Abstract
Advances in ambulatory intracranial EEG devices have enabled objective analyses of circadian and multiday seizure periodicities, and seizure clusters in humans. This study characterizes circadian and multiday seizure periodicities, and seizure clusters in dogs with naturally occurring focal epilepsy, and considers the implications of an animal model for the study of seizure risk patterns, seizure forecasting and personalized treatment protocols. In this retrospective cohort study, 16 dogs were continuously monitored with ambulatory intracranial EEG devices designed for humans. Detailed medication records were kept for all dogs. Seizure periodicity was evaluated with circular statistics methods. Circular non-uniformity was assessed for circadian, 7-day and approximately monthly periods. The Rayleigh test was used to assess statistical significance, with correction for multiple comparisons. Seizure clusters were evaluated with Fano factor (index of dispersion) measurements, and compared to a Poisson distribution. Relationships between interseizure interval (ISI) and seizure duration were evaluated. Six dogs met the inclusion criteria of having at least 30 seizures and were monitored for an average of 65 weeks. Three dogs had seizures with circadian seizure periodicity, one dog had a 7-day periodicity, and two dogs had approximately monthly periodicity. Four dogs had seizure clusters and significantly elevated Fano factor values. There were subject-specific differences in the dynamics of ISI and seizure durations, both within and between lead and clustered seizure categories. Our findings show that seizure timing in dogs with naturally occurring epilepsy is not random, and that circadian and multiday seizure periodicities, and seizure clusters are common. Circadian, 7-day, and monthly seizure periodicities occur independent of antiseizure medication dosing, and these patterns likely reflect endogenous rhythms of seizure risk.
Keywords: EEG, epilepsy, animal models, intracranial EEG, ambulatory EEG, seizure prediction
Abbreviated summary Dogs can accommodate ambulatory intracranial EEG devices designed for humans. Gregg et al. report that endogenous circadian and multiday seizure periodicities, and seizure clusters are common in dogs with naturally occurring epilepsy. Canine epilepsy can advance the development of seizure forecasting, personalized treatment protocols, and chronotherapy.
Graphical Abstract
Graphical Abstract.
Introduction
People with epilepsy consistently report that the unpredictability of seizures is a primary factor in epilepsy-related disability (Dumanis et al., 2017). However, reports of apparently nonrandom timing and predictability of seizures dates back centuries, and questions about the temporal distribution of seizures persist. Early speculative explanations for the periodic timing of seizures suggested a lunar effect—in 1748 one author writes, ‘the moon’s influence is necessarily greater on the nervous fluid or animal spirits, than on the blood, or any other fluid in the animal body. … Of this class none seem more remarkable than epileptic diseases, … [so that] some fits do constantly return every new and full moon’ (Mead, 1748).
In the early 20th century, such notional explanations were replaced by objective descriptions of the temporal patterns of seizures, based on detailed case records (Gowers, 1901) and seizure logs from a supervised residential facility for people with epilepsy (Langdon-Down and Russell Brain, 1929; Griffiths and Fox, 1938). That work described subject-specific circadian rhythms as well as multiday rhythms with weekly, monthly (notably in men), 5-weekly, 3½ monthly and annual seizure periodicities. Patient-generated seizure diaries are notoriously unreliable (Hoppe et al., 2007; Cook et al., 2013), and until recently the accurate calculation of seizure cycles over long time scales had been impossible (Elger and Mormann, 2013). The development of devices that provide objective, chronic measures of seizure activity in ambulatory patients have led to greater interest in seizure forecasting and the circadian and multiday periodicities in epilepsy. New devices combined with circular statistical methods have provided validation of circadian and multiday seizure cycles in humans (Karoly et al., 2016; Baud et al., 2018; Karoly et al., 2018). Furthermore, seizure timing is shown to often have a phase preference relative to the circadian and multiday oscillations in interictal epileptiform activity in humans, and rats with pharmacologically induced epilepsy (Baud et al., 2018, 2019). A limitation of these human studies of circadian and multiday seizure cycles, however, is the absence of data on patient medication regimens and the potential role of medication timing and adherence on seizure periodicities. In clinical epilepsy care it is common, and best practice, to adjust antiseizure medications in response to continued seizures. The quantitative impact of medication changes on seizure dynamics is unknown.
In 1901, Sir W. R. Gowers described seizure clusters, another feature of the non-random timing of seizures. He called these clusters ‘groups of attacks’, and suggested that ‘seizures beget seizures’ (Gowers, 1901). Seizure clusters continue to be a clinically important area of study (Osorio et al., 2009; Haut, 2015). A discussion of the excitatory and inhibitory neuronal and network mechanisms in ictogenesis and seizure clusters is beyond the scope of this article. However, a relevant question for this article is whether seizure clusters are driven by an inherent self-triggering capacity (‘seizures beget seizures’) in which an initial seizure promotes subsequent seizures. An alternative explanation is that seizure clusters simply reflect fluctuations in seizure risk, in which seizures recur in high-risk phases. In this article, we report on the co-occurrence of seizure periodicities and seizure clusters, which supports this latter explanation, although these are not mutually exclusive mechanisms.
The temporal distribution of seizures is important for understanding seizure dynamics, seizure risk factors, seizure forecasting and for the accurate evaluation of any therapy, e.g. behavioural, pharmacological or electrical stimulation. Progress requires continuous, long-term data and appropriate statistical models for analyzing the temporal patterns of seizures. Animal models are important to study the mechanisms involved in the timing of seizures because they can be studied in a controlled fashion. Animals are not necessarily subject to the medication and neurostimulation changes required in human clinical care, factors which can affect the stationarity of EEG signals and seizure rates. Reliable measurement of seizure periodicities could enable the anticipation of periods of increased seizure risk, with implications for patient care and seizure forecasting (Karoly et al., 2017b; Baud et al., 2018).
Over the past decade, there have been significant advances in long-term ambulatory intracranial EEG (iEEG) devices. Two investigational devices designed for human epilepsy are the NeuroVista Seizure Advisory System (Cook et al., 2013), and the Medtronic Summit System RC+S (Rechargeable Cell with Sensing) (Kremen et al., 2018; Stanslaski et al., 2018), which provide full bandwidth (250–500 Hz sampling rates) continuous ambulatory iEEG recordings.
Naturally occurring canine epilepsy shares many characteristics with human epilepsy (Berendt et al., 1999), and dogs are large enough to accommodate iEEG devices designed for humans (Davis et al., 2011; Coles et al., 2013; Kremen et al., 2018). This study relies on ambulatory iEEG data recorded in dogs with naturally occurring epilepsy using the NeuroVista and the Medtronic RC+S devices. We show that canine circadian and multiday seizure cycles, and seizure clusters can be characterized over months-long time scales with ambulatory iEEG devices. Additionally, we show that seizure periodicities and seizure clusters are common in dogs, as they are in humans, and can occur independently from antiseizure medication effects. Our findings suggest that dogs with naturally occurring focal epilepsy are a good platform for the study of the non-random patterns of seizure risk and can aid the development of seizure forecasting methods. Dogs provide a model system for the development of adaptive therapies, for example, chronotherapy with which therapies are delivered based on changing seizure risk profiles.
Materials and methods
Participants and data collection
Sixteen dogs with naturally occurring focal epilepsy were implanted and monitored with long-term mobile iEEG devices (Fig. 1). Fourteen dogs were monitored with the NeuroVista Seizure Advisory System described previously (Davis et al., 2011; Cook et al., 2013; Howbert et al., 2014; Brinkmann et al., 2015), and four dogs were monitored with the Medtronic Summit Research System RC+S device described previously (Kremen et al., 2018; Stanslaski et al., 2018). Two dogs underwent recording with both devices—the initial monitoring was with the NeuroVista device, followed by explanation, and then implantation of the RC+S device. All dogs were cared for in a research kennel environment. Inclusion criteria were that dogs were had at least 30 recorded seizures distributed over at least 8 seizure days. Daily antiseizure medications were used in an effort to prevent prolonged seizures and minimize morbidity and mortality. The Mayo Clinic, University of California, Davis, and the University of Minnesota Institutional Animal Care and Use Committees approved the study.
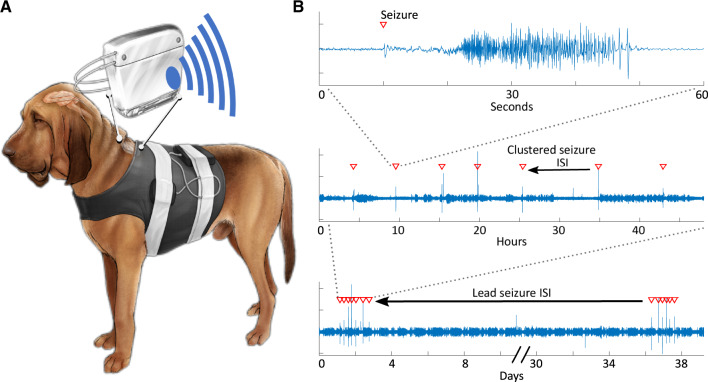
Figure 1.
Temporal distribution of seizures at multiple time scales in canine epilepsy. (A) Schematic of a dog with epilepsy and an implanted ambulatory iEEG device. (B) Raw iEEG tracings from a single referential contact displayed at multiple time scales, which shows a seizure, a seizure cluster and a pair of seizure clusters separated by a prolonged ISI. Red triangles indicate seizure onset.
The NeuroVista device provides continuous iEEG recordings from 16 electrodes with sampling rate of 400 Hz, and has no electrical stimulation functionality. The Medtronic RC+S can deliver electrical stimulation to 16 contacts and has 4 dynamically selectable (from a total of 16) bipolar sensing channel pairs, with sampling rates of 250 or 500 Hz. No electrical brain stimulation was delivered during this study. Device specifications and details of human and canine recordings have been described previously (Davis et al., 2011; Cook et al., 2013; Howbert et al., 2014; Brinkmann et al., 2015; Kremen et al., 2018).
Experimental design
This study hypothesized that, similar to human epilepsy, dogs with naturally occurring focal epilepsy have non-random temporal distributions of seizures with a range of periodicities, and clustering. Electrographic seizures were identified by previously validated, high-sensitivity automated detections (Baldassano et al., 2017; Kremen et al., 2018), followed by visual review and confirmation by a board certified epileptologist (G.W.).
Seizure periodicities
Circular statistics were used to assess the significance of periodic data. Circular plots and circular histograms display the phase of events relative to a defined period (e.g. 24-h, 7-days, 1-month). To evaluate a 24-h cycle, one sweep around the circular plot is 24 h. The time of day of seizure onset can then be plotted around a 24-h circle for circadian rhythms, or a 7-day circle for circaseptan (weekly) rhythms, and circular histograms can show the phase preference and spread of events for the respective period. The amplitude of the mean resultant vector, or R-value, provides a measure of the circular non-uniformity for a given period. To calculate the R-value, the timing of each event is plotted on a circular graph, with an arbitrary unitless amplitude (radius). Vectors are plotted from the origin to each event around the circle—vector summation generates a resultant mean vector, and the normalized magnitude, the R-value, is a measure of the consistency of event timing. Randomly distributed events relative to a cycle will have a resultant R-value of 0, while if all events occur at the same phase in the cycle the R-value equals 1.
Seizure periodicities were assessed for 24-h, 7-day and approximately monthly periods. Lead seizures were defined as being separated from a preceding seizure by at least 24-h, and clustered seizures are defined as seizures that occur within 24 h of a preceding seizure (Haut et al., 2005) (Fig. 1).
To prevent seizure clusters from biasing results for 7-day and monthly period (and periods ≥ 48 h), only lead seizures were included in those analyses. Inclusion of clustered seizures in the assessment of multiday periodicities would place multiple events from a single cluster around the same phase of the cycle, which would increase the non-uniformity of seizure occurrences, and inflate the R-value. For circadian rhythms (and rhythms ≤48 h), seizures occurring within 1 h (rather than 24 h) of a preceding seizure were withheld from analysis for the same reason. Note that 24-h exclusion period for circadian rhythms could spuriously suggest a circadian periodicity. Relative risk scores were calculated to quantify seizure risk (number of seizures per unit time) in the quartile centered at the peak phase of the mean resultant vector, relative to seizure risk of the remaining three quartiles.
Seizure clusters
As above, clustered seizures were defined as seizures occurring within 24 h of a preceding seizure, consistent with a common clinical definition and prior studies (Dreifuss et al., 1998; Haut, 2015; Karoly et al., 2018). A clustered seizure is believed to be a seizure whose occurrence is influenced by a preceding seizure (Haut, 2015).
The Poisson model: provides a discrete probability distribution of the number of times a random event will occur in a given amount of time, where λ is both the mean and variance of the distribution. The Poisson model was used to evaluate the randomness of the temporal distribution of seizures for dogs with and without seizure clusters, with λ defined as the mean number of seizures per day (total number of seizures divided by the duration of monitoring in days). Dogs were classified to have seizure clusters if the temporal distribution of their seizures had a significant deviation from the Poisson distribution consistent with clustering, as quantified by the Fano factor (see ‘Statistical analysis’ section). Plots that compare the patient seizure events to a Poisson distribution can be used to visualize distributions that are consistent with seizure clustering. Histograms of seizure frequency were generated to assess the likelihood of a clustered seizure to occur relative to the time elapsed since the prior seizure, as previously described (Osorio et al., 2009). For each dog, all clustered seizures were evaluated, while lead seizures were withheld.
Circular statistics were used to evaluate the significance of seizure periodicity for seizures that occur within clusters. For each patient the median within-cluster interseizure interval (ISI) was determined (lead seizures withheld). Median within-cluster ISI periodicity was evaluated using the circular histogram and the R-value. Consistency of between-cluster periodicity was visualized with box-plots for which the central mark is the median, the box edges are the 25th and 75th percentiles, and the whiskers extend to the most extreme non-outlier datapoints; outliers are plotted individually. Outliers were defined to be more extreme than 2.7 times the standard deviation (covers 99.3% of normally distributed data).
Features of lead seizures and clustered seizures were compared to evaluate if these groups had unique characteristics. The durations of lead and clustered seizures were compared. The correlation of preceding ISI and seizure duration were evaluated for lead seizures and clustered seizures. Rare seizures with durations greater than 30 min were removed from ISI-seizure duration analysis, except for dog 3 whose seizures typically that lasted greater than 30 min. Previous studies in humans (Ferastraoaru et al., 2016) report that seizures within clusters have shorter duration than isolated seizures and terminal seizures. To explore this result in the canine model, seizures were further subcategorized: lead seizures were separated into isolated seizures (occur independently from a cluster) and lead seizures [first seizure in a cluster; marked with ‘(prime) for clarity’]. Clustered seizures were separated into terminal seizures (final seizure in a cluster) and ‘clustered’ seizures (occur within a cluster, excluding lead’ and terminal seizures; marked with ‘for clarity’).
Statistical analyses
For assessments of seizure periodicity, statistical significance was determined with the Rayleigh test (Berens, 2009). The Rayleigh test determines how large the R-value must be to reject the null hypothesis that events are uniformly distributed with respect to the period length. The Rayleigh test assumes that the sample is drawn from a von Mises distribution, the corollary of a Gaussian distribution for circular data, and is particularly suited to detect unimodal deviations from circular uniformity. The Benjamini–Hochberg false discovery rate (FDR) procedure was used to correct for multiple comparisons, and the FDR was set at 0.1 (Benjamini and Hochberg, 1995). The Benjamini–Hochberg FDR procedure compares rank-ordered P-values to a pre-specified critical value threshold to account for multiple comparisons and determine statistical significance.
The Fano factor, or index of dispersion (Fano, 1947), measures the dispersion of a probability distribution, and can quantify event clustering. It has been used to characterize neural spiking (Litwin-Kumar and Doiron, 2012), and seizure clusters (Karoly et al., 2017a). The Fano factor is the ratio of the variance of event rates relative to the mean. For a Poisson process, the variance and mean are equal, and the Fano factor = 1. A process that demonstrates clustering has a Fano factor > 1, and is said to be over-dispersed. Regularly spaced periodic events have a Fano factor < 1. The Fano factor is dependent on the intervals over which event rates are evaluated, and we evaluated the Fano factor for days, weeks and months-long intervals. Fano factor P-values were calculated using a previously described method based on the gamma distribution to test if the observed temporal distribution of events was generated by a Poisson process (Eden and Kramer, 2010).
Seizure durations and ISIs both within and between the lead seizure population and clustered seizure population were evaluated with unpaired two-sample t-tests, and the Pearson’s correlation coefficient (Pearson’s r). Further assessment of seizure durations after additional subclassification into isolated, ‘lead’, ‘clustered’ and terminal seizures was performed with one-way analysis of variance testing (ANOVA). Statistical significance was defined as P < 0.05. All analyses were performed in MATLAB (version 2017 b, Mathwords Inc, Natick, MA, USA). Some analyses used the MATLAB Circular Statistics Toolbox (Berens, 2009).
Data availability
All data and MATLAB scripts are available at https://msel.mayo.edu/research.html.
Results
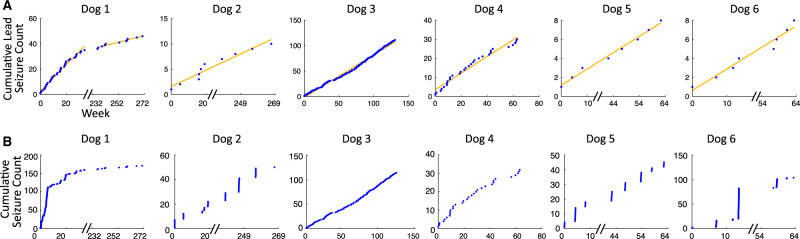
Six dogs met inclusion criteria (five males), and were monitored with the NeuroVista iEEG device (n = 3), NeuroVista device transitioned to RC+S (n = 2) or Medtronic RC+S device (n = 1). There was one beagle (Dog 3), one coonhound (Dog 6) and four mixed-breed dogs. Details of canine monitoring are reported in Table 1. Dogs were monitored for an average of 65 weeks (standard deviation 35 weeks), between 13 August 2009 and 31 August 2018. Subject-specific lead seizure rates were stable throughout the recording for five dogs (Fig. 2A). Dog 1 had a 2-year non-recording period between NeuroVista explanation and RC+S device implantation. This dog exhibited a stable lead seizure rate while monitored by the NeuroVista device, and a stable but different rate while monitored by the RC+S device (Table 2). Dogs 1, 2, 5 and 6 had seizure clustering, some of whom can be visually distinguished from dogs without seizure clustering (Fig. 2B). Three dogs died during monitoring. Dog 2 died secondary to a device related infection. Dog 5 died secondary to a traumatic head injury. Dog 6 died as a complication of surgery during implantation of the RC+S system (subsequent to NeuroVista monitoring).
Table 1.
Subject characteristics and features of seizure timing in canine epilepsy. The ISI columns lists mean and standard deviation. Statistically significant periodicities after FDR correction are in bold font. A trend towards significance that did not survive FDR correction is in italic font. For the monthly periodicity column, the cycle duration is listed parenthetically
| Seizure Count |
ISI |
Periodicity (Rayleigh test P-value) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Device | Recording Time (weeks) | All | Lead | Lead seizures (days) | Clustered seizures (hr) | Circadian | Circaseptan | Approximately monthly | |
| Dog 1 | M | NV → RC+S | 69 | 171 | 46 | 10.6 (14.6) | 5.7 (5.9) | 0.001 | 0.61 | 0.0007 (27 days) |
| Dog 2 | M | NV → RC+S | 47 | 50 | 10 | 41.1 (30.1) | 3.4 (5.6) | 0.14 | 0.18 | 0.046 (28¼ days) |
| Dog 3 | F | RC+S | 131 | 114 | 111 | 8.3 (5.5) | 16.4 (6.4) | 0.009 | 0.72 | 0.80 (28 days) |
| Dog 4 | M | NV | 63 | 32 | 30 | 15.1 (16.5) | 10.9 (15.3) | <5 × 10−5 | 0.18 | 0.97 (28 days) |
| Dog 5 | M | NV | 49 | 45 | 8 | 31.7 (5.7) | 5.9 (2.9) | 0.32 | 0.036 | 0.016 (27 days) |
| Dog 6 | M | NV | 30 | 104 | 8 | 21.6 (15.6) | 1.7 (3.8) | 0.88 | 0.028 | 0.96 (28 days) |
| Dog 7 | M | NV | 7 | 0 | 0 | |||||
| Dog 8 | M | NV | 38 | 2 | 2 | |||||
| Dog 9 | M | NV | 0 | 0 | 0 | |||||
| Dog 10 | F | NV | 33 | 12 | 4 | |||||
| Dog 11 | M | NV | 6 | 90 | 4 | |||||
| Dog 12 | M | NV | 17 | 0 | 0 | |||||
| Dog 13 | M | NV | 5 | 0 | 0 | |||||
| Dog 14 | M | NV | 4 | 0 | 0 | |||||
| Dog 15 | M | NV | 18 | 0 | 0 | |||||
| Dog 16 | F | RC+S | 47 | 17 | 6 | |||||
NV = NeuroVista
Statistically significant results are in bold font (P < 0.05).
Figure 2.
Lead seizure counts and total seizure counts over time. (A) Cumulative lead seizure counts over time. Subject specific seizure rates [equal to the slope of the least squares line (orange line)], were stable throughout the recording for five dogs. Dog 1 had a 2-year non-recording period between NeuroVista explanation and RC+S device implantation, over which time there was a change in seizure rate. (B) Total seizure counts plotted relative to time. Dogs 1, 2, 5 and 6 had significant seizure clustering. Non-recording time periods were removed from analysis and are indicated by hash-marks.
Table 2.
Seizure rate stability over time. Pearson’s r provides a measure of the linear correlation between seizure occurrences and time
| Seizure rate (seizures per week) | Pearson's r | P | |
|---|---|---|---|
| Dog 1 | 1.04 | 0.26 | 0.98 | 0.96 | <10−5 | <10−5 |
| Dog 2 | 0.16 | 0.93 | <10−5 |
| Dog 3 | 0.84 | 0.99 | <10−5 |
| Dog 4 | 0.44 | 0.96 | <10−5 |
| Dog 5 | 0.17 | 0.99 | <10−5 |
| Dog 6 | 0.23 | 0.96 | 2.7 × 10−5 |
Statistically significant results are in bold font (P < 0.05).
Circadian and multiday seizure periodicities
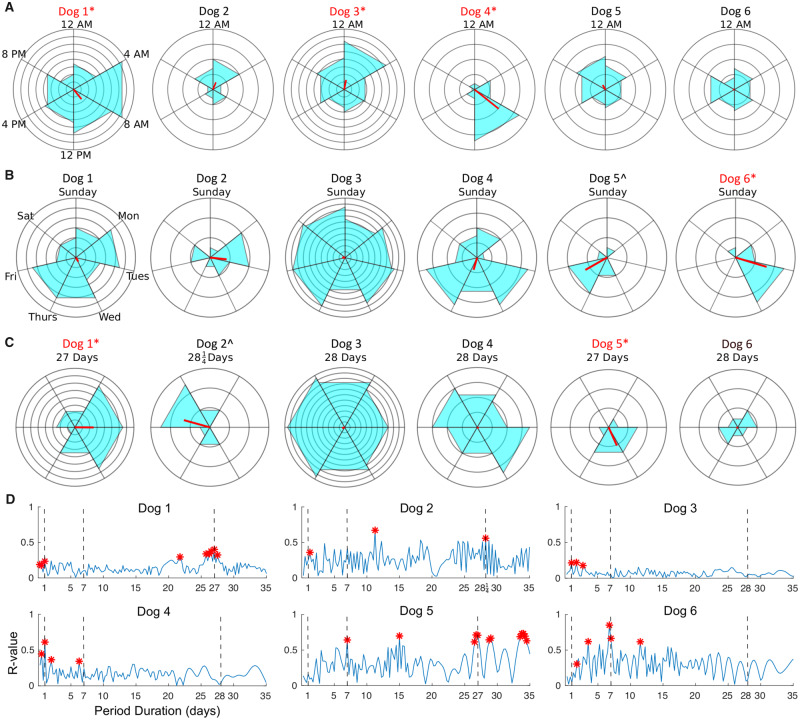
Five out of the six subjects had statistically significant periodicity of seizure timing—three dogs had circadian seizure periodicity, one dog had circaseptan seizure periodicity and two dogs had approximately monthly seizure periodicity (Table 1; Fig. 3). Dogs 1, 3 and 4 had subject-specific, circadian seizure periodicities, with increased relative risk of seizure occurrence of 5.1 (at 9:25 AM), 4.8 (at 12:46 AM) and 12.5 (at 8:34 AM) times higher than baseline (Fig. 3A).
Figure 3.
Circadian, circaseptan and monthly seizure periodicity. Circular histograms of all dogs for (A) 24-h, (B) 7-day and (C) approximately monthly period durations. The red bar is the resultant vector, or R-value. Concentric rings demarcate the number of seizures (five seizures per concentric ring in (A), two seizure per ring in (B) and (C). Dogs with statistically significant periodicity are marked in red font and by ‘asterisk’. In (B) and (C), ‘hash’ next to Dog 5 and Dog 2, respectively, indicates a trend towards significant periodicity that did not survive FDR correction. (D) The R-value is plotted for cycle durations of 6 h to 35 days in steps of quarter-days. (A) Results for lead seizures only. Statistically significant R-values as determined by the Rayleigh test (P < 0.05 without correction for multiple comparisons) are marked in red.
Dog 6 had a circaseptan seizure periodicity with a corresponding relative risk of 5, and peak phase in the early morning of Wednesdays around 2:00 AM. Dog 5 had a trend towards a circaseptan periodicity which did not survive FDR correction, with peak phase on Friday afternoons around 4:00 PM (Fig. 3B). Dog 1 had an approximately monthly seizure periodicity with relative risk of 2.1, as did Dog 5 with a relative risk of 21. Dog 4 had a trend towards circaseptan periodicity (Fig. 3B), and Dog 2 had a trend towards a monthly periodicity (Fig. 3C), which did not survive FDR correction.
Other multiday cycle durations were not evaluated as part of the primary analysis. However, Fig. 3D shows the amplitude of the resultant vector (lead seizures only) for cycle durations of 6 h to 35 days in steps of quarter-days to help situate the daily, weekly, and monthly periodicity results within the larger context of the distribution of apparent seizure periodicities. Results in Fig. 3D are displayed without correction for multiple comparisons; with Bonferroni correction (n = 140) the only significant periodicity is a circadian rhythm for Dog 4. See Supplementary Fig. 1 for the same periodicity analysis but including all seizures.
Seizure periodicities should be considered in the context of antiseizure medication regimen. Dog 3 did not receive daily antiseizure medications. The only chronic antiseizure medication exposure for Dogs 1, 2, 5 and 6, was twice daily dosing of phenobarbital. Dog 4 was treated with a combination of Phenobarbital, Levetiracetam, Zonisamide and Potassium Bromide during monitoring. Medication records are unavailable for the initial period of monitoring for Dog 5 and 6; however, the 63% of lead seizures for Dog 5, and half of the lead seizures for Dog 6 were recorded with contemporaneous medication records. See Supplementary Tables 1–6 for detailed medication records for each dog. Some dogs received benzodiazepine rescue medication for prolonged seizures or seizure clusters, however detailed records of rescue medications are not available.
Seizure clusters
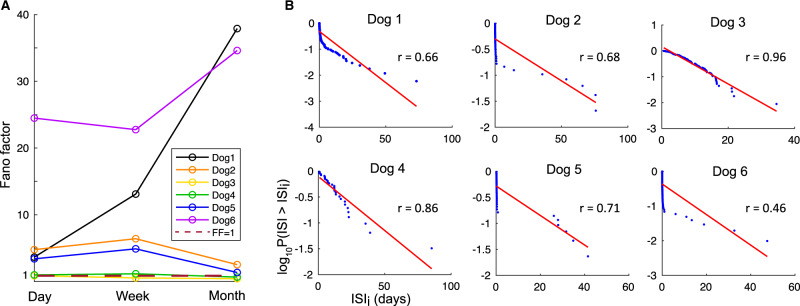
Four dogs (Dogs 1, 2, 5 and 6) had over-dispersed seizure rates by Fano factor analysis consistent with seizure clustering (Fig. 4A, Table 3), and the temporal distribution of seizures for these dogs deviated from Poisson distribution (Fig. 4B, Supplementary Table 7). Correspondingly, these dogs could have visually apparent seizure clustering on the cumulative seizure count plots (Fig. 2B). A high proportion of seizures occurred within clusters—66%, 67%, 82% and 79%, for Dogs 1, 2, 5 and 6, respectively. Furthermore, a high proportion of lead seizures progressed to seizure clustering – 39%, 80%, 2.7%, 6.7%, 100%, and 75% for Dogs 1 through 6.
Figure 4.
Seizure clustering. (A) Fano factor for each dog for day, week and month-long intervals. (B) Blue circles are the logarithm of the proportion of seizures with ISI > xi, relative to ISI xi, for each subject. Linearity of the distribution in consistent with a Poisson process, shown in red (Taubøll et al., 1991). A negative deviation from Poisson distribution (down and to the left) indicates seizure clustering. The dogs whose temporal distribution of seizures best fit a Poisson process (Pearson’s r in graph) also have non-significant Fano factor values (Dogs 3 and 4), and vice versa.
Table 3.
Fano factor and seizure clustering. Columns list the Fano factor value and associated P-value
| Fano factor |
||||||
|---|---|---|---|---|---|---|
| Day |
Week |
Month |
||||
| P | P | P | ||||
| Dog 1 | 3.7 | <10−5 | 13.1 | <10−5 | 37.9 | <10−5 |
| Dog 2 | 4.8 | <10−5 | 6.4 | <10−5 | 2.52 | 0.005 |
| Dog 3 | 0.9 | 0.99 | 0.54 | 0.99 | 0.42 | 0.99 |
| Dog 4 | 0.99 | 0.57 | 1.2 | 0.20 | 0.66 | 0.81 |
| Dog 5 | 3.4 | <10−5 | 4.9 | <10−5 | 1.4 | 0.21 |
| Dog 6 | 24.5 | <10−5 | 22.8 | <10−5 | 34.6 | <10−5 |
Statistically significant results are in bold font (P < 0.05).
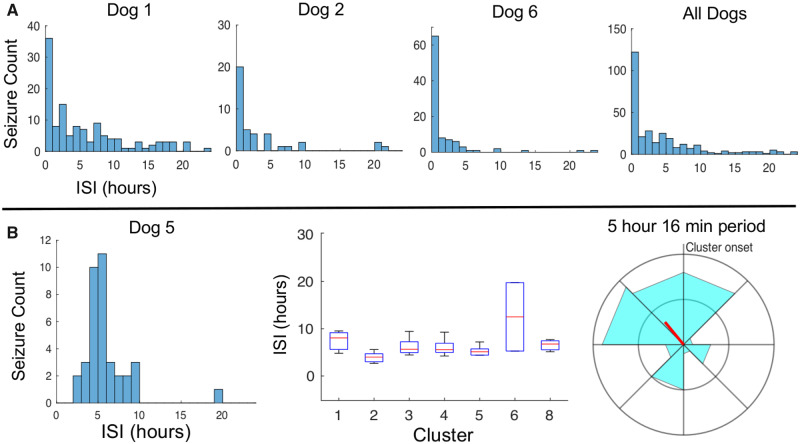
Histograms of clustered seizures indicate the likelihood of seizure occurrence relative to the time elapsed since the last seizure. For three dogs (Dogs 1, 2 and 6), clustered seizures had progressive reduction in seizure likelihood with increasing time elapsed since the last seizure (Fig. 5A). Dog 5 had within cluster seizure periodicity (P = 0.0075 by Rayleigh test), with median ISI 5 h and 16 min (Fig. 5B). Dogs 3 and 4 had very few clustered seizures (Supplementary Fig. 2).
Figure 5.
Dynamics of clustered seizures. All dogs with seizure clusters. (A) Histograms of clustered seizure counts relative to ISI (Dog 1, n = 125; Dog 2, n = 40; Dog 6, n = 96; and All Dogs, n = 298 seizures). (B) Dog 5 had periodicity of clustered seizures apparent in the histogram (n = 37 seizures). The box-plot shows ISI data for each of the dog’s seizure clusters that contained at least three seizures (cluster 1, n = 4; cluster 2, n = 10; cluster 3, n = 4; cluster 4, n = 7; cluster 5, n = 6; cluster 6, n = 7; cluster 8, n = 4). The circular histogram period length was defined as the clustered seizure median ISI, which is 5 h and 16 min; five seizures per concentric ring.
The relationships between seizure duration and ISI both within and between lead seizure and clustered seizure categories was complex and patient specific (Supplementary Fig. 3, Supplementary Table 8). Lead seizure durations were significantly longer than clustered seizure durations for Dog 1. Lead seizure ISI could have significant positive correlation with seizure duration (Dog 1, Dog 3). Clustered seizure ISIs could have a significant positive correlation with seizure duration (Dog 1) or negative correlation (Dog 6). ANOVA testing that compared the subcategories of lead’ seizures, isolated seizures, clustered’ seizures, and terminal seizures resulted in a significant result for one dog (Dog; P < 10−5) (Supplementary Fig. 4, Supplementary Table 9). A comparison of isolated and terminal seizure durations versus lead’ and clustered’ seizure durations demonstrated longer durations for isolated and terminal seizures for Dog 1 only (P < 10−5). Of note, rare outlier seizure durations that were >30 min were withheld from analysis of seizure durations (two seizures for Dog 1), except for Dog 3 whose typical seizures were prolonged.
Discussion
In this study, we show that non-random seizure temporal patterns with significant periodicities are common in dogs with naturally occurring focal epilepsy. Three of the six dogs had circadian seizure periodicity, one of the six dogs had circaseptan (7-day) seizure periodicity, and two dogs had approximately monthly seizure periodicity. Four dogs had seizure clustering (seizures that recur within 24 h of a preceding seizure). Circadian, circaseptan, and monthly seizure periodicity (Karoly et al., 2018), and seizure clustering (Taubøll et al., 1991) have previously been demonstrated in humans as well. All of the dogs in the study exhibited at least one non-random seizure timing feature—circadian, circaseptan or monthly seizure rhythm, or seizure clustering. Our findings suggest that dogs can serve as a platform to study the mechanisms that influence seizure timing, and advance seizure forecasting, and chronotherapy protocols that deliver medication or neurostimulation based on evolving seizure risk.
Seizure periodicities and seizure clusters in human epilepsy have been reported in the literature, and yet questions remain about the medication timing, brain networks, behavioural states and physiological mechanisms behind these patterns. Prior ambulatory iEEG-based human studies of seizure periodicities did not track medication use, which left unresolved questions about the impact of daily medications and medication adherence on circadian and multiday rhythms (Karoly et al., 2016, 2018; Baud et al., 2018). Our findings, with detailed contemporaneous medication records, provide support to the hypothesis that seizure periodicities reflect endogenous rhythms of seizure risk.
Franz Halberg introduced the term ‘circadian’ (from Latin circa, meaning ‘around’, and diēm, meaning ‘day’) in the 1950s to describe biological oscillations that have ∼24-h periodicity. Since that time circadian rhythms have been extensively studied in humans and many species of animals, plants, fungi and bacteria. Circadian rhythms modulate an array of physiological processes including sleep cycles, body temperature and behavioural state (Aschoff, 1965), autonomic and hormonal activity (Reppert and Weaver, 2002) and brain activity as measured by EEG (Gundel and Hilbig, 1983). Extensive work has demonstrated cellular pacemaker mechanisms behind underlying biological rhythms (Jagota et al., 2000). Circadian seizure periodicity was present in three of the six dogs studied, and the relative risk of seizure occurrence at peak phase of the cycle ranged between 4.8 and 12.5 times the baseline risk. The ability of this periodicity to stratify epochs of increased seizure risk could inform whether to engage in activities at different phases of the day, and direct the timing of medication and neurostimulation.
The peak phase of circadian seizure cycles appears to be subject specific; however, the sample size of this study limits generalizability. Subject-specific periodicity is consistent with prior human studies that reported the peak phase of circadian seizure periodicities to be distributed throughout the day (Karoly et al., 2018) (with relative predominance between 6:00 AM and 9:00 AM), and may depend on the brain regions generating seizures (Durazzo et al., 2008).
In addition to circadian rhythms, circaseptan rhythms have been a focus of prior investigations, and some authors have proposed the presence of endogenous circaseptan rhythms in humans and single-celled organisms (Halberg et al., 1965; Schweiger et al., 1986). Weekly cycles have been demonstrated in pathological processes such as myocardial infarction (Willich et al., 1994) and stroke (Kelly-Hayes et al., 1995), as well as mood fluctuations (Almagor and Ehrlich, 1990). In this study, Dog 6 had a circaseptan rhythm with a 5-fold higher relative risk of seizures at peak phase. Dog 5 had a trend toward a circaseptan rhythm that did not survive FDR correction. More work is needed to clarify if circaseptan seizure cycles reflect a purely endogenous periodicity, or are behaviourally driven and related to changes in sleep patterns, activities or stress between weekday and weekend routines.
Dogs 1 and 5 had approximately monthly seizure rhythms with 2-fold to 20-fold higher relative risk of seizures at peak phase, and Dog 2 had a trend towards a monthly rhythm that did not survive FDR correction. Monthly rhythms of seizure risk have been well described in women with catamenial epilepsy (Herzog et al., 2004) and attributed to monthly hormonal changes. However, there is considerable evidence that monthly seizure rhythms are common in both women and men (Griffiths and Fox, 1938; Karoly et al., 2016, 2018; Baud et al., 2018), which cannot be fully explained by catamenial cycling. All of the dogs with a monthly seizure cycle in this study were male. Furthermore, the period between canine estrus cycles is typically 5–6 months.
The dogs in this study were cared for in an academic veterinary kennel setting, which provided detailed medication records, and assured medication adherence. Daily medications were used to avoid prolonged seizures and minimize morbidity and mortality. Dog 3 did not receive any daily antiseizure medications, which indicates that this dog’s circadian seizure periodicity is not an artefact of daily oscillations in medication levels. Dog 1 received twice daily doses of phenobarbital throughout the study. The half-life of phenobarbital in dogs is ∼72.3 h (Pedersoli et al., 1987), and it seems unlikely that the small fluctuations in phenobarbital levels would induce a circadian seizure periodicity. We cannot exclude the possibility of entrainment of an endogenous rhythm or a shift in the peak phase by daily fluctuations in drug levels. Dog 4 was treated with a combination of phenobarbital, potassium bromide (half-life 15.2 days) (March et al., 2002), levetiracetam (half-life 3.6 h) (Isoherranen et al., 2001) and zonisamide (half-life 13 h) (Orito et al., 2008), and the influence of medications on this dog’s circadian seizure periodicity is uncertain. The lack of a relative peak at the opposite phase (12-h from peak) of the circadian circular histogram (Fig. 3A), argues against a purely medication driven periodicity, given twice-daily dosing. Medication records were unavailable for the first half of Dog 6’s lead seizures; however, the circaseptan periodicity is preserved in the second half of lead seizures, which occurred without medications, suggesting that medication did not induce the periodicity (Supplementary Fig. 5 shows circular histogram of Dog 6’s lead seizures off of antiseizure medication).
Given the lack of medication records and undetermined influence of medications on seizure periodicities in prior human iEEG-based studies (Baud et al., 2018; Karoly et al., 2018), these findings notably support the hypothesis that endogenous rhythms can drive periodic changes in seizure risk. Further work is needed to evaluate how seizure periodicities and clusters respond to personalized seizure risk-based treatments. One could imagine improved seizure control with reduced side effect profile by preferentially delivering medications when seizure risk is high. However, it is possible that modulated pharmacotherapy could simply induce a phase shift in the timing of seizures.
Other multiday seizure cycle durations, which were not part of the primary analyses, are presented to situate circadian, circaseptan and monthly periodicities within the larger context of apparent periodicities (Fig. 3D for lead seizures; Supplementary Fig. 1 for all seizures). We favour the use of lead seizures to evaluate these periodicities. Closely spaced seizures within a cluster will fall next to each other on a circular plot regardless of the period duration being tested. When all seizures are used for subjects with a single or few dominant clusters the results can be confounding and show many significant periodicities, or even that all tested periods are significant (Supplementary Fig. 1, Dog 6).
Zeitgebers (from German, ‘time giver’) are environmental signals that synchronize or entrain biological rhythms to the external world. Examples of Zeitgebers are daily sunlight and temperature changes that synchronize circadian rhythms to the 24-h rotation of the earth. Without Zeitgebers the circadian biological rhythms in humans and animals can desynchronize from a 24-h period and drift by minutes or hours per day, and compound over time (Aschoff, 1965). There are known Zeitgebers than entrain daily, weekly and annual (season changes) biological and behavioural rhythms. The dogs in our study were exposed to daily Zeitgebers (cycles of light/dark, mealtimes and interactions with caregivers), as well as weekly Zeitgebers (changes in meal schedule, and interactions with caregivers between weekdays and weekends).
An important concern for the analysis of free-running endogenous multiday rhythms is to maintain synchronization between the biological rhythm and the observer-selected period of interest. Desynchronization may occur for a number of reasons: (i) the period being tested differs from the endogenous rhythm (even small differences will compound over multiple cycles), (ii) the endogenous process is non-stationary, (iii) the endogenous rhythm has an outlier period or (iv) the endogenous rhythm is skewed. Under these conditions, the biological rhythm will drift from the observer-defined period. Given the lack of an associated Zeitgeber for longer time-scales, it is important to consider desynchronization between periods being tested and endogenous rhythms. More work is needed to ensure synchronization over time and for prospective studies.
Seizure clusters were common in this cohort (67% of the subjects), which is higher than the 25–50% prevalence reported for people with epilepsy (Milton et al., 1987; Taubøll et al., 1991). The temporal distribution of seizures in seizure-cluster-dogs had marked deviation from Poisson distribution and elevated Fano factor values (Fig. 4, Table 3). Quantifying seizure clustering provides information that can improve estimates of seizure probabilities over different time intervals.
For three dogs, clustered seizures had characteristic temporal distributions: there was progressive reduction in seizure likelihood as time elapsed since the last seizure increased. In other words, within a seizure cluster, the longer one has gone since the prior seizure, the less likely one is to have a subsequent seizure. This feature of seizure clusters has also been demonstrated in human data (Osorio et al., 2009). One dog demonstrated significant periodicity of clustered seizures, with ISI duration of 5 h and 16 min.
Two potential mechanisms for seizure clustering are (i) a self-triggering mechanism whereby a single spontaneous seizure influences seizure-likelihood for a following period of time (‘seizures beget seizures’) (Gowers, 1901), and (ii) fluctuations in seizure threshold produce sustained periods when conditions for a seizure are favourable, independent from any given seizure. It is appealing to attribute seizure clusters to periodic fluctuations in seizure threshold (mechanism ii) given the co-occurrence of multiday seizure periodicities and seizure clusters in our cohort (significant multiday periodicities for Dogs 1, 5 and 6, with a trend towards a monthly periodicity in Dog 2, all of whom have seizure clusters). This finding, however, does not preclude a role for seizures to influence subsequent seizure risk. Further work is needed to clarify the impact that either mechanism has on seizure clusters. Inducing seizures at different phases of an animal’s endogenous rhythm could help distinguish fluctuations in seizure threshold from a self-triggering mechanism.
Seizure clusters are well described in humans and have significant impact on the clinical safety and wellbeing of patients (Haut, 2015). Seizure clusters can evolve into status epilepticus (Mitchell, 2002), result in more emergency department visits (Haut, 2006), prolong hospitalizations (Spatola et al., 2013) and increase the risk of post-ictal psychosis (Kanner et al., 1996). Rescue medication protocols are in use to prevent or abort seizure clusters and the associated morbidity (Abou-Khalil et al., 2013). A high proportion of people with epilepsy have seizure clusters, which underscores the importance of medication and neurostimulation protocols to address these periods of increased seizure risk.
To our knowledge, there are no neurostimulation protocols in use designed to adaptively cover the sustained period of increased seizure risk in individuals prone to seizure clusters. FDA-approved neurostimulation devices for epilepsy either provide responsive neurostimulation after seizure onset (Morrell and Group, 2011), or lack seizure sensing function and provide open-loop, duty cycle stimulation (e.g. cycling stimulation on for 1 min and off for 5 min) (Fisher et al., 2010). Dogs with epilepsy could be used as a model system to develop novel closed-loop neurostimulation protocols to prevent or moderate seizure clusters, a neurostimulation corollary to existing medication protocols for patients predisposed to seizure clusters.
The relationship between ISI and seizure duration, both within and between the lead seizure category and clustered seizure category, was complex and subject specific (Supplementary Fig. 3, Supplementary Table 8). Our findings do not demonstrate a consistent relationship between ISI and seizure duration. When seizures were subdivided into lead’ (lead seizure not including isolated seizures), isolated, clustered’ (clustered seizures not including terminal seizures) and terminal seizure categories only Dog 1 had significant differences in seizure durations (Supplementary Fig. 4, Supplementary Table 9). For Dog 1 isolated and terminal seizures were longer than lead’ and clustered’ seizures. This finding is consistent with human work that suggests the shorter duration of lead’ and clustered’ seizures may fail to activate the inhibitory mechanisms necessary to prevent a seizure cluster (Ferastraoaru et al., 2016). This was not found in the other three dogs with seizure clusters.
This study is limited by the number of subjects with long-term ambulatory iEEG recordings. Although the number of subjects is relatively small, the data sets are large and span multiple months of iEEG recording with > 30 seizures for each subject. Evaluation of the stability of subject-specific circadian and multiday seizure rhythms over time will benefit from even longer recording durations in the future. Some dogs had relatively infrequent seizures, which impacts our ability to establish patterns of seizure periodicity. Dog 1 had variation in seizure rate over time, and this may cause challenges for prospective studies. Very long cycles, such as annual cycles, could not be evaluated due to insufficient recording duration. We did not track the behavioural state of dogs in this study, so associations between seizures and sleep or other states were not assessed. We used objective quantification of electrographic seizures for the study, given the limitations of assessing non-motor symptoms of seizures in dogs. The study was retrospective, and a future goal would be to use knowledge about the temporal dynamics of seizure events to prospectively forecast periods of increased seizure risk, and to trial personalized medication and neurostimulation treatment protocols.
To our knowledge, this is the first study to objectively characterize circadian and multiday seizure periodicities, and seizure clusters in dogs with naturally occurring epilepsy. Seizure periodicities and seizure clusters are common in dogs, as they are in humans, and dogs may serve as a model system to evaluate the physiological and behavioural mechanisms that contribute to the non-random temporal distributions of seizures. A better understanding of seizure periodicities and seizure clusters can inform personalized profiles of seizure risk, and may advance seizure forecasting models. Dogs can accommodate neurostimulation devices designed for humans, and may enable the development of novel chronotherapy protocols where neurostimulation and medications are adjusted based on seizure risk.
Supplementary Material
Acknowledgements
We would like to acknowledge the support of Nate Nelson and the veterinary staff at Mayo Clinic, UC-Davis, and University of Minnesota.
Glossary
- ANOVA
analysis of variance
- FDR
false discovery rate
- iEEG
intracranial electroencephalography
- ISI
interseizure interval
Funding
This research was supported by American Epilepsy Society Research & Training Fellowship for Clinicians (N.M.G.), National Institutes of Health (U01-NS073557, R01-NS92882, UH2-NS95495) and the Epilepsy Foundation Epilepsy Innovation Institute My Seizure Gauge.
Competing interests
Drs Worrell and Brinkmann have rights to receive future royalties from the licensing of technology related in this research. Mayo Clinic has a financial interest related to this research. Mayo Clinic is co-owner of Cadence Neuroscience Inc, the development of which has been assisted by Drs Worrell and Brinkmann. The remaining authors declare no competing financial interests.
References
- Abou-Khalil B, Wheless J, Rogin J, Wolter KD, Pixton GC, Shukla RB, et al. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia 2013; 54: 1968–76. [DOI] [PubMed] [Google Scholar]
- Almagor M, Ehrlich S.. Personality correlates and cyclicity in positive and negative affect. PR 1990; 66: 1159–69. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Circadian rhythms in man. Science 1965; 148: 1427–32. [DOI] [PubMed] [Google Scholar]
- Baldassano SN, Brinkmann BH, Ung H, Blevins T, Conrad EC, Leyde K, et al. Crowdsourcing seizure detection: algorithm development and validation on human implanted device recordings. Brain J Neurol 2017; 140: 1680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud MO, Ghestem A, Benoliel JJ, Becker C, Bernard C.. Endogenous multidien rhythm of epilepsy in rats. Exp Neurol 2019; 315: 82–7. [DOI] [PubMed] [Google Scholar]
- Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun 2018; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B 1995; 57: 289–300. [Google Scholar]
- Berendt M, Hogenhaven H, Flagstad A, Dam M.. Electroencephalography in dogs with epilepsy: similarities between human and canine findings. Acta Neurol Scand 1999; 99: 276–83. [DOI] [PubMed] [Google Scholar]
- Berens P. CircStat: A MATLAB toolbox for circular statistics. J Stat Softw 2009; 31: 21. [Google Scholar]
- Brinkmann BH, Patterson EE, Vite C, Vasoli VM, Crepeau D, Stead M, et al. Forecasting seizures using intracranial EEG measures and SVM in naturally occurring canine epilepsy. PloS One 2015; 10: e0133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles LD, Patterson EE, Sheffield WD, Mavoori J, Higgins J, Michael B, et al. Feasibility study of a caregiver seizure alert system in canine epilepsy. Epilepsy Res 2013; 106: 456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MJ, O'Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol 2013; 12: 563–71. [DOI] [PubMed] [Google Scholar]
- Davis KA, Sturges BK, Vite CH, Ruedebusch V, Worrell G, Gardner AB, et al. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG. Epilepsy Res 2011; 96: 116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM, Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med 1998; 338: 1869–75. [DOI] [PubMed] [Google Scholar]
- Dumanis SB, French JA, Bernard C, Worrell GA, Fureman BE.. Seizure forecasting from idea to reality. Outcomes of the my seizure gauge epilepsy innovation institute workshop. eNeuro 2017; 4: ENEURO.0349-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TS, Spencer SS, Duckrow RB, Novotny EJ, Spencer DD, Zaveri HP.. Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology 2008; 70: 1265–71. [DOI] [PubMed] [Google Scholar]
- Eden UT, Kramer MA.. Drawing inferences from Fano factor calculations. J Neurosci Methods 2010; 190: 149–52. [DOI] [PubMed] [Google Scholar]
- Elger CE, Mormann F.. Seizure prediction and documentation–two important problems. Lancet Neurol 2013; 12: 531–2. [DOI] [PubMed] [Google Scholar]
- Fano U. Ionization yield of radiations. II. The fluctuations of the number of ions. Phys Rev 1947; 72: 26–9. [Google Scholar]
- Ferastraoaru V, Schulze-Bonhage A, Lipton RB, Dumpelmann M, Legatt AD, Blumberg J, et al. Termination of seizure clusters is related to the duration of focal seizures. Epilepsia 2016; 57: 889–95. [DOI] [PubMed] [Google Scholar]
- Fisher Rthe SANTE Study GroupSalanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010; 51: 899–908. [DOI] [PubMed] [Google Scholar]
- Gowers WR. Epilepsy and other chronic convulsive diseases: their causes, symptoms, and treatment. London: J. & A. Churchill; 1901. [Google Scholar]
- Griffiths GM, Fox J.. Rhythm in epilepsy. Lancet 1938; 232: 409–16. [Google Scholar]
- Gundel A, Hilbig A.. Circadian acrophases of powers and frequencies in the waking EEG. Int J Neurosci 1983; 22: 125–33. [DOI] [PubMed] [Google Scholar]
- Halberg F, Engeli M, Hamburger C, Hillman D.. Spectral resolution of low-frequency, small-amplitude rhythms in excreted 17-ketosteroids; probable androgen-induced circaseptan desynchronization. Acta Endocrinol 1965; 50 (Suppl 103): 1–54. [DOI] [PubMed] [Google Scholar]
- Haut SR. Seizure clustering. Epilepsy Behav 2006; 8: 50–5. [DOI] [PubMed] [Google Scholar]
- Haut SR. Seizure clusters: characteristics and treatment. Curr Opin Neurol 2015; 28: 143–50. [DOI] [PubMed] [Google Scholar]
- Haut SR, Shinnar S, Moshe SL.. Seizure clustering: risks and outcomes. Epilepsia 2005; 46: 146–9. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol 2004; 56: 431–4. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Poepel A, Elger CE.. Epilepsy: accuracy of patient seizure counts. Arch Neurol 2007; 64: 1595–9. [DOI] [PubMed] [Google Scholar]
- Howbert JJ, Patterson EE, Stead SM, Brinkmann B, Vasoli V, Crepeau D, et al. Forecasting seizures in dogs with naturally occurring epilepsy. PloS One 2014; 9: e81920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoherranen N, Yagen B, Soback S, Roeder M, Schurig V, Bialer M.. Pharmacokinetics of levetiracetam and its enantiomer (R)-alpha-ethyl-2-oxo-pyrrolidine acetamide in dogs. Epilepsia 2001; 42: 825–30. [DOI] [PubMed] [Google Scholar]
- Jagota A, de la Iglesia HO, Schwartz WJ.. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci 2000; 3: 372–6. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Stagno S, Kotagal P, Morris HH.. Postictal psychiatric events during prolonged video-electroencephalographic monitoring studies. Arch Neurol 1996; 53: 258–63. [DOI] [PubMed] [Google Scholar]
- Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain J Neurol 2016; 139: 1066–78. [DOI] [PubMed] [Google Scholar]
- Karoly PJ, Goldenholz DM, Freestone DR, Moss RE, Grayden DB, Theodore WH, et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol 2018; 17: 977–85. [DOI] [PubMed] [Google Scholar]
- Karoly PJ, Nurse ES, Freestone DR, Ung H, Cook MJ, Boston R.. Bursts of seizures in long-term recordings of human focal epilepsy. Epilepsia 2017; 58: 363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly PJ, Ung H, Grayden DB, Kuhlmann L, Leyde K, Cook MJ, et al. The circadian profile of epilepsy improves seizure forecasting. Brain J Neurol 2017; 140: 2169–82. [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M, Wolf PA, Kase CS, Brand FN, McGuirk JM, D’Agostino RB.. Temporal patterns of stroke onset. The Framingham Study. Stroke J Cerebral Circul 1995; 26: 1343–7. [DOI] [PubMed] [Google Scholar]
- Kremen V, Brinkmann B, Kim I, Guragain H, Nasseri M, Magee A, et al. Integrating brain implants with local and distributed computing devices: a next generation epilepsy management system. IEEE J Transl Eng Health Med 2018; 5: 2500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon-Down M, Russell Brain W.. Time of day in relation to convulsions in epilepsy. Lancet 1929; 213: 1029–32. [Google Scholar]
- Litwin-Kumar A, Doiron B.. Slow dynamics and high variability in balanced cortical networks with clustered connections. Nat Neurosci 2012; 15: 1498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March PA, Podell M, Sams RA.. Pharmacokinetics and toxicity of bromide following high-dose oral potassium bromide administration in healthy Beagles. J Vet Pharmacol Ther 2002; 25: 425–32. [DOI] [PubMed] [Google Scholar]
- Mead R. A treatise concerning the influence of the sun and moon upon human bodies: and the diseases thereby produced. By Richard Mead … Translated from the Latin … by Thomas Stack. London: Printed for J. Brindley; 1748.
- Milton JG, Gotman J, Remillard GM, Andermann F.. Timing of seizure recurrence in adult epileptic patients: a statistical analysis. Epilepsia 1987; 28: 471–8. [DOI] [PubMed] [Google Scholar]
- Mitchell WG. Status epilepticus and acute serial seizures in children. J Child Neurol 2002; 17 (Suppl 1): S36–43. [DOI] [PubMed] [Google Scholar]
- Morrell MJGroup RNSSiES. . Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011; 77: 1295–304. [DOI] [PubMed] [Google Scholar]
- Orito K, Saito M, Fukunaga K, Matsuo E, Takikawa S, Muto M, et al. Pharmacokinetics of zonisamide and drug interaction with phenobarbital in dogs. J Vet Pharmacol Ther 2008; 31: 259–64. [DOI] [PubMed] [Google Scholar]
- Osorio I, Frei MG, Sornette D, Milton J.. Pharmaco-resistant seizures: self-triggering capacity, scale-free properties and predictability?. Eur J Neurosci 2009; 30: 1554–8. [DOI] [PubMed] [Google Scholar]
- Pedersoli WM, Wike JS, Ravis WR.. Pharmacokinetics of single doses of phenobarbital given intravenously and orally to dogs. Am J Vet Res 1987; 48: 679–83. [PubMed] [Google Scholar]
- Reppert SM, Weaver DR.. Coordination of circadian timing in mammals. Nature 2002; 418: 935–41. [DOI] [PubMed] [Google Scholar]
- Schweiger HG, Berger S, Kretschmer H, Morler H, Halberg E, Sothern RB, et al. Evidence for a circaseptan and a circasemiseptan growth response to light/dark cycle shifts in nucleated and enucleated Acetabularia cells, respectively. Proc Natl Acad Sci USA 1986; 83: 8619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatola M, Alvarez V, Rossetti AO.. Benzodiazepine overtreatment in status epilepticus is related to higher need of intubation and longer hospitalization. Epilepsia 2013; 54: e99–102. [DOI] [PubMed] [Google Scholar]
- Stanslaski S, Herron J, Chouinard T, Bourget D, Isaacson B, Kremen V, et al. A chronically implantable neural coprocessor for investigating the treatment of neurological disorders. IEEE Trans Biomed Circuits Syst 2018; 12: 1230–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubøll E, Lundervold A, Gjerstad L.. Temporal distribution of seizures in epilepsy. Epilepsy Res 1991; 8: 153–65. [DOI] [PubMed] [Google Scholar]
- Willich SN, Lowel H, Lewis M, Hormann A, Arntz HR, Keil U.. Weekly variation of acute myocardial infarction. Increased Monday risk in the working population. Circulation 1994; 90: 87–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and MATLAB scripts are available at https://msel.mayo.edu/research.html.