Abstract
Background
It is unclear whether blood pressure should be altered actively during the acute phase of stroke. This is an update of a Cochrane review first published in 1997, and previously updated in 2001 and 2008.
Objectives
To assess the clinical effectiveness of altering blood pressure in people with acute stroke, and the effect of different vasoactive drugs on blood pressure in acute stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched in February 2014), the Cochrane Database of Systematic reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 2), MEDLINE (Ovid) (1966 to May 2014), EMBASE (Ovid) (1974 to May 2014), Science Citation Index (ISI, Web of Science, 1981 to May 2014) and the Stroke Trials Registry (searched May 2014).
Selection criteria
Randomised controlled trials of interventions that aimed to alter blood pressure compared with control in participants within one week of acute ischaemic or haemorrhagic stroke.
Data collection and analysis
Two review authors independently applied the inclusion criteria, assessed trial quality and extracted data. The review authors cross‐checked data and resolved discrepancies by discussion to reach consensus. We obtained published and unpublished data where available.
Main results
We included 26 trials involving 17,011 participants (8497 participants were assigned active therapy and 8514 participants received placebo/control). Not all trials contributed to each outcome. Most data came from trials that had a wide time window for recruitment; four trials gave treatment within six hours and one trial within eight hours. The trials tested alpha‐2 adrenergic agonists (A2AA), angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor antagonists (ARA), calcium channel blockers (CCBs), nitric oxide (NO) donors, thiazide‐like diuretics, and target‐driven blood pressure lowering. One trial tested phenylephrine.
At 24 hours after randomisation oral ACEIs reduced systolic blood pressure (SBP, mean difference (MD) ‐8 mmHg, 95% confidence interval (CI) ‐17 to 1) and diastolic blood pressure (DBP, MD ‐3 mmHg, 95% CI ‐9 to 2), sublingual ACEIs reduced SBP (MD ‐12.00 mm Hg, 95% CI ‐26 to 2) and DBP (MD ‐2, 95%CI ‐10 to 6), oral ARA reduced SBP (MD ‐1 mm Hg, 95% CI ‐3 to 2) and DBP (MD ‐1 mm Hg, 95% CI ‐3 to 1), oral beta blockers reduced SBP (MD ‐14 mm Hg; 95% CI ‐27 to ‐1) and DBP (MD ‐1 mm Hg, 95% CI ‐9 to 7), intravenous (iv) beta blockers reduced SBP (MD ‐5 mm Hg, 95% CI ‐18 to 8) and DBP (‐5 mm Hg, 95% CI ‐13 to 3), oral CCBs reduced SBP (MD ‐13 mmHg, 95% CI ‐43 to 17) and DBP (MD ‐6 mmHg, 95% CI ‐14 to 2), iv CCBs reduced SBP (MD ‐32 mmHg, 95% CI ‐65 to 1) and DBP (MD ‐13, 95% CI ‐31 to 6), NO donors reduced SBP (MD ‐12 mmHg, 95% CI ‐19 to ‐5) and DBP (MD ‐3, 95% CI ‐4 to ‐2) while phenylephrine, non‐significantly increased SBP (MD 21 mmHg, 95% CI ‐13 to 55) and DBP (MD 1 mmHg, 95% CI ‐15 to 16).
Blood pressure lowering did not reduce death or dependency either by drug class (OR 0.98, 95% CI 0.92 to 1.05), stroke type (OR 0.98, 95% CI 0.92 to 1.05) or time to treatment (OR 0.98, 95% CI 0.92 to 1.05). Treatment within six hours of stroke appeared effective in reducing death or dependency (OR 0.86, 95% CI 0.76 to 0.99) but not death (OR 0.70, 95% CI 0.38 to 1.26) at the end of the trial. Although death or dependency did not differ between people who continued pre‐stroke antihypertensive treatment versus those who stopped it temporarily (worse outcome with continuing treatment, OR 1.06, 95% CI 0.91 to 1.24), disability scores at the end of the trial were worse in participants randomised to continue treatment (Barthel Index, MD ‐3.2, 95% CI ‐5.8, ‐0.6).
Authors' conclusions
There is insufficient evidence that lowering blood pressure during the acute phase of stroke improves functional outcome. It is reasonable to withhold blood pressure‐lowering drugs until patients are medically and neurologically stable, and have suitable oral or enteral access, after which drugs can than be reintroduced. In people with acute stroke, CCBs, ACEI, ARA, beta blockers and NO donors each lower blood pressure while phenylephrine probably increases blood pressure. Further trials are needed to identify which people are most likely to benefit from early treatment, in particular whether treatment started very early is beneficial.
Plain language summary
Drug interventions for deliberately altering blood pressure in acute stroke
Background: In people who have just had a stroke (a sudden brain attack due to either blockage or rupture of an artery in the brain), very high and very low blood pressures may be harmful. Therefore, drugs that raise low blood pressure or lower high blood pressure might be beneficial. Up to 50% of people admitted with acute stroke are taking blood pressure tablets on hospital admission and it is not clear whether these medications should be continued or discontinued in the acute situation. This review looked at those trials that deliberately altered blood pressure or compared continuing or stopping blood pressure‐lowering tablets taken before stroke.
Study characteristics: This review is up‐to‐date to May 2014. We included 26 trials involving 17,011 participants: 24 trials assessed lowering blood pressure, one trial tested raising blood pressure, and two trials assessed what to do with drugs taken before stroke. All studies took place in hospitals that were used to treating people with stroke. Not all trials contributed information to all outcomes, and we have used data that were available in publications.
Key results: There is insufficient evidence to say that lowering blood pressure saves lives or reduces disability in people with acute stroke. Immediately restarting blood pressure‐lowering drugs taken before the stroke may increase disability.
Conclusion: More research is needed to identify those people who are most likely to benefit from altering blood pressure in acute stroke, the time window in which the treatment is likely to be of benefit, what types of stroke are likely to respond favourably, and the environment in which such treatment may be best given in routine practice.
Background
Description of the condition
Stroke is the third most common cause of death and the most common cause of disability in the western world. Acute stroke, whether due to infarction or haemorrhage, is associated with high blood pressure in 75% of patients, of whom 50% have a previous history of high blood pressure (Britton 1986; Oppenheimer 1992). After a stroke, blood pressure falls in most patients over a week although a third of patients remain hypertensive (Wallace 1981; Britton 1986; Harper 1994). A number of small studies have assessed the relationship between blood pressure (Marshall 1959; Adams 1965; Droller 1965; Bourestom 1967; Marquarsden 1969; Carlberg 1993) and outcome. A meta‐analysis of these studies found that elevated blood pressure was associated with a poor outcome (Willmot 2004). Data from 17,398 participants in the International Stroke Trial identified a U‐shaped relationship such that both low and high blood pressure were associated independently with increased early death and later death or dependency (Leonardi‐Bee 2002), a finding that has been replicated by others (Castillo 2004; Vemmos 2004). High blood pressure is also associated with an increased early recurrence of stroke (Leonardi‐Bee 2002; Sprigg 2006).
The mechanisms underlying high blood pressure in stroke are complex but pre‐existing hypertension, hospitalisation stress, activation of the sympathetic renin‐angiotensin‐aldosterone, cortisol and natriuretic peptide neuroendocrine systems, and the Cushing reflex (raised blood pressure secondary to raised intracranial pressure) all contribute (Myers 1982). In ischaemic stroke, high blood pressure also appears to adversely affect outcome through increasing the risk of cerebral oedema, but not haemorrhagic transformation (Leonardi‐Bee 2002). Haematoma expansion is related to high blood pressure in people with intracerebral haemorrhage (ICH) although this relationship may be confounded by stroke severity and time to presentation (Fujii 1994; Kazui 1997; Fujii 1998; Bath 2003).
Description of the intervention
Although debated more than 29 years ago, it still remains unclear whether high blood pressure should (Spence 1985) or should not (Yatsu 1985) be treated acutely following stroke. Recent guidelines recommend that acute lowering of blood pressure should be delayed for several days or even weeks unless blood pressure is greater than 220/120 mmHg, blood pressure is greater than 200/100 mmHg with end organ involvement (hypertensive encephalopathy, aortic dissection, cardiac ischaemia, pulmonary oedema, acute renal failure), or blood pressure is greater than 200/120 mmHg with primary ICH, are present (O'Connell 1994; EUSI 2004; AHA‐HS 2010; RCP 2012; AHA‐IS 2013). Though the evidence is weaker, guidelines now recommend that patients who have elevated blood pressure and are otherwise eligible for treatment with recombinant tissue plasminogen activator may have their blood pressure lowered so that systolic blood pressure (SBP) is less than or equal to 185 mmHg and diastolic blood pressure (DBP) is less than or equal to 110 mmHg before thrombolysis using intravenous labetalol, nitroprusside or nicardipine and it should be maintained below 180/105 mmHg for at least the first 24 hours after therapy (AHA‐IS 2013). Unfortunately, such guidelines are inconsistent and are based on theoretical arguments and individual case reports, and not on the results of systematic overviews or large intervention trials of blood pressure manipulation in acute stroke. Nevertheless, a number of case reports and series have suggested that active lowering of blood pressure in people with primary intracranial haemorrhage and ischaemic stroke may improve (Dandapani 1995; Chamorro 1998) or worsen (Graham 1975; Britton 1980; Fischberg 2000) outcome.
Low blood pressure is not common in acute stroke but it, like high blood pressure, is associated with a poor outcome (Leonardi‐Bee 2002). Possible reasons for low blood pressure include potentially reversible conditions such as hypovolaemia, sepsis, impaired cardiac output secondary to cardiac failure, arrhythmias or cardiac ischaemia, and aortic dissection (Sprigg 2005). Guidelines recommend that causes of hypotension in the setting of acute stroke should be sought with the view to correcting reversible causes such as hypovolaemia and cardiac arrhythmias (AHA‐IS 2013). Since cerebral autoregulation is lost following stroke (Strandgaard 1973; Burke 1986; Paulson 1990), such that cerebral blood flow becomes dependent on systemic blood pressure, some researchers have hypothesised that blood pressure should be increased to improve cerebral perfusion (Sandercock 1992) and a case series (Rordorf 1997) and a pilot randomised trial of phenylephrine (Hillis 2003) reporting this approach have been published.
How the intervention might work
Although the different drugs assessed work in a variety of ways, all lower (or elevate ‐ phenylephrine) blood pressure.
Why it is important to do this review
We are reviewing this topic in three parts (Bath 1997).
Part 1: this review
Assessment of trials in which the primary aim of the intervention was to alter blood pressure in people with acute stroke with the aim of improving clinical outcome.
A Cochrane review of blood pressure intervention in stroke published in 2001 (BASC 2001) updated the original review published in 1997. It was again updated in 2008 to include more information from 13 trials published between 2003 and 2008 including a total of 1153 participants (BASC 2009). With a relatively small amount of data, there was insufficient evidence to evaluate the effect of altering blood pressure during the acute phase of stroke.
The present review includes all new trials completed and published since 2008. The total number of participants is now 17,011, a 14‐fold increase since the review in 1997 and 2008. Although many of the data are from trials testing blood pressure alteration in the acute phase (≤ 48 hours), some recent trials have examined specific questions such as lowering blood pressure in ICH (INTERACT pilot 2008; INTERACT‐2 2013), with angiotensin receptor antagonists (SCAST 2011), or glyceryl nitrate (ENOS 2014) or in the pre‐hospital setting (PIL‐FAST 2013; RIGHT 2013). Furthermore, two trials (COSSACS 2010; ENOS 2014) have investigated whether to continue or stop temporarily pre‐stroke antihypertensive therapy. This systematic review includes these data and provides up‐to‐date evidence.
Part 2: vasoactive drugs for acute stroke
Assessment of trials where vasoactive drugs were administered to people with acute stroke and where clinical outcome was measured. Drugs include: alpha receptor antagonists, angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor inhibitors (ARA), beta receptor antagonists, calcium channel blockers (CCB), diuretics, magnesium, naftidrofuryl, nitric oxide donors (nitrates), papaverine, pentoxifylline, prostacyclin, serotonin receptor antagonists, sympathomimetics, theophylline (and mimetics), thromboxane A2 antagonists, vinpocetine, and their derivatives. Aggregated patient data are analysed separately for drugs which lower and elevate blood pressure (BASC 2000). Work on this analysis is ongoing.
Part 3: analysis of individual patient data from the trials identified in parts 1 and 2
Work on this analysis is ongoing through the international Blood pressure in Acute Stroke Collaboration. In brief, individual patient data from the trials included in Part 1 and Part 2 are being collated with the intention of extending analyses, particularly in subgroups of participants and interventions.
Objectives
To assess the clinical effectiveness of altering blood pressure in people with acute stroke, and the effect of different vasoactive drugs on blood pressure in acute stroke.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) of vasoactive drugs in acute ischaemic stroke or acute intracerebral haemorrhage (ICH) where the aim of the trial was to alter blood pressure, and drug therapy was initiated within one week of stroke onset. We excluded uncontrolled studies, confounded controlled studies where two or more active interventions were compared, and studies of people with subarachnoid haemorrhage.
Types of participants
Adults (age 18 or older) of either sex with acute ischaemic stroke or ICH who were eligible for randomisation to either active treatment, or placebo or open control.
Types of interventions
We sought RCTs evaluating single or multiple agents of deliberate blood pressure lowering or elevation in acute stroke, regardless of dosage or route of treatment, compared against placebo or open control. We also included trials with two groups receiving different doses of the same BP lowering agent, and studies assessing effects of continuing or stopping pre‐existing antihypertensive treatment.
Types of outcome measures
Primary outcomes
Combined death or disability/dependency at end of trial (≥ one month after stroke). We defined death or dependency as the modified Rankin Scale (mRS) > 2 (or > 3 as available).
Secondary outcomes
Blood pressure when first measured after randomisation.
Early case fatality (< one month).
Late case fatality (≥ one month).
Early neurological deterioration (< one month). As there is no consensus on how early neurological deterioration should be standardised, we used the trial‐specific definition as a decrease in the Scandinavian Stroke Scale (SSS) of > 5 points or a decrease in consciousness part of the SSS by > 2 points (ENOS 2014), increase in the National Institutes of Health Stroke Scale (NIHSS) of 2 (Koch 2008) or more (CHHIPS 2009; COSSACS 2010; INTERACT‐2 2013) or decline of 2 or more points in Glasgow Coma Scale (GCS) (INTERACT‐2 2013).
Late disability or dependency (Barthel Index ≥ one month).
Baseline and on‐treatment blood pressure and heart rate.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. Our methods comprised electronic searches and assessment of studies referenced in published systematic and non‐systematic reviews. We applied no language restrictions.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, (last searched by the Managing Editor in February 2014), the Cochrane Database of Systematic reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 2), MEDLINE (Ovid) (1966 to May 2014) (Appendix 1), EMBASE (Ovid) (1974 to May 2014) (Appendix 2), Science Citation Index (ISI, Web of Science, 1981 to May 2014) (Appendix 3) and the Stroke Trials Registry (www.strokecenter.org/trials/) (searched May 2014).
Searching other resources
We searched reviews of acute stroke relating to drugs that may alter blood pressure, including: calcium channel blockers (CCBs) (Horn 2001), nitric oxide (Bath 2002), pentoxifylline (Bath 2004a) and prostacyclin (Bath 2004b). In addition, we searched reference lists of included trials and relevant papers. We contacted principal investigators and researchers when we required additional information. For a previous version of this review (BASC 2001), we contacted the following pharmaceutical companies: Bayer (nimodipine), Napp (pentoxifylline), Novartis (isradipine), Lipha Sante (naftidrofuryl), Hoffmann la Roche (N‐methyl‐D‐aspartate), Hoechst (flunarizine) and UCB Pharma (piracetam).
Data collection and analysis
We extracted data using a standard proforma; KK entered data into Review Manager (RevMan 2012) and PB checked the data.
Selection of studies
For this update, one review author (KK) screened the records obtained from the electronic searches and excluded obviously irrelevant studies. We obtained the full paper copy of the remaining studies and both review authors (KK and PB) selected trials for inclusion criteria detailed previously. We resolved any disagreements by discussion.
Data extraction and management
We extracted data from published and unpublished material where available. We recorded information on the method of randomisation, concealment of allocation, blinding of treatment administration, analysis (intention‐to‐treat, efficacy analysis), stroke type (ischaemia, haemorrhage, or mixed), drug dose, route of administration (oral, sublingual, intravenous, transdermal) and timing, blood pressure and heart rate before and during treatment, numbers of deaths, functional disability, quality of life, and length of stay.
Assessment of risk of bias in included studies
We assessed the methodological quality of the trials using the following criteria.
Method of randomisation.
Balance of prognostic factors.
Allocation concealment.
Blinding of treatment administration.
Intention‐to‐treat analysis.
Blinding of outcome assessment.
Follow‐up.
We used the quality criteria to derive an overall assessment bias score as 'low risk' (all criteria met), moderate risk (one or more criteria unclear) and high risk (one or more criteria absent) (Higgins 2011).
Measures of treatment effect
We calculated the weighted estimate of the typical treatment effect across trials using RevMan 5 (RevMan 2012), odds ratios (OR) using the Mantel‐Haenszel random‐effects model for binary data, and mean difference (MD) using the inverse variance method for continuous data, each with 95% confidence intervals (CI).
Unit of analysis issues
The primary outcome was based on the modified Rankin Scale (mRS 0 to 6, where death = 6) assessed using the binary outcome of combined death or dependency (mRS > 1 or > 2 depending on trial definition). The Barthel Index (BI) (disability measure of activities of daily living) was also assessed (BI 100 to ‐5, where death = ‐5). Where functional outcome was not assessed, we excluded the trial from analysis of functional outcome.
Dealing with missing data
We attempted to collect missing data from trial investigators. In instances where on‐treatment blood pressure data were not provided or could not be obtained from study authors, we obtained data (mean, SD) from graphs in the trial publication; where the SD was not presented graphically, we used baseline data, a conservative strategy. We excluded trials from individual analyses when summary data were omitted in the trial publication.
Assessment of heterogeneity
We assessed heterogeneity between RCTs' results using the I2 statistic based on the DerSimonian‐Laird formula.
Assessment of reporting biases
We examined reporting bias using funnel plots (Figure 1; Figure 2).
1.
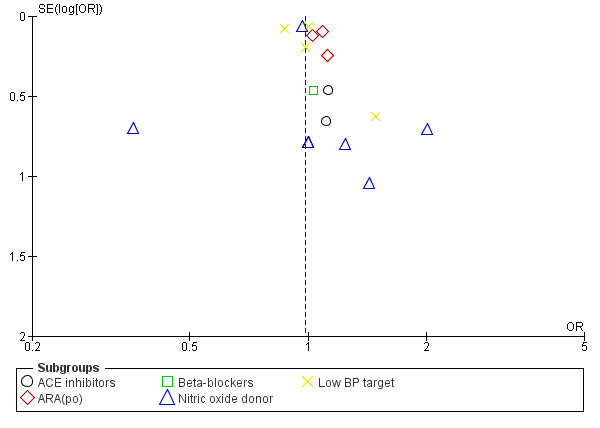
Funnel plot of comparison: 1 Blood pressure lowering therapy in acute stroke, outcome: 1.1 Death or dependency, end of trial by intervention.
2.
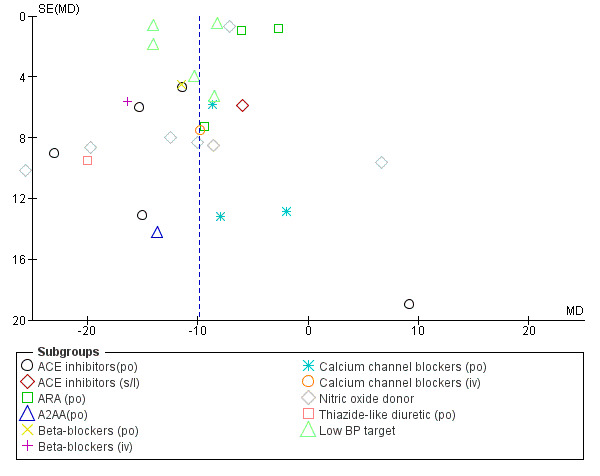
Funnel plot of comparison: 1 Blood pressure lowering therapy in acute stroke, outcome: 1.23 SBP, first after randomisation, by intervention.
Data synthesis
We performed statistical analysis using RevMan (RevMan 2012). We reported outcomes as ORs with 95% CIs for dichotomous data, and MD with 95% CI for continuous data. We used a random‐effects model to analyse individual results regardless of whether there was heterogeneity or not; this is a conservative strategy and takes account that the trials had heterogenous designs and participant populations.
Subgroup analysis and investigation of heterogeneity
We assessed the primary outcomes in the following pre‐specified subgroups.
Class or type of intervention.
Type of stroke: ischaemic stroke or ICH.
Stroke location: cortical or subcortical ischaemic stroke; deep or superficial ICH. (The definition of deep haemorrhage was not defined in all the trials and we therefore used the data as given in the trials.)
Timing of intervention: ultra‐acute (≤ four hours) and pre‐hospital, hyper‐acute (≤ six hours) and in hospital, acute (≤ 48 hours), sub‐acute (≤ 168 hours).
We considered an I2 greater than 50% to infer significant heterogeneity. If significant heterogeneity was present, we looked for potential causes, e.g. differences in trial design and study participants.
Sensitivity analysis
We based the analyses on all trials. We did not perform any sensitivity analyses.
Results
Description of studies
The 26 included trials are summarised in Characteristics of included studies, including details on baseline characteristics (Figure 3).
3.
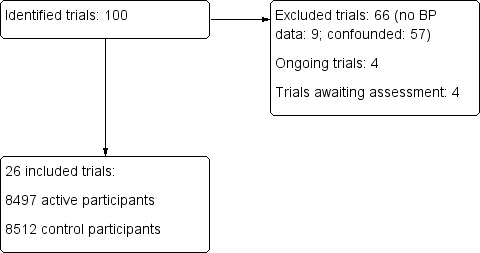
Results of database search
Results of the search
The quantity of outcome data varied between studies:
outcomes not universally collected;
some data still to be published.;
'raw data' available by personal communication.
If a trial used more than one dose of a particular drug then the trial identifier is written as author followed by year and dose of the drug. Referencing the whole trial was given by author and year. For example the Fagan 1988 trial comprises: (Fagan 1988 120 mg; Fagan 1988 240 mg).
Included studies
We identified 26 trials that fulfilled the inclusion criteria (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; ACCESS 2003; Hillis 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; ACCOST 2006; Willmot 2006; Eveson 2007; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; PIL‐FAST 2013; RIGHT 2013; ICH‐ADAPT 2013; INTERACT‐2 2013; TAST 2013; VENTURE 2013; ENOS 2014). Three trials compared more than one drug against the control group (Lisk 1993; CHHIPS 2009; ICH‐ADAPT 2013) and two studies compared different doses (Fagan 1988 120 mg/Fagan 1988 240 mg; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg).
We obtained trial protocols and group data from published material for the following studies (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Hillis 2003; ACCESS 2003; Eames 2005; Eveson 2007; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; RIGHT 2013; TAST 2013; ENOS 2014), whilst individual patient data were provided by seven sets of authors (Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; ENOS 2014). We obtained unpublished SBP, DBP and heart rate data for active and control groups by contacting the authors for three trials (Hillis 2003; Eveson 2007; COSSACS 2010).
A variety of strategies and drug classes were used to lower blood pressure.
Alpha‐2‐adrenoceptor agonist, oral centrally‐acting (clonidine: two participants): one trial (Lisk 1993).
Angiotensin converting enzyme‐inhibitor (ACE‐I) (captopril, perindopril or lisinopril: 152 participants): five trials (Lisk 1993; Dyker 1997; Eveson 2007; CHHIPS 2009; PIL‐FAST 2013).
Angiotensin receptor antagonist (ARA), oral (candesartan or telmisartan: 4190 participants): six trials (ACCESS 2003; ACCOST 2006; PRoFESS 2009; SCAST 2011; TAST 2013; VENTURE 2013).
Beta‐receptor antagonist (ß‐RA) (labetalol: 56 participants): one trial (CHHIPS 2009).
Calcium channel blocker (CCB) (nimodipine or nicardipine: 75 participants): three trials (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Uzuner 1995).
Diuretic, oral thiazide‐like (bendrofluazide: 18 participants): one trial (Eames 2005).
Nitric oxide (NO) donor (transdermal glyceryl trinitrate (GTN): 4197 participants): five trials (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; RIGHT 2013; ENOS 2014).
Intensive versus guideline blood pressure targets (7421 participants): five trials (INTERACT pilot 2008; Koch 2008; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013).
Continue versus stop pre‐stroke antihypertensive drugs (2860 participants): two trials (COSSACS 2010; ENOS 2014).
One strategy was used to raise blood pressure.
Sympathomimetic, intravenous (phenylephrine: nine participants): one trial (Hillis 2003).
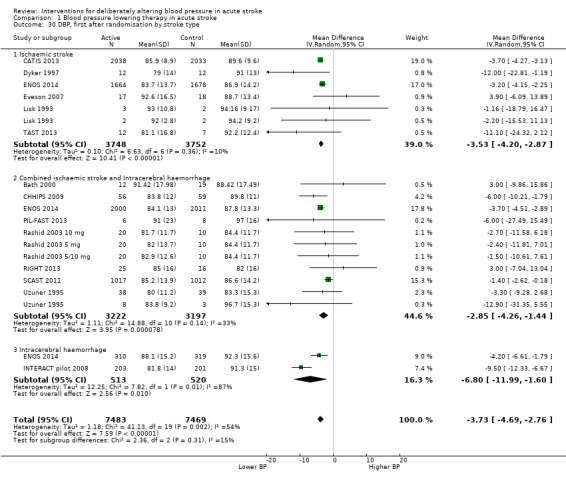
The trials recruited participants with only ischaemic stroke, mixed stroke (ischaemic stroke and ICH), or only ICH.
Ischaemic stroke: 12 trials (Fagan 1988 120 mg; Fagan 1988 240 mg; Lisk 1993; Dyker 1997; ACCESS 2003; Hillis 2003; Eames 2005; ACCOST 2006; Eveson 2007; PRoFESS 2009; CATIS 2013; TAST 2013; VENTURE 2013). In the Fagan study (Fagan 1988 120 mg; Fagan 1988 240 mg) participants were recruited with presumed ischaemic stroke based on history and neurological examination.
Mixed stroke: 10 trials (Uzuner 1995; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; CHHIPS 2009; COSSACS 2010; SCAST 2011; PIL‐FAST 2013; RIGHT 2013; ENOS 2014).
ICH: four trials (INTERACT pilot 2008; Koch 2008; ICH‐ADAPT 2013; INTERACT‐2 2013).
Trials recruited participants at different time frames after stroke:
Ulta‐acute (< four hours of onset)/pre‐hospital: two trials (PIL‐FAST 2013; RIGHT 2013).
Hyper‐acute (< six hours)/hospital: two trials (INTERACT pilot 2008; INTERACT‐2 2013).
Acute (< 48 hours): 11 trials (Uzuner 1995; ACCESS 2003; CHHIPS 2009; COSSACS 2010; SCAST 2011; Eveson 2007; Koch 2008; CATIS 2013; ICH‐ADAPT 2013; VENTURE 2013; ENOS 2014).
Sub‐acute (< 168 hours): 10 trials (Lisk 1993; Dyker 1997; Bath 2000; Hillis 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; ACCOST 2006; Willmot 2006; PRoFESS 2009; TAST 2013).
Timing unclear: one trial (Fagan 1988 120 mg/Fagan 1988 240 mg).
Trials variously defined enrolment blood pressure levels.
Hypertension (SBP > 120 to 170 and ≤ 220 mm Hg): 21 trials (Lisk 1993; Dyker 1997; Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; SCAST 2011; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; TAST 2013; VENTURE 2013; ENOS 2014).
Normotension (systolic BP < 140 mmHg): two trials (Hillis 2003; ACCOST 2006).
No BP criteria: three trials (Fagan 1988 120 mg/Fagan 1988 240 mg; Uzuner 1995; COSSACS 2010).
Trials treated participants for varying lengths of time.
For one day: one trial (ICH‐ADAPT 2013).
For up to two days: three trials (Uzuner 1995; Hillis 2003; Koch 2008).
For up to three days: one trial (Lisk 1993).
For seven to 12 days: 13 trials (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; ACCESS 2003; Eames 2005; Willmot 2006; INTERACT pilot 2008; SCAST 2011; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; RIGHT 2013; VENTURE 2013; ENOS 2014).
For 14 days: five trials (Dyker 1997; Eveson 2007; CHHIPS 2009; COSSACS 2010; CATIS 2013).
For 21 days: one trial (Fagan 1988 120 mg/Fagan 1988 240 mg).
For 28 days: one trial (ACCOST 2006).
For three months: one trial (TAST 2013).
For up to 2.5 years: one trial (PRoFESS 2009); outcomes at one to three months are used and longer‐term follow‐up data are ignored.
The trials recruited from one or more centres.
Single centre: 14 trials (Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; Hillis 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; ACCOST 2006; Willmot 2006; Eveson 2007; Koch 2008; PIL‐FAST 2013; RIGHT 2013; TAST 2013).
Multicentre: 12 trials (Fagan 1988 120 mg; Fagan 1988 240 mg; ACCESS 2003; INTERACT pilot 2008; CHHIPS 2009; COSSACS 2010; PRoFESS 2009; SCAST 2011; ICH‐ADAPT 2013; CATIS 2013; INTERACT‐2 2013; VENTURE 2013; ENOS 2014).
A total of 17,011 participants received placebo or control treatment across the studies. Several trials compared two or more active treatment groups (8497 participants) with one control group (8512 participants) (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg).
One study reported on 19 participants from a larger RCT (Fagan 1988 120 mg/Fagan 1988 240 mg); further information on the main study is not available.
One trial was performed in two stages: this review includes the first phase, a double‐blind comparison of candesartan versus placebo (ACCOST 2006), and excludes the second open‐label comparison of candesartan and an ACE‐I.
One study expressly included patients with either ICH, who were given intravenous nimodipine (treatment: eight participants; placebo: three participants), or ischaemic stroke, who were given oral nimodipine (treatment: 38 participants; placebo: 39 participants) (Uzuner 1995); 10 participants (treatment: two participants; placebo: eight participants) treated with antihypertensive agents for malignant hypertension, and two participants treated with intravenous nimodipine for subarachnoid haemorrhage were excluded.
Data from two trials were only available from published abstracts (ACCOST 2006; VENTURE 2013).
Blood pressure measurements
Sixteen studies reported the method by which blood pressure was measured, including equipment (manufacturer, model) and patient posture (Lisk 1993; Dyker 1997; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; RIGHT 2013; TAST 2013; ENOS 2014). The Fagan trial only reported the average blood pressure measurements at, and for one hour after, morning dosing over seven days of treatment (Fagan 1988 120 mg/Fagan 1988 240 mg); in the absence of individual patient data, it is not possible to determine the blood pressure at selected time points during treatment. Furthermore, this trial co‐administered beta blockers to some participants, although these were always given at least two hours before or after nimodipine. In ACCESS 2003, during the first three days blood pressure measurements were performed by nurses as part of routine clinical care; on day seven, automatic 24‐hour blood pressure recording was performed. The other nine trials (Fagan 1988 120 mg/Fagan 1988 240 mg; Uzuner 1995; Hillis 2003; ACCOST 2006; INTERACT pilot 2008; INTERACT‐2 2013; ICH‐ADAPT 2013; PIL‐FAST 2013; VENTURE 2013) made no mention of patient posture or how blood pressure was measured.
Three trials recorded systolic but not diastolic BP after the first intervention: (Koch 2008; ICH‐ADAPT 2013; INTERACT‐2 2013).
Outcomes
The trials reported a variety of outcomes.
mRS or BI or both at ≥ one month: 15 trials (Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; INTERACT‐2 2013; RIGHT 2013; ENOS 2014). Historically, trials dichotomised outcome as death or dependency, defined as mRS > 2, or mRS > 3, or BI < 60. Ordinal analysis of ordered categorical data is statistically more efficient and provides information on severity of outcome (Bath 2012) and recent trials have used ordinal analysis of mRS data (INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; SCAST 2011; CATIS 2013; INTERACT‐2 2013; TAST 2013; ENOS 2014).
Case fatality at ≥ one month: 15 trials (Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; INTERACT‐2 2013; RIGHT 2013; ENOS 2014).
Early neurological impairment (e.g. NIHSS, SSS) at < one month: 11 trials (Lisk 1993; Dyker 1997; Hillis 2003; Eames 2005; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; COSSACS 2010; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013).
Hospital length of stay: four trials (CHHIPS 2009; CATIS 2013; RIGHT 2013; ENOS 2014).
Excluded studies
We excluded 66 studies because they lacked randomisation, were irrelevant to the questions addressed in the current review, or failed to provide blood pressure or outcome assessments (see Characteristics of excluded studies).
Other studies
Eight studies are either awaiting assessment (MAPAS 2009; ATTACI 2010; STABLE‐ICAS 2010; ESH‐CHL‐SHOT 2013) or are ongoing (ATACH‐2 2011; ENCHANTED 2011; SETIN‐HYPERTENSION 2012; FAST‐BP 2013) (Figure 3).
Risk of bias in included studies
Computed tomography was used prior to entry in 10 trials to identify people with ICH (Uzuner 1995; INTERACT pilot 2008; Koch 2008; ICH‐ADAPT 2013; INTERACT‐2 2013) or to exclude people with ICH (Lisk 1993; Dyker 1997; ACCESS 2003; Hillis 2003; Eames 2005). Another study attempted to exclude ICH through information from the history and neurological examination (Fagan 1988 120 mg/Fagan 1988 240 mg); it may therefore have inadvertently included some participants with ICH.
Statistical analysis
Four trials compared more than one treatment against a common control group (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; CHHIPS 2009). The most appropriate analysis in this situation involves dividing the control group participants equally between treatment groups to prevent control participants being counted more than once and thereby artificially narrowing the confidence intervals.
Allocation
We classified allocation concealment as 'low risk', 'high risk' or 'unclear risk' according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Blinding
The method of randomisation was given for 23 trials (Dyker 1997; Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Hillis 2003; Eames 2005; ACCOST 2006; Willmot 2006; Eveson 2007; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; CATIS 2013; RIGHT 2013; TAST 2013; VENTURE 2013; ENOS 2014). Two authors were unable to describe the method of randomisation (Lisk 1993; Uzuner 1995) and one did not respond to our communication (Fagan 1988 120 mg/Fagan 1988 240 mg).
Participants and investigators were blinded to treatment as follows.
Double‐blind (participant and investigator blinded): 13 trials (Fagan 1988 120 mg; Lisk 1993; Dyker 1997; Bath 2000; ACCESS 2003; CHHIPS 2009; Eames 2005; SCAST 2011; ACCOST 2006; Eveson 2007; PRoFESS 2009; PIL‐FAST 2013; TAST 2013).
Single‐blind (participant blinded): four trials (Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; RIGHT 2013; ENOS 2014).
Open‐label: six trials (Rashid 2003 5 mg; Rashid 2003 5/10 mg; Rashid 2003 10 mg; Koch 2008; COSSACS 2010; ICH‐ADAPT 2013; INTERACT‐2 2013; VENTURE 2013).
Incomplete outcome data
Sixteen trials were analysed by intention‐to‐treat (ITT) (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Dyker 1997; Bath 2000; Rashid 2003 5 mg /Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011CATIS 2013; INTERACT‐2 2013; RIGHT 2013; ENOS 2014).
One study excluded 10 participants from the analysis because they had been treated with antihypertensive agents for concurrent accelerated (malignant) hypertension (Uzuner 1995).
Cardiovascular data were analysed on a per‐protocol basis and outcome data by intention‐to‐treat in one trial of lisinopril (Eveson 2007).
Selective reporting
We assessed selective reporting as low risk, high risk or unclear risk according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not see evidence of selective reporting in any of the trials.
Other potential sources of bias
One trial randomised participants before neuroimaging and those having a non‐ischaemic stroke were subsequently withdrawn from the study (Eveson 2007). Two trials did not state the method of analysis (Hillis 2003; Eames 2005). We did not find any other potential risks to the validity of the included studies.
Effects of interventions
The Results section is split into three parts.
Comparisons of BP lowering with control.
Comparisons of continuing versus stopping temporarily pre‐stroke antihypertensive drugs.
Comparisons of BP elevation with control.
Blood pressure lowering
Clinical outcomes
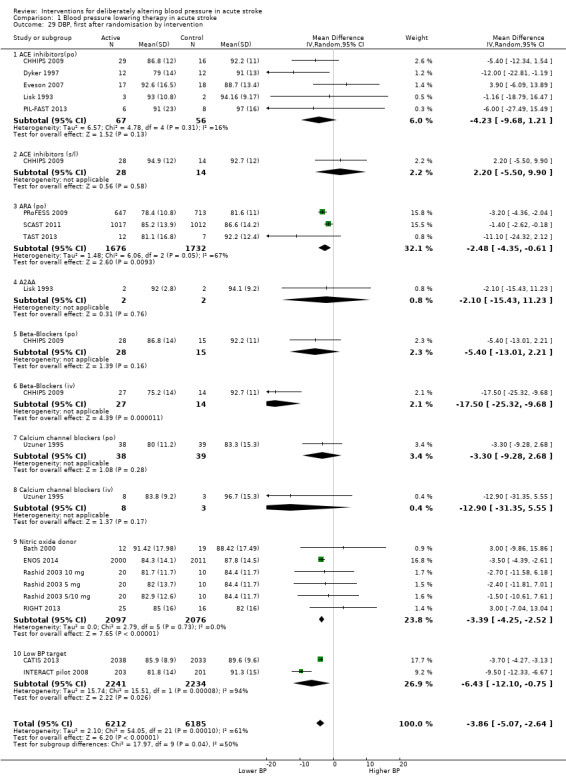
Twenty‐one trials provided data on one or more outcomes relating to treatment with:
ACE‐I (lisinopril): (Eveson 2007; CHHIPS 2009; PIL‐FAST 2013);
ARA (candesartan, telmisartan): (ACCESS 2003; PRoFESS 2009; SCAST 2011; TAST 2013; VENTURE 2013);
ß‐RA (labetalol): (CHHIPS 2009);
CCB (nimodipine): (Uzuner 1995);
NO donor (glyceryl trinitrate): (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; RIGHT 2013; ENOS 2014);
intensive blood pressure lowering: (INTERACT pilot 2008; Koch 2008; CHHIPS 2009; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013);
blood pressure elevation (phenylephrine): (Hillis 2003).
Death or dependency, end of trial
Drug class
Combined death or dependency was assessed using the mRS at the end of follow‐up. Data were available for 15,489 participants recruited into 14 trials (Analysis 1.1). We observed no significant difference between blood pressure lowering and control (OR 0.98; 95% CI 0.92 to 1.05), and heterogeneity was minimal. No individual comparison within drug classes or BP lowering strategies was significant (Analysis 1.1).
1.1. Analysis.
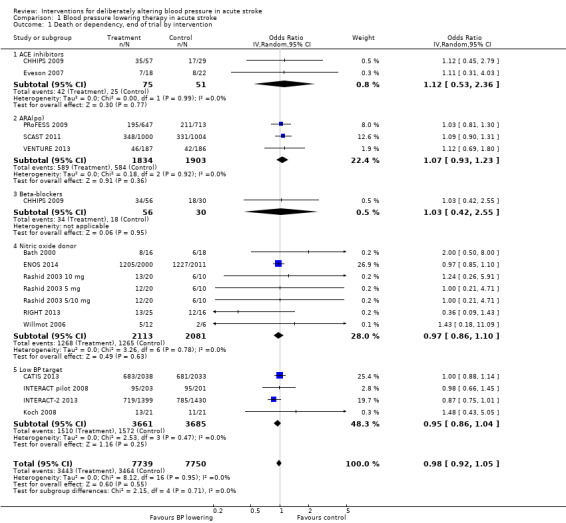
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 1 Death or dependency, end of trial by intervention.
Stroke type
The effect of lowering blood pressure did not vary by stroke subtype (ischaemic stroke, mixed stroke, ICH) across 14 trials (Analysis 1.2).
1.2. Analysis.
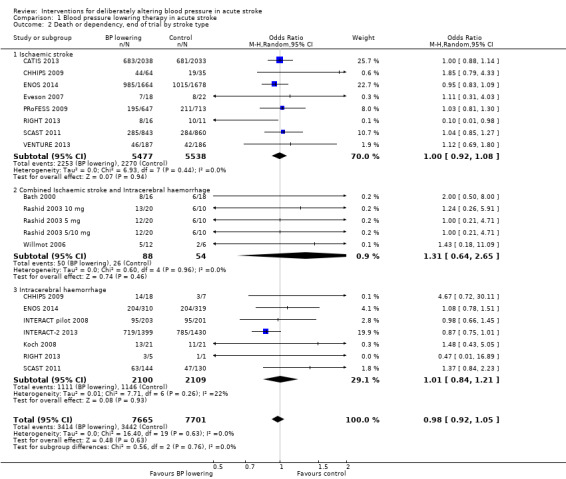
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 2 Death or dependency, end of trial by stroke type.
Stroke location
The effect of lowering blood pressure did not vary by stroke location (ICH deep or not, ischaemic stroke cortical or subcortical) across six trials with 11951 participants (Analysis 1.2). Although there was insufficient evidence to assess heterogeneity, blood pressure lowering in deep ICH almost reached significance (OR 0.86; 95% CI 0.73 to 1.00, P value = 0.06) (Analysis 1.3).
1.3. Analysis.
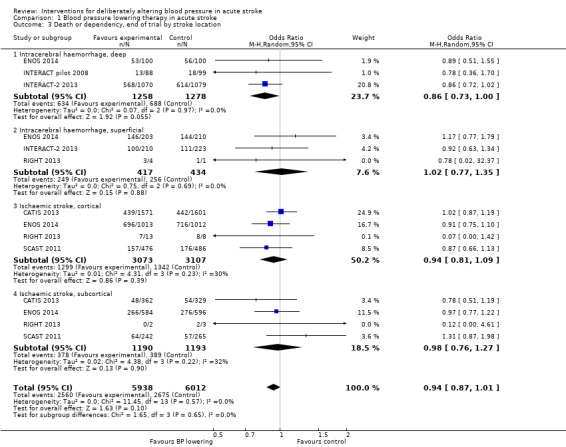
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 3 Death or dependency, end of trial by stroke location.
Time to treatment
Data from 15 trials involving 15,520 participants were available. There was significant reduction in death or dependency if treatment was administered during the hyperacute period and in hospital (OR 0.87; 95% CI 0.76 to 0.99, P value = 0.03). Recruitment of participants later than this was associated with no benefit (Analysis 1.4).
1.4. Analysis.
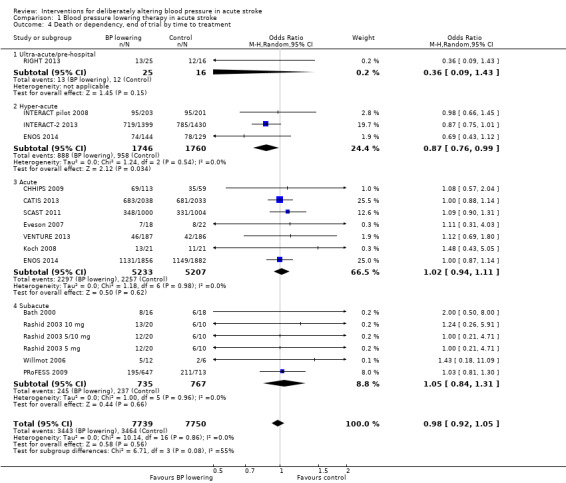
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 4 Death or dependency, end of trial by time to treatment.
Death, early and end of trial
There was no overall effect of treatment on early death or death at end of trial (Analysis 1.5; Analysis 1.8). When considering subgroups, no differences existed when analysed by drug class (Analysis 1.5; Analysis 1.8), stroke type (Analysis 1.6; Analysis 1.9), or time to treatment (Analysis 1.7; Analysis 1.10).
1.5. Analysis.
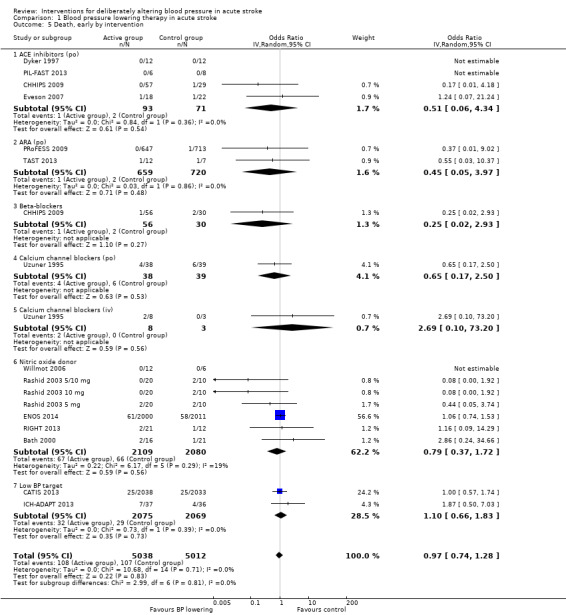
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 5 Death, early by intervention.
1.8. Analysis.
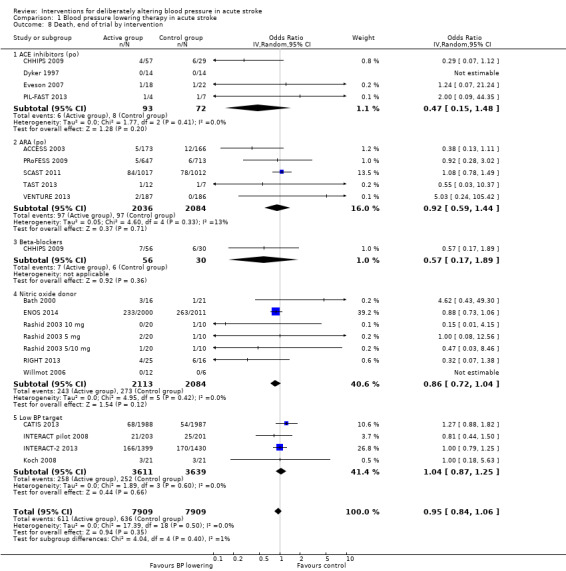
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 8 Death, end of trial by intervention.
1.6. Analysis.
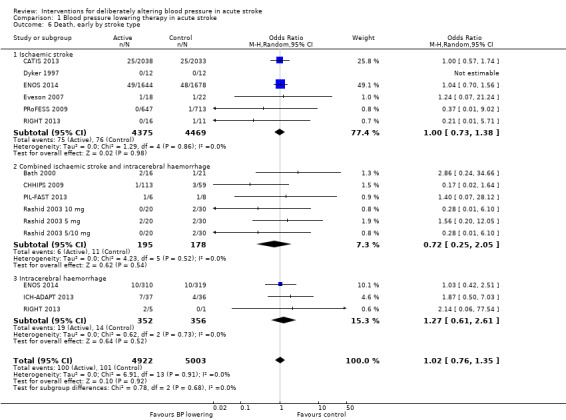
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 6 Death, early by stroke type.
1.9. Analysis.
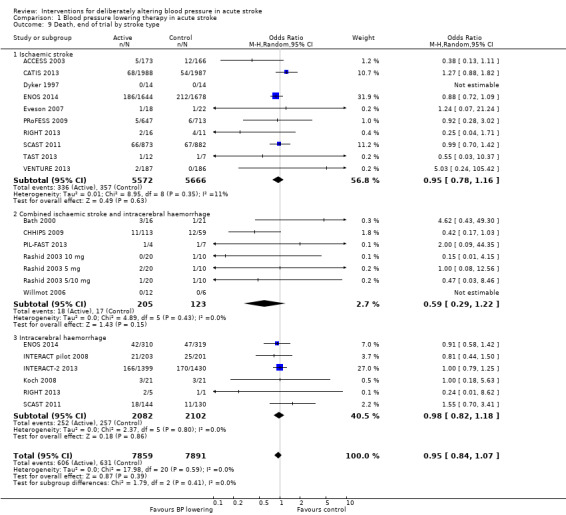
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 9 Death, end of trial by stroke type.
1.7. Analysis.
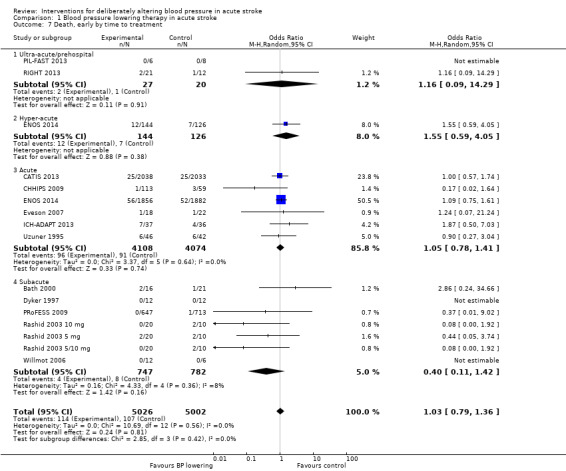
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 7 Death, early by time to treatment.
1.10. Analysis.
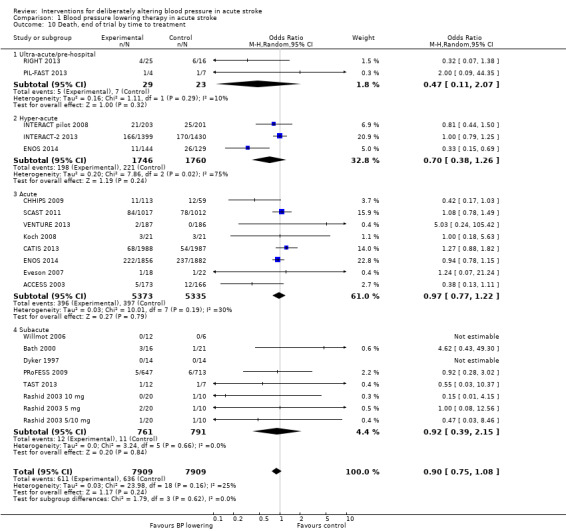
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 10 Death, end of trial by time to treatment.
Barthel Index (disability), end of trial
We assessed the BI at the end of follow‐up in two trials with 4350 participants (Analysis 1.11). Although we observed no significant difference between blood pressure lowering and control (OR 0.63; 95% CI ‐3.28 to ‐4.54) (Analysis 1.11) and did not vary with stroke type (Analysis 1.12), BI scores were lower if treatment was started within six hours of stroke onset (Analysis 1.13).
1.11. Analysis.
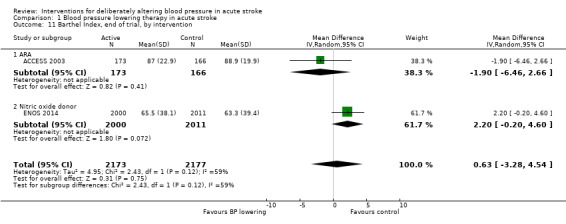
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 11 Barthel Index, end of trial, by intervention.
1.12. Analysis.
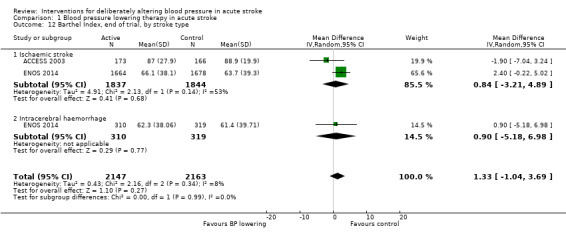
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 12 Barthel Index, end of trial, by stroke type.
1.13. Analysis.
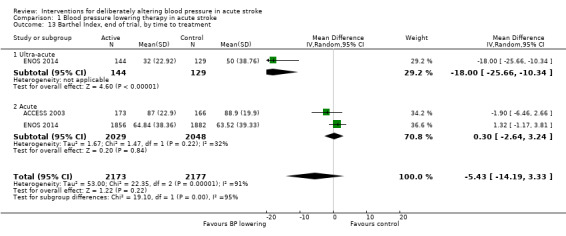
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 13 Barthel Index, end of trial, by time to treatment.
Neurological deterioration, early
There was no overall difference in the rate of early neurological deterioration across seven trials (Analysis 1.14). Subgroup differences were not present when analysed by drug class or intensity (Analysis 1.14), or stroke type (Analysis 1.15). However, subgroup differences were apparent when assessed by time to treatment (Analysis 1.16); specifically, an increase in neurological deterioration was seen in participants with acute stroke (≤ 48 hours post stroke) (OR 1.39; 95% CI 1.07 to 1.81, P value = 0.01) but not when trials specifically treated earlier during the ultra‐acute and hyper‐acute periods.
1.14. Analysis.
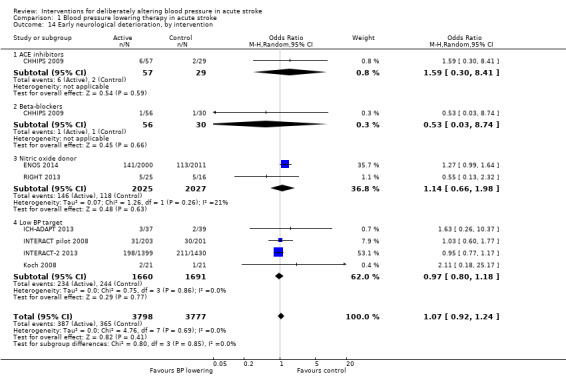
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 14 Early neurological deterioration, by intervention.
1.15. Analysis.
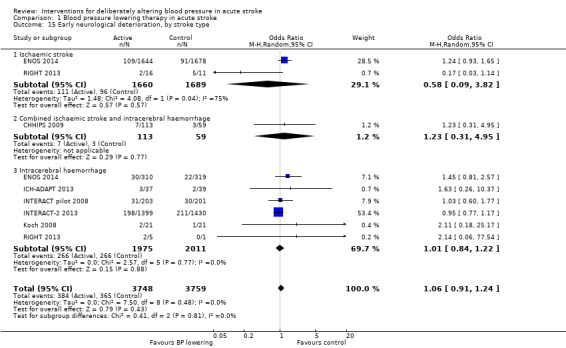
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 15 Early neurological deterioration, by stroke type.
1.16. Analysis.
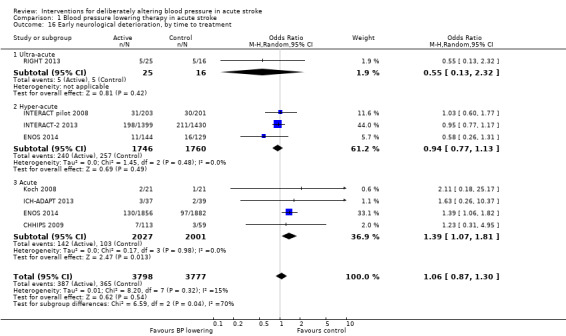
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 16 Early neurological deterioration, by time to treatment.
Quality of life
Quality of life, assessed using the EQ‐5D and transformed into a Health Utility Status, was assessed in three trials (Analysis 1.17; Analysis 1.18). Health Utility scores were higher/better with blood pressure lowering (MD 0.02; 95% CI 0.01 to 0.04). However, heterogeneity was apparent between studies (I2 = 76.0%) with a significant result in INTERACT‐2 2013, but not ENOS 2014. When broken down into stroke types, participants with ICH in INTERACT‐2 2013 treated with blood pressure lowering tended to report a better quality of life (Analysis 1.18). When assessed by time to treatment, Health Utility Status scores were higher in participants treated ≤ six hours (MD 0.06; 95% CI 0.03 to 0.08), but not when treated later (Analysis 1.19).
1.17. Analysis.
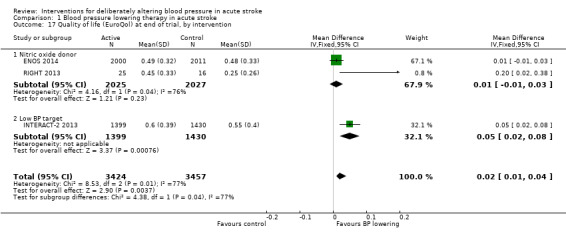
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 17 Quality of life (EuroQol) at end of trial, by intervention.
1.18. Analysis.
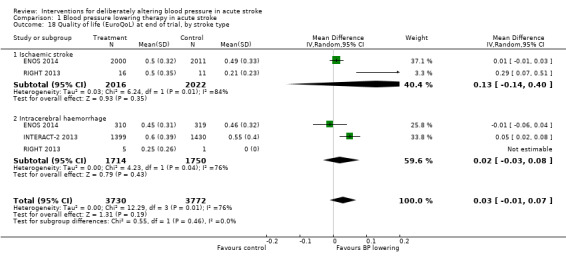
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 18 Quality of life (EuroQoL) at end of trial, by stroke type.
1.19. Analysis.
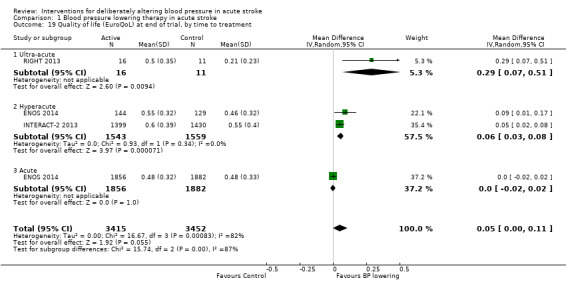
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 19 Quality of life (EuroQoL) at end of trial, by time to treatment.
Length of stay
Length of stay was not influenced by lowering blood pressure and there were no subgroup differences by type of intervention, stroke type or time to treatment (Analysis 1.20; Analysis 1.21; Analysis 1.22).
1.20. Analysis.
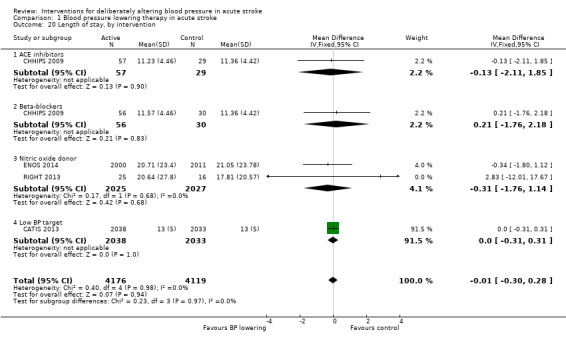
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 20 Length of stay, by intervention.
1.21. Analysis.
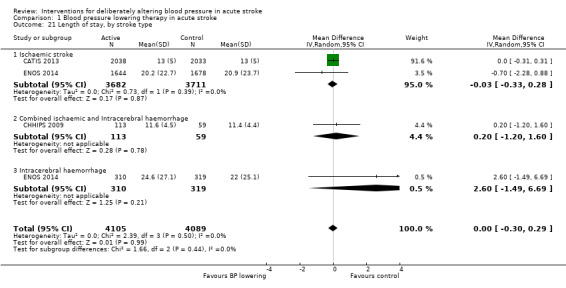
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 21 Length of stay, by stroke type.
1.22. Analysis.
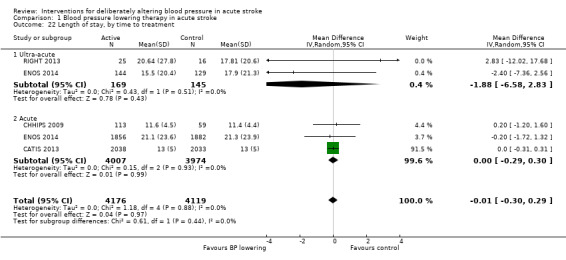
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 22 Length of stay, by time to treatment.
Haemodynamic measures
Blood pressure
The effect of different blood pressure‐lowering strategies on blood pressure after the first dose are summarised in Analysis 1.23 and Analysis 1.29, and in Table 1. Altogether, 24 trials studied 15,432 participants; most participants received an ARA, a NO donor or intensive blood pressure lowering. The focus for the following comments are on systolic rather than diastolic blood pressure. The magnitude of blood pressure reduction varied between ‐4.6/‐2.5 mmHg for oral ARA (primarily candesartan and telmisartan) and ‐13.7/‐7.9 mmHg for ACE‐Is. When assessed by stroke type, heterogeneity was present; slightly larger reductions in blood pressure were seen in participants with ICH (‐11.8/‐5.1 mmHg) with mixed stroke intermediate (‐7.9/‐3.0 mmHg) and ischaemic stroke least (‐7.0/‐3.1 mmHg) (Analysis 1.22; Analysis 1.30). Similarly, the magnitude of reduction varied by time to randomisation or treatment (I2 = 89%); a graded decrease was seen by time with the largest reduction occurring in participants treated during the ultra‐acute/pre‐hospital (‐16.0/‐15.0 mmHg), hyper‐acute/hospital (‐13.4/‐7.5 mmHg), acute (‐8.2/‐2.8 mmHg) and sub‐acute (‐7.3/‐4.9 mmHg) periods.
1.23. Analysis.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 23 SBP, first after randomisation, by intervention.
1.29. Analysis.
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 29 DBP, first after randomisation by intervention.
1. Blood pressure changes after first dose.
| Type | Trials | Participants | SBP | 95% CI | I2 % | DBP | 95% CI | I2% |
| Type of intervention | ||||||||
| ACE‐I, oral | 5 | 123 | ‐13.7 | ‐20.0 to ‐7.3 | 0 | ‐4.2 | ‐9.7 to 1.2 | 16 |
| ACE‐I, sublingual | 1 | 42 | ‐6.0 | ‐17.6 to 5.6 | ‐ | +2.2 | ‐5.5 to 9.9 | ‐ |
| ARA | 3 | 3408 | ‐4.6 | ‐8 to ‐1 | 74 | ‐2.5 | ‐4.3 to ‐0.6 | 67 |
| α‐2 adrenoceptor agonist | 1 | 4 | ‐13.7 | ‐41.5 to 14.1 | ‐ | ‐2.1 | ‐15.4 to 11.2 | ‐ |
| ß‐receptor antagonist, oral | 1 | 44 | ‐11.5 | ‐20.3 to ‐2.7 | ‐ | ‐5.4 | ‐13 to 2.2 | ‐ |
| ß‐receptor antagonist, iv | 1 | 41 | ‐16.4 | ‐27.4 to ‐5.4 | ‐ | ‐17.5 | ‐25.3 to ‐9.7 | ‐ |
| Calcium channel blocker, oral | 3 | 106 | ‐7.6 | ‐17.2 to 1.9 | 0 | ‐3.3 | ‐9.3 to 2.7 | ‐ |
| Calcium channel blocker, iv | 1 | 11 | ‐9.7 | ‐24.4 to 4.9 | ‐ | ‐12.9 | ‐31.4 to 5.6 | ‐ |
| Nitric oxide donor | 5 | 4192 | ‐9.3 | ‐14.5 to ‐4 | 26 | ‐3.6 | ‐4.4 to ‐2.8 | 0 |
| Diuretic, thiazide‐like | 1 | 40 | ‐20.0 | ‐38.6 to ‐1.4 | ‐ | ‐ | ‐ | ‐ |
| Low BP target | 5 | 7421 | ‐11.4 | ‐15.3 to ‐7.5 | 93 | ‐6.9 | ‐15.7 to 1.9 | 94 |
| Overall | 27 | 15432 | ‐11.2 | ‐13.7, ‐8.7 | 86 | ‐3.9 | ‐5.4 to ‐2.6 | 61 |
ACE‐I: angiotensin converting enzyme inhibitors ARA: angiotensin receptor antagonist BP: blood pressure DBP: diastolic blood pressure SBP: systolic blood pressure iv: intravenous
1.30. Analysis.
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 30 DBP, first after randomisation by stroke type.
The effect of the various types of intervention over the first week are summarised in Table 2.
2. Blood pressure changes by type of intervention.
| SBP | First | Day 1 | Day 7 |
| Type of intervention | |||
| ACE‐I, oral | ‐21.0 | ‐7.9 | ‐26 |
| ACE‐I, sublingual | ‐6.0 | ‐12 | ‐ |
| Angiotensin receptor antagonist | ‐4.6 | ‐0.5 | ‐6 |
| Alpha‐2 adrenoceptor agonist | ‐13.7 | ‐21.8 | ‐ |
| Beta‐receptor antagonist, oral | ‐11.5 | ‐14 | ‐ |
| Beta‐receptor antagonist, iv | ‐16.4 | ‐5 | ‐ |
| Calcium channel blocker, oral | ‐7.6 | ‐13.2 | ‐ |
| Calcium channel blocker, iv | ‐9.8 | ‐31.9 | ‐ |
| Nitric oxide donor | ‐9.3 | ‐7 | ‐1 |
| Diuretic, thiazide‐like | ‐20.0 | ‐ | ‐15 |
| Low BP target | ‐11.4 | ‐10 | ‐8 |
| Overall | ‐11.2 | ‐7.7 | ‐7 |
ACE‐I: angiotensin converting enzyme inhibitors BP: blood pressure SBP: systolic blood pressure iv: intravenous
Heart rate
Thirteen trials reported heart rate measurements (Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; PRoFESS 2009; ICH‐ADAPT 2013; RIGHT 2013; TAST 2013; ENOS 2014). The heart rate for glyceryl trinitrate increased at day one (MD 4.5 bpm; 95% CI 2 to 8) (Analysis 1.37) (Table 3). Intravenous CCBs reduced heart rate at day one (Uzuner 1995) (Analysis 1.37).
1.37. Analysis.
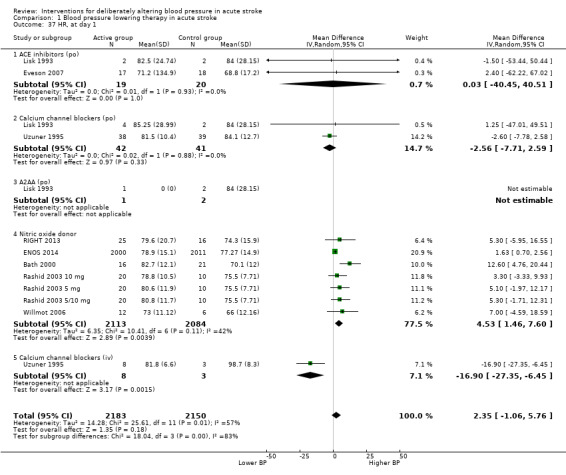
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 37 HR, at day 1.
3. Heart rate changes at day 1.
| Type of intervention | Trials | Participants | Day 1 | 95% CI |
| ACE (oral) | 2 | 39 | 0.03 | ‐40.4 to 40.5 |
| Alpha‐2 adrenoceptor agonist | 1 | 3 | ‐ | ‐ |
| Calcium channel blocker, oral | 2 | 83 | ‐2.6 | ‐7.7 to 2.6 |
| Calcium channel blocker, iv | 1 | 11 | ‐16.9 | ‐27.4 to ‐6.5 |
| Nitric oxide donor | 5 | 4197 | 4.5 | 1.5 to 7.6 |
ACE: angiotensin converting enzyme iv: intravenous
Continuing versus stopping pre‐stroke antihypertensive drugs
Two trials tested whether pre‐stroke antihypertensives should be continued in the immediate post‐stroke period, or stopped temporarily (COSSACS 2010; ENOS 2014). The total number of participants numbered 2860.
Clinical outcomes
Death or dependency, end of trial
There was no significant difference in mRS at day 90 between those participants assigned to continue or stop antihypertensives (OR 1.06; 95% CI 0.91 to 1.24) (Analysis 2.1). Within subgroups, the effect of continuing versus stopping antihypertensives did not differ by stroke types or time to treatment across both trials (Analysis 2.2 and Analysis 2.3).
2.1. Analysis.
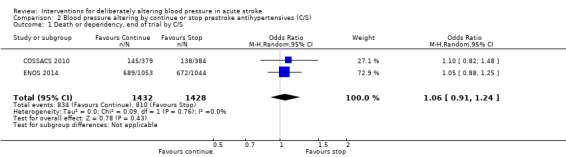
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 1 Death or dependency, end of trial by C/S.
2.2. Analysis.
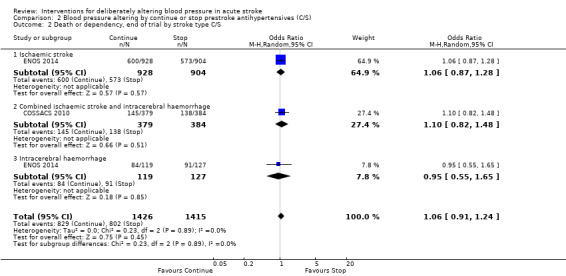
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 2 Death or dependency, end of trial by stroke type C/S.
2.3. Analysis.
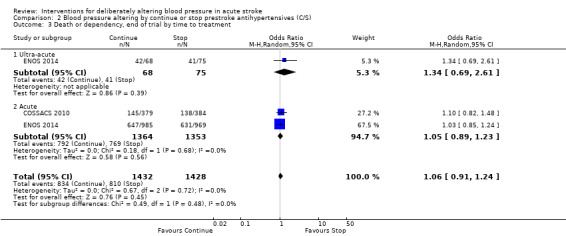
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 3 Death or dependency, end of trial by time to treatment.
Death, early and at the end of trial
The rates of death at the end of treatment, and at the end of trial, did not differ between the treatment groups (Analysis 2.4 and Analysis 2.7). No significant differences were observed by stroke types or time to treatment (Analysis 2.5; Analysis 2.6; Analysis 2.8; Analysis 2.9).
2.4. Analysis.
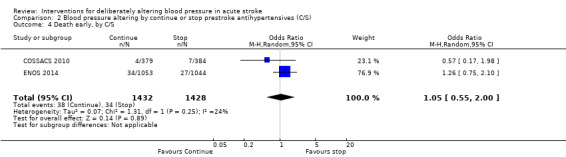
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 4 Death early, by C/S.
2.7. Analysis.
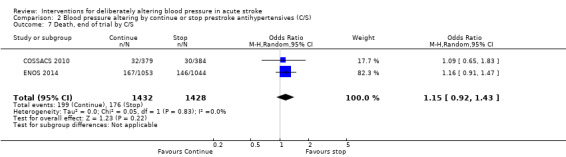
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 7 Death, end of trial by C/S.
2.5. Analysis.
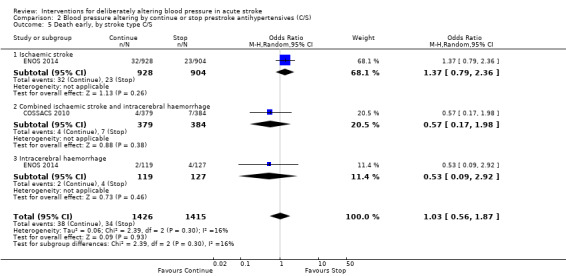
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 5 Death early, by stroke type C/S.
2.6. Analysis.
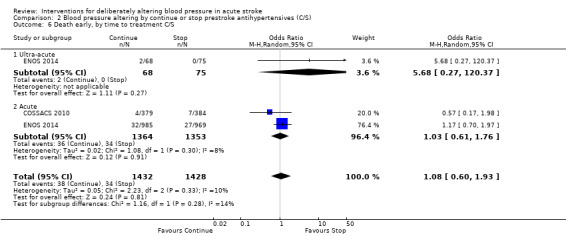
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 6 Death early, by time to treatment C/S.
2.8. Analysis.
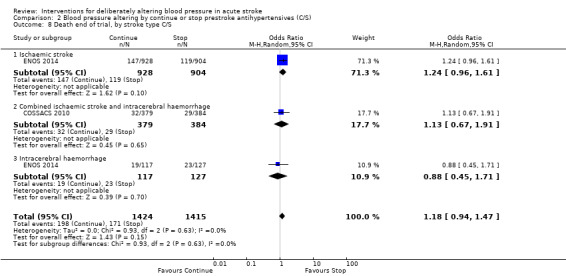
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 8 Death end of trial, by stroke type C/S.
2.9. Analysis.
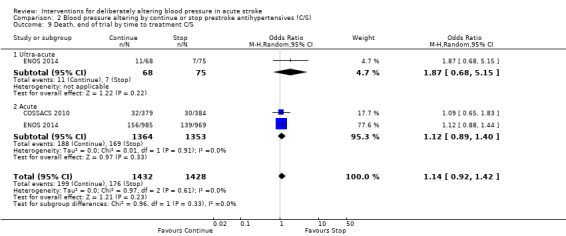
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 9 Death, end of trial by time to treatment C/S.
Barthel Index
Barthel Index scores were lower in participants assigned to continue treatment (MD ‐3.18; 95% CI ‐0.55 to ‐5.80, P value = 0.02) (Analysis 2.10).
2.10. Analysis.
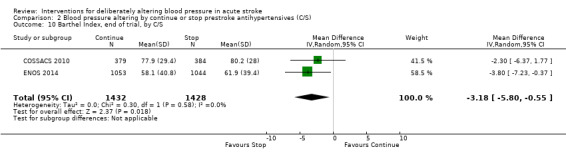
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 10 Barthel Index, end of trial, by C/S.
Neurological deterioration, early
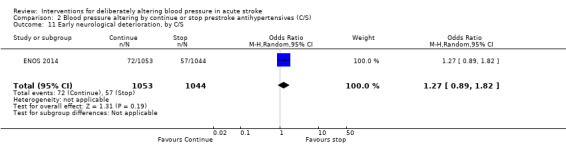
The rate of death at the end of treatment did not differ between the treatment groups (Analysis 2.11).
2.11. Analysis.
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 11 Early neurological deterioration, by C/S.
Quality of life
Quality of life, assessed using the EQ‐5D and transformed into a Health Utility Status, was lower (i.e. worse) in participants randomised to continue pre‐stroke antihypertensive drugs (MD ‐0.03; 95% CI ‐0.05 to ‐0.01, P value = 0.008) (Analysis 2.12).
2.12. Analysis.
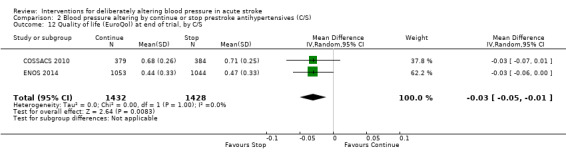
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 12 Quality of life (EuroQol) at end of trial, by C/S.
Haemodynamic measures
Blood pressure
Blood pressure was lower by ‐7.9/‐1.2 mmHg at the first measurement after randomisation in participants randomised to continue treatment with the reduction in systolic BP much greater in COSSACS 2010 than in ENOS 2014 (Analysis 2.14). By the end of treatment, blood pressure was lower by ‐11.3/‐6.4 mmHg in the continue group.
2.14. Analysis.
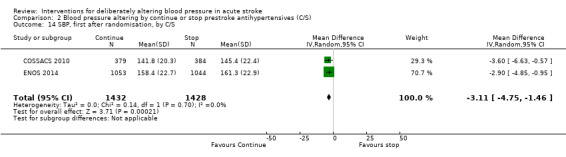
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 14 SBP, first after randomisation, by C/S.
Heart rate
Data were only available for ENOS 2014. Heart rate was significantly lower by 3.2 bpm by end of treatment in those who were randomised to continue treatment (Analysis 2.21).
2.21. Analysis.
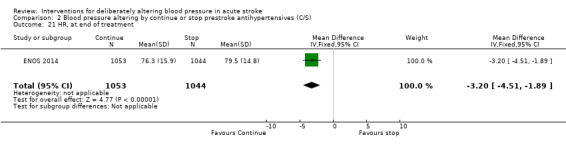
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 21 HR, at end of treatment.
Blood pressure elevation therapy in acute stroke
Phenylephrine
Phenylephrine non‐significantly increased systolic blood pressure at 24 hours (MD 21 mmHg; 95% CI ‐13 to 55 mmHg), but had no significant effect on diastolic blood pressure (Hillis 2003) (Analysis 3.4). Insufficient data were available on clinical outcomes.
3.4. Analysis.
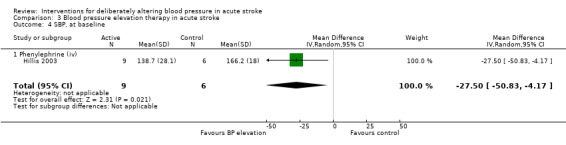
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 4 SBP, at baseline.
Discussion
Twenty‐six trials, involving 17,011 participants with stroke, assessed the effects of deliberate blood pressure alteration.
Blood pressure lowering
The results come from 24 trials that studied 15,432 participants.
Clinical outcomes
Overall, lowering blood pressure did not improve outcome, whether assessed as death, combined death or dependency, neurological deterioration or quality of life. These findings were maintained irrespective of type of intervention (drug class, intensity of lowering) or type of stroke. However, when assessed by time to treatment, very early blood pressure lowering (before hospital presentation or within six hours of stroke onset) was associated with reduced death or dependency, and improved quality of life (INTERACT‐2 2013; RIGHT 2013).
Haemodynamic effects
All the studied antihypertensive drug classes lowered blood pressure during the period of treatment. Reductions in blood pressure after the first treatment varied between ‐4.6/‐2.5 mmHg for oral ARA and ‐21.0/‐7.9 mmHg for ACE‐I. Slightly larger reductions in blood pressure were seen in participants with ICH (‐11.8/‐5.1 mmHg) than in those with ischaemic stroke (‐7.0/‐3.1 mmHg). The largest reductions were seen if treatment was started very early before hospital presentation (‐16.0/‐15.0 mmHg); smaller reductions occurred if treatment was started beyond 48 hours after stroke onset (‐7.3/‐4.9 mmHg).
Discussion
A variety of hypotheses can be postulated for why functional outcome was better if blood pressure lowering was started very early after stroke. First, the magnitude of blood pressure lowering may be important since the greatest improvement in outcome occurred when treatment was started early. Second, the type of intervention may be important since improved outcome was seen with early intensive blood pressure lowering (INTERACT‐2 2013), and early nitrate administration (RIGHT 2013; ENOS 2014). Conversely, apparent hazard was seen with ARA drugs (SCAST 2011; Jusufovic 2014).
Perhaps surprisingly, stroke type may not be particularly relevant since differential effects on outcome were not seen for ischaemic stroke versus ICH.
In summary, very early treatment with an appropriate agent or target blood pressure may be the most important strategy to test in the future, irrespective of stroke type. Importantly, systolic blood pressure should not be lowered excessively (> 20%), at least in ischaemic stroke, since trials of intravenous CCBs found that these worsened outcome (Bridgers 1991; Wahlgren 1994).
Continue versus stop pre‐stroke antihypertensive drugs
The results come from two trials that studied 2860 participants (COSSACS 2010; ENOS 2014).
Clinical outcomes
The findings were mixed with some comparisons, in particular dependency (mRS), death, and neurological deterioration, neutral for the comparison of continue versus stop pre‐stroke antihypertensive drugs. In contrast, measures of disability (BI) and quality of life (EQ‐5D, transformed into a Health Utility Status) were worse in participants randomised to continue treatment immediately.
Haemodynamic effects
Immediately continuing antihypertensive drugs taken before stroke was associated with a lower blood pressure by ‐7.9/‐1.2 mmHg at the first measurement after randomisation, and ‐11.3/‐6.4 mmHg by end‐of‐treatment.
Discussion
The discrepancy in findings for two measures of functional outcome, mRS and BI, is challenging to explain. First, it may represent chance such that no difference exists between the interventions. Second, it could reflect outcome bias since it is not possible to test this question in a double‐blind placebo‐controlled design. Nevertheless, both trials used blinded outcome assessment for both mRS and BI. Further, since a majority of stroke physicians tend to continue treatment in routine practice (Bath 2000b), the result seen across the two results is counter‐intuitive. Last, the difference may be real in which case mRS, usually considered to be the optimal functional outcome in stroke trials (Lees 2012), failed to detect a difference in contrast to a comparison of BI scores.
If continuing drugs immediately is, indeed, hazardous, then the two trials do not identify the cause. Drugs that attenuate stress hormones, in particular that down‐regulate the renin‐angiotensin‐aldosterone‐system (RAAS) were commonly taken before stroke, e.g. ACE‐I, ARA and ß‐receptor antagonists. Initiating these drugs in the acute phase of stroke has been associated with harm (BEST 1988; SCAST 2011), so it can be postulated that continuing these during the acute phase of stroke is potentially harmful. Alternatively, continuing drugs in people who are dysphagic and who do not have safe enteral access for feeding may be hazardous through aspiration of these drugs and then the development of pneumonia. The ENOS trial gives some support for this hypothesis (ENOS 2014).
The main implication for clinicians is that it is reasonable to withhold BP‐lowering drugs until patients are medically and neurologically stable, and have suitable oral or enteral access, after which drugs can then be reintroduced.
General
An important problem with some of the trials was the absence of detailed information on how blood pressure was measured. Hence, the quality of blood pressure readings is unknown. It is essential that future trials describe in detail how blood pressure is measured by including the following information.
Equipment: manufacturer, model, measurement method (mercury, anaeroid or oscillometry, and manual or automatic), and whether the equipment has been independently validated, and if so by whom.
Measurer: who measured blood pressure, and how they were trained, assessed, re‐trained and re‐assessed.
Measurements: the number of readings at each time point, site of measurement (brachial, finger, etc) and what position the person was in (supine, sitting, standing).
Little is known about the effect of blood pressure altering in older people with acute stroke, who comprise the largest group of patients, including those with ischaemic stroke who need thrombolysis. Of the trials, only two had mean age over 75 years contributing to a total of 98 participants (CHHIPS 2009; RIGHT 2013). The number and proportion of older people is likely to increase with population ageing. As the variation in response by individuals to blood pressure modulating agents increases with age, e.g. related to concurrent isolated systolic hypertension or cardiac dysfunction, it may be inappropriate to assume that the effects of changing blood pressure seen in younger populations will necessarily be the same in older ones.
This review is Part 1 of the Blood pressure in Acute Stroke Collaboration and reports only those trials that specifically set out to alter blood pressure in people with acute stroke. Part 2 of the project assesses all RCTs in acute stroke where vasoactive drugs were administered and includes all those studies covered in Part 1. Although progress has been made in the number and quality of stroke trials in the last few years, a substantial number of questions remain. The number of participants included in this review is very small in comparison to the global burden of stroke (about 15,000,000 per year worldwide). At present, any benefit of treatment is small, and additional data are required to recommend changes to routine clinical practice. The centres that took part in the trials were interested and familiar with the management of acute stroke. To extrapolate these results in routine clinical practice to less specialist centres could result in greater hazard or completely negate any potential benefit. Therefore, there is a need for new centres to participate in trials. Further evidence is needed on:
how to select participants;
the influence of age, time of onset, stroke subtype, severity, choice of drug, dose, route of administration and blood pressure variability, on response to active changes in blood pressure.
Recent guidelines based on the non‐systematic analysis of (largely) observational data recommend that hypertension should not be treated for up to two weeks after an ischaemic stroke unless severe hypertension, hypertensive encephalopathy, heart failure, cardiac ischaemia, aortic dissection, or continued intracerebral bleeding are present (O'Connell 1994; EUSI 2004; AHA‐HS 2010; AHA‐IS 2013). Persistent hypertension after two weeks should be treated since the risk of stroke in people with cerebrovascular disease is dependent on systolic and diastolic blood pressure (Rodgers 1996). In PROGRESS 2001, perindopril (with or without indapamide) reduced the risk of stroke among both hypertensive and non‐hypertensive participants with a history of stroke or transient ischaemic attack. Further, evidence from the Heart Outcomes Prevention Evaluation (HOPE) trial suggests that an ACE‐I may reduce stroke and other vascular events in people with prior cerebrovascular disease (HOPE 2000). Overall, lowering blood pressure in people with chronic stroke reduces the subsequent risk of recurrence (Rashid 2003).
Summary of main results
In this updated review, blood pressure lowering did not improve functional outcome. However, early initiation of treatment might be beneficial, and further studies are required to test this specific question. Immediately continuing pre‐stroke antihypertensive drugs appears to be associated with a worse functional outcome and lower quality of life; hence, it is reasonable to delay treatment until patients are stable and have oral or enteral access to allow safe administration of drugs.
Overall completeness and applicability of evidence
The included trials are an excellent start to answering the question of optimal blood pressure management in acute stroke. The lack of a definitive result based on data from more than 16,000 participants confirms that the question is complex and future trials need to refine trial design, especially focusing on very early treatment.
Quality of the evidence
The quality of the included studies was variable. Methods of randomisation and allocation concealment were not always clear. Not all trials contributed to each outcome. Details of blood pressure recording including equipment, number of readings and patient positioning were not provided in some studies. Trials in the last decade were more standardised compared with earlier ones when reporting baseline stroke characteristics, primary and secondary outcomes. Outcome assessment was blinded in recent large RCTs and clearly reported in trial protocols. Trials were largely not representative of unselected stroke populations around the world ‐ participants tended to be younger, have fewer comorbidities and be conscious, so that poor outcomes were less common than might be expected.
Potential biases in the review process
This review follows an extensive literature search by both the Cochrane Stroke Review Group, and the authors, and without any language restrictions. Hence the risk of study inclusion bias is low.
Agreements and disagreements with other studies or reviews
The limited data mean that we can draw no firm conclusions, as shown in other reviews.
Authors' conclusions
Implications for practice.
The lack of definitive results for blood pressure lowering, and very limited data for raising blood pressure, mean that no firm recommendations can be made.
There is no evidence to support the routine policy of immediately continuing prestroke antihypertensive drugs; treatment may be re‐started once patients have stabilised medically and neurologically, and once safe feeding or enteral access is available.
The very limited data related to blood pressure elevation mean that no recommendations can be made.
Implications for research.
Large randomised controlled trials (RCTs) of blood pressure lowering are needed to:
test the effect of ultra‐acute/pre‐hospital lowering of blood pressure in RCTs;
test the effect of hyper‐acute/hospital lowering of blood pressure in RCTs. Two trials are important examples of ongoing studies (ATACH‐2 2011; ENCHANTED 2011);
determine the effects on long‐term survival (≥ one year);
determine the effects on quality of life and cost‐effectiveness.
An individual patient data (IPD) meta‐analysis is required to:
identify subgroups of patients who are likely to benefit or be harmed, e.g. by age, sex, race‐ethnicity group, baseline blood pressure, history of hypertension, stroke type (ischaemic stroke, ICH);
identify what type of treatment is required, e.g. drug class, route, dose of administration.
The ongoing 'Blood pressure in Acute Stroke Collaboration' is performing an IPD meta‐analysis and includes many of the quoted trials.
Feedback
'BEST' Trial
Summary
The 'Beta‐blockade in acute stroke trial (BEST)' done in Nottingham in the 1980s (which compared atenolol, propranolol and placebo) is not included in this review. Although lowering blood pressure was not the major aim of the trial, lower blood pressures did occur in the intervention group. Should the study not be included in the review, or be explicitly excluded?
Reference: Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JR. Low dose beta blockade in acute stroke ("BEST" trial): an evaluation. Br Med J 1988;296:737‐741.
Reply
The BEST trial was not included since the trial's primary aim was not about altering blood pressure, but rather assessing the effect of beta blockers in acute stroke. However, BEST is included in our related review published in the Cochrane Database of Systematic Reviews: Vasoactive drugs for acute stroke. We have drawn this distinction since there are methodological differences between studies which aim to alter blood pressure, and those which may, or not, have measured blood pressure as part of their protocol.
Contributors
Shah Ebrahim
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2014 | New search has been performed | The searches have been updated to May 2014. We have added 14 new trials (including 15,858 participants). This brings the total number of included studies to 26, involving 17,011 participants. We have added new subgroup analyses. |
| 8 July 2014 | New citation required but conclusions have not changed | Change in authorship. Whilst substantially more information is now available compared with the previous version, the core questions remain unanswered so the basic conclusion that more data are needed remains unchanged. |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 24 October 2008 | New search has been performed | Contact details updated. |
| 1 April 2008 | New search has been performed | Review was updated:
(1) Addition of seven completed trials (ACCESS 2003; Hillis 2003; Rashid 2003; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008) involving 943 patients;
(2) Addition of nine ongoing or planned trials (COSSACS 2002; CHIPPS 2005; SCAST 2005; ACCOST 2006; ATACH 2006; TAST 2006; Geeganage 2007; Intracerebral haemorrhage ADAPT‐E 2007; INTERACT2 2008). The previous version of the review included five trials involving 210 patients. The conclusions of this review have not changed with the addition of the new data. Change of authors. |
| 25 March 2008 | Amended | Converted to new review format. |
Acknowledgements
Data collation and analysis, and review writing for this version of the review: K Krishnan, PMW Bath.
Trialists:
DR Lisk, JC Grotta: (summary and individual data) (Lisk 1993).
N Uzuner: (summary and individual data) (Uzuner 1995).
AG Dyker, K Lees: (summary and individual patient data) (Dyker 1997).
PMW Bath: (summary and individual patient data) (Bath 2000; Rashid 2003 10 mg; Rashid 2003 5/10 mg; Rashid 2003 5 mg; Willmot 2006; RIGHT 2013; TAST 2013; ENOS 2014).
AB Hillis: (summary data) (Hillis 2003).
D Eveson: (summary data) (Eveson 2007).
FJ Bath‐Hextall was a co‐author involved with the following aspects of the first version of the review: design, development of search strategies, carrying out searches, input of data, analysis, and writing.
The late C Geeganage was a co‐author involved with the following aspects of the second version of the review: development of search strategies, carrying out searches, input of data, analysis, and writing.
We are grateful to the Editorial Board of the Cochrane Stroke Group and external peer reviewers for commenting on this review. All the analyses and their interpretation reflect the opinions of the authors; no pharmaceutical company was involved in this review.
Appendices
Appendix 1. MEDLINE search strategy
1. blood pressure.tw. 2. hypertension.tw 3. acute/ 4. stroke.tw. 5. or/1‐4 6. and/1‐4 7. 1‐4.kf. 8.1‐4.ti. 9. trials 10. 1‐4 and 9 11. 1‐4 and 9.ti. 12. ischaemic stroke.tw/ti. 13. haemorrhagic stroke.tw/ti. 14. intracerebral haemorrhage.tw./ti. 15. blood pressure lowering/ 16. blood pressure increase/ 17. 1‐4 or 12 18. 1‐4 or 13 19. 1‐4 or 14 20. 1‐4 and 15 21.1‐4 and 16 22. cerebr 23. 1‐4 or 22 24. vasoactive/ 25. 12‐16 or 24 26. nitrate.tw. 27. glyceryl trinitrate/GTN.tw 28. nitric Oxide Donors.tw. 29. 1‐4 and/or 26‐28 30. 9, 12‐13, 16 and/or 26‐28 31. thiazide.tw. 32. bendrofluazide.tw. 33. bendroflumethiazide.tw. 34. hydrochrlothiazide/HCT.tw. 35. 31‐34 and/or 1‐4 36. 31‐34 and/or 12‐15 37. beta blockers.tw 38. atenolol.tw. 39. propanalol.tw. 40. 37‐39 and/or 1‐4 41. 37‐39 and or 12‐15 42. calcium channel blockers.tw. 43. nimodipine.tw. 44. nicardipine.tw. 45. amilodipine.tw. 46. felodipine.tw. 47. isradipine.tw. 48. nifedipine.tw. 49. nisolodipine.tw. 50. 42‐49 and or 1‐4 51. 42‐49 and or 12‐15 52. angiotensin‐converting enzyme inhibitors/ACE inhibitors.tw 53. captopril.tw. 54. enalapril.tw. 55. lisinopril.tw. 56. perindopril.tw. 57. ramipril.tw. 58. 52‐57 and or 1‐4 59. 52‐57 and or 12‐15 60. angiotensin receptor blockers/antagonists.tw. 61. candesartan.tw 62. losartan.tw. 63. telmisartan.tw 64. valsartan.tw. 65. clonidine.tw. 65. 60‐65 and or 1‐4 66. 60‐65 and or 12‐15 67. vasoconstrictors.tw. 68. dopamine.tw. 69. dobutamine.tw. 70. noradrenaline.tw. 71. phenylephrine.tw. 72. 67‐71 and or 3, 4, 9, 16 73. cerebral blood flow 74. autoregulation 75. stroke outcome 76. 73‐75 and or 1‐4 77. 73‐75 and or 12‐16
Appendix 2. EMBASE search strategy
1. blood pressure.tw. 2. hypertension.tw 3. acute/ 4. stroke.tw. 5. or/1‐4 6. and/1‐4 7. 1‐4.kf. 8.1‐4.ti. 9. trials 10. 1‐4 and 9 11. 1‐4 and 9.ti. 12. ischaemic stroke.tw/ti. 13. haemorrhagic stroke.tw/ti. 14. intracerebral haemorrhage.tw./ti. 15. blood pressure lowering/ 16. blood pressure increase/ 17. 1‐4 or 12 18. 1‐4 or 13 19. 1‐4 or 14 20. 1‐4 and 15 21.1‐4 and 16 22. cerebr 23. 1‐4 or 22 24. vasoactive/ 25. 12‐16 or 24 26. nitrate.tw. 27. glyceryl trinitrate/GTN.tw 28. nitric Oxide Donors.tw. 29. 1‐4 and/or 26‐28 30. 9, 12‐13, 16 and/or 26‐28 31. thiazide.tw. 32. bendrofluazide.tw. 33. bendroflumethiazide.tw. 34. hydrochrlothiazide/HCT.tw. 35. 31‐34 and/or 1‐4 36. 31‐34 and/or 12‐15 37. beta blockers.tw 38. atenolol.tw. 39. propanalol.tw. 40. 37‐39 and/or 1‐4 41. 37‐39 and or 12‐15 42. calcium channel blockers.tw. 43. nimodipine.tw. 44. nicardipine.tw. 45. amilodipine.tw. 46. felodipine.tw. 47. isradipine.tw. 48. nifedipine.tw. 49. nisolodipine.tw. 50. 42‐49 and or 1‐4 51. 42‐49 and or 12‐15 52. angiotensin‐converting enzyme inhibitors/ACE inhibitors.tw 53. captopril.tw. 54. enalapril.tw. 55. lisinopril.tw. 56. perindopril.tw. 57. ramipril.tw. 58. 52‐57 and or 1‐4 59. 52‐57 and or 12‐15 60. angiotensin receptor blockers/antagonists.tw. 61. candesartan.tw 62. losartan.tw. 63. telmisartan.tw 64. valsartan.tw. 65. clonidine.tw. 65. 60‐65 and or 1‐4 66. 60‐65 and or 12‐15 67. vasoconstrictors.tw. 68. dopamine.tw. 69. dobutamine.tw. 70. noradrenaline.tw. 71. phenylephrine.tw. 72. 67‐71 and or 3, 4, 9, 16 73. cerebral blood flow 74. autoregulation 75. stroke outcome 76. 73‐75 and or 1‐4 77. 73‐75 and or 12‐16
Appendix 3. Science Citation Index search strategy
1. blood pressure.TI. 2. hypertension.TI 3. acute/ 4. stroke.TS. 5. OR/1‐4 6. AND/1‐4 7. 1‐4.TI. 8.1‐4.TI. 9. trials 10. 1‐4 AND 9 11. 1‐4 AND 9.ti. 12. ischaemic stroke.TI/TS. 13. haemorrhagic stroke.TI/TS. 14. intracerebral haemorrhage.TI./TS. 15. blood pressure lowering/ 16. blood pressure increase/ 17. 1‐4 OR 12 18. 1‐4 OR 13 19. 1‐4 OR 14 20. 1‐4 AND 15 21.1‐4 AND 16 22. cerebr 23. 1‐4 OR 22 24. vasoactive/ 25. 12‐16 OR 24 26. nitrate.TI./TS 27. glyceryl trinitrate/GTN.TI/TS 28. nitric Oxide Donors.TI./TS 29. 1‐4 AND/OR 26‐28 30. 9, 12‐13, 16 AND/OR 26‐28 31. thiazide.TI. 32. bendrofluazide.TI. 33. bendroflumethiazide.TI. 34. hydrochrlothiazide/HCT.TI. 35. 31‐34 AND/OR 1‐4 36. 31‐34 AND/OR 12‐15 37. beta blockers.TI 38. atenolol.TI. 39. propanalol.TI. 40. 37‐39 AND/OR 1‐4 41. 37‐39 AND/OR 12‐15 42. calcium channel blockers.TI. 43. nimodipine.TI. 44. nicardipine.TI. 45. amilodipine.TI. 46. felodipine.TI. 47. isradipine.TI. 48. nifedipine.TI. 49. nisolodipine.TI. 50. 42‐49 AND/OR 1‐4 51. 42‐49 AND/OR 12‐15 52. angiotensin‐converting enzyme inhibitors/ACE inhibitors.TI 53. captopril.TI. 54. enalapril.TI. 55. lisinopril.TI. 56. perindopril.TI. 57. ramipril.TI. 58. 52‐57 AND/ OR 1‐4 59. 52‐57 AND/OR 12‐15 60. angiotensin receptor blockers/antagonists.TI. 61. candesartan.TI. 62. losartan.TI. 63. telmisartan.TI. 64. valsartan.TI. 65. clonidine.TI. 65. 60‐65 AND/OR 1‐4 66. 60‐65 AND/OR 12‐15 67. vasoconstrictors.TI. 68. dopamine.TI. 69. dobutamine.TI. 70. noradrenaline.TI. 71. phenylephrine.TI. 72. 67‐71 AND/OR 3, 4, 9, 16 73. cerebral blood flow 74. autoregulation 75. stroke outcome 76. 73‐75 AND/OR 1‐4 77. 73‐75 AND/OR 12‐16
Data and analyses
Comparison 1. Blood pressure lowering therapy in acute stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency, end of trial by intervention | 16 | 15489 | Odds Ratio (IV, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 1.1 ACE inhibitors | 2 | 126 | Odds Ratio (IV, Random, 95% CI) | 1.12 [0.53, 2.36] |
| 1.2 ARA(po) | 3 | 3737 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.93, 1.23] |
| 1.3 Beta‐blockers | 1 | 86 | Odds Ratio (IV, Random, 95% CI) | 1.03 [0.42, 2.55] |
| 1.4 Nitric oxide donor | 7 | 4194 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 1.5 Low BP target | 4 | 7346 | Odds Ratio (IV, Random, 95% CI) | 0.95 [0.86, 1.04] |
| 2 Death or dependency, end of trial by stroke type | 16 | 15366 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 2.1 Ischaemic stroke | 8 | 11015 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.92, 1.08] |
| 2.2 Combined Ischaemic stroke and Intracerebral haemorrhage | 5 | 142 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.64, 2.65] |
| 2.3 Intracerebral haemorrhage | 7 | 4209 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.84, 1.21] |
| 3 Death or dependency, end of trial by stroke location | 6 | 11950 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.87, 1.01] |
| 3.1 Intracerebral haemorrhage, deep | 3 | 2536 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.00] |
| 3.2 Intracerebral haemorrhage, superficial | 3 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.77, 1.35] |
| 3.3 Ischaemic stroke, cortical | 4 | 6180 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.09] |
| 3.4 Ischaemic stroke, subcortical | 4 | 2383 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.27] |
| 4 Death or dependency, end of trial by time to treatment | 16 | 15489 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.1 Ultra‐acute/pre‐hospital | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.09, 1.43] |
| 4.2 Hyper‐acute | 3 | 3506 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 0.99] |
| 4.3 Acute | 7 | 10440 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.94, 1.11] |
| 4.4 Subacute | 6 | 1502 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.84, 1.31] |
| 5 Death, early by intervention | 16 | 10050 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.74, 1.28] |
| 5.1 ACE inhibitors (po) | 4 | 164 | Odds Ratio (IV, Random, 95% CI) | 0.51 [0.06, 4.34] |
| 5.2 ARA (po) | 2 | 1379 | Odds Ratio (IV, Random, 95% CI) | 0.45 [0.05, 3.97] |
| 5.3 Beta‐blockers | 1 | 86 | Odds Ratio (IV, Random, 95% CI) | 0.25 [0.02, 2.93] |
| 5.4 Calcium channel blockers (po) | 1 | 77 | Odds Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.50] |
| 5.5 Calcium channel blockers (iv) | 1 | 11 | Odds Ratio (IV, Random, 95% CI) | 2.69 [0.10, 73.20] |
| 5.6 Nitric oxide donor | 7 | 4189 | Odds Ratio (IV, Random, 95% CI) | 0.79 [0.37, 1.72] |
| 5.7 Low BP target | 2 | 4144 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.66, 1.83] |
| 6 Death, early by stroke type | 13 | 9925 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.76, 1.35] |
| 6.1 Ischaemic stroke | 6 | 8844 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 6.2 Combined ischaemic stroke and intracerebral haemorrhage | 6 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.25, 2.05] |
| 6.3 Intracerebral haemorrhage | 3 | 708 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.61, 2.61] |
| 7 Death, early by time to treatment | 15 | 10028 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.36] |
| 7.1 Ultra‐acute/prehospital | 2 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.09, 14.29] |
| 7.2 Hyper‐acute | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.59, 4.05] |
| 7.3 Acute | 6 | 8182 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.41] |
| 7.4 Subacute | 7 | 1529 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.11, 1.42] |
| 8 Death, end of trial by intervention | 20 | 15818 | Odds Ratio (IV, Random, 95% CI) | 0.95 [0.84, 1.06] |
| 8.1 ACE inhibitors (po) | 4 | 165 | Odds Ratio (IV, Random, 95% CI) | 0.47 [0.15, 1.48] |
| 8.2 ARA (po) | 5 | 4120 | Odds Ratio (IV, Random, 95% CI) | 0.92 [0.59, 1.44] |
| 8.3 Beta‐blockers | 1 | 86 | Odds Ratio (IV, Random, 95% CI) | 0.57 [0.17, 1.89] |
| 8.4 Nitric oxide donor | 7 | 4197 | Odds Ratio (IV, Random, 95% CI) | 0.86 [0.72, 1.04] |
| 8.5 Low BP target | 4 | 7250 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.87, 1.25] |
| 9 Death, end of trial by stroke type | 20 | 15750 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 9.1 Ischaemic stroke | 10 | 11238 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 9.2 Combined ischaemic stroke and intracerebral haemorrhage | 7 | 328 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.22] |
| 9.3 Intracerebral haemorrhage | 6 | 4184 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 10 Death, end of trial by time to treatment | 20 | 15818 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.75, 1.08] |
| 10.1 Ultra‐acute/pre‐hospital | 2 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.11, 2.07] |
| 10.2 Hyper‐acute | 3 | 3506 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.26] |
| 10.3 Acute | 8 | 10708 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 10.4 Subacute | 8 | 1552 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.15] |
| 11 Barthel Index, end of trial, by intervention | 2 | 4350 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐3.28, 4.54] |
| 11.1 ARA | 1 | 339 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐6.46, 2.66] |
| 11.2 Nitric oxide donor | 1 | 4011 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐0.20, 4.60] |
| 12 Barthel Index, end of trial, by stroke type | 2 | 4310 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐1.04, 3.69] |
| 12.1 Ischaemic stroke | 2 | 3681 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐3.21, 4.89] |
| 12.2 Intracerebral haemorrhage | 1 | 629 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐5.18, 6.98] |
| 13 Barthel Index, end of trial, by time to treatment | 2 | 4350 | Mean Difference (IV, Random, 95% CI) | ‐5.43 [‐14.19, 3.33] |
| 13.1 Ultra‐acute | 1 | 273 | Mean Difference (IV, Random, 95% CI) | ‐18.0 [‐25.66, ‐10.34] |
| 13.2 Acute | 2 | 4077 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐2.64, 3.24] |
| 14 Early neurological deterioration, by intervention | 7 | 7575 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.24] |
| 14.1 ACE inhibitors | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.30, 8.41] |
| 14.2 Beta‐blockers | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.03, 8.74] |
| 14.3 Nitric oxide donor | 2 | 4052 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.66, 1.98] |
| 14.4 Low BP target | 4 | 3351 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 15 Early neurological deterioration, by stroke type | 7 | 7507 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 15.1 Ischaemic stroke | 2 | 3349 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.09, 3.82] |
| 15.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.31, 4.95] |
| 15.3 Intracerebral haemorrhage | 6 | 3986 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.84, 1.22] |
| 16 Early neurological deterioration, by time to treatment | 7 | 7575 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.87, 1.30] |
| 16.1 Ultra‐acute | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.32] |
| 16.2 Hyper‐acute | 3 | 3506 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.77, 1.13] |
| 16.3 Acute | 4 | 4028 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [1.07, 1.81] |
| 17 Quality of life (EuroQol) at end of trial, by intervention | 3 | 6881 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [0.01, 0.04] |
| 17.1 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 17.2 Low BP target | 1 | 2829 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.02, 0.08] |
| 18 Quality of life (EuroQoL) at end of trial, by stroke type | 3 | 7502 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 18.1 Ischaemic stroke | 2 | 4038 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.14, 0.40] |
| 18.2 Intracerebral haemorrhage | 3 | 3464 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.03, 0.08] |
| 19 Quality of life (EuroQoL) at end of trial, by time to treatment | 3 | 6867 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.00, 0.11] |
| 19.1 Ultra‐acute | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.29 [0.07, 0.51] |
| 19.2 Hyperacute | 2 | 3102 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.03, 0.08] |
| 19.3 Acute | 1 | 3738 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 20 Length of stay, by intervention | 4 | 8295 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.30, 0.28] |
| 20.1 ACE inhibitors | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐2.11, 1.85] |
| 20.2 Beta‐blockers | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐1.76, 2.18] |
| 20.3 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.76, 1.14] |
| 20.4 Low BP target | 1 | 4071 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.31, 0.31] |
| 21 Length of stay, by stroke type | 3 | 8194 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.29] |
| 21.1 Ischaemic stroke | 2 | 7393 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.28] |
| 21.2 Combined ischaemic and Intracerebral haemorrhage | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.20, 1.60] |
| 21.3 Intracerebral haemorrhage | 1 | 629 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐1.49, 6.69] |
| 22 Length of stay, by time to treatment | 4 | 8295 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.30, 0.29] |
| 22.1 Ultra‐acute | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐6.58, 2.83] |
| 22.2 Acute | 3 | 7981 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.29, 0.30] |
| 23 SBP, first after randomisation, by intervention | 24 | 15432 | Mean Difference (IV, Random, 95% CI) | ‐9.83 [‐12.11, ‐7.56] |
| 23.1 ACE inhibitors(po) | 5 | 123 | Mean Difference (IV, Random, 95% CI) | ‐13.68 [‐20.03, ‐7.32] |
| 23.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐17.55, 5.55] |
| 23.3 ARA (po) | 3 | 3408 | Mean Difference (IV, Random, 95% CI) | ‐4.59 [‐7.71, ‐1.48] |
| 23.4 A2AA(po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐13.67 [‐41.48, 14.14] |
| 23.5 Beta‐blockers (po) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐11.5 [‐20.29, ‐2.71] |
| 23.6 Beta‐blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐16.40 [‐27.40, ‐5.40] |
| 23.7 Calcium channel blockers (po) | 3 | 106 | Mean Difference (IV, Random, 95% CI) | ‐7.62 [‐17.21, 1.96] |
| 23.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐9.76 [‐24.42, 4.90] |
| 23.9 Nitric oxide donor | 7 | 4192 | Mean Difference (IV, Random, 95% CI) | ‐9.25 [‐14.54, ‐3.96] |
| 23.10 Thiazide‐like diuretic (po) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐20.0 [‐38.60, ‐1.40] |
| 23.11 Low BP target | 5 | 7421 | Mean Difference (IV, Random, 95% CI) | ‐11.39 [‐15.30, ‐7.49] |
| 24 SBP, first after randomisation by stroke type | 22 | 15659 | Mean Difference (IV, Random, 95% CI) | ‐8.77 [‐11.04, ‐6.50] |
| 24.1 Ischaemic stroke | 10 | 9256 | Mean Difference (IV, Random, 95% CI) | ‐6.99 [‐8.61, ‐5.38] |
| 24.2 Combined ischaemic stroke and intracerebral haemorrhage | 9 | 2466 | Mean Difference (IV, Random, 95% CI) | ‐7.89 [‐12.43, ‐3.36] |
| 24.3 Intracerebral haemorrhage | 4 | 3937 | Mean Difference (IV, Random, 95% CI) | ‐11.77 [‐15.25, ‐8.30] |
| 25 SBP, first after randomisation by time to treatment | 17 | 15211 | Mean Difference (IV, Random, 95% CI) | ‐9.67 [‐12.14, ‐7.20] |
| 25.1 Ultra‐acute/prehospital | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐15.98 [‐30.43, ‐1.53] |
| 25.2 Hyper‐acute | 3 | 3506 | Mean Difference (IV, Random, 95% CI) | ‐13.38 [‐15.41, ‐11.35] |
| 25.3 Acute | 6 | 10120 | Mean Difference (IV, Random, 95% CI) | ‐7.23 [‐9.83, ‐4.63] |
| 25.4 Subacute | 7 | 1530 | Mean Difference (IV, Random, 95% CI) | ‐7.26 [‐10.02, ‐4.50] |
| 26 SBP, at day 1 | 18 | 14203 | Mean Difference (IV, Random, 95% CI) | ‐8.33 [‐10.97, ‐5.69] |
| 26.1 ACE inhibitors (po) | 5 | 120 | Mean Difference (IV, Random, 95% CI) | ‐7.90 [‐16.83, 1.03] |
| 26.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐25.60, 1.60] |
| 26.3 ARA (po) | 2 | 2368 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.52, 1.51] |
| 26.4 A2AA (po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐21.83 [‐66.12, 22.46] |
| 26.5 Beta‐blockers(po) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐14.00 [‐27.28, ‐0.72] |
| 26.6 Beta‐blockers (iv) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐18.44, 8.44] |
| 26.7 Calcium channel blockers (po) | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐13.23 [‐43.36, 16.91] |
| 26.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐31.90 [‐64.73, 0.93] |
| 26.9 Nitric oxide donor | 7 | 4183 | Mean Difference (IV, Random, 95% CI) | ‐12.10 [‐19.06, ‐5.14] |
| 26.10 Low BP target | 3 | 7304 | Mean Difference (IV, Random, 95% CI) | ‐10.04 [‐12.47, ‐7.61] |
| 27 SBP, at day 7 | 11 | 15151 | Mean Difference (IV, Random, 95% CI) | ‐6.74 [‐9.39, ‐4.10] |
| 27.1 ACE inhibitors | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐26.0 [‐43.00, ‐7.00] |
| 27.2 ARA(po) | 4 | 3747 | Mean Difference (IV, Random, 95% CI) | ‐5.74 [‐7.44, ‐4.03] |
| 27.3 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐34.25, 4.25] |
| 27.4 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.63, 0.31] |
| 27.5 Low BP target | 3 | 7304 | Mean Difference (IV, Random, 95% CI) | ‐7.62 [‐11.69, ‐3.56] |
| 28 SBP, at end of treatment | 20 | 15684 | Mean Difference (IV, Random, 95% CI) | ‐8.02 [‐10.07, ‐5.97] |
| 28.1 ACE inhibitors (po) | 4 | 123 | Mean Difference (IV, Random, 95% CI) | ‐17.37 [‐23.42, ‐11.31] |
| 28.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐12.20, 9.20] |
| 28.3 ARA (po) | 5 | 3785 | Mean Difference (IV, Random, 95% CI) | ‐7.37 [‐10.19, ‐4.56] |
| 28.4 A2AA (po) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.5 Beta‐blockers (po) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐13.17, 6.77] |
| 28.6 Beta‐blockers (iv) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐13.00 [‐25.21, ‐4.79] |
| 28.7 Calcium channel blockers (po) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐17.12, 1.52] |
| 28.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐9.20 [‐25.73, 7.33] |
| 28.9 Nitric oxide donor | 4 | 4101 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐2.50, 0.39] |
| 28.10 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.0 [‐11.74, 17.74] |
| 28.11 Low BP target | 5 | 7421 | Mean Difference (IV, Random, 95% CI) | ‐10.06 [‐13.58, ‐6.55] |
| 29 DBP, first after randomisation by intervention | 17 | 12397 | Mean Difference (IV, Random, 95% CI) | ‐3.86 [‐5.07, ‐2.64] |
| 29.1 ACE inhibitors(po) | 5 | 123 | Mean Difference (IV, Random, 95% CI) | ‐4.23 [‐9.68, 1.21] |
| 29.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐5.50, 9.90] |
| 29.3 ARA (po) | 3 | 3408 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐4.35, ‐0.61] |
| 29.4 A2AA | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐15.43, 11.23] |
| 29.5 Beta‐Blockers (po) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐13.01, 2.21] |
| 29.6 Beta‐Blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐17.5 [‐25.32, ‐9.68] |
| 29.7 Calcium channel blockers (po) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐9.28, 2.68] |
| 29.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐31.35, 5.55] |
| 29.9 Nitric oxide donor | 6 | 4173 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐4.25, ‐2.52] |
| 29.10 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐6.43 [‐12.10, ‐0.75] |
| 30 DBP, first after randomisation by stroke type | 16 | 14952 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐4.69, ‐2.76] |
| 30.1 Ischaemic stroke | 6 | 7500 | Mean Difference (IV, Random, 95% CI) | ‐3.53 [‐4.20, ‐2.87] |
| 30.2 Combined ischaemic stroke and Intracerebral haemorrhage | 10 | 6419 | Mean Difference (IV, Random, 95% CI) | ‐2.85 [‐4.26, ‐1.44] |
| 30.3 Intracerebral haemorrhage | 2 | 1033 | Mean Difference (IV, Random, 95% CI) | ‐6.80 [‐11.99, ‐1.60] |
| 31 DBP, first after randomisation by time to treatment | 16 | 10977 | Mean Difference (IV, Random, 95% CI) | ‐3.80 [‐5.06, ‐2.54] |
| 31.1 Ultra‐acute/prehospital | 2 | 55 | Mean Difference (IV, Random, 95% CI) | 1.39 [‐7.71, 10.48] |
| 31.2 Hyper‐acute | 2 | 677 | Mean Difference (IV, Random, 95% CI) | ‐6.48 [‐12.55, ‐0.41] |
| 31.3 Acute | 6 | 10076 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.26, ‐1.97] |
| 31.4 Subacute | 7 | 169 | Mean Difference (IV, Random, 95% CI) | ‐3.88 [‐7.98, 0.22] |
| 32 DBP, at day 1 | 16 | 11361 | Mean Difference (IV, Random, 95% CI) | ‐3.05 [‐4.20, ‐1.91] |
| 32.1 ACE inhibitors (po) | 4 | 96 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐8.95, 2.47] |
| 32.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐9.70, 5.70] |
| 32.3 ARA (po) | 2 | 2368 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐3.47, 1.48] |
| 32.4 A2AA (po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐26.77, 27.11] |
| 32.5 Beta‐blockers (po) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐8.99, 6.99] |
| 32.6 Beta‐blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐13.21, 3.21] |
| 32.7 Calcium channel blockers (po) | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐6.10 [‐14.08, 1.89] |
| 32.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐31.35, 5.55] |
| 32.9 Nitric oxide donor | 7 | 4197 | Mean Difference (IV, Random, 95% CI) | ‐3.31 [‐4.17, ‐2.44] |
| 32.10 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐5.34 [‐9.02, ‐1.65] |
| 33 DBP, at day 7 | 10 | 12686 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐3.96, ‐1.83] |
| 33.1 ACE inhibitors | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐18.0 [‐33.10, ‐2.90] |
| 33.2 ARA(po) | 5 | 4152 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐3.24, ‐1.70] |
| 33.3 Nitric oxide donor | 1 | 4011 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.00, ‐0.20] |
| 33.4 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐16.00, 6.00] |
| 33.5 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐4.49 [‐6.18, ‐2.80] |
| 34 DBP, at end of treatment | 15 | 13050 | Mean Difference (IV, Random, 95% CI) | ‐3.95 [‐5.15, ‐2.75] |
| 34.1 ACE inhibitors (po) | 4 | 123 | Mean Difference (IV, Random, 95% CI) | ‐6.06 [‐11.18, ‐0.95] |
| 34.2 ARA (po) | 6 | 4190 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐5.61, ‐1.61] |
| 34.3 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐7.70, 7.70] |
| 34.4 Beta‐blockers (po) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐7.48, 9.28] |
| 34.5 Beta‐Blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐16.40 [‐24.22, ‐8.58] |
| 34.6 Calcium channel blockers (po) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐9.40 [‐15.50, ‐3.30] |
| 34.7 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐21.35, 15.55] |
| 34.8 Nitric oxide donor | 1 | 4011 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.00, ‐0.20] |
| 34.9 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐13.03, 5.03] |
| 34.10 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐4.24 [‐5.62, ‐2.86] |
| 35 HR at baseline | 15 | 5841 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.86, 0.99] |
| 35.1 ACE inhibitors (po) | 3 | 61 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐5.31, 5.75] |
| 35.2 ARA(po) | 2 | 1379 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.57, 0.90] |
| 35.3 A2AA (po) | 1 | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.4 Calcium channel blockers (po) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐9.47, 2.27] |
| 35.5 Nitric oxide donor | 7 | 4197 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐1.26, 5.77] |
| 35.6 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐10.50, 2.50] |
| 35.7 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐4.21, 14.81] |
| 35.8 Low BP target | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐10.53, 4.53] |
| 36 HR, first after randomisation | 6 | 4196 | Mean Difference (IV, Random, 95% CI) | 2.74 [‐1.13, 6.61] |
| 36.1 ACE inhibitors (po) | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐9.89, 8.72] |
| 36.2 ARA(po) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Calcium channel blockers (po) | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 1.28 [‐21.89, 24.46] |
| 36.4 A2AA (po) | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.5 Nitric oxide donor | 3 | 4061 | Mean Difference (IV, Random, 95% CI) | 3.95 [‐1.03, 8.93] |
| 36.6 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 10.0 [0.71, 19.29] |
| 37 HR, at day 1 | 10 | 4333 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐1.06, 5.76] |
| 37.1 ACE inhibitors (po) | 2 | 39 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐40.45, 40.51] |
| 37.2 Calcium channel blockers (po) | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.56 [‐7.71, 2.59] |
| 37.3 A2AA (po) | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.4 Nitric oxide donor | 7 | 4197 | Mean Difference (IV, Random, 95% CI) | 4.53 [1.46, 7.60] |
| 37.5 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐16.90 [‐27.35, ‐6.45] |
| 38 HR, at day 7 | 4 | 5449 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.24, 1.19] |
| 38.1 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.14, 6.14] |
| 38.2 ARA(po) | 1 | 1360 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.07, 1.27] |
| 38.3 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.20, 1.63] |
| 39 HR, at end of treatment | 4 | 4124 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.21, 1.61] |
| 39.1 ACE inhibitors | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐62.22, 67.02] |
| 39.2 Thiazide‐ like diuretic | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.14, 6.14] |
| 39.3 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.20, 1.63] |
1.24. Analysis.
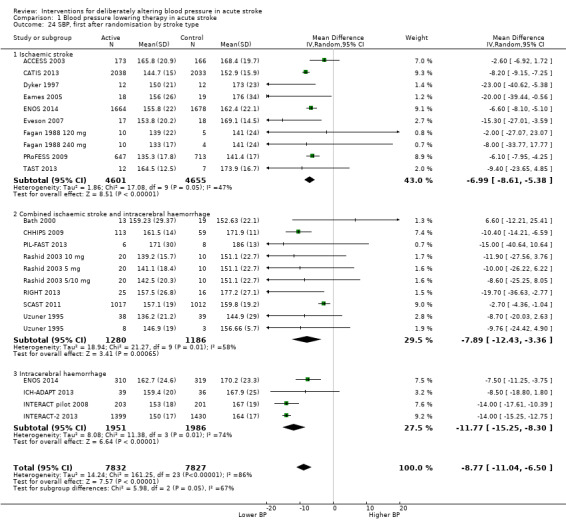
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 24 SBP, first after randomisation by stroke type.
1.25. Analysis.
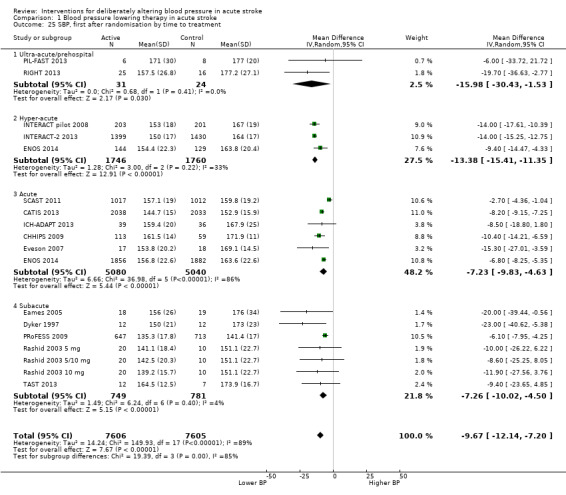
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 25 SBP, first after randomisation by time to treatment.
1.26. Analysis.
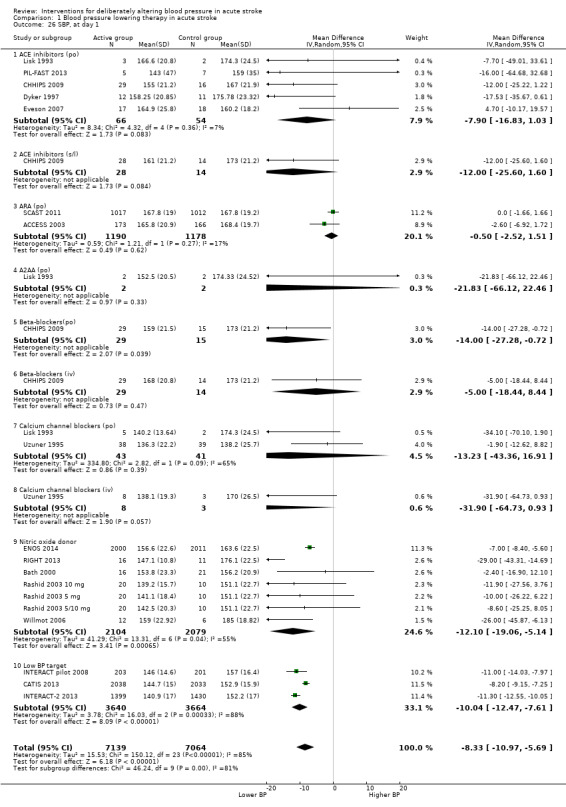
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 26 SBP, at day 1.
1.27. Analysis.
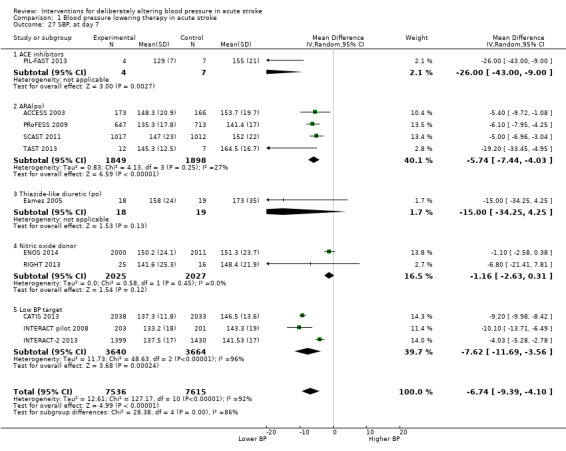
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 27 SBP, at day 7.
1.28. Analysis.
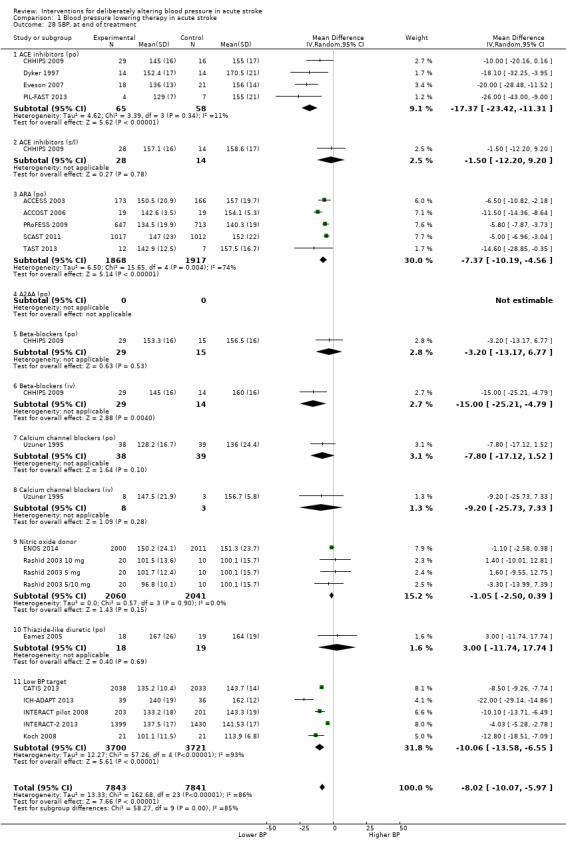
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 28 SBP, at end of treatment.
1.31. Analysis.
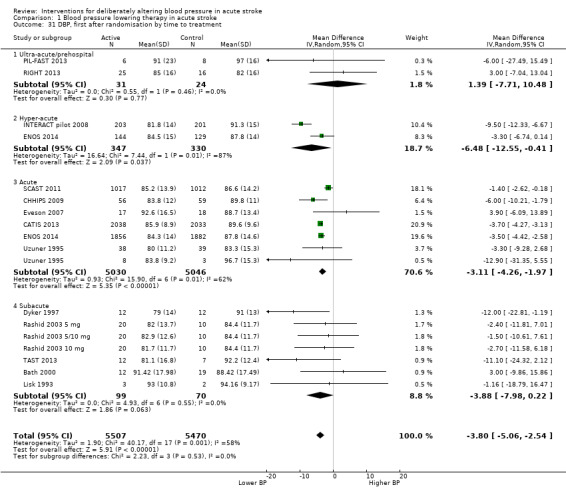
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 31 DBP, first after randomisation by time to treatment.
1.32. Analysis.
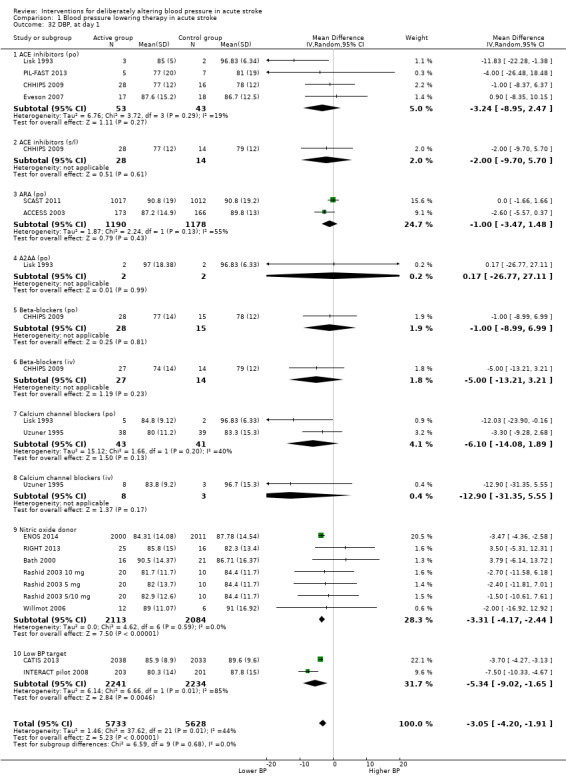
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 32 DBP, at day 1.
1.33. Analysis.
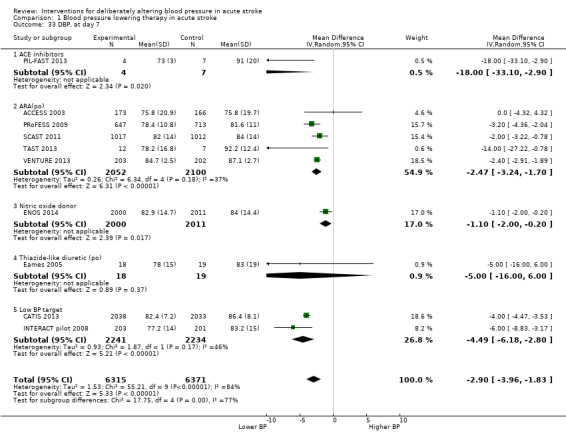
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 33 DBP, at day 7.
1.34. Analysis.
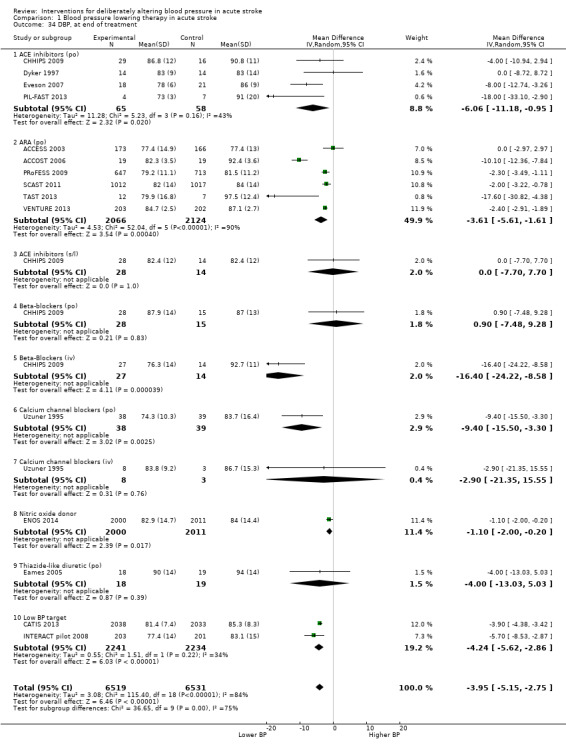
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 34 DBP, at end of treatment.
1.35. Analysis.
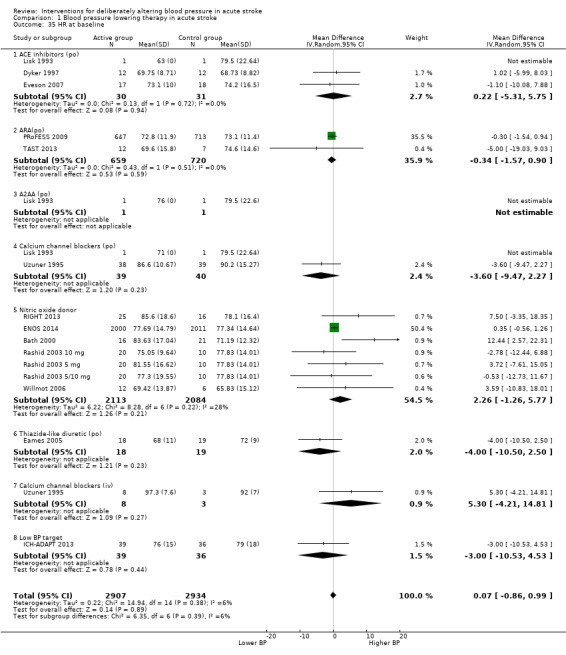
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 35 HR at baseline.
1.36. Analysis.
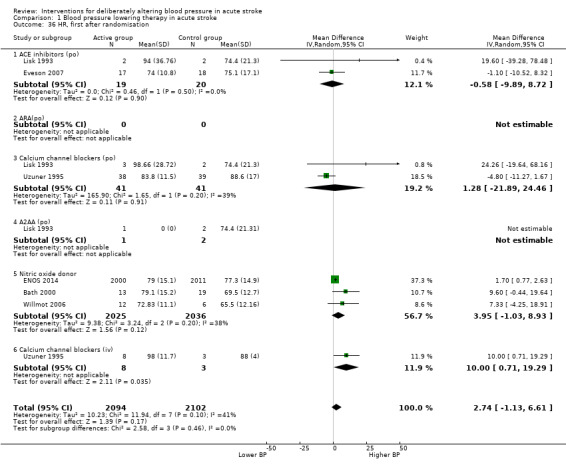
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 36 HR, first after randomisation.
1.38. Analysis.
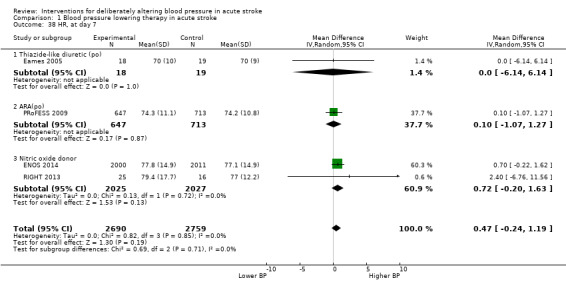
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 38 HR, at day 7.
1.39. Analysis.
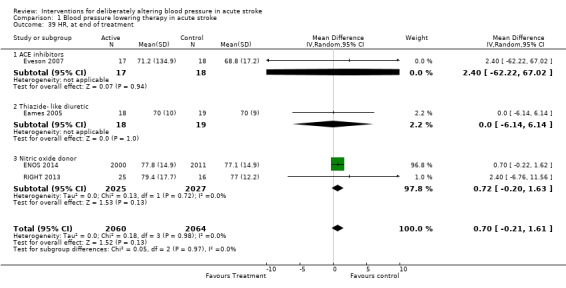
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 39 HR, at end of treatment.
Comparison 2. Blood pressure altering by continue or stop prestroke antihypertensives (C/S).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency, end of trial by C/S | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 2 Death or dependency, end of trial by stroke type C/S | 2 | 2841 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 2.1 Ischaemic stroke | 1 | 1832 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.87, 1.28] |
| 2.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
| 2.3 Intracerebral haemorrhage | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.55, 1.65] |
| 3 Death or dependency, end of trial by time to treatment | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 3.1 Ultra‐acute | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.69, 2.61] |
| 3.2 Acute | 2 | 2717 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 4 Death early, by C/S | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.55, 2.00] |
| 5 Death early, by stroke type C/S | 2 | 2841 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.56, 1.87] |
| 5.1 Ischaemic stroke | 1 | 1832 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.79, 2.36] |
| 5.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.98] |
| 5.3 Intracerebral haemorrhage | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.09, 2.92] |
| 6 Death early, by time to treatment C/S | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.60, 1.93] |
| 6.1 Ultra‐acute | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 5.68 [0.27, 120.37] |
| 6.2 Acute | 2 | 2717 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.61, 1.76] |
| 7 Death, end of trial by C/S | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.92, 1.43] |
| 8 Death end of trial, by stroke type C/S | 2 | 2839 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.47] |
| 8.1 Ischaemic stroke | 1 | 1832 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.96, 1.61] |
| 8.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.67, 1.91] |
| 8.3 Intracerebral haemorrhage | 1 | 244 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.71] |
| 9 Death, end of trial by time to treatment C/S | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.92, 1.42] |
| 9.1 Ultra‐acute | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [0.68, 5.15] |
| 9.2 Acute | 2 | 2717 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.89, 1.40] |
| 10 Barthel Index, end of trial, by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐3.18 [‐5.80, ‐0.55] |
| 11 Early neurological deterioration, by C/S | 1 | 2097 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.89, 1.82] |
| 12 Quality of life (EuroQol) at end of trial, by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 13 Length of stay, by C/S | 1 | 2097 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.87, 3.27] |
| 14 SBP, first after randomisation, by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.75, ‐1.46] |
| 15 SBP, at day 1 by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.75, ‐1.46] |
| 16 SBP, at end of treatment by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐11.30 [‐15.20, ‐7.40] |
| 17 DBP, at baseline by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.65, 0.27] |
| 18 DBP at day 1, by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.24, ‐0.08] |
| 19 DBP, at end of treatment by C/S | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐9.28, ‐3.61] |
| 20 HR, at day 1 | 1 | 2097 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.70, 1.90] |
| 21 HR, at end of treatment | 1 | 2097 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐4.51, ‐1.89] |
2.13. Analysis.
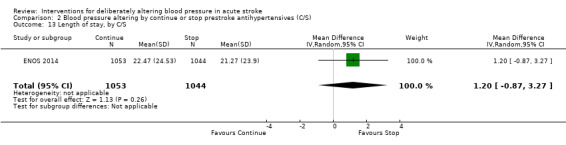
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 13 Length of stay, by C/S.
2.15. Analysis.
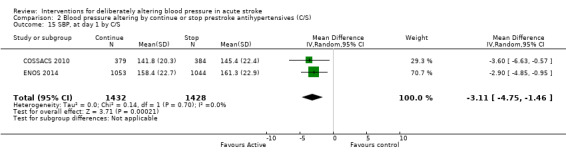
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 15 SBP, at day 1 by C/S.
2.16. Analysis.
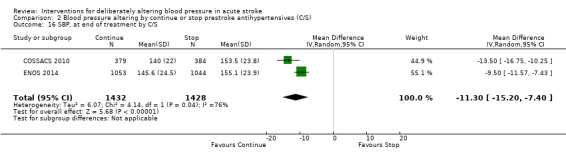
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 16 SBP, at end of treatment by C/S.
2.17. Analysis.
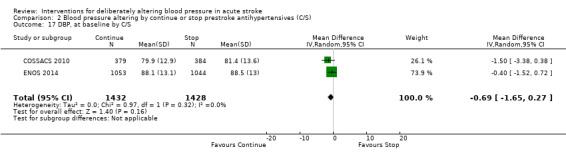
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 17 DBP, at baseline by C/S.
2.18. Analysis.
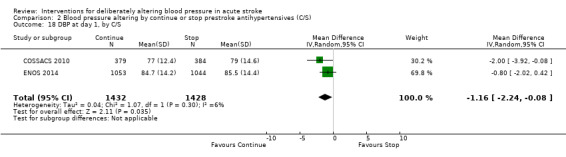
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 18 DBP at day 1, by C/S.
2.19. Analysis.
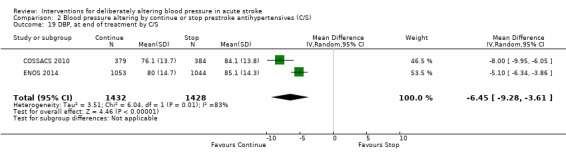
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 19 DBP, at end of treatment by C/S.
2.20. Analysis.
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 20 HR, at day 1.
Comparison 3. Blood pressure elevation therapy in acute stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death early, by intervention | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Phenylephrine (iv) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death early, by stroke type | 1 | 15 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Ischaemic stroke | 1 | 15 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death end of trial, by intervention | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Phenylephrine (iv) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 SBP, at baseline | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐27.5 [‐50.83, ‐4.17] |
| 4.1 Phenylephrine (iv) | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐27.5 [‐50.83, ‐4.17] |
| 5 SBP, first after randomisation | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 20.60 [‐13.31, 54.51] |
| 6 SBP, at day 1 | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 20.60 [‐13.31, 54.51] |
| 7 DBP, at baseline | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐8.30 [‐19.13, 2.53] |
| 8 DBP, first after randomisation | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐14.86, 15.86] |
| 9 DBP, at day 1 | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐14.86, 15.86] |
3.1. Analysis.
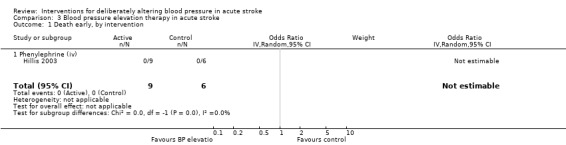
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 1 Death early, by intervention.
3.2. Analysis.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 2 Death early, by stroke type.
3.3. Analysis.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 3 Death end of trial, by intervention.
3.5. Analysis.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 5 SBP, first after randomisation.
3.6. Analysis.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 6 SBP, at day 1.
3.7. Analysis.
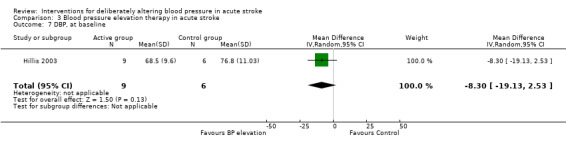
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 7 DBP, at baseline.
3.8. Analysis.
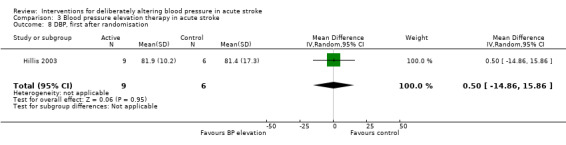
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 8 DBP, first after randomisation.
3.9. Analysis.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 9 DBP, at day 1.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACCESS 2003.
| Methods | Double‐blind, placebo‐controlled Method of randomisation not known | |
| Participants | Germany, multicentre 339 participants. T: 173, C: 166 Age T: 68 years, C: 67.8 years Male T: 50%, C: 52% Inclusion: IS 100% CT Enrolment within 24 to 36 hours after admission | |
| Interventions | T: candesartan 4 mg po on day 1 and dose was increased to 8 or 16 mg if BP exceeded 160 mmHg SBP or 100 mmHg DBP C: matching placebo Rx: 7 days | |
| Outcomes | BP measured by a nurse or automatically Case fatality and disability using BI 3 months | |
| Notes | Exclusion: age > 85 years, > 70% stenosis of internal carotid artery, disorders in consciousness, cardiac failure, unstable angina, malignant hypertension, and high grade aortic or mitral stenosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
ACCOST 2006.
| Methods | Double‐blind, placebo‐controlled Method of randomisation not known |
|
| Participants | UK, single centre 38 participants, T: 19, C: 19 Age: not given Inclusion: IS with BP > 120/70 Enrolment within 72 hours |
|
| Interventions | T: candesartan 4 mg once daily C: matching placebo Rx: 28 days Dose was increased to 8 mg or 2 placebo if BP criteria not met. Target BP not reported |
|
| Outcomes | BP methodology not given All‐cause mortality and mortality due to vascular causes 90 days: NIHSS, mRS, BI |
|
| Notes | Exclusion criteria: not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was an abstract only. The author reported the study as being prospective, double‐blind and placebo‐controlled |
| Allocation concealment (selection bias) | Unclear risk | No further information available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | This was an abstract only. The authors described the study as being double‐blind, although it was unclear who was blinded and how |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was an abstract only. The authors described the study as being double‐blind, although it was unclear who was blinded and how |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was an abstract only. The authors described the study as being double‐blind, although it was unclear who was blinded and how |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This was an abstract only. 38 participants were enrolled into this trial, insufficient data to assess attrition |
| Selective reporting (reporting bias) | Unclear risk | This was an abstract only |
| Other bias | Unclear risk | This was an abstract only |
Bath 2000.
| Methods | Double‐blind, placebo‐controlled Randomisation by computer (with minimisation on age and mean arterial BP, baseline SSS, hours from onset presence of a visible stroke lesion on CT) | |
| Participants | UK, single centre 37 participants. T: 16, C: 21 Age T: 76 years, C: 72 years Male T: 6, C: 12 Inclusion: IS or ICH 100% CT Enrolment within 5 days | |
| Interventions | T: transdermal GTN (Schwarz Pharma) 5 mg once daily C: matching placebo Rx: 12 days | |
| Outcomes | 24‐hour ambulatory BP (Spacelabs 90207, measured 3 times/hour during the day, hourly during the night) at days 0, 1 and 8 Rankin scale, BI and case fatality at 3 months | |
| Notes | Exclusion: taking part in another trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
CATIS 2013.
| Methods | Single blind, blinded end‐point Randomisation central and stratified by participating hospitals and use of antihypertensives. Randomisation schedules generated using SAS PROC PLAN in SAS and concealed until eligible participant was ready for enrolment |
|
| Participants | China, multicentre 4071 participants, T: 2038, C: 2033 Males T: 62.1 years, C: 61.8 years Male T: 1317, C: 1287 Inclusion: IS confirmed by CT or MRI with SBP between 140 to 220 mmHg Enrolment within 48 hours FU: losses ‐ T: 50 participants, C: 46 participants |
|
| Interventions | T: early intensive lowering of BP (target BP 140/90 mmHg) C: stop pre‐existing antihypertensive drugs Rx: 14 days |
|
| Outcomes | BP measured supine using a standard sphygmomanometer and using 1 of 4 cuff sizes (paediatric, regular, adult, large adult, or thigh) based on arm circumference. After randomisation, 3 BP measurements every 2 hours on day 1, every 4 hours on day 2 and 3 and three times a day until hospital discharge or death Primary outcomes: BP at days 1, 7, 14; death and major disability at day 14 Secondary outcomes: death and major disability at day 14 and 90 |
|
| Notes | Exclusion: severe heart failure, myocardial infarction, unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, or resistant hypertension, deep coma, treatment with iv rtPA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Central randomisation ensured allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Treating clinicians and nurses were not blinded to treatment group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although participants were masked to treatment allocation, treating clinicians and nurses were not blinded to treatment group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few participants (96 among 4071) lost to follow up; no differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported; no difference in reporting between groups |
| Other bias | Low risk | No other biases found |
CHHIPS 2009.
| Methods | Double‐blind, placebo‐controlled Block randomisation (6 per block) by secure Internet centrally to receive either active treatment or placebo in 2:1 ratio |
|
| Participants | UK, multicentre 179 participants, T: 113, C: 59 Labetalol arm: 56 participants Mean age T: 74 years; C: 74 years Males T: 34 (61%), C: 31 (53%) Lisinopril arm: 57 participants Mean age: 75 years; C: 74 years Males T: 30 (53%), C: 31 (53%) Inclusion: neuroradiologically confirmed stroke patients 12 hours of stroke onset, hypertensive (SBP > 160), non dysphagic ischaemic and haemorrhagic stroke within 36 hours of stroke onset and hypertensive, dysphagic ischaemic and haemorrhagic stroke patients within 36 hours of stroke onset |
|
| Interventions | Non‐dysphagic patients: T: oral labetalol 50 mg or lisinopril 5 mg orally; C: matching oral placebo Dysphagic patients: either of: T: iv labetalol 50 mg C: sublingual placebo T: 5 mg sl lisinopril C: intravenous placebo T: sublingual placebo C: intravenous placebo Rx: 14 days |
|
| Outcomes | BP changes at 24 hours and 2 weeks. BP measured in the brachial artery every 30 minutes for 8 hours post treatment using a validated A&D UA‐767 blood pressure monitor and appropriate cuff Primary outcomes: death or dependency mRS and BI at 14 days following stroke onset Secondary outcomes: NIHSS at 72 hours, mRS and NIHSS day 14, stroke recurrence over 2 weeks, death, quality of life, discharge disposition at 3 months |
|
| Notes | Exclusion: hypertensive encephalopathy, co‐existing cardiac or vascular emergency, SBP > 200 mm Hg and/or diastolic blood pressure > 120 mm Hg with ICH, pre‐existing antihypertensive treatment in patients without dysphagia, NIHSS section 1a ≥ 2 points, premorbid mRS > 3, any coexisting life‐threatening condition with a life expectancy of < 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Central randomisation ensured allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatment assignment was blinded. Both active and placebo tablets were identical in shape, size and colour. Similarly, the vials of labetalol and placebo were identical in size, shape and colour |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment assignment was blinded. Both active and placebo tablets were identical in shape, size and colour. Similarly, the vials of labetalol and placebo were identical in size, shape and colour |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported; no differences between trial groups |
| Other bias | Low risk | No other biases found |
COSSACS 2010.
| Methods | Prospective, open‐label, blinded‐endpoint Randomisation (1:1) with a block size of 4 centrally by secure Internet Allocation to continue or stop treatment was done by computer with stratification by age at entry (< 75 years and > 75 years) |
|
| Participants | UK, multicentre 763 participants. T: 379, C: 384 Mean age T: 74 years, C: 74 years Males T: 210, C: 216 Inclusion: non‐dysphagic ischaemic or haemorrhagic stroke within 48 hours of last dose of antihypertensive drugs |
|
| Interventions | T: continue pre‐existing antihypertensive medications C: stop pre‐existing antihypertensive drugs Rx: 14 days |
|
| Outcomes | BP measured by use of a UA‐767 BP monitor (A&D Medical, San Jose, CA, USA). BP calculated throughout the treatment period as mean of 2 sets of 3 supine readings taken 10 minutes apart Primary outcome: death or dependency at 2 weeks Secondary outcomes: BP changes at admission and 2 weeks; NIHSS, BI at 2 weeks; death, recurrent stroke, quality of life at 2 weeks Discharge disposition at 2 weeks and 6 months |
|
| Notes | Exclusion: hypertensive encephalopathy; coexisting cardiac or vascular urgency; SBP greater than 200 mm Hg or DBP greater than 120 mm Hg associated with known primary ICH; contraindications to stopping or indications for continuing antihypertensive treatment; dysphagia; impaired consciousness (NIHSS section 1a score ≥ 2 points); women of childbearing potential; premorbid dependency (mRS > 3 points); any coexisting life‐threatening condition with an estimated life expectancy of less than 6 months; and no evidence of stroke on neuroimaging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer and the method of random sequence generation was described |
| Allocation concealment (selection bias) | Low risk | Central web‐based allocation ensured allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether treatment was either to stop or continue |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether treatment was either to stop or continue. However all participants were to receive best medical care and there was no difference in care between treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported; no differences between trial groups |
| Other bias | Low risk | No other biases found |
Dyker 1997.
| Methods | Double‐blind, placebo‐controlled Method of randomisation not known | |
| Participants | UK, single centre 28 participants. T: 14, C: 14 Mean age 70 years Males T: 9, C: 8 Inclusion: ischaemic strokes with mild to moderate hypertension (170 to 250/95 to 120 mmHg) 100% CT on entry Enrolment within 1 week Patients admitted on prescribed antihypertensive therapy had treatment discontinued for at least 48 hours before entry into the study | |
| Interventions | T: perindopril 4 mg po once daily C: matching placebo Rx: 15 days | |
| Outcomes | BP measured semi‐automatically pre‐treatment and hourly at 10 hours repeated at 24 hours and at 2 weeks Neurological impairment: NIHSS made at baseline and day 15 | |
| Notes | Exclusion: severe carotid disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Eames 2005.
| Methods | Double‐blind, placebo‐controlled, parallel group study Block randomisation (4 per block) | |
| Participants | UK, single centre 37 participants. T: 18, C: 19 Age: 68 years Male: 86% Inclusion: neuroradiologically diagnosed ischaemic stroke with 24 hour BP > 130/80 mmHg or daytime mean BP > 135/85 mmHg Enrolment within 96 hours of stroke onset | |
| Interventions | T: bendrofluazide 2.5 mg po daily C: matching placebo Rx: 7 days | |
| Outcomes | Casual and non‐invasive beat‐to‐beat arterial BP level, cerebral blood flow velocity, ECG and transcutaneous carbon dioxide levels within 70 ∓ 20 hours of cerebral infarction and 7 days later were measured. 24‐hour BP monitoring with Spacelabs 90207 and brachial artery BP with validated semi‐automatic BP monitor (Omron 711) | |
| Notes | Exclusion: history of previous stroke, dysphagia, symptoms lasting < 24 hours, or presented > 76 hours after symptom onset (to allow for 24 hour BP monitoring to be performed prior to randomisation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
ENOS 2014.
| Methods | Single‐blind, parallel‐group, partial factorial study Randomisation via password protected, data‐encrypted website, with: stratification by prior antihypertensive treatment and country; minimisation by sex, age, stroke severity, time to treatment and total anterior circulation syndrome |
|
| Participants | International (23 countries), multicentre (173 sites) 4011 participants. T: 2000; C: 2011 Age T: 70 years, C: 70 years Male T: 1147 (57%), C:1150 (57%) Inclusion: haemorrhagic or ischaemic stroke; motor deficit in arm and/or leg; SBP between 140 to 220 mmHg Enrolment: < 48 hours of onset |
|
| Interventions | Factor 1: T: Transdermal GTN 5 mg C: No GTN Blinding with a gauze dressing applied over GTN patch or equivalent area of skin Factor 2 (in relevant participants): T: Continue pre‐stroke antihypertensive therapy C: Stop pre‐stroke antihypertensive therapy Rx: 7 days |
|
| Outcomes | Primary outcome: mRS at day 90 Secondary outcomes:
|
|
| Notes | Exclusions: GCS < 8; pure sensory stroke; preceding dependency (mRS 3 to 5); confounding neurological or psychiatric disease; stroke mimic; severe liver or renal dysfunction; severe concomitant medical conditions: pregnant or breastfeeding; planned surgical intervention; previous participation in ENOS 2014; definite need for, or contradiction to, nitrates and/or prestroke antihypertensive therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central web‐based allocation ensured allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | GTN was given in a single‐blind design as no manufacturer was able to supply placebo patches. Participants were blinded with placement of a gauze dressing over an area of skin out of view (e.g. back or shoulders) with or without GTN patch a underneath. The continue versus stop arm was open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | GTN was given in a single‐blind design as no manufacturer was able to supply placebo patches. Participants were blinded with placement of a gauze dressing over an area of skin out of view (e.g. back or shoulders) with or without GTN patch a underneath. The continue versus stop arm was open‐label. All participants were to receive best medical care and there was no difference in care between treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported; no differences between trial groups |
| Other bias | Low risk | No other biases found |
Eveson 2007.
| Methods | Double‐blind, placebo‐controlled, parallel‐group study Randomisation by numbered identical study packs | |
| Participants | UK, single centre 40 participants. T: 18, C: 22 Age T: 73 years, C: 75 years Male: 63% Inclusion: acute IS within previous 24 hours with mean SBP level ≥ 140 mmHg or DBP level ≥ 90 mmHg Randomisation done before neuroimaging; participants with non‐IS were withdrawn from the study | |
| Interventions | T: lisinopril 5 mg po once daily C: matching placebo Rx: 14 days Dose was increased to 10 mg or 2 placebo on day 7 if SBP ≥ 140 mmHg or DBP ≥ 90mmHg | |
| Outcomes | Casual brachial artery BP monitoring at 5‐minute intervals during a 30‐minute period with a validated monitor (A&D UA 767) NIHSS score at day 14, BI and mRS at day 14 and day 90 | |
| Notes | Exclusion: severe carotid stenosis, significant aortic stenosis, cardiac failure, MI within past 6 months, dysphagia, dehydration, adverse reactions to ACEI, and pre‐stroke mRS score > 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
Fagan 1988 120 mg.
| Methods | Double‐blind, placebo‐controlled Randomisation technique not stated Intention‐to‐treat analysis FU: no losses | |
| Participants | USA, multicentre 19 participants, T: 10, C: 9 Age > 45 years, no genders given IS diagnosed on history and neurological examination Enrolment times not given | |
| Interventions | T: nimodipine (Miles Pharmaceuticals, USA) 120 mg/day (20 mg 4 hourly) po C: matching placebo Rx for 21 days | |
| Outcomes | Brachial BP before and 30 and 60 minutes after each morning dose for 7 days BP methodology not stated | |
| Notes | Exclusion: concurrent calcium channel antagonists, antihypertensive agents (other than beta blockers) Part of a larger unpublished trial to evaluate the safety and efficacy of nimodipine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
Fagan 1988 240 mg.
| Methods | Double‐blind, placebo‐controlled Randomisation technique not stated Intention‐to‐treat analysis FU: no losses | |
| Participants | USA, multicentre 19 participants, T: 10, C: 9 Age > 45 years, no genders given IS diagnosed on history and neurological examination Enrolment times not given | |
| Interventions | T: nimodipine (Miles Pharmaceuticals, USA) 240 mg/day (40 mg 4 hourly) po C: matching placebo Rx: for 21 days | |
| Outcomes | Brachial BP before and 30 and 60 minutes after each morning dose for 7 days BP methodology not stated | |
| Notes | Exclusion: concurrent calcium channel antagonists,antihypertensive agents (other than beta blockers) Part of a larger unpublished trial to evaluate the safety and efficacy of nimodipine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
Hillis 2003.
| Methods | Pilot randomised controlled trial Method of randomisation: 2:1 to BP elevation or conventional management FU: no losses | |
| Participants | USA, single centre 15 participants T: 9, C: 6 Age T: 59 years, C: 68 years Male T: 2, C: 2 Inclusion: IS > 20% diffusion perfusion mismatch; quantifiable, stable or worsening aphasia; hemispatial neglect or hemiparesis Enrolment: up to 7 days from the onset of stroke symptoms Prior antihypertensive medication was discontinued prior to initiation | |
| Interventions | T: intravenous phenylephrine was titrated to reach 10% to 20% increase MAP and continued for maximum of 72 hours. After 24 hours the participants were started on midodrine (up to 10 mg), fludrocortisone(up to 0.2 mg) and sodium chloride tablets with simultaneous weaning of intravenous phenylephrine. By 4 weeks, midodrine, fludrocortisone and sodium chloride were tapered providing no clinical deterioration C: conventional management | |
| Outcomes | BP measurement method not given NIHSS and cognitive tests on day 1, day 3 and 6 to 8 weeks | |
| Notes | Exclusion: CI or inability to tolerate MRI, cardiac ejection fraction < 25%, recent congestive heart failure, myocardial ischaemia, unstable angina, bradycardia, allergy to gadolinium, haemorrhage seen on initial CT, agitation requiring ongoing sedation, or MAP > 140 with no intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
ICH‐ADAPT 2013.
| Methods | Open‐label, blinded‐endpoint randomised trial Block randomisation (6 per block), stratified by onset to treatment time (≤ 6 and 6 to 24 hours) Treatment allocation using a random number generator before trial commencement Treatment assignment in sealed, opaque envelopes at the site |
|
| Participants | Canada, multicentre 75 participants, T: 39, C: 36 Mean age T: 70.7 years, C: 68.7 years Male T: 26, C: 28 Inclusion: spontaneous ICH confirmed by CT and elevated BP ≥ 150 mm Hg (≥ 2 readings, ≥ 5 minutes apart) Enrolment: within 24 hours of symptom onset Rx: 1 day FU: no losses |
|
| Interventions | T: early intensive BP lowering of BP (SBP target < 150 mm Hg) with iv Labetalol/hydralazine/enalapril C: guideline based management of BP (target SBP 180 mm Hg) At 24 hours, both groups received perindopril 4 mg daily and or previous antihypertensives po or ng as per investigators discretion |
|
| Outcomes | Primary: perihaematoma relative CBF, as measured with CT perfusion 2 hours after initiation of antihypertensive therapy Secondary: continuous non‐invasive BP and HR monitoring for minimum 24 hours (BP methodology not stated); NIHSS at 2 hours; mRS, NIHSS at 24 hours, day 30 and 90; BI at day 30 and 90 |
|
| Notes | Exclusion: secondary ICH, planned surgical resection, or CIs to CT perfusion e.g. contrast allergy or renal impairment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Randomisation by sequential numbered packs (allocation have been generated randomly) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether treatment was either to stop or continue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was open‐label, therefore participants and clinicians knew whether the assigned treatment was intensive or guideline‐recommended management of BP |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Images were measured centrally by readers blinded to treatment and clinical outcomes Clinical assessment were performed by investigators masked to image analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between treatment groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported; no differences between trial groups |
| Other bias | Low risk | No other biases found |
INTERACT pilot 2008.
| Methods | Open, blinded outcome, randomised trial Randomisation done with minimisation through a password protected Internet‐based system Intention‐to‐treat analysis | |
| Participants | International, multicentre
404 participants, T: 203, C: 201
Age 63 years
Male 65%
Inclusion: spontaneous ICH confirmed by CT and elevated SBP ( ≥ 2 measurements of 150 to 220 mmHg, recorded ≥ 2 minutes apart)
100% CT
Enrolment: within 6 hours of ICH onset FU: no losses |
|
| Interventions | T: early intensive lowering of BP (target SBP 140 mmHg) C: standard guideline based management of BP (target SBP 180 mmHg) Both groups received oral as well as intravenous agents for lowering BP Rx: for 7 days | |
| Outcomes | Proportional change in haematoma volume at 24 hours BP methodology not stated | |
| Notes | Exclusion: indication for intensive lowering of BP, CI to intensive lowering of BP, ICH secondary to structural cerebral abnormality or use of thrombolytic agent, IS within 30 days, deep coma (3 to 5 on the GCS), pre‐stroke disability or medical illness, and early planned decompressive neurosurgical intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
INTERACT‐2 2013.
| Methods | Open, blinded outcome, randomised trial Randomisation done with minimisation through a password protected Internet based system | |
| Participants | International, multicentre 2839 participants, T: 1382, C: 1412 Mean age T: 63 years, C:64.1 years Male T: 64.2%, C: 61.7% Inclusion: spontaneous ICH confirmed by CT and elevated SBP ( ≥ 2 measurements of 150 to 220 mmHg, recorded ≥ 2 minutes apart) 100% CT Enrolment: within 6 hours of ICH onset FU: no losses |
|
| Interventions | T: intensive lowering of BP (target SBP 140 mmHg) C: standard guideline based management of BP (target SBP 180 mmHg) Both groups received oral as well as intravenous agents for lowering BP Rx: for 7 days | |
| Outcomes | Primary: mRS at day 90 Secondary: combined death and dependency at day 90 in participants treated < 4 hours of ICH onset; recurrent stroke; haematoma expansion and cerebral oedema at 24 and 72 hours; BP during 7 days of treatment (BP methodology not stated); length of stay; discharge disposition; BI, QoL (EuroQoL), MMSE, Zung depression at day 90 (BP methodology not stated) |
|
| Notes | Exclusion: indication for intensive lowering of BP, CI to intensive lowering of BP, ICH secondary to structural cerebral abnormality or use of thrombolytic agent, IS within 30 days, deep coma (3 to 5 on the GCS), pre‐stroke disability or medical illness, and early planned decompressive neurosurgical intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by secure Internet system |
| Allocation concealment (selection bias) | Low risk | Central web‐based allocation ensured allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether the assigned treatment was intensive or guideline‐recommended management of BP |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether the assigned treatment was intensive or guideline‐recommended management of BP. All participants were to receive best medical care and there was no differences in care between the 2 treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No difference between trial groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported; no differences between trial groups |
| Other bias | Low risk | No other biases found |
Koch 2008.
| Methods | Open‐label, blinded outcome, randomised trial Allocation concealment by numbered envelopes in random sequence |
|
| Participants | USA, single centre 42 participants: T: 21, C: 21 Mean age T: 61 years, C: 60 years Male T: 9, C: 14 Inclusion: CT confirmed spontaneous supratentorial ICH Enrolment: within 8 hours of ICH onset Rx: 2 days |
|
| Interventions | T: intensive lowering of BP (target MAP < 110 mm Hg) C: guideline based management of BP (target MAP 110 to 130 mm Hg) |
|
| Outcomes | BP; monitored every 15 minutes for the first 3 hours, every 30 minutes from 3 to 6 hours, and hourly from 6 to 48 hours. BP measured with automated cuff sphygmomanometry NIHSS, GCS at 24 and 48 hours Haematoma and oedema growth between baseline and 24 hours mRS day 90 |
|
| Notes | Exclusion: inability to consent, head injury, comatose, coagulopathy (platelet count < 50,000 or INR ≥ 1.8), MAP < 110 mm Hg, ICH secondary to other causes (arteriovenous malformations, trauma, aneurysm) or needing surgical evacuation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation has been stratified by centre, but method of random sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Allocation have been done randomly |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether the assigned treatment was intensive or guideline‐recommended management of BP |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatment was open‐label, therefore participants and clinicians knew whether the assigned treatment was intensive or guideline‐recommended management of BP |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; no differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported; no difference between treatment groups |
| Other bias | Low risk | No other biases found |
Lisk 1993.
| Methods | Double‐blind, placebo‐controlled Randomisation technique not stated Intention‐to‐treat analysis FU: no losses | |
| Participants | USA, single centre Mean age 66 years, range 46 to 83 years, 4 male, 12 female IS, 14 participants had MCA territory infarct 100% CT pre‐entry Enrolment within 72 hours Baseline SBP 170 to 220 mmHg and DBP 95 to 120 mmHg, or mean BP 120 to 140 mmHg History of previous hypertension (current treatment or clinical evidence of end organ damage) | |
| Interventions | T 1: nicardipine hydrochloride 20 mg po tds (5 participants) T 2: captopril 12.5 mg po tds (3 participants) T 3. clonidine hydrochloride 0.1 mg tds (2 participants) C: matching dextrose/starch capsule tds (6 participants) Rx: for 3 days | |
| Outcomes | Supine BP at baseline then every 10 minutes for first hour, then hourly for 6 hours, then 4 hourly BP measured using an automatic monitor (Space Labs, model IEC 601‐1) Neurological impairment: NIHSS at baseline and daily SPECT at baseline and at 3 days | |
| Notes | Exclusion: coma, significant neurological deficit from previous stroke, brain stem stroke, acute MI, severe heart failure or cardiac conduction defect, history of angioedema or collagen vascular disease, liver dysfunction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
PIL‐FAST 2013.
| Methods | Double‐blind parallel‐group external pilot controlled trial Both active and control group tablets were blister packed and placed in identical boxes. Each box had a unique study number according to the randomisation code (intervention and control in 1:1 ratio) |
|
| Participants | UK, single centre 14 participants. T: 6 C: 8 Median age 73 years Male T: 7, C: 7 Inclusion: conscious ("A" on AVPU scale), ≥ 40 years with new unilateral arm weakness thought to be due to be acute stroke and SBP > 160 mm Hg on 2 consecutive seated or lying readings taken 5 to 10 minutes apart Enrolment within 3 hours of symptom onset FU: not completed for 1 participant |
|
| Interventions | T: lisinopril (Modepharma) 5 mg sublingual and second dose of 5 mg given po, sublingual or via nasogastric tube C: matched placebo (Haupt Pharma Wuelfing) Rx: 7 days |
|
| Outcomes | BP measured seated or supine 5 to 10 minutes apart before randomisation Primary: feasibility‐recruitment rate, compliance with data collection Secondary: change in BP for 7 days (BP measurement methodology during study schedule not given); NIHSS at days 3 and 7; BI, mRS, renal function, death at day 7 |
|
| Notes | Exclusion: age < 40 years; females, pregnant, lactating or at risk of pregnancy; females < 56 years of age consented by a relative; suspected stroke without unilateral arm weakness; unable to establish whether stroke onset time was within the last 3 hours; SBP < 160 mm Hg; reduced level of consciousness below "A" on AVPU scale; patient not being transported to PIL‐FAST trial site; absence of participant or next of kin consent; known to be taking ACE inhibitor or angiotensin II receptor blocker medication already; known sensitivity to lisinopril or other ACE inhibitor medication; pulse > 120 bpm; seizure; hypoglycaemia; unable to walk independently prior to stroke; obvious understanding or memory problems when next of kin is absent; significant head trauma or brain surgery in the last 3 months; known renal failure, liver failure (or currently jaundiced); uncontrolled heart failure (breathlessness at rest); receiving palliative care for known malignancy; participating in a clinical trial assessing a study drug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list created by independent statistician |
| Allocation concealment (selection bias) | Low risk | Both lisinopril and placebo were supplied in identical boxes and each box was packaged into a secondary trial pack. Each pack carried a unique study number linked to the randomisation code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators centrally masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, both groups have similar dropout rates; clinical reasoning given |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other biases evident |
PRoFESS 2009.
| Methods | Double‐blind, 2 x 2 factorial trial Randomisation done by central telephone system |
|
| Participants | International (35 countries), multicentre (695 centres) 1360 participants. T: 647 , C: 713 Mean age T: 66.8 years, C: 67.1 years Male T: 64.9%, C: 65.1% Inclusion: IS Enrolment within 72 hours FU: no losses |
|
| Interventions | T: telmisartan 80 mg once daily C: placebo Rx: 2.5 years |
|
| Outcomes | Primary: BP, HR at days 7, 30 and 90 (BP and HR recorded using validated semiautomatic monitor ‐ Omron 705CP) Secondary: mRS day 30, haemorrhagic transformation of the infarct, cerebral oedema, recurrent stroke, MI, composite vascular events (vascular death, non‐fatal stroke, or MI), death at days 7, 30 and 90 |
|
| Notes | Exclusion: mRS > 3, using or needing ARA at time of randomisation, known severe renal insufficiency or renal artery stenosis, hyperkalaemia, uncorrected volume or sodium depletion, known severe coronary artery disease or recent MI, patients scheduled for carotid endarterectomy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Central allocation ensured allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Endpoint adjudication committee blinded; independent safety committee blinded |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | None |
Rashid 2003 10 mg.
| Methods | Open‐label, blinded‐endpoint dose comparison controlled trial Randomisation by minimisation (age, gender, SSS, mean arterial pressure) FU: no losses | |
| Participants | UK, single centre 90 participants. T: 20, C: 30 Mean age T: 70.8 years, C: 73.9 years Male T: 28, C: 13 Inclusion: IS or ICH Enrolment within 72 hours of ictus Clinical stroke subtype at baseline and CT scanning within a week of stroke onset Any antihypertensive medication was stopped at the time of admission and recommenced after 10 days once the trial treatment phase was completed | |
| Interventions | T: transdermal GTN 10 mg once daily C: no patch Rx: 10 days | |
| Outcomes | 24 hour ambulatory BP monitoring during day and hourly during night at days 0, 1, 4, 5 and 10 mRS, BI, QoL at 3 months | |
| Notes | Exclusion: SBP > 230 mmHg or < 100 mmHg, DBP > 130 mmHg or < 60 mmHg, HR > 130 bpm or < 50 bpm, mild stroke, coma, pre‐morbid dependence, or presence of illnesses that could confound neurological or functional evaluation (such as pre‐existing neurologic or psychiatric disorders) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Rashid 2003 5 mg.
| Methods | Open‐label, blinded‐endpoint dose comparison controlled trial Randomisation by minimisation (age, gender, SSS, mean arterial pressure) FU: no losses | |
| Participants | UK, single centre 90 participants. T: 20, C: 30 Mean age T: 70.8 years, C: 73.9 years Male T: 28, C: 13 Inclusion: IS or ICH Enrolment within 72 hours of ictus Clinical stroke subtype at baseline and CT scanning within a week of stroke onset Any antihypertensive medication was stopped at the time of admission and recommenced after 10 days once the trial treatment phase was completed | |
| Interventions | T: transdermal GTN 5 mg once daily C: no patch Rx: 10 days | |
| Outcomes | 24 hour ambulatory BP monitoring during day and hourly during night at days 0, 1, 4, 5 and 10 mRS, BI, QoL at 3 months | |
| Notes | Exclusion: SBP > 230 mmHg or < 100 mmHg, DBP > 130 mmHg or < 60 mmHg, HR > 130 bpm or < 50 bpm, mild stroke, coma, pre‐morbid dependence, or presence of illnesses that could confound neurological or functional evaluation (such as pre‐existing neurologic or psychiatric disorders) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Rashid 2003 5/10 mg.
| Methods | Open‐label, blinded‐endpoint dose comparison controlled trial Randomisation by minimisation (age, gender, SSS, mean arterial pressure) FU: no losses | |
| Participants | UK, single centre 90 participants. T: 20, C: 30 Mean age T: 70.8 years, C: 73.9 years Male T: 28, C: 13 Inclusion: IS or ICH Enrolment within 72 hours of ictus Clinical stroke subtype at baseline and CT scanning within a week of stroke onset Any antihypertensive medication was stopped at the time of admission and recommenced after 10 days once the trial treatment phase was completed | |
| Interventions | T: transdermal GTN 5/10 mg once daily C: no patch Rx: 10 days | |
| Outcomes | 24 hour ambulatory BP monitoring during day and hourly during night at days 0, 1, 4, 5 and 10, mRS, BI, QoL at 3 months | |
| Notes | Exclusion: SBP > 230 mmHg or < 100 mmHg, DBP > 130 mmHg or < 60 mmHg, HR > 130 bpm or < 50 bpm, mild stroke, coma, pre‐morbid dependence, or presence of illnesses that could confound neurological or functional evaluation (such as pre‐existing neurologic or psychiatric disorders) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
RIGHT 2013.
| Methods | Single‐blind, blinded‐endpoint Randomisation (1:1) Intervention in double non‐opaque envelopes carried in ambulance: outer envelope ‐ case report form; inner envelope ‐ gauze dressing +/‐ GTN patch |
|
| Participants | UK, single centre 41 participants. T: 25, C: 16 Mean age T: 79 years, C: 81 years Male T: 15 (60%), C: 7 (43.8%) Inclusion: positive FAST test Enrolment within 4 hours of symptom onset (wake‐up stroke defined as onset at bedtime) FU: no losses |
|
| Interventions | T: GTN patch C: No GTN patch Blinding: gauze dressing covering patch or similar area of skin |
|
| Outcomes | Primary: SBP at 2 hours (BP measured in the ambulance using a semiautomatic sphygmomanometer and in hospital with Omron 705 CP or 705 CP II) Secondary: 15 minutes: SBP, DBP, HR; Day 7: SSS, recurrent stroke, death, hypotension, neurological deterioration (5 point reduction in SSS); Day 90: mRS, BI, EQ‐5D, EQ‐VAS, MMSE, Zung Depression Scale |
|
| Notes | Exclusion: definite need or CI for GTN; GCS ≤ 8; blood glucose < 2.5 mmol/L; non‐ambulatory prior to symptom onset | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple randomisation equally distributed with equal distribution between treatment and control groups |
| Allocation concealment (selection bias) | Low risk | Adequate. The opaque envelope containing the gauze dressing with or without GTN was opened only after informed consent was obtained; thus the research paramedic did not know or was not able to guess treatment allocation unless the opaque envelope was opened |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | GTN was given in a single‐blind design as no manufacturer was able to supply placebo patches. Participants were blinded with placement of a gauze dressing over an area of skin out of view (e.g. back or shoulders) with or without GTN patch a underneath |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | GTN was given in a single‐blind design as no manufacturer was able to supply placebo patches. Participants were blinded with placement of a gauze dressing over an area of skin out of view (e.g. back or shoulders) with or without GTN patch a underneath. All participants were to receive best medical care and there was no difference in care between treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by investigators blinded to all clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants have been accounted for; no differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other biases found |
SCAST 2011.
| Methods | Double‐blind, placebo‐controlled, blinded‐endpoint Randomisation (1:1) by secure Internet website with both participants and investigators masked to treatment allocation |
|
| Participants | International (9 countries), multicentre (146 sites) 2029 participants. T: 1017, C: 1012 Mean age T: 70.8 years, C: 71.0 years Male T: 60%, C: 56% Inclusion: IS or ICH Enrolment within 30 hours of stroke onset and elevated SBP > 140 mm Hg FU: 25 losses |
|
| Interventions | T: candesartan (Astra Zeneca), doses increasing from 4 mg on day 1 to 16 mg on days 3 to 7 C: placebo Rx: 7 days |
|
| Outcomes | BP measured twice with validated automated blood pressure monitor (UA‐767 Plus 30, A&D Medical, San Jose, CA, USA). Primary: composite of vascular death, nonfatal MI or non‐fatal stroke in first 6 months; mRS at 6 months Secondary: SSS at day 7 and BI; death from all causes; vascular death; recurrent stroke; MI; stroke progression |
|
| Notes | Exclusion: CI to, or current treatment with ARA; markedly reduced consciousness (SSS consciousness score ≤ 2); clear indication for an ARA during treatment period; clear indication for antihypertensive treatment during the acute phase of stroke; premorbid modified mRS ≥ 4; life expectancy of 12 months or less; patient unavailable for follow‐up; pregnancy or breastfeeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by secure Internet system |
| Allocation concealment (selection bias) | Low risk | Both candesartan and placebo tablets were identical in appearance. Central web‐based allocation ensured allocation concealment. If Internet was not available, investigators used the drug pack with the lowest pack number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported; no differences between trial groups |
| Other bias | Unclear risk | No other biases found |
TAST 2013.
| Methods | Double‐blind, blinded outcome Randomisation (2:1) by computerised minimisation (age, sex, SBP, SSS, time to first Xenon scan and cortical features according to OCSP classification); randomisation and minimisation were carried out by a single investigator who had no contact with the trial participants or trial data. Randomisation sequence was generated by trial pharmacist |
|
| Participants | UK, single centre 19 participants. T: 12, C: 7 Mean age T: 71.9 years, C: 68.3 years Male: T: 10 (83%), C: 4 (57%) Inclusion:CT confirmed or suspected IS Enrolment within 5 days and elevated SBP > 140 mm Hg FU: no losses |
|
| Interventions | T: telmisartan 80 mg daily administered orally or by nasogastric tube C: placebo Rx: 90 days |
|
| Outcomes | Primary: change in ipsilateral hemispheric CBF Secondary: BP; CBF velocity; CPP; ZFP; mRS at day 90. BP was measured using OmronHEM‐ 705CP (Omron, 705IT, Kyoto, Japan) semiautomatic sphygmomanometer with participants supine or sitting; measurements were taken in the unaffected arm and done in duplicate with the average of the two readings recorded in the database |
|
| Notes | Exclusion: CI to telmisartan or xenon CT scanning, or no enteral access | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised after core data entry was complete by computer and minimised on key prognostic variables |
| Allocation concealment (selection bias) | Unclear risk | All validations made with treatment allocation blinded |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; no differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other biases evident |
Uzuner 1995.
| Methods | Double‐blind, placebo‐controlled Randomisation technique not stated Per‐protocol analysis FU: no losses | |
| Participants | Turkey, single centre 100 participants. T: 50, C: 50 100% CT pre‐entry Enrolment within 24 hours IS: 41 male, 36 female Mean age 63 years ICH: 3 male, 8 female Mean age 65 years | |
| Interventions | T IS: nimodipine 180 mg/day (60 mg tds) po T ICH: nimodipine 2 mg/h iv C: matching po or iv placebo Rx: for 2 days | |
| Outcomes | BP and HR at baseline, 5, 15, 30 and 60 minutes, then every hour for 23 hours (day 1), then every 2 hours for 24 hours (day 2). BP measured supine using unstated automatic device; LOS; GCS | |
| Notes | Exclusion: 10 participants (T 2, C 8) treated with antihypertensive agents for malignant hypertension and 2 participants with subarachnoid haemorrhage (treated with iv nimodipine) were excluded from our analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
VENTURE 2013.
| Methods | Prospective, open‐labelled, blinded‐endpoint Randomisation by computer |
|
| Participants | South Korea, multicentre (30 sites) 405 participants, T: 203, C: 202 |
|
| Interventions | T: valsartan 80 mg po once daily titrated up to 320 mg C: no valsartan Inclusion: IS and SBP 150 to 185 mm Hg Enrolment: within 24 hours Rx: 7 days |
|
| Outcomes | BP methodology not known Primary: mRS at 90 days Secondary: early neurological deterioration during the first 7 days; death at day 90 |
|
| Notes | Exclusion: not known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was an abstract only. The authors describe the study as being randomised open‐labelled and blinded‐endpoint |
| Allocation concealment (selection bias) | Unclear risk | No further information available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was published as an abstract only. The authors describe the study as being open‐labelled and blinded‐endpoint |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was published as an abstract only. The authors describe the study as being open‐labelled and blinded‐endpoint |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was published as an abstract only. The authors describe the study was prospective, open‐labelled and blinded‐endpoint |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient sufficient data were provided in the abstract to assess attrition |
| Selective reporting (reporting bias) | Unclear risk | This was an abstract only |
| Other bias | Unclear risk | This was an abstract only |
Willmot 2006.
| Methods | Participant‐ and measurement‐blinded RCT Randomisation by computer (with minimisation on age, sex, baseline SBP, baseline SSS, hours from onset, presence of a visible stroke lesion on CT) FU: no losses | |
| Participants | UK, single centre 18 participants. T: 12, C: 6 Age T: 69 years, C: 70 years Male T: 2, C: 3 Inclusion: IS or ICH, previously independent adult patients with a clinical stroke syndrome and limb weakness 100% CT Enrolment: within 5 days of ictus Prior antihypertensive medication was discontinued at the time of admission | |
| Interventions | T: transdermal GTN 5 mg (Transiderm‐Nitro5, Novartis Pharmaceuticals) once daily C: no patch Rx: 7 days | |
| Outcomes | BP was measured immediately before the baseline xenon CT scan and immediately after the post‐treatment scan Peripheral SBP and DBP was measured in the non‐hemiparetic arm with a validated digital readout oscillometric device (Omron HEM‐705CP, Omron Corp, Toyoko, Japan) Central BP was assessed by applanation tonometry of the left radial artery and using the pulse wave analysis (PWA) system (Sphygmocor, Sydney, Australia) | |
| Notes | Exclusion: requirement for or CI to nitrate therapy, had a definite need for prior antihypertensive therapy or vasoactive drugs, unable to co‐operate with scanning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
ACEI: Angiotensin converting enzyme Inhibitors ARA: Angiotensin receptor antagonist AVPU: Alert, Voice, Pain, Unresponsive (AVPU) scale BI: Barthel Index BP: blood pressure bpm: beats per minute CI: contraindication C: control group CBF: cerebral blood flow CPP: cerebral perfusion pressure CT: computed tomography DBP: diastolic blood pressure ECG: electrocardiogram EQ‐5d: European Quality of life‐5 dimensions questionnaire FU: follow up GCS: Glasgow coma scale GTN: glycerol trinitrate HR: heart rate ICH: intracerebral haemorrhage INR: International Normalised Ratio IS: ischaemic stroke iv: intravenous LOS: length of stay in hospital MAP: mean arterial pressure MCA: middle cerebral artery MI: myocardial infarction MMSE: mini mental state examination MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale OCSP: Oxford Community Stroke Project classification po: orally QoL: quality of life RCT: randomised controlled trial rtPA: recombinant tissue plasminogen activator Rx: treatment SBP: systolic blood pressure SSS: Scandinavian stroke scale T: treatment group tds: three times daily
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACCELERATE 2013 | Single‐arm, non‐blinded trial with all participants enrolled to receive iv clevidipine |
| Alem 2005 | Recruitment of participants was within 3 months and not in the acute stage; compared bendrofluazide and indapamide |
| ATACH 2006 | Prospective open‐label study; used single agent intravenous nicardipine comparing 3 tiers of BP lowering |
| Beer 2012 | Trial aim not to alter BP, but assess the effect of irbesartan on infarct size and cerebral blood flow |
| BEST 1988 | Trial aim not about altering BP, but to assess the effect of beta blockers in acute stroke |
| BIAS 2012 | Trial aim not about altering BP, but to assess the effect of neuro and cardioprotective effects of propranolol |
| BLAST 2007 | Withdrawn before recruitment |
| Bougousslavsky 1990 | Trial aim not to alter BP, but to assess the effect of nimodipine on functional outcome |
| Bridgers 1991 | Aim of the trial was not to alter BP, but to test the effect of nimodipine in acute stroke |
| Bursztyn 1985 | Head‐to‐head comparison of nifedipine + betablocker + thiazide versus nifedipine alone |
| Canwin 1993 | Trial aim not about altering BP, but to assess the effect of nimodipine in acute stroke |
| CAPON 1983 | Concealment, treatment losses, exclusion criteria, stroke criteria not available |
| Carlsson 1993 | BP and outcome data not available |
| Chandra 1995 | Head‐to‐head comparison study comparing intravenous versus oral nimodipine |
| CHERISH 2010 | Head‐to‐head comparison study comparing cilnidipine versus losartan |
| Csiba 2012 | No data available |
| Dalal 1995 | Tested nimodipine as a neuroprotectant |
| FIST 1996 | Aim not to alter BP, but assessed the effect of flunarizine on functional outcome |
| Gelmers 1984 | Not placebo controlled |
| Gelmers 1988 | Trial tested nimodipine in neurological outcome and survival |
| German‐Austrian 1994 | Trial aim not to alter BP, but to assess effect of nimodipine in neurological and functional outcome |
| Hartmann 2005 | Head‐to‐head comparison study comparing urapidil versus nifedipine |
| HASTE 2010 | No trial design or data available |
| Heiss 1990 | Study compared morphological and functional effect between nimodipine and placebo |
| Infield 1999 | Assessed the effect of nimodipine on cerebral perfusion and outcome |
| Inzhutova 2007 | Study aim not to alter BP, but to assess effect on humoral endothelial dysfunction markers in ischaemic stroke |
| Kaste 1994 | Trial tested the effect of nimodipine on functional outcome, not BP |
| Koenig 2006 | A retrospective chart review of induced hypertension |
| Kornhuber 1993 | Study assessed the effect of flunarizine on functional outcome, not BP |
| Kwon 2013 | Head‐to head comparison between amlodipine and losartan |
| Lamsudin 1995 | Assessed functional outcome with nimodipine, not BP |
| Limburg 1990 | Trial tested the effect of death and dependency of flunarizine |
| Lowe 1989 | Comparison of death and disability with oral nimodipine versus placebo |
| Marin Gámez 1988 | Published in Spanish. Very little known about treatment group, large number of participants excluded after randomisation |
| Martinez‐Vila 1990 | Trial tested the effect of nimodipine on mortality and functional outcome |
| Marzan 2004 | A retrospective evaluation of induced hypertension |
| Matias Guiu 1988 | Study tested nicardipine on cognitive impairment |
| Meier 1991 | BP data not available |
| Mohr 1992 | Aim not to alter BP, but tested the effect of nimodipine on death and functional outcome |
| MOSES 2005 | Head‐to‐head comparison study comparing eprosartan and nitrendipine |
| Nag 1998 | BP data reported 4 weeks after treatment |
| Naidech 2003 | BP data not available |
| Nakamura 2007 | Head‐to‐head comparison study comparing perindopril versus candesartan versus conventional antihypertensive therapy |
| Nazir 2004 | SBP and DBP data not available |
| Nazir 2005 | SBP and DBP data not available |
| NEST 1993 | Significant number of participants excluded after randomisation and outcomes not presented in the publication |
| NICE 2010 | Trial aim not to alter BP, but to evaluate nimodipine in preventing cognitive impairment after acute ischaemic stroke |
| Ning 2007 | Trial aim not to alter BP, but to assess the effect of nimodipine in treating perifocal oedema and neurological function after ICH |
| Oczkowski 1989 | Not relevant |
| PACI 1989 | Tested effect of nimodipine on functional outcome and mortality, not BP |
| Popa 1995 | Pre‐stroke antihypertensive drugs discontinued (nifedipine, clonidine, furosemide or acetazolamide or both) |
| Powers 1999 | BP data not available |
| Roitberg 2008 | Head‐to‐head comparison between nicardipine and nitroprusside |
| Rordorf 1997 | Retrospective study of induced hypertension |
| Rosenbaum 1990 | Feasibility and safety study |
| Rosselli 1992 | Article published in Italian; only abstract available, insufficient details |
| Sherman 1986 | Trial tested effect of nimodipine versus placebo on clinical outcome, not BP |
| Shibuya 2005 | Study compared clinical outcome between fasudil and placebo |
| Sze 1998 | Not relevant; trial tested the effect of nimodipine on memory |
| TOPS 2013 | Non‐randomised study investigating the effects of olmesartan post‐stroke |
| TRUST 1990 | Primary aim not to alter BP |
| Wang 2006 | Non‐randomised study |
| Wimalaratna 1994 | Study aim not to alter BP |
| Wityk 2008 | No comparator, all participants received intervention to induce hypertension |
| Yao 1991 | Article published in Chinese; nimodipine trial; preliminary study, insufficient details |
| Yordanov 1984 | No details of control group |
BP: blood pressure DBP: diastolic blood pressure ICH: intracerebral haemorrhage iv: intraveous SBP: systolic blood pressure
Characteristics of studies awaiting assessment [ordered by study ID]
ATTACI 2010.
| Methods | Prospective, single centre |
| Participants | Elderly people (> 65 years) with ischaemic stroke |
| Interventions | Antihypertensive medications added to existing BP medications either morning, afternoon or bedtime |
| Outcomes | BP |
| Notes | Size: 200 participants Contact person: Dr N Hosomi Department of Clinical Neuroscience and Therapeutics, Hiroshima University Graduate School of Biomedical and Health Sciences, Hiroshima, Japan |
ESH‐CHL‐SHOT 2013.
| Methods | Factorial 3 x 2 arm, phase 4 study |
| Participants | People with recent stroke or TIA |
| Interventions | Randomisation to one of 3 BP targets:
Random allocation to 1 of 2 lipid‐lowering targets:
|
| Outcomes | Recurrent stroke Time to recurrent stroke Composite vascular events: cardiovascular death, non‐fatal stroke, non‐fatal MI and cardiac failure Cognitive impairment and dementia |
| Notes | Size: 7500 participants Funding: Instituto Auxologico Italiano, Italy |
MAPAS 2009.
| Methods | Single centre, randomised, open‐label, parallel assignment |
| Participants | Non‐thrombolysed acute ischaemic stroke patients within 6 hours of ictus onset |
| Interventions | T1: infusion up to 1 litre of saline and/or norepinephrine T2: infusion of esmolol or sodium nitroprusside |
| Outcomes | Primary: mRS at day 90 Secondary: treatment feasibility of the antihypertensive treatment, comparing the SBP range for the 24‐hour period |
| Notes | Size: 240 Funding: Hospital de Clinicas de Porto Alegre, Brazil |
STABLE‐ICAS 2010.
| Methods | Phase 4 study |
| Participants | People with subacute ischaemic stroke due to symptomatic severe intracranial atherosclerosis |
| Interventions | Intensive BP lowering (SBP < 120 mm Hg) compared with modest BP control (SBP < 140mm Hg) |
| Outcomes | Primary outcomes: ischaemic lesion volume change in the whole forebrain on fluid attenuation inversion recovery (FLAIR) magnetic resonance imaging (MRI); difference between final ischaemic lesions volume and base ischaemic lesions of both hemisphere on FLAIR MRI Secondary outcomes: Ischaemic lesion volume change in the territory of symptomatic intracranial disease on FLAIR MRI difference between final ischaemic lesions volume and base ischaemic lesions in the territory of symptomatic intracranial disease on FLAIR MRI; participants with new ischaemic lesion in the whole forebrain on FLAIR MRI; cardiovascular events; vascular death; number of adverse events and adverse drug reactions |
| Notes | Size: 156 participants Funding: Asan Medical Center, South Korea |
BP: blood pressure LDL‐C: low‐density lipoprotein cholesterol MI: myocardial infarction mRS: modified Rankin Scale SBP: systolic blood pressure TIA: transient ischaemic attack
Characteristics of ongoing studies [ordered by study ID]
ATACH‐2 2011.
| Trial name or title | Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH II) |
| Methods | Multicentre, prospective, open‐label, phase 3 randomised trial |
| Participants | People with supratentorial intracerebral haemorrhage |
| Interventions | Early intensive BP lowering with nicardipine iv, or management according to current American Heart Association guidelines |
| Outcomes | Primary outcome: combining death and dependency, according to a 4 to 6 score on the mRS at 90 days Secondary outcomes: all cause and cause‐specific early neurological deterioration during the first 24 hours; haematoma expansion at 24 hours; quality of life at 3 months |
| Starting date | 2011 |
| Contact information | Prof Adnan I Qureshi University of Minnesota, MMC 295, 420 Delaware St. SE., Minneapolis MN 55455, USA |
| Notes | Size: 1280 participants Funding: National Institutes of Health, USA |
ENCHANTED 2011.
| Trial name or title | Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED) |
| Methods | Prospective, international, multicentre, open‐label, blinded‐endpoint quasi‐factorial randomised trial |
| Participants | People with ischaemic stroke within 4.5 hours of ictus and SBP ≤ 185mm Hg |
| Interventions |
|
| Outcomes | Primary outcome: death and dependency, according to a 2 to 6 score on the mRS Secondary outcomes: early neurological deterioration during the first 72 hours; symptomatic intracerebral haemorrhage during the first 7 days; day 90: discharge: quality of life; length of stay; disposition; mortality |
| Starting date | 2012 |
| Contact information | Prof Craig Anderson, The George Institute, PO Box M 201, Misssenden Road, Sydney NSW 2050, Australia |
| Notes | Size: 3300 Sponsor: National Health and Medical Research Council of Australia (NHMRC) |
FAST‐BP 2013.
| Trial name or title | Field Administration of Stroke Therapy‐Blood Pressure Lowering (FAST‐BP) |
| Methods | Single centre, prospective, open‐label, safety and feasibility study |
| Participants | People with ischaemic stroke or intracerebral haemorrhage |
| Interventions | T1: GTN 5 mg/24 hour patch T2: GTN 10 mg/24 hour patch T3: GTN 5 mg/24 hour patch plus single metered dose 0.4 mg sublingual GTN |
| Outcomes | Primary outcome: mean BP change 15 minutes after treatment Secondary outcomes: early neurological deterioration (two point or greater worsening in GCS) during the first hour; SBP less than 120 mm Hg; serious adverse events at 90 days |
| Starting date | 2013 |
| Contact information | Dr Nerses Sanossian, Keck School of Medicine of University of South California, 1520 San Pablo St, Los Angeles, CA90033, USA |
| Notes | Size: 45 Sponsor: University of California Los Angeles, USA |
SETIN‐HYPERTENSION 2012.
| Trial name or title | Safety and Efficacy of Therapeutic Induced Hypertension in Acute Non‐cardioembolic Ischemic Stroke |
| Methods | Multicentre, prospective, randomised, open‐label, phase 3 study |
| Participants | Acute ischaemic stroke, confirmed by DWI within 24 hours of onset of ictus, or people who show a 2‐point or more increase in NIHSS including one or more increase in the motor score of the affected arm or leg or clear evidence of symptom worsening judged by the investigator confirmed by DWI performed within 24 hours of symptom aggravation |
| Interventions | T: iv phenylephrine C: no phenylephrine |
| Outcomes | Primary outcome: NIHSS between day 0 and day 7 Secondary outcomes: Day 7: infarct growth or new ischaemic lesion on MRI; Day 90: mRS, BI; symptomatic intracerebral haemorrhage or cerebral oedema, MI, death from any cause during the first 3 months; intracerebral haemorrhage on follow up MRI and side effects (headache, arrhythmia, chest pain, dysuria, or gastrointestinal haemorrhage) up to 3 months |
| Starting date | 2012 |
| Contact information | Prof Oh Young Bang Samsung Medical Centre, South Korea |
| Notes | Size: 170 Sponsor: Samsung Medical Centre |
BI: Barthel Index BP: blood pressure DWI: Diffusion‐weighted imaging GCS: Glasgow Coma Score GTN: glyceryl trinitrate iv: intravenous MI: myocardial infarction MRI: magnetic resonance imaging mRS: modified Rankin Score NIHSS: National Institutes of Health Stroke Scale rtPA: recombinant tissue plasminogen activator SBP: systolic blood pressure
Differences between protocol and review
None relevant.
Contributions of authors
PMW Bath was involved with the design, development of search strategies, analysis and writing. He is the study guarantor. K Krishnan was involved with searches for studies, input of data into the latest version, analysis of the latest version, and writing.
Sources of support
Internal sources
No sources of support supplied
External sources
South Thames NHS Executive (1995‐1997), UK.
Wolfson Foundation (1993‐1998), UK.
Trent NHS Executive (1998‐2000), UK.
The Stroke Association (1998‐2015), UK.
Medical Research Council (2006‐2014), UK.
British Heart Foundation (2006‐2008), UK.
Declarations of interest
PMWB was involved with eight completed trials (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; PRoFESS 2009; SCAST 2011; RIGHT 2013; TAST 2013; ENOS 2014), each of which are included in this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ACCESS 2003 {published data only}
- Kulschewski A, Luders S, Venneklaas U, Schoder J. Blood‐pressure rhythm in acute cerebral ischemia. Analyses from the ACCESS‐Study: Acute Candesartan Cilexetil Evaluation in Stroke Survivors. Deutsche Medizinische Wochenschrift 2003;128 Suppl 3:S160. [Abst.V48] [Google Scholar]
- Michel P, Bogousslavsky J, Kernan WN, Schrader J, Luders S, Kulschewski A, et al. ACCESS Study: blood pressure effect?. Stroke 2003;34(12):e237‐8. [DOI] [PubMed] [Google Scholar]
- Schrader J, Luders S, Kulschewski A, Berger J, Zidek W, Treib J, et al. The ACCESS study: evaluation of acute candesarten cilexetil therapy in stroke survivors. Stroke 2003;34:1699‐703. [DOI] [PubMed] [Google Scholar]
ACCOST 2006 {published and unpublished data}
- O'Brien RE, Palmer L, Johnston D, O'Connell JE, Hildreth AJ, Gray CS. The Acute Candesartan Cilexetil Outcomes Stroke Trial (ACCOST): Candesartan gradually lowers blood pressure following acute ischaemic stroke. Proceedings of the 2nd International Conference on Hypertension, Lipids, Diabetes & Stroke Prevention. 6‐8 March 2008; Prague, Czech Republic. 2008.
Bath 2000 {published and unpublished data}
- Bath PMW, Pathansali R, Bath FJ. Effect of nitric oxide on blood pressure in acute stroke. The Challenge of Stroke. Lancet Conference, Montreal. 1998:40‐1.
- Bath PMW, Pathansali R, Iddenden R, Bath FJ. The effect of nitric oxide, given as transdermal glyceryl trinitrate, on blood pressure in acute stroke. Cerebrovascular Diseases 1999;9 Suppl 1:101. [DOI] [PubMed] [Google Scholar]
- Bath, PMW, Pathansali R, Iddenden R, Bath FJ. The effect of transdermal glyceryl trinitrate, a nitric oxide donor on blood pressure and platelet function in acute stroke. Cerebrovascular Diseases 2000;11:265‐72. [DOI] [PubMed] [Google Scholar]
CATIS 2013 {published data only}
- He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, et al. CATIS Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA 2014;311(5):479‐89. [DOI] [PubMed] [Google Scholar]
CHHIPS 2009 {published data only}
- Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al. Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial. Health Technology Assessment 2009;13(9: iii, ix‐xi):1‐73. [DOI] [PubMed] [Google Scholar]
- Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post‐stroke (CHHIPS): a randomised, placebo‐controlled, double‐blind pilot trial. Lancet Neurology 2009;8(1):48‐56. [DOI] [PubMed] [Google Scholar]
- The CHHIPS Trial Group. CHHIPS (Controlling Hypertension and Hypotension Immediately Post‐Stroke) pilot trial: rationale and design. Journal of Hypertension 2005;23:649‐55. [DOI] [PubMed] [Google Scholar]
COSSACS 2010 {published data only}
- Cossacs Trial Group. COSSACS (Continue or Stop post‐Stroke Antihypertensive Collaborative Study): rationale and design. Journal of Hypertension 2005;23(2):455‐8. [DOI] [PubMed] [Google Scholar]
- Robinson TG, Potter JF, Ford GA, Bulpitt CJ, Chernova J, Jagger C, COSSACS Investigators. Effects of antihypertensive treatment after acute stroke in the Continue Or Stop post‐Stroke AntihypertensivesCollaborative Study (COSSACS): a prospective, randomised,open, blinded‐endpoint trial. Lancet Neurology 2010;9(8):767‐75. [DOI] [PubMed] [Google Scholar]
Dyker 1997 {published and unpublished data}
- Dyker AG, Grosset DG, Lees K. Perindopril reduces blood pressure but not cerebral blood flow in patients with recent cerebral ischemic stroke. Stroke 1997;28:580‐3. [DOI] [PubMed] [Google Scholar]
- Dyker AG, Grosset DG, Lees KR. Effect of perindopril on blood pressure and cerebral blood flow after acute ischaemic stroke. European Journal of Neurology 1995;2 Suppl 2:7. [Google Scholar]
Eames 2005 {published data only}
- Eames PJ, Robinson TG, Panerai RB, Potter JF. Bendrofluazide fails to reduce elevated blood pressure levels in the immediate post‐stroke period. Cerebrovascular Diseases 2005;19:253‐9. [DOI] [PubMed] [Google Scholar]
ENOS 2014 {published data only}
- Bath PMW, Woodhouse L, Scutt P, Krishnan K, Wardlaw JM, Berezcki D, et al. Glyceryl trinitrate vs. control, and continue versus stop temporarily prior antihypertensive therapy in acute stroke: main results from the Efficacy of Nitric Oxide in Stroke (ENOS) trial. Lancet (in press) 2014.
- The ENOS Trial Investigators. Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122). International Journal of Stroke 2006;1:2459. [DOI] [PubMed] [Google Scholar]
Eveson 2007 {published and unpublished data}
- Eveson DJ, Robinson TG, Potter JF. Lisinopril for the treatment of hypertension within the first 24 hours of acute ischemic stroke and follow up. American Journal of Hypertension 2007;20:270‐7. [DOI] [PubMed] [Google Scholar]
Fagan 1988 120 mg {published data only}
- Fagan SC, Gengo FM, Bates V, Levine SR, Kinkel WR. Effect of nimodipine on blood pressure in acute ischemic stroke in humans. Stroke 1988;19:401‐2. [DOI] [PubMed] [Google Scholar]
Fagan 1988 240 mg {published data only}
- Fagan SC, Gengo FM, Bates V, Levine SR, Kinkel WR. Effect of nimodipine on blood pressure in acute ischemic stroke in humans. Stroke 1988;19:401‐2. [DOI] [PubMed] [Google Scholar]
Hillis 2003 {published and unpublished data}
- Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp NJ, et al. A pilot randomised trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular Diseases 2003;16:236‐48. [DOI] [PubMed] [Google Scholar]
ICH‐ADAPT 2013 {published data only}
- Butcher K, Jeerakathil T, Emery D, Dowlatshahi D, Hill MD, Sharma M, et al. The Intracerebral Haemorrhage Acutely Decreasing Arterial Pressure Trial: Intracerebral haemorrhage ADAPT. International Journal of Stroke 2010;5:227‐33. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, Intracerebral haemorrhage ADAPT Investigators. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke 2013;44:620‐6. [DOI] [PubMed] [Google Scholar]
INTERACT‐2 2013 {published data only}
- Anderson C. INTERACT2 the second intensive blood pressure reduction in acute cerebral haemorrhage trial. ClinicalTrials.gov 2008. [NCT00716079]
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, INTERACT2 Investigators. Rapid blood‐pressure lowering in patients with acute intracerebral haemorrhage. New England Journal of Medicine 2013;368(25):2355‐65. [DOI] [PubMed] [Google Scholar]
- Delcourt C, Huang Y, Wang J, Heeley E, Lindley R, Stapf C, et al. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2). International Journal of Stroke 2010;5:110‐6. [DOI] [PubMed] [Google Scholar]
INTERACT pilot 2008 {published data only}
- Anderson C. INTERACT: Intensive blood pressure reduction in acute cerebral haemorrhage trial. http://www.thegeorgeinstitute.org/research/neurological‐diseases 2005. [DOI] [PubMed]
- Anderson CS, Butcher K, Huang Y, Morgenstern L. INTERACT (Intensive blood pressure reduction in acute cerebral haemorrhage trial): rationale, design, and early results. Journal of the Neurological Sciences 2005;238 Suppl 1:S381. [Google Scholar]
- Anderson CS, Huang Y, Wang J, Morgenstern L, Butcher K, Neal B, et al. INTERACT (Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) rationale and design. Proceedings of the International Stroke Conference. 2006.
- Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurology 2008;7(5):391‐9. [DOI] [PubMed] [Google Scholar]
Koch 2008 {published data only}
- Koch S, Romano JG, Forteza AM, Otero CM, Rabinstein AA. Rapid blood pressure reduction in acute intracerebral hemorrhage: feasibility and safety. Neurocritical Care 2008;8:316‐21. [DOI] [PubMed] [Google Scholar]
Lisk 1993 {published and unpublished data}
- Lisk DR, Grotta JC, Lamki LM, Tran HD, Taylor JW, Molony DA, et al. Should hypertension be treated after acute stroke? A randomized controlled trial using single photon emission computed tomography. Archives of Neurology 1993;50:855‐62. [DOI] [PubMed] [Google Scholar]
PIL‐FAST 2013 {published data only}
- Shaw L, Price C, McLure S. Paramedic Initiated Lisinopril For Acute StrokeTreatment (PIL‐FAST): study protocol for a pilot randomised controlled trial. Trials 2011;12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Price C, McLure S, Howel D, McColl E, Younger P, et al. Paramedic Initiated Lisinopril For Acute StrokeTreatment (PIL‐FAST): results from the pilot randomised controlled trial. Emergency Medical Journal 2013:1‐6. [DOI] [PMC free article] [PubMed]
PRoFESS 2009 {published data only}
- Bath PMW, Martin RH, Palesch Y, Cotton D, Yusuf S, Sacco R, PRoFESS Study Group. Effects of telmisartan on functional outcome, recurrence and blood pressure in patients with acute mild ischemic stroke: a PRoFESS subgroup analysis. Stroke 2009;40:3541‐6. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Diener HC, Yusuf S, Cotton D, Ôunpuu S, Lawton WA, the PRoFESS Study Group. Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke. New England Journal of Medicine 2008;359:1238‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rashid 2003 10 mg {published and unpublished data}
- Rashid P, Weaver C, Leonardi‐Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac haemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
Rashid 2003 5/10 mg {published and unpublished data}
- Rashid P, Weaver C, Leonardi‐Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac haemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
Rashid 2003 5 mg {published and unpublished data}
- Rashid P, Weaver C, Leonardi‐Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac haemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
RIGHT 2013 {published data only}
- Ankolekar S, Fuller M, Cross I, Renton C, Cox P, Sprigg N, et al. Feasibility of an Aabulance‐based stroke trial, and safety of glyceryl trinitrate in ultra‐acute stroke: the Rapid Intervention with Glyceryl trinitrate in Hypertensive stroke Trial (RIGHT). Stroke 2013;44(11):3120‐8. [ISRCTN66434824] [DOI] [PubMed] [Google Scholar]
- Ankolekar S, Gare S, Geeganage C, Fuller M, Stokes L, Sprigg N, et al. Determining the feasibility of ambulance‐based randomised controlled trials in patients with ultra‐acute stroke: study protocol for the "Rapid Intervention with GTN in Hypertensive stroke Trial" (RIGHT). Stroke Research and Treatment 2012;2012:385753. [ISRCTN66434824] [DOI] [PMC free article] [PubMed] [Google Scholar]
SCAST 2011 {published data only}
- Sandset EC, Bath PMW, Boysen G, Jatuzis D, Kõrv J, Lüders S, SCAST Study Group. The angiotensin‐receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo‐controlled, double‐blind trial. Lancet 2011;377:741‐50. [DOI] [PubMed] [Google Scholar]
- Sandset EC, Murray G, Boysen G. Angiotensin receptor blockade in acute stroke. The Scandinavian Candesartan Acute Stroke Trial: rationale, methods and design of a multicentre, randomised and placebo‐controlled clinical trial. International Journal of Stroke 2010;5:423‐7. [NCT00120003] [DOI] [PubMed] [Google Scholar]
TAST 2013 {published data only}
- Sare GM, Ghadami A, Ankolekar S, England T, Hammonds F, Adrian M, et al. Effect of telmisartan on cerebral and systemic haemodynamics in patients with recent ischaemic stroke: a randomised controlled trial. ISRN Stroke 2013;2013:1‐9. [Google Scholar]
Uzuner 1995 {published and unpublished data}
- Uzuner N, Gucuyener D, Ozdemir G. The interaction between nimodipine and systemic blood pressure and pulse rate. Unpublished manuscript.
- Uzuner N, Ozdemir G, Gucuyener D. The interaction between nimodipine and systemic blood pressure and pulse rate. Pan‐European consensus meeting on stroke management. 8‐10 November 1995, Helsingborg, Sweden. 1995.
VENTURE 2013 {published data only}
Willmot 2006 {published and unpublished data}
- Willmot M, Ghadami A, Clarke W, Weaver C, Wardlaw J, Bath P. Effect of glyceryl tri‐nitrate, a nitric oxide donor, on blood pressure and cerebral blood flow in acute/subacute stroke. Journal of the Neurological Sciences 2005;238 Suppl 1:Abst OPL104. [Google Scholar]
- Willmot M, Ghadami A, Whysall B, Clarke W, Wardlaw J, Bath PMW. Transdermal glyceryl trinitrate lowers blood pressure and maintains cerebral blood flow in recent stroke. Hypertension 2006;47:1209‐15. [DOI] [PubMed] [Google Scholar]
- Willmot M, Ghadami A, Whysall B, Weaver C, Bath P. Glyceryl trinitrate, a nitric oxide donor, lowers blood pressure but not cerebral blood flow in patients with acute/subacute stroke. Cerebrovascular Diseases 2005;19 Suppl 2:31. [Google Scholar]
References to studies excluded from this review
ACCELERATE 2013 {published data only}
- Graffagnino C, Bergese S, Love J, Schneider D, Lazaridis C, LaPointe M, et al. Clevidipine rapidly and safely reduces blood pressure in acute intracerebral hemorrhage: the ACCELERATE trial. Cerebrovascular Diseases 2013;36(3):173‐80. [DOI] [PubMed] [Google Scholar]
Alem 2005 {published data only}
- Alem M, Milia P, Muir S, Lees K, Walters M. Comparison of the effects of diuretics on blood pressure and arterial stiffness in stroke patients. Cerebrovascular Diseases 2005;19 Suppl 2:142 . [DOI] [PubMed] [Google Scholar]
ATACH 2006 {published data only}
- Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) Trial: final results. Critical Care Medicine 2010;38(2):637‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beer 2012 {published data only}
- Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB. A randomized placebo controlled trial of early treatment of acute ischemic stroke with atorvastatin and irbesartan. International Journal of Stroke 2012;7:104‐11. [DOI] [PubMed] [Google Scholar]
BEST 1988 {published data only}
- Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JRA. Low dose blockade in acute stroke ("BEST" trial): an evaluation. British Medical Journal 1988;296:737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
BIAS 2012 {published data only}
- Haverkamp W. Beta blockers In Acute ischaemic Stroke (BIAS). ClinicalTrials.gov 2012. [NCT01061190]
BLAST 2007 {published data only}
- Albers G, Schwartz N. Blood pressure Lowering in Acute Stroke Trial (BLAST). ClinicalTrials.gov 2011. [NCT01400256]
Bougousslavsky 1990 {published data only}
- Bogousslavsky J, Regli F, Zumstein V, Kobberling W. Double‐blind study of nimodipine in non‐severe stroke. European Neurology 1990;30:23‐6. [DOI] [PubMed] [Google Scholar]
Bridgers 1991 {published data only}
- Bridgers SL, Koch G, Munera C, Karwon M, Kurtz NM. Intravenous nimodipine in acute stroke: interim analysis of randomized trials. Stroke 1991;22:153. [Google Scholar]
Bursztyn 1985 {published data only}
- Bursztyn M, Grossman E, Rosenthal T. Long‐acting nifedipine in moderate and severe hypertensive patients with serious concomitant diseases. American Heart Journal 1985;110(1 Pt 1):96‐101. [DOI] [PubMed] [Google Scholar]
Canwin 1993 {published data only}
- Norris JW, LeBrun LH, Anderson BA, The Canwin Study Group. Intravenous nimodipine in acute ischaemic stroke. Cerebrovascular Diseases 1994;4:194‐6. [Google Scholar]
CAPON 1983 {unpublished data only}
- Nimodipine for acute ischaemic stroke. Bayer AG, Wuppertal, Germany 1983.
Carlsson 1993 {unpublished data only}
- Carlsson A. Management of high blood pressure in stroke patients. Thesis 1993.
Chandra 1995 {published data only}
- Chandra B. A new form of management of stroke. Journal of Stroke and Cerebrovascular Diseases 1995;5:241‐3. [DOI] [PubMed] [Google Scholar]
CHERISH 2010 {published data only}
- Hong KS, Kang DW, Bae HJ, Kim YK, Park JM, Rha JH, et al. Effect of cilnidipine vs losartan on cerebral blood flow in hypertensive patients with a history of ischemic stroke. Acta Neurologica Scandinavica 2009;121(1):51‐7. [DOI] [PubMed] [Google Scholar]
Csiba 2012 {unpublished data only}
- Zhang J, Yang J, Zhang C, Jiang X, Zhou H, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001928.pub2] [DOI] [PubMed] [Google Scholar]
Dalal 1995 {published data only}
- Dalal PM, Dalal KP. Use of calcium channel blockers in acute ischemic cerebrovascular disease. Journal of the Association of Physicians of India 1995;43(6):394‐7. [PubMed] [Google Scholar]
FIST 1996 {published data only}
- Franke CL, Palm R, Dalby M, Hantson L, Eriksson B, Lang‐Jenssen L, et al. Flunarizine in stroke treatment (FIST). A double‐blind, placebo‐controlled trial in Scandinavia and the Netherlands. Acta Neurologic Scandinavica 1996;93:56‐60. [DOI] [PubMed] [Google Scholar]
Gelmers 1984 {published data only}
- Gelmers HJ. The effects of nimodipine on the clinical course of patients with acute ischemic stroke. Acta Neurologica Scandinavica 1984;69:232‐9. [DOI] [PubMed] [Google Scholar]
Gelmers 1988 {published data only}
- Gelmers HJ, Gorter K, Weerdt CJ, Wiezer HJA. A controlled trial of nimodipine in acute ischemic stroke. New England Journal of Medicine 1988;318:203‐7. [DOI] [PubMed] [Google Scholar]
German‐Austrian 1994 {published data only}
- Kramer G, Tettenborn B, Schmutzhard E, Aichner F, Schwartz A. Nimodipine in acute ischemic stroke. Results of the nimodipine German‐Austrian stroke trial. Cerebrovascular Diseases 1994;4:182‐8. [Google Scholar]
Hartmann 2005 {published data only}
- Hartmann A, Pavlidis C, Dettmers C, Rommel T. The effect of antihypertensive drugs on intracranial pressure and cerebral blood flow in patients with acute stroke. Cerebrovascular Diseases 2005;19 Suppl 2:2. [Google Scholar]
HASTE 2010 {unpublished data only}
- Suri FK. Hypertension in Acute Stroke Treatment (HASTE). http://www.strokecenter.org/trials/clinicalstudies/hypertension‐in‐acute‐stroke‐treatment/description 2010.
Heiss 1990 {published data only}
- Heiss WD, Holthoff V, Pawlik G, Neveling M. Effect of nimodipine on regional cerebral glucose metabolism in patients with acute ischemic stroke as measured by positron emission tomography. Journal of Cerebral Blood Flow and Metabolism 1990;10:127‐32. [DOI] [PubMed] [Google Scholar]
Infield 1999 {published data only}
- Infeld B, Davis SM, Donnan GA, Yasaka M, Lichtenstein M, Mitchell PJ, et al. Nimodipine and perfusion changes after stroke. Stroke 1999;30:1417‐23. [DOI] [PubMed] [Google Scholar]
Inzhutova 2007 {published data only}
- Inzhutova AI, Petrova MM, Salmina AB. The therapy of complicated forms of essential hypertension with ACE inhibitor perindopril. Klinicheskaia Meditsina 2007;85(11):58‐61. [PubMed] [Google Scholar]
Kaste 1994 {published data only}
- Kaste M, Fogelholm R, Erila T, Palomaki H, Murros K, Rissanen A, et al. A randomized, double‐blind, placebo controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke 1994;25:1348‐53. [DOI] [PubMed] [Google Scholar]
Koenig 2006 {published data only}
- Koenig MA, Geocadin RG, Grouchy M, Glasgow J, Vimal S, Restrepo L, et al. Safety of induced hypertension therapy in patients with acute ischemic stroke. Neurocritical Care 2006;4:3‐7. [DOI] [PubMed] [Google Scholar]
Kornhuber 1993 {published data only}
- Kornhuber HH, Hartung J, Herrlinger JD, Hertel G, Hulser P‐J, Prange H, et al. Flunarizine in ischemic stroke: a randomised, multicentre, placebo‐controlled, double‐ blind study. Neurology Psychiatry and Brain Research 1993;1:173‐80. [Google Scholar]
Kwon 2013 {published data only}
- Kwon HM, Shin JW, Lim JS, Hong YH, Lee YS, Nam H, et al. Comparison of effects of amlodipine and losartan on the blood pressure and its variation in hypertensive patients with acute stroke: a randomized double‐blind non‐inferiority trial. Proceedings of the International Stroke Conference, Honolulu, Hawaii. 2013:Abstract: LB P17.
Lamsudin 1995 {published data only}
- Lamsudin R, Misbach J, Andradi, Petru Cahyadi A, Hadirioto S, Sumaryanto F. A controlled trial of nimodipine in acute ischaemic stroke. Indonesian Journal of Clinical Epidemiology and Biostatistics 1997;4(2):46‐50. [Google Scholar]
Limburg 1990 {published data only}
- Limburg M, Hijdra A. Flunarizine in acute ischemic stroke: a pilot study. European Neurology 1990;30(3):121‐2. [DOI] [PubMed] [Google Scholar]
Lowe 1989 {published data only}
- Zhang J, Yang J, Zhang C, Jiang X, Zhou H, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001928.pub2] [DOI] [PubMed] [Google Scholar]
Marin Gámez 1988 {published data only}
- Marín Gámez N, Soto Mas JA, Aguilar Martínez JL, Bermúdez García JM, Salim A, Ramos Jiménez A, et al. A controlled double blind clinical trial of nicardipine versus placebo in acute focal cerebral ischaemia [Ensayo clinicocontrolado a doble ciego: nicardipina frente a placebo en laisquemia cerebral focal aguda]. Medicina Clinica 1988;90:690‐2. [PubMed] [Google Scholar]
Martinez‐Vila 1990 {published data only}
- Martinez‐Vila E, Guillen F, Villanueva JA, Matias‐Guiu J, Bigorra J, Gil P, et al. Placebo‐controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction. Stroke 1990;21:1023‐8. [DOI] [PubMed] [Google Scholar]
Marzan 2004 {published data only}
- Marzan AS, Hungerbühler HJ, Studer A, Baumgartner RW, Georgiadis D. Feasibility and safety of norepinephrine‐induced arterial hypertension in acute ischemic stroke. Neurology 2004;62:1193‐5. [DOI] [PubMed] [Google Scholar]
Matias Guiu 1988 {published data only}
- Matias‐Guiu J, Molto JM, Galiano L, Insa R, Falip R, Martin R. Pilot double‐blind placebo controlled trial of nicardipine versus placebo for cognitive impairment in minor stroke patients. Journal of Neurology 1992;239(2):S39. [Google Scholar]
Meier 1991 {published data only}
- Meier F, Wessel G, Thiele R, Gottschild D, Brandstatt H. Induced hypertension as an approach to treating acute cerebrovascular ischaemia: possibilities and limitations. Experimental Pathology 1991;42:257‐63. [DOI] [PubMed] [Google Scholar]
Mohr 1992 {published data only}
- The American Nimodipine Study Group. Clinical trial of nimodipine in acute ischemic stroke. Stroke 1992;23(1):3‐8. [DOI] [PubMed] [Google Scholar]
MOSES 2005 {published data only}
- Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, the MOSES Study Group. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention. Stroke 2005;36:1218‐24. [DOI] [PubMed] [Google Scholar]
Nag 1998 {published data only}
- Nag D, Garg RK, Varma M. A randomized double‐blind controlled study of nimodipine in acute cerebral ischemic stroke. Indian Journal of Physiology and Pharmacology 1998;42:555‐8. [PubMed] [Google Scholar]
Naidech 2003 {published data only}
- Naidech A, Khasani S, Lafaye K, Martin J, Weisberg L. Chart review and pilot study of blood pressure control in acute ischemic stroke. Journal of the Louisiana State Medical Society 2003;155:99‐102. [PubMed] [Google Scholar]
Nakamura 2007 {published data only}
- Nakamura T, Uchiyama S, Tsutsumi Y, Shimizu Y, Iwata M. Renin‐angiotensin system blockade safely reduces blood pressure without affecting cerebral blood flow in patients with acute ischemic stroke. Stroke 2007;38(2):517. [DOI] [PubMed] [Google Scholar]
Nazir 2004 {published data only}
- Nazir FS, Overell JR, Bolster A, Hilditch TE, Reid JL, Lees KR. The effect of losartan on global and focal cerebral perfusion and on renal function in hypertensives in mild early ischaemic stroke. Journal of Hypertension 2004;22:989‐95. [DOI] [PubMed] [Google Scholar]
Nazir 2005 {published data only}
- Nazir FS, Overell JR, Bolster A, Hilditch TE, Lees KR. Effect of perindopril on cerebral and renal perfusion on normotensives in mild early ischaemic stroke: a randomised controlled trial. Cerebrovascular Diseases 2005;19:77‐83. [DOI] [PubMed] [Google Scholar]
NEST 1993 {published data only}
- Hennerici M, Kramer G, North PM, Schmitz H, Tettenborn D, Nimodipine European Stroke Trial Group (NEST). Nimodipine in the treatment of acute MCA ischemic stroke. Cerebrovascular Diseases 1994;4:189‐93. [Google Scholar]
NICE 2010 {published data only}
- Wang P, Wang Y, Feng T, Zhao X, Zhou Y, Wang Y, et al. Rationale and design of a double‐blind, placebo‐controlled, randomised trial to evaluate the safety and efficacy of nimodipine in preventing cognitive impairment in ischaemic cerebrovascular events (NICE). BMC Neurology 2012;12:88. [DOI: 10.1186/1471-2377-12-88] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ning 2007 {published data only}
- Ning X, Liu Q, Zhao H. Nimodipine for treatment of perifocal edema following aspiration and drainage of patients with cerebral haemorrhage. Neural Regeneration Research 2007;2(5):310‐3. [Google Scholar]
Oczkowski 1989 {published data only}
- Oczkowski WJ, Hachinski VC, Bogousslavsky J, Barnett HJ, Carruthers SG. A double‐blind, randomized trial of PY108‐068 in acute ischemic cerebral infarction. Stroke 1989;20(5):604‐8. [DOI] [PubMed] [Google Scholar]
PACI 1989 {published data only}
- Paci A, Ottaviano P, Trenta A, Iannone G, Santis L, Lancia G, et al. Nimodipine in acute ischemic stroke: a double‐blind controlled study. Acta Neurologica Scandinavica 1989;80:282‐6. [DOI] [PubMed] [Google Scholar]
Popa 1995 {published data only}
- Popa G, Voiculescu V, Popa C, Stanescu A, Nistorescu A, Jipescu I. Stroke and hypertension, antihypertensive therapy withdrawal. Romanian Journal of Neurology and Psychiatry 1995;33:29‐36. [PubMed] [Google Scholar]
Powers 1999 {published data only}
- Powers WJ, Adams RE, D Yundt K, Manno EM, Diebert E, Zazulia A, et al. Acute pharmacological hypotension after intracerebral hemorrhage does not change cerebral blood flow. Stroke 1999;30:242. [Google Scholar]
Roitberg 2008 {published data only}
- Roitberg BZ, Hardman J, Urbaniak K, Merchant A, Mangubat EZ, Alaraj A, et al. Prospective randomized comparison of safety and efficacy of nicardipine and nitroprusside drip for control of hypertension in the neurosurgical intensive care unit. Neurosurgery 2008;63(1):115‐21. [DOI] [PubMed] [Google Scholar]
Rordorf 1997 {published data only}
- Rordorf G, Cramer SC, Efird JT, Schwamm LH, Buonanno F, Koroshetz WJ. Pharmacological elevation of blood pressure in acute stroke. Clinical effects and safety. Stroke 1997;28(11):2133‐8. [DOI] [PubMed] [Google Scholar]
Rosenbaum 1990 {published data only}
- Rosenbaum D, Zabramski J, Frey J, Yatsu F, Marler J, Spetzler R, et al. Early treatment of ischemic stroke with a calcium antagonist. Stroke 1991;22:437‐41. [DOI] [PubMed] [Google Scholar]
Rosselli 1992 {published data only}
- Rosselli A, Landini G, Castagnoli A, Vannucchi L, Mugnaini C, Calacoci S, et al. The nimodipine therapy of acute focal cerebral ischemia (minor stroke). A clinical study with an assessment of regional cerebral blood flow by SPECT. Recenti Progressi in Medicina 1992;83(2):85‐8. [PubMed] [Google Scholar]
Sherman 1986 {published data only}
- Sherman DG, Easton JD, Hart RG, Sherman CP. Acute Brain Ischaemia: Medical and Surgical Therapy. Raven Press, 1986:257‐62. [Google Scholar]
Shibuya 2005 {published data only}
- Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E. Effects of fasudil in acute ischemic stroke: results of a prospective placebo‐controlled double‐blind trial. Journal of the Neurological Sciences 2005;238:31‐9. [DOI] [PubMed] [Google Scholar]
Sze 1998 {published data only}
- Sze KH, Sim TC, Wong E, Cheng S, Woo J. Effect of nimodipine on memory after cerebral infarction. Acta Neurologica Scandinavica 1998;97:386‐92. [DOI] [PubMed] [Google Scholar]
TOPS 2013 {published data only}
- Akioka N. Toyama antihypertensive therapy with Olmesartan in Post‐Stroke patients (TOPS) study. UMIN Clinical Trials Registry 2013. [UMIN000009790]
TRUST 1990 {published data only}
- TRUST Study Group. Randomised, double‐blind placebo‐controlled trial of nimodipine in acute stroke. Lancet 1990;336:1205‐9. [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang D. IV double and triple concentrated nicardipine for stroke and intracerebral haemorrhage. ClinicalTrials.gov 2006. [NCT00325793]
Wimalaratna 1994 {published data only}
- Wimalaratna HSK, Capildeo R. Nimodipine in acute ischaemic cerebral hemisphere infarction. Cerebrovascular Diseases 1994;4:179‐81. [Google Scholar]
Wityk 2008 {published data only}
- Wityk R. Induced hypertension for acute ischemic stroke. ClinicalTrials.gov. [NCT00227448]
Yao 1991 {published data only}
- Yao J. Preliminary study of nimodipine in acute cerebral infarction. Chinese Journal of Nervous and Mental Diseases 1991;17(1):47. [Google Scholar]
Yordanov 1984 {unpublished data only}
- Zhang J, Yang J, Zhang C, Jiang X, Zhou H, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001928.pub2] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ATTACI 2010 {published data only}
- Hosomi N, Sueda Y, Masugata H, Dobashi H, Murao K, Ueno M, et al. The optimal timing of antihypertensive medication administration for morning hypertension in patients with cerebral infarction. Hypertension Research 2012;35(7):720‐4. [DOI] [PubMed] [Google Scholar]
ESH‐CHL‐SHOT 2013 {published data only}
- Zanchetti A. Optimal blood pressure and cholesterol targets for preventing recurrent stroke in hypertensives ESH‐CHL‐SHOT. ClinicalTrials.gov 2013. [NCT01563731]
MAPAS 2009 {published data only}
- MAPAS trial investigators. Manipulation of Arterial Pressure in Acute ischemic Stroke. ClinicalTrials.gov 2009. [NCT00848770]
STABLE‐ICAS 2010 {published data only}
- Strategy for adequate blood pressure lowering in patients with intracranial atherosclerosis (STABLE‐ICAS). ClinicalTrials.gov 2012. [NCT01104311]
References to ongoing studies
ATACH‐2 2011 {published data only}
- Qureshi, AI. Antihypertensive treatment of acute cerebral hemorrhage (ATACH): rationale and design. Neurocritical Care 2007;6:56‐66. [DOI] [PubMed] [Google Scholar]
ENCHANTED 2011 {published data only}
- Anderson C, ENCHANTED investigators. Enhanced control of hypertension and thrombolysis stroke study: a multicentre randomised trial. Current Controlled Trials 2011. [ISRCTN82387104]
FAST‐BP 2013 {published data only}
- Sanossian N, FAST‐BP investigators. Field Administration of Stroke Therapy‐Blood Pressure lowering (FAST‐BP). ClinicalTrials.gov 2013. [NCT01811693]
SETIN‐HYPERTENSION 2012 {published data only}
- Bang OY. Safety and efficacy of therapeutic induced hypertension in acute non‐cardioembolic ischemic stroke. ClinicalTrials.gov 2012. [NCT01600235]
Additional references
Adams 1965
- Adams GF. Prospects for patients with strokes, with special reference to the hypertensive hemiplegic. British Medical Journal 1965;ii:253‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
AHA‐HS 2010
- Morgenstern LB, Hemphill III JC, Anderson C, Becker C, Broderick JP, Connolly ES, et al. Guidelines for the management of spontaneous intracerebral haemorrhage. Stroke 2010;41:2108‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
AHA‐IS 2013
- Jauch EC, Saver JL, Adams Jr HP, Bruno A, Connors B, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke. Stroke 2013;44:870‐947. [DOI] [PubMed] [Google Scholar]
BASC 2000
- Blood Pressure in Acute Stroke Collaboration. Vasoactive drugs for acute stroke. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002839] [DOI] [Google Scholar]
Bath 1997
- Bath FJ, Bath PMW. What is the correct management of blood pressure in acute stroke? The Blood pressure in Acute Stroke Collaboration. Cerebrovascular Diseases 1997;7:205‐13. [Google Scholar]
Bath 2000b
Bath 2002
- Bath PMW, Willmot M, Leonardi‐Bee J, Bath‐Hextall FJ. Nitric oxide donor donors (nitrates), L‐arginine, or nitric oxide synthase inhibitors for acute stroke. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000398] [DOI] [PubMed] [Google Scholar]
Bath 2003
- Bath P, Chalmers J, Powers W, Beilin L, Davis S, Lenfant C, et al. International Society of Hypertension (ISH): statement on the management of blood pressure in acute stroke. Journal of Hypertension 2003;21(4):665‐72. [DOI] [PubMed] [Google Scholar]
Bath 2004a
- Bath PMW, Bath‐Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000162.pub2] [DOI] [PubMed] [Google Scholar]
Bath 2004b
- Bath PMW. Prostacyclin and analogues for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2012
- Bath PMW, Lees KR, Schellinger PD, Altman H, Bland M, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke 2012;43(4):1171‐8. [DOI] [PubMed] [Google Scholar]
Bourestom 1967
- Bourestom NC. Predictors of long term recovery in cerebrovascular disease. Archives of Physical Medicine and Rehabilitation 1967;48:415‐9. [PubMed] [Google Scholar]
Britton 1980
- Britton M, Faire U, Helmers C. Hazards of therapy for excessive hypertension in acute stroke. Acta Medica Scandinavica 1980;207:253‐7. [DOI] [PubMed] [Google Scholar]
Britton 1986
- Britton M, Carlsson A, Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986;17:861‐4. [DOI] [PubMed] [Google Scholar]
Burke 1986
- Burke AM, Younkin D, Gordon J, Goldberg H, Graham T, Kushner M, et al. Changes in cerebral blood flow and recovery from acute stroke. Stroke 1986;17:173‐8. [DOI] [PubMed] [Google Scholar]
Carlberg 1993
- Carlberg B, Asplund K, Hagg E. The prognostic value of admission blood pressure in patients with acute stroke. Stroke 1993;24:1372‐5. [DOI] [PubMed] [Google Scholar]
Castillo 2004
- Castillo J, Leira R, García MM, Serena J, Blanco M, Dávalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke 2004;35(2):520‐6. [DOI] [PubMed] [Google Scholar]
Chamorro 1998
- Chamorro A, Vila N, Ascaso C, Elices E, Schonewille W, Blanc R. Blood pressure and functional recovery in acute ischemic stroke. Stroke 1998;29:1850‐3. [DOI] [PubMed] [Google Scholar]
Dandapani 1995
- Dandapani B, Suzuki S, Kelley RE, Reyes‐Iglesias Y, Duncan RC. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke 1995;26:21‐4. [DOI] [PubMed] [Google Scholar]
Droller 1965
- Droller H. The outlook in hemiplegia. Geriatrics 1965;20:630‐6. [Google Scholar]
EUSI 2004
- Toni D, Chamorro A, Kaste M, Lees K, Wahlgren NG, Hacke W. Acute treatment of ischaemic stroke. Cerebrovascular Diseases 2004;17 Suppl 2:30‐46. [DOI] [PubMed] [Google Scholar]
Fischberg 2000
- Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. Journal of Emergency Medicine 2000;19:339‐46. [DOI] [PubMed] [Google Scholar]
Fujii 1994
- Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage. Journal of Neurosurgery 1994;80:51‐7. [DOI] [PubMed] [Google Scholar]
Fujii 1998
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 1998;29:1160‐6. [DOI] [PubMed] [Google Scholar]
Graham 1975
- Graham DI. Ischaemic stroke brain damage after cerebral perfusion failure after treatment of severe hypertension. British Medical Journal 1975;4:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harper 1994
- Harper G, Castleden CM, Potter JF. Factors effecting changes in blood pressure after acute stroke. Stroke 1994;25:1726‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HOPE 2000
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. New England Journal of Medicine 2000;342:145‐53. [DOI] [PubMed] [Google Scholar]
Horn 2001
- Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke 2001;32:570‐6. [DOI] [PubMed] [Google Scholar]
Jusufovic 2014
Kazui 1997
- Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke 1997;28:2370‐5. [DOI] [PubMed] [Google Scholar]
Lees 2012
- Lees KR, Bath PM, Schellinger PD, Kerr DM, Fulton R, Hacke W, et al. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke 2012;43(4):1163‐70. [DOI] [PubMed] [Google Scholar]
Leonardi‐Bee 2002
- Leonardi‐Bee J, Bath PMW, Phillips SJ, Sandercock PAG. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315‐20. [DOI] [PubMed] [Google Scholar]
Marquarsden 1969
- Marquarsden J. The natural history of acute cerebrovascular disease: a retrospective study of 769 patients. Acta Neurologica Scandinavica 1969;45 Suppl 38:118‐24. [PubMed] [Google Scholar]
Marshall 1959
- Marshall J, Shaw DA. The natural history of cerebrovascular disease. British Medical Journal 1959;i:1614‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 1982
- Myers M, Norris J, Hachinski V, Weingert M, Sole M. Cardiac sequelae of acute stroke. Stroke 1982;13:838‐42. [DOI] [PubMed] [Google Scholar]
O'Connell 1994
- O'Connell JE, Gray CS. Treating hypertension after stroke. BMJ 1994;308:1523‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oppenheimer 1992
- Oppenheimer S, Hachinski V. Complications of acute stroke. Lancet 1992;339:721‐4. [DOI] [PubMed] [Google Scholar]
Paulson 1990
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovascular and Brain Metabolism Reviews 1990;2:161‐92. [PubMed] [Google Scholar]
PROGRESS 2001
- PROGRESS Collaborative group. Randomised trial of a perindopril‐based blood pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033‐41. [DOI] [PubMed] [Google Scholar]
Rashid 2003
- Rashid P, Leonardi‐Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741‐8. [DOI] [PubMed] [Google Scholar]
RCP 2012
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke. 4th Edition. London: Royal College of Physicians, 2012. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rodgers 1996
- Rodgers A, MacMahon S, Gamble G, Slattery J, Sandercock P, Warlow C, for the United Kingdom Transient Ischaemic stroke Attack Collaborative Group. Blood pressure and risk of stroke in patients with cerebrovascular disease. BMJ 1996;313:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandercock 1992
- Sandercock P, Willems H. Medical treatment of acute ischaemic stroke. Lancet 1992;339:537‐9. [DOI] [PubMed] [Google Scholar]
Spence 1985
- Spence JD, Maestro RF. Hypertension in acute ischaemic strokes. Treat. Archives of Neurology 1985;42:1000‐2. [DOI] [PubMed] [Google Scholar]
Sprigg 2005
- Sprigg N, Bath PMW. Management of blood pressure in acute stroke. Practical Neurology 2005;5:218‐23. [Google Scholar]
Sprigg 2006
- Sprigg N, Gray LJ, Bath PMW, Boysen G, Deyn PP, Friss P, et al. Relationship between outcome and baseline blood pressure and other haemodynamic measures in acute ischaemic stroke: data from the TAIST trial. Journal of Hypertension 2006;24(7):1413‐8. [DOI] [PubMed] [Google Scholar]
Strandgaard 1973
- Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. British Medical Journal 1973;3:507‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vemmos 2004
- Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, et al. U‐shaped relationship between mortality and admission blood pressure in patients with acute stroke. Journal of Internal Medicine 2004;255:257‐65. [DOI] [PubMed] [Google Scholar]
Wahlgren 1994
- Wahlgren NG, MacMahon DG, Keyser J, Indredavik B, Ryman T, for the INWEST Study Group. Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke. Cerebrovascular Diseases 1994;4:204‐10. [Google Scholar]
Wallace 1981
- Wallace JD, Levy LL. Blood pressure after stroke. JAMA 1981;246:2177‐80. [PubMed] [Google Scholar]
Willmot 2004
- Willmot M, Leonardi‐Bee J, Bath PMW. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18‐24. [DOI] [PubMed] [Google Scholar]
Yatsu 1985
- Yatsu FM, Zivin J. Hypertension in acute ischaemic strokes. Not to treat. Archives of Neurology 1985;42:999‐1000. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
BASC 2001
- Blood Pressure in Acute Stroke Collaboration. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000039] [DOI] [PubMed] [Google Scholar]
BASC 2009
- Geeganage C, Bath PMW. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000039.pub2] [DOI] [PubMed] [Google Scholar]


