Abstract
Extracellular vesicles (EVs) have emerged as key regulators of cell-cell communication during inflammatory responses to lung injury induced by diverse pulmonary toxicants including cigarette smoke, air pollutants, hyperoxia, acids and endotoxin. Many lung cell types, including epithelial cells and endothelial cells, as well as infiltrating macrophages generate EVs. EVs appear to function by transporting cargo to recipient cells that, in most instances, promotes their inflammatory activity. Biologically active cargo transported by EVs include miRNAs, cytokines/chemokines, damage-associated molecular patterns (DAMPs), tissue factor (TF)s and caspases. Findings that EVs are taken up by target cells such as macrophages, and that this leads to increased proinflammatory functioning provide support for their role in the development of pathologies associated with toxicant exposure. Understanding the nature of EVs responding to toxic exposures and their cargo may lead to the development of novel therapeutic approaches to mitigating lung injury.
Keywords: macrophages, inflammation, epithelial cells, ozone, cigarette smoke, LPS
1. Introduction
Because the respiratory track is continuously exposed to the external environment, it is highly sensitive to the adverse effects of inhaled gases, acid aerosols, particles, and pathogens. The lung is also sensitive to systemically administered drugs and other agents, as well as radiation. Exposure to toxic levels of these xenobiotics results in acute lung injury. This can progress to chronic lung disease depending on the nature of the agent, the dose, and duration of exposure. A characteristic response to lung injury is an accumulation of inflammatory cells including neutrophils and macrophages in the tissue. These cells, which are largely derived from blood and bone marrow precursors, function to rid the body of foreign materials and debris and restore normal tissue structure and function. Cell-cell communication between infiltrating neutrophils and macrophages, and parenchymal cells in the lung (e.g., epithelial cells, endothelial cells and fibroblasts) is essential for a successful initiation and resolution of the inflammatory response.
Extracellular vesicles (EVs) have emerged as key players in cell-cell communication during inflammatory responses. EVs are a heterogeneous group of cell-derived membranous structures that are classified as exosomes, microvesicles (MVs), or apoptotic bodies (ABs) according to their mechanism of formation, cargo, and approximate size. Whereas exosomes and MVs are released by healthy cells, apoptotic bodies are generated by cells undergoing programmed cell death. Apoptotic bodies are the largest and most heterogeneous of the EVs, ranging in size from 1–5 μm, and are produced by membrane blebbing during apoptotic disassembly [1]. MVs range in size from 200–500 nm and are formed by the outward budding and fission of the plasma membrane [1–3]. Exosomes are the smallest of the EVs ranging in size from 30 nm-150 nm; they are generated from the endosome and express an evolutionally conserved set of proteins including CD81, CD63, CD9, MHC I and II, Alix, and Tsg101 [4, 5]. The significance of EVs lies in their ability to transfer cargo (e.g., DNA, proteins, lipids, mRNAs and miRNAs) to other cells, thereby influencing recipient cell function. The cargo varies depending on the cellular origin of the EVs and the inflammatory state of the tissue, which changes following exposure to environmental stressors such as cigarette smoke, air pollutants, hyperoxia and other pulmonary toxicants. EVs have been isolated from blood, urine, bronchoalveolar lavage fluid (BAL), and saliva and accumulating evidence suggests that they play a role in both normal physiological processes and in disease pathogenesis by transporting cargo that either exacerbate or attenuate inflammatory responses in recipient cells [6, 7].
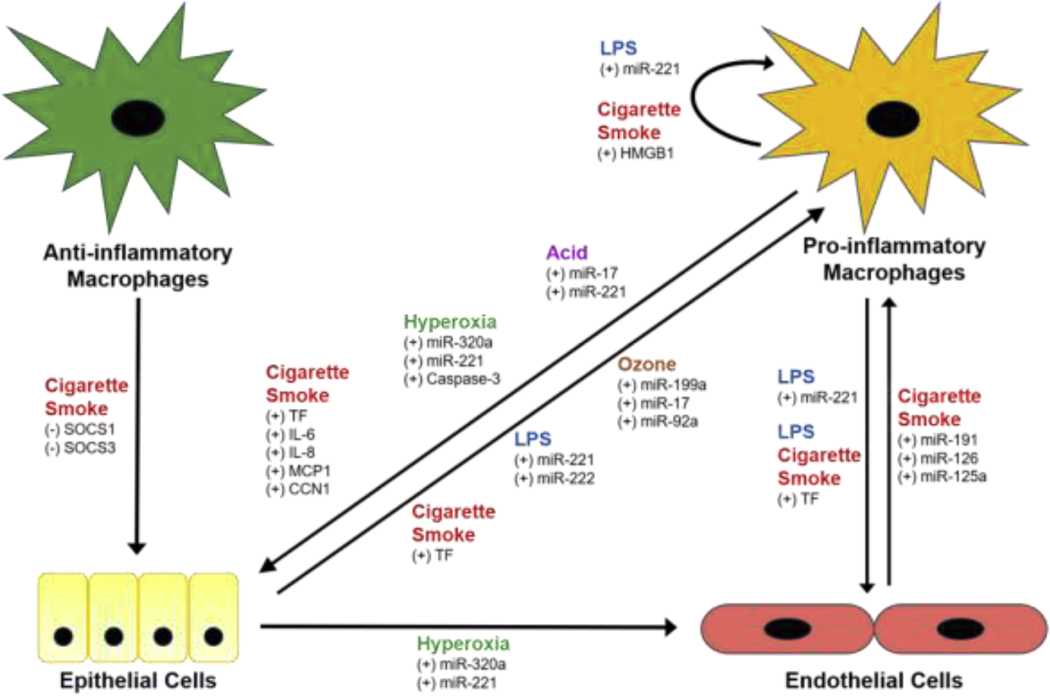
Although most cells types within the lung are capable of releasing EVs, the major cellular sources following exposure to pulmonary toxicants are macrophages, epithelial cells, and endothelial cells. Macrophages are located throughout the lung most prominently in the alveolar and interstitial spaces and function as the first line of immune defense against inhaled toxicants. In addition to phagocytizing foreign materials and secreting cytotoxic mediators, resident macrophages play a key role in initiating inflammatory responses in the lung. Inflammatory macrophages have been classified as proinflammatory and anti-inflammatory. These macrophage subpopulations sequentially appear in the lung after exposure to toxicants, consistent with initiation and resolution of inflammation. EVs are released by both macrophage cell types in response to injury; cargo they carry has been shown to target epithelial and endothelial cells in the lung (Figure 1).
Figure 1.
Cell-cell communication between lung cell types in response to pulmonary toxicants. Exposure to cigarette smoke, ozone, hyperoxia, acid, and LPS stimulates (+) or inhibits (−) the release of extracellular vesicles (EVs) from anti-inflammatory and pro-inflammatory macrophages, epithelial cells, and endothelial cells in the lung. The EVs transport cargo (e.g., proteins, miRNAs) to recipient cells thereby influencing their function through uni- and bidirectional paracrine and autocrine signaling mechanisms.
Epithelial cells in the respiratory tract are increasingly recognized to be active effectors contributing to both innate and adaptive immune responses. Together with alveolar macrophages, they function as immune sentinels, responding to inhaled pathogens and other foreign materials by upregulating pattern recognition receptors, initiating defensive signaling cascades, recruiting and activating leukocyte-mediated defenses, killing microbes, and restoring host homeostasis [8]. In the lower respiratory tract, two types of epithelial cells have been identified; type I cells which provide a gas exchange surface and are highly sensitive to inhaled toxicants, and type II cells which synthesize and release surfactants and replace type I cells following lung injury. Evidence suggests that type I epithelial cells are major producers of EVs which mainly contribute to the proinflammatory environment of the lung.
Pulmonary endothelial cells line the blood vasculature. In addition to providing a passive surface for gas exchange, they regulate the synthesis and metabolism of vasoactive compounds such as nitric oxide and endothelin-1, which regulate vascular tone. They also play an active role in hemostasis, as well as inflammation [9]. Damage to endothelial cells during stress and injury results in alterations in hemodynamics, permeability, gas exchange, and intercellular signaling. Endothelial cells have been identified as a source of EVs that influence inflammatory macrophage activity and systemic coagulant activity and are themselves targets of EVs derived from epithelial cells and macrophages.
In this review, we provide an overview of the impact of exposure to pulmonary toxicants on EV release from macrophages, epithelial cells and endothelial cells, focusing on cargo carried by MVs and its impact on target cells and disease pathogenesis.
2. Cigarette Smoke
First-hand cigarette smoke is attributed to approximately five million deaths per year in developed countries, while an additional 600,000 deaths are suspected to be caused by second-hand smoke [10]. Cigarette smoke is a complex mixture containing thousands of different chemical species, many of which are highly reactive including carbonyls, nitrosamines, and quinones [11]. The respiratory system is a major target of cigarette smoke where it is known to cause acute and chronic lung injury including pneumonia, chronic obstructive pulmonary disease, and lung cancer [12, 13]. Cigarette smoke-induced lung injury is thought to be caused by oxidative stress, inflammation, and protease-antiprotease imbalance in the lung [11]. The inflammatory response is caused by activation of epithelial cells, endothelial cells and resident macrophages, which release chemotactic molecules and inflammatory mediators that recruit neutrophils, eosinophils, monocytes and lymphocytes to the site of injury [11, 14, 15]. Accumulating evidence suggests that EVs play a key role in both cigarette smoke-induced epithelial cell activation and inflammation [16].
EVs generated following cigarette smoke exposure are derived from pulmonary epithelial cells and macrophages and, to a lesser extent, endothelial cells. Airway epithelial cells are the first targets of cigarette smoke and have been shown to release increased numbers of EVs after exposure. The increase has been attributed to thiol-reactive compounds present in cigarette smoke [17]. These EVs are largely directed towards proinflammatory/cytotoxic macrophages causing them to upregulate cell adhesion molecules, promoting their migratory and proliferative activity [18, 19]. Cargo that has been identified in epithelial cell-derived EVs following cigarette smoke exposure includes adhesion molecules (e.g., CD11b, ICAM-1, ELAM-1, etc.), cytokines (e.g., IL-6, IL-8, MCP-1, CCN1 family members (e.g., CYR61, CTGF, NOV family1, CD63, CD81, and tissue factor [7, 20, 21]. These proteins stimulate the release of proinflammatory cytokines from macrophages. CCN1 is also thought to promote inflammation by stimulating IL-8 production [3]. EVs identified as CD63 and CD81 positive contain tissue factor which induces activation of inflammatory macrophages by altering the expression of cell surface thiols. Epithelial cells also release EVs containing miR-210, which inhibits macrophage autophagy and reduces fibroblast differentiation into myofibroblasts [7].
Increased numbers of EVs have been identified in the serum of smokers and are thought to primarily originate from pulmonary epithelial cells [22]. These EVs contain high levels of lipoproteins APOLI, LPA APOB, CETP, CLU, SAA4 and coagulation cascade proteins C1QA, C1Qc, C1S, CFB, CLU, and SERPINA5 [22, 23]. Increases in EVs in plasma and serum of humans can modulate coagulant signaling and cardiovascular function, leading to increased risk of cardiovascular disease in smokers.
Cigarette smoke also modifies the release of EVs from macrophages. Whereas proinflammatory macrophages release increased numbers of EVs after cigarette smoke exposure, EV release from anti-inflammatory macrophages is generally suppressed. For example, cigarette smoke stimulates proinflammatory macrophages to release EVs containing tissue factor Xa, a procoagulant molecule that induces expression of coagulation cascade proteins in red blood cells, endothelial cells and epithelial cells [24]. Macrophages also release EVs containing HMGB1 which promotes proinflammatory activation of macrophages in an autocrine and paracrine manner [19]. Following cigarette smoke exposure, macrophages release 3-fold more MMP-14 containing EVs relative to macrophages from nonexposed subjects. This is important as MMP-14 has gelatinolytic and collagenolytic activity and is involved in tissue remodeling and fibrogenesis [25]. In contrast, the number of anti-inflammatory macrophage-derived EVs containing SOCS1 and SOCS3 decreased after cigarette smoke exposure. Under homeostatic conditions, SOCS1 and SOCS3 transported to epithelial cells in EVs reduce inflammatory cytokine signaling. Suppression of anti-inflammatory macrophage release of SOCS-containing EVs prolongs inflammation [18].
Endothelial cell derived EVs released following cigarette smoke exposure are less well-characterized. Endothelial EVs containing miR-191, miR-126 and miR-125a have been identified which promote proinflammatory macrophage activation, as measured by uptake of apoptotic bodies. Another study showed that cigarette smoke increases endothelial EVs containing high levels of spermine in their outer membranes. Spermine activates extracellular calcium-sensing receptors and promotes smooth muscle constriction, contributing to cigarette induced pulmonary hypertension [26]. Overall, the EV mediated response of cigarette smoke exposure plays a key role in the development of pulmonary inflammation and chronic disease pathologies.
3. Air Pollution
Exposure to ambient air pollution is a significant public health concern worldwide. It is the ninth leading risk factor for mortality and is responsible for 3.2 million deaths each year [27, 28]. Inhalation of air pollutants contributes to acute and chronic cardiopulmonary diseases including asthma, chronic obstructive pulmonary disease, lung cancer and respiratory infection in healthy and susceptible populations [27]. Air pollution is a complex mixture of particulate matter (PM), metals, carbon monoxide, ozone and sulfur and nitrogen oxides [27]. Of the criteria air pollutants, PM and ozone impart the most widespread cardiopulmonary health effects [27].
PM itself is a complex mixture of organic chemicals, metals, acids and soil or dust particles [29]. The health effects of PM are attributed to the composition and size of particles which are classified as PM10, PM2.5, or PM0.1 based on their aerodynamic diameter [30]. It has been suggested that PM10 is more pro-inflammatory due to the presence of more soluble and insoluble components including LPS, whereas PM2.5 and PM0.1 are less inflammatory but cause greater cardiovascular impairment [31]. Nonetheless, PM exposure causes increased oxidative stress and activation of endothelial cells, macrophages, and epithelial cells in the lung leading to the release of pro-inflammatory mediators and local and systemic inflammation [32, 33].
PM exposure in humans has been reported to be associated with increases in EVs in plasma and serum, which modulates coagulant signaling and cardiovascular function [29, 34–37]. In one report, an increase in endothelial cell-derived EVs was noted in serum following episodic exposure to PM2.5, while another study found increases in platelet and red blood cell-derived EVs in individuals chronically exposed to PM10 [35, 36]. Short-term exposure of overweight/obese subjects to PM10 was associated with increased levels of EVs which were mostly derived from CD14+ monocytes/macrophages and CD61+ platelets [37, 38]. Increased release of EVs from monocytes, endothelial cells, epithelial cells, and macrophages has also been described after in vitro exposure to PM [38–40].
EVs released in response to PM exposure are thought to modulate cardiovascular function by transporting EV-bound tissue factor and miRNAs. PM exposure caused increases in EV-bound tissue factor release from mononuclear and endothelial cells in vitro [29]. An analysis of serum from humans exposed to PM10 identified increased circulating tissue factor that was presumably bound to EVs of monocyte origin, but no conclusive evidence was presented [35]. Observational studies have indicated strong associations between PM exposure and EV miRNA content. Thus, chronic PM2.5 exposure was positively associated with circulating EVs containing miR-199a/b, miR-223–3p, let-7g-5p, miR-126–3p, miR-130a-3p, miR146a-5p, miR-150–5p, miR-191–5p and miR-23a-3p [41, 42]. A positive association between plasma EVs containing miR-203b, miR-200c and miR-30d was also noted after exposure of steel plant workers to PM, which was attributed to the metallic component, whereas another study reported a significant association with miR-128 and miR-302c [39, 43]. In obese patients, PM exposure was correlated with a down-regulation of EV miRNAs including let-7c-5p, miR-331–3p, miR-185–5p, miR-106–5p and miR-652–3p [37].
A few studies have mechanistically connected EV miRNAs or EV-bound tissue factor content with phenotypic changes in target cells. One study reported that EVs released from THP-1 macrophages and peripheral blood mononuclear cells (PBMC) following exposure to PM2.5 in vitro caused increased release of IL-6 and TNFα, but reduced release of IL-8 from co-cultured BEAS-2Bs epithelial cells [40]. Most studies correlated changes in miRNA levels with markers of inflammation, coagulation parameters, or to downstream mRNA targets known to be involved in cardiovascular function. In one report, an association was noted between long-term ambient PM2.5 levels and EV-derived miRNA-let-7g-5p, miRNA-126–3p, miRNA-130a-3p, miRNA146a-5p, miRNA-150–5p, miRNA-191–5p and miRNA-23a-3p, each of which regulates genes involved with cardiovascular disease, based on linkage analysis using Ingenuity Pathway Analysis [42]. Additionally, a mediation analysis identified five EV miRNAs (let-7c-5p, miR-331–3p, miR-185–5p, miR-106–5p, miR-652–3p) that were predicted to be associated with elevated fibrinogen levels in overweight/obese subjects exposed to PM10 [37]. Occupational exposure of steel plant workers to PM was also positively associated with changes in miRNA-203b, miRNA-200c and miRNA-30d in EVs which were also related to changes in inflammatory markers and coagulation function [43].
Less is known about the potential role of EVs and EV-miRNAs in ozone-induced lung toxicity. A recent study by our group found that EVs recovered from BAL after ozone treatment of mice, contained increased amounts of miRNA cargo, specifically miR-17, miR-92a, and miR199a [44]. Flow cytometric analysis of these EVs indicated that most originated from CD326+ epithelial cells. Importantly, confocal imaging demonstrated that the EVs were actively taken up by alveolar macrophages. Intratracheal administration of EVs collected from ozone-exposed mice to naïve mice resulted in a significant increase in expression of inducible nitric oxide synthase and IL-6 in lung tissue and CXCL-1, CXCL-2 and IL-1β in alveolar macrophages. The increase in IL-1β in alveolar macrophages was attributed to EV-mediated transfer of miR-199a-3p [44]. Taken together, these studies revealed a novel mechanism whereby EVs potentiate ozone-induced proinflammatory signaling by transporting miR-199a-3p from lung epithelial cells to alveolar macrophages promoting their proinflammatory activity.
4. Hyperoxia
Hyperoxic acute lung injury is caused by exposure to high concentrations of oxygen (>50%) for an extended period and may occur in response to administration of supplemental oxygen [45]. Hyperoxia generates reactive oxygen species in the lung that induce lipid peroxidation and mitochondrial damage in epithelial cells resulting in impaired alveolar-capillary membrane integrity and an influx of inflammatory cells [45–48]. The mechanisms of intercellular cross talk involved in the pathogenesis of hyperoxia-induced lung inflammation and injury are not well established, but growing evidence points to a role for EVs [6, 49].
Multiple studies have identified increased numbers of EVs in BAL from mice exposed to hyperoxia the majority of which were found to be MVs [6, 49]. This was in contrast to BAL from air-exposed mice which only contained exosomes [6]. Flow cytometric analyses confirmed that the majority of EVs originated from epithelial cells after hyperoxia exposure, while alveolar macrophages were the primary source of EVs in air-exposed mice [6, 49]. Hyperoxia resulted in enrichment of protein and miRNA cargo within EVs including caspases, miR-92a-3p, miR-320a, miR-33a-5p, miR221–3p, miR-145–5p, miR-342–3p, miR-10a-5p and miR-422a [6, 49].
Functional analyses revealed that inhalation of MVs isolated from hyperoxia-exposed mice caused an increase in macrophages in BAL of naïve mice [6]. These results were confirmed in vitro where MVs collected from supernatants of hyperoxia-exposed BEAS-2B epithelial cells promoted the migration of THP-1 macrophages and increased their secretion of TNFα and IL-1β [6]. Subsequent studies showed that the functional changes in macrophages were due to MV-mediated transport of miR-221 and miR-320a [6].
Caspase-3, an essential endoprotease involved in apoptosis and inflammation, has also been identified in epithelial cell-derived MVs following hyperoxia, along with caspase-1, caspase8 and caspase-12 [49, 50]. Caspase-3 enriched EVs were found to regulate macrophage function and augment a pro-inflammatory response by upregulating rho-associated protein kinase 1 (ROCK1) and macrophage inflammatory protein 2 (MIP-2) [49]. Interestingly, epithelial cell derived EVs have also been detected in serum following hyperoxia exposure suggesting systemic effects [49]. Taken together, these data suggest that epithelial-derived EVs released in response to hyperoxia facilitate macrophage activation and inflammation.
5. Acids
Exposure to acids which can occur when acid gastric contents are aspirated under general anesthesia causes injury to the airways and extensive alveolar epithelial damage (4). This is characterized by increased permeability of the alveolar/capillary barrier resulting in alveolar hemorrhage and edema which initiates a predominantly neutrophilic acute inflammatory response that may progress to fibrosis [51–53]. As with other lung injury models associated with inflammation, there is a growing appreciation for the potential role for EVs in mediating intercellular communication contributing to pathophysiological responses.
Using a hydrochloric acid inhalation-induced lung injury model, Lee et al. [54] demonstrated that most MVs released into BAL were derived from epithelial cells and were readily taken up by alveolar macrophages [54]. The epithelial-derived MVs were enriched with miRNAs including miR-17, miR-92a, miR-221 and miR-320a [54]. Functional analyses revealed that miR-17 and miR-221 upregulated integrin β1, a key protein responsible for activating genes that stimulate monocyte differentiation and mediate cell adhesion in recipient macrophages [54, 55]. Macrophage uptake of MVs was correlated with increases in TNFα, IL-1β and IL-6 levels in BAL, suggesting a mechanism of pro-inflammatory activation following acid inhalation [54].
6. Lipopolysaccharide
Pulmonary administration of lipopolysaccharide (LPS), also known as endotoxin, is a widely used experimental model of acute lung injury or its more severe form, acute respiratory distress syndrome. LPS binds to the CD14/TLR4/MD2 receptor complex on epithelial cells, macrophages and neutrophils in the lung thereby initiating inflammatory responses [56]. LPS-induced inflammation leads to multiple organ dysfunction consistent with acute lung injury and is thought to be a consequence of epithelial and endothelial damage, production of proinflammatory and cytotoxic mediators and extensive neutrophilic influx into the lungs [57].
LPS-induced lung injury in mice is associated with increases in EVs in BAL that originate from alveolar macrophages [54]. Functionally, alveolar macrophage-derived EVs promote the recruitment of macrophages to the lung, augment their production of cytokines and inflammatory mediators, upregulate TLR expression and contribute to the development of lung inflammation and injury [54]. EVs isolated from the lungs of LPS treated animals have been reported to consist predominantly of apoptotic bodies containing miR-221 and miR-222 which stimulate lung epithelial cell proliferation by modulating cyclin-dependent kinase inhibitor 1B pathways [58].
LPS-induced lung injury can progress to a systemic hyperinflammatory response resulting in sepsis. Sepsis is the leading cause of death in critically ill patients and treatment protocols are not well-defined [59]. Nair et al. [60] reported an increase in macrophage-derived EVs in the blood of LPS-exposed mice that developed sepsis. These EVs contained high concentrations of macrophage histones, which were postulated to directly bind TLR-4 receptors on circulating immune cells contributing to the development of sepsis [60]. Altogether, these studies highlight the importance of EVs and in particular their cargo in LPS-induced acute lung injury and sepsis.
7. Conclusions
The role of EVs in mediating communication between the different cell types in the lung is increasingly being recognized as key in orchestrating inflammatory responses to diverse pulmonary toxicants. The nature of the cargo carried by EVs varies with their cellular origin, the type of inflammatory insult and the recipient cell. In general, it appears that most EV cargo promotes inflammation, tissue injury, and disease pathogenesis. This suggests that proinflammatory EVs offer a potential new target for the development of therapeutics aimed at mitigating the untoward effects of pulmonary toxicants.
Table 1.
Pulmonary Toxicants and Extracellular Vesicles
| ORIGIN | CARGO | TARGET | RESPONSE | REFERENCE |
|---|---|---|---|---|
| Cigarette Smoke | ||||
| Epithelial cells | TF | Proinflammatory macrophages Epithelial cells | Stimulation inflammation | [61] |
| CCN1 (CYR61, CTGF, NOV family 1), IL-6, IL-8, MCP-1 | Proinflammatory macrophages | Increase IL-8 release and stimulate migration | [20] | |
| Proinflammatory macrophages | TF-Xa | Proinflammatory macrophages | Increase coagulation cascade pathways | [24] |
| TF | Proinflammatory macrophages Epithelial cells | Increase inflammation | [61] | |
| CCN1 (CYR61, CTGF, NOV family 1), IL-6, IL-8, MCP-1 | Proinflammatory macrophages | Increase IL-8 release and stimulate migration | [20] | |
| Anti-inflammatory macrophages | SOCS1, | Epithelial cells | Decrease cytokine signaling | [18] |
| SOCS3 | ||||
| Endothelial cells | miR-191, | Proinflammatory macrophages | Stimulate phagocytosis of Abs | [62] |
| miR-126, | ||||
| miR-125a | ||||
| Air Pollution-PM | ||||
| ND | miRNA-203b, | ND | Increase inflammation and coagulation | [43] |
| miRNA-200c, | ||||
| miRNA-30d | ||||
| Air Pollution - Ozone | ||||
| Epithelial cells | miR-199a-3p | Proinflammatory macrophages | Increase IL-1β release | [44] |
| Hyperoxia | ||||
| Epithelial Cells Proinflammatory macrophages | miR-320a, | Proinflammatory macrophages Epithelial cells | Increase TNFα and IL- 1β release Increase VEGF release | [6, 49] |
| miR-221 | ||||
| Caspase-3 | Proinflammatory macrophages | ROCK1 and MIP-2 dependent activation | ||
| Acid | ||||
| Epithelial cells | miR-17, | Proinflammatory macrophages | Upregulate β1 and stimulate migration | [54] |
| miR-221 | ||||
| LPS | ||||
| Epithelial Cells Proinflammatory macrophages | miR-221, | Endothelial cells Proinflammatory macrophages | Stimulate proliferation | [58] |
| miR-222 | ||||
Abbreviations. ABs, apoptotic bodies; APOB, apolipoprotein B; BAL, bronchioalveolar lavage fluid; CCN, cellular communication network factor; CTGF, connective tissue growth factor; CYR, cysteine-rich; EVs, extracellular vesicles; HMGB1, high mobility group box 1; IL, interleukin; LPA, lipoprotein A; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; MVs, microvesicles; ND, not determined; NOV, nephroblastoma overexpressed; PM, particulate matter; ROCK, Rho-associated protein kinase; SOCS, suppressor of cytokine signaling; TF, tissue factor; TF-Xa, tissue factor Xa; TLR, toll-like receptor; TNF, tumor necrosis factor
Highlights.
Extracellular vesicles (EVs) are released from lung macrophages, epithelial cells and endothelial cells
Exposure to pulmonary toxicants stimulates the release of EVs
EVs contain miRNA and protein cargo that influences target cell function
Cell-cell communication via EVs is important in inflammation and tissue injury
Acknowledgments
Funding
This work was supported by National Institute of Health [grant numbers AR055073, ES004738, ES007148, ES030984, ES005022, GM111313, GM1275696, and HL142758].
Abbreviations:
- ABs
apoptotic bodies
- APOB
apolipoprotein B
- APOLI
apolipoprotein L1
- BAL
bronchoalveolar lavage fluid
- C1QA
complement C1q A chain
- C1Qc
complement C1q C chain
- C1S
complement component
- CCN
cellular communication network factor
- CETP
cholesterol ester transfer protein
- CFB
complement factor B
- CLU
clusterin
- CTGF
connective tissue growth ractor
- DAMPs
damage-associated molecular patterns
- EVs
extracellular besicles
- HMGB1
high mobility group box 1
- ICAM
intracellular adhesion molecule
- LPA
lipoprotein A
- LPS
lipopolysaccharide
- MIP
macrophage inflammatory protein
- MMP
matrix metalloproteinase
- MVs
microvesicles
- NOV
nephroblastoma overexpressed
- PBMC
peripheral blood mononuclear cells
- PM
particulate matter
- ROCK
rho-associated protein kinase
- SAA4
serum amyloid A4
- SERPINA5
serpin family A member 5
- SOCS
suppressor of cytokine signaling
- TF
tissue factor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- Tsg
tumor susceptibility gene
Biography

Jaclynn Andres is currently a graduate student in the Joint Graduate Program in Toxicology at Rutgers University. Prior to beginning her graduate studies, she worked as a technician for the United States Medical Research Institute of Chemical Defense developing medical countermeasures for chemical warfare agents. She also worked as a research technician for Weill Cornell Medicine in Immunology. Her current doctoral research, under the direction of Dr. Debra Laskin, is focused on inflammatory mechanisms of pulmonary injury induced by chemical toxicants.

Ley Cody Smith is a postdoctoral fellow in the Department of Pharmacology and Toxicology in the Ernest Mario School of Pharmacy at Rutgers University. The focus of his research is the role of macrophage subpopulations in chemical-induced lung injury and repair and is performed under the mentorship of Dr. Andrew Gow and Dr. Debra Laskin. Dr. Smith received his master’s and doctorate in toxicology from the University of Florida, under the mentorship of Dr. Tara Sabo-Attwood. He is author/co-author of 11 publications. Dr. Smith has served in trainee leadership roles in the Society of Toxicology and the Society of Environmental Toxicology and Chemistry.

Alexa Murray is currently completing her doctoral dissertation in the Joint Graduate Program in Toxicology at Rutgers University. Prior to beginning her graduate studies, she worked as a Research Technician at The Scripps Research Institute in La Jolla, CA in Immunology. Her doctoral research, under the direction of Dr. Debra Laskin, is focused on inflammatory mechanisms of pulmonary injury induced by chemical toxicants. She is active in the Society of Toxicology and has served as student representative for the Immunotoxicology Specialty Section.

Yang Jin obtained her MD from Peking University Medical Health Center in China and her Ph.D. from Rutgers University in New Jersey. She completed her research post-doctoral training at the National Institutes of Health, her internal medicine residency at the University of Rochester, and her Pulmonary/Critical Care Medicine fellowship at the University of Pittsburgh. She is currently Associate Professor of Medicine at Boston University. Her research interests focus on the role of non-coding RNAs, extracellular vesicles and their underlying mechanisms in the pathogenesis of lung injury and systemic inflammation. She has more than 55 publications.

Rita Businaro received her MD and Ph.D. degrees from Sapienza University of Rome with a medical specialization in General Pathology. She completed her postdoctoral training at the Wistar Institute of Anatomy and Biology at the University of Pennsylvania where she studied cancer and immunology. She is currently Professor of Professor of Human Anatomy at the School of Medicine, Sapienza University of Rome and Vice-Chair of I level degree in TSRM, Polo Pontino. She is a member of the Technico-Pedagogical Commission of the Department of Medico-Surgical Sciences and Biotechnologies at Sapienza University, and Vice-President the Italian Fulbright Association. Her research is focused on inflammatory mediators and cellular players in atherosclerosis development and progression, comorbidity of Alzheimer’s disease and cerebrovascular disorders, nutraceuticals with anti-inflammatory activity, and the development of new methods for regenerative medicine. She has over 150 publications.

Jeffrey Laskin received his Ph.D. in Experimental Therapeutics from Roswell Park Cancer Institute, the State University of New York at Buffalo, NY. He completed a post-doctoral fellowship at the College of Physicians and Surgeons at Columbia University before joining the faculty of Rutgers University. He is currently a Distinguished Professor in the Rutgers School of Public Health. He is Director of the Division of Toxicology at the Environmental and Occupational Health Sciences Institute (EOHSI) and Deputy Director of the Joint Graduate Program in Toxicology at Rutgers University. He is D also irector of the Rutgers University CounterACT Research Center of Excellence, a major research effort to develop the most promising scientific discoveries that lead to improved medical countermeasures to protect against a chemical attack. Dr. Laskin’s research focuses on mechanisms of chemical-induced skin, lung and liver toxicity. He is an expert in mechanisms of chemical toxicity and redox chemistry. He has over 300 publications.

Debra Laskin received her Ph.D. degree in Pharmacology and Toxicology from the Medical College of Virginia, Virginia Commonwealth University. She completed her postdoctoral training at the Wistar Institute of Anatomy and Biology at the University of Pennsylvania where she studied carcinogenesis and immunology. She is currently Distinguished Professor and Chair of the Department of Pharmacology and Toxicology and the Roy A. Bowers Endowed Chair at Rutgers University School of Pharmacy. She is Deputy Director of the Rutgers Center for Environmental Exposures and Diseases and a member of the Rutgers Cabinet of the Environmental and Occupational Health Sciences Institute. Her research is focused on nonspecific immunity and inflammation; specifically, the role of activated macrophages and inflammatory mediators in the pathophysiology of xenobiotic-induced tissue injury. She is also studying biochemical and molecular mechanisms underlying macrophage activation and function. She has over 275 publications.
Footnotes
Conflict of interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J, Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes, J. Extracell. Vesicles 2 (2013), 20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Devaux F, Herrmann A, Ohlwein N, Kozlov MM, How lipid flippases can modulate membrane structure, Biochim. Biophys. Acta 1778 (2008), 1591–1600. [DOI] [PubMed] [Google Scholar]
- [3].Tuck S, Extracellular vesicles: budding regulated by a phosphatidylethanolamine translocase, Curr. Biol 21 (2011), R988–R990. [DOI] [PubMed] [Google Scholar]
- [4].Andreu Z, Yáñez-Mó M, Tetraspanins in extracellular vesicle formation and function, Front. Immunol 5 (2014), 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G, Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles, J. Cell Sci 126 (2013), 5553–5565. [DOI] [PubMed] [Google Scholar]
- [6].Lee H, Zhang D, Zhu Z, Cruz CSD, Jin Y, Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicles-containing microRNAs, Sci. Rep 6 (2016), 35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fujita Y, Araya J, Ito S, Kobayashi K, Kosaka N, Yoshioka Y, Kadota T, Hara H, Kuwano K, Ochiya T, Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis, J. Extracell. Vesicles 4 (2015), 28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leiva-Juárez MM, Kolls JK, Evans SE, Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense, Mucosal Immunol. 11 (2018), 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Block ER, Pulmonary endothelial cell pathobiology: implications for acute lung injury, Am. J. Med. Sci 304 (1992), 136–144. [DOI] [PubMed] [Google Scholar]
- [10].World Health Organization, World Health Organization global report on mortality attributable to tobacco, (2012). [Google Scholar]
- [11].Colombo G, Clerici M, Giustarini D, Portinaro NM, Aldini G, Rossi R, Milzani A, Dalle-Donne I, Pathophysiology of tobacco smoke exposure: recent insights from comparative and redox proteomics, Mass Spectrom. Rev 33 (2014), 183–218. [DOI] [PubMed] [Google Scholar]
- [12].Caminati A, Harari S, Smoking-related interstitial pneumonias and pulmonary Langerhans cell histiocytosis, Proc. Am. Thorac. Soc 3 (2006), 299–306. [DOI] [PubMed] [Google Scholar]
- [13].Patel RR, Ryu JH, Vassallo R, Cigarette smoking and diffuse lung disease, Drugs 68 (2008), 1511–1527. [DOI] [PubMed] [Google Scholar]
- [14].Ortega E, Hueso F, Collazos M, Pedrera M, Barriga C, Rodriguez A, Phagocytosis of latex beads by alveolar macrophages from mice exposed to cigarette smoke, Comp. Immunol. Microbiol. Infect. Dis 15 (1992), 137–142. [DOI] [PubMed] [Google Scholar]
- [15].Tardif J, Borgeat P, Laviolette M, Inhibition of human alveolar macrophage production of leukotriene b4 by acute in vitro and in vivo exposure to tobacco smoke, Am J Respir Cell Mol Biol 2 (1990), 155–161. [DOI] [PubMed] [Google Scholar]
- [16].Ryu A, Kim DH, Kim E, Lee MY, The potential roles of extracellular vesicles in cigarette smoke-associated diseases, Oxid. Med. Cell. Longev 2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benedikter BJ, Volgers C, van Eijck H, Wouters EF, Savelkoul H, Reynaert NL, Haenen GR, Rohde GG, Weseler AR, Stassen FR, Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants, Free Radic. Biol. Med 108 (2017), 334–344. [DOI] [PubMed] [Google Scholar]
- [18].Bourdonnay E, Zasłona Z, Penke LRK, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling, J. Exp. Med 212 (2015), 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y, Li G, Liu Y, Werth V, Williams KJ, Liu ML, Translocation of endogenous danger signal HMGB1 from nucleus to membrane microvesicles in macrophages, J. Cell Physiol 231 (2016), 2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moon H-G, Kim S-H, Gao J, Quan T, Qin Z, Osorio JC, Rosas IO, Wu M, Tesfaigzi Y, Jin Y, CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke, Am. J. Physiol. Lung Cell Mol. Physiol 307 (2014), L326–L337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cordazzo C, Petrini S, Neri T, Lombardi S, Carmazzi Y, Pedrinelli R, Paggiaro P, Celi A, Rapid shedding of proinflammatory microparticles by human mononuclear cells exposed to cigarette smoke is dependent on Ca 2+ mobilization, Inflamm. Res 63 (2014), 539–547. [DOI] [PubMed] [Google Scholar]
- [22].Sundar IK, Li D, Rahman I, Proteomic analysis of plasma-derived extracellular vesicles in smokers and patients with chronic obstructive pulmonary disease, ACS Omega 4 (2019), 10649–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maremanda K, Sundar IK, Rahman I, Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice, Toxicol. Appl. Pharmacol (2019), 114–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li M, Yu D, Williams KJ, Liu M-L, Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages, Arterioscler. Thromb. Vasc. Biol 30 (2010), 1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li C-J, Liu Y, Chen Y, Yu D, Williams KJ, Liu M-L, Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke, Am. J. Pathol 182 (2013), 1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhu L, Xiao R, Zhang X, Lang Y, Liu F, Yu Z, Zhang J, Su Y, Lu Y, Wang T, Spermine on endothelial extracellular vesicles mediates smoking-induced pulmonary hypertension partially through calcium-sensing receptor, Arterioscler. Thromb. Vasc. Biol 39 (2019), 482–495. [DOI] [PubMed] [Google Scholar]
- [27].Kurt OK, Zhang J, Pinkerton KE, Pulmonary health effects of air pollution, Curr. Opin. Pulm. Med, 22 (2016), 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010, Lancet 380 (2012), 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Neri T, Pergoli L, Petrini S, Gravendonk L, Balia C, Scalise V, Amoruso A, Pedrinelli R, Paggiaro V Bollati, Particulate matter induces prothrombotic microparticle shedding by human mononuclear and endothelial cells, Toxicol. In Vitro 32 (2016), 333–338. [DOI] [PubMed] [Google Scholar]
- [30].Clifford S, Mazaheri M, Salimi F, Ezz WN, Yeganeh B, Low-Choy S, Walker K, Mengersen K, Marks GB, Morawska L, Effects of exposure to ambient ultrafine particles on respiratory health and systemic inflammation in children, Environ. Int 114 (2018), 167–180. [DOI] [PubMed] [Google Scholar]
- [31].Cho SH, Tong H, McGee JK, Baldauf RW, Krantz QT, Gilmour MI, Comparative toxicity of size-fractionated airborne particulate matter collected at different distances from an urban highway, Environ. Health Perspect 117 (2009), 1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress, Circ. Res 102 (2008), 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laskin DL, Malaviya R, Laskin JD, Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants, Toxicol. Sci 168 (2018), 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Benedikter BJ, Wouters EF, Savelkoul H, Rohde GG, Stassen FR, Extracellular vesicles released in response to respiratory exposures: implications for chronic disease, J. Toxicol. Environ, Part B 21 (2018), 142–160. [DOI] [PubMed] [Google Scholar]
- [35].Bind MA, Lepeule J, Zanobetti A, Gasparrini A, Baccarelli AA, Coull BA, Tarantini L, Vokonas S, Koutrakis P, Schwartz J, Air pollution and gene-specific methylation in the normative aging study: association, effect modification, and mediation analysis, Epigenetics 9 (2014), 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pope III CA, Bhatnagar A, McCracken J, Abplanalp W, Conklin DJ, O’toole T, Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation, Circ. Res 119 (2016), 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pergoli L, Cantone L, Favero C, Angelici L, Iodice S, Pinatel E, Hoxha M, Dioni L, Letizia M, Albetti B, Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation, Part. Fibre Toxicol 14 (2017), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bonzini M, Pergoli L, Cantone L, Hoxha M, Spinazzè A, Del Buono L, Favero C, Carugno M, Angelici L, Broggi L, Short-term particulate matter exposure induces extracellular vesicle release in overweight subjects, Environ. Res 155 (2017), 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bollati V, Angelici L, Rizzo G, Pergoli L, Rota F, Hoxha M, Nordio F, Bonzini M, Tarantini L, Cantone L, Microvesicle associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells, J. Appl. Toxicol 35 (2015), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martin J, Héliot A, Tremolet G, Landkocz Y, Dewaele D, Cazier F, Ledoux F, Courcot D, Cellular response and extracellular vesicles characterization of human macrophages exposed to fine atmospheric particulate matter, Environ. Pollut 254 (2019), 112933. [DOI] [PubMed] [Google Scholar]
- [41].Rodosthenous RS, Kloog I, Colicino E, Zhong J, Herrera LA, Vokonas P, Schwartz J, Baccarelli AA, Prada D, Extracellular vesicle-enriched microRNAs interact in the association between long-term particulate matter and blood pressure in elderly men, Environ. Res 167 (2018), 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodosthenous RS, Coull BA, Lu Q, Vokonas S, Schwartz JD, Baccarelli AA, Ambient particulate matter and microRNAs in extracellular vesicles: A pilot study of older individuals, Part. Fibre Toxicol 13 (2015), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pavanello S, Bonzini M, Angelici L, Motta V, Pergoli L, Hoxha M, Cantone L, Pesatori AC, Apostoli P, Tripodi A, Extracellular vesicle-driven information mediates the long-term effects of particulate matter exposure on coagulation and inflammation pathways, Toxicol. 259 (2016), 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Camino J, Lee H, Zhang D, Sunil V, Vayas K, Laskin JD, Laskin DL, Jin Y, Ozone-associated ROS induces microvesicle containing miRNAs which regulate epithelial cell death., The Toxicologist 168 (2019), 30. [Google Scholar]
- [45].Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Hyperoxia causes angiopoietin 2–mediated acute lung injury and necrotic cell death, Nat. 12 (2006), 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Warner BB, Stuart LA, Papes RA, Wispé JR, Functional and pathological effects of prolonged hyperoxia in neonatal mice, Am. J. Physiol. Lung Cell Mol. Physiol 275 (1998), L110–L117. [DOI] [PubMed] [Google Scholar]
- [47].Alam MA, nee Betal SG, Aghai ZH, Bhandari V, Hyperoxia causes miR199a-5pmediated injury in the developing lung, Pediatr. Res (2019), 1–10. [DOI] [PubMed] [Google Scholar]
- [48].Robba C, Ball L, Pelosi P, Between hypoxia or hyperoxia: not perfect but more physiologic, J. Thorac 10 (2018), S2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Moon H, Cao Y, Yang J, Lee J, Choi H, Jin Y, Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway, Cell Death 6 (2015), e2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McIlwain DR, Berger T, Mak TW, Caspase functions in cell death and disease, CSH Perspect. Biol 5 (2013), a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Knight R, Druskovich G, Tait AR, Johnson KJ, The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury, Anesthesiology 77 (1992), 772–778. [DOI] [PubMed] [Google Scholar]
- [52].Matute-Bello G, Frevert CW, Martin TR, Animal models of acute lung injury, Am. J. Physiol. Lung Cell Mol. Physiol 295 (2008), L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yano T, Deterding RR, Simonet WS, Shannon JM, Mason RJ, Keratinocyte growth factor reduces lung damage due to acid instillation in rats, Am. J. Respir. Cell Mol. Biol 15 (1996), 433–442. [DOI] [PubMed] [Google Scholar]
- [54].Lee H, Zhang D, Wu J, Otterbein LE, Jin Y, Lung epithelial cell–derived microvesicles regulate macrophage migration via microRNA-17/221–induced integrin β1 recycling, J. Immunol 199 (2017), 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Reyes-Reyes M, Mora N, Gonzalez G, Rosales C, Β1 and β2 integrins activate different signaling pathways in monocytes, Biochem. J 363 (2002), 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Donovan C, Royce SG, Vlahos R, Bourke JE, Lipopolysaccharide does not alter small airway reactivity in mouse lung slices, PloS one 10 (2015), e0122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli G, Lipopolysaccharide-induced lung injury in mice. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage, Pulm. Pharmacol. Ther 13 (2000), 61–69. [DOI] [PubMed] [Google Scholar]
- [58].Zhu Z, Zhang D, Lee H, Menon AA, Wu J, Hu K, Jin Y, Macrophage derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA 221/222, J. Leukoc. Biol 101 (2017), 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q, Sun J, Lin Y, Zhang M, Huang R, Resveratrol reduces acute lung injury in a LPS‑ induced sepsis mouse model via activation of SIRT1, Mol. Med. Rep 7 (2013), 1889–1895. [DOI] [PubMed] [Google Scholar]
- [60].Nair RR, Mazza D, Brambilla F, Gorzanelli A, Agresti A, Bianchi ME, LPS-challenged macrophages release microvesicles coated with histones, Front. Immunol 9 (2018), 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stassen FR, van Eijck H, Savelkoul H, Wouters EF, Rohde GG, Briedé JJ, Reynaert NL, de Kok TM, Benedikter BJ, Cell type and exposure-specific modulation of CD63/CD81-positive and tissue factor-positive extracellular vesicle release in response to respiratory toxicants, Oxid. Med. Cell. Longev 2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Serban KA, Rezania S, Petrusca DN, Poirier C, Cao D, Justice MJ, Patel M, Tsvetkova I, Kamocki K, Mikosz A, Structural and functional characterization of endothelial microparticles released by cigarette smoke, Sci. Re 6 (2016), 31596. [DOI] [PMC free article] [PubMed] [Google Scholar]