Abstract
Background
The effectiveness of clomiphene citrate has been demonstrated in the treatment of subfertility associated with infrequent or irregular ovulation. The physiologic effects and clinical benefits in ovulatory women with unexplained subfertility are less clear. The drug is associated with an increased risk of multiple pregnancy and a suggestion of potentially increased ovarian cancer risks. In light of these concerns, defining the effectiveness of clomiphene citrate for ovulatory women with unexplained subfertility is extremely important.
Objectives
To determine the effectiveness of clomiphene citrate in improving pregnancy outcomes in women with unexplained subfertility, used in a dose range of 50 to 250 mg for up to 10 days. The primary outcome was live births.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register (June 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2), MEDLINE (1966 to June 2009), EMBASE (1980 to June 2009) and reference lists of articles.
Selection criteria
Only randomised controlled trials were included. Quasi‐randomised designs were excluded.
Data collection and analysis
Fourteen potentially relevant trials were identified of which seven were included in this review. All trials were assessed for risk of bias using standardised Menstrual Disorders and Subfertility Group methodology.
Main results
Data relating to 1159 participants from seven trials were collated. There was no evidence that clomiphene citrate was more effective than no treatment or placebo for live birth (odds ratio (OR) 0.79, 95% CI 0.45 to 1.38; P = 0.41) or for clinical pregnancy per woman randomised both with intrauterine insemination (IUI) (OR 2.40, 95% CI 0.70 to 8.19; P = 0.16), without IUI (OR 1.03, 95% CI 0.64 to 1.66; P = 0.91) and without IUI but using human chorionic gonadotropin (hCG) (OR 1.66, 95% CI 0.56 to 4.80; P = 0.35). It should be noted that heterogeneity between studies ranged from 34% to 58% using the I2 statistic.
Authors' conclusions
There is no evidence of clinical benefit of clomiphene citrate for unexplained fertility. When making this treatment choice, potential side effects should be discussed. These include the increased risk of multiple pregnancy and the concern that use for more that 12 cycles has been associated with a three‐fold increase in risk of ovarian cancer.
Plain language summary
Clomiphene citrate for unexplained subfertility in women
Clomiphene citrate is a fertility drug that can increase the number of eggs released for possible fertilisation. It is used by women who do not ovulate regularly and by some who do but still have not become pregnant. Clomiphene citrate does not appear to increase the chance of pregnancy in women who ovulate regularly but have failed to conceive after more than a year of unprotected intercourse and so are considered to be subfertile. An associated risk of treatment with clomiphene citrate is a 10% chance of multiple pregnancy. The results of this review of trials should be used with caution due to the heterogeneity between some of the studies.
Background
Description of the condition
Ovulatory dysfunction is one of the primary causes of reproductive failure in subfertile couples. Women with ovulatory dysfunction do not ovulate regularly or do ovulate but fail to become pregnant. Some women ovulate regularly but fail to conceive after more than a year of unprotected intercourse and so are considered to be subfertile.
Description of the intervention
Clomiphene citrate is a drug used for women with ovulatory dysfunction, such as luteal phase deficiency and anovulation where there is no ovulatory cycle, or oligo‐ovulation where there are irregular or infrequent ovulatory cycles. Clomiphene citrate usually comes as a 50 mg tablet taken orally on the fifth through to the ninth day of the cycle. Clomiphene citrate is generally well tolerated. Side effects associated with its use include hot flashes, mood swings, headaches and visual disturbances. Many of the side effects are transitory and typically abate after the cessation of treatment. The quality and quantity of cervical mucus production in clomiphene citrate treatment cycles may sometimes be reduced (Maxson 1984) but rarely to an extent that would affect the capture, transport or survival of sperm. A variety of publications have raised the question of increased ovarian cancer risks associated with clomiphene use (Rossing 1994; Whittemore 1992). The more rigorous of these studies (Rossing 1994) suggests that in women taking clomiphene for more than 12 cycles the incidence of invasive epithelial cancer increases approximately three‐fold. However, it remains unclear whether this association is causal or secondary to an inherent increased risk of ovarian cancer associated with the disease processes leading to subfertility.
How the intervention might work
The effectiveness of clomiphene citrate in the treatment of subfertility associated with oligo‐ovulation has been described (Hughes 1996). It has been shown to increase the number of follicles produced per cycle and, therefore, increases the potential number of eggs available for fertilisation (Randall 1991). Clomiphene appears to act as an estrogen receptor blocker and leads to elevation of endogenous follicle stimulating hormone (FSH) production, which in turn stimulates multiple follicular development. As a result, the multiple pregnancy rate is elevated to approximately 8% to 10%. Clomiphene citrate is cleared through the liver and excreted in the stools.
Why it is important to do this review
In oligo‐ovulatory women clomiphene citrate increases the likelihood of ovulation approximately 10 fold and pregnancy approximately six‐fold (Hughes 1996). However, in the absence of a clear indication such as oligo‐ovulation, the use of clomiphene may be considered somewhat controversial. Despite this, clomiphene is widely prescribed in ovulatory women with unexplained infertility. Understanding the effectiveness of clomiphene in this patient group is, therefore, extremely important.
Objectives
To determine the effectiveness of clomiphene citrate use in improving pregnancy outcomes for women with unexplained subfertility.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were included. Quasi‐randomised trials were excluded.
Types of participants
Women with unexplained subfertility. This was defined as: a) no evidence of tubal disease as demonstrated by normal hysterosalpingogram (HSG) or laparoscopy; and b) normal ovulatory function demonstrated by biphasic basal body temperature chart, luteal phase serum progesterone level on ovulation or disappearance of a dominant follicle on ultrasound, or both; and c) normal sperm parameters based on criteria acceptable at the time of publication, e.g. WHO standards. The specific criteria used to define population samples within individual studies are included in the table 'Characteristics of included studies'.
Types of interventions
Clomiphene citrate, administered orally, compared with placebo or no treatment.
Types of outcome measures
Primary outcomes
The primary outcome measures were:
live birth, per woman randomised;
multiple pregnancy, per woman randomised.
Secondary outcomes
Secondary outcome measures were:
ongoing pregnancy, per woman randomised;
clinical pregnancy, per woman randomised;
ectopic pregnancy, per woman randomised;
miscarriage, per woman randomised;
incidence of ovarian hyperstimulation syndrome (OHSS), per woman randomised;
other patient‐reported adverse effects.
Search methods for identification of studies
Electronic searches
We searched for all publications which described (or might have described) randomised controlled trials of clomiphene citrate for unexplained subfertility. The original search was performed in 1995 and the search was updated in 1999, 2005, 2006 and 2009.
We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register of trials (June 2009) (Appendix 1). See the Review Group details for more information on the Specialised Register.
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2) searched in all fields (Appendix 4).
MEDLINE (1966 to June 2009) (Appendix 2).
EMBASE (1980 to June 2009) (Appendix 3).
PsycINFO (1806 to June week 1 2009) (Appendix 5).
Searching other resources
The citation lists of relevant publications, review articles and included studies were also searched. Authors were contacted to obtain further information, where necessary.
Data collection and analysis
Selection of studies
Potentially relevant trials were screened independently by two authors (EH, JC). Any differences of opinion were resolved by a consensus meeting or a third author, if necessary. JB updated the search in October 2006 and June 2009 and added any additional relevant studies.
When crossover studies were identified data were included in the review from the pre‐crossover period, where possible. If data could not be extracted separately, the study was excluded from meta‐analysis.
Data extraction and management
Data were extracted into a pre‐designed data extraction form by two independent researchers. Disagreement was resolved by consensus or a third review author if necessary. All data were extracted as dichotomous values per woman randomised.
Assessment of risk of bias in included studies
Risk of bias in included studies was assessed by two independent researchers for sequence generation, allocation concealment, blinding, incomplete data and selective reporting. These details were summarised and entered into the 'Risk of bias' table for each included study and graphically represented in Figure 1 and Figure 2. Pregnancies occurring before and after treatment were to be included in the analysis based on the randomisation protocol.
1.
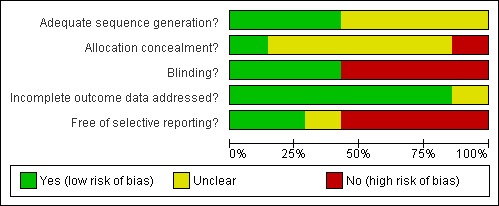
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
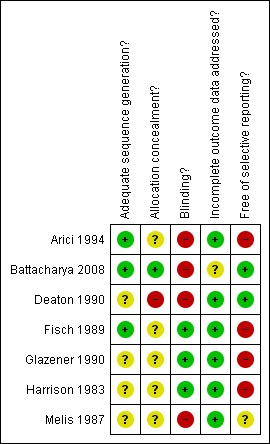
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
The Peto odds ratio (OR) was the chosen summary statistic for all outcomes (Peto 1987).
Unit of analysis issues
Data were excluded from analysis if they were reported per cycle as this can not be pooled. There was the issue of crossover design, which was taken into consideration by including only the first arm of the trial in the analysis, that is prior to crossover.
Dealing with missing data
Where data were missing the trial authors were contacted, where possible. No further data have been received at this point in time.
Assessment of heterogeneity
Clinical heterogeneity was assessed through careful evaluation of populations, interventions and outcomes within each study. Statistical homogeneity was assessed using the Chi2 test and the I2 statistic.
Assessment of reporting biases
There were insufficient trials to be able to produce a funnel plot for this review. The review authors attempted to obtain relevant papers from peer reviewed journals and conference proceedings.
Data synthesis
Meta‐analyses were conducted for the outcomes of live birth, miscarriage, multiple pregnancy, ectopic pregnancy and adverse effects.
Subgroup analysis and investigation of heterogeneity
There were insufficient studies to conduct subgroup analysis. Despite the potential threats to validity from the use of co‐interventions, it was the opinion of the review authors that the combination of data from the included studies is reasonable because endometriosis‐associated and unexplained infertility have similar spontaneous conception rates (Collins 1991). However, the clinical heterogeneity between populations should be kept in mind in extrapolating from these results.
Sensitivity analysis
There were insufficient studies to conduct sensitivity analyses.
Results
Description of studies
Results of the search
Fourteen potential studies were identified. Seven trials met the inclusion criteria, seven trials were excluded (see 'Characteristics of excluded studies' table). All included studies were published in peer reviewed journals between 1983 and 2008.
Included studies
Seven studies were included in the review. Three trials used a parallel design (Battacharya 2008; Fisch 1989; Melis 1987) and four studies (Arici 1994; Deaton 1990; Glazener 1990; Harrison 1983) used a crossover design where pre‐crossover data could be extracted. Arici 1994 used a crossover design for which the first‐arm data could not be extracted; the review authors were also unable to separate couples with unexplained subfertility from other diagnoses.
Studies were conducted in the USA (Arici 1994; Deaton 1990), Canada (Fisch 1989), UK and Ireland (Battacharya 2008; Glazener 1990; Harrison 1983) and Italy (Melis 1987). The duration of treatment in the included studies was reported as three months (Glazener 1990), four cycles (Deaton 1990; Fisch 1989) and six months or cycles (Battacharya 2008; Harrison 1983; Melis 1987). Arici 1994 reported on pregnancy per cycle.
The mean female age ranged from 29.3 to 33 (SD 4.4) years with a reported range of 20 to 41 years. The mean duration of infertility ranged from 3.5 (SD 1.7) to 5.4 years (range 2 to 15 years). All but one study (Fisch 1989) included women with primary and secondary infertility; Fisch et al included only primary infertility. The number of cycles in the studies ranged from four to six.
There was clinical heterogeneity between studies in terms of the population included. Surgically treated endometriosis was present in approximately 40% of women in Deaton 1990. The distribution of such participants between groups after randomisation was not reported. Fisch 1989 included only participants with primary infertility. Although that study focused on this specific group it was the most rigorously designed of all the studies included and used a parallel design with central randomisation.
The dose ranges of clomiphene and the times of administration were similar between studies. Three studies administered 50 mg (Arici 1994; Battacharya 2008; Deaton 1990) and four administered 100 mg for four days (Fisch 1989; Glazener 1990; Harrison 1983; Melis 1987).
Clomiphene was administered on days two to six of the menstrual cycle in the studies reported by Battacharya 2008 and Glazener 1990 and on days five to nine in four other trials (Arici 1994; Deaton 1990; Fisch 1989; Melis 1987); if the menstrual cycle was less than 27 days clomiphene was administered on days four to eight of the cycle by Deaton 1990. Harrison (1983) reported administering clomiphene for four days but gave no details of which days these were (Harrison 1983).
There was some discrepancy in the use of human chorionic gonadotropin (hCG), an agent for triggering ovulation. In one study, the treatment group but not the control group received hCG (Deaton 1990). The dose of hCG varied from 5000 U (Fisch 1989; Harrison 1983) to 10,000 U (Arici 1994; Deaton 1990). No details were provided in three trial reports (Battacharya 2008; Glazener 1990; Melis 1987).
The main outcomes reported were pregnancy, live birth, miscarriage and multiple pregnancies. These are described further in the 'Effects of Interventions' section of this review. Ovarian hyperstimulation syndrome was not reported by any author.
Excluded studies
Seven studies were excluded after reviewing the full text (see 'Characteristics of excluded studies'). The main reasons for exclusion were that the trial population failed to meet the entry criteria for this review; and a conference proceeding had been superseded by a full paper.
Risk of bias in included studies
Allocation
Computer‐generated randomisation was described by Fisch 1989 and Arici 1994. Although Fisch 1989 described that allocation of study codes and medication was from one co‐coordinating centre they did not detail how this was achieved.
Battacharya 2008 reported that an independent statistician generated the randomisation allocation sequence, which was stratified by centre. Allocation was balanced by age, parity and duration of subfertility and was by a central telephone method.
Allocation was not described in four trials (Deaton 1990; Glazener 1990; Harrison 1983; Melis 1987).
Concealment was described adequately only by Battacharya 2008, who used a centralised telephone allocation method. Concealment was not described in the remaining studies.
Blinding
There was no blinding in four studies (Arici 1994; Battacharya 2008; Deaton 1990; Melis 1987). Harrison 1983 reported that patients were blinded to treatment, identical in appearance, and Fisch 1989 reported a double‐blind, double dummy design. Glazener 1990 also reported a double‐blind study with identical placebo although they did not specify exactly who was blinded.
Incomplete outcome data
Minimal loss was reported by Battacharya 2008 and Harrison 1983. The remaining studies reported attrition ranging from 12% to 48%; this would constitute a high risk for bias.
Selective reporting
There is a risk of selective reporting in this review. There was a failure to report on major outcomes in sufficient detail to allow group comparisons. Five trials (Arici 1994; Fisch 1989; Glazener 1990; Harrison 1983; Melis 1987) did not report on live birth as an outcome. In addition, Melis 1987 failed to report on a definition of pregnancy or adverse effects. Arici 1994 and Glazener 1990 failed to report on adverse effects.
Other potential sources of bias
Co‐intervention clearly was present in Deaton 1990 as only the intervention group received additional treatments with hCG and IUI.
Effects of interventions
Live birth
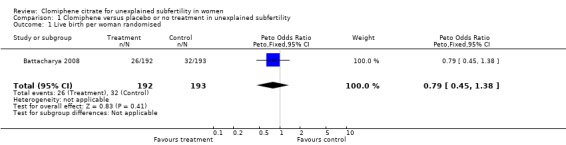
Live birth was reported by four trials (Battacharya 2008; Deaton 1990; Fisch 1989; Harrison 1983) however the data reported by Deaton 1990 and Harrison 1983 could not be separated for the arm before crossover took place; therefore, the data could not be extracted for analysis. Battacharya 2008 reported on live birth: 26/192 women in the clomiphene group and 32/193 women in the control group. The difference was not statistically significant (OR 0.79, 95% CI 0.45 to 1.38; P = 0.41) (Figure 3). Live birth was also described as an outcome by Fisch 1989 but details as to which group the women had been allocated to were not available in the paper.
3.
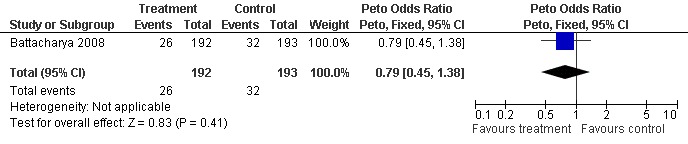
Forest plot of comparison: 1 Clomiphene versus placebo or no treatment in unexplained subfertility, outcome: 1.1 Live birth per woman randomised.
Pregnancy
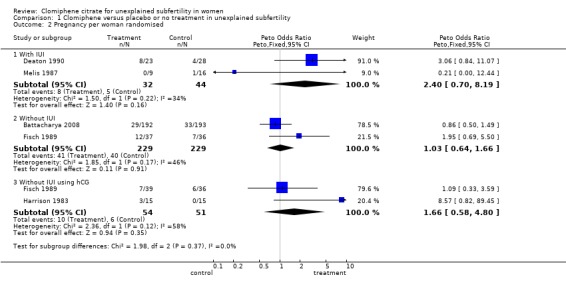
One study (Fisch 1989) reported a statistically significant improvement in pregnancy rate over placebo following clomiphene (P < 0.05) but this difference was based on the absence of pregnancy in the placebo group of 36 patients during the four cycles of observation. In the six months that followed, seven pregnancies occurred in this group. These data have been taken into account in the meta‐analysis.
The ORs for clinical pregnancy per patient were 2.40 (95% CI 0.70 to 8.19; P = 0.16) for clomiphene citrate with IUI; 1.03 (95% CI 0.64 to 1.66; P = 0.91) without IUI; and 1.66 (95% CI 0.58 to 4.80; P = 0.35) without IUI but with hCG. It should be noted that the heterogeneity between studies ranged from 34% to 58% measured using the I2 statistic (Figure 4).
4.
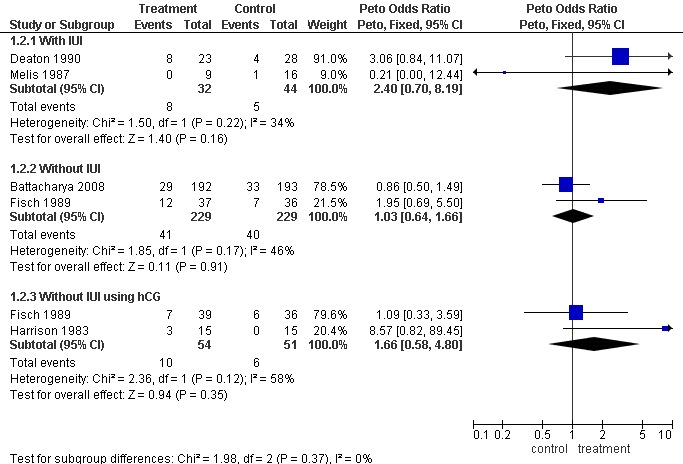
Forest plot of comparison: 1 Clomiphene versus placebo or no treatment in unexplained subfertility, outcome: 1.2 Pregnancy per woman randomised.
Miscarriage
Miscarriage was reported in three of the studies (Battacharya 2008; Deaton 1990; Harrison 1983). The data reported by Harrison 1983 and Deaton 1990 were not separable before crossover and therefore could not be extracted for analysis. Battacharya 2008 did not report a significant difference in miscarriage rate between the treatment and control groups (OR 0.71, 95% CI 0.31 to 1.61; P = 0.41) (Figure 5).
5.
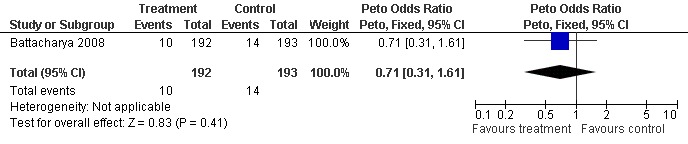
Forest plot of comparison: 1 Clomiphene versus placebo or no treatment in unexplained subfertility, outcome: 1.3 Miscarriage per woman randomised.
Multiple pregnancy The crude rate of twin pregnancy was reported by only one study (Fisch 1989). It was not clear whether one of these pregnancies was conceived after clomiphene or placebo (Fisch 1989). Multiple births and ectopic pregnancies were reported by Deaton 1990 but data could not be separated from the first and second arms of the crossover and could not, therefore, be included in the analysis. Battacharya 2008 reported on the incidence of multiple pregnancies and no significant differences were identified between treatment and placebo groups (OR 1.01, 95% CI 0.14 to 7.19; P = 1.0).
Ectopic pregnancy
Ectopic pregnancy was reported only by Battacharya 2008 (OR 0.33, 95% CI 0.01 to 8.23; P = 0.5).
Adverse effects
Battacharya 2008 was the only paper to report on adverse effects (Figure 6). There were significant differences between clomiphene and expectant management for abdominal pain (OR 9.89, 95% CI 3.81 to 25.69; P < 0.00001); nausea (OR 6.11, 95% CI 2.07 to 18.10; P = 0.001); headache (OR 6.47, 95% CI 2.64 to 15.83; P < 0.0001); hot flushes (OR 8.75, 95% CI 3.02 to 25.36; P < 0.0001) and bloating (OR 81.28, 95% CI 4.94 to 1337.02; P = 0.002). There were no differences in anxiety, depression, vaginal bleeding and vomiting.
6.
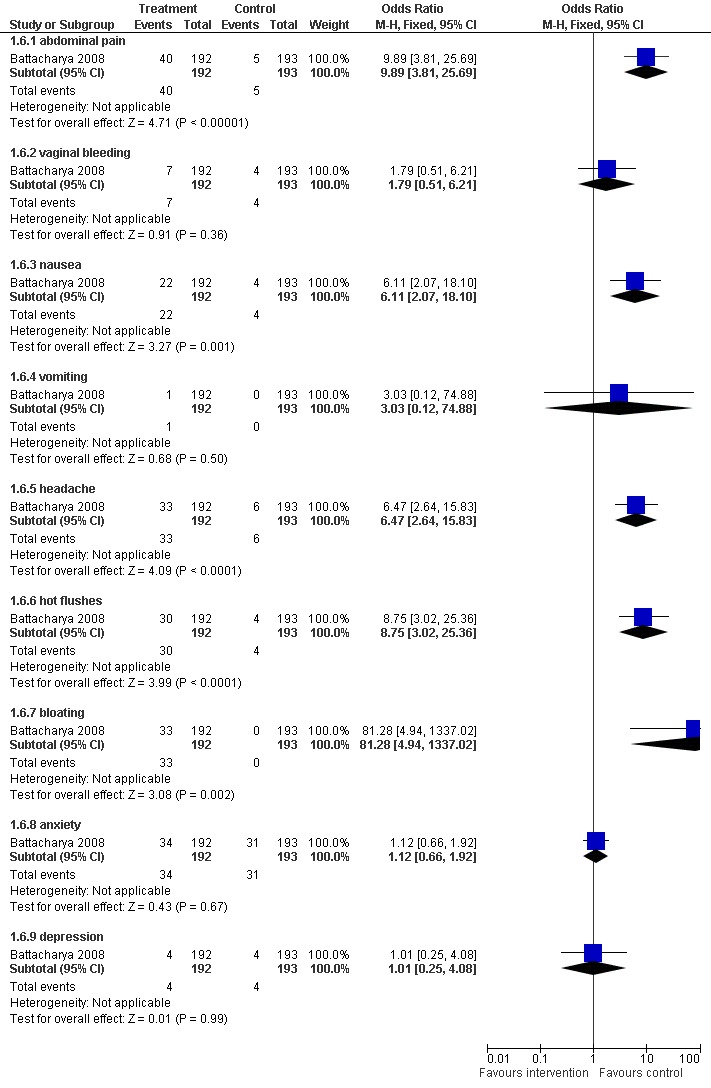
Forest plot of comparison: 1 Clomiphene versus placebo or no treatment in unexplained subfertility, outcome: 1.6 Adverse effects.
No studies reported ovarian hyperstimulation syndrome.
Discussion
In counselling women regarding the effectiveness of clomiphene, the data presented here suggest no evidence of treatment benefit on pregnancy rate. Realistic expectations should thus be clearly defined prior to treatment, by presenting both absolute and relative risks.
Summary of main results
Seven studies were identified. The data from the trials included in this review pose some concerns. Their methodological quality was variable and some clinical heterogeneity existed. Details of the methods and quality can be referred to in the 'Characteristics of included studies' and 'Risk of bias' tables.
Overall completeness and applicability of evidence
The studies lack information on adverse effects, only reported in one study (Battacharya 2008). The primary outcome of live birth was only reported in one study (Battacharya 2008).
Quality of the evidence
The quality of the evidence was varied. Adequate sequence generation was described by three trials (Arici 1994; Battacharya 2008; Fisch 1989). Only Battacharya 2008 adequately described allocation concealment. The data may not be generalisable.
Potential biases in the review process
Important clinical differences between included studies in terms of populations and interventions should be remembered in generalising to other settings. Surgically treated endometriosis was present in 40% of the patients reported by Deaton 1990. Although the effectiveness of clomiphene in these women may be different from other groups, data from a large cohort study of women attending Canadian university fertility clinics suggested a similar effect when clomiphene was used for infertility associated with early stages of endometriosis (Collins 1991).
Another concern is the potential for adverse effects. Only one trial reported adverse effects. There were no identified cases of ovarian hyperstimulation syndrome. The rate of miscarriage did not differ from the expected rate and could not be extrapolated between crossover arms. The same occurred for multiple pregnancies and other adverse events such as ectopic pregnancies. Multiple pregnancy risk (10%), transient hot flushes (approximately 11%), and the visual disturbances of blurred vision and diplopia (1% to 3%) are reported from other sources (Compendium 1998). Although concerns have been raised over a possible association between fertility drugs and ovarian cancer, the researchers did not focus on any specific drugs (Whittemore 1992). A case‐cohort study has suggested a link with clomiphene when used for more than 12 months (Rossing 1994). Thus, if clomiphene has been unsuccessful after six to nine months it should probably be discontinued.
Authors' conclusions
Implications for practice.
These data suggest that there is no evidence that clomiphene citrate has an effect on pregnancy rate in women with unexplained subfertility. The data should be used with caution due to the heterogeneity among the studies. Further research is unlikely to change the findings and therefore clomiphene would not be recommended as a treatment for unexplained subfertility.
Implications for research.
There is unlikely to be further evidence which would change these findings and, therefore, this review will not be updated.
What's new
| Date | Event | Description |
|---|---|---|
| 20 July 2009 | New citation required but conclusions have not changed | Some studies previously excluded on the basis of being crossover design and unable to extract first arm have been moved to included studies and added to the 'Risk of bias' tables. Some lesser adverse events have been reported in this review which were not pre‐specified in the methods section. Review will no longer be updated. |
| 6 July 2009 | New search has been performed | New search run. One study identified which superseded a previously included abstract. Review reformatted and closed to further updating due to lack of new evidence likely to alter findings. Some studies previously excluded as crossover design added to included trials. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 7 November 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review was updated in October to November 2006 with the conclusions amended, and again in 2009.
Acknowledgements
The authors would like to acknowledge the support of the Cochrane Menstrual Disorders and Subfertility Group (MDSG) and its editors.
Appendices
Appendix 1. Menstrual Disorders and Subfertility Group (MDSG) search terms for specialist register
MDSG search strings for EH252 23.06.09
Keywords CONTAINS "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or "idiopathic subfertility" or "idiopathic‐unexplained" or Title CONTAINS "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or "idiopathic subfertility" or "idiopathic‐unexplained"
AND
Keywords CONTAINS "*Clomiphene" or "Clomiphene citrate" or Title CONTAINS "*Clomiphene" or "Clomiphene citrate"
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1950 to June Week 2 2009> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Clomiphene/ (4491) 2 clomifene.tw. (81) 3 clomiphene.tw. (3848) 4 clomid$.tw. (167) 5 or/1‐4 (5546) 6 (unexplain$ adj3 infertil$).tw. (1279) 7 (unexplain$ adj3 subfertil$).tw. (65) 8 (idiopathic adj3 subfertil$).tw. (49) 9 (idiopathic adj3 infertil$).tw. (573) 10 or/6‐9 (1906) 11 10 and 5 (208) 12 randomized controlled trial.pt. (273274) 13 controlled clinical trial.pt. (79487) 14 randomized.ab. (182938) 15 placebo.tw. (116152) 16 clinical trials as topic.sh. (143977) 17 randomly.ab. (132738) 18 trial.ti. (79684) 19 (crossover or cross‐over or cross over).tw. (43084) 20 or/12‐19 (647472) 21 (animals not (humans and animals)).sh. (3294684) 22 20 not 21 (599421) 23 22 and 11 (64) 24 (2006$ or 2007$ or 2008$ or 2009$).ed. (2476825) 25 24 and 23 (18) 26 from 25 keep 1‐18 (18)
Appendix 3. EMBASE search strategy
Database: EMBASE <1980 to 2009 Week 25> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp clomifene/ or exp clomifene citrate/ (6438) 2 clomifene.tw. (111) 3 clomiphene.tw. (2928) 4 clomid.tw. (702) 5 or/1‐4 (6817) 6 (unexplain$ adj3 infertil$).tw. (1274) 7 (unexplain$ adj3 subfertil$).tw. (51) 8 (idiopathic adj3 subfertil$).tw. (48) 9 (idiopathic adj3 infertil$).tw. (557) 10 or/6‐9 (1871) 11 10 and 5 (294) 12 Clinical Trial/ (544573) 13 Randomized Controlled Trial/ (169996) 14 exp randomization/ (26878) 15 Single Blind Procedure/ (8243) 16 Double Blind Procedure/ (72817) 17 Crossover Procedure/ (21433) 18 Placebo/ (127767) 19 Randomi?ed controlled trial$.tw. (33726) 20 Rct.tw. (2803) 21 random allocation.tw. (641) 22 randomly allocated.tw. (10318) 23 allocated randomly.tw. (1358) 24 (allocated adj2 random).tw. (562) 25 Single blind$.tw. (7555) 26 Double blind$.tw. (85595) 27 ((treble or triple) adj blind$).tw. (140) 28 placebo$.tw. (111319) 29 prospective study/ (82941) 30 or/12‐29 (715383) 31 case study/ (6142) 32 case report.tw. (120749) 33 abstract report/ or letter/ (501753) 34 or/31‐33 (626277) 35 30 not 34 (690449) 36 35 and 11 (108) 37 (2008$ or 2009$).em. (867314) 38 36 and 37 (8) 39 from 38 keep 1‐8 (8)
Appendix 4. CENTRAL search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <2nd Quarter 2009> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Clomiphene/ (290) 2 clomifene.tw. (7) 3 clomiphene.tw. (518) 4 clomid$.tw. (18) 5 or/1‐4 (565) 6 (unexplain$ adj3 infertil$).tw. (190) 7 (unexplain$ adj3 subfertil$).tw. (15) 8 (idiopathic adj3 subfertil$).tw. (10) 9 (idiopathic adj3 infertil$).tw. (58) 10 or/6‐9 (263) 11 10 and 5 (58) 12 limit 11 to yr="2006 ‐Current" (16) 13 from 12 keep 1‐16 (16)
Appendix 5. PsycINFO search strategy
Database: PsycINFO <1806 to June Week 1 2009> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Clomiphene/ (0) 2 clomifene.tw. (0) 3 clomiphene.tw. (29) 4 clomid$.tw. (0) 5 or/1‐4 (29) 6 (unexplain$ adj3 infertil$).tw. (21) 7 (unexplain$ adj3 subfertil$).tw. (1) 8 (idiopathic adj3 subfertil$).tw. (0) 9 (idiopathic adj3 infertil$).tw. (10) 10 or/6‐9 (31) 11 10 and 5 (1) 12 from 11 keep 1 (1)
Data and analyses
Comparison 1. Clomiphene versus placebo or no treatment in unexplained subfertility.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth per woman randomised | 1 | 385 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.45, 1.38] |
| 2 Pregnancy per woman randomised | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 With IUI | 2 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [0.70, 8.19] |
| 2.2 Without IUI | 2 | 458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.64, 1.66] |
| 2.3 Without IUI using hCG | 2 | 105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.58, 4.80] |
| 3 Miscarriage per woman randomised | 1 | 385 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.31, 1.61] |
| 4 Multiple pregnancies | 1 | 385 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.14, 7.19] |
| 5 Ectopic pregnancy per woman randomised | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.23] |
| 6 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 abdominal pain | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.89 [3.81, 25.69] |
| 6.2 vaginal bleeding | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.51, 6.21] |
| 6.3 nausea | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.11 [2.07, 18.10] |
| 6.4 vomiting | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.88] |
| 6.5 headache | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.47 [2.64, 15.83] |
| 6.6 hot flushes | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.75 [3.02, 25.36] |
| 6.7 bloating | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 81.28 [4.94, 1337.02] |
| 6.8 anxiety | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.66, 1.92] |
| 6.9 depression | 1 | 385 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.25, 4.08] |
1.1. Analysis.
Comparison 1 Clomiphene versus placebo or no treatment in unexplained subfertility, Outcome 1 Live birth per woman randomised.
1.2. Analysis.
Comparison 1 Clomiphene versus placebo or no treatment in unexplained subfertility, Outcome 2 Pregnancy per woman randomised.
1.3. Analysis.

Comparison 1 Clomiphene versus placebo or no treatment in unexplained subfertility, Outcome 3 Miscarriage per woman randomised.
1.4. Analysis.
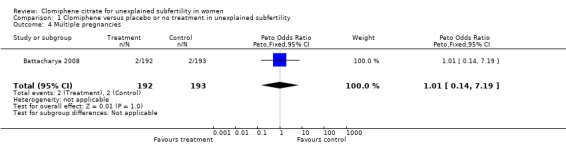
Comparison 1 Clomiphene versus placebo or no treatment in unexplained subfertility, Outcome 4 Multiple pregnancies.
1.5. Analysis.
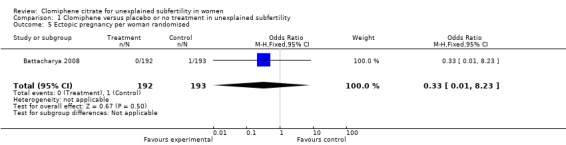
Comparison 1 Clomiphene versus placebo or no treatment in unexplained subfertility, Outcome 5 Ectopic pregnancy per woman randomised.
1.6. Analysis.
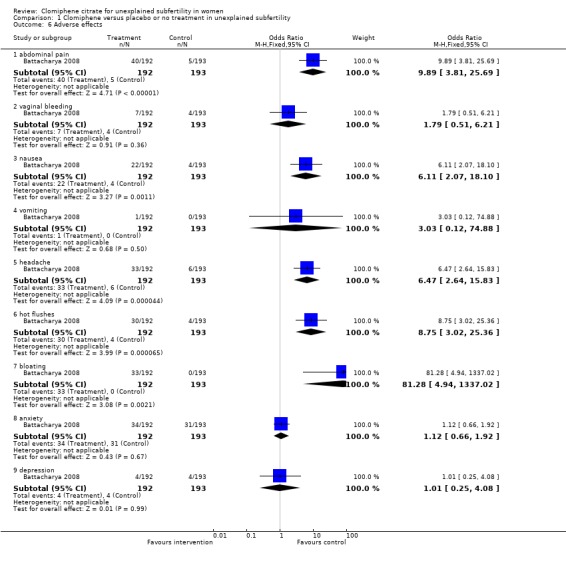
Comparison 1 Clomiphene versus placebo or no treatment in unexplained subfertility, Outcome 6 Adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arici 1994.
| Methods | Randomised crossover trial | |
| Participants | US study in private infertility clinic 75 couples enrolled of which 19 were excluded (9 never returned, 5 declined randomisation, 3 had other fertility factors and there were 2 pregnancies before treatment ‐ one in each arm) 56 randomised Mean age was 33 years (range 24‐41) Mean duration of infertility 3.5 years (range 1‐15) 26 of the couples had unexplained subfertility Inclusion for this subgroup: normal semen analysis; negative antisperm antibodies; normal hysterosalpingogram; regular ovulatory cycles; normal laparoscopic findings |
|
| Interventions | Natural cycle IUI ‐ no adjuvant therapy. IUI on day of luteinising hormone (LH) peak versus Clomiphene citrate 50 mg/day on days 5‐9 with 10,000 U IM hCG when one or more follicles reached 18mm mean diameter as determined by ultrasound scan. IUI performed 32 hours after hCG injection |
|
| Outcomes | Pregnancy per cycle | |
| Notes | Data presented per cycle rather than per woman randomised and not separable by unexplained infertility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised using computer‐generated random number tables |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition before and during study accounted for: 75 couples enrolled of which 19 were excluded (9 never returned, 5 declined randomisation, 3 had other fertility factors and there were 2 pregnancies before treatment ‐ one in each arm) Of 56 randomised only 29 completed. There were nine pregnancies, 3 moved out of area, 6 failed to return and 18 refused crossover |
| Free of selective reporting? | High risk | Did not report on live birth, miscarriage or adverse effects. Data reported per cycle and the review authors were unable to separate those with unexplained subfertility |
Battacharya 2008.
| Methods | Multicentre (n=5) 3‐arm randomised trial. Attrition was 18/580 | |
| Participants | UK study Inclusion: women with infertility for 2+ years; bilateral tubal patency with confirmed ovulation, patent fallopian tubes and partners with motile sperm; couples with minimum sperm motility of 20%; or minimal endometriosis (rAFS stage 1) Mean age 31.7±3.4 years for expectant management and 31.8±3.5 years for clomiphene. Main cause of infertility was primary infertility in 70% of expectant management and 74% of clomiphene women |
|
| Interventions | Expectant management (n=193): 6 months with no clinic visits or medical interventions scheduled, regular intercourse advised
versus
Clomiphene 50mg from days 2 to 6 of a cycle (n=194), couples advised to have intercourse on days 12‐18 of the cycle
versus
Unstimulated IUI (n=193) Treated for 6 months |
|
| Outcomes | Pregnancy, miscarriage, live birth, multiple pregnancies, anxiety and depression | |
| Notes | Spontaneous pregnancies included. Women who became pregnant but had a miscarriage within 6 months of randomisation were allowed to have further treatment within their randomised arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | An independent statistician generated the randomisation allocation sequence. Stratified by centre. Allocation balanced by age, parity and duration of subfertility |
| Allocation concealment? | Low risk | A ‐ patients were randomised by a central telephone system |
| Blinding? All outcomes | High risk | No blinding due to nature of interventions |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 4 participants lost to follow up, some women received treatment other than randomised for but did continue to be analysed in their allocated arm as per intention to treat (ITT) |
| Free of selective reporting? | Low risk | All a priori outcomes addressed: harms and benefits |
Deaton 1990.
| Methods | Random allocation, method not described. Allocation concealment not described, unblinded crossover design. Sixteen couples removed from study details in quality table | |
| Participants | American study, 67 couples enrolled. Mean female age 33±4.0 years, mean duration of infertility 3.5±1.7 years. Primary (60%) and secondary infertility (40%). 27 out of 67 women underwent surgical treatment for endometriosis ‐ 24 were minimal or mild ‐ prior to enrolment. Ovulation confirmed by BBT or endometrial biopsy; tubal status normal on HSG; sperm normal by WHO standards; PCT 'normal'. Total of 67 couples enroled, 51 included in analysis Women with tubal disease or unwilling to be randomised were excluded, also if sperm or ovulatory abnormalities were encountered during the study | |
| Interventions | Clomiphene (CC) 50 mg orally cycle days 5 to 9 unless cycle length was <27 days and then CC administered on days 4 to 8, hCG 10,000 administered IM then timed IUI versus Control group had intercourse around time of ovulation Study continued for 4 treatment or 4 control cycles. Couples followed for 8 cycles in total or until pregnancy, whichever occurred first No details of washout period after active treatment arm | |
| Outcomes | Pregnancy (diagnosis not defined), live birth, ectopic pregnancy, multiple birth, miscarriage | |
| Notes | Co‐intervention with hCG and IUI in treatment group. Crossover design but pre and post‐crossover data separable; 27 women had endometriosis treated surgically shortly before randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomised to either four control or four treatment cycles' |
| Allocation concealment? | High risk | C ‐ inadequate |
| Blinding? All outcomes | High risk | Open‐labelled study |
| Incomplete outcome data addressed? All outcomes | Low risk | Sixteen out of 67 were withdrawn from the study, 10 because of abnormal semen analysis or anovulatory cycle An additional 6 withdrew because of stress or inability to follow protocol |
| Free of selective reporting? | Low risk | Reported on the main outcomes |
Fisch 1989.
| Methods | Randomised, double‐blind trial. Treatment allocated by one centre using a computer‐generated randomisation list. No details of allocation concealment. Four‐arm, parallel factorial design assessing CC and hCG luteal phase support. Multicentre trial in five Canadian University centres. Attrition: 148/177 women were analysed, details in quality table | |
| Participants | Canadian study of women attending University Centres. Mean female age 30 (range 20‐41) years, mean duration of infertility 4.3±1.4 years (range 2‐12). Primary subfertility only of 2 years or greater. Ovulation confirmed by BBT, luteal serum progesterone or endometrial biopsy; tubal status normal on laparoscopy; sperm normal by WHO standards. Endometriosis patients excluded. n= 177 couples enrolled, 148 included in analysis | |
| Interventions | Clomiphene 100 mg in 2x tablets taken orally on cycle days 5 to 9 followed by saline injections IM on cycle days 19,22,25 and 28 (n=37)
versus
Clomiphene 100 mg in 2x tablets taken orally on cycle days 5 to 9 followed by hCG injections IM 5000 IU on cycle days 19,22,25 and 28 (n=39)
versus
Placebo 2x tablets taken orally on cycle days 5 to 9 followed by saline injections IM on cycle days 19,22,25 and 28 (n=36)
versus
Placebo 2x tablets taken orally on cycle days 5 to 9 followed by hCG injections IM 5000 IU on cycle days 19,22,25 and 28 (n=36) Interventions were given for 4 consecutive cycles After 6 months of follow up women were offered the IVF programme |
|
| Outcomes | Pregnancy confirmed by ultrasound evidence of fetal heart activity or trophoblast on pathological examination, chemical pregnancies excluded. Miscarriage, still birth and multiple pregnancies | |
| Notes | Couples were followed for 6 consecutive cycles post‐treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Treatment allocated by one centre using a computer‐generated randomisation list |
| Allocation concealment? | Unclear risk | B ‐ unclear, co‐ordination of codes and drug distribution from one centre but no details of how concealment was maintained |
| Blinding? All outcomes | Low risk | Double‐blind double dummy trial |
| Incomplete outcome data addressed? All outcomes | Low risk | 177 women enrolled, 22 were excluded for incomplete data or missed medication (n=11), 7 dropped out, 2 had endometriosis found on review and 2 were found to have secondary infertility. Seven pregnancies occurred after enrolment but before study medication leaving 148 women for analysis |
| Free of selective reporting? | High risk | Did not report on live birth |
Glazener 1990.
| Methods | Randomised, double‐blind placebo‐controlled crossover study | |
| Participants | UK study, volunteers attending clinic because of at least one year Inclusion: normal menstrual cycles (21‐35 days); normal serum prolactin and thyroid hormone; normal coital frequency (at least twice weekly); normal post‐coital sperm‐mucus penetration Those failing to conceive after a few months had a laparoscopy to exclude pelvic disease and tubal damage 118 women with unexplained infertility. Median age 28 (range 19‐44) years); median duration of infertility 28 months (range 12‐102) |
|
| Interventions | 3‐month duration for each arm Clomiphene 100mg versus identical placebo on days 2‐6 for 3 cycles and then crossover |
|
| Outcomes | Conception rates | |
| Notes | Unable to separate data for first arm of crossover | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomised', no other details |
| Allocation concealment? | Unclear risk | No details |
| Blinding? All outcomes | Low risk | Double blind, identical placebo. No details of who was blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | All women accounted for |
| Free of selective reporting? | High risk | Report on multiple pregnancies, no details of live birth or ongoing pregnancies. No details of miscarriage or adverse effects |
Harrison 1983.
| Methods | Random allocation, method not described. Allocation concealment not described. Crossover design. Patients were blinded to treatment. Three women dropped out of the trial during second six months, see quality table for details. No details of washout period | |
| Participants | Irish study of women attending one of two infertility clinics. Mean age 29.3 years, mean duration of infertility 5.4 years (range 2‐14). Primary and secondary infertility; ovulation confirmed by luteal phase serum progesterone; tubal status normal on hysterosalpingogram or laparoscopy; sperm 'normal'; post‐coital test 'normal', 30 couples | |
| Interventions | Clomiphene 100 mg daily x 4 days + hCG 5000 IU day 12; placebo two tablets x 4 days + hCG 5000 IU day 12. Women given up to 6 treatment or placebo cycles pre‐crossover. Total number of patients was 30, 15 in each group prior to crossover | |
| Outcomes | Pregnancy (method of diagnosis not defined), miscarriage, patient‐reported side effects, hormonal and haematological analysis | |
| Notes | Up to 12 treatment cycles. Crossover design: pre and post‐data separable. All patients received cycles of clomiphene alone before randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No details |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details |
| Blinding? All outcomes | Low risk | Women blinded to treatment, which was identical |
| Incomplete outcome data addressed? All outcomes | Low risk | Three women withdrew during second 6 months. One had a cerebral haemorrhage (placebo arm), one had depression and one adopted a child (on active arm) |
| Free of selective reporting? | High risk | Did not report on live birth |
Melis 1987.
| Methods | Random allocation, method not described. No details of allocation concealment. Unblinded. Attrition: n=25/131, details in quality table | |
| Participants | Italian study. 131 infertile couples included in the study, 28 had a diagnosis of unexplained infertility as opposed to male subfertility. Mean female age not given, mean duration of infertility 3.7±4.1 years (range 2 to 10). Primary and secondary infertility but proportions not given Ovulation confirmed by luteal phase serum progesterone, endometrial biopsy and ultrasound; tubal status normal on hysterosalpingogram and/or laparoscopy; sperm 'normal'; post‐coital test 'normal' Menstrual cycle abnormalities were excluded, as were abnormalities of the female genital tract | |
| Interventions | CC 100 mg/day from cycle days 5 to 9, followed by cervico‐vaginal AIH timed by BBT and cervical score (n=9) Control patients received AIH alone (n=16) Treatment repeated for a maximum of 6 cycles | |
| Outcomes | Pregnancy (diagnosis not defined) | |
| Notes | Timing of AIH may have been sub optimal, based on BBT and cervical mucus. All women had received 6 cycles of CC before entering the study perhaps eliminating those in whom this treatment is relatively effective | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No details |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details |
| Blinding? All outcomes | High risk | Not blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | 25/131 withdrawn (10 from AIH alone and 15 from AIH and CC). Three women withdrew from unexplained subfertility although no details of which women from which group |
| Free of selective reporting? | Unclear risk | Did not report on live birth, did not define pregnancy. Did not report on any adverse effects |
BBT: basal body temperature
PCT: post‐coital test
po: oral
IVF: in vitro fertilisation
AIH: artifical insemination by husband
CC: clomiphene citrate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2004 | All women received clomiphene and were randomised to intrauterine insemination or timed intercourse |
| Battacharya 2006 | Conference proceeding superseded by full paper included in review |
| Check 1992 | Population included only women with oligo‐anovulation or luteal phase defect. Clomiphene compared with human menopausal gonadotropin |
| Fujii 1997 | Excluded because of concern over selection bias based on the reported method of allocation: alternating chart numbers. Also follow ups may have differed between treatment and control groups since women with symptoms or signs of 'anti‐estrogenic' nature were withdrawn from treatment and follow up after two cycles. Cycle fecundity in spontaneous cycles 21%. This exceeds the expected rate for FSH/IUI by a factor of 2 and approaches rate seen with IVF. Expected cycle fecundity with no treatment in unexplained infertility is 1% to 2% |
| Karlstrom 1993 | Compared clomiphene with human menopausal gonadotropin (hMG) |
| Martinez 1990 | Population included male factor and unexplained subfertility patients. Data were presented in a way which did not allow for separate analysis by patient and treatment group |
| Parker 1992 | Population not women with unexplained subfertility but donor sperm recipients |
Contributions of authors
Julie Brown conducted the latest search and edited the review to bring it up to the latest standards of the MDSG.
Sources of support
Internal sources
No sources of support supplied
External sources
Royal Commission on New Reproductive Technologies, Not specified.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arici 1994 {published data only}
- Arici A, Byrd W, Bradshaw K, Kutteh W, Marshburn P, Carr B. Evaluation of clomiphene citrate and human chorionic gonadotropin treatment: a prospective, randomized, crossover study during intrauterine insemination cycles. Fertility and Sterility 1994;61:314‐8. [DOI] [PubMed] [Google Scholar]
Battacharya 2008 {published data only}
- Battacharya S, Harrild K, Mollison J, Wordsworth S, Tay C, Harrold A, et al. Clomifene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained fertility: pragmatic randomised controlled trial. BMJ 2008;337:a716. [DOI: 10.1136/bmj.a716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deaton 1990 {published data only}
- Deaton J, Gibson M, Blackmer K, Nakajima S, Badger G, Brumsted J. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertility and Sterility 1990;54:1083‐8. [PubMed] [Google Scholar]
Fisch 1989 {published data only}
- Fisch P, Casper R, Brown S, Wrixon W, Collins J, Reid R, Simpson C. Unexplained infertility: evaluation of treatment with clomiphene citrate and human chorionic gonadotropin. Fertility and Sterility 1989;51(5):828‐32. [DOI] [PubMed] [Google Scholar]
Glazener 1990 {published data only}
- Glazener CMA, Coulson C, Lambert PA, Watt E, Hinton RA, Kelly NG, Hull MGR. Clomiphene treatment for women with unexplained infertility: placebo‐controlled study of hormonal responses and conception rates. Gynecological Endocrinology 1990;4:75‐83. [DOI] [PubMed] [Google Scholar]
Harrison 1983 {published data only}
- Harrison RF, O'Moore R. The use of clomiphene citrate with and without human chorionic gonadotropin. Irish Medical Journal 1983;76(6):273‐4. [PubMed] [Google Scholar]
Melis 1987 {published data only}
- Melis G, Paoletti A, Strigini F, Fabris F, Canale D, Fiorette P. Pharmacologic induction of multiple follicular development improves the success rate of artificial insemination with husband's semen in couples with male‐related or unexplained infertility. Fertility and Sterility 1987;47:441‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2004 {published data only}
- Agarwal S, Mittal S. A randomised prospective trial of intrauterine insemination versus timed intercourse in superovulated cycles with clomiphene. The Indian Journal of Medical Research 2004;120(6):519‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Battacharya 2006 {published and unpublished data}
- Battacharya S, Harrild K, Harrold A, Lyall H, McQueen D, Tay C. A randomised trial of expectant management, clomiphene and intrauterine insemination (IUI) in the treatment of infertility. Fertility and Sterility 2006;86 Suppl 1(3):43. [Google Scholar]
Check 1992 {published data only}
- Check J, Davies E, Adelson H. A randomized prospective study comparing pregnancy rates following clomiphene citrate and human menopausal gonadotrophin therapy. Human Reproduction 1992;7:801‐5. [DOI] [PubMed] [Google Scholar]
Fujii 1997 {published data only}
- Fuji S, Fukui A, Fukushi Y, Kagiya A, Sato S, Saito Y. The effects of clomiphene citrate on normally ovulating women. Fertility and Sterility 1997;68(6):997‐9. [DOI] [PubMed] [Google Scholar]
Karlstrom 1993 {published data only}
- Karlstrom P, Bergh T, Lundkvist O. A prospective randomized trial of artificial insemination versus intercourse in cycles stimulated with human menopausal gonadotropin or clomiphene citrate. Fertility and Sterility 1993;59:554‐9. [PubMed] [Google Scholar]
Martinez 1990 {published data only}
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JPW, Schoemaker J. Intrauterine insemination does and clomiphene citrate does not improve fecundity in couples with infertility due to male or idiopathic factors: A prospective, randomized, controlled study. Fertility and Sterility 1990;53(5):847‐53. [DOI] [PubMed] [Google Scholar]
Parker 1992 {published data only}
- Parker JA, Kaplan BR, Nisker JA, Tummon IS, Powers SD, Yuzpe AA. Empiric administration of clomiphene citrate to women with normal ovulatory cycles in a therapeutic donor insemination program (abstract). Fertility and Sterility 1992:97. [Google Scholar]
Additional references
Collins 1991
- Collins JA, Milner RA. The effect of treatment on pregnancy among couples with unexplained infertility. International Journal of Fertility 1991;36(3):140‐52. [PubMed] [Google Scholar]
Compendium 1998
- Canadian Pharmacists Association. Compendium of Pharmaceuticals and Specialties. Thirty‐third. Canada: Canadian Pharmacists Association, 1998:332‐3. [Google Scholar]
Hughes 1996
- Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate vs placebo for ovulation induction in oligo‐amenorrhoeic women. Cochrane Database of Systematic Reviews 1996, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxson 1984
- Maxson WS, Pittaway DE, Herbert CM, Garner CH, Wentz AC. Antiestrogenic effect of clomiphene citrate: correlation with serum estradiol concentrations. Fertility and Sterility 1984;42:356‐9. [PubMed] [Google Scholar]
Peto 1987
- Peto R. Why do we need systematic overviews of randomized trials. Statistics in Medicine 1987;6:233‐40. [DOI] [PubMed] [Google Scholar]
Randall 1991
- Randall JM, Templeton AA. Transvaginal sonographic assessment of follicular and endometrial growth in spontaneous and clomiphene citrate cycles. Fertility and Sterility 1991;56:208‐12. [DOI] [PubMed] [Google Scholar]
Rossing 1994
- Rossing MA, Weiss NS. Ovarian tumours in a cohort of infertile women. New England Journal of Medicine 1994;331:771‐6. [DOI] [PubMed] [Google Scholar]
Whittemore 1992
- Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: Collaborative analysis of 12 U.S. case controlled studies. American Journal of Epidemiology 1992;136:1184‐203. [DOI] [PubMed] [Google Scholar]


