Highlights
-
•
Co-translational folding of NBD1 is disrupted by CF mutations.
-
•
FRET analysis of transmembrane constructs reveals misfolding by CF mutations.
-
•
The impact of the F508del mutation on CFTR is species-dependent.
-
•
Two potentiators with distinct actions restore CFTR activity to rare CF mutations.
Keywords: CFTR Cl− channel, F508del-CFTR, Rare CF mutations, Protein folding, CFTR correction, CFTR potentiation
Abstract
The treatment of cystic fibrosis (CF) has been transformed by orally-bioavailable small molecule modulators of the cystic fibrosis transmembrane conductance regulator (CFTR), which restore function to CF mutants. However, CFTR modulators are not available to all people with CF and better modulators are required to prevent disease progression. Here, we review selectively recent advances in CFTR folding, function and pharmacology. We highlight ensemble and single-molecule studies of CFTR folding, which provide new insight into CFTR assembly, its perturbation by CF mutations and rescue by CFTR modulators. We discuss species-dependent differences in the action of the F508del-CFTR mutation on CFTR expression, stability and function, which might influence pharmacological studies of CFTR modulators in CF animal models. Finally, we illuminate the identification of combinations of two CFTR potentiators (termed co-potentiators), which restore therapeutically-relevant levels of CFTR activity to rare CF mutations. Thus, mechanistic studies of CFTR folding, function and pharmacology inform the development of highly effective CFTR modulators.
1. Introduction
Thirty years after the identification and cloning of the cystic fibrosis transmembrane conductance regulator (CFTR) [1], a transformational drug therapy for most people with cystic fibrosis (CF) received regulatory approval [2,3]. The development of Trikafta™ (elexacaftor-tezacaftor-ivacaftor) (Vertex Pharmaceuticals) is the culmination of great efforts to understand the structure of CFTR, its synthesis in epithelial cells, function as a ligand-gated anion channel and modulation by small molecules [4]. Despite the enormous progress, CFTR modulators are not yet available to all people with CF, while there is evidence of disease progression in CFTR modulator-treated patients (for review, see [4]). Thus, there remains a pressing need to understand even better CFTR, its biosynthesis in cells, structure-function relationships and physiological roles. This knowledge will inform the development of next generation small molecule CFTR modulators, leading to life-long personalised treatments for CF.
Here, we review selectively some recent technical innovations to understand better how CF mutations perturb the assembly of CFTR, highlight species-dependent differences in CFTR expression, stability and function relevant to CF animal models and identify combinations of two small molecule CFTR potentiators that restore function to rare CF mutations, which have so far proven unresponsive to CFTR modulators. This review is based on symposia presentations from the 16th European Cystic Fibrosis Society Basic Science Conference, Dubrovnik, Croatia, 27–30th March 2019. Although CFTR folding, function and pharmacology are broad topics that each merit their own review, here they are combined to highlight the importance of mechanistic insight for CF drug discovery and development. For more comprehensive reviews of CFTR folding, function and pharmacology, we refer the Reader to [5], [6], [7], [8], [9].
2. CFTR folding: mechanism of CFTR cotranslational folding
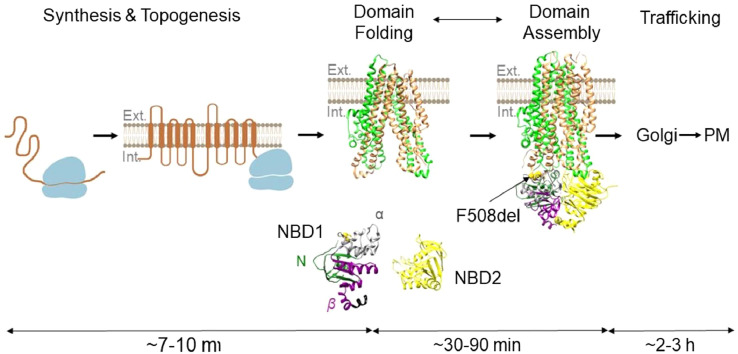
Cotranslational folding is a highly dynamic process that takes place as the nascent polypeptide is progressively synthesized from N- to C-terminus in a complex biological environment. This process is influenced by the adjacent ribosome [10,11], the translation rate [12,13], cellular chaperones [14,15], and the cytosolic folding environment [16]. For CFTR, cotranslational folding requires the precise insertion of 12 transmembrane α-helices into the lipid bilayer, tertiary folding and packing of three cytosolic domains and two membrane-spanning domains (MSDs), and assembly of these domains into a mature tertiary structure (Fig. 1). Many of these steps occur during the 7–10 minutes required to complete CFTR peptide synthesis and thus are truly cotranslational events [17]. The final steps of post-translational folding, including domain packing and assembly, are likely orchestrated after synthesis by cytosolic folding machinery prior to exit from the endoplasmic reticulum and delivery to the plasma membrane and take 30–90 min [17].
Fig. 1.
CFTR folding in the cell. The schematic shows the time scale of CFTR synthesis, folding and domain assembly in cells. The sub-domains of NBD1 (N-terminus (N), K377 – S492; α-helical (α), Q493 – D565; α/β-core (β), A566 – Q637) and the position of the F508del mutation are indicated. Abbreviations: NBD, nucleotide-binding domain; PM, plasma membrane. The figure was created, in part, with BioRender.com using CFTR structures (PDB id: 5UAK and 6MSM) and therefore lacks the unstructured R domain.
CFTR maturation relies crucially on proper folding of individual domains, and inherited mutations that disrupt any steps in this process lead to a misfolded protein that is degraded by the ubiquitin proteasome pathway (UPP) via a process known as Endoplasmic Reticulum Associated Degradation (ERAD) [5,17]. Although, the first nucleotide-binding domain (NBD1) is a particular hot-spot for trafficking mutations and plays a major role in overall CFTR folding efficiency [18], [19], [20], it is unknown when and how such mutations exert their effect. To determine whether trafficking mutations influence NBD1 folding cotranslationally or posttranslationally, Skach and coworkers [11], [21] developed an assay using Fluorescence (Förster) Resonance Energy Transfer (FRET) between two fluorophores that were cotranslationally incorporated into the growing nascent polypeptide to identify the stage of synthesis at which specific compaction events occur.
Using this FRET assay, Khushoo et al. [21] and Kim et al. [11] showed that NBD1 folds cotranslationally during synthesis on the ribosome via the sequential compaction of N-terminal, α-helical, and α/β-core subdomains (Fig. 1). The timing of these folding events is finely tuned by properties of the ribosome that delay collapse of the α-subdomain until synthesis of a 4-stranded parallel β-sheet core is completed. For example, premature release of the polypeptide from the ribosome results in rapid folding of the α-subdomain and irreversible failure of the core β-sheet to form. Because the timing of these folding events is critical, Kim et al. [11] tested whether the rate of translation influences coupling of the α-subdomain and β-core folding by introducing synonymous codon changes that were predicted to increase the translation rate precisely when the α-subdomain exited the ribosome (CFTR residues 525–593, “Fast-CFTR”), while keeping the amino acid sequence unchanged. These synonymous changes had little effect on CFTR synthesis or processing efficiency. However, they altered CFTR biogenesis in such a way as to induce a delayed aggregation of NBD1 and hence, full-length immature CFTR protein. Moreover, these synonymous codon changes also induced structural changes in epitopes on NBD1 and full-length CFTR that were related to the rate of cotranslational folding and were independent of CFTR sequence. Thus, an altered local epitope conformation within the native peptide sequence was cotranslationally imprinted and preserved throughout CFTR processing and intracellular trafficking [11]. Indirect analysis of the translation rate by ribosome profiling further confirmed ribosomal pausing within the region of synonymous codon changes that was abolished in the “Fast-CFTR” construct, indicating that translation rate can impact the efficiency of the overall folding outcome. Consistent with these data, in silico analyses of synonymous single nucleotide polymorphisms (sSNPs) identified sSNPs which have the potential to change CFTR structure, so called “non-silent” synonymous mutations [22] (see also Kirchner et al. [23]). Taken together, the data suggest that restoration of cotranslational folding dynamics might provide an important therapeutic strategy for CF and other folding disorders.
3. CFTR folding: analysis of transmembrane helices with single-molecule FRET
CF mutations located in the MSDs frequently cause misfolding (e.g. [24,25]). Analysis of their effects on CFTR folding are mostly investigated indirectly by evaluating protein maturation rates in cells. However, such analyses preclude insight into how CF mutations cause misfolding of transmembrane helices and how CFTR correctors reverse misfolding. A significant challenge in the development of such assays is the inherent complexity of studying the folding of full-length CFTR protein. The full-length protein with its 1,480 amino acids is notoriously difficult to obtain in sufficient quantities and purity for in vitro analysis, a problem confounded by CF mutations, which destabilise CFTR. Efforts to overcome this lack of protein stability recently culminated in a functional CFTR construct with six stabilising mutations in NBD1 [26]. Nevertheless, effective characterisation of the contribution of individual CF mutations to CFTR misfolding remains a challenge using classical biochemical and biophysical techniques due to the large size of the CFTR protein [27]. Such techniques are often limited in their ability to resolve the structural heterogeneities of misfolded protein states.
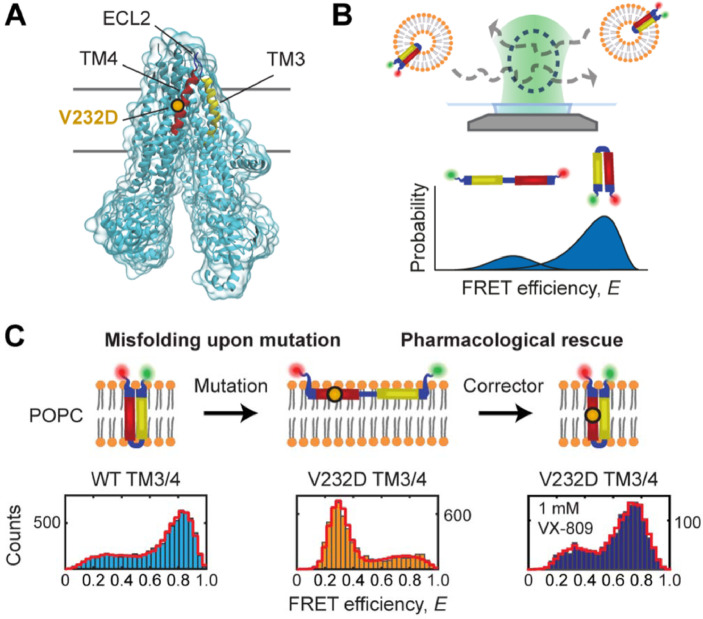
To gain molecular-level insights into CFTR misfolding and drug rescue of misfolded states, Krainer and Treff et al. [28] developed a single-molecule FRET-based approach that exploits helical-hairpin constructs derived from full-length CFTR as minimalist in vitro systems (Fig. 2A and B). Helical hairpins, comprising two transmembrane (TM) helices and their intervening loop region, are readily prepared in sufficient amounts for biophysical analysis [29,30]. They represent the smallest units that can be inserted autonomously by the translocon since CFTR topogenesis in the ER is based on pairwise integration of helical segments [17]. In tandem with single-molecule FRET [31,32], which serves as a spectroscopic ruler to probe the end-to-end distances of hairpins reconstituted in lipid bilayers (Fig. 2B), these minimalist folding units constitute versatile platforms to characterise biophysically the molecular events of CFTR folding.
Fig. 2.
A minimal helical-hairpin motif provides molecular-level insights into misfolding and pharmacological rescue of CFTR. (A) Structure of CFTR (PDB id: 5UAK) highlighting the position of the V232D mutation in TM3/4 (yellow/red) and the intervening second extracellular loop (ECL2). (B) Schematic of the single-molecule FRET approach for investigating hairpin conformations. Shown are single fluorescently labeled TM3/4 hairpin molecules reconstituted into phospholipid vesicles freely diffusing through the observation volume of the confocal microscope. Obtained FRET efficiency histograms report on coexisting conformational hairpin states and their relative occupancies. (C) FRET efficiency histograms of wild-type (WT) TM3/4 (light blue), V232D TM3/4 (orange) and V232D TM3/4 in the presence of lumacaftor (VX-809; dark blue). Figure adapted from Krainer and Treff et al. [28] under Creative Commons Attribution License. (For interpretation of the references to colour in this figure legend, the Reader is referred to the web version of this article).
Krainer and Treff et al. [28] applied this hairpin approach to study misfolding of the CF mutation V232D in TM4 (Fig. 2A) and the impact of the clinically-approved CFTR corrector lumacaftor [33] by exploiting a helix/loop/helix hairpin construct [34] comprising TM3 and TM4 of CFTR. The folding of TM3/4 was tracked by monitoring the FRET efficiency of single-molecule hairpin molecules reconstituted in lipid-bilayer membranes (Fig. 2C). When compared with the wild-type hairpin, the TM3/4 V232D mutant hairpin was misfolded in lipid membranes resembling the thickness of the endoplasmic reticulum, assuming an open structure associated with the interfacial region of the membrane [28]. These data suggest a model for V232D pathogenesis whereby partitioning of the TM3/4 V232D into the interfacial region traps CFTR as a partially folded intermediate at the membrane (Fig. 2C). They also rationalise cell-based analyses of CFTR misprocessing by the V232D mutation [35], arguing that the minimal hairpin motif might be used as a proxy to study the in vivo/in vitro correlates of CFTR misfolding.
Using the TM3/4 hairpin, Krainer and Treff et al. [28] investigated the action of lumacaftor on the CF mutation V232D [35,36]. Strikingly, lumacaftor reversed the open hairpin structure of TM3/4 V232D to restore a compact structure similar to that of the wild-type hairpin (Fig. 2C). This result suggests that the action of lumacaftor stabilises the native or near-native state of CFTR. In summary, this minimal in vitro approach constitutes a versatile platform to characterise the molecular events that link CF to structural effects of mutations, opening new avenues for the development of mechanism-based therapeutics that target CFTR and other misfolding-prone helical membrane proteins.
4. CFTR function: species-dependent differences inform CF mutation studies
Comparative studies of CFTR orthologues have demonstrated that CFTR misfolding is highly species dependent and identified structural motifs important for CFTR processing and plasma membrane stability [37,38]. Following identification of the CFTR gene [1], CFTR orthologues have been found in many jawed vertebrates (for CFTR phylogenic relationships, see [39]) and recently in the jawless vertebrate lamprey [40]. To understand better the pathogenesis of CF and evaluate new therapeutics for the disease, CF animal models have been developed using zebrafish, mice, rats, ferrets, pigs, sheep and rabbits [41], [42], [43], [44], [45], [46], [47]. By contrast, understanding of the single-channel behaviour of CFTR orthologues from these species and the impact of CF mutations upon them is incomplete.
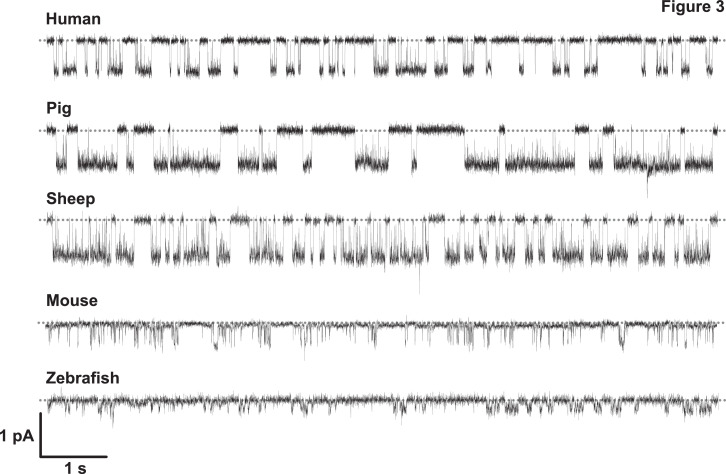
Comparison of the first cryo-EM structures of CFTR revealed high degrees of structural similarity despite only 54% amino acid identity between human and zebrafish CFTR [48], [49], [50], [51]. Thus, the striking differences in function between human and zebrafish CFTR were unexpected and not accounted for by codon optimisation [6, 52]. These differences include, first, zebrafish CFTR has an inwardly rectifying current–voltage (I–V) relationship unlike the ohmic I–V relationship of human CFTR [52]. Second, the gating pattern of zebrafish CFTR is dominated by prolonged channel closures with only brief channel openings (Fig. 3), despite similar ATP dependence to human CFTR [52]. Third, zebrafish CFTR exhibits marked differences in pharmacology, including the failure of ATP analogues to enhance channel activity and altered sensitivity to channel inhibitors [52]. Interestingly, the single-channel behaviour of zebrafish CFTR shows some similarities to mouse CFTR, the mammalian orthologue with greatest amino acid sequence difference from human CFTR (Fig. 3) [52], [53], [54].
Fig. 3.
The single-channel behaviour of CFTR orthologues. Representative single-channel recordings of human, pig, sheep, mouse and zebrafish CFTR in excised inside-out membrane patches from Chinese hamster ovary (CHO) cells expressing the indicated CFTR variants or a C127 cell expressing human CFTR. The recordings were acquired using the experimental conditions described in Cai et al. [57] except that voltage was –100 mV for zebrafish CFTR. Dotted lines indicate where channels are closed and downward deflections correspond to changes in current following channel opening. Note that the sub-conductance state of mouse CFTR is not apparent without further filtering of single-channel records [54,58]. Figure adapted in part from Cai et al. [57] and Bose et al. [58] under Creative Commons Attribution License.
Ostedgaard et al. [37] first demonstrated that the impact of the F508del mutation on CFTR is species dependent. Like human F508del-CFTR, ferret and sheep F508del-CFTR fail to mature, whereas mouse, pig, rabbit and especially chicken F508del-CFTR produce substantially more mature CFTR protein [38,55,56]. Less is known about the species dependence of F508del-CFTR thermostability, which is potentially confounded by cell type-dependent differences in the expression of regulatory molecules, such as protein phosphatases. However, the available data suggest a gradation of severity with human F508del-CFTR exhibiting marked thermoinstability, sheep intermediate and chicken and mouse F508del-CFTR little or none [38,57,58]. Interestingly, in all species studied, the severity of the F508del-CFTR gating defect is noticeably reduced when compared with that of human F508del-CFTR [37,38,55,57,58]. Taken together, the data suggest that subtle structural differences between CFTR homologues, including the I539T revertant mutation [59] and proline residues at dynamic locations in NBD1 [38], account for the spectrum of effects of the F508del mutation on CFTR processing, thermostability and channel gating.
Using a CF ferret with the G551D mutation, Sun et al. [60] demonstrated that in utero and postnatal administration of the clinically-approved CFTR potentiator ivacaftor [61] rescues disease progression. However, species dependent differences in pharmacology caution that small molecule CFTR modulators should be tested on CFTR orthologues expressed in heterologous cells before they are used in CF animal models. For example, fifteen CFTR correctors, including lumacaftor, rescued both human and mouse F508del-CFTR [58]. By contrast, twelve CFTR potentiators had differential effects on human and mouse F508del-CFTR with eight, including ivacaftor, enhancing human, but not mouse F508del-CFTR activity and one compound, genistein, potentiating human and mouse F508del-CFTR by distinct mechanisms [58].
A potential explanation for the different effects of ivacaftor on human and mouse F508del-CFTR is provided by the identification of a binding site for ivacaftor and a second potentiator GLPG1837 [62,63]. The tyrosine residue at position 304 in TM5 of human CFTR, which forms a hydrogen bond with the bound potentiator, is replaced by a phenylalanine residue in mouse CFTR [62,63]. Transfer of this tyrosine residue to mouse CFTR (i.e. mouse F304Y-CFTR), confers potentiation by ivacaftor and GLPG1837 on mouse CFTR [63]. However, the different effects of ivacaftor on mouse models of autoimmune disease [64] might require a different explanation. One possibility is the action of ivacaftor on the solute carriers (SLCs) SLC6A14, SLC26A3 and SLC26A9, which modify CFTR function and hence, the severity of CF [65]. Alternatively, acute and chronic ivacaftor might have different effects on mouse CFTR [58,64,66]. Future studies should address these possibilities.
5. CFTR pharmacology: development of novel therapeutic paradigms for rare CF mutations
Despite advances in understanding how CF mutations alter CFTR folding and function and the development of robust model systems for modulator evaluation, there remains an unmet need for new therapeutics. Small molecule CFTR modulators remain the only viable therapeutic approach to correct the ion transport defect in CF epithelia. To date, four CFTR modulator therapies, all developed by Vertex Pharmaceuticals, are in clinical use: ivacaftor (KalydecoⓇ) for G551D-CFTR and 37 additional gating mutations [67], [68], [69]; lumacaftor-ivacaftor combination therapy (OrkambiⓇ) for F508del-CFTR homozygous subjects [70], and tezacaftor-ivacaftor combination therapy (SymdekoⓇ/SymkeviⓇ) for homozygous F508del-CFTR subjects, or subjects with F508del-CFTR and a residual function allele [71,72]. The latest combination therapy to receive regulatory approval (Trikafta™ (elexacaftor-tezacaftor-ivacaftor)) promises to benefit ~90% of CF subjects, including individuals homozygous for F508del-CFTR and those with F508del-CFTR and a minimal-function mutation [2,3]. However, no therapeutic modalities are currently available for ~10% of CF subjects with rare, or hard-to-treat mutations.
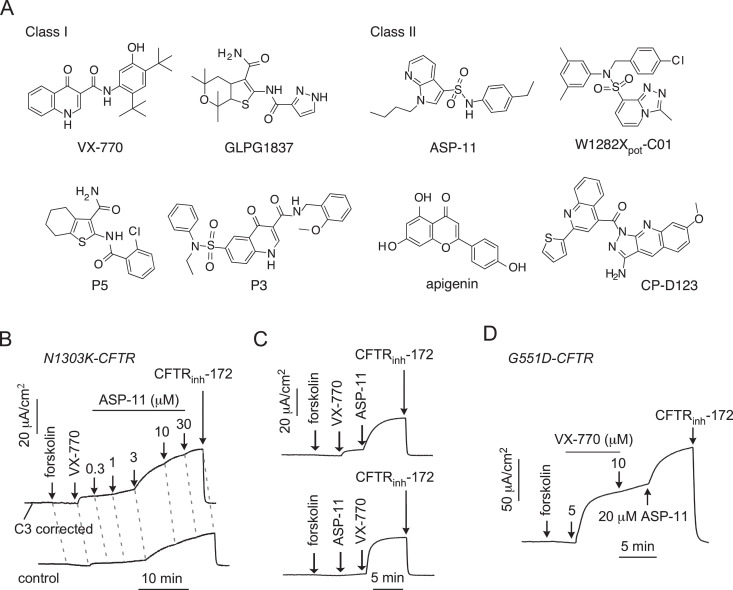
Motivated to develop novel therapeutic paradigms for CF mutations that are currently not served by CFTR modulators, high-throughput screening (HTS) was undertaken with CFTR1281 (the truncated polypeptide generated by the W1282X-CFTR mutation). Although arylsulfonyl-piperazine and spiro-piperidine-quinazolinone corrector scaffolds with ~5-fold greater efficacy than tezacaftor were identified, efforts to discover new potentiator scaffolds encountered an apparent potentiation ceiling for a single compound [73]. However, when ivacaftor was combined with new potentiators discovered by HTS, including arylsulfonamide-pyrrolopyridines (e.g. ASP-11; Fig. 4), CFTR1281 channel activity was enhanced by ~8-fold relative to ivacaftor alone, essentially restoring wild-type-like channel activity [73]. Strikingly, similar synergistic enhancement of channel activity was observed with the ivacaftor / ASP-11 potentiator combination (termed co-potentiation) in Fischer rat thyroid (FRT) cells expressing N1303K-CFTR (Fig. 4) [74]. Consistent with previous studies of CFTR modulators in primary epithelial cells (e.g. [75]), some variability in the response to co-potentiation was observed using epithelial cells endogenously expressing W1282X-CFTR possibly due to protective cellular processes including nonsense-mediated decay and ERAD [73,76,77]. However, co-potentiation with ivacaftor / ASP-11 restored therapeutically relevant levels of channel activity to N1303K-CFTR homozygous primary nasal epithelial cells (i.e. the magnitude of the potentiated short-circuit current was equivalent to that achieved in CF airway epithelial cells (genotype F508del/F508del) after correction with lumacaftor and potentiation by ivacaftor) [74,76]. Using HTS, Phuan et al. [76] identified additional chemical scaffolds (piperidinepyridoindoles, phenylazepines, tetrahydroquinolines and pyrazoloquinolines) that potently co-potentiate with ivacaftor the channel activity of CFTR1281 in FRT cells and N1303K-CFTR in primary nasal epithelial cell cultures.
Fig. 4.
Structure and activity of potentiators and co-potentiators. (A) Chemical structures of the class I potentiators ivacaftor (VX-770), GLPG1837, P3 and P5 (left) and the class II potentiators ASP-11, W1282Xpot-C01, CP-D123 and apigenin (right). (B–D) Representative recordings of short-circuit current from FRT epithelia expressing N1303K-CFTR (B and C) and G551D-CFTR (D) demonstrate the enhancement of CFTR-mediated Cl− currents by ivacaftor and ASP-11 when used together. CFTR-mediated Cl− currents were recorded using the experimental conditions described in Phuan et al. [74] and in B, the magnitude of N1303K-CFTR Cl− current was augmented by pretreating epithelia with the CFTR corrector C3 (VRT-325 [84]; 3 μM for 24 h at 37 °C). Figure adapted from Phuan et al. [74] under the Creative Commons Attribution License.
To explain co-potentiation mechanism of action, Phuan et al. [76] grouped CFTR potentiators into two classes. Class I potentiators include ivacaftor and GLPG1837, which interact with a common binding site at the MSD-lipid interface [62,63] as well as P3 (SF-03 ([78]) and P5 (ΔF508act-02 [79]) [76] (Fig. 4). Class II potentiators include ASP-11, the flavonoid apigenin and newly identified co-potentiator scaffolds [76] (Fig. 4). Although the binding site of class II potentiators remains to be identified, in silico docking studies using the flavonoid genistein suggest that it might be located at the NBD1:NBD2 dimer interface [80]. Phuan et al. [76] demonstrated that co-potentiation occurs when two compounds from different classes are used; when both compounds are from the same class there is no enhancement of CFTR activity. Intriguingly, Phuan et al. [76] found that some CF mutations (e.g. G551D, W1282X and N1303K) are co-potentiation responsive, but other CF mutations (e.g. R334W, A561E and M1101K) were unresponsive. Using a similar strategy, Veit et al. [81] demonstrated that ivacaftor and apigenin potentiate other CF mutations including R352Q, S549N and S1251N. Taken together, these studies identify a novel therapeutic paradigm, and de-risk modulator development for rare CF mutations.
6. Future directions
We foresee a prominent role for mechanistic studies to understand CFTR folding, function and pharmacology. We anticipate the application of multi-disciplinary methods to innovative model systems and the use of machine learning to provide insightful analysis of data. Already, Wang and Balch [82] have employed machine learning to investigate genotype-phenotype-CFTR structure relationships.
7. Conclusions
The important data that have emerged from clinical trials of triple combination therapies [2,3] suggest that CFTR modulators have wide utility in the treatment of CF. However, CFTR modulators are not available for all CF mutations and modulators with greater efficacy are required to prevent disease progression. A bottleneck in the development of effective CFTR modulators has been a lack of insight into how CF mutations cause CFTR misfolding. Encouragingly, new insights into CFTR structure combined with innovative single-molecule studies using FRET are proving a powerful combination to elucidate the molecular basis of CFTR misfolding and the mechanism of action of small molecules that repair CFTR structure. These approaches are complemented by high-resolution single-channel recording, which provides unique insights into CFTR behaviour, illuminating the action of CF mutations and CFTR modulators. Finally, just as combinations of CFTR correctors have proved decisive in restoring the plasma membrane expression and stability of mutant CFTR [83], the identification of potent efficacious potentiator combinations [73,74,76,81] heralds a strategy to restore wild-type levels of activity to CF mutants. Translation of these potentiator combinations to the clinic should now be a priority.
Author contributions
All authors drafted the review manuscript or revised it critically for important intellectual content. All authors approved the final version of the review manuscript.
SJ Bose, G Krainer, DRS Ng, M Schenkel, H Shishido and JS Yoon are co-first authors of this work.
PM Haggie, M Schlierf, DN Sheppard and WR Skach are co-last authors of this work.
Declaration of Competing Interest
HS, JSY and WRS are employees of the Cystic Fibrosis Foundation. DNS is the recipient of a Vertex Innovation Award from Vertex Pharmaceuticals (Europe) Ltd. All the other authors have no conflicts of interest to declare.
Acknowledgments
We thank our laboratory colleagues and collaborators for stimulating discussions and assistance. Work in the authors’ laboratories discussed in this manuscript was supported by the Cystic Fibrosis Foundation (HS, JSY and WRS), an Exploration grant of the Boehringer Ingelheim Foundation (BIS), the Deutsche Forschungsgemeinschaft (grant no. SCHL 1896/3-1) and the European Social Fund and the Free State of Saxony (GK, MaS and MS), Cystic Fibrosis Trust (SJB, DRSN and DNS) and the National Institutes of Health, the Cystic Fibrosis Foundation, Emily's Entourage and Cystic Fibrosis Research, Inc (PMH). SJB was the recipient of an Industrial CASE Studentship from the Medical Research Council (grant no. MR/L015919/1).
Footnotes
This paper is part of a Supplement supported by The European Cystic Fibrosis Society (ECFS).
References
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleizen B, Hunt JF, Callebaut I, Hwang TC, Sermet-Gaudelus I, Hafkemeyer S. CFTR: new insights into structure and function and implications for modulation by small molecules. J Cyst Fibros. 2019 Nov 21 doi: 10.1016/j.jcf.2019.10.021. pii: S1569-1993(19)30936-1[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Farinha CM, Canato S. From the endoplasmic reticulum to the plasma membrane: mechanisms of CFTR folding and trafficking. Cell Mol Life Sci. 2017;74:39–55. doi: 10.1007/s00018-016-2387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang T-C, Yeh J-T, Zhang J, Yu Y-C, Yeh H-I, Destefano S. Structural mechanisms of CFTR function and dysfunction. J Gen Physiol. 2018;150:539–570. doi: 10.1085/jgp.201711946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mijnders M, Kleizen B, Braakman I. Correcting CFTR folding defects by small-molecule correctors to cure cystic fibrosis. Curr Opin Pharmacol. 2017;34:83–90. doi: 10.1016/j.coph.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Jih KY, Lin WY, Sohma Y, Hwang TC. CFTR potentiators: from bench to bedside. Curr Opin Pharmacol. 2017;34:98–104. doi: 10.1016/j.coph.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanrahan JW, Matthes E, Carlile G, Thomas DY. Corrector combination therapies for F508del-CFTR. Curr Opin Pharm. 2017;34:105–111. doi: 10.1016/j.coph.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Holtkamp W, Kokic G, Jager M, Mittelstaet J, Komar AA, Rodnina MV. Cotranslational protein folding on the ribosome monitored in real time. Science. 2015;350:1104–1107. doi: 10.1126/science.aad0344. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Yoon JS, Shishido H, Yang Z, Rooney LA, Barral JM. Translational tuning optimizes nascent protein folding in cells. Science. 2015;348:444–448. doi: 10.1126/science.aaa3974. [DOI] [PubMed] [Google Scholar]
- 12.Sander IM, Chaney JL, Clark PL. Expanding Anfinsen's principle: contributions of synonymous codon selection to rational protein design. J Am Chem Soc. 2014;136:858–861. doi: 10.1021/ja411302m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhr F, Jha S, Thommen M, Mittelstaet J, Kutz F, Schwalbe H. Synonymous codons direct cotranslational folding toward different protein conformations. Mol Cell. 2016;61:341–351. doi: 10.1016/j.molcel.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wruck F, Katranidis A, Nierhaus KH, Buldt G, Hegner M. Translation and folding of single proteins in real time. Proc Natl Acad Sci U S A. 2017;114:E4399–E4407. doi: 10.1073/pnas.1617873114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willmund F, del Alamo M, Pechmann S, Chen T, Albanese V, Dammer EB. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell. 2013;152:196–209. doi: 10.1016/j.cell.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuffee SR, Elcock AH. Diffusion, crowding & protein stability in a dynamic molecular model of the bacterial cytoplasm. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Skach WR. Mechanisms of CFTR folding at the endoplasmic reticulum. Front Pharmacol. 2012;3:201. doi: 10.3389/fphar.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendoza JL, Schmidt A, Li Q, Nuvaga E, Barrett T, Bridges RJ. Requirements for efficient correction of ΔF508 CFTR revealed by analyses of evolved sequences. Cell. 2012;148:164–174. doi: 10.1016/j.cell.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabeh WM, Bossard F, Xu H, Okiyoneda T, Bagdany M, Mulvihill CM. Correction of both NBD1 energetics and domain interface is required to restore ΔF508 CFTR folding and function. Cell. 2012;148:150–163. doi: 10.1016/j.cell.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Aleksandrov AA, An J, Cui L, Yang Z, Brouillette CG. Restoration of NBD1 thermal stability is necessary and sufficient to correct ΔF508 CFTR folding and assembly. J Mol Biol. 2015;427:106–120. doi: 10.1016/j.jmb.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khushoo A, Yang Z, Johnson AE, Skach WR. Ligand-driven vectorial folding of ribosome-bound human CFTR NBD1. Mol Cell. 2011;41:682–692. doi: 10.1016/j.molcel.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartoszewski R, Kroliczewski J, Piotrowski A, Jasiecka AJ, Bartoszewska S, Vecchio-Pagan B. Codon bias and the folding dynamics of the cystic fibrosis transmembrane conductance regulator. Cell Mol Biol Lett. 2016;21:23. doi: 10.1186/s11658-016-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchner S, Cai Z, Rauscher R, Kastelic N, Anding M, Czech A. Alteration of protein function by a silent polymorphism linked to tRNA abundance. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han ST, Rab A, Pellicore MJ, Davis EF, McCague AF, Evans TA. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight. 2018;3 doi: 10.1172/jci.insight.121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Willigen M, Vonk AM, Yeoh HY, Kruisselbrink E, Kleizen B, van der Ent CK. Folding-function relationship of the most common cystic fibrosis-causing CFTR conductance mutants. Life Sci Alliance. 2019;2 doi: 10.26508/lsa.201800172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Hildebrandt E, Jiang F, Aleksandrov AA, Khazanov N, Zhou Q. Structural stability of purified human CFTR is systematically improved by mutations in nucleotide binding domain 1. Biochim Biophys Acta Biomembr. 2018;1860:1193–1204. doi: 10.1016/j.bbamem.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehbi H, Gasmi-Seabrook G, Choi MY, Deber CM. Positional dependence of non-native polar mutations on folding of CFTR helical hairpins. Biochim Biophys Acta. 2008;1778:79–87. doi: 10.1016/j.bbamem.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Krainer G, Treff A, Hartmann A, Stone TA, Schenkel M, Keller S. A minimal helical-hairpin motif provides molecular-level insights into misfolding and pharmacological rescue of CFTR. Commun Biol. 2018;1:154. doi: 10.1038/s42003-018-0153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y-H, Stone TA, Chin S, Glibowicka M, Bear CE, Deber CM. Structural effects of extracellular loop mutations in CFTR helical hairpins. Biochim Biophys Acta Biomembr. 2018;1860:1092–1098. doi: 10.1016/j.bbamem.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Therien AG, Glibowicka M, Deber CM. Expression and purification of two hydrophobic double-spanning membrane proteins derived from the cystic fibrosis transmembrane conductance regulator. Protein Expr Purif. 2002;25:81–86. doi: 10.1006/prep.2001.1612. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann A, Krainer G, Keller S, Schlierf M. Quantification of millisecond protein-folding dynamics in membrane-mimetic environments by single-molecule Förster resonance energy transfer spectroscopy. Anal Chem. 2015;87:11224–11232. doi: 10.1021/acs.analchem.5b03207. [DOI] [PubMed] [Google Scholar]
- 32.Krainer G, Keller S, Schlierf M. Structural dynamics of membrane-protein folding from single-molecule FRET. Curr Opin Struct Biol. 2019;58:124–137. doi: 10.1016/j.sbi.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Van Goor F, Hadida S, Grootenhuis PDJ, Burton B, Stack JH, Straley KS. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Therien AG, Grant FEM, Deber CM. Interhelical hydrogen bonds in the CFTR membrane domain. Nat Struct Biol. 2001;8:597–601. doi: 10.1038/89631. [DOI] [PubMed] [Google Scholar]
- 35.Loo TW, Clarke DM. The cystic fibrosis V232D mutation inhibits CFTR maturation by disrupting a hydrophobic pocket rather than formation of aberrant interhelical hydrogen bonds. Biochem Pharmacol. 2014;88:46–57. doi: 10.1016/j.bcp.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Ren HY, Grove DE, De La Rosa O, Houck SA, Sopha P, Van Goor F. VX-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. Mol Biol Cell. 2013;24:3016–3024. doi: 10.1091/mbc.E13-05-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostedgaard LS, Rogers CS, Dong Q, Randak CO, Vermeer DW, Rokhlina T. Processing and function of CFTR-ΔF508 are species-dependent. Proc Natl Acad Sci U S A. 2007;104:15370–15375. doi: 10.1073/pnas.0706974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleksandrov AA, Kota P, Cui L, Jensen T, Alekseev AE, Reyes S. Allosteric modulation balances thermodynamic stability and restores function of ΔF508 CFTR. J Mol Biol. 2012;419:41–60. doi: 10.1016/j.jmb.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose SJ, Scott-Ward TS, Cai Z, Sheppard DN. Exploiting species differences to understand the CFTR Cl− channel. Biochem Soc Trans. 2015;43:975–982. doi: 10.1042/BST20150129. [DOI] [PubMed] [Google Scholar]
- 40.Cui G, Hong J, Chung-Davidson YW, Infield D, Xu X, Li J. An ancient CFTR ortholog informs molecular evolution in ABC transporters. Dev Cell. 2019;51 doi: 10.1016/j.devcel.2019.09.017. 421-30.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagnat M, Navis A, Herbstreith S, Brand-Arzamendi K, Curado S, Gabriel S. Cse1l is a negative regulator of CFTR-dependent fluid secretion. Curr Biol. 2010;20:1840–1845. doi: 10.1016/j.cub.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 43.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS ONE. 2014;9:e91253. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho H-J. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan Z, Perisse IV, Cotton CU, Regouski M, Meng Q, Domb C. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI insight. 2018;3 doi: 10.1172/jci.insight.123529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, Zoso A. Animal and model systems for studying cystic fibrosis. J Cyst Fibros. 2018;17(2S):S28–S34. doi: 10.1016/j.jcf.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F, Zhang Z, Csanády L, Gadsby DC, Chen J. Molecular structure of the human CFTR ion channel. Cell. 2017;169 doi: 10.1016/j.cell.2017.02.024. 85-95.e8. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Liu F, Chen J. Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc Natl Acad Sci U S A. 2018;115:12757–12762. doi: 10.1073/pnas.1815287115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Chen J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell. 2016;167 doi: 10.1016/j.cell.2016.11.014. 1586-97.e9. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Liu F, Chen J. Conformational changes of CFTR upon phosphorylation and ATP binding. Cell. 2017;170 doi: 10.1016/j.cell.2017.06.041. 483-91.e8. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Yu Y-C, Yeh J-T, Hwang T-C. Functional characterization reveals that zebrafish CFTR prefers to occupy closed channel conformations. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0209862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lansdell KA, Delaney SJ, Lunn DP, Thomson SA, Sheppard DN, Wainwright BJ. Comparison of the gating behaviour of human and murine cystic fibrosis transmembrane conductance regulator Cl− channels expressed in mammalian cells. J Physiol. 1998;508:379–392. doi: 10.1111/j.1469-7793.1998.379bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott-Ward TS, Cai Z, Dawson ES, Doherty A, Da Paula AC, Davidson H. Chimeric constructs endow the human CFTR Cl− channel with the gating behavior of murine CFTR. Proc Natl Acad Sci U S A. 2007;104:16365–16370. doi: 10.1073/pnas.0701562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.French PJ, van Doorninck JH, Peters RH, Verbeek E, Ameen NA, Marino CR. A ΔF508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. J Clin Invest. 1996;98:1304–1312. doi: 10.1172/JCI118917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher JT, Liu X, Yan Z, Luo M, Zhang Y, Zhou W. Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2012;287:21673–21685. doi: 10.1074/jbc.M111.336537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Z, Palmai-Pallag T, Khuituan P, Mutolo MJ, Boinot C, Liu B. Impact of the F508del mutation on ovine CFTR, a Cl− channel with enhanced conductance and ATP-dependent gating. J Physiol. 2015;593:2427–2446. doi: 10.1113/JP270227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bose SJ, Bijvelds MJC, Wang Y, Liu J, Cai Z, Bot AGM. Differential thermostability and response to cystic fibrosis transmembrane conductance regulator potentiators of human and mouse F508del-CFTR. Am J Physiol Lung Cell Mol Physiol. 2019;317:L71–L86. doi: 10.1152/ajplung.00034.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.deCarvalho ACV, Gansheroff LJ, Teem JL. Mutations in the nucleotide binding domain 1 signature motif region rescue processing and functional defects of cystic fibrosis transmembrane conductance regulator ΔF508. J Biol Chem. 2002;277:35896–35905. doi: 10.1074/jbc.M205644200. [DOI] [PubMed] [Google Scholar]
- 60.Sun X, Yi Y, Yan Z, Rosen BH, Liang B, Winter MC. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med. 2019;11:e7531. doi: 10.1126/scitranslmed.aau7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Goor F, Hadida S, Grootenhuis PDJ, Burton B, Cao D, Neuberger T. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu F, Zhang Z, Levit A, Levring J, Touhara KK, Shoichet BK. Structural identification of a hotspot on CFTR for potentiation. Science. 2019;364:1184–1188. doi: 10.1126/science.aaw7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeh H-I, Qiu L, Sohma Y, Conrath K, Zou X, Hwang T-C. Identifying the molecular target sites for CFTR potentiators GLPG1837 and VX-770. J Gen Physiol. 2019;151:912–928. doi: 10.1085/jgp.201912360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng M, Szymczak M, Ahuja M, Zheng C, Yin H, Swaim W. Restoration of CFTR activity in ducts rescues acinar cell function and reduces inflammation in pancreatic and salivary glands of mice. Gastroenterology. 2017;153:1148–1159. doi: 10.1053/j.gastro.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chin S, Hung M, Won A, Wu Y-S, Ahmadi S, Yang D. Lipophilicity of the cystic fibrosis drug, ivacaftor (VX-770), and its destabilizing effect on the major CF-causing mutation: F508del. Mol Pharmacol. 2018;94:917–925. doi: 10.1124/mol.118.112177. [DOI] [PubMed] [Google Scholar]
- 66.Cook DP, Rector MV, Bouzek DC, Michalski AS, Gansemer ND, Reznikov LR. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. Am J Respir Crit Care Med. 2016;193:417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. New Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an arg117his-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–533. doi: 10.1016/S2213-2600(15)00201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. New Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for phe508del. New Engl J Med. 2017;377:2013–2023. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 72.Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. New Engl J Med. 2017;377:2024–2035. doi: 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haggie PM, Phuan P-W, Tan J-A, Xu H, Avramescu RG, Perdomo D. Correctors and potentiators rescue function of the truncated W1282X-cystic fibrosis transmembrane regulator (CFTR) translation product. J Biol Chem. 2017;292:771–785. doi: 10.1074/jbc.M116.764720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phuan P-W, Son J-H, Tan J-A, Li C, Musante I, Zlock L. Combination potentiator ('co-potentiator') therapy for CF caused by CFTR mutants, including N1303K, that are poorly responsive to single potentiators. J Cyst Fibros. 2018;17:595–606. doi: 10.1016/j.jcf.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matthes E, Goepp J, Martini C, Shan J, Liao J, Thomas DY. Variable responses to CFTR correctors in vitro: estimating the design effect in precision medicine. Front Pharmacol. 2018;9:1490. doi: 10.3389/fphar.2018.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phuan PW, Tan JA, Rivera AA, Zlock L, Nielson DW, Finkbeiner WE. Nanomolar-potency 'co-potentiator' therapy for cystic fibrosis caused by a defined subset of minimal function CFTR mutants. Sci Rep. 2019;9:17640. doi: 10.1038/s41598-019-54158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laselva O, Eckford PDW, Bartlett C, Ouyang H, Gunawardena TNA, Gonska T. Functional rescue of c.3846G>A (W1282X) in patient-derived nasal cultures achieved by inhibition of nonsense mediated decay and protein modulators with complementary mechanisms of action. J Cyst Fibros. 2019 Dec 9 doi: 10.1016/j.jcf.2019.12.001. pii: S1569-1993(19)30980-4[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 78.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF. Phenylglycine and sulfonamide correctors of defective ΔF508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 79.Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K. Nanomolar affinity small molecule correctors of defective ΔF508-CFTR chloride channel gating. J Biol Chem. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 80.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PubMed] [Google Scholar]
- 81.Veit G, Da Fonte DF, Avramescu RG, Premchandar A, Bagdany M, Xu H. Mutation-specific dual potentiators maximize rescue of CFTR gating mutants. J Cyst Fibros. 2019 Oct 30 doi: 10.1016/j.jcf.2019.10.011. pii: S1569-1993(19)30924-5[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C, Balch WE. Bridging genomics to phenomics at atomic resolution through variation spatial profiling. Cell Rep. 2018;24 doi: 10.1016/j.celrep.2018.07.059. 2013-28.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veit G, Xu H, Dreano E, Avramescu RG, Bagdany M, Beitel LK. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat Med. 2018;24:1732–1742. doi: 10.1038/s41591-018-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]