Abstract
Background
In the randomized controlled RESTORE-IMI 1 clinical trial (NCT02452047), imipenem/cilastatin (IMI) with relebactam (IMI/REL) was as effective as colistin plus IMI for the treatment of imipenem-nonsusceptible gram-negative infections. Differences in nephrotoxicity were observed between treatment arms. As there is no standard definition of nephrotoxicity used in clinical trials, we conducted analyses to further understand the renal safety profile of both treatments.
Methods
Nephrotoxicity was retrospectively evaluated using 2 acute kidney injury assessment criteria (Kidney Disease Improving Global Outcomes [KDIGO] and Risk, Injury, Failure, Loss, and End-stage Kidney Disease [RIFLE]). Additional outcomes included time to onset of protocol-defined nephrotoxicity and incidence of renal adverse events.
Results
Of 47 participants receiving treatment, 45 had sufficient data to assess nephrotoxicity (IMI/REL, n = 29; colistin plus IMI, n = 16). By KDIGO criteria, no participants in the IMI/REL but 31.3% in the colistin plus IMI group experienced stage 3 acute kidney injury. No IMI/REL-treated participants experienced renal failure by RIFLE criteria, vs 25.0% for colistin plus IMI. Overall, the time to onset of nephrotoxicity varied considerably (2–22 days). Fewer renal adverse events (12.9% vs 37.5%), including discontinuations due to drug-related renal adverse events (0% vs 12.5%), were observed in the IMI/REL group compared with the colistin plus IMI group, respectively.
Conclusions
Our analyses confirm the findings of a preplanned end point and provide further evidence that IMI/REL had a more favorable renal safety profile than colistin-based therapy in patients with serious, imipenem-nonsusceptible gram-negative bacterial infections.
ClinicalTrials.gov Identifier
Keywords: acute kidney injury, colistin, IMI/REL, KDIGO criteria, RIFLE criteria
Infections due to carbapenem-resistant gram-negative bacteria such as Enterobacteriaceae (new taxonomy: Enterobacterales) and Pseudomonas aeruginosa pose a serious threat to global public health due to increased mortality, hospital length of stay, and cost compared with carbapenem-susceptible infections [1–5]. Potential treatment options for carbapenem-resistant infections include polymyxins (polymyxin B and colistin [polymyxin E]), tigecycline, aminoglycosides, ceftazidime-avibactam, and meropenem-vaborbactam [6–10]; however, polymyxins, including colistin, are associated with toxicities and other important safety concerns that can further complicate the management of patients with drug-resistant bacterial infections who are medically complex (eg, patients with multiple comorbidities, patients at risk of renal injury or who have preexisting renal insufficiency, and/or patients taking concomitant nephrotoxic medications) [11].
Nephrotoxicity is commonly associated with polymyxin-based therapy [12, 13]. The reported rates of nephrotoxicity increase with higher concentrations or doses and longer durations of therapy [11, 14–16]. Current guidelines recommend that polymyxins be used in combination with other agents to maximize the clinical effect [8, 17, 18]. However, rates of nephrotoxicity are high with colistin and colistin-based combination therapies, ranging from 12% to 29% in some studies [12, 16, 19, 20]. Further complicating the clinical management of these serious bacterial infections is that nephrotoxicity with polymyxins (especially colistin) can occur soon after initiation of treatment [21, 22].
Recently, there has been an increased focus on the development of novel therapies that can address increased incidences of antibacterial-resistant infections and offer improved safety over older drugs such as colistin. Relebactam (REL) is a β-lactamase inhibitor active against Ambler Class A and C enzymes that can restore imipenem activity against many imipenem-nonsusceptible strains of gram-negative bacteria [9, 10]. In the phase 3 RESTORE-IMI 1 trial (NCT02452047), the combination of imipenem/cilastatin (IMI) with relebactam (IMI/REL) had comparable efficacy to colistin plus IMI in the primary end point of favorable overall response and was better tolerated, including a lower incidence of protocol-defined treatment-emergent nephrotoxicity (10.3% [3/29] vs 56.3% [9/16]; P = .002) [23].
To date, no single biomarker or set definition of drug-induced nephrotoxicity has been established for use in drug development trials [24, 25]. Here we present an additional exploratory, post hoc analysis of renal safety data from the phase 3 RESTORE-IMI 1 trial, including an evaluation of nephrotoxicity using 2 acute kidney injury (AKI) assessment criteria commonly cited in the literature (Kidney Disease Improving Global Outcomes [KDIGO] and Risk, Injury, Failure, Loss, and End-stage Kidney Disease [RIFLE]) (Table 1) [26, 27].
Table 1.
Criteria for Assessment of Treatment-Emergent Nephrotoxicity
| Protocol-Defined Criteriaa | |
|---|---|
| Baseline Renal Function Category | Serum Cr |
| Normal (serum Cr <1.2 mg/dL) | Doubling of Cr up to >1.2 mg/dL OR ≥50% reduction in CrCl |
| Preexisting dysfunction (serum Cr ≥1.2 mg/dL) | Increases in Cr ≥1 mg/dL OR ≥20% reduction in CrCl OR Initiation of renal replacement therapy |
| KDIGO Criteriab | |
| Stagec | Serum Cr |
| 1 | 1.5–1.9 times baseline OR ≥0.3 mg/dL (≥26.5 µmol/L) increase |
| 2 | 2.0–2.9 times baseline |
| 3 | 3.0 times baseline OR Increase ≥4.0 mg/dL (≥353.6 µmol/L) OR Initiation of renal replacement therapy |
| RIFLE Criteria | |
| Classificationc,d | GFR Criteria |
| Risk | Increased serum Cr × 1.5 OR GFR decrease >25% |
| Injury | Increased serum Cr × 2 OR GFR decrease >50% |
| Failure | Increased serum Cr × 3 OR GFR decrease 75% OR Serum creatinine ≥4 mg/dL (acute rise ≥0.5 mg/dL) |
Abbreviations: AKI, acute kidney injury; Cr, creatinine; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease.
aCriteria assessed a priori.
bCriteria assessed retrospectively.
cIf a participant met criteria for >1 AKI stage/classification, the participant was included only once, in the “worst-case” stage/classification.
dLoss of kidney function (>4 weeks) and end-stage kidney disease (>3 months) classifications were not included due to the study duration being less than the defined timelines.
METHODS
Study Design and Participants
RESTORE-IMI-1 (Protocol MK-7655A-013) was a phase 3, randomized, active comparator–controlled, double-blind study to evaluate the efficacy and safety of IMI/REL compared with colistin plus IMI in participants enrolled at 16 sites in 11 countries between October 2015 and September 2017. The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. The approach of Merck & Co., Inc., to the conduct of clinical trials is in accordance with ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practice and the applicable regulatory requirement(s). Written patient consent was obtained before screening. The full methodology was published previously [23].
Briefly, eligible participants were aged ≥18 years and required hospitalization and treatment with intravenous (IV) antibiotics for serious gram-negative infections (complicated intra-abdominal infection [cIAI], complicated urinary tract infection [cUTI], hospital-acquired bacterial pneumonia [HABP], or ventilator-associated bacterial pneumonia [VABP]) caused by pathogens confirmed to be imipenem-nonsusceptible, but susceptible to imipenem/relebactam and colistin. Participants were excluded if they had an APACHE II score >30, creatinine clearance (CrCl) <15 mL/min, or if they required hemodialysis or peritoneal dialysis.
Eligible participants were randomized (stratified by infection type) 2:1 to IV IMI/REL (500 mg/250 mg every 6 hours; IMI dose includes 500 mg imipenem and 500 mg cilastatin) plus placebo or IV colistin (as colistimethate sodium; loading dose to achieve 300 mg colistin base activity [CBA], followed by maintenance doses every 12 hours based on CrCl up to 150 mg CBA) + IMI (500 mg every 6 hours) (Supplementary Figure 1). Doses for all 3 study drugs were adjusted based on renal function (Supplementary Table 1), and the duration of therapy was determined by infection type and did not exceed 21 days.
Measurements and Criteria for AKI
Blood for laboratory safety tests (hematology and chemistry) was collected for analysis at a central laboratory at randomization (day 1) before administration of the first dose of IV therapy, on day 3, and every 3 days thereafter until the end of therapy (EOT), and then at the following visits: EOT, early follow-up (5–9 days after EOT), day 28 postrandomization, and safety follow-up (14 days after EOT) (Supplementary Figure 2). Urine was collected for analysis on day 1 and at EOT.
Data contributing to the assessment of nephrotoxicity were collected from both central and local laboratories (local collection was performed as needed to ensure timely patient management). Treatment-emergent nephrotoxicity was assessed a priori by protocol-defined criteria and by guideline-based definitions of AKI (KDIGO and RIFLE), which were assessed retrospectively (Table 1) [20, 27]. Information on renal adverse events (AEs) and renal AEs leading to discontinuation was collected from the first dose of IV study therapy through 14 days after EOT. AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA; version 20.0). Renal AEs that may be clinically relevant indicators of potential renal injury were derived from the renal and urinary disorders and investigations system organ classes. The relationship of any AE to study medication was determined by the investigator assessment during IV therapy through safety follow-up.
Statistical Analysis
The safety population was used for all safety assessments and was defined as all randomized participants who received ≥1 dose of IV study treatment according to the actual treatment received. Descriptive statistics were used to summarize baseline demographics, AEs, and treatment-emergent nephrotoxicity. A planned comparison of treatment-emergent nephrotoxicity rates based on protocol-defined criteria was performed using 2-sided P values obtained using the Fisher exact test. Analyses of additional end points were conducted to further assess and confirm the prespecified nephrotoxicity end point. Although these new end points were not prespecified, the 2-sided P values from the Fisher exact test have been included here for consistency. As the latter comparisons were not preplanned, these P values are simply to provide additional evidence in support of the preplanned end point.
RESULTS
A total of 47 participants were in the safety population, including 31 participants treated with IMI/REL and 16 participants treated with colistin plus IMI. The study population in each treatment group was balanced with respect to sex, weight, APACHE II score, primary diagnosis, and CrCl (Table 2). The majority of participants in the IMI/REL and colistin plus IMI treatment groups had a primary diagnosis of cUTI, followed by HABP/VABP and cIAI. At baseline, the majority of participants (74.5%) had normal renal function or mild renal impairment (ie, CrCl ≥ 60 mL/min), as calculated by the Cockcroft-Gault equation.
Table 2.
Baseline Demographics and Clinical Characteristics (Safety Population)
| IMI/REL (n = 31) | Colistin + IMI (n = 16) | Total (n = 47) | |
|---|---|---|---|
| Sex, No. (%) | |||
| Male | 20 (64.5) | 10 (62.5) | 30 (63.8) |
| Female | 11 (35.5) | 6 (37.5) | 17 (36.2) |
| Age | |||
| <65 y, No. (%) | 19 (61.3) | 7 (43.8) | 26 (55.3) |
| ≥65 y, No. (%) | 12 (38.7) | 9 (56.3) | 21 (44.7) |
| Median (range) | 59 (19–77) | 66 (22–80) | 59 (19–80) |
| Weight, kg | |||
| Median (range) | 76 (49.0–140.0) | 73.5 (52.8–117.0) | 75.8 (49–140.0) |
| APACHE II score, No. (%) | |||
| ≤15 | 22 (71.0) | 12 (75.0) | 34 (72.3) |
| >15 | 9 (29.0) | 4 (25.0) | 13 (27.7) |
| Primary diagnosis, No. (%) | |||
| HABP | 1 (3.2) | 1 (6.3) | 2 (4.3) |
| VABP | 9 (29.0) | 5 (31.3) | 14 (29.8) |
| cIAI | 5 (16.1) | 3 (18.8) | 8 (17.0) |
| cUTI (urinary tract abnormalities) | 9 (29.0) | 3 (18.8) | 12 (25.5) |
| cUTI (acute pyelonephritis) | 7 (22.6) | 4 (25.0) | 11 (23.4) |
| CrCl, No. (%) | |||
| ≥90 mL/min | 10 (32.3) | 6 (37.5) | 16 (34.0) |
| <90 to ≥60 mL/min | 14 (45.2) | 5 (31.3) | 19 (40.4) |
| <60 to ≥30 mL/min | 5 (16.1) | 3 (18.8) | 8 (17.0) |
| <30 to ≥15 mL/min | 1 (3.2) | 2 (12.5) | 3 (6.4) |
| Not available | 1 (3.2) | 0 (0.0) | 1 (2.1) |
Abbreviations: APACHE II, Acute Physiologic Assessment and Chronic Health Evaluation II; cIAI, complicated intra-abdominal infection; CrCl, creatinine clearance; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; VABP, ventilator-associated bacterial pneumonia.
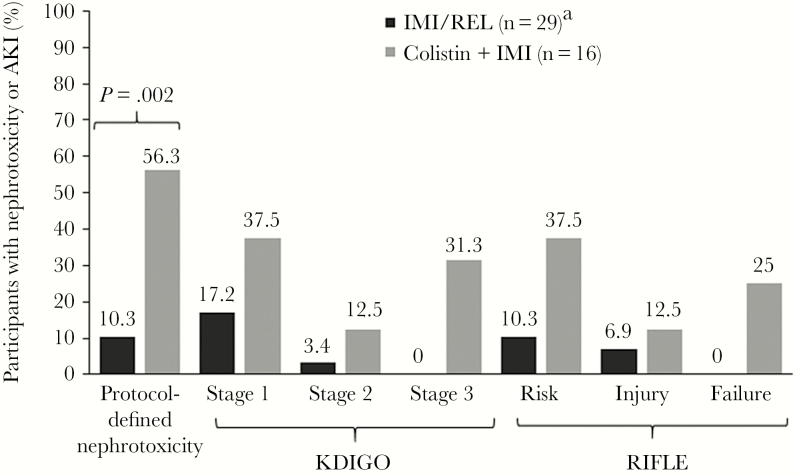
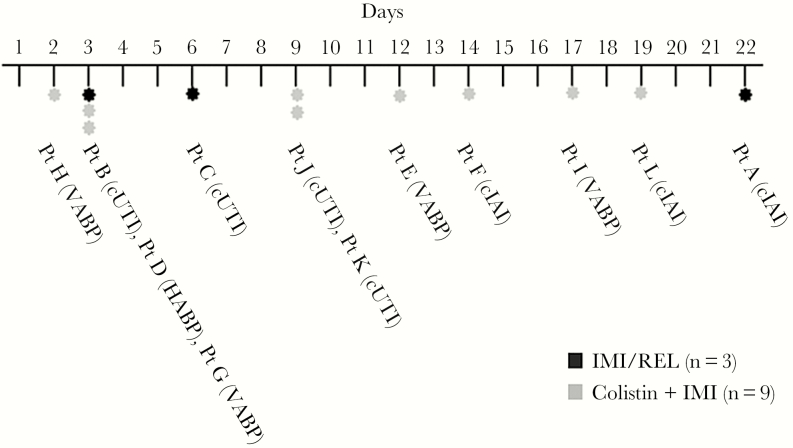
Of the 47 participants in the safety population, 45 had complete data for assessment of nephrotoxicity; 2 participants in the IMI/REL group had missing creatinine values and were excluded. As was reported previously, a significantly smaller percentage of participants in the IMI/REL group than in the colistin plus IMI group experienced protocol-defined treatment-emergent nephrotoxicity (10.3% [3/29] vs 56.3% [9/16]; treatment difference, –45.9%; 95% confidence interval, –69.1% to –18.4%; P = .002) (Figure 1) [23]. A post hoc review of the timing of protocol-defined nephrotoxicity onset shows a wide range of onset, with no discernible pattern related to primary diagnosis or treatment arm (Figure 2). For IMI/REL, the first occurrence of protocol-defined nephrotoxicity occurred as early as day 3 and as late as day 22. In the colistin plus IMI group, the first occurrence of nephrotoxicity occurred as early as day 2 and as late as day 19. The magnitude of CrCl reduction at any point in participants experiencing protocol-defined nephrotoxicity ranged from 20.1% to 54.2% in IMI/REL-treated participants and 0.3% to 86.8% in participants treated with colistin plus IMI.
Figure 1.
Participants with protocol-defined nephrotoxicity or AKI by KDIGO or RIFLE criteria. If the patient met criteria for >1 AKI stage, they were only included once, in the worst-case stage. aTwo participants in the IMI/REL group with missing creatinine values were excluded from the nephrotoxicity and AKI evaluations.Abbreviations: AKI, acute kidney injury; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; KDIGO, Kidney Disease: Improving Global Outcomes; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney Disease.
Figure 2.
Time to nephrotoxicity event. Abbreviations: cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; HABP, hospital-acquired bacterial pneumonia; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; VABP, ventilator-associated bacterial pneumonia.
When evaluated according to KDIGO criteria, the proportion of patients with AKI (stage 1–3) was significantly lower for the IMI/REL group compared with the colistin plus IMI group (20.7% [6/29] vs 81.3% [13/16]; treatment difference, –60.6%; P < .001) (Figure 1). No participants in the IMI/REL group were classified as stage 3 AKI compared with 31.3% of participants in the colistin plus IMI group). The proportion of patients with stage 1 and 2 AKI was also smaller in the IMI/REL group compared with colistin plus IMI. Assessment according to RIFLE criteria found that nephrotoxicity was significantly lower for the IMI/REL group compared with the colistin plus IMI group (17.2% [5/29] vs 75.0% [12/16]; treatment difference, –57.8%; P < .001). No participants in the IMI/REL group experienced renal failure compared with 25.0% of participants in the colistin plus IMI group. In addition, a lower proportion of patients in the IMI/REL group experienced risk or injury per RIFLE criteria compared with colistin plus IMI.
By MedDRA terms, a total of 10 participants experienced ≥1 renal AE: 4 in the IMI/REL group (12.9%) and 6 in the colistin plus IMI group (37.5%) (Table 3). The most common renal AE was “blood creatinine increased,” occurring with greater frequency in the colistin plus IMI group (IMI/REL 0% vs colistin plus IMI 25.0%). There was 1 AE of AKI and 1 AE of renal failure observed in 2 patients in the IMI/REL group. There were no drug-related renal AEs leading to discontinuation of treatment in the IMI/REL group, but there were 2 in the colistin plus IMI group (1 patient each due to “blood creatinine decreased” and “CrCl decreased”).
Table 3.
Renal AEsa (Safety Population)
| IMI/REL | Colistin + IMI | |||
|---|---|---|---|---|
| Renal AEs | n/m | % | n/m | % |
| Patients with ≥1 renal AE | 4/31 | 12.9 | 6/16 | 37.5 |
| Blood creatinine increased | 0/31 | 0 | 4/16 | 25.0 |
| Blood urea increased | 0/31 | 0 | 1/16 | 6.3 |
| Creatinine clearance decreased | 2/31 | 6.5 | 2/16 | 12.5 |
| Glomerular filtration rate decreased | 0/31 | 0 | 1/16 | 6.3 |
| Acute kidney injury | 1/31 | 3.2 | 0/16 | 0 |
| Renal failure | 1/31 | 3.2 | 0/16 | 0 |
| Drug-related renal AEs leading to discontinuation of treatmentb | n/m | % | n/m | % |
| Blood Cr increased | 0/31 | 0 | 1/16 | 6.3 |
| CrCl decreased | 0/31 | 0 | 1/16 | 6.3 |
Abbreviations: AE, adverse event; Cr, creatinine; CrCl, creatinine clearance; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; n/m, number of participants with the observation of interest/number of evaluable participants.
aAEs were classified according to the Medical Dictionary for Regulatory Activities, version 20.0.
bBased on investigator assessment.
Key characteristics for the 10 participants who experienced ≥1 renal AE are shown in Supplementary Table 2. Treatment-emergent renal AEs occurred in participants of a broad age range (40 to 80 years), and time to onset varied widely in both treatment groups (IMI/REL: 3 to 19 days; colistin + IMI: 2 to 19 days). There was no discernible pattern to potential factors that may have contributed to renal AEs. Most participants, but not all, had a history of renal impairment, AKI, or chronic kidney disease or received a potentially nephrotoxic medication (eg, colistin, vancomycin, aminoglycoside) within 7 days of starting study medication.
Two patients in the colistin plus IMI treatment arm required initiation of dialysis during the study period, which was initiated on day 3. One patient died the following day due to cardiac arrest, which was deemed unrelated to the study medication. The other patient received dialysis until day 8, when the patient died from hypoxic brain injury. This death was also deemed unrelated to the study medication. No patients who received IMI/REL required dialysis during therapy.
DISCUSSION
In the phase 3 RESTORE-IMI 1 study, IMI/REL was as effective as colistin plus IMI in several outcome measures (overall response, clinical response, and 28-day mortality) but was better tolerated, including a significantly lower incidence of protocol-defined nephrotoxicity [23]. Our additional analyses of safety data from that trial further supported the conclusion of improved renal safety with IMI/REL compared with colistin-based therapy [23]. Evaluation of renal safety data using 2 widely accepted assessment criteria demonstrated that no participants who received IMI/REL experienced stage 3 AKI by KDIGO guidelines or failure by RIFLE criteria and that IMI/REL resulted in fewer incidents of AKI in the less severe strata for both KDIGO and RIFLE. Overall, nephrotoxicity rates determined by KDIGO guidelines and RIFLE criteria were higher in the colistin plus IMI group. Furthermore, renal AEs, based on MedDRA terms, were infrequent in the IMI/REL treatment group, and no IMI/REL-treated participants discontinued treatment due to drug-related renal AEs. Capturing AEs by investigator assessment is subjective and may not be supported with laboratory data; however, the overall trends observed using investigator assessment generally aligned with improved renal safety.
A review of relevant characteristics of the 10 patients who experienced renal AEs showed no discernible pattern of potential contributing factors. The majority (n = 8) of these patient cases were medically complex (serious infections with multiple comorbidities including prior history of renal impairment and/or prior use of nephrotoxic medications), but there were also 2 participants who had no history of renal impairment and/or no prior use of nephrotoxic medications who developed renal AEs. This underscores the importance of considering the renal safety profile of antibacterial agents selected for management of the full spectrum of patient cases.
Nephrotoxicity rates reported in the literature for colistin-based therapy vary widely from 0% to 76%, depending on the study design, patient population, and the criteria or definition used for nephrotoxicity [8, 11, 12, 16, 20, 21, 28–31]. The wide range in nephrotoxicity rates reported in the literature may also be attributed to the lack of standard dosing regimens for colistin across studies. In the RESTORE-IMI 1 study, the dosing of colistin was optimized based on contemporary pharmacokinetic/pharmacodynamic modeling, consistent with current treatment guidelines, to ensure that the given dose was sufficient to achieve efficacy, while balancing concerns about nephrotoxicity [8, 32, 33]. Colistin dosing was also standardized to ensure that study participants would receive a uniform dose (adjusted for renal function), to support a robust comparison of safety and efficacy against IMI/REL. Despite colistin dose optimization, a more favorable renal safety profile was observed for IMI/REL in this exploratory post hoc analysis, with observed rates of 25% and 31% in the colistin plus IMI treatment group for stage 3 AKI (KDIGO) and failure (RIFLE), respectively. Both KDIGO and RIFLE criteria have demonstrated significant predictive utility for mortality risk in critically ill patients [34–36]. In our study, the results using both criteria were generally consistent, and these results were comparable to the primary study analysis [23]. This suggests that patient evaluation using either KDIGO or RIFLE criteria should provide reliable indications of nephrotoxicity and that, regardless of definition, incidences of nephrotoxicity were lower in patients treated with IMI/REL compared with those treated with colistin-based therapy.
As in RESTORE-IMI 1, recent clinical studies of other new antibacterial agents compared with colistin-based therapy have suggested that colistin has a higher rate of nephrotoxicity than these newer therapies [37, 38]. The consistency of this observation across recent clinical trials is notable for clinicians managing complex infections.
A limitation of this study is the small sample size (n = 45 participants with complete data for the current analysis), which prevented formal statistical between-group comparisons of AE incidence, AKI risk factors, and time to (AKI) event. Although nephrotoxicity was significantly less frequent with IMI/REL than colistin plus IMI, it is notable that nephrotoxicity occurred across a very wide time frame after the initiation of either antibacterial therapy regimen (ie, days 2 to 19 for colistin plus IMI and days 3 to 22 for IMI/REL). The RESTORE-IMI 1 study design did not include a preplanned time-to-event analysis; this may have prevented detection of a discernible pattern of nephrotoxic events related to initiation of therapy (3/29 IMI/REL-treated participants and 9/16 colistin plus IMI–treated participants). In a prospective observational cohort study, Dalfino et al. observed a median time to onset of AKI (by Acute Kidney Injury Network criteria) of 5 days, with an interquartile range of 3 to 14 days, in 31 colistin-treated participants with severe sepsis or shock [30]. In a larger retrospective cohort study, including 64 colistin-treated participants who developed nephrotoxicity, the mean time to peak serum creatinine for colistin-treated participants was 6.5 days [28]. Future observational studies with larger sample sizes can provide more insight into the timing and patterns of nephrotoxic events associated with colistin and other potentially nephrotoxic antibacterial agents.
For patients with renal impairment and/or those in whom potential de novo nephrotoxicity might make medical management of these challenging infections even more complex, it is appropriate to choose antibacterial agents to minimize renal toxicity. In adult patients with serious, imipenem-nonsusceptible gram-negative bacterial infections, IMI/REL has a more favorable renal safety profile than colistin-based therapy, regardless of the assessment criteria applied.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing and/or editorial assistance was provided by Robert Schupp, PharmD, CMPP, of The Lockwood Group, Stamford, Connecticut, USA.
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA (MSD); MSD funded the medical writing support.
Potential conflicts of interest. J.M.’s institution (University Clinics Heidelberg) received research support from MSD. H.W.B. has been a (scientific advisory board) consultant to MSD. K.S.K. received research support from MSD and has been a consultant for MSD, Melinta, Achaogen, Allergan, and Shionogi. T.M.F. has been a consultant to MSD, Motif Bio, Nabriva, Paratek, and Shionogi. M.L.B., N.V., A.A., H.-K.J., R.W.T., J.D., D.D.D., J.R.B., N.A.K., and A.P. are employees of MSD and may own stock and/or hold stock options in the company. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. All authors are responsible for the work described in this paper. All authors were involved in at least 1 of the following: conception, design of work or acquisition, analysis, interpretation of data; and drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Prior presentation. This research was presented in part at IDWeek 2018 in San Francisco, California, USA.
Data availability. MSD’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
References
- 1. Cai B, Echols R, Magee G, et al. . Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017; 4(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed 17 August 2018.
- 3. Judd WR, Ratliff PD, Hickson RP, et al. . Clinical and economic impact of meropenem resistance in Pseudomonas aeruginosa-infected patients. Am J Infect Control 2016; 44:1275–9. [DOI] [PubMed] [Google Scholar]
- 4. Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zilberberg MD, Nathanson BH, Sulham K, et al. . Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 2017; 17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fritzenwanker M, Imirzalioglu C, Herold S, et al. . Treatment options for carbapenem-resistant Gram-negative infections. Dtsch Arztebl Int 2018; 115:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munoz-Price LS, Poirel L, Bonomo RA, et al. . Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuji BT, Pogue JM, Zavascki AP, et al. . International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019; 39:10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Temkin E, Torre-Cisneros J, Beovic B, et al. . Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 2017; 61:e01964–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. . Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7:439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aggarwal R, Dewan A. Comparison of nephrotoxicity of colistin with polymyxin B administered in currently recommended doses: a prospective study. Ann Clin Microbiol Antimicrob 2018; 17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durante-Mangoni E, Signoriello G, Andini R, et al. . Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 2013; 57:349–58. [DOI] [PubMed] [Google Scholar]
- 13. Okoduwa A, Ahmed N, Guo Y, et al. . Nephrotoxicity associated with intravenous polymyxin B once- versus twice-daily dosing regimen. Antimicrob Agents Chemother 2018; 62:e00025–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elias LS, Konzen D, Krebs JM, Zavascki AP. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother 2010; 65:2231–7. [DOI] [PubMed] [Google Scholar]
- 15. Fresenius Kabi USA LLC. GENTAMICIN - gentamicin sulfate injection, solution 2015. Available at: http://editor.fresenius-kabi.us/PIs/US-PH-Gentamicin-Inj-USP-FK-45855I-11-2015-PI.pdf. Accessed 20 August 2018.
- 16. Paul M, Daikos GL, Durante-Mangoni E, et al. . Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18:391–400. [DOI] [PubMed] [Google Scholar]
- 17. Hawkey PM, Warren RE, Livermore DM, et al. . Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 2018; 73:iii2–78. [DOI] [PubMed] [Google Scholar]
- 18. Kalil AC, Metersky ML, Klompas M, et al. . Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abdelsalam MFA, Abdalla MS, El-Abhar HSE. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin-meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist 2018; 15:127–35. [DOI] [PubMed] [Google Scholar]
- 20. Lodise TP, Fan W, Griffith DC, Dudley MN, Sulham KA. A retrospective cohort analysis shows that coadministration of minocycline with colistin in critically ill patients is associated with reduced frequency of acute renal failure. Antimicrob Agents Chemother 2018; 62:e01165–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phe K, Lee Y, McDaneld PM, et al. . In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 2014; 58:2740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pogue JM, Lee J, Marchaim D, et al. . Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–84. [DOI] [PubMed] [Google Scholar]
- 23. Motsch J, de Oliveria C, Stus V, et al. . RESTORE-IMI 1: a multicentre, randomised, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. European Medicines Agency. Letter of support for drug-induced renal tubular injury biomarker(s) 2016. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2017/01/WC500219666.pdf. Accessed 20 August 2018.
- 25. Fuchs TC, Hewitt P. Biomarkers for drug-induced renal damage and nephrotoxicity-an overview for applied toxicology. AAPS J 2011; 13:615–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. Accessed 17 August 2018.
- 27. Bellomo R, Ronco C, Kellum JA, et al. ; Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akajagbor DS, Wilson SL, Shere-Wolfe KD, et al. . Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis 2013; 57:1300–3. [DOI] [PubMed] [Google Scholar]
- 29. Benattar YD, Omar M, Zusman O, et al. . The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis 2016; 63:1605–12. [DOI] [PubMed] [Google Scholar]
- 30. Dalfino L, Puntillo F, Ondok MJ, et al. . Colistin-associated acute kidney injury in severely ill patients: a step toward a better renal care? A prospective cohort study. Clin Infect Dis 2015; 61:1771–7. [DOI] [PubMed] [Google Scholar]
- 31. Omrani AS, Alfahad WA, Shoukri MM, et al. . High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann Clin Microbiol Antimicrob 2015; 14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garonzik SM, Li J, Thamlikitkul V, et al. . Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nation RL, Garonzik SM, Li J, et al. . Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016; 62:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levi TM, de Souza SP, de Magalhães JG, et al. . Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev Bras Ter Intensiva 2013; 25:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo X, Jiang L, Du B, et al. ; Beijing Acute Kidney Injury Trial (BAKIT) workgroup A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014; 18:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai TY, Chien H, Tsai FC, et al. . Comparison of RIFLE, AKIN, and KDIGO classifications for assessing prognosis of patients on extracorporeal membrane oxygenation. J Formos Med Assoc 2017; 116:844–51. [DOI] [PubMed] [Google Scholar]
- 37. McKinnell JA, Dwyer JP, Talbot GH, et al. ; CARE Study Group Plazomicin for infections caused by carbapenem-resistant enterobacteriaceae. N Engl J Med 2019; 380:791–3. [DOI] [PubMed] [Google Scholar]
- 38. Shields RK, Nguyen MH, Chen L, et al. . Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 2017; 61:e00883–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.