Summary
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent liver disease worldwide, and a major cause of liver cirrhosis and hepatocellular carcinoma. NAFLD is intimately linked with other metabolic disorders characterized by insulin resistance. Metabolic diseases are driven by chronic inflammatory processes, in which macrophages perform essential roles. The polarization status of macrophages is itself influenced by metabolic stimuli such as fatty acids, which in turn affect the progression of metabolic dysfunction at multiple disease stages and in various tissues. For instance, adipose tissue macrophages respond to obesity, adipocyte stress and dietary factors by a specific metabolic and inflammatory programme that stimulates disease progression locally and in the liver. Kupffer cells and monocyte-derived macrophages represent ontologically distinct hepatic macrophage populations that perform a range of metabolic functions. These macrophages integrate signals from the gut-liver axis (related to dysbiosis, reduced intestinal barrier integrity, endotoxemia), from overnutrition, from systemic low-grade inflammation and from the local environment of a steatotic liver. This makes them central players in the progression of NAFLD to steatohepatitis (non-alcoholic steatohepatitis or NASH) and fibrosis. Moreover, the particular involvement of Kupffer cells in lipid metabolism, as well as the inflammatory activation of hepatic macrophages, may pathogenically link NAFLD/NASH and cardiovascular disease. In this review, we highlight the polarization, classification and function of macrophage subsets and their interaction with metabolic cues in the pathophysiology of obesity and NAFLD. Evidence from animal and clinical studies suggests that macrophage targeting may improve the course of NAFLD and related metabolic disorders.
Keywords: NASH; immunometabolism; steatohepatitis, Kupffer cells; innate immunity; liver immunology
Key points
Macrophages play a key role in the chronic inflammatory processes that drive NAFLD and other metabolic diseases.
Crosstalk between macrophages and metabolic stimuli is crucial to the development of metabolic dysfunction, inflammation and disease progression in NAFLD.
Kupffer cells and monocyte-derived macrophages integrate signals from the gut-liver axis, overnutrition, systemic low-grade inflammation and steatosis, driving the progression of NAFLD to NASH and fibrosis.
Increasing evidence from both animal and clinical studies suggests that macrophage targeting may be an effective therapeutic strategy for NAFLD and related metabolic disorders.
Alt-text: Unlabelled Box
Introduction
In parallel to the obesity pandemic, non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide.[1], [2] NAFLD, particularly in its inflammatory form of non-alcoholic steatohepatitis (NASH), can progress to fibrosis, cirrhosis and NASH-induced hepatocellular carcinoma. The prevalence of NAFLD is projected to increase further over the next 10–15 years, with a higher proportion of patients developing fibrosis,3 the main determinant of both liver-related and overall mortality.[4], [5] Tremendous advances in basic and translational research have provided insights into the pathogenic mechanisms driving the progression of NAFLD, in which inflammatory processes that are controlled by macrophages, a key component of innate immunity, play a major role.6 Historically, NAFLD has been viewed as a consequence of insulin resistance and the metabolic syndrome.7 In particular, depending on selection criteria, the prevalence of NAFLD in type 2 diabetic patients ranges from 30 to almost 100%.[8], [9], [10], [11] Nevertheless, NAFLD is not only a manifestation of, but can also precede the development of other components of the metabolic syndrome, which has sparked a debate over the direction of the causal relations involved.12 Indeed, insulin resistance and ectopic fat accumulation interconnect NAFLD with other metabolic disorders such as obesity, type 2 diabetes mellitus and atherosclerosis. While numerous pathways are involved in this pathophysiological process, chronic inflammation and macrophages have been implicated as drivers of disease at multiple stages and in various tissues during the development of metabolic disease.13
Metabolic inflammation is critically different from the paradigmatic acute inflammation seen in bacterial infections, where a strong immune response is followed by elimination of the pathogen and rapid resolution to baseline (if successful). Instead, metabolic inflammation is characterized by a persistent, low-grade, sterile inflammation, often termed metaflammation. From this perspective, inflammatory mediators can be thought of as metabolic hormones that regulate insulin signalling.14
In the last decades, macrophages have emerged as key regulators of inflammation-mediated insulin resistance.15 The functional diversity of macrophages is reflected by the concept of “inflammation-promoting M1 macrophages” and “inflammation-suppressing M2 macrophages”. Beyond this rigid (and over-simplifying) M1 vs. M2 paradigm, macrophages can adapt and react to a wide variety of stimuli, including metabolic signals.16 Upon stimulation, macrophages undergo a complex and context-dependent metabolic reprogramming that determines their polarization status. These findings have led to the emergence of immunometabolism as a research field, which investigates the crosstalk between metabolism and immune cells.[17], [18]
Further progress in our understanding of the functional and tissue-specific heterogeneity of macrophages, as well as their crosstalk with metabolism could direct the development of therapies for metabolic disorders. In this review, we describe the relationship between inflammation (with a focus on macrophages) and insulin resistance, as well as the regulation of macrophage activation by metabolic processes. We elaborate on their contribution to the initiation and progression of fatty liver disease, highlighting putative macrophage-mediated mechanisms that link NAFLD and cardiovascular disease, and discussing the potential of macrophages as therapeutic targets in fatty liver disease.
Inflammation, macrophages and insulin resistance
Overnutrition leads to a positive energy balance and the accumulation of fat in the adipose tissue, which evokes an immune response that evolutionarily constitutes a physiological attempt to restore homeostasis. Nevertheless, in the long term this response is maladaptive and leads to insulin resistance and the loss of metabolic flexibility.19
Initial evidence for the link between obesity, inflammation and insulin resistance was provided by seminal studies showing increased expression and production of tumor necrosis factor-α (TNF-α) in the adipose tissue of obese rodents and patients.[20], [21] TNF-α was subsequently demonstrated to derive mainly from adipose tissue macrophages (ATMs). These cells represent the predominant immune population in the adipose tissue, accumulate further in obesity and are to a large extent responsible for perpetuating adipose tissue inflammation[22], [23] (Fig. 1).
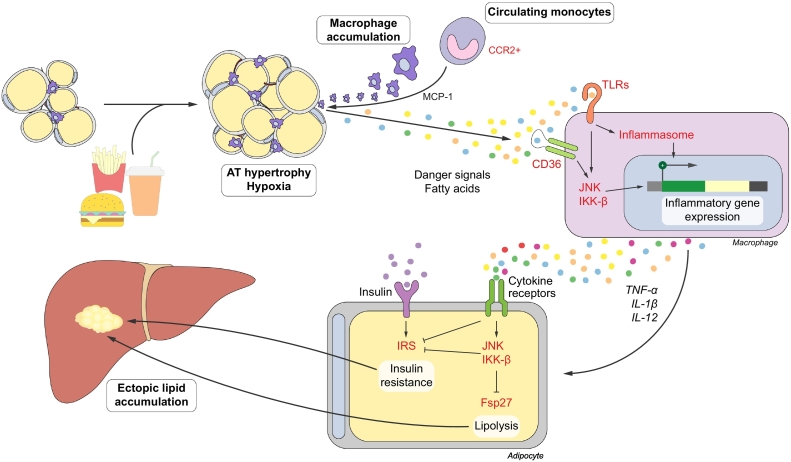
Fig. 1.
Macrophages in obese adipose tissue. In obesity, chemokines stimulate the infiltration of macrophages into the AT. Various danger molecules and fatty acids promote a pro-inflammatory phenotype in ATMs, which contributes to the development of a persistent, low-grade inflammation. Pro-inflammatory mediators secreted by ATMs induce insulin resistance by signalling through the JNK and IKK-β pathways. Inhibition of lipid droplet-associated peptides, such as fsp27, enhance lipolysis. Downstream, insulin resistance and lipolysis lead to ectopic lipid accumulation in various organs, including the liver. AT, adipose tissue; ATMs, adipose tissue macrophages; IRS, insulin receptor substrates; TLRs, Toll-like receptors.
The inflammatory process in obesity is affected through a plethora of cytokines, receptors and signalling molecules. Apart from direct binding of cytokines to their corresponding receptors, as in the case of TNF-α, inflammation is often initiated by activation of pattern recognition receptors, such as Toll-like receptors (TLRs), of which TLR4 is the most well-studied in this context.24 Furthermore, adipose tissue expansion in obesity often exceeds the compensatory increase in oxygen delivery through angiogenesis, causing adipose tissue hypoxia and adipocyte cell death.25 Dying cells release inflammatory cytokines and danger signals, which aggravate inflammation and activate ATMs. These various mediators then converge on inflammatory signalling pathways such as the activation of c-jun N-terminal kinase (JNK) and IκB kinase β (IKK-β). These pathways directly affect insulin signalling in adipocytes by phosphorylating the insulin receptor and insulin receptor substrates, and indirectly by modulating metabolic genes and altering energy homeostasis.[26], [27]
This link between inflammation and metabolism is remarkably conserved between organisms. Eiger, the drosophila TNF-α orthologue, is produced by the specialized fat body and binds on its receptors Wengen and Grindelwald to suppress insulin action through JNK and IKK-β pathways.[28], [29] Notably, knockdown of Grindelwald in the fat body reversed the insulin resistance induced by a high-sugar diet in these flies.28
Multiple macrophage-targeting approaches have underscored their importance in the development of insulin resistance.15 For instance, knocking out JNK, IKK-β or TLR4 in macrophages or hematopoietic cells improved glucose tolerance and adipose tissue inflammation in mice.[24], [30], [31] These approaches simultaneously ameliorated hepatic inflammation and/or liver steatosis, corroborating the role of macrophages and adipose tissue inflammation in the progression of NAFLD. Furthermore, inhibiting ATM infiltration has yielded promising results. Deleting the chemokine macrophage chemoattractant protein-1 (MCP-1), also known as CCL2, strongly reduced insulin resistance,32 and inhibition of its receptor CCR2 in diet-induced obesity improved glucose tolerance as well.[33], [34], [35] Similar results were obtained upon inhibition of other effectors of macrophage recruitment, such as the CCL5-CCR5 axis.[36], [37] Accordingly, ablation of pro-inflammatory CD11c+ macrophages38 or clodronate-mediated depletion of ATMs[39], [40] improved glucose tolerance in obese mice.
Metabolic reprogramming coordinates macrophage polarization
The rapid response of macrophages to infection and inflammation is an energy-intensive process. Macrophages therefore undergo a reprogramming of their own metabolism upon stimulation. This metabolic switch is context-dependent and influences the outcome of the inflammatory response, which also holds true in metabolic disease. The relevance of these metabolic adaptations of macrophages is corroborated by the fact that classical M1 or alternatively activated M2 macrophages show striking differences in their cellular metabolism.
Early studies already indicated that classical M1 macrophages, differentiated in vitro by lipopolysaccharide (LPS) or interferon gamma (IFNγ) stimulation, rely on aerobic glycolysis to satisfy the increased need for ATP and metabolic precursors.41 More recent reports have revealed a much more complex interplay between metabolic programming and polarization. Enhanced flux through the pentose phosphate pathway further secures building blocks for biosynthesis, while mitochondrial oxidative phosphorylation and fatty acid oxidation are repressed in LPS-stimulated macrophages[42], [43], [44] (Fig. 2A). This rewiring is in essence identical to the Warburg effect observed in proliferating tumour cells almost a century ago.45
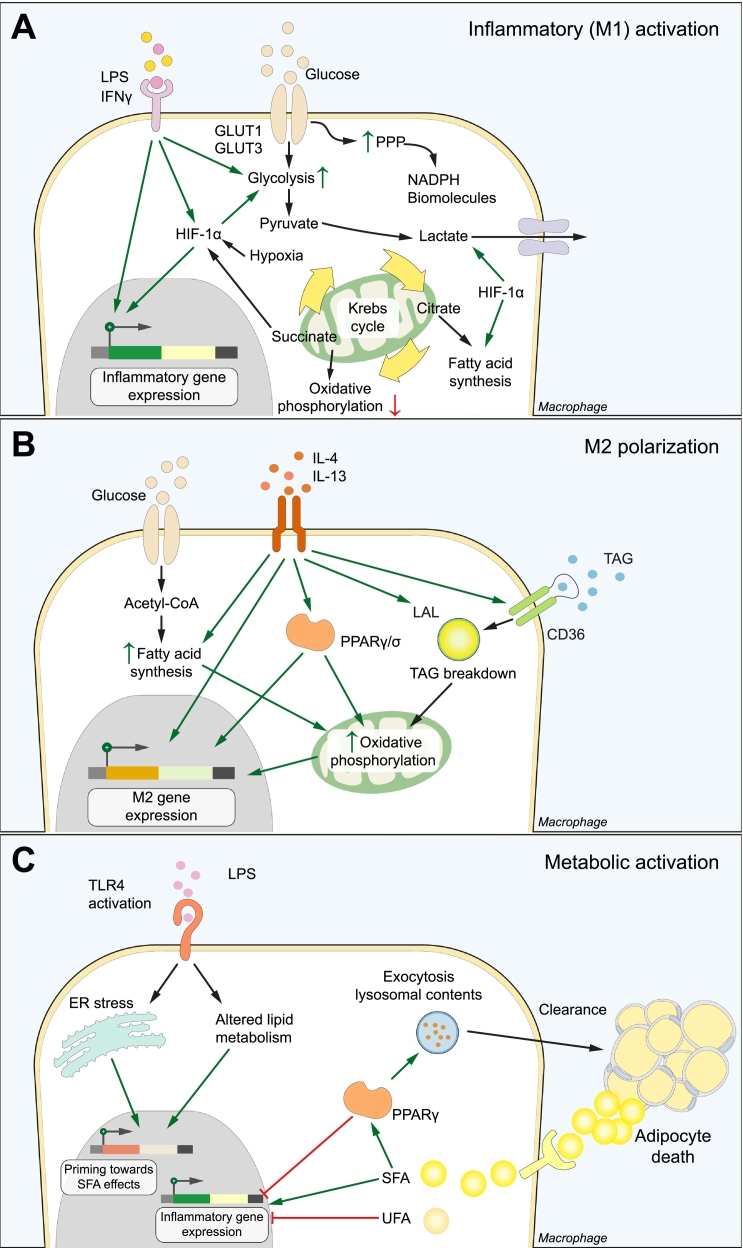
Fig. 2.
Metabolic coordination of macrophage activation. (A) Inflammatory (M1) activation. LPS and/or IFNγ stimulation increases glucose consumption, which is converted to lactate instead of entering the Krebs cycle as pyruvate. The pentose phosphate pathway and fatty acid synthesis are activated to generate biomolecules. These metabolic alterations depend in part on HIF-1α, which stimulates glucose entry and conversion to lactate and directly promotes M1 gene expression. (B) M2 polarization depends on increased β-oxidation and oxidative phosphorylation of fatty acids. This is fuelled by the uptake and lysosomal breakdown of TAGs and the de novo synthesis from glucose. PPARγ and PPARδ bind these intracellular lipids and are key effectors of the metabolic and phenotype switch upon IL-4 stimulation. (C) Macrophages display a metabolically activated phenotype upon stimulation with SFAs. In the adipose tissue, one mechanism of SFA uptake is through the digestion of dead adipocytes at crown-like structures. Binding of LPS primes macrophages towards the pro-inflammatory effects of SFAs, leading to a distinct macrophage phenotype that secretes inflammatory mediators such as IL-1β. PPARγ has a dual role in stimulating the clearance and breakdown of dead adipocytes, and inhibiting the production of inflammatory cytokines. ER, endoplasmic reticulum; LPS, lipopolysaccharide; PPP, pentose phosphate pathway; SFA, saturated fatty acid; TAGs, triacylglycerides; UFA, unsaturated fatty acid.
Hypoxia-inducible factor (HIF)-1α is a master regulator of glycolysis in macrophages. Besides linking hypoxia with macrophage activation, HIF-1α is also activated through hypoxia-independent mechanisms.46 For instance, the metabolic rewiring induced by LPS and/or IFNγ generates elevated levels of the citric acid cycle intermediate succinate. Succinate inhibits the oxygen-sensing prolyl hydroxylases, leading to HIF-1α stabilization and a state of pseudo-hypoxia.[43], [47] Downstream, HIF-1α increases the expression of the glucose transporters GLUT1 and GLUT3, and stimulates the conversion of pyruvate to and subsequent secretion of lactate.[48], [49] The enzyme pyruvate dehydrogenase kinase, the expression of which is also regulated by HIF-1α, channels glucose metabolism away from oxidative phosphorylation towards glycolysis.[42], [50] Notably, inhibition of either glycolysis or HIF-1α signalling suppresses pro-inflammatory gene production and impairs bacterial killing by macrophages, demonstrating the functional importance of metabolic rewiring.[43], [46], [51], [52]
In line with the hypoxia-induced adipose tissue dysfunction during obesity,25 ATM HIF-1α is involved in the development of insulin resistance. Deletion of myeloid HIF-1α in obese mice reduced adipose tissue inflammation and restored glucose tolerance. Paradoxically, macrophage HIF-1α suppressed adipose tissue angiogenesis, which in turn stimulated hypoxia in a vicious circle.53 Furthermore, macrophage HIF-1α was increased in mice and patients with NASH, inducing pro-inflammatory cytokines and aggravating hepatic steatosis and inflammation.54 In contrast, macrophage HIF-2α was reported to ameliorate insulin resistance and adipose tissue inflammation through induction of M2 polarization.55
Alternatively activated M2 macrophages, obtained in vitro after interleukin (IL)-4 stimulation, rely on mitochondrial fatty acid oxidation instead[56], [57] (Fig. 2B). The lysosomal breakdown of exogenous triacylglycerol substrates is the main fatty acid source supporting this metabolic programme, with de novo fatty acid synthesis as a complementary pathway.56 Importantly, inhibition of fatty acid oxidation has been shown to suppress M2 polarization.[56], [57] Moreover, in vitro LPS + IFNγ-stimulated macrophages inhibit fatty acid phosphorylation, which confers resistance to M2 repolarization,44 implying macrophage metabolism at the centre of their functional plasticity. Accordingly, transcription factors that modulate lipid metabolism, such as peroxisome proliferator-activated receptors (PPARs), are key effector molecules regulating this switch.
PPARs, members of the nuclear receptor superfamily, act as fatty acid sensors and modulate lipid transport, storage and expenditure. PPARα is mainly expressed in metabolically active tissues such as the liver, skeletal muscle and brown adipose tissue.58 In addition to their roles in other cells, including adipocytes, PPARγ and PPARβ/δ are expressed in macrophages in a stimulus- and tissue-dependent manner.[58], [59] Although PPARγ but not PPARδ was required for the metabolic switch upon stimulation with IL-4,60 ablation of either isoform has been shown to impair IL-4-stimulated alternative macrophage activation.[60], [61], [62]
These findings are of translational significance in the context of obesity and NAFLD. Both adipose tissue and hepatic macrophages displayed an impaired M2 polarization upon PPARγ or PPARδ deletion in a high-fat diet mouse model, resulting in insulin resistance and hepatic steatosis.[60], [61], [62], [63] Furthermore, the response to pioglitazone and rosiglitazone, PPARγ agonists with well-known therapeutic effects on insulin resistance and NAFLD, was impaired following macrophage PPARγ deletion.[61], [63]
Fatty acids direct adipose tissue macrophage polarization in obesity
Although most data on the interplay between macrophage metabolism and polarization have been obtained in M1 and M2 polarized macrophages, this classical paradigm is too simplistic and cannot fully describe the many distinct phenotypes observed in tissue macrophages.64 Among other stimulants, metabolites can induce unique activation states.16 One exemplar characterized in recent years is the effect of fatty acids on macrophage polarization. The plasticity of macrophages in tissues such as the liver or fat makes it difficult to accurately capture their ontogeny, polarization and function by characteristic markers, although some phenotypic features have been consistently reported in mouse models (Table 1).
Table 1.
Selected markers in mouse macrophage subsets.
| M1 macrophages | M2 macrophages | MMe ATMs | Kupffer cells | Liver MoMFs |
|---|---|---|---|---|
| CD80 | Mannose receptor (CD206) | ABCA1 | F4/80high | CD11bhigh |
| CD11c | CD301 | PLIN2 | CD11bint | F4/80int/high |
| Inducible NO synthase | IL-4 receptor (CD124) | LAMP2 | Tim4+ | Ly6C+/- |
| TNF-α | Arginase 1 | NPC1 | Clec4f+ | CCR2+ |
| S100A8/A9 (calprotectin) | CD68+ | CX3CR1+/- |
Expression of the macrophage polarization markers is context-dependent and these should ideally be used in combination. MMe ATMs: metabolically activated adipose tissue macrophages; MoMFs, monocyte-derived macrophages.
In the healthy adipose tissue, ATMs display an M2-like phenotype and produce anti-inflammatory cytokines such as IL-10. The development of obesity leads to a shift in polarization, which has classically been described as a pro-inflammatory M1 polarization that contributes to metabolic dysregulation.[65], [66] However, an unbiased transcriptomic analysis could not confirm a typical M1 signature in obese adipose tissue.67 Specifically, ATMs from obese mice or humans were shown to display a ‘metabolically activated’ (MMe) phenotype that is distinct from M1 or M2 macrophages (Fig. 2C). This phenotypic switch can be reproduced in vitro using saturated fatty acids (SFAs) such as palmitate.68 Genes involved in lipid metabolism and lysosome biogenesis, such as perilipin-2 (Plin2) and lysosome-associated membrane protein 2 (Lamp2), were found to be significantly upregulated in these macrophages.[67], [68] Glycolysis and oxidative phosphorylation are similarly activated after stimulation with SFAs69, indicating a unique metabolic signature in MMe macrophages.
This metabolic reprogramming of ATMs is closely linked to the increased production of pro-inflammatory cytokines in obesity.[69], [70] SFAs promote the expression of inflammatory cytokines and chemokines such as IL-1β, IL-6 and MCP-1, whereas unsaturated fatty acids counteract these effects.[71], [72], [73] PPARγ promotes lipid metabolism while negatively regulating pro-inflammatory gene expression in MMe macrophages as well,68 suggesting the possibility of uncoupling these key functions as a strategy to reduce insulin resistance.
One source of SFA delivery to macrophages is through digestion of dead adipocytes.74 Because adipocytes are generally much larger than macrophages, classical phagocytosis is not possible. Instead, ATMs form hydrolytic synapses with dead adipocytes, in which they secrete lysosomal contents, mostly at sites known as crown-like structures.74 The resulting uptake of fatty acids has been shown to reproduce the metabolically activated ATM phenotype.70 Interestingly, these data have been validated using a single-cell sequencing approach. Hill et al. were able to distinguish between different ATM populations in high-fat diet-induced obesity. CD9+ ATMs resided within crown-like structures, were lipid-laden and upregulated lysosomal metabolism and inflammatory genes. Conversely, Ly6C+ ATMs were dispersed throughout the adipose tissue and were phenotypically distinct, expressing genes involved in tissue organization and angiogenesis.75
Further evidence for the importance of cellular metabolism in directing macrophage polarization by fatty acids comes from a recent study showing that, contrary to the accepted paradigm, SFAs do not directly activate TLR4. Intriguingly however, TLR4 activation by other signals resulted in an altered intracellular lipid composition, which was necessary for SFA-induced inflammation.76 In the context of obesity, SFAs may thus act as a ‘second hit’ in macrophages primed towards a pro-inflammatory response.
Adipose tissue macrophages fuel hepatic lipid accumulation
Insulin resistance in adipose tissue and the subsequent inability to store the surplus energy as fatty acids lead to ectopic lipid accumulation. Given the impact of ATMs on insulin resistance, it is not surprising that many of the macrophage-targeting approaches to counteract insulin resistance cited in previous sections, such as inhibition of macrophage infiltration or of pro-inflammatory signalling, also ameliorated hepatic lipid accumulation.15 Beside their promotion of insulin resistance, pro-inflammatory macrophages also directly stimulate lipolysis, which is key in increasing lipid flux to the skeletal muscle and liver.77 Mechanistically, pro-inflammatory cytokines secreted by ATMs downregulate lipid droplet-associated peptides, thereby decreasing lipid droplet stability and permitting lipolysis.[78], [79] Indeed, adipose triglyceride lipase inhibition was shown to simultaneously reduce insulin resistance and hepatic steatosis.80 In mice, transplanting visceral adipose tissue from obese mice increased hepatic macrophage accumulation and worsened steatohepatitis, compared with adipose tissue transplanted from lean mice.81 This effect was dependent on CD11c+ inflammatory ATMs that secrete manifold cytokines including chemotactic signals for neutrophils and macrophages.81
Furthermore, inflammation can interfere with beige adipogenesis, a process critical for energy dissipation through thermogenesis and prevention of ectopic lipid accumulation.82 Activated ATMs were recently shown to express α4 integrin, which can bind vascular cell adhesion molecule-1 (VCAM-1) on adipocytes. These adhesive interactions downregulate uncoupling protein-1, a key mediator of adipose tissue thermogenesis, whereas genetic or pharmacological α4 inhibition reduced macrophage retention and improved insulin sensitivity. Adipocyte VCAM-1 induction by ATMs and, conversely, ATM TNF-α induction by VCAM-1 binding constitutes a self-sustaining pro-inflammatory loop.83 The upregulation of VCAM-1 expression in the adipose tissue of patients with NAFLD and fibrosis compared to those without84 suggests this mechanism may contribute to the link between obesity, ATM activation and NAFLD progression.
The importance of ATMs in NAFLD was further corroborated in humans, as both the adipose tissue expression of pro-inflammatory genes as well as the number of ATMs were associated with the progression of NAFLD to NASH and fibrosis.[85], [86]
Hepatic macrophage subsets play distinct roles in metabolism
The liver contains the largest proportion of macrophages in any solid organ.87 These hepatic macrophages are highly heterogeneous with respect to cellular origin, functionality and interaction with other liver cells. Specifically, the resident Kupffer cells (KCs) can be distinguished from bone marrow-derived monocytes, which receive cues from the local micro-environment that can prompt their differentiation into infiltrating macrophages (Table 1). KCs are self-renewing macrophages that line the sinusoidal endothelium and scavenge cellular debris, pathogens and gut-derived products. In the healthy liver, they promote tolerance towards these potential particulate antigens, in part via an expansion of regulatory T-cells.88 KCs originate from yolk-sac derived progenitor cells, which then give rise to archetypical pre-macrophages that colonize the embryo during early organogenesis and activate tissue-specific transcriptional programmes, in this case under the control of inhibitor of DNA binding 3 (ID3).[89], [90]
Under homeostatic conditions, monocyte-derived macrophages are present in relatively low numbers, where they fulfil metabolic functions.87 In reaction to any type of liver injury, however, hepatocytes as well as non-parenchymal cells secrete chemokines, which can induce a massive infiltration of monocytes, which, in mice, express Ly6C. The phenotype of these macrophages is again highly plastic, with classical pro-inflammatory and restorative macrophages at the edges of a continuous spectrum.
Whereas KCs do not derive from monocytes in the steady state, several reports have now shown that upon their experimental depletion, monocytes can differentiate into bona fide KCs (moKCs), repopulating the now available KC niche.[91], [92] Notably, a similar process occurs in experimental NASH, although these moKCS appeared to be rather short-lived after recovery,93 and it is unclear at this point if they are capable of self-renewal.
While these subsets have been difficult to distinguish in vivo, transcriptomic approaches have recently enabled the identification of selective markers. Mouse KCs are characterized by C-type lectin domain family 4 member F (Clec4f) and T-cell immunoglobulin and mucin domain containing 4 (Tim4) surface expression.[92], [93] Infiltrating macrophage marker expression is correlated with their polarization status, with pro-inflammatory monocyte-derived macrophages typically positive for CCR2, while more restorative macrophages upregulate CX3CR1.94 Although these subsets have not been characterized to the same extent in humans, markers such as CD14 and CD68 have been proposed to distinguish these populations, and this remains an area of active investigation.95 For instance, a recent report using single-cell RNA sequencing of healthy human liver cells identified 2 CD68 + macrophage populations, of which one displayed features of an inflammatory phenotype (Lysozyme, S100A8/A9, CD74), while the other population may represent a more regulatory subset (Marco, CD5L, CD163).96
In keeping with their specialized scavenging capacity, KCs perform accessory roles in lipid and iron metabolism.97 Although lipid handling is a function of macrophages in general, the KC expression profile is particularly enriched for these genes compared to other tissue-resident macrophages.92 The liver X receptor α (LXRα), a nuclear receptor critical for lipid metabolism, is differentially expressed by KCs early on in development.90 LXRα seems essential for the maintenance of KC identity, as targeted deletion leads to a selective loss of KCs and their replacement by moKCs.98 In macrophages, LXRα regulates cholesterol efflux and reverse cholesterol transport from peripheral tissues to the liver via high-density lipoprotein (HDL) particles. Moreover, LXRα provides another link between macrophage metabolism and inflammation, as its activation reduces the production of pro-inflammatory mediators.[99], [100] LXRα modulation to stimulate lipid export from vessels is therefore an appealing pharmacological target in cardiovascular and metabolic disease. However, early LXRα agonists simultaneously stimulated fatty acid synthesis, resulting in hepatic steatosis and hypercholesterolemia.101 New LXRα agonists have recently been developed that do not exhibit these side effects. Interestingly, they achieve this in part by selectively activating LXRα in macrophages, including KCs, while having almost no biological effect on hepatocytes, in contrast to the older compounds.102
In addition, the liver plays a major role in whole-body iron metabolism. KCs are well-positioned for this task and express multiple genes involved in the uptake and processing of iron under homeostatic conditions.[92], [103] In circumstances of increased iron delivery however, such as haemolytic anaemia, monocyte-derived macrophages infiltrate the liver and differentiate to express ferroportin-1. These macrophages protect against the deleterious effects of iron deposition in both the liver and other iron-sensitive organs such as the kidney.104 Of note, NALFD and insulin resistance are associated with serum and hepatic iron overload in a subset of patients,105 which has been termed the dysmetabolic iron overload syndrome. Studies have suggested that iron accumulation specifically in macrophages correlates with increased cell death and fibrosis in NAFLD, possibly mediated through oxidative stress,[106], [107] although conflicting data exist.108
Inflammatory macrophages drive non-alcoholic fatty liver disease progression
Although KCs induce tolerogenic responses under homeostatic conditions, they express multiple pattern recognition receptors and are capable of immune stimulation upon infectious or sterile liver injury[88], [95] (Fig. 3). KCs of mice fed a high-fat diet accumulated toxic lipids, resulting in altered expression of genes involved in lipid metabolism and the production of pro-inflammatory mediators,109 similar to the mechanism in ATMs discussed earlier. KC-mediated inflammation is also fuelled by increased binding of bacterial products reaching the liver via the portal vein, secondary to the gut dysbiosis and impaired intestinal barrier observed in obesity.110 In addition, hepatocyte damage due to lipid overload results in the release of danger signals. For example, histidine-rich glycoprotein (HRG), produced by hypoxic hepatocytes,111 induces macrophage M1 polarization, whereas HRG knock-out mice were protected from experimental steatohepatitis.112 The formation of altered lipid products such as malondialdehyde through oxidative stress is another consequence of hepatic fat accumulation which promotes macrophage activation.113
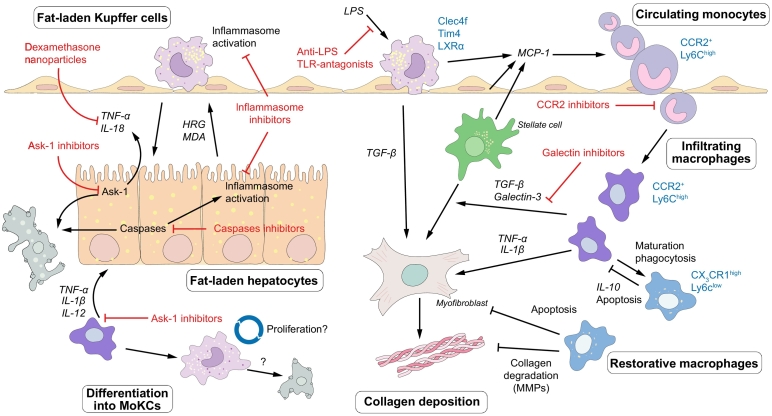
Fig. 3.
Macrophage-mediated inflammation as a therapeutic target in NASH progression. In NASH, KCs are activated by bacterial LPS and by danger signals from fat-laden hepatocytes, leading to the production of pro-inflammatory mediators that promote disease progression. Chemokines induce the infiltration of monocytes, which differentiate into monocyte-derived macrophages. These cells contribute to the progression of NASH by aggravating hepatic steatosis, activating stellate cells and promoting fibrosis. Ly6Chigh macrophages can mature into Ly6Clow restorative macrophages that endorse disease resolution through secretion of anti-inflammatory cytokines and collagen degrading factors. Monocyte-derived macrophages can differentiate into KCs (monocyte-derived KCs, MoKC), although it is unclear at this point if this population is capable of long-term self-renewal or if they are short-lived. Selected drugs that target inflammatory pathways and which are currently under investigation are indicated. HRG, histidine-rich glycoprotein; KC, Kupffer cell; LPS, lipopolysaccharide; MDA, malonyldialdehyde; MMPs, matrix metalloproteinases; MoKC, monocyte-derived Kupffer cell; NASH, non-alcoholic steatohepatitis; TLR, Toll-like receptor.
KC-derived IL-1β was shown to aggravate hepatocyte triglyceride accumulation through downregulation of PPARα, leading to reduced fatty acid oxidation.114 Importantly, KC depletion decreased steatosis and hepatic insulin resistance,[114], [115] providing direct evidence that KCs act as drivers of early NAFLD.13
Bone marrow-derived pro-inflammatory Ly6C+ monocytes infiltrate the murine liver following the initiation of injury, constituting a major pathophysiological mechanism in NASH progression. In analogy to the recruitment to dysfunctional adipose tissue, various studies have identified the MCP-1/CCR2 axis as the main effector of monocyte homing to the liver.[35], [116], [117] MCP-1 is secreted by activated KCs, but also hepatocytes, stellate cells and sinusoidal endothelial cells. The latter respond to inflammation or lipid accumulation by upregulating leukocyte adhesion molecules and facilitating VCAM-1/very late antigen-4 (VLA-4)-dependent monocyte adhesion and migration.118 Nevertheless, other chemokine pathways have been identified that also regulate myeloid cell recruitment, including CCL25/CCR9 and CCL1/CCR8.[119], [120]
The recruitment of infiltrating monocyte-derived macrophages is also observed in human fatty liver disease, especially in patients with more advanced stages of fibrosis, where these cells accumulate mainly in clusters around the portal areas.[121], [122] Indeed, both KCs and inflammatory macrophages are profibrogenic during active NASH. They promote the activation and differentiation of hepatic stellate cells to myofibroblasts by secreting mediators such as transforming growth factor β.[119], [123] Furthermore, macrophage-derived IL-1β and TNF-α promote stellate cell survival.124 Nevertheless, infiltrating monocytes and macrophages can mature and assume a restorative phenotype characterized by reduced Ly6C and increased CX3CR1 surface protein expression (Ly6ClowCX3CR1high) and the expression of matrix degrading metalloproteinases.[94], [125] These fibrolytic macrophages accumulate preferentially upon the cessation of injury by ingestion of apoptotic material, although studies inhibiting autophagy also suggest this mechanism to be relevant to limit scar formation during active fibrogenesis.[125], [126] Accordingly, the timing of macrophage depletion influences the outcome of the fibrotic process, as depletion of fibrolytic macrophages during the recovery phase inhibits the resolution of fibrosis.125
Related to this, the balance between macrophage polarization states is a crucial determinant in the progression of steatohepatitis. Anti-inflammatory macrophages can affect IL-10-driven apoptosis of their M1 counterparts, which has been linked to reduced disease severity in patients with alcoholic and non-alcoholic fatty liver disease.127 Moreover, the beneficial effects of macrophage IL-10 include stimulating lipid catabolism and thereby limiting inflammation in hepatocytes.128 Interestingly, Arginase-2 knock-out mice lacked M2 polarized macrophages and developed spontaneous steatohepatitis, which was again mitigated by KC depletion.129 As discussed, this anti-inflammatory programme is under the transcriptional control of PPARγ and δ,[61], [62] the former of which was notably activated in fibrolytic Ly6Clow macrophages as well.125
NAFLD and atherosclerosis: a putative role for macrophages in cardiovascular risk elevation
A large body of evidence now indicates that NAFLD increases the risk for fatal and non-fatal cardiovascular events. Moreover, NAFLD correlates with markers of (subclinical) atherosclerosis such as endothelial dysfunction, coronary arterial calcifications and carotid artery intima-media thickness (reviewed in 12). However, the underlying pathophysiological mechanisms have not been fully elucidated, despite a number of potential pathways being put forward.130 Macrophage-mediated inflammation is one plausible overarching mechanism, given the crucial role of macrophages in NAFLD progression as well as in atherosclerosis development.
Foam cells had been observed over a century ago by Virchow, and were later identified as macrophages that had accumulated cholesterol esters.131 Classical scavenger receptors that promote lipid uptake, such as macrophage scavenger receptor-1 (Msr1) and CD36, are implicated in macrophage proliferation, inflammatory gene expression and cell death in atherosclerosis,132 indicating a profound influence of lipid handling on macrophage behaviour. Interestingly, haematopoietic deletion of Msr1 and/or CD36 has also been shown to reduce the severity of inflammation and fibrosis in experimental NASH, in part through alterations in the intracellular fat distribution in foamy macrophages.[133], [134]
Related to this, plaque and hepatic macrophages are regulators of whole-body cholesterol flux. The efflux of macrophage cholesterol to HDL particles, mediated by LXRα, rather than the absolute HDL concentration, inversely correlates with the incidence of cardiovascular events in patients.135 This efflux is counteracted by the enzyme cholesterol ester transfer protein (CETP), which regulates the transport of cholesterol from HDL to atherogenic very-low-density and low-density lipoproteins. Circulating CETP derives mainly from KCs, and depletion of KCs has been shown to ameliorate the lipid profile in mice fed a western diet.136
The risk of cardiovascular disease correlates with the severity of the systemic inflammation that is characteristic of metabolic disease.132 In support of this concept, studies have demonstrated an increased cardiovascular risk across a range of chronic inflammatory disorders such as rheumatoid arthritis.137 NAFLD, and especially NASH, are associated with elevated circulating levels of inflammatory markers secreted by activated KCs or monocyte-derived macrophages and might therefore aggravate metabolic inflammation. This has been confirmed in a number of human studies, although the results for individual cytokines are sometimes discordant. For example, IL-6 serum levels have been reported as elevated in NAFLD138 or selectively in patients with NASH,139 whereas this was not confirmed in another report.86 Multiple studies have suggested elevations of TNF-α and IL-8 in NAFLD.[86], [138], [140] Similar increases have been reported for the adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and VCAM-1, which mediate leukocyte infiltration and serve as markers of endothelial dysfunction.[84], [141] Moreover, the macrophage marker soluble CD163 correlated with the severity of histological fatty liver disease in 2 independent cohorts and was predictive of liver fibrosis.142
Taken together, these data suggest that NAFLD contributes to a pro-atherogenic environment. These data also highlight a shared interaction between lipid metabolism and macrophage activation in NAFLD and atherosclerosis. Nevertheless, it is inevitably difficult to establish the causality of elevated inflammatory markers on the incidence of cardiovascular events, and to assess the precise hepatic contribution to systemic cytokine levels in metabolic diseases.
Macrophages and innate immunity are therapeutic targets in NAFLD
Macrophages, and innate immune responses in general, represent attractive therapeutic targets for the treatment of fatty liver disease, given their role as drivers of hepatic steatosis, inflammation and fibrosis, and their contribution to the metabolic syndrome. Several of the pathways involved can be targeted by novel or repurposed drugs, and various compounds have indeed advanced to clinical evaluation (Table 2; Fig. 3).143
Table 2.
Selected compounds under investigation for NAFLD/NASH, acting on macrophages and innate immunity components.
| Compound | Molecular target | Study population | Primary outcome | Trial number | Trial status/results |
|---|---|---|---|---|---|
| Phase III | |||||
| Cenicriviroc (AURORA) | CCR2/5 inhibitor | Histological NASH with stage F2-F3 fibrosis | Improvement in fibrosis without worsening NASH | NCT03028740 | Recruiting |
| Selonsertib (STELLAR-3) | ASK-1 inhibitor | Histological NASH with stage F3 fibrosis | Improvement in fibrosis without worsening NASH | NCT03053050 | Active, not recruiting |
| Selonsertib (STELLAR-4) | ASK-1 inhibitor | Histological NASH cirrhosis | Improvement in fibrosis without worsening NASH | NCT03053063 | Active, not recruiting |
| Phase II | |||||
| Emricasan (Encore-NF) | Caspase inhibitor | Histological NASH with stage F1-F3 fibrosis | Improvement in fibrosis without worsening NASH | NCT02686762 | Active, not recruiting |
| Emricasan (Encore-LF) | Caspase inhibitor | NASH cirrhosis + history of variceal bleeding and/or moderate/severe ascites | Event-free survival | NCT03205345 | Active, not recruiting |
| Emricasan (Encore-PH) | Caspase inhibitor | NASH cirrhosis with HVPG > 12 mm | Change in HVPG | NCT02960204 | Active, not recruiting |
| GR-MD-02 (NASH-CX) | Galectin-3 inhibitor | Compensated NASH cirrhosis with HVPG > 6 mm | Improvement in HVPG | NCT02462967 | Completed |
| IMM-124E | Polyclonal anti-LPS | Histological NASH | Improvement in liver fat content on MRI | NCT02316717 | No significant changes in liver fat content, decreased serum ALT and LPS |
| JKB-121 | TLR4 antagonist | Histological NASH | Improvement in liver fat content on MRI and/or serum ALT | NCT02442687 | No significant differences compared to placebo |
| SGM-1019 | Inflammasome inhibitor | Histological NASH with stage F1-F3 fibrosis | Safety and tolerability | NCT03676231 | Recruiting |
| Phase II trials with drug combinations | |||||
| Cenicriviroc + Tropifexor (TANDEM) | CCR2/5 inhibitor + FXR agonist | Histological NASH with stage F2-F3 fibrosis | Safety; improvement in fibrosis | NCT03517540 | Recruiting |
| Selonsertib + GS-0976 + GS-9674 (ATLAS) | ASK-1 inhibitor + ACC inhibitor + FXR agonist | Histological NASH with stage F3-F4 fibrosis or Fibroscan stiffness value ≥14.5 kPa or ELF™ score ≥ 9.8 | Safety; improvement in fibrosis without worsening NASH | NCT03449446 | Active, not recruiting |
ALT, alanine aminotransferase; ELF, Enhanced Liver Fibrosis; HVPG, hepatic venous pressure gradient; LPS, lipopolysaccharide; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Inhibiting the chemokine-regulated infiltration of blood monocytes is one therapeutic strategy supported by preclinical studies. For instance, pharmacological inhibition of MCP-1 with the RNA aptamer mNOX-E36 attenuated murine steatohepatitis.116 Regarding this mode of action, the dual chemokine receptor CCR2/CCR5 inhibitor cenicriviroc has been studied in most depth so far. Cenicriviroc was able to reduce hepatic monocyte infiltration and fibrosis in mouse steatohepatitis models.[122], [144] Results from the CENTAUR phase II trial showed that although cenicriviroc did not lead to resolution of NASH, it doubled the percentage of patients in whom fibrosis was improved by at least 1 stage after 1 year, compared with placebo.145 Based on these results, a phase III trial is currently underway. Other chemokine-axis inhibitors are under development for the treatment of diabetes or related disorders, and trials evaluating these compounds in NAFLD are eagerly awaited.95
Macrophages, especially KCs, readily phagocytose circulating nanoparticles such as liposomes. Liposomal delivery of dexamethasone, a derivative of corticosterone, reduced experimental liver fibrosis. The tailored delivery of nanoparticles to macrophages is therefore an interesting possibility, although practical issues such as the most effective drug delivery systems, active compounds and dosing regimen have not been established.146
Galectin-3 is a protein secreted by macrophages which stimulates fibrogenesis in the lung, kidney and liver.147 It functions as a mitogen for fibroblasts and stimulates the formation of lipid oxidation products which serve as danger molecules.[147], [148] Galectin-3 ablation attenuated experimental steatohepatitis,148 and inhibitors are being tested in clinical trials for their potential to reduce fibrosis and its complications.
Macrophage activation entails the activation of intracellular effector pathways such as the inflammasome, which regulates the production of IL-1β and IL-18 and contributes to insulin resistance.149 A clinical trial including over 10,000 patients found that targeting subclinical inflammation with the IL-1β antagonist canakinumab reduced the number of cardiovascular events in secondary prevention.150 This clinical benefit correlated strongly with the magnitude of initial reduction in C-reactive protein levels. However, despite these anti-inflammatory effects, there was no reduction in incident diabetes.151
Inflammasome activation is also involved in chronic liver disease, forming the rationale for the clinical evaluation of the inflammasome inhibitor SGM-1019 in NAFLD. Caspase inhibitors are of particular interest as they could prevent both hepatocyte cell death and caspase 1-mediated inflammasome activation.152 The pan-caspase inhibitor emricasan decreased serum alanine aminotransferase and apoptotic fragments after 28 days in an exploratory trial in patients with NAFLD,153 and it is currently being investigated in multiple phase II trials. Similarly, inhibition of apoptosis signal-regulating kinase 1 (ASK1), an upstream mediator of inflammation in both hepatocytes and macrophages, is a promising approach. Selonsertib, a small molecule ASK1 inhibitor improved liver fibrosis but not histological inflammation or ballooning in a 24-week proof-of-concept study,154 and is now being evaluated in 2 phase III trials, enrolling patients with NASH and advanced fibrosis or cirrhosis. Given the shared activation of these inflammatory pathways in various macrophage subsets in the liver and adipose tissue, these drugs presumably have pleiotropic effects in various stages of the pathogenesis of metabolic disease.
Of note, many of the drugs currently undergoing clinical evaluation for the treatment of NASH have direct and/or indirect effects on macrophages and innate immune responses, such as farnesoid X receptor or PPAR agonists, that not only impact hepatocyte metabolism but also inflammatory processes.143 It is currently unclear, yet a subject of intense investigation, to what extent combining different modes of action would act synergistically to improve metabolic disorders and halt NAFLD progression.
Conclusion
Obesity and metabolic diseases can be characterized as low-grade inflammatory disorders, in which pro-inflammatory molecules can function as metabolic hormones and impact on insulin signalling. Macrophages integrate metabolic cues to adapt their phenotype to the local environment, which is especially relevant in tissues sensitive to nutrient changes, such as adipose tissue and the liver. Indeed, fatty acids induce a unique polarization status in macrophages, which contribute to ectopic lipid accumulation as well as local and systemic inflammation. KCs and monocyte-derived macrophages represent ontologically distinct phagocyte populations in the liver that perform a range of metabolic functions in homeostasis and disease. Hepatic macrophages are central players in the progression of NAFLD and may link this condition with cardiovascular disease.
Thus, macrophage targeting holds promise as a therapeutic strategy in metabolic disease. Recent advances in this field, as described in this review, might guide the development of new treatment options to counter these increasingly prevalent diseases. Various approaches, such as inhibiting monocyte infiltration or influencing their polarization, are already advancing from preclinical to clinical trials. Studies combining innovative approaches, such as single-cell sequencing and mouse lines targeting specific macrophage subsets, will further help unravel the complex pathophysiology of NAFLD and the roles of macrophages therein.
Financial support
SL is supported by the Research Foundation – Flanders (fellowship 11W4716N and grant for study abroad V426818N). FT is supported by the German Research Foundation (DFG; Ta434/3-1, Ta434/5-1 and SFB/TRR57).
Conflict of interest
SL has no disclosures to report. FT’s laboratory has received funding from Allergan, Galapagos, Inventiva and Bristol Myers Squibb.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We cordially thank all members of our labs and collaborating scientists for helpful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2019.02.004.
Supplementary data
Supplementary material
References
- 1.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 6.Ritz T, Krenkel O, Tacke F. Dynamic plasticity of macrophage functions in diseased liver. Cell Immunol. 2018;330:175–182. doi: 10.1016/j.cellimm.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 8.Jinjuvadia R, Antaki F, Lohia P, Liangpunsakul S. The association between nonalcoholic fatty liver disease and metabolic abnormalities in the United States population. J Clin Gastroenterol. 2017;51:160–166. doi: 10.1097/MCG.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 10.Masarone M, Rosato V, Aglitti A, Bucci T, Caruso R, Salvatore T. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Devisscher L, Verhelst X, Colle I, Van Vlierberghe H, Geerts A. The role of macrophages in obesity-driven chronic liver disease. J Leukoc Biol. 2016;99:693–698. doi: 10.1189/jlb.5RU0116-016R. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 15.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krenkel O, Tacke F. Macrophages in Nonalcoholic Fatty Liver Disease: A Role Model of Pathogenic Immunometabolism. Semin Liver Dis. 2017;37:189–197. doi: 10.1055/s-0037-1604480. [DOI] [PubMed] [Google Scholar]
- 18.Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 2017;26:142–156. doi: 10.1016/j.cmet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 22.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefere S, Van Steenkiste C, Verhelst X, Van Vlierberghe H, Devisscher L, Geerts A. Hypoxia-regulated mechanisms in the pathogenesis of obesity and non-alcoholic fatty liver disease. Cell Mol Life Sci. 2016;73:3419–3431. doi: 10.1007/s00018-016-2222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 27.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal N, Delanoue R, Mauri A, Basco D, Pasco M, Thorens B. The Drosophila TNF Eiger is an adipokine that acts on insulin-producing cells to mediate nutrient response. Cell Metab. 2016;23:675–684. doi: 10.1016/j.cmet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 29.DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A. 2009;106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 31.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan TJ, Miao Z, Zhao BN, Ertl LS, Wang Y, Krasinski A. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metab Clin Exp. 2013;62:1623–1632. doi: 10.1016/j.metabol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17:219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 35.Parker R, Weston CJ, Miao Z, Corbett C, Armstrong MJ, Ertl L. CC chemokine receptor 2 promotes recruitment of myeloid cells associated with insulin resistance in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2018;314:G483–G493. doi: 10.1152/ajpgi.00213.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keophiphath M, Rouault C, Divoux A, Clement K, Lacasa D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:39–45. doi: 10.1161/ATVBAHA.109.197442. [DOI] [PubMed] [Google Scholar]
- 37.Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–1690. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bu L, Gao M, Qu S, Liu D. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. AAPS J. 2013;15:1001–1011. doi: 10.1208/s12248-013-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newsholme P, Gordon S, Newsholme EA. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J. 1987;242:631–636. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 50.Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M. HIF-1alpha-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7:11635. doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Lu Y, Martinez J, Bi Y, Lian G, Wang T. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1alpha-dependent. Proc Natl Acad Sci U S A. 2016;113:1564–1569. doi: 10.1073/pnas.1518000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S. HIF-1alpha in Myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes. 2016;65:3649–3659. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Ribeiro M, Iracheta-Vellve A, Lowe P, Ambade A, Satishchandran A. Macrophage-specific HIF-1alpha contributes to impaired autophagic flux in non-alcoholic steatohepatitis. Hepatology. 2019;69(2):545–563. doi: 10.1002/hep.30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB. Macrophage HIF-2alpha ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63:3359–3371. doi: 10.2337/db13-1965. [DOI] [PubMed] [Google Scholar]
- 56.Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuchs CD, Traussnigg SA, Trauner M. Nuclear Receptor Modulation for the Treatment of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2016;36:69–86. doi: 10.1055/s-0036-1571296. [DOI] [PubMed] [Google Scholar]
- 59.Gautier EL, Chow A, Spanbroek R, Marcelin G, Greter M, Jakubzick C. Systemic analysis of PPARgamma in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J Immunol. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Catrysse L, van Loo G. Adipose tissue macrophages and their polarization in health and obesity. Cell Immunol. 2018;330:114–119. doi: 10.1016/j.cellimm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–953. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20:3149–3161. doi: 10.1016/j.celrep.2017.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan KL, Pillon NJ, Sivaloganathan DM, Costford SR, Liu Z, Theret M. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated Protein Kinase (AMPK) J Biol Chem. 2015;290:16979–16988. doi: 10.1074/jbc.M115.646992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pardo V, Gonzalez-Rodriguez A, Guijas C, Balsinde J, Valverde AM. Opposite cross-talk by oleate and palmitate on insulin signaling in hepatocytes through macrophage activation. J Biol Chem. 2015;290:11663–11677. doi: 10.1074/jbc.M115.649483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robblee MM, Kim CC, Porter Abate J, Valdearcos M, Sandlund KL, Shenoy MK. Saturated fatty acids engage an IRE1alpha-dependent pathway to activate the NLRP3 inflammasome in myeloid cells. Cell Rep. 2016;14:2611–2623. doi: 10.1016/j.celrep.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res. 2016;57:980–992. doi: 10.1194/jlr.M064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced Inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27:1096–1110. doi: 10.1016/j.cmet.2018.03.014. e5. [DOI] [PubMed] [Google Scholar]
- 77.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014;27:63–93. doi: 10.1017/S095442241400002X. [DOI] [PubMed] [Google Scholar]
- 79.Ranjit S, Boutet E, Gandhi P, Prot M, Tamori Y, Chawla A. Regulation of fat specific protein 27 by isoproterenol and TNF-alpha to control lipolysis in murine adipocytes. J Lipid Res. 2011;52:221–236. doi: 10.1194/jlr.M008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schweiger M, Romauch M, Schreiber R, Grabner GF, Hutter S, Kotzbeck P. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun. 2017;8:14859. doi: 10.1038/ncomms14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bijnen M, Josefs T, Cuijpers I, Maalsen CJ, van de Gaar J, Vroomen M. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut. 2018;67:1317–1327. doi: 10.1136/gutjnl-2016-313654. [DOI] [PubMed] [Google Scholar]
- 82.Villarroya F, Cereijo R, Villarroya J, Gavalda-Navarro A, Giralt M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 2018;27:954–961. doi: 10.1016/j.cmet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 2017;18:654–664. doi: 10.1038/ni.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lefere S, Van de Velde F, Devisscher L, Bekaert M, Raevens S, Verhelst X. Serum vascular cell adhesion molecule-1 predicts significant liver fibrosis in non-alcoholic fatty liver disease. Int J Obes (Lond) 2017;41:1207–1213. doi: 10.1038/ijo.2017.102. [DOI] [PubMed] [Google Scholar]
- 85.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 86.du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology. 2015;149:635–648. doi: 10.1053/j.gastro.2015.05.044. e14. [DOI] [PubMed] [Google Scholar]
- 87.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 88.Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62:279–291. doi: 10.1002/hep.27793. [DOI] [PubMed] [Google Scholar]
- 89.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353 doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beattie L, Sawtell A, Mann J, Frame TCM, Teal B, de Labastida Rivera F. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol. 2016;65:758–768. doi: 10.1016/j.jhep.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Devisscher L, Scott CL, Lefere S, Raevens S, Bogaerts E, Paridaens A. Non-alcoholic steatohepatitis induces transient changes within the liver macrophage pool. Cell Immunol. 2017;322:74–83. doi: 10.1016/j.cellimm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 94.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2 + monocytes at a site of sterile injury. J Exp Med. 2015;212:447–456. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 96.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scott CL, Guilliams M. The role of Kupffer cells in hepatic iron and lipid metabolism. J Hepatol. 2018;69(5):1197–1199. doi: 10.1016/j.jhep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott CL, T'Jonck W, Martens L, Todorov H, Sichien D, Soen B. The Transcription Factor ZEB2 Is Required to Maintain the Tissue-Specific Identities of Macrophages. Immunity. 2018;49:312–325. doi: 10.1016/j.immuni.2018.07.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 100.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muse ED, Yu S, Edillor CR, Tao J, Spann NJ, Troutman TD. Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc Natl Acad Sci U S A. 2018;115:E4680–E4689. doi: 10.1073/pnas.1714518115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nairz M, Theurl I, Swirski FK, Weiss G. "Pumping iron"-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch Eur J Physiol. 2017;469:397–418. doi: 10.1007/s00424-017-1944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945–951. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Britton LJ, Subramaniam VN, Crawford DH. Iron and non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8112–8122. doi: 10.3748/wjg.v22.i36.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maliken BD, Nelson JE, Klintworth HM, Beauchamp M, Yeh MM, Kowdley KV. Hepatic reticuloendothelial system cell iron deposition is associated with increased apoptosis in nonalcoholic fatty liver disease. Hepatology. 2013;57:1806–1813. doi: 10.1002/hep.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 109.Leroux A, Ferrere G, Godie V, Cailleux F, Renoud ML, Gaudin F. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57:141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 110.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 111.Morello E, Sutti S, Foglia B, Novo E, Cannito S, Bocca C. Hypoxia-inducible factor 2alpha drives nonalcoholic fatty liver progression by triggering hepatocyte release of histidine-rich glycoprotein. Hepatology. 2018;67:2196–2214. doi: 10.1002/hep.29754. [DOI] [PubMed] [Google Scholar]
- 112.Bartneck M, Fech V, Ehling J, Govaere O, Warzecha KT, Hittatiya K. Histidine-rich glycoprotein promotes macrophage activation and inflammation in chronic liver disease. Hepatology. 2016;63:1310–1324. doi: 10.1002/hep.28418. [DOI] [PubMed] [Google Scholar]
- 113.Busch CJ, Hendrikx T, Weismann D, Jackel S, Walenbergh SM, Rendeiro AF. Malondialdehyde epitopes are sterile mediators of hepatic inflammation in hypercholesterolemic mice. Hepatology. 2017;65:1181–1195. doi: 10.1002/hep.28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 115.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 117.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miyachi Y, Tsuchiya K, Komiya C, Shiba K, Shimazu N, Yamaguchi S. Roles for cell-cell adhesion and contact in obesity-induced hepatic myeloid cell accumulation and glucose intolerance. Cell Rep. 2017;18:2766–2779. doi: 10.1016/j.celrep.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 119.Chu PS, Nakamoto N, Ebinuma H, Usui S, Saeki K, Matsumoto A. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. Hepatology. 2013;58:337–350. doi: 10.1002/hep.26351. [DOI] [PubMed] [Google Scholar]
- 120.Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Russeler V. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55:898–909. doi: 10.1002/hep.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 122.Krenkel O, Puengel T, Govaere O. Abdallah adipose tissue, Mossanen JC, Kohlhepp M, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 123.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C. Hepatic recruitment of the inflammatory Gr1 + monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 124.Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lodder J, Denaes T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 128.Han YH, Kim HJ, Na H, Nam MW, Kim JY, Kim JS. RORalpha Induces KLF4-Mediated M2 Polarization in the Liver Macrophages that Protect against Nonalcoholic Steatohepatitis. Cell Rep. 2017;20:124–135. doi: 10.1016/j.celrep.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 129.Navarro LA, Wree A, Povero D, Berk MP, Eguchi A, Ghosh S. Arginase 2 deficiency results in spontaneous steatohepatitis: a novel link between innate immune activation and hepatic de novo lipogenesis. J Hepatol. 2015;62:412–420. doi: 10.1016/j.jhep.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 131.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bieghs V, Verheyen F, van Gorp PJ, Hendrikx T, Wouters K, Lutjohann D. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, Binder CJ. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology. 2010;138:2477–2486. doi: 10.1053/j.gastro.2010.02.051. 86 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, van der Tuin S, Tjeerdema N, van Dam AD, Rensen SS, Hendrikx T. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology. 2015;62:1710–1722. doi: 10.1002/hep.27985. [DOI] [PubMed] [Google Scholar]
- 137.Kerekes G, Nurmohamed MT, Gonzalez-Gay MA, Seres I, Paragh G, Kardos Z. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol. 2014;10:691–696. doi: 10.1038/nrrheum.2014.121. [DOI] [PubMed] [Google Scholar]
- 138.Coulon S, Francque S, Colle I, Verrijken A, Blomme B, Heindryckx F. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine. 2012;59:442–449. doi: 10.1016/j.cyto.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 139.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 140.Jamali R, Arj A, Razavizade M, Aarabi MH. Prediction of Nonalcoholic Fatty Liver Disease Via a Novel Panel of Serum Adipokines. Medicine. 2016;95 doi: 10.1097/MD.0000000000002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sookoian S, Castano GO, Burgueno AL, Rosselli MS, Gianotti TF, Mallardi P. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis. 2010;209:585–591. doi: 10.1016/j.atherosclerosis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 142.Kazankov K, Barrera F, Moller HJ, Rosso C, Bugianesi E, David E. The macrophage activation marker sCD163 is associated with morphological disease stages in patients with non-alcoholic fatty liver disease. Liver Int. 2016;36:1549–1557. doi: 10.1111/liv.13150. [DOI] [PubMed] [Google Scholar]
- 143.Tacke F, Weiskirchen R. An update on the recent advances in antifibrotic therapy. Expert Rev Gastroenterol Hepatol. 2018:1–10. doi: 10.1080/17474124.2018.1530110. [DOI] [PubMed] [Google Scholar]
- 144.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F. Antifibrotic effects of the dual CCR2/CCR5 antagonist Cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bartneck M, Warzecha KT, Tacke F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobi Surg Nutr. 2014;3:364–376. doi: 10.3978/j.issn.2304-3881.2014.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 149.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 151.Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J Am Coll Cardiol. 2018;71:2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 152.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 153.Shiffman M, Freilich B, Vuppalanchi R, Watt K, Chan JL, Spada A. Randomised clinical trial: emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49:64–73. doi: 10.1111/apt.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2017 doi: 10.1002/hep.29514. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material