Abstract
Eosinophils mediate airway hyperresponsiveness by increasing vagally mediated reflex bronchoconstriction. Here, we tested whether circulating or airway eosinophils change nerve function. Airway resistance in response to aerosolized 5-hydroxytryptamine (5-HT, 10–300 mM) was measured in wild-type mice or transgenic mice that overexpress IL5 in T cells (+IL5T), overexpress IL5 in airway epithelium (+IL5AE), or overexpress IL5 but are devoid of eosinophils (+IL5AE/−Eos). Inflammatory cells in bronchoalveolar lavage (BAL), blood, and bone marrow were quantified. Blood eosinophils were increased in +IL5T and +IL5AE mice compared with wild-type mice. +IL5T mice had increased eosinophils in bone marrow while +IL5AE mice had increased eosinophils in BAL. Eosinophils surrounding large airways were significantly increased only in +IL5AE mice. With intact vagal innervation, aerosolized 5-HT significantly increased airway resistance in +IL5AE mice. 5-HT-induced bronchoconstriction was blocked by vagotomy or atropine, demonstrating that it was mediated via a vagal reflex. Airway resistance was not increased in +IL5AE/−Eos mice, demonstrating that it required lung eosinophils, but was not affected by increased bone marrow or blood eosinophils or by increased IL5 in the absence of eosinophils. Eosinophils did not change M3 function on airway smooth muscle, since airway responses to methacholine in vagotomized mice were not different among strains. Eosinophils surrounding large airways were sufficient, even in the absence of increased IL5 or external insult, to increase vagally mediated reflex bronchoconstriction. Specifically blocking or reducing eosinophils surrounding large airways may effectively inhibit reflex hyperresponsiveness mediated by vagus nerves in eosinophilic asthma.
Keywords: airway hyperresponsiveness, anti-eosinophilia treatment, asthma, eosinophils, neural reflex
INTRODUCTION
Eosinophils are bone marrow-derived granulocytes whose maturation, migration, and survival are controlled by IL5 (52, 53). Eosinophilia is present in more than 50% of asthmatics (3). Activated eosinophils release their granule contents, including major basic protein (MBP), eosinophil peroxidase, eosinophil cationic protein, and eosinophil-derived neurotoxin, along with cytokines and chemokines. Thus, activated eosinophils contribute to airway inflammation, airway smooth muscle cell proliferation (24), and airway epithelial damage (22, 30). In addition, eosinophils also induce airway hyperresponsiveness by altering airway nerve architecture (11) and function (1). Circulating eosinophils have been used as a key marker to both predict asthma severity (3) and guide treatment (3, 27, 37).
Anti-IL5 therapy has been developed to treat severe eosinophilic asthma. Blocking IL5 effectively depletes eosinophils in bone marrow and circulation in all species tested, including mice (16) and humans (38), while overexpression of IL5 enhances eosinophil recruitment (53). Monoclonal antibodies targeting IL5 (reslizumab, mepolizumab) or its receptor (benralizumab) are promising therapeutic options for patients with severe asthma. In phase III studies, reslizumab reduces exacerbation rates by 50% in patients who have severe asthma and a high blood eosinophil count (5). In addition, anti-IL5 treatments slightly improve forced expiratory volume during the first second (FEV1) (2, 14, 15, 34), a measurement for lung function. However, reducing blood eosinophils does not always predict improved lung function. For example, in a recent clinical trial (12), mepolizumab significantly reduces circulating eosinophils in 97% of recruited patients with severe eosinophilic asthma but improves lung function in only 76% of these patients. This clinical trial raises a question as to whether peripheral blood eosinophil count is the best indicator for predicting efficiency of anti-IL5 treatment for severe eosinophilic asthma. Thus, further research is needed on biomarkers for prognosis and predicting treatment response.
An important mechanism in asthma is increased reflex bronchoconstriction. This is evidenced by the effectiveness of anticholinergic drugs in asthma (25) and vagotomy in animal models of asthma (18, 19, 44, 47, 54). One well described mechanism for increased reflex induced bronchoconstriction is eosinophil-mediated increased acetylcholine release from the parasympathetic nerves. Acetylcholine release is normally limited by inhibitory, prejunctional M2 muscarinic receptors on parasympathetic nerves (20). After antigen challenge, eosinophils are actively recruited to the airway nerves (8), where they release major basic protein, an endogenous antagonist for M2 receptors (26). Depleting eosinophils (44, 55), blocking eosinophil recruitment to the nerves (17, 21), or blocking major basic protein (13) each prevent airway hyperreactivity following antigen challenge. Thus, eosinophils increase acetylcholine release from parasympathetic nerves via loss of M2 receptor function (26).
Eosinophils also increase the afferent limb of the reflex arc by stimulating sensory nerve growth in the epithelium (11). Thus, in the lungs, eosinophils enhance both arms of the neural reflex, contributing to airway hyperresponsiveness in asthma (1, 11).
Given that in human asthma, peripheral blood eosinophil reduction does not synchronize with improvement of lung function after anti-IL5 interventions and airway hyperresponsiveness does not typically correct after anti-IL5 treatment (7, 12, 14), we used transgenic mice to study whether increased eosinophils, compartmentalized in lungs versus distributed peripherally in blood, enhance reflex induced bronchoconstriction.
METHODS
Animals.
All experiments were performed with 12– 20-wk-old male and female mice on a C57BL/6J background. Characteristics of transgenic mice used are in Table 1. Wild-type C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) and bred in house. We additionally used transgenic mice bred and characterized by Jamie Lee. These included NJ1638 mice that overexpress IL5 in peripheral T cells (+IL5T) and have increased eosinophils in peripheral blood (33, 41) and NJ1726 that overexpress IL5 in airway epithelium (+IL5AE) and have increased eosinophils in the lung, including the peribronchial space and airway lumen (32). We also used PHIL mice that express diphtheria toxin A under the eosinophil peroxidase promoter and are specifically devoid of eosinophils (−Eos) but otherwise have a full complement of leukocytes (31). To distinguish the role of eosinophils induced by increased IL5 from increased IL5 alone, mice were created by crossbreeding NJ1726 and PHIL transgenic mice (10) to create NJ1726/PHIL (+IL5AE/−Eos) mice.
Table 1.
Eosinophil distribution in mouse strains used
| Eosinophils Present In: | WT C57/BL6 Littermates |
+IL5T IL5 in T Cells |
+IL5AE IL5 in Airway Epithelium |
+IL5AE/–Eos IL5 in Airway Epithelial Cells × PHIL Mice with Diphtheria Toxin A Under EPO (High IL5 in Lung, But No Eosinophils) |
|---|---|---|---|---|
| Bone marrow (Fig. 2) | + | +++ | + | 0 |
| Blood (Fig. 3) | + | +++ | +++ | 0 |
| BAL (Fig. 4) | +++ | 0 | ||
| Increase in airway resistance to 300 mM inhaled 5-HT (Fig. 6A) | 0.1 cmH2O·mL−1·s−1 | 0.05 cmH2O·mL−1·s−1 | 0.65* cmH2O·mL−1·s−1 | 0.05 cmH2O·mL−1·s−1 |
5-HT, 5-hydroxytryptamine; BAL, bronchoalveolar lavage; WT, wild type.
P < 0.05.
+IL5T, +IL5AE, +IL5AE/−Eos, and −Eos mice were bred in-house, and male +IL5AE/−Eos and −Eos mice were backcrossed with female C57BL/6J mice since female −Eos mice do not reproduce well. The genotypes of transgenic mice and their wild-type littermates were determined by PCR analysis of ear DNA. Wild-type littermates were used as controls in experiments. Mice were maintained in ventilated microisolator cages housed in the pathogen-free animal facility at Oregon Health and Science University. All protocols and studies involving animals were performed in accordance with the U.S. Animal Welfare Act. Protocols were approved by Institutional Animal Care and Use Committee of Oregon Health and Science University.
Measuring bronchoconstriction.
Mice (body weight 20–25 g, male and female) were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Anesthesia of −Eos mice required slightly less ketamine (90 mg/kg) and xylazine (9 mg/kg). Mice were intubated, mechanically ventilated (100% oxygen, tidal volume 0.2 mL and frequency of 120 breaths/min), and paralyzed with succinylcholine (20 mg/kg im). Blood oxygen saturation and heart rate were monitored by Pulse Oximeter (model no. 000734 Bioseb, In Vivo Research Instruments) and 3-lead electrocardiogram, respectively. Body temperature was maintained between 36°C and 37°C by heating lamp and monitored by a rectal thermometer. In some animals, vagus nerves were isolated on both sides in the cervical region and cut with microdissection scissors (bilateral vagotomy). In control animals, bilateral cervical vagus nerves were isolated but not cut.
Inspiratory flow and airway pressure were measured and recorded with a pneumotachograph (part no. MLT1L) and differential pressure transducer (part no. MLT0670) from AD Instruments (Colorado Springs, CO). Data were captured using a PowerLab 4/SP analog-to-digital converter and analyzed with the PowerLab Chart software.
Measuring bronchoconstriction using increased airway resistance.
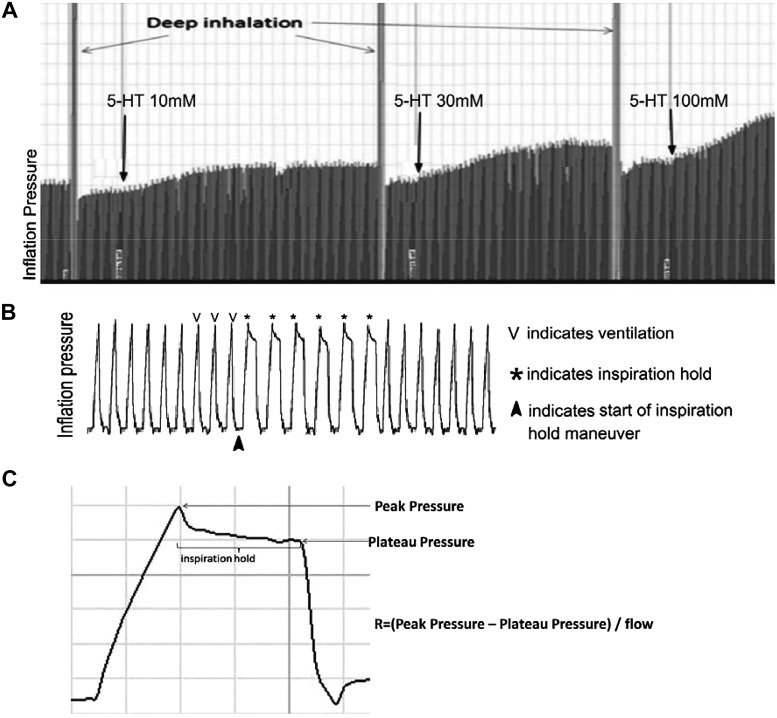
To measure airway resistance, two deep inflations (sighs) at 25 cmH2O were given, followed by an inspiration hold maneuver, stopping inspiratory flow for 225 ms at peak inspiration for 6 breaths in a row. For each breath, both peak pressure and end-inflation pressure (plateau pressure) were recorded, and resistance was calculated as the average (Ppeak−Pplateau)/inspiratory flow of these six breaths (Fig. 1).
Fig. 1.
Measuring airway resistance in a mouse model. Mice were anaesthetized, paralyzed, and ventilated with constant volume and flow. Respiratory rate was maintained and the same tidal volume was used for each animal. A: inflation pressure before and after each dose of aerosolized 5-hydroxytryptamine (5-HT) was measured. B: inspiration hold was applied before and after each dose of 5-HT and a magnified view of inflation pressure is shown. Changes in inflation pressure were measured during maneuvers where the lungs were inflated and held open (to see the plateau pressure). C: airway resistance was calculated.
Baseline airway resistance was measured at the start of each experiment before any treatments. It was measured again 10 s before and 35 s after each dose of either aerosolized saline, 5-hydroxtryptamine (5-HT; 0–300 mM), or methacholine (0–1,000 mM), averaging across 6 breaths each time. Bronchoconstriction was measured as the difference between airway resistance after aerosolized challenge and that immediately before challenge; this increase in airway resistance is graphed in cmH2O·mL−1·s−1. Two deep inflations were delivered between each aerosolized treatment to ensure recovery of baseline between treatments.
Measuring neuronal contribution to bronchoconstriction.
The neuronal contribution to bronchoconstriction was measured by comparing changes in airway resistance in response to inhaled 5-HT (10 μL, 10–300 mM, Sigma). We have previously demonstrated that inhaled 5-HT produces reproducible reflex bronchoconstriction (48). We used vagotomy and atropine (3 mg/kg ip) to test whether the responses were vagally mediated. A dose-response curve to 5-HT was generated in each animal twice, once with the vagi intact and again after either both vagi were cut to eliminate reflex (neuronal) bronchoconstriction or after atropine in vagus-intact animals.
Measuring M3 muscarinic receptor function.
Dose response curves to aerosolized methacholine (10 μL of 10–1,000 mM) were generated in vagotomized mice before and after atropine (3 mg/kg ip).
Bronchoalveolar lavage, blood, and bone marrow supernatant.
Mice were killed by overdose of anesthetic, and inflammatory cells were harvested from blood, lungs, and bone marrow. Whole blood was taken from the abdominal inferior vena cava, spread on a slide, and stained with Hemacolor to obtain a differential cell count. Red blood cells were lysed with 0.1 N HCl, and total peripheral white blood cells were counted on a hemocytometer. Cells were collected from the lungs and right femur by flushing each three times with 0.5 mL aliquots of PBS. In each case, cells were washed, resuspended in PBS, and counted on a hemocytometer or were spun onto slides and stained with Hemacolor (Hemacolor Stain Set, EMD Millipore 65044) to obtain a differential cell count.
Immunohistochemical quantification of lung tissue eosinophils.
Lungs from wild-type, IL5/T, and IL5/AE mice were fixed in Zamboni’s and embedded in paraffin. Serial 6-μm sections were cut, deparaffinized in xylene, and hydrated in graded alcohol solutions. Eosinophils were stained using a rat anti-MBP antibody (1:500, gift from Dr. Elizabeth Jacobsen, Mayo Clinic, Scottsdale, AZ), which recognizes an epitope unique to mouse eosinophil MBP-1 (41). Lung tissue was counterstained with methyl green.
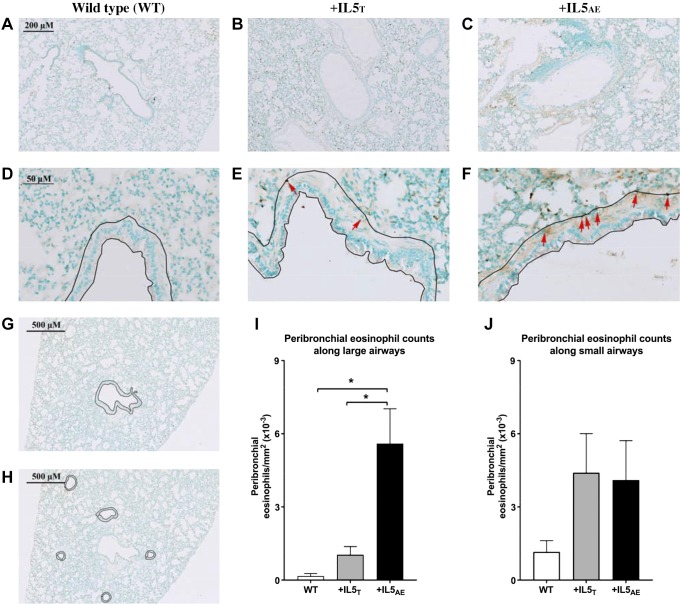
Airways were identified visually by having epithelium and basement membrane. Eosinophils were counted only in the peribronchial area around airways (Fig. 5). Large airways were defined as having a distinct smooth muscle layer and a peribronchial area >1,500 mm2. Eosinophils in large airways were counted in an area including the epithelium, basement membrane, smooth muscle, and additional 15 μm outside the airway smooth muscle. The boundary of this area is shown in Fig. 5, D–G. Small airways lacked smooth muscle, so eosinophils in these airways were counted in an area including the epithelium, basement membrane, and additional 5 μm outside the basement membrane (Fig. 5H). Each experimental group contained three to four animals. For each animal, eosinophils were averaged from three to four large airways and four to six small airways drawn from a minimum of three slides/animal, with each slide representing a different lobe of the lung. Peribronchial eosinophils were counted by blinded investigator. Eosinophils are presented as eosinophils/mm2.
Fig. 5.
Peribronchial eosinophils are significantly increased in large airways of mice overexpressing IL5 in airway epithelium (+IL5AE) compared with mice overexpressing IL5 in T cells (+IL5T) or in wild-type (WT) mice. Representative photomicrographs of lungs from wild-type mice (A and D), +IL5T (B, E, G, and H), and +IL5AE mice (C and F) are shown with an area from large airways. Eosinophils are stained using an anti-major basic protein antibody (brown; some are identified with red arrows for reference), and lung tissue is counterstained with methyl green. In large airways, the area 15 μm above airway smooth muscle is traced (D–F). In small airways, the area traced is 5 μm above the adventitia (H). This significant increase is only observed in large airways which have a smooth muscle layer and peribronchial area >1,500 mm2 (I), but not in small airways (J). The peribronchial eosinophils are not significantly different. N = 3–4 mice per group; n = 3–6 airways per animal. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s multiple comparison test. *P < 0.05.
Statistical analysis.
All data are expressed as mean ± SE. Dose-response curves measuring bronchoconstriction were compared using two-way ANOVA with a Bonferroni post hoc test. Cell counts in bronchoalveolar lavage (BAL), blood, lung tissue, and bone marrow were analyzed using a one-way ANOVA, also with a Bonferroni post hoc test. Bronchoconstriction with or without atropine was compared using unpaired t test. P values less than 0.05 were considered statistically significant. Data were analyzed using the GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
RESULTS
Eosinophils cell counts in bone marrow, blood, BAL, and lung tissue.
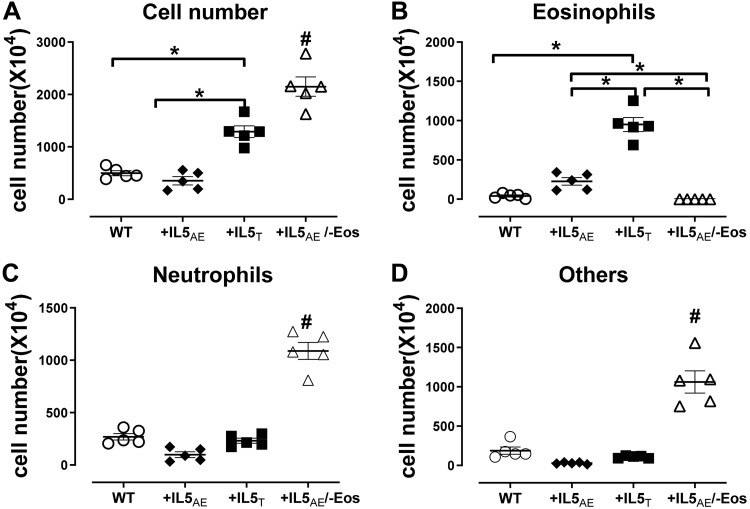
In bone marrow, leukocytes were increased in mice overexpressing IL5 in T cells (+IL5T) versus wild-type mice and mice overexpressing IL5 in lung only (+IL5AE) (Fig. 2A). This increase was composed entirely of eosinophils (Fig. 2, B–D). Overexpression of IL5 in lung (+IL5AE) had no significant effect on any bone marrow cells (Fig. 2B). In mice overexpressing IL5 in lung but depleted of eosinophils (+IL5AE/−Eos), total leukocytes in bone marrow were significantly increased compared with all other groups (Fig. 2A). This increase was composed of mainly neutrophils (Fig. 2C) and other cell types (Fig. 2D), but it was not reflected by any increase in circulating cells (Fig. 3).
Fig. 2.
Bone marrow cell counts by cell types in different mouse strains. It shows significantly higher total cells (A) and eosinophils (B) in mice with increased blood eosinophilia (+IL5T) than in wild type mice (WT) or mice with lung eosinophilia (+IL5AE). Total cells, neutrophils, and other cells, but not eosinophils, are high in mice overexpressing IL5 but without eosinophils (+IL5AE/−Eos) (A, C, and D) as compared with the other three strains. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s multiple comparison test. *P < 0.05 between the 2 groups in bracket. #P < 0.05 compared with all other groups.
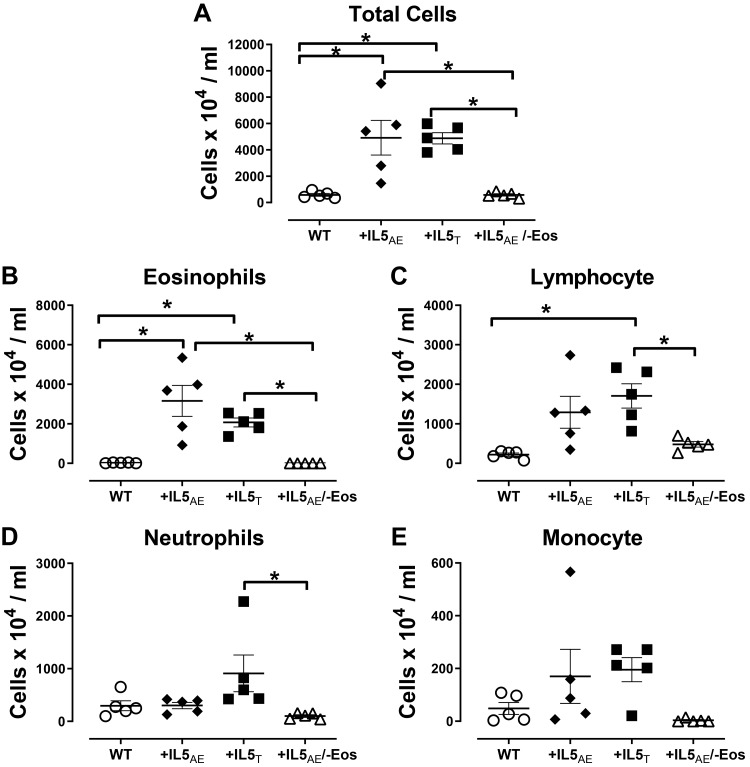
Fig. 3.
Leukocyte counts in blood by cell type. It shows significantly higher total leukocytes and eosinophils in both +IL5AE and +IL5T mice compared with wild-type (WT) mice or mice overexpressing IL5 without eosinophils (+IL5AE/−Eos) (A and B). Lymphocyte levels (C) in +IL5T mice are higher than in wild-type mice or mice without eosinophils (+IL5AE/−Eos). Neutrophil levels (D) in +IL5T mice are significantly higher than mice without eosinophils (+IL5AE/−Eos). Monocyte levels are not significantly different among different strains of mice (E). Data were analyzed by one-way ANOVA with post hoc Bonferroni’s multiple comparison test. *P < 0.05 between the 2 groups in bracket.
In blood, overexpression of IL5 in T cells (+IL5T) or lung (+IL5AE) significantly increased circulating eosinophils compared with wild-type mice (Fig. 3). Circulating lymphocytes were also increased by IL5 versus wild-type mice (Fig. 3C). Circulating neutrophils (Fig. 3D) and monocytes (Fig. 3E) in mice with IL5 overexpression in either blood or lung were not significant different compared with wild-type mice. Total blood cell count in mice with IL5 overexpression in lung but lacking eosinophils (+IL5AE/−Eos) was not significantly different from that in wild-type mice (Fig. 3).
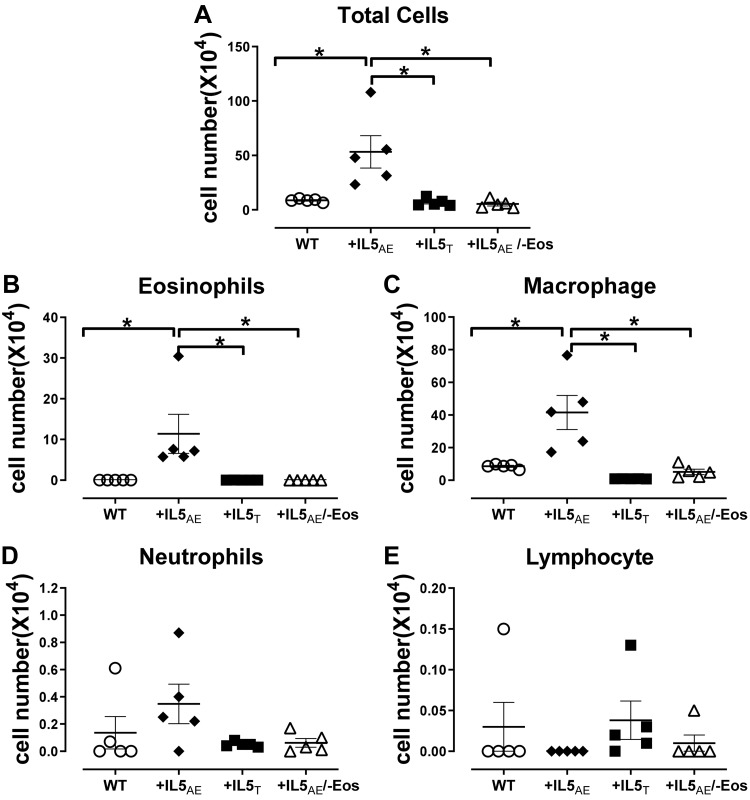
In bronchoalveolar lavage, inflammatory cells were increased only in mice with overexpression of IL5 in airway epithelial cells (+IL5AE) (Fig. 4). This increase comprised eosinophils and macrophages (Fig. 4, B and C). Neither eosinophils nor macrophages were increased in mice overexpressing IL5 but lacking eosinophils (+IL5AE/−Eos).
Fig. 4.
Bronchoalveolar lavage cell counts by cell types in different mouse strains. It shows significantly higher total cell number (A), eosinophils (B), and macrophage cells (C) in +IL5AE mice as compared with wild-type (WT) or +IL5T mice or mice without eosinophils (+IL5AE/−Eos). However, the neutrophils cells (D) and lymphocytes (E) are not different among these strains of mice. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s multiple comparison test. *P < 0.05 between the 2 groups in bracket.
Eosinophils were present in lungs of every animal examined, independent of genetic background. Around small airways specifically, eosinophils were increased in both transgenic, overexpressing IL5 animals, but there was no significant difference among the three mouse groups (Fig. 5J). However, in large airways, eosinophils were significantly increased in mice overexpressing IL5 in airway epithelium (+IL5AE) versus mice overexpressing IL5 in T cells (+IL5T) and versus wild-type mice (Fig. 5I).
5-HT induced bronchoconstriction in mice with overexpression of eosinophils in lungs.
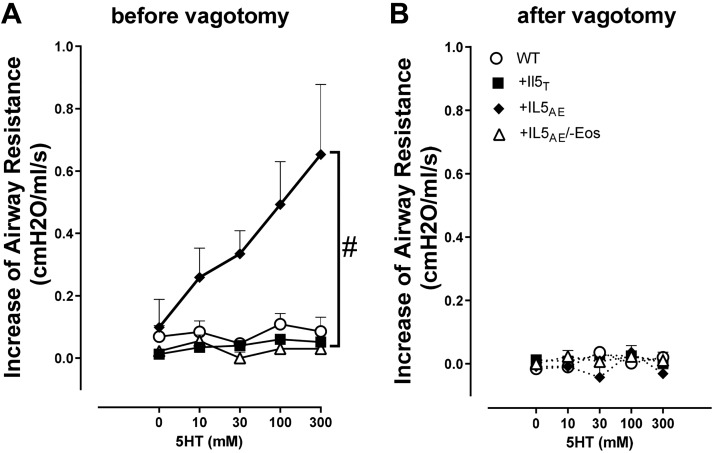
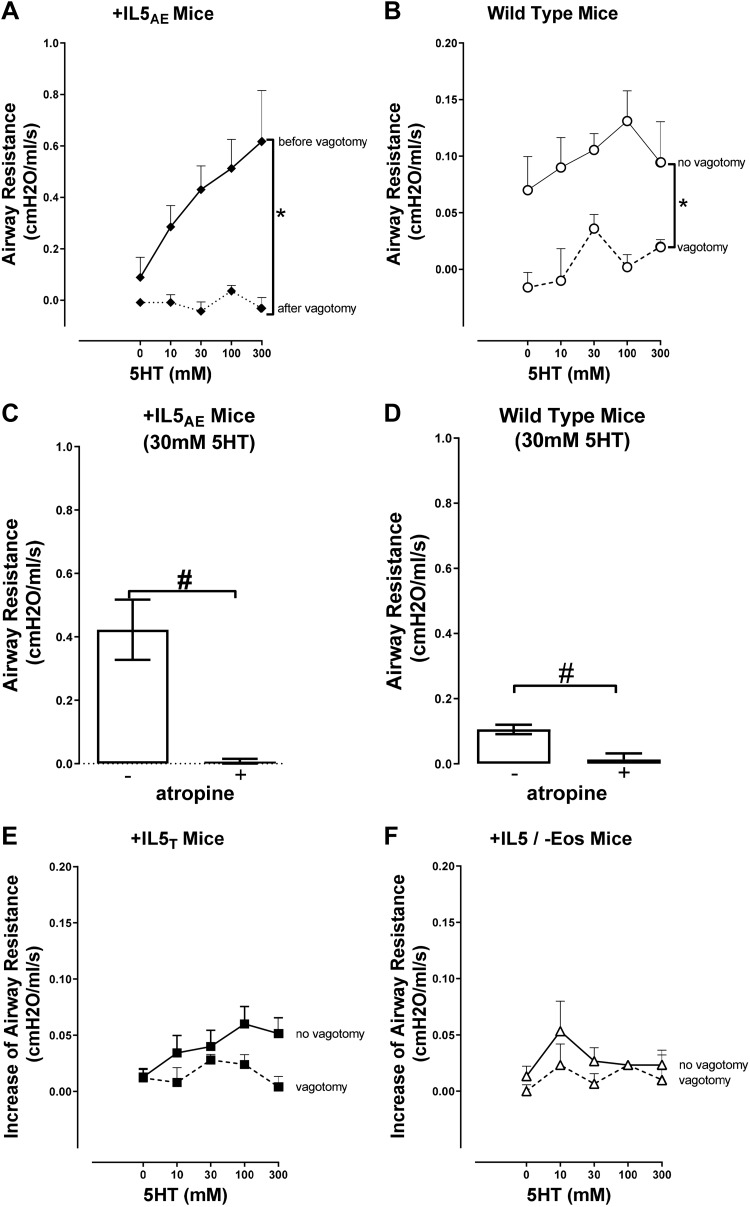
Aerosolized 5-HT induced dose-dependent bronchoconstriction measured as an increase in airway resistance (Figs. 6 and 7). With the vagus nerve intact, 5-HT-induced bronchoconstriction was significantly greater in mice overexpressing IL5 in the lungs (+IL5AE) than in the other three strains (Fig. 6A). This difference disappeared when the vagi were cut (Fig. 6B) so that 5-HT-induced bronchoconstriction was not different among any of the strains. When examined individually on relevant scales, 5-HT-induced bronchoconstriction was dose dependent in mice overexpressing IL5 in lungs (+IL5AE) (Fig. 7A) and in normal wild-type mice (Fig. 7B). In both these strains, 5-HT-induced bronchoconstriction was completely blocked by vagotomy (Fig. 7, A and B) and also by atropine (Fig. 7, C and D), indicating its dependence on parasympathetic nerves activation and the release of acetylcholine. In mice expressing IL5 in the blood (+IL5T), 5-HT also appeared to induce dose-related bronchoconstriction that was inhibited by vagotomy (Fig. 7E), although to a much smaller extent. In contrast, in mice lacking eosinophils, 5-HT had no measurable effect on airway resistance, without or with vagotomy (Fig. 7F).
Fig. 6.
5-Hydroxytryptamine (5-HT)-induced bronchoconstriction is increased in mice with overexpression of eosinophils in lungs. With the vagi being intact (A), 5-HT-induced bronchoconstriction is significantly greater in mice with pulmonary eosinophilia (+IL5AE; closed diamonds) compared with all other strains tested. With the vagi cut (B), there is no difference among different strains. Bronchoconstriction was measured as an increase in airway resistance. n = 4–9. Data were analyzed by two-way ANOVA with Bonferroni’s multiple comparison test. #P < 0.05 compared with all other groups. Closed diamonds represent mice with pulmonary eosinophilia (+IL5AE); open circles represent wild-type (WT) mice; closed squares represent mice with blood eosinophilia (+IL5T); open triangles represent mice deficient in eosinophils but with high IL5 (+IL5AE/−Eos).
Fig. 7.
Hyperresponsiveness to 5-hydroxytryptamine (5-HT) is mediated by a neural reflex. 5-HT-induced bronchoconstriction in +IL5AE mice and in wild-type (WT) mice is significantly reduced after vagotomy (A and B) and by atropine (C and D). Vagotomy has no significant effect on 5-HT-induced bronchoconstriction in mice with blood eosinophilia (+IL5T) (E) or eosinophils-deficient mice (+IL5AE/−Eos) (F). Bronchoconstriction was measured as an increase in airway resistance and data were analyzed by two-way ANOVA with Bonferroni’s multiple comparison test. Bronchoconstriction with or without atropine was analyzed by unpaired t test. *P < 0.005 between the 2 groups in bracket; n = 4–9. #P < 0.05 between the 2 groups in bracket; n = 3–9. Data in Fig. 6 are re-presented here in A, B, E, and F to better illustrate the pre/post vagotomy difference.
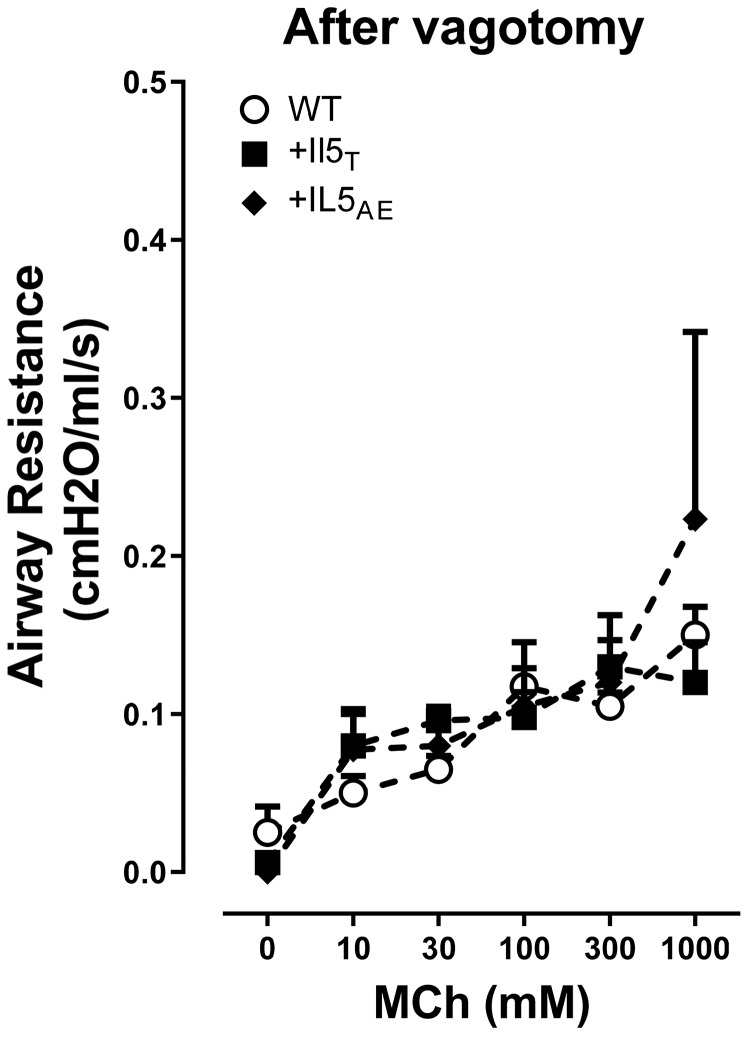
No change in M3 muscarinic receptor function in mice with pulmonary eosinophilia.
To test the role of M3 muscarinic receptors on airway smooth muscle, bronchoconstriction in response to aerosolized methacholine was measured in vagotomized mice (Fig. 8). Inhaled methacholine (10–1,000 mM) caused similar dose-dependent bronchoconstriction in all strains of mice (Fig. 8), demonstrating that overexpression of IL5 in lung airway epithelium or T cells does not affect airway smooth muscle contraction. Atropine (3 mg/kg ip) completely blocked methacholine-induced bronchoconstriction (data not shown).
Fig. 8.
M3 muscarinic receptor-induced bronchoconstriction in vagotomized mice. Airway contraction induced by aerosol methacholine (MCh) showed no difference among vagotomized mice, including wild-type mice, +IL5AE mice, and +IL5T mice. Changes of airway resistance data were analyzed by two-way ANOVA with Bonferroni’s multiple comparison test (n = 4–5).
DISCUSSION
Our data show that bronchoconstriction induced by 5-HT is potentiated specifically by increased eosinophils around large airways, even in the absence of external challenges such as antigen, ozone, or viral exposure. This hyperresponsiveness to 5-HT is entirely mediated by a neural reflex. Using mice that overexpress IL5 in T cells (33) or in airway epithelium (32), we are able, for the first time to show that eosinophils, compartmentalized around large airways in the lung versus distributed peripherally in blood or around small airways, potentiate bronchoconstriction, i.e., eosinophils surrounding large airways in the lungs increase bronchoconstriction, while eosinophilia restricted to the circulation, or in lung, but not around large airways do not.
While mice are widely used in lung research, including measurement of airway resistance (6, 43, 45), several important differences in our methodological approach are important to highlight. We used a novel, trans-oral method for intubating mice that did not involve cutting the trachea, as this could potentially sever vagal nerves supplying the lungs at the beginning of the experiment (9, 35). We measured bronchoconstriction before and after cutting both vagus nerves to quantify the contribution of the vagal reflex to bronchoconstriction induced by aerosolized 5-HT and measured bronchoconstriction before and after atropine to quantify the contribution of muscarinic receptors. Finally, we cut both vagus nerves before measuring bronchoconstriction induced by inhaled methacholine since there is an underappreciated, but substantial, vagally mediated reflex component to inhaled and intravenous methacholine, as we (50) and others (36) have previously shown. To ensure we were measuring the effect of methacholine only on airway smooth muscle, these experiments were all carried out in vagotomized mice.
Aerosolized 5-HT induced significant dose-dependent bronchoconstriction in two strains of mice [mice with high IL5 in lungs (+IL5AE) and wild-type mice] and induced smaller dose-related bronchoconstriction that was not significant in mice with IL5 in the blood (+IL5T). In all three strains, bronchoconstriction induced by aerosolized 5-HT was abolished (Fig. 7, A and B) or reduced (Fig. 7E) by bilateral vagotomy, indicating that it was reflex mediated. It was also significantly blocked by atropine (in +IL5AE and wild-type mice, Fig. 7, C and D), a muscarinic receptor antagonist, demonstrating that in mice with eosinophils, 5-HT-induced bronchoconstriction is mediated by release of acetylcholine from parasympathetic nerves onto muscarinic receptors.
The role of M3 receptors (39, 46) and 5-HT (4, 49) receptors on airway smooth muscle was tested in vagotomized mice. Aerosolized methacholine and aerosolized 5-HT each caused dose-related bronchoconstriction that was identical in all strains after vagotomy, showing no difference in the response of airway smooth muscle to inhaled agonists in the absence of intact nerves. Thus, changes in M3 or 5-HT receptors on airway smooth muscle were not responsible for hyperresponsiveness to 5-HT in mice with airway eosinophilia.
The important role of airway eosinophils in 5-HT-induced bronchoconstriction is clearly demonstrated when comparing the magnitude of 5-HT-induced responses. Mice with lung eosinophils (+IL5AE) have the highest eosinophils surrounding large airways (Fig. 5) and the strongest 5-HT-induced bronchoconstriction response (Fig. 6) compared with that of other mouse strains. Hyperreactivity is mediated by eosinophils, not IL5, since mice overexpressing IL5 that are devoid of eosinophils (+IL5AE/−Eos) were not hyperreactive (Fig. 7F). In contrast, mice with IL5 only in blood (+IL5T) did not have a significant increase in eosinophils around large airways (Fig. 5), confirming that the majority of lung eosinophils in +IL5T mice are likely limited to the lung vasculature (41). Large airways are surrounded by smooth muscle and are the major contributor to airway resistance (29). Airway smooth muscle contraction is under the control of parasympathetic nerves, which are the dominant autonomic nerves in the airways. These nerves are known to supply smooth muscle in mouse lungs and do not supply smaller airways (51). Thus, eosinophils recruited to large airways via IL5 in airway epithelium cause parasympathetic nerve dysfunction, leading to increased smooth muscle contraction and increased airway contraction to inhaled stimuli (8, 26). This may explain the airway hyperresponsiveness in mice overexpressing IL5 in the lung. Therefore, peribronchial eosinophils around large airways play a key role in eosinophil-potentiated airway response to 5-HT.
It is known that eosinophils and airway nerves have an intimate relationship. Nerves make eotaxin, which can recruit eosinophils (28). Once near airway nerves, eosinophils can adhere via interactions between CD11/CD18 and ICAM-1 on nerves (40) and release major basic protein, an endogenous antagonist for neuronal M2 receptors that normally limit vagally induced bronchoconstriction (26). We have recently shown that eosinophils also increase sensory-nerve density in airway epithelium (11). All of these could potentiate 5-HT induced bronchoconstriction. Here, we show that the mere presence of eosinophils in the lung, in the absence of any other external insult (i.e., animals were not antigen challenged or exposed to ozone or to viral infection, etc.), is sufficient to cause vagally mediated hyperreactivity.
Our data show that eosinophils in airway smooth muscle surrounding large airways in lung, not circulating eosinophils or IL5, mediate increased reflex bronchoconstriction that requires activation of parasympathetic nerves. This is important because it may explain why reducing blood eosinophils is not always synchronized with improvement of lung function, as shown in some clinical trials (7, 12, 14). As mentioned in a recent review of 13 clinical trials related to anti-IL5 treatment (14), there were small improvements in symptoms and lung function tests with anti-IL5 interventions, but these changes were too small to be clinically detected by patients, and a relationship between blood-eosinophil reduction and symptoms was not established. In these clinical trials (7, 12, 14), anti-IL5 treatments all markedly reduced blood eosinophils but failed to show solid evidence supporting significant reduction of pulmonary eosinophils. It is known that the number of eosinophils in lungs is not simply proportional to the number of eosinophils in blood. The expression of eosinophil chemokines or intercellular adhesion molecules in lungs (23, 28, 40) may lead to eosinophils concentrated in lung tissues. Additionally, the life span of tissue eosinophils is longer than circulation eosinophils (42). Thus, our data provide evidence to support that sputum eosinophils may be a better standard for diagnosis of eosinophilic asthma and a more reliable prognostic indicator for anti-IL5/anti-eosinophilia treatment in severe eosinophil asthma.
GRANTS
This research was supported by grants from NIH HL131525, HL113023, AR061567, HL124165, HL 065228 and ES017592.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.N., D.B.J., and A.D.F. conceived and designed research; Z.N. and J.N.M. performed experiments; Z.N., J.N.M., and A.D.F. analyzed data; Z.N., D.B.J., and A.D.F. interpreted results of experiments; Z.N. and J.N.M. prepared figures; Z.N., J.N.M., and A.D.F. drafted manuscript; Z.N., J.N.M., D.B.J., and A.D.F. edited and revised manuscript; Z.N., J.N.M., D.B.J., and A.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the kind gift of the transgenic mice (NJ1726 and NJ1638) by James Lee, Ph.D (Mayo Clinic, Scottsdale, AZ) and the kind gift of anti-MBP antibody by Elizabeth A. Jacobsen, Ph.D (Mayo Clinic, Scottsdale, AZ). The authors also thank Lauren Hales Beck for technical assistance and Becky Proskocil, Ph.D, for reviewing and editing the manuscript.
REFERENCES
- 1.Ayala LE, Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma? Chest 96: 1285–1291, 1989. doi: 10.1378/chest.96.6.1285. [DOI] [PubMed] [Google Scholar]
- 2.Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 150: 789–798, 2016. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med 323: 1033–1039, 1990. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 4.Campos-Bedolla P, Vargas MH, Segura P, Carbajal V, Calixto E, Figueroa A, Flores-Soto E, Barajas-López C, Mendoza-Patiño N, Montaño LM. Airway smooth muscle relaxation induced by 5-HT2A receptors: role of Na+/K+-ATPase pump and Ca2+-activated K+ channels. Life Sci 83: 438–446, 2008. doi: 10.1016/j.lfs.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 3: 355–366, 2015. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 6.Chong L, Zhang W, Yu G, Zhang H, Zhu L, Li H, Shao Y, Li C. High-fat-diet induces airway hyperresponsiveness partly through activating CD38 signaling pathway. Int Immunopharmacol 56: 197–204, 2018. doi: 10.1016/j.intimp.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 150: 799–810, 2016. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol Physiol Lung Cell Mol Physiol 273: L93–L103, 1997. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 9.Das S, MacDonald K, Chang HY, Mitzner W. A simple method of mouse lung intubation. J Vis Exp (73): e50318, 2013. doi: 10.3791/50318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake MG, Bivins-Smith ER, Proskocil BJ, Nie Z, Scott GD, Lee JJ, Lee NA, Fryer AD, Jacoby DB. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol 55: 387–394, 2016. doi: 10.1165/rcmb.2015-0405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake MG, Scott GD, Blum ED, Lebold KM, Nie Z, Lee JJ, Fryer AD, Costello RW, Jacoby DB. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci Transl Med 10: eaar8477, 2018. doi: 10.1126/scitranslmed.aar8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med 18: 119, 2018. doi: 10.1186/s12890-018-0689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 100: 2254–2262, 1997. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 9: CD010834, 2017. doi: 10.1002/14651858.CD010834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FitzGerald JM, Bleecker ER, Menzies-Gow A, Zangrilli JG, Hirsch I, Metcalfe P, Newbold P, Goldman M. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med 6: 51–64, 2018. doi: 10.1016/S2213-2600(17)30344-2. [DOI] [PubMed] [Google Scholar]
- 16.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 183: 195–201, 1996. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fryer AD, Costello RW, Yost BL, Lobb RR, Tedder TF, Steeber DA, Bochner BS. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J Clin Invest 99: 2036–2044, 1997. doi: 10.1172/JCI119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 102: 267–271, 1991. doi: 10.1111/j.1476-5381.1991.tb12164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 286: L963–L969, 2004. doi: 10.1152/ajplung.00343.2003. [DOI] [PubMed] [Google Scholar]
- 20.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 83: 973–978, 1984. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest 116: 228–236, 2006. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J Allergy Clin Immunol 81: 776–781, 1988. doi: 10.1016/0091-6749(88)90931-1. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez-A C, Siegelman MH, Cybulsky M, Gutierrez-Ramos JC. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Invest 98: 2332–2345, 1996. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halwani R, Vazquez-Tello A, Sumi Y, Pureza MA, Bahammam A, Al-Jahdali H, Soussi-Gounni A, Mahboub B, Al-Muhsen S, Hamid Q. Eosinophils induce airway smooth muscle cell proliferation. J Clin Immunol 33: 595–604, 2013. doi: 10.1007/s10875-012-9836-3. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby DB, Fryer AD. Anticholinergic therapy for airway diseases. Life Sci 68: 2565–2572, 2001. doi: 10.1016/S0024-3205(01)01053-0. [DOI] [PubMed] [Google Scholar]
- 26.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest 91: 1314–1318, 1993. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med 161: 64–72, 2000. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 28.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med 179: 881–887, 1994. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaminsky DA. What does airway resistance tell us about lung function? Respir Care 57: 85–99, 2012. doi: 10.4187/respcare.01411. [DOI] [PubMed] [Google Scholar]
- 30.Kato M, Ishioka T, Kita H, Kozawa K, Hayashi Y, Kimura H. Eosinophil granular proteins damage bronchial epithelial cells infected with respiratory syncytial virus. Int Arch Allergy Immunol 158, Suppl 1: 11–18, 2012. doi: 10.1159/000337752. [DOI] [PubMed] [Google Scholar]
- 31.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305: 1773–1776, 2004. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 32.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med 185: 2143–2156, 1997. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol 158: 1332–1344, 1997. [PubMed] [Google Scholar]
- 34.Liu Y, Zhang S, Li DW, Jiang SJ. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PLoS One 8: e59872, 2013. [Erratum in PLoS One 8, 2013.] doi: 10.1371/journal.pone.0059872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald KD, Chang HY, Mitzner W. An improved simple method of mouse lung intubation. J Appl Physiol (1985) 106: 984–987, 2009. doi: 10.1152/japplphysiol.91376.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAlexander MA, Gavett SH, Kollarik M, Undem BJ. Vagotomy reverses established allergen-induced airway hyperreactivity to methacholine in the mouse. Respir Physiol Neurobiol 212-214: 20–24, 2015. doi: 10.1016/j.resp.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meijer RJ, Postma DS, Kauffman HF, Arends LR, Koëter GH, Kerstjens HA. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy 32: 1096–1103, 2002. doi: 10.1046/j.1365-2222.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 38.Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol 111: 714–719, 2003. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 39.Minette PA, Barnes PJ. Muscarinic receptor subtypes in lung. Clinical implications. Am Rev Respir Dis 141: S162–S165, 1990. doi: 10.1164/ajrccm/141.3_Pt_2.S162. [DOI] [PubMed] [Google Scholar]
- 40.Nie Z, Nelson CS, Jacoby DB, Fryer AD. Expression and regulation of intercellular adhesion molecule-1 on airway parasympathetic nerves. J Allergy Clin Immunol 119: 1415–1422, 2007. doi: 10.1016/j.jaci.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol 178: 7879–7889, 2007. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 42.Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res 2: 87–101, 2010. doi: 10.4168/aair.2010.2.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peebles RS Jr, Sheller JR, Johnson JE, Mitchell DB, Graham BS. Respiratory syncytial virus infection prolongs methacholine-induced airway hyperresponsiveness in ovalbumin-sensitized mice. J Med Virol 57: 186–192, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Proskocil BJ, Bruun DA, Lorton JK, Blensly KC, Jacoby DB, Lein PJ, Fryer AD. Antigen sensitization influences organophosphorus pesticide-induced airway hyperreactivity. Environ Health Perspect 116: 381–388, 2008. doi: 10.1289/ehp.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol (1985) 96: 2200–2206, 2004. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- 46.Roffel AF, Elzinga CR, Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm Pharmacol 3: 47–51, 1990. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 47.Schultheis AH, Bassett DJ, Fryer AD. Ozone-induced airway hyperresponsiveness and loss of neuronal M2 muscarinic receptor function. J Appl Physiol (1985) 76: 1088–1097, 1994. doi: 10.1152/jappl.1994.76.3.1088. [DOI] [PubMed] [Google Scholar]
- 48.Scott G. Sensory Neuroplasticity in Asthma (PhD thesis). Portland, OR: Oregon Health and Science University School of Medicine, 2012. [Google Scholar]
- 49.Szarek JL, Zhang JZ, Gruetter CA. Mechanisms of 5-hydroxytryptamine-induced contraction of isolated rat intrapulmonary bronchi. Pulm Pharmacol 8: 273–281, 1995. doi: 10.1006/pulp.1995.1037. [DOI] [PubMed] [Google Scholar]
- 50.Wagner EM, Jacoby DB. Methacholine causes reflex bronchoconstriction. J Appl Physiol (1985) 86: 294–297, 1999. doi: 10.1152/jappl.1999.86.1.294. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe T, Nakamura R, Takase Y, Susaki EA, Ueda HR, Tadokoro R, Takahashi Y. Comparison of the 3-D patterns of the parasympathetic nervous system in the lung at late developmental stages between mouse and chicken. Dev Biol 444, Suppl 1: S325–S336, 2018. doi: 10.1016/j.ydbio.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med 167: 1737–1742, 1988. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med 167: 43–56, 1988. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yost BL, Gleich GJ, Fryer AD. Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol (1985) 87: 1272–1278, 1999. doi: 10.1152/jappl.1999.87.4.1272. [DOI] [PubMed] [Google Scholar]
- 55.Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol 289: L627–L635, 2005. doi: 10.1152/ajplung.00377.2004. [DOI] [PubMed] [Google Scholar]