Abstract
Idiopathic pulmonary fibrosis (IPF) is characterized by a profound remodeling of the collagen in the extracellular matrix (ECM), where the fibers become both denser and more highly aligned. However, it is unknown how this reconfiguration of the collagen matrix affects disease progression. Here, we investigate the role of specific alterations in collagen fiber organization on cell migration dynamics by using biomimetic image-based collagen scaffolds representing normal and fibrotic lung, where the designs are derived directly from high-resolution second harmonic generation microscopy images. The scaffolds are fabricated by multiphoton-excited (MPE) polymerization, where the process is akin to three-dimensional printing, except that it is performed at much greater resolution (∼0.5 microns) and with collagen and collagen analogs. These scaffolds were seeded with early passaged primary human normal and IPF fibroblasts to enable the decoupling of the effect of cell-intrinsic characteristics (normal vs. IPF) versus ECM structure (normal vs. IPF) on migration dynamics. We found that the highly aligned IPF collagen structure promoted enhanced cell elongation and F-actin alignment along with increased cell migration speed and straightness relative to the normal tissues. Collectively, the data are consistent with an enhanced contact guidance mechanism on the aligned IPF matrix. Although cell intrinsic effects were observed, the aligned collagen matrix morphology had a larger effect on these metrics. Importantly, these biomimetic models of the lung cannot be synthesized by conventional fabrication methods. We suggest that the MPE image-based fabrication method will enable additional hypothesis-based testing studies of cell-matrix interactions in the context of tissue fibrosis.
Keywords: biomimetic, collagen fibers, migration, multiphoton excitation
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF), a devastating disorder that kills approximately 40,000 Americans each year, is characterized by deposition of a dense, aberrantly organized collagen matrix. It is well established that alterations in the biomechanical characteristics of fibrotic extracellular matrix (ECM; including IPF ECM), such as increased tissue stiffness, can modify availability of profibrotic growth factors, cell behaviors, and phenotype determination (14, 16, 26, 29, 34, 44). Thus, the ECM and its cellular constituents can be critical drivers of fibrotic progression.
In addition to the biomechanical features of profibrotic ECM, the composition of fibrillar structural proteins that constitute the IPF matrix provide biochemical cues that are important in cell fate determination (30, 45, 56). Fibrillar collagens are the primary components of the newly deposited, abnormal ECM in IPF (7, 23, 28, 41). New collagen deposition requires cell-mediated assembly of a nascent FN matrix (24, 47, 54) and is then post-translationally cross-linked by tissue transglutaminases and lysyl oxidases (27). Integrin-mediated interactions with the collagen matrix can modulate fibroblast functions, including FN matrix assembly (54), proliferative capacity (56), and survival, whereas cell-autonomous differences in IPF cells have been observed (31, 32). Although derangements in collagen matrix assembly, accumulation, and organization are key features of the IPF, there is surprisingly little quantitative, definitive information on how collagen organization may contribute to the underlying cell-matrix interactions and pathogenesis of this disorder.
We previously utilized the collagen-specific optical microscopy technique of second harmonic generation (SHG) imaging to quantitatively assess nano/microscale differences between the organization of the collagen stroma in IPF versus nonfibrotic lung (53). Overall, the IPF collagen is denser and comprised of more highly aligned wavy fibers than in the normal matrix, where the fibers are straighter and less oriented (43). Cells are able to recognize three-dimensional (3D) spatial and biochemical domains at these size scales (4, 9, 18, 46, 52, 55), but it has so far been difficult to conduct specific experiments in vitro to identify the importance of normal and altered collagen organization in promoting these dynamics. This is because the most biomimetic scaffolds need to be comprised of collagen (and/or analogs) with 3D features of submicron dimensions to recapitulate the fibrillar structure. Conventional fabrication techniques often used in tissue engineering lack all these attributes. For example, well-established methods (57) such as electrospinning and soft and hard lithographies all have limitations in spatial and/or materials control and cannot reproduce the complex fiber morphology found in lung. Collagen gel models have been useful tools for studying ECM alterations in cancer (40), but there is limited control in the structure beyond the average distribution of fiber sizes and density; i.e., user-defined 3D morphologies are not achievable. Three-dimensional tissues generated by decellularization can provide topographical, mechanical, and biochemical cues (3). However, these structures cannot be readily altered for hypothesis testing of matrix conditions such as collagen morphology and matrix stiffness, and the process can have deleterious effects on the tissue structure.
To overcome these limitations, we have developed and characterized multiphoton-excited (MPE) fabrication. The methodology is analogous to multiphoton-excited fluorescence microscopy that obtains 3D resolved images, but here MPE employs photochemistry to create cross-linked structures. Due to intrinsic 3D control, the method is truly freeform and is compatible with use of any protein in solution as the starting scaffold materials (38, 49). MPE fabrication can be considered a form of 3D printing; however, the minimum feature sizes are much smaller (0.5 vs. 50 μm). We have previously characterized the instrumentation and methodology for the approach (2, 38, 39). Using this method, we have cross-linked collagen into 3D structures with lateral and axial features on the order of 300 and 700 nm, respectively (49). These resolutions are ideal for creating models of the fibrillar ECM, as they correspond to the size scales of collagen fiber diameters to which cells relate during adhesion and migration. Through detailed analysis of morphology and cytoskeletal architecture, we have shown how topography and ECM cues, together and separately, affect the morphology, migration, and proliferation of fibroblasts, ovarian cancer cells, and mesenchymal stem cells (MSCs) (1, 10, 37, 50). We have expanded the capabilities of the method to create scaffolds directly from SHG or fluorescence microscopy images (2), such that essentially any fibrillar pattern can be reproduced from the corresponding protein. This approach affords the ability to hypothesis test the role of the cell phenotype and matrix morphology on cell motile behaviors.
In this study, we apply this image-based approach to create models that faithfully recapitulate the nano/microstructured collagen topography observed in normal and IPF lung. Using these scaffolds, we have examined the effect of aberrant collagen organization in modifying fibroblast behavior relevant to the progression of fibrosis. In comparing the behavior of normal and IPF fibroblasts in these models, we found some intrinsic cell differences; however, the resulting cell shape, migration dynamics, and actin cytoskeletal alignment are governed largely by the highly aligned fibrotic matrix.
MATERIALS AND METHODS
Cell culture.
Deidentified tissue samples of normal (nonfibrotic) and fibrotic (IPF) lungs were obtained from thoracic surgical resection specimens (nonfibrotic) or transplantation explants (IPF) at the Carbone Cancer Center Translational Science BioCore at the University of Wisconsin-Madison via Institutional Review Board approval. The normal tissues were from pathologist-defined normal adjacent tissue from biopsies of patients without fibrotic lung disease.
Human lung fibroblasts (HLF) were isolated as described previously (8, 13). To obtain normal (nonfibrotic) fibroblasts, we utilized lobectomy or biopsy specimens from patients undergoing lung resection and who did not have interstitial lung disease. All specimens were examined by a pathologist to confirm that no underlying lung disease (emphysema, idiopathic lung diseases, etc.) was present on histology.
Briefly, to isolate fibroblasts, tissue specimens were placed in DMEM with 100 U/mL streptomycin, 250 ng/mL amphotericin B, 100 U/mL penicillin, and 10 µg/mL ciprofloxacin. Alveolated lung tissue was minced and plated onto 10-cm plates in Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin, 2 mM l-glutamine, 10 µg/mL ciprofloxacin, and 10% fetal bovine serum (FBS) using an aseptic technique. Expanded populations of fibroblasts were subsequently subcultured after 4–5 days, resulting in the development of a homogenous fibroblast population. Trypsin-Versene was used to detach cells from the culture plate for counting or reseeding the cell lines. For all experiments, subcultures were used from passages 2 to 5.
Scaffold models and photochemistry.
Scaffold designs are based upon SHG microscopy images of collagen in normal and IPF lung tissues (53). Due to overlapping fibers within the focal volume, image processing techniques such as filtering and discretizing are applied to create the final model. The laser-scanning fabrication instrument has been described in detail previously (2, 49). Briefly, a femtosecond ti:sapphire laser (Coherent MIRA, Coherent, Santa Clara, CA) is coupled to an upright microscope. The laser scanning for patterning is controlled by LabVIEW software (available upon request). We termed the scanning approach modulated raster scanning, where the scanning galvos are scanned at their maximum rate, and then the intensity at each pixel is controlled by a high-speed electro-optic modulator (Conoptics, Danbury, CT). Specifically, the grayscale level in the SHG image is linearly related to the “open” time at each pixel, where for example, if the grayscale values are 0 or 256 (for 8 bits), then the pixel open time would be 0 or 100%, respectively, with a linear relationship for all intermediate values. We have shown that this approach better replicates fibrillar structure than other scanning methods (2). For example, stl-based models commonly used in conventional 3D printing are not readily capable of reproducing the highly spatially varying fibrillar collagen patterns.
The scaffolds are created from a mixture of 75% gelatin methacrylate (GelMA) (33) and 24% rat tail collagen I (vol/vol); 100% collagen is not compatible with our available photochemistries, and GelMA is widely used in collagen scaffolds (21). The collagen mixture was cross-linked via two-photon-excited photochemistry. Here, we use the water-soluble Irgacure sodium 4-[2-(4-morpholino)benzoyl-2-dimethylamino]-butylbenzenesulfonate (MBS) (22), where the two-photon excitation wavelength is 760 nm. This photoactivator is highly biocompatible, as it breaks down into the fragments in the photochemical reaction, and normal cell adhesion and proliferation is observed.
Sample preparation.
The slides were prepared first with a rubber hybridization chamber secured to a silanized microscope slide (37). A monolayer of 30 mg/mL bovine serum albumin (BSA) formed on the slide before the final collagen solution was applied. The collagen solution used was a 1:3 ratio (25 µL of collagen and 75 µL of GelMA). The GelMA and collagen were kept strictly below 40°C. Scaffolds were kept in phosphate-buffered solution (PBS) until cell seeding. The dimensions of a single pattern were each 100 × 100 × 8 µm, where these were then tiled together for larger areas for cell migration assays.
Prior to cell seeding, the devices were sterilized by washing with 1× PBS containing 100 U/mL penicillin-streptomycin (Invitrogen). All the cell lines were seeded at density of 35K cell/mL and incubated overnight in 10% FBS to afford cell attachment and spreading before imaging. Culture dishes containing fabricated structures and cells were flooded, with their respective media supplemented with penicillin-streptomycin in advance of time-lapse imaging of migration.
Time-lapse imaging.
The fibroblast and IPF cells were seeded on separate normal or diseased patterns, giving four permutations: normal fibroblast on normal stroma, normal fibroblast on IPF stroma, IPF on normal stroma, and IPF on IPF stroma, with four separate models for both IPF and normal scaffolds. This affords decoupling of the roles of cell phenotype from matrix morphology on the resulting cell dynamics. Cells were imaged by phase contrast microscopy (10×, 0.5 NA; Nikon Eclipse Ti inverted microscope) under live-cell imaging conditions (Pathology Devices; constant 37°C, 5% CO2, humidification). Images were captured every 30 min over 72 h. Cells became overly crowded at longer durations, precluding analyses of single cell-matrix interactions. There were at least four independent runs for all combinations with different cell batches.
F-actin staining.
For analysis of the distribution of actin stress fibers, fibroblasts were grown on fabricated structures between 16 and 24 h and then fixed with 4% paraformaldehyde in PBS for 15 min. Following two washes with 1× PBS, the cells were permeabilized with 0.3% Triton X-100 for 10 min and stained with Texas Red conjugated phalloidin for 30 min. Fluorescent images were collected with a sensitive charge-coupled device camera (QImaging R2000R) using a 40× 0.8NA water immersion lens. Three sets of measurements were made for each cell/scaffold combination, with eight to 12 cells analyzed for each scaffold.
Data analysis and statistical analysis.
The image stacks from each time lapse were analyzed with Imaris software (Bitplane) to determine instantaneous velocities and motility attributes. The stacks were first processed with ImageJ using the Enhance Local Contrast (CLAHE) macro before importing to Imaris software. The cells were manually tracked to plot cell migration distance, speed, and directionality and calculate straightness. Cell shape characteristics were determined with ImageJ software. Analysis of these data was run via Origin 2015 (OriginLab) to determine statistical significance with ANOVA and two-sample t-test analysis. Origin 2015 was also used to perform ANOVA and two-sample t-tests for the cell shape analyses.
CurveAlign (ImageJ plugin) was used to obtain a distribution of F-actin angles within stained images of fibroblasts for the four cell/matrix combinations. This algorithm is based on the curvelet transform and extracts fiber angles relative to a fixed axis. These distributions were converted to radial plots by Oriana software (Kovach computing services), and statistical differences were analyzed by the Watson U2 tests. The alignment parameter κ was further used to characterize the distribution, where this is extracted from the probability density function of the angle distribution, and higher values represent greater alignment (19).
RESULTS
Characterization of MPE-fabricated collagen stroma.
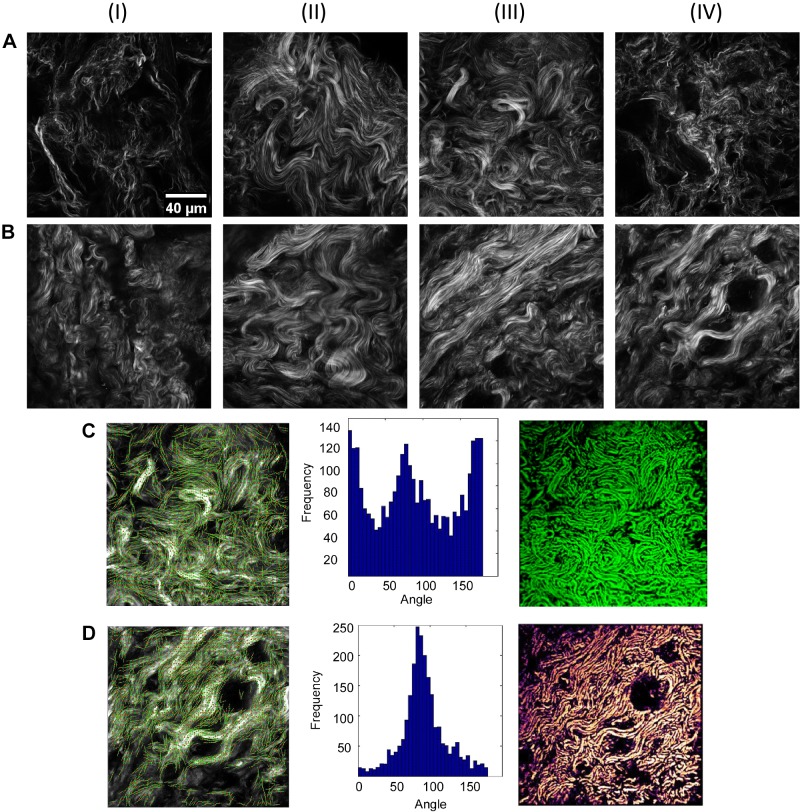
We used an existing library of SHG images of normal and fibrotic lung to serve as design “blueprints” for the MPE-fabricated models. Randomly selected images to be used for the designs are shown in Fig. 1, A and B. We previously showed that these were morphologically distinct by machine learning-based texture analysis (53). In this work, we examined six normal and three IPF lungs, where the total numbers of single images for each were 495 and 270, respectively. There were classified at >95% accuracy using two-dimensional wavelets and principle component analysis. Here, we note that, within the 100 × 100 μm field of view, representative images from normal lung tissue contained a relatively heterogeneous distribution of fiber angles In contrast, wavier collagen fibers within IPF tissue appeared to co-align to a large degree within each field of view.
Fig. 1.
Second harmonic generation (SHG) images of normal (A) and idiopathic pulmonary fibrosis (B) lung extracellular matrix used as the design blueprints. As examples, C and D show the design process for AIII and BIV, respectively, where the marked identified fibers are given in the left column, the extracted fiber angle distributions from CurveAlign analysis are given in the middle column, and the two-photon-excited fluorescence images of the multiphoton-excited fabricated models are given in the right column. Field sizes of the images = 100 × 100 μm.
We note that precise features in these images are difficult to identify. This is because the SHG imaging was performed on tissue sections that are thicker (∼100 μm) than that of a typical histological slide (5–10 μm). As a result, there is likely spatial heterogeneity within the samples that we imaged, with many of the SHG images from parenchymal areas of the lung that have limited/modest amounts of readily discernable alveoli (although in some cases, alveoli are identifiable, e.g., Fig. 1, AI and AIV). This may be due to relative compression of the tissue or the presence of collagen that is more adjacent to airways/vasculature. Despite this limitation, our previous work has shown that a classifier based on a machine-learning approach was highly accurate in classifying normal collagen from IPF-derived collagen in the same thickness of tissue without manual selection of visually discernable structures (53).
To design the blueprints for fabrication from these images, we first discretized the images using the eigenvalues of the Hessian matrix, where this is necessary because there are overlapping fibers within the focal volume. We then extracted the collagen fiber angle distribution with the SHG images by the ImageJ plugin CurveAlign. An example from normal (Fig. 1AIII) and IPF (Fig. 1BIV) tissue with the corresponding delineated collagen angles overlapped with the collagen fibers is shown in the left-hand column of Fig. 1, C and D, respectively, and the respective histogram is shown in the corresponding middle columns. The resulting fabricated normal and IPF scaffolds (right-hand column, Fig. 1, C and D) were imaged via two-photon-excited fluorescence, where the contrast is from added rhodamine B. Comparative images show that there is a high degree of concordance between SHG images of the lung tissue with that of the corresponding MPE-fabricated collagen structure. We quantify the concordance in terms of fidelity, which we define as the both pixel co-localization as well as the respective grayscale level. We obtained fidelities of ≥90% between the discretized model based on the SHG image and the fluorescence of the scaffold. This is similar to our previous efforts on other tissues (2). This entire process was repeated for the other images in Fig. 1, A and B. The similarity between the design and resulting scaffold justifies the use of these scaffolds as biomimetic models of normal and fibrotic lung.
Migration dynamics.
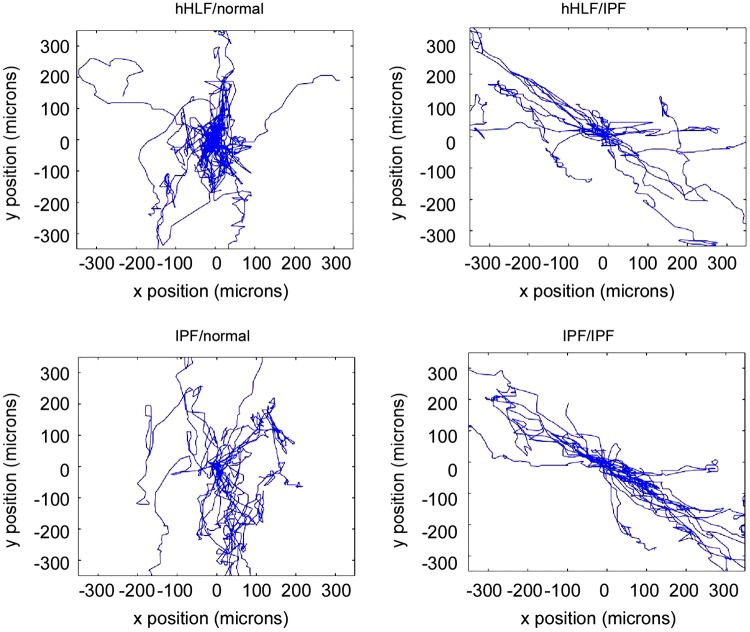
After creating a library of fabricated collagen stromal models, we then examined how this topography influenced cell-matrix interactions of primary cultures of adult human lung fibroblasts. Specifically, we examined the migratory behavior of both normal human lung fibroblasts (nHLF)- and IPF-derived fibroblasts on both normal and IPF models (4 combinations). This allows decoupling of the role of cell phenotype and matrix morphology on the resulting cell migration dynamics. For each cell/scaffold combination, we observed the migration trajectories from time lapse imaging and plotted these in the x-y plane. Superimposed migration trajectories for ∼20 cells for each of the combinations over 72 h are shown in Fig. 2.
Fig. 2.
Migration trajectories for each cell/collagen type combination: normal human lung fibroblasts (nHLF) on normal stroma; nHLF on idiopathic pulmonary fibrosis (IPF) stroma; IPF-derived fibroblasts on normal stroma; and IPF-derived fibroblasts on IPF stroma, where the results of ∼20 cells are shown for each case.
These data show that the migration pattern of nHLF on the normal model appears constrained, staying close to the original location and with random migration directionality on the fibers. Conversely, the nHLF cells migrated on the IPF model primarily along a well-defined axis. The IPF/IPF combination appeared to have the longest trajectories, whereas the IPF fibroblasts had longer displacements on the normal model than the nHLF cells with fairly random directionality. These results suggest that the trajectories are governed more by the fiber pattern than the cell phenotype.
We quantified these observations by assessing the straightness of the migration path (Imaris software) for the four combinations (Fig. 3A). Straightness is related to persistence, as it is sometimes used as a measure of trajectory, where this is defined by the ratio of straight line of flight distance traveled over total distance traveled. Cumulative cell numbers from at least four completely independent trails (and multiple wells) for these quantitative analyses were between 100 and 200. The straightness of migration was almost exclusively dependent on the type of fabricated collagen stroma on which the fibroblasts were plated (normal or IPF), with little influence from the fibroblast type (nHLF or IPF derived). Specifically, there was a significant increase in straightness (P < 0.05) seen on IPF collagen patterns relative to the normal matrix for both the IPF and nHLF fibroblasts, which were not statistically different from each other. In contrast, the straightness for both cell types on the normal matrix was lower than on the IPF matrix (P < 0.05).
Fig. 3.
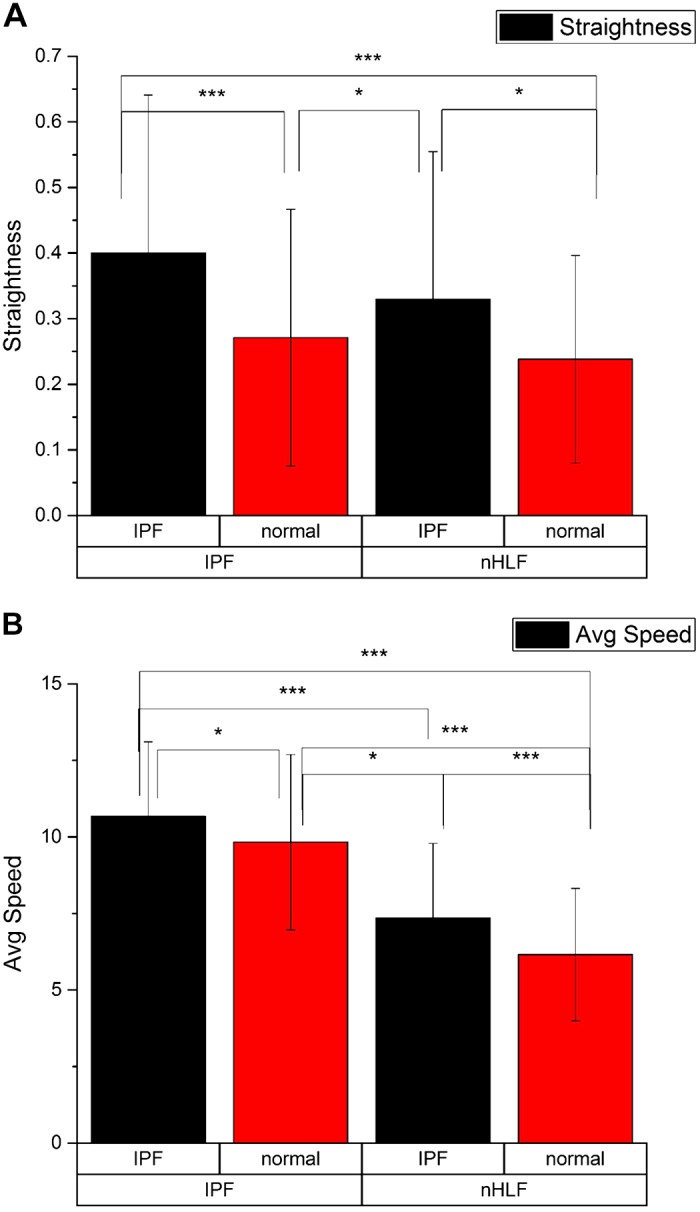
Straightness of cell migration (A) and average speed (μm/h) (B) for the four combinations. The bottom row and top row of the x-axis labels correspond to the cell type and matrix model, respectively. *P < 0.05; ***P < 0.001. Cumulative cell numbers in the analyses were between 100 and 200 with at least three independent experiments with multiple wells for each combination.
We further measured the instantaneous velocities for the four combinations (Fig. 3B). First, we note that the IPF fibroblasts migrated faster than normal fibroblasts for both matrix types (P < 0.001). In addition, the IPF fibroblasts migrated faster on the IPF scaffold than on the normal matrix, showing that the aligned fibers further promoted migration. However, both nHLF and IPF fibroblasts migrated faster (P < 0.05) on the highly aligned IPF collagen scaffold than on the normal lung scaffold, suggesting an added influence from the collagen configuration. Taken together, these data indicate that the altered, more aligned collagen matrix seen in IPF exerts a large influence on migration directionality but that cell-intrinsic differences may exist vis-à-vis the migration speed on these matrices.
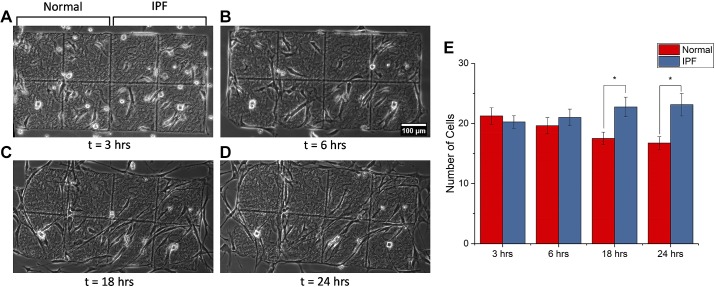
We can also question the consequences of the enhanced migration. To investigate this, as a first step, we created scaffolds comprised of both normal and IPF regions to determine whether the latter promotes migratory ingrowth of IPF fibroblasts. The data are given in Fig. 4, which shows representative phase contrast images over 24 h (Fig. 4, A–D) and then plots the number of cells on both regions at these time points (Fig. 4E). We found that the IPF morphology does preferentially promote migration into these locations.
Fig. 4.
Examination of migration of idiopathic pulmonary fibrosis (IPF) fibroblasts on mixed scaffolds having both IPF and normal regions. A–D: representative phase contrast images at different time points. Scale bar, 100 μm. E: number of cells at each location at each time point, where P < 0.05 (*).
Cell morphology.
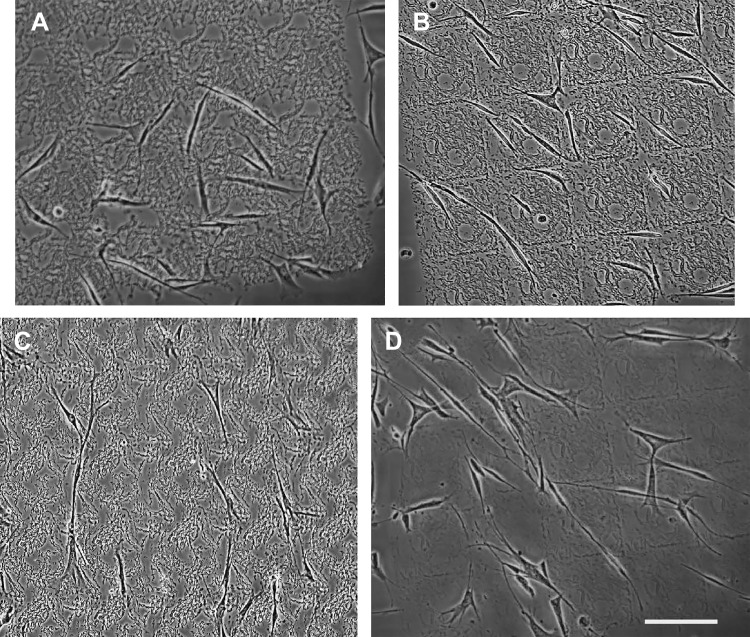
Because ECM composition is known to exert a strong influence on cell morphology and phenotype, we next examined the role of the collagen stromal architecture of normal and IPF scaffolds on the resulting fibroblast shape. Early-passage primary cultures of lung fibroblasts were allowed to adhere and spread for ∼12 h on the respective collagen scaffolds (representative phase contrast images are shown in Fig. 5). Maximum length and spread area of each of the four combinations were determined via Image J and are shown with statistical differences in Fig. 6. Interestingly, spread area was highly dependent on the interaction between cell type and collagen scaffold, with IPF lung fibroblasts on IPF collagen scaffold combination (Fig. 6B) having the largest spread area of the four combinations (P < 0.05). In contrast, nHLF (Fig. 6, A and B) consistently displayed lower spread areas (P < 0.001). Although there was a statistically significant increase (P < 0.05) in spread area of IPF fibroblasts compared with nHLF cells on the normal collagen scaffold, the magnitude of this effect was small. Although the maximum cell length showed a strong interaction between IPF fibroblasts and IPF collagen scaffolds (resulting in the longest cells, P < 0.05), there was a more prominent influence (P < 0.05) on cell length by scaffold composition that spanned either cell type (IPF or nHLF). Conversely, IPF fibroblasts retained a significant increase in cell length compared with nHLF on the normal collagen scaffold (P < 0.05). In total, this suggested that cell length was similarly influenced by cell type and collagen scaffold composition.
Fig. 5.
Representative phase-contrast images used for the cell shape analyses. A: normal human lung fibroblasts (nHLF) on normal matrix. B: nHLF on idiopathic pulmonary fibrosis (IPF) matrix. C: IPF fibroblasts on normal matrix. D: IPF fibroblasts on IPF matrix. Scale bar, 100 μm.
Fig. 6.

Resulting maximum length (A) and spread area (B) for the four cell/model combinations, where the bottom row and top row of the x-axis labels correspond to the cell type and matrix model, respectively. *P < 0.05; ***P < 0.001.
To begin to investigate the molecular origin of the shape changes, we performed the experiments using ROCK inhibition (iY27632 at 10 μM). This inhibition should result in further spreading and polarization, as ROCK itself enhances contraction. To best show the effects of polarization, we used the metrics of alignment of cells relative to the fiber axis and circularity. The alignment data for IPF fibroblasts on IPF and normal matrix are shown in Fig. 7, A and B, respectively. After 6 h of adhesion (sufficient time to attach and spread) on the IPF matrix, we found that the cells were more polarized (where a lower angle corresponds to higher alignment) with ROCK inhibition. A similar trend at these time points was observed on the normal matrix, but the differences were not significant. The circularity data were similar where cells were more elliptical following ROCK inhibition, with a more pronounced effect on the IPF matrix (not shown).
Fig. 7.
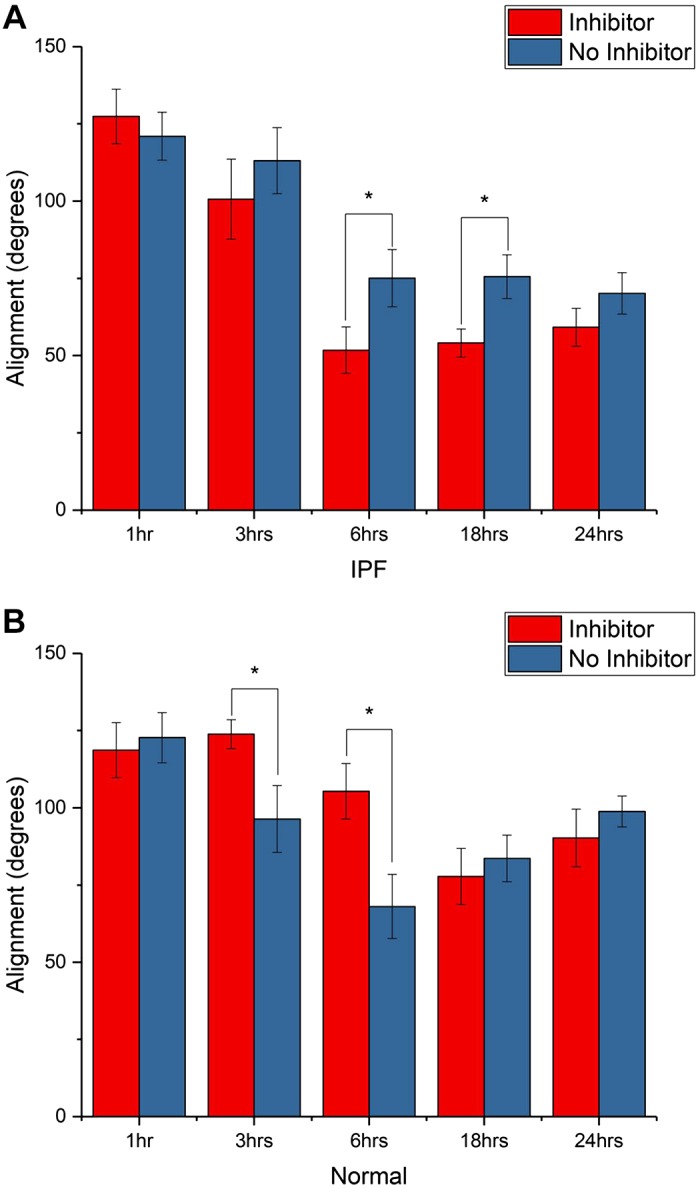
Alignment of idiopathic pulmonary fibrosis (IPF) fibroblasts with and without ROCK inhibitor in IPF (A) and normal (B) matrix. The inhibitor resulted in greater spreading (after 6 h of attachment) for both scaffolds but was significant for the IPF scaffold, where P < 0.05 (*).
Actin cytoskeleton staining.
We determined the radial distribution of actin stress fibers as further analysis of the cell response to the matrix morphology. Here, cells were fixed following time lapse imaging and stained for F-actin (Texas Red-conjugated phalloidin). The fluorescence images were analyzed to compare the radial distribution of stress fibers in each of the four cell-matrix combinations, where these are shown in Fig. 8, A–D. The Watson’s U2 test was used to determine the statistical significances between the four combinations, and these are tabulated in Table 1. Cells plated on normal collagen scaffold had a broad distribution of actin filament orientations, whereas there was a narrower range of orientations within cells on IPF collagen scaffold. Specifically, normal fibroblasts had a narrower distribution of stress fibers on the IPF versus normal matrix (P < 0.005). The latter was also broader than the IPF/IPF combination (P < 0.005). These differences may reflect the linearization of cell orientation on IPF matrix (Fig. 6). Moreover, there appeared to be little cell-intrinsic differences in F-actin orientation (Fig. 8), where neither the nHLF and IPF cells on IPF matrix nor nHLF and IPF on normal matrix were different. Thus, these data suggest that the collagen fiber alignment is the primary determinant of the angular distribution of actin stress fibers.
Fig. 8.
Radial distribution of F-actin stress fibers of normal human lung fibroblasts (nHLF) on normal (A) and idiopathic pulmonary fibrosis (IPF) (B) matrix, where κ = 2.85 and κ = 1.74, respectively. Corresponding data for IPF fibroblasts on normal (C) and IPF (D) matrix, where κ = 2.13 and κ = 1.93, respectively.
Table 1.
Watson U2 statistics for angular distributions
| HLF on IPF | HLF on Normal | IPF on IPF | IPF on Normal | |
|---|---|---|---|---|
| HLF on IPF | <0.005 | 0.5 > P > 0.2 | <0.01 | |
| HLF on normal | 0.344 | <0.005 | 0.5 > P > 0.2 | |
| IPF on IPF | 0.124 | 0.344 | <0.02 | |
| IPF on normal | 0.296 | 0.09 | 0.24 |
HLF, human lung fibroblasts; IPF, idiopathic pulmonary fibrosis.
We further quantify the distribution and alignment through the κ-parameter from the radial probability distribution function, where higher values correspond to greater alignment (1, 19). Although there are some fluctuations, the widest distribution (least aligned) was for the nHLF/normal combination (κ = 1.74), whereas the nHLF had the greatest alignment (2.85). This analysis further shows the that collagen fiber alignment largely governs the angular distribution of actin stress fibers.
DISCUSSION
In this study, we have utilized MPE-based fabrication to construct scaffolds that faithfully recapitulate the collagen topography of normal and fibrotic lung. The models were based directly on SHG images of the collagen fiber structure, where this modality is ideal, as it can image through 3D tissues slices (approximately several hundreds of μm in thickness) and has better contrast and resolution than either bright-field or polarization microscopy (11). Specifically, the SHG images clearly reveal highly aligned, wavy fibers in the IPF tissue. We note that prior work using SE and TEM found aligned collagen fibrils in normal lung; however, we are examining cell function on much larger fiber size scales (more than tens of μm in length).
We show here that this image-based model approach is a feasible strategy to probe how the altered collagen fiber organization of fibrotic lung influences cell behavior. Specifically, our observations suggest that the highly aligned wavy collagen fibers of IPF lung strongly affect cell elongation (more elongated), migration speed (faster migration), and directionality (increased straightness). It is possible that these properties could enhance fibroblast influx to areas of remodeling lung, thereby propagating expansion of tissue fibrosis. As a model system, we created scaffolds containing normal and IPF regions that indeed exhibited this behavior (Fig. 4).
This work builds upon previous studies that have shown that artificial micrometer and nanoscale topographies influence cell migration patterns (17, 35). For example, Petroll et al. (36) observed that fibroblasts assumed an elongated morphology and tightly aligned with collagen matrix during the healing process in injured corneas, suggesting a tight link between collagen organization and fibroblast behavior during wound healing. Moreover, Kim et al. (20) described the influence of differential spacing of nanogrooved surfaces on dermal wound healing, suggesting that fibroblasts were sensitive to artificial nanotopography with respect to migration and directionality.
In concordance with the effect of collagen organization on cell motile behaviors, internal actin organization was highly dependent on collagen configuration. This suggests that adhesive structures and the associated actin cytoskeleton are likely involved in conforming the cell to the underlying collagen topography. Hamilton et al. (15) observed that the focal adhesions of fibroblasts plated on linear nanotopographical patterns were strongly aligned parallel to the nanogrooves, in contrast to the randomly dispersed orientations of focal adhesions on nonpatterned surfaces. However, their studies did not identify strong development of actin stress fibers, precluding analysis of their orientation. Our system, which uses biomimetic collagen topographies, was conducive to the formation of actin stress fibers aligned with the fibers, enabling the analysis of the resulting radial distribution.
Finally, we observed combined effects between cell-intrinsic properties and matrix cues with regard to cell spread area and migration speed. Migration speed was largely a cell-intrinsic phenomenon, with IPF cells exhibiting higher levels, which was analogous to our previous findings identifying increased migration speeds in ovarian cancer cells compared with normal epithelial cells (1, 10). In contrast, IPF cells have a highly spread phenotype only on IPF-derived collagen stromal patterns. This could indicate that not only does fibrotic matrix induce aberrant cell behaviors (increased migratory capacity and directionality), but normal matrix may constrain properties of IPF fibroblasts.
It is known that the IPF lung fibroblasts have cell-intrinsic differences in gene expression, growth rate, apoptotic potential, migratory capacity, and invasiveness (25, 34, 42, 51). However, in some cases, these features were identified in cells removed from their in vivo matrix context (cultured on tissue culture plastic), and thus the interaction between matrix dependent cues and these cell-intrinsic differences remains unknown. Our work begins to fill in this gap, suggesting that IPF fibroblast morphology is strikingly polarized when cultured on the more aligned diseased collagen scaffolds. This suggests that disruption of appropriate matrix cues and signaling derived from collagen topography could be an important way to modify the phenotype and perhaps the fibrogenic potential of IPF lung fibroblasts.
We can begin to question the underlying mechanism of this enhanced migration and cell polarization by examining ROCK inhibition. This resulted in greater spreading and alignment of IPF fibroblasts on both IPF and normal matrix, but the effect was significantly greater on the former, more aligned matrix. This observation, coupled with greater actin stress fiber alignment, increased focal adhesion expression (not shown), and enhanced migratory influx on IPF scaffolds, suggests that the enhanced migration aspects are due to a contact guidance mechanism. As will be detailed below, our system as of yet does not provide stiffness cues; thus a more aligned, polarized fiber morphology could be expected to reflect this behavior.
Although we are using a biomimetic system that allows us to probe the effect of collagen fiber morphology on lung fibroblast behavior, this system does not fully recapitulate the native ECM context. For example, GelMA is a necessary component of the fabricated scaffolds, but it is not completely reflective of the in vivo condition, because whereas GelMA is a widely used biomaterial, the integrin binding sites are different than that of triple helical collagen. We somewhat mitigate this by using 25% collagen in the mixture to provide the GFOGER binding sites (12). Furthermore, these thin scaffolds likely reflect the high stiffness of the underlying glass substrate, which is nonphysiological. However, this approach does allow us to directly isolate the role of different collagen morphology without combined effects of different stiffness. A further strength of our system is the use of human lung fibroblasts derived from either normal or IPF lung in these biomimetic contexts, thereby directly probing the behavior of a major effector cell for IPF pathogenesis.
We can put our work in the context of a recent study by Southern et al. (48), where they examined migration on ex vivo fibrotic (bleomycin) mouse lung. They found more random migration on fibrotic regions, seemingly in contradiction to our efforts here. However, importantly, they observed effects that may be highly related to underlying differences in both matrix stiffness and morphology between normal and fibrotic lung.
Further iterations of this overall approach by our group will seek to improve upon this system and address current limitations. For example, whereas MPE fabrication of collagen matrices recapitulates the patterning of in vivo collagen observed by SHG, it does not allow for the formation post-translational modifications, e.g., cross-linking, that could modify the behavior of fibroblasts in vivo. Thus, going forward, we will build models that incorporate underlying stiffness and further change the cross-link density in the collagen fibers (5, 6). Additionally, although we have directed this study to collagen morphology, as it is the largest component of the lung ECM, it is also possible to add additional components such as elastin and fibronectin.
In conclusion, we have utilized SHG imaging of IPF lung coupled with MPE-based fabrication to create highly biomimetic scaffolds to interrogate fibroblast behaviors in response to disordered collagen organization. We have found that highly aligned collagen topography in fibrotic lung has a strong influence on fibroblast morphology and motile behaviors that suggest an important role for collagen organization in modifying the development of lung fibrosis. The enhanced migration dynamics on the IPF matrix relative to the normal models are consistent with a contact guidance mechanism. Despite not completely recapitulating the lung ECM, the MPE method has significant advantages over more established techniques such as hard and soft lithographies in patterning in 3D and with collagen and collagen analogs. Importantly, this system represents a further step in the direction of recapitulating the in vivo cell-matrix conditions of IPF lung in an ex vivo experimental system, thereby enabling further studies that can probe how other matrix characteristics (biophysical, compositional) may modify lung fibroblast behavior, along with hypothesis-based testing of targeted interventions (including pharmacological) that may modify profibrotic fibroblast behavior.
GRANTS
This work was supported by NIH National Heart, Lung, and Blood Institute (NHLBI) Grant 1R21 HL126190-01a1 (to P. J. Campagnola and N. Sandbo), National Cancer Institute Grant R01 CA206561-01 (to P. J. Campagnola); NHLBI Grant HL136795 (to N. Sandbo), and the University of Wisconsin Comprehensive Cancer Center Grant P30 CA014520 (for the fibroblasts).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.C. conceived and designed research; M.T., S.A., H.-Y.C., J.L., and K.B. performed experiments; M.T., S.A., and H.-Y.C. analyzed data; N.S. and P.J.C. interpreted results of experiments; M.T., S.A., and P.J.C. prepared figures; M.T. drafted manuscript; N.S. and P.J.C. edited and revised manuscript; M.T., H.-Y.C., N.S., and P.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Wisconsin (UW) Carbone Cancer Center (Madison, WI) for use of its Shared Services for tissue acquisition to derive primary fibroblast cultures.
REFERENCES
- 1.Ajeti V, Lara-Santiago J, Alkmin S, Campagnola PJ. Ovarian and breast cancer migration dynamics on laminin and fibronectin bidirectional gradient fibers fabricated via multiphoton excited photochemistry. Cell Mol Bioeng 10: 295–311, 2017. doi: 10.1007/s12195-017-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajeti V, Lien CH, Chen SJ, Su PJ, Squirrell JM, Molinarolo KH, Lyons GE, Eliceiri KW, Ogle BM, Campagnola PJ. Image-inspired 3D multiphoton excited fabrication of extracellular matrix structures by modulated raster scanning. Opt Express 21: 25346–25355, 2013. doi: 10.1364/OE.21.025346. [DOI] [PubMed] [Google Scholar]
- 3.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13: 27–53, 2011. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey KE, Floren ML, D’Ovidio TJ, Lammers SR, Stenmark KR, Magin CM. Tissue-informed engineering strategies for modeling human pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 316: L303–L320, 2019. doi: 10.1152/ajplung.00353.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu S, Campagnola PJ. Properties of crosslinked protein matrices for tissue engineering applications synthesized by multiphoton excitation. J Biomed Mater Res A 71A: 359–368, 2004. doi: 10.1002/jbm.a.30175. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Wolgemuth CW, Campagnola PJ. Measurement of normal and anomalous diffusion of dyes within protein structures fabricated via multiphoton excited cross-linking. Biomacromolecules 5: 2347–2357, 2004. doi: 10.1021/bm049707u. [DOI] [PubMed] [Google Scholar]
- 7.Bateman ED, Turner-Warwick M, Adelmann-Grill BC. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax 36: 645–653, 1981. doi: 10.1136/thx.36.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernau K, Torr EE, Evans MD, Aoki JK, Ngam CR, Sandbo N. Tensin 1 is essential for myofibroblast differentiation and extracellular matrix formation. Am J Respir Cell Mol Biol 56: 465–476, 2017. doi: 10.1165/rcmb.2016-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia SN, Chen CS. Tissue engineering at the micro-scale. Biomed Microdev 2: 131–144, 1999. doi: 10.1023/A:1009949704750. [DOI] [Google Scholar]
- 10.Chen X, Brewer MA, Zou C, Campagnola PJ. Adhesion and migration of ovarian cancer cells on crosslinked laminin fibers nanofabricated by multiphoton excited photochemistry. Integr Biol 1: 469–476, 2009. doi: 10.1039/b906310b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7: 654–669, 2012. doi: 10.1038/nprot.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell 101: 47–56, 2000. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 13.Esnault S, Bernau K, Torr EE, Bochkov YA, Jarjour NN, Sandbo N. RNA-sequencing analysis of lung primary fibroblast response to eosinophil-degranulation products predicts downstream effects on inflammation, tissue remodeling and lipid metabolism. Respir Res 18: 188, 2017. doi: 10.1186/s12931-017-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268, 2006. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton DW, Oates CJ, Hasanzadeh A, Mittler S. Migration of periodontal ligament fibroblasts on nanometric topographical patterns: influence of filopodia and focal adhesions on contact guidance. PLoS One 5: e15129, 2010. doi: 10.1371/journal.pone.0015129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon H, Hidai H, Hwang DJ, Healy KE, Grigoropoulos CP. The effect of micronscale anisotropic cross patterns on fibroblast migration. Biomaterials 31: 4286–4295, 2010. doi: 10.1016/j.biomaterials.2010.01.103. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Directing cell migration with asymmetric micropatterns. Proc Natl Acad Sci USA 102: 975–978, 2005. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA 102: 15895–15900, 2005. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HN, Hong Y, Kim MS, Kim SM, Suh KY. Effect of orientation and density of nanotopography in dermal wound healing. Biomaterials 33: 8782–8792, 2012. doi: 10.1016/j.biomaterials.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 21.Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J, Melchels FPW. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol 34: 394–407, 2016. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima K, Ito M, Morishita H, Hayashi N. A novel water-soluble photoinitiator for the acrylic photopolymerization type resist system. Chem Mater 10: 3429–3433, 1998. doi: 10.1021/cm9801688. [DOI] [Google Scholar]
- 23.Laurent GJ, Cockerill P, McAnulty RJ, Hastings JR. A simplified method for quantitation of the relative amounts of type I and type III collagen in small tissue samples. Anal Biochem 113: 301–312, 1981. doi: 10.1016/0003-2697(81)90081-6. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Van Den Diepstraten C, D’Souza SJ, Chan BM, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol 163: 1045–1056, 2003. doi: 10.1016/S0002-9440(10)63464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63: 2304–2316, 2006. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madri JA, Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol 11: 353–366, 1980. doi: 10.1016/S0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol 21: 933–941, 2011. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med 177: 638–645, 2008. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nho RS, Hergert P, Kahm J, Jessurun J, Henke C. Pathological alteration of FoxO3a activity promotes idiopathic pulmonary fibrosis fibroblast proliferation on type i collagen matrix. Am J Pathol 179: 2420–2430, 2011. doi: 10.1016/j.ajpath.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nho RS, Peterson M, Hergert P, Henke CA. FoxO3a (Forkhead Box O3a) deficiency protects idiopathic pulmonary fibrosis (IPF) fibroblasts from type I polymerized collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas. PLoS One 8: e61017, 2013. doi: 10.1371/journal.pone.0061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31: 5536–5544, 2010. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635, 2014. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett 7: 2122–2128, 2007. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 36.Petroll WM, Kivanany PB, Hagenasr D, Graham EK. Corneal fibroblast migration patterns during intrastromal wound healing correlate with ECM structure and alignment. Invest Ophthalmol Vis Sci 56: 7352–7361, 2015. doi: 10.1167/iovs.15-17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pins GD, Bush KA, Cunningham LP, Campagnola PJ. Multiphoton excited fabricated nano and micropatterned extracellular matrix proteins direct cellular morphology. J Biomed Mater Res 78A: 194–204, 2006. doi: 10.1002/jbm.a.30680. [DOI] [PubMed] [Google Scholar]
- 38.Pitts JD, Campagnola PJ, Epling GA, Goodman SL. Submicron multiphoton free-form fabrication of proteins and polymers: studies of reaction efficiencies and applications in sustained release. Macromolecules 33: 1514–1523, 2000. doi: 10.1021/ma9910437. [DOI] [Google Scholar]
- 39.Pitts JD, Howell AR, Taboada R, Banerjee I, Wang J, Goodman SL, Campagnola PJ. New photoactivators for multiphoton excited three-dimensional submicron cross-linking of proteins: bovine serum albumin and type 1 collagen. Photochem Photobiol 76: 135–144, 2002. doi:. [DOI] [PubMed] [Google Scholar]
- 40.Provenzano PP, Eliceiri KW, Inman DR, Keely PJ. Engineering three-dimensional collagen matrices to provide contact guidance during 3D cell migration. Curr Protoc Cell Biol 47: 10.17.1–10.17.11, 2010. doi: 10.1002/0471143030.cb1017s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghu G, Striker LJ, Hudson LD, Striker GE. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis 131: 281–289, 1985. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 42.Ramos C, Montaño M, García-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24: 591–598, 2001. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 43.Saldiva PH, Delmonte VC, de Carvalho CR, Kairalla RA, Costa Auler JO Jr. Histochemical evaluation of lung collagen content in acute and chronic interstitial diseases. Chest 95: 953–957, 1989. doi: 10.1378/chest.95.5.953. [DOI] [PubMed] [Google Scholar]
- 44.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature 474: 343–349, 2011. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith LR, Cho S, Discher DE. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology (Bethesda) 33: 16–25, 2018. doi: 10.1152/physiol.00026.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sniadecki NJ, Desai RA, Ruiz SA, Chen CS. Nanotechnology for cell-substrate interactions. Ann Biomed Eng 34: 59–74, 2006. doi: 10.1007/s10439-005-9006-3. [DOI] [PubMed] [Google Scholar]
- 47.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 13: 3546–3559, 2002. doi: 10.1091/mbc.e02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Southern BD, Grove LM, Rahaman SO, Abraham S, Scheraga RG, Niese KA, Sun H, Herzog EL, Liu F, Tschumperlin DJ, Egelhoff TT, Rosenfeld SS, Olman MA. Matrix-driven myosin II mediates the pro-fibrotic fibroblast phenotype. J Biol Chem 291: 6083–6095, 2016. doi: 10.1074/jbc.M115.712380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sridhar M, Basu S, Scranton VL, Campagnola PJ. Construction of a laser scanning microscope for multiphoton excited optical fabrication. Rev Sci Instrum 74: 3474–3477, 2003. doi: 10.1063/1.1584079. [DOI] [Google Scholar]
- 50.Su PJ, Tran QA, Fong JJ, Eliceiri KW, Ogle BM, Campagnola PJ. Mesenchymal stem cell interactions with 3D ECM modules fabricated via multiphoton excited photochemistry. Biomacromolecules 13: 2917–2925, 2012. doi: 10.1021/bm300949k. [DOI] [PubMed] [Google Scholar]
- 51.Suganuma H, Sato A, Tamura R, Chida K. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 50: 984–989, 1995. doi: 10.1136/thx.50.9.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol (1985) 98: 1892–1899, 2005. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 53.Tilbury K, Hocker J, Wen BL, Sandbo N, Singh V, Campagnola PJ. Second harmonic generation microscopy analysis of extracellular matrix changes in human idiopathic pulmonary fibrosis. J Biomed Opt 19: 086014, 2014. doi: 10.1117/1.JBO.19.8.086014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torr EE, Ngam CR, Bernau K, Tomasini-Johansson B, Acton B, Sandbo N. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J Biol Chem 290: 6951–6961, 2015. doi: 10.1074/jbc.M114.606186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Wong Po Foo C, Warrier A, Poo MM, Heilshorn SC, Zhang X. Gradient lithography of engineered proteins to fabricate 2D and 3D cell culture microenvironments. Biomed Microdevices 11: 1127–1134, 2009. doi: 10.1007/s10544-009-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 279: 33024–33034, 2004. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 57.Zhang LJ, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4: 66–80, 2009. doi: 10.1016/j.nantod.2008.10.014. [DOI] [Google Scholar]