Abstract
Background
Planned caesarean delivery for women thought be in preterm labour may be protective for baby, but could also be quite traumatic for both mother and baby. The optimal mode of delivery of preterm babies for both cephalic and breech presentation remains, therefore, controversial.
Objectives
To assess the effects of a policy of planned immediate caesarean delivery versus planned vaginal birth for women in preterm labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (5 August 2013).
Selection criteria
Randomised trials comparing a policy of planned immediate caesarean delivery versus planned vaginal delivery for preterm birth.
Data collection and analysis
Two review authors independently assessed trials for inclusion. Two review authors independently extracted data and assessed risk of bias. Data were checked for accuracy.
Main results
We included six studies (involving 122 women) but only four studies (involving only 116 women) contributed data to the analyses.
Infant
There were very little data of relevance to the three main (primary) outcomes considered in this review: There was no significant difference between planned immediate caesarean section and planned vaginal delivery with respect to birth injury to infant (risk ratio (RR) 0.56, 95%, confidence interval (CI) 0.05 to 5.62; one trial, 38 women) or birth asphyxia (RR 1.63, 95% CI 0.84 to 3.14; one trial, 12 women). The only cases of birth trauma were a laceration of the buttock in a baby who was delivered by caesarean section and mild bruising in another allocated to the group delivered vaginally.
The difference between the two groups with regard to perinatal deaths was not significant (0.29, 95% CI 0.07 to 1.14; three trials, 89 women) and there were no data specifically relating to neonatal admission to special care and/or intensive care unit.
There was also no difference between the caesarean or vaginal delivery groups in terms of markers of possible birth asphyxia (RR 1.63, 95% CI 0.84 to 3.14; one trial, 12 women) or Apgar score less than seven at five minutes (RR 0.83, 95% CI 0.43 to 1.60; four trials, 115 women) and no difference in attempts at breastfeeding (RR 1.40, 95% 0.11 to 17.45; one trial, 12 women). There was also no difference in neonatal fitting/seizures (RR 0.22, 95% CI 0.01 to 4.32; three trials, 77 women), hypoxic ischaemic encephalopathy (RR 4.00, 95% CI 0.20 to 82.01;one trial, 12 women) or respiratory distress syndrome (RR 0.55, 95% CI 0.27 to 1.10; three trials, 103 women). There were no data reported in the trials specifically relating to meconium aspiration. There was also no significant difference between the two groups for abnormal follow‐up in childhood (RR 0.65, 95% CI 0.19 to 2.22; one trial, 38 women) or delivery less than seven days after entry (RR 0.95, 95% CI 0.73 to 1.24; two trials, 51 women).
Mother
There were no data reported on maternal admissions to intensive care. However, there were seven cases of major maternal postpartum complications in the group allocated to planned immediate caesarean section and none in the group randomised to vaginal delivery (RR 7.21, 95% CI 1.37 to 38.08; four trials, 116 women).
There were no data reported in the trials specifically relating to maternal satisfaction (postnatal). There was no significant difference between the two groups with regard to postpartum haemorrhage. A number of non‐prespecified secondary outcomes were also considered in the analyses. There was a significant advantage for women in the vaginal delivery group with respect to maternal puerperal pyrexia (RR 2.98, 95% CI 1.18 to 7.53; three trials, 89 women) and other maternal infection (RR 2.63, 95% CI 1.02 to 6.78; three trials, 103 women), but no significant differences in wound infection (RR 1.16, 95% CI 0.18 to 7.70; three trials, 103 women), maternal stay more than 10 days (RR 1.27, 95% CI 0.35 to 4.65; three trials, 78 women) or the need for blood transfusion (results not estimable).
Authors' conclusions
There is not enough evidence to evaluate the use of a policy of planned immediate caesarean delivery for preterm babies. Further studies are needed in this area, but recruitment is proving difficult.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Delivery, Obstetric; Obstetric Labor, Premature; Birth Injuries; Birth Injuries/etiology; Cesarean Section; Cesarean Section/adverse effects; Elective Surgical Procedures; Infant, Premature; Infant, Small for Gestational Age; Perinatal Mortality; Randomized Controlled Trials as Topic
Plain language summary
Caesarean section versus vaginal delivery for preterm birth for women with a single baby (not multiple birth)
There is not enough evidence to show the effects of a policy of planned immediate caesarean delivery rather than a policy of planned vaginal delivery for the birth of premature babies.
Caesarean section is an operation performed to deliver a baby through a cut in the abdomen and womb. Planned caesarean delivery for women thought to be in preterm labour may be protective for the baby, also preventing an intrapartum emergency surgery with its associated complications, but could also be traumatic for both the mother and her baby. More often than not, women thought to be in preterm labour deliver weeks later, often at term. There is, therefore, a real possibility that a policy of planned caesarean section may increase the number of babies born preterm.
We included six randomised studies (involving 122 pregnant women) but only four studies (involving 116 women) contributed to the analyses. Our review found that there is not enough reliable evidence to compare planned caesarean delivery with planned vaginal delivery. Sometimes a planned caesarean cannot happen because labour progresses too quickly and sometimes, even though vaginal delivery is planned, complications arising during labour may make a caesarean section necessary. The review found that not enough women have been recruited into trials and, therefore, the decision how best to deliver a preterm baby, either cephalic or breech presentation, remains opinion and current practice within a hospital, rather than being evidence‐based.
All four trials were stopped early, due to difficulties with recruiting women. There were no data on serious maternal complications including admissions to intensive care unit. However, there were seven cases of major maternal postpartum complications in the group allocated to planned caesarean section (wound dehiscence, deep vein thrombosis, endotoxic shock and puerperal sepsis) and none in the group randomised to vaginal delivery. Excess blood loss from the birth canal after childbirth (postpartum haemorrhage) was not clearly different between the two groups, nor was birth asphyxia or respiratory distress syndrome or injury to the baby at birth.
Background
The optimal mode of delivery for women thought to be in preterm labour is controversial. Claims that planned preterm caesarean delivery reduces the chances of fetal or neonatal death and birth trauma have been met by counter claims that such a policy leads to risk of serious morbidity for both mother and baby.
Malloy 1991 found no evidence that caesarean section can be protective for preterm neonates, especially for very low birthweight infants (less than 1500 g). The same author published further evidence that, for intermediate or late low‐risk preterm neonates (32 to 36 weeks), primary caesarean section may in fact increase risk of neonatal mortality and morbidity, such as pulmonary hypoplasia, necrotizing enterocolitis or sepsis (Malloy 2009).
Caesarean section is known to be associated with an increased risk of respiratory morbidity in neonates, because hormonal and physiological changes associated with labour are necessary for lung maturation in neonates (Cohen 1985; Hansen 2008). Gravenhorst 1993 on the other hand, found a trend towards reduced neonatal mortality rate in preterm (before 32 weeks) or very low weight (less than 1500 g) babies with breech presentation born by caesarean section compared with vaginal delivery. There is also a question to what extent the data from the Term Breech Trial (Hannah 2000) may be applicable to preterm births. The Term Breech Trial (Hannah 2000) showed a reduction in perinatal mortality from 13/1000 to 3/1000 (risk ratio (RR) 0.23, 95% confidence interval (CI) 0.07 to 0.81) and a reduction in serious neonatal morbidity from 3.8 to 1.4 per cent with planned caesarean section (RR 0.36, 95% CI 0.19 to 0.65). No differences were reported between the two groups regarding the health of the infant at three months follow‐up (Hannah 2002, RR 1.00, 95% CI 0.82 to 1.23) and equal rates of death or neurodevelopmental delay (Hannah 2004, RR 1.09, 95% CI 0.52 to 2.30) were evidenced at follow‐up two years after the trial.
In the Term Breech Trial (Hannah 2000) maternal complications were similar in both groups, but the data about possible excess maternal morbidity and mortality (or lack of it) for women who undergo planned caesarean section are quite complex. In the 2005 WHO global survey on maternal and perinatal health (Villar 2007), the risk for caesarean section compared to vaginal delivery was three to five times higher for maternal death, twice higher for hysterectomy, and again twice higher for being admitted to intensive care and hospital stay of more than seven days. Postnatal infection (pyrexia, endometritis, puerperal sepsis), thrombosis and pulmonary embolism, and excessive blood loss are indeed higher after caesarean section (DOH 1996; Petitti 1985). However, a lot of these complications may be a consequence of underlying causes that lead to the need for caesarean section, particularly when the procedure is carried out as an intrapartum emergency. Planned caesarean section may be, therefore, seen as a way of preventing an intrapartum emergency surgery and its associated complications.
Another problem, particularly for preterm caesarean sections, is that the lower segment (where the uterine incision is usually performed) may not have formed. Consequently, a vertical incision of the upper part of the uterus may be necessary. In this instance, more complications can occur, including heavier blood loss (Shah 1990), and the need to perform future deliveries by caesarean section, because of the lack of strength of the womb scar. Furthermore, caesarean section for first birth seems to be associated with an increased risk of placenta praevia and placental abruption in the second pregnancy (Yanq 2007).
The concept of planned caesarean section for preterm birth implies that it is possible to diagnose preterm birth accurately and perform caesarean section very early in labour or just before labour starts. This is clearly not the case as, more often than not, women thought to be in preterm labour deliver weeks later, often at term. There is, therefore, a real possibility that a policy of planned caesarean section may increase the number of babies born preterm.
The risk associated with excess preterm births may be reduced with corticosteroids given before delivery (Roberts 2006) and magnesium sulphate for neuroprotection (Crowther 2009).
This review aims to address the issue of best mode of delivery for preterm births in singleton pregnancy. The mode of delivery in multiple pregnancies is addressed in a separate Cochrane review (Stock 2012).
Objectives
To assess the effects of a policy of planned immediate caesarean delivery as opposed to aiming for vaginal birth for women thought to be in labour and at high risk of delivering a preterm baby.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised trial which compared a policy of planned immediate caesarean delivery versus a policy aiming for vaginal birth for women at high risk of delivering a preterm baby. We did not include quasi‐randomised controlled trials.
Types of participants
Women presenting or thought to be in preterm labour (less than 37 weeks), irrespective of fetal presentation.
Types of interventions
Comparison of two policies to deliver preterm baby once the labour starts, or a decision is made that baby needs to be delivered:
aiming to deliver preterm baby by planned immediate caesarean delivery; or
aiming to deliver baby vaginally irrespective of the presentation (cephalic, breech).
We acknowledge the ambiguity with the term "planned" caesarean section. A planned caesarean section normally means that it is scheduled before the onset of labour (NICE 2011). However, for the purposes of this review, we refer to the term "planned immediate caesarean section", meaning a caesarean section which is planned only after the labour has started. When a woman presents in preterm labour, the issue is whether to deliver immediately by caesarean section to prevent preterm vaginal birth, or to aim to deliver vaginally with recourse to caesarean section if problems develop (Penn 1996). Planned immediate caesarean section in the context of this review implies performing caesarean section as soon as the spontaneous preterm birth is thought to be inevitable. However, this decision to perform caesarean section may be delayed in order to give corticosteroids to the mother before birth of the baby.
Types of outcome measures
The outcome measures used in previous versions of this review have now changed to those listed below. In addition, a number of outcomes not pre‐specified have been analysed in this review, as indicated.
Please refer to Differences between protocol and review to see outcomes previously used.
Primary outcomes
Infant
Birth injury to infant
Birth asphyxia (occurs when a baby does not receive enough oxygen before, during, or just after birth) as defined by the trialists
Mother
Mother requires admission to intensive care/major maternal postpartum complications (as defined by trialists)
Secondary outcomes
Infant
Perinatal death
Neonatal admission to special care and/or intensive care unit
Hypoxic ischaemic encephalopathy (a condition of injury to the brain)
Breastfeeding at discharge
Breastfeeding at three months
Cord pH below normal range
Abnormal follow‐up in childhood
Neonatal fitting/seizures
Meconium aspiration (means the newborn inhales a mixture of meconium and amniotic fluid, either in the uterus or just after delivery)
Apgar score less than seven at five minutes
Respiratory distress syndrome
Delivery less than seven days after entry
Neonatal infection (proven) (outcome not prespecified)
Intracranial pathology (outcome not prespecified)
Intracranial haemorrhage (outcome not prespecified)
Other birth trauma (outcome not prespecified)
Head entrapment (outcome not prespecified)
Necrotising enterocolitis (outcome not prespecified)
Cord prolapse (outcome not prespecified)
Need for mechanical ventilation (outcome not prespecified)
Ventilation (days) (outcome not prespecified)
Supplemental oxygen (days) (outcome not prespecified)
Neonatal jaundice (outcome not prespecified)
Mother
Maternal satisfaction (postnatal)
Postpartum haemorrhage (excess blood loss from the birth canal after childbirth, as defined by trialists)
Maternal puerperal pyrexia (outcome not prespecified)
Maternal wound infection (outcome not prespecified)
Other maternal infection (outcome not prespecified)
Maternal stay more than 10 days (outcome not prespecified)
Need for blood transfusion (outcome not prespecified)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (5 August 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors Stefania Livio (SL) and Stephen Milan (SM)) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. There was agreement but had there been any disagreement this would have been resolved through discussion or, if required, we would have consulted our remaining review author (Zarko Alfirevic (ZA)). We also included data provided from the previous version of the review (Grant 2010).
Data extraction and management
We designed a form to extract data. For eligible studies, we re‐extracted data on the additional outcomes included in this review. Two review authors (SL; SM) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author (ZA). We entered data into Review Manager software (RevMan 2012) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (SL and SM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged that studies were at low risk of bias if they were blinded, or that the lack of blinding would be unlikely to affect results.
We assessed the methods as:
low, high or unclear risk of bias participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis, stating whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, missing data were included in the analyses.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated” analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias and assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we considered the direction of the bias and whether it was likely to impact on the findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We had planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods, if that had been necessary.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials if any had been identified.
In future updates, if cluster‐randomised trials are identified, we will adjust their sample sizes using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We plan to synthesise the relevant information from both cluster‐randomised trials and individually‐randomised trials. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We planned to note levels of attrition if this had been an issue, exploring the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were performed, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial being the number randomised minus any participants whose outcomes are known to be missing.
Where data were clearly reported in terms of intervention received rather than on an intention‐to‐treat basis (Wallace 1984), and the intention‐to‐treat data were unavailable, the trial was omitted from the analyses (Analysis 1.2 and Analysis 1.16).
1.2. Analysis.
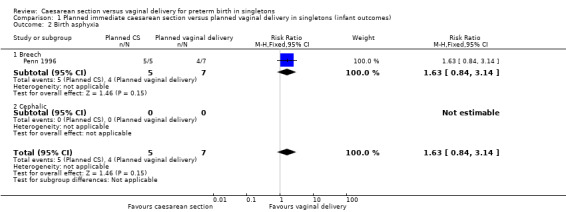
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 2 Birth asphyxia.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there had been 10 or more studies in the meta‐analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. In such an event we planned to assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we had planned to use the test proposed by Egger 1997, and for dichotomous outcomes we would have used the test proposed by Harbord 2006. If asymmetry had been detected in any of these tests or was suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there had been clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used a random‐effects analysis to produce it.
We carried out the following subgroup analyses:
fetal presentation breech;
fetal presentation cephalic.
All outcomes were used in subgroup analysis. The data were combined to obtain total summary statistics from the two subgroups. We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012).
Sensitivity analysis
We planned to conduct sensitivity analyses if there had been a clear risk of bias associated with the quality of some of the included trials.
Results
Description of studies
Six studies (involving 122 women) were considered as potentially eligible for inclusion (Lumley 1985; MacLennan 1986; Penn 1996; Viegas 1985; Wallace 1984; Zlatnik 1993) and two trials were excluded (Dietl 1987; McColgin 1990). SeeCharacteristics of included studies, Characteristics of excluded studies.
It was noted in the previous version of this review (Grant 2010) that three included trials were restricted to cephalic presentations (Lumley 1985; MacLennan 1986; Wallace 1984) whereas the other three included trials were breech presentations (Penn 1996; Viegas 1985; Zlatnik 1993). In Grant 2010, data were included from MacLennan 1986 which had recruited only two women, who were both randomised to the same group (caesarean delivery). The data for those two women were added to the data for the four women recruited to a separate Australian trial (Lumley 1985) which also stopped at a very early stage having recruited only two women to the caesarean section group and two to the planned vaginal birth groups. In this version of the review, we decided not to include the data from Lumley 1985 and MacLennan 1986 because of the extreme paucity of data. We had intended to include the data from Lumley 1985, but this data were not available from the paper. The original data had been obtained by correspondence with the author and we did not have access to this.
Consequently, four trials (involving 116 women) contribute data to our analyses (Penn 1996; Viegas 1985; Wallace 1984; Zlatnik 1993).
Risk of bias in included studies
Please refer to Figure 1; Figure 2, for a summary of 'Risk of bias' assessments.
1.
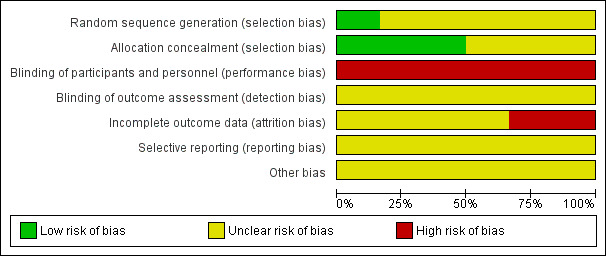
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
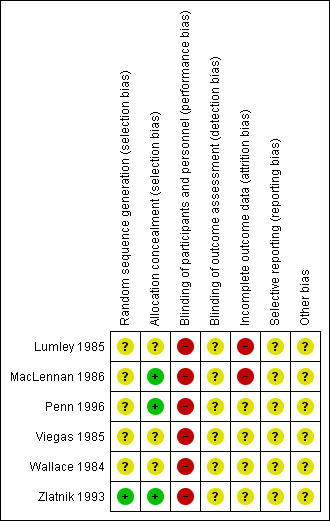
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
It was noted in Grant 2010 that 'the quality of the data in the review has been significantly improved by the extra information provided by the authors' and wherever possible we have retained the data from Penn 1996; Viegas 1985; Wallace 1984; Zlatnik 1993 as it was reported in Grant 2010. As was noted in Grant 2010 'analyses have been based on all women randomised with no known withdrawals after trial entry, other than for longer‐term follow‐up. It is not possible to 'blind' the policies being compared, and this should be taken into account when reviewing the data'.
Allocation
One of the six trials included in this review described adequate methods of randomisation (Zlatnik 1993), however, post‐randomisation consent was used in Zlatnik 1993. The other trials (Lumley 1985; MacLennan 1986; Penn 1996; Viegas 1985; Wallace 1984) were reported as randomised although the method of allocation was not specified.
Allocation concealment was assessed as low risk of bias in three trials (MacLennan 1986; Penn 1996; Zlatnik 1993) and unclear in the remaining trials (Lumley 1985; Viegas 1985; Wallace 1984).
Blinding
It is not possible to blind the policies being compared. All studies were therefore assessed as high risk of bias for blinding of participants and personnel. Insufficient information was reported for a definitive evaluation of outcome assessment and so all studies were assessed as unclear risk of bias for blinding of outcome assessment.
Incomplete outcome data
Two trials were at high risk of bias for incomplete outcome (Lumley 1985; MacLennan 1986), both having extreme paucity of data due to poor recruitment and stopping early. The remaining trials were assessed as unclear risk of bias (Penn 1996; Viegas 1985; Wallace 1984; Zlatnik 1993).
Selective reporting
All trials were assessed as unclear risk of bias for selective outcome reporting (Lumley 1985; MacLennan 1986; Penn 1996; Viegas 1985; Wallace 1984; Zlatnik 1993).
Other potential sources of bias
All trials were assessed as unclear risk of bias for other potential sources of bias (Lumley 1985; MacLennan 1986; Penn 1996; Viegas 1985; Wallace 1984; Zlatnik 1993).
Effects of interventions
A total of only 116 women were recruited to the four trials contributing to the analyses reported in this review.
In the previous version of this review, data were included from a trial (MacLennan 1986) that had recruited only two women. Both women were randomised to the same group (caesarean delivery). The data for those two women were added to the data for the four women recruited to a separate Australian trial (Lumley 1985) which also stopped at a very early stage. In this version of the review because of the extreme paucity of data, and because the original data from these trials is not now available, these trials are not included in the analysis.
For the infant
Primary outcomes
There were very little data relating to the three main (primary) outcomes considered in this review. There was no significant difference between planned immediate caesarean section and planned vaginal delivery with respect to birth injury to infant (risk ratio (RR) 0.56, 95% confidence interval (CI) 0.05 to 5.62; one trial, 38 women, (Analysis 1.1)) or birth asphyxia (RR 1.63, 95% CI 0.84 to 3.14; one trial, 12 women, (Analysis 1.2 )). The only cases of birth trauma were a laceration of the buttock in a baby who was delivered electively by caesarean section (Viegas 1985) and mild bruising in another allocated to the expectant group and delivered vaginally (Penn 1996).
1.1. Analysis.
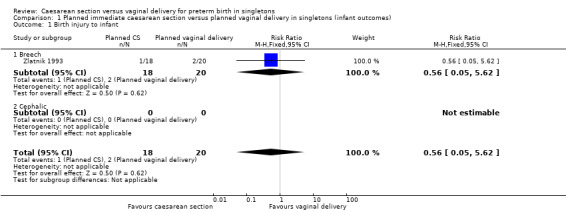
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 1 Birth injury to infant.
Secondary outcomes
The difference between the two groups with regard to perinatal deaths was not significant (RR 0.29, 95% CI 0.07 to 1.14; three trials, 89 women (Analysis 1.3)) and there were no data specifically relating to neonatal admission to special care and/or intensive care unit.
1.3. Analysis.
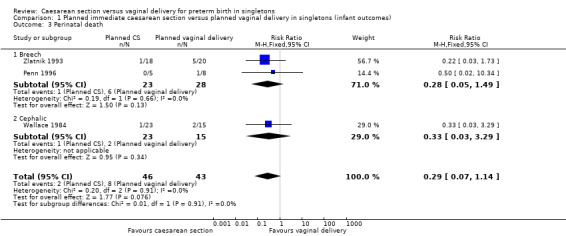
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 3 Perinatal death.
There was no difference between the caesarean or vaginal delivery groups in hypoxic ischaemic encephalopathy (a condition of injury to the brain) (RR 4.00, 95% CI 0.20 to 82.01; one trial, 12 women (Analysis 1.5)), in attempts at breastfeeding (RR 1.40, 95% CI 0.11 to 17.45; one trial, 12 women (Analysis 1.6)) or in terms of markers of possible birth asphyxia, i.e. cord pH being below the normal range (RR 9.00, 95% CI 0.56 to 143.89; two trials, 33 women (Analysis 1.7).
1.5. Analysis.
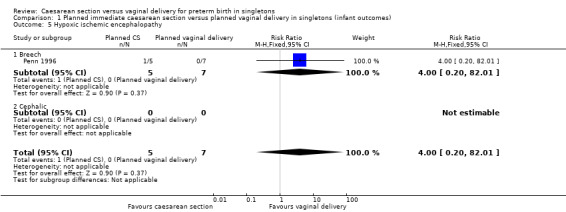
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 5 Hypoxic ischemic encephalopathy.
1.6. Analysis.
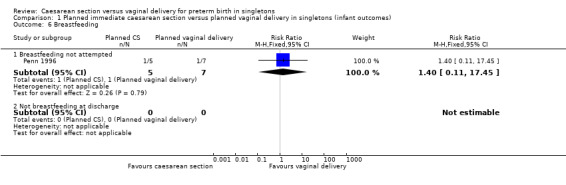
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 6 Breastfeeding.
1.7. Analysis.
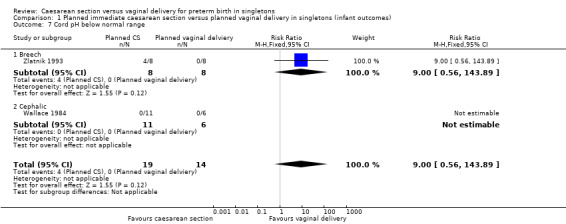
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 7 Cord pH below normal range.
There was also no significant difference between the two groups for abnormal follow‐up in childhood (RR 0.65, 95% CI 0.19 to 2.22; one trial, 38 women (Analysis 1.8)), in neonatal fitting/seizures (RR 0.22, 95% CI 0.01 to 4.32; three trials, 77 women (Analysis 1.9)), in low Apgar score at five minutes (RR 0.83, 95% CI 0.43 to 1.60; four trials, 115 women (Analysis 1.11)), respiratory distress syndrome (RR 0.55, 95% CI 0.27 to 1.10; three trials, 103 women (Analysis 1.12)) or delivery less than seven days after entry (average RR 0.95, 95% CI 0.73 to 1.24; two trials, 51 women; Heterogeneity: Tau² = 0.02; Chi² = 1.66, df = 1 (P = 0.20); I² = 40% (Analysis 1.13).
1.8. Analysis.
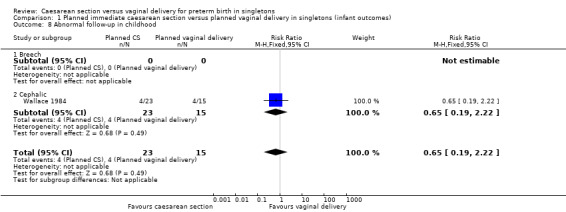
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 8 Abnormal follow‐up in childhood.
1.9. Analysis.
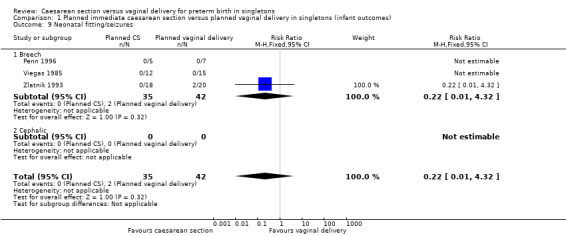
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 9 Neonatal fitting/seizures.
1.11. Analysis.
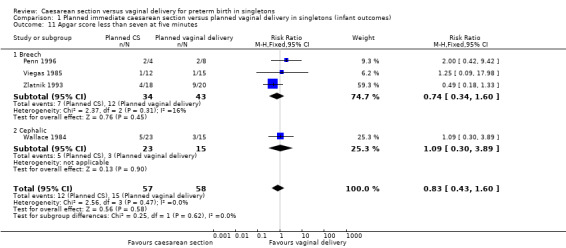
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 11 Apgar score less than seven at five minutes.
1.12. Analysis.
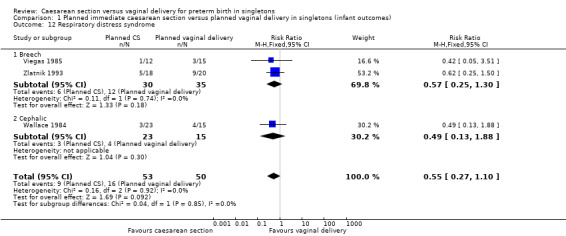
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 12 Respiratory distress syndrome.
1.13. Analysis.
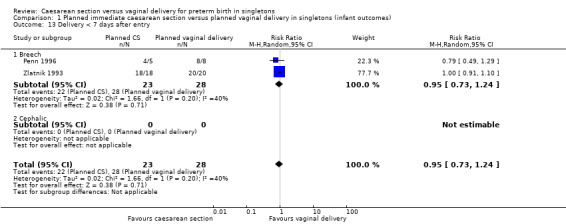
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 13 Delivery < 7 days after entry.
There were no data reported in the trials specifically relating to meconium aspiration (where the newborn inhales a mixture of meconium and amniotic fluid, either in the uterus or just after delivery).
Non prespecified secondary outcomes
We have also included a number of non‐prespecified outcomes: cord prolapse (Analysis 1.20); need for mechanical ventilation (Analysis 1.21); ventilation (days) (Analysis 1.22); supplemental oxygen (days) (Analysis 1.23); neonatal jaundice (Analysis 1.24) ‐ none of them showed important differences between the two groups.
1.20. Analysis.
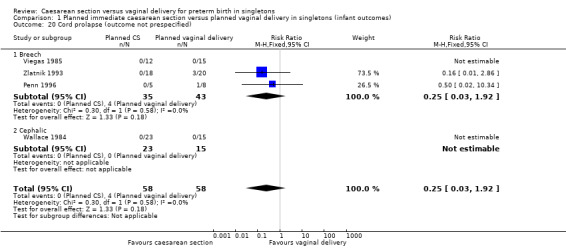
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 20 Cord prolapse (outcome not prespecified).
1.21. Analysis.
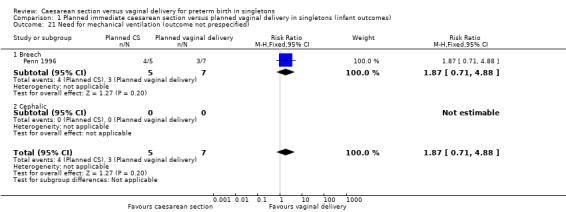
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 21 Need for mechanical ventilation (outcome not prespecified).
1.22. Analysis.
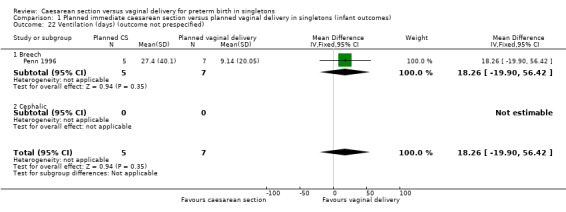
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 22 Ventilation (days) (outcome not prespecified).
1.23. Analysis.
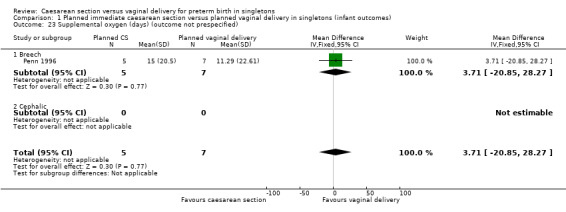
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 23 Supplemental oxygen (days) (outcome not prespecified).
1.24. Analysis.
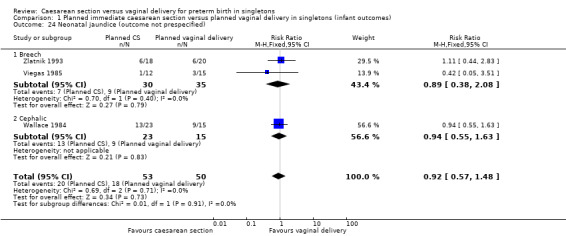
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 24 Neonatal jaundice (outcome not prespecified).
For the mother
Primary outcomes
There were no data reported on maternal admissions admission to intensive care. However, there were significantly more cases of major maternal postpartum complications (wound dehiscence, deep vein thrombosis, endotoxic shock and puerperal sepsis) in the group allocated to planned immediate caesarean section compared with the group randomised to vaginal delivery (RR 7.21, 95% CI 1.37 to 38.08; four trials, 116 women, (Analysis 2.2)).
2.2. Analysis.
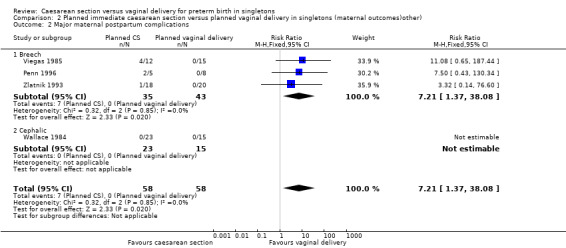
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 2 Major maternal postpartum complications.
Secondary outcomes
The included studies did not report data on maternal satisfaction (postnatal) (Analysis 2.3).
There was no significant difference between the two groups with regard to postpartum haemorrhage (excess blood loss from the birth canal after childbirth) (RR 3.69, 95% CI 0.16 to 83.27; four trials, 105 women (Analysis 2.4)).
2.4. Analysis.
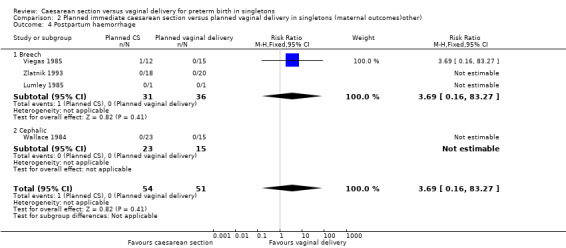
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 4 Postpartum haemorrhage.
Non prespecified secondary outcomes
A number of non‐prespecified secondary outcomes were also considered in the analyses. There was a significant advantage for women in the vaginal delivery group with respect to maternal puerperal pyrexia (RR 2.98, 95% CI 1.18 to 7.53; three trials, 89 women, (Analysis 2.5)) and other maternal infection (RR 2.63, 95% CI 1.02 to 6.78, three trials, 103 women, (Analysis 2.7)), although only just significant, P = 0.05), but no significant differences in wound infection (Analysis 2.6), maternal stay more than 10 days (Analysis 2.8) or the need for blood transfusion (Analysis 2.9).
2.5. Analysis.
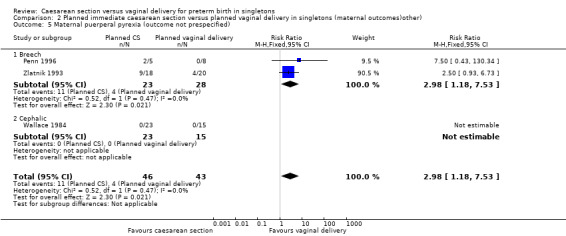
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 5 Maternal puerperal pyrexia (outcome not prespecified).
2.7. Analysis.
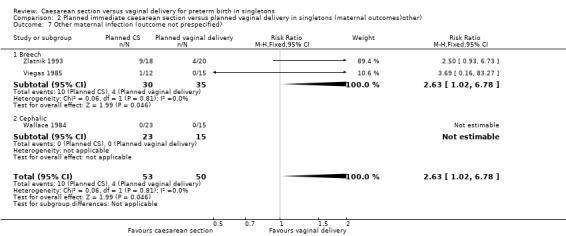
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 7 Other maternal infection (outcome not prespecified).
2.6. Analysis.
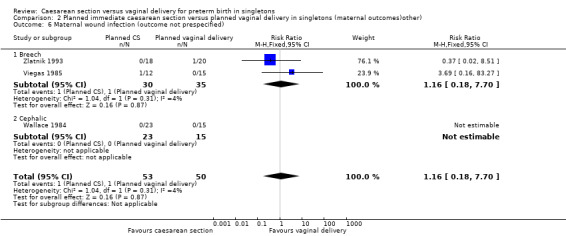
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 6 Maternal wound infection (outcome not prespecified).
2.8. Analysis.
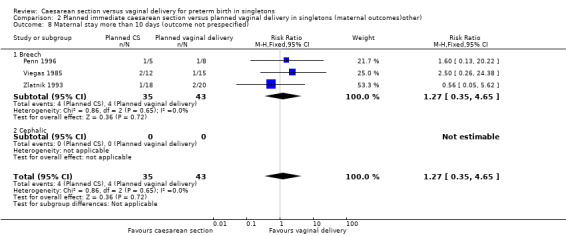
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 8 Maternal stay more than 10 days (outcome not prespecified).
2.9. Analysis.
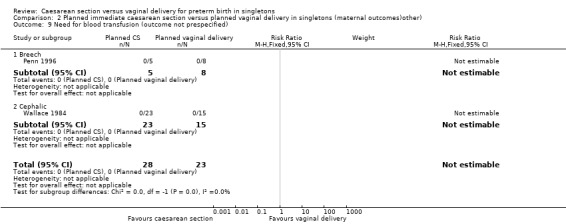
Comparison 2 Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other), Outcome 9 Need for blood transfusion (outcome not prespecified).
In terms of compliance with allocated intervention, 20% (9/46) of women allocated to elective caesarean section were actually delivered vaginally, usually because the delivery was too rapid to allow a caesarean to be performed. Twenty‐one per cent (9/43) of women allocated to vaginal delivery were delivered by caesarean section, usually because of fetal distress.
Subgroup Analyses
There was no clear evidence for subgroup differences between breech and cephalic presentations for the following outcomes examined: perinatal death (test for subgroup differences P = 0.91, I² = 0% (Analysis 1.3)); Apgar score less than seven at five minutes (test for subgroup differences P = 0.62, I² = 0% (Analysis 1.11)); neonatal infection (test for subgroup differences P = 0.51, I² = 0% (Analysis 1.14)); intracranial pathology (test for subgroup differences P = 0.38, I² = 0% (Analysis 1.15)); or neonatal jaundice (test for subgroup differences P = 0.91, I² = 0% (Analysis 1.24)).
1.14. Analysis.
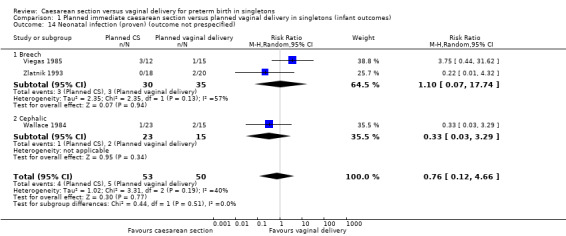
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 14 Neonatal infection (proven) (outcome not prespecified).
1.15. Analysis.
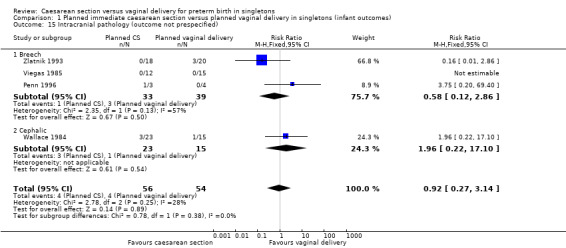
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 15 Intracranial pathology (outcome not prespecified).
Discussion
Summary of main results
Only 116 women were recruited to the four trials contributing to the analyses reported in this review. All four trials were stopped early, due to difficulties with recruitment. Therefore, any firm conclusions regarding the relative merits of planned immediate caesarean section versus planned vaginal delivery should not be drawn from this review in order to guide current clinical practice.
The need for more 'intention‐to‐treat' data from properly controlled trials is essential.
Overall completeness and applicability of evidence
Eighteen of 89 babies (20%) were not delivered by the planned mode (Penn 1996; Wallace 1984; Zlatnik 1993). This reflects rapid progress of preterm labour and results in vaginal birth despite caesarean section being planned. On the other hand, problems developing during labour may require caesarean section despite aiming for vaginal birth. Any attempt to perform 'on treatment' analysis should be resisted, as it introduces considerable bias. The bias is likely to favour vaginal birth as quick, appropriately monitored preterm births tend to be uncomplicated, particularly for the mother, whilst intrapartum emergency caesarean sections when vaginal births were planned are likely to be particular risky for both mother and baby.
It is also noteworthy that the infants who fared worst in Zlatnik's study (as judged by cord blood pH and perinatal death) were those whose mothers were delivered too rapidly to be recruited to the trial (Zlatnik 1993). The problem was not due to vaginal delivery, but undiagnosed labour which was therefore not monitored.
Quality of the evidence
All the included studies were randomised controlled trials. However, in Penn 1996; Viegas 1985; and Wallace 1984, details of random sequence generation are not reported. It is important to note that in the case of all four trials, data collection was stopped early.
Potential biases in the review process
Whilst a systematic process for including and excluding studies in this review was adhered to in relation to the prespecified criteria, the final selection of studies is of course open to interpretation or criticism. For further details, please see Characteristics of included studies; Characteristics of excluded studies.
Agreements and disagreements with other studies or reviews
No new trials have been published since the previous version of this review (Grant 2010). However, we have not included the data from two trials which were previously included (Lumley 1985; MacLennan 1986).
Authors' conclusions
Implications for practice.
Given that very few women have been recruited to trials of planned immediate caesarean section versus planned vaginal delivery for preterm birth, and that the quality of the trials conducted is generally unclear, we recommend that firm conclusions regarding the relative merits of planned immediate caesarean section versus planned vaginal delivery should not be drawn from this evidence to guide practice for preterm births.
Implications for research.
Failure to successfully recruit to all trials carried out thus far highlights the challenges with recruitment in this area. We hope that this review will contribute further to the equipoise that, surely, must exist. We are confident that, with adequate resources and growing recognition about how important it is to base clinical decisions on sound evidence, well designed randomised trials are possible.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2013 | New search has been performed | Search updated. No new trials identified. |
| 28 August 2013 | New citation required but conclusions have not changed | Review updated. |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 24 April 2012 | New search has been performed | Search updated. No new trials identified. Data from two included studies (Lumley 1985; MacLennan 1986) are no longer included in the analyses because of the extreme paucity of data. The background and methods sections have also been updated. |
| 24 April 2012 | New citation required but conclusions have not changed | A new team of review authors prepared this update. Change of title used in previous versions of this review from "Elective caesarean section versus expectant management for delivery of the small baby" to "Caesarean section versus vaginal delivery for preterm birth in singletons". SeeDifferences between protocol and review. |
| 11 February 2009 | Review declared as stable | It is very unlikely that future RCTs on this topic will be mounted because of difficulties in recruitment. |
| 27 January 2009 | New search has been performed | Search updated. No new trials identified. |
| 18 February 2008 | Amended | Converted to new review format. |
| 1 June 2006 | New search has been performed | Search updated. No new trials identified. |
| 25 February 2004 | New search has been performed | Search updated. No new trials identified. |
| 31 March 2001 | New search has been performed | Search updated. The review was updated with unpublished data from an included trial (Zlatnik 1993), and the background, results, discussion and conclusions substantially amended. The title was changed from 'Elective versus selective caesarean section for delivery of the small baby' to 'Elective caesarean section versus expectant management for delivery of the small baby'. |
| 13 December 2000 | New citation required and conclusions have changed | Substantive amendment. |
| 1 December 2000 | New search has been performed | Search updated. The review was updated by inclusion of data from one further trial (Penn 1996), resulting in amendments to all the text. |
Acknowledgements
We are most grateful for the contribution of Adrian Grant and Cathryn Glazener, authors on the previous versions of this review, and to Sonja Henderson, Frances Kellie, Lynn Hampson, Jill Hampson, Denise Atherton and Leanne Jones for their guidance and support.
As part of the pre‐publication editorial process, the first version of this review was commented on by three peers (an editor and two referees who are external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser (Alfirevic 2012).
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth injury to infant | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 5.62] |
| 1.1 Breech | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 5.62] |
| 1.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Birth asphyxia | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.84, 3.14] |
| 2.1 Breech | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.84, 3.14] |
| 2.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Perinatal death | 3 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.14] |
| 3.1 Breech | 2 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.05, 1.49] |
| 3.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.29] |
| 4 Neonatal admission to special care and/or intensive care unit | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Breech | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Hypoxic ischemic encephalopathy | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.20, 82.01] |
| 5.1 Breech | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.20, 82.01] |
| 5.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Breastfeeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Breastfeeding not attempted | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.11, 17.45] |
| 6.2 Not breastfeeding at discharge | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Cord pH below normal range | 2 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.56, 143.89] |
| 7.1 Breech | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.56, 143.89] |
| 7.2 Cephalic | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Abnormal follow‐up in childhood | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.19, 2.22] |
| 8.1 Breech | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.19, 2.22] |
| 9 Neonatal fitting/seizures | 3 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.32] |
| 9.1 Breech | 3 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.32] |
| 9.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Meconium aspiration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Breech | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Apgar score less than seven at five minutes | 4 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.60] |
| 11.1 Breech | 3 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.34, 1.60] |
| 11.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.30, 3.89] |
| 12 Respiratory distress syndrome | 3 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.27, 1.10] |
| 12.1 Breech | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.30] |
| 12.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.13, 1.88] |
| 13 Delivery < 7 days after entry | 2 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.24] |
| 13.1 Breech | 2 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.24] |
| 13.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal infection (proven) (outcome not prespecified) | 3 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.12, 4.66] |
| 14.1 Breech | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 17.74] |
| 14.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.29] |
| 15 Intracranial pathology (outcome not prespecified) | 4 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.27, 3.14] |
| 15.1 Breech | 3 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.12, 2.86] |
| 15.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.22, 17.10] |
| 16 Intracranial hemorrhage (outcome not prespecified) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Breech | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Other birth trauma (outcome not prespecified) | 4 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.17, 8.13] |
| 17.1 Breech | 3 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.17, 8.13] |
| 17.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Head entrapment (outcome not prespecified) | 4 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Breech | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Necrotosing entercolitis (outcome not prespecified) | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [0.39, 114.78] |
| 19.1 Breech | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [0.39, 114.78] |
| 19.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Cord prolapse (outcome not prespecified) | 4 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.92] |
| 20.1 Breech | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.92] |
| 20.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Need for mechanical ventilation (outcome not prespecified) | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.71, 4.88] |
| 21.1 Breech | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.71, 4.88] |
| 21.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Ventilation (days) (outcome not prespecified) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 18.26 [‐19.90, 56.42] |
| 22.1 Breech | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 18.26 [‐19.90, 56.42] |
| 22.2 Cephalic | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Supplemental oxygen (days) (outcome not prespecified) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [‐20.85, 28.27] |
| 23.1 Breech | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [‐20.85, 28.27] |
| 23.2 Cephalic | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Neonatal jaundice (outcome not prespecified) | 3 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.48] |
| 24.1 Breech | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.08] |
| 24.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.55, 1.63] |
1.17. Analysis.
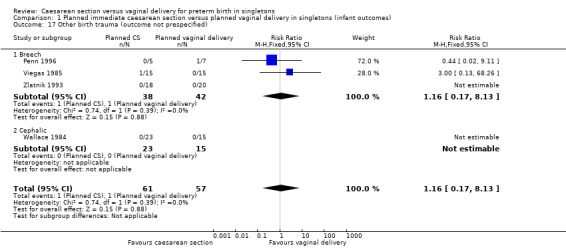
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 17 Other birth trauma (outcome not prespecified).
1.18. Analysis.
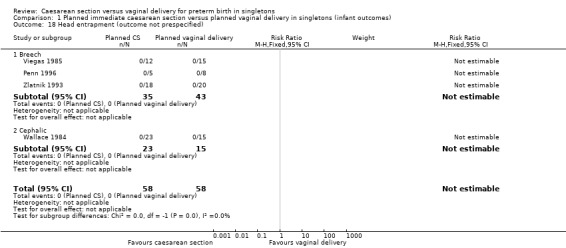
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 18 Head entrapment (outcome not prespecified).
1.19. Analysis.
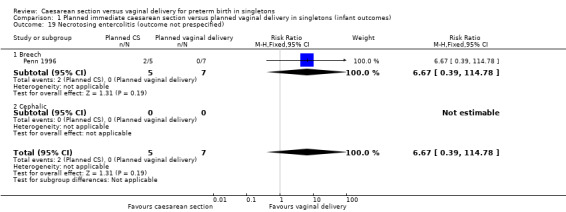
Comparison 1 Planned immediate caesarean section versus planned vaginal delivery in singletons (infant outcomes), Outcome 19 Necrotosing entercolitis (outcome not prespecified).
Comparison 2. Planned immediate caesarean section versus planned vaginal delivery in singletons (maternal outcomes)other).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mother requires admission to intensive care | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Breech | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Major maternal postpartum complications | 4 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.21 [1.37, 38.08] |
| 2.1 Breech | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.21 [1.37, 38.08] |
| 2.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal satisfaction (postnatal) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Breech | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Cephalic | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Postpartum haemorrhage | 4 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.69 [0.16, 83.27] |
| 4.1 Breech | 3 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.69 [0.16, 83.27] |
| 4.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal puerperal pyrexia (outcome not prespecified) | 3 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.18, 7.53] |
| 5.1 Breech | 2 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.18, 7.53] |
| 5.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal wound infection (outcome not prespecified) | 3 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.18, 7.70] |
| 6.1 Breech | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.18, 7.70] |
| 6.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Other maternal infection (outcome not prespecified) | 3 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.02, 6.78] |
| 7.1 Breech | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.02, 6.78] |
| 7.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal stay more than 10 days (outcome not prespecified) | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.35, 4.65] |
| 8.1 Breech | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.35, 4.65] |
| 8.2 Cephalic | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Need for blood transfusion (outcome not prespecified) | 2 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Breech | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Cephalic | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lumley 1985.
| Methods | "Randomised"; method not specified. | |
| Participants | Inclusion: in labour; single fetus in cephalic or breech presentation between 26‐31 weeks; no congenital abnormality at ultrasound examination; no other contraindication to caesarean or vaginal delivery; consent to participate. Setting (period of recruitment): Queen Victoria Medical Centre, Melbourne, Australia (1980). Number of randomised participants: elective 2, selective 2. |
|
| Interventions | I: spontaneous birth vs II: CS as soon as labour starts. | |
| Outcomes |
|
|
| Notes | Trial terminated after 5 months because of recruitment difficulties (only 4 women randomised). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported for definitive evaluation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial stopped early. |
| Selective reporting (reporting bias) | Unclear risk | Details of women who were not randomised included in trial report. |
| Other bias | Unclear risk | After the study had been running for 5 months, only 4 women had been randomised. However, the birth register indicated there had been 33 deliveries between 26 and 31 weeks. 16 women were withdrawn at the discretion of consultants. |
MacLennan 1986.
| Methods | Randomised trial: randomisation by central telephone allocation. | |
| Participants | Inclusion: spontaneous preterm labour; cephalic (or breech) presentation between 24‐32 weeks; no contraindication to caesarean or vaginal delivery. Setting (period of recruitment): Queen Victoria Hospital, Adelaide, Australia (1987). Number of randomised participants: 2. | |
| Interventions | I: vaginal delivery vs II: elective caesarean section. | |
| Outcomes |
|
|
| Notes | Trial terminated because of recruitment difficulties. In both cases, the presentation was cephalic. Unpublished data were provided by the principal investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Central telephone allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported for definitive evaluation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial stopped early. |
| Selective reporting (reporting bias) | Unclear risk | Only 2 women randomised. |
| Other bias | Unclear risk | Only 2 women had been randomised when the trial stopped. The intended sample size as > 500. Recruitment to trial proved too difficult. |
Penn 1996.
| Methods | Multicentre randomised controlled trial with telephone randomisation. Minimisation algorithm used to balance groups for gestational age, centre and rupture of membranes. | |
| Participants | Inclusion: women in spontaneous preterm labour between 26 and 32 completed weeks' gestation with singleton breech fetus; no clear indication for CS or vaginal delivery. Exclusion: intrauterine death or congenital fetal malformation, clear indications for CS or vaginal delivery. Setting: 26 centres in England (teaching and district general hospitals). Recruitment ended June 1991. Number of randomised participants: elective 5, selective 8. | |
| Interventions | I: Vaginal delivery. II: Caesarean delivery. | |
| Outcomes |
|
|
| Notes | Trial terminated after 17 months because of low recruitment due to clinicians' reluctance to randomise eligible women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation co‐ordinated by the Clinical Trial Service in Oxford. A minimisation algorithm was used to provide a balance between trial groups in terms of: gestational age, participating centre, and presence or absence of ruptured membranes at trial entry. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported for definitive evaluation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis. Some limitations with incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Details of all 13 participants included in trial report. |
| Other bias | Unclear risk | Low recruitment levels. Trial terminated after 2 years preliminary work when only 13 women had been randomised in 6 hospitals (3 teaching hospitals and 3 district general hospitals). |
Viegas 1985.
| Methods | Random allocation; method not stated. | |
| Participants | Inclusion: single pregnancy; in established preterm labour in breech presentation between 28‐36 weeks; no contraindication to caesarean or vaginal delivery; no congenital malformations; no severe pre‐eclampsia or IUGR; consent to participate. Setting (period of recruitment): 5 large delivery units in Singapore (1982‐3). Number of randomised participants: elective 12, selective 15. | |
| Interventions | I: vaginal delivery vs II: elective caesarean delivery. | |
| Outcomes |
|
|
| Notes | Individual patient data supplied by the first author. The data for randomised women reanalyzed by 1 of the review authors on an 'intention‐to‐treat' basis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported for definitive evaluation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomised data not reported separately from descriptive study group. |
| Selective reporting (reporting bias) | Unclear risk | Randomised data not reported separately from descriptive study group. |
| Other bias | Unclear risk | The RCT (N = 23) is reported with data from a descriptive study group (N = 50) and there is no separation between the two. Therefore it is not possible to isolate the randomised controlled trial data. |
Wallace 1984.
| Methods | Randomised trial; method not stated. | |
| Participants | Inclusion: established preterm labour in cephalic presentation between 26‐33 weeks; single pregnancy; no contraindication to caesarean or vaginal delivery; no congenital anomalies. Setting (period of recruitment): Los Angeles County/University of Southern California Medical Center (1981). Number of randomised participants: elective 23, selective 15. | |
| Interventions | I: vaginal delivery vs II: caesarean delivery. | |
| Outcomes |
|
|
| Notes | Trial terminated after 6 months because the proportion (63%) of babies with birthweight > 1500 g was "unacceptably high". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported for definitive evaluation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 women excluded for malpresentation after randomisation. Vaginal delivery group includes 3 randomised to caesarean section who delivered vaginally prior to surgery. |
| Selective reporting (reporting bias) | Unclear risk | Data for 14 cases eligible for the < 1500 g group reported separately. |
| Other bias | Unclear risk | High frequency of infants with weight > 1500 g. 38 women participated in total of which only 14 were eligible for the <1500 g group. After enrolling 40 women study discontinued. Trialists had planned to enrol 175 in each group. |
Zlatnik 1993.
| Methods | Randomised trial with sealed envelope method and post randomisation consent. | |
| Participants | Inclusion: established preterm labour in breech presentation between 28‐36 weeks; single fetus; delivery not imminent; no fetal abnormality on abdominal X‐ray examination; no contraindication to caesarean or vaginal delivery; no tocolytics used in labour; consent to participate. Setting (period of recruitment): University of Iowa Hospitals, USA (1978‐83). Number of randomised participants: elective 18, selective 20. | |
| Interventions | I: vaginal delivery vs II: caesarean delivery. | |
| Outcomes |
|
|
| Notes | Trial terminated after 52 months because of recruitment difficulties. Unpublished data were provided by the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported for definitive evaluation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No apparent issues with selective reporting. |
| Other bias | Unclear risk | There were no significant differences between 2 groups in: maternal age, length of gestation, parity, or estimated or actual birthweight. Accrual of participants proved too difficult and authors acknowledge the sample size was too small. |
CS: caesarean section IUGR: intrauterine growth restriction min: minutes NICU: neonatal intensive care unit vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dietl 1987 | No information or data available from the authors. |
| McColgin 1990 | No information or data available from the authors. |
Differences between protocol and review
Title
Change of title used in previous versions of this review from 'Elective caesarean section versus expectant management for delivery of the small baby' to 'Caesarean section versus vaginal delivery for preterm birth'.
Types of participants
Change of text used in previous versions of this review from 'women presenting in labour and thought to be carrying a small or immature baby, irrespective of fetal presentation' to 'women presenting or thought to be preterm labour (< 37 weeks) irrespective of fetal presentation'.
Types of interventions
Change of text used in previous versions of this review from 'a policy of elective caesarean delivery in comparison with expectant management with recourse to caesarean section if a clear clinical indication arose' to:
'Comparison of two policies to deliver preterm baby once the labour starts, or a decision is made that baby needs to be delivered:
aiming to deliver preterm baby by planned immediate caesarean delivery; or
aiming to deliver baby vaginally irrespective of the presentation (cephalic, breech).
We acknowledge the ambiguity with the term "planned" caesarean section. A planned caesarean section normally means that it is scheduled before the onset of labour (NICE 2011). However, for the purposes of this review, we refer to the term "planned immediate caesarean section", meaning a caesarean section which is planned only after the labour has started. When a woman presents in preterm labour, the issue is whether to deliver immediately by caesarean section to prevent preterm vaginal birth, or to aim to deliver vaginally with recourse to caesarean section if problems develop (Penn 1996). Planned immediate caesarean section in the context of this review implies performing caesarean section as soon as the spontaneous preterm birth is thought to be inevitable. However, this decision to perform caesarean section may be delayed in order to give corticosteroids to the mother before birth of the baby.'
Types of outcome measures
Change of outcomes used in previous versions of this review from:
One or more of a range of outcome measures assessing the effects of the policies on: length of pregnancy; complications in labour (for example, cord prolapse, head entrapment); neonatal condition at birth (for example, Apgar score, cord pH, need for intubation or mechanical ventilation, admission to special care facility); subsequent neonatal morbidity (for example, seizures, infection, jaundice, intracranial pathology, trauma); fetal or neonatal mortality; long‐term follow‐up in childhood; serious maternal morbidity or mortality (for example, postpartum haemorrhage, anaemia, need for blood transfusion, infection and prolonged hospital stay); and breastfeeding.
to:
Primary outcomes
Infant
Birth injury to infant
Birth asphyxia (occurs when a baby does not receive enough oxygen before, during, or just after birth) as defined by the trialists
Mother
Mother requires admission to intensive care/major maternal postpartum complications
Secondary outcomes
Infant
Perinatal death
Neonatal admission to special care and/or intensive care unit
Hypoxic ischaemic encephalopathy (a condition of injury to the brain)
Breastfeeding at discharge
Breastfeeding at three months
Cord pH below normal range
Abnormal follow‐up in childhood
Neonatal fitting/seizures
Meconium aspiration (means the newborn inhales a mixture of meconium and amniotic fluid, either in the uterus or just after delivery)
Apgar score at five minutes
Respiratory distress syndrome
Delivery less than seven days after entry
Neonatal infection (proven) (outcome not prespecified)
Intracranial pathology (outcome not prespecified)
Intracranial haemorrhage (outcome not prespecified)
Other birth trauma (outcome not prespecified)
Head entrapment (outcome not prespecified)
Necrotising enterocolitis (outcome not prespecified)
Cord prolapse (outcome not prespecified)
Need for mechanical ventilation (outcome not prespecified)
Ventilation (days) (outcome not prespecified)
Supplemental oxygen (days) (outcome not prespecified)#
Neonatal jaundice (outcome not prespecified)
Mother
Maternal satisfaction* (postnatal)
Postpartum haemorrhage (excess blood loss from the birth canal after childbirth) NB Including need for transfusion
Maternal puerperal pyrexia (outcome not prespecified)
Maternal wound infection (outcome not prespecified)
Other maternal infection (outcome not prespecified)
Maternal stay more than 10 days (outcome not prespecified)
Need for blood transfusion (outcome not prespecified)
Contributions of authors
Stefania Livio wrote the Background and Stephen Milan completed the Methods and Results sections. Data extraction for new analyses, completion of risk of bias and characteristics of included studies sections was performed by Stefania Livio and Stephen Milan. Stephen Milan conducted the new analyses. Zarko Alfirevic, Stefania Livio and Stephen Milan wrote the discussion and authors' conclusions.
The 2013 update was approved by all authors.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Lumley 1985 {published and unpublished data}
- Lumley J, Lester A, Renou P, Wood C. A failed RCT to determine the best method of delivery for very low birth weight infants. Controlled Clinical Trials 1985;6:120‐7. [DOI] [PubMed] [Google Scholar]
MacLennan 1986 {unpublished data only}
- MacLennan AH. Randomised trial of the mode of delivery of the very premature infant (trial abandoned). Personal communication 28th October 1986.
Penn 1996 {published data only}
- Penn ZJ, Steer PJ, Grant AM. A multicentre randomised controlled trial comparing elective and selective caesarean section for the delivery of the preterm breech infant. British Journal of Obstetrics and Gynaecology 1996;103(7):684‐9. [DOI] [PubMed] [Google Scholar]
Viegas 1985 {published and unpublished data}
- Viegas OAC, Ingemarsson I, Sim LP, Singh K, Cheng M, Ratnam SS, et al. Collaborative study on preterm breeches: vaginal delivery versus caesarean section. Asia Oceania Journal of Obstetrics and Gynaecology 1985;11:349‐55. [DOI] [PubMed] [Google Scholar]
Wallace 1984 {published and unpublished data}
- Wallace RL, Schifrin BS, Paul RH. The delivery route for very‐low‐birth‐weight infants. Journal of Reproductive Medicine 1984;29:736‐40. [PubMed] [Google Scholar]
Zlatnik 1993 {published and unpublished data}
- Zlatnik FJ. The Iowa premature breech trial. American Journal of Perinatology 1993;10(1):60‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dietl 1987 {unpublished data only}
- Dietl J. Effect of delivery route on outcome of infants at early gestational age. Personal communication 1987.
McColgin 1990 {unpublished data only}
- McColgin S. Delivery of the vertex VLBW infant: vaginal vs Caesarean section delivery. Personal communication 1990.
Additional references
Cohen 1985
- Cohen M, Carson BS. Respiratory morbidity benefit of awaiting onset of labor after elective cesarean section. Obstetrics & Gynecology 1985;65(6):818‐24. [PubMed] [Google Scholar]
Crowther 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
DOH 1996
- Department of Health, Welsh Office, Scottish Office Department of Health, Department of Health and Social Services NI. In: Rubery E, Bourdillon P, Hibbard B editor(s). Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: HMSO, 1996. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gravenhorst 1993
- Gravenhorst JB, Schreuder AM, Veen S, Brand R, Verloove‐Vanhorick SP, Verweij RA, et al. Breech delivery in very preterm and very low birthweight infants in The Netherlands. British Journal of Obstetrics and Gynaecology 1993;100(5):411‐5. [DOI] [PubMed] [Google Scholar]
Hannah 2000
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet 2000;356:1375‐83. [DOI] [PubMed] [Google Scholar]
Hannah 2002
- Hannah ME, Hannah WJ, Hodnett ED, Chalmers B, Kung R, Willan A, et al. Outcomes at 3 months after planned cesarean vs planned vaginal delivery for breech presentation at term: the international randomized Term Breech Trial. JAMA 2002;287(14):1822‐31. [DOI] [PubMed] [Google Scholar]
Hannah 2004
- Whyte H, Hannah ME, Saigal S, Hannah WJ, Hewson S, Amankwah K, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. American Journal of Obstetrics and Gynecology 2004;191(3):864‐71. [DOI] [PubMed] [Google Scholar]
Hansen 2008
- Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ 2008;336(7635):85‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Malloy 1991
- Malloy MH, Onstad L, Wright E. The effect of cesarean delivery on birth outcome in very low birth weight infants. National Institute of Child Health and Human Development Neonatal Research Network. Obstetrics & Gynecology 1991;77(4):498‐503. [PubMed] [Google Scholar]
Malloy 2009
- Malloy MH. Impact of cesarean section on intermediate and late preterm births: United States, 2000–2003. Birth 2009;36(1):26‐33. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Caesarean Section. NICE clinical guideline 132. http://guidance.nice.org.uk/CG132/NICEGuidance/pdf/English (accessed 24 April 2012) 2011 November. [PubMed]
Petitti 1985
- Petitti D. Maternal mortality and morbidity in cesarean section. Clinical Obstetrics and Gynecology 1985;28(4):763‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Shah 1990
- Shah YG, Ronner W, Eckl CJ, Stinson SK. Acute maternal morbidity following classical cesarean delivery of the preterm infant. Obstetrics & Gynecology 1990;76:16‐9. [PubMed] [Google Scholar]
Stock 2012
- Stock SJ, Bricker L, Norman JE. Immediate versus deferred delivery of the preterm baby with suspected fetal compromise for improving outcomes. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD008968.pub2] [DOI] [PubMed] [Google Scholar]
Villar 2007
- Villar J, Carroli G, Zavaleta N, Donner A, Wojdyla D, Faundes A, et al. Maternal and neonatal individual risks and benefits associated with caesarean delivery: multicentre prospective study. BMJ 2007;335(7628):1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yanq 2007
- Yang Q, Wen SW, Oppenheimer L, Chen XK, Black D, Gao J, et al. Association of caesarean delivery for first birth with placenta praevia and placental abruption in second pregnancy. BJOG: an international journal of obstetrics and gynaecology 2007;114(5):609‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alfirevic 2012
- Alfirevic Z, Milan SJ, Livio S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000078.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 1996
- Grant A. Elective or selective caesarean section of the small baby? A systematic review of the controlled trials. British Journal of Obstetrics and Gynaecology 1996;103(12):1197‐200. [DOI] [PubMed] [Google Scholar]
Grant 2000
- Grant A. Elective versus selective caesarean section for delivery of the small baby. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
Grant 2001
- Grant A. Elective versus selective caesarean section for delivery of the small baby. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI] [PubMed] [Google Scholar]
Grant 2010
- Grant A, Glazer CMA. Elective caesarean section versus expectant management for delivery of the small baby. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD000078] [DOI] [PubMed] [Google Scholar]


