Key Points
Question
Does an artificial intelligence smartphone coaching application (app) improve blood pressure and hypertension-associated behaviors?
Findings
In this randomized clinical trial of 297 adults with uncontrolled hypertension, participants randomized to a smartphone coaching app did not have lower blood pressure at 6 months compared with those receiving a blood pressure tracking app. The study was not large enough to detect small but potentially important intervention effects.
Meaning
Benefits of combining this mobile coaching app with home monitoring were not established in this trial.
This randomized clinical trial investigates the effect on blood pressure of an artificial intelligence smartphone coaching application (app) designed to promote home monitoring and behavioral changes vs a blood pressure tracking smartphone app.
Abstract
Importance
Mobile applications (apps) may help improve hypertension self-management.
Objective
To investigate the effect of an artificial intelligence smartphone coaching app to promote home monitoring and hypertension-related behaviors on systolic blood pressure level compared with a blood pressure tracking app.
Design, Setting, and Participants
This was a 2-group, open, randomized clinical trial. Participants with uncontrolled hypertension were recruited in 2016 and 2017 and were followed up for 6 months. Data analysis was performed from April 2019 to December 2019.
Interventions
Intervention group participants received a smartphone coaching app to promote home monitoring and behavioral changes associated with hypertension self-management plus a home blood pressure monitor. Control participants received a blood pressure tracking app plus a home blood pressure monitor.
Main Outcomes and Measures
The primary study outcome was systolic blood pressure at 6 months. Secondary outcomes included self-reported antihypertensive medication adherence, home monitoring and self-management practices, measures of self-efficacy associated with blood pressure, weight, and self-reported health behaviors.
Results
There were 333 participants randomized, and 297 completed the follow-up assessment. Among the participants who completed the study, the mean (SD) age was 58.9 (12.8) years, 182 (61.3%) were women, and 103 (34.7%) were black. Baseline mean (SD) systolic blood pressure was 140.6 (12.2) mm Hg among intervention participants and 141.8 (13.4) mm Hg among control participants. After 6 months, the corresponding mean (SD) systolic blood pressures were 132.3 (15.0) mm Hg and 135.0 (13.9) mm Hg, with a between-group adjusted difference of −2.0 mm Hg (95% CI, −4.9 mm Hg to 0.8 mm Hg; P = .16). At 6 months, self-confidence in controlling blood pressure was greater in the intervention group (0.36 point on a 5-point scale; 95% CI, 0.18 point to 0.54 point; P < .001). There were no significant differences between the 2 groups in other secondary outcomes. The adjusted difference in self-reported physical activity was 26.7 minutes per week (95% CI, −5.4 minutes per week to 58.8 minutes per week; P = .10). Subgroup analysis raised the possibility that intervention effects differed by age.
Conclusions and Relevance
Among individuals with uncontrolled hypertension, those randomized to a smartphone coaching app plus home monitor had similar systolic blood pressure compared with those who received a blood pressure tracking app plus home monitor. Given the direction of the difference in systolic blood pressure between groups and the possibility for differences in treatment effects across subgroups, future studies are warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT03288142
Introduction
Hypertension affects approximately 46% of adults in the United States and is a major cause of death and disability from heart and vascular diseases.1 Despite the efficacy of antihypertensive medications and positive effects of lifestyle changes on blood pressure, hypertension control rates are far from optimal.1
Interventions to promote blood pressure self-monitoring reduce blood pressure when monitoring leads to treatment intensification.2,3,4,5,6 Blood pressure may also improve with dietary changes or increased exercise.7 Mobile health interventions such as smartphone applications (apps) may support hypertension self-management, and many such apps are commercially available.8,9,10 However, high-quality evidence of their effectiveness is limited.8,9,11,12 In particular, it is not known whether stand-alone mobile health interventions can successfully support the behaviors that lead to better blood pressure control. Studies11,12,13,14 of apps or interventions that used text messages for hypertension that were not integrated into clinical care delivery have not clearly demonstrated improved blood pressure. A stand-alone app tested by Morawski and colleagues11 improved medication adherence slightly but did not change systolic blood pressure. In that study,11 the app did not sync with the home blood pressure monitor (HBPM), provide individualized coaching based on measured values, or address nonpharmacologic behaviors, such as weight loss, diet, or physical activity.
To address this gap, we sought to test whether providing a smartphone hypertension coaching app directed at multiple hypertension care behaviors—referred to as the hypertension personal control program (HPCP)—along with an HBPM would lead to lower systolic blood pressure and improvement in other aspects of hypertension self-management compared with providing a blood pressure tracking app and HBPM among adults with uncontrolled hypertension.
Methods
Study Design
The Improving Hypertension Using a Smartphone-Enabled Personal Control Program, or “Smart Hypertension Control Study,” was a nonblinded randomized clinical trial among hypertensive adults receiving care from outpatient clinics affiliated with a large health care system. Northwestern University’s institutional review board approved the study. Participants provided written informed consent. This study follows the Consolidated Standards of Reporting Trials Extension (CONSORT Extension) reporting guideline.
Enrollment took place between September 28, 2017, and September 28, 2018. Data collection was completed April 9, 2019. The design and methods have been previously published.15 The trial protocol is provided in Supplement 1. Participants were randomized to the intervention group (smartphone coaching app plus an HBPM) or the control group (blood pressure tracking app and HBPM) and were followed up for 6 months. The primary outcome was systolic blood pressure at 6 months adjusted for baseline systolic blood pressure, sex, and age.
Setting and Participants
We recruited patients from 4 primary care clinics affiliated with Northwestern Medical Group in downtown Chicago, Illinois. We recruited adults aged 18 to 84 years whose intake blood pressure was greater than or equal to 135 mm Hg systolic and 85 mm Hg diastolic (either value) and less than 180 mm Hg systolic and 110 mm Hg diastolic (both values). Participants with or without antihypertensive medication use were eligible. Because of variation in how Bluetooth technology was used among non-iOS smartphone systems, participants were required to have a compatible iOS (Apple) device. Full inclusion and exclusion criteria have been published elsewhere.15
Recruitment and Randomization
We searched electronic health record data to identify potentially eligible participants at participating practices whose most recent in-office blood pressure was at least 145 mm Hg systolic or 95 mm Hg diastolic. We used these higher criteria to identify individuals who would be more likely to meet study inclusion criteria at the research study visit. Primary care physicians could indicate which patients not to contact. Recruitment involved both mail and telephone. Potential participants could also be referred by clinicians or respond to flyers distributed in practices. After a telephone eligibility assessment, volunteers presented in person for determination of blood pressure eligibility.
After enrollment, participants were randomized 1:1 using a centralized computer-generated assignment sequence uploaded a priori to Northwestern University’s Research Electronic Data Capture platform.16 We performed randomization in 4 strata by age (<65 or ≥65 years) and baseline systolic blood pressure (<145 or ≥145 mm Hg) in blocks of 5. The study staff members enrolling participants did not have prior access to the allocation sequence.
Interventions
Tracking App With HBPM (Control Group)
Control participants received an HBPM (7 Series Wireless Upper Arm Blood Pressure Monitor Model BP761N, Omron; or global Model HEM-7320T, Omron Healthcare Co Ltd), were instructed how to perform self-monitoring, and were asked to demonstrate use of the device. Information provided to participants explained how to interpret home blood pressure results and to respond to extreme values but did not make specific recommendations about when to share information with clinicians. Initially, we described home blood pressure mean of 135 mm Hg systolic and 85 mm Hg diastolic or greater as elevated and changed this to 130 mm Hg systolic and 80 mm Hg diastolic or greater after the 2017 release of the American College of Cardiology and American Heart Association hypertension guideline recommendations.7 We assisted participants with installation of a smartphone app that uses Bluetooth to connect to the HBPM and can track the HBPM data app (Omron Wellness, Omron Healthcare, Inc).
Hypertension Coaching App and HBPM (Intervention Group)
Intervention group participants received all interventions provided to the control group except the Omron smartphone app. Instead, they installed the HPCP coaching app (a beta version of HTN Pro, Lark Technologies, Inc).
The HPCP coaching app was described previously.15 In short, it is a conversational artificial intelligence smartphone app that uses cognitive behavioral therapy techniques to provide support and coaching to promote hypertension self-management and healthy behaviors, including diet, physical activity, medication adherence, blood pressure measurement, sleep, and stress management. Recommendations were tailored according to participants’ prior data and responses. The app uses encouragement, reminders, and tracking to promote home monitoring. It prompted participants to measure blood pressure daily for the first week, then weekly thereafter. It recommended retaking readings if outlier values were recorded and to call their medical professional for extreme values. The HPCP informed users of changes over time and provided appropriate coaching based on proprietary algorithms. The app encouraged users to contact medical practitioners if blood pressure remained greater than 130 mm Hg systolic or 80 mm Hg diastolic for more than 1 month.
The HPCP prompted medication users to set up reminders, asked about adherence, and provided coaching around barriers to adherence. Over time, the HPCP provided education on hypertension-related topics, including weight loss and its association with hypertension. The HPCP encouraged users to make choices consistent with the Dietary Approaches to Stop Hypertension diet.17 The HPCP allowed users to set physical activity goals and provided coaching on progress toward these goals. Activity was tracked using the smartphone’s motion detector or was entered manually. The HPCP tracked sleep, provided coaching based on sleep habits, and included education on stress management. The HPCP sent notifications to users to remind them to log blood pressure, medications, weight, meals and snacks, and physical activity. Users could initiate conversations any time. Content was customized on the basis of the user’s values and inputs. Additional description is reported in eAppendix 1 in Supplement 2.
Measurements and Outcomes
We performed in-person assessments at baseline and 6 months. Research staff measured blood pressure in a standardized fashion using an automated device (Digital Automatic Blood Pressure Monitor HEM-907XL, Omron Healthcare Co Ltd). We performed 3 recordings, and the mean of the second and third readings was the study blood pressure. Height was measured at baseline. Weight was measured at baseline and 6 months. We assessed the following items by survey: adherence to antihypertensive medications (characterized as no missed doses vs any missed doses using a 4-day recall)18; number of antihypertensive medication classes used; number of medication intensifications or substitutions during the study period; self-confidence using HBPM, controlling blood pressure, knowing when medication changes were needed, and performing nonpharmacologic behaviors to control blood pressure (on a 5-point scale, ranging from 1 [not at all confident] to 5 [extremely confident]); Dietary Approaches to Stop Hypertension diet compliance Questionnaire19; days per week eating several other food categories; self-reported at-least-moderate physical activity (days per week times minutes per day); and self-reported sleep duration. We assessed the frequency of office visits and telephone and email contacts from data in the Northwestern Medicine electronic health record. We measured HBPM usage (months used and uses per month) from data recorded in the Lark or Omron apps uploaded to the respective company’s server. We encouraged patients in both groups to synchronize their HBPM with their smartphone app during the initial and follow-up visits.
Sample Size and Power
The primary study outcome was systolic blood pressure at 6 months with prespecified adjustment for baseline systolic blood pressure, sex, and age. We conservatively estimated power according to the independent 2-sample t test; 150 participants in each group completing the study would provide 80% power to detect a 0.3 SD effect size across the 2 groups at the 5% level of significance. If the observed SD were 15 mm Hg, we would have adequate power to detect a difference of 5 mm Hg across the 2 groups. A study of this size was feasible to perform with the available study resources. With prespecified adjustments, we anticipated increased precision, allowing for 80% power to detect a slightly smaller effect size. We initially sought to enroll 350 participants and anticipated approximately 85% retention. Because actual retention was higher, we ended recruitment after 333 participants had enrolled.
Statistical Analysis
All analyses used intention-to-treat principles and followed the previously described analysis plan unless otherwise stated.15 For the primary analysis, we compared the difference in systolic blood pressure at 6 months between the 2 treatment groups using a linear regression model, adjusting for baseline age (years), sex, and baseline systolic blood pressure. We performed similar analyses for the secondary outcomes of continuous variables, adjusting for age, sex, and baseline value of the respective variable. We used logistic or Poisson regression for other outcomes, adjusting for age, sex, and the baseline value of the outcome, as previously stated.15 To keep the probability of a type I error less than .05 with the multiple hypotheses tested in the secondary analyses, we applied the method of Benjamini and Hochberg.20 We compared the effects of treatment group assignment on the primary outcome in subgroup analyses stratified by baseline systolic blood pressure, body mass index (BMI; calculated as the weight in kilograms divided by height in meters squared), sex, age, and general self-efficacy, which is measured on a scale of 10 to 40 according to the sum of responses to 10 questions, with each question having options from 1 (not at all true) to 4 (exactly true).21 We tested for heterogeneity of treatment effects across subgroups by examining the significance of treatment group times subgroup interaction terms in models that included randomization group and subgroup variables. Data analysis was performed from April 2019 to December 2019 using SAS statistical software version 9.4 (SAS Institute).
Results
Participants
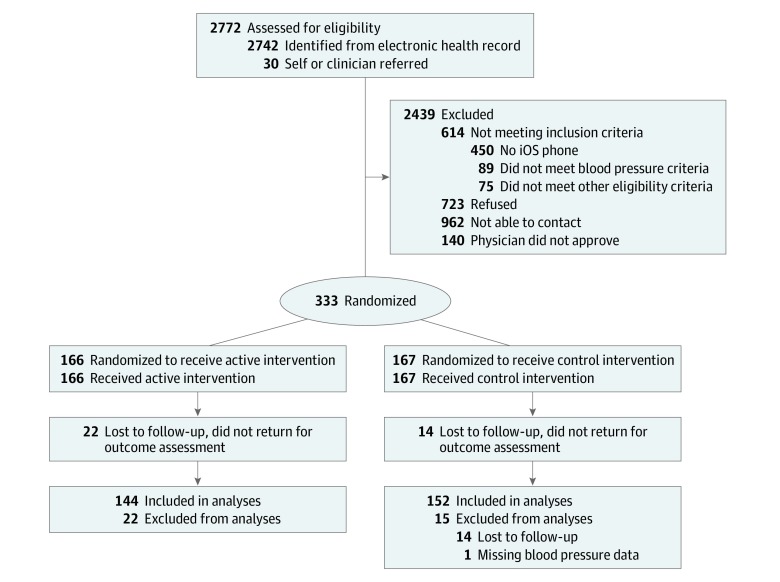
We identified 2742 patients receiving care from 183 primary care physicians who met the initial electronic health record screening criteria, and 30 additional participants were directly referred by their clinician or responded to a recruitment flyer. The flow of participants is depicted in Figure 1. There were 333 participants randomized (166 to the intervention group and 167 to the control group). In total, 297 (89.2%) participants completed the follow-up assessment and were included in analyses (144 intervention group and 153 control group). Participants who did not complete follow-up are described in eTable 1 in Supplement 2. The primary efficacy analysis included 152 control group participants (1 control group participant did not have follow-up blood pressure values successfully stored).
Figure 1. Recruitment, Randomization, and Participant Flow Diagram.
Baseline Characteristics
Participants’ mean (SD) age was 58.9 (12.8) years, 182 (61.3%) were women, 103 (34.7%) were black, 13 (4.4%) were Asian, and 23 (7.7%) were of Hispanic ethnicity. Study groups were similar: most participants were white (73 [50.7%] in the intervention group vs 80 [52.3%] in the control group), most spoke English as their primary language (141 [97.9%] in the intervention group vs 144 [94.1%] in the control group), and most had 4 years or more of college education (103 [71.5%] in the intervention group vs 107 [69.9%] in the control group) (Table 1).
Table 1. Baseline Characteristics.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Hypertension Coaching Application and Home Monitor: Intervention (n = 144) | Tracking Application and Home Monitor: Control (n = 153) | |
| Age, mean (SD), y | 59.6 (12.4) | 58.3 (13.2) |
| Female | 91 (63.2) | 91 (59.5) |
| Ethnicity, Hispanic or Latino | 10 (6.9) | 13 (8.5) |
| Race | ||
| Asian | 4 (2.8) | 9 (5.9) |
| Black | 56 (38.9) | 47 (30.7) |
| White | 73 (50.7) | 80 (52.3) |
| Other or unknown | 11 (7.6) | 17 (11.1) |
| Education | ||
| Less than high school graduate | 3 (2.1) | 2 (1.3) |
| Grade 12 or General Education Development examination | 10 (6.9) | 16 (10.5) |
| College 1-3 y | 27 (18.8) | 28 (18.3) |
| College ≥4 y | 103 (71.5) | 107 (69.9) |
| Unknown | 1 (0.7) | 0 |
| Primary language | ||
| English | 141 (97.9) | 144 (94.1) |
| Other | 3 (2.1) | 8 (5.2) |
| Refused to identify | 0 | 1 (0.7) |
| Comorbidities | ||
| Asthma or chronic obstructive pulmonary disease | 26 (18.1) | 30 (19.6) |
| Diabetes | 28 (19.4) | 26 (17.0) |
| Coronary heart disease | 5 (3.5) | 10 (6.5) |
| Stroke | 4 (2.8) | 6 (3.9) |
| Heart failure | 1 (0.7) | 6 (3.9) |
| Body mass index, mean (SD)a | 31.9 (8.3) | 31.7 (7.8) |
| Current smoker | 4 (2.8) | 8 (5.2) |
| Generalized self-efficacy score, mean (SD)b | 33.7 (4.4) | 33.8 (4.4) |
| No. of antihypertensive agents used | ||
| 0 | 34 (23.6) | 54 (35.3) |
| 1 | 61 (42.4) | 44 (28.8) |
| 2 | 35 (24.3) | 40 (26.1) |
| ≥3 | 14 (9.7) | 15 (9.8) |
Body mass index is calculated as the weight in kilograms divided by height in meters squared.
Self-efficacy is measured on a scale of 10 to 40 according to the sum of responses to 10 questions, with each question having options from 1 (not at all true) to 4 (exactly true).21
Blood Pressure
Baseline mean (SD) systolic blood pressure was 140.6 (12.2) mm Hg for intervention participants and 141.8 (13.4) mm Hg for control participants. After 6 months, the mean (SD) systolic blood pressures in the intervention group decreased by 8.3 (13.8) mm Hg to 132.3 (15.0) mm Hg in the intervention group and by 6.8 (13.7) mm Hg to 135.0 (13.9) mm Hg in the control group. The between-group adjusted mean difference was −2.0 mm Hg (95% CI, −4.9 mm Hg to 0.8 mm Hg; P = .16). Diastolic blood pressure (mean [SD], 85.1 [9.6] mm Hg in the intervention group vs 85.6 [9.8] mm Hg in the control group) and rates of blood pressure less than 140 mm Hg systolic and 90 mm Hg diastolic (72 [50.0%] participants in the intervention group vs 78 [51.3%] participants in the control group) were similar in the study groups at 6 months (Table 2).
Table 2. Blood Pressure Outcomes.
| Variable | Hypertension Coaching App and Home Monitor, Intervention (n = 144) | Tracking App and Home Monitor, Control (n = 152) | Adjusted Difference, Mean (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Change | Baseline | Month 6 | Change | |||
| Systolic blood pressure, mean (SD), mm Hga | 140.6 (12.2) | 132.3 (15.0) | −8.3 (13.8) | 141.8 (13.4) | 135.0 (13.9) | −6.8 (13.7) | −2.0 (−4.9 to 0.8) | .16 |
| Diastolic blood pressure, mean (SD), mm Hg | 89.4 (8.7) | 85.1 (9.6) | −4.3 (8.4) | 89.2 (9.2) | 85.6 (9.8) | −3.6 (9.5) | −0.5 (−2.4 to 1.4) | .61 |
| Blood pressure <140/90 mm Hg, participants, No. (%) | 36 (25.0) | 72 (50.0) | 36 (25.0) | 41 (27.0) | 78 (51.3) | 37 (24.3) | 0.9 (0.6 to 1.4)b | .66 |
Primary study outcome.
Odds ratio (95% CI).
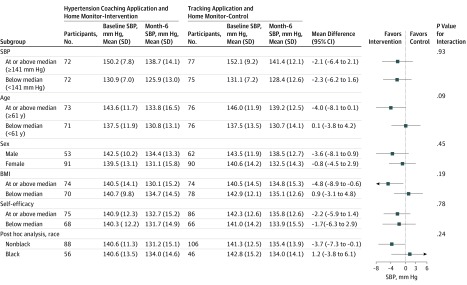
Study group effects on the primary outcome across subgroups (prespecified, baseline systolic blood pressure, age, sex, BMI, and general self-efficacy; post hoc, race) are shown in Figure 2. There was no statistically significant heterogeneity in intervention effect across these subgroups (P values for intervention by subgroup interaction terms were >.05 in all cases). The adjusted mean between-group differences in systolic blood pressure at 6 months in the intervention and control groups were −4.0 mm Hg (95% CI, −8.1 mm Hg to 0.1 mm Hg) for participants at or above the median age and 0.1 mm Hg (95% CI, −3.8 mm Hg to 4.2 mm Hg) for participants below the median age. Corresponding mean differences for BMI were −4.8 mm Hg (95% CI, −8.9 mm Hg to −0.6 mm Hg) for participants at or above the median BMI and 0.9 mm Hg (95% CI, −3.1 mm Hg to 4.8 mm Hg) for participants below the median BMI. The mean differences were −3.6 mm Hg (95% CI, −8.1 mm Hg to 0.9 mm Hg) for men, −0.8 (−4.5 mm Hg to 2.9 mm Hg) for women, −3.7 mm Hg (95% CI, −7.3 mm Hg to −0.1 mm Hg) for nonblack participants, and 1.2 mm Hg (95% CI, −3.8 mm Hg to 6.1 mm Hg) for black participants.
Figure 2. Subgroup Analyses of the Difference Between Intervention and Control in Systolic Blood Pressure at 6 Months.
BMI indicates body mass index (calculated as the weight in kilograms divided by height in meters squared); and SBP, systolic blood pressure.
Secondary Study Outcomes
Secondary study outcomes are shown in Table 3. There was a statistically significant difference in mean self-confidence in controlling blood pressure score (0.36 point on a 5-point scale; 95% CI, 0.18 to 0.54 point; P < .001) after accounting for the multiple hypotheses tested for all secondary outcomes.20 There were no additional statistically significant differences in any additional secondary outcomes. Self-reported minutes per week of moderate or strenuous physical activity was greater in the intervention group but this difference was not statistically significant, with an adjusted mean difference of 26.7 minutes per week (95% CI, −5.4 minutes per week to 58.8 minutes per week; P = .10). In both study groups, HBPM use was frequent and medication intensifications or substitutions during the study period were infrequent.
Table 3. Secondary Outcomes.
| Outcome | Mean (SD) | Treatment Effect (95% CI)a | P Value | |||
|---|---|---|---|---|---|---|
| Hypertension Personal Control Program and Home Monitoring Device | Tracking Application and Home Monitoring Device | |||||
| Baseline | Month 6 | Baseline | Month 6 | |||
| Self-reported full adherence to antihypertensive medications, participants, No. with finding/total No. (%) | 85/108 (78.7) | 87/108 (80.6) | 76/93 (81.7) | 76/93 (81.7) | OR, 1.01 (0.5 to 2.2) | .99 |
| Antihypertensive agents used, No. | 1.2 (1.0) | 1.3 (1.0) | 1.1 (1.1) | 1.3 (1.2) | RR, 1.0 (0.8 to 1.1) | .56 |
| Antihypertensive medication intensification, No. of additions, dose increases, or substitutions | NA | 0.3 (0.6) | NA | 0.4 (0.8) | RR, 0.8 (0.5 to 1.2) | .21 |
| Months when a home blood pressure reading is obtained, No. | NA | 4.9 (1.8) | NA | 5.0 (1.8) | RR, 1.0 (0.9 to 1.1) | .55 |
| Frequency of home blood pressure measurements per month derived from home blood pressure monitor for months when home monitoring occurred, median (interquartile range) | NA | 16 (8.5 to 29) | NA | 17 (9 to 32.5) | RR, 1.0 (0.8 to 1.2) | .81 |
| Self-efficacy score | ||||||
| Confidence in using home monitor | 4.9 (0.5) | 4.8 (0.6) | 4.9 (0.4) | 4.7 (0.7) | 0.09 (−0.05 to 0.23) | .21 |
| Confidence in controlling blood pressureb | 3.9 (1.1) | 4.4 (0.8) | 4.0 (0.9) | 4.1 (0.9) | 0.4 (0.2 to 0.5) | <.001c |
| Confidence in judging when medication change neededb | 3.9 (1.3) | 4.0 (1.3) | 3.9 (1.3) | 4.0 (1.2) | −0.03 (−0.3 to 0.3) | .86 |
| Confidence in doing nonmedication behaviors to control blood pressureb | 4.4 (0.9) | 4.4 (0.9) | 4.5 (0.7) | 4.4 (0.9) | 0.12 (−0.07 to 0.31) | .22 |
| Body mass indexd | 31.9 (8.3) | 32.0 (8.2) | 31.7 (7.8) | 31.9 (7.7) | −0.15 (−0.51 to 0.20) | .39 |
| Dietary Approaches to Stop Hypertension–Questionnaire scoree | 37.6 (12.1) | 38.7 (12.1) | 37.4 (11.6) | 39.3 (11.8) | −0.7 (−2.8 to 1.4) | .52 |
| Consumption of processed meats, d/wk | 1.6 (1.7) | 1.3 (1.6) | 1.5 (1.7) | 1.5 (1.8) | −0.2 (−0.6 to 0.1) | .15 |
| Consumption of fried foods, d/wk | 1.2 (1.3) | 1.0 (1.3) | 1.2 (1.5) | 1.1 (1.4) | −0.03 (−0.31 to 0.24) | .81 |
| Consumption of sugar-sweetened beverages, d/wk | 2.1 (2.8) | 1.7 (2.5) | 1.9 (2.7) | 1.7 (2.4) | −0.1 (−0.5 to 0.3) | .66 |
| Consumption of candy, baked goods, or ice cream, d/wk | 3.2 (2.4) | 2.9 (2.3) | 2.8 (2.2) | 2.9 (2.2) | −0.2 (−0.6 to 0.2) | .35 |
| Self-reported physical activity (min/wk of at least moderate exercise), d/wk | 172.5 (173.8) | 177.6 (169.2) | 159.1 (184.0) | 143.1 (156.4) | 26.7 (−5.4 to 58.8) | .10 |
| Self-reported sleep duration, h/night | 6.5 (1.3) | 6.6 (1.4) | 6.5 (1.3) | 6.5 (1.2) | 0.1 (−0.2 to 0.4) | .51 |
| Health system contacts (telephone, office, and patient portal encounters), No. | NA | 7.0 (11.5) | NA | 6.7 (6.5) | RR, 1.0 (0.8 to 1.4) | .91 |
Abbreviations: NA, not applicable; OR, adjusted odds ratio; RR, adjusted incidence rate ratio.
Data are adjusted between-group differences at 6 months unless specified as an OR or RR.
Reponses were on a 5-point scale ranging from (1) not at all confident to (5) extremely confident.
Rejects the null hypothesis controlling the false discovery rate using the method of Benjamini and Hochberg.20
Body mass index is calculated as the weight in kilograms divided by height in meters squared.
Sum of responses represents intake of foods associated with the Dietary Approaches to Stop Hypertension diet. Range is from 0 to 77 with a higher score indicating better diet. Scores 32 and below indicate a low-quality diet, scores 33 to 51 indicate a medium-quality diet, and scores greater than or equal to 52 indicate a high-quality diet.19
App Usage
Information about the number of conversations participants had with the HPCP are provided in eAppendix 2 and eTable 2 in Supplement 2. A post hoc analysis of HPCP usage stratified by age showed that participants at or above the median age had more conversations with the app and performed more blood pressure measurements than did participants below the median age (eAppendix 2 and eTable 3 in Supplement 2).
Discussion
In a population with mild uncontrolled hypertension, a beta version of a smartphone coaching app to promote home monitoring and other behaviors associated with hypertension plus an HBPM did not lower systolic blood pressures at 6 months compared with a tracking app and an HBPM. Intervention group participants had greater self-confidence in controlling blood pressure and may have increased exercise more compared with the control group. There are several observations worth noting that may further aid in the interpretation of these results.
Antihypertensive medication intensification was infrequent, self-reported adherence was high, and neither differed by group. There was also no evidence that the coaching app increased contacts with primary care physicians for medication intensification. Prior successful interventions using home monitoring linked this monitoring to cointerventions whereby clinicians2,3,4,5 or patients6 could increase medication when readings remained elevated. A prior trial11 of a stand-alone smartphone app to address hypertension did not show significant blood pressure improvement, whereas interventions using apps on a tablet computer or smartphone along with clinician contact have shown favorable results.22,23 Future research should examine whether more directive messages from a coaching app could lead to more rapid treatment intensification. In addition, research directed at the subgroups with the largest observed effects could be pursued.
The degree of blood pressure elevation observed here was lower than in many prior studies. We did not exclude participants with only low levels of blood pressure elevation because a large portion of the population with hypertension has blood pressures that are only mildly elevated,7 and a scalable coaching solution might be a particularly useful way to reach a large group with less severe hypertension. It is possible that many participants thought that the degree of elevation was not high enough to warrant taking action. Further adoption of the 2017 American College of Cardiology and American Heart Association hypertension guideline7 may lead to more attention to blood pressures in the range observed here.
This study compared a coaching app plus HBPM with an active comparator that included a blood pressure tracking app plus HBPM rather than to usual care. Even though baseline blood pressure elevations were mild, blood pressure decreased by a mean of 6.8 mm Hg systolic and 3.6 mm Hg diastolic in the control group. It is likely that, at least in part, this decrease was associated with regression to the mean for individuals whose enrollment blood pressure was greater than their true average, but it is also possible that there were blood pressure effects due to the control intervention. The high usage of HBPM in both groups supports this possibility.
Limitations
The results of this trial should be considered in the context of several limitations. First, because of the nature of the intervention, participant blinding was impossible. We could not blind research staff because the same staff enrolled participants and conducted follow-up. To minimize the risk of bias, we used allocation concealment before randomization and a standardized assessment of blood pressure outcomes with an automated device. Second, some outcomes were self-reported. We do not know whether receiving coaching from an app would compel respondents to give socially desirable answers to questions regarding behaviors such as exercise. Third, we did not specifically select participants who were likely to use a health-coaching app. Greater effects may have been observed if we had selectively enrolled participants most likely to benefit from this type of intervention.
Fourth, the sample size was not large enough to detect systolic blood pressure differences smaller than approximately 5 mm Hg, or to detect differences between subgroups that may be clinically important. The observed difference in the primary outcome by study groups is consistent with no effect, or with a treatment effect that was smaller than what the study was powered to detect. Similarly, subgroup analyses raise the question of whether treatment effects were not uniform across subgroups. A larger trial would be needed to address these questions. Fifth, the app used in the study was a beta version that underwent several refinements (most recently in early 2019). It is possible that results would be different if the study were repeated with the current version of the app. Sixth, the artificial intelligence and machine learning technology used here in this app gains information with larger numbers of users contributing data. Therefore, the performance of the app and clinical outcomes could differ if the number of users increased. Seventh, even though we recruited patients whose blood pressure in the electronic health record was greater than 145 mm Hg systolic and 95 mm Hg diastolic and greater than 135 mm Hg systolic and 85 mm Hg diastolic at the screening visit, the study population had mild blood pressure elevations and we cannot exclude the possibility that some patients may have, on average, had well-controlled hypertension. Eighth, the generalizability of these findings to other populations—including non-iOS device users—is not known.
Conclusions
Individuals with hypertension randomized to a smartphone coaching app plus HBPM had similar mean blood pressure at 6 months compared with controls randomized to receive a tracking app plus an HBPM. Given the direction of the difference in systolic blood pressure and some secondary outcomes between groups, and the possibility for differences in treatment effects across age subgroups, future studies are warranted.
Trial Protocol
eAppendix 1. Additional Description of the Hypertension Coaching App
eAppendix 2. Engagement and Usage of the HPCP
eTable 1. Characteristics of Participants Who Did Not Complete Follow Up
eTable 2. Engagement, Blood Pressure Measurement, and Meal Logging, N = 158 Intervention Group Participants
eTable 3. Engagement, Blood Pressure Measurement, and Meal Logging by Age, N =158 Intervention Group Participants
Data Sharing Statement
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):-. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Margolis KL, Asche SE, Dehmer SP, et al. Long-term outcomes of the effects of home blood pressure telemonitoring and pharmacist management on blood pressure among adults with uncontrolled hypertension: follow-up of a cluster randomized clinical trial. JAMA Netw Open. 2018;1(5):e181617. doi: 10.1001/jamanetworkopen.2018.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185-194. doi: 10.7326/0003-4819-159-3-201308060-00008 [DOI] [PubMed] [Google Scholar]
- 4.Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389. doi: 10.1371/journal.pmed.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens. 2015;28(10):1209-1221. doi: 10.1093/ajh/hpv008 [DOI] [PubMed] [Google Scholar]
- 6.McManus RJ, Mant J, Franssen M, et al. ; TASMINH4 Investigators . Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(10124):949-959. doi: 10.1016/S0140-6736(18)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 8.Thangada ND, Garg N, Pandey A, Kumar N. The emerging role of mobile-health applications in the management of hypertension. Curr Cardiol Rep. 2018;20(9):78. doi: 10.1007/s11886-018-1022-7 [DOI] [PubMed] [Google Scholar]
- 9.McLean G, Band R, Saunderson K, et al. ; DIPSS Co-Investigators . Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi: 10.1097/HJH.0000000000000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar N, Khunger M, Gupta A, Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9(2):130-136. doi: 10.1016/j.jash.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Morawski K, Ghazinouri R, Krumme A, et al. Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Intern Med. 2018;178(6):802-809. doi: 10.1001/jamainternmed.2018.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Searcy RP, Summapund J, Estrin D, et al. Mobile health technologies for older adults with cardiovascular disease: current evidence and future directions. Curr Geriatr Rep. 2019;8(1):31-42. doi: 10.1007/s13670-019-0270-8 [DOI] [Google Scholar]
- 13.Kim JY, Wineinger NE, Steinhubl SR. The influence of wireless self-monitoring program on the relationship between patient activation and health behaviors, medication adherence, and blood pressure levels in hypertensive patients: a substudy of a randomized controlled trial. J Med Internet Res. 2016;18(6):e116. doi: 10.2196/jmir.5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buis L, Hirzel L, Dawood RM, et al. Text messaging to improve hypertension medication adherence in African Americans from primary care and emergency department settings: results from two randomized feasibility studies. JMIR Mhealth Uhealth. 2017;5(2):e9. doi: 10.2196/mhealth.6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persell SD, Karmali KN, Stein N, et al. Design of a randomized controlled trial comparing a mobile phone-based hypertension health coaching application to home blood pressure monitoring alone: the Smart Hypertension Control Study. Contemp Clin Trials. 2018;73:92-97. doi: 10.1016/j.cct.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Moore TJ, Obarzanek E, et al. ; DASH Collaborative Research Group . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117-1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 18.DeMasi RA, Graham NM, Tolson JM, et al. Correlation between self-reported adherence to highly active antiretroviral therapy (HAART) and virologic outcome. Adv Ther. 2001;18(4):163-173. doi: 10.1007/BF02850110 [DOI] [PubMed] [Google Scholar]
- 19.Warren-Findlow J, Reeve CL, Racine EF. Psychometric validation of a brief self-report measure of diet quality: the DASH-Q. J Nutr Educ Behav. 2017;49(2):92-99. doi: 10.1016/j.jneb.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Satist Soc. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 21.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, eds. Measures in Health Psychology: A User's Portfolio. Windsor, UK: NFER-NELSON; 1995:35-37. [Google Scholar]
- 22.Moore JO, Marshall MA, Judge DC, et al. Technology-supported apprenticeship in the management of hypertension: a randomized controlled trial. J Clin Outcomes Manag. 2014;21(3):110-122. [Google Scholar]
- 23.Milani RV, Lavie CJ, Bober RM, Milani AR, Ventura HO. Improving hypertension control and patient engagement using digital tools. Am J Med. 2017;130(1):14-20. doi: 10.1016/j.amjmed.2016.07.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Additional Description of the Hypertension Coaching App
eAppendix 2. Engagement and Usage of the HPCP
eTable 1. Characteristics of Participants Who Did Not Complete Follow Up
eTable 2. Engagement, Blood Pressure Measurement, and Meal Logging, N = 158 Intervention Group Participants
eTable 3. Engagement, Blood Pressure Measurement, and Meal Logging by Age, N =158 Intervention Group Participants
Data Sharing Statement