google Scholar]