This case-control study evaluates the association between QT-prolonging medications and autopsy-defined sudden arrhythmic death vs nonarrhythmic sudden death in an autopsy cohort.
Key Points
Question
What is the association of QT-prolonging medications with the risk of sudden cardiac death when adjudicated by autopsy as arrhythmic or nonarrhythmic death?
Findings
In this countywide, case-control autopsy study of 525 presumed sudden cardiac deaths defined by standard consensus criteria and 104 matched control deaths due to trauma, QT-prolonging medications were associated with increased odds of presumed sudden cardiac death compared with deaths due to trauma; however, autopsy demonstrated that this increased risk was specific for nonarrhythmic but not arrhythmic cause of death.
Meaning
Studies using consensus criteria for sudden cardiac death that presume arrhythmic cause may overestimate the association of QT-prolonging medications with the risk of sudden arrhythmic death.
Abstract
Importance
QT-prolonging medications (QTPMs) are a reported risk factor for sudden cardiac death (SCD) when defined by consensus criteria that presume an arrhythmic cause. The effect of QTPM on autopsy-defined sudden arrhythmic death (SAD) is unknown.
Objective
To evaluate the association between QTPM and autopsy-defined SAD vs nonarrhythmic cause of sudden death.
Design, Setting, and Participants
This prospective countywide case-control study included World Health Organization–defined (presumed) SCD cases who underwent autopsy as part of the San Francisco Postmortem Systematic Investigation of Sudden Cardiac Death Study (POST SCD) to determine arrhythmic or nonarrhythmic cause, and control deaths due to trauma (hereinafter referred to as trauma controls) in San Francisco County, California, from February 1, 2011, to March 1, 2014. Multivariate regression was used to evaluate the association of QTPM with the risk of presumed SCD, autopsy-defined SAD, and non-SAD compared with trauma controls. Medication exposure, determined by prescription lists and postmortem toxicologic findings, was used to calculate a summative QTPM exposure score (range, 0-20). Data were analyzed from September 1, 2018, to June 15, 2019.
Exposure
QT-prolonging medication exposure, as measured by QTPM score (1 indicated low; 2-4, moderate; and >4, high).
Main Outcomes and Measures
Death due to trauma, presumed SCD, and autopsy-defined non-SAD and SAD with no postmortem findings of extracardiac cause.
Results
A total of 629 patients (mean [SD] age, 61.4 [15.7] years; 439 men [69.8%]) were included, 525 with presumed SCDs and 104 traumatic death controls. Individuals with presumed SCDs had higher exposure and were more likely to be taking any QTPM (291 [55.4%] vs 28 [26.9%]; P < .001) than trauma controls. Use of QTPMs was associated with increased risk of presumed SCD in low (odds ratio [OR], 2.25 [95% CI, 1.03-4.96]; P = .04) and high (OR, 6.70 [95% CI, 1.47-30.67]; P = .01) exposure groups. After autopsy adjudication, use of QTPMs was associated with increased risk of non-SAD (low-risk OR, 2.88 [95% CI, 1.18-6.99; P = .02]; moderate-risk OR, 2.62 [95% CI, 1.20-5.73; P = .02]; and high-risk OR, 14.22 [95% CI, 2.91-69.30; P = .001]) but not SAD in all exposure groups. This association was attenuated by the exclusion of occult overdose non-SADs in the highest exposure group.
Conclusions and Relevance
These findings confirm the association between QTPMs and presumed SCD; however, after autopsy, this risk was specific for nonarrhythmic causes of sudden death. Studies using consensus SCD criteria may overestimate the association of QTPMs with the risk of SAD.
Introduction
Sudden cardiac death (SCD) is one of the most feared manifestations of cardiovascular disease and is responsible for as many as 5% to 15% of total deaths in the United States.1,2 Heart rate–corrected QT interval prolongation has long been established as an important risk factor for SCD, conveying a 2- to 3-fold increased risk in certain populations.3,4 As such, use of QT interval–prolonging medication (QTPM) has represented a traditional target for prevention of SCD.5 Cautious use of QTPMs has been bolstered by a number of studies linking use of key drug classes such as antipsychotics and antibiotics to SCD.6,7 The collective evidence linking QTPMs and SCD has had lasting clinical and regulatory practice implications, including removal of several QTPMs from the market and the widely adopted practice of routine electrocardiographic monitoring on initiation or increase of a known QTPM regimen.8,9
A key limitation of prior evidence is that it is based on definitions of SCD that are adjudicated without postmortem data.10 Sudden cardiac death has historically been defined by consensus criteria, including the American College of Cardiology/American Heart Association/Heart Rhythm Society definition11 and the widely used World Health Organization (WHO) criteria12: sudden unexpected death within 1 hour of symptom onset or within 24 hours of having been observed alive and symptom free. These criteria were proposed with the expressed aim to capture sudden arrhythmic death (SAD), the only type of sudden death rescuable by defibrillator. However, because autopsy confirmation of these out-of-hospital deaths is rare, these criteria presume an arrhythmic cause of death.11,13,14 In the prospective San Francisco Postmortem Systematic Investigation of Sudden Cardiac Death (POST SCD) Study, the investigators demonstrated that nearly half of presumed SCDs defined by WHO criteria were found to have an easily identified nonarrhythmic cause on autopsy, including occult overdose, cardiac tamponade, intracranial hemorrhage, aneurysm rupture, and pulmonary embolism.13
To our knowledge, no other study has reliably adjudicated presumed SCDs for arrhythmic causes using systematic autopsy; thus, the association of QTPMs with risk of autopsy-defined SAD is unknown.11,12 Therefore, we sought to assess the risk of QTPMs on autopsy-defined SAD in the POST SCD Study.13
Methods
Setting and Study Population
We analyzed data from the POST SCD Study, a prospective countywide autopsy study of all deaths attributed to WHO-defined SCD in San Francisco County from February 1, 2011, to March 1, 2014.13 This study was approved by the University of California, San Francisco, institutional review board, which approved a waiver of informed consent because all patients were dead. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
In the POST SCD Study, all out-of-hospital unattended deaths in San Francisco County, reported to the medical examiner by law, were screened daily for inclusion. Paramedic records and investigator scene reports were reviewed to identify all out-of-hospital deaths due to cardiac arrest among individuals aged 18 to 90 years for subsequent detailed autopsy by a single board-certified forensic pathologist (E.M.), including comprehensive gross and histological examination of the internal organs of the thorax, abdomen, and cranial vault, with particular attention to the heart for evidence of cardiovascular abnormalities.
Postmortem chemical analyses were performed for all patients, and toxicologic analyses (blood and urine) for those younger than 75 years or 75 years and older without an obvious cause of death after initial examination. Comprehensive premortem medical records, including current prescription medication lists, were obtained from all 10 county hospitals, paramedic records, and out-of-county medical records for all cases.
A multidisciplinary committee, including the assistant medical examiner (E.M.), a cardiac pathologist (P.U.), a neurologist, and 2 cardiac electrophysiologists (J.E.O. and Z.H.T.), reviewed comprehensive premortem medical records, forensic investigator reports, paramedic records, cardiac implantable electronic device interrogations,15 and detailed autopsy, toxicologic, and histologic findings for all out-of-hospital deaths due to cardiac arrest to adjudicate (1) whether cardiac arrest deaths met WHO criteria for SCD; (2) the mechanism of death, that is, sudden arrhythmic death (SAD) or nonarrhythmic sudden death (non-SAD); and (3) the single cause of death (eg, acute coronary syndrome, intracranial hemorrhage, and occult overdose). Out-of-hospital deaths due to cardiac arrest determined to have met WHO criteria—hereinafter referred to as presumed SCD—were included in this study. As a control group, we included a population of demographically similar individuals with nonsuicide, nonhomicide, accidental deaths due to trauma that were referred for medicolegally mandated autopsy during the study period (hereinafter referred to as trauma controls). These deaths underwent identical postmortem investigation.
Adjudication of Cause of Death
Autopsy-defined SAD was death for which no identifiable alternative cause (eg, acute cerebrovascular accident, tamponade, vascular rupture, pulmonary embolism, lethal toxicologic finding/occult overdose, acute heart failure with pulmonary edema) was found despite comprehensive postmortem investigation, including histologic, toxicologic, and chemical evaluations. Per usual forensic toxicology protocol, overdose cause of death required lethal serum or tissue levels in the absence of other pathologic findings that could explain sudden death. Cases with intermediate toxicologic levels were not included as overdose deaths and were considered SADs unless another nonarrhythmic cause was found. Full details of the original POST SCD Study design and methods, including a flowchart of the study population and exclusion criteria, have been published previously.13
QTPM Exposure Definition
Comprehensive medication lists were compiled from medical records, including electronic medical record–generated prescriptions, physician notes, and medical examiner reports of medications found on scene. All medication lists were reviewed and adjudicated by a clinical pharmacist (T.F.S.). In addition, qualitative and quantitative blood and urine toxicologic analyses, sensitive to most major QTPM drug classes and their metabolites, were performed.
Exposure to a QTPM was defined as the presence of a medication or metabolite, isolated at any concentration by serum or urine toxicologic findings, or present on the most recent verified medication list. In the event of disagreement, toxicologic results were used; in cases of medications not sensitively screened by toxicologic analysis, adjudicated medication lists were used.
We used the CredibleMeds website,16 a continually updated database of QTPM risk, to classify medications according to QT prolongation risk consistent with prior studies.17,18,19 Because the database classifies medications according to varying risk of QT prolongation and torsades de pointes and to quantify the additive effects of multiple QTPMs demonstrated in epidemiologic and molecular studies, a score was derived for each patient as the sum of all medications using the following CredibleMeds categories: 3 points for known risk; 2 points for possible risk; 1 point for conditional risk; and 0 points for no risk.20,21,22
A total QTPM exposure score was calculated for each patient representing the sum of the CredibleMeds scores for each detected QTPM (eTable in the Supplement), then categorized as none (0), low (1), moderate (2-4), or high (>4). This categorization required exposure to more than 1 high-risk QTPM for inclusion in the high exposure group and aimed to evenly distribute patients in each category. Medication lists were available at the time of adjudication, but investigators were blinded to QTPM exposure scores.
Statistical Analysis
Data were analyzed from September 1, 2018, to June 15, 2019. Baseline characteristics were presented as median and interquartile range (IQR) or percentage and compared using χ2 and unpaired t tests as appropriate. Logistic models were used to assess the associations of the categorized QTPM exposure score with presumed SCD, autopsy-defined SAD, and non-SAD vs trauma, adjusting for potential confounders. Seemingly unrelated regressions were used to assess the equality of the associations with QTPM across the comparisons of SAD and non-SAD with trauma.23 The following sensitivity analyses were performed: (1) using a continuous QTPM score, with checks for nonlinear effects using a 3-knot restricted cubic spline transformation of the score; (2) excluding SCDs due to occult overdose; and (3) excluding psychiatric medications in calculating the QTPM exposure score. All analyses were implemented using STATA, version 15.1 (StataCorp LLP). Two-tailed P < .05 indicated significance.
Results
A total of 629 patients (mean [SD] age at time of death, 61.4 [15.7] years; 439 men [69.8%] and 190 women [30.2%]) included 525 WHO-defined (presumed) SCDs and 104 trauma controls for analysis. Compared with trauma controls, individuals with presumed SCDs were older (mean [SD] age, 62.6 [14.7] vs 55.1 [19.4] years) and had more medical comorbidities, including coronary artery disease (100 [19.0%] vs 11 [10.6%]; P = .04), hypertension (290 [55.2%] vs 28 [26.9%]; P = .001), and depression (93 [17.7%] vs 7 [6.7%]; P = .005) (Table 1). Among presumed SCDs, 293 (55.8%) were autopsy-defined SAD and 232 (44.2%) were non-SAD; details of these findings have been reported previously.13 Medication lists were available for all patients, and comprehensive toxicologic findings identified additional QTPMs in 62 patients (9.9%).
Table 1. Demographic Characteristics and Premortem Conditions in Control Deaths Due to Trauma, Presumed SCDs, and Autopsy-Defined SAD or Non-SAD.
| Characteristic | All Presumed SCD (n = 525) | QTPM Exposure | Trauma Controls (n = 104) | P Value, Trauma Controls All Presumed SCD | Presumed SCD | P Value, SAD vs Non-SAD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| None (n = 234) | Low (n = 112) | Moderate (n = 122) | High (n = 57) | Autopsy-Defined SAD (n = 293) | Non-SAD (n = 232) | |||||
| Age, mean (SD), y | 62.6 (14.7) | 61.5 (15.6) | 65.0 (14.1) | 64.3 (14.3) | 60.3 (11.6) | 55.1 (19.4) | <.001 | 63.9 (14.5) | 60.9 (14.8) | .01 |
| Male, No. (%) | 362 (69.0) | 169 (72.2) | 78 (69.6) | 80 (65.6) | 35 (61.4) | 77 (74.0) | .30 | 220 (75.1) | 142 (61.2) | <.001 |
| Race/ethnicity, No. (%) | ||||||||||
| Asian | 110 (21.0) | 55 (23.5) | 26 (23.2) | 23 (18.9) | 6 (10.5) | 28 (26.9) | .02 | 68 (23.2) | 42 (18.1) | .12 |
| Black | 81 (15.4) | 20 (8.5) | 22 (19.6) | 27 (22.1) | 12 (21.1) | 9 (8.7) | 35 (11.9) | 46 (19.8) | ||
| White | 279 (53.1) | 129 (55.1) | 52 (46.4) | 65 (53.3) | 33 (57.9) | 50 (48.1) | 160 (54.6) | 119 (51.3) | ||
| Hispanic | 40 (7.6) | 23 (9.8) | 6 (5.4) | 5 (4.1) | 6 (10.5) | 16 (15.4) | 21 (7.2) | 19 (8.2) | ||
| Other | 15 (2.9) | 7 (3.0) | 6 (5.4) | 2 (1.6) | 0 | 1 (1.0) | 9 (3.1) | 6 (2.6) | ||
| History, No. (%) | ||||||||||
| Coronary artery disease | 100 (19.0) | 29 (12.4) | 32 (28.6) | 28 (23.0) | 11 (19.3) | 11 (10.6) | .04 | 70 (23.9) | 30 (12.9) | .001 |
| Congestive heart failure | 68 (13.0) | 7 (3.0) | 27 (24.1) | 26 (21.3) | 8 (14.0) | 7 (6.7) | .07 | 47 (16.0) | 21 (9.1) | .02 |
| Tobacco use | 211 (40.2) | 73 (31.2) | 55 (49.1) | 54 (44.3) | 29 (50.9) | 20 (19.2) | <.001 | 115 (39.2) | 96 (41.4) | .62 |
| Hypertension | 290 (55.2) | 88 (37.6) | 87 (77.7) | 78 (63.9) | 37 (64.9) | 28 (26.9) | .001 | 175 (59.7) | 115 (49.6) | .02 |
| Diabetes | 117 (22.3) | 47 (20.1) | 33 (29.5) | 24 (19.7) | 13 (22.8) | 13 (12.5) | .02 | 72 (24.6) | 45 (19.4) | .16 |
| Chronic kidney disease | 58 (11.0) | 7 (3.0) | 23 (20.5) | 19 (15.6) | 9 (15.8) | 9 (8.7) | .47 | 33 (11.3) | 25 (10.8) | .86 |
| Depression | 93 (17.7) | 17 (7.3) | 12 (10.7) | 32 (26.2) | 32 (56.1) | 7 (6.7) | .005 | 37 (12.6) | 56 (24.1) | <.001 |
| Schizophrenia | 36 (6.9) | 3 (1.3) | 6 (5.4) | 18 (14.8) | 9 (15.8) | 4 (3.8) | .25 | 16 (5.5) | 20 (8.6) | .15 |
| Any psychiatric disordera | 143 (27.2) | 28 (12.0) | 23 (20.5) | 53 (43.4) | 39 (68.4) | 17 (16.3) | .02 | 61 (20.8) | 82 (35.3) | <.001 |
Abbreviations: QTPM, QT-prolonging medication; SAD, sudden arrhythmic death; SCD, sudden cardiac death.
Includes a prior diagnosis of anxiety, bipolar disorder, borderline personality disorder, depression, insomnia, mood disorder, obsessive-compulsive disorder, posttraumatic stress disorder, psychosis, or schizophrenia.
QTPM Exposure Among Presumed SCDs
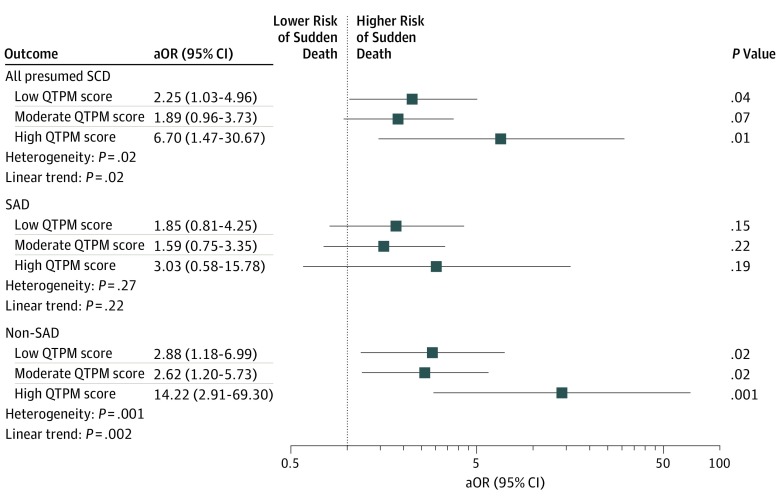
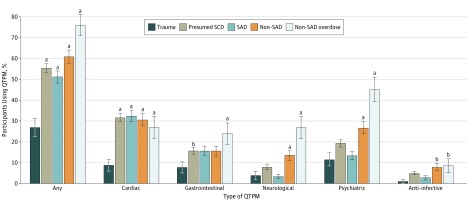
Compared with trauma controls, presumed SCDs had higher QTPM exposure scores (median, 1 [IQR, 0-2] vs 0 [IQR, 0-1]; P < .001) and were more likely to be taking any QTPM (291 [55.4%] vs 28 [26.9%]; P < .001) (Table 2). Compared with no QTPM exposure, low and high QTPM exposure scores were associated with an increased risk of presumed SCD (adjusted odds ratio [aOR] for low, 2.25 [95% CI, 1.03-4.96; P = .04]; aOR for high, 6.70 [95% CI, 1.47-30.67; P = .02]) (Figure 1), with evidence of dose-response (P = .02 for linear trend). We observed no significant increased risk for scores in the moderate range (aOR, 1.89 [95% CI, 0.96-3.73; P = .07]). Individuals with presumed SCD were more likely to be taking cardiac (166 [31.6%] vs 9 [8.7%]; P < .001) and gastrointestinal tract (78 [14.9%] vs 7 [6.7%]; P = .02) QTPM, with similar rates of neurological (25 [4.8%] vs 3 [2.9%]; P = .40), psychiatric (88 [16.8%] vs 10 [9.6%]; P = .07), and anti-infective (26 [5.0%] vs 1 [1.0%]; P = .07) QTPM exposure (Figure 2).
Table 2. QTPM Exposure and Class by Autopsy-Adjudicated Outcome.
| Exposure/Class | Presumed Sudden Cardiac Death (n = 525) | Trauma Controls (n = 104) | P Value, Trauma Controls vs SCD | Presumed SCD | Occult Overdose SCD (n = 71) | P Value, Occult Overdose vs Trauma Controls | ||
|---|---|---|---|---|---|---|---|---|
| SAD (n = 293) | Non-SAD (n = 232) | P Value, SAD vs Non-SAD | ||||||
| QT score, median (IQR) | 1 (0-2) | 0 (0-1) | <.001 | 1 (0-2) | 1 (0-4) | <.001 | 3 (1-5) | <.001 |
| Any QTPM, No. (%) | 291 (55.4) | 28 (26.9) | <.001 | 150 (51.2) | 141 (60.8) | .13 | 54 (76.1) | <.001 |
Abbreviations: IQR, interquartile range; QTPM, QT-prolonging medication; SAD, sudden arrhythmic death; SCD, sudden cardiac death.
Figure 1. Risk of Autopsy-Defined Causes of Sudden Cardiac Death (SCD) Compared With Deaths Due to Trauma (Trauma Controls) .
Data were stratified by QT-prolonging medication (QTPM) exposure (low, moderate, and high). Odds ratios were adjusted for baseline age, sex, race, tobacco use, history of coronary artery disease, heart failure, chronic renal disease, diabetes, hypertension, depression, and any psychiatric diagnosis (aORs). SAD indicates sudden arrhythmic death.
Figure 2. Proportion of Patients Exposed to QT-Prolonging Medication (QTPM) by Drug Class and Autopsy-Defined Cause of Death.
Whiskers denote standard error of mean. Comparison of sudden arrhythmic deaths (SAD) and nonarrhythmic sudden deaths (non-SAD) with control deaths due to trauma (trauma controls) was performed using χ2 test. Neurological QPTMs included antispasmodics, cholinesterase inhibitors, and opiates, including methadone. Psychiatric QTPMs included selective serotonin reuptake inhibitors, first- and second-generation antipsychotics, and tricyclic and atypical antidepressants. Anti-infective QPTMs included antiretrovirals, antibiotics, and antifungals. SCD indicates sudden cardiac death.
aP < .01 vs trauma controls.
bP < .05 vs trauma controls.
QTPM Exposure Among Autopsy-Defined SADs and Non-SADs
Non-SADs had greater exposure to QTPMs than SADs (median score, 1 [IQR, 0-4] vs 1 [IQR, 0-2]; P < .001), and a higher proportion had exposure to any QTPM (141 of 232 [60.8%] vs 150 of 293 [51.2%]; P = .03) (Table 2). Compared with trauma controls, QTPM exposure as categorized by QTPM scores was associated with increased risk of non-SAD in a dose-response manner (aOR for low, 2.88 [95% CI, 1.18-6.99; P = .02]; aOR for moderate, 2.62 [95% CI, 1.20-5.73; P = .02]; aOR for high, 14.22 [95% CI, 2.91-69.30; P = .001]; P = .002 for linear trend). In contrast, categorized QTPM scores did not have a statistically significant association with SAD compared with trauma controls (aOR for low, 1.85 [95% CI, 0.81-4.25; P = .15]; aOR for moderate, 1.59 [95% CI, 0.75-3.35; P = .22]; aOR for high, 3.03 [95% CI, 0.58-15.78; P = .19) (P = .22 for linear trend) (Figure 1). The comparison using seemingly unrelated regressions provided evidence that the estimates for non-SAD and SAD differ (P = .02 overall; P = .003 for high QTPM). The non-SAD group was more likely to be taking neurological (18 [7.8%] vs 7 [2.4%]; P = .004), psychiatric (50 [21.6%] vs 38 [13.0%]; P = .009), and anti-infective (18 [7.8%] vs 8 [2.7%]; P = .008) QTPM compared with the SAD group, with similar rates of use for cardiac (71 [30.6%] vs 95 [32.4%]; P = .65) and gastrointestinal (33 [14.2%] vs 45 [15.4%]; P = .72) QTPM (Figure 2).
QTPM Exposure by Autopsy Causes of Death
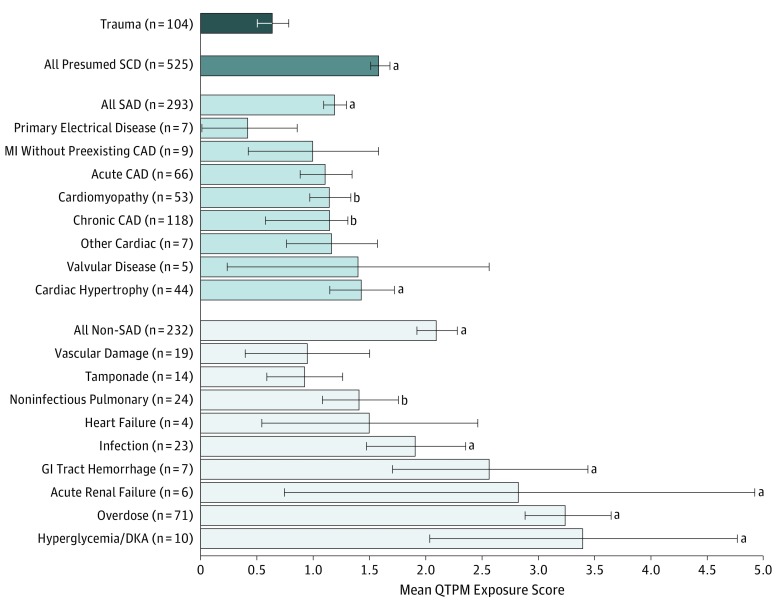
Among autopsy-defined SADs, QTPM exposure was highest among individuals with cardiac hypertrophy, chronic coronary artery disease, and cardiomyopathy as the underlying cause of arrhythmic death. Those with primary electrical disease/normal heart findings had the lowest exposure (Figure 3). Among non-SADs, QTPM exposure was highest among individuals with diabetic ketoacidosis, overdose, acute renal failure, gastrointestinal tract hemorrhage, and infection including pneumonia.
Figure 3. Exposure to QT-Prolonging Medication (QTPM) by Selected Autopsy-Defined Cause of Death.
Whiskers denote standard error of means. CAD indicates coronary artery disease; DKA, diabetic ketoacidosis; GI, gastrointestinal; MI, myocardial infarction; SAD, sudden arrhythmic death; and SCD, sudden cardiac death.
aP < .01 vs trauma controls.
bP < .05 vs trauma controls.
QPTMs and Occult Overdose SCDs
Presumed SCDs found to be due to overdose by systematic postmortem toxicologic findings (ie, occult overdose SCDs) accounted for 71 of the 232 non-SADs (30.6%). Individuals with occult overdose SCDs had greater QTPM exposure and were more likely to be taking QTPMs (mean [SD] exposure, 3.3 [3.2]; QTPM exposure, 54 [76.0%]) than trauma controls (mean [SD] exposure, 0.7 [1.4]; QTPM exposure, 28 [26.9%]) or those with non-overdose SCDs (mean [SD] exposure, 1.2 [1.4]; QTPM exposure, 265 [47.5%]; P < .001 for all comparisons) (Table 2). Individuals with occult overdose SCDs were more likely to be taking neurological QTPMs (8 of 71 [11.3%] vs 25 of 525 [4.8%]; P = .02) and psychiatric QTPMs (22 of 71 [31.0%] vs 88 of 525 [16.8%]; P = .004), including methadone (19 of 71 [26.8%] vs 30 of 525 [5.7%]; P < .001), and more likely to use illicit substances (cocaine: 23 of 71 [32.4%] vs 37 of 525 [7.0%]; methamphetamine: 11 of 71 [15.5%] vs 15 of 525 [2.9%]; P < .001 for both) than those with presumed SCDs as a whole. Overdose SCDs had similar rates of exposure to cardiac, gastrointestinal, and anti-infective QTPMs (Figure 2).
Sensitivity Analyses
Findings were similar when evaluating the QTPM score as a continuous variable. Compared with trauma controls, QTPM score was associated with increased risk of presumed SCD (aOR, 1.27 [95% CI, 1.06-1.51; P = .01]), with no evidence for nonlinearity (P = .31). After postmortem investigation, QTPM score was associated with increased risk of non-SAD (aOR, 1.43 [95% CI, 1.16-1.75; P < .001]) but not SAD (aOR, 1.15 [95% CI, 0.94-1.40; P = .16]), again with no evidence of nonlinear effects (P ≥ .30 for both).
Exclusion of occult overdose deaths attenuated the association of QTPM score categories with presumed SCDs, especially in the high-exposure group, which had the highest proportion of overdose deaths (aOR for low score, 2.06 [95% CI, 0.93-4.57; P = .08]; aOR for moderate score, 1.57 [95% CI, 0.77-3.19; P = .21]; aOR for high score, 3.81 [95% CI, 0.81-18.06; P = .09]). Exclusion of psychiatric QTPM attenuated the association of QTPM score with presumed SCD (aOR for low score, 3.07 [95% CI, 1.34-7.03; P = .008]; aOR for moderate score, 2.37 [95% CI, 1.06-5.29; P = .04]; aOR for high score, 1.00 [95% CI, 1.00-1.00]) and non-SAD (aOR for low score, 4.09 [95% CI, 1.59-10.48; P = .004]; aOR for moderate score, 2.97 [95% CI, 1.20-7.34; P = .02]; aOR for high score, 1.00 [95% CI, 1.00-1.00]) in the high-exposure groups, which were disproportionately affected by mental health disorders (Table 1).
Discussion
In this analysis of the POST SCD study, a comprehensive postmortem investigation of all incident presumed SCDs as defined by conventional criteria countywide during a 3-year period found that after adjustment for potential confounders, QTPM exposure was associated with an increased risk of presumed SCD in an exposure-response manner, consistent with prior studies.24,25 As with prior studies, at face value, these findings are concerning for QTPMs conveying an increased risk of SCD that is presumed due to fatal arrhythmias. However, after systematic postmortem investigation, we found that the increased risk was specific for deaths found to have a nonarrhythmic cause.
In the POST SCD Study, nearly half of presumed SCDs were nonarrhythmic; herein we determined that these nonarrhythmic deaths had higher overall exposure to QTPM, in many cases prescribed for an indication associated with the autopsy-defined cause of death (eg, psychiatric QTPM and illicit drug overdose). The range of exposure to QTPM increased the odds of nonarrhythmic death from 2.8- to 14.2-fold in a statistically significant, exposure-dependent manner. In contrast, not even the highest exposure to QTPM translated to a statistically significant increased odds of autopsy-defined SAD. Unrelated regression analysis supported a true difference between these comparisons rather than lack of power in the SAD group leading to such findings. Overall, we believe that these findings reflect the predisposition for higher exposure to QTPMs among individuals at risk for non-SAD, leading to confounding by indication and an overestimation of the risk of QTPM for presumed SCD not defined by autopsy.
Our study is the first, to our knowledge, to assess the association of QTPMs with SCD risk adjudicated with a nearly 100% rate of autopsy. A prior uncontrolled study evaluating SCD in young patients (aged 1-49 years; mean age, 38 years)26 achieved a 55% autopsy rate and found that QTPMs were associated with increased risk of SAD compared with non-SAD. However, this prior study did not perform toxicologic analysis to rule out overdose as occult cause of presumed SCD, particularly important in a younger population, and its lower autopsy rates may lead to bias because only half of SCDs were confirmed. In our study, we found QTPM use to be the highest in this overdose subgroup. Although continuous rhythm monitoring has been proposed as a more ideal standard than autopsy for SAD,27 ostensibly fatal arrhythmias may actually be due to nonarrhythmic cause. In the POST SCD Study, autopsy identified cases in which an implantable cardioverter defibrillator detected and treated ventricular fibrillation but failed to prevent sudden death because an inciting subarachnoid hemorrhage had caused neurocardiogenic ventricular fibrillation.28
In the POST SCD Study, occult overdoses (13.5% of SCDs) were the most common noncardiac cause of presumed SCDs.13 Exclusion of these occult overdose SCDs attenuated the risk of SCD in the highest QTPM exposure strata; these patients were disproportionately taking QTPMs, including psychiatric medications and opiates. Exclusion of psychiatric QTPMs resulted in similar attenuation, because the comorbid burden of mental health disorders was disproportionately higher in those with the highest QTPM exposure. This finding suggests that misclassified occult overdose deaths may partially explain the previously reported increase in SCD risk with some QTPMs.19,24 Occult overdoses misclassified as SCDs may also account for the previously reported association between psychiatric and neurological QTPMs, including antipsychotics and methadone, with presumed SCD.24,25,29
QT prolongation has long served as a surrogate marker of medication cardiotoxicity, by which drugs prolong ventricular repolarization and predispose to ventricular arrhythmias, including torsades de pointes.5,30 As a result of epidemiologic associations and a history of public drug withdrawals based on postapproval studies, QT prolongation has become a major focus in drug safety and development, with many candidate agents being abandoned owing to signals in QT prolongation.5,31 However, the link between the effect of prolonged repolarization, as measured by prolonged QT interval, and the risk of torsades de pointes and other fatal ventricular tachyarrhythmias is imperfect.5,31 The risk of arrhythmia is not a linear function of the QT interval nor the extent of change with therapy, and some drugs prolong QT intervals yet rarely cause torsades de pointes.5,31,32 Despite its limitations, QT prolongation continues to be an important metric of drug safety and is among the most common reasons for drug withdrawal and black-box warnings in the United States.5 Our results caution the use of consensus criteria in evaluating the association of QTPMs and SCD and highlight the importance of comprehensive postmortem evaluation, including toxicologic effects, in the evaluation of sudden deaths.
Because our results do not exclude increased risk of SAD with QTPMs, regulatory policy and patient safety measures surrounding QTPM continue to be important. However, our study indicates that most of the association with sudden death was nonarrhythmic and mechanistically unrelated to QT prolongation. Clinicians routinely use screening electrocardiography during treatment, with discontinuation of QTPM if the QT interval is prolonged, in many cases limiting the use of first-line therapies. Outside specific highly-monitored settings (eg, hospitalized initiation of dofetilide treatment),33 it remains unclear whether routine screening and QT interval–guided tailoring of therapy is beneficial or sufficient to alter the risk of sudden death. Current drug information label recommendations from the US Food and Drug Administration are drug specific and equivocal but generally recommend assessment of baseline risk factors for torsades de pointes and close monitoring of electrolyte levels, with variable recommendations for measurement of baseline QT intervals.
Our findings suggest the current best estimate of the risk of SAD associated with QTPM is confounded and may be significantly overestimated. Given that our study lacks QT interval data, clinicians should continue to exercise caution in monitoring patients with QTPM exposure, because the greatest exposure was highly associated with sudden death, albeit predominantly due to nonarrhythmic causes. However, clinicians should be aware that interventions focused solely on prevention of arrhythmic death are unlikely to entirely address the risk of sudden death in this population. Our results suggest that efforts focused on reducing risks of comorbid conditions, including close monitoring for illicit drug use and sequelae of diabetes, psychiatric diseases, and renal failure, are important to reduce overall risk of sudden mortality in patients prescribed QTPMs.
Limitations
This study was limited by the use of electronic medical records and forensic investigator–generated medication lists, which may incompletely capture medication exposures at the time of death. Pharmacy filling records were unavailable, and therefore we are unable to reliably ascertain the duration and compliance of therapy; thus, toxicologic results were preferentially applied. Although comprehensive toxicologic analysis does not capture all routinely used pharmaceuticals, because the same toxicologic assay was performed in all patients, the effect of incomplete medication capture is likely balanced and minimal. We also proposed a novel scoring system to quantify QTPM exposure, because no other validated measure exists. This system was used to account for the variable risk of QT prolongation and torsades de pointes associated with medications and to quantify the demonstrated additive effects of multiple QTPMs, which is incompletely captured by binomial classification of QTPM exposure. It is unclear whether this approach optimally quantifies additive or potentially synergistic effects of multiple QTPM.22 Owing to the population-based nature of the POST SCD Study, not all patients had electrocardiograms because they had been performed only as part of clinical care and were variably timed in relation to QTPM exposure and sudden death. Thus, we were unable to reliably correlate QTPM exposure with measured QT corrected intervals and arrhythmic events. Although no single electrocardiographic measurement can fully stratify risk for sudden death, future studies may attempt to correlate QT corrected interval and autopsy-defined SAD and non-SAD. In addition, this study was performed in a single city in the United States; rates of QTPM use, illicit drug use, and intensity of QT interval monitoring may differ geographically, and thus results may not be generalizable to all populations. However, the rate of overdose sudden deaths in San Francisco County is similar to other urban communities, such as King County (Seattle), Washington.34
Conclusions
Leveraging the comprehensive autopsy adjudication performed in the novel POST SCD cohort, we confirmed the association of QTPMs with risk of presumed SCD; however, after comprehensive postmortem investigation, we found this risk was specific for nonarrhythmic causes. Studies using consensus SCD criteria that presume arrhythmic cause may result in confounded and/or exaggerated risk of SAD, especially antipsychotics, methadone, and anti-infectives, and interventions focused solely on prevention of arrhythmic death may not fully address the risk of sudden mortality. Although our study cannot exclude increased risk of SAD with QTPMs, these results suggest our current approach to QT monitoring merits further evaluation to prevent actual SADs.
eTable. Prescription Drug Use by Number of Subjects, QTPM Risk, and Autopsy-Adjudicated Cause of Sudden Death
References
- 1.Chugh SS, Jui J, Gunson K, et al. . Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large US community. J Am Coll Cardiol. 2004;44(6):1268-1275. doi: 10.1016/j.jacc.2004.06.029 [DOI] [PubMed] [Google Scholar]
- 2.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158-2163. doi: 10.1161/hc4301.098254 [DOI] [PubMed] [Google Scholar]
- 3.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83(6):1888-1894. doi: 10.1161/01.CIR.83.6.1888 [DOI] [PubMed] [Google Scholar]
- 4.Straus SMJM, Kors JA, De Bruin ML, et al. . Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362-367. doi: 10.1016/j.jacc.2005.08.067 [DOI] [PubMed] [Google Scholar]
- 5.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013-1022. doi: 10.1056/NEJMra032426 [DOI] [PubMed] [Google Scholar]
- 6.Wu CS, Tsai YT, Tsai HJ; Wu Chi-Shin . Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Assoc. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert RK, Schuller JL; COPD Clinical Research Network . Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med. 2014;189(10):1173-1180. doi: 10.1164/rccm.201402-0385CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89(11):1363-1372. doi: 10.1136/heart.89.11.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287(17):2215-2220. doi: 10.1001/jama.287.17.2215 [DOI] [PubMed] [Google Scholar]
- 10.Tseng ZH. Sudden cardiac deaths: WHO says they are always arrhythmic? JAMA Cardiol. 2018;3(7):556-558. doi: 10.1001/jamacardio.2018.1008 [DOI] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. . 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138(13):e272-e391. doi: 10.1161/CIR.0000000000000548 [DOI] [PubMed] [Google Scholar]
- 12.WHO Scientific Group on Sudden Cardiac Death and World Health Organization. Sudden Cardiac Death: Report of a WHO Scientific Group. Geneva: World Health Organization; 1985. [PubMed] [Google Scholar]
- 13.Tseng ZH, Olgin JE, Vittinghoff E, et al. . Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD Study. Circulation. 2018;137(25):2689-2700. doi: 10.1161/CIRCULATIONAHA.117.033427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinkle LE Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65(3):457-464. doi: 10.1161/01.CIR.65.3.457 [DOI] [PubMed] [Google Scholar]
- 15.Tseng ZH, Hayward RM, Clark NM, et al. . Sudden death in patients with cardiac implantable electronic devices. JAMA Intern Med. 2015;175(8):1342-1350. doi: 10.1001/jamainternmed.2015.2641 [DOI] [PubMed] [Google Scholar]
- 16.Woosley RL, Heise CW, Gallo T, et al. CredibleMeds.org, QTdrugs List. Oro Valley, AZ: AZCERT, Inc. https://www.crediblemeds.org/. Published 2019. Accessed September 1, 2018.
- 17.Alburikan KA, Aldemerdash A, Savitz ST, et al. . Contribution of medications and risk factors to QTc interval lengthening in the Atherosclerosis Risk in Communities (ARIC) study. J Eval Clin Pract. 2017;23(6):1274-1280. doi: 10.1111/jep.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woosley RL, Romero K, Heise CW, et al. . Adverse drug event causality analysis (ADECA): a process for evaluating evidence and assigning drugs to risk categories for sudden death. Drug Saf. 2017;40(6):465-474. doi: 10.1007/s40264-017-0519-0 [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Szepietowska B, Polonsky B, et al. . Risk of cardiac events associated with antidepressant therapy in patients with long QT syndrome. Am J Cardiol. 2018;121(2):182-187. doi: 10.1016/j.amjcard.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala M, Vicentini A, Brambilla P, et al. . QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry. 2005;4(1):1. doi: 10.1186/1744-859X-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friemel A, Zünkler BJ. Interactions at human ether-à-go-go–related gene channels. Toxicol Sci. 2010;114(2):346-355. doi: 10.1093/toxsci/kfq011 [DOI] [PubMed] [Google Scholar]
- 22.Margulis M, Sorota S. Additive effects of combined application of multiple hERG blockers. J Cardiovasc Pharmacol. 2008;51(6):549-552. doi: 10.1097/FJC.0b013e31817532ee [DOI] [PubMed] [Google Scholar]
- 23.Hausman JA. Specification tests in econometrics. Econometrica. 1978;46:1251-1271. doi: 10.2307/1913827 [DOI] [Google Scholar]
- 24.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235. doi: 10.1056/NEJMoa0806994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straus SM, Sturkenboom MC, Bleumink GS, et al. . Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26(19):2007-2012. doi: 10.1093/eurheartj/ehi312 [DOI] [PubMed] [Google Scholar]
- 26.Risgaard B, Winkel BG, Jabbari R, et al. . Sudden cardiac death: pharmacotherapy and proarrhythmic drugs: a nationwide cohort study in Denmark. JACC Clin Electrophysiol. 2017;3(5):473-481. doi: 10.1016/j.jacep.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee NA, Albert CM. Sudden arrhythmic death. Circ Arrhythm Electrophysiol. 2019;12(7):e007474. doi: 10.1161/CIRCEP.119.007474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim AS, Moffatt E, Ursell PC, Devinsky O, Olgin J, Tseng ZH. Sudden neurologic death masquerading as out-of-hospital sudden cardiac death. Neurology. 2016;87(16):1669-1673. doi: 10.1212/WNL.0000000000003238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zambon A, Polo Friz H, Contiero P, Corrao G. Effect of macrolide and fluoroquinolone antibacterials on the risk of ventricular arrhythmia and cardiac arrest: an observational study in Italy using case-control, case-crossover and case-time-control designs. Drug Saf. 2009;32(2):159-167. doi: 10.2165/00002018-200932020-00008 [DOI] [PubMed] [Google Scholar]
- 30.Drew BJ, Ackerman MJ, Funk M, et al. ; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-1060. doi: 10.1161/CIRCULATIONAHA.109.192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock EP, Finkle J, Fingert HJ, et al. . Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J. 2009;157(5):827-836.e1. doi: 10.1016/j.ahj.2009.02.020 [DOI] [PubMed] [Google Scholar]
- 32.Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated proarrhythmic effects: a review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994;121(7):529-535. doi: 10.7326/0003-4819-121-7-199410010-00009 [DOI] [PubMed] [Google Scholar]
- 33.Tikosyn [package insert]. New York, NY: Pfizer; 2014.
- 34.Centers for Disease Control and Prevention (CDC) Overdose deaths involving prescription opioids among Medicaid enrollees: Washington, 2004-2007. MMWR Morb Mortal Wkly Rep. 2009;58(42):1171-1175. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Prescription Drug Use by Number of Subjects, QTPM Risk, and Autopsy-Adjudicated Cause of Sudden Death