Abstract
Background
The aim of this study is to present a national surveillance report on pediatric central nervous system (CNS) tumors in Canada during the period between 2001 and 2015.
Methods
All pediatric patients with a diagnosis of primary CNS tumors were collected by the Cancer in Young People in Canada (CYP-C) surveillance system that includes every patient less than 15 years of age with a tumor seen in one of the 17 pediatric oncology centres in Canada. This registry included malignant and benign CNS tumors. We calculated the age-adjusted incidence rates (AAIRs) per 100 000 person-years for CNS tumors overall and by age group, major histology subgroups, and geographical distribution over the country.
Results
Overall, 3306 patients less than 15 years old had been diagnosed with a CNS tumor in Canada in 2001–2015 with a 1.23:1 male to female ratio. The overall AAIR is 3.80. The three most frequent groups of tumors were low-grade gliomas (36.4%), high-grade gliomas (22.3%), and embryonal tumors (18.7%) with incidence rates of 1.41, 0.86, and 0.72 per 100 000 person-years, respectively. The incidence rate of pediatric CNS tumors is stable during the period 2001–2015 in Canada and no significant differences were seen between malignant and benign tumors over the country.
Conclusions
These data represent all the pediatric patients 0–14 years old with a CNS tumor in the Canadian population. Incidence rates by age group, sex, and subgroups of tumors are similar to those seen in the literature.
Keywords: brain tumors, central nervous system tumors, children, epidemiology, incidence
Key Points.
The incidence rate of pediatric CNS tumors is stable during the period 2001–2015 in Canada.
The distribution of tumor histologies varies with age.
There is no significant difference in incidence rate between malignant versus benign tumors over the country.
Importance of the Study.
Cancer in Young People in Canada (CYP-C) is a national pediatric cancer population-based registry that had been created to promote surveillance in oncology in the young population of Canada. Our study focuses on pediatric central nervous system tumors from this registry that include full data on malignant as well as benign tumors in all provinces over 2001–2015. Incidence rate, subtypes of tumor histology, geographical distribution in the country, and clinical outcomes are all important data to better understand the epidemiology of such disease and to promote research, development of novel therapies, and preventing care. Also, these oncology surveillances allow us to compare with other similar populations.
Central nervous system (CNS) tumors are the most frequent solid tumors in childhood. Nowadays, through the 0–14 years age group, the average annual incidence rate (IR) of malignant CNS tumors, adjusted by age of diagnosis, is evaluated to be 2.28 per 100 000 population in the world, with the United States and Canada having the highest rate with 3.03 and 2.70 per 100 000, respectively.1 According to the Cancer in Young People in Canada (CYP-C) database, between 2001 and 2006, 23.4% of cancers diagnosed below the age of 15 years in Canada were CNS tumors.2 Furthermore, CNS tumors represent the most common cause of cancer death in pediatric and significantly high rate of morbidity in survivors.3,4
Epidemiological studies are essential to assist clinicians in providing accurate and up-to-date information to patients and families affected by this prevalent condition. However, we observe important variations between epidemiological studies. These differences are related to diverse factors that have an impact on the incidence and prognosis of CNS tumors, such as age of the patient, type of tumor, treatment modalities, and time frame studied. In addition, national registries do not collect the same level of details in terms of diagnosis and treatment. Many of them do not include data on benign tumors, such as low-grade gliomas (LGGs), rending comparison between populations difficult from an epidemiological point of view.
Central Brain Tumor Registry of the United States (CBTRUS) has been used as a reference for clinician but it is not known if there is a difference between these two populations. Indeed, in their last 2011–2015 report, CNS tumors were becoming the most common cancer site, now before leukemia group, in the patient’s age 0–14 years, with an average annual age-adjusted incidence rate (AAAIR) of 5.65 per 100 000 population.4
In terms of the geographical distribution of CNS tumors, the Canadian Brain Tumour Registry report on malignant brain tumors from 2009 to 2013 and objective that the highest age-adjusted rates estimated were among residents of Quebec and Ontario.5 However, only2 years of data were available for the province of Quebec. A subsequent report had been published for the period 2010–2015 reflecting provincial incidence in four provinces (BC, AB, MB, ON) that demonstrated clearly the difference in the incidence of major tumor histologies between adults and children.6 Meningioma was the most frequent histology tumor in women and glioblastoma in men in adulthood as oppose to neuroepithelial tumors in childhood.
As we are aware, no recent epidemiology study with a focus on pediatric CNS tumors was conducted in Canada, with full data on malignant as well as benign tumors in all provinces for more than a decade. Consequently, we used the CYP-C national pediatric cancer population-based database in order to describe the characteristics of the patients, tumors type, incidence of CNS tumors by age group, and geographical distribution along the country. The aim of this study is to provide descriptive statistics on the IRs and trends for the common histologic subtypes of primary CNS tumors among the Canadian pediatric population during a 15 years period.
Methods
Patient Population
Patient’s data were collected by the CYP-C surveillance system that includes every patient less than 15 years of age with a tumor seen in 1 of the 17 pediatric oncology centres in Canada. We analyzed all pediatric patients who received a diagnosis of primary CNS tumors in Canada between 2001 and 2015. This includes malignant as well as benign tumors of the brain or spine. The variables analyzed are as follows: year of diagnosis, age at diagnosis, gender, ethnicity, postal code, type of tumor, the staging of the disease, and treatment modalities including surgery, chemotherapy, and radiotherapy. Patients who were Canadian residents since less than a month prior to diagnosis were excluded, as well as those who received a diagnosis before January 1, 2001 or after December 31, 2015. Some other exclusions and limitations to the database are described in CYP-C: A Report from the Enhanced Childhood Cancer Surveillance System.2 We had received approval from the Ethics Board Committee as well as CYP-C Management Committee.
CNS Tumor Definition and Classification
Tumors are detailed as per the International Classification of Childhood Cancer, 3rd Edition (ICCC-3).7 During the period 2001–2015, CYP-C registry used CNS grade as per description in WHO 2000 Classification of Tumours of Central Nervous System8 and the version later updated in 2007.9 In order to assess the incidence, different subgroups were created according to the tumor, the diagnosis period, and the age at diagnosis. Tumors were classified into 7 subgroups: LGG, high-grade glioma (HGG), embryonal tumors (including medulloblastoma, Primitive Neuroectodermal Tumors [PNET] and Atypical Teratoid Rhabdoid Tumors [ATRT]), ependymoma, craniopharyngioma, germ cell tumor, and others. We did include 9421/3 (pilocytic astrocytoma) and 9400/3 (astrocytoma NOS low grade) in the group of LGG as they correspond to indolent glial tumors and behave as low grade in term of survival. Morphology ICD-O-3 codes included in each major histology subgroups used for this present analysis are described in the table in the Supplementary Material.
Statistical and Geographical Analysis
Three diagnosis periods were determined within the 15 years period analyzed: 2001–2005, 2006–2010, and 2011–2015. Estimates for the child population between the ages of 0 to 14 years in Canada, the provinces and territories and health regions are based on data from the 5-year censuses conducted between 2001 and 2016. For this study, we used the intercensal estimates developed by Statistics Canada for the years between each census.10
Patients were separated in three age groups based on the age of their initial diagnosis: 0–4 years, 5–9 years, and 10–14 years. For each year, for a given population defined by sex, age, and health region or province, the age-adjusted IR with 95% confidence limits was estimated using Poisson or negative binomial models in case of overdispersion. The results of these analyses were joined to the mapping file. All statistical analyses were performed using SAS 9.4 software while geo-referencing and mapping were performed using ArcGIS Pro 2.0.1 software.
The residence of each patient was estimated using the postal code of the residence at the time of diagnosis in the database. The postcodes have been geocoded using ESRI’s ArcGIS World Geocoding Service. Assignment to a province or a health region was determined by spatial joining between the geographical coordinates of the estimated place of residence and the geospatial files that mapped the limits of these provincial and regional boundaries. Geographical areas counting 10 patients and less in Canadian provinces or in Quebec Health regions had been combined together respectively for statistical analysis. This is important to respect the privacy of personal health information when small numbers of patients are described in the CYP-C registry.
Results
Characteristics of the Patients
Overall, 3306 Canadian patients had been followed in 1 of the 17 pediatric oncology centres in the country for a pediatric CNS tumor diagnosed between 2001 and 2015. Tumors in the CNS are more prevalent amongst males with 1.23:1 male to female ratio, males representing 55% of the cohort (n = 1823). The population was mainly Caucasian (n = 1992). Asian ethnicity represents only 7.9% of the patients (n = 260) in our whole cohort. We observe that the Asian ethnicity was significantly in higher proportion (20.7%, 23/111) in the malignant germ cell tumors than the Asian ethnicity proportion (8.0%, 237/2964) in all other tumors (P < .001). Ethnicity was unknown in 7.0% of cases of our cohort (231/3306).
The most represented age group was 0–4 years (38.7%), followed by the 5–9 years (32.7%) and the 10–14 years (28.6%). Their respective IRs were 4.65, 3.79, and 3.11 per 100 000 person-years (P <.0001). The overall incidence of CNS tumors was stable over the 15 years of observation with an average of 220 new cases per year with an average incidence of 3.80 (95% CI 3.65–3.97) per 100 000 person-years.
Characteristics of the Tumors
The predominant tumor location is found to be the supratentorial area in 65% of our cohort (n = 2158). Infratentorial region is involved in 26% of patients (n = 858), three-quarter of them having less than 10 years of age at diagnosis. Only 101 patients, representing 3% of the entire cohort, had a spine as a primary tumor, half of them are in the older group of 10–14 years. Tumor location was not specified in 6% of the patients (n = 189).
Overall, 55% of our patients had a malignant tumor (n = 1810). Tumors were localized at the time of diagnosis in 88% of the cohort (n = 2910). Therefore, we observed the incidence of 1.7% of metastasis in the benign tumor group (n = 24) versus 14.0% in malignant tumor group (n = 242). From 266 patients presented with metastasis disease at presentation, almost half of them were from medulloblastoma tumors (n = 121). In the registry, 132 cases had an unknown staging disease.
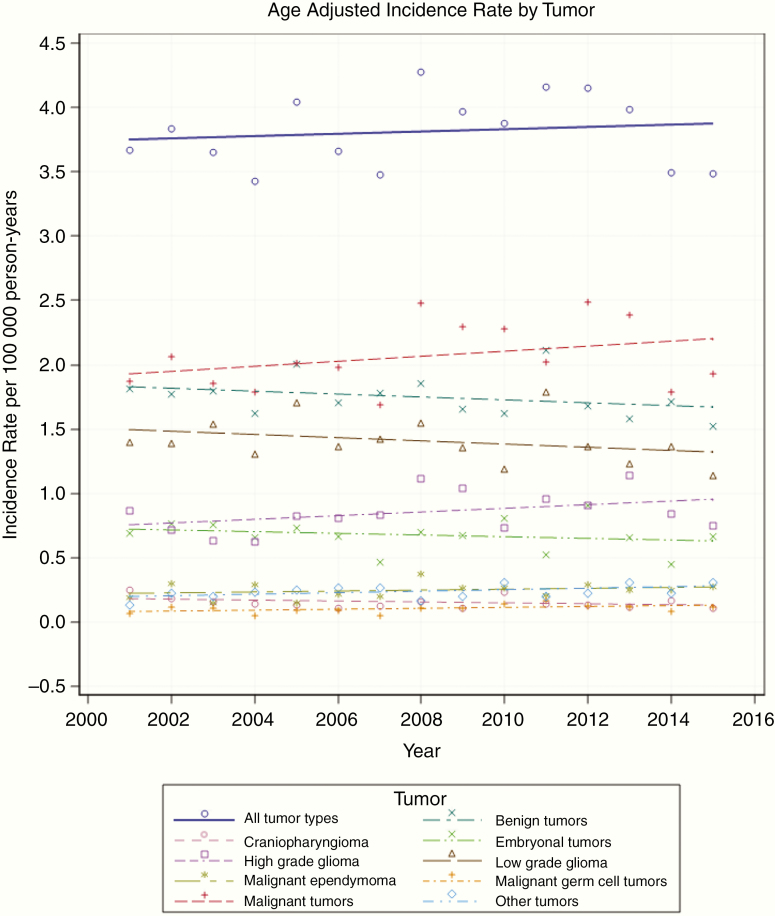
The IR for malignant tumors and benign tumors is 2.05 and 1.75 per 100 000 person-years, respectively (Figure 1). Between 2001 and 2015, we observed a not significant trend for decreasing IR for benign tumors (IR 1.81 in 2001 to 1.53 in 2015, P = .21) and a not significant increase of IR for malignant tumors during the same period (IR 1.85 in 2001 to 1.96 in 2015, P = .73).
Fig. 1.
Incidence rate according to tumor type and year of diagnosis.
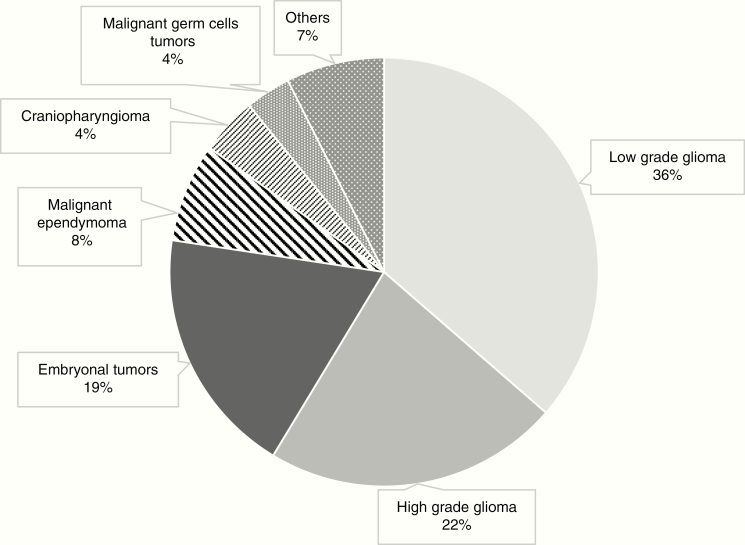
The three most frequent groups of tumors were LGGs (36.4%), HGGs (22.3%), and embryonal tumors (18.7%) with IRs of 1.41, 0.86, and 0.72 per 100 000 person-years, respectively. Figure 2 represents the most common CNS tumors in the Canadian pediatric population during the era 2001–2015.
Fig. 2.
Histology spectrum of pediatric CNS tumors in Canada.
Malignant tumors had a higher IR in the youngest group of patients, IR 2.85 for patients less than 5 years old in comparison with 1.49 for the older group 10–14 years old (P < .0001). Mean age at diagnosis differed according to a group of tumors ranging from 1.39 years for children with ATRT to 9.73 years for those with malignant germ cell tumors. Tumor’s repartition by age group is given in Table 1. Germ cell tumors and craniopharyngioma seem to become more prevalent with age. The opposite tendency is suggested by the prevalence observed for embryonal tumors and ependymoma decreasing with age.
Table 1.
Number of Pediatric CNS Tumors by Major Histology Grouping and Age Group at Diagnosis During the Period 2001–2015 in Canada
| Histology | Age at Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–14 years | 0–4 years | 5–9 years | 10–14 years | ||||||
| Mean age (95% CI) | n | IR | n | IR | n | IR | n | IR | |
| Low-grade glioma | 6.93 (6.69–7.17) | 1203 | 1.41 | 402 | 1.48 | 412 | 1.46 | 389 | 1.29 |
| High-grade glioma | 6.36 (6.07–6.65) | 736 | 0.86 | 278 | 1.02 | 265 | 0.94 | 193 | 0.64 |
| Embryonal tumors | 5.46 (5.15–5.77) | 619 | 0.72 | 292 | 1.07 | 213 | 0.76 | 114 | 0.38 |
| Medulloblastoma | 6.25 (5.90–6.60) | 425 | 0.50 | 158 | 0.58 | 177 | 0.63 | 90 | 0.30 |
| PNET | 5.38 (4.59–6.17) | 114 | 0.13 | 61 | 0.22 | 29 | 0.10 | 24 | 0.08 |
| ATRT | 1.39 (0.98–1.80) | 80 | 0.09 | 73 | 0.27 | 7 | 0.03 | 0 | 0.00 |
| Ependymoma malignant | 4.70 (4.18–5.23) | 247 | 0.29 | 152 | 0.56 | 48 | 0.17 | 47 | 0.16 |
| Other tumors | 6.04 (5.45–6.64) | 245 | 0.25 | 118 | 0.38 | 50 | 0.15 | 77 | 0.23 |
| Craniopharyngioma | 8.36 (7.76–8.97) | 143 | 0.17 | 23 | 0.08 | 64 | 0.23 | 56 | 0.19 |
| Malignant germ cell | 9.73 (9.02–10.45) | 113 | 0.13 | 13 | 0.05 | 28 | 0.10 | 72 | 0.24 |
| Total | 6.49 (6.31–6.60) | 3306 | 3.80 | 1278 | 4.65 | 1080 | 3.79 | 948 | 3.11 |
Characteristics of the Treatments
Surgical management of tumors was similarly used for benign tumors and malignancies. Approximately a quarter of patients having a complete resection of their tumor upfront. Repeated surgery is performed in two-third of the patients. By opposition, radiotherapy and chemotherapy were preferred for the therapy of patients with malignant tumors, represented 85% and 83%, respectively. Radiation therapy is significantly less employed in the youngest patients, 29% instead of 46% and 42% for the 0–4 years, 5–9 years, and 10–14 years, respectively (P < .0001). The opposite is seen for chemotherapy with 23%, 18%, and 14%, respectively, by age group (P < .0001). Only 420 (12.7%) patients are registered on a clinical trial, among them, 81% were patients with malignant tumors, mainly HGGs and medulloblastoma. Participating patients were mainly residents from Ontario and Quebec. On trial, treatment modalities included radiation and/or chemotherapy in 66% and 37% of patients registered, respectively.
Geographic Distribution in Canada
One of the main goals of our study was to evaluate IRs by a Canadian province and more precisely within Quebec and its health regions. Our cohort was mainly composed of patients from Ontario (43.5%), Quebec (21.7%), British Columbia (11.9%), and Alberta (9.3%). The other provinces and territories were also represented, but composed only 13.6% of our population.
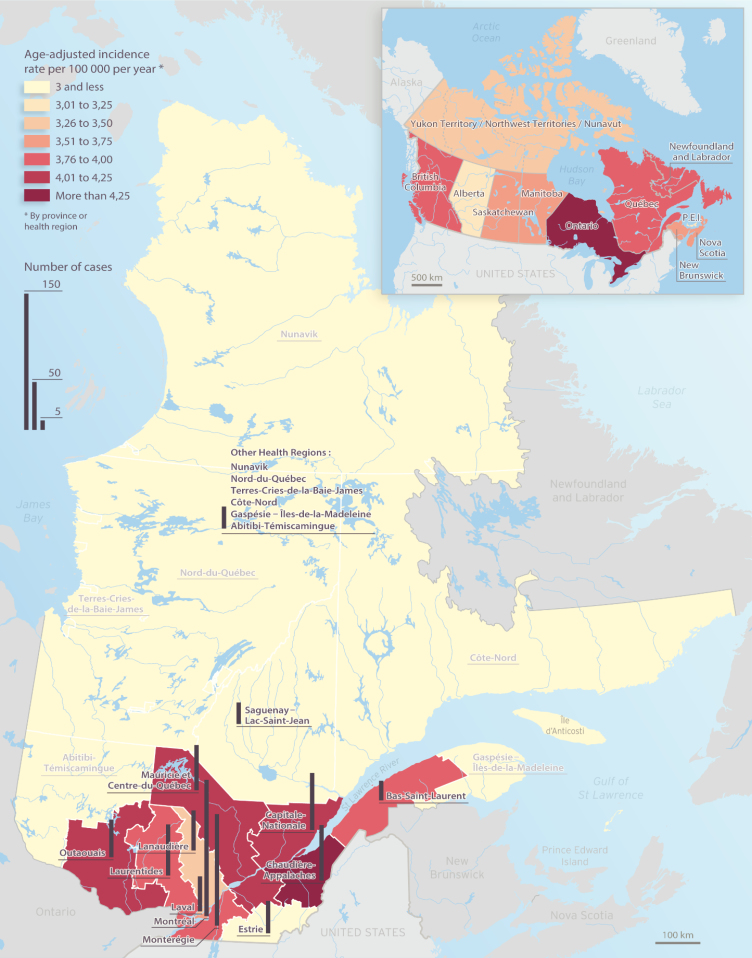
When benign tumors are concerned, the most frequent IRs are seen in New Brunswick (1.98), Newfoundland and Labrador (1.94), Ontario (1.84), and Quebec (1.82). Malignant tumors had IR slightly greater than 2.00 per 100 000 person-years for 2 of our provinces: Ontario (2.38) and Nova Scotia (2.02). However, when respective results are analyzed considering average AAAIR, regardless of specific tumor type, provinces that presented the most important IR of CNS pediatric tumors were Ontario (4.26), Newfoundland and Labrador (3.95), British Columbia (3.78), and Quebec (3.76). Table 2 summarizes the age-adjusted IRs of pediatric CNS tumors by regions in Canada. Figure 3 shows the geographic distribution of the estimated IR for the Canadian provinces and Quebec regions.
Table 2.
Number of Patients and Age-Adjusted Incidence Rates of Pediatric CNS Tumors by Geographical Regions
| Provinces and Territories | No. of Patients (%) | IR Benign | IR Malignant | Global Age-Adjusted Incidence (Benign and Malignant CNS Tumors) |
|---|---|---|---|---|
| Alberta | 308 (9.3) | 1.25 (1.05–1.49) | 1.72 (1.48–1.99) | 2.99 (2.67–3.36) |
| British Columbia | 394 (11.9) | 1.77 (1.53–2.04) | 1.97 (1.72–2.26) | 3.78 (3.41–4.19) |
| Prince Edward Island | 11 (0.3) | 1.39 (0.58–3.35) | 1.65 (0.74–3.67) | 3.06 (1.70–5.53) |
| Manitoba | 123 (3.7) | 1.77 (1.38–2.27) | 1.69 (1.32–2.18) | 3.48 (2.91–4.16) |
| New Brunswick | 66 (2.0) | 1.98 (1.42–2.76) | 1.72 (1.21–2.45) | 3.72 (2.92–4.74) |
| Nova Scotia | 80 (2.4) | 1.60 (1.15–2.23) | 2.02 (1.51–2.71) | 3.65 (2.92–4.55) |
| Ontario | 1438 (43.5) | 1.84 (1.70–1.99) | 2.38 (2.22–2.56) | 4.26 (4.01–4.52) |
| Quebec | 717 (21.7) | 1.82 (1.64–2.02) | 1.92 (1.73–2.13) | 3.76 (3.47–4.07) |
| Saskatchewan | 108 (3.2) | 1.79 (1.37–2.34) | 1.74 (1.33–2.27) | 3.55 (2.93–4.29) |
| Newfoundland and Labrador | 47 (1.4) | 1.94 (1.29–2.91) | 1.99 (1.33–2.97) | 3.95 (2.96–5.26) |
| Nunavut/Territoires Nord-Ouest/Yukon | 14 (0.4) | 1.77 (0.84–3.70) | 1.70 (0.81–3.56) | 3.48 (2.06–5.87) |
| Total | 3306 (100) | 1.75 (1.66–1.84) | 2.05 (1.94–2.15) | 3.80 (3.65–3.95) |
Fig. 3.
Age-adjusted incidence rate for CNS tumors by Quebec Health region and Canadian province/territory, 2001–2015, adjusted for the 2000 US age distribution.
Geographic Distribution in Quebec
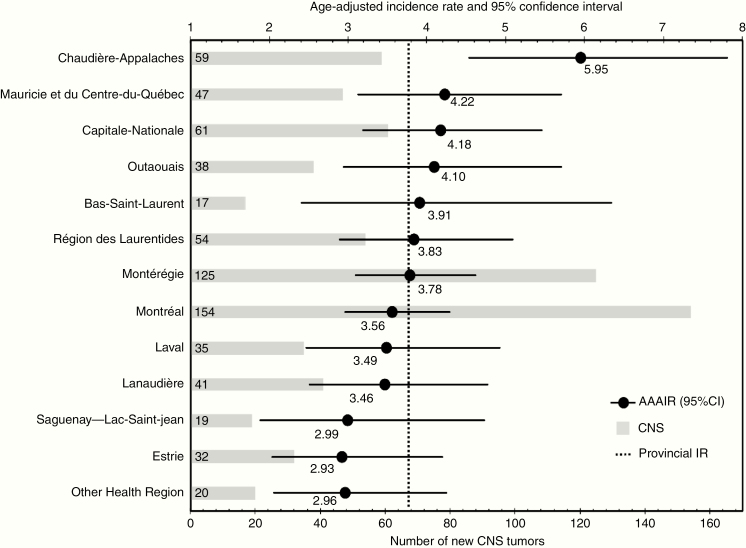
AAAIR was also calculated for every Quebec Health Region in order to assess regional variability (Figure 4). Between 2001 and 2015, 717 patients were diagnosed with a CNS tumor in our province, so the AAAIR was 3.76 per 100 000 person-years. Five regions presented higher AAIR: Chaudière-Appalaches (5.95), Mauricie and Centre-Du-Québec (4.22), Capitale-Nationale (4.18), and Outaouais (4.10), but only the incidence in the Chaudière-Appalaches region is statistically higher than the IR in the province of Quebec.
Fig. 4.
Age-adjusted incidence rate of pediatric CNS tumors by Health Region in Quebec province.
Discussion
This retrospective cohort study described the incidence of primary CNS tumors among the Canadian pediatric population during the period 2001–2015. Within our Canadian cohort, a predominance of male relative to female (1.23:1) can be observed, as described in the literature with a male to female ratio of 1.29:1.11 We observe that the difference was more pronounced in the group of embryonal tumors (sex ratio: 1.6) and less in LGGs or HGGs (sex ratio: 1.1). Ethnicity data were missing in a quarter of our population, limiting the analysis. However, we did find a significant increase in the proportion of Asian ethnicity patients into the malignant germ cell tumors group compared to other groups of tumors. This finding had been reported also from 554 intracranial germ cell tumors in patients less than 30 years old living in the United States over the period 1992–2010 in the SEER registry.12
IRs for the whole group of CNS tumors were stable in Canada from 2001 to 2015, despite improvement in the diagnosis through time with the accessibility of MRI. In comparison, the CBTRUS database from 2000 to 2010 revealed a significant increase in the incidence of malignant CNS tumors, but no significant modification in the incidence of benign CNS tumors within the pediatric population.13 In our study, we did see a trend for increasing IR for malignant tumors and decreasing IR for benign tumors but that was not statistically significant. The variable having the greatest impact on IRs is the age, with significantly higher IRs of CNS tumors from 0 to 4 years old and lower IR from 5 years to 15 years old. This evolution is similarly described in the European population with age-groups distribution comparable to ours: 37% of 0–4 years old, 35% of 5–9 years old, and 29% of 10–14 years old from 1978 to 1989.14 The same pattern is presented in the American epidemiological studies, but IRs tend to be higher than in our population with 6.11, 5.30, and 5.55 per 100 000 person-years, respectively, from 2011 to 2015 by each age group.4
Our patients mainly present malignancies. In comparison, from 2011 to 2015, CBTRUS reported 67% of malignant CNS tumors (AAIR of 3.80 per 100 000) and 33% of benign tumors (1.85 per 100 000) from their 17 273 patients 0–14 years old.4 This difference between both populations (10% more malignant tumors in the United States) could be significant, but is more likely to be attributed to a different tumor classification. We did place 9421/3 (pilocytic astrocytoma) and 9400/3 (astrocytoma NOS low grade) into a benign category, corresponding to 910 patients (27.5% of the cohort) because they behave favorably in survival. Until 2000, pilocytic astrocytoma was registered as a malignancy (code 3), then code changed to uncertain behavior (code 1) but still entered as code 3 in the registry to maintain consistency. Another limitation for the histologic group analysis is the code 9380 for HGG including optic pathway glioma as well that are usually LGG. This heterogeneous group represents 512 patients, 15.4% of our entire cohort, contributing to overestimated HGG cohort. With the recent WHO 2016 revision including biomarkers for more precision in the diagnosis of brain tumors, molecular data will be collected in the CYP-C registry version 2.15
We observe as well that within our cohort, 9% of patients having metastasis at initial diagnosis was discovered in the benign tumor group. Some reports described dissemination from pediatric low grade and atypical choroid plexus papilloma16–18 but the true incidence is still difficult to know even from registries. Accuracy of this issue could be underestimated as complete tumor staging with a spine MRI is not always done for this population.
In term of distribution of tumors by histology, CBTRUS reports that gliomas accounted for approximately 52% of tumors, and that the most common brain tumors in children less than 15 years of age are pilocytic astrocytoma, malignant glioma NOS, and embryonal tumors in 18%, 14%, and 13%, respectively.4 This distribution is comparable to our Canadian pediatric cohort. Instead, in the age group of 15–19 years old report in CBTRUS, almost a third were diagnosed with pituitary and craniopharyngeal duct tumors and another third having gliomas. To better acknowledge all our Canadian pediatric patients, we adopt to include all of them up to 19 years of age now prospectively in the CYP-C database.
Pediatric CNS tumors are such a heterogeneous group of tumors and these explain diverse treatment modalities used. Radiation therapy is often postponed or even avoided in youngest patients because of potential side effects such as learning difficulties, vascular events, and second malignancies. Instead, chemotherapy modality is favored to treat malignant tumors as well as unresectable or recurrent benign tumors. Only 12.7% of our cohort was registered on a clinical trial. Overall, 27.5% of all the children having cancer from CYP-C database in Canada had been enrolled in a therapeutic trial during the similar period 2001–2012, up to 48.8% for acute lymphoblastic leukemia in contrast to only 10.3% for the CNS tumors group.19 In this study, brain tumor had been identified as a risk factor for non-enrollment, astrocytoma had the lowest rate of enrollment, and the two most common reasons cited were non-trial availability and physician decision. Potential long distances for many pediatric patients living far from their cancer center can also be an issue, development of a clinical research system with satellite centers could support the participation in clinical trials.20
We objective no significant difference in IR between malignant versus benign tumors over the country. The provinces with highest AAAIR were Ontario, New Foundland and Labrador, British Columbia, and Quebec. However, the rate instability resulting from the small number of cases in some provinces and territories requires caution in the interpretation of the analysis, so we combined areas where 10 cases and less were seen. Only the first three digits of the postal code for each patient were available in British Columbia limiting the analysis into this province.
In the province of Quebec, the Health Region of Chaudiere-Appalaches is the only one showing a significantly higher AAAIR. We believe that this is an important finding that public health authorities should take into consideration. This region, bordered to the north by the St. Lawrence River and to the south by the border with the United States, is highly diversified in terms of land use density (inhabitants/hectare) and land use (agriculture, industrial, and residential). We postulate that there may be inequalities within this region in the geographical distribution of incidence of pediatric brain tumors. We consider that it would be interesting to investigate the existence of statistically significant spatial cluster at the local and sub-regional levels, allowing to identify areas where the number of cases per child at risk is higher than in the study area overall.21–23 However, we need to be careful about the interpretation of this data. The analyses presented in this article are limited by the low number of events and the largest of each Health Region areas in the province. Further study to confirm the tendency of incidence in pediatric cancer, including all types of tumors, in the province of Quebec will be pursued. Hypothesis of a particular genetic condition and/or environmental issues is always rising questions when the incidence of cancer is more seen in a specific geographical area.
Conclusion
This retrospective study reports descriptive statistics on the patients less than 15 years old with a diagnosis of CNS tumors in Canada during a 15 years period. This cohort is similar to what had been described in the United States and Europe in terms of patients and tumors characteristics. Our results show stable IR of pediatric CNS tumors during the period 2001–2015 in Canada. Geographical patterns in the country and in the province of Quebec suggest areas for further study to better understand the causes of these tumors and environmental risk factors.
Funding
This work was supported by the funding from the Canadian Institutes of Health Research. The CYP-C is fully funded by the Public Health Agency of Canada.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of study participants, participating pediatric oncology centres, members of the Cancer in Young People in Canada (CYP-C) Management and Steering Committees, the Pediatric Oncology Group of Ontario (POGO), and the five POGO Hospital Partners. We wish to thank all the data managers at the 17 CYP-C sites for their dedicated work in maintaining the CYP-C data quality.
This paper is being submitted as the first part of the Canadian epidemiology study from CYP-C registry for CNS tumors. The second part of the analysis on the prognosis of pediatric CNS tumors in the Canadian population is ongoing.
Contribution or task: V.L., A.K.T., B.L., D.S., N.J., and S.P. met the following criteria: (1) substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; (2) drafting the work or revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest statement. The authors have no conflicts of interest.
References
- 1. Leece R, Xu J, Ostrom QT, et al. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol. May 2017;19(11):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Public Health Agency of Canada. Release notice - cancer in young people in Canada: a report from the enhanced childhood cancer surveillance system. Health Promot Chronic Dis Prev Can. 2017;37(11):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker EV, Davis FG; CBTR founding affiliates Malignant primary brain and other central nervous system tumors diagnosed in Canada from 2009 to 2013. Neuro Oncol. 2019;21(3):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith T, Yuan Y, Walker E, Davis F.. Brain Tumor Registry in Canada (BTRC): Incidence Report 2010–2015. Brain Tumor Foundation of Canada; https://braintumourregistry.ca/full-report/. Accessed August 2019. [Google Scholar]
- 7. Fritz A, Jack A, Shanmugaratnam K, et al. International Classification of Diseases for Oncology, 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 8. Kleihues P, Cavanee W, eds. Tumours of the Nervous System: World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000. [Google Scholar]
- 9. Louis D, Wiestler O, Cavanee W, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 10. Statistics Canada. Annual Demographic Estimates: Canada, Provinces and Territories, 2016 (Catalogue no. 91-215-X). Ottawa: Minister of Industry; 2016. CANSIM Table 051-0001 released in September 2016. www.statcan.gc.ca/pub/91-215-x/91-215-x2016000-eng.pdf. Accessed August 12, 2019. [Google Scholar]
- 11. Keene DL, Johnston DL. Epidemiology of central nervous system tumors. In: Bouffet E, ed. Pediatric Neuro-oncology. New York: Springer-Verlag; 2015:9–12. [Google Scholar]
- 12. Poynter JN, Fonstad R, Tolar J, Spector LG, Ross JA. Incidence of intracranial germ cell tumors by race in the United States, 1992–2010. J Neurooncol. 2014;120(2):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gittleman HR, Ostrom QT, Rouse CD, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magnani C, Aareleid T, Viscomi S, Pastore G, Berrino F; EUROCARE Working Group Variation in survival of children with central nervous system (CNS) malignancies diagnosed in Europe between 1978 and 1992: the EUROCARE study. Eur J Cancer. 2001;37(6):711–721. [DOI] [PubMed] [Google Scholar]
- 15. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 16. Tsang DS, Murphy ES, Ezell SE, Lucas JT Jr, Tinkle C, Merchant TE. Craniospinal irradiation for treatment of metastatic pediatric low-grade glioma. J Neurooncol. 2017;134(2):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bian SX, McAleer MF, Vats TS, Mahajan A, Grosshans DR. Pilocytic astrocytoma with leptomeningeal dissemination. Childs Nerv Syst. 2013;29(3):441–450. [DOI] [PubMed] [Google Scholar]
- 18. Scala M, Morana G, Milanaccio C, Pavanello M, Nozza P, Garrè ML. Atypical choroid plexus papilloma: spontaneous resolution of diffuse leptomeningeal contrast enhancement after primary tumor removal in 2 pediatric cases. J Neurosurg Pediatr. 2017;20(3):284–288. [DOI] [PubMed] [Google Scholar]
- 19. Pole JD, Barber R, Bergeron RÉ, et al. Most children with cancer are not enrolled on a clinical trial in Canada: a population-based study. BMC Cancer. 2017;17(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexander S, Greenberg M, Malkin D, et al. Pediatric oncology clinical trial participation where the geography is vast: development of a clinical research system for tertiary and satellite centers in Ontario, Canada. Pediatr Blood Cancer. 2018;65(4):1–4. [DOI] [PubMed] [Google Scholar]
- 21. Hjalmars U, Kulldorff M, Wahlquist Y, Lannering B. Increased incidence rates but no space-time clustering of childhood astrocytoma in Sweden, 1973-1992: a population-based study of pediatric brain tumors. Cancer. 1999;85(9):2077–2090. [PubMed] [Google Scholar]
- 22. Wheeler DC. A comparison of spatial clustering and cluster detection techniques for childhood leukemia incidence in Ohio, 1996–2003. Int J Health Geogr. 2007;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amin R, Bohnert A, Holmes L, Rajasekaran A, Assanasen C. Epidemiologic mapping of Florida childhood cancer clusters. Pediatr blood cancer. 2010;54(4):511–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.