Abstract
Background
Acute otitis media (AOM) is a common illness during childhood, for which antibiotics are frequently prescribed.
Objectives
To determine the effectiveness of a short course of antibiotics (less than seven days) in comparison to a long course of antibiotics (seven days or greater) for the treatment of AOM in children.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, issue 4) which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE, EMBASE, MEDLINE In‐Process & Other Non‐Indexed Citations, CINAHL, BIOSIS Previews, OCLC Papers First and Proceedings First, Proquest Dissertations and Theses (inception to November 2009); International Pharmaceutical Abstracts, the NLM Gateway, ClinicalTrials.gov and Current Controlled Trials (inception to August 2008).
Selection criteria
Trials were included if they met the following criteria: participants aged one month to 18 years; clinical diagnosis of ear infection; no previous antimicrobial therapy; and randomisation to treatment with less than seven days versus seven days or more of antibiotics.
Data collection and analysis
The primary outcome of treatment failure was defined as the absence of clinical resolution, relapse or recurrence of AOM during one month following initiation of therapy. Treatment outcomes were extracted from individual studies and combined in the form of a summary odds ratio (OR). A summary OR of 1.0 indicates that the treatment failure rate following less than seven days of antibiotic treatment was similar to the failure rate following seven days or more of treatment.
Main results
This update included 49 trials containing 12,045 participants. Risk of treatment failure was higher with short courses of antibiotics (OR 1.34, 95% CI 1.15 to 1.55) at one month after initiation of therapy (21% failure with short‐course treatment and 18% with long‐course; absolute difference of 3% between groups). There were no differences found when examining treatment with ceftriaxone for less than seven days (30% failure in those receiving ceftriaxone and 27% in short‐acting antibiotics administered for seven days or more) or azithromycin for less than seven days (18% failure in both those receiving azithromycin and short‐acting antibiotics administered for seven days or more) with respect to risk of treatment failure at one month or less. Significant reductions in gastrointestinal adverse events were observed for treatment with short‐acting antibiotics and azithromycin.
Authors' conclusions
Clinicians need to evaluate whether the minimal short‐term benefit from longer treatment of antibiotics is worth exposing children to a longer course of antibiotics.
Keywords: Child, Humans, Acute Disease, Age Factors, Anti‐Bacterial Agents, Anti‐Bacterial Agents/adverse effects, Anti‐Bacterial Agents/therapeutic use, Azithromycin, Azithromycin/therapeutic use, Ceftriaxone, Ceftriaxone/adverse effects, Ceftriaxone/therapeutic use, Drug Administration Schedule, Otitis Media, Otitis Media/drug therapy, Time Factors
Plain language summary
Short course antibiotics for healthy children with uncomplicated acute otitis media
Acute otitis media (AOM), or middle ear infection, is a common childhood illness, with more than half of all children having at least one infection by the time they are seven. Although otitis media often resolves without treatment, it is frequently treated with antibiotics. The length of treatment varies widely. This review of 49 trials found that treating children with a short course (less than seven days) of antibiotics, compared to treatment with a long course (seven days or greater) of antibiotics, increases the likelihood of treatment failure in the short term. No differences are seen one month later. The amount of gastrointestinal adverse events decreased with a shorter course of antibiotics.
Summary of findings
Summary of findings for the main comparison. Short‐acting antibiotic > 48 hours short‐term treatment for acute otitis media.
| Short‐acting antibiotic > 48 hours short‐term treatment for acute otitis media | ||||||
| Patient or population: patients with acute otitis media Settings: Intervention: Short‐acting antibiotic > 48 hours short‐term treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Short‐acting antibiotic > 48 hours short‐term treatment | |||||
| Treatment failure at 1 month or less | Study population | OR 1.34 (1.15 to 1.55) | 5093 (16) | |||
| 175 per 1000 | 221 per 1000 (196 to 247) | |||||
| Medium risk population | ||||||
| 157 per 1000 | 200 per 1000 (176 to 224) | |||||
| Treatment failure at 8 to 19 days | Study population | OR 1.37 (1.15 to 1.64) | 3932 (11) | |||
| 144 per 1000 | 187 per 1000 (162 to 216) | |||||
| Medium risk population | ||||||
| 117 per 1000 | 154 per 1000 (132 to 179) | |||||
| Treatment failure at 20 to 30 days | Study population | OR 1.16 (0.94 to 1.42) | 2476 (9) | |||
| 203 per 1000 | 228 per 1000 (193 to 266) | |||||
| Medium risk population | ||||||
| 172 per 1000 | 194 per 1000 (163 to 228) | |||||
| Treatment failure at 3 months or less | Study population | OR 1.18 (0.98 to 1.41) | 2068 (7) | |||
| 364 per 1000 | 403 per 1000 (359 to 447) | |||||
| Medium risk population | ||||||
| 364 per 1000 | 403 per 1000 (359 to 447) | |||||
| Treatment failure at 90 days | Study population | OR 1.16 (0.65 to 2.06) | 207 (2) | |||
| 327 per 1000 | 360 per 1000 (240 to 500) | |||||
| Medium risk population | ||||||
| 311 per 1000 | 344 per 1000 (227 to 482) | |||||
| Treatment failure at 30 to 45 days | Study population | OR 1.18 (0.97 to 1.43) | 1861 (5) | |||
| 368 per 1000 | 407 per 1000 (361 to 454) | |||||
| Medium risk population | ||||||
| 364 per 1000 | 403 per 1000 (357 to 450) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Ceftriaxone for acute otitis media.
| Ceftriaxone for acute otitis media | ||||||
| Patient or population: patients with acute otitis media Settings: Intervention: Ceftriaxone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Ceftriaxone | |||||
| Treatment failure at 1 month or less | Study population | OR 1.07 (0.86 to 1.33) | 1709 (8) | |||
| 270 per 1000 | 284 per 1000 (241 to 330) | |||||
| Medium risk population | ||||||
| 254 per 1000 | 267 per 1000 (226 to 312) | |||||
| Treatment failure at 3 months or less | Study population | OR 0.89 (0.66 to 1.21) | 701 (3) | |||
| 402 per 1000 | 374 per 1000 (307 to 449) | |||||
| Medium risk population | ||||||
| 379 per 1000 | 352 per 1000 (287 to 425) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. Azithromycin 3 to 5 days short‐term treatment for acute otitis media.
| Azithromycin 3 to 5 days short‐term treatment for acute otitis media | ||||||
| Patient or population: patients with acute otitis media Settings: Intervention: Azithromycin 3 to 5 days short‐term treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Azithromycin 3 to 5 days short‐term treatment | |||||
| Treatment failure at 1 month or less | Study population | OR 1.02 (0.87 to 1.2) | 4354 (19) | |||
| 185 per 1000 | 188 per 1000 (165 to 214) | |||||
| Medium risk population | ||||||
| 61 per 1000 | 62 per 1000 (53 to 72) | |||||
| Treatment failure at 8 to 19 days | Study population | OR 1.27 (1.04 to 1.55) | 4347 (18) | |||
| 95 per 1000 | 118 per 1000 (98 to 140) | |||||
| Medium risk population | ||||||
| 56 per 1000 | 70 per 1000 (58 to 84) | |||||
| Treatment failure at 20 to 30 days | Study population | OR 0.98 (0.82 to 1.17) | 2708 (11) | |||
| 265 per 1000 | 261 per 1000 (228 to 297) | |||||
| Medium risk population | ||||||
| 273 per 1000 | 269 per 1000 (235 to 305) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Acute otitis media (AOM) is one of the most common childhood health problems, a leading cause of visits to a physician and the most frequent reason children receive antibiotics or undergo surgery. The cost estimates for AOM in the US are between $3 and $5 billion per year. Most children will have at least one episode of AOM, with the peak incidence period being between 6 and 11 months (Rovers 2004).
AOM is defined as the presence of a middle ear effusion in conjunction with signs and symptoms of inflammation in the middle ear, such as otalgia, otorrhoea, fever or irritability. There is a high rate of spontaneous resolution for AOM, but if left untreated it can occasionally lead to complications, such as acute mastoiditis. Hearing loss can lead to behavioural changes and communication delay (Rovers 2004).
Description of the intervention
The standard duration of therapy in North America is 10 days (Froom 1990). However, the optimal duration of treatment is not known and varies worldwide. Half of the practitioners in Great Britain prescribe a five‐day course compared to the majority of practitioners in the Netherlands who prescribe six to seven days of antibiotic therapy. Many opt for no treatment (Froom 1990).
How the intervention might work
The decline during the 1940s and 1950s in suppurative complications of AOM throughout North America and Europe has been attributed to antibiotic therapy (Berman 1995). Evidence suggests that antibiotic‐treated and untreated children living in high‐income countries have similar long‐term outcomes (Burke 1991; Mygind 1981; van Buchem 1981). However, a meta‐analysis pooling the results of four studies suggests that although the spontaneous rate of resolution was high with placebo or no drug (81%) (95% confidence interval (CI) 69% to 94%), antimicrobial therapy increased resolution by 13.7% (95% CI 8.2% to 19.2%) (Rosenfeld 1994). A meta‐analysis by Del Mar documented that compared to placebo, antibiotics reduce pain at two to seven days after the start of treatment (Del Mar 1997; Sanders 2009). The absolute benefit of this finding is 5.6% fewer children experience pain at two to seven days. Long‐term outcomes were similar with and without treatment.
Why it is important to do this review
Practitioners in North America may be hesitant to discontinue prescribing antibiotics, despite findings from the medical literature that support management of AOM without antibiotics. Moreover, the therapy duration for optimal outcome continues to be a question. Expert opinion has recommended five days of antimicrobial treatment for uncomplicated otitis media in children over the age of six years (Paradise 1995; Paradise 1997). The quality of scientific evidence to support a policy for shorter courses of antibiotic treatment has been assessed (Pichichero 1997), but lacks a systematic, quantitative evaluation. Concern regarding resistant bacteria from the overuse of antibiotics (Cohen 1992; Murray 1994) and poor compliance is increasing, as is the cost of health care. Therefore, it is desirable to determine the shortest duration of antibiotic treatment that would result in favorable outcomes. We felt that a systematic review would add some objectivity to this debate.
Objectives
To determine the effectiveness of a short course of antibiotics (less than seven days) in comparison to a long course (seven days or longer) of antibiotics for the treatment of children with AOM. Subgroup analyses of children less than two years old, children with a perforated ear drum, and children with a history of recurrent otitis media were pursued to address concerns that these groups may have less favorable outcomes.
Methods
Criteria for considering studies for this review
Types of studies
For inclusion in the review, we considered randomized controlled trials (RCTs) of the empiric treatment of AOM, comparing two antibiotic regimens of different durations, as described in the title or abstract of the clinical trial. When the duration of antibiotic treatment was not specified in the title or abstract, we retrieved the trial to verify the treatment duration in both arms. We placed no language restrictions on the selection of clinical trials (Gregoire 1995).
Types of participants
We included children aged one month to 18 years, with a clinical diagnosis of AOM and no history of immediate antibiotic use, immune deficiency, chronic disease or head and neck abnormalities.
Types of interventions
We compared antibiotic therapy of a treatment arm for less than seven days (defined as the short course), with a treatment arm greater than or equal to seven days (defined as the long course). The antibiotic may be the same or different in the two treatment arms. Studies using the antibiotic ceftibuten were excluded from this 2009 update due to a lack of clinical relevance as the antibiotic is not commonly used by clinicians.
Types of outcome measures
Primary outcomes
Treatment failure, which included lack of clinical resolution, relapse or recurrence of AOM during a one‐month period following the initiation of therapy. Clinical resolution meant that the presenting signs or symptoms of AOM had improved or resolved.
Secondary outcomes
The cumulative number of treatment failures, relapses and recurrences reported at time of diagnosis and again at a final evaluation point between one to three months. Middle ear effusion was not classified as a treatment failure because of its documented persistence during the course of the disease, regardless of treatment. We sought data on the number of children with persistent middle ear effusion at both of the evaluation points.
Search methods for identification of studies
Electronic searches
The original search was developed and run in 1997 (see Appendix 1 for details of the search). The search was then updated and run in August 2008 for the years 1997 to 2007 (see search strategies in Appendix 2), and updated again in November 2009.
In this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, issue 4) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1966 to November Week 1, 2009); EMBASE (1974 to November 2009); MEDLINE In‐Process & Other Non‐Indexed Citations (1966 to Week 1, 2009); International Pharmaceutical Abstracts (1970 to August Week 1, 2008); BIOSIS Previews (1969 to November 2009); CINAHL (1981 to November 2009); the NLM Gateway (1998 to August 2008); OCLC Papers First and Proceedings First (1997 to November 2009); ClinicalTrials.gov (1998 to August 2008); Proquest Dissertations and Theses (1861 to November 2009); and Current Controlled Trials (1997 to August 2008). We searched the following databases without any date restrictions in September 2007: the National Research Register; CRISP; the TRIP Database; Scirus; and Google Scholar. We imposed no language or publication restrictions.
We ran the following search strategy in MEDLINE and CENTRAL. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid version (Lefebvre 2008). The search strategy also incorporated the child search strategy developed by Boluyt (Boluyt 2008). We adapted this search strategy to search the other databases.
MEDLINE (Ovid)
1 exp Otitis Media/ 2 otitis media.tw. 3 (OM or OME or AOM).tw. 4 (otorrhea* or otorrhoea*).tw. 5 (ear* adj3 (infect* or acute*)).tw. 6 or/1‐5 7 exp treatment outcome/ 8 exp Anti‐Infective Agents/ 9 (antibacter* or anti‐bacter* or anti bacter* or antibiotic* or anti‐biotic* or anti biotic* or bacteriocid* or antimicrob* or anti‐microb* or anti microb*).tw. 10 (amoxicillin* or amoxycillin* or penicillin* or cefprozil* or clarithromycin* or cefpodoxime* or cefaclor* or ceftriaxone* or azthromycin* cefixime*).tw. 11 or/7‐10 12 6 and 11
Searching other resources
We retrieved clinical trial references and published reviews on the treatment of AOM in order to identify further trials. We then contacted primary authors for information on such trials.
Data collection and analysis
Selection of studies
In the first publication of this review (Kozyrskyj 2000) clinical trials examining the antibiotic treatment of AOM were retrieved from the medical literature search and evaluated by the review authors according to the selection criteria previously described. Decisions to include the trial were made independently. Interreviewer agreement was assessed by the kappa statistic using PC Agree Software (Stephen Walter, McMaster University, Hamilton, Ontario) and consensus was reached regarding trial inclusion.
In this updated review, two review authors (KH, NH) evaluated for inclusion in the review the clinical trials retrieved during the search update. A third review author (TK) was consulted when consensus could not be reached.
Data extraction and management
At least two review authors (KH, LB) independently performed data extraction, followed by a consensus conference to resolve any differences. We developed a standardized form to facilitate data extraction. Data extracted from each trial included:
the number of children with no clinical resolution of AOM;
relapse or recurrence of AOM;
the number of children with persistent middle ear effusion; and
the number of withdrawals from each arm of the trial.
We recorded the data as follows:
Primary outcome: evaluation points included time points until one month after initiation of therapy.
Secondary outcome: evaluation point between one to three months after initiation of therapy.
Important subgroups defined a priori for which data were sought are:
antibiotic use for two days or less;
children aged less than two years; and
perforated tympanic membrane (Appelman 1991; Hendrickse 1988; Kaleida 1991; van Buchem 1981).
Descriptive data collected included treatment site, patient baseline characteristics, co‐interventions, and inclusion, exclusion and outcome criteria to summarise the generalisability of included studies and to facilitate sensitivity analyses. We also recorded drug dose, route and treatment duration.
Change in statistical methods from original version of review
Two studies (Gooch 1996; Hoberman 1997) in the current review contained two arms comparing long‐course versus short‐course antibiotics. In the first version of this review (Kozyrskyj 2000) the short‐course arm was counted twice against each long‐course arm of that study. In this review update, we have modified our methods and combined both long‐course arms into a single comparison against the short‐course arm, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This prevents overweighing the short‐course arm of these trials in the meta‐analysis.
Assessment of risk of bias in included studies
We used the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2008) to assess for bias in this updated review. Two review authors (KH, MO) independently evaluated the risk of bias of included trials and then came to a consensus. When consensus could not be reached a third review author was consulted (TK).
Measures of treatment effect
We compared the treatment failure rate following a short course of antibiotics to the treatment failure rate following a long course of antibiotics using odds ratios (ORs) for individual trial outcomes. We determined a summary OR with 95% CI for trials pooled by antibiotic type using the Peto fixed‐effect model.
Unit of analysis issues
We performed analysis on trials grouped by the pharmacokinetic behaviour of the antibiotic used in the short‐course treatment arm, as follows.
Short‐acting oral antibiotics, for example, penicillin, amoxicillin, cefaclor and cefuroxime.
Oral azithromycin.
Intramuscular ceftriaxone.
We also conducted additional meta‐analyses in the short‐acting antibiotic group for treatment duration less than 48 hours and more than 48 hours.
Assessment of heterogeneity
We assessed statistical heterogeneity (Higgins 2008; Laupacis 1988). When significant heterogeneity existed, we examined trials for specific potential clinical differences (Thompson 1994). We also calculated summary ORs using the DerSimonian and Laird random‐effects model (DerSimonian 1986). We determined the summary risk difference (difference in the failure rate between the short and long‐course antibiotics) and a 95% CI for pooled group data.
Sensitivity analysis
We performed a sensitivity analyses to assess whether the results of the primary outcome for all antibiotics was sensitive to blinding, allocation concealment and publication bias. Additionally a sensitivity analysis was undertaken to assess those trials that used the same antibiotic in both the short and long courses of the trial.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Updated electronic searches and handsearches from January 2000 through to November 2009 retrieved 4175 additional citations. In this updated review, we included 49 studies containing 12,045 participants. Refer to the Characteristics of included studies for detailed information on individual trials.
Included studies
We included 22 new trials, resulting in 49 eligible trials for this updated review (Adam 2000; Al‐Ghamdi 1999; Arguedas 2005; Arrieta 2003; Block 2000; Block 2003; Block 2004; Catania 2004; Cohen 1998; Cohen 1999; Cohen 2000; Dagan 2000a; Dagan 2000b; de Jose 1998; Dunne 2003; Guven 2006; Hoberman 2005; Kara 1998; Oguz 2003; Pessey 1999; Varsano 1997; Wang 2004). Fourty‐eight of the included studies were classified as randomized controlled trials (RCTs). Randomisation was not mentioned in one study (Mohs 1993). Included sample sizes ranged from 17 participants (Puczynski 1987) to 868 participants (Hoberman 1997). The median sample size was 215 participants (Dagan 2000b). Studies were primarily conducted in North America, South America, Europe and the Middle East (Israel and Turkey). Single trials were carried out in both Egypt (Mohs 1993) and China (Wang 2004). All trials enrolled children; 39 trials enrolled children less than one year old; eight trials began enrolment at two years of age; and two trials evaluated children three years of age or less. The maximum age of enrolment was 14 years (Kafetzis 1997).
The following antibiotics were evaluated in the included studies (number of studies in brackets): amoxicillin or amoxicillin/clavulanate (32); azithromycin (21); cefaclor (8); cefdinir (2); cefixime (2); cefpodoxime (4); cefprozil (2); cefuroxime (3); ceftriaxone (9); clarithromycin (1); penicillin (3); and trimethoprim‐sulfamethoxazole (1). Short‐course interventions were primarily a three to five‐day regimen. Two studies compared a single dose of azithromycin (Arguedas 2005; Block 2003) and two studies compared a short course of the antibiotic regimen at less than or equal to 48 hours (Meistrup‐Larsen 1983; Puczynski 1987).
Short regimens of ceftriaxone or azithromycin were analyzed separately from all other antibiotics. Twenty trials employed tympanocentesis. Of these, four trials (Hendrickse 1988; Pessey 1999; Ploussard 1984; Puczynski 1987) studied short regimens of antibiotics and 15 trials evaluated azithromycin (Arguedas 1996; Arguedas 1997; Arguedas 2005; Aronovitz 1996; Arrieta 2003; Dagan 2000a; Dagan 2000b; Daniel 1993; de Jose 1998; Guven 2006; Hoberman 2005; Khurana 1996; Oguz 2003; Petalozza 1992; Principi 1995).
For the majority of studies, clinical cure or improvement at the end of treatment and at a follow‐up period of 10 days to one month after beginning the study were primary outcomes. Please refer to the Characteristics of included studies table for details on individual study outcomes.
Excluded studies
We excluded two short‐acting antibiotic trials because outcomes were not reported as treatment failures (Bain 1985; Jones 1986). One study (Jones 1986) reported the number of days symptoms were recorded in diaries and the percentage resolution of ear signs, and the other study (Bain 1985) reported eardrum signs and symptoms. Neither of these studies presented data that could contribute to treatment failure as defined in this review. We excluded an additional two studies because the antibiotic used, ceftibuten, was deemed to be not clinically relevant due to its limited use in clinical practice (Roos 2000; Simon 1997), and excluded one study due to the duration of the antibiotic treatment being the same in both arms (Suzuki 2009).
Risk of bias in included studies
A detailed summary of the risk of bias assessment for each study is available in the Characteristics of included studies table.
Allocation
Adequate sequence generation was performed in 12 studies and was unclear in 37. Allocation concealment was adequate in six studies, inadequate in one study and unclear in 42 studies.
Blinding
Appropriate blinding was employed in 15 studies, there was no blinding in 24 studies and unclear blinding in 10 studies. Allocation concealment was also judged as adequate in only one of the appropriately blinded studies (Green 1993).
Incomplete outcome data
In 33 studies incomplete outcome data were appropriately addressed. Six studies did not address the incomplete outcome data appropriately and in 10 studies it was unclear if the data were appropriately reported.
Selective reporting
Thirty‐eight included studies were judged free from a risk of selective reporting bias; five studies had a high risk of selective reporting bias; and six studies were unclear in risk of selective reporting bias.
Other potential sources of bias
Fifteen studies were judged as low risk for other sources of bias. In 31 studies the risk of other sources of bias was unclear, and in three studies the risk was judged as high. Unreported sources of funding were the primary reason for an unclear risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Treatment failure
Treatment failure has been previously defined as lack of clinical resolution, relapse or recurrence of AOM during a one‐month period following the initiation of therapy. See summary of findings tables for each intervention (Table 1; Table 2; Table 3).
Outcome of short‐acting antibiotics in the short course (less than seven days)
Two trials were included in the meta‐analysis for short‐acting antibiotics administered for a period of less than 48 hours, and 17 trials were included in the meta‐analysis of short‐acting antibiotics administered for more than 48 hours.
i) Short‐acting antibiotics given for less than 48 hours
A summary odds ratio (OR) of 2.99 (95% CI 1.04 to 8.54) was found for treatment failures at less than one month (Analysis 1.1). Two trials evaluating a total of 118 children were included in this meta‐analysis (Meistrup‐Larsen 1983; Puczynski 1987). The trial by Puczynski was prematurely terminated (Puczynski 1987).
1.1. Analysis.

Comparison 1 Short‐acting antibiotic =< 48 hours in short treatment arm, Outcome 1 Treatment failure at 1 month or less.
ii) Short‐acting antibiotics given for more than 48 hours
Risk of treatment failure, defined as lack of clinical resolution, relapse or recurrence of AOM at one month or less, was higher with short courses of antibiotics (OR 1.34, 95% CI 1.15 to 1.55) (Analysis 2.1). Significant results were found when treatment failure was compared at a follow‐up period of 19 days or less (OR 1.37, 95% CI 1.15 to 1.64) (Analysis 2.2). At 21 days there was no difference in the risk of treatment failure between short and long‐course antibiotics (Analysis 2.3). At one month or less, 22 children needed treatment with short regimens of antibiotics to cause an additional treatment failure compared to longer regimens of antibiotics.
2.1. Analysis.

Comparison 2 Short‐acting antibiotic > 48 hours short‐term treatment, Outcome 1 Treatment failure at 1 month or less.
2.2. Analysis.

Comparison 2 Short‐acting antibiotic > 48 hours short‐term treatment, Outcome 2 Treatment failure at 8 to 19 days.
2.3. Analysis.
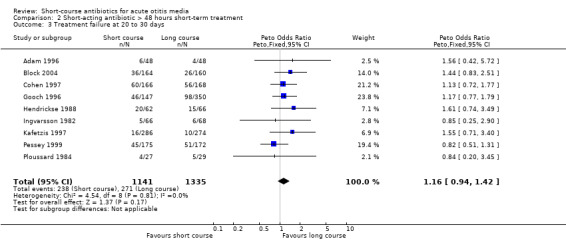
Comparison 2 Short‐acting antibiotic > 48 hours short‐term treatment, Outcome 3 Treatment failure at 20 to 30 days.
Subgroup analysis examining treatment failure in children less than two years and two years of age or older was performed. Six trials containing 614 children found non‐significant results for treatment failure at one month or less in short versus long‐course regimens of antibiotics (Analysis 3.1). Significant differences in outcomes between five‐day and 10‐day treatment among children less than two years old were reported in one trial, but no extractable subgroup data were presented (Hoberman 1997). Non‐significant results were repeated in seven trials containing 1183 participants when examining children two years of age or older (Analysis 4.1).
3.1. Analysis.

Comparison 3 Short‐acting antibiotic > 48 hours short‐term treatment, < 2 yrs old, Outcome 1 Treatment failure at 1 month or less.
4.1. Analysis.

Comparison 4 Short‐acting antibiotic > 48 hours short‐term treatment, => 2 yrs old, Outcome 1 Treatment failure at 1 month or less.
Hendrickse reported outcome data for perforated and non‐perforated eardrums (Hendrickse 1988). The OR for treatment failure in children with perforated eardrums (n = 27) was 3.62 (95% CI 0.81 to 16.06) (Analysis 5.1) and 1.06 (95% CI 0.40 to 2.75) (Analysis 6.1) in children with non‐perforated eardrums (n = 101).
5.1. Analysis.

Comparison 5 Short‐acting antibiotic > 48 hours short‐term treatment, perforated eardrum, Outcome 1 Treatment failure at 1 month or less.
6.1. Analysis.

Comparison 6 Short‐acting antibiotic > 48 hours short‐term treatment, non‐perforated eardrum, Outcome 1 Treatment failure at 1 month or less.
Outcome of ceftriaxone in the short course (less than seven days)
Eight trials (n = 1709) were included in the meta‐analysis for treatment failure at one month or less. No significant results were found (OR 1.07, 95% CI 0.86 to 1.33) (Analysis 17.1), and this lack of significance was repeated when treatment failure at three months or less was examined (Analysis 17.2).
17.1. Analysis.

Comparison 17 Ceftriaxone, Outcome 1 Treatment failure at 1 month or less.
17.2. Analysis.

Comparison 17 Ceftriaxone, Outcome 2 Treatment failure at 3 months or less.
Outcome of azithromycin in the short course (less than seven days)
A comparison of regimens of short‐course azithromycin for treatment failure at one month or less yielded an OR of 1.02 (95% CI 0.87 to 1.20). This meta‐analysis included 19 trials and 4354 children (Analysis 19.1). Examination of a follow‐up period of 8 to 19 days (18 trials, n = 4347) found a significant OR of 1.27 (95% CI 1.04 to 1.55) (Analysis 19.2). Forty‐four children needed treatment with short‐course azithromycin so that one child may experience an additional treatment failure compared to children treated with longer courses of antibiotics. This significant result was not repeated when 20 to 30‐day follow‐up periods were examined (Analysis 19.3).
19.1. Analysis.

Comparison 19 Azithromycin 3 to 5 days short‐term treatment, Outcome 1 Treatment failure at 1 month or less.
19.2. Analysis.

Comparison 19 Azithromycin 3 to 5 days short‐term treatment, Outcome 2 Treatment failure at 8 to 19 days.
19.3. Analysis.

Comparison 19 Azithromycin 3 to 5 days short‐term treatment, Outcome 3 Treatment failure at 20 to 30 days.
The odds of treatment failure with azithromycin was 1.92 (95% CI 0.73 to 5.04) (Analysis 20.1) in children less than two years (n = 138, 17.4%) and 1.34 (95% CI 0.61 to 2.94) (Analysis 21.1) in older children (n = 656) (Principi 1995; Schaad 1993).
20.1. Analysis.

Comparison 20 Azithromycin 3 to 5 days short‐term treatment, < 2 years old, Outcome 1 Treatment failure at 1 month or less.
21.1. Analysis.

Comparison 21 Azithromycin 3 to 5 days short‐term treatment, => 2 years old, Outcome 1 Treatment failure at 1 month or less.
Sensitivity analysis
Assessment of risk of bias
A sensitivity analysis of allocation concealment and blinding from the risk of bias assessment and treatment failure at one month or less was completed. An OR of 1.30 (95% CI 1.09 to 1.54) was found in short‐acting antibiotics for treatment failure in 14 studies (Analysis 12.1) where the risk of bias for allocation concealment was unclear and 1.45 (95% CI 1.08 to 1.93) (Analysis 12.2) in three studies where the risk of bias was low for allocation concealment. Thirteen studies had a high or unclear risk of bias in blinding and an OR of 1.18 (95% CI 1.00 to 1.40) (Analysis 12.3) compared to four studies with a low risk of bias and an OR of 2.03 (95% CI 1.48 to 2.77) (Analysis 12.4).
12.1. Analysis.

Comparison 12 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: treatment failure at 1 month or less and risk of bias, Outcome 1 Sensitivity analysis: allocation concealment unclear risk of bias.
12.2. Analysis.

Comparison 12 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: treatment failure at 1 month or less and risk of bias, Outcome 2 Sensitivity analysis: allocation concealment low risk of bias.
12.3. Analysis.

Comparison 12 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: treatment failure at 1 month or less and risk of bias, Outcome 3 Sensitivity analysis: blinding high and unclear risk of bias.
12.4. Analysis.

Comparison 12 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: treatment failure at 1 month or less and risk of bias, Outcome 4 Sensitivity analysis: blinding low risk of bias.
Due to a lack of difference between the risk of bias assessment for studies examining ceftriaxone, a sensitivity analysis was not completed for this outcome.
Seventeen studies using azithromycin as the antibiotic in the short course were judged to have a high or unclear risk of bias for allocation concealment. The OR was 1.04 (95% CI 0.88 to 1.22) (Analysis 26.1). Two studies had a low risk of bias for allocation concealment and had an OR of 0.13 (95% CI 0.02 to 0.90) (Analysis 26.2). The large difference between these two values should be interpreted with caution given the low number of studies included in the meta‐analysis of low risk of bias for allocation concealment. For blinding, 15 studies had a high or unclear risk of bias and an OR of 1.15 (95% CI 0.94 to 1.40) (Analysis 26.3) and four studies with a low risk of bias for blinding and an OR of 0.80 (95% CI 0.60 to 1.07) (Analysis 26.4).
26.1. Analysis.

Comparison 26 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: risk of bias, Outcome 1 Sensitivity analysis: allocation concealment unclear or high risk of bias.
26.2. Analysis.

Comparison 26 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: risk of bias, Outcome 2 Sensitivity analysis: allocation concealment low risk of bias.
26.3. Analysis.

Comparison 26 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: risk of bias, Outcome 3 Sensitivity analysis: blinding high and unclear risk of bias.
26.4. Analysis.

Comparison 26 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: risk of bias, Outcome 4 Sensitivity analysis: blinding low risk of bias.
Use of same antibiotic in both arms
A total of 10 studies utilised the same antibiotic in both the short and long courses of therapy. Nine studies including 3321 participants presented data for treatment failure at one month or less; this analysis resulted in an OR of 1.65 (95% CI 1.35 to 2.01) (Analysis 10.1). A sensitivity analysis was also undertaken to compare the two trials that used the same antibiotic (amoxicillin‐clavulanate) in both arms. This analysis resulted in an OR of 1.99 (95% CI 1.44 to 2.74) (Analysis 13.1) compared to an OR of 1.20 (95% CI 1.02 to 1.42) when excluding the above trials from the analysis (Analysis 13.2). The effect was larger with amoxicillin‐clavulanate, however caution must be used when interpreting these results due to the number of subgroup analyses.
10.1. Analysis.

Comparison 10 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms, Outcome 1 Treatment failure at 1 month or less.
13.1. Analysis.

Comparison 13 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis amoxil‐clav. both arms, Outcome 1 Amoxil‐clav. 5 versus 10 days, treatment failure 1 month or less.
13.2. Analysis.

Comparison 13 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis amoxil‐clav. both arms, Outcome 2 Excluding amoxil‐clav. 5 versus 10 days, treatment failure 1 month or less.
Publication bias
We produced funnel plots for the antibiotic comparisons, short‐acting antibiotics (Figure 1), ceftriaxone (Figure 2) and azithromycin (Figure 3). Upon visual inspection, funnel plots suggested publication bias for the azithromycin comparison but not for the other antibiotic groups. Additional statistical tests (Duval 2000; Egger 1997) of significance did not reveal publication bias in any of the groups. A sensitivity analysis of studies declaring industry funding as compared to those not reporting a funding source was not significant (Analysis 27.1 and Analysis 28.1).
1.

Funnel plot of comparison: 2 Short‐acting antibiotic > 48 hours short‐term treatment, outcome: 2.1 Treatment failure at 1 month or less.
2.

Funnel plot of comparison: 21 Ceftriaxone, outcome: 21.1 Treatment failure at 1 month or less.
3.

Funnel plot of comparison: 23 Azithromycin 3 to 5 days short‐term treatment, outcome: 23.1 Treatment failure at 1 month or less.
27.1. Analysis.

Comparison 27 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: industry funding, Outcome 1 Treatment failure 1 month or less.
28.1. Analysis.

Comparison 28 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: funding not reported, Outcome 1 Treatment failure 1 month or less.
Gastrointestinal adverse events
Outcome of short‐acting antibiotics in the short course (less than seven days)
In 13 trials containing 4918 children a significant reduction in gastrointestinal adverse events was observed (Analysis 16.1). The OR was found to be 0.72 (95% CI 0.60 to 0.87) which translates to one less child experiencing an adverse event for every 29 children given a short regimen of antibiotics. A sensitivity analysis of the five trials that did not use amoxicillin‐clavulanate provided non‐significant results (Analysis 11.1).
16.1. Analysis.

Comparison 16 Short‐acting antibiotic, > 48 hours short‐term treatment, adverse GI effects, Outcome 1 Gastrointestinal adverse effects.
11.1. Analysis.

Comparison 11 Short‐acting antibiotic > 48 hours short‐term treatment, excluding amoxicillin‐clavulanate, Outcome 1 Gastrointestinal adverse effects.
Outcome of ceftriaxone in the short course (less than seven days)
One trial was included in the analysis of ceftriaxone adverse events (Barnett 1997). In this study (n = 402) more children experienced adverse events in the short course ceftriaxone group (OR 2.89, 95% CI 1.70 to 4.91). This OR translates to an additional child experiencing an adverse gastrointestinal event for every seven to eight children treated compared to long courses of antibiotics (Analysis 17.3).
17.3. Analysis.

Comparison 17 Ceftriaxone, Outcome 3 Gastrointestinal adverse effects.
Outcome of azithromycin in the short course (less than seven days)
In 14 trials containing 3722 children, significantly fewer gastrointestinal events were experienced in the short course of azithromycin comparison group (Analysis 29.1). The OR was 0.36 (95% CI 0.28 to 0.46) which translates to one less child experiencing an adverse event for every 14 children treated compared to longer courses of antibiotics.
29.1. Analysis.

Comparison 29 Azithromycin 3 to 5 days short‐term treatment, adverse GI effects, Outcome 1 Gastrointestinal adverse effects.
Discussion
Summary of main results
A reduction in the treatment of AOM from 10 to five days of short‐acting antibiotics may slightly increase the risk of a child experiencing signs and symptoms, relapse or re‐infection at eight to 19 days (OR 1.37, 95% CI 1.15 to 1.64). By 30 days following initiation of therapy, a longer course of short‐acting antibiotics is comparable to a five‐day course in terms of these outcomes (OR 1.17, 95% CI 0.95 to 1.43). When studies comparing the same antibiotics in both arms were analyzed the results favoured the use of longer regimens of therapy (OR 1.65, 95% CI 1.35 to 2.01). Notably 22 studies published after 1998 were added to the updated meta‐analysis and brought the 30‐day findings closer to equivalence. Studies that had low risk of bias for the component of blinding had significantly higher risks of treatment failure (OR 2.03, 95% CI 1.48 to 2.77) compared to those with high risk of bias for blinding (OR 1.19, 95% CI 1.01 to 1.41). The long‐term comparability between a short and long course of antibiotics is biologically plausible, on the basis of: 1) spontaneous resolution of untreated AOM (Del Mar 1997; Rosenfeld 1994); 2) early eradication of pathogens after three to five days of treatment (Howie 1969); 3) poorer penetration of the antibiotic into the ear with continued administration as inflammation decreases (Canafax 1991); and 4) treatment of children without AOM because of diagnostic uncertainty (Froom 1990).
Overall completeness and applicability of evidence
Appreciating that a shortened course of antibiotics may protect the child from developing resistant microorganisms (Kozyrskyj 1998), we report these results 12 years later when many clinical practice guidelines are proposing no antibiotic treatment or 'watchful waiting' as a first‐line approach to treating AOM. The American Academy of Pediatrics recommends reserving antibiotic therapy for children that are less than or equal to two years with severe disease (Pediatric Guidelines 2004). More recently the Canadian Paediatric Society recommended a 'wait and watch' approach for children over six months with uncomplicated, non‐severe disease (Forgie 2009). This is consistent with a Cochrane Review examining the effectiveness of antibiotics for AOM (Sanders 2009) and an individual patient meta‐analysis (Koopman 2008; Spiro 2008; Vouloumanou 2009). If antibiotics are used, our results indicate that a long course of treatment can minimise the risks of treatment failure or recurrence post‐treatment, but may not make a difference in the long term.
This updated review also found no significant differences when examining short courses of ceftriaxone. However, in the one study where adverse effects were examined, they were much higher in the ceftriaxone group. As administering ceftriaxone also requires an injection, this does not seem to be a good option for management of AOM.
Quality of the evidence
Higher risk of treatment failure occurred with three to five days of azithromycin (OR 1.27, 95% CI 1.04 to 1.55) at 8 to 19 days after the initiation of treatment. This significant result was not repeated when examining treatment failure at 20 to 30 days (OR 0.98, 95% CI 0.82 to 1.17). Outcome differences noted between the evaluations at eight to 19 days and 20 to 30 days likely reflects bias in the timing of evaluation. Children treated with a long course of antibiotics had fewer days to experience an outcome than those treated with five days when the time to evaluation was 8 to 19 days, as opposed to 30 days (Pichichero 1997).
Potential biases in the review process
Potential weaknesses of meta‐analysis techniques are that they incorporate existing biases and introduce new biases, some of which have predicted discordance of results between meta‐analyses and single large RCTs (Borzak 1995; Egger 1997). To minimise bias during study selection, we used pre‐determined inclusion criteria, and most trials were assessed in a blinded fashion, although recent evidence suggests that blinded evaluations are not necessary (Berlin 1997). We assessed publication bias in our funnel plot of sample size versus ORs (Higgins 2008) and with additional statistical tests (Duval 2000; Egger 1997) of significance. Publication bias was not evident. The issue of trial heterogeneity was addressed in our grouping of antibiotics according to pharmacokinetic profile (Schentag 1995).
In this review two studies (Gooch 1996; Hoberman 1997) contained two arms comparing longer course versus a short course of antibiotic. In the original review the short‐course arm was counted twice against each long‐course arm of that study. In the update of this review, we have modified our methods and combined both long‐course arms in a single comparison against the short‐course arm. In an effort to test whether this new method would change our results we compared the previous meta‐analysis against a new analysis where we used the new statistical method but did not add the additional studies which are a part of this update. This new method did not have a great effect on our results.
Agreements and disagreements with other studies or reviews
Most of the 'short‐acting' antibiotics in this study are commonly prescribed in primary practice and our sensitivity analyses indicated that comparisons between different short‐acting antibiotics did not alter treatment outcomes. Comparability was also demonstrated between ceftriaxone and a longer course of antibiotics, although the sample size of the consolidated trials was smaller. The equivalence observed between a three or five‐day course of azithromycin and a 10‐day course of other antibiotics was unchanged in sensitivity analyses, but these could not be performed for outcomes at 8 to 19 days. It is difficult to support the use of azithromycin or ceftriaxone based on their cost, and concern over indiscriminate use of broad‐spectrum antibiotics (Rosenfeld 1996). Dagan et al documented increasing rates of erythromycin resistance to Streptococcus pneumoniae (S. pneumoniae) over the period that azithromycin was prescribed more often to children. The increased prescription of amoxicillin‐clavulanate did alter resistance to penicillin (Dagan 2006). Ceftriaxone's intramuscular mode of administration may further limit its role (Eppesl 1997). Incidence of diarrhea and vomiting may be increased with antibiotic use in children with AOM (Del Mar 1997). Findings did not demonstrate that a shortened course of antibiotics decreased the likelihood of gastrointestinal effects, except when compared to a longer course of amoxicillin‐clavulanate.
Authors' conclusions
Implications for practice.
Evidence is increasing for a wait and watch approach to AOM. We believe that this is the most prudent approach for most children who are older than six months or do not have serious or complicated disease. If treatment is warranted, the clinician must decide if treatment for 7 to 10 days is worth the slightly reduced risk of treatment failure in the short term (< 21 days). Shorter courses can also be safely used, resulting in few side effects and, perhaps, a lower risk of antibiotic resistant bacteria. Shorter courses may also be associated with higher levels of compliance.
Implications for research.
In light of the increasing use of the wait and watch approach, it would be helpful to have trials that randomise participants to short versus long‐course treatment with oral antibiotics after an unsuccessful observation period.
Feedback
'Short course antibiotics for acute otitis media' has severe errors of analysis and procedure and needs to be withdrawn, 29 November 2006
Summary
1. Data are counted twice as follows Study Short course Long course Boulesteix, 1995 11/124 11/118 Cohen, 1997 26/186 31/184 Hendrickse, 1988 14/74 6/77 Hoberman, 1997a 57/197 24/178 Hoberman, 1997b 57/197 40/189 Hoberman 1997a and b are the same study and so the short course data have been counted twice.
2. For both the Hoberman and Boulesteix studies the treatments being compared differ not only in terms of duration (short versus long) but also in terms of other aspects (for example, formulation, dose and dosing schedule). Thus as regards the comparison of short versus long they are biased.
Reply
1. Two studies (Gooch 1996; Hoberman 1997) in the current review contained two arms comparing long course versus short course of antibiotics. In the original review the short arm was counted twice against each long arm of that study. In the update of this review, we have modified our methods and combined both long‐course arms in a single comparison against the short‐course arm, as recommended in the Cochrane Handbook (Higgins 2008).
The remaining articles (Boulesteix 1995; Cohen 1997; Hendrickse 1988) were checked to ensure that all patients enrolled in the study were accounted for only once. These three trials clearly indicate that each patient is only accounted for in one of the groups (short or long‐course).
Hoberman 1997b has been removed from the updated review.
2. These minor differences were considered but were not deemed clinically important to the current question, and we don't believe this introduces any bias to the comparison.
Reply approved and added to review September 2009 by: Anita Kozyrskyj, Terry P Klassen, and Michael Moffatt
Contributors
Stephen Senn Comment submitted 30 November 2006. Added to review 22 Decemer 2008.
What's new
| Date | Event | Description |
|---|---|---|
| 19 June 2012 | Review declared as stable | As of 19 June 2012, this Cochrane Review is no longer being updated, as there is high‐quality evidence that treating children with acute otitis media with a short course (less than seven days) of antibiotics, compared to treatment with a long course (seven days or greater) of antibiotics, increases the likelihood of treatment failure in the short term, meaning further research is unlikely to change our confidence in the estimate of effect in our primary outcome. The review authors recommend that it is no longer necessary to update this review. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 9 September 2010 | Amended | Contact details updated. |
| 18 November 2009 | New citation required but conclusions have not changed | The searches were conducted in November 2009, with a total of 22 new trials added since the original review in 2000 (Adam 2000; Al‐Ghamdi 1999; Arguedas 2005; Arrieta 2003; Block 2000; Block 2003; Block 2004; Catania 2004; Cohen 1998; Cohen 1999; Cohen 2000; Dagan 2000a; Dagan 2000b; de Jose 1998; Dunne 2003; Guven 2006; Hoberman 2005; Kara 1998; Oguz 2003; Pessey 1999; Varsano 1997; Wang 2004). Two of the studies included in the first version of this review (Kozyrskyj 2000) were excluded due to the outcome not being reported as a treatment failure (Bain 1985; Jones 1986) and an additional three studies have been excluded; two were excluded due to use of ceftibuten which has been deemed not to be clinically relevant (Roos 2000; Simon 1997); and one for the duration of antibiotic therapy being equivalent in both arms (Suzuki 2009). The 22 studies that were included in this updated review brought the 30‐day findings closer to equivalence (primary outcome). This would suggest that any new studies would not change the current outcomes and the review authors recommend that it is no longer necessary to update this review. |
| 11 November 2009 | New search has been performed | Searches conducted. |
| 22 December 2008 | Feedback has been incorporated | Feedback added. |
| 12 March 2008 | Amended | Converted to new review format. |
| 30 January 2000 | New search has been performed | Searches conducted. |
Acknowledgements
Support for this work was provided by the Canadian Cochrane Network and by the Department of Community Health Sciences at the University of Manitoba. We would like to acknowledge the previous review authors Hildes‐Ripstein GE, Longstaffe SE, Wincott JL and Sitar DS for their work on the original document. Thanks to Mary Cheang, MSc for statistical assistance. In the update of this review thank you to Maria Ospina (MO) and Ben Vandermeer (BV) from the Alberta Research Centre for Health Evidence (ARCHE) for assistance with the risk of bias evaluation and statistical analysis. Also from ARCHE we would like to thank Nicola Hooton (NH) and Liza Bialy (LB) for assisting with screening, data extraction and author support; and librarians Carol Fiesen, Tamara Durec and Lisa Tjosvold. We would also like to thank Sarah Thorning, Acute Respiratory Tract Infections Group's Trials Search Co‐ordinator who assisted with the last update and implemented the search strategy. Finally, we wish to thank the following people for commenting on the draft of this updated review: Dilip Raghavan, Sharon Sanders, Brian Westerberg, Max Bulsara and Peter Morris.
Appendices
Appendix 1. Previous searches
The research librarian, in collaboration with the research team, developed and implemented search strategies designed to identify the highest quality of evidence for this review. The English and non‐English language medical literature was searched using the MEDLINE and EMBASE databases to identify published clinical trials of AOM during the time period, January 1966 to July 1997. The Science Citation Index was utilised to identify additional trials which had cited relevant papers. The Current Contents/Life Sciences Index was searched for recently published AOM trials, not yet abstracted in MEDLINE.
Search updates were conducted with a modified search strategy using the following electronic resources for the years 1997 to August 2008 (exceptions stated in brackets) and no language restriction: MEDLINE, EMBASE, MEDLINE In‐Process & Other Non‐Indexed Citations, Cochrane Central Register of Controlled Trials (which contains the Cochrane Acute Respiratory Infections Group; this group handsearches journals pertinent to their content area and adds relevant trials to the registry), and International Pharmaceutical Abstracts, BIOSIS Previews (ISI Web of KnowledgeSM [3.0]), CINAHL Full Text (via EBSCOhost). The NLM (National Library of Medicine) Gateway, BioMed Central and OCLC PapersFirst and ProceedingsFirst were searched for identification of meeting abstracts. Additionally, trials registers such as Current Controlled Trials, ClinicalTrials.gov, the National Research Register, CRISP (Computer Retrieval of Information on Scientific Projects), the TRIP Database (Turning Research Into Practice), Scirus, Proquest Dissertations and Theses ‐ Full Text (1861 to 2008), and Google Scholar were searched for additional unpublished controlled trials and reports. The reference lists of relevant reviews and included studies were reviewed, and authors of included studies were contacted, as required (e.g. to clarify the source of population in cases of multiple publications or to seek additional data).
For the search strategies, a combination of subject headings and keywords were adapted for each electronic resource using the following terms: ‘otitis media’, ‘acute otitis media’, ‘ear infection’, ‘otorrhea’, ‘anti‐infective agents’, ‘treatment outcome’, ‘antibacterial’, ‘antibiotic’, ‘bacteriocide’ and ‘antimicrobial’.
Appendix 2. Search strategies for 1997‐2008 update
| Electronic databases | Search strategies |
|
MEDLINE OVID Version: rel10.5.2
1950 to August Week 2008
Searched: 1 August 2008
Results: 1892 Limits: Date: 1997 to 2008 |
1. exp Otitis Media/ 2. (acute adj5 (OM or otitis media or ear or infection?)).mp. 3. (OM or OME or AOM).ti,ab. 4. otorrh?ea.ti,ab. 5. or/1‐4 6. exp Anti‐Infective Agents/ 7. exp treatment outcome/ 8. (antibacter$ or anti‐bacter$ or "anti bacter$" or antibiotic$ or anti‐biotic$ or "anti biotic$" or bacteriocid$ or antimicrob$ or anti‐microb$ or "anti microb$").mp. 9. or/6‐8 10. clinical trial.pt. 11. randomized controlled trial.pt. 12. randomi?ed.ti,ab. 13. placebo.ti,ab. 14. dt.fs. 15. randomly.ti,ab. 16. trial.ti,ab. 17. groups.ti,ab. 18. or/10‐17 19. animals/ 20. humans/ 21. 19 not (19 and 20) 22. 18 not 21 23. and/5,9,22 24. exp Infant/ 25. exp Child/ 26. Adolescent/ 27. Minors/ 28. exp Puberty/ 29. exp Pediatrics/ 30. infant$.mp. 31. infancy.mp. 32. newborn$.mp. 33. baby.mp. 34. babies.mp. 35. neonat$.mp. 36. preterm$.mp. 37. prematur$.mp. 38. postmatur$.mp. 39. child$.mp. 40. kid.mp. 41. kids.mp. 42. toddler$.mp. 43. adolescen$.mp. 44. teen$.mp. 45. boy$.mp. 46. girl.mp. 47. minor$.mp. 48. pubert$.mp. 49. pubescen$.mp. 50. prepubescen$.mp. 51. pediatric$.mp. 52. paediatric$.mp. 53. peadiatric$.mp. 54. or/24‐52 55. and/5,9,22,54 56. limit 55 to yr="1997 ‐ 2007" |
| Ovid MEDLINE In‐Process & Other
Non‐Indexed Citations
OVID Version: rel10.5.2
1 August 2008
Searched: 1 August 2008
Results: 6 Limits: Date: 1997 to 2008 |
1. (acute adj5 (OM or otitis media or ear or infection?)).mp. 2. (OM or OME or AOM).ti,ab. 3. otorrh?ea.ti,ab. 4. or/1‐3 5. (antibacter$ or anti‐bacter$ or "anti bacter$" or antibiotic$ or anti‐biotic$ or "anti biotic$" or bacteriocid$ or antimicrob$ or anti‐microb$ or "anti microb$").mp. 6. (treatment adj5 outcome?).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 7. or/5‐6 8. "controlled clinical trial$".mp. 9. "randomi?ed controlled trial$".mp. 10. "research design$".mp. 11. "clinical research".mp. 12. "random allocation".mp. 13. randomi?ed.ti,ab. 14. ("double blind" adj3 method$).mp. 15. ("single blind" adj3 method$).mp. 16. ((clin$ or control$) adj25 trial$).mp. 17. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 18. placebo$.ti,ab. 19. randomly.ti,ab. 20. or/8‐19 21. infant$.mp. 22. infancy.mp. 23. newborn$.mp. 24. baby.mp. 25. babies.mp. 26. neonat$.mp. 27. preterm$.mp. 28. prematur$.mp. 29. postmatur$.mp. 30. child$.mp. 31. kid.mp. 32. kids.mp. 33. toddler$.mp. 34. adolescen$.mp. 35. teen$.mp. 36. boy$.mp. 37. girl.mp. 38. minor$.mp. 39. pubert$.mp. 40. pubescen$.mp. 41. prepubescen$.mp. 42. pediatric$.mp. 43. paediatric$.mp. 44. peadiatric$.mp. 45. or/21‐44 46. and/4,7,20,45 47. limit 46 to yr="1997 ‐ 2007" |
|
EMBASE OVID Version: rel10.5.2 1996 to 2008 Searched: 1 August 2008 Results: 2993 Limits: Date: 1997 to 2008 |
1. exp Otitis Media/ 2. (acute adj5 (OM or otitis media or ear or infection?)).mp. 3. (OM or OME or AOM).ti,ab. 4. otorrh?ea.ti,ab. 5. or/1‐4 6. exp Antiinfective Agent/ 7. exp treatment outcome/ 8. (antibacter$ or anti‐bacter$ or "anti bacter$" or antibiotic$ or anti‐biotic$ or "anti biotic$" or bacteriocid$ or antimicrob$ or anti‐microb$ or "anti microb$").mp. 9. or/6‐8 10. exp clinical trial/ 11. randomi?ed.ti,ab. 12. placebo.ti,ab. 13. dt.fs. 14. randomly.ti,ab. 15. trial.ti,ab. 16. groups.ti,ab. 17. or/10‐16 18. exp child/ 19. exp newborn/ 20. exp adolescent/ 21. exp puberty/ 22. exp pediatrics/ 23. infant$.mp. 24. infancy.mp. 25. newborn$.mp. 26. baby.mp. 27. babies.mp. 28. neonat$.mp. 29. preterm$.mp. 30. prematur$.mp. 31. postmatur$.mp. 32. child$.mp. 33. kid.mp. 34. kids.mp. 35. toddler$.mp. 36. adolescen$.mp. 37. teen$.mp. 38. boy$.mp. 39. girl.mp. 40. minor$.mp. 41. pubert$.mp. 42. pubescen$.mp. 43. prepubescen$.mp. 44. pediatric$.mp. 45. paediatric$.mp. 46. peadiatric$.mp. 47. or/18‐46 48. and/5,9,17,47 49. limit 48 to yr="1997 ‐ 2007" |
|
International Pharmaceutical
Abstracts OVID Version: rel10.5.2 1970 to August 2008 Searched: 1 August 2008 Results: 45 Limits: Date: 1997 to 2008 |
1. (acute adj5 (OM or otitis media or ear or infection?)).mp. 2. (OM or OME or AOM).ti,ab. 3. otorrh?ea.ti,ab. 4. or/1‐3 5. (antibacter$ or anti‐bacter$ or "anti bacter$" or antibiotic$ or anti‐biotic$ or "anti biotic$" or bacteriocid$ or antimicrob$ or anti‐microb$ or "anti microb$").mp. 6. (treatment adj5 outcome?).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 7. or/5‐6 8. "controlled clinical trial$".mp. 9. "randomi?ed controlled trial$".mp. 10. "research design$".mp. 11. "clinical research".mp. 12. "random allocation".mp. 13. randomi?ed.ti,ab. 14. ("double blind" adj3 method$).mp. 15. ("single blind" adj3 method$).mp. 16. ((clin$ or control$) adj25 trial$).mp. 17. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 18. placebo$.ti,ab. 19. randomly.ti,ab. 20. or/8‐19 21. infant$.mp. 22. infancy.mp. 23. newborn$.mp. 24. baby.mp. 25. babies.mp. 26. neonat$.mp. 27. preterm$.mp. 28. prematur$.mp. 29. postmatur$.mp. 30. child$.mp. 31. kid.mp. 32. kids.mp. 33. toddler$.mp. 34. adolescen$.mp. 35. teen$.mp. 36. boy$.mp. 37. girl.mp. 38. minor$.mp. 39. pubert$.mp. 40. pubescen$.mp. 41. prepubescen$.mp. 42. pediatric$.mp. 43. paediatric$.mp. 44. peadiatric$.mp. 45. or/21‐44 46. and/4,7,20,45 47. limit 46 to yr="1997 ‐ 2007" |
| EBM Reviews Cochrane Central Register of Controlled Trials
2nd Quarter 2008
OVID Version: rel10.5.2
Searched: 1 August 2008
Results: 425 Limits: Date: 1997 to 2008 |
1. exp Otitis Media/ 2. (acute adj5 (OM or otitis media or ear or infection?)).mp. 3. (OM or OME or AOM).ti,ab. 4. otorrh?ea.ti,ab. 5. or/1‐4 6. exp Anti‐Infective Agents/ 7. exp treatment outcome/ 8. (antibacter$ or anti‐bacter$ or "anti bacter$" or antibiotic$ or anti‐biotic$ or "anti biotic$" or bacteriocid$ or antimicrob$ or anti‐microb$ or "anti microb$").mp. 9. or/6‐8 10. exp Infant/ 11. exp Child/ 12. Adolescent/ 13. Minors/ 14. exp Puberty/ 15. exp Pediatrics/ 16. infant$.mp. 17. infancy.mp. 18. newborn$.mp. 19. baby.mp. 20. babies.mp. 21. neonat$.mp. 22. preterm$.mp. 23. prematur$.mp. 24. postmatur$.mp. 25. child$.mp. 26. kid.mp. 27. kids.mp. 28. toddler$.mp. 29. adolescen$.mp. 30. teen$.mp. 31. boy$.mp. 32. girl.mp. 33. minor$.mp. 34. pubert$.mp. 35. pubescen$.mp. 36. prepubescen$.mp. 37. pediatric$.mp. 38. paediatric$.mp. 39. peadiatric$.mp. 40. or/10‐38 41. and/5,9,40 limit 41 to yr="1997 ‐ 2007" |
|
CINAHL Plus with Full Text (EBSCOhost) 1981‐present Searched: 1 August 2008 Results: 532 Limits: Date: 1997 to 2008 Age Groups: Infant, Newborn 0‐1 month, Infant, 1‐23 months, Child, Preschool 2‐5 years, Child, 6‐12 years, Adolescence, 13‐18 years |
# Query S7( S5 and S1 ) S6( S5 and S1 ) S5( S4 or S3 or S2 ) S4( antibacter* or anti‐bacter* or "anti bacter*" or antibiotic* or anti‐biotic* or "anti biotic*" or bacteriocid* or antimicrob* or anti‐microb* or "anti microb*" ) S3(MH "Treatment Outcomes+") S2(MH "Antiinfective Agents+") S1(MH "Otitis Media+") or ( acute w5 OM OR acute w5 "otitis media" OR actue w5 ear OR acute w5 infection ) or ( ottorrh?ea or OM or OME or AOM ) |
|
BIOSIS Previews® (ISI Web of KnowledgeSM [3.0]) 1969 to present Searched: 1 August 2008 Results: 1161 Limits: 1997 to 2008 |
Sets History #6 #5 AND #4 #5 TS=clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*) #4#3 OR #2 OR #1 #3 TS=ottorrhea #2 TS=(OM OR OME OR OME OR AOM) #1 TS="otitis media" OR TS=(acute SAME OM OR acute SAME "otitis media" OR acute SAME "ear infection") |
|
OCLC PapersFirst (OCLC FirstSearch) Searched: 1 August 2008 Results: 25 + 3 Limits: 1997 to 2008 |
(kw: otitis w media) and (kw: antibiotic* OR kw: antibacter* OR kw: bacteriocid* OR kw: antimicrob*) |
|
OCLC ProceedingsFirst (OCLC FirstSearch) Searched: 1 August 2008 Results: 1 Limits: 1997 to 2008 |
(kw: otitis w media) and (kw: antibiotic* OR kw: antibacter* OR kw: bacteriocid* OR kw: antimicrob*) |
|
ProQuest® Dissertations & Theses Full Text 1861 to present Searched: 1 August 2008 Results: 62 = 3 |
(("otitis media" OR "ear infection" or ottorrhea) AND (acute)) |
OVID databases: RCT filter adapted from: Cochrane Highly Sensitive Search Strategy (2005) Revision from Glanville JM, Lefebvre C, Miles JNV, Camosso‐Stefinovic J. How to identify randomized controlled trials in Medline: ten years on. J Med Libr Assoc 2006; 94(2):130‐6
Appendix 3. Search Strategies 2009 Update
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1950 to Present> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 otitis media.tw. 2 (OM or OME or AOM).tw. 3 (ear* adj3 (infect* or acute)).tw. 4 (otorrhea or otorrhoea).tw. 5 or/1‐4 6 (antibacter* or anti‐bacter* or anti bacter* or antibiotic* or anti‐biotic* or anti biotic* or bacteriocid* or antimicrob* or anti‐microb* or anti microb*).tw. 7 (amoxicillin* or amoxycillin* or penicillin* or cefprozil* or clarithromycin* or cefpodoxime* or cefaclor* or ceftriaxone* or azithromycin* or cefixime*).tw. 8 (treatment* adj5 outcome*).tw. 9 or/6‐8 10 5 and 9 11 (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur* or child* or kid or kids or toddler* or adolescen* or teen* or boy* or girl* or minor* or pubert* or pubescen* or prepubescen* or pediatric* or paediatric* or schoolchild* or school age* or preschool* or kindergar* or nursery school* or primary school* or secondary school* or elementary school* or high school* or highschool*).tw. (1599556) 12 10 and 11 13 (random* or placebo* or clinical trial* or research design* or doubl* blind* or singl* blind*).tw. (692296) 14 12 and 13
EMBASE.com 23. #18 AND #22 22. #19 OR #20 OR #21 21. ((singl* OR doubl*) NEAR/2 blind*):ab,ti 20. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti 19. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 18. #12 AND #17 17. #13 OR #14 OR #15 OR #16 16. ((age* OR nursery OR primary OR secondary OR elementary OR high) NEAR/2 school*):ti,ab 15. schoolchild*:ab,ti OR preschool*:ab,ti OR kindergar*:ab,ti OR highschool*:ab,ti 14. infant*:ab,ti OR infancy:ab,ti OR newborn*:ab,ti OR baby*:ab,ti OR babies*:ab,ti OR neonat*:ab,ti OR preterm*:ab,ti OR prematur*:ab,ti OR postmatur*:ab,ti OR child*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti OR adolescen*:ab,ti OR teen*:ab,ti OR boy*:ab,ti OR girl*:ab,ti OR minor*:ab,ti OR pubert*:ab,ti OR pubescen*:ab,ti OR prepubescen*:ab,ti OR pediatric*:ab,ti OR paediatric*:ab,ti 13. 'child'/exp OR 'newborn'/exp OR 'adolescent'/exp OR 'puberty'/exp OR 'pediatrics'/exp 12. #6 AND #11 11. #7 OR #8 OR #9 OR #10 10. amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR penicillin*:ab,ti OR cefprozil*:ab,ti OR clarithromycin*:ab,ti OR cefpodoxime*:ab,ti OR cefaclor*:ab,ti OR ceftriaxone*:ab,ti OR azithromycin*:ab,ti OR cefixime*:ab,ti 9. antibacter*:ab,ti OR 'anti‐bacterial':ab,ti OR 'anti‐bacterials':ab,ti OR 'anti bacterial':ab,ti OR 'anti bacterials':ab,ti OR antibiotic*:ab,ti OR 'anti‐biotic':ab,ti OR 'anti‐biotics':ab,ti OR 'anti biotic':ab,ti OR bacteriocid*:ab,ti OR antimicrob*:ab,ti OR 'anti‐microbial':ab,ti OR 'anti‐microbials':ab,ti OR 'anti microbial':ab,ti OR 'anti microbials':ab,ti 8. 'antiinfective agent'/exp 7. 'treatment outcome'/exp 6. #1 OR #2 OR #3 OR #4 OR #5 5. otorrhoea:ab,ti OR otorrhea:ab,ti 4. om:ab,ti OR ome:ab,ti OR aom:ab,ti 3. ((infect* OR acute*) NEAR/5 ear*):ab,ti 2. 'otitis media':ab,ti 1. 'otitis media'/exp
Data and analyses
Comparison 1. Short‐acting antibiotic =< 48 hours in short treatment arm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 2 | 118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [1.04, 8.54] |
Comparison 2. Short‐acting antibiotic > 48 hours short‐term treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 16 | 5093 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [1.15, 1.55] |
| 2 Treatment failure at 8 to 19 days | 11 | 3932 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [1.15, 1.64] |
| 3 Treatment failure at 20 to 30 days | 9 | 2476 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.94, 1.42] |
| 4 Treatment failure at 3 months or less | 7 | 2068 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.98, 1.41] |
| 5 Treatment failure at 90 days | 2 | 207 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.65, 2.06] |
| 6 Treatment failure at 30 to 45 days | 5 | 1861 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
2.4. Analysis.

Comparison 2 Short‐acting antibiotic > 48 hours short‐term treatment, Outcome 4 Treatment failure at 3 months or less.
2.5. Analysis.

Comparison 2 Short‐acting antibiotic > 48 hours short‐term treatment, Outcome 5 Treatment failure at 90 days.
2.6. Analysis.

Comparison 2 Short‐acting antibiotic > 48 hours short‐term treatment, Outcome 6 Treatment failure at 30 to 45 days.
Comparison 3. Short‐acting antibiotic > 48 hours short‐term treatment, < 2 yrs old.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 5 | 570 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.76, 1.57] |
Comparison 4. Short‐acting antibiotic > 48 hours short‐term treatment, => 2 yrs old.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 6 | 1064 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
Comparison 5. Short‐acting antibiotic > 48 hours short‐term treatment, perforated eardrum.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 1 | 27 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.62 [0.81, 16.06] |
Comparison 6. Short‐acting antibiotic > 48 hours short‐term treatment, non‐perforated eardrum.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.40, 2.75] |
Comparison 7. Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity Analysis: include chronic OM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 6 | 1713 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [1.09, 1.72] |
| 2 Treatment failure at 20 to 30 days | 5 | 1149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.89, 1.54] |
7.1. Analysis.

Comparison 7 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity Analysis: include chronic OM, Outcome 1 Treatment failure at 1 month or less.
7.2. Analysis.

Comparison 7 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity Analysis: include chronic OM, Outcome 2 Treatment failure at 20 to 30 days.
Comparison 8. Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity Analysis: exclude chronic OM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 4 | 1298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.73, 1.85] |
| 2 Treatment failure at 20 to 30 days | 2 | 656 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.79, 3.04] |
8.1. Analysis.

Comparison 8 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity Analysis: exclude chronic OM, Outcome 1 Treatment failure at 1 month or less.
8.2. Analysis.

Comparison 8 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity Analysis: exclude chronic OM, Outcome 2 Treatment failure at 20 to 30 days.
Comparison 9. Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: outcome only if "cured".
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 9 | 2955 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [1.09, 1.58] |
| 2 Treatment failure at 20 to 30 days | 6 | 1749 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.98, 1.58] |
9.1. Analysis.

Comparison 9 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: outcome only if "cured", Outcome 1 Treatment failure at 1 month or less.
9.2. Analysis.
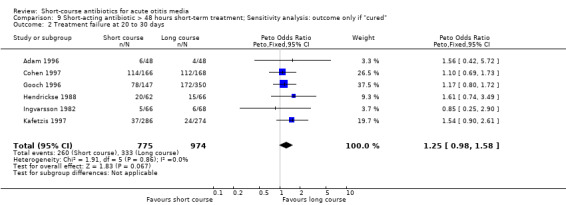
Comparison 9 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: outcome only if "cured", Outcome 2 Treatment failure at 20 to 30 days.
Comparison 10. Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 9 | 3321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.35, 2.01] |
| 2 Treatment failure at 8 to 19 days | 6 | 2153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [1.54, 2.52] |
| 3 Treatment failure at 20 to 30 days | 4 | 1319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.92, 1.76] |
| 4 Treatment failure at 3 months or less | 5 | 1492 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [1.00, 1.53] |
| 5 Treatment failure at 90 days | 2 | 207 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.65, 2.06] |
| 6 Treatment failure at 30 to 45 days | 3 | 1285 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [1.00, 1.57] |
10.2. Analysis.
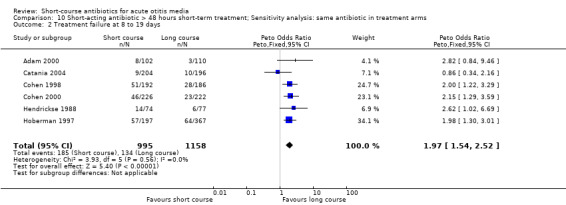
Comparison 10 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms, Outcome 2 Treatment failure at 8 to 19 days.
10.3. Analysis.

Comparison 10 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms, Outcome 3 Treatment failure at 20 to 30 days.
10.4. Analysis.
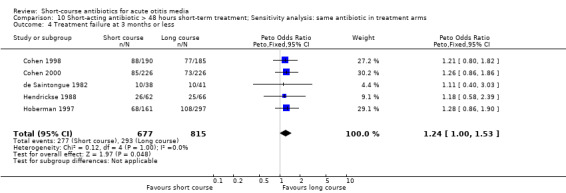
Comparison 10 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms, Outcome 4 Treatment failure at 3 months or less.
10.5. Analysis.

Comparison 10 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms, Outcome 5 Treatment failure at 90 days.
10.6. Analysis.

Comparison 10 Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: same antibiotic in treatment arms, Outcome 6 Treatment failure at 30 to 45 days.
Comparison 11. Short‐acting antibiotic > 48 hours short‐term treatment, excluding amoxicillin‐clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal adverse effects | 5 | 1088 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.61, 1.85] |
Comparison 12. Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis: treatment failure at 1 month or less and risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis: allocation concealment unclear risk of bias | 13 | 3953 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [1.09, 1.54] |
| 2 Sensitivity analysis: allocation concealment low risk of bias | 3 | 1140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [1.08, 1.93] |
| 3 Sensitivity analysis: blinding high and unclear risk of bias | 12 | 3927 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [1.00, 1.40] |
| 4 Sensitivity analysis: blinding low risk of bias | 4 | 1166 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [1.48, 2.77] |
Comparison 13. Short‐acting antibiotic > 48 hours short‐term treatment; Sensitivity analysis amoxil‐clav. both arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amoxil‐clav. 5 versus 10 days, treatment failure 1 month or less | 2 | 942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [1.44, 2.74] |
| 2 Excluding amoxil‐clav. 5 versus 10 days, treatment failure 1 month or less | 14 | 4151 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [1.02, 1.42] |
Comparison 14. Short‐acting antibiotic > 48 hours short‐term treatment, => 2 yrs old; Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 5 | 656 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.68, 1.47] |
14.1. Analysis.

Comparison 14 Short‐acting antibiotic > 48 hours short‐term treatment, => 2 yrs old; Sensitivity analysis, Outcome 1 Treatment failure at 1 month or less.
Comparison 15. Short‐acting antibiotic > 48 hours short‐term treatment, < 2 yrs old; Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 5 | 548 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.82, 1.73] |
15.1. Analysis.

Comparison 15 Short‐acting antibiotic > 48 hours short‐term treatment, < 2 yrs old; Sensitivity analysis, Outcome 1 Treatment failure at 1 month or less.
Comparison 16. Short‐acting antibiotic, > 48 hours short‐term treatment, adverse GI effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal adverse effects | 13 | 4918 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.60, 0.87] |
Comparison 17. Ceftriaxone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 8 | 1709 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 2 Treatment failure at 3 months or less | 3 | 701 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.66, 1.21] |
| 3 Gastrointestinal adverse effects | 1 | 402 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.89 [1.70, 4.91] |
Comparison 18. Azithromycin single‐dose short‐term treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 25 to 32 days | 2 | 608 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 2 Treatment failure at 25 to 32 days, =< 2 yrs old | 2 | 368 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.43, 1.09] |
| 3 Treatment failure at 25 to 32 days, > 2 yrs old | 2 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.53, 2.20] |
| 4 Gastrointestinal adverse effects | 2 | 658 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.45, 0.96] |
18.1. Analysis.

Comparison 18 Azithromycin single‐dose short‐term treatment, Outcome 1 Treatment failure at 25 to 32 days.
18.2. Analysis.

Comparison 18 Azithromycin single‐dose short‐term treatment, Outcome 2 Treatment failure at 25 to 32 days, =< 2 yrs old.
18.3. Analysis.

Comparison 18 Azithromycin single‐dose short‐term treatment, Outcome 3 Treatment failure at 25 to 32 days, > 2 yrs old.
18.4. Analysis.

Comparison 18 Azithromycin single‐dose short‐term treatment, Outcome 4 Gastrointestinal adverse effects.
Comparison 19. Azithromycin 3 to 5 days short‐term treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 19 | 4354 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 2 Treatment failure at 8 to 19 days | 18 | 4347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [1.04, 1.55] |
| 3 Treatment failure at 20 to 30 days | 11 | 2708 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.82, 1.17] |
Comparison 20. Azithromycin 3 to 5 days short‐term treatment, < 2 years old.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 2 | 138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [0.73, 5.04] |
Comparison 21. Azithromycin 3 to 5 days short‐term treatment, => 2 years old.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 2 | 656 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.61, 2.94] |
Comparison 22. Azithromycin 3‐5 days short‐term treatment; Sensitivity analysis: include chronic OM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 7 | 1688 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 2 Treatment failure at 20 to 30 days | 4 | 740 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.57, 1.21] |
22.1. Analysis.

Comparison 22 Azithromycin 3‐5 days short‐term treatment; Sensitivity analysis: include chronic OM, Outcome 1 Treatment failure at 1 month or less.
22.2. Analysis.

Comparison 22 Azithromycin 3‐5 days short‐term treatment; Sensitivity analysis: include chronic OM, Outcome 2 Treatment failure at 20 to 30 days.
Comparison 23. Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: exclude chronic OM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 6 | 1193 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.89, 1.80] |
| 2 Treatment failure at 20 to 30 days | 3 | 688 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.86, 1.86] |
23.1. Analysis.
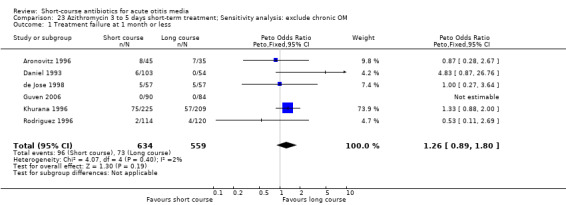
Comparison 23 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: exclude chronic OM, Outcome 1 Treatment failure at 1 month or less.
23.2. Analysis.

Comparison 23 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: exclude chronic OM, Outcome 2 Treatment failure at 20 to 30 days.
Comparison 24. Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: outcome only if "cured".
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 1 month or less | 11 | 2355 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.61, 0.90] |
| 2 Treatment failure at 20 to 30 days | 5 | 902 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
24.1. Analysis.

Comparison 24 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: outcome only if "cured", Outcome 1 Treatment failure at 1 month or less.
24.2. Analysis.

Comparison 24 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: outcome only if "cured", Outcome 2 Treatment failure at 20 to 30 days.
Comparison 25. Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: similar spectrum antibiotic in treatment arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 20 to 30 days | 6 | 1254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Treatment failure at 1 month or less | 9 | 2205 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.89, 1.44] |
25.1. Analysis.

Comparison 25 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: similar spectrum antibiotic in treatment arms, Outcome 1 Treatment failure at 20 to 30 days.
25.2. Analysis.

Comparison 25 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: similar spectrum antibiotic in treatment arms, Outcome 2 Treatment failure at 1 month or less.
Comparison 26. Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis: allocation concealment unclear or high risk of bias | 17 | 4165 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.22] |
| 2 Sensitivity analysis: allocation concealment low risk of bias | 2 | 189 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.02, 0.90] |
| 3 Sensitivity analysis: blinding high and unclear risk of bias | 15 | 3348 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.94, 1.40] |
| 4 Sensitivity analysis: blinding low risk of bias | 4 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.60, 1.07] |
Comparison 27. Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: industry funding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure 1 month or less | 8 | 1747 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 2 Treatment failure at 8 to 19 | 7 | 1667 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [1.10, 1.87] |
| 3 Treatment failure at 20 to 30 days | 6 | 1469 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.74, 1.17] |
27.2. Analysis.

Comparison 27 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: industry funding, Outcome 2 Treatment failure at 8 to 19.
27.3. Analysis.

Comparison 27 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: industry funding, Outcome 3 Treatment failure at 20 to 30 days.
Comparison 28. Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: funding not reported.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure 1 month or less | 11 | 2616 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.86, 1.38] |
| 2 Treatment failure at 8 to 19 days | 11 | 2680 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.80, 1.46] |
| 3 Treatment failure at 20 to 30 days | 5 | 1239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
28.2. Analysis.

Comparison 28 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: funding not reported, Outcome 2 Treatment failure at 8 to 19 days.
28.3. Analysis.

Comparison 28 Azithromycin 3 to 5 days short‐term treatment; Sensitivity analysis: funding not reported, Outcome 3 Treatment failure at 20 to 30 days.
Comparison 29. Azithromycin 3 to 5 days short‐term treatment, adverse GI effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal adverse effects | 14 | 3722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.28, 0.46] |
Comparison 30. Methods ‐ combined control group versus twice counting tx.
30.1. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 1 Twice counting ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure at 1 month or less.
30.2. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 2 Combined control groups ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure 1 month or less.
30.3. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 3 Twice counting ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure at 1 to 19 days.
30.4. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 4 Combined control groups ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure 1 to 19 days.
30.5. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 5 Twice counting ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure 20 to 30 days.
30.6. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 6 Combined control groups ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure at 20 to 30 days.
30.7. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 7 Twice counting ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure at 30 to 40 days.
30.8. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 8 Combined control groups ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure 30 to 40 days.
30.9. Analysis.
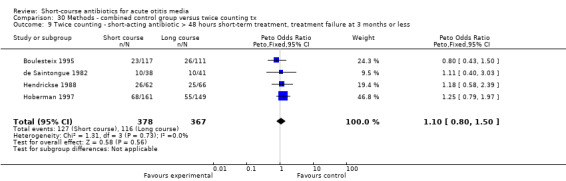
Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 9 Twice counting ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure at 3 months or less.
30.10. Analysis.

Comparison 30 Methods ‐ combined control group versus twice counting tx, Outcome 10 Combined control groups ‐ short‐acting antibiotic > 48 hours short‐term treatment, treatment failure at 3 months or less.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adam 1996.
| Methods | RCT | |
| Participants | German multicentre trial of children 3 months to 6 years old | |
| Interventions | Cefpodoxime 40 mg to 60 mg twice daily for 5 days versus cefaclor 40 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 3 days and no relapse at 3 weeks after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Adam 2000.
| Methods | Double‐blind RCT | |
| Participants | German children aged 2 to 14 years | |
| Interventions | Cefixime 8 mg/kg/day for 5 days versus same treatment for 10 days | |
| Outcomes | Complete recovery or improvement of symptoms by day 11, clinical response rate at day 6, rate of relapse at day 28 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Other bias | Unclear risk | Funding not reported |
Al‐Ghamdi 1999.
| Methods | RCT | |
| Participants | Saudi children aged 6 months to 6 years | |
| Interventions | Single intramuscular dose 50 mg/kg ceftriaxone versus 40 mg/kg/day Augmentin 3 times daily for 10 days | |
| Outcomes | Resolution of symptoms at 10 days after study entry; improved otoscopic and tympanometric measurements at 60 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Did not report baselines or funding |
Arguedas 1996.
| Methods | RCT | |
| Participants | Children 6 months to 12 years old in an ambulatory unit in Costa Rica | |
| Interventions | Azithromycin 10 mg/kg once daily for 3 days versus amoxil‐clavulanate 40 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 11 to 13 days, 28 to 30 days and 55 to 60 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Other bias | Low risk | Industry funding |
Arguedas 1997.
| Methods | RCT | |
| Participants | Children 6 months to 12 years old seen at an ambulatory unit in Costa Rica | |
| Interventions | Azithromyzin 10 mg/kg once daily for 3 days versus clarithromycin 15 mg/kg/day twice daily for 10 days | |
| Outcomes | Resolution of symptoms with or without the presence of middle ear fluid at 10 to 11 days and 28 to 32 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Other bias | Low risk | Industry funding |
Arguedas 2005.
| Methods | Double‐blind, double‐dummy multinational RCT | |
| Participants | Children 6 to 30 months of age in multiple countries | |
| Interventions | Azithromycin single dose 30 mg/kg versus amoxicillin 90 mg/kg/day for 10 days in 2 divided doses | |
| Outcomes | Absence or improvement of symptoms at 12 to 14 days and at end of study (day 25 to 28) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | High risk | |
| Other bias | High risk | Interim analysis, industry funding |
Aronovitz 1996.
| Methods | RCT | |
| Participants | US multicentre trial of children 2 to 15 years old | |
| Interventions | Azithromycin 5 to 10 mg/kg once daily for 5 days versus amoxil‐clavulanate 40 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 11 days and 30 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | No funding declared |
Arrieta 2003.
| Methods | Double‐blind, double‐dummy, multicentre trial | |
| Participants | US and Latin American children aged 6 months to 6 years | |
| Interventions | Azithromycin 20 mg/kg/day once daily for 3 days versus amoxicillin/clavulanate 45/6.4 mg/kg/day twice daily for 10 days plus additional amoxil 45 mg/kg/day twice daily for 10 days | |
| Outcomes | Clinical response (improvement or cure) among clinically evaluated patients at day 28 to 32; clinical response at day 12 to 16 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Corresponding author from Pfizer, unclear independence of authors, industry funding |
Barnett 1997.
| Methods | RCT | |
| Participants | Boston, US multicentre trial of children 3 months to 3 years old | |
| Interventions | Ceftriaxone 50 mg/kg intramuscularly x one dose versus trimethoprim‐sulfamethoxazole twice daily for 10 days | |
| Outcomes | Absence of signs and symptoms at 14 days and 28 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | Personnel not blinded |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Industry funding |
Block 2000.
| Methods | Comparative, randomized, investigator blinded multicentre trial | |
| Participants | US children aged 6 months through 12 years | |
| Interventions | Cefdinir 14 mg/kg/day twice daily for 5 days versus cefprozil 30 mg/kg twice daily for 10 days | |
| Outcomes | Cure or failure at end of treatment visit, cure 11 to 16 days post‐therapy, long‐term follow‐up days 38 to 45 after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | Only investigators blinded |
| Other bias | Low risk | Industry funded |
Block 2003.
| Methods | Double‐blind, placebo‐controlled, RCT | |
| Participants | US children aged 6 months to 12 years | |
| Interventions | Single dose of 30 mg/kg azithromycin versus amoxicillin/clavulanate 45 mg/kg twice daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms on day 12 to 16 and day 28 to 32 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Industry funding |
Block 2004.
| Methods | Randomised, investigator blinded multicentre study | |
| Participants | US children aged 6 months through 6 years | |
| Interventions | Cefdinir 14 mg/kg twice daily for 5 days versus amoxicillin/clavulanate 45/6.4 mg/kg twice daily for 10 days | |
| Outcomes | Cure at end of treatment (7 to 9 days or 12 to 14 days) and days 25 to 28 of the study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | Only investigator blinded |
| Selective reporting (reporting bias) | High risk | |
| Other bias | Low risk | Industry funding |
Boulesteix 1995.
| Methods | RCT | |
| Participants | French multicentre trial of children 6 months to 6 years old | |
| Interventions | Cefpodoxime 4 mg/kg twice daily for 5 days versus cefixime 4 mg/kg twice daily for 8 days | |
| Outcomes | Absence or improvement of signs and symptoms at 8 to 10 days and no recurrence at 30 to 40 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not declared |
Catania 2004.
| Methods | RCT | |
| Participants | Italian children 2 to 6 years | |
| Interventions | Cefaclor 40 mg/kg/day for 5 days versus cefaclor 40 mg/kg/day for 10 days | |
| Outcomes | Clinical improvement or failure at end of therapy and 15 to 20 days after end of therapy. Relapse at 15 to 20 days after end of therapy | |
| Notes | Italian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | No funding declared |
Chamberlain 1994.
| Methods | RCT | |
| Participants | Children 18 months to 6 years old in Washington, DC | |
| Interventions | Ceftriaxone 50 mg/kg intramuscularly x one dose versus cefaclor 40 mg/kg/day for 10 days | |
| Outcomes | Absence or improvement of symptoms, and normal or improved tympanogram at 7 to 10 days and monthly for 90 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Cohen 1997.
| Methods | RCT | |
| Participants | French multicentre trial of children 4 months to 3 years old | |
| Interventions | Cefpodoxime 8 mg/kg/day twice daily for 5 days versus amoxil‐clavulanate 80 mg/kg/day 3 times daily for 8 days | |
| Outcomes | Absence or improvement of signs and symptoms and no additional antibiotics at 9 to 14 days and 20 to 30 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not declared |
Cohen 1998.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | French trial of children aged 4 to 30 months | |
| Interventions | Amoxicillin/clavulanate 80/10 mg/kg/day 3 times daily for 5 days versus same treatment for 10 days | |
| Outcomes | Resolution or improvement of signs and symptoms at 12 to 14 days and 28 to 42 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | High risk | |
| Other bias | Unclear risk | The only significant difference at baseline was diarrhea, but GI symptoms are an outcome; industry funding |
Cohen 1999.
| Methods | Randomised open trial | |
| Participants | French trial of children aged 4 to 30 months | |
| Interventions | Ceftriaxone 50 mg/kg intramuscularly x one dose versus amoxicillin/clavulanate 80/10 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Resolution or improvement of signs and symptoms at 12 to 14 days and 28 to 42 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | Unclear for the 28 to 42‐day follow‐up period |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Cohen 2000.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | French trial of children aged 4 to 30 months | |
| Interventions | Cefpodoxime proxetil 8 mg/kg/day for 5 days versus same regimen for 10 days | |
| Outcomes | Resolution or improvement of signs and symptoms at 12 to 14 days and 28 to 42 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Dagan 2000a.
| Methods | Single‐blind, RCT | |
| Participants | Multicentre trial (Israel, US, Dominican Republic) of children aged 6 to 48 months | |
| Interventions | Azithromycin, 10 mg/kg on day 1 then 5 mg/kg on days 2 to 5 versus amoxicillin/clavulanate 45/6.4 mg/kg in 2 divided doses for 10 days | |
| Outcomes | Resolution of signs and symptoms on day 12 to 14 and day 22 to 28, bacteriologic cure days 4 to 6 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Dagan 2000b.
| Methods | Open label, RCT | |
| Participants | Israeli trial of children aged 3 to 36 months old | |
| Interventions | Azithromycin 10 mg/kg/day for 3 days versus cefaclor 40 mg/kg/day in 3 divided doses for 10 days | |
| Outcomes | Resolution or improvement of signs and symptoms on day 10 and day 17 +/‐ 2 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Daniel 1993.
| Methods | RCT | |
| Participants | European multicentre trial of children 2 to 8 years old | |
| Interventions | Azithromycin 10 mg/kg daily for 3 days versus amoxil‐clavulanate 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 10 to 12 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | Potential bias in that in the short course, azithromycin patients were placed on different antibiotics if failing, whereas for co‐amoxil they stayed on co‐amoxil; industry funding |
de Jose 1998.
| Methods | RCT | |
| Participants | Spanish children 6 months to 12 years old | |
| Interventions | Azithromycin 10 mg/kg/day once daily for 3 days versus amoxicillin/clavulanate 40 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Clinical cure, improvement or failure at end of treatment | |
| Notes | Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | Outcome assessors not blinded |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
de Saintongue 1982.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | British multicentre trial of children 2 to 10 years old | |
| Interventions | Amoxicillin 125/250 mg 3 times daily for 3 days + placebo for 7 days versus amoxicillin 125/250 mg 3 times daily for 10 days | |
| Outcomes | Resolution of signs and symptoms at 13 to 16 days after and recurrence within 12 weeks of study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Dunne 2003.
| Methods | Randomised controlled double‐blind trial | |
| Participants | US trial of children 6 months to 12 years | |
| Interventions | Azithromycin 10 mg/kg/day for 3 days versus co‐amoxiclav 45 mg/kg/day twice a day for 10 days | |
| Outcomes | Absence of symptoms at day 24 to 28, absence or improvement of symptoms at day 10 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Industry funding, first author from Pfizer |
Gooch 1996.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | US multicentre trial of children 3 months to 12 years old | |
| Interventions | Cefuroxime 30 mg/kg/day twice daily for 5 days + placebo twice daily for 5 days versus cefuroxime 30 mg/kg/day twice daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 14 to 18 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Green 1993.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Children 5 months to 5 years old seen in emergency departments or medical centres in California, North Carolina, US | |
| Interventions | Ceftriaxone 50 mg/kg intramuscularly x 1 dose + placebo 3 times daily for 10 days versus placebo intramuscularly x 1 dose + amoxicillin 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence of symptoms at 10 days and no recurrence at 11 to 30 days and 31 to 90 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Guven 2006.
| Methods | RCT | |
| Participants | Turkish children between 6 months and 12 years | |
| Interventions | Azithromycin (10 mg/kg/24 hours, per oral) once daily for 3 days versus amoxicillin‐clavulanate (45 mg/kg/24 hours to 6.4 mg/kg/24 hours, peroral in 2 divided doses) for 10 days | |
| Outcomes | Clinical cure, failure or improvement at the end of treatment and clinical cure, failure, improvement, relapse or re‐infection at 26 to 28 days following study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Unclear risk | Regimen was not equivalent |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Funding not declared |
Hendrickse 1988.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Children 1 month to 12 years old seen at a medical centre in Dallas, US | |
| Interventions | Cefaclor 40 mg/kg/day twice daily for 5 days + placebo for 5 days versus cefaclor 40 mg/kg/day twice daily for 10 days | |
| Outcomes | Resolution of middle ear fluid, or healing of tympanic membrane with resolution of signs and symptoms at 10 days, 30 days, 60 days and 90 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Hoberman 1997.
| Methods | RCT | |
| Participants | US and Canadian multicentre trial of children 2 months to 12 years old | |
| Interventions | Amoxil‐clavulanate (new formulation) twice daily for 5 days versus amoxil‐clavulanate (new formulation) twice daily for 10 days or amoxil‐clavulanate (old formulation) 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms with or without middle ear effusion at 12 to 14 days and 32 to 38 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Unclear risk | Only investigators blinded |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Industry funding and authors from SmithKline Beecham, baseline differences in exposure to cigarette smoke |
Hoberman 2005.
| Methods | Investigator blinded, randomized controlled multicentre trial | |
| Participants | Multicentre trial of children 6 to 30 months of age in various countries | |
| Interventions | Azithromycin 10 mg/kg for 1 day followed by 5 mg/kg/d for 5 days versus amoxicillin/clavulanate 90/6.4 mg/kg/d in 2 divided doses for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at day 4 to 6, 12 to 14 and 21 to 25 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Unclear risk | Investigator blinded |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Industry funding |
Ingvarsson 1982.
| Methods | RCT | |
| Participants | Swedish children 6 months to 7 years old | |
| Interventions | Penicillin‐V 25 mg/kg twice daily for 5 days versus penicillin‐V 25 mg/kg twice daily for 10 days | |
| Outcomes | Improved signs and symptoms at 10 to 12 days and normal eardrum at 28 to 30 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | Not mentioned and no placebo |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Kafetzis 1997.
| Methods | RCT | |
| Participants | Greek multicentre trial of children 2 to 172 months old | |
| Interventions | Cefprozil 30 mg/kg/day twice daily for 5 days versus cefprozil 30 mg/kg/day twice daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 7 to 14 days and 28 to 32 days after study entry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Kara 1998.
| Methods | RCT | |
| Participants | Turkish children aged 6 months to 6 years | |
| Interventions | Ceftriaxone 50 mg/kg single intramuscular dose versus cefuroxime axetil 30 mg/kg 3 times daily for 10 days versus amoxicillin 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Clinical cure rate at 10 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | Funding not reported, baseline characteristics not reported |
Khurana 1996.
| Methods | RCT | |
| Participants | US multicentre trial of children 6 months to 12 years old | |
| Interventions | Azithromycin 5 to 10 mg/kg once daily for 5 days versus amoxil‐clavulanate 40 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 45 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
McLinn 1996.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | US multicentre trial of children 1 to 15 years old | |
| Interventions | Azithromycin 5 to 10 mg/kg once daily/placebo twice daily for 5 days + placebo 3 times daily for 5 days versus amoxil‐clavulanate 40 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 11 days and 30 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Meistrup‐Larsen 1983.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Children 1 to 10 years old in Copenhagen | |
| Interventions | Penicillin‐V 55 mg/kg twice daily for 2 days + placebo for 5 days versus penicillin‐V 55 mg/kg twice daily for 7 days | |
| Outcomes | Absence of symptoms and no otorrhoea or contralateral otitis at 6 to 7 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported, doses prepared in the home |
Mohs 1993.
| Methods | Comparative, open trial (randomisation not mentioned) | |
| Participants | Multicentre trial (South America and Egypt) of children 2 to 12 years old | |
| Interventions | Azithromycin 10 mg/kg once daily for 3 days versus amoxicillin 10 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 11 to 13 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Oguz 2003.
| Methods | Randomised, single‐blind, open study | |
| Participants | Turkish children aged 6 months to 12 years | |
| Interventions | Azithromycin 10 mg/kg/day for 3 days versus cefaclor 40 mg/kg/day in 3 divided doses for 10 days | |
| Outcomes | Resolution of all clinical and otoscopic findings at 3 to 5, 10 and 30 days after study entry; relapse at 10 and 30 days | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Pessey 1999.
| Methods | Randomised, open, multinational study | |
| Participants | Children aged 6 to 36 months | |
| Interventions | Cefuroxime axetil 30 mg/kg/day twice daily for 5 days versus amoxicillin/clavulanate 40 mg/kg/day 3 times daily for 10 days versus amoxicillin/clavulanate 80 mg/kg/day 3 times daily for 8 days | |
| Outcomes | Absence of symptoms 1 to 4 days after therapy completion; no relapse 21 to 28 days after therapy completion | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | Open study |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Corresponding author from Glaxo, funding not reported |
Petalozza 1992.
| Methods | RCT | |
| Participants | Italian children 11 months to 9 years old | |
| Interventions | Azithromycin 10 mg/kg once daily for 3 days versus amoxil‐clavulanate 50 mg/kg/day twice daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 12 to 14 days and 30 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Openly randomized |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Ploussard 1984.
| Methods | RCT | |
| Participants | Children 5 months to 5 years old in Alabama, US | |
| Interventions | Cefaclor 40 mg/kg 3 times daily for 5 days versus amoxicillin 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 10 to 16 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Principi 1995.
| Methods | RCT | |
| Participants | Multicentre trial (Europe, South America) of children 6 months to 12 years old | |
| Interventions | Azithromycin 10 mg/kg once daily for 3 days versus amoxil‐clavulanate 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 10 to 14 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Puczynski 1987.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Children > 2 years old enrolled May 1984 to February 1985 in Illinois, US | |
| Interventions | Amoxicillin 100 mg x 1 dose followed by placebo 3 times daily for 10 days versus placebo x 1 dose followed by amoxicillin 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence of fever and otalgia, decreased irritability and improved appearance of tympanic membrane at 48 to 72 hours and 10 to 14 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | Low risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | Stopped early with small sample size due to treatment failures in experimental group, funding not reported |
Rodriguez 1996.
| Methods | RCT | |
| Participants | Multicentre trial of children 6 months to 12 years old in Guatemala | |
| Interventions | Azithromycin 10 mg/kg once daily for 3 days versus cefaclor 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 10 to 14 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Schaad 1993.
| Methods | RCT | |
| Participants | Swiss multicentre trial of children 2 to 12 years old | |
| Interventions | Azithromycin 10 mg/kg once daily for 3 days versus amoxil‐clavulanate 40 mg/kg 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at 12 to 16 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
Varsano 1988.
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Children 6 months to 8 years old seen at a medical clinic in Israel | |
| Interventions | Ceftriaxone 50 mg/kg intramuscularly x 1 dose + placebo 3 times daily for 7 days versus placebo intramuscularly x 1 dose + amoxicillin 12.5 mg/kg 3 times daily for 7 days | |
| Outcomes | Absence of signs and symptoms with or without middle ear effusion at 7 to 10 days and no recurrence at 30 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | Industry funded |
Varsano 1997.
| Methods | RCT | |
| Participants | Israeli children aged 4 months to 6 years | |
| Interventions | Ceftriaxone 50 mg/kg single intramuscular dose versus 37.5/9.4 mg/kg/day amoxicillin/clavulanate for 10 days | |
| Outcomes | Resolution of symptoms and no recurrence at 11 days, no relapse at 30, 60 and 90 days after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | High risk | Separate analysis performed for simple and recurrent AOM. Findings only reported in cases where differences reached statistical significance |
| Other bias | Unclear risk | Industry funded |
Wang 2004.
| Methods | Prospective comparative randomized trial | |
| Participants | Chinese children between 3 months and 6 years of age | |
| Interventions | Ceftriaxone 50 mg/kg single intramuscular dose versus amoxicillin/clavulanate 45 mg/kg/day 3 times daily for 10 days | |
| Outcomes | Absence or improvement of signs and symptoms at day 11 and day 28 after study entry | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) Treatment failure at 1 month or less | High risk | |
| Incomplete outcome data (attrition bias) Treatment failure at 1 month or less | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funding not reported |
d: day; GI: gastrointestinal; RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bain 1985 | Outcomes not reported as treatment failures |
| Gehanno 1990 | Duration of antibiotic in both treatment arms was less than 7 days |
| Harrison 1993 | Duration of antibiotic therapy equivalent in both arms |
| Jacobson 1979 | Duration of antibiotic therapy equivalent in both arms |
| Jenner 1987 | Duration of antibiotic in both treatment arms was less than 7 days |
| Johnson 1991 | Duration of antibiotic therapy equivalent in both arms |
| Jones 1986 | Outcomes not reported as treatment failures |
| MacLouglin 1996 | Duration of antibiotic therapy equivalent in both arms |
| Mandel 1995 | Duration of antibiotic in both treatment arms 7 days or greater |
| Murph 1993 | Duration of antibiotic therapy equivalent in both arms |
| O'Doherty 1996 | Trial participants predominantly adults |
| Roos 2000 | Antibiotic ceftibuten was deemed to be not clinically relevant |
| Rubenstein 1965 | Antibiotic therapy no longer current |
| Scott 1990 | No comparison of different durations of antibiotic |
| Simon 1997 | Antibiotic ceftibuten was deemed to be not clinically relevant |
| Spencer 1993 | Duration of antibiotic therapy equivalent in both arms |
| Stickler 1964 | Antibiotic therapy no longer current |
| Stickler 1967 | Antibiotic therapy no longer current |
| Suzuki 2009 | Duration of antibiotic therapy equivalent in both arms |
| Varsano 1994 | Insufficient data reported in abstract |
Contributions of authors
Anita Kozyrskyj (AK), Terry Klassen (TK) and Michael Moffatt (MM) first conceived the review, presenting it as a meta‐analysis in a journal (Kozyrskyj 1998). It was updated for The Cochrane Library with assistance from Krystal Harvey (KH) and Liza Bialy (LB).
Sources of support
Internal sources
Alberta Research Centre for Health Evidence, Canada.
External sources
No sources of support supplied
Declarations of interest
None.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Adam 1996 {published data only}
- Adam D. Five‐day therapy with cefpodoxime vs. ten‐day treatment with cefaclor in infants with acute otitis media. Infection 1995;23:398‐9. [Google Scholar]
Adam 2000 {published data only}
- Adam D. Efficacy and tolerability of 5‐day vs. 10‐day treatment with cefixime suspension in children with acute otitis media. Drugs of Today 2000;36 (Suppl E):29‐33. [Google Scholar]
Al‐Ghamdi 1999 {published data only}
- Al‐Ghamdi YS, El‐Kafrawi M, Aglan YI, Saleh MA. Efficacy of single‐dose intramuscular ceftriaxone for treatment of acute otitis media in children. Saudi Medical Journal 1999;20(1):41‐5. [PubMed] [Google Scholar]
Arguedas 1996 {published data only}
- Arguedas A, Loaiza C, Herrara M, Mohs E. Comparative trial of 3‐day azithromycin vs. 10‐day amoxycillin/clavulante potassium in the treatment of children with acute otitis media with effusion. International Journal of Antimicrobial Agents 1996;6:233‐8. [DOI] [PubMed] [Google Scholar]
Arguedas 1997 {published data only}
- Arguedas A, Loaiza C, Rodriguez F, Herrera ML, Mohs E. Comparative trial of 3 days of azithromycin versus 10 days of clarithromycin in the treatment of children with acute otitis media with effusion. Journal of Chemotherapy 1997;9:44‐50. [DOI] [PubMed] [Google Scholar]
Arguedas 2005 {published data only}
- Arguedas A, Emparanza P, Schwartz RH, Soley C, Guevara S, Caprariis PJ, et al. A randomized, multicenter, double blind, double dummy trial of single dose azithromycin versus high dose amoxicillin for treatment of uncomplicated acute otitis media. Pediatric Infectious Disease Journal 2005;24(2):153‐61. [DOI] [PubMed] [Google Scholar]
Aronovitz 1996 {published data only}
- Aronovitz G. A multicenter, open label trial of azithromycin vs. amoxicillin/clavulanate for the management of acute otitis media in children. Pediatric Infectious Disease Journal 1996;Suppl 15:5‐9. [DOI] [PubMed] [Google Scholar]
Arrieta 2003 {published data only}
- Arrieta A, Arguedas A, Fernandez P, Block SL, Emperanza P, Vargas SL, et al. High‐dose azithromycin versus high‐dose amoxicillin‐clavulanate for treatment of children with recurrent or persistent acute otitis media. Antimicrobial Agents & Chemotherapy 2003;47(10):3179‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnett 1997 {published data only}
- Barnett ED, Teele DW, Klein JO, Cabral HJ, Kharasch SJ. Comparison of ceftriaxone and trimethoprim‐sulfamethoxazole for acute otitis media. Pediatrics 1997;99:23‐8. [DOI] [PubMed] [Google Scholar]
Block 2000 {published data only}
- Block SL, Kratzer J, Nemeth MA, Tack KJ. Five‐day cefdinir course vs. ten‐day cefprozil course for treatment of acute otitis media. Pediatric Infectious Disease Journal 2000;19(Suppl 12):147‐52. [DOI] [PubMed] [Google Scholar]
Block 2003 {published data only}
- Block SL, Arrieta A, Seibel M, McLinn S, Eppes S, Murphy MJ. Single‐dose (30 mg/kg) azithromycin compared with 10‐day amoxicillin/clavulanate (45 mg/kg per day) for the treatment of uncomplicated acute otitis media. Current Therapeutic Research, Clinical & Experimental 2003;64(Suppl A):30‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Block 2004 {published data only}
- Block SL, Busman TA, Paris MM, Bukofzer S. Comparison of five‐day cefdinir treatment with ten‐day low dose amoxicillin/clavulanate treatment for acute otitis media. Pediatric Infectious Disease Journal 2004;23(9):834‐8. [DOI] [PubMed] [Google Scholar]
Boulesteix 1995 {published data only}
- Boulesteix J, Dubreuil C, Moutot M, Rezvani Y, Rosembaum. Cefpodoxime proxetil five days versus cefixime eight days in the treatment of acute otitis media in children [Cefpodoxime proxetil 5 jours versus cefixime 8 jours, dans le traitement des otites moyennes aigues de l'enfant]. Médecine et Maladies Infectieuses 1995;25:534‐9. [Google Scholar]
Catania 2004 {published data only}
- Catania S, Gallo A. Clinical efficacy and tolerability of short course therapy with cefaclor compared with long‐term therapy for treatment of acute otitis media in children [Il ceflacor nella terapia dell'otite media acuta in eta pediatrica: confronto dell'efficacia e della tollerabilita di un trattamento breve rispetto a quello convenzionale]. Le Infezioni in Medicina 2004;4:259‐65. [PubMed] [Google Scholar]
Chamberlain 1994 {published data only}
- Chamberlain JM, Boenning DA, Waisman Y, Ochsenschlager DW, Klein BL. Single‐dose ceftriaxone vs. 10 days of cefaclor for otitis media. Clinical Pediatrics 1994;33:642‐6. [DOI] [PubMed] [Google Scholar]
Cohen 1997 {published data only}
- Cohen R, Rocque F, Boucherat M, Levy C, Langue J, Bourrillon A. Randomized trial comparing 5‐day cefpodoxime proxetil and 8‐day amoxicillin‐clavulanate treatment of acute otitis media in children [Etude randomisee cefpodoxime proxetil 5 jours versus amoxicilline‐acide calvulanique 8 jours dans le traitment de l'otite moyenne aigue de l'enfant]. Médecine et Maladies Infectieuses 1997;27:596‐602. [Google Scholar]
Cohen 1998 {published data only}
- Cohen R, Levy C, Boucherat M, Langue J, Rocque F. A multicenter, randomized, double‐blind trial of 5 versus 10 days of antibiotic therapy for acute otitis media in young children. Journal of Pediatrics 1998;133(5):634‐9. [DOI] [PubMed] [Google Scholar]
Cohen 1999 {published data only}
- Cohen R, Navel M, Grunberg J, Boucherat M, Geslin P, Derriennic M, et al. One dose ceftriaxone vs. ten days of amoxicillin/clavulanate therapy for acute otitis media: Clinical efficacy and change in nasopharyngeal flora. Pediatric Infectious Disease Journal 1999;18(5):403‐9. [DOI] [PubMed] [Google Scholar]
Cohen 2000 {published data only}
- Cohen R, Levy C, Boucherat M, Langue J, Autret E, Gehanno P, et al. Five vs. ten days of antibiotic therapy for acute otitis media in young children. Pediatric Infectious Disease Journal 2000;19(5):458‐63. [DOI] [PubMed] [Google Scholar]
Dagan 2000a {published data only}
- Dagan R, Johnson CE, McLinn S, Abughali N, Feris J, Beibovitz E, et al. Bacteriologic and clinical efficacy of amoxicillin/clavulanate vs. azithromycin in acute otitis media. Pediatric Infectious Disease Journal 2000;19(2):95‐104. [DOI] [PubMed] [Google Scholar]
Dagan 2000b {published data only}
- Dagan R, Leibovitz E, Fliss DM, Leiberman A, Jacobs MR, Craig W, et al. Bacteriologic efficacies of oral azithromycin and oral cefaclor in treatment of acute otitis media in infants and young children. Antimicrobial Agents & Chemotherapy 2000;44(1):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniel 1993 {published data only}
- Daniel RR. Comparison of azithromycin and co‐amoxiclav in the treatment of otitis media in children. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):65‐71. [DOI] [PubMed] [Google Scholar]
de Jose 1998 {published data only}
- Jose MI, Garcia de Miguel MJ, Aristegui J, Rodrigo C, Castillo F, Borque C, et al. International multicenter study comparing azithromycin and amoxicillin‐clavulanic acid in the treatment of pediatric acute otitis media [Estudio sobre la eficacia, seguridad y tolerancia de azitromicina frente a amoxicilina‐clavulanico en el tratamiento de otitis media aguda]. Acta Pediatrica Espanola 1998;56(6):350‐5. [Google Scholar]
de Saintongue 1982 {published data only}
- Chaput de Saintongue DM, Levine DF, Temple Savage I, Burgess GWS, Sharp J, Mayhew SR, et al. Trial of three‐day and ten‐day courses of amoxycillin in otitis media. British Medical Journal 1982;284:1078‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunne 2003 {published data only}
- Dunne MW, Latiolais T, Lewis B, Pistorius B, Bottenfield G, Moore WH, et al. Randomized, double‐blind study of the clinical efficacy of 3 days of azithromycin compared with co‐amoxiclav for the treatment of acute otitis media. Journal of Antimicrobial Chemotherapy 2003;52(3):469‐72. [DOI] [PubMed] [Google Scholar]
Gooch 1996 {published data only}
- Gooch WM, Blair E, Puopolo A, Paster ZR, Schwartz RH, Miller HC, et al. Effectiveness of five days of therapy with cefuroxime axetil suspension for treatment of acute otitis media. Pediatric Infectious Disease Journal 1996;15:157‐64. [DOI] [PubMed] [Google Scholar]
Green 1993 {published data only}
- Green SM, Rothrock SG. Single‐dose intramuscular ceftriaxone for acute otitis media in children. Pediatrics 1993;91:23‐30. [PubMed] [Google Scholar]
Guven 2006 {published data only}
- Guven M, Bulut Y, Sezer T, Aladag I, Eyibilen A, Etikan I. Bacterial etiology of acute otitis media and clinical efficacy of amoxicillin‐clavulanate vs. azithromycin. International Journal of Pediatric Otorhinolaryngology 2006;70(5):915‐23. [DOI] [PubMed] [Google Scholar]
Hendrickse 1988 {published data only}
- Hendrickse WA, Kusmiesz H, Shelton S, Nelson JD. Five vs. ten days of therapy for acute otitis media. Pediatric Infectious Disease Journal 1988;7:14‐23. [DOI] [PubMed] [Google Scholar]
Hoberman 1997 {published data only}
- Hoberman A, Paradise JL, Burch DJ, Valinski WA, Hedrick JA, Aronovits GH, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin) for treatment of acute otitis media in children. Pediatric Infectious Disease Journal 1997;16(5):463‐70. [DOI] [PubMed] [Google Scholar]
Hoberman 2005 {published data only}
- Hoberman A, Dagan R, Leibovitz E, Rosenblut A, Johnson CE, Huff A, et al. Large dosage amoxicillin/clavulanate, compared with azithromycin, for the treatment of bacterial acute otitis media in children. Pediatric Infectious Disease Journal 2005;24(6):525‐32. [DOI] [PubMed] [Google Scholar]
Ingvarsson 1982 {published data only}
- Ingvarsson L, Lundgren K. Penicillin treatment of acute otitis media in children. Acta Oto‐Laryngologica 1982;94:283‐7. [DOI] [PubMed] [Google Scholar]
Kafetzis 1997 {published data only}
- Kafetzis DA, Astra H, Mitropoulos L. Five‐day versus ten‐day treatment of acute otitis media with cefprozil. European Journal of Clinical Microbiology & Infectious Diseases 1997;16:283‐6. [DOI] [PubMed] [Google Scholar]
Kara 1998 {published data only}
- Kara CO, Ozuer MZ, Kilic I, Yalcin AN, Ergin H. Comparison of amoxicillin with second and third generation cephalosporins in the treatment of acute otitis media. Infezioni in Medicina 1998;6(2):93‐5. [PubMed] [Google Scholar]
Khurana 1996 {published data only}
- Khurana CM. A multicenter, randomized, open label comparison of azithromycin and amoxicillin/clavulanate in acute otitis media among children attending day care or school. Pediatric Infectious Disease Journal 1996;15(Suppl 9):24‐9. [DOI] [PubMed] [Google Scholar]
McLinn 1996 {published data only}
- McLinn S. A multicenter, double blind comparison of azithromycin and amoxicillin/clavulanate for the treatment of acute otitis media in children. Pediatric Infectious Disease Journal 1996;15(Suppl 9):20‐3. [DOI] [PubMed] [Google Scholar]
Meistrup‐Larsen 1983 {published data only}
- Meistrup‐Larsen KI, Sorensen H, Johnsen NJ, Thomsen J, Mygind N, Sederberg‐Olsen J. Two vs. seven days penicillin treatment for acute otitis media. Acta Oto‐Laryngologica 1983;96:99‐104. [DOI] [PubMed] [Google Scholar]
Mohs 1993 {published data only}
- Mohs E, Rodriguez‐Solares A, Rivas E, Hoshy ZE. A comparative study of azithromycin and amoxycillin in paediatric patients with acute otitis media. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):73‐9. [DOI] [PubMed] [Google Scholar]
Oguz 2003 {published data only}
- Oguz F, Unuvar E, Suoglu Y, Erdamar B, Dundar G, Katircioglu S, et al. Etiology of acute otitis media in childhood and evaluation of two different protocols of antibiotic therapy: 10 days cefaclor vs. 3 days azithromycin. International Journal of Pediatric Otorhinolaryngology 2003;67(1):43‐51. [DOI] [PubMed] [Google Scholar]
Pessey 1999 {published data only}
- Pessey JJ, Gehanno P, Thoroddsen E, Dagan R, Leibovitz E, Machac J, et al. Short course therapy with cefuroxime axetil for acute otitis media: results of a randomized multicenter comparison with amoxicillin/clavulanate. Pediatric Infectious Disease Journal 1999;18(10):854‐9. [DOI] [PubMed] [Google Scholar]
Petalozza 1992 {published data only}
- Petalozza G, Cioce C, Facchini M. Azithromycin in upper respiratory tract infections: a clinical trial in children with otitis media. Scandinavian Journal of Infectious Diseases 1992;83:22‐5. [PubMed] [Google Scholar]
Ploussard 1984 {published data only}
- Ploussard JH. Evaluation of five days of cefaclor vs ten days of amoxicillin therapy in acute otitis media. Current Therapeutic Research 1984;36:641‐5. [Google Scholar]
Principi 1995 {published data only}
- Principi N. Multicentre comparative study of the efficacy and safety of azithromycin compared with amoxicillin/clavulanic acid in the treatment of paediatric patients with otitis media. European Journal of Clinical Microbiology & Infectious Diseases 1995;14:669‐7. [DOI] [PubMed] [Google Scholar]
Puczynski 1987 {published data only}
- Puczynski MS, Stankiewicz JA, O'Keefe JP. Single dose amoxicillin treatment of acute otitis media. Laryngoscope 1987;97:16‐8. [DOI] [PubMed] [Google Scholar]
Rodriguez 1996 {published data only}
- Rodriguez AF. An open study to compare azithromycin with cefaclor in the treatment of children with acute otitis media. Journal of Antimicrobial Chemotherapy 1996;37(Suppl C):63‐9. [DOI] [PubMed] [Google Scholar]
Schaad 1993 {published data only}
- Schaad UB. Multicentre evaluation of azithromycin in comparison with co‐amoxiclav for the treatment of acute otitis media in children. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):81‐8. [DOI] [PubMed] [Google Scholar]
Varsano 1988 {published data only}
- Varsano I, Frydman M, Amir J, Alpert G. Single intramuscular dose of ceftriaxone as compared to 7‐day amoxicillin therapy for acute otitis media in children. Chemotherapy 1988;34(Suppl 1):39‐46. [DOI] [PubMed] [Google Scholar]
Varsano 1997 {published data only}
- Varsano I, Volovitz B, Horev Z, Robinson J, Laks Y, Rosenbaum I, et al. Intramuscular ceftriaxone compared with oral amoxicillin‐clavulanate for treatment of acute otitis media in children. European Journal of Pediatrics 1997;156(11):858‐63. [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang CY, Lu CY, Hsieh YC, Lee CY, Huang LM. Intramuscular ceftriaxone in comparison with oral amoxicillin‐clavulanate for the treatment of acute otitis media in infants and children. Journal of Microbiology, Immunology & Infection 2004;37(1):57‐62. [PubMed] [Google Scholar]
References to studies excluded from this review
Bain 1985 {published data only}
- Bain J, Murphy E, Ross F. Acute otitis media: clinical course among children who received a short course of high dose antibiotic. British Medical Journal (Clinical Research Edition) 1985;291:1243‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehanno 1990 {published data only}
- Gehanno P, Taillebe M, Denis P, Jacquet P, Hoareau J, Gojon D, et al. Short‐course cefotaxime compared with five‐day co‐amoxyclav in acute otitis media in children. Journal of Antimicrobial Chemotherapy 1990;26(Suppl A):29‐36. [DOI] [PubMed] [Google Scholar]
Harrison 1993 {published data only}
- Harrison CJ, Chartrand SA, Pichichero ME. Microbiologic and clinical aspects of a trial of once daily cefixime compared with twice daily cefaclor for treatment of acute otitis media in infants and children. Pediatric Infectious Disease Journal 1993;12:62‐9. [DOI] [PubMed] [Google Scholar]
Jacobson 1979 {published data only}
- Jacobson JA, Metcalf TJ, Parkin JL, Wenerstrom LG, Matsen JM. Evaluation of cefaclor and amoxycillin in the treatment of acute otitis media. Postgraduate Medical Journal 1979;55(Suppl 4):39‐41. [PubMed] [Google Scholar]
Jenner 1987 {published data only}
- Jenner PN, Tierney HE, Carle WK. A general practice study of a new approach to the use of antibiotics in the treatment of acute otitis media in children aged three and over. British Journal of Clinical Practice 1987;41:820‐6. [PubMed] [Google Scholar]
Johnson 1991 {published data only}
- Johnson CE, Carlin SA, Super DM, Rehmus JM, Roberts DG, Christopher NC, et al. Cefixime compared with amoxicillin for treatment of acute otitis media. Journal of Pediatrics 1991;119:117‐22. [DOI] [PubMed] [Google Scholar]
Jones 1986 {published data only}
- Jones R, Bain J. Three‐day and seven‐day treatment in acute otitis media: a double‐blind antibiotic trial. Journal of the Royal College of General Practitioners 1986;36:356‐8. [PMC free article] [PubMed] [Google Scholar]
MacLouglin 1996 {published data only}
- MacLoughlin GJF, Barreto DG, Torre C, Pinetta EA, Castilllo F, Palma L. Cefpodoxime proxetil suspension compared with cefaclor suspension for treatment of acute otitis media in paediatric patients. Journal of Antimicrobial Chemotherapy 1996;37:565‐73. [DOI] [PubMed] [Google Scholar]
Mandel 1995 {published data only}
- Mandel EM, Casselbrant ML, Rockette HE, Bluestone CD, Kurs‐Lasky M. Efficacy of 20‐ vs. 10‐day antimicrobial treatment for acute otitis media. Pediatrics 1995;96:5‐13. [PubMed] [Google Scholar]
Murph 1993 {published data only}
- Murph JR, Dusdieker LB, Booth B, Murph WE. Is treatment of acute otitis media with once‐a‐day amoxicillin feasible?. International Pediatrics 1993;32(9):528‐34. [DOI] [PubMed] [Google Scholar]
O'Doherty 1996 {published data only}
- O'Doherty B. An open comparative study of azithromycin versus cefaclor in the treatment of patients with upper respiratory tract infections. Journal of Antimicrobial Chemotherapy 1996;37(Suppl C):71‐81. [DOI] [PubMed] [Google Scholar]
Roos 2000 {published data only}
- Roos K, Larsson P. Efficacy of ceftibuten in 5 vs 10 days treatment of recurrent acute otitis media in children. International Journal of Pediatric Otorhinolaryngology 2000;55:109‐15. [DOI] [PubMed] [Google Scholar]
Rubenstein 1965 {published data only}
- Rubenstein MM, McBean JB, Hedgecock LD, Stickler GB. The treatment of acute otitis media in children. 3. A third clinical trial. American Journal of Diseases of Children 1965;109:308‐13. [DOI] [PubMed] [Google Scholar]
Scott 1990 {published data only}
- Scott HV, Pannowitz D, Ketelbey JW. Cefixime:clinical trial against otitis media and tonsillitis. New Zealand Medical Journal 1990;103:25‐6. [PubMed] [Google Scholar]
Simon 1997 {published data only}
- Simon MW. Five‐ vs. 10‐day treatment of acute otitis media with ceftibuten in infants and children. Advances in Therapy 1997;14(6):312‐7. [Google Scholar]
Spencer 1993 {published data only}
- Spencer RC, Hannington J, Fraser S, Mason T. Cefpodoxime proxetil versus co‐amoxiclav in the treatment of acute infections of the ear, nose and throat in children‐a multicentre randomized study. Antibiotic Abstract 851. 18th International Congress of Chemotherapy (June 27 ‐ July 2). Stockholm, Sweden, 1993:264.
Stickler 1964 {published data only}
- Stickler GB, McBean JB. The treatment of acute otitis media in children.II. A second clinical trial. JAMA 1964;187:85‐9. [DOI] [PubMed] [Google Scholar]
Stickler 1967 {published data only}
- Stickler GB, Rubenstein MM, McBean JB, Hedgecock LD, Hugstad A, Griffing T. Treatment of acute otitis media in children. IV. A fourth clinical trial. American Journal of Diseases of Children 1967;114:123‐30. [DOI] [PubMed] [Google Scholar]
Suzuki 2009 {published data only}
- Suzuki K, Baba S, Totsuka K, Hori S, Ubukata K, Nakashima M, et al. Double‐blind comparative study of tebipenem pivoxil and high‐dose cefditoren pivoxil in children with acute otitis media. Japanese Journal of Chemotherapy 2009;57(Suppl 1):167‐85. [Google Scholar]
Varsano 1994 {published and unpublished data}
- Varsano I, Volovitz B, Horev Z, Robinson J, Laks Y, Rosenbaum I, et al. Single IM dose of ceftriaxone (CRO) compared to 10 days amoxicillin‐clavulanate augmentin (AUG) for therapy of acute otitis media (AOM) in children. Sixth International Congress for Infectious Disease. Czech Republic, 1994; Vol. Session 117:1059.
Additional references
Appelman 1991
- Appelman CLM, Claessen QPL, Touw‐Otten FWMM, Hordijk GJ, Melker RE. Co‐amoxiclav in recurrent acute otitis media;placebo controlled study. BMJ 1991;303:1450‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses. Lancet 1997;350:185‐6. [DOI] [PubMed] [Google Scholar]
Berman 1995
- Berman S. Otitis media in developing countries. Pediatrics 1995;96:126‐31. [PubMed] [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen T, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatrics and Adolescent Medicine 2008;162(2):111‐16. [DOI] [PubMed] [Google Scholar]
Borzak 1995
- Borzak S, Ridker PM. Discordance between meta‐analyses and large‐scale randomized, controlled trials. Annals of Internal Medicine 1995;123:873‐7. [DOI] [PubMed] [Google Scholar]
Burke 1991
- Burke P, Bain J, Robinson D, Dunleavey J. Acute red ear in children: controlled trial of non‐antibiotic treatment in general practice. BMJ 1991;303:558‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canafax 1991
- Canafax DM, Giebink GS. Clinical and pharmacokinetic basis for the antimicrobial treatment of acute otitis media. Otolaryngologic Clinics of North America 1991;24:859‐74. [PubMed] [Google Scholar]
Cohen 1992
- Cohen ML. Epidemiology of drug resistance: implications for a post‐antimicrobial era. Science 1992;257:1050‐5. [DOI] [PubMed] [Google Scholar]
Dagan 2006
- Dagan R, Barkai G, Leibovitz E, Dreifuss E, Greenberg D. Will reduction of antibiotic use reduce antibiotic resistance? The pneumococcus paradigm. Pediatric Infectious Disease Journal 2006;25(10):981‐6. [DOI] [PubMed] [Google Scholar]
Del Mar 1997
- Mar C, Glasziou P, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta‐analysis. BMJ 1997;314:1526‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. A nonparametric trim and fill method of accounting for publication bias in meta‐analysis. JAMA 2000;95(449):89‐98. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eppesl 1997
- Eppes SC, Klein JD, Lewis LL. Ceftriaxone for acute otitis media. Pediatrics 1997;100:157. [PubMed] [Google Scholar]
Forgie 2009
- Forgie S, Zhanel G, Robinson J, Canadian Paediatric Society, Infectious Disease and Immunization Committee. Management of acute otitis media. Paediatric Child Health (Available at http://www.cps.ca/ENGLISH/statements/ID/ID09‐01.htm) 2009; Vol. 14, issue 7:457‐60. [PMC free article] [PubMed]
Froom 1990
- Froom J, Culpepper L, Grob P, Bartelds A, Bowers P, Bridges‐Webb C, et al. Diagnosis and antibiotic treatment of acute otitis media: report from International Primary Care Network. BMJ 1990;300(6724):582‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregoire 1995
- Gregoire G, Derderian F, Lorier J. Selecting the language of the publications included in a meta‐analysis: is there a Tower of Babel bias?. Journal of Clinical Epidemiology 1995;48:159‐63. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Howie 1969
- Howie VM, Ploussard JH. The "in vitro sensitivity test" bacteriology of middle ear exudate. Pediatrics 1969;44:940‐4. [PubMed] [Google Scholar]
Kaleida 1991
- Kaleida PH, Casselbrant ML, Rockette HE, Paradise JL, Bluestone CD, Blatter MM, et al. Amoxicillin or myringotomy or both for acute otitis media: results of a randomized clinical trial. Pediatrics 1991;87:466‐74. [PubMed] [Google Scholar]
Koopman 2008
- Koopman L, Hoes AW, Glasziou PP, Appelman CL, Burke P, McCormick DP, et al. Antibiotic therapy to prevent the development of asymptomatic middle ear effusion in children with acute otitis media: a meta‐analysis of individual patient data. Archives of Otolaryngology ‐ Head & Neck Surgery 2008;134(2):128‐32. [DOI] [PubMed] [Google Scholar]
Kozyrskyj 1998
- Kozyrskyj AL, Hildes‐Ripstein GE, Longstaffe SEA, Wincott JL, Sitar DS, Klassen TP, et al. Treatment of acute otitis media with a shortened course of antibiotics. JAMA 1998;279(21):1736‐42. [DOI] [PubMed] [Google Scholar]
Laupacis 1988
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1998;318:1728‐33. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world‐wide ‐ the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerging Themes in Epidemiology 2008;5:13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1994
- Murray BE. Can antibiotic resistance be controlled?. New England Journal of Medicine 1994;330(17):1229‐30. [DOI] [PubMed] [Google Scholar]
Mygind 1981
- Mygind N, Meistrup‐Larsen KI, Thomsen J, Thomsem VF, Josefsson K, Sorensen H. Penicillin in acute otitis media: a double‐blind placebo‐controlled trial. Clinical Otolaryngology 1981;6:5‐13. [DOI] [PubMed] [Google Scholar]
Paradise 1995
- Paradise JL. Managing otitis media: a time for change. Pediatrics 1995;96:712‐5. [PubMed] [Google Scholar]
Paradise 1997
- Paradise JL. Short‐course antimicrobial treatment for acute otitis media. Not best for infants and young children. JAMA 1997;278:1640‐2. [PubMed] [Google Scholar]
Pediatric Guidelines 2004
- Subcommittee on Management of Acute Otitis Media, American Academy of Pediatrics, American Academy of Family Physicians. Clinical practice guidelines: diagnosis and management of acute otitis media. Pediatrics 2004;113(5):1451‐65. [DOI] [PubMed] [Google Scholar]
Pichichero 1997
- Pichichero ME, Cohen R. Shortened course of antibiotic therapy for acute otitis media, sinusitis and tonsillopharyngitis. Pediatric Infectious Disease Journal 1997;16:680‐95. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1994
- Rosenfeld RM, Vertrees JE, Carr J, Cipolle RJ, Uden DL, Giebink GS, et al. Clinical efficacy of antimicrobial drugs for acute otitis media:meta‐analysis of 5400 children from thirty‐three randomized trials. Journal of Pediatrics 1994;124:355‐67. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1996
- Rosenfeld RM. An evidence‐based approach to approach to treating otitis media. Pediatric Clinics of North America 1996;43:1165‐81. [DOI] [PubMed] [Google Scholar]
Rovers 2004
- Rovers MM, Schilder AGM, Zielhuis GA, Rosenfeld RM. Otitis media. The Lancet 2004;363:465‐73. [DOI] [PubMed] [Google Scholar]
Sanders 2009
- Sanders S, Glasziou PP, Mar C, Rovers M. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000219.pub2] [DOI] [PubMed] [Google Scholar]
Schentag 1995
- Schentag JJ. Antibiotic treatment of acute otitis media in children: dosing considerations. Pediatric Infectious Disease Journal 1995;14:S30‐3. [Google Scholar]
Spiro 2008
- Spior DM, Arnold DH. The concept and practice of a wait‐and‐see approach to acute otitis media. Current Opinion in Pediatrics 2008;20:72‐8. [DOI] [PubMed] [Google Scholar]
Thompson 1994
- Thompson SG. Why sources of heterogeneity in meta‐analysis should be investigated. BMJ 1994;309:1351‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Buchem 1981
- Buchem FL, Dunk JHM, Van't Hof MA. Therapy of acute otitis media: myringotomy, antibiotic or neither. Lancet 1981;II:883‐7. [DOI] [PubMed] [Google Scholar]
Vouloumanou 2009
- Vouloumanou EK, Karageorgopoulos DE, Kazantzi MS, Kapaskelis AM, Falagas ME. Antibiotics versus placebo or watchful waiting for acute otitis media: a meta‐analysis of randomized controlled trials. Journal of Antimicrobial Chemotherapy 2009;64:16‐24. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kozyrski 1998
- Kozyrski AL, Hildes‐Ripstein E, Longstaffe SA, Wincott JL, Sitar DS, Klassen TP, et al. Treatment of acute otitis media with a shortened course of antibiotics. JAMA 1998;279(21):1736‐42. [DOI] [PubMed] [Google Scholar]
Kozyrskyj 2000
- Kozyrskyj AL, Hildes‐Ripstein GE, Longstaffe SEA, Wincott JL, Sitar DS, Klassen TP, et al. Short course antibiotics for acute otitis media. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001095] [DOI] [PubMed] [Google Scholar]


