Abstract
Airway surface dehydration is a pathological feature of cystic fibrosis (CF) lung disease. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR), a cyclic AMP-regulated Cl− channel controlled in part by the adenosine A2B receptor. An alternative CFTR-independent mechanism of fluid secretion is regulated by ATP via the P2Y2 receptor (P2Y2R) that activates Ca2+-regulated Cl− channels (CaCC/TMEM16) and inhibits Na+ absorption. However, due to rapid ATP hydrolysis, steady-state ATP levels in CF airway surface liquid (ASL) are inadequate to maintain P2Y2R-mediated fluid secretion. Therefore, inhibiting airway epithelial ecto-ATPases to increase ASL ATP levels constitutes a strategy to restore airway surface hydration in CF. Using [γ32P]ATP as radiotracer, we assessed the effect of a series of ATPase inhibitory compounds on the stability of physiologically occurring ATP concentrations. We identified the polyoxometalate [Co4(H2O)2(PW9O34)2]10− (POM-5) as the most potent and effective ecto-ATPase inhibitor in CF airway epithelial cells. POM-5 caused long-lasting inhibition of ATP hydrolysis in airway epithelia, which was reversible upon removal of the inhibitor. Importantly, POM-5 markedly enhanced steady-state levels of released ATP, promoting increased ASL volume in CF cell surfaces. These results provide proof of concept for ecto-ATPase inhibitors as therapeutic agents to restore hydration of CF airway surfaces. As a test of this notion, cell-free sputum supernatants from CF subjects were studied and found to have abnormally elevated ATPase activity, which was markedly inhibited by POM-5.
Keywords: cystic fibrosis, ecto-ATPases, extracellular ATP, polyoxometalates, purinergic receptors
INTRODUCTION
The mucus gel layer covering the airway surface periciliary layer (PCL) traps inhaled materials and also acts as a reservoir for water, buffering hydration of the PCL for needed cell-surface lubrication and efficient ciliary beating. Airway surface liquid dehydration and the production of hyperconcentrated mucus lead to airway obstruction and are pathological features of cystic fibrosis (CF), chronic obstructive lung disease (COPD), and asthma. Airway surface hydration is maintained by water fluxes driven by the balance between active Cl− secretion versus Na+ absorption.
CF is caused by a genetically deficient production/function of the CF transmembrane conductance regulator (CFTR) protein, a cyclic AMP-regulated airway epithelial Cl− channel. In normal airways, fluid transport is regulated in part by airway surface liquid (ASL) concentrations of adenosine (Ado) acting on the airway epithelial Gs-coupled A2B receptor (A2BR), which promotes Cl− secretion via cyclic AMP-regulated CFTR activation (20, 21). The Gq-coupled P2Y2 receptor (P2Y2R), which is activated by ATP, provides additional mechanisms for CFTR activation via protein kinase C (17) and Ca2+ stimulation of adenylyl cyclase (26). However, due to the genetically deficient production/function of CFTR, these mechanisms are inoperative in CF. An alternative CFTR-independent pathway for water transport is provided by the P2Y2R via the Ca2+-activated Cl− channel CaCC/TMEM16 and inhibition of Na+ absorption (19).
Mechanisms that sense surface hydration tune the nucleotide release and extracellular hydrolysis pathways to regulate ATP and Ado levels in ASL (5). ATP is released onto the luminal surface from 1) ciliated cells via the apical membrane channel pannexin 1 (32, 35) and 2) goblet cell mucin granules via vesicular nucleotide transporter (VNUT)-mediated transport (36). Once released, ATP can interact with P2Y2R but is also rapidly converted to Ado by cell-surface ecto-nucleotidases. While a body of literature, including our own studies, indicates that ATP release rates are not altered in CF (14, 21, 28, 34, 40, 42), ecto-ATPases in CF maintain ASL ATP levels below threshold values for P2Y2R activation (21, 27). Consequently, the CFTR-independent mechanism downstream of P2Y2R cannot optimally regulate ASL hydration throughout the lung in CF (21).
Restoration of ASL hydration is a goal of CF therapies but remains difficult to achieve due to the complexity of the regulatory systems that maintain ASL homeostasis. One strategy to restore ASL volume in CF airway surfaces is to raise the effective level of nucleotides in airway surfaces. One approach to achieve this goal was to aerosolize relatively ecto-ATPase-resistant dinucleotides (denufosol) onto CF airway surfaces. This approach was limited by the receptor desensitization produced by delivery of high concentrations of these agents onto airway surfaces (5). An alternative approach to raising steady-state ATP levels on airway surfaces is to inhibit the metabolism of endogenously released ATP. However, effective inhibitors of ATP hydrolysis in CF airways have not been identified, due, in part, to the complex array of ATP metabolizing ectoenzyme activities expressed on airway surfaces.
Three major families of ecto-nucleotidases hydrolyze extracellular ATP: 1) ecto-nucleoside triphosphate diphosphohydrolases (ENTPDs); 2) ecto-nucleotide pyrophosphatases/phosphodiesterases (ENPPs); and 3) nonspecific alkaline phosphatase (NSAP) (43). ENTPDs are two-transmembrane domain proteins with large extracellular loops harboring catalytic sites. ENTPDs hydrolyze ATP and ADP, producing AMP and inorganic phosphate (Pi) as final hydrolysis products. ENTPDs are composed of four subtypes (ENTPD1 or CD39, ENTPD2, ENTPD3, and ENTPD8), and each hydrolyzes ATP and ADP at different rates (43). ENPPs remove pyrophosphate (PPi) from ATP, producing AMP. ENPP1 and ENPP3 contain a single transmembrane domain, while ENPP2 is a secreted enzyme. NSAP is a glycosylphosphatidylinositol-anchored protein that dephosphorylates ATP, ADP, and AMP, generating adenosine (43). Members of these three families of ecto-ATPases are expressed in airway epithelia (4, 29, 30). In addition to cell-associated activities, ATPases also can be secreted from submucosal glands (10) or shed into the airways by epithelial or inflammatory cells, e.g., with exosomes (7), likely contributing as “soluble” enzymes to ATP depletion in ASL in vivo (2, 18, 37). Thus, secreted/shed ATPases further shield the P2Y2R from bulk phase nucleotides, greatly diminishing the effectiveness of released ATP to hydrate intraluminal mucus in CF.
In the present study, we assessed ATP hydrolysis under first-order kinetics, i.e., using trace amounts of [γ32P]ATP [(ATP) << Km], which most closely predicts the decay pattern of physiologic concentrations of ATP occurring in ASL (21). Using this approach, we screened a series of ecto-ATPase inhibitor structures to identify molecules that potently and effectively reduce ATP hydrolysis in CF airway epithelia and CF lung secretions. We then tested the hypothesis that, by enhancing steady-state levels of released ATP, ATPase inhibitors promote increased ASL volume production in CF cells.
METHODS
Cell cultures and incubations.
Primary (P0) human bronchial epithelial cells (HBEC) from normal subjects and CF subjects were obtained from the University of North Carolina (UNC) CF Center Tissue Culture Core. Cells were differentiated into air-liquid interface (ALI) preparations for 5–7 days on collagen-coated 12-mm Transwell permeable supports (Costar) and subsequently maintained for 4–5 wk, as previously described (21, 35). All cultures used exhibited a ∆508/∆508 CFTR genotype, which is the most common CFTR mutation.
Sputum and subjects.
Induced sputum was collected from 15 healthy and 11 CF subjects (Table 1) and cell-free sputum supernatants were obtained as previously described (2, 15). Studies were approved by the UNC Institutional Review Board (IRB no. 06-0979), and all participants provided written informed consent.
Table 1.
Subject demographics
| Demography | Controls | CF |
|---|---|---|
| N | 15 | 11* |
| Age, yr | 25.9 ± 6.1 | 30.3 ± 10.8 |
| Sex, % male | 73.3 | 41.7 |
| Race, % Caucasian | 73.3 | 100 |
| FVC, % predicted† | 102.0 ± 10.6 | 65.8 ± 19.8 |
| FEV1, % predicted† | 83.7 ± 34.3 | 49.5 ± 15.4 |
| FEV1/FVC† | 81.2 ± 5.6 | 70.9 ± 11.6 |
| FEF 25–75, % predicted† | 83.8 ± 19.0 | 25.1 ± 13.8 |
| Sputum PMNs, cells/mg plug‡ | 334 ± 314 | 18,645 ± 12,766 |
Values are means ± SD.
Genotype data indicate that 9 cystic fibrosis (CF) sputum donors expressed either the ∆508/∆508 CFTR mutation or ∆508/other mutation; the other 2 subjects expressed G551/R334 and S549/S549, respectively.
Values are prebronchodilator for controls and postbronchodilator for subjects with CF.
PMN data available for 13 controls and 8 subjects with CF.
FVC, force vital capacity; FEV1, force expiratory volume (1 s); FEF 25–75, force expiratory flow at 25%–75% FVC; PMN, polymorphonuclear leukocytes (neutrophils).
ATP hydrolysis.
Measurements with HBECs were performed as previously described (21). Briefly, cultures were maintained in (basolateral) ALI medium and the apical surface rinsed (3×) with PBS and preincubated for 1 h with 250 μL Hanks’ balanced salt solution (HBSS) supplemented with 2 mM CaCl2 and 2 mM MgCl2 and buffered with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; 25 mM pH 7.4). To assess ATP hydrolysis under first-order kinetics, incubations were initiated by the addition of 2 μL 0.1 μCi [γ32P]ATP (Perkin-Elmer; S.A. > 3,000 Ci/mmol); no ATP mass was added to these incubations At the times indicated, 10 μL mucosal solutions were transferred to 1.5-mL Eppendorf tubes containing 100 μL 1 mM ATP, heated at 95°C for 2 min to inactivate potential ATPase activities shed from cells, cooled on ice, and analyzed by HPLC (see below). To assess the rates of hydrolysis of various ATP concentrations, 0.03–1.0 mM ATP was added to cells concurrently with [γ32P]ATP and aliquots were collected at 2, 5, 15, 30, and 60 min. The initial rate of ATP hydrolysis was calculated from incubations that exhibited <10% [γ32P]ATP hydrolysis. For experiments with ATPase inhibitors, 25 μL vehicle or drugs (10×) were added to cells 10 min prior to addition of [γ32P]ATP. To measure ATP hydrolysis in sputum supernatants, 5 μL 0.1 μCi [γ32P]ATP was added to 45 μL ice-cold sputum supplemented with 2 mM CaCl2, 2 mM MgCl2, and 25 mM HEPES pH 7.4 and vehicle/inhibitor as indicated. Samples were incubated at 37°C for 15 min. Reactions were terminated by transferring samples to ice followed by the addition of 100 μL 1 mM ATP and heated at 95°C, as above.
32P-labeled species were quantified via a Waters HPLC using a Symmetry C15 5-μm (4.6 × 150 mm) column eluted with 1 mL/min of a mobile phase consisting of 100 mM KH2PO4 and 8 mM tetrabutylammonium bisulfate in 20% methanol pH 5.3. Elution times of 32P-labeled species were 32Pi, 2.3 min; 32PPi, 3.1 min; and [32P]ATP, 5.3 min.
ATP release.
To promote acute ATP release, HBECs were subjected to hypotonic cell swelling, as previously described (35). Briefly, the apical surface was rinsed and preincubated undisturbed for 1 h in 100 μL luminal HBSS. In the absence/presence of 100 μM polyoxometalate-5 (POM-5), 100 μL saline or H2O were added to cells. After 5 min, 100 μL of the apical solution was removed, transferred to tubes containing 100 μL ice-cold H2O, heated at 95°C as above, and stored at −80°C until further analysis. To induce chronic shear stress-promoted ATP release, cultures were subjected to phasic motion for 1 h in 60 μL HBSS in the presence or absence of 100 μM POM-5 (5). At the end of the incubation, 30 μL ASL were transferred to tubes containing 170 μL ice-cold H2O and processed as above.
Quantification of adenine nucleotide concentrations.
Concentrations of ATP, ADP, AMP, and adenosine were measured by a modification of the etheno-derivatization technique previously described (21). Briefly, 200 μL samples were incubated with 0.5 M 2Cl-acetaldehyde in citrate-phosphate buffer pH 4.0 (45 min, 80°C), and the resulting fluorescent etheno species were separated by HPLC (injection volume = 50 μL) via an RP-18c Chromolith column (11). Ethenylated (e) species were eluted as follows: eAMP, 2.47 min; eAdo, 3.22 min; eADP, 4.13 min; eATP, 6.99 min.
ASL height.
Cells were maintained at an air-liquid interface overnight under phasic motion (0.5 dyn/cm2, 14 cycles/min) (5). Afterward, PBS or PBS containing 1 mM POM-5 was applied via an Aeroneb Laboratory Nebulizer [final (POM-5) = 100 μM; volume applied = 100 nL], and ASL height was measured by confocal microscopy, as in Ref. 5.
Real-time PCR analysis.
Total RNA was isolated using the Zymo Direct-zol RNA mini prep kit and 500 ng RNA were reverse-transcribed into cDNA using Superscript II. RT-PCR for ENTPD1, ENTPD2, ENTPD3, ENTPD8, and NSAP was performed using TaqMan Gene Expression Assays from Applied Biosystems. GAPDH was used as a reference gene.
Transepithelial resistance (RT) was measured via an EVOM2 epithelial volt ohm meter, as described (9).
Reagents.
4,4ʹ-Diisothiocyano-2,2ʹ-stilbenedisulfonic acid (DIDS), pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), β,γ-MetATP, and levamisol were purchased from Sigma-Aldrich (St. Louis, MO). DIDS was obtained from Toronto Research Chemicals (Toronto, ON, Canada). POM-1 (Na6[H2W12O40].21H2O), NF 279, PSB 06126, PSB 069, and ARL 67156 were from Tocris Biosciences (Minneapolis, MN). POM-4 (K6H2[TiW11CoO40].13H2O), POM-5 (K10[Co4(H2O)2(PW9O342)2].22H2O), and POM-6 ((NH4)18[NaSb9W21O86].14H2O) were prepared according to published procedures (22, 24). Other chemicals were from sources reported previously (21).
Statistics.
Except when indicated otherwise, comparisons between sample groups were tested with independent t tests (JMP Pro-12). Because of overall small sample sizes, no adjustments for multiple comparisons were made. P value < 0.05 was considered significant. All results are presented as mean ± SD.
RESULTS
ATP hydrolysis in HBEC ASL.
The stability of radiotracer amounts of [γ32P]ATP was assessed in ASL bathing non-CF and CF HBEC. [γ32P]ATP hydrolysis exhibited first-order kinetics with similar rate constants (k) in non-CF and CF cells and comparable half-live values (non-CF, t1/2 = 1.9 min; CF, t1/2 = 1.4 min) (Fig. 1). In both CF and non-CF HBEC, 32Pi was the sole product of [γ32P]ATP hydrolysis, i.e., at no time was the formation of 32PPi observed. The absence of 32PPi accumulation was not due to PPi degradation (PPi→2Pi), as 32PPi added to cells remained unaltered after 60 min (not shown). These results suggest that ecto-ATPases other than ENPPs (e.g., ENTPDs and/or NSAP) are responsible for the rapid ATP depletion observed in non-CF and CF HBEC ASL.
Fig. 1.
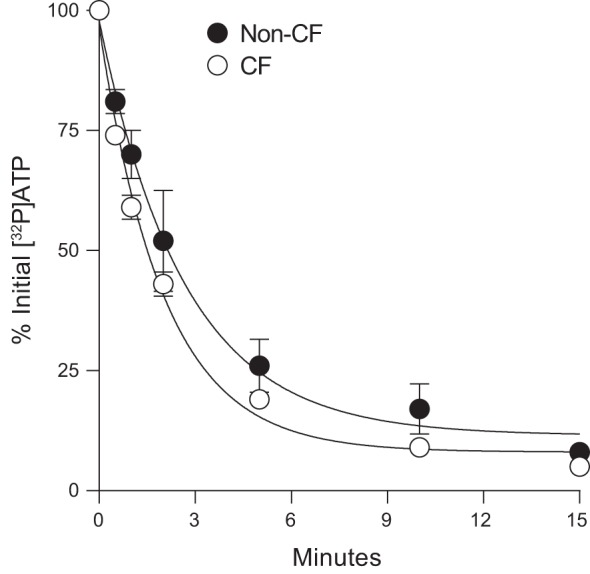
Decay of [γ32P]ATP on human bronchial epithelial cells (HBEC). Incubations were initiated by the addition of 0.1 μCi [γ32P]ATP to 250 μL HBSS bathing the luminal surface of non-cystic fibrosis (CF) and CF HBEC cultures. Aliquots were removed at the time indicated and the resulting 32P-labeled species were quantified by HPLC, as described in methods. The data represent the mean ± SD from three experiments. Exponential decay lines were generated, and first-order rate constant values were calculated (non-CF, k = 0.37 min−1; CF, k = 0.49 min−1) using Sigma Plot 10 regression fitting.
ENTPD and NSAP expression in HBEC.
The relative abundance of ENTPDs and NSAP was assessed via real-time PCR analysis. ENTPD3 and NSAP were the predominant ATPase genes amplified in control and CF cells. ENTPD1 and ENTPD2 were also amplified in control and CF HBEC but with apparently lower abundance than ENTPD3 (Fig. 2). ENTPD8 expression was below detection limit, i.e., the amplification signal was undistinguished from background after 35 PCR cycles. No differences were observed between control and CF cells in the levels of expression of these genes (Fig. 2).
Fig. 2.
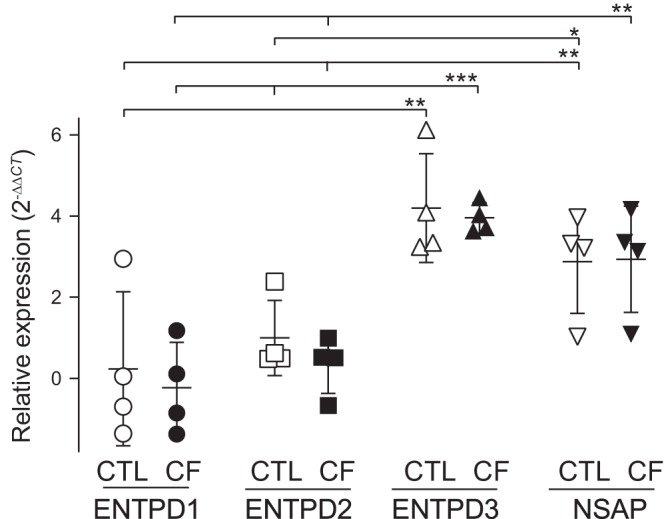
RT-PCR of ecto-nucleoside triphosphate diphosphohydrolase (ENTPDs) and nonspecific alkaline phosphatase (NSAP) in human bronchial epithelial cells (HBEC). mRNA quantification was performed in HBEC cultures from four non-cystic fibrosis (CF) (control, CTL) and four CF donors. Values were log transformed to achieve normality, and mean differences between CF and non-CF HBEC data were assessed with linear mixed models, with a sample as random effect factor (R package, nlme). The data were plotted using the 2(−∆∆CT) transformation [CT, threshold cycles (33)]. The mean value for CTL ENTPD1 expression was used as relative baseline value. ENTPD3 and NSAP were significantly different from ENTPD1 and ENTPD2. *P < 0.05; **P < 0.001; ***P < 0.0001.
Screening for inhibitors of ATP hydrolysis in airway epithelia.
Ecto-ATPase inhibitors described in the literature derive from the following chemical classes: 1) nucleotides and analogs (e.g., ARL 67156, β,γ-MetATP); 2) sulfonated dyes/anthraquinone derivatives (e.g., Reactive Blue, PSB 06126) (3); 3) suramin (a hexasulfonated naphthylurea) and analogs (e.g., NF 279) (25); and 4) polyoxometalates (POMs) (22). Additional molecules exhibiting ATPase inhibitory activity include the stilbene disulfonate DIDS (12) and pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (16). Lastly, levamisol (imidazothiazole derivative) is an effective although weak inhibitor of NSAP (30). The reported selectivity of these inhibitors toward ecto-ATPases is summarized in Table 2.
Table 2.
Reported selectivity of ATPase inhibitors
| Inhibitor | Enzyme Selectivity | Reference |
|---|---|---|
| ARL 67156 | ENTPD1 > ENTPD3 | (24, 25) |
| β,γ-MetATP | ENPP* | (18) |
| PSB 06126 | ENTPD3r | (3) |
| NF 279 | ENTPD1, ENTPD2, ENTPD3, ENTPD8 | (26) |
| DIDS | Unidentified ATPase† | (12) |
| PPADS | ENTPD1r, ENPPsr | (16) |
| Levamisol | NSAP‡ | (31) |
| POM-1 | ENTPD, ENTPD2, ENTPD3, ENPP1 | (23) |
| POM-4 | ENTPD1, ENTPD2, ENTPD3, ENPP1, ENPP2, ENPP3, NSAP | (23) |
| POM-5 | ENTPD1, ENTPD2, ENTPD3, ENPP1 | (23) |
| POM-6 | ENTPD2, ENTPD3, ENPP1 | (23) |
Endogenously expressed in rat PC12.
Endogenously expressed in rat parotid.
Endogenously expressed in human bronchial epithelial cells.
Rat, recombinant; all the others are human, recombinant.
ENPP, ecto-nucleotide pyrophosphatase/phosphodiesterase; ENTPD, ecto-nucleoside triphosphate diphosphohydrolase; NSAP, nonspecific alkaline phosphatase; POM, polyoxometalate; PPADS, pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid.
As an initial screen to identify candidate molecules that inhibit ATP hydrolysis in HBEC ASL, the effects of members of the above mentioned chemical groups on [γ32P]ATP hydrolysis were investigated. Because of the limited availability of primary cultures of CF cells, our initial screen used HBEC from non-CF donors.
As shown in Fig. 3, nearly 80% of the initial [γ32P]ATP administrated to cells was hydrolyzed within 5 min. Levamisol had little to no effect on this hydrolysis, ruling out contributions of NSAP. The nonspecific ENPP inhibitor β,γ-MetATP also had negligible effects on [γ32P]ATP hydrolysis. NF 279, which was shown to inhibit ENTPDs (25), markedly reduced ATP hydrolysis after the initial 5–15 min, but 97% [γ32P]ATP was hydrolyzed after 60 min. PPADS, a P2XR antagonist shown to inhibit rat ENTPD1 and ENTPD2 (16), also delayed but did not prevent ATP hydrolysis. Similarly, ARL 67156, a relatively weak inhibitor of ENTPDase1 and ENTPD3 that has no effect on ENTPD2 (23, 24), showed a partial and transient effect. A more robust inhibition of [32P]ATP hydrolysis was observed with PSB 06126, DIDS and, most sustainable, POM-1 (Fig. 3). The DIDS analog SITS displayed no effect at all on ATP hydrolysis (not shown). Attempts to test for the effect of the PSB 06126 analog PSB-069 were unsuccessful due to its poor solubility in aqueous solutions (3).
Fig. 3.
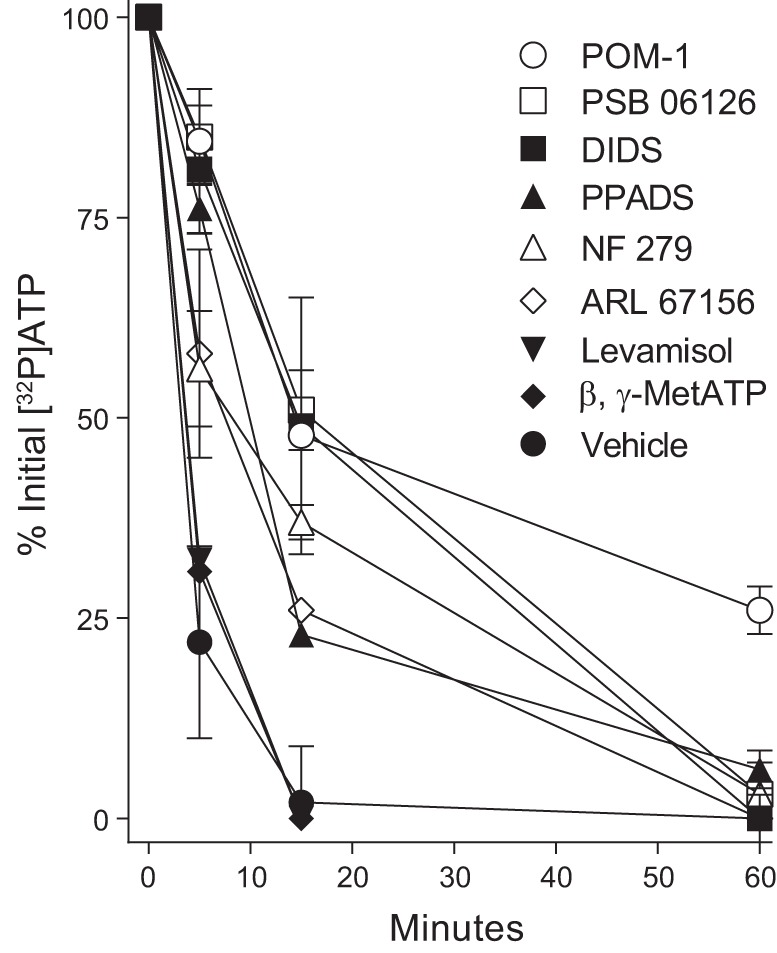
Effect of ATPase inhibitors on ATP hydrolysis in human bronchial epithelial cells. Cells were incubated with [γ32P]ATP as in Fig. 1 in the absence or presence of the indicated drug. All inhibitors were at 100 μM final concentration, except levamisol (10 mM). The data indicate the mean ± SD from 2–4 independent experiments. POM, polyoxometalate; PPADS, pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid.
POM-5 is a potent and effective inhibitor of ATP hydrolysis in HBEC.
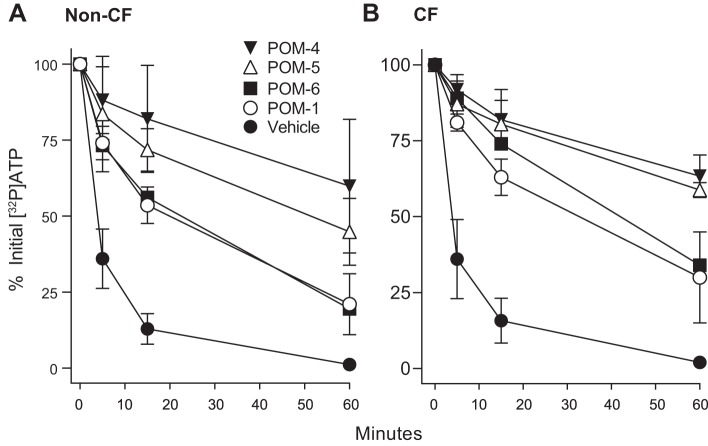
Recently, various POMs have been evaluated and shown to exhibit similar or more potent inhibition, relative to POM-1, on recombinant ecto-ATPases (22). Accordingly, the effects of POM-1, POM-4, POM-5, and POM-6 were examined in both non-CF and CF HBEC. POM-4 and POM-5 exhibited more robust and long-lasting inhibition of ATP hydrolysis than POM-1 and POM-6 in both non-CF and CF HBEC; 50–80% of the initial [32P]ATP was recovered after 60 min in the presence of POM-4 or POM-5. No major differences were observed between non-CF and CF HBEC regarding POM inhibition (Fig. 4).
Fig. 4.
Effect of polyoxometalates (POMs) on ATP hydrolysis on non-cystic fibrosis (CF) (A) and CF (B) human bronchial epithelial cells. Cells were incubated with [γ32P]ATP as in previous figures. POMs (100 μM) were added to cells 10 min prior to [γ32P]ATP. The data represent the mean ± SD from at least two independent experiments.
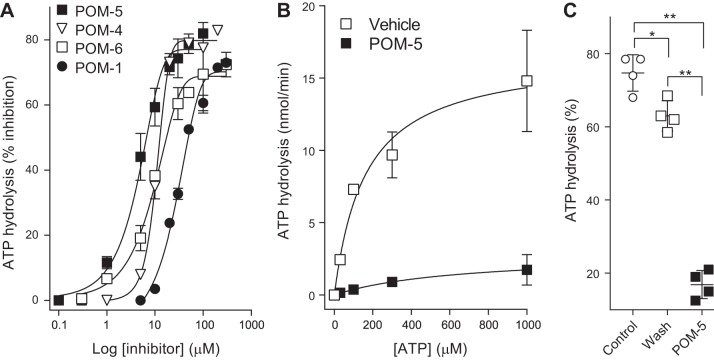
Inhibitor concentration-effect relationships for [32P]ATP hydrolysis on HBEC indicated that POM-5 was a more potent inhibitor (IC50 = 4.8 μM) than POM-6 (IC50 = 9.8 μM) and POM-4 (IC50 = 10.6 μM), whereas POM-1 exhibited relatively weaker inhibition (IC50 = 32.1 μM) (Fig. 5A). To gain further insight into the nature of POM-5 inhibition, the initial rates of hydrolysis of various ATP concentrations were determined in the absence or presence of 30 μM POM-5. In naïve cells, ATP hydrolysis rates increased exponentially with substrate concentration, but changes in hydrolysis rates were blunted by 30 μM POM-5 (Fig. 5B). The minor effect of substrate concentration on POM-5 inhibition suggests that POM-5 either acts as a noncompetitive inhibitor or exhibits an affinity for ENTPDs that is considerably higher than ATP, as reported with POM-1 in cerebellar slices (41). In addition, as shown with POM-1 in cerebellum (41), POM-5 inhibition of ATP hydrolysis in HBEC was largely reversible upon removing the inhibitor from cells. Incubation of HBEC with 30 μM POM-5 resulted in robust inhibition of [32P]ATP hydrolysis, but hydrolysis returned to near (although not identical to) control values in cells that were incubated with 30 μM POM-5, then washed and incubated with [32P]ATP (Fig. 5C).
Fig. 5.
Polyoxometalate (POM)-5 is a potent, noncompetitive, reversible inhibitor of ATP hydrolysis in human bronchial epithelial cells. A: hydrolysis of [γ32P]ATP was assessed for 15 min in the presence of the indicated concentration of POM-1, POM-4, POM-5, or POM-6. B: the initial rates of hydrolysis of 30, 100, 300, and 1,000 μM ATP were measured in the absence or presence of 30 μM POM-5. The data in A and B represent the mean ± SD from two experiments performed with duplicate samples. The sigmoidal curves (A) and exponential rise regression lines (B) were fitted using Sigma Plot 10. C: inhibition of ATP hydrolysis is greatly reversed upon removal of POM-5: open squares, cells were preincubated (30 min) with POM-5 and then washed and incubated with [32P]ATP in the absence of POM-5 for 15 min; closed squares, naïve cells were incubated with [32P]ATP in the presence of 30 μM POM-5. The data are expressed as % ATP hydrolysis relative to cells that were not exposed to POM-5. Values (mean ± SD; n = 4 per condition) are representative of three independent experiments. *P < 0.05; **P < 0.001.
POM-5 markedly reduces the hydrolysis of released ATP.
The effectiveness of POM-5 to prevent the metabolism of released ATP was assessed in HBEC subjected to acute (5 min) hypotonic cell swelling (27, 35). To assess the pattern of nucleotide/nucleoside distribution following hypotonicity-induced ATP release, the entire spectrum of adenine nucleotides/nucleosides in ASL was quantified using the etheno-derivatization technique (21). In static, vehicle-treated HBECs, concentrations of ADP (~145 ± 46 nM) and AMP (255 ± 47 nM) were markedly higher than ATP (7 ± 3 nM) and Ado (8 ± 17 nM) (Fig. 6). ATP represented <2% of the total extracellular nucleotide/nucleoside pool in static cells. Hypotonic challenge produced a 3-fold increase of total extracellular nucleotides in both vehicle-treated and POM-5-treated cells (net increase of ~800 nM). However, striking differences in nucleotide distributions were observed with POM-5 treatment. In the absence of POM-5, levels of Ado, AMP, and ADP markedly increased (to 114 ± 18 nM, 433 ± 54 nM, and 702 ± 103 nM, respectively), but ATP levels remained in the low nM range (13 ± 5 nM). In contrast, in the presence of POM-5, ATP levels increased >50-fold (isotonic, 10 ± 3 nM; hypotonic, 552 ± 110 nM) and represented nearly 60% of the newly released adenine purine pool (Fig. 6). These findings indicate that POM-5 greatly (although not completely) reduced the ability of ENTPDs to hydrolyze ATP upon its release from cells.
Fig. 6.
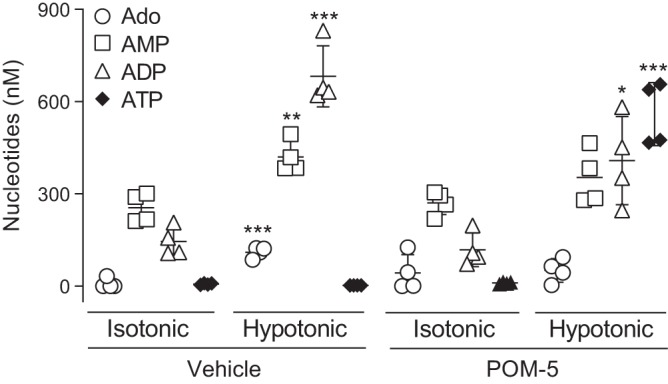
Polyoxometalate (POM)-5 reduces the hydrolysis of released ATP. Human bronchial epithelial cells were subjected to hypotonic cell swelling for 5 min in the presence of 100 μM POM-5 (or vehicle, PBS), and adenosine (Ado), AMP, ADP, and ATP were quantified by etheno-derivatization and HPLC analysis, as indicated in methods. The scatter plots indicate the mean ± SD, n = 4 per condition. *P = 0.009; **P = 0.0033; ***P < 0.0005 (isotonic vs. hypotonic). Similar data were obtained with two independent experiments.
POM-5 improves ASL volume regulation in CF HBEC cells.
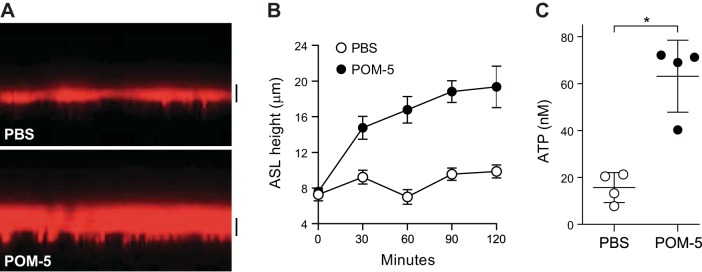
The ultimate goal of our study was to identify reagents that, by inhibiting ATP hydrolysis, would increase P2Y2R-mediated fluid transport in CF cells. Therefore, the effect of POM-5 on ASL volume regulation was investigated in CF HBEC. Cells were incubated overnight under oscillatory shear stress that mimics the phasic motion of the lung in vivo (6, 39, 40), following which 100 nL 1 mM POM-5 was apically administrated via nebulization [final (POM-5) = 100 μM], as described (5). Confocal microscopy was used to measure ASL heights as an index of ATP-mediated fluid secretion (Fig. 7A and Ref. 5). As we previously reported (40), shear stress-stimulated CF HBEC exhibited an ASL height of ~5–7 μm (Fig. 7, A and B), which is markedly lower than the ~14-μm height measured in normal HBEC under similar conditions (40). Notably, POM-5 increased ASL height in phasic motion-stimulated CF HBEC to ~15–20 μm, and ASL height remained elevated after 2 h in the presence of POM-5 (Fig. 7B). In parallel measurements, a >3-fold increase of ASL ATP concentration was observed in CF HBEC subjected to phasic motion in the presence, but not absence, of POM-5 (Fig. 7C).
Fig. 7.
Polyoxometalate (POM)-5 improves airway surface liquid (ASL) volume regulation in cystic fibrosis (CF) human bronchial epithelial cells (HBEC). ASL height and nucleotide levels were measured in CF HBEC subjected to phasic motion, as described in methods. A: representative confocal microscopy image taken at 60 min in the absence (PBS) or presence of 100 μM POM-5. Bar = 10 μm. B: quantification of ASL height in CF HBEC. The data represent the mean ± SD from 10 images/culture (4 cultures/condition; *P < 0.05) and are representative of two independent experiments. C: ATP release was measured in cells bathed with 60 μL PBS with/without 100 μM POM-5 for 60 min under phasic motion. The scatter plots indicate the mean ± SD (*P < 0.05) from four CF HBEC cultures and are representative of two independent experiments.
Control measurements indicated that POM-5 did not affect the transepithelial resistance (Table 3). Furthermore, the fact that no radioactivity was detected in the basolateral medium after 1 h of the combined addition of [32P]PATP and POM-5 to the mucosal bath (not shown) suggests that POM-5 did not affect the monolayer integrity.
Table 3.
POM-5 has no effect on transepithelial cell resistance
|
RT, Ω/cm2 |
||
|---|---|---|
| Minutes | PBS | 100 μM POM-5 |
| 0 | 1,097 ± 127 | 1,013 ± 12 |
| 5 | 816 ± 188 | 1,383 ± 237 |
| 30 | 1,170 ± 244 | 956 ± 108 |
| 60 | 780 ± 141 | 988 ± 106 |
| 120 | 885 ± 140 | 1,004 ± 25 |
Values are means ± SD; n = 3/group. Transepithelial resistance (RT) was measured in human bronchial epithelial cells via an EVOM2 epithelial volt ohm meter, as described (9). POM, polyoxometalate.
Increased ATPase activity in CF airway secretions.
Studies with cell cultures may not completely describe the mechanisms of nucleotide-regulated ASL hydration in vivo. For example, we have previously reported that sputum from subjects with CF exhibited enhanced ATP hydrolysis rates relative to controls (37). This observation suggests that ecto-ATPases secreted/shed from airway epithelial and/or inflammatory cells in CF airways in vivo also contribute to ATP depletion in ASL, further reducing the effectiveness of released nucleotides to hydrate intraluminal mucus in CF lungs. Therefore, we investigated associations between ATPase activities and nucleotide/nucleoside distribution in sputum supernatants from control and CF subjects.
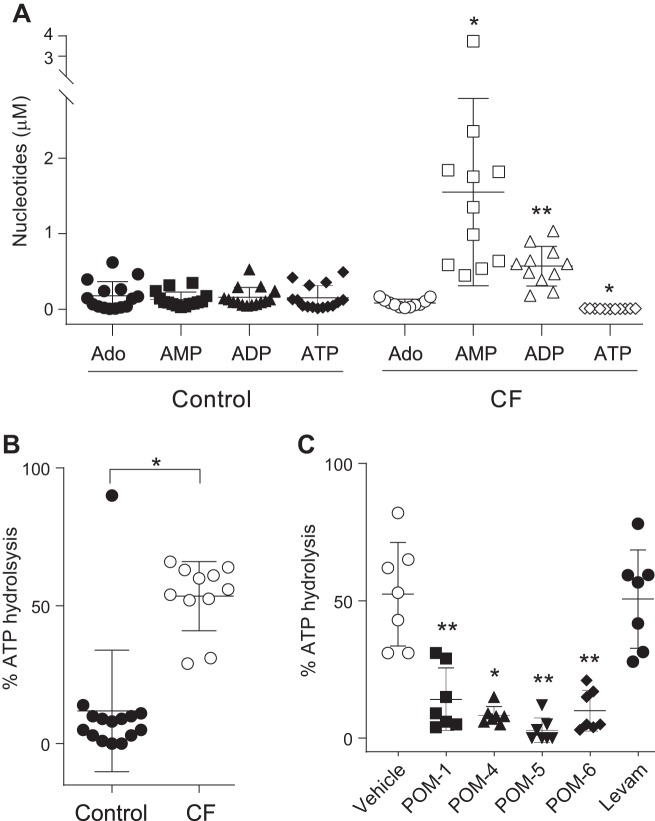
The total quantity of purines in CF sputa (2.4 ± 1.4 μM, mean ± SD) was fourfold higher than in control sputa (0.6 ± 0.4 μM), suggesting that nucleotide release is upregulated in CF airways, e.g., via VNUT nucleotide transporter upregulation (28). However, while AMP and ADP levels were elevated in CF sputa, ATP concentration in CF samples (0.006 ± 0.001 μM) was markedly reduced (>20× lower) relative to healthy controls (0.16 ± 0.05 μM) (Fig. 8A). Notably, ATP comprised 26% of the total nucleotide pool in control samples but only 0.25% of the total pool in CF (Fig. 8A). These data suggest that the reduced levels of ATP observed in CF secretions likely reflect increased ATP hydrolysis.
Fig. 8.
Enhanced ATPase activity in cystic fibrosis (CF) sputum. A: nucleotide levels in control (n = 15) and CF (n = 11) sputum supernatants are expressed as mean ± SD. *P = 0.004 and **P = 0.0002, relative to control. B: [32P]ATP was added to sputum supernatants from 15 controls and 11 subjects with CF for 15 min (37°C), and the resulting % hydrolysis is expressed as mean ± SD. *P < 0.0005. C: effect of polyoxometalates (POMs) and levamisol on ATP hydrolysis in CF sputum. The data (mean ± SD from 7 CF sputum samples) represent the % ATP hydrolysis observed in the presence of vehicle, 100 μM of the indicated POM, or 10 mM levamisol (Levam), *P = 0.0007 and **P ≤ 0.0001 against vehicle (paired t test). Ado, adenosine.
Direct measurements of [γ32P]ATP hydrolysis in sputum supported this hypothesis. Incubation of CF sputum supernatants with [γ32P]ATP for 15 min resulted in robust (53.5 ± 12.5%, mean ± SD) conversion of [γ32P]ATP to 32Pi. In contrast, 11.9 ± 22% conversion was observed in control sputa (Fig. 8B). Similar to our findings with HBEC, 32Pi was the sole product of [γ32P]ATP hydrolysis in sputum (not shown). Importantly, ATPase activity in CF sputa was nearly abolished by POM-5 and markedly reduced by POM-1, POM-4, and POM-6 but was not affected by levamisol (Fig. 8C). Collectively, these data suggest that ATP hydrolysis in CF sputum is driven by members of the ENTPD family of ecto-ATPases.
DISCUSSION
By rapidly dephosphorylating ATP (and ADP), ecto-ATPases generate AMP and facilitate the formation of adenosine, leading to A2BR-promoted cyclic AMP-regulated CFTR activity. This pathway results in sustained regulation of water fluxes in normal airway epithelia. However, adenosine/A2BR-mediated signaling fails to promote airway hydration in CF (21). Furthermore, by reducing ASL ATP levels, ecto-ATPases limit the ability of airway epithelia to promote fluid secretion via P2Y2R-mediated CaCC Cl− channel activity and inhibition of epithelial Na+ channel, dampening an otherwise key compensatory mechanism for fluid secretion in CF cells. Therefore, we hypothesized that inhibition of ATPase activities would facilitate ASL hydration in CF.
We tested this hypothesis by utilizing a [32P]ATP hydrolysis assay that, by closely predicting the decay of naturally occurring ASL ATP, allowed us to 1) identify molecules that potently and efficaciously reduced ATP hydrolysis in CF airway epithelial cells and increased the stability of released ATP and 2) demonstrated that inhibition of ATP hydrolysis led to increased ASL volume secretion onto CF airway epithelial cells.
As described in the present study (Fig. 1), ATP hydrolysis on airway surfaces exhibited first-order kinetics with rate constant and half-life values that were similar in CF and non-CF cells (Fig. 1). These data suggest that the CFTR defect inherent to CF cells does not directly affect the stability of physiologically occurring ATP levels in ASL. Our real-time PCR analyses, indicating no difference in expression levels of ENTPDs and NSAP between CF and non-CF cells, support the functional data.
An important finding of our study was the identification of POMs as potent inhibitors of ATP hydrolysis in airway epithelial cells. Polyoxometalates are anionic metal-oxides of early transition metals that, due to their negative charge, bear resemblance to nucleotides. This enables them to selectively block enzymes such as nucleotidases (38). Using membrane preparations isolated from COS-7 cells overexpressing human ecto-ATPases, Lee and collaborators (22) identified POM-5 as the most potent inhibitor ENTPD1, ENTPD2, and ENTPD3; POM-5 also inhibited ENPP1 but had no effect on ENPP2, ENPP3, and NSAP. POM-4 was the second most potent inhibitor of ENTPD1, ENTPD2, and ENTPD3 and also inhibited ENPP1, ENPP2, ENPP3, and NSAP. POM-1 inhibited ENTPD1, ENTPD2, and ENTPD3, but with markedly lower affinity (20–200-fold higher Ki values) than POM-4 and POM-5 (22). POM-6 inhibited ENTPD2 and ENTPD3 with affinities similar to POM-1 but was a weak and partial inhibitor of ENTPD1 [38% inhibition at 20 μM (22)]. None of these POMs inhibited ENTPD8 (22). Our results indicating 1) potent and strong inhibitory action of POM-5; 2) lack of effect of the NSAP inhibitor levamisol; and 3) absence of formation of 32PPi following the addition of [32P]ATP to cells led us to hypothesize that ENTPD1, ENTPD2, and/or ENTPD3 were responsible for ATP hydrolysis on HBEC. Furthermore, PSB 06126, a potent and highly selective inhibitor of rat ENTPD3 (3), markedly delayed (but did not prevent) [32P]ATP hydrolysis in HBEC (Fig. 3), suggesting that ENTPD3 is one important (but not the only) contributor. Unambiguous elucidation of the roles of ENTPD1, ENTPD2, and ENTPD3 in the regulation of ATP concentrations in ASL will require the development of selective inhibitors of these enzymes and/or the use of molecular approaches to knockdown/knockout these molecules.
Importantly, >60% of the initial [32P]ATP was recovered intact in ASL in the presence of POM-4 and POM-5 after 1 h (Fig. 3), suggesting that these POMs markedly prolong the stability of physiologically relevant concentrations of ATP. Indeed, POM-5 enhanced ATP levels resulting from acute cell swelling-promoted ATP release (Fig. 6). Notably, in response to prolonged, physiologically relevant stimulation, i.e., shear stress-promoted ATP release, POM-5 increased ATP levels and produced rehydration of ASL in CF HBEC (Fig. 7). This observation provides a proof of concept for the use of ENTPD inhibitors as therapeutic agents to restore ASL volume regulation in CF cells.
The mechanisms of nucleotide-regulated ASL hydration in inflamed CF airways are more complex than those described in CF cells cultured in the absence of an inflammatory/infectious environment. To mimic the in vivo CF environment, HBEC have been chronically exposed to CF-relevant inflamed environments, e.g., sterile supernatants of mucopurulent material (SMM) from CF lungs. Notably, SMM-exposed cell cultures exhibited mucus hyperproduction (1, 13) and enhanced expression of ENTPD3 (13). Although not assessed here, the enhanced ENTPD3 activity associated with SMM-inflamed HBEC is predicted to be inhibited by POMs, most potently by POM-5 (22). However, we did study CF sputum. Our data showed that cell-free sputum supernatants from subjects with CF exhibited markedly (>20×) decreased concentrations of endogenous ATP and increased levels of AMP and ADP, consistent with increased ATPase activity (Fig. 8). These supernatant data suggest that, in addition to cell-bound activities, ATPases either secreted from submucosal glands, as we previously reported (10), or shed with exosomes contribute to ATP depletion in CF airways. In this regard, Clayton and coworkers (7) demonstrated that plasma membrane-tethered ecto-nucleotidases associated with secreted exosomes accounted for 20% of the total ATP-hydrolytic activity in pleural effusions from patients with mesothelioma.
Similar to HBEC, [γ32P]ATP hydrolysis in CF sputum resulted in formation of 32Pi (but not 32PPi), was markedly reduced in the presence of POMs, and was not affected by levamisol, strongly suggesting that ATP hydrolysis in CF lung secretions is driven by ENTPDs. Based on the elevated number of neutrophils present in CF sputa (Table 1), one speculation is that ENTPD1, which is abundantly expressed in inflammatory cells (8, 31), is shed from neutrophils and contributes to ATP hydrolysis in CF lung secretions, as reported in COPD (18). This hypothesis is apparently at odds with our data indicating strong inhibition of ATP hydrolysis with POM-6, which inhibits ENTPD2 and 3 but, as mentioned above, had only a partial effect on ENTPD1 (22). As discussed above for HBEC, the molecular identification of the enzyme(s) responsible for ATP hydrolysis in lung secretions awaits the development of ENTPD subtype-specific inhibitors.
In summary, we have identified polyoxometalates that markedly reduce the hydrolysis of ATP by airway epithelial cells and inflamed CF sputum. By increasing the stability of released ATP in ASL, POM-5 restored ASL volume regulation in CF cells, suggesting that ecto-ATPase inhibitors are candidate tools to improve ASL/mucus hydration in CF. An ancillary observation in our study is that ATPase activity is elevated in CF lung secretions, suggesting that secreted/shed ATPases contribute to the positive feedback cycle of ATP degradation, airway/mucus dehydration, obstruction, and inflammation that characterizes the CF airways.
GRANTS
This study was supported by NIH Grants R56 HL136909, R01 HL125280, UH3 HL123645, P01 HL110873, R01 HL136961, P30 DK065988-13, P01 HL108808, and Specialized Centers of Clinically Oriented Research (SCCOR) P50-HL-084934, and by Cystic Fibrosis Foundation Grants LAZARO18P0, LAZARO19GO, and BOUCHE15R0.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.v.H., H.S., R.C.B., and E.R.L. conceived and designed research; C.v.H., B.B., W.H.A., L.C.M., H.D., N.E.A., S.D., H.S., R.C.B., and E.R.L. performed experiments; C.v.H., B.B., W.H.A., A.C., L.C.M., W.K.O., H.D., H.S., R.C.B., and E.R.L. analyzed data; C.v.H., H.S., R.C.B., and E.R.L. interpreted results of experiments; C.v.H., W.H.A., A.C., W.K.O., H.D., and E.R.L. prepared figures; C.v.H., H.S., R.C.B., and E.R.L. drafted manuscript; C.v.H., B.B., W.H.A., A.C., L.C.M., S.D., H.S., R.C.B., and E.R.L. edited and revised manuscript; C.v.H., B.B., W.H.A., A.C., L.C.M., W.K.O., H.D., N.E.A., S.D., H.S., R.C.B., and E.R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Scott Randell and the University of North Carolina Tissue Core for supplying HBEC cultures, to Dr. José L. Boyer for helpful comments, and to Eric Roe for editorial assistance. We thank Karin Landrock for technical support in the synthesis of POM-4, POM-5, and POM-6.
REFERENCES
- 1.Abdullah LH, Coakley R, Webster MJ, Zhu Y, Tarran R, Radicioni G, Kesimer M, Boucher RC, Davis CW, Ribeiro CMP. Mucin production and hydration responses to mucopurulent materials in normal versus cystic fibrosis airway epithelia. Am J Respir Crit Care Med 197: 481–491, 2018. doi: 10.1164/rccm.201706-1139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med 192: 182–190, 2015. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baqi Y, Weyler S, Iqbal J, Zimmermann H, Müller CE. Structure-activity relationships of anthraquinone derivatives derived from bromaminic acid as inhibitors of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). Purinergic Signal 5: 91–106, 2009. doi: 10.1007/s11302-008-9103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burch LH, Picher M. E-NTPDases in human airways: regulation and relevance for chronic lung diseases. Purinergic Signal 2: 399–408, 2006. doi: 10.1007/s11302-006-9001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal 6: ra46, 2013. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol 580: 577–592, 2007. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 187: 676–683, 2011. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 8.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem 283: 28480–28486, 2008. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne CB, Ribeiro CM, Boucher RC, Johnson LG. Acute mechanism of medium chain fatty acid-induced enhancement of airway epithelial permeability. J Pharmacol Exp Ther 305: 440–450, 2003. doi: 10.1124/jpet.102.047654. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med 6: 969–982, 2000. doi: 10.1007/BF03401831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donoso VM, Hernández F, Villalón T, Acuña-Castillo C, Pablo Huidobro-Toro J. Pharmacological dissection of the cellular mechanisms associated to the spontaneous and the mechanically stimulated ATP release by mesentery endothelial cells: roles of thrombin and TRPV. Purinergic Signal 14: 121–139, 2018. doi: 10.1007/s11302-017-9599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowd FJ, Li LS, Zeng W. Inhibition of rat parotid ecto-ATPase activity. Arch Oral Biol 44: 1055–1062, 1999. doi: 10.1016/S0003-9969(99)00100-4. [DOI] [PubMed] [Google Scholar]
- 13.Fausther M, Pelletier J, Ribeiro CM, Sévigny J, Picher M. Cystic fibrosis remodels the regulation of purinergic signaling by NTPDase1 (CD39) and NTPDase3. Am J Physiol Lung Cell Mol Physiol 298: L804–L818, 2010. doi: 10.1152/ajplung.00019.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol Cell Physiol 272: C1058–C1066, 1997. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 15.Henderson AG, Anderson WH, Ceppe A, Coakley RD, Button B, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Fuller F, Almond M, Qaqish B, Kesimer M, Boucher RC. Mucus hydration in subjects with stable chronic bronchitis: a comparison of spontaneous and induced sputum. COPD 15: 572–580, 2018. doi: 10.1080/15412555.2019.1566892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann C, Heine P, Pradel G, Kim YC, Jacobson KA, Zimmermann H. Inhibition of ecto-apyrase and ecto-atpase by pyridoxal phosphate-related compounds. Drug Dev Res 51: 153–158, 2000. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia Y, Mathews CJ, Hanrahan JW. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem 272: 4978–4984, 1997. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- 18.Lazar Z, Müllner N, Lucattelli M, Ayata CK, Cicko S, Yegutkin GG, De Cunto G, Müller T, Meyer A, Hossfeld M, Sorichter S, Horvath I, Virchow CJ, Robson SC, Lungarella G, Idzko M. NTPDase1/CD39 and aberrant purinergic signalling in the pathogenesis of COPD. Eur Respir J 47: 254–263, 2016. doi: 10.1183/13993003.02144-2014. [DOI] [PubMed] [Google Scholar]
- 19.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9: 262–267, 2009. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br J Pharmacol 106: 774–782, 1992. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SY, Fiene A, Li W, Hanck T, Brylev KA, Fedorov VE, Lecka J, Haider A, Pietzsch HJ, Zimmermann H, Sévigny J, Kortz U, Stephan H, Müller CE. Polyoxometalates–potent and selective ecto-nucleotidase inhibitors. Biochem Pharmacol 93: 171–181, 2015. doi: 10.1016/j.bcp.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sévigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152: 141–150, 2007. doi: 10.1038/sj.bjp.0707361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller CE, Iqbal J, Baqi Y, Zimmermann H, Röllich A, Stephan H. Polyoxometalates–a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett 16: 5943–5947, 2006. doi: 10.1016/j.bmcl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Munkonda MN, Kauffenstein G, Kukulski F, Lévesque SA, Legendre C, Pelletier J, Lavoie EG, Lecka J, Sévigny J. Inhibition of human and mouse plasma membrane bound NTPDases by P2 receptor antagonists. Biochem Pharmacol 74: 1524–1534, 2007. doi: 10.1016/j.bcp.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell 21: 2639–2648, 2010. doi: 10.1091/mbc.e09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada SF, Ribeiro CM, Sesma JI, Seminario-Vidal L, Abdullah LH, van Heusden C, Lazarowski ER, Boucher RC. Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways. Am J Respir Cell Mol Biol 49: 814–820, 2013. doi: 10.1165/rcmb.2012-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picher M, Boucher RC. Biochemical evidence for an ecto alkaline phosphodiesterase I in human airways. Am J Respir Cell Mol Biol 23: 255–261, 2000. doi: 10.1165/ajrcmb.23.2.4088. [DOI] [PubMed] [Google Scholar]
- 30.Picher M, Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem 279: 20234–20241, 2004. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- 31.Pulte ED, Broekman MJ, Olson KE, Drosopoulos JH, Kizer JR, Islam N, Marcus AJ. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res 121: 309–317, 2007. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3: 71–85, 2013. [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science 271: 1876–1879, 1996. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 35.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286: 26277–26286, 2011. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, Lazarowski ER. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol 304: C976–C984, 2013. [Erratum in Am J Physiol Cell Physiol 306: C415, 2014.] doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sesma JI, Weitzer CD, Livraghi-Butrico A, Dang H, Donaldson S, Alexis NE, Jacobson KA, Harden TK, Lazarowski ER. UDP-glucose promotes neutrophil recruitment in the lung. Purinergic Signal 12: 627–635, 2016. doi: 10.1007/s11302-016-9524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephan H, Kubeil M, Emmerling F, Muller CE. Polyoxometalates as versatile enzyme inhibitors. Eur J Inorg Chem 2013: 1585–1594, 2013. doi: 10.1002/ejic.201201224. [DOI] [Google Scholar]
- 39.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol 68: 543–561, 2006. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 40.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280: 35751–35759, 2005. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall MJ, Wigmore G, Lopatár J, Frenguelli BG, Dale N. The novel NTPDase inhibitor sodium polyoxotungstate (POM-1) inhibits ATP breakdown but also blocks central synaptic transmission, an action independent of NTPDase inhibition. Neuropharmacology 55: 1251–1258, 2008. doi: 10.1016/j.neuropharm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J Biol Chem 273: 14053–14058, 1998. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8: 437–502, 2012. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]