Abstract
TMEM16A (anoctamin 1) is an important calcium-activated chloride channel in airway smooth muscle (ASM). We have previously shown that TMEM16A antagonists such as benzbromarone relax ASM and have proposed TMEM16A antagonists as novel therapies for asthma treatment. However, TMEM16A is also expressed on airway epithelium, and TMEM16A agonists are being investigated as novel therapies for cystic fibrosis. There are theoretical concerns that agonism of TMEM16A on ASM could lead to bronchospasm, making them detrimental as airway therapeutics. The TMEM16A agonist Eact induced a significant contraction of human ASM and guinea pig tracheal rings in an ex vivo organ bath model. Pretreatment with two different TMEM16A antagonists, benzbromarone or T16Ainh-A01, completely attenuated these Eact-induced contractions. Pretreatment with Eact alone augmented the maximum acetylcholine contraction. Pretreatment of A/J mice in vivo with nebulized Eact caused an augmentation of methacholine-induced increases in airway resistance measured by the forced oscillatory technique (flexiVent). Pretreatment with the TMEM16A antagonist benzbromarone significantly attenuated methacholine-induced increases in airway resistance. In in vitro cellular studies, TMEM16A was found to be expressed more abundantly in ASM compared with epithelial cells in culture (8-fold higher in ASM). Eact caused an increase in intracellular calcium in human ASM cells that was completely attenuated by pretreatment with benzbromarone. Eact acutely depolarized the plasma membrane potential of ASM cells, which was attenuated by benzbromarone or nifedipine. The TMEM16A agonist Eact modulates ASM contraction in both ex vivo and in vivo models, suggesting that agonism of TMEM16A may lead to clinically relevant bronchospasm.
Keywords: ANO1, Eact, flexiVent, organ bath
INTRODUCTION
Calcium-activated chloride currents are important components of airway smooth muscle (ASM) contraction (17). In 2008, three separate laboratories discovered that calcium-activated chloride currents are mediated by the TMEM16 (anoctamin) family of proteins in a wide variety of cells, including smooth muscle (2, 4, 29). We have previously demonstrated that the calcium-activated chloride channel TMEM16A is expressed on ASM and plays an important role in ASM contraction (12). Several selective and potent TMEM16A antagonists have been identified (16, 27), and we have demonstrated that many of these TMEM16A antagonists acutely relax ASM contracted by a variety of mediators and potentiate relaxation induced by β-agonists (6). We have shown that the TMEM16A antagonist benzbromarone relaxes both central and peripheral airways through membrane hyperpolarization and attenuation of calcium flux (6).
TMEM16A is present in other organs and tissues in addition to ASM (12, 35), including vascular smooth muscle (22), uterine smooth muscle (7), interstitial cells of Cajal in the gastrointestinal system (15), sensory neurons (5), and airway epithelial cells (15, 16). Because TMEM16A is present on many cell types, treating bronchospasm with systemic TMEM16A antagonists could lead to off-target effects. However, targeting ASM to treat acute bronchospasm via direct inhalation could circumvent many concerns of off-target effects, but the airway epithelium would be exposed to these ligands during inhalation therapy. There is some concern that blockade of TMEM16A on airway epithelium with modulation of airway epithelial Cl− flux could lead to increased or tenacious mucus as seen in cystic fibrosis. However, in asthmatic models, TMEM16A antagonists have actually been shown to decrease mucus secretion (16). TMEM16A is more abundantly expressed in mucus-secreting cells compared with ciliated epithelial cells, and TMEM16A expression is increased in airway epithelial cells in asthmatic models compared with controls (16). These results suggest the possibility that TMEM16A antagonists could have a dual beneficial therapeutic effect on ASM tone and mucus secretion in asthmatics.
Despite these beneficial therapeutic effects seen with TMEM16A antagonists, TMEM16A agonists are being suggested as possible treatment for cystic fibrosis (19, 21, 24, 30). Cystic fibrosis is the most common lethal autosomal recessive disease in Caucasians caused by a defect in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (20). There are many different known mutations of the CFTR gene, and treatment with mutation-specific approaches using CFTR correctors, potentiators, and combinations of both have demonstrated some clinical improvement (10, 11, 20, 33). Because of the large number of mutations of CFTR, an alternative mutation-independent strategy has been proposed in which other anion transporters are activated on epithelial surfaces to compensate for the defective CFTR, such as TMEM16A (21). It is hypothesized that activation of TMEM16A could circumvent the defect of the CFTR channel function. Because the main defect in cystic fibrosis is the lack of chloride secretion resulting from a defect in cAMP-dependent chloride secretion, it has been hypothesized that direct and sustained activation of the calcium-activated chloride channel TMEM16A would be a beneficial target in airway epithelium in cystic fibrosis patients (19). Theoretically, TMEM16A agonists could be useful adjuvants to therapy by helping to normalize airway surface liquid and mucus by increasing chloride and bicarbonate secretion (21).
TMEM16A agonists would need to be delivered via inhalation, since systemic administration could cause potentially harmful effects. Inhalation would provide excellent delivery to the airway epithelium, but ASM would also be exposed. There is a theoretical concern that TMEM16A agonists could cause acute bronchospasm by activation of TMEM16A on ASM (21, 24); however, this has not been specifically investigated.
After screening over 100,000 compounds, Namkung et al. identified Eact as the most potent small-molecule activator of TMEM16A (26). They demonstrated that Eact caused a contraction in mouse ileal smooth muscle ex vivo that was inhibited by the TMEM16A antagonist T16Ainh-A01. However, the effect of this TMEM16A agonist on ASM has not been investigated.
We hypothesize that Eact would cause acute contraction of ASM. Using this specific and potent activator of TMEM16A, we sought to determine whether TMEM16A agonism by Eact would cause acute bronchoconstriction of ASM. If so, caution should be exercised when evaluating TMEM16A agonists as therapeutics for airway diseases such as cystic fibrosis.
METHODS
Reagents.
All reagents were obtained from Fisher Scientific unless otherwise specified. Tetrodotoxin was purchased from Calbiochem, EMD Biosciences (La Jolla, CA). Eact and T16Ainh-A01 were purchased from Tocris (Minneapolis, MN).
Cultured human ASM cells and epithelial cells.
Primary human ASM cells were prepared from studies using deidentified human tissue (reviewed by the Columbia University Institutional Review Board and deemed not human subjects research). Tissues were obtained from organ donor discarded surgical waste subsequent to lung transplant surgery. Smooth muscle tissue was dissected and enzymatically dissociated using a papain-collagenase kit (Worthington). Cell lines were grown in Dulbecco’s modified Eagle’s medium-F-12 media (Gibco, Grand Island, NY) with 10% FBS and antibiotics in a 37°C incubator with 5% CO2-95% air.
Primary airway epithelial cells were purchased from Lonza (normal human bronchial epithelial cells) and grown in Bronchial Epithelial Growth Medium with all supplements recommended by the manufacturer.
Quantitative polymerase chain reaction for TMEM16A.
ASM and epithelial cells were grown to confluence in 100-mm dishes and serum deprived for 24 h. Cells were harvested for RNA using the Qiagen RNeasy Plus Mini Kit. RNA (2 µg) was reverse transcribed into cDNA using the Super Script VILO cDNA synthesis kit (Invitrogen, Life Technologies, Carlsbad, CA). Quantitative PCR was performed as described previously (13). Briefly, gene specific primers for TMEM16A and GAPDH (housekeeping gene) were designed (Table 1) that spanned at least one large intron to avoid confounding results of amplifying contaminating genomic DNA. cDNA, primers (0.4 µM), and Power SYBR Green Master Mix (Applied Biosystems) were combined in a 20-µl reaction. The Applied Biosystems 7500 PCR machine was used with an initial denaturing step at 95°C for 10 min followed by 40 cycles of 92°C for 15 s and 65°C for 1 min. Amplification plots were generated by Applied Biosystems software. ΔCt was calculated as (Ct of TMEM16A) – (Ct of GAPDH).
Table 1.
Primers for TMEM16A and GAPDH
| Forward | Reverse | |
|---|---|---|
| Human ANO1 (320 bp) | CAGAGCCAAAGACATCGGAATCTGGTACAATAT | CCCAGAAGTCCTTGGAGATGTCGTACTTGTTTT |
| Human GAPDH (231 bp) | CCAGGGCTGCTTTTAACTCTGGTAAAGTGGATA | CATCGCCCCACTTGATTTTGGAGGGA |
Guinea pig tracheal rings.
Animal studies were approved by the Columbia University Institutional Animal Care and Use Committee. As described previously (6, 8), male Hartley guinea pigs (~400 g) were anesthetized with intraperitoneal pentobarbital sodium (100 mg/kg). Tracheas were removed and transferred to ice‐cold Krebs‐Henseleit (KH) buffer of the following composition (in mM): 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.24 MgSO4, 1.3 NaH2PO4, 25 NaHCO3, and 5.6 glucose, pH 7.4. Closed guinea pig tracheal rings containing two incomplete cartilaginous rings were isolated and denuded of epithelium. The rings were suspended in 4-mL water-jacketed (37°C) organ baths (Radnoti Glass Technology, Monrovia, CA) and connected to Grass FT03 force transducers (Grass Telefactor, West Warwick, RI) using silk sutures. The transducers were coupled to the computer with BioPac hardware, and data were collected using Acqknowledge 7.3.3 software (BioPac Systems, Goleta, CA). Organ baths were bubbled with 95% oxygen and 5% carbon dioxide, and KH buffer was exchanged every 15 min for 1 h during equilibration of tracheal rings at 1 g resting tension. All baths received 10 µM indomethacin to block the synthesis of endogenous prostanoids, 1 µM tetrodotoxin to block neuronal effects, and 10 µM of pyrilamine to block effects of endogenous histamine release. Rings were first contracted with 10 µM N-vanillylnonanamide (capsaicin analog) to activate and deplete neurotransmitter release from nonadrenergic noncholinergic (NANC) nerves. Rings were then contracted with two cycles of acetylcholine (ACh) dose responses (100 nM–100 µM) to measure the maximum response and ACh EC50 of ACh for each ring. Tracheal rings were pretreated with vehicle (0.2% DMSO) or a TMEM16A antagonist (50 µM benzbromarone or 50 µM T16AinhA01) for 15 min before the TMEM16A agonist Eact (50 µM) was added to the buffer bathing the tracheal rings. The muscle force present after 30 min was measured and expressed as a percentage of the maximum ACh contraction for each ring.
Human tracheal strips.
Studies using deidentified human tissue were reviewed by the Columbia University Institutional Review Board and were deemed not human subjects research. Tissues were obtained from organ donor discarded surgical waste subsequent to lung transplant surgery. Tissues were collected and placed in DMEM media (Gibco) and bubbled overnight in 5% carbon dioxide and 95% oxygen at 4°C. The following morning, the smooth muscle tissue was dissected and denuded of epithelium. The strips were anchored in the organ bath as described above using KH buffer of the same composition. The strips were allowed to equilibrate for 1 h with buffer exchanges every 15 min at a resting tension of 1.5 g and were treated with 1 µM tetrodotoxin and 10 µM pyrilamine. Strips were first contracted with 10 µM capsaicin and then with three cycles of ACh dose responses (100 nM–1 mM) to measure maximum contraction to ACh. As above, human ASM strips were pretreated with vehicle (0.2% DMSO) or a TMEM16A antagonist (50 µM benzbromarone or 50 µM T16AinhA01) for 15 min before the addition of Eact (50 µM). The muscle force present after 30 min was measured and expressed as a percent of maximum ACh contraction for each strip. In separate studies to investigate whether Eact potentiated an ACh contraction, human ASM strips were repetitively contracted with an EC50 concentration of ACh and then pretreated with vehicle (0.2% DMSO) or Eact (50 µM) before a subsequent treatment with an EC50 concentration of ACh. The initial peak contraction induced by ACh EC50 was compared with the ACh EC50 contraction after vehicle or Eact.
In vivo mouse airway resistance.
A/J mice were anesthetized with pentobarbital sodium (50 mg/kg ip) and tracheotomized with an 18-gauge cannula. Mice were mechanically ventilated (tidal volume 10 mg/kg, 150 breaths/min, positive end expiratory pressure 3 mmHg) with a flexiVent (SciReq, Montreal, QC, Canada) with an FX1 module and an in-line nebulizer and paralyzed with succinylcholine (10 mg/kg ip). A/J mice received either vehicle (25% ethanol in PBS for benzbromarone or 50% ethanol in PBS for Eact), 5 mM Eact, or 2.5 mM benzbromarone via nebulization (10 s nebulization, 50% duty cycle) 5 min before a nebulized methacholine challenge (0, 3.125, 6.25, 12.5, and 25 mg/ml). Central airway resistance (Rn) was measured throughout by the forced oscillation technique. EKG and temperature monitoring were performed throughout the experiment. Rn values for each mouse at each methacholine dose represent an average of three measurements.
Fura 2 calcium assay.
Primary human ASM cells were cultured on black-walled 96-well plates to 100% confluence and washed four times with warmed modified (37°C) Hanks’ balanced salt solution (HBSS) buffer (concentration in mM: 137.93 NaCl, 5.33 KCl, 2 CaCl2, 1 MgSO4, 2.38 HEPES, and 5.5 glucose, pH 7.4). Cells were loaded with 5 µM fura 2-AM in HBSS for 45 min and washed an additional three times with HBSS. Cells were then pretreated with vehicle (0.1% DMSO) or benzbromarone (10 µM) for 15 min after which they were placed in a FlexStation 3 microplate reader. Baseline fluorescence was measured, and vehicle (0.1% DMSO), bradykinin (10 μM), histamine (10 μM), ACh (10 μM), or Eact (1–50 μM) was pipetted into the wells of the plate using the automated injection feature of the FlexStation 3. Fluorescence was read every 4 s for 400 s using excitation wavelengths of 340 and 380, an emission wavelength of 510, and a cutoff filter of 495. Fluorescence values were reported as ∆F according to the calculation: ∆F = (340 nm)F/(380 nm)F – (340 nm)F0 /(380 nm)F0. For calcium-free fura 2-AM studies, cells were prepared as above, but, after fura 2-AM loading, they were washed in calcium-free HBSS (supplemented with 1 mM EGTA), and the remainder or the study was carried out in calcium-free HBSS.
Inositol phosphate assay.
Primary human ASM cells were grown to confluence in 24-well plates. Inositol phosphate (IP) synthesis was measured as described previously (25). Briefly, cells were loaded with 5 μCi/ml myo-[3H]inositol in inositol-free DMEM overnight and then washed two times with HBSS containing 10 mM LiCl. Cells were treated with either 10 μM bradykinin or Eact (10, 25, 50, or 100 μM) for 30 min. Reactions were terminated with cold methanol, and newly synthesized [3H]IPs were separated from [3H]inositols by methanol/chloroform extraction and column chromatography and were quantified by liquid scintillation counting (14).
FLIPR membrane potentiometric dye assay.
Primary human ASM cells were grown to confluence on black-walled 96-well plates and washed with Kreb’s buffer (in mM: 140 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 11 HEPES, and 10 d-glucose, pH 7.4) four times. FLIPR blue dye was reconstituted in buffer (0.75 mg/ml) and incubated for 30 min at 37°C. All drugs were reconstituted in dye solution. Cells were pretreated with vehicle (0.1% DMSO), 10 μM benzbromarone, or 10 μM nifedipine for 5 min. Fluorescence was measured with a FlexStation 3 microplate reader using excitation wavelength of 530 nm and emission wavelength of 565 nm while 25 μM Eact was injected (or positive controls 40 mM potassium gluconate or 100 μM NS-1619).
Statistics.
Data were analyzed using one-way ANOVA. The Bonferroni correction was applied for multiple comparisons. Statistical significance was established at P < 0.05, and all values are expressed as means ± SE. Nonpaired t test was used for ΔCt values in qRT-PCR and flexiVent experiments. flexiVent experiments were analyzed by comparing differences in the area under the curve for each mouse. For organ bath experiments, “n” refers to the number of guinea pig tracheal rings or human ASM strips. For cellular assays, n refers to a well of a 96-well plate.
RESULTS
mRNA encoding TMEM16A is more abundantly expressed in ASM than epithelium.
Primary human bronchial epithelial cells and primary human ASM cells were isolated and grown to confluence. Primary cells were harvested for RNA and reverse transcribed to cDNA. Quantitative PCR demonstrated that mRNA encoding TMEM16A was more abundant in primary ASM compared with primary bronchial epithelial cells. ΔCt for primary normal human bronchial epithelial (NHBE) cells [ΔCt = Ct (TMEM16A) – Ct (GAPDH)] was 11.7 ± 0.5 (n = 4 different lines from 4 different patients), whereas the ΔCt for primary ASM was 8.7 ± 0.5 (n = 4 different lines from 4 different patients, P = 0.006; Fig. 1). When comparing the quantitative expression of TMEM16A of NHBE and ASM, this represents an 8.0-fold higher expression of mRNA encoding TMEM16A in ASM.
Fig. 1.
TMEM16A mRNA is more abundantly expressed in human airway smooth muscle compared with airway epithelium. Quantitative PCR of primary normal human bronchial epithelial (NHBE) and primary human airway smooth muscle (ASM) cells demonstrated a lower abundance in NHBE compared with ASM (reflected in higher ΔCt for NHBE). ΔCt = Ct (TMEM16A) – Ct (GAPDH), n = 4, **P = 0.006 (unpaired t test).
The TMEM16A agonist Eact contracts guinea pig tracheal rings.
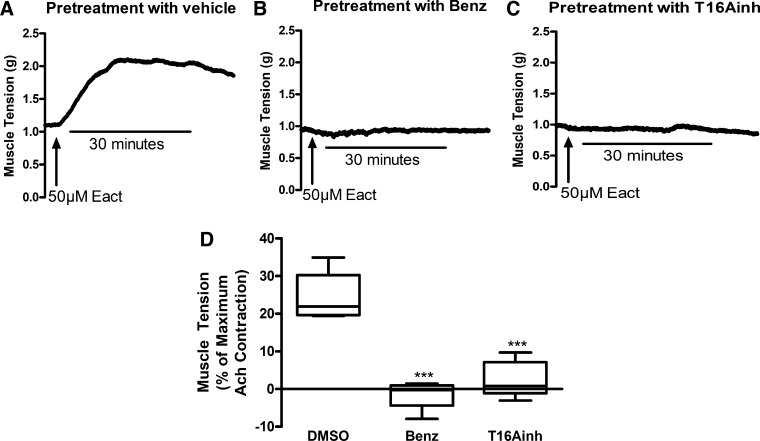
Guinea pig tracheal rings were pretreated with either vehicle (0.1% DMSO), 50 μM benzbromarone, or 50 μM T16Ainh-AO1 and were then treated with 50 μM Eact. In tracheal rings pretreated with only vehicle, Eact induced a significant contraction (23.9 ± 1.7% of the maximum ACh contraction at 30 min, n = 6; Fig. 2, A and D). Pretreatment with the TMEM16A antagonist benzbromarone completely attenuated this contraction (−1.4 ± 1.7% of the maximum ACh contraction at 30 min, n = 5, P < 0.001; Fig. 2, B and D). Pretreatment with another TMEM16A antagonist (T16Ainh-A01) also completely attenuated this contraction (2.6 ± 2.1% of the maximum ACh contraction at 30 min, n = 5, P < 0.001; Fig. 2, C and D).
Fig. 2.
Eact causes contraction of guinea pig tracheal rings ex vivo. Representative tracings of guinea pig tracheal rings pretreated with vehicle (0.2% DMSO, A), benzbromarone (Benz, 50 µM, B), or T16Ainh-A01 (T16Ainh, 50 µM, C) with subsequent exposure to 50 µM Eact 15 min later. D: 50 µM Eact caused a significant contraction of guinea pig tracheal rings (23.9 ± 2.4% of the maximum acetylcholine contraction). Pretreatment with 50 µM benzbromarone or 50 µM T16Ainh-A01 significantly inhibited the contraction (−1.4 ± 1.7 and 2.6 ± 2.1%, respectively, ***P < 0.001, n = 5–6). One-way ANOVA with post hoc Bonferroni’s test.
The TMEM16A agonist Eact contracts human ASM.
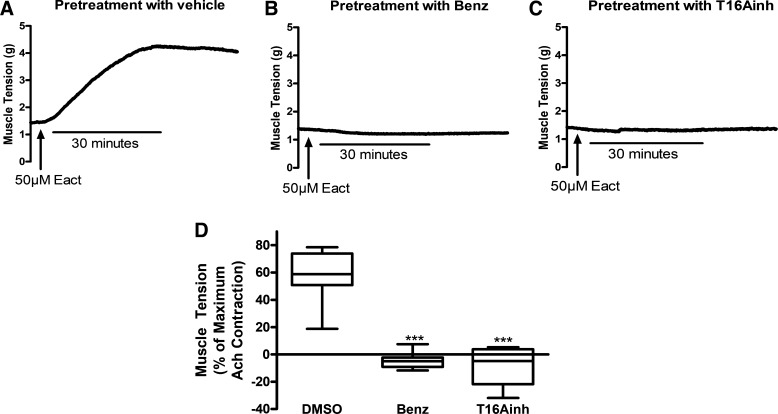
Because Eact was found to be procontractile in guinea pig tracheal rings, these experiments were then repeated in human ASM studied ex vivo in organ baths for clinical relevance. Human ASM strips were pretreated with either vehicle (0.1% DMSO), 50 μM benzbromarone, or 50 μM T16Ainh-AO1 and then treated with 50 μM Eact as in the experiments with guinea pig tracheal rings. In human ASM pretreated with only vehicle, Eact caused a significant contraction (60.0 ± 4.6% of the maximum ACh contraction at 30 min, n = 13 smooth muscle strips, 4 different patients; Fig. 3, A and D). This was an even more robust contraction (relative to ACh) than observed in guinea pig tracheal rings. Pretreatment with the TMEM16A antagonist benzbromarone completely attenuated this contraction (−4.6 ± 2.2% of the maximum ACh contraction at 30 min, n = 8, P < 0.001; Fig. 3, B and D). Pretreatment with another TMEM16A antagonist (T16Ainh-A01) also completely attenuated this contraction (−7.6 ± 5.4% of the maximum ACh contraction at 30 min, n = 6, P < 0.001; Fig. 3, C and D).
Fig. 3.
Eact causes contraction of human airway smooth muscle (ASM) ex vivo. Representative tracings of human ASM pretreated with vehicle (0.2% DMSO, A), 50 µM benzbromarone (Benz, B), or 50 µM T16Ainh-A01 (T16Ainh, C) and subsequently exposed to 50 µM Eact 15 min later. D: 50 µM Eact caused a significant contraction of human ASM (55.4 ± 6.3% of the maximum acetylcholine contraction, n = 13, 4 different patients). Pretreatment with 50 µM benzbromarone or 50 µM T16Ainh-A01 significantly inhibited the contraction (−4.6 ± 2.2 and −7.6 ± 5.4%, respectively, ***P < 0.001, N = 6–13, 4 patients). One-way ANOVA with post hoc Bonferroni’s test.
Eact augments the peak of an ACh contraction in human ASM.
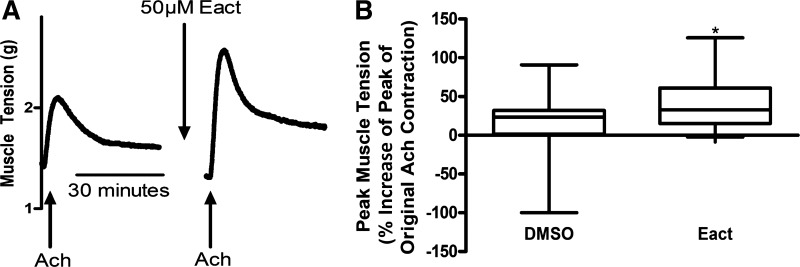
After demonstrating that Eact alone induces a contraction in human ASM, we next sought to investigate whether Eact could augment an ACh contraction. Human ASM strips were first contracted with an EC50 of ACh as a baseline, then pretreated with either vehicle (0.1% DMSO) or 50 μM Eact, and then contracted with another EC50 of ACh. Eact potentiated the peak of the ACh contraction but not the subsequent maintained tension after the initial peak of contraction. The vehicle control (0.1% DMSO) treatment showed a 13.0 ± 11.7% increase in the peak from original contraction, whereas the Eact treated showed a 41.7 ± 8.3% increase in the peak contraction (n = 13–19, 6 different patients, P = 0.048; Fig. 4).
Fig. 4.
Eact potentiates the peak of an acetylcholine (ACh) contraction ex vivo. A: representative tracings of human airway smooth muscle contracted with ACh EC50 and then pretreated with 50 µM Eact to demonstrate potentiation of an ACh contraction. B: 50 µM Eact pretreatment showed a significant increase in the peak of a ACh contraction compared with treatment with vehicle (0.2% DMSO; 41.7 ± 8.3% increase in the peak contraction compared with 13.0 ± 11.7%, n = 13–19, 6 different patients, *P < 0.05). Unpaired t test.
Inhaled Eact augments methacholine-induced bronchoconstriction in vivo.
Eact was subsequently tested in a mouse in vivo model for further clinical relevance. Pretreatment of A/J mice in vivo with 5 mM Eact via nebulization caused an augmentation of methacholine-induced increases in airway resistance measured by the forced oscillatory technique [flexiVent (n = 6, P = 0.005; Fig. 5A)]. Pretreatment with 2.5mM benzbromarone via nebulization significantly attenuated methacholine-induced increases in airway resistance (n = 7, P = 0.01; Fig. 5B).
Fig. 5.
Eact causes an increase in airway hyperresponsiveness in vivo. A: pretreatment with 5 mM aerosolized Eact before a graded inhaled methacholine challenge caused an increase in central airway resistance (Rn, n = 6, **P = 0.005). B: pretreatment with 2.5 mM aerosolized benzbromarone caused a decrease in Rn (n = 7, **P = 0.01). EtOH, ethanol. Unpaired t test of area under the curve.
Eact causes an increase in intracellular calcium but not IP synthesis.
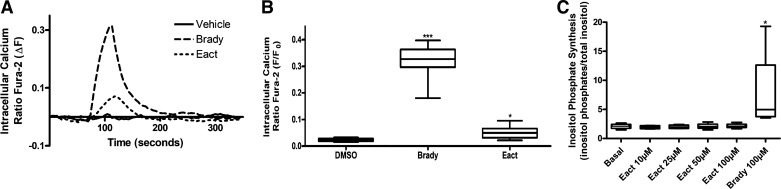
Eact was then studied in primary cultured human ASM cells to further investigate the cellular mechanisms involved in smooth muscle contraction caused by Eact. Cells were loaded with the calcium indicator fura 2-AM and treated with either vehicle or increasing concentrations of Eact. Eact caused an increase in intracellular calcium compared with vehicle starting at 10 μM, with the largest increase at 25 μM [10 μM Eact 0.62 ± 0.05, 25 μM 0.71 ± 0.07, 50 μM 0.56 ± 0.09, fura 2 fluorescence ratio (∆F), n = 10, P < 0.001; Fig. Fig. 6, A and B]. Cells were also treated with different positive controls (bradykinin, histamine, and ACh) that all demonstrated an increase with intracellular calcium as seen with Eact (Fig. 6, C and D). Finally, cells were pretreated with either vehicle (DMSO) or 10 μM benzbromarone and then treated with 25 μM Eact, which showed attenuation of the increase in intracellular calcium (0.45 ± 0.06 vs. 0.11 ± 0.01 with benzbromarone pretreatment, n = 24–30, 3 different primary ASM lines, P < 0.001; Fig. 6, C and D).
Fig. 6.
Eact causes an increase in intracellular calcium concentrations ([Ca2+]i). A: representative tracings of fura 2 calcium fluorescence in human primary airway smooth muscle (ASM) cells demonstrating calcium increases induced by different concentrations of Eact (1, 10, 25, and 50 µM). B: Eact demonstrated an increase in [Ca2+]i starting at 10 µM (***P < 0.01, n = 10). ns, Not significant. C: representative tracings of increase of [Ca2+]i with positive controls bradykinin, histamine, and acetylcholine (ACh, all 10 µM). Eact (25 µM) induced an increase in [Ca2+]i, but, if pretreated with benzbromarone (Benz, 10 µM), this increase was attenuated. D: 25 µM Eact increased [Ca2+]i, which was attenuated with pretreatment with benzbromarone (10 µM, 0.45 ± 0.06 vs. 0.11 ± 0.01 fura 2 ∆F, ***P < 0.001, n = 24–30). Bradykinin (brady), histamine, and acetylcholine (ACh) (positive controls) all caused an increase in [Ca2+]i (*P < 0.05 and ***P < 0.001). All treatments were done in 3 different primary ASM cell lines. One-way ANOVA with post hoc Bonferroni’s test.
We next sought to determine whether Eact would elicit an increase in intracellular calcium flux involving the sarcoplasmic reticulum (SR). Studies were performed in the absence of external calcium. Eact (25 μM) caused a small but significant increase in intracellular calcium compared with vehicle (0.02 ± 0.001 with vehicle, 0.05 ± 0.01 with 25 μM Eact, n = 18, 3 different primary ASM lines, P < 0.05; Fig. 7, A and B). Bradykinin as a positive control caused a larger increase (0.32 ± 0.01, P < 0.001). Therefore, Eact causes a slight increase in intracellular calcium in the absence of extracellular calcium.
Fig. 7.
Eact causes minor increases in intracellular calcium concentrations ([Ca2+]i) with 0 external calcium and does not increase inositol trisphosphate (IP3) synthesis. A: representative tracings of fura 2 calcium fluorescence in human primary airway smooth muscle (ASM) cells in 0 external Ca2+. B: bradykinin (Brady) caused a significant increase in [Ca2+]i (0.32 ± 0.01, n = 18, P < 0.001), whereas Eact caused a significant but small increase in [Ca2+]i (0.05 ± 0.01, n = 18, *P < 0.05 and ***P < 0.001). C: Eact did not cause an increase in IP3 synthesis, whereas bradykinin increased IP3 synthesis (positive control, n = 4, *P < 0.05). All treatments were done in 3–4 different primary ASM cell lines. One-way ANOVA with post hoc Bonferroni’s test.
In separate studies, we investigated whether Eact caused an increase in IP synthesis. Eact did not cause an increase in IP synthesis (at multiple concentrations of 10, 25, 50, and 100 μM) compared with bradykinin control (Fig. 7C). Therefore, Eact is not increasing intracellular calcium via increase in inositol trisphosphate (IP3) or through a phospholipase C pathway.
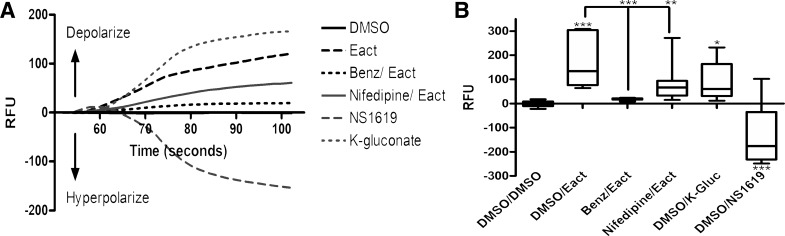
Eact depolarizes ASM cells.
Human ASM cells were loaded with the membrane potentiometric dye FLIPR. Eact (25 μM) caused an acute depolarization compared with DMSO vehicle control [−3.1 ± 3.0 relative fluorescence units (RFU) for DMSO control vs. 182.2 ± 28.5 RFU for 25 μM Eact, n = 14, P < 0.001; Fig. 8]. Pretreatment with the TMEM16A antagonist benzbromarone completely attenuated this depolarization (18.3 ± 1.7 RFU, n = 8, P < 0.001). Pretreatment with the L-type voltage-dependent calcium channel blocker nifedipine also attenuated the depolarization by Eact (76.8 ± 14.0 RFU, n = 19, P < 0.01), which suggests Eact’s effects are mediated by depolarization at the plasma membrane. Positive controls that are known to depolarize (potassium gluconate) and hyperpolarize (NS-1619) ASM cells demonstrated an increase and decrease, respectively, in RFU as expected.
Fig. 8.
Eact depolarizes airway smooth muscle (ASM) cells. A: representative tracings of FLIPR membrane potentiometric dye demonstrating depolarization [increase in relative fluorescence units (RFU)] and hyperpolarization (decrease in RFU). Benz, benzbromarone. B: 25 μM Eact caused an acute depolarization compared with DMSO vehicle (−3.1 ± 3.0 RFU for DMSO control vs. 182.2 ± 28.5 RFU for 25 μM Eact, n = 14, ***P < 0.001). Pretreatment with the TMEM16A antagonist benzbromarone (18.3 ± 1.7 RFU, n = 8, P < 0.001) or the L-type voltage-dependent calcium channel blocker nifedipine (76.8 ± 14.0 RFU, n = 19, **P < 0.01) completely attenuated this depolarization. One-way ANOVA with post hoc Bonferroni’s test, *P < 0.05.
DISCUSSION
In the present study we have demonstrated that the TMEM16A agonist Eact causes an acute contraction of ASM in both human ASM and guinea pig tracheal rings in an ex vivo organ bath model. This contraction was especially robust in human ASM, with an amplitude of 60% of a maximum ACh contraction at 30 min after drug treatment. This is the first demonstration that the TMEM16A agonist Eact could cause clinically significant bronchoconstriction in human ASM.
Eact has previously been shown to contract mouse ileal segments (26). Unlike ASM, mouse ileal smooth muscle segments have spontaneous rhythmic smooth muscle contractile activity under baseline conditions. In these previous studies, Eact only showed an increase in the amplitude of contractions after atropine was added to inhibit baseline contractions induced by the endogenous release of ACh. In contrast, in the current study, Eact induced significant ASM contractions when added to tissues at baseline resting tension. Therefore, although Eact has been shown to contribute to contraction in other smooth muscle beds, our study is unique in that Eact induce contractions from a resting baseline. In addition, we were also able to demonstrate that Eact augments the peak of an ACh contraction.
We further demonstrated the specificity of Eact for the TMEM16A channel by demonstrating that two different TMEM16A antagonists (benzbromarone and T16Ainh-A01) completely attenuated the Eact-induced contractions in both human and guinea pig ASM. Specificity of Eact has also been investigated in mouse ileal smooth muscle by demonstrating reversal of the Eact effects with T16Ainh-A01 (26). The same study also investigated Eact’s specificity using TMEM16A-expressing Fischer rat thyroid cells, which demonstrated a large increase in chloride current with Eact that was reversed with T16Ainh-A01. Subsequently, many other groups have used Eact as a specific activator of TMEM16A (3, 18, 28, 31), including a study with pulmonary endothelial cells (1). This study investigated TMEM16A’s role in the pathogenesis of pulmonary arterial hypertension and demonstrated that activation of pulmonary endothelial cells with Eact decreased proliferation, an effect of Eact that was significantly reduced with TMEM16A-specific siRNA knockdown. Therefore, multiple studies demonstrate specificity of Eact for TMEM16A using pharmacologic and genetic approaches.
In contrast, one study has suggested an alternative molecular target for Eact (23). TMEM16A is expressed in the dorsal root ganglion where it modulates pain and itch responses (9, 23, 32), and Eact has been shown to activate dorsal root ganglion neurons to induce a pain response. The Eact pain response was suppressed by the TMEM16A antagonist T16Ainh-A01 in some studies (9), but a recent study by Liu et al. concluded that the pain and itch response induced with Eact is mediated by direct activation of transient receptor potential vanilloid 1 (TRPV1) channels, not TMEM16A (23). In this study, Eact responses were not blocked by the TMEM16A antagonist T16Ainh-A01 but were dramatically attenuated with the TRPV1 antagonist AMG-9810. Although multiple studies have demonstrated Eact’s specificity for TMEM16A activation, this study was the first to demonstrate that Eact directly activates the TRPV1 channel in sensory nociceptors.
Our laboratory has investigated the role of TRPV1 in ASM (34). Yocum et al. demonstrated that TRPV1 is expressed on ASM but concluded that contraction of ASM seen with the TRPV1 agonist capsaicin is likely indirect and is neurally mediated. Although capsaicin induces contraction of tracheal rings, it is significantly inhibited by the addition of the sodium channel inhibitor bupivacaine or the NK-2 receptor antagonist GR-159897. These findings suggest that ASM contraction by activation of TRPV1 is likely mediated by the release of tachykinins from C-fiber excitatory NANC nerves in the airway rather than a direct effect on ASM. This conclusion was further supported by studies demonstrating that, upon a second exposure of tracheal rings to capsaicin, considerable tachyphylaxis was seen, suggesting a depletion of tachykinins from NANC neurons.
We routinely pretreat ex vivo ASM preparations with capsaicin to deplete NANC nerve endings of neurotransmitters to eliminate the confounding effects of these nerves during pharmacologic studies on ASM. In the present study, both guinea pig tracheal rings and human ASM strips were pretreated with capsaicin. In ASM from both species, a subsequent exposure to capsaicin does not induce a substantial contraction because of this neural depletion, and thus it is very unlikely that our Eact-induced contraction is because of direct activation of the TRPV1 receptor on ASM or NANC nerves. In addition, our complete blockade of Eact-induced ASM contraction with two different structurally distinct TMEM16A antagonists makes the possibility that the Eact-induced contraction is the result of an off-target effect of TRPV1 activation even less likely.
The current study also demonstrated that an inhaled pretreatment with Eact augmented a methacholine-induced increase in airway resistance in mice in vivo. This is the first demonstration that Eact can augment bronchoconstriction in vivo and that Eact can have effects via an inhalation route. This differs from our ex vivo data in that inhaled Eact did not cause a contraction from baseline but rather increased a response to methacholine, which is consistent with our ex vivo data demonstrating potentiation of the peak of an ACh contraction. This difference of Eact’s effect on baseline contractile tone in vivo versus ex vivo could be because of species differences, since the ex vivo studies were performed in human and guinea pig models, less efficient delivery via inhalation, or less sensitive detection of ASM contraction in in vivo models. However, this could still be of great clinical significance, since the theoretical treatment with TMEM16A agonists could lead to worsening bronchospasm in cystic fibrosis patients.
We also demonstrated that inhaled pretreatment with the TMEM16A antagonist benzbromarone led to an attenuation of the methacholine-induced contraction in vivo. Zhang et al. have shown that benzbromarone attenuates methacholine contraction in ovalbumin-sensitized mice, but these mice were treated intravenously (35). Our study is the first to show that benzbromarone can be effective via inhalation delivery. Because of widespread TMEM16A expression, this finding is important, since inhalational delivery could help to avoid side effects as demonstrated with classic medications for bronchoconstriction, inhaled corticosteroids, and inhaled β2-agonists.
Our in vitro cellular studies in ASM demonstrate that the mRNA encoding TMEM16A is expressed approximately eightfold higher in ASM compared with airway epithelium. This has been suggested with immunohistochemistry from murine asthmatic models (16), but our study is the first to quantify the differences with both primary ASM and epithelium from multiple patients. Previously, we have demonstrated that Eact causes plasma membrane depolarization in ASM cells while the TMEM16A antagonist benzbromarone causes a hyperpolarization (6). In our current study, we showed that Eact causes an increase in intracellular calcium in human primary ASM cells. These ASM cell-based studies are consistent with a role of Eact in activating TMEM16A to induce increases in intracellular calcium concentrations and plasma membrane depolarization, which may mechanistically contribute to the contractile responses seen in ASM ex vivo and in vivo. We further investigated the mechanism of action of Eact by demonstrating that Eact had a significant but minimal effect on intracellular calcium when external calcium was depleted. This suggests that Eact plays a more important role in calcium flux at the plasma membrane rather than the SR. Eact was not found to have any effect on IP3 synthesis, which causes an increase in intracellular calcium through the SR. Finally, we also expanded on prior studies that demonstrated that Eact caused an acute depolarization of ASM cells. Pretreatment with nifedipine, an L-type voltage-dependent calcium channel blocker, completely attenuated the depolarization caused by Eact, suggesting Eact may be causing an acute contraction through calcium flux from extracellular sources rather than internal SR stores.
Our primary conclusion is that the TMEM16A agonist Eact causes an acute contraction of ASM and an increase in airway hyperresponsiveness. These results should be taken into consideration when developing TMEM16A agonists as therapeutic agents in treating other pulmonary diseases such as cystic fibrosis. Although these studies were not performed in a cystic fibrosis model, TMEM16A is highly expressed in ASM. Thus, the demonstrated airway hyperresponsive effects of inhaled Eact in vivo could be very clinically significant.
GRANTS
This work was supported by National Institutes of Health Grants HL-132203 (J. Danielsson), HL-122340 (C. W. Emala, Sr.), GM-065281 (C. W. Emala, Sr.), HD-082251 (G. Gallos), and HL-140102 (G. T. Yocum) and the Louis V. Gerstner Jr. Scholars Program (J. Danielsson and G. T. Yocum).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D., A.S.K., G.T.Y., G.G., and C.W.E. conceived and designed research; J.D., A.S.K., Y.Z., and D.X. performed experiments; J.D., A.S.K., Y.Z., D.X., and C.W.E. analyzed data; J.D., A.S.K., G.T.Y., Y.Z., D.X., G.G., and C.W.E. interpreted results of experiments; J.D. and A.S.K. prepared figures; J.D., A.S.K., G.G., and C.W.E. drafted manuscript; J.D., A.S.K., G.T.Y., Y.Z., D.X., G.G., and C.W.E. edited and revised manuscript; J.D., A.S.K., G.T.Y., Y.Z., D.X., G.G., and C.W.E. approved final version of manuscript.
REFERENCES
- 1.Allawzi AM, Vang A, Clements RT, Jhun BS, Kue NR, Mancini TJ, Landi AK, Terentyev D, O-Uchi J, Comhair SA, Erzurum SC, Choudhary G. Activation of anoctamin-1 limits pulmonary endothelial cell proliferation via p38-mitogen-activated protein kinase-dependent apoptosis. Am J Respir Cell Mol Biol 58: 658–667, 2018. doi: 10.1165/rcmb.2016-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao R, Lifshitz LM, Tuft RA, Bellvé K, Fogarty KE, ZhuGe R. A close association of RyRs with highly dense clusters of Ca2+-activated Cl- channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle. J Gen Physiol 132: 145–160, 2008. doi: 10.1085/jgp.200709933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burris SK, Wang Q, Bulley S, Neeb ZP, Jaggar JH. 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. Br J Pharmacol 172: 2459–2468, 2015. doi: 10.1111/bph.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 5.Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, Raouf R, Wood JN, Oh U. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 15: 1015–1021, 2012. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 6.Danielsson J, Perez-Zoghbi J, Bernstein K, Barajas MB, Zhang Y, Kumar S, Sharma PK, Gallos G, Emala CW. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology 123: 569–581, 2015. doi: 10.1097/ALN.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danielsson J, Vink J, Hyuga S, Fu XW, Funayama H, Wapner R, Blanks AM, Gallos G. Anoctamin channels in human myometrium: a novel target for tocolysis. Reprod Sci 25: 1589–1600, 2018. doi: 10.1177/1933719118757683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielsson J, Yim P, Rinderspacher A, Fu XW, Zhang Y, Landry DW, Emala CW. Chloride channel blockade relaxes airway smooth muscle and potentiates relaxation by β-agonists. Am J Physiol Lung Cell Mol Physiol 307: L273–L282, 2014. doi: 10.1152/ajplung.00351.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deba F, Bessac BF. Anoctamin-1 Cl(-) channels in nociception: activation by an N-aroylaminothiazole and capsaicin and inhibition by T16A[inh]-A01. Mol Pain 11: 55, 2015. 10.1186/s12990-015-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Stefano D, Villella VR, Esposito S, Tosco A, Sepe A, De Gregorio F, Salvadori L, Grassia R, Leone CA, De Rosa G, Maiuri MC, Pettoello-Mantovani M, Guido S, Bossi A, Zolin A, Venerando A, Pinna LA, Mehta A, Bona G, Kroemer G, Maiuri L, Raia V. Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy 10: 2053–2074, 2014. doi: 10.4161/15548627.2014.973737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajac I, De Boeck K. New horizons for cystic fibrosis treatment. Pharmacol Ther 170: 205–211, 2017. doi: 10.1016/j.pharmthera.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Gallos G, Remy KE, Danielsson J, Funayama H, Fu XW, Chang HY, Yim P, Xu D, Emala CW Sr. Functional expression of the TMEM16 family of calciumfig-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 305: L625–L634, 2013. doi: 10.1152/ajplung.00068.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallos G, Townsend E, Yim P, Virag L, Zhang Y, Xu D, Bacchetta M, Emala CW. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol 304: L191–L197, 2013. doi: 10.1152/ajplung.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L405–L411, 1999. doi: 10.1152/ajplung.1999.276.3.L405. [DOI] [PubMed] [Google Scholar]
- 15.Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci USA 106: 21413–21418, 2009. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA 109: 16354–16359, 2012. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen LJ, Sims SM. Ca2+-dependent Cl− current in canine tracheal smooth muscle cells Am J Physiol Cell Physiol 269: C163–C169, 1995. doi: 10.1152/ajpcell.1995.269.1.C163. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Yu B, Yang H, Ma T. Shikonin inhibits intestinal calcium-activated chloride channels and prevents rotaviral diarrhea. Front Pharmacol 7: 270, 2016. doi: 10.3389/fphar.2016.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Wolf L, Schreiber R. Airway epithelial cells--functional links between CFTR and anoctamin dependent Cl- secretion. Int J Biochem Cell Biol 44: 1897–1900, 2012. doi: 10.1016/j.biocel.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Kym PR, Wang X, Pizzonero M, Van der Plas SE. Recent progress in the discovery and development of small-molecule modulators of CFTR. Prog Med Chem 57: 235–276, 2018. doi: 10.1016/bs.pmch.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Salomon JJ, Sheppard DN, Mall MA, Galietta LJ. Bypassing CFTR dysfunction in cystic fibrosis with alternative pathways for anion transport. Curr Opin Pharmacol 34: 91–97, 2017. doi: 10.1016/j.coph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Li RS, Wang Y, Chen HS, Jiang FY, Tu Q, Li WJ, Yin RX. TMEM16A contributes to angiotensin II-induced cerebral vasoconstriction via the RhoA/ROCK signaling pathway. Mol Med Rep 13: 3691–3699, 2016. doi: 10.3892/mmr.2016.4979. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Feng J, Luo J, Yang P, Brett TJ, Hu H. Eact, a small molecule activator of TMEM16A, activates TRPV1 and elicits pain- and itch-related behaviours. Br J Pharmacol 173: 1208–1218, 2016. doi: 10.1111/bph.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros 14: 561–570, 2015. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Mikami M, Perez-Zoghbi JF, Zhang Y, Emala CW Sr. Attenuation of murine and human airway contraction by a peptide fragment of the cytoskeleton regulatory protein gelsolin. Am J Physiol Lung Cell Mol Physiol 316: L105–L113, 2019. doi: 10.1152/ajplung.00368.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J 25: 4048–4062, 2011. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh SJ, Hwang SJ, Jung J, Yu K, Kim J, Choi JY, Hartzell HC, Roh EJ, Lee CJ. MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol Pharmacol 84: 726–735, 2013. doi: 10.1124/mol.113.087502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi J, Wang Y, Liu Y, Zhang F, Guan B, Zhang H. Development and validation of HTS assay for screening the calcium-activated chloride channel modulators in TMEM16A stably expressed CHO cells. Anal Bioanal Chem 406: 1713–1721, 2014. doi: 10.1007/s00216-013-7550-5. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sondo E, Caci E, Galietta LJ. The TMEM16A chloride channel as an alternative therapeutic target in cystic fibrosis. Int J Biochem Cell Biol 52: 73–76, 2014. doi: 10.1016/j.biocel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Sui Y, Li L, Su W, Hao F, Zhu Q, Di W, Gao H, Ma T. Anoctamin 1 calcium-activated chloride channel downregulates estrogen production in mouse ovarian granulosa cells. Endocrinology 155: 2787–2796, 2014. doi: 10.1210/en.2013-2155. [DOI] [PubMed] [Google Scholar]
- 32.Takayama Y, Uta D, Furue H, Tominaga M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci USA 112: 5213–5218, 2015. doi: 10.1073/pnas.1421507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu HX, Zhu M, Xiong XF, Wei J, Zhuo KQ, Cheng DY. Efficacy and safety of CFTR corrector and potentiator combination therapy in patients with cystic fibrosis for the F508del-CFTR homozygous mutation: a systematic review and meta-analysis. Adv Ther 36: 451–461, 2018. doi: 10.1007/s12325-018-0860-4. [DOI] [PubMed] [Google Scholar]
- 34.Yocum GT, Chen J, Choi CH, Townsend EA, Zhang Y, Xu D, Fu XW, Sanderson MJ, Emala CW. Role of transient receptor potential vanilloid 1 in the modulation of airway smooth muscle tone and calcium handling. Am J Physiol Lung Cell Mol Physiol 312: L812–L821, 2017. doi: 10.1152/ajplung.00064.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CH, Li Y, Zhao W, Lifshitz LM, Li H, Harfe BD, Zhu MS, ZhuGe R. The transmembrane protein 16A Ca(2+)-activated Cl- channel in airway smooth muscle contributes to airway hyperresponsiveness. Am J Respir Crit Care Med 187: 374–381, 2013. doi: 10.1164/rccm.201207-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]