This randomized clinical trial evaluates the efficacy and safety of 2 doses of a pharmaceutical formulation of cannabidiol vs placebo for adjunctive treatment of convulsive seizures in patients with Darvet syndrome.
Key Points
Question
Is adjunctive cannabidiol at doses of 10 and 20 mg/kg/d superior to placebo in reducing convulsive seizure frequency in patients with Dravet syndrome?
Findings
This double-blind clinical trial randomized 199 children with Dravet syndrome to cannabidiol (10 or 20 mg/kg/d) or matched placebo for 14 weeks. Convulsive seizure frequency compared with baseline was reduced by 48.7% in the 10-mg/kg/d cannabidiol group and 45.7% in the 20-mg/kg/d cannabidiol group vs 26.9% in the placebo group.
Meaning
Both doses of adjunctive cannabidiol were similarly efficacious in reducing convulsive seizures associated with Dravet syndrome.
Abstract
Importance
Clinical evidence supports effectiveness of cannabidiol for treatment-resistant seizures in Dravet syndrome, but this trial is the first to evaluate the 10-mg/kg/d dose.
Objective
To evaluate the efficacy and safety of a pharmaceutical formulation of cannabidiol, 10 and 20 mg/kg/d, vs placebo for adjunctive treatment of convulsive seizures in patients with Dravet syndrome.
Design, Setting, and Participants
This double-blind, placebo-controlled, randomized clinical trial (GWPCARE2) recruited patients from April 13, 2015, to November 10, 2017, with follow-up completed on April 9, 2018. Of 285 patients screened from 38 centers in the United States, Spain, Poland, the Netherlands, Australia, and Israel, 86 were excluded, and 199 were randomized. Patients were aged 2 to 18 years with a confirmed diagnosis of Dravet syndrome and at least 4 convulsive seizures during the 4-week baseline period while receiving at least 1 antiepileptic drug. Data were analyzed from November 16 (date of unblinding) to December 13 (date of final outputs), 2018, based on intention to treat and per protocol.
Interventions
Patients received cannabidiol oral solution at a dose of 10 or 20 mg/kg per day (CBD10 and CBD20 groups, respectively) or matched placebo in 2 equally divided doses for 14 weeks. All patients, caregivers, investigators, and individuals assessing data were blinded to group assignment.
Main Outcomes and Measures
The primary outcome was change from baseline in convulsive seizure frequency during the treatment period. Secondary outcomes included change in all seizure frequency, proportion with at least a 50% reduction in convulsive seizure activity, and change in Caregiver Global Impression of Change score.
Results
Of 198 eligible patients (mean [SD] age, 9.3 [4.4] years; 104 female [52.5%]), 66 were randomized to the CBD10 group, 67 to the CBD20 group, and 65 to the placebo group, and 190 completed treatment. The percentage reduction from baseline in convulsive seizure frequency was 48.7% for CBD10 group and 45.7% for the CBD20 group vs 26.9% for the placebo group; the percentage reduction from placebo was 29.8% (95% CI, 8.4%-46.2%; P = .01) for CBD10 group and 25.7% (95% CI, 2.9%-43.2%; P = .03) for the CBD20 group. The most common adverse events were decreased appetite, diarrhea, somnolence, pyrexia, and fatigue. Five patients in the CBD20 group discontinued owing to adverse events. Elevated liver transaminase levels occurred more frequently in the CBD20 (n = 13) than the CBD10 (n = 3) group, with all affected patients given concomitant valproate sodium.
Conclusions and Relevance
Adjunctive cannabidiol at doses of 10 and 20 mg/kg/d led to similar clinically relevant reductions in convulsive seizure frequency with a better safety and tolerability profile for the 10-mg/kg/d dose in children with treatment-resistant Dravet syndrome. Dose increases of cannabidiol to greater than 10 mg/kg/d should be tailored to individual efficacy and safety.
Trial Registration
ClinicalTrials.gov Identifier: NCT02224703
Introduction
Dravet syndrome is a rare treatment-resistant developmental and epileptic encephalopathy1,2 caused by pathogenic variants in SCN1A (OMIM 182389),3 the gene encoding the sodium channel α subunit 1 in approximately 80% of diagnosed patients.4,5 An estimated 1 in 15 500 children have SCN1A-related Dravet syndrome.6 Onset generally occurs by 15 months of age in infants with normal development5,7 and typically begins with febrile hemiclonic or generalized status epilepticus often triggered by fever.8 Multiple seizure types evolve, including hemiclonic, tonic-clonic, focal impaired awareness, absence, myoclonic, and rarely atonic seizures.1,9 Development is normal in the first year of life but then slows, leading to intellectual disability of variable severity.10 Patients with Dravet syndrome have a high mortality risk due to sudden unexpected death in epilepsy and status epilepticus.11,12 Worse seizure control is associated with more comorbidities and lower quality of life.13 Despite treatment with multiple antiepileptic drugs (AEDs), patients with Dravet syndrome often remain treatment resistant.8,14,15
Highly purified cannabidiol (approved as Epidiolex in the United States and Epidyolex in the European Union) was the first medicine to receive approval in the United States for treating seizures in Dravet syndrome.16 Valproate sodium and clobazam are commonly used to manage seizures; however, neither is approved for Dravet syndrome.17 Stiripentol is approved in Europe for patients with Dravet syndrome taking clobazam and valproate and has recently been approved in the United States for patients with Dravet syndrome taking clobazam.18
In previous randomized clinical trials, cannabidiol demonstrated efficacy with an acceptable safety profile as add-on antiepileptic treatment in patients with Dravet syndrome at a dose of 20 mg/kg/d19 and in patients with Lennox-Gastaut syndrome at doses of 10 and 20 mg/kg/d.20,21 Although doses as high as 20 mg/kg/d are approved for Dravet and Lennox-Gastaut syndromes, the US prescribing information indicates 10 mg/kg/d as the recommended maintenance dose,16 although data on this dose were unavailable for Dravet syndrome. This trial is, to our knowledge, the first to evaluate the efficacy and safety of 2 doses of cannabidiol (10 and 20 mg/kg/d) in children with Dravet syndrome.
Methods
Trial Design
This multicenter, double-blind, randomized, placebo-controlled, parallel-group trial involved 43 clinical centers, 38 of which enrolled patients (in the United States [n = 23], Spain [n = 7], Poland [n = 3], the Netherlands [n = 2], Australia [n = 2], and Israel [n = 1]). Patients were recruited from April 13, 2015, to November 10, 2017, and were followed for up to 20 weeks after randomization. Follow-up was completed on April 9, 2018. The trial consisted of a 4-week baseline period, 14-week treatment period (2-week dose escalation [titration], followed by 12 weeks of stable dosing [maintenance]), a taper period of as long as 10 days, and a 4-week safety follow-up period) (Figure 1). The trial protocol was approved by each center’s institutional review board or independent ethics committee and was conducted in accordance with the principles of the Declaration of Helsinki22 and the International Conference on Harmonization Tripartite Guideline on Good Clinical Practice. The original trial protocol, deviations, amendments, and reason for changes to the protocol are provided in Supplement 1. All parents or legal guardians provided written informed consent. Assent was obtained where possible from adolescents. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
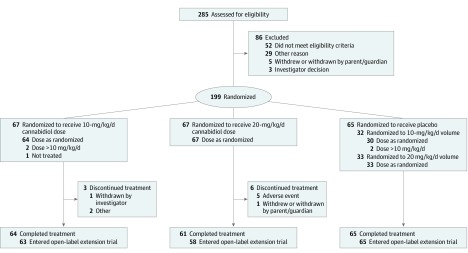
Figure 1. Flow of Patients Through the GWPCARE2 Trial.
Patients in the placebo group were pooled for a combined total of 65 patients; 32 were assigned to receive a volume equivalent to the 10-mg/kg/d cannabidiol dose and 33 were assigned to receive a volume equivalent to the 20-mg/kg/d cannabidiol dose. Among the 86 patients excluded, 3 had multiple reasons for exclusion. One patient randomized to the 10-mg/kg/d dose was not treated and was subsequently withdrawn by the investigator.
Patients
Patients aged 2 to 18 years (inclusive) with a confirmed diagnosis of Dravet syndrome were eligible if they had seizures incompletely controlled by current AEDs, were taking at least 1 AED, and had at least 4 convulsive seizures during the 4-week baseline period. Use of medications and nonpharmacological interventions for epilepsy (including the ketogenic diet and vagus nerve stimulation) had to be stable for 4 weeks before screening and throughout the trial. Patients were excluded if they had a clinically significant unstable illness (other than epilepsy) in the 4 weeks before screening, alcohol or substance abuse, use of recreational or medicinal cannabis in the previous 3 months, and current use of felbamate for less than 1 year. Details of eligibility criteria are provided in eTable 1 in Supplement 2.
Procedures
The independent Epilepsy Study Consortium confirmed the Dravet syndrome diagnosis and verified seizure types of screened patients. Patients began the 4-week screening/baseline period at visit 1. After instruction on identification of countable seizure types at the screening visit, caregivers recorded the number and type of convulsive and nonconvulsive seizures each day using an interactive voice response system. Patients who met the eligibility criteria were randomized at visit 2 and received plant-derived, highly purified cannabidiol (Epidiolex in the United States and Epidyolex in the European Union; 100-mg/mL oral solution) or matching placebo solution (excipients only) provided in identical 100-mL amber glass bottles. Patients were randomized at a ratio of 2:2:1:1 with a block size of 6 and were stratified by age group (2-5, 6-12, and 13-18 years). A randomization schedule was computer generated by an independent statistician and held centrally. An interactive web response system was used to allocate patients to receive cannabidiol at a dose of 10 or 20 mg/kg/d or placebo in volumes equivalent to the 2 cannabidiol doses. The placebo groups were pooled for reporting efficacy and safety results. Patients, caregivers, investigators, individuals assessing the data, and the sponsor were unaware of the allocation of the patient (cannabidiol or placebo groups) and remained unaware until trial completion; however, they were not blinded to the volume of each received.
Trial medication was administered twice daily in 2 equally divided doses starting at 2.5 mg/kg/d (or equivalent volume of placebo), reaching the 10-mg/kg/d dose on day 7 and the 20-mg/kg/d dose on day 11. Investigators were instructed to maintain doses of concomitant medications; however, dose adjustments were permitted for adverse events. Information on trial medication use, concomitant medications, and adverse events was recorded in a paper diary. Clinic visits took place at 2, 4, 8, and 14 weeks after randomization. Additional safety telephone calls took place at 6 and 10 weeks, after tapering of the trial medication (where applicable) and 4 weeks after the final dose (eFigure 1 in Supplement 2). Patients who completed the treatment period were eligible to enter an open-label extension trial under a separate protocol. An adjudication committee was used to determine any potential signals of abuse.
Outcome Measures
The primary outcome was the change in convulsive seizure frequency during the 14-week treatment period compared with baseline. Convulsive seizures were defined as tonic-clonic, tonic, clonic, or atonic; nonconvulsive seizures were defined as myoclonic, partial, or absence. Although the study protocol and Epilepsy Study Consortium used the term absence seizures, different types of absence seizures in Dravet syndrome may occur, including absence seizures with eyelid myoclonias. Key secondary outcomes were the (1) change in total (all types) seizure frequency during the treatment period; (2) proportion of patients with at least a 50% reduction from baseline in convulsive seizure frequency during the treatment period; and (3) change in Caregiver Global Impression of Change (CGIC) score from baseline for overall condition.
Other secondary outcomes included the (1) proportion of patients experiencing worsening or improvement in convulsive seizure frequency; (2) proportion of patients with at least a 25%, 75%, and 100% reduction in convulsive seizure frequency; (3) change in nonconvulsive seizure frequency; (4) change in seizure frequency by individual seizure type; (5) change in CGIC from baseline in seizure duration (decreased; stayed the same; increased); (6) change in sleep disruption; (7) change in Epworth Sleepiness Scale23 score; (8) change in Quality of Life in Childhood Epilepsy24 score; and (9) change in Vineland Adaptive Behavior Scales II score. Safety was assessed by evaluation of adverse events and clinical laboratory parameters.
Statistical Analysis
Data were analyzed from November 16 (date of unblinding) to December 13 (date of final outputs), 2018. Based on a 2-sided nonparametric Mann-Whitney-Wilcoxon test with a significance level of 5%, we calculated that a sample size of 62 patients per group would provide 80% power to detect a difference in response distributions (eMethods in Supplement 2). The primary outcome was analyzed using negative binomial regression on the sum of convulsive seizure counts during the treatment period. The estimated ratio of least squares means for treatment to baseline period and 95% CIs were calculated for each treatment group and transformed into percentage reductions ([1 – ratio] × 100). The estimated ratio of each cannabidiol group to placebo and 95% CIs were presented along with the P value testing the null hypothesis that this ratio was 1. The treatment effect estimates (active vs placebo) are calculated on a logarithmic scale. Hence, the difference between active and placebo on the log scale is equivalent to a ratio of active to placebo on the original scale. Using the ratios of treatment period to baseline period, the treatment effect is then calculated as the ratio of active to placebo. Ratios have been presented as percentage reductions to aid interpretation. For each ratio and upper and lower bound of the 95% CI, the percentage reduction was presented. The primary and key secondary outcomes were tested in order with their type I error controlled by use of a hierarchical gate-keeping procedure, wherein each successive outcome was tested only if the prior comparison was statistically significant (statistical analysis plan in Supplement 1).25
Data up to the time of withdrawal were included in the outcome analyses, and no imputation for missing data was performed. Two-sided P < .05 was considered significant. Prespecified sensitivity analyses were performed for the primary and key secondary outcomes using alternative statistical methods on the intention-to-treat (ITT) as well as the per-protocol analysis set (primary and first key secondary only) (eMethods and eFigures 2-4 in Supplement 2). For other (nonkey) secondary outcomes, there was no adjustment to P values to account for multiple comparisons; all other secondary outcomes were considered exploratory. Analyses were conducted using SAS software, version 9.3 (SAS Institute, Inc).
Results
Patients
A total of 198 eligible patients (mean [SD] age, 9.3 [4.4] years; 104 female [52.5%] and 94 male [47.5%]) constituted the ITT analysis set; of these, 65 received placebo, 66 received the 10-mg/kg/d dose of cannabidiol (CBD10 group), and 67 received the 20-mg/kg/d dose of cannabidiol (CBD20 group). Among the 285 patients screened, 86 were excluded, and the remaining 199 were randomized. Nine patients were withdrawn (3 in the CBD10 group and 6 in the CBD20 group); 5 of 6 in the CBD20 group discontinued because of adverse events. A total of 190 patients completed the treatment period, and 186 (97.9%) entered the open-label extension trial (Figure 1).
Baseline demographics were similar across all treatment groups. A similar proportion of male and female patients were in the ITT analysis set; 176 (88.9%) were white, and the highest proportion came from the United States (93 [47.0%]). Patients in each group previously received a median of 4 (range, 0-19) AEDs and were taking a median of 3 (range, 1-5) concomitant AEDs; the most commonly used were valproate (139 [70.2%]) and clobazam (126 [63.6%]). Seventeen patients (8.6%) used the ketogenic diet and 27 (13.6%) had received vagus nerve stimulators. The most common seizure type during screening was generalized tonic-clonic (150 [75.8%]), followed by myoclonic (100 [50.5%]), absence (any type; 83 [41.9%]), and complex partial (80 [40.4%]) types. Patients had a median of 12 (interquartile range [IQR], 6-33) convulsive seizures during the 4-week baseline period, with patients in the placebo group having a numerically higher baseline median number of convulsive seizures (median, 17 [IQR, 7-51]) than the CBD10 (median, 14 [IQR, 6-31] and CBD20 (9 [IQR, 6-21]) groups (Table 1).
Table 1. Baseline Demographic and Clinical Characteristics Among Patients in the ITT Analysis Seta.
| Characteristic | Treatment Group | ||
|---|---|---|---|
| Placebo (n = 65) | CBD10 (n = 66)a | CBD20 (n = 67)b | |
| Age, mean (SD) [range], y | 9.6 (4.6) [2.2-18.1] | 9.2 (4.3) [2.3-17.7] | 9.3 (4.3) [2.2-18.9] |
| Age group, No. (%), y | |||
| 2-5 | 18 (28) | 19 (29) | 20 (30) |
| 6-12 | 28 (43) | 31 (47) | 31 (46) |
| 13-18 | 19 (29) | 16 (24) | 16 (24) |
| Female, No. (%) | 34 (52) | 39 (59) | 31 (46) |
| No. of AEDs, median (range) | |||
| Previous AEDs | 4 (0-11) | 4 (0-19) | 4 (0-11) |
| Concomitant AEDs | 3 (1-5) | 3 (1-5) | 3 (1-4) |
| Most common concomitant AEDs, No. (%)c | |||
| Valproate (all forms) | 48 (74) | 44 (67) | 47 (70) |
| Clobazam | 41 (63) | 45 (68) | 40 (60) |
| Stiripentol | 24 (37) | 25 (38) | 22 (33) |
| Levetiracetam | 14 (22) | 19 (29) | 21 (31) |
| Topiramate | 17 (26) | 11 (17) | 18 (27) |
| Baseline seizure frequency per 28 d, median (IQR) | |||
| Convulsive seizures | 17 (7-51) | 14 (6-31) | 9 (6-21) |
| All seizures | 46 (16-217) | 35 (10-104) | 26 (10-194) |
Abbreviations: AED, antiepileptic drug; CBD10, 10-mg/kg/d dose of cannabidiol; CBD20, 20-mg/kg/d dose of cannabidiol; IQR, interquartile range; ITT, intention-to-treat.
One patient randomized to the CBD10 group was not treated and was withdrawn by the principal investigator.
One patient randomized to the CBD20 group had an incomplete list of current AEDs in the database; at the time of the database lock the patient was reported to take stiripentol, clobazam, and topiramate; however, it was later determined that the patient was also taking felbamate, carbamazepine, and levetiracetam.
Indicates more than 20% of patients in any group.
Primary Outcome
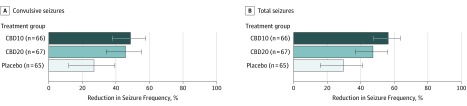
The percentage reduction from baseline in convulsive seizure frequency during the 14-week treatment period was 48.7% for the CBD10 group, 45.7% for the CBD20 group, and 26.9% for the placebo group. The percentage reduction from placebo was 29.8% (95% CI, 8.4%-46.2%; P = .01) for the CBD10 group and 25.7% (95% CI, 2.9%-43.2%; P = .03) for the CBD20 group (Figure 2). The results of the sensitivity analyses were consistent with the results of the primary analysis (eFigure 2 in Supplement 2). Similarly, the percentage reduction from baseline in convulsive seizure frequency during the 12-week maintenance period was 49.2% for the CBD10 group, 48.6% for the CBD20 group, and 28.6% for the placebo group (eFigure 2B in Supplement 2). The differences between treatment groups favored cannabidiol over placebo during the first 4 weeks of the maintenance period (CBD10 group, 0.63 [95% CI, 0.47-0.84]; CBD20 group, 0.70 [95% CI, 0.52-0.94]) and was maintained for the duration of treatment (eFigure 2A in Supplement 2).
Figure 2. Percentage Reductions in Convulsive and Total Seizure Frequency During the Treatment Period.
The estimated percentage reduction in seizure frequency and 95% CIs are shown for each treatment group. Cannabidiol doses of 10 mg/kg/d (CBD10 group) and 20 mg/kg/d (CBD20 group) were associated with greater reductions in convulsive (primary end point) and total seizure frequency compared with placebo. Convulsive seizures include tonic, clonic, tonic-clonic, and atonic types; total seizures include convulsive and nonconvulsive seizures (myoclonic, countable partial, and other partial or absence types). The percentage reduction in convulsive seizures from placebo was 29.8% (95% CI, 8.4%-46.2%; P = .01) for the CBD10 group and 25.7% (95% CI, 2.9%-43.2%; P = .03) for the CBD20 group; for total seizures, 38.0% for the CBD10 group (95% CI, 20.1%-51.9%; P < .001) and 25.1% for the CBD20 group (95% CI, 3.5%-41.9%; P = .03).
Key Secondary Outcomes
The percentage reduction from baseline in total seizure frequency during the treatment period was 56.4% for the CBD10 group, 47.3% for the CBD20 group, and 29.7% for the placebo group. The percentage reduction from placebo was 38.0% for the CBD10 group (95% CI, 20.1%-51.9%; P < .001) and 25.1% for the CBD20 group (95% CI, 3.5%-41.9%; P = .03) (Figure 2). Sensitivity analyses showed similar results (eFigure 3 in Supplement 2).
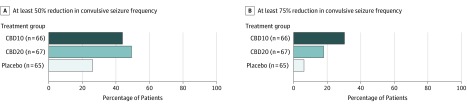
The proportion of patients achieving at least a 50% reduction from baseline in convulsive seizure frequency during the treatment period was 43.9% (n = 29) for the CBD10 group (P = .03), 49.3% (n = 33) for the CBD20 group (P = .007), and 26.2% (n = 17) for the placebo group (Figure 3). The proportion of patients achieving at least a 75% reduction from baseline in convulsive seizure frequency (other secondary) was 30.3% (n = 20) for the CBD10 group, 17.9% (n = 12) for the CBD20 group, and 6.2% (n = 4) for the placebo group (Figure 3). Compared with placebo, caregivers of cannabidiol-treated patients were significantly more likely to report an improvement in overall condition. The patients reporting slightly improved, much improved, or very much improved as measured by the CGIC scale at last visit included 27 of 65 patients in the placebo group, 45 of 66 in the CBD10 group (P < .001), and 40 of 66 in the CBD20 group (P = .03) (eFigure 4 in Supplement 2).
Figure 3. Reduction in Convulsive Seizure Frequency During the Treatment Period.
Cannabidiol doses of 10 mg/kg/d (CBD10 group) and 20 mg/kg/d (CBD20 group) resulted in a higher proportion of patients achieving at least a 50% reduction in convulsive seizure frequency compared with placebo, and the differences were statistically significant (odds ratio [OR] for CBD10 group, 2.21 [95% CI, 1.06-4.62; P = .03]; OR for CBD20 group, 2.74 [95% CI, 1.32-5.70; P = .007]). A higher proportion of patients treated with cannabidiol compared with placebo achieved at least a 75% reduction in convulsive seizure frequency (OR for CBD10 group, 6.63 [95% CI, 2.12-20.73]; OR for CBD20 group, 3.33 [95% CI, 1.10-10.92]; P values are not shown because this was not a key secondary outcome and type I error was not controlled for).
Other Secondary Outcomes
All other secondary outcomes are provided in eTable 2 in Supplement 2. Among those who completed the trial, freedom from convulsive seizures was achieved by 6 patients (1 in the placebo group, 2 in the CBD10 group, and 3 in the CBD20 group) during the 12-week maintenance period, of whom 5 patients (1 in the placebo group, 2 in the CBD10 group, 2 in the CBD20 group) achieved freedom from convulsive seizures during the 14-week treatment period. During the duration of the trial, there was no notable difference between both cannabidiol doses or placebo for assessments of sleep disruption, daytime sleepiness, quality of life, adaptive behaviors, or cognitive function (eTable 2 in Supplement 2); however, the number of patients who completed the adaptive behavior and cognitive function measures was low and limited the interpretability of results.
Adverse Events
All-cause treatment-emergent adverse events occurred in 176 of 198 patients (88.9%): 58 of 65 (89.2%) in the placebo group, 56 of 64 (87.5%) in the CBD10 group, and 62 of 69 (89.9%) in the CBD20 group. Of 176 patients with adverse events, 162 (92.0%) had events judged by the investigator to be mild or moderate in severity (mild in 98, moderate in 64, and severe in 14). The 5 most common adverse events occurring in at least 10% of patients in any group included decreased appetite, diarrhea, somnolence, pyrexia, and fatigue (Table 2). Serious adverse events occurred in 40 patients (10 in the placebo group, 13 in the CBD10 group, and 17 in the CBD20 group). Adverse events resolved by the end of the trial in 61 of 118 patients (51.7%) in both cannabidiol groups and 35 of 58 patients (60.3%) in the placebo group. No deaths occurred.
Table 2. Common Adverse Events Among Patients in the Safety-Analysis Seta.
| Adverse Event | Treatment Group, No. (%) | ||
|---|---|---|---|
| Placebo (n = 65) | CBD10 (n = 64) | CBD20 (n = 69)b | |
| Decreased appetite | 11 (17) | 11 (17) | 20 (29) |
| Mild | 9 (14) | 7 (11) | 14 (20) |
| Moderate | 2 (3) | 4 (6) | 5 (7) |
| Severe | 0 | 0 | 1 (1) |
| Diarrhea | 8 (12) | 11 (17) | 18 (26) |
| Mild | 7 (11) | 10 (16) | 14 (20) |
| Moderate | 1 (2) | 1 (2) | 3 (4) |
| Severe | 0 | 0 | 1 (1) |
| Somnolence | 9 (14) | 16 (25) | 16 (23) |
| Mild | 9 (14) | 11 (17) | 9 (13) |
| Moderate | 0 | 4 (6) | 7 (10) |
| Severe | 0 | 1 (2) | 0 |
| Pyrexia | 11 (17) | 15 (23) | 15 (22) |
| Mild | 9 (14) | 12 (19) | 11 (16) |
| Moderate | 2 (3) | 3 (5) | 3 (4) |
| Severe | 0 | 0 | 1 (1) |
| Fatigue | 7 (11) | 5 (8) | 15 (22) |
| Mild | 7 (11) | 4 (6) | 8 (12) |
| Moderate | 0 | 1 (2) | 7 (10) |
| Vomiting | 4 (6) | 4 (6) | 11 (16) |
| Mild | 3 (5) | 4 (6) | 8 (12) |
| Moderate | 1 (2) | 0 | 3 (4) |
| Mild nasopharyngitis | 5 (8) | 4 (6) | 8 (12) |
| Status epilepticus | 9 (14) | 5 (8) | 7 (10) |
| Mild | 3 (5) | 0 | 0 |
| Moderate | 4 (6) | 2 (3) | 5 (7) |
| Severe | 2 (3) | 3 (5) | 2 (3) |
| ALT level increasedc | 0 | 3 (5) | 9 (13) |
| Mild | 0 | 1 (2) | 7 (10) |
| Moderate | 0 | 2 (3) | 2 (3) |
| AST level increasedc | 0 | 3 (5) | 8 (12) |
| Mild | 0 | 1 (2) | 5 (7) |
| Moderate | 0 | 2 (3) | 2 (3) |
| Severe | 0 | 0 | 1 (1) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBD10, 10 mg/kg/d cannabidiol; CBD20, 20 mg/kg/d cannabidiol.
The table shows the all-causality treatment-emergent adverse events (Medical Dictionary for Regulatory Activities preferred term) that occurred in more than 10% of patients in any treatment group from the safety analysis set. The severity of adverse events was determined by the investigators and was not independently adjudicated.
Of the 66 patients randomized to the CBD10 group, 2 patients titrated above the target dose and were therefore assigned to the CBD20 group for all safety analyses.
Of the 16 patients with liver transaminase elevations greater than 3 times the upper limit of the reference range, 12 reported ALT and/or AST increased as an adverse event.
Increases in liver transaminase levels greater than 3 times the upper limit of the reference range occurred in 16 of 133 patients (12.0%) from both cannabidiol groups (3 of 44 [6.8%] in the CBD10 and 13 of 47 [27.7%] in the CBD20 groups), all of whom were taking concomitant valproate, and in no patients in the placebo group. All elevations resolved after discontinuation from the trial (1 patient in the CBD10 group and 2 in the CBD20 group), spontaneously (1 patient in the CBD10 group and 3 in the CBD20 group), after dose reduction of concomitant valproic acid and/or clobazam (3 patients in the CBD20 group), after dose reduction of cannabidiol (1 patient in the CBD20 group), or after completion and once enrolled in the open-label extension trial (1 patient in the CBD10 group 4 in the CBD20 group). There were no patients who met the Hy’s Law criteria for severe drug-induced liver injury.
Notably, a higher incidence of adverse events associated with somnolence, rash, and pneumonia occurred with concomitant clobazam use (eTable 3 in Supplement 2). Across cannabidiol and placebo groups, clobazam dosage was adjusted in 20 of 126 patients (15.9%), and valproate dosage was adjusted in 19 of 139 patients (13.7%) (eTable 3 in Supplement 2). Of those with dose adjustments during the trial, 1 patient in the CBD10 group initiated clobazam therapy owing to prolonged seizures, and 1 patient in the CBD20 group initiated valproate therapy owing to a serious adverse event of status epilepticus. For other AEDs, changes were infrequent.
Discussion
In this randomized clinical trial of 2 doses of highly purified cannabidiol in children with highly treatment-resistant Dravet syndrome, both doses significantly reduced convulsive seizure frequency compared with placebo. Results for all 3 key secondary outcomes—percentage reduction in total seizure frequency, proportion of patients achieving at least a 50% reduction in convulsive seizure frequency, and improvement in overall condition on the CGIC scale—also significantly favored the cannabidiol groups vs placebo. These results are consistent with those of 3 prior trials of this formulation, 1 in Dravet syndrome evaluating only the 20-mg/kg/d dose19 and 2 in Lennox-Gastaut syndrome.20,21 Given the availability of many formulations of cannabidiol that vary in purity, excipients, and consistency, results from this trial should not be extrapolated to other cannabidiol-containing products.
Efficacy was similar between the 2 cannabidiol doses; however, the safety and tolerability profile was better for the 10- vs 20-mg/kg/d dose, suggestive of a more favorable benefit-risk profile at the lower dose. Because the efficacy of doses lower than 10 mg/kg/d was not tested in this trial, it is unknown but possible that lower doses may provide optimal benefit-risk for some patients. Several of the most common adverse events, including decreased appetite, diarrhea, and fatigue, occurred more frequently in the CBD20 group, as did serious adverse events, adverse events leading to discontinuation, and elevations of liver transaminase levels of greater than 3 times the upper limit of the reference range.
Consistent with the described pharmacokinetic drug-drug interaction between cannabidiol and clobazam, leading to an approximately 3-fold increase in exposure to clobazam’s active metabolite N-desmethyl clobazam and an approximately 1.5-fold increase in cannabidiol’s active metabolite 7-hydroxy cannabidiol,26 somnolence was markedly greater in patients receiving clobazam compared with those who did not and led to dose reductions of clobazam more frequently in the cannabidiol groups than the placebo group. Multiple post hoc or uncontrolled analyses have suggested the cannabidiol treatment effect exists without clobazam, although it may be enhanced by the presence of clobazam.27,28,29,30 The interaction P value for clobazam use in this trial was not statistically significant, suggesting that clobazam use did not significantly affect the treatment outcome. A meta-analysis of the clobazam subgroup data from this trial combined with 3 prior randomized clinical trials sponsored by GW Pharmaceuticals is under way.
As in prior studies, the 20-mg/kg/d cannabidiol dose and valproate were independent risk factors for elevated liver transaminase levels. Notably, only 6.8% of patients receiving valproate in the CBD10 group had transaminase elevations greater than 3 times the upper limit of the reference range compared with 27.7% of patients receiving valproate in the CBD20 group. Although there is an interaction between cannabidiol and valproate influencing a risk of drug-induced liver injury, cannabidiol does not have a notable effect on the plasma levels of valproate or its major hepatotoxic metabolite in healthy individuals26 and patients with epilepsy,31 suggesting the interaction is not likely pharmacokinetic in nature. The potential for drug-drug interactions between cannabidiol and other medications highlights the need for careful monitoring of adverse events by a clinician.
The placebo response rate for the change in convulsive seizure frequency was higher at 26.9% than 13% in the first Dravet trial (GWPCARE1).19 The reason for the higher placebo response is unclear but might be partly related to the numerically higher median baseline convulsive and total seizure frequency in the placebo group vs both cannabidiol groups and high patient and caregiver expectations of efficacy for cannabidiol. It may also reflect the increased placebo response in epilepsy trials reported in the last 2 decades.32 Despite the large placebo response, the treatment effect was statistically significant, replicating and expanding on the previous positive results.
Limitations
The trial has several limitations. First, given that this study was limited to children and adolescents, with 88.9% of patients white, results are limited in their generalizability to adults and other ethnic populations. Second, because cannabidiol was added to regimens that included multiple AEDs at varying doses, it was not possible to assess the effects of specific drug combinations. Third, although 2 doses were tested providing useful dose-ranging information, the safety and efficacy of doses lower than 10 mg/kg/d in this population remain unknown. Finally, because this study lasted only 14 weeks, it will be important to continue to evaluate the long-term safety and efficacy of cannabidiol.
Conclusions
Findings from this trial are an important addition to the emerging clinical trial data on cannabidiol in the acute and long-term treatment of pediatric developmental and epileptic encephalopathies. Our key finding is the significant and clinically meaningful reduction of seizures with an acceptable safety profile for both cannabidiol doses in patients with highly treatment-resistant Dravet syndrome. There was no appreciable difference in the efficacy between the 2 active treatment doses, but because individual responses vary, dose escalation to 20 mg/kg/d in patients requiring better seizure control may still be warranted if safety and tolerability allow.
Trial Protocol
eTable 1. Eligibility Criteria
eTable 2. Other Secondary Outcomes
eTable 3. Adverse Events of Special Interest in Patients With and Without Clobazam and AED Dose Adjustments From the Safety Analysis Set
eFigure 1. Trial Schematic
eFigure 2. Sensitivity Analyses of the Primary End Point and Percentage Reductions in Convulsive and Total Seizure Frequency During the Maintenance Period
eFigure 3. Sensitivity Analyses of Change in Total Seizure Frequency Compared to Baseline
eFigure 4. Caregiver Global Impression of Change in Overall Condition at Last Visit
eMethods. Statistical Analysis for Sample Size, Other Secondary Outcomes, and Sensitivity Analyses
Data Sharing Statement
References
- 1.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(suppl 2):3-9. doi: 10.1111/j.1528-1167.2011.02994.x [DOI] [PubMed] [Google Scholar]
- 2.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521. doi: 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68(6):1327-1332. doi: 10.1086/320609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harkin LA, McMahon JM, Iona X, et al. ; Infantile Epileptic Encephalopathy Referral Consortium . The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130(pt 3):843-852. doi: 10.1093/brain/awm002 [DOI] [PubMed] [Google Scholar]
- 5.Depienne C, Trouillard O, Saint-Martin C, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46(3):183-191. doi: 10.1136/jmg.2008.062323 [DOI] [PubMed] [Google Scholar]
- 6.Symonds JD, Zuberi SM, Stewart K, et al. Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019;142(8):2303-2318. doi: 10.1093/brain/awz195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dravet C, Oguni H. Dravet syndrome (severe myoclonic epilepsy in infancy). Handb Clin Neurol. 2013;111:627-633. doi: 10.1016/B978-0-444-52891-9.00065-8 [DOI] [PubMed] [Google Scholar]
- 8.Wirrell EC, Laux L, Donner E, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American consensus panel. Pediatr Neurol. 2017;68:18-34.e3. doi: 10.1016/j.pediatrneurol.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 9.Genton P, Velizarova R, Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011;52(suppl 2):44-49. doi: 10.1111/j.1528-1167.2011.03001.x [DOI] [PubMed] [Google Scholar]
- 10.Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia. 2010;51(6):1043-1052. doi: 10.1111/j.1528-1167.2009.02466.x [DOI] [PubMed] [Google Scholar]
- 11.Cooper MS, Mcintosh A, Crompton DE, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43-47. doi: 10.1016/j.eplepsyres.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 12.Myers KA, McMahon JM, Mandelstam SA, et al. Fatal cerebral edema with status epilepticus in children with Dravet syndrome: report of 5 cases. Pediatrics. 2017;139(4):e20161933. doi: 10.1542/peds.2016-1933 [DOI] [PubMed] [Google Scholar]
- 13.Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60(1):63-72. doi: 10.1111/dmcn.13591 [DOI] [PubMed] [Google Scholar]
- 14.Chiron C. Current therapeutic procedures in Dravet syndrome. Dev Med Child Neurol. 2011;53(suppl 2):16-18. doi: 10.1111/j.1469-8749.2011.03967.x [DOI] [PubMed] [Google Scholar]
- 15.Chiron C, Dulac O. The pharmacologic treatment of Dravet syndrome. Epilepsia. 2011;52(suppl 2):72-75. doi: 10.1111/j.1528-1167.2011.03007.x [DOI] [PubMed] [Google Scholar]
- 16.Epidiolex [package insert]. Carlsbad, CA: Greenwich Biosciences, Inc; 2018.
- 17.Wirrell EC. Treatment of Dravet syndrome. Can J Neurol Sci. 2016;43(suppl 3):S13-S18. doi: 10.1017/cjn.2016.249 [DOI] [PubMed] [Google Scholar]
- 18.Diacomit [package insert]. Gentilly, France: Biocodex; 2018.
- 19.Devinsky O, Cross JH, Laux L, et al. ; Cannabidiol in Dravet Syndrome Study Group . Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011-2020. doi: 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 20.Devinsky O, Patel AD, Cross JH, et al. ; GWPCARE3 Study Group . Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888-1897. doi: 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 21.Thiele EA, Marsh ED, French JA, et al. ; GWPCARE4 Study Group . Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096. doi: 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 24.Sabaz M, Cairns DR, Lawson JA, Nheu N, Bleasel AF, Bye AM. Validation of a new quality of life measure for children with epilepsy. Epilepsia. 2000;41(6):765-774. doi: 10.1111/j.1528-1157.2000.tb00240.x [DOI] [PubMed] [Google Scholar]
- 25.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800-802. doi: 10.1093/biomet/75.4.800 [DOI] [Google Scholar]
- 26.Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(8):1009-1031. doi: 10.1002/cpdd.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246-1251. doi: 10.1111/epi.13060 [DOI] [PubMed] [Google Scholar]
- 28.Devinsky O, Nabbout R, Miller IO, et al. Long-term safety and efficacy of cannabidiol treatment in Dravet syndrome: results of an open-label extension trial (GWPCARE5) [Abstract]. Paper presented at: American Epilepsy Society (AES) Annual Meeting; December 2, 2017; Washington DC. [Google Scholar]
- 29.Thiele E, Devinsky O, Checketts D, Knappertz V. Cannabidiol treatment responder analysis in patients with Lennox-Gastaut syndrome on and off clobazam [Abstract]. Paper presented at: American Epilepsy Society (AES) Annual Meeting; December 2, 2017; Washington DC. [Google Scholar]
- 30.Szaflarski JPBE, Cutter G, DeWolfe J, et al. ; UAB CBD Program . Seizure frequency and severity in an open-label CBD study for the treatment of epilepsy [Abstract]. Paper presented at: American Epilepsy Society (AES) Annual Meeting; December 2, 2017; Washington DC. [Google Scholar]
- 31.Tayo BB-ME, Gunning B, Cabrera C, et al. Exploration of the potential for plasma protein binding displacement and drug-drug interactions of valproate in combination with cannabidiol [Abstract]. Paper presented at: American Epilepsy Society (AES) Annual Meeting; December 1, 2018; New Orleans, LA. [Google Scholar]
- 32.Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials: a systematic review and meta-analysis. Epilepsia. 2011;52(2):219-233. doi: 10.1111/j.1528-1167.2010.02915.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Eligibility Criteria
eTable 2. Other Secondary Outcomes
eTable 3. Adverse Events of Special Interest in Patients With and Without Clobazam and AED Dose Adjustments From the Safety Analysis Set
eFigure 1. Trial Schematic
eFigure 2. Sensitivity Analyses of the Primary End Point and Percentage Reductions in Convulsive and Total Seizure Frequency During the Maintenance Period
eFigure 3. Sensitivity Analyses of Change in Total Seizure Frequency Compared to Baseline
eFigure 4. Caregiver Global Impression of Change in Overall Condition at Last Visit
eMethods. Statistical Analysis for Sample Size, Other Secondary Outcomes, and Sensitivity Analyses
Data Sharing Statement