Abstract
Background
Aripiprazole is a relatively new antipsychotic drug, said to be the prototype of a new third generation of antipsychotics; the so‐called dopamine‐serotonin system stabilisers. In this review we examine how the efficacy and tolerability of aripiprazole differs from that of typical antipsychotics.
Objectives
To evaluate the effects of aripiprazole compared with other typical antipsychotics for people with schizophrenia and schizophrenia‐like psychoses.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (November 2007) which is based on regular searches of BIOSIS, CENTRAL, CINAHL, EMBASE, MEDLINE and PsycINFO. We inspected references of all identified studies for further trials. We contacted relevant pharmaceutical companies, drug approval agencies and authors of trials for additional information.
Selection criteria
We included all randomised trials comparing aripiprazole with typical antipsychotics in people with schizophrenia or schizophrenia‐like psychosis.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated relative risks (RR) and their 95% confidence intervals (CI) on an intention‐to‐treat basis, based on a random effects model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate. For continuous data, we calculated weighted mean differences (WMD) again based on a random effects model. We have contacted representatives of Bristol Myers Squibb pharmaceuticals (UK) for additional data.
Main results
We included nine randomised trials involving 3122 people comparing aripiprazole with typical antipsychotic drugs. None of the studies reported on relapse ‐ our primary outcome of interest. Attrition from studies was high and data reporting poor. Participants given aripiprazole were comparable to those receiving typical drugs in improving global state and mental state. Aripiprazole provided a significant advantage over typical antipsychotics in terms of fewer occurrences of extra‐pyramidal symptom (n=968, 3 RCT, RR 0.46 CI 0.3 to 0.9, NNT 13 CI 17 to 10), and particularly akathisia (n=897, 3 RCT, RR 0.39 CI 0.3 to 0.6, NNT 11 CI 14 to 9). Fewer participants given aripiprazole developed hyperprolactinaemia (n=300, 1 RCT, RR 0.07 CI 0.03 to 0.2, NNT 2 CI 3 to 1). Aripiprazole presented a lesser risk of sinus tachycardia (n=289, 1 RCT, RR 0.09 CI 0.01 to 0.8, NNT 22 CI 63 to 13) and blurred vision (n=308, 1 RCT, RR 0.19 CI 0.1 to 0.7, NNT 14 CI 25 to 10); but enhanced risk of occurrence of dizziness (n=957, 3 RCT, RR 1.88 CI 1.1 to 3.2, NNH 20 CI 33 to 14) and nausea (n=957, 3 RCT, RR 3.03 CI 1.5 to 6.1, NNH 17 CI 25 to 13). Attrition rates were high in both groups, although significantly more participants in the aripiprazole group completed the study in the long term (n=1294, 1 RCT, RR 0.81 CI 0.8 to 0.9 NNT 8 CI 5 to 14).
Authors' conclusions
Aripiprazole differs little from typical antipsychotic drugs with respect to efficacy, however it presents significant advantages in terms of tolerability. Clearly reported pragmatic short, medium and long term randomised controlled trials are required to replicate and validate these findings and determine the position of aripiprazole in everyday clinical practice.
Plain language summary
Aripiprazole versus typical antipsychotic drugs for schizophrenia
Schizophrenia is a severe mental illness which mostly affects people in early adulthood. The symptoms of schizophrenia are perceptions without cause (hallucinations), fixed false beliefs (delusions) and/or apathy, slowing and less movement or thought. People with this condition are usually treated with antipsychotic medication but there are a significant number of people receiving this treatment who don’t respond, or develop uncomfortable adverse effects. Aripiprazole is a new medication which acts differently in the brain to other antipsychotics and may benefit people who have been resistant to treatment so far. This review compares aripiprazole to the older ‘typical’ antipsychotics.
The data for nine clinical trials containing a total of 3622 patients were analysed. In the trials of less than 12 weeks that reported improvement of general well‐being and mental state, there was no statistically significant difference between typical antipsychotics and aripiprazole. However, when looking at adverse effects, people on aripiprazole were less likely to suffer from movement side effects, blurred vision, high levels of the hormone prolactin or increased heart rate. These people were also less likely to withdraw their consent to being in the study in short (less than 12 weeks) and longer (more than 12 weeks) trials. Conversely, people on typical antipsychotics were significantly less likely to feel dizzy or nauseous. These trials were all quite different from each other ‐ they had varying settings, enrolled different groups of people, were for varying lengths of times (from 24 hours to 52 weeks) and compared aripiprazole to different first generation antipsychotics. This made it difficult to compare outcomes from trial to trial. In addition, a lot of the data were not able to be used because measurements were not given in full. This medication looks promising but there needs to be more trials, particularly longer‐term well‐planned trials.
(Plain language summary prepared for this review by Janey Antoniou of RETHINK, UK www.rethink.org).
Background
'Conventional' antipsychotic drugs such as chlorpromazine and haloperidol have traditionally been used as a first line treatment for people with schizophrenia (Kane 1990, Kane 1993). However, approximately 5‐25% of people with schizophrenia show poor response to these treatments (Christison 1991, Davis 1977, Meltzer 1992). In addition, adverse effects such as movement disorders and sedation often make compliance with these medications problematic (Kane 1990). Although their efficacy in alleviating positive schizophrenic symptoms (such as delusions and hallucinations) is reasonably clear, their effect on the negative symptoms of schizophrenia (such as apathy and poverty of speech) is limited (Crow 1980, Andreasen 1985).
'Atypical' or 'second generation' antipsychotics include drugs such as clozapine, olanzapine, risperidone, quetiapine, amisulpride and ziprasidone. Initially, these were said to differ from typical antipsychotics in that they were found not to cause movement disorders (catalepsy) in rats at clinically effective doses. Other more clinically‐important distinguishing factors include their reputed effects on both the positive and negative symptoms of schizophrenia (although this is far from certain), their more limited adverse effect profiles, particularly in relation to movement disorders, and the possibility that these drugs may improve cognitive function (Gelder 2001). These atypical drugs however are far from ideal, and problematic adverse effects do occur. With the exception of ziprasidone, most atypicals are associated with marked weight gain and disruption of glucose metabolism that may lead to diabetes. Clozapine, which is used for people whose illness is 'treatment‐resistant', may induce life‐threatening decreases in white blood cells (agranulocytosis) as well as heart problems (acute myocarditis, cardiomyopathy) and diabetes (Rivas‐Vasquez 2003).
Aripiprazole is a relatively new antipsychotic which is currently being marketed by Otsuka Pharmaceutical Co.Ltd and Bristol Myers Squibb. It is said to be the prototype of a new third generation of antipsychotics; the so‐called dopamine‐serotonin system stabilisers. Aripiprazole is claimed to be at least as effective as haloperidol for the positive symptoms of schizophrenia such as fixed, false beliefs (delusions) and perceptions without cause (hallucinations). It is also claimed to be effective against the negative symptoms of schizophrenia, such as apathy and slowing and paucity of movement and thought. It has also been suggested that aripiprazole may be associated with fewer movement disorders (parkinsonism, dystonias, tardive dyskinesia), less weight gain and reduced negative effects on the heart (QTc interval abnormalities) and on glucose metabolism compared with standard antipsychotics (Rivas‐Vasquez 2003). In addition, aripiprazole is said to be useful in both the acute and maintenance phases of schizophrenia, and to have cognition enhancing effects.
Technical background Aripiprazole is reported to exert its antipsychotic effects by acting as a partial agonist at D2 dopamine and 5‐HT1a serotonin receptors, and as an antagonist at 5‐HT2 serotonin receptors. It has been postulated that, through the above receptor site actions, and hence dopamine and serotonin system stabilisation, a partial D2 agonist would be able to act as an antagonist in pathways where an abundance of dopamine was producing psychosis, yet it would stimulate receptors as an agonist at sites in which low dopaminergic tone would produce side effects (e.g. areas mediating motor movement and prolactin release) (Rivas‐Vasquez 2003). Aripiprazole, however, also has an affinity for other receptors including D3, D4, 5‐HT2c, 5‐HT7, alpha‐1 adrenergic and H1 histamine receptors. This may explain adverse effects associated with this compound such as somnolence, headache, gastrointestinal upset and light‐headedness (FDA 2002a).
The recommended target dose for aripiprazole is 10‐15 mg/day (dose range 10‐30 mg). Phase III trials were initially conducted in Japan in 1995, and the drug was granted Approvable Status by the FDA (USA) on the 15th November 2002, for the treatment of schizophrenia (FDA 2002a). Aripiprazole has since been licensed in most countries worldwide, including in the UK for treatment of schizophrenia.
In an earlier Cochrane review (El‐Sayeh 2006), the reviewers concluded that aripiprazole is as effective as typical and atypical antipsychotic drugs for schizophrenia, although data are far too few to allow any conclusions to be drawn. Aripiprazole does not offer significant advantages over these two classes of drugs in terms of outcomes with the exception of causing less hyperprolactinaemia and rises in QTc interval than risperidone. Aripiprazole may be the first of a new generation of atypical drugs but its clinical effects do not seem to be very different from those of older drugs. More studies are needed to replicate and validate these findings. The current review is envisaged as one of a triad of three separate reviews comparing aripiprazole with placebo, typical and atypical antipsychotic drugs respectively, on the contextual background of the original singular review.
Objectives
To review the effects of aripiprazole compared with typical antipsychotics for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials. Where a trial was described as 'double‐blind', but it was only implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see types of outcome measures) when these 'implied randomisation' studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
We included people with schizophrenia and other types of schizophrenia‐like psychosis (e.g. schizophreniform and schizoaffective disorders), irrespective of the diagnostic criteria used. There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
1. Aripiprazole: any dose or form of application. 2. 'Typical' antipsychotic drugs such as haloperidol or chlorpromazine, any dose or form of application.
Types of outcome measures
We grouped outcomes into short term (up to 12 weeks), medium term (13 to 26 weeks) and long term (over 26 weeks).
Primary outcomes
1. Global state 1.1 Relapse
Secondary outcomes
1. Death ‐ suicide and natural causes
2. Global state 2.1 No clinically important change in global state (as defined by individual studies) 2.2 Average endpoint global state score 2.3 Average change in global state scores
3. Service outcomes 3.1 Hospitalisation 3.2 Time to hospitalisation
4. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 4.1 No clinically important change in general mental state 4.2 Average endpoint general mental state score 4.3 Average change in general mental state scores 4.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 4.5 Average endpoint specific symptom score 4.6 Average change in specific symptom scores
5. General functioning 5.1 No clinically important change in general functioning 5.2 Average endpoint general functioning score 5.3 Average change in general functioning scores 5.4 No clinically important change in specific aspects of functioning, such as social or life skills 5.5 Average endpoint specific aspects of functioning, such as social or life skills 5.6 Average change in specific aspects of functioning, such as social or life skills
6. Behaviour 6.1 No clinically important change in general behaviour 6.2 Average endpoint general behaviour score 6.3 Average change in general behaviour scores 6.4 No clinically important change in specific aspects of behaviour 6.5 Average endpoint specific aspects of behaviour 6.6 Average change in specific aspects of behaviour
7. Adverse effects ‐ general and specific (particularly movement disorders, and those known to occur more commonly with aripiprazole such as anxiety, somnolence, disturbances of the gastrointestinal tract and headache) 7.1 Clinically important general adverse effects 7.2 Average endpoint general adverse effect score 7.3 Average change in general adverse effect scores 7.4 Clinically important specific adverse effects 7.5 Average endpoint specific adverse effects 7.6 Average change in specific adverse effects
8. Engagement with services
9. Satisfaction with treatment 9.1 Leaving the studies early 9.2 Recipient of care not satisfied with treatment 9.3 Recipient of care average satisfaction score 9.4 Recipient of care average change in satisfaction scores 9.5 Carer not satisfied with treatment 9.6 Carer average satisfaction score 9.7 Carer average change in satisfaction scores
10. Quality of life 10.1 No clinically important change in quality of life 10.2 Average endpoint quality of life score 10.3 Average change in quality of life scores 10.4 No clinically important change in specific aspects of quality of life 10.5 Average endpoint specific aspects of quality of life 10.6 Average change in specific aspects of quality of life
11. Economic outcomes 11.1 Direct costs 11.2 Indirect costs
12. Cognitive functioning 12.1 No clinically important change in cognitive functioning 12.2 Average endpoint cognitive functioning score 12.3 Average change in cognitive functioning scores 12.4 No clinically important change in specific aspects of cognitive functioning 12.5 Average endpoint specific aspects of cognitive functioning 12.6 Average change in specific aspects of cognitive functioning
13. Leaving the study early.
Search methods for identification of studies
Electronic searches
1. Update search We searched The Cochrane Schizophrenia Group Trials Register (November 2007) using the phrase:
(*aripiprazole* or * abilitat* or *abilify* in title, abstract, index terms of REFERENCE) or (aripiprazole in interventions of STUDY)
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
2 Previous electronic searches We searched The Cochrane Schizophrenia Group Trials Register (May 2007) using the phrase:
(*aripiprazole* or * abilitat* or *abilify* in title, abstract, index terms of REFERENCE) or (aripiprazole in interventions of STUDY)
Searching other resources
1. Reference searching We inspected the references of all identified studies for more trials.
2. Personal contact We contacted the first author of each included study for information regarding unpublished trials.
3. Drug companies We contacted the manufacturers of aripiprazole (Bristol‐Myers Squibb) for additional data.
4. We searched The US Food and Drugs Administration website ‐ http://www.fda.gov using the word 'aripiprazole' and also 'abilify'.
Data collection and analysis
[For definitions of terms used in this, and other sections, please refer to the Glossary].
1. Selection of trials Citation information downloaded from electronic sources included details of author, institution or journal of publication. We independently inspected all reports. We resolved any disagreement by discussion, and where doubt remained, we acquired the full article for further inspection. Once the full articles were obtained, we independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and these trials were added to the list of those awaiting assessment.
2. Assessment of methodological quality We assessed the methodological quality of included studies using the criteria described in the Cochrane Handbook (Higgins 2005), which is based on the degree of allocation concealment. Poor concealment has been associated with overestimation of treatment effect (Schulz 1995). Category A includes studies in which allocation has been randomised and concealment is explicit. Category B studies are those which have randomised allocation but in which concealment is not explicit. Category C studies are those in which allocation has neither been randomised nor concealed. Only trials that are stated to be randomised (categories A or B of the handbook) were included in this review. The categories are defined below:
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment).
We assessed the methodological quality of included trials in this review using the Jadad Scale (Jadad 1996). The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scale includes three items:
1. Was the study described as randomised? 2. Was the study described as double‐blind? 3. Was there a description of withdrawals and drop outs?
Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomisation or the blinding/masking procedures described are inadequate. For this review we used a cut‐off of two points on the Jadad scale to check the assessment made by the handbook criteria. However, the Jadad Scale was not used to exclude trials.
3. Data collection JB independently extracted data from selected trials, and HGE re‐extracted data from two different samples (10%). Where disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data but added the trial to the list of those awaiting assessment.
4. Data synthesis 4.1 Data types We assessed outcomes using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such as "little change", "moderate change" or "much change") or dichotomous (for example, either "no important changes or "important change" in a person's behaviour) measures. Currently RevMan does not support categorical data so we were unable to analyse this.
4.2 Incomplete data We did not include trial outcomes where more than 40% of people were not reported in the final analysis.
4.3 Dichotomous ‐ yes/no ‐ data We analysed data using an intention to treat analysis. If more than 60% of people completed the study, everyone allocated to the intervention was counted whether they complete the follow up or not. We assumed that those who dropped out had the negative outcome, with the exception of death.
Where possible, efforts were made to convert outcome measures to binary data. This was done by identifying cut off points on rating scales and dividing participants accordingly into "clinically improved" or "not clinically improved". It was generally assumed that if there had been a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005a, Leucht 2005b). It was recognised that for many people, especially those with chronic or severe illness, a less rigorous definition of important improvement (e.g. 25% on the BPRS) would be equally valid. If individual patient data were available, the 50% cut‐off was used for the definition in the case of non‐chronically ill people and 25% for those with chronic illness. If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
We calculated the relative risk (RR) and its 95% confidence interval (CI) based on the random effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number‐needed‐to‐harm (NNH) as the inverse of the risk difference.
4.4 Continuous data 4.4.1 Normally distributed data: Continuous data on outcomes in trials relevant to mental health issues are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data we applied the following standards to continuous final value endpoint data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from zero, the standard deviation, when multiplied by two, should be less than the mean (otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution ‐ Altman 1996); in cases with data that are greater than the mean they were entered into 'Other data' table as skewed data and noted in the text of the review. If a scale starts from a positive value (such as PANSS, which can have values from 30 to 210) the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skewness is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. We reported non‐normally distributed data (skewed) in the 'other data types' tables.
For change data (mean change from baseline on a rating scale) it is impossible to tell whether data are non‐normally distributed (skewed) or not, unless individual patient data are available. After consulting the ALLSTAT electronic statistics mailing list, we entered change data in RevMan analyses and reported the finding in the text to summarise available information. In doing this, we assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew.
4.4.2 Final endpoint value versus change data Where both final endpoint data and change data were available for the same outcome category, only final endpoint data were presented. We acknowledge that by doing this much of the published change data may be excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. We are contacting authors of studies reporting only change data for endpoint figures.
4.4.3 Rating scales A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid, and are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore continuous data from rating scales were included only if the measuring instrument had been described in a peer‐reviewed journal. Furthermore, we stipulate that the instrument should either be a self report or be completed by an independent rater or relative (not the therapist), and that the instrument could be considered a global assessment of an area of functioning. However, as it is expected that therapists would frequently also be the rater, such data was included but commented on as 'prone to bias'.
Whenever possible we took the opportunity to make direct comparisons between trials that use the same measurement instrument to quantify specific outcomes. Where continuous data were presented from different scales rating the same effect, both sets of data were presented and the general direction of effect inspected.
4.4.4 Summary statistic For continuous outcomes we estimated the weighted mean difference (WMD) between groups based on the random effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity.
4.5 Crossover design We expected that some trials would use a crossover design. In order to exclude the potential additive effect in the second or later stages on these trials, only data from the first stage were analysed.
4.6 Cluster trials Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering is not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation co‐efficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering had been incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation co‐efficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported we assumed it to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intra‐class correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
5. Investigation for heterogeneity Firstly, we considered all the included studies within any comparison to judge for clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. We supplemented this by using primarily the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 50%, we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2003). Where heterogeneity was present, reasons for this were investigated. If it substantially altered the results, we did not summate data, but presented the data separately and investigated reasons for heterogeneity.
6. Addressing publication bias We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias.
7. Sensitivity analyses Results for high doses (however 'high' were defined in the study or, if such a definition was not presented, greater than 15 mg aripiprazole per day) were compared to those for lower doses with regard to the primary outcome of relapse.
8. General Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for aripiprazole.
Results
Description of studies
For substantive descriptions of the studies please see Included and Excluded Studies tables.
1. Excluded studies We excluded sixteen studies from the review. Three were review articles (Argo 2004, Carson 2002, Petrie 1997). Two studies were quasi‐randomised (Kim 2006, Wu 2005). Two studies (Kelemen 2006, Swanson 2006) reported data of comparisons between typical and atypical antipsychotic drugs with no data available for the individual drugs. We will try to obtain further data about groups allocated to individual drugs (i.e. aripiprazole). We excluded Casey 2002 because, although randomised, it evaluated one switch regimen to aripiprazole versus another and also reported no data that could be included. Carson 2004 is a naturalistic study which evaluated the effectiveness of switching to aripiprazole with prior stratified exposure to various antipsychotics with no identified comparator drug. Shim 2006 evaluates the effect of adjunctive treatment with aripiprazole versus placebo for haloperidol‐induced hyperprolactinaemia. We excluded Xia 2005 due to discrepant data reporting which rendered the reported data unusable. We excluded Andrezina 2006 because it reports a sub‐population post‐hoc analysis of a previously reported RCT which has been included in this review. Two studies (Allen 2007 and Talbott 2007) conducted post‐hoc analyses of pooled data from three randomised controlled trials. We excluded Jung 2007 because treatment with haloperidol and adjunctive aripiprazole was compared with haloperidol with adjunctive placebo. Kim 2007 evaluates the switch from existing antipsychotics to aripiprazole and other antipsychotics, and aripiprazole is not compared with any specific antipsychotic drug.
2. Awaiting assessment Daniel 2006, Currier 2006 and Daniel 2007 report minimal data which could not be used. We have contacted Bristol‐Myers Squibb and the Otsuka Pharmaceutical Company for more data. We are waiting for Ao 2006, Cheng 2006, Lin 2006, Lu 2006, Wang 2006, Xu 2006, Zhang 2005, Zhang 2006 and Zhuang 2006 which are published in Chinese to be translated so we can assess these for future updates of this review.
3.Ongoing studies Barbui 2006 is presently recruiting people with schizophrenia in a trial comparing aripiprazole, clozapine and haloperidol.
4. Included studies We identified nine studies that for inclusion. All were described as randomised and all (Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Kane 2003, Kujawa 2002, Oren 2005) but two as being double‐blind. McCue 2006 was an open‐label study, though the rating scale outcome assessor was blind to the allocation of the intervention. The blinding status was not described for Wang 2005.
4.1 Length of trials Schizophrenia is an illness that affects young people and is lifelong. Eight studies reported data on short‐term follow‐up (up to 12 weeks) of which two studies involved the administration of intramuscular aripiprazole and had extremely short follow‐up periods of up to 24 hours (Daniel 2004,Oren 2005). One study reported data on long‐term follow‐up (over 26 weeks) (Kujawa 2002).
4.2 Study size Kujawa 2002 with 1294 people was the largest study. Wang 2005 randomised 72 participants. All the remaining studies included between 100 and 500 participants. 4.3 Participants All included studies involved participants with clearly operationalised diagnoses. Five trials included people with a sole diagnosis of schizophrenia (Csernansky 2003, Daniel 2000, Kane 2003, Kujawa 2002, Wang 2005). The remaining four studies included patients with schizophrenia or schizoaffective disorder (Carson 2000, Daniel 2004, McCue 2006, Oren 2005). Males comprised a significant majority of the participants. Six studies excluded participants with a prior history of refractoriness to anti‐psychotic medication (Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Oren 2005, Kujawa 2002). McCue 2006 excluded participants with a clear history of response or lack of response to a particular antipsychotic drug and who, in the judgement of the treating psychiatrist, would best be treated accordingly. Kane 2003 required participants to be resistant to at least two periods of treatment (each lasting at least six weeks) with adequate doses of antipsychotic agents (one of which had to be a typical antipsychotic) during the two years prior to the study, but excluded people with previous unsatisfactory response to perphenazine or clozapine. Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Kujawa 2002, McCue 2006, and Oren 2005 included people who were acutely ill.
4.4 Setting Six of the nine studies were described as occurring in hospital or inpatient settings (Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, McCue 2006, Oren 2005). Kujawa 2002 took place in out‐patient or mixed settings. We could find no explicit information on the settings of the remaining studies. All trials involved adult participants > 18 years of age. All studies included participants of both genders. Only Daniel 2004 does not describe the sex distribution of the participants. All included studies involved adult participants, ≥18 years of age.
4.5 Interventions 4.5.1 Experimental drugs The trialists used aripiprazole in a wide range of doses from 1 mg per day to 30 mg per day. In most studies drugs were given orally, apart from two studies where medication was given intramuscularly (Daniel 2004,Oren 2005).
4.5.2 Comparison drugs Comparators were placebo, conventional antipsychotics {haloperidol (5‐20 mg/day), perphenazine (8‐64 mg/day) and chlorpromazine (50‐500 mg/day)} and the newer generation antipsychotics {olanzapine (5‐40 mg/day); risperidone (2‐9 mg/day); quetiapine (50‐1200 mg/day); ziprasidone (40‐240 mg/day)}. We have only used outcome data from the typical drugs arm in the analysis.
4.6 Outcomes Our primary outcome of relapse was not clearly reported in any study. All other outcomes in this review were of secondary importance to us but we do recognise that they may be of primary interest to others.
Daniel 2004, Kane 2003, Kujawa 2002, and Oren 2005 reported usable data on death.
We have used mean change in CGI‐S score and mean CGI‐I score as global state outcome measures. Only Kane 2003 reported usable data in this respect. Daniel 2004 reported mean change data from the CGI‐S score and CGI‐I score from baseline, but we were unable to derive a summated change data for all the participants randomised to the different dose groups of aripiprazole. Consequently we have excluded these data from the analysis. We have used discontinuation from the study due to an adverse event including psychosis, non‐compliance with study protocol, lack of efficacy and marked deterioration as global state outcomes.
We have used clinically significant response to treatment with antipsychotic medication and BPRS total score and PANSS score as mental state outcome measures. Six studies (Carson 2000, Daniel 2004, Kane 2003, McCue 2006, Oren 2005, Wang 2005) reported usable data about clinically significant response to treatment. Carson 2000 and Kane 2003 defined response to treatment as a CGI‐I score of 1 or 2, or a ≥30% decrease from baseline in PANSS total score. Daniel 2004 and Oren 2005 defined clinical response as a reduction ≥ 40% in PEC score from baseline to 2 hours after first injection. McCue 2006 defined clinically significant response as a sufficient improvement in the patient's mental state within the study period to no longer necessitate acute in‐patient care. Wang 2005 defined >80% decrease in PANSS score as cure, >50% decrease as markedly improved, >25% decrease as improved and <25% decrease as showing no effect. We chose the 50% cut‐off as indicative of clinically significant response. The validity of this dichotomising from these measures as used in the respective studies was nevertheless not investigated. The other studies did not report any usable data in this respect. Kane 2003 and McCue 2006 reported usable data about changes in BPRS total scores; and only Kane 2003 reported usable data about changes in PANSS total scores. Daniel 2004 reported data about change in mean BPRS score from baseline, but we were unable to use the data for analysis due to our inability to derive a summated change result from the groups of participants randomised to different doses of aripiprazole. Only Daniel 2004 reported data about behaviour in terms of change in ACES score and CABS score. However we have been unable to use the data in our analysis due to our inability to derive a summated effect from the groups of participants randomised to different doses of aripiprazole.
Four studies (Carson 2000, Daniel 2004, Kane 2003, Oren 2005) reported usable data on specific adverse effects and extrapyramidal side‐effects, but only those spontaneously reported in more than 5% of participants. This design precludes reporting of important less frequent effects. Only Carson 2000 reported usable data on clinically significant weight gain which was defined as > 7% increase from baseline. It was unclear however how the level of significant weight gain (7 percent) was decided upon. We found no mention of this figure in the methodology section of the included study reports and if it was decided on after the data were inspected, any results would be prone to bias. Carson 2000, Csernansky 2003, Daniel 2000 and Kane 2003 reported usable data about satisfaction with services in the form of leaving the study early due to withdrawal of consent.
Only Kane 2003 reported usable data about quality of life. The Quality of Life Scale data was dichotomised and a clinically significant improvement in this scale was defined as being at least a 20% improvement from baseline.
Carson 2000, Kane 2003, McCue 2006 and Oren 2005 reported usable data about indirect economic costs in the form of concomitantly administered medication, apart from those to which the participants in the study were allocated during randomisation.
All but Wang 2005 reported usable data on leaving the study early due to any reason.
4.6.1 Outcome scales: only details of the scales that provided usable data are shown below. Reasons for exclusions of data are given under 'Outcomes' in the 'Included studies' table.
4.6.1.1 Global state 4.6.1.1.1 Clinical Global Impression Scale ‐ CGI Scale (Guy 1976) This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven‐point scoring system is usually used with low scores showing decreased severity and/or overall improvement. A CGI‐I (CGI‐Improvement) score was also validated for use in this review. Csernansky 2003 and Kane 2003 reported usable data from this scale.
4.6.1.2 Mental state 4.6.1.2.1 Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986) This schizophrenia scale has 30 items, each of which can be defined on a seven‐point scoring system varying from 1 ‐ absent to 7 ‐ extreme. This scale can be divided into three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity. Kane 2003 reported usable data from this scale.
4.6.1.2.2 PANSS excited component score ‐ PEC score (Lindenmeyer 2004) This scale consists of five items on the PANSS total scale (hostility, lack of cooperation, excitement, poor impulse control, and tension), with each item scored on a scale of 1(absent)to 7(extreme). It is a validated instrument that allows assessment of the antiagitation effects of antipsychotic treatment and is particularly useful in acute treatment settings. It is a physician‐rated observation that avoids the need for interaction between the patient and physician that could exacerbate agitation. Csernansky 2003, Kane 2003 and McCue 2006 reported usable data from this scale.
4.6.1.2.3 Brief psychiatric rating scale ‐ BPRS (Overall & Gorham1962) This is a semi structured interview schedule composed of 18 symptom constructs measuring positive symptoms, general psychopathology and affective symptoms. The original scale has 16 items, but a revised 18 item scale is commonly used and some versions may include up to 25 items. The items are rated on a 7‐point scale and include self‐report and interviewer‐rated observations. Scores can range from 0‐126. Although its psychometric properties in terms of reliability, validity and sensitivity have been extensively examined (Hedlund 1980), the clinical implications of BPRS scores are not always clear. Csernansky 2003, Kane 2003 and McCue 2006 reported usable data from this scale.
4.6.1.3 Quality of life 4.6.1.3.1 Quality of Life Scale ‐QLS (Carpenter 1984) This is a semi structured interview administered and rated by trained clinicians. It contains 21 items rated on a 7‐point scale based on the interviewer’s judgement of patient functioning. A total QLS and four sub‐scale scores are calculated, with higher scores indicating less impairment. Kane 2003 reported usable data from this scale.
4.7 Missing outcomes Our primary outcome of relapse was not clearly reported in any study. No usable outcomes were found for the following categories: service outcomes, general functioning, engagement with services and cognitive functioning.
4.8 Redundant data In five studies (Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Oren 2005) the trialists compare aripiprazole with both haloperidol and placebo, with the data related to the latter being rendered redundant. McCue 2006 compares aripiprazole with haloperidol, olanzapine, quetiapine, risperidone and ziprasidone with the data related to the atypical drugs being redundant for the purposes of this review. The trialists have reported continuous measures of global state (CGI), repeated measures of mental state (BPRS, BPRS‐PANNS), and adverse effects (Simpson‐Angus scale, Barnes Akathisia scale, Abnormal Involuntary Movement scale) and other outcome measures including changes in body weight, serum prolactin, and QTc interval changes but no variances have been reported. We were unable to include these data in our analyses. This poor reporting is by no means unique to any particular study (see Included Studies table, Outcomes). Other problems have included the use of non‐externally validated outcome scales such as the TESS (Wang 2005). We have asked various representatives of Bristol Myers Squibb pharmaceuticals (UK) for further clarification on numerous data that were not adequately reported thereby rendering them as unsuitable for inclusion in the analysis. At the time of publishing this update, we have not received any additional data from Bristol Myers Squibb pharmaceuticals. Enormous efforts were invested in studies rating and recording data that are then reported in such a way as to render them useless for reviews such as this. Participants in trials may be appalled to know how much of their data has been rendered useless.
4.9 Pharmaceutical industry support Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Kane 2003, Kujawa 2002 and Oren 2005 were supported by Otsuka Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb Company. McCue 2006 was not supported by any pharmaceutical company. We were unable to ascertain any pharmaceutical company support for Wang 2005.
Risk of bias in included studies
1. Randomisation All included studies were said to be randomised. Only one study (Oren 2005) details a centralised call‐in system organised by the study centres using permuted block randomisation where those in charge of allocation were blind to the participant list. None of the other included studies explicitly detailed the method of randomisation. This concealment of allocation has repeatedly been shown to be of key importance in excluding selection biases (Jüni 2001). Therefore all trials were category B (moderate risk of bias ‐ some doubt about the results ‐ see Methods), apart from Oren 2005 which qualified for category A. This was confirmed by correspondingly poor ratings on the Jadad Scale (see Included Studies table).
2. Blindness Seven studies were described as being double‐blind (Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Kane 2003, Kujawa 2002, Oren 2005). In Kane 2003 the randomised double‐blind treatment phase with aripiprazole was preceded by a 4‐6 week open label atypical antipsychotic treatment phase to confirm treatment‐resistance, and then by a 2‐10 day single‐blind placebo‐washout phase. One trial (McCue 2006) was described as open‐label, but the assessor of the outcome rating scales was blind to the intervention allocation. Wang 2005 did not describe blinding. None of the studies reported testing of the blinding. If blinding is recognised to be of such importance for minimising observation bias, it could be expected that testing of this blinding would be a priority.
3. Loss to follow up Some of the included studies reported data in terms of a last observation carried forwards (LOCF) analysis and an OC analysis (observed cases, defined as those completing the trial). Although LOCF analyses are commonly used to account for missing observations, this technique could introduce bias as considerable assumptions are made about people who did not stay in the study. We saw no reporting of attempts to validate assumptions by following up people who did drop out early. In Csernansky 2003, Daniel 2000, Kujawa 2002 dropout rates were more than 40%. Using LOCF, more than 40% of the data on certain outcomes are assumed and we feel that this amount of fabricated data is too great. We have only included data from these studies about leaving the study early; discontinuation due to adverse events, non‐compliance with study protocol, lack of efficacy and marked deterioration; and death in the analyses. For studies with ≥60% total completion rates we have tried to undertake an intention‐to‐treat analysis. Where no data were available for proportions of 60%, we have assumed a negative outcome other than death (see Methods).
The reasons for loss to follow up are reasonably well reported and we have recorded these in the outcomes.
4. Data reporting Overall much of the data we found could not be used because of poor reporting. Findings which are presented as graphs, in percentiles or just reported as p‐values are often of little use to a reviewer. Many studies failed to provide standard deviations when reporting mean changes on a particular outcome measure. We are seeking further data from the first authors of relevant trials.
Effects of interventions
1. The Search The initial search in (May 2007) identified 211 citations from 135 studies. Of these we were only able to include nine studies in the review. The update search (Nov 2007) identified 103 citations. We did not find any additional studies that we could include from the update search. Many studies are multiply reported in different media. We have contacted various professionals from Bristol Myers Squibb pharmaceuticals (UK) for further studies and data. At the time of publishing this review, we have not received any further information. 2. COMPARISON 1: ARIPIPRAZOLE versus TYPICAL antipsychotic drugs (short term < / = 12 weeks). 2.1 Death No deaths were reported in the studies in either comparator group (n=955, 3 RCT, RR not estimable).
2.2 Global state ‐ dichotomous outcomes We prestated relapse as being our primary outcome of interest. None of the included studies reported usable data for relapse.
2.3 Global state ‐ continuous outcomes 2.3.1 Improvement in CGI‐S scale score Only one study (Kane 2003) provided usable data for improvement in CGI‐S scale score. Aripiprazole did not show any statistically significant greater improvement in CGI‐S scale scores compared to perphenazine (n=300, 1 RCT, WMD 0.00 CI ‐0.3 to 0.3).
2.3.2 CGI‐I score Only Kane 2003 provided usable data for CGI‐I scale scores. Aripiprazole did not improve CGI‐I scale scores to a statistically significant degree compared with perphenazine (n=300, 1 RCT, WMD ‐0.20 CI ‐0.5 to 0.1).
2.4 Mental state ‐ dichotomous outcomes 2.4.1 Clinically significant response to treatment A total of 56% of participants given aripiprazole and 54% of participants given typical antipsychotics failed to achieve a clinically significant response to treatment, and the results were significantly heterogeneous (n=1447, 6 RCT, RR 1.01 CI 0.8 to 1.3, I2 70.7%). Subgroup analysis of the studies excluding the two smallest studies in the group (McCue 2006 and Wang 2005) found the results homogenous, but with no statistically significant difference between aripiprazole and typical drugs (n=1263, 4 RCT, RR 0.97 CI 0.9 to 1.1).
2.5 Mental state ‐ continuous outcomes 2.5.1 Improvement in BPRS total score There were no statistically significant differences between aripiprazole and typical antipsychotic drugs in terms of improvement in BPRS total scores (n=412, 2 RCT, WMD 1.07 CI ‐2.1 to 4.2).
2.5.2 Improvement in PANSS total score Only Kane 2003 provided usable data for improvement in total PANSS scores. Participants receiving aripiprazole showed greater improvement in PANSS total scores than those on typical antipsychotic drugs but the differences were not statistically significant (n=300, 1 RCT, WMD 0.70 CI ‐4.1 to 5.5).
2.6 Adverse effects: extra‐pyramidal symptoms 2.6.1 EPS‐related adverse event Twelve percent of participants given aripiprazole developed an EPS‐related adverse event compared with 20% of those on typical antipsychotics. The results though statistically significant were heterogeneous (n=968, 3 RCT, RR 0.46 CI 0.3 to 0.9, I² 69.1%, NNT 13 CI 10 to 17). If the study with the most extreme result (Oren 2005) was excluded from the analysis the results became homogenous but remained significantly in favour of aripiprazole (n=608, 2 RCT, RR 0.58 CI 0.4 to 0.8, NNT 11 CI 8 to 17).
2.6.2 Akathisia Rates of akathisia were significantly less common in participants receiving aripiprazole (5%) compared with typical antipsychotics (14%) (n=897, 3 RCT, RR 0.39 CI 0.3 to 0.6, NNT 11 CI 9 to 14).
2.6.3 Hypertonia We found no significant difference for the frequency of hypertonia between aripiprazole (3%) and typical antipsychotics (4%) (n=597, 2 RCTs, RR 0.49 CI 0.04 to 6.3).
2.6.4 Tremor Only Carson 2000 reported usable data about incidence of tremor as an adverse side‐effect. We found no significant difference between participants given aripiprazole (3%) and those receiving typical antipsychotics (7%) (n=308, 1 RCT, RR 0.36 CI 0.1 to 1.1).
2.7 Adverse effects: other symptoms 2.7.1 Abdominal pain Only Carson 2000 reported usable data for abdominal pain as an adverse effect. We found no significant differences between aripiprazole (7%) and typical antipsychotic drugs (6%) (n=308, RR 1.27 CI 0.5 to 3.2).
2.7.2 Abnormal total CPK levels Kane 2003 recorded abnormal CPK levels and we found no significant difference between aripiprazole (8%) compared with perphenazine (5%) (n=300, RR 1.63 CI 0.7 to 4.0).
2.7.3 Agitation About 7% of participants receiving aripiprazole and 9% of those given typical drugs suffered agitation during the trial period, but the differences were not statistically significant (n=949, 3 RCT, RR 0.99 CI 0.6 to 1.5).
2.7.4 Anxiety Outcomes for anxiety were equivocal between aripiprazole and typical antipsychotics (n=608, 2 RCTs, RR 0.95 CI 0.7 to 1.4).
2.7.5 Asthenia (tiredness) Only Carson 2000 reported asthenia as an adverse effect and we found data to be equivocal between aripiprazole (4%) and haloperidol (5%) (n=308, RR 0.92 CI 0.3 to 2.7).
2.7.6 Blurred vision Only Carson 2000 reported usable data for incidence of blurred vision as an adverse effect. We found blurred vision to be significantly less common in participants given aripiprazole (2%) compared with the haloperidol group (8%) (n=308, RR 0.19 CI 0.1 to 0.7, NNT 14 CI 10 to 25).
2.7.7 Clinically significantly raised prolactin levels In Kane 2003 aripiprazole (4%) caused significantly fewer cases of clinically significantly raised prolactin levels compared to perphenazine (54%) (n=300, 1 RCT, RR 0.07 CI 0.03 to 0.2, NNT 2 CI 1 to 3).
2.7.8 Dizziness Aripiprazole caused dizziness in significantly more participants (10%) than typical drugs (5%) (n=957, 3 RCT, RR 1.88 CI 1.1 to 3.2, NNH 20 CI 14 to 33).
2.7.9 Dyspepsia Dyspepsia was reported as an adverse effect in only Kane 2003. Ten percent of participants randomised to aripiprazole experienced dyspepsia compared to 6% of those to perphenazine, but the differences were not statistically significant (n=300, 1 RCT, RR 1.69 CI 0.8 to 3.7).
2.7.10 Headache Headaches were a common adverse effect amongst those on aripiprazole (16%) and typical drugs (11%), though the results were not statistically significantly different (n=1257, 4 RCT, RR 1.32 CI 0.8 to 2.1).
2.7.11 Increase in CPK levels Kane 2003 reported cases of increased CPK levels from baseline among the aripiprazole group (6%) and typical drugs (3%) and the small differences were not statistically significant (n=300, RR 2.13 CI 0.7 to 6.8).
2.7.12 Insomnia Insomnia appeared almost as often in those on aripiprazole (17%) as in those on typical antipsychotic drugs (18%), but the results were not significantly different (n=968, 3 RCT, RR 0.84 CI 0.5 to 1.3).
2.7.13 Nausea Nausea occurred significantly more frequently in those receiving aripiprazole (9%) compared with those on typical antipsychotic drugs (3%) (n=957, 3 RCT, RR 3.03 CI 1.5 to 6.1, NNH 17 CI 13 to 25).
2.7.14 Orthostatic hypotension Only Carson 2000 reported usable data for orthostatic hypotension. We found no greater incidence of asthenia among people randomised to aripiprazole (4%) compared with those on haloperidol (1%) (n=308, RR 4.59 CI 0.6 to 35.7).
2.7.15 Pain at injection site Aripiprazole (2%) did not cause a significantly greater occurrence of local pain at the injection site compared with those receiving typical drug injections (1%) (n=649, 2 RCT, RR 2.80 CI 0.7 to 11.3).
2.7.16 Psychosis Only Kane 2003 reported usable data about the incidence of psychosis during the study. We found no significant difference between the two comparator groups in the incidence of psychosis (n=300, RR 1.58 CI 0.7 to 3.5).
2.7.17 QTc abnormalities About 1% of participants given aripiprazole and typical antipsychotic drugs developed QTc abnormalities, but the results were not statistically significant (n=1257, 4 RCT, RR 0.37 CI 0.03 to 4.4).
2.7.18 Sinus tachycardia We found significantly fewer cases of sinus tachycardia in the aripiprazole (0.4%) group compared with typical drugs (5%) (Daniel 2004, n=289, RR 0.09 CI 0.01 to 0.8, NNT 22 CI 13 to 63).
2.7.19 Somnolence We found no significant difference for the frequency of somnolence between aripiprazole (6%) and typical antipsychotic (8%) (n=1257, 4 RCT, RR 0.66 CI 0.4 to 1.0).
2.7.20 Tachycardia Incidences of tachycardia were not significantly more common in participants given aripiprazole (4%) compared with typical drugs (2%) (Daniel 2004, n= 289, RR 2.30 CI 0.3 to 17.8).
2.7.21 Vomiting Incidences of vomiting were equally common (7%) in participants receiving aripiprazole and typical antipsychotic drugs (n=597, 2 RCT, RR 1.30 CI 0.7 to 2.5).
2.7.22 Weight gain Carson 2000 defined clinically significant weight gain as >7% increase in body weight from baseline. Aripiprazole caused fewer participants (5%) to gain weight compared with haloperidol (10%) but the differences were not statistically significant (n=308, 1RCT, RR 0.56 CI 0.3 to 1.3).
2.8 Satisfaction with treatment: leaving study early due to withdrawal of consent Significantly fewer participants given aripiprazole (12%) left the study early due to withdrawal of consent compared with those on typical drugs (15%) (n=919, 4 RCT, RR 0.70 CI 0.5 to 1.0, NNH 33 CI 20 to 100).
2.9 Quality of Life: clinically significant improvement in QLS score Kane 2003 described clinically significant improvement in QLS score as ≥20% increase from baseline score. We found significantly greater numbers (36%) of participants given aripiprazole compared to those on perphenazine (21%) were noted to have significantly improved QLS score (n=300, RR 0.82 CI 0.7 to 0.9, NNT 7 CI 4 to 31).
2.10 Concomitant drug treatment Fewer participants receiving aripiprazole (13%) required concomitant antimuscarinic medication compared with those on typical drugs (33%), but the results were heterogeneous and the differences were not statistically significant (n=412, 2 RCT, RR 0.13 CI 0.00 to 9.6, I² 89.4%). In Carson 2000 we found fewer cases of concomitant use of additional antipsychotic medication amongst the aripiprazole group (3%) compared with the typical antipsychotics (6%) but the results were not statistically significant (n=308, 1 RCT, RR 0.51 CI 0.2 to 1.5). In Oren 2005 we found that patients given aripiprazole (8%) did not require concomitant benzodiazepines significantly more often compared with typical drugs (12%) (n=360, 1 RCT, RR 0.67 CI 0.4 to 1.3). 2.11 Leaving study early 2.11.1 Discontinuation due to any reason Nineteen percent of participants left the study early in both comparator groups and there were no statistically significant differences between the two groups (n=1686, 7 RCT, RR 0.92 CI 0.8 to 1.1).
2.11.2 Discontinuation due to an adverse event including psychosis Six percent of participants in the aripiprazole group and 7% of those given typical antipsychotic drugs discontinued from the study due to an adverse event with psychosis being the most common adverse effect which led to discontinuation. The difference in discontinuation rates were not statistically significant (n=1326, 6 RCT, RR 1.06 CI 0.7 to 1.7).
2.11.3 Discontinuation due to lack of efficacy We did not find a significantly greater discontinuation rate in groups allocated to aripiprazole (7%) compared with typical antipsychotic drugs (6%) due to lack of treatment efficacy (n=1214, 5 RCT, RR 1.16 CI 0.7 to 1.8).
2.11.4 Discontinuation due to marked deterioration Four percent of participants receiving aripiprazole and 3% of those on typical antipsychotics discontinued from the study due to marked deterioration, but the differences were not statistically significant (n=423, 3 RCT, RR 0.90 CI 0.3 to 2.5).
2.11.5 Discontinuation due to non‐compliance with study protocol Only 1% of participants given aripiprazole discontinued from the trials due to non‐compliance with study protocol compared to none of those allocated to the typical antipsychotic group, and were not statistically significant (n=311, 2 RCT, RR 1.79 CI 0.2 to 16.9).
3. COMPARISON 2: ARIPIPRAZOLE versus TYPICALS ( medium / long term > 12 weeks ). Only Kujawa 2002 reported long term outcomes beyond 12 weeks. 3.1 Death There were no statistically significant differences in the occurrence of death as reported for 0.5% of participants receiving aripiprazole and 0.2% of those in the haloperidol group (Kujawa 2002) (n=1294, 1 RCT, RR 2.01 CI 0.2 to 17.9).
3.2 Global state ‐ dichotomous outcomes We found no reported usable data for relapse, our primary outcome.
3.3 Leaving study early 3.3.1 Discontinuation due to any reason Significantly fewer participants given aripiprazole (57%) left the study early for any reason compared with those on haloperidol (70%) (n=1294, 1 RCT, RR 0.81 CI 0.8 to 0.9 NNT 8 CI 5 to 14).
3.3.2 Discontinuation due to an adverse event including psychosis Significantly fewer participants given aripiprazole (25%) discontinued from the study due to an adverse event including psychosis compared with those on typical antipsychotic drugs (32%) (n=1294, 1 RCT, RR 0.78 CI 0.7 to 0.9, NNT 14 CI 8 to 100).
3.3.3 Discontinuation due to lack of efficacy Fewer (7%) participants given aripiprazole discontinued from the study due to lack of efficacy compared with (9%) of those on typical antipsychotic drugs, but the differences were not statistically significant (n=1294, 1 RCT, RR 0.83 CI 0.6 to 1.2).
3.3.4 Discontinuation due to marked deterioration More participants given aripiprazole (17%) discontinued from the study than those given typical antipsychotic drugs (13%), but differences were not statistically significant (n=1294, 1 RCT, RR 1.24 CI 0.9 to 1.6).
3.3.5 Discontinuation due to non‐compliance with study protocol Significantly fewer participants given aripiprazole (8%) discontinued from the study due to non‐compliance with study protocol compared with those on typical antipsychotic drugs (19%) (n=1294, 1 RCT, RR 0.44 CI 0.3 to 0.6 NNT 9 CI 7 to 14).
Discussion
1. Small number of studies The condition of schizophrenia is difficult to study and co‐operation from the study population is rare. Aripiprazole is a relatively new drug. This may be one of the reasons for the relative scarcity of controlled clinical trials comparing aripiprazole with typical drugs.
2. Quality of trials Only Oren 2005 described adequate methods of randomised allocation and double‐blinding and scored three on the Jadad scale (Jadad 1996). None of the other trials described the randomisation procedure and did not score highly on the Jadad scale indicating moderate risk of bias. Wang 2005 did not describe randomisation allocation and blinding, nor withdrawals and drop‐outs; and scored one on the Jadad scale.
3. Publication bias The data included in this review were insufficient to enter into a funnel plot and so we could not address the possibility of publication bias.
4. Generalisability of findings Three (Carson 2000, Csernansky 2003, McCue 2006) of the nine included studies occurred exclusively in North America. Kane 2003 took place in a North American and Canadian multicentre setting. Daniel 2004 was conducted in multiple centres worldwide. Although it could not be definitely determined from the limited descriptions of the remaining three multicentre studies (Daniel 2000, Oren 2005, Kujawa 2002) it is likely that they took place in a similar environment. Wang 2005 appears to have been conducted in China. Clinicians should take this into consideration when thinking of using aripiprazole in very different settings of care. Six (Carson 2000, Csernansky 2003, Daniel 2000, Daniel 2004, Oren 2005, McCue 2006) of the nine included studies were conducted in an in‐patient setting. Kujawa 2002 used an out‐patient setting. The setting of the remaining two studies (Kane 2003, Wang 2005) remains unclear. The majority of trials involved inpatient participants with little in the way of physical and psychiatric co‐morbidity and with well‐defined schizophrenia or schizoaffective disorder. Such people are a minority in everyday care, where people who are not in hospital and suffer from less well defined illness combined with problems such as depression and substance abuse are the norm.
The drugs of comparison used in the trials in this review further limit the applicability of its findings. These were of a nature, or used at such a dose, that they distance these trials from everyday practice even more. The reader is subsequently left with the difficult decision as to whether the findings of studies with such over‐defined participants, interventions and outcomes and standardised highly developed inpatient settings can still inform routine clinical practice.
5. Limited data, confusing data The collection and quality of the available data varied. We were often unable to use data because of high drop‐out rates. The enormous degree of loss to follow up is common in similar studies of other compounds, but rare in everyday practice. This also casts doubt on the applicability of findings to routine care. The design of the studies is clearly encouraging loss to follow up. People leave for many reasons, often not specified, and their last observation is carried forward to the end. These data are nevertheless acceptable to the regulatory authorities. Until this ends, pharmaceutical companies will see little reason to change their practices, as compounds such as aripiprazole will gain licenses for clinical use even if 40% of their data is unavailable.
Often the trialists do not have an obligation to follow people up for longer than the period during which they took the medication. One possible consequence of this might be that an extreme adverse reaction to discontinuation of an intervention, such as death, would go unreported if it occurred a week after the treatment stopped. Data beyond the discontinuation of the medications used within the trial may have been collected but were not reported. This data should be reported since it is not just the individual compounds in a trial which are randomised but the intention to give those individual compounds.
Where scales were employed the degree of improvement was proportional to the change in number of points recorded on each scale. This does not necessarily reflect a satisfactory degree of improvement as would be defined by carers, patients or their families. Trialists tended to report mean figures without giving standard deviations. This renders averages meaningless for re‐analysis and of no use to reviewers.
Several trials only reported adverse effects which occurred in at least 5% of participants which could exclude potentially serious less frequent outcomes. Randomised trials are limited in their ability to highlight important rare outcomes, so further restricting what is reported seems odd. We note that the design of the trials did not limit reporting of positive outcomes to only those that occurred at least 5% of the time.
We found it disappointing that no outcome data were available on service outcomes, general functioning, behaviour, engagement with services and cognitive functioning. We used relapse as our primary outcome but none of the studies reported relapse data. Other outcomes were of secondary importance to this review, but we have reported what we can in order to present the most complete data set possible for the reader. Along with the poor reporting of data, the limited outcomes recorded greatly lessens not so much the applicability, but the value of this review.
Currier 2006, Daniel 2006 and Daniel 2007 which may have been of use in the review could not be included due to lack of usable data. Ao 2006, Cheng 2006, Lin 2006, Lu 2006, Wang 2006, Xu 2006, Zhang 2005, Zhang 2006 and Zhuang 2006 may have provided valuable data, but we were unable to translate the papers from Chinese. We intend to make further attempts to translate these papers and obtain other vital information for future updates of this review.
6. Duration of trials With the exception of one trial (Kujawa 2002), no studies reported outcomes over 26 weeks and all others lasted less than 12 weeks. Aripiprazole is a relatively new compound, so it is not surprising that long term studies are rare at this point but it is to be hoped that they are planned. The difference in duration between trials, 24 hours to 52 weeks influences the outcomes measured. Even if an intervention works in the short term (by 12 weeks) there is no guarantee that this means that the compound has long term benefits.
7.COMPARISON 1: ARIPIPRAZOLE versus TYPICAL ANTIPSYCHOTICS (short term < / = 12 weeks). 7.1 Death No deaths occurred among participants allocated to treatment with either aripiprazole or typical antipsychotic drugs in the short term.
7.2 Global state No data were available for relapse. Aripiprazole and perphenazine appeared equally potent in decreasing the severity of the illness and improving the global clinical impression.
7.3 Mental state There was little evidence to support the superiority or inferiority of aripiprazole compared to typical drugs in effecting a clinically significant response to treatment, and in improving mental state as defined by changes in BPRS and PANSS scale scores. This, along with similar improvement in clinical global impressions suggests that aripiprazole is an efficacious antipsychotic drug.
7.4 Adverse effects: extra‐pyramidal symptoms Aripiprazole caused significantly less instances of an extra‐pyramidal symptom related adverse event than typical antipsychotics. Considering haloperidol's propensity to cause movement disorders (Joy 2004), this is not surprising. However the results were heterogeneous, and the NNT (NNT 13 CI 10 to 17) could be regarded as clinically significant given the distressing nature of these adverse effects. Excluding the study with the most extreme result (Oren 2005) from the analysis produced homogenous data which remained significantly in favour of aripiprazole (NNT 11 CI 8 to 17). Aripiprazole presented a significant advantage over typical drugs with regard to akathisia as a treatment emergent adverse effect (NNT 11 CI 9 to 14). There were no greater risks caused by aripiprazole compared with typical drugs in the occurrence of tremor and hypertonia. No objective rating scales appear to have been used consistently in all the included studies to measure these symptoms, even though for extrapyramidal side effects these are commonly used in research. The outcome scores of the Simpson‐Angus scale, Abnormal Involuntary Movement scale and Barnes Akathisia scale reported in some studies could not be used in the analysis due to unavailability of standard deviations and mean scores.
7.5 Adverse effects: other symptoms 7.5.1 Clinically important specific adverse effects The adverse effects were those spontaneously reported in at least 5% of people, and, as stated earlier, this method of recording may exclude potentially serious, less frequent problems. Dizziness (NNH 20 CI 14 to 33) and nausea (NNH 17 CI 13 to 25) were significantly more common among those taking aripiprazole compared with those on typical drugs. Given the unpleasant nature of these symptoms these findings could be regarded as clinically significant. Only Carson 2000 reported an advantage with aripiprazole over haloperidol in causing blurred vision as an adverse side‐effect (NNT 14 CI 10 to 25). There was no statistically significant difference between aripiprazole and typical drugs in terms of occurrence of anxiety, agitation, asthenia, headache, psychosis, insomnia or somnolence. Vomiting, abdominal pain and dyspepsia were equally common in both comparator groups. Weight gain data were not significantly more common among patients allocated to aripiprazole compared with typical drugs. Local pain at the injection site occurred as commonly with aripiprazole as typical antipsychotic drugs. More well‐designed and reported studies are required to further determine any differential emergence of adverse effects in the two comparator groups.
7.5.2 Physiological (serum) measures Serum CPK levels were not differentially affected by aripiprazole and typical antipsychotic drugs. Kane 2003 suggests a clinically significant advantage for aripiprazole over perphenazine in causing raised serum prolactin levels (NNT 2 CI 1 to 3). However mean prolactin levels were elevated in both groups at baseline. Mean prolactin levels decreased during treatment with aripiprazole in more people than with perphenazine. This finding may have important clinical implications with reference to the problems caused by hyperprolactinaemia, such as galactorrhoea and osteoporosis. 7.5.3 Cardio‐vascular effects Aripiprazole produced sinus tachycardia in fewer people than the typical antipsychotic drugs (NNT 22 CI 13 to 63). Even though the number‐needed‐to‐treat is high, the nature of the symptom makes the difference clinically significant. It is important to note that these results were derived from only one study. Participants receiving aripiprazole were not more likely than those given typical drugs to experience orthostatic hypotension, QTc abnormalities and tachycardia due to unspecified reasons.
7.6 Satisfaction with treatment: leaving study early due to withdrawal of consent More people given aripiprazole appeared to be satisfied with treatment and withdrew their consent for the study less frequently than did those on typical antipsychotic drugs (NNH 33 CI 20 to 100). However the clinical applicability of these findings is limited in the absence of information about specific reasons for dissatisfaction.
7.7 Quality of Life: clinically significant improvement in QLS score More people on aripiprazole perceived a change for the better in their perceived quality of life compared to those on perphenazine (NNT 7 CI 4 to 31). This may be interpreted as a significant observation with important implications for patient satisfaction and compliance, but the wide confidence interval reduces the clinical significance of the finding. However the mean change in Quality of Life scale scores were more favourably inclined towards perphenazine. These findings may be an example of the pharmaceutical industry presenting the data in a more favourable light than any real gains achieved.
7.8 Concomitant drug treatment Participants given aripiprazole did not require significantly greater amounts of additional concomitant antimuscarinic, antipsychotic or benzodiazepine medication compared with those receiving typical antipsychotic drugs. However we were unable to identify clear methods about the determination of the doses of the comparator drugs; nor ascertain any objective criteria for administration of concomitant adjunctive medication. The clinical applicability of these findings remains limited. 7.9 Leaving study early Participants who were given aripiprazole did not discontinue from the study in the short term more often due to an adverse event, development of psychosis, lack of efficacy or marked deterioration, or non‐compliance with study protocol compared to those treated with typical antipsychotic drugs. Haloperidol was the comparator typical antipsychotic in all the studies included in this analysis apart from Kane 2003 which compares aripiprazole to perphenazine. These data are homogenous and suggest that aripiprazole is an effective antipsychotic drug. But the equally moderate numbers of participants in both comparator groups who left the study early due to any reason suggest that aripiprazole may not offer any significant advantage over typical antipsychotic drugs in terms of tolerability in the short term.
8. COMPARISON 2: ARIPIPRAZOLE versus TYPICALS ( medium / long term > 12 weeks ). 8.1 Death Deaths were rare and do not appear to be significantly more or less common among participants taking aripiprazole compared with those on typical drugs. Further well‐designed and reported studies of longer duration will hopefully shed light on this very important outcome.
8.2 Global state There were no data available for relapse or changes in global functioning in the long term. 8.3 Leaving study early Large numbers of participants in both the aripiprazole and typical groups left the study early due to any reason, but significantly fewer participants in the aripiprazole arm (57%) left the study early compared with those on typical antipsychotic drugs (70%) (n=1294, 1 RCT, RR 0.81 CI 0.8 to 0.9 NNT 8 CI 5 to 14). Aripiprazole appeared to be at a clinically significant advantage compared to typical drugs in terms of the attrition rate due to any reason (NNT 8 CI 5 to 14). Participants on aripiprazole did not discontinue from the study more often due to lack of efficacy or marked deterioration as compared to those on typical antipsychotic drugs. Aripiprazole appeared to confer an advantage over typical antipsychotic drugs in terms of discontinuation due to the occurrence of an adverse event including psychosis (NNT 14 CI 8 to 100), but the results may not be clinically significant due to the relatively large number‐needed‐to‐treat and wide confidence interval. Aripiprazole appears to afford a clinically significant advantage over typical antipsychotic drugs in terms of potential non‐compliance in the long‐term, as evident in discontinuation rates due to non‐compliance with study protocol (NNT 9 CI 7 to 14).
Data from studies where huge levels of attrition are deemed acceptable are clinically meaningless and clinicians and recipients of care are left with no reliable information upon which to base decisions. When leaving the study early was attributed to the occurrence of adverse effects, marked deterioration or lack of efficacy there were no significant differences between groups. These findings indicate that aripiprazole may confer greater advantage over the older drugs in encouraging long‐term compliance.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia Aripiprazole is not clearly more or less effective than typical antipsychotics in terms of improving global outcomes or mental state. However it confers a significant advantage over older drugs in terms of fewer occurrences of extra‐pyramidal symptom related adverse events, but it is more likely to cause dizziness and nausea. Hyperprolactinaemia and associated complications of unpleasant breast pain and secretion and osteoporosis do not seem to be a significant concern with aripiprazole; while they remain adverse effects commonly seen with the older drugs. There appears to be a lesser chance of developing sinus tachycardia whilst taking aripiprazole even though it does not present any significant advantages over the typical drugs in causing QTc abnormalities.
2. For clinicians Aripiprazole appears to be as efficacious and effective as the comparator typical antipsychotic drugs in the included studies. It presents a significantly favourable adverse effect profile when compared to the older drugs. This suggests a significant advantage over typical drugs in ensuring compliance. However aripiprazole has been compared to haloperidol, perphenazine, and chlorpromazine in the studies included in this review and a sensitivity analysis could not be conducted due to lack of adequate relevant data; it cannot therefore be definitively concluded that it is as effective as or better tolerated than all typical antipsychotic drugs at various dose ranges. More studies are needed to replicate and validate these findings.
3. For managers / policy makers No data has emerged about service outcomes. Data about economic outcomes are minimal and insignificant. Even though aripiprazole appears to be an efficacious and effective drug there is inadequate data about medium and long‐term outcomes. In the context of finite resources, the lack of good quality data leaves managers and policy makers with difficult decisions to make.
Implications for research.
1. General As with all similar studies, public registration of a study before anyone is randomised would ensure that participants could be confident that people would know that the study had at least taken place. Unique study numbers would help researchers to identify single studies from multiple publications and reduce the risk of duplicating the reporting of data. Compliance with CONSORT (Moher 2001), both on the part of authors and editors, would help to clarify methodology and ensure outcome data were more transparent and usable. Failure to comply produces loss of data and confusion in the results, neither of which help clinicians, patients or managers.
Intention‐to‐treat analysis should be performed on all outcomes and all trial data should be made easily accessible. A minimal requirement should be that all data should, at least, be presented as numbers. In addition, continuous data should be presented with means, standard deviations (or standard errors) and the number of participants. Data from graphs, 'p' values of differences and statements of significant or non‐significant differences are of limited value. Unfortunately, in spite of the large numbers of participants randomised, we were unable to use most of the data in the trials included in this review due to the high drop‐out rates observed as well as poor data reporting.
2. Specific As an antipsychotic agent with a novel mechanism of action, aripiprazole is an interesting compound, but pragmatic, real world, randomised controlled trials should be carried out to determine its value in standard clinical practice. Studies of medium and long‐term risks, including mortality, behaviour, satisfaction with treatment and cost‐effectiveness‐in comparison to typical and atypical antipsychotics, are a priority. Pragmatic entry criteria and non‐blinding with hard endpoints may be helpful in decreasing the study attrition (Roland 1998).
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2014 | Amended | Please note, this review is currently being updated by the authors. This will involve splitting the review into four new titles: Aripiprazole versus chlorpromazine, Aripiprazole versus perphenazine, Aripiprazole versus sulpiride and Aripiprazole versus haloperidol. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 11 November 2009 | Amended | Contact details updated. |
| 18 December 2008 | Amended | Plain language summary added |
| 21 October 2008 | Amended | Converted to new review format. |
| 14 May 2008 | New search has been performed | new search |
| 13 May 2008 | New citation required and conclusions have changed | updated following new search in Nov 2007 |
| 22 April 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Prof. CE Adams and Tessa Grant for their support during the development of the protocol and full review. We thank Judith Wright for her assistance in developing the search strategy for this review, and for her help in conducting the electronic searches.
Data and analyses
Comparison 1. Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 955 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Global state | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Improvement in CGI‐S score | 1 | 300 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.28, 0.28] |
| 2.2 CGI‐I score | 1 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 3 Mental state | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Lack of clinically significant response to treatment | 6 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.83, 1.24] |
| 3.2 Lack of clinically significant response to treatment (subgroup excluding the two smallest studies) | 4 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 4 Mental state | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Improvement in BPRS total score | 2 | 412 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐2.09, 4.22] |
| 4.2 Improvement in PANSS total score | 1 | 300 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐4.13, 5.53] |
| 5 Adverse effects : extra‐pyramidal symptoms | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 EPS‐related adverse event | 3 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.25, 0.87] |
| 5.2 Akathisia | 3 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.60] |
| 5.3 Hypertonia | 2 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.04, 6.28] |
| 5.4 Tremor | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.12] |
| 6 Adverse effects : other symptoms | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Abdominal pain | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.51, 3.19] |
| 6.2 Abnormal total CPK levels | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.66, 4.01] |
| 6.3 Agitation | 3 | 955 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.63, 1.54] |
| 6.4 Anxiety | 2 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.65, 1.40] |
| 6.5 Asthenia (Tiredness) | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.32, 2.67] |
| 6.6 Blurred vision | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.71] |
| 6.7 Clinically significantly raised prolactin levels | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.03, 0.16] |
| 6.8 Dizziness | 3 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.11, 3.21] |
| 6.9 Dyspepsia | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.77, 3.69] |
| 6.10 Headache | 4 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.84, 2.06] |
| 6.11 Increase in CPK levels | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.67, 6.78] |
| 6.12 Insomnia | 3 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.30] |
| 6.13 Nausea | 3 | 963 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [1.51, 6.05] |
| 6.14 Orthostatic hypotension | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 4.59 [0.59, 35.73] |
| 6.15 Pain at injection site | 2 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.69, 11.31] |
| 6.16 Psychosis | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.71, 3.50] |
| 6.17 QTc abnormalities | 4 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.03, 4.39] |
| 6.18 Sinus tachycardia | 1 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.80] |
| 6.19 Somnolence | 4 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.00] |
| 6.20 Tachycardia | 1 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.30, 17.79] |
| 6.21 Vomiting | 2 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.67, 2.51] |
| 6.22 Weight gain | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.28] |
| 7 Satisfaction with treatment : leaving study early due to withdrawal of consent | 4 | 919 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.50, 0.99] |
| 8 Quality of life : incidence of non‐improvement of QLS score | 1 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 9 Concomitant drug treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Antimuscarinic medication | 2 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.00, 9.63] |
| 9.2 Antipsychotics | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.17, 1.54] |
| 9.3 Benzodiazepines | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.36, 1.27] |
| 10 Leaving study early | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Discontinuation due to any reason | 7 | 1686 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.10] |
| 10.2 Discontinuation due to adverse event including psychosis | 6 | 1326 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.68, 1.65] |
| 10.3 Discontinuation due to lack of efficacy | 5 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.74, 1.82] |
| 10.4 Discontinuation due to marked deterioration | 3 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.33, 2.46] |
| 10.5 Discontinuation due to non‐compliance with study protocol | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.19, 16.93] |
1.1. Analysis.
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 1 Death.
1.2. Analysis.
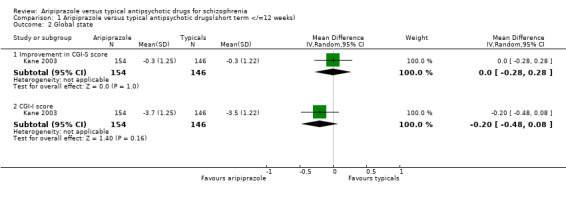
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 2 Global state.
1.3. Analysis.
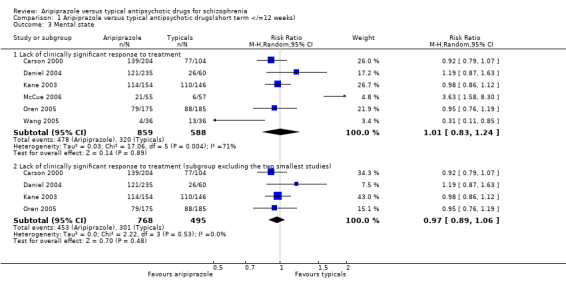
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 3 Mental state.
1.4. Analysis.
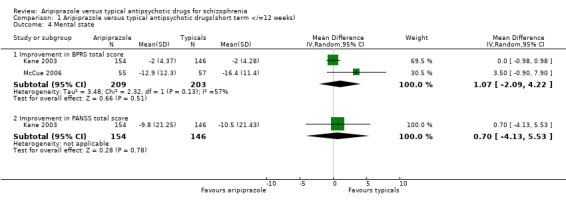
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 4 Mental state.
1.5. Analysis.
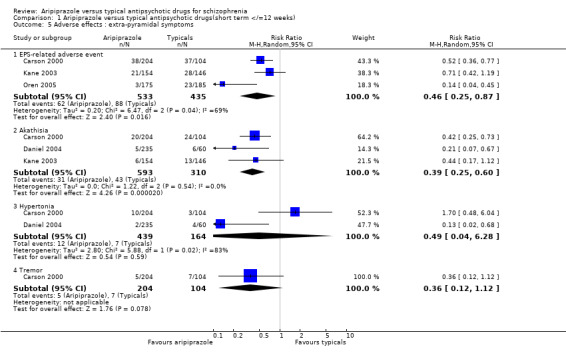
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 5 Adverse effects : extra‐pyramidal symptoms.
1.6. Analysis.
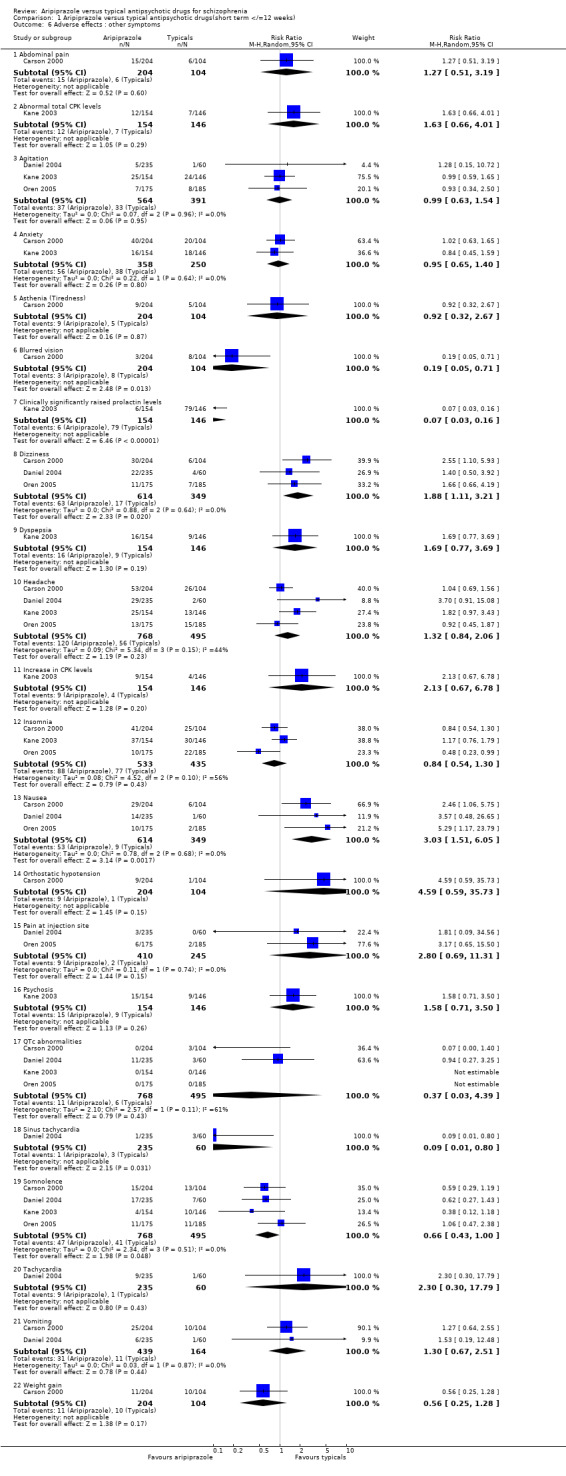
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 6 Adverse effects : other symptoms.
1.7. Analysis.
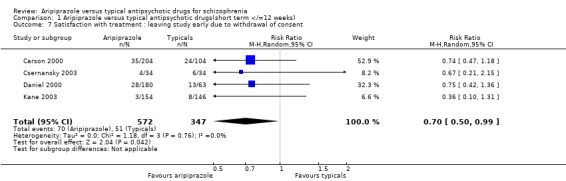
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 7 Satisfaction with treatment : leaving study early due to withdrawal of consent.
1.8. Analysis.
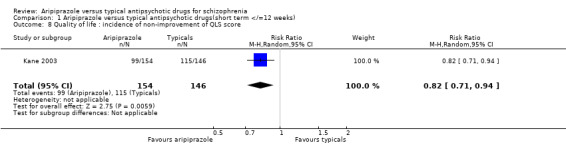
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 8 Quality of life : incidence of non‐improvement of QLS score.
1.9. Analysis.
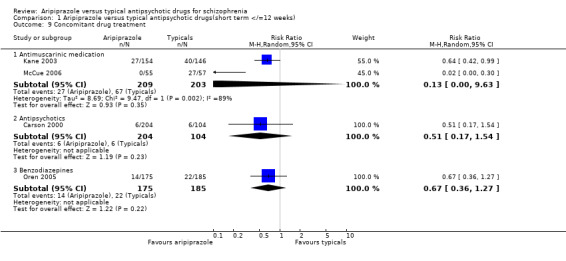
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 9 Concomitant drug treatment.
1.10. Analysis.
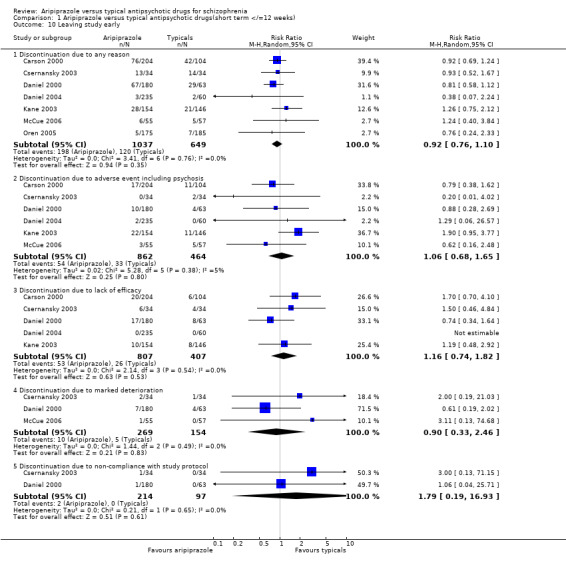
Comparison 1 Aripiprazole versus typical antipsychotic drugs(short term </=12 weeks), Outcome 10 Leaving study early.
Comparison 2. Aripiprazole versus typical antipsychotic drugs(medium / long term > 12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.23, 17.94] |
| 2 Leaving study early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Discontinuation due to any reason | 1 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.75, 0.89] |
| 2.2 Discontinuation due to adverse event including psychosis | 1 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.93] |
| 2.3 Discontinuation due to lack of efficacy | 1 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.23] |
| 2.4 Discontinuation due to marked deterioration | 1 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.93, 1.64] |
| 2.5 Discontinuation due to non‐compliance with study protocol | 1 | 1294 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.33, 0.59] |
2.1. Analysis.
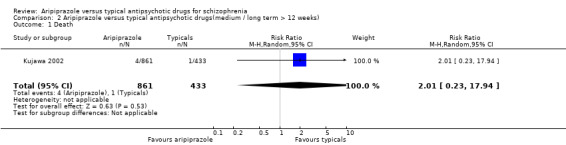
Comparison 2 Aripiprazole versus typical antipsychotic drugs(medium / long term > 12 weeks), Outcome 1 Death.
2.2. Analysis.
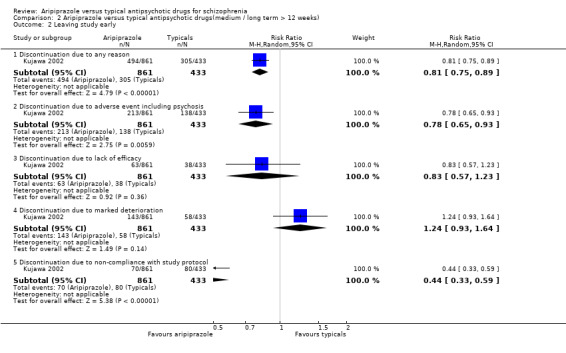
Comparison 2 Aripiprazole versus typical antipsychotic drugs(medium / long term > 12 weeks), Outcome 2 Leaving study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carson 2000.
| Methods | Allocation: randomised, method not described. Blindness: double, no further details. Duration: 4 weeks, preceded by > 5 day washout period. Design: parallel‐group, multicentre. Setting: hospital in‐patients. Consent: obtained. Loss: described. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N=414. Age: 18‐65 years, mean˜39 years. Sex: male 288, female 126. History: acute relapse; previously diagnosed with schizophrenia or schizoaffective disorder; not refractory to antipsychotics; no treatment with long‐acting neuroleptic within one treatment cycle plus one week prior to randomisation; PANSS total score >60 and >4 on any 2 items on psychotic items subscale at screening and end of placebo washout; randomised within 4 weeks after starting treatment for current episode; had been an out‐patient for at least one 3‐month period during past year; Exclusions: other psychiatric disorders; first episode of schizophrenia or schizoaffective disorder; history of violence or suicidal ideation/self harm; significant neurological abnormality other than tardive dyskinesia or EPS; psychoactive drug or alcohol abuse/dependence within one month of study; treatment with an investigational drug within 4 weeks prior to washout period; any acute or unstable medical condition; potential need for concomitant medication that might cause unwanted interactions. | |
| Interventions | 1. Aripiprazole: dose 15 mg/day. N=102. 2. Aripiprazole: dose 30 mg/day. N=102. 3. Haloperidol: dose 10 mg/day. N=104. 4. Placebo. N=106. | |
| Outcomes | Leaving the study early.
Adverse effects.
Economic outcomes. Unable to use ‐ Death: suicide and natural causes (incomplete data). Global state: CGI (no SDs). Mental state: BPRS, BPRS‐PANSS derived (no SDs). General functioning: CGI (no SDs). Adverse effects: SAS, BAS, AIMS, other outcome measures including mean changes in body weight, changes in serum prolactin levels, and QTc interval changes, changes in vital signs and serum physiological measures( no usable/standard deviation data). |
|
| Notes | Overall 40% discontinuation rate. No information given on standard deviations. Data reported in both OC and LOCF analyses. Jadad=2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Csernansky 2003.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 4 weeks, preceded by 3‐7 day placebo washout period. Design: parallel, multicentre. Setting: inpatient, USA. Consent: not described. Loss: described. | |
| Participants | Diagnosis: schizophrenia (DSM‐III‐R). N=103. Age: 18‐65 years, average ˜36. Sex: male 91, female 12. History: acute relapse, BPRS score >30 & score of >4 on 2 of 4 positive symptoms, evidence of previous response to antipsychotic medication. Exclusions: >moderate motor symptoms as measured by SAS, AIMS and Barnes Akathisia scale; primary diagnoses other than schizophrenia; substance dependence within past 2 months; cardiac patients, acute/unstable medical conditions. | |
| Interventions | 1. Aripiprazole (OPC‐14597): dose 5 mg/day on days 1+2, 10 mg/day on days 3+4, 15 mg/day on days 5+6, 20 mg/day on days 7‐12, 30 mg/day on days 13‐28. N=34. 2. Haloperidol: dose 5 mg/day on days 1+2, 10 mg/day on days 3+4, 15 mg/day on days 5+6, 20 mg/day 7‐28. N=34. 3. Placebo. N=35. | |
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: BPRS score (no SDs); >40% discontinuation rate. Global state: CGI‐S score >40% discontinuation rate. Economic outcome: concomitant medication >40% discontinuation rate. |
|
| Notes | Overall 48.5% discontinuation rate. Limited information on standard deviations. Considered a 'negative' study by the FDA. Data reported in OC and LOCF analyses. Jadad=2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Daniel 2000.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 4 weeks, preceded by 3‐7 day washout period. Design: parallel, multicentre, dose‐ranging. Setting: inpatient. Consent: not described. Loss: described. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=307. Age: 18‐65 years, average ˜38. Sex: male 247, female 60. History: acute relapse, BPRS score >36 & score of > 4 on 2 criteria, no antipsychotic medication taken for >72 hours prior to randomisation(4 weeks for a long‐acting agent). Exclusions: first episode of schizophrenia; refractory to conventional antipsychotics; moderate to severe EPS, dyskinesia or akathisia; substance abuse or dependence; cardiac disease; acute or unstable medical condition; pregnancy, lactation, women not using adequate contraception. | |
| Interventions | 1. Aripiprazole: dose 2 mg/day. N=59. 2. Aripiprazole: dose 10 mg/day. N=60. 3. Aripiprazole: dose 30 mg/day. N=61. 4. Haloperidol: dose 10 mg/day after 5mg/day 1+2). N=63. 5. Placebo. N=64. | |
| Outcomes | Leaving the study early. Unable to use ‐ Global state: CGI score (no SDs); >40% discontinuation rate. Mental state: BPRS score (no SDs); >40% discontinuation rate. General functioning: CGI (no SDs); >40% discontinuation rate. Adverse effects: reported adverse effects, extra‐pyramidal side effects, mean weight gain, mean prolactin levels (no usable data); >40% discontinuation rate. Economic outcomes: use of concomitant medication >40% discontinuation rate. |
|
| Notes | Over 40% overall discontinuation rate. No data given on standard deviations. Marked sex differential in participants. Data reported in both LOCF and OC analyses. Jadad=2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Daniel 2004.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 24hrs. Design: parallel, multicentre, dose‐ranging. Setting: inpatient. Consent: obtained. Loss: described. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, schizophreniform disorder (DSM IV). N=357. Age: >18 years. Sex: male 214, female 143. History: acute agitation; PEC score >15 and <32, and a score >4 on at least 2 items; deemed appropriate for intra‐muscular therapy. Exclusions: neurological or psychiatric condition other than schizophrenia, schizoaffective disorder or schizophreniform disorder; known non‐responders to anti‐psychotic medication; significant medical condition; psychoactive substance dependence within 2 months of study start; required involuntary restraint; suicidal. | |
| Interventions | 1. Aripiprazole IM: dose 1mg/day. N=57. 2. Aripiprazole IM: dose 5.25 mg/day. N=63. 3. Aripiprazole IM: dose 9.75 mg/day. N=57. 4. Aripiprazole IM: dose 15 mg/day. N=58. 5. Haloperidol IM: dose 7.5 mg/day. N=60. 6. Placebo IM. N=62. | |
| Outcomes | Global state: discontinuation from study due to lack of efficacy, marked deterioration, or adverse event including psychosis.
Mental state: response to treatment.
Adverse effects: reported in > 5% participants, extrapyramidal symptoms.
Death. Unable to use ‐ Global state: CGI score (inadequate data/unusable). Mental state: BPRS score (inadequate data/ unusable). Behaviour: PEC score (inadequate data/ unusable), CAB score‐ mean change (inadequate data/ unusable), ACES‐ (inadequate data/ unusable). Adverse effects: laboratory abnormalities (no usable data), mean changes in Simpson‐Angus scale (no usable data), Barnes Akathisia scale (no usable data). Satisfaction with treatment: leaving study early due to withdrawal of consent (no usable data). Economic outcomes: requiring additional dose of antipsychotic medication, benzodiazepines (incomplete data). Leaving study early (inadequate data/ unusable). |
|
| Notes | Limited data reported on standard deviations. Data reported in LOCF analyses. Jadad=2. Only adverse effects occurring in over 5% participants recorded. Not all outcomes reported at 24hrs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kane 2003.
| Methods | Allocation: randomised, method not described. Blindness: double (during treatment phase). Duration: 6 weeks, preceded by 2‐14 day patient screening, 2 day neuroleptic washout, 4‐6 weeks confirmation of treatment resistance, 2‐10 day neuroleptic washout. Design: multicentre, parallel. Setting: unknown. Consent: obtained. Loss: described. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=300. Age: >18 years, mean˜42.1 years. Sex: male 208, female 92. History: treatment resistant during 2 years prior to study, PANSS total score of >/= 75 & score of >/= 4 on 2 or more specified PANSS items; CGI‐I score > 4, treated as out‐patient for at least 1 continuous 3‐month period during the 2 years prior to study entry. Exclusions: DSM IV diagnosis of schizoaffective disorder, residual schizophrenia or bipolar disorder; delirium, dementia, amnesia or other cognitive disorders; any acute or unstable medical condition; refractory to previous clozapine therapy; prior perphenazine treatment without adequate response; likelihood to require prohibited concomitant therapy during study; current or recent psychoactive drug or alcohol abuse or dependence; suicidal attempts or serious suicidal thoughts; known allergy or hypersensitivity to study drugs; treatment with an investigational drug within 4 weeks of the washout period; previous enrolment in an aripiprazole clinical study; pregnant or lactating women. | |
| Interventions | 1. Aripiprazole: dose 15‐30 mg/day, average dose 28.8 mg/day. N=154. 2. Perphenazine: dose 8‐64 mg/day, average dose 39.1 mg/day. N=146. | |
| Outcomes | Global state: change in CGI‐S score, mean CGI‐I score; discontinuation due to adverse event including psychosis, lack of efficacy.
Mental state: response, change in PANSS score, change in BPRS score.
Adverse effects.
Quality of life: QLS scores.
Satisfaction with treatment: withdrawal of consent.
Economic outcomes: concomitant medication.
Leaving the study early.
Death. Unable to use ‐ Adverse affects: mean change in AIMS, BAS, SAS (no usable data); mean change in prolactin levels and body weight (no SD); abnormalities of vital signs and laboratory findings (no usable data). Quality of life: mean change in QLS scores. |
|
| Notes | Limited data given on standard deviations. Data reported in both LOCF and OC analyses. Jadad=2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kujawa 2002.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 52 weeks (preceded by variable washout period‐ >5 days). Design: parallel, active‐controlled prospective, dual study‐multicentre. Setting: out‐patient, USA and Europe. Consent: described. Loss: described. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N=1294. Age: 18‐65, average ˜37 years. Sex: male 758, female 536. History: acute relapse; baseline PANSS of >/=60 with >/=4 on any 2 psychotic items subscale; history of prior response to antipsychotic medication(other than clozapine); continuous outpatient treatment for at least 3 months in the past year; non‐pregnant, non‐lactating women and men; medically stable on physical examination, ECG,routine laboratory testing. Exclusions: pregnancy and lactation, treatment resistance, suicidal ideation/risks, first episode of schizophrenia, other psychiatric or neurological disorder, substance dependence, concomitant medication that might interfere with metabolism or analysis, participants in previous aripiprazole study or used an investigational medication within 4 weeks. | |
| Interventions | 1. Aripiprazole: dose 30 mg/day, with possibility of one‐off dose decrease to 20 mg for tolerability. N=861. 2. Haloperidol: dose 5 mg/day for days 1‐3; dose 10 mg/day from day 4 onwards; with possibility to decrease to 7 mg for tolerability. N=433. | |
| Outcomes | Global state: discontinuation due to lack of efficacy, adverse event, marked deterioration.
Engagement with services: discontinuation due to lack of compliance(>60% discontinuation rate).
Leaving the study early.
Death. Unable to use ‐ Global state: CGI score (no usable data, >60% discontinuation rate). Mental state: PANSS total score, PANSS negative subscale score, MADRAS total score, PANSS depression item, PANSS depression/anxiety cluster, PANSS negative subscale score (no usable data/SDs, >60% discontinuation rate). Adverse effects: SAS, BAS, AIMS; specific symptoms; changes in body weight, serum prolactin levels, vital signs; ECG changes (>60% discontinuation rate). Economic outcomes: concomitant medication (>60% discontinuation rate). |
|
| Notes | Greater than 60% discontinuation rate. Limited data on standard deviations given. Different patient numbers analysed for safety and efficacy. Data reported in both LOCF and OC analyses. Jadad=2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
McCue 2006.
| Methods | Allocation:randomised, method adequate and well described. Blindness: open label (rating scale assessor blind). Duration: 3 weeks. Design: pragmatic clinical trial; single centre. Setting: In‐patient ‐ hospital. Consent: obtained. Loss: described. | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder or schizophreniform disorder (DSM‐IV). N=327*. Age: >18 years. Sex: male 198, female 121. History: acute mental illness requiring hospital admission. Exclusion: pregnant or lactating women; diagnosis of bipolar disorder, major depressive disorder or substance‐induced psychotic disorder; clear history of response or lack of response to a particular antipsychotic drug; medical condition posing significant clinical risk. | |
| Interventions | 1. Aripiprazole mean maximum daily dose 21.8 mg (SD‐8.1);
range 10‐45. N=55.
2. Haloperidol mean maximum daily dose 16.0 mg (SD‐7.6); range 4‐30. N=57.
3. Olanzapine mean maximum daily dose 19.1 mg (SD‐7.1); range 5‐40. N=52.
4. Quetiapine mean maximum daily dose 652.5 mg (SD‐280.8); range 50‐1200. N=52.
5. Risperidone mean maximum daily dose 5.2 mg (SD‐1.8); range 2‐9. N=61.
6. Ziprasidone mean maximum daily dose 151.2 mg (SD‐32.4); range 40‐240. N=50. Haloperidol, lorazepam and diphenhydramine administered together IM for agitation. Diphenhydramine at patient's request for sleep. Benztropine for extra‐pyramidal side‐effects After second week an anti‐depressant, mood stabiliser or anxiolytic could be added for significant mood symptoms or impulsivity. |
|
| Outcomes | Global state: discontinuation due to adverse event, marked deterioration.
Mental state: response rate, mean change in BPRS total score.
Economic outcome: concomitant anticholinergic.
Leaving the study early. Unable to use ‐ Adverse effect: mean changes from baseline in SAS and BAS (no usable data). |
|
| Notes | Jadad = 2. * Eight participants not accounted for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Oren 2005.
| Methods | Allocation: randomised, adequately concealed by centralise call‐in system. Blindness: double. Duration: 24 hours. Design: parallel, multicentre. Setting: hospital in‐patient. Consent: obtained Loss: described. | |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐IV). N=448. Age: >/=18 years. Sex: male 273, female 175. History: acute agitation, voluntarily hospitalised, able to comprehend and comply with protocol, PEC score of >/=15 and </=32, and at least 2 components >/=4(moderate), eligible for parenteral treatment, discontinued all psychotropic medication for duration of study, women of child‐bearing potential must have been using adequate contraception during study and for 8 weeks after. Exclusions: schizophreniform disorder, other psychiatric diagnoses, significant risk of suicide, significant neurological diagnosis or unstable medical conditions, any substance or alcohol dependence within 2 months before the study, any substance‐induced psychiatric disorder or behavioural disturbance, history of neuroleptic malignant syndrome from antipsychotic agents, use of benzodiazepines or anticholinergics within 4 hours before first injection of study medication, lack of response to previous antipsychotic medication. | |
| Interventions | 1. Aripiprazole IM: dose 9.75 mg/day. N=175.
2. Placebo IM. N=88.
3. Haloperidol IM: dose 6.5 mg/day. N=185. Allowed concomitant medication of up to 4mg lorazepam or equivalent. |
|
| Outcomes | Global state: discontinuation from study due to lack of efficacy, marked deterioration, or adverse event including psychosis.
Mental state: response to treatment.
Adverse effects.
Economic outcomes: requiring additional benzodiazepines.
Leaving the study early.
Death.
Adverse effects: reported in > 5% participants, extrapyramidal symptoms. Unable to use ‐ Global state: CGI‐ mean score (no SD). Mental state: BPRS scores (no SD). Behaviour: PEC score‐ mean change (no usable data/SD), CAB score (no SD), ACES (no data). Adverse effects: mean changes in SAS, BAS, clinically significant changes in vital signs and laboratory findings(no usable data). Economic outcomes: mean number of injections per group and amount of additional concomitant medication required. |
|
| Notes | Limited information given on standard deviations. Data was analysed in LOCF. Jadad=3. Adverse effects reported occurring in >/=5% participants. Not all outcomes recorded over 24 hrs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wang 2005.
| Methods | Allocation: randomised, method not described. Blindness: not described. Duration: 8 weeks. Loss: all randomised patients accounted for in outcome data. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N=72. Age: 18‐50 years, average 25 years. History: no details. Exclusions: no details. | |
| Interventions | 1. Aripiprazole: 5‐25 mg/day. N=36. 2. Chlorpromazine: 50‐500 mg/day. N=36. | |
| Outcomes | Mental state: response rate. Unable to use ‐ Mental state: changes in PANSS score (no usable data). Adverse effects: TESS scores (invalidated scale). |
|
| Notes | Paper in Chinese language ‐ unable to translate entire text. Jadad = 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
AIMS ‐ Abnormal involuntary movement scale ACES‐ Agitation‐calmness behaviour scale BAS ‐ Barnes Akathisia scale BPRS ‐ Brief psychiatric rating scale CGI ‐ CIinical global impression CGI‐I‐ CGI‐improvment scale CGI‐S‐ CGI severity of illness score CAB‐ Corrigan agitated behaviour scale DSM‐IV ‐ Diagnostic and Statistical Manual Edition IV ECG ‐ Electrocardiogram LOCF ‐ Last observation carried forward N ‐ number of participants OC ‐ Observed cases PANSS ‐ Positive and negative symptom scale PEC‐ PANSS excited component scale QLS ‐ Quality of life scale SAS ‐ Simpson Angus scale SD ‐ Standard deviation TESS ‐ Treatment emergent symptom scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2007 | Allocation: not randomised (post‐hoc analysis of pooled data from 3 RCT's). |
| Andrezina 2006 | Allocation: randomised. Participants: schizophrenia. Interventions: sub‐population post‐hoc analysis of a previously reported RCT which has been included in this review. |
| Argo 2004 | Allocation: not randomised. |
| Carson 2002 | Allocation: not randomised. |
| Carson 2004 | Allocation: not randomised (naturalistic study). |
| Casey 2002 | Allocation: randomised. Participants: schizophrenia or schizoaffective disorder. Interventions: aripiprazole at different switching regimens as interventions ‐ no other comparator drug. |
| Jung 2007 | Allocation: not described. Participants: schizophrenia. Interventions: haloperidol with adjunctive aripiprazole compared with haloperidol with adjunctive placebo. |
| Kelemen 2006 | Allocation: not described. Participants: schizophrenia. Interventions: allocation to typical and atypical drugs ‐ no data available for individual drugs. |
| Kim 2006 | Allocation: quasi‐randomisation. |
| Kim 2007 | Allocation: randomisation. Participants: schizophrenia or schizoaffective disorder. Interventions: switch from existing antipsychotics to aripiprazole and other antipsychotics. Aripiprazole not compared with any specific antipsychotic. |
| Petrie 1997 | Allocation: not randomised, review. |
| Shim 2006 | Allocation: not described. Participants: schizophrenia, with hyperprolactinaemia after taking haloperidol. Interventions: aripiprazole versus placebo as adjunctive treatment for people receiving haloperidol. |
| Swanson 2006 | Allocation: not randomised. |
| Talbott 2007 | Allocation: not randomised (post‐hoc analysis of pooled data from 3 RCT's). |
| Wu 2005 | Allocation: quasi‐randomisation. |
| Xia 2005 | Allocation: randomised. Participants: schizophrenia. Interventions: allocation to aripiprazole and chlorpromazine. Outcomes: no usable data. |
Characteristics of ongoing studies [ordered by study ID]
Barbui 2006.
| Trial name or title | CHAT(Clozapine Haloperidol Aripiprazole Trial) Randomized Evaluation of the Effectiveness of Clozapine and Aripiprazole Versus Clozapine and Haloperidol in the Treatment of Schizophrenia |
| Methods | |
| Participants | Individuals with Schizophrenia (DSM‐IV) with an incomplete response to treatment with 400mg or more per day of clozapine over at least 6 months. Age 18 and above. |
| Interventions | Combination treatment with clozapine plus aripiprazole compared to combination treatment with clozapine plus haloperidol |
| Outcomes | Withdrawal from allocated treatment within 3 to 12 months of follow‐up. Time to withdrawal from allocated treatment. Severity of illness. Adverse events and metabolic syndrome within 3 to 12 months. Biologic parameters measured at month 3 and 12. Concurrent use of adjunctive medication within 3 to 12 months. Subjective tolerability of antipsychotic drugs measured at month 3 and 12. Deliberate self‐harm within 3 and 12 months. |
| Starting date | Currently recruiting |
| Contact information | Clinical Trials.gov identifier NCT00395915 Corrado Barbui, corrado.barbui@univr.it 0039 045 8126418 |
| Notes |
Contributions of authors
Jayanti Bhattacharjee ‐ protocol and review development, and writing
Hany George El‐Sayeh ‐ protocol and review development, and writing
Sources of support
Internal sources
Academic Unit of Psychiatry, University of Leeds, UK.
External sources
No sources of support supplied
Declarations of interest
Jayanti Bhattacharjee ‐ None known Hany George El‐Sayeh ‐ None known
Edited (no change to conclusions)
References
References to studies included in this review
Carson 2000 {published data only}
- Anutosh S, Ali MW, Ingenito G, Carson WH. Controlled study of aripiprazole and haloperidol in schizophrenia. European Psychiatry 2002;Suppl 1:103s. [Google Scholar]
- Aquino P, CE Adams, T Crow, I Wood. Personal communication. Personal Communication 2006. [XXIInd C.I.N.P. [CD‐ROM]: Conifer, Excerpta Medica Medical Communications BV, 2000 PO1124]
- Carson WH, Ali M, Dunbar G, Ingenito G, Saha AR. A double‐blind, placebo‐controlled trial of aripiprazole and haloperidol. Schizophrenia Research 2001;1‐2:221‐2. [MEDLINE: ] [Google Scholar]
- Carson WH, Ali M, Saha AR, Dunbar GC, Ingenito G. A double‐blind, placebo‐controlled trial of aripiprazole and haloperidol in patients with schizophrenia or schizoaffective disorder. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan; Puerto Rico. 2000. [MEDLINE: ]
- Carson WH, Kane JM, Ali M, Dunbar GC, Ingenito G. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl 3):S309. [Google Scholar]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 1. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:1‐50.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 3. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:111‐175.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 4. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm Vol. 2002:176‐232.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug administration CDER, 2002:50‐110. [XXIInd C.I.N.P.[CD‐ROM]: Conifer, Excerpta Medica Medical Communications BV, 2000 PO1124]
- European Medicines Agency. Abilify H‐C‐471; European Public Assessment Report; Scientific discussion. http://www.emea.europa.eu/humandocs/. 7 2007. [IMEA]
- Kane J, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. Schizophrenia Research 2000;1:39. [MEDLINE: ] [Google Scholar]
- Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. Journal of Clinical Psychiatry 2002;9:763‐71. [DOI] [PubMed] [Google Scholar]
- Kane JM, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, USA. 2000.
- Kane JM, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA, USA. 2002.
- Kane JM, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. International Journal of Neuropsychopharmacology 2000;Suppl 1:S124. [Google Scholar]
Csernansky 2003 {published data only}
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 1. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:1‐50.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 2. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:51‐110.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 3. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:111‐175.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:176‐232. [PO1.76.]
- European Medicines Agency. Abilify H‐C‐471; European Public Assessment Report; Scientific discussion. http://www.emea.europa.eu/humandocs/. 7 2007. [IMEA]
Daniel 2000 {published data only}
- Daniel DG, Saha AR, Ingenito G, Carson WH, Dunbar G. Aripiprazole, a novel antipsychotic: overview of a phase II study result. International Journal of Neuropsychopharmacology 2000;Suppl 1:S157. [Google Scholar]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 2. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:50‐110. [XXIInd C.I.N.P. [CD‐ROM] : Conifer, Excerpta Medica Medical Communications BV, 2000 PO1242]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 1. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:1‐50.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 3. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:111‐175.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:176‐232. [XXIInd C.I.N.P.[CD‐ROM] : Conifer, Excerpta Medica Medical Communications BV,2000 PO1242]
- European Medicines Agency. Abilify H‐C‐471; European Public Assessment Report; Scientific discussion. http://www.emea.europa.eu/humandocs/. 7 2007. [IMEA]
- Saha AR, Petrie JL, Ali MW. Safety and efficacy profile of aripiprazole, a novel antipsychotic. Schizophrenia Research 1999;1‐3:295. [MEDLINE: ] [Google Scholar]
Daniel 2004 {published data only}
- Daniel DG, Stock EG, Wilber CH, Marcus RN, Carson Jr WH, Manos G, Iwamoto T. Intramuscular aripiprazole in acutely agitated psychotic patients. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York, Usa. 2004. [APA Conference Abstracts NR612]
- Gismondi R, Daniel D, Stock E, Wilber R, Carson W, Manos G, Iwamoto T. Intramuscular aripiprazole treatment for acute agitation in patients with psychosis. Journal of the European College of Neuropsychopharmacology 2004;14(Suppl.3):S261. [P.2.066] [Google Scholar]
- Kungel M, Daniel D, Stock E, Wilber R, Marcus R, Carson W, Manos G, Iwamoto T. Efficacy and safety of intramuscular aripiprazole in acutely agitated patients with psychosis. Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004. [PO1.81.]
- Modell S, Daniel D, Stock E, Wilber R, Marcus R, Carson W, Manos G, Iwamoto T. Intramuscular aripiprazole treatment for acute agitation in patients with psychosis. XXIVth Collegium Internationale Neuro‐Psychopharmacologicum Congress; 2004 June 20‐24, Paris, France 2004. [P12.203]
- Tran‐Johnson TK, Sack DA, Marcus RN, Auby P, McQuade RD, Oren DA. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomised double‐blind,placebo‐controlled trial. Journal of Clinical Psychiatry 2007;68(1):111‐9. [MEDLINE: ; EMBASE 2007077930] [DOI] [PubMed] [Google Scholar]
- Yocca F, Daniel D, Stock E, Oren D, Marcus R, Carson W, Manos G, Iwamoto T. Efficacy and safety of intramuscular aripiprazole treatment for acute agitation in patients with psychosis. 43rd Annual Meeting of the American College of Neuropsychopharmacology ; 2004 Dec 12‐16, San Juan, Puerto Rico. 2004. [APA2006L1_4195]
Kane 2003 {published data only}
- Gismondi R, Meltzer H, Kujawa M, Carson W, Stringfellow J, Iwamoto T, Marcus R, Stock E. Aipiprazole versus perphenazine in treatment‐resistant schizophrenia. XXIVth Collegium Internationale Neuro‐Psychopharmacologicum Congress ; 2004 June 20‐24, Paris, France 2004. [P12.P203]
- Kane J, Carson Wh, Kujawa M, Stringfellow J, Marcus R, Sanchez R, Iwamoto T, Meltzer H. Aripiprazole in Treatment‐Resistant Schizophrenia: a 6‐Week Double‐ Blind Comparison Study Versus Perphenazine. Schizophrenia Research 2004;67(1):155‐6. [ISI 000188788100371] [Google Scholar]
- Kane J, William HCJ, Kujawa MJ, Stringfellow J, Marcus RN, Sanchez R, Meltzer HY. Aripiprazole vs. perphenazine in treatment‐resistant schizophrenia. 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003. [[NR537]]
- Kane JM, Meltzer HY, Carson WH Jr, McQuade RD, Marcus RN, Sanchez R, Aripiprazole Study Group. Aripiprazole for treatment‐resistant schizophrenia : results of a multicenter, randomised, double‐blind, comparison study versus perphenazine. Journal of Clinical Psychiatry 2007;68(2):213‐23. [MEDLINE: ] [PubMed] [Google Scholar]
- McQuade R, Jody D, Kane J, Carson W, Kujawa M, Stringfellow J, Marcus R, Sanchez R, Meltzer HY. Efficacy and safety of aripiprazole versus perphenazine in treatment‐resistant schizophrenia. 16th European College of Neuropsychopharmacology Congress ; 2003 Sep 20‐24; Prague, Czech Republic. 2003. [P.2.105]
- McQuade R, Jody D, Kane J, Carson W, Kujawa M, Stringfellow J, Marcus R, Sanchez R, Meltzer HY. Efficacy and safety of aripiprazole versus perphenazine in treatment‐resistant schizophrenia. Journal of the European College of Neuropsychopharmacology 2003;13(4):S326. [P.2.105] [Google Scholar]
- Meltzer HY, Kujawa MJ, Carson Jr WH, Stringfellow J, Iwamoto T, Marcus RN, Stock EG. Aripiprazole and perphenazine in severe treatment‐resistant schizophrenia. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York, Usa. 2004. [APA Conference Abstracts NR622]
- Modell S, Jody D, Kujawa M, Carson W, Stringfellow J, Iwamoto T, Marcus R, Stock E. Efficacy of aripiprazole and perphenazine in svere schiziphrenia resistant to treatment with atypical antipsychotics. Journal of the European College of Neuropsychopharmacology 2004;14(Suppl.3):S265. [P.2.076] [Google Scholar]
- Sanchez R, Meltzer HY, Marcus RN, Stringfellow J, Carson WH, Kane JM. Aripiprazole versus Perphenazine in Treatment ‐ Resistant Schizophrenia. Schizophrenia Bulletin 2005;31:502. [116046] [Google Scholar]
Kujawa 2002 {published data only}
- Archibald DG, Manos G, Stock E, Jody D, Tourkodimitris S, Marcus R, Iwamoto T, Yamamoto Y. Effects of Long‐Term Aripiprazole Therapy on the Negative Symptoms of Schizophrenia. Schizophrenia Research 2004;67(1):155. [ISI 000188788100369] [Google Scholar]
- Archibald DG, Manos G, Tourkodimitris S, Iwamoto T, Carson WH, Stock E, Marcus R. Reduction in negative symptoms of schizophrenia during long‐term therapy with aripiprazole. Schizophrenia Research 2003;60:271. [MEDLINE: ; PMID 12701664] 12701664 [Google Scholar]
- Crandall D, Pikalov A, Kostic D, Kaplita S, Berman R, McQuade R, Radhakrishnan M. Is sedation needed for effective reduction of acute schizophrenia symptoms ?. 158th Annual Meeting of the American Psychiatric Association ; 2005 May 21‐26; Atlanta, Georgia, USA. 2005. [NR 264]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 3. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:111‐75. [P.4.E.032]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets, medical review part 1. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:1‐50.
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 4. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:176‐232. [P.4.E.032]
- Dubitsky GM, Harris R, Laughren T, Hardeman s. Abilify (aripiprazole) tablets ; medical review part 2. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration, 2002:50‐110. [P.4.E.032]
- European Medicines Agency. Abilify H‐C‐471; European Public Assessment Report; Scientific discussion. http://www.emea.europa.eu/humandocs/. 7 2007. [IMEA]
- Kane JM, Swyzen W, Wu X, McQuade R, Gutierrez‐Esteinou R, Tran Q, Marcus R. Long‐term symptomatic remission in schizophrenia patients treated with aripiprazole or haloperidol. 159th Annual Meeting of the American Psychiatric Association ; 2006 May 20‐25, Toronto, Canada. 2006. [APA2006L1_4223]
- Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, Archibald D, Ingenito G, Marcus R, Pigott T. Efficacy and safety of aripiprazole vs. haloperidol for long‐term maintenance treatment following acute relapse of schizophrenia. International Journal of Neuropsychopharmacology 2003;6(4):325‐37. [MEDLINE: ; EMBASE: 2004054004] [DOI] [PubMed] [Google Scholar]
- Kostic D, Manos G, Stock E, Jody D, Archibald D, Tourkodimitris S, Marcus R. Long‐term effects of aripiprazole on the negative symptoms of schizophrenia. Journal of the European College of Neuropsychopharmacology 2003;13(4):S328. [P.2.111] [Google Scholar]
- Kostic D, Marcus RN, Stock EG, Carson WH, Tourkodimitris S, Archibald DG. Maintenance of response in chronic schizophrenia : effects of aripiprazole and haloperidol on affective symptoms. Schizophrenia Bulletin. 2005; Vol. 31:492.
- Kujawa M, Saha AR, Ingenito GG, Ali MW, Luo X, Archibald DG, Carson WH. Aripiprazole for long‐term maintenance treatment of schizophrenia. International Journal of Neuropsychopharmacology 2002;suppl 1:S186. [DOI] [PubMed] [Google Scholar]
- Kujawa MJ, Saha AR, Ingenito GG, Ali MW, Luo X, Archibald DG, William Jr.HC. Aripiprazole for long‐term maintenance treatment in schizophrenia. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA, USA. 2002. [NR 376]
- Manos G, Stock EG, Jody D, Archibald DG, Tourkodimitris S, Marcus RN. Long‐term effects of aripiprazole on the negative symptoms of schizophrenia. 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, USA. 2003.
- Marder SR, Archibald DG, Manos G, Stock EG, Jody DN, Tourkodimitris S, Marcus RN, Iwamoto T, Yamomoto Y, Carson WH. Long‐term effects of aripiprazole therapy on the negative symptoms of schizophrenia. XXIVth Collegium Internationale Neuro‐Psychopharmacologicum Congress ; 2004 June 20‐24 , Paris, France 2004. [P12.P203]
- McQuade R, Carson W, Saha A, Ingenito G, Ali M, Archibald D. Aripiprazole for long‐term maintenance treatment of schizophrenia. Journal of the European College of Neuropsychopharmacology 2002;12(Supplement 3):S288. [Hallo P.2.078] [DOI] [PubMed] [Google Scholar]
- McQuade RD, Kujawa M, Saha AR, Ingenito GG, Ali MW, Luo X, Archibald DG, Carson WH. Aripiprazole for long‐term maintenance treatment of schizophrenia. Schizophrenia Research 2003;60:295. [MEDLINE: ; NR 376] [Google Scholar]
- Octavio I, Stock E, Archibald D, Tourkodimitris S, Kujawa M, Marcus R, Carson W, Iwamoto T. Long‐term effects of aripiprazole on affective symptoms of schizophrenia. Journal of the European College of Neuropsychopharmacology 2004;14(Suppl.3):S261. [P.2.067] [Google Scholar]
- Octavio I, Stock EG, Archibald DG, Tourkodimitris S, Kujawa MJ, Marcus R, Carson WH, Iwamoto T. Long‐term effects of aripiprazole on affective symptoms of schizophrenia. XXIVth Collegium Internationale Neuro‐Psychopharmacologicum Congress ; 2004 June 20‐24, Paris, France 2004. [P12.P203]
- Saha AR, Carson WH, Mcquade RD, Stock EG, Inada I, Ali MW. Long‐term aripiprazole therapy in schizophrenia. XIIth World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama, Japan. 2002.
- Stock E, Archibald Dg, Tourkodimitris S, Kujawa M, Marcus R, Carson W, Iwamoto T. Long‐Term Effects of Aripiprazole and Haloperidol on Affective Symptoms of Schizophrenia. Schizophrenia Research 2004;67(1):158‐9. [ISI 000188788100378] [Google Scholar]
- Stock EG, Achibald DG, Tourkodimitris S, Kuwaja MJ, Marcus RN, Carson Jr WH. Long‐term effects of aripiprazole on affective symptoms of schizophrenia. 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, USA. 2003.
McCue 2006 {published data only}
- McCue RE, Waheed R, Urcuyo L, Orendain G, Joseph MD, Charles R, Hasan SM. Comparative effectiveness of second‐generation antipsychotics and haloperidol in acute schizophrenia. British Journal of Psychiatry 2006;189(Nov):433‐40. [MEDLINE: ; EMBASE 2006539005] [DOI] [PubMed] [Google Scholar]
Oren 2005 {published data only}
- Andrezina R, Josiassen RC, Marcus RN, Oren DA, Manos G, Stock E, Carson WH, Iwamoto T. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder ; a double‐blind, placebo‐controlled comparison with intramuscular haloperidol. Psychopharmacology 2006;188(3):281‐92. [MEDLINE: ; EMBASE 2006450840] [DOI] [PubMed] [Google Scholar]
- Citrome LL, Vester‐Blokland E, Archibald D, McQuade R, Kostic D, Pikalov A, Oren D. Benefits of a second dose of intramuscular (im) aripiprazole to control agitation in patients with schizophrenia or bipolar 1 disorder. 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006. [APA2006L1_4658]
- Dillenschneider A, Oren D, Marcus R, Kostic D, McQuade R, Iwamoto T, Manos G. Comparison of intramuscular aripiprazole and haloperidol with placebo in acutely agitated schizophrenia patients. Journal of the European College of Neuropsychopharmacology 2005;15(Suppl.3):S509. [P.3.122] [Google Scholar]
- McQuade R, Auby P, Oren D, Marcus R, Kostic D, Iwamoto T, Manos G. Intramuscular aripiprazole in acute schizophrenia : a double‐blind, placebo‐controlled comparison with IM haloperidol. 13th Biennial winter workshop on Schizophrenia Research; 2006 February 4‐10, Davos, Switzerland 2006. [ISI 000188788100378]
- Oren D, Marcus R, Kostic D, McQuade R, Iwamoto T, Archibald D. Efficacy and Safety of Intramuscular Aripiprazole, Haloperidol or Placebo in Acutely Agitated Patients with Schizophrenia or Schizoaffective Disorder. Schizophrenia Bulletin 2005;31:399. [116266] [Google Scholar]
- Oren D, Marcus R, Kostic D, McQuade R, Iwamoto T, Archibald D. Efficacy and safety of intramuscular aripiprazole, haloperidol or placebo in acutely agitated patients with schizophrenia or schizoaffective disorder. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26, Atlanta, GA, USA 2005. [P01.83.]
- Yocca F, Marcus R, Oren D, Manos G, Carson W, Iwamoto T, Stock E. Intramuscular aripiprazole in acute schizophrenia: A pivotal phase‐three study. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta, GA, USA 2005. [116266]
Wang 2005 {published data only}
- Wang G‐P, Xie R, Pei G‐X. A comparative study between aripiprazole and chlorpromazine in the treatment of schizophrenia. Shandong Archives of Psychiatry 2005;18(4):250‐1. [CAJ MEDI0510; WANFANG] [Google Scholar]
References to studies excluded from this review
Allen 2007 {published data only}
- Allen M, Talbott S, Eudicone J, McQuade R, Pikalov A. Similar rates of agitation, anxiety and insomnia in sedating and non‐sedating antipsychotics: evaluating clinical trial results with aripiprazole, haloperidol and olanzapine. Schizophrenia Bulletin 2007;33(2):418. [ISIP:000244506601017] [Google Scholar]
Andrezina 2006 {published data only}
- Andrezina R, Marcus RN, Oren DA, Manos G, Stock E, Carson WH, McQuade RD. Intramuscular aripiprazole or haloperidol and transition to oral therapy in patients with agitation associated with schizophrenia: sub‐analysis of a double‐blind study. Current Medical Research and Opinion 2006;22(11):2209‐19. [EMBASE 2006574726] [DOI] [PubMed] [Google Scholar]
Argo 2004 {published data only}
- Argo TR, Carnahan RM, Perry PJ. Aripiprazole, a novel atypical antipsychotic drug. Pharmacotherapy 2004;24(2):212‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carson 2002 {published data only}
- Carson WH, Ingenito GG, McQuade RD, Stock EG, Iwamoto T. [Schizophrenia; safety/tolerability of aripiprazole]. XIIth World Congress of Psychiatry; Aug 24‐29; Yokohama, Japan. 2002.
Carson 2004 {published data only}
- Carson W, Marcus R, Jody D, Liston H, Pans M, Riera L, Iwamoto T. The effectiveness of switch to aripiprazole stratified by prior antipsychotic. American Psychiatric Association Annual Meeting; 2004 May 1‐6, New York, NY, USA 2004. [APA2006L1_4195]
Casey 2002 {published data only}
- Casey D, Saha AR, Ali MW, Jody DN, Kujawa MJ, Stock EG, Ingenito GG. Switching to aripiprazole monotherapy. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S187. [P.4.E.034] [Google Scholar]
- Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D, Ingenito GG. Switching patients to aripiprazole from other antipsychotic agents : a multicenter randomised study. Psychopharmacology 2003;166(4):391‐9. [EMBASE 2003167418] [DOI] [PubMed] [Google Scholar]
- Casey DE, Saha AR, Ali MW, Jody D, Kujawa MJ, Stock EG, Ingenito GG. Switching to aripiprazole monotherapy. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennysylvania, USA. 2002. [NR 310]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 1. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:1‐50. [EMBASE 2003167418]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 2. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:50‐110. [2003167418]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 3. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:111‐75. [EMBASE 2003167418]
- Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets ; medical review part 4. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm. U.S. Food and Drug Administration CDER, 2002:176‐232. [EMBASE 2003167418]
- Kujawa M, Saha AR, Ali MW, Jody DN, McQuade RD, Ingenito GG. Switching to aripiprazole monotherapy. Schizophrenia Research 2003;60:290. [Hallo P.2.091] [Google Scholar]
- Medori R, Gharbia N, Saha A, Ali M, Stock E, Ingenito G. Switching to aripiprazole monotherapy. Journal of the European College of Neuropsychopharmacology 2002;12(Suppl 3):S292. [Hallo P.2.091] [Google Scholar]
Jung 2007 {published data only}
- Jung D, Shin J, Kelly DL, Seo Y, Liu K, Sohn J, Conley RR, Shim J. Drug interactions between aripiprazole and haloperidol: double blind, placebo controlled study. Schizophrenia Bulletin 2007;33(2):434‐5. [ISIP:000244506601063] [Google Scholar]
Kelemen 2006 {published data only}
- Kelemen O, Nagy O, Mattyassy A, Kiss I, Janka Z, Keri S. Do second‐generation antipsychotics disrupt decision‐making abilities in schizophrenia ?. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S430. [P.3.d.002] [Google Scholar]
Kim 2006 {published data only}
- Kim JG, Cho DH, Lee SJ, Lee JK, Wang KS, Seo YI, Choi SJ, Joo MJ, Kim HD, Lee KH, Kwon YJ, Han KR. The comparison of efficacy between aripiprazole and haloperidol in treatment of chronic schizophrenia and schizoaffective disorder : results for 16‐week clinical study. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S417. [P.3.C.040] [Google Scholar]
Kim 2007 {published data only}
- Kim C, Chung S, Lee J, Kwon J, Lee Y, Kim C, Oh K, Jeon Y, Ham B, Kim D, Jung H. Efficacy and safety of aripiprazole a 12 weeks, multi‐center, open‐label switching to aripiprazole study in stable outpatients with schizophrenia or schizoaffective disorder. Schizophrenia Bulletin 2007;33(2):437. [ISIP:000244506601068] [Google Scholar]
Petrie 1997 {published and unpublished data}
- Petrie JL, Saha JR, McEvoy JP. Aripiprazole, a new typical antipsychotic: phase 2 clinical trial result. 10th European College of Neuropsychopharmacology Congres; Sep 13‐17; Vienna, Austria.. 1997.
Shim 2006 {published data only}
- Shim JC, Kelly DL, Jung DU, Seo YS, Kim YH, Conley RR. Adjunctive treatment with a dopamine partial agonist, aripiprazole for antipsychotic‐induced hyperprolactinaemia. Journal of the European College of Neuropsychopharmacology 2006;16(Suppl 4):S405. [P.3.C.017] [DOI] [PubMed] [Google Scholar]
Swanson 2006 {published data only}
- Swanson JW, Swartz MS, Dorn RA. Effectiveness of atypical antipsychotics for substance abuse in schizophrenia patients. 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada.. 2006. [APA2006L1_4195]
Talbott 2007 {published data only}
- Talbott S, Buckley P, Eudicone J, McQuade R, Van‐Tran Q. Akathisia in schizophrenia patients treated with aripiprazole, haloperidol, or olanzapine‐analyses of the first 12 weeks of three double‐blind, long‐term trials. 11th International Congress on Schizophrenia Research; 2007 Mar 28‐Apr 1, Colorado Springs, Colorado, USA. 2007. [EMEA]
Wu 2005 {published data only}
- Wu J‐D, Li Y‐D, Song Z‐W. Effect of aripiprazole or haldol on intelligence and memory in the first‐onset schizophrenia. Chinese Journal of Rehabilitation Theory and Practice 2006;12(1):64‐5. [CAJ MEDI0603S1] [Google Scholar]
Xia 2005 {published data only}
- Xia S‐Y, Hua P, Nie Y‐B. A treatment study of schizophrenia with aripiprazole and chlorpromazine. Practical Clinical Medicine 2005;6(10):33‐4. [CAJ MEDI0512] [Google Scholar]
References to studies awaiting assessment
Ao 2006 {published data only}
- Ao X, Shao Q, Song H‐M. Comparative study between aripiprazole and chlorpromazine in the treatment of female patients with schizophrenia. Title only available in chinese characters 2006;18(17):719‐20. [Library number =81082A] [Google Scholar]
Cheng 2006 {published data only}
- Cheng P. Title only available in Chinese characters. Nervous Diseases and Mental Health 2006;6(3):203‐4. [Library number =84069X] [Google Scholar]
Currier 2006 {published data only}
- Currier GW, Crandall D, Archibald D, Nyilas M, Kostic D, Pikalov A, Oren D. Intramuscular (im) aripiprazole controls agitation in patients with schizophrenia or bipolar disorder without excessive sedation. 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006. [APA2006L1_4234]
Daniel 2006 {published data only}
- Daniel DG, Crandall D, Manos G, McQuade RD, Gutierrez‐Esteinou R, Pikalov AA, Oren D. Transitioning from intramuscular (im) to oral aripiprazole in patients with schizophrenia. 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006. [APA2006L1_4335]
Daniel 2007 {published data only}
- Daniel D, Crandall D, Manos G, McQuade R. Transitioning from intramuscular (IM) to oral aripiprazole in patients with schizophrenia TTransitioning from intramuscular (IM) to oral aripiprazole in patients with schizophrenia. Schizophrenia Bulletin 2007;33(2):426‐7. [ISIP:000244506601041] [Google Scholar]
Lin 2006 {published data only}
- Lin H‐L, Shi W‐H, Wang J‐H. Effects of aripiprazole and chlorpromazine on the cognitive function in first‐episode schizophrenia patients. Title in Chinese characters 2006;15(5):440‐2. [Library number =98293A] [Google Scholar]
Lu 2006 {published data only}
- Lu Z‐D, Cao Y‐N, Wang D. A comparative study of aripiprazole and chlorpromazine in the treatment of schizophrenia. Title in Chinese characters 2006;19(2):90‐2. [Library number =98411X] [Google Scholar]
Wang 2006 {published data only}
- Wang L‐G, Wang D‐M, Su Y. A comparative study of aripiprazole and haloperidol in the treatment of patient with treatment‐resistant schizophrenia. Title in Chinese characters 2006;6(1):38‐9. [Library number =84069X] [Google Scholar]
Xu 2006 {published data only}
- Xu D‐G, Zhao Z‐A, Huang W‐P. The clinic study of the change medicine ways about aripiprazole. Medical Journal of Chinese Peoples Health 2006;18(9):737‐8. [Library number =81082A] [Google Scholar]
Zhang 2005 {published data only}
- Zhang Z. Title only available in Chinese characters. Nervous Diseases and Mental Health 2005;5(5):374‐5. [Library number =84069X] [Google Scholar]
Zhang 2006 {published data only}
- Zhang Z. Title only available in Chinese characters. Medical Journal of Chinese Peoples Health 2006;18(1):11‐2. [Library number =81082A] [Google Scholar]
Zhuang 2006 {published data only}
- Zhuang J. Title only available in Chinese characters. Only available in Chinese characters 2006;16(2):111. [Library number =98038X] [Google Scholar]
References to ongoing studies
Barbui 2006 {published data only}
- Barbui C, Tansella M. Randomized evaluation of the effectiveness of clozapine and aripiprazole versus clozapine and haloperidol in the treatment of schizophrenia. An independent, pragmatic, multicentre, parallel‐group, superiority trial. http://www.clinicaltrials.gov 2006. [ID: NCT00395915]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1985
- Andreasen NC. Negative syndrome in schizophrenia: strategies for long‐term management. Advances in Biochemical Psychopharmacology 1985;40:1‐7. [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Carpenter 1984
- Heinrichs DW, Hanlon ET, Carpenter WT Jr. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome.. Schizophrenia Bulletin 1984;10:388‐96. [DOI] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Christison 1991
- Christison GW, Kirch DG, Wyatt RJ. When symptoms persist: choosing among alternative somatic symptoms for schizophrenia. Schizophrenia Bulletin 1991;17:217‐45. [DOI] [PubMed] [Google Scholar]
Crow 1980
- Crow TJ. Positive and negative schizophrenic symptoms and the role of dopamine. British Journal of Psychiatry 1980;137:383‐6. [PubMed] [Google Scholar]
Davis 1977
- Davis JM, Casper R. Antipsychotic drugs: clinical pharmacology and therapeutic use. Drugs 1977;14:260‐82. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analysis of binary data. In: Abstracts of 8th InternationalCochrane Colloquim; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behaviour. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomised trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
El‐Sayeh 2006
- El‐Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 2. [10.1002/14651858.CD004578.pub 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2002a
- U.S. Federal Drug Administration CDER. Abilify (aripiprazole) tablets, medical review. www.fda.gov/cder/foi/nda/2002/21‐436_Abilify.htm 2002.
Gelder 2001
- Gelder M, Lopez‐Ibor jnr JJ, Andreason NC. Antipsychotic and anticholinergic drugs. New Oxford Textbook of Psychiatry. Vol. 2, Oxford: Oxford University Press, 2000:1314‐16. [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy U. ECDEU assessment manual for psychopharmacology. Revised. Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Hedlund 1980
- Hedlund JL, Vieweg BW. The Brief Psychiatric Rating Scale (BPRS): a comprehensive review. Journal of Operational Psychiatry 1980;11:48‐65. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd., 2005.
Jadad 1996
- Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled clinical trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Joy 2004
- Joy CB, Adams CE, Lawrie SM. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003082.pub2] [DOI] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kane 1990
- Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica 1990;358(suppl):151‐7. [DOI] [PubMed] [Google Scholar]
Kane 1993
- Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica 1993;46:585‐93. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry 2005;187:366‐71. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
Lindenmeyer 2004
- Lindenmeyer JP, Brown E, Baker RW, Schuh LM, Shao L, Tohen M, Ahmed S, Stauffer VL. An excitement subscale of the Positive and Negative Syndrome Scale. Schizophrenia research 2004;68:331‐337. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Meltzer 1992
- Meltzer HY. Treatment of the neuroleptic‐nonresponsive schizophrenic patient. Schizophrenia Bulletin 1992;18:515‐42. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Rivas‐Vasquez 2003
- Rivas‐Vasquez RA. Aripiprazole: A novel antipsychotic with dopamine stabilising properties. Professional Psychology: Research and Practice 2003;34(1):108‐11. [Google Scholar]
Roland 1998
- Roland M, Torgerson DJ. What are pragmatic trials?. BMJ 1998;316(7127):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC. Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):3‐92. [PubMed] [Google Scholar]


