Abstract
In asthma, acute bronchospasm is driven by contractile forces of airway smooth muscle (ASM). These forces can be imaged in the cultured ASM cell or assessed in the muscle strip and the tracheal/bronchial ring, but in each case, the ASM is studied in isolation from the native airway milieu. Here, we introduce a novel platform called tissue traction microscopy (TTM) to measure ASM contractile force within porcine and human precision-cut lung slices (PCLS). Compared with the conventional measurements of lumen area changes in PCLS, TTM measurements of ASM force changes are 1) more sensitive to bronchoconstrictor stimuli, 2) less variable across airways, and 3) provide spatial information. Notably, within every human airway, TTM measurements revealed local regions of high ASM contraction that we call “stress hotspots”. As an acute response to cyclic stretch, these hotspots promptly decreased but eventually recovered in magnitude, spatial location, and orientation, consistent with local ASM fluidization and resolidification. By enabling direct and precise measurements of ASM force, TTM should accelerate preclinical studies of airway reactivity.
Keywords: airway, ASM, contraction, stretch
INTRODUCTION
In preclinical investigation, airway smooth muscle (ASM) contraction is typically measured using the tracheal/bronchial ring, the ASM strip, or the isolated and cultured ASM cell. While these systems have been valuable tools, they lack the physical and biochemical cues of the native ASM microenvironment. Consequently, the results obtained using these models are not necessarily confirmed in animal and human studies. Emerging approaches to simulate airway complexity include “airway-on-a-chip” and “lung-on-a-chip” technologies (1, 6, 15, 36); however, these approaches are limited to a small subset of interacting cells and do not fully recapitulate the native lung anatomy and airway biology. Similar limitations also apply to three-dimensional bio-printed airway tissues (28).
To improve the preclinical investigation of ASM contractile response in a more realistic physiological microenvironment, we investigated precision-cut lung slices (PCLS) (32). In PCLS, the morphological organization of cells, extracellular matrix (ECM), airways, and vessels are nearly identical to that in the in vivo lung. The cells and the ECM are directly accessible for measurements, while the functional responses of vessels and airways are preserved. Additional practical advantages of PCLS include ease of preparation, ease of storage via cryopreservation (30), and suitability for study of many species, including human. In PCLS, responses to neural stimulation (34, 35) and mechanical stretch (10, 11, 24) have been possible to ascertain, highlighting the marked physiological relevance of this system. To study disease processes, PCLS can be prepared from animal models of disease. Alternatively, PCLS prepared from normal animals can be exposed to disease-inducing conditions (2). Given their versatility and unique advantages, PCLS are ideally suited to link the disparate length scales that are currently problematic in asthma research.
Here, we introduce a novel biomechanical technology called tissue traction microscopy (TTM) that is enabled by an adhesive and compliant silicone substrate to which the PCLS can be adhered. Compared with other existing traction microscopy methods (18), TTM is unprecedented in scope because it is not limited to cultured cells. Rather, it elucidates cellular force changes within the native airway ECM in the more physiological settings of living animal/human tissues. Compared with other existing PCLS methods, TTM is not limited by constraints due to airway morphology, geometry, or spatial location within the PCLS. Any airway with an intact lumen can be readily quantified. Applying TTM to porcine and human PCLS, we measured the change in ASM forces (contraction/relaxation) together with airway area (constriction/dilation) and demonstrate the suitability of these measurements for mechanistic investigation.
MATERIALS AND METHODS
Preparation of porcine and human PCLS.
Lungs were isolated from three newborn pigs and four nonasthmatic human lungs that were not suitable for transplantation. The lungs were filled with 2% UltraPure low melting point agarose (Invitrogen, Carlsbad, CA); this specific agarose formulation and percentage are key to enabling reproducible attachment of PCLS to the adhesive substrates. From the distal regions of the agarose-filled lungs, blocks of tissue were cut and sliced using a vibratome (VF-300; Precisionary Instruments) to create 250–300 µm-thick slices. The slices were cryopreserved (5, 30) for up to 12 mo. All animal studies were reviewed and approved by the University of Iowa Animal Care and Use Committee. Human lungs were purchased from the International Institute for the Advancement of Medicine.
Preparation of deformable and adhesive substrates.
A polymer solution of polydimethylsiloxane (PDMS) NuSil Gel-8100 (NuSil Silicone Technologies, Carpinteria, CA) (44) was formulated with a base to crosslinker ratio of 1:1 Part A:Part B. 500 µL of this solution was placed in the center of a 35-mm dish with a 20-mm circular glass bottom (D35-20-1-N; Cellvis, Sunnyvale, CA) and incubated at 90°C for 2 days. The cured product is a deformable substrate with a final thickness of approximately 1 mm and Young’s Modulus of 0.36 kPa (44). A second PDMS mixture at 1.15:1 was then spin-coated (2,500 rpm for 60 s) onto the first layer to yield a second layer of approximately 1-µm thickness. This second layer provides an adhesive layer for the PCLS to conformally attach; its weight ratio (i.e., part A relative to part B) needs to be precisely within 1.145 to 1.150 to obtain the optimal tackiness to promote PCLS adhesion. To achieve this ratio, we recommend using larger working volumes (5–10 mL) of both part A and part B. We also recommend mixing part A and part B in a 50-mL flat-bottom tube at a slow rotational speed (~1/2 rotations/s). The composite was cured at 90°C for an additional 5–10 days to fully solidify the top layer. Four milliliters of a fluorescent bead solution mixed at a ratio of 4 µL of 1-µm red fluorescent beads (F8851; Thermo Fisher Scientific, Waltham, MA) per mL of HBSS (21-023-CV; Corning, Tewksbury, MA) was then added to each dish for 1 h. The dishes were then rinsed once with ultrapure water and stored at room temperature until the day of the experiment.
Preparation of PCLS for substrate adhesion.
On the day of the experiment, PCLS were thawed (30) and incubated in media for 1–4 h for porcine PCLS and overnight for human PCLS. Following post-thaw incubation, porcine PCLS were then placed in a 24-well plate, treated with 10−4 M ACh in HBSS and imaged in bright field using an inverted microscope for 30 min, at a 1-min time increment. From the bright-field images, we quantified lumen area (ImageJ, National Institutes of Health, Bethesda, MD). Only PCLS with airways that exhibited a measurable lumen area change were carried forward for further experimentation. However, we did not exclude any airways on the basis of size, shape, location in the lung, location in the PCLS, proximity to a nearby airway, and wall thickness. Every airway whose lumen area could be measured were included. These selected PCLS were washed three times with HBSS to reset the airway to its initial, prestimulated state.
Adhesion of PCLS to the substrate.
The PCLS were placed upon the PDMS substrates, lightly dried with an airflow at a pressure of 2 psi (~14 kPa) imposed for 2.5–3 min from a tube of 4-mm inner diameter held 3.5 cm above the slice, followed by immediate immersion in room-temperature HBSS.
Measurements of ASM contraction and relaxation.
Using an inverted fluorescence microscope, two images were obtained in quick succession: 1) bright-field image of the airway and 2) fluorescent image of fiducial bead markers embedded in the substrate immediately underneath the airway (Fig. 1A). Such image pairs were obtained in the adherent PCLS before treatment (pretreatment), and following compound addition (posttreatment). The pretreatment fluorescent image was used as reference from which posttreatment displacement maps were calculated. With the additional knowledge of substrate stiffness (44), ASM contractile maps were calculated using unconstrained Fourier transform traction cytometry (7). By considering only those ASM contraction values that lie interior to a manually traced airway contour, the final traction map and the net contractile moment tending to either contract or dilate the substrate (7) were obtained. The net contractile moment is a convenient metric of the overall airway contractile strength. From the bright-field images, we quantified lumen area (ImageJ, NIH, Bethesda, MD).
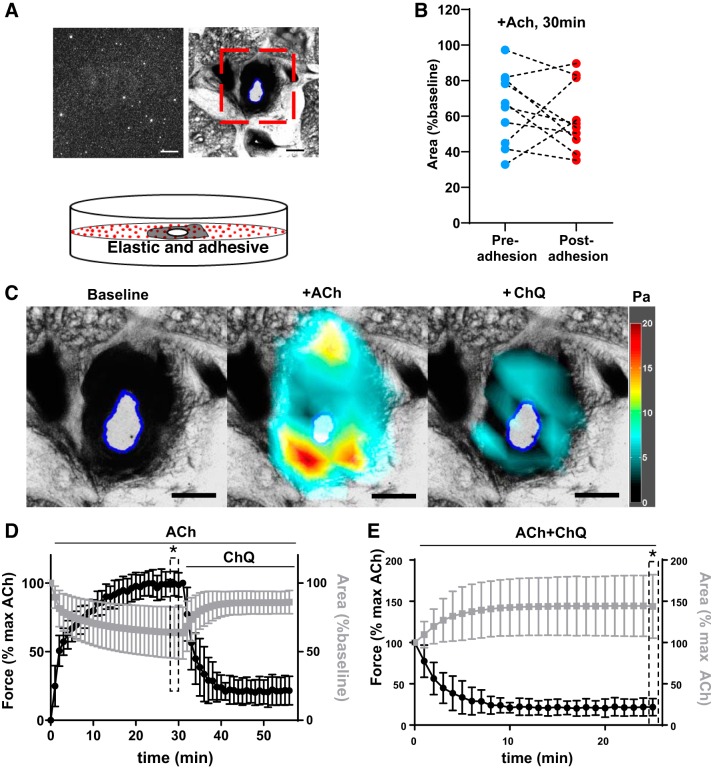
Fig. 1.
Overview of tissue traction microscopy to elucidate airway smooth muscle (ASM) contraction. A: schematic of the experimental setup. Shown in inset are representative images of ASM and airway in precision-cut lung slices (PCLS) (top right) and surface-bound fluorescent beads (top left). Red box indicates the region of interest. B: adhesion of the PCLS to the substrate does not change (P = 0.48, paired t test) the magnitude of airway constriction with 10−4 M ACh at 30 min (each airway was imaged pre- and postadherence; shown are pre/post values for each airway as individual connected points). C: representative bright-field images and overlaid stress maps in response to 10−4 M ACh (30 min) and 10−3 M chloroquine (ChQ; 25 min). Scale bar = 500 μm. D and E: time-dependent lumen area changes (gray line) and ASM contraction changes (black line) in response to 10−4 M ACh followed by 10−3 M ChQ. D: the lumen area for each airway is normalized to its pretreatment baseline value. E: both lumen area and ASM contractile moment are normalized to the value at 10−4 M ACh at 30 min, denoted in the plot as “max ACh.” All data are reported as average and SD (n = 13 airways). *P < 0.001 (F-test two samples for variance).
Imposition of cyclic stretch.
A hollow circular indenter (inner diameter = 2 mm, outer diameter = 3 mm) was centered above the airway of adherent PCLS. Depression of the indenter imposes a radial stretch to the lung tissue encircled by the indenter (17, 24).
Measurements of cell viability in the PCLS.
Cell metabolic activity was determined using the Cell Titer 96 AQueous One Solution Reagent (MTS) (Promega, Madison, WI) while cellular cytotoxicity measurements was performed using the lactate dehydrogenase (LDH)-releasing assay (CytoTox 96 nonradioactive cytotoxicity assay; Promega, Madison, WI), as we have described previously (30). These measurements were performed using culture supernatant obtained from PCLS that were either placed directly into HBSS-filled wells of a multiwell plate (“plastic”) or first adhered upon the PDMS substrate (“substrate”) before rehydration with HBSS. Each measurement set (i.e., MTS vs. LDH) used frozen-thawed PCLS from a different human lung donor. For a given set, the PCLS were distributed equally between the plastic and the substrate groups.
RESULTS
Adhesion of PCLS does not impede airway constriction.
Our methods enabled prompt and conformal adhesion between PCLS and the substrate, as corroborated by the strong coupling of displacements within PCLS and the substrate (Supplemental Fig. S1; all Supplemental Materials are available at https://doi.org/10.6084/m9.figshare.8983709). PDMS substrate adhesion did not impede microscopy (Fig. 1A), magnitude, and rate of airway constriction (Fig. 1B and Supplemental Fig. S2), or overall viability (Supplemental Fig. S3).
ASM force measurements are less variable across airways.
Porcine PCLS (n = 13 airways from three different lungs using 3–7 airways per lung) were stimulated with 10−4 M ACh for 30 min followed by 10−3 M chloroquine (ChQ) for 25 min. In response, the airways constricted and then partially dilated (lumen identified by the blue line, Fig. 1C), while the ASM contracted and then partially relaxed (stress maps, Fig. 1C). Because we did not exclude any airways on the basis of size, shape, or lung/PCLS location, the extent of airway constriction was variable from airway to airway. However, the corresponding ASM contraction was less variable (Fig. 1D) (F-test two samples for variance revealed a five times greater variability for normalized lumen area than normalized force). To fully reveal the scope of bronchodilation, we replotted all data as a % of the maximum ACh reponse (Fig. 1E). We observed that the variability in lumen area change was now especially pronounced (Fig. 1E). Indeed, the F-test with two samples for variance revealed a 13 times greater variability for normalized lumen area than normalized force.
We considered airway size as a source of variability. We grouped airways into two bins on the basis of their initial lumen size: 1) larger than 500 µm and 2) smaller than 500 µm. Within each airway size bin, we observed that agonist-induced changes in lumen area and ASM contraction continued to remain variable (Supplemental Fig. S4, B and D). Moreover, initial airway size was not related to either the extent of airway constriction (Supplemental Fig. S4E) or the magnitude of ASM contraction (Supplemental Fig. S4F).
ASM force measurements are sensitive to bronchoconstrictor stimuli.
Human PCLS (n = 4 airways from one donor lung) were stimulated with increasing doses of histamine (10−8 M to 10−5 M). For each dose, changes in force and lumen area were determined following 15 min of treatment. Force changes were detected at lower concentrations (Fig. 2).
Fig. 2.
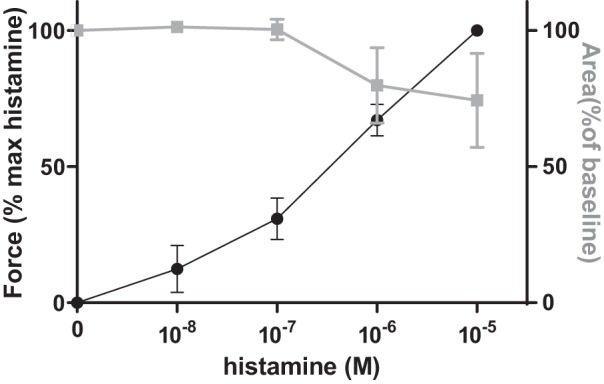
Tissue traction microscopy (TTM) measurements of airway smooth muscle force changes are sensitive to histamine stimulation. Human precision-cut lung slices (n = 4 airways from 1 donor lung) were stimulated with increasing doses of histamine (10−8 M to 10−5 M). At each dose, TTM measurements were obtained after 15 min of treatment. When compared with lumen area changes (gray line), force changes (black line) were detected at lower histamine concentrations. All data are reported as average and SE.
Cyclic stretch dilates the airway by reducing ASM contractile force.
Human PCLS (n = 3 airways obtained from two human donor lungs) were stimulated with 10−6 M histamine for 25 min followed by 10−11 M formoterol for 25 min. The airways narrowed in area in response to histamine and then dilated in response to formoterol (Fig. 3, gray curve). With superimposed cyclic stretch (mean amplitude = 4% then 19%; frequency = 0.25 Hz, and duration = 5 min each amplitude), the airways dilated further. Across both agonist stimulation and cyclic stretch, these lumen area changes (Fig. 3, gray curve) were inversely correlated (r2 = 0.96 by linear regression) with ASM force changes (Fig. 3, black curve). Following stretch cessation, the ASM force recovered to the prestretch value (Fig. 4, A and F, and Supplemental Fig. S5).
Fig. 3.
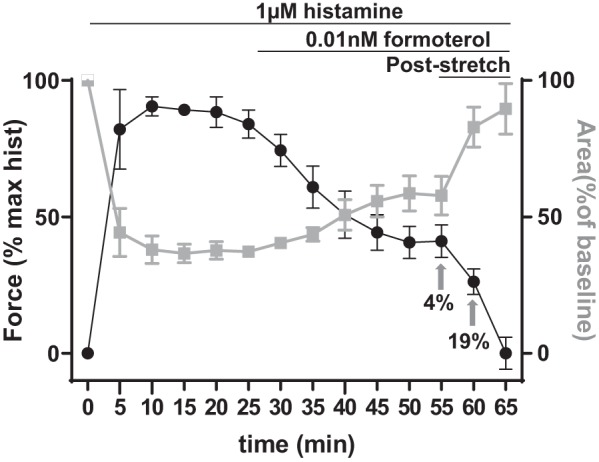
Tissue traction microscopy reveals an inverse relationship between airway smooth muscle (ASM) contraction and airway lumen area. In response to 10−6 M histamine followed by 10−11 M formoterol, the airways of human precision-cut lung slices (n = 3 obtained from 2 human donor lungs) constricted and then dilated (gray curve) while the ASM contracted and then partially relaxed (black curve). The lumen dilated and the ASM relaxed further immediately after cyclic stretches resembling tidal and deep breathing (amplitude = 4% or 19% cyclic stretch at a frequency of 0.25 Hz for a duration of 5 min each). Data are reported as average and SE.
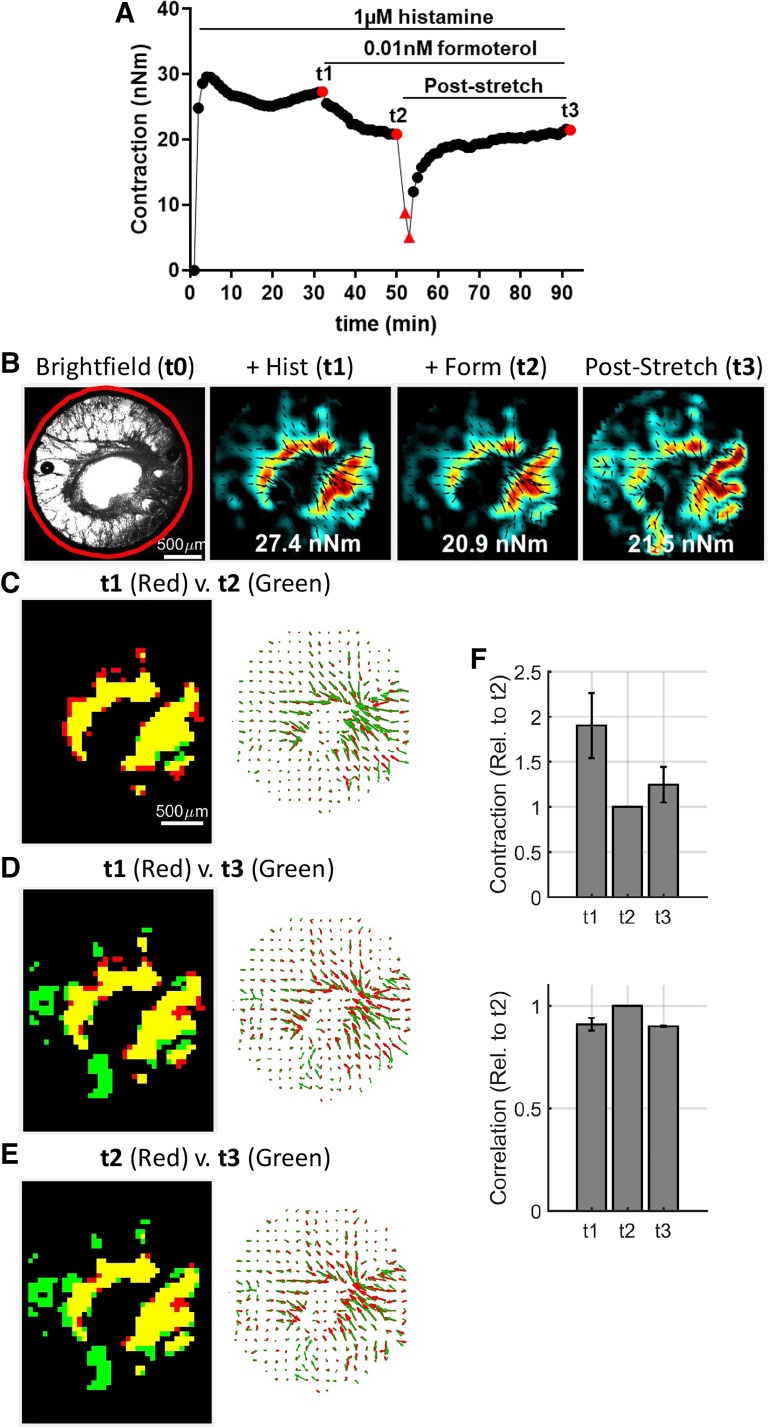
Fig. 4.
Tissue traction microscopy reveals a heterogeneous spatial distribution of airway smooth muscle contraction around the airway lumen. A and B: representative time course and traction maps of an airway in response to sequential stimuli with histamine, formoterol, and transient stretch. Red triangles indicate measurements obtained after the imposition of either 4% or 19% mean cyclic stretch, imposed sequentially, at a frequency of 0.25 Hz for a duration of 5 min each. Further comparisons were made at a single snapshot, indicated in the force trace as red circles: histamine only at time = t1, with formoterol at time = t2, and after stretch recovery at time = t3. Indicated in inset of each traction map is the numerical value of the corresponding net contractile moment. C–E: high stress regions (>75% percentile) were identified from each stress map at t1, t2, and t3 and compared for overlap in spatial location (left) and orientation (right). F: across all airways (n = 4 obtained from 3 human donor lungs), the average magnitude (top) recovered to the formoterol-induced prestretch value. Furthermore, both magnitude and location of the stresses were strongly correlated (close to 1), as indicated by the peak value of the cross-correlation coefficient (bottom). All data are reported as average and SE. For correlation analysis, the traction maps were compared using the normxcorr2 function of the commercially available Matlab software (Mathworks, Natick, MA).
Hotspots of ASM contraction around the airway lumen.
Further analysis of single snapshots of ASM contraction maps (at time = t1, t2, and t3 in Fig. 4B) revealed a heterogenous distribution of contractile forces within the airway wall. From this distribution, we identified regions bearing highest ASM contraction, i.e., >75% percentile, called “stress hotspots”. The likely locus of these hotspots are regions of increased muscle mass. In response to stretch, these hotspots promptly decreased but eventually recovered to a similar extent as the mean stress response (Supplemental Fig. S5). In addition, they retained their spatial location and local orientation throughout the experiment (Fig. 4, C–E). Beyond this representative case, when analyzed across all airways (n = 4 airways obtained from three human donors, with lumen diameter ranging from 0.5 to 0.7 mm), pairwise comparisons of the contraction maps performed yielded a cross-correlation coefficient that was close to 1, implying that for a given airway, the magnitude and location of the stress hotspots were highly conserved.
DISCUSSION
Using an adhesive and compliant silicone substrate elastomer, we have developed TTM to elucidate cellular force changes in porcine and human PCLS. We measured contractile force changes in ASM and demonstrated its utility for sensitive, less variable, and spatially resolved measurements of airway reactivity.
TTM provides three major advantages over existing approaches to quantify ASM responses. First, it directly interrogates ASM mechanical behavior within the native physical and cellular microenvironment of the intraparenchymal airway. Second, it retains key components of biomechanical signaling and contractile proteins that are rapidly lost when ASM cells are isolated and cultured (12). Finally, it applies traction force microscopy, for the first time, to human organ slices. This innovation has the potential to uncover the mechanisms by which mechanical changes in individual ASM cell within their native ECM might promote dysfunction at the level of the airway within intact tissue. These unique advantages, coupled with the ability to impose simulated breathing through periodic stretch, should enable new applications in asthma (3, 9, 14, 30, 34, 38), fibrosis (26, 39), toxicity (33, 43), airway remodeling (9, 31), airway-parenchymal interactions (14, 42), and COPD (42).
Conventional studies are typically focused on airways that are matched on the basis of size, shape, location in the lung, location in the PCLS, proximity to a nearly airway, and wall thickness. In contrast, we have included every airway whose lumen area can be measured. While this introduces variability in airway constriction (Fig. 1D) and dilation (Fig. 1E), it highlights the strength of TTM to perform airway reactivity measurements without the burden of preselection. Importantly, when simultaneous changes in ASM force dynamics are considered, the variability in response is reduced. A likely reason for this reduced variability is that ASM force is less affected by extrinsic factors other than the internal contractile machinery of the ASM itself. This homogeneity of force response, together with the ability to automate traction analysis (27, 44), makes TTM an attractive platform for quantifying airway responsiveness.
ASM contraction alone provides an incomplete description of the mechanical forces in the airway. With each successive breath, the airway and ASM are subjected to considerable cyclic stretch. In response to commensurate stretch imposed in vitro, the cultured ASM cell or the excised muscle strip devoid of parenchyma, significantly decrease their contractile forces through the physical process of cytoskeletal fluidization (17, 19, 40). Here, we directly quantify the impact of this ASM fluidization response in enabling human airway dilation (Fig. 3). Furthermore, we observed that the acute fluidization response is enhanced by successively increasing strain. Nevertheless, it is important to recognize that as soon as stretching is halted, the ASM returns into its constricted state. These findings identify ASM force changes as a key effector of cyclic stretch-induced bronchodilation and reconstriction following cessation of cyclic stretch, previously demonstrated in ACh-stimulated human PCLS (24).
We demonstrated that the stretch-induced ASM force relaxation was additive to formoterol-induced bronchodilation (Fig. 3). These data are salient because they reveal a new therapeutic window for bronchodilation. Molecular effectors that augment this window for stretch-induced ASM relaxation, such as zyxin (29), cofilin (21), actin-myosin-actin connectivity (23), and signaling elements (8), could, thus, be developed as singular or combinatorial bronchodilator therapies. The stretch-induced ASM force relaxation was also reversible (Fig. 3), suggesting the mechanism of stretch-induced pharmacological bronchodilation postulated previously (4) is likely attributable to changes in ASM contraction itself, rather than passive structural remodeling in the matrix components of the airway wall.
We have made five simplifying assumptions to facilitate the present study. First, by measuring traction force parallel to the plane of the PCLS, we implicitly assumed that the forces are two-dimensional (41). Because the ASM is oriented at a mean angle of ~13° relative to the transverse plane of the airway (25), the forces are predominantly parallel to the surface, and out of plane displacements do not strongly manifest in a 250-µm-thick PCLS. In support, we did not detect any vertical substrate deformation that led to fluorescent beads moving out of plane. Second, our proposed measurements are restricted to PCLS adherent upon a soft substrate (Young’s Modulus = 0.36 kPa). This choice of stiffness ensures that the airway narrowing is minimally restricted by the resistance of the substrate, and our comparisons of adhered and nonadhered airway constriction in the same airways suggest the PDMS substrate does not hinder contraction (Fig. 1B and Supplemental Fig. S2). While substratum stiffness substantially affects properties of adherent isolated cultured cells, we believe that cells within the PCLS are insulated from this effect because they reside within their natural tissue environment. Third, we assume that the adhesive layer itself has a negligible impact on overall substrate stiffness. For the current experimental configuration, analytical modeling based on a previously derived apparent shear modulus of a composite material (37) supports this assumption (Supplemental Fig. S6). Fourth, the imposed strain was not adjusted on an airway-by-airway basis. Accordingly, for a given depth of breathing, the actual strain varied between airways. However, this variation was relatively small, at all strain magnitudes (standard deviation of 1.2% for 4% mean strain, and 3.6% for 19% mean strain). Additionally, our methodology imposes stretch by pulling on the parenchymal tethers while a related methodology imposes stretch via a pressure difference prescribed across the airway wall of the explanted airway segment (13). This experimental difference might explain the qualitatively similar but quantitatively diminished effects of cyclic strain observed in the explanted airway segment. Finally, our methodology lacks the ability to precisely control/modulate the load impeding ASM shortening before agonist treatment or stretch imposition. Such a lack of control over the preload implies that the absolute magnitude of ASM contraction (in nNm), which is ASM length-dependent, will vary from PCLS-to-PCLS (as is observed in Supplemental Fig. S4C). To overcome this weakness, future studies should examine stiffer substrates as an approach to impose isometric loading to the muscle. Alternatively, a force boundary could be imposed on the PCLS to mimic transpulmonary pressure. But until this is accomplished, measurements of ASM contraction in the ASM segment [e.g., (16, 20)] or the airway segment [e.g., (4, 22)] that can precisely control both the preload and ASM length are needed.
We propose two future directions for TTM. First, it seems logical to expand TTM for repeated drug exposure measurements in the same adhered airway. If successful, not only would this enable paired comparisons of drug responsiveness within the same airway of the PCLS but also maximize use of precious PCLS samples, especially from human subjects. Second, it would be ideal to improve the spatial resolution of TTM to perform simultaneous measurements of ASM mass, thickness, and density. This enhanced capacity will enable more focused studies of the mechanisms underlying the ASM stress hotspots.
In conclusion, we have introduced TTM, a novel approach to quantify ASM contractile forces within intraparenchymal airways of porcine and human PCLS. The key advantages of this approach are its high physiological relevance, less variance across airways, and spatiotemporal sensitivity. TTM should thus enable a broad scope of applications in preclinical studies of airway reactivity.
GRANTS
This work was supported, in part, by National Institutes of Health National Heart, Lung, and Blood Institute Grants R21HL123522 (R. Krishnan and B. Suki), UH3 HL123816 (J. Solway and R. Krishnan), P01 HL091842 (D. A. Stoltz), and K08 HL135443 (Y. Bai), National Institute of General Medical Sciences Grant T32 GM007337 (D. P. Cook), Cystic Fibrosis Foundation (Research Development Program) (D. P. Cook), and Natural Sciences and Engineering Research Council of Canada RGPIN/05843-2014 and EQPEQ/472339-2015 (to A. J. Ehrlicher).
DISCLOSURES
R. Krishnan is a consultant for SCIREQ Inc., to enable development of new methods using precision-cut lung slices. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.R.-M., Y.B., A.J.E., D.P.C., D.A.S., X.A., and R.K. conceived and designed research; S.R.-M., Y.B., and R.K. performed experiments; S.R.-M., Y.B., N.S., B.S., and R.K. analyzed data; S.R.-M., Y.B., N.S., J.S., X.A., and R.K. interpreted results of experiments; S.R.-M., Y.B., N.S., and R.K. prepared figures; S.R.-M., Y.B., N.S., and R.K. drafted manuscript; S.R.-M., Y.B., N.S., A.J.E., D.P.C., B.S., D.A.S., J.S., X.A., and R.K. edited and revised manuscript; S.R.-M., Y.B., N.S., A.J.E., D.P.C., B.S., D.A.S., J.S., X.A., and R.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Amanda Tatler at University of Birmingham (Birmingham, UK), Dr. Cecile Bidan at the Max Planck Institute for Colloids and Interfaces (Potsdam, Germany), Drs. Jarred Mondonedo and Samer Bou Jawde at Boston University (Boston, MA), and David Brunet and Drs. Liah Fereydoonzad and Percival Graham at Scireq, Inc. (Montreal, QC, Canada), for helpful discussions and for technical contributions.
REFERENCES
- 1.Ahmad AA, Wang Y, Gracz AD, Sims CE, Magness ST, Allbritton NL. Optimization of 3-D organotypic primary colonic cultures for organ-on-chip applications. J Biol Eng 8: 9, 2014. doi: 10.1186/1754-1611-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, Lindner M, Peschel B, Königshoff M, Wagner DE. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol 312: L896–L902, 2017. doi: 10.1152/ajplung.00084.2017. [DOI] [PubMed] [Google Scholar]
- 3.An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA Jr, Liggett SB. TAS2R activation promotes airway smooth muscle relaxation despite β2-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol 303: L304–L311, 2012. doi: 10.1152/ajplung.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansell TK, Noble PB, Mitchell HW, McFawn PK. Pharmacological bronchodilation is partially mediated by reduced airway wall stiffness. Br J Pharmacol 171: 4376–4384, 2014. doi: 10.1111/bph.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y, Krishnamoorthy N, Patel KR, Rosas I, Sanderson MJ, Ai X. Cryopreserved human precision-cut lung slices as a bioassay for live tissue banking. A viability study of bronchodilation with bitter-taste receptor agonists. Am J Respir Cell Mol Biol 54: 656–663, 2016. doi: 10.1165/rcmb.2015-0290MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release 190: 82–93, 2014. doi: 10.1016/j.jconrel.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 8.Cleary RA, Wang R, Wang T, Tang DD. Role of Abl in airway hyperresponsiveness and airway remodeling. Respir Res 14: 105, 2013. doi: 10.1186/1465-9921-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croisier H, Tan X, Chen J, Sneyd J, Sanderson MJ, Brook BS. Ryanodine receptor sensitization results in abnormal calcium signaling in airway smooth muscle cells. Am J Respir Cell Mol Biol 53: 703–711, 2015. doi: 10.1165/rcmb.2014-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dassow C, Wiechert L, Martin C, Schumann S, Müller-Newen G, Pack O, Guttmann J, Wall WA, Uhlig S. Biaxial distension of precision-cut lung slices. J Appl Physiol (1985) 108: 713–721, 2010. doi: 10.1152/japplphysiol.00229.2009. [DOI] [PubMed] [Google Scholar]
- 11.Davidovich N, Huang J, Margulies SS. Reproducible uniform equibiaxial stretch of precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol 304: L210–L220, 2013. doi: 10.1152/ajplung.00224.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall IP, Kotlikoff M. Use of cultured airway myocytes for study of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 268: L1–L11, 1995. doi: 10.1152/ajplung.1995.268.1.L1. [DOI] [PubMed] [Google Scholar]
- 13.Harvey BC, Parameswaran H, Lutchen KR. Can breathing-like pressure oscillations reverse or prevent narrowing of small intact airways? J Appl Physiol (1985) 119: 47–54, 2015. doi: 10.1152/japplphysiol.01100.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiorns JE, Bidan CM, Jensen OE, Gosens R, Kistemaker LE, Fredberg JJ, Butler JP, Krishnan R, Brook BS. Airway and parenchymal strains during bronchoconstriction in the precision cut lung slice. Front Physiol 7: 309, 2016. [Erratum in Front Physiol 8: 117, 2017.] doi: 10.3389/fphys.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ijpma G, Liang CQ, Kachmar L, Panariti A, Benedetti A, Lavoie JP, Lauzon AM. Maintenance of contractile function of isolated airway smooth muscle after cryopreservation. Am J Physiol Lung Cell Mol Physiol 315: L724–L733, 2018. doi: 10.1152/ajplung.00064.2018. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan R, Park CY, Lin YC, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, Butler JP, Fredberg JJ. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS One 4: e5486, 2009. doi: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan R, Tambe D, Steward RL, Hardin C, Butler J. Traction microscopy. In: Cells, Forces, and the Microenvironment. Boca Raton, FL: CRC Press, 2015. [Google Scholar]
- 19.Krishnan R, Trepat X, Nguyen TT, Lenormand G, Oliver M, Fredberg JJ. Airway smooth muscle and bronchospasm: fluctuating, fluidizing, freezing. Respir Physiol Neurobiol 163: 17–24, 2008. doi: 10.1016/j.resp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan B, Deng L, Donovan GM, Chin LY, Syyong HT, Wang L, Zhang J, Pascoe CD, Norris BA, Liu JC, Swyngedouw NE, Banaem SM, Paré PD, Seow CY. Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L1–L10, 2015. doi: 10.1152/ajplung.00222.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan B, Krishnan R, Park CY, Watanabe RA, Panganiban R, Butler JP, Lu Q, Cole WC, Fredberg JJ. Transient stretch induces cytoskeletal fluidization through the severing action of cofilin. Am J Physiol Lung Cell Mol Physiol 314: L799–L807, 2018. doi: 10.1152/ajplung.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPrad AS, Szabo TL, Suki B, Lutchen KR. Tidal stretches do not modulate responsiveness of intact airways in vitro. J Appl Physiol (1985) 109: 295–304, 2010. doi: 10.1152/japplphysiol.00107.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavoie TL, Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Seow CY, Mitchell RW, Solway J. Disrupting actin-myosin-actin connectivity in airway smooth muscle as a treatment for asthma? Proc Am Thorac Soc 6: 295–300, 2009. doi: 10.1513/pats.200808-078RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, Dowell ML. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am J Respir Crit Care Med 186: 225–232, 2012. doi: 10.1164/rccm.201202-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei M, Ghezzo H, Chen MF, Eidelman DH. Airway smooth muscle orientation in intraparenchymal airways. J Appl Physiol (1985) 82: 70–77, 1997. doi: 10.1152/jappl.1997.82.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Milara J, Ballester B, Morell A, Ortiz JL, Escrivá J, Fernández E, Perez-Vizcaino F, Cogolludo A, Pastor E, Artigues E, Morcillo E, Cortijo J. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study. Thorax 73: 519–529, 2018. doi: 10.1136/thoraxjnl-2017-210728. [DOI] [PubMed] [Google Scholar]
- 27.Park CY, Zhou EH, Tambe D, Chen B, Lavoie T, Dowell M, Simeonov A, Maloney DJ, Marinkovic A, Tschumperlin DJ, Burger S, Frykenberg M, Butler JP, Stamer WD, Johnson M, Solway J, Fredberg JJ, Krishnan R. High-throughput screening for modulators of cellular contractile force. Integr Biol 7: 1318–1324, 2015. doi: 10.1039/C5IB00054H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JY, Ryu H, Lee B, Ha DH, Ahn M, Kim S, Kim JY, Jeon NL, Cho DW. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 11: 015002, 2018. doi: 10.1088/1758-5090/aae545. [DOI] [PubMed] [Google Scholar]
- 29.Rosner SR, Pascoe CD, Blankman E, Jensen CC, Krishnan R, James AL, Elliot JG, Green FH, Liu JC, Seow CY, Park JA, Beckerle MC, Paré PD, Fredberg JJ, Smith MA. The actin regulator zyxin reinforces airway smooth muscle and accumulates in airways of fatal asthmatics. PLoS One 12: e0171728, 2017. doi: 10.1371/journal.pone.0171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosner SR, Ram-Mohan S, Paez-Cortez JR, Lavoie TL, Dowell ML, Yuan L, Ai X, Fine A, Aird WC, Solway J, Fredberg JJ, Krishnan R. Airway contractility in the precision-cut lung slice after cryopreservation. Am J Respir Cell Mol Biol 50: 876–881, 2014. doi: 10.1165/rcmb.2013-0166MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royce SG, Nold MF, Bui C, Donovan C, Lam M, Lamanna E, Rudloff I, Bourke JE, Nold-Petry CA. Airway remodeling and hyperreactivity in a model of bronchopulmonary dysplasia and their modulation by IL-1 receptor antagonist. Am J Respir Cell Mol Biol 55: 858–868, 2016. doi: 10.1165/rcmb.2016-0031OC. [DOI] [PubMed] [Google Scholar]
- 32.Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther 24: 452–465, 2011. doi: 10.1016/j.pupt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer UG, Vogel S, Aumann A, Hess A, Kolle SN, Ma-Hock L, Wohlleben W, Dammann M, Strauss V, Treumann S, Gröters S, Wiench K, van Ravenzwaay B, Landsiedel R. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol Appl Pharmacol 276: 1–20, 2014. doi: 10.1016/j.taap.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Schlepütz M, Rieg AD, Seehase S, Spillner J, Perez-Bouza A, Braunschweig T, Schroeder T, Bernau M, Lambermont V, Schlumbohm C, Sewald K, Autschbach R, Braun A, Kramer BW, Uhlig S, Martin C. Neurally mediated airway constriction in human and other species: a comparative study using precision-cut lung slices (PCLS). PLoS One 7: e47344, 2012. doi: 10.1371/journal.pone.0047344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlepütz M, Uhlig S, Martin C. Electric field stimulation of precision-cut lung slices. J Appl Physiol (1985) 110: 545–554, 2011. doi: 10.1152/japplphysiol.00409.2010. [DOI] [PubMed] [Google Scholar]
- 36.Stucki AO, Stucki JD, Hall SR, Felder M, Mermoud Y, Schmid RA, Geiser T, Guenat OT. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 15: 1302–1310, 2015. doi: 10.1039/C4LC01252F. [DOI] [PubMed] [Google Scholar]
- 37.Suki B, Hu Y, Murata N, Imsirovic J, Mondoñedo JR, de Oliveira CLN, Schaible N, Allen PG, Krishnan R, Bartolák-Suki E. A microfluidic chamber-based approach to map the shear moduli of vascular cells and other soft materials. Sci Rep 7: 2305, 2017. doi: 10.1038/s41598-017-02659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan X, Sanderson MJ. Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br J Pharmacol 171: 646–662, 2014. doi: 10.1111/bph.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatler AL, Barnes J, Habgood A, Goodwin A, McAnulty RJ, Jenkins G. Caffeine inhibits TGFβ activation in epithelial cells, interrupts fibroblast responses to TGFβ, and reduces established fibrosis in ex vivo precision-cut lung slices. Thorax 71: 565–567, 2016. doi: 10.1136/thoraxjnl-2015-208215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature 447: 592–595, 2007. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nat Phys 5: 426–430, 2009. doi: 10.1038/nphys1269. [DOI] [Google Scholar]
- 42.Van Dijk EM, Culha S, Menzen MH, Bidan CM, Gosens R. Elastase-induced parenchymal disruption and airway hyper responsiveness in mouse precision cut lung slices: toward an ex vivo COPD model. Front Physiol 7: 657, 2017. doi: 10.3389/fphys.2016.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson CY, Damiani F, Ram-Mohan S, Rodrigues S, de Moura Queiroz P, Donaghey TC, Rosenblum Lichtenstein JH, Brain JD, Krishnan R, Molina RM. Screening for chemical toxicity using cryopreserved precision cut lung slices. Toxicol Sci 150: 225–233, 2016. doi: 10.1093/toxsci/kfv320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshie H, Koushki N, Kaviani R, Tabatabaei M, Rajendran K, Dang Q, Husain A, Yao S, Li C, Sullivan JK, Saint-Geniez M, Krishnan R, Ehrlicher AJ. Traction force screening enabled by compliant PDMS elastomers. Biophys J 114: 2194–2199, 2018. doi: 10.1016/j.bpj.2018.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]