Abstract
Aim
Prescription opioid analgesic use (OAU) is associated with increased risk of cardiovascular disease (CVD). OAU is more common in patients with than without posttraumatic stress disorder (PTSD), and PTSD is associated with higher CVD risk. We determined whether PTSD and OAU have an additive or interactive association with incident CVD.
Methods and Results
Veterans Health Affairs patient medical record data from 2008 to 2015 was used to identify 2,861 patients 30–70 years of age, free of cancer, CVD and OAU for 12 months before index date. We defined a 4-level exposure variable: 1) no PTSD/no OAU, 2) OAU alone, 3) PTSD alone and 4) PTSD+OAU. Cox proportional hazard models estimated the association between the exposure variable and incident CVD. The mean age was 49.0 (±11.0), 85.7% were male and 58.3% were white, 34.4% had no PTSD/no OAU, 32.9% had PTSD alone, 10.6% had OAU alone, and 22.1% had PTSD+OAU. Compared to patients with no PTSD/no OAU, those with PTSD alone were not at increased risk of incident CVD (HR=0.82; 95%CI:0.63–1.17); however, OAU alone and PTSD+OAU were both significantly associated with incident CVD (HR= 1.99; 95%CI:1.36–2.92 and HR=2.20; 95%CI: 1.61–3.02). There was no significant additive or interactive PTSD and OAU association with incident CVD.
Conclusion
OAU is associated with nearly a two-fold increased risk of CVD in patients with and without PTSD. Despite no additive or interactive effects, the high prevalence of OAU in PTSD may represent a novel contributor to the elevated CVD burden among patients with PTSD.
Keywords: PTSD, opioids, cardiovascular disease, epidemiology, medical records, cohort
One sentence take home summary
Although prescription opioid use and PTSD do not have a statistically significant additive or interactive association with incident cardiovascular disease (CVD), the near two fold increased risk of CVD associated with prescription opioid use, and the much higher prevalence of opioid use in patients with PTSD, point to the possibility that opioids contribute to the CVD burden in this patient population.
INTRODUCTION
A growing body of epidemiological evidence indicates prescription opioid analgesic use (OAU) among patients with non-cancer chronic pain is an independent risk factor for incident cardiovascular disease (CVD). Analysis of the UK General Practice Database indicated that compared to no use of opioids, current opioid use, (but not past use) was significantly associated with incident myocardial infarction (MI) and the risk of MI increased with longer duration of opioid prescriptions.(1) After robust control for confounding, a study using Medicare claims data found that prescription opioid use was associated with a 71% increased risk of CVD events as compared to NSAIDs over a 12 month observation period.(2) Compared to NSAIDs, opioid use was associated with increased risk of MI (HR=2.3; 95%CI:1.3–3.8), heart failure (HR=1.6; 95%CI:1.1–2.4); coronary revascularization (HR=5.3; 95%CI:2.4–11.9) and cardiac death (1.9; 95%CI:1.15–3.2) but was not associated with incident stroke (HR=0.9; 95%CI:0.6–1.5).(2) A dose-response effect was observed in that the number needed to harm (i.e., number of patients prescribed opioids to produce one cardiovascular event) after 30 days of OAU vs. NSAIDS was 214, but after 365 days the number was 7.4.(2) Carman et. al.(3) obtained similar results from an analysis of medical claims which revealed that after age and gender matching, higher morphine equivalent dose (MED) was associated with increased risk of CVD.
Patients with PTSD may be particularly vulnerable to OAU-related CVD. PTSD has been associated with increased risk for CVD,(4, 5) and patients with vs. without PTSD are more likely to receive a prescription for opioid analgesics, to be prescribed higher doses and for longer intervals, and not surprisingly are more likely to exhibit opioid misuse.(6) Among new Veterans Health Affairs (VHA) patients followed for a year, 17.8% of those with PTSD vs. 6.5% without psychiatric diagnoses received an opioid for pain.(6)
Since 2010, the VHA has been successful in reducing the prevalence of opioid prescribing,(7) however, available evidence indicates PTSD remains a risk factor for receiving prescription opioids.(8) In summary, studies to date have determined the separate effects of OAU and of PTSD on CVD as described above. Both as a logical next step and to advance understanding, the aim of the present study was to determine whether PTSD and OAU have additive or interactive effects in the relationship with incident CVD. Even if no additive or interactive effect exists, the high prevalence of OAU among patients with PTSD warrants determining if OAU contributes to the elevated burden of CVD in those with PTSD. After controlling for confounding, we used a large cohort of patients with PTSD to determine if the risk of CVD was greater in patients with both PTSD and OAU compared to patients with PTSD alone, opioid use alone and those without either exposure.
METHODS
Subjects
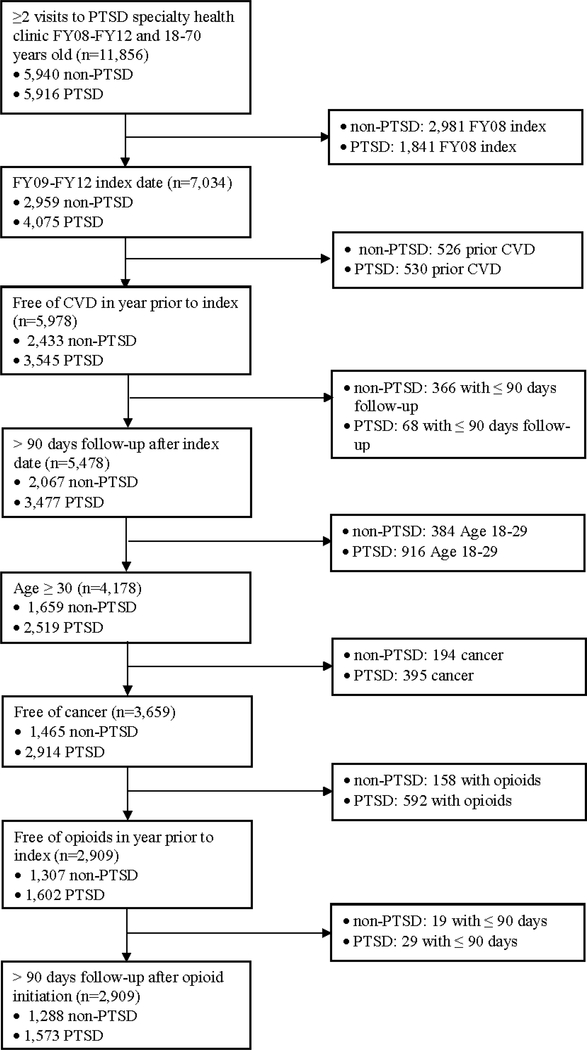
Electronic medical record data were obtained from 11,856 VHA patients between 18–70 years of age. Of these, 5,940 did not have PTSD and 5,916 had PTSD. Patients with PTSD were sampled from five VHA clinics distributed across the United States if they had ≥ 2 visits to the medical center’s PTSD specialty health clinic. Controls were randomly sampled from the same medical centers and were free of PTSD from FY2008 to FY2012. Cases and controls had 2 or more visits between fiscal year (FY) 2008–2012 with follow-up continuing until September 30, 2015. Thus, all patients had the possibility of at least 3 years of follow-up time. We excluded patients < 30 years of age for this analysis because these young patients were unlikely to develop CVD during follow-up. The study procedures were reviewed and approved by review boards of participating institutions.
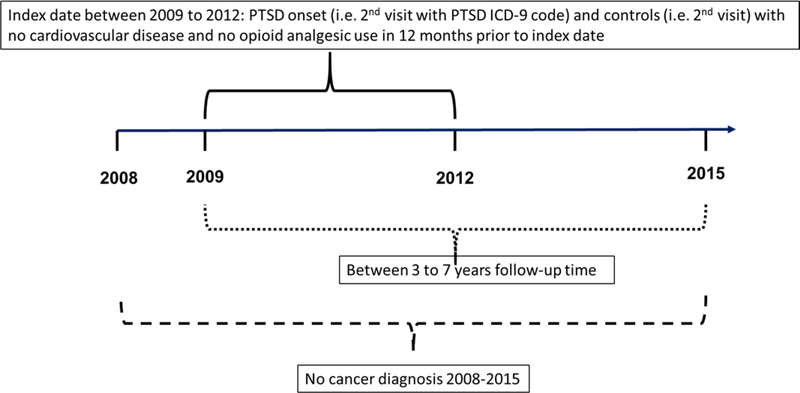
The index date for those with PTSD was the second visit with a PTSD diagnosis and for patients without PTSD it was the second visit for any reason at one of the five VHA’s. To control for unknown duration of PTSD and allow for a 12 month washout prior to index, we excluded patients with PTSD in FY2008 and required the index date to occur from FY2009 to FY2012. Patients were free of OAU and CVD in the year prior to index. To exclude cancer pain, patients were free of cancer for the entire observation period. To allow for a biologically plausible association between PTSD, OAU and incident CVD, we required >90 days follow-up after index and opioid initiation. This resulted in a final analytic sample of 1,288 controls and 1,573 cases with PTSD. The sampling process is shown in Figure 1. The methodological design is illustrated in Figure 2.
Figure 1.
Sample selection process
Figure 2.
PTSD, OAU and incident CVD Retrospective Cohort Design
Variable definitions
Detailed variable definitions for PTSD, OAU, incident CVD, and all potential confounding variables are shown in Appendix A. PTSD was defined by an ICD-9 code for PTSD on 2 or more separate visits within the same 12-month period or one inpatient visit. This diagnostic algorithm has an 82% positive predictive value compared to a PTSD Checklist Score ≥50(9) and 79.4% agreement with Structured Clinical Interview for DSM-IV PTSD diagnosis.(10)
Following our prior methods,(11–13) new OAU was defined by a prescription for any of the following medications at any dose and duration after index: codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, morphine, oxycodone, oxymorphone, and pentazocine. We did not have sufficient sample size to estimate the potential joint effect of PTSD and different OAU dose or duration of use. Therefore, we treated new OAU as a binary (yes/no) variable. We used PTSD and OAU status to create a 4-level exposure variable: 1) no PTSD/no OAU, 2) OAU alone, 3) PTSD alone and 4) PTSD with OAU.
Following methods validated in VHA patient data,(14, 15) and in other administrative data,(16) incident CVD was defined using ICD-9 codes and Current Procedural Terminology (CPT) codes for cardiovascular re-vascularization procedures. CVD diagnoses included hypertensive heart disease, myocardial infarction, ischemic heart disease, diseases of pulmonary circulation and ‘other’ heart disease. We used a broad CVD definition to maximize the power to detect this outcome; therefore we computed sensitivity analyses that defined CVD diagnoses as myocardial infarction or ischemic heart disease.
Potential confounding variables were selected based on theory and/or prior evidence that they are associated with PTSD, OAU, and incident CVD. Potential confounding variables modeled as time dependent were measured from FY2008 to start of OAU or CVD/censor date for those without OAU and included depression, anxiety disorders, alcohol and drug abuse/dependence, sleep disorders, obesity, hypertension, hyperlipidemia, type 2 diabetes and chronic pain conditions. Time invariant potential confounders included smoking status (current/former/never) prior to OAU or CVD/censor date for those without OAU, body mass index at time of CVD/censor date (for descriptive statistics only), and age, race, gender and marital status. To control for detection bias and to account for potential non-VA health care use, we adjusted for access to VHA health coverage only vs. access to non-VHA health insurance and volume of primary health care utilization. Both were treated as time invariant.
Analytic approach
All analyses were performed with SAS v9.4 (SAS Institute, Cary, NC) at an alpha level of 0.05. The distributions of potential confounding variables by the 4-level exposure variable were computed and significant associations were assessed by chi-square tests for categorical variables or one-way ANOVA for continuous variables. We reported the maximum standardized mean difference percent (SMD%)(17) from all pairwise exposure group comparisons as an indicator of meaningful effect size differences in prevalence and means. Potential confounders with an SMD>10%, indicative of meaningful imbalance,(17) were included in final adjusted models.
Age-adjusted and fully adjusted Cox proportional hazard models were computed to obtain hazard ratios and 95% confidence intervals for the association of PTSD and OAU with CVD. The unit of follow-up time was in days and follow-up time was from index date to onset of CVD diagnosis or censor date, defined as last clinic encounter for patients who remained without CVD.
The proportional hazard assumption was tested by examining a time-dependent interaction term of the 4-group PTSD and OAU variable and log(follow-up time), where a significant (p<.05) test indicates assumption violation and different hazard trends over time. The proportional hazard assumption was met in all models (p=0.150 age-adjusted; p=0.184 fully adjusted). Prior to computing adjusted analyses, we tested for multicollinearity and found there was none. The highest correlation was between PTSD and depression diagnoses (r=0.54) and between drug and alcohol abuse/dependence (r=0.52).
The joint effects of OAU and PTSD on CVD were tested and presented on both the multiplicative and additive scales. Statistical or multiplicative interaction, or classic effect modification, was assessed via the ratio and 95% confidence interval of stratum specific hazard ratios for the relationship between OAU and incident CVD for those with and without PTSD. Additive interaction, which is thought to better reflect biological interaction,(18) was tested using the relative excess risk due to interaction (RERI) measure and 95% confidence interval.(19) RERI is the risk in excess of what is expected if the effects of OAU and PTSD were purely additive. A significant RERI > 0 indicates a departure from additivity or a synergistic additive effect where there is observed excess risk than what is expected.
PTSD and depression are highly comorbid and both are associated with increased odds of OAU and incident CVD. Therefore, the associations between PTSD and OAU and incident CVD may be masked by depression. We, thus, computed a priori subgroup sensitivity analysis among patients without depression.
RESULTS
Cohort characteristics are shown in Table 1. On average, patients were 49.0 (±11.0) years of age, 85.7% were male, 58.3% were white and 49% were married. The most prevalent comorbid condition was arthritis (57.6%) and the least prevalent was type 2 diabetes (14.3%).
Table 1.
Sample characteristics, veterans age 30–70 years, free of CVD and OAU for 12 months prior to index (n=2,861)
| Variable | n(%) or mean (±sd) |
|---|---|
| PTSD and OAU 4 level variable: | |
| No PTSD/no OAU | 984 (34.4) |
| PTSD only | 942 (32.9) |
| OAU only | 304 (10.6) |
| PTSD and OAU | 631 (22.1) |
| Age, mean (±sd) (years) | 49.0 (±11.0) |
| Age category | |
| 30–39 | 707 (24.7) |
| 40–49 | 748 (26.1) |
| 50–59 | 718 (25.1) |
| 60–70 | 688 (24.1) |
| Male gender | 2452 (85.7) |
| Race | |
| White | 1667 (58.3) |
| Black | 749 (26.2) |
| Other | 222 (7.8) |
| Missing | 223 (7.8) |
| Marital status | |
| Not married | 1342 (46.9) |
| Married | 1403 (49.0) |
| Missing | 116 (4.1) |
| VHA only insurance | 1716 (60.0) |
| High primary HCU | 600 (21.0) |
| Comorbidities* | |
| Arthritis | 1648 (57.6) |
| Back pain | 1412 (49.4) |
| Musculoskeletal pain | 1081 (37.8) |
| Headache pain | 577 (20.2) |
| Neuropathy | 412 (14.4) |
| Depression | 1230 (43.0) |
| Other anxiety | 497 (17.4) |
| Sleep disorder | 1106 (38.7) |
| Alcohol abuse/dependence | 767 (26.8) |
| Drug abuse/dependence | 462 (16.2) |
| Hypertension | 1344 (47.0) |
| Hyperlipidemia | 1345 (47.0) |
| Type 2 Diabetes | 408 (14.3) |
| Obesity† | 1471 (51.4) |
| BMI, mean (±sd) ‡ | 30.0 (±5.8) |
| BMI category ‡ | |
| < 25 | 464 (16.2) |
| 25 to < 30 | 962 (33.6) |
| ≥ 30 | 1187 (41.5) |
| Missing | 248 (8.7) |
| Smoking | |
| Never | 1262 (44.1) |
| Former | 438 (15.3) |
| Current | 1161 (40.6) |
CVD=cardiovascular disease; PTSD=post-traumatic stress disorder; OAU=opioid analgesic use; FY=fiscal year; VHA= Veterans Health Affairs; HCU=healthcare utilization; BMI= Body Mass Index
Comorbidities occur prior to OAU, unless otherwise noted
BMI≥30 or ICD-9-CM code
Last BMI prior to CVD or last visit date, 2,613 overall
During follow-up the percent of each type of opioid dispensed was: codeine (15.8%), fentanyl (1.0%), hydrocodone (80.1%), hydromorphone (1.7%), levorphanol (0.0%), meperidine (0.3%), morphine (5.9%), oxycodone (36.8%), oxymorphone (0.0%), and pentazocine (0.0%).
About one-third of patients were free of PTSD and OAU, 32.9% had PTSD only, 10.6% OAU only and 22.1% had PTSD and OAU.
The distributions of potential confounders by the 4-level PTSD-OAU variable are shown in Table 2. All covariates were significantly (p<0.0001; type 2 diabetes p=0.045) associated with the PTSD-OAU variable and in all cases the maximum SMD was > 10%.
Table 2.
Covariate relationships with PTSD*OAU, veterans age 30–70 years, free of CVD and OAU for 12 months prior to index (n=2,861)
| Variable, n(%) or mean (±sd) | None(n=984) | PTSD only(n=942) | OAU only (n=304) | PTSD+OAU(n=631) | p-value | max |SMD%|‡ |
|---|---|---|---|---|---|---|
| Age, mean (±sd) (years) | 50.5 (±11.0) | 48.0 (±11.4) | 50.4 (±10.3) | 47.4 (±10.6) | <.0001 | 28.8% |
| Male gender, n(%) | 821 (83.4) | 832 (88.3) | 273 (89.8) | 526 (83.4) | .001 | 19.0% |
| Race, n(%) | ||||||
| White | 557 (56.6) | 532 (56.5) | 207 (68.1) | 371 (58.8) | 24.1% | |
| Black | 193 (19.6) | 293 (31.1) | 66 (21.7) | 197 (31.2) | <.0001 | 26.9% |
| Other | 81 (8.2) | 74 (7.9) | 17 (5.6) | 50 (7.9) | 10.4% | |
| Missing | 153 (15.6) | 43 (4.6) | 14 (4.6) | 13 (2.1) | 49.0% | |
| Marital status, n(%) | ||||||
| Not married | 389 (39.5) | 426 (45.2) | 176 (57.9) | 351 (55.6) | 37.4% | |
| Married | 482 (49.0) | 514 (54.6) | 128 (42.1) | 279 (44.2) | <.0001§ | 25.1% |
| Missing | 113 (11.5) | < 5 | 0 (0.0) | < 5 | 50.9% | |
| VHA only insurance, n(%) | 621 (63.1) | 504 (53.5) | 215 (70.7) | 376 (59.6) | <.0001 | 36.1% |
| High primary HCU, n(%) | 108 (11.0) | 182 (19.3) | 77 (25.3) | 233 (36.9) | <.0001 | 63.8% |
| Comorbidities* | ||||||
| Arthritis, n(%) | 387 (39.3) | 633 (67.2) | 171 (56.3) | 457 (72.4) | <.0001 | 70.7% |
| Back pain, n(%) | 315 (32.0) | 554 (58.8) | 134 (44.1) | 409 (64.8) | <.0001 | 69.5% |
| Musculoskeletal pain, n(%) | 267 (27.1) | 405 (43.0) | 99 (32.6) | 310 (49.1) | <.0001 | 46.5% |
| Headache pain, n(%) | 73 (7.4) | 269 (28.6) | 43 (14.1) | 192 (30.4) | <.0001 | 61.4% |
| Neuropathy, n(%) | 93 (9.5) | 171 (18.2) | 36 (11.8) | 112 (17.8) | <.0001 | 25.4% |
| Depression, n(%) | 117 (11.9) | 630 (66.9) | 49 (16.1) | 434 (68.8) | <.0001 | 142.3% |
| Other anxiety, n(%) | 62 (6.3) | 254 (27.0) | 26 (8.6) | 155 (24.6) | <.0001 | 57.8% |
| Sleep disorder, n(%) | 210 (21.3) | 536 (56.9) | 63 (20.7) | 297 (47.1) | <.0001 | 79.9% |
| Alcohol abuse/dependence, n(%) | 107 (10.9) | 360 (38.2) | 46 (15.1) | 254 (40.3) | <.0001 | 71.5% |
| Drug abuse/dependence, n(%) | 59 (6.0) | 198 (21.0) | 40 (13.2) | 165 (26.2) | <.0001 | 57.1% |
| Hypertension, n(%) | 397 (40.4) | 501 (53.2) | 139 (45.7) | 307 (48.7) | <.0001 | 25.9% |
| Hyperlipidemia, n(%) | 376 (38.2) | 527 (55.9) | 119 (39.1) | 323 (51.2) | <.0001 | 36.1% |
| Type 2 Diabetes | 133 (13.5) | 152 (16.1) | 30 (9.9) | 93 (14.7) | .045 | 18.7% |
| Obesity†, n(%) | 413 (42.0) | 539 (57.2) | 138 (45.4) | 381 (60.4) | <.0001 | 37.5% |
| Smoking, n(%) | ||||||
| Never | 530 (53.9) | 359 (38.1) | 128 (42.1) | 245 (38.8) | 32.0% | |
| Former | 151 (15.4) | 162 (17.2) | 46 (15.1) | 79 (12.5) | <.0001 | 13.2% |
| Current | 303 (30.8) | 421 (44.7) | 130 (42.8) | 307 (48.6) | 37.1% |
CVD=cardiovascular disease; PTSD=post-traumatic stress disorder; OAU=opioid analgesic use; SMD=standardized mean difference; FY=fiscal year; VHA= Veterans Health Affairs; HCU=healthcare utilization; BMI= Body Mass Index
Comorbidities occur prior to OAU, unless otherwise noted
BMI≥30 or ICD-9-CM code
Maximum SMD% out of all possible group pairwise comparisons
Exact p-value
Patients with PTSD tended to be younger than those without PTSD. Male gender and white race were more prevalent among those with OAU only. Painful conditions were most common among those with PTSD and OAU, followed by PTSD only and OAU only with the lowest prevalence among patients without PTSD and OAU. Depression was most prevalent among patients with PTSD only (70%) and in patients with PTSD and OAU (68.8%) and much less common among those with OAU only (16.1%) and least common among patients free of PTSD and OAU (11.9%). A similar distribution pattern was observed for patients with anxiety disorder, sleep disorder, alcohol abuse/dependence and drug abuse/dependence.
Hypertension, hyperlipidemia and type 2 diabetes were slightly more prevalent among patients with PTSD only. Obesity was most common among those with PTSD and OAU, followed by those with PTSD alone. Current smoking was slightly more prevalent among patients with PTSD and OAU.
Among the 464 incident CVD cases, 10.3% were hypertensive heart disease, 6.7% were myocardial infarction, 31.9% ischemic heart disease, 3.5% diseases of pulmonary circulation and 56.0% ‘other’ heart disease diagnoses. Overall, 7.3% had more than one type of CVD diagnoses (data not shown). The overall CVD incidence was 40.1/1000 person years (PY).
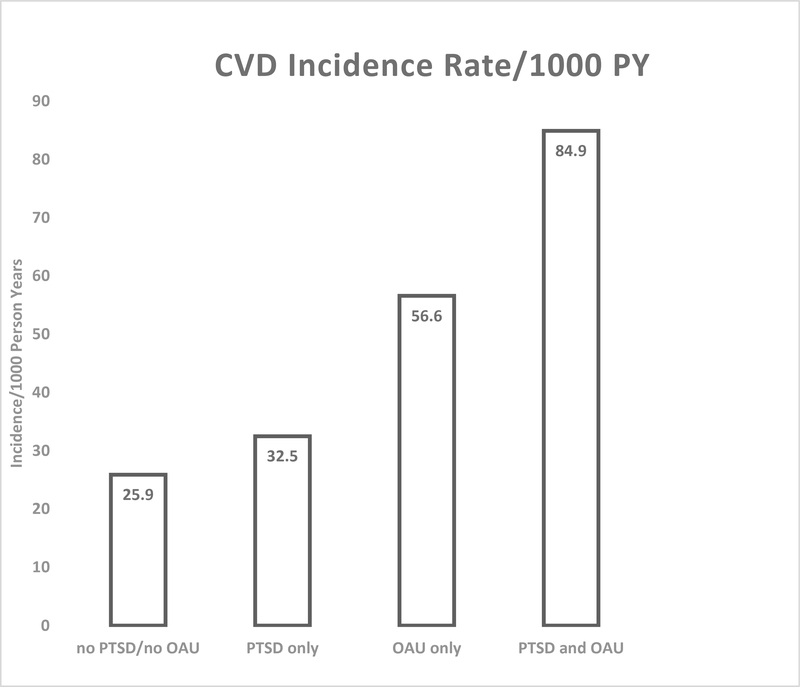
As shown in Figure 3, the age – adjusted CVD incidence rate increased from 25.9/1000PY in patients without PTSD and OAU, to 32.5/1000PY among those with PTSD only, to 56.6/1000PY among those with OAU only, to 84.9/1000PY among patients with PTSD and OAU (p<0.0001).
Figure 3.
Age-adjusted CVD incidence by 4-level PTSD-OAU exposure
Results of age- and fully-adjusted Cox proportional hazard models estimating the joint association of OAU and PTSD on CVD are shown in Table 3. As shown in the fully adjusted Model 2, there was almost three times the risk of CVD for OAU relative to non-OAU among those with PTSD (HR=2.70;95%CI:2.07–3.52), compared to two times the risk among those without PTSD (HR=1.99; 95%CI:1.36–2.92). This 35% increase in hazard ratios among those with PTSD compared to those without PTSD was not statistically significant, indicating no significant multiplicative interaction (ratio of HR = 1.35; 95% CI: 0.86–2.12).
Table 3.
Results from Cox proportional hazard models estimating the association between PTSD and OAU and incident CVD (n=2,861)
| Model 1 – Age-adjusted | Model 2 – Fully adjusted | |
|---|---|---|
| HR (95%CI) | HR (95%CI) | |
| OAU within PTSD strata | ||
| No PTSD: OAU vs. no OAU | 2.35 (1.61–3.43) | 1.99 (1.36–2.92) |
| PTSD: OAU vs. no OAU | 2.81 (2.16–3.66) | 2.70 (2.07–3.52) |
| OAU*PTSD | ||
| None | 1.00 | 1.00 |
| PTSD only | 1.24 (1.01–1.54) | 0.82 (0.63–1.17) |
| OAU only | 2.35 (1.61–3.43) | 1.99 (1.36–2.92) |
| PTSD+OAU | 3.49 (2.66–4.59) | 2.20 (1.61–3.02)* |
| Estimate (95% CI) | Estimate (95% CI) | |
| Multiplicative interaction | ||
| Ratio of OAU HR’s within PTSD strata | 1.20 (0.77–1.87) | 1.35 (0.86–2.12) |
| Additive interaction | ||
| RERI | 0.90 (−0.17, 1.98) | 0.39 (−0.45, 1.24) |
Note: CVD=cardiovascular disease; OAU=opioid analgesic use; HR=hazard ratio; CI=confidence interval; RERI=relative excess risk due to interaction
fully adjusted model with hazard ratios for all covariates shown in Appendix B.
Significant estimates are bolded
Also, in the fully adjusted model, compared to patients with no PTSD and no OAU, those with OAU only and those having PTSD and OAU had about twice the risk of incident CVD (HR=1.99; 95%CI:1.36–2.92 and HR=2.20; 95%CI:1.61–3.02, respectively). PTSD only was not associated with incident CVD (HR=0.82; 95% CI: 0.63–1.17). Hazard ratios for all covariates in the full model are shown in Appendix B. RERI was not significantly different than 0, indicating the lack of an additive interaction (RERI=0.39; 95% CI: −0.45, 1.24).
After removing patients with depression, the results from Cox proportional hazard models estimating the association between PTSD, OAU and incident CVD did not change our conclusions regarding the absence of both multiplicative and additive effects of PTSD and OAU on CVD. However, the magnitude of the association between having PTSD and OAU, compared to having no PTSD and no OAU, and incident CVD was reduced. Compared to patients without PTSD and OAU, having both PTSD and OAU was associated with a 67% increased risk of CVD (HR=1.67; 95%CI:1.01–2.78). Similarly, the stratum specific estimate for OAU vs. no OAU in patients with PTSD was reduced (HR=1.94; 95%CI:1.16–3.24).
All models shown in Table 3 were recomputed in sensitivity analysis which defined incident CVD by a diagnosis for myocardial infarction or ischemic heart disease. Results of sensitivity analysis did not change our findings (data no shown).
DISCUSSION
Among a large cohort of VHA patients free of prevalent CVD and prevalent OAU 12 months prior to index date, we observed patients without PTSD who started a new opioid prescription had a 99% increased risk of incident CVD. Having both OAU and PTSD was associated with 120% greater risk of incident CVD, compared to patients who had neither exposure. However, there was no statistically significant additive or interactive effect of PTSD and OAU.
The association between OAU and incident CVD is consistent with existing research. Among Medicare recipients, OAU compared to NSAID use, was associated with a 77% increased risk for CVD events,(2) and a 28% higher risk of CVD was observed in prescription opioid users vs. non-users in the UK General Practice Research Database.(1)
To our knowledge, there are no reports of the potential combined effect of PTSD and OAU on incident CVD. While we did not find evidence for a significant interactive or additive association with incident CVD, the high OAU prevalence in patients with PTSD may contribute to more cases of CVD in those with vs. without PTSD. In our sample, the number of patients who were exposed to both OAU and PTSD was about double that with OAU alone (22.1% vs. 10.6%). This is consistent with our prior research in which the prevalence of PTSD in patients who started a new opioid prescription was significantly higher than in those who remained opioid free (35.5% vs. 20.4%).(13) The association between PTSD and increased odds of receiving a prescription opioid has been observed in other VHA patient samples.(6)
The age adjusted association between PTSD and incident CVD was significant (HR=1.24; 95%CI:1.01–1.54) but in the full model PTSD was no longer associated with risk of CVD. This result is consistent with recent evidence that a combination of physical conditions, psychiatric conditions and smoking explained the association between PTSD and incident CVD.(20) These conditions are all more prevalent among patients with PTSD which may explain why PTSD does not remain associated with incident CVD after adjusting for hypertension, hyperlipidemia, type 2 diabetes, depression and smoking, particularly when modeled as time dependent covariates.
Although speculative, there are several possible physiological and behavioral mechanisms that may underlie the OAU – incident CVD association. Opioids are associated cardiovascular inflammation(21) and decreased myocardial oxygen.(22) Androgen deficiency related to chronic OAU may contribute to CVD risk.(23) OAU can worsen mood, itself a risk for CVD, and lead to correlated behavioral risk factors such as increased smoking, lack of exercise, and subsequently worsen or contribute to persistent hypertension and obesity. Our study was not designed to identify mechanisms and further research on this topic is warranted.
Limitations
We did not use propensity scores (PS) and matching or inverse probability of treatment weighting to balance the distribution of confounding variables across the 4 exposure groups, however, controlling for time-dependent variables that onset prior to OAU should result in similar point estimates that would be obtained by PS adjustment and PS stratified analysis.(24),(25) We did not include severity of PTSD in our analysis. It is possible that more severe PTSD is present among patients with OAU and more severe PTSD has been shown to increase risk of CVD.(4) This could bias our estimate of the association between PTSD, OAU and incident CVD. Therefore, in post-hoc analysis we computed the mean PTSD Symptom Checklist (PCL) score in patients with PTSD alone and those with PTSD and OAU in a subset of patients with PCL scores. Using the last available PCL score prior to incident CVD or end of follow-up for those who did not develop CVD, we found the mean PCL score was 55.8 (±16.4) among patients with PTSD only and 57.8 (±15.4) among those with PTSD and OAU. While this difference was statistically significant (p=0.03), it is far from clinically meaningful. We are unable to determine if patients used their opioid prescription and we did not have sufficient sample size to measure dose-response relationships that may exist between longer and higher dose OAU and incident CVD. Results from VHA patient data may not generalize to non-VHA patient samples, however, studies of chronic OAU and new onset depression and research on any OAU and depression recurrence conducted in VHA patient samples have replicated in private sector patient data.(11, 13) Last, while we adjusted for a large number of confounding factors, it is possible that unmeasured confounding influenced results. To determine if an unmeasured confounder was likely to completely explain our results, we computed the E-value which is the minimum magnitude of association than an unmeasured confounder would have with both the exposure and outcome to account for the hazard ratios estimating the association between the 4-group exposure variable and incident CVD.(26) For the association between OAU and incident CVD the E-value was 2.59 and for the association between PTSD+OAU and incident CVD, the E-value was 2.83. Therefore, an unmeasured confounder would need to have an association of at least 2.83 with both OAU or PTSD+OAU and incident CVD. As shown in Table 3, the strongest association between common risk factors for CVD was observed for hypertension (HR=1.42; 1.14–1.76). In addition, although unavailable in our data, genetic risk is not large enough (HR range=1.28 to 1.74)(27) to account for our results. Thus, it seems unlikely that unmeasured confounding could explain away our findings.
Conclusions
Our results expand on existing evidence that OAU is associated with incident CVD. Although we did not find an additive or interactive effect of dual OAU and PTSD exposure, the number needed to harm,(28) based on fully adjusted models, is lower in those with PTSD. Compared to patients without OAU and PTSD, the number needed to harm among those with OAU alone is 75 patients while only 59 patients are needed if they have both OAU and PTSD. While reducing opioid prescribing should lessen risk for CVD overall, avoiding or limiting opioid prescriptions for patients with PTSD may be particularly important because OAU is nearly three times higher in patients with PTSD compared to those without mental illness.(6) Further research is needed to determine if other common comorbid conditions associated with OAU and CVD, such as depression, have an additive or interactive association with incident CVD. Because OAU is much more common in depression and other anxiety disorders, educating patients with chronic mental illness, as well as physicians, about opioid related CVD risk could enhance safe opioid prescribing.
Receipt of opioids for non-cancer pain is influenced by patients and not solely attributable to provider behavior. Some patients use opioids to medicate stress and poor mood and, to a degree, self-select into chronic OAU.(29) To the degree that patients self-select into chronic OAU, and decisions to initiate OAU are modifiable, then OAU can be added to the list of health behaviors that can be modified to delay and reduce incident CVD in patients with non-cancer pain. Our results provide an additional rationale for developing and implementing non-opioid pain management therapies, especially for patients groups with PTSD who already have numerous conditions that increase CVD risk.
Table 4.
Results from Cox proportional hazard models estimating the association between PTSD and OAU and incident CVD among patients without depression (n=1,631)
| Fully adjusted | |
|---|---|
| HR (95%CI) | |
| OAU within PTSD strata | |
| No PTSD: OAU vs. no OAU | 2.00 (1.33–3.02) |
| PTSD: OAU vs. no OAU | 1.94 (1.16–3.24) |
| OAU*PTSD | |
| None | 1.00 |
| PTSD only | 0.86 (0.62–1.20) |
| OAU only | 2.00 (1.33–3.02) |
| PTSD+OAU | 1.67 (1.01–2.78) |
| Estimate (95% CI) | |
| Multiplicative interaction | |
| Ratio of OAU HR’s within PTSD strata | 0.97 (0.51–1.84) |
| Additive interaction | |
| RERI | −0.19 (−1.27, 0.89) |
Note: CVD=cardiovascular disease; OAU=opioid analgesic use; HR=hazard ratio; CI=confidence interval; RERI=relative excess risk due to interaction; fully adjusted model controlled for all covariates shown in Table 2.
Significant estimates are bolded
ACKNOWLEDGEMENTS
The views expressed do not necessarily reflect those of the Veterans Administration.
Funding: National Heart Lung and Blood Institute, PTSD Treatment: Effects on Health Behavior, Cardiovascular and Metabolic Disease, R01HL125424.
Funding: NHLBI, R01HL125424
Appendix
Appendix A.
Variable definitions
| PTSD (baseline/fixed) – Presence of 309.81 at one of five VHA PTSD specialty clinics on ≥2 outpatient visits or one inpatient in FY2008 to FY2012. Index date is the second PTSD date or the second visit date for those without PTSD. |
| New OAU (time dependent) – new fill for OAU after index date and greater than 90 days prior to study end date |
|
Cardiovascular Disease - ≥1 ICD9 diagnosis code or revascularization CPT/ICD9 procedure codes a. ICD-9 diagnosis codes for any of the following conditions: 1. hypertensive heart disease: 402x, 403x, 404x, 405x 2. MI: 410x, 411x 3. Ischemic heart disease: 412x, 413x, 414x 4. disease of pulm circulation: 415x, 416x, 417x 5. other heart disease: 420x – 429x b. CPT codes (outpatient clinic stop files): 35450–35459; 35470–35475; 35480–35495; 92980, 92981, 92984, 92995, 92996; 33510–33536; 33572; 37220–37235; 92920–92944; 0234T-0238T; G0290, G0291; S2211; S2222; S2204-S2209 c. ICD-9 procedure codes (inpatient procedure files): 36.0x – 36.3x All patients with a revascularization code also had an ICD9 code for cardiovascular disease |
| Study end date - Date of cardiovascular disease or last encounter with VHA system |
| Depression (time dependent) – (296.2x, 296.3x, 311). Presence of a single inpatient code or ≥2 outpatient codes within a 12 month period prior to OAU/study end date. |
| Other anxiety (time dependent) –Composite of panic disorder, OCD, social phobia, GAD, anxiety NOS (300.00, 300.01, 300.02, 300.23, 300.3). Presence of a single inpatient code or ≥2 outpatient codes within a 12 month period prior to OAU/study end date. |
| Alcohol abuse/dependence (time dependent) – (303.9x, 305.0x). At least a single code prior to OAU/study end date |
|
Drug abuse/dependence (time dependent) = composite of any drug abuse/dependence. At least a single code for the following prior to OAU/study end date: sedative (304.1x, 305.4x), cocaine (304.2x, 305.6x), cannabis (304.3x, 305.2x), amphetamine (304.4x, 305.7x), hallucinogens (304.5x, 305.3x), ‘other’ (304.6x, 305.9x), opioid (304.0x, 305.5x), opioid with other SUD (304.7x), other SUD excluding opioid (304.8x), unspecified drug abuse/dependence (304.9x). |
| Sleep disorder (time dependent) = At least a single code for 307.4x, 327.x, 780.5x, 333.94 prior to OAU/study end date |
|
Smoke status (fixed) –This is indicated as Current, Former, Never. 1. Current – “current smoker” in health factors or ICD9 code for nicotine dependence (V15.82, 305.1) prior to OAU/study end date. 2. Former – never has a “current smoking” indicator and has an indicator for “former smoker” in health factors prior to OAU/study end date. 3. Never = all else. |
| Obese any (time dependent) – Presence of BMI ≥30 or ICD9 (278.00, 278.01) code for obesity prior to OAU/study end date |
| Body Mass Index (fixed – descriptive only) - last BMI prior to study end date. |
| Hypertension (time dependent) - (401.x) At least a single code prior to OAU/study end date. |
| Hyperlipidemia (time dependent) – (272.0, 272.1, 272.2, 272.4). At least a single code prior to OAU/study end date. |
| Type 2 Diabetes (time dependent) – (250.x0, 250.x2, 357.2, 362.0x, 366.41) Presence of ≥2 codes in any 24 month period prior to OAU/study end date. |
|
Pain conditions (time dependent) – at least one code for the following prior to OAU/study end date. a) Arthritis (710.0–710.4, 710.8, 710.9, 711x, 713x-717x, 718.0x-718.3x, 718.5x-718.9x, 719x, 720.0, V13.4) b) Back pain (720.1, 720.2, 720.8x, 720.9, 721x, 722x, 723.0–723.3, 723.5–723.7, 723.9, 724x, 756.1x c) Musculoskeletal pain (725x, 726.0x-726.6x, 726.71, 726.72, 726.90, 727.00, 727.03, 727.04, 727.05, 727.06, 727.09, 727.2, 727.3, 727.49, 727.50, 727.51, 727.6x, 727.89, 727.9, 729.0, 729.1, 729.4, 729.5, 729.7x, 729.89, 729.9, 729.91, 729.92, 781.99, 830x-848x, 905.6, 905.7, V43.6x, V43.7, V48.3, V49.6x, V49.7x) d) Headache (307.81, 339x, 346.0x-346.5x, 346.7x-346.9x, 784.0) e) Neuropathy (053.13, 072.12, 337.0x, 337.1, 353x-357x, 377.33, 377.34, 377.41) |
| Demographic Information (all fixed covariates) |
| Age – age at initial visit to one of five VHA PTSD specialty clinics |
| Gender – male vs. female |
| Race – most commonly occurring in record (white vs. black vs. other) |
| Marital status – most commonly occurring in record (married vs. other) |
| VHA only insurance – most commonly occurring in record (yes vs no) |
| Primary healthcare utilization - number of unique primary care clinic stops per total months in entire VA system. Total months is calculated from first visit date to any VA facility in FY08 to FY15 to the study end date. Primary Care stop codes: 170, 172, 301, 322, 323, 348, 350. From entire sample of 17–80 year olds, quartiled visits/months and top quartile = high. |
Appendix
Appendix B.
Results from Cox proportional hazard models estimating the association between PTSD and OAU on risk of CVD. Estimates for fully adjusted proportional hazards model (n=2,861)
| Variable | HR (95% CI) |
|---|---|
| PTSD*OAU | |
| None | 1.00 |
| PTSD only | 0.82 (0.63–1.17) |
| OAU only | 1.99 (1.36–2.92) |
| PTSD+OAU | 2.20 (1.61–3.02) |
| Age | 1.04 (1.03–1.05) |
| Male gender | 1.50 (1.04–2.17) |
| Race | |
| White | 1.00 |
| Black | 1.11 (0.90–1.36) |
| Other | 0.74 (0.48–1.13) |
| Marital status | |
| Not married | 1.00 |
| Married | 0.87 (0.72–1.07) |
| VHA only insurance | 0.84 (0.69–1.02) |
| High primary HCU | 1.72 (1.40–2.11) |
| Arthritis | 1.10 (0.89–1.36) |
| Back pain | 1.06 (0.87–1.30) |
| Musculoskeletal pain | 1.25 (1.02–1.54) |
| Headache pain | 0.87 (0.67–1.13) |
| Neuropathy | 1.00 (0.77–1.30) |
| Depression | 0.96 (0.76–1.21) |
| Other anxiety | 0.93 (0.72–1.20) |
| Sleep disorder | 1.30 (1.06–1.60) |
| Alcohol abuse/dependence | 1.10 (0.85–1.41) |
| Drug abuse/dependence | 1.32 (1.01–1.75) |
| Hypertension | 1.42 (1.14–1.76) |
| Hyperlipidemia | 1.10 (0.89–1.36) |
| Type 2 Diabetes | 1.03 (0.81–1.32) |
| Obesity | 1.28 (1.05–1.57) |
| Smoking | |
| Never | 1.00 |
| Former | 1.01 (0.77–1.32) |
| Current | 1.13 (0.91–1.40) |
HR=hazard ratio; CI=confidence interval
Significant estimates are bolded
Footnotes
Disclosures:
Jeffrey F. Scherrer receives compensation as Editor of Oxford Press’ Family Practice. Joanne Salas reports no conflicts of interest. Patrick Lustman reports no conflicts of interest. Carissa van den Berk-Clark reports no conflicts of interest. Paula Schnurr reports no conflicts of interest. Peter Tuerk reports no conflicts of interest. Beth Cohen reports no conflicts of interest. Sonya Norman reports no conflicts of interest, F. David Schneider reports no conflicts of interest. Kathleen Chard reports no conflicts of interest.
Prior presentations: none
Access to Data and Data Analysis: Jeffrey F. Scherrer and Joanne Salas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Joanne Salas conducted the analysis.
REFERENCES
- 1.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. Journal of internal medicine. 2013;273(5):511–26. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Archives of internal medicine. 2010;170(22):1968–76. [DOI] [PubMed] [Google Scholar]
- 3.Carman WJ, Su S, Cook SF, Wurzelmann JI, McAfee A. Coronary heart disease outcomes among chronic opioid and cyclooxygenase-2 users compared with a general population cohort. Pharmacoepidemiol Drug Saf. 2011;20(7):754–62. [DOI] [PubMed] [Google Scholar]
- 4.Kubzansky LD, Koenen KC, Spiro A 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of general psychiatry. 2007;64(1):109–16. [DOI] [PubMed] [Google Scholar]
- 5.Koenen KC, Sumner JA, Gilsanz P, Glymour MM, Ratanatharathorn A, Rimm EB, et al. Post-traumatic stress disorder and cardiometabolic disease: improving causal inference to inform practice. Psychol Med. 2017;47(2):209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–7. [DOI] [PubMed] [Google Scholar]
- 7.Hadlandsmyth K, Mosher H, Vander Weg MW, Lund BC. Decline in Prescription Opioids Attributable to Decreases in Long-Term Use: A Retrospective Study in the Veterans Health Administration 2010–2016. Journal of general internal medicine. 2018;33(6):818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisi AJ, Corcoran KL, DeRycke EC, Bastian LA, Becker WC, Edmond SN, et al. Opioid Use Among Veterans of Recent Wars Receiving Veterans Affairs Chiropractic Care. Pain medicine (Malden, Mass). 2018;19(suppl_1):S54–S60. [DOI] [PubMed] [Google Scholar]
- 9.Gravely AA, Cutting A, Nugent S, Grill J, Carlson K, Spoont M. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self-reported PTSD Checklist scores. Journal of rehabilitation research and development. 2011;48(1):21–30. [DOI] [PubMed] [Google Scholar]
- 10.Holowka DW, Marx BP, Gates MA, Litman HJ, Ranganathan G, Rosen RC, et al. PTSD diagnostic validity in Veterans Affairs electronic records of Iraq and Afghanistan veterans. J Consult Clin Psychol. 2014;82(4):569–79. [DOI] [PubMed] [Google Scholar]
- 11.Scherrer JF, Salas J, Copeland LA, Stock EM, Ahmedani BK, Sullivan MD, et al. Prescription Opioid Duration, Dose, and Increased Risk of Depression in 3 Large Patient Populations. Annals of family medicine. 2016;14(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherrer JF, Salas J, Sullivan MD, Schneider FD, Bucholz KK, Burroughs T, et al. The influence of prescription opioid use duration and dose on development of treatment resistant depression. Prev Med. 2016;91:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherrer JF, Salas J, Copeland LA, Stock EM, Schneider FD, Sullivan M, et al. Increased Risk of Depression Recurrence After Initiation of Prescription Opioids in Noncancer Pain Patients. The journal of pain: official journal of the American Pain Society. 2016;17(4):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiology and Drug Safety. 2016;25(4):467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashner TM. Agreement between administrative files and written medical records: a case of the Department of Veterans Affairs. Medical care. 1998;36:1324–36. [DOI] [PubMed] [Google Scholar]
- 16.Hussain MA, Mamdani M, Saposnik G, Tu JV, Turkel-Parrella D, Spears J, et al. Validation of Carotid Artery Revascularization Coding in Ontario Health Administrative Databases. Clinical and investigative medicine Medecine clinique et experimentale. 2016;39(2):E73–8. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statistics in medicine. 2015;34(28):3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, Wolters KluwerHealth/LippincottWilliams & Wilkins, 2012. [Google Scholar]
- 19.VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology. 2011;22(5):713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherrer JF, Salas J, Cohen BE, Schnurr PP, Schneider FD, Chard KM, et al. Comorbid Conditions Explain the Association between Posttraumatic Stress Disorder and Incident Cardiovascular Disease. Journal of the American Heart Association In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vozoris NT, Wang X, Austin PC, Lee DS, Stephenson AL, O’Donnell DE, et al. Adverse cardiac events associated with incident opioid drug use among older adults with COPD. European journal of clinical pharmacology. 2017;73(10):1287–95. [DOI] [PubMed] [Google Scholar]
- 22.Sonntag H, Larsen R, Hilfiker O, Kettler D, Brockschnieder B. Myocardial blood flow and oxygen consumption during high-dose fentanyl anesthesia in patients with coronary artery disease. Anesthesiology. 1982;56(6):417–22. [DOI] [PubMed] [Google Scholar]
- 23.Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain physician. 2012;15(3 Suppl):ES145–56. [PubMed] [Google Scholar]
- 24.Ali MS, Groenwold RH, Belitser SV, Souverein PC, Martin E, Gatto NM, et al. Methodological comparison of marginal structural model, time-varying Cox regression, and propensity score methods: the example of antidepressant use and the risk of hip fracture. Pharmacoepidemiol Drug Saf. 2016;25 Suppl 1:114–21. [DOI] [PubMed] [Google Scholar]
- 25.Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol. 2017;69(3):345–57. [DOI] [PubMed] [Google Scholar]
- 26.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA: the journal of the American Medical Association. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Abraham G, Havulinna AS, Bhalala OG, Byars SG, De Livera AM, Yetukuri L, et al. Genomic prediction of coronary heart disease. European heart journal. 2016;37(43):3267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stang A, Poole C, Bender R. Common problems related to the use of number needed to treat. Journal of clinical epidemiology. 2010;63(8):820–5. [DOI] [PubMed] [Google Scholar]
- 29.Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. General hospital psychiatry. 2014;36(1):99–104. [DOI] [PubMed] [Google Scholar]