Abstract
Polyoxometalates (POMs) provide rigid and highly symmetric coordination sites and can be used as a strategy for the stabilization of magnetic ions. Herein, we report a new member of the Keggin archetype, the Cr-centered Keggin anion [α-CrW12O40]5– (CrW12), with the unusual tetrahedral coordination of CrIII reported for the first time in POMs conferring unattended magnetic properties. POM chemistry has recently presented excellent examples of single-molecule and single-ion magnets (SMMs and SIMs) as well as molecular spin qubits; however, the majority of POM-based SIMs reported to date contain lanthanoid ions. CrW12, as the first example of a chromium(III) SIM, exhibits slow relaxation of magnetization and quantum tunneling with a single-ion magnetic behavior even above 10 K with an energy barrier for the reversal of the magnetization of 3.0 K. The first 3d-metal SIM based on a nonlacunary Keggin anion is the foundation for a new research area in POM chemistry.
Chromium(III) ions in inorganic compounds exhibit a strong tendency to adopt an octahedral coordination1 owing to high ligand field stabilization energy (LFSE) for a 3d3 ion with a 4A2 ground state. The equivalent tetrahedral complexes, which have a ground state that is degenerate, are not stable, and the tetrahedral coordination has been confirmed only in blue-colored diopsides and CrIII[μ3-O4] cubanes.2 The confinement of CrIII inside certain molecular cages, such as polyoxometalates (POMs), can help to stabilize a tetrahedral coordination geometry. A handful of chromium-containing POMs are known to date, with all exhibiting an octahedral coordination of CrIII.3−5 In 1962, Brown reported the preparation of 12-tungstochromic(III) acid H5[α-CrW12O40]·nH2O with CrIII ions in a tetrahedral ligand field.6 The conclusion of the formation of [α-CrW12O40]5– was solely based on the appearance of a weak band assigned to (Cr–O)Td at 8300 cm–1 in the near-infrared spectrum of [α-CrW12O40]5– and was subsequently questioned.7 To the best of our knowledge, the crystal structure of [α-CrW12O40]5– with a tetrahedral {CrO4} unit in POMs has not been documented yet.
The incorporation of magnetic units into the POM architectures is of considerable interest, since POMs as ligands may shield the magnetic core from interaction with other molecules, creating a single-molecule magnet (SMM).8 SMMs based on POMs reported so far can be divided into three groups, representing: (1) a number of 3d-transition metal ions, which can be connected through oxo-bridges forming magnetic clusters of variable nuclearities and high symmetries;9 (2) a number of lanthanoid ions giving rise to lanthanoid complexes in which 4f-magnetic ions are submitted to the crystal field created by POM ligands;8 (3) a mixed-valence framework hosting a number of electrons that are usually delocalized over all the framework structure.10 The possibility of constructing nanomagnets using a single lanthanoid ion has been demonstrated for both polyoxotungstates ([Ln(W5O18)2]9–,11 [Ln(β2-SiW11O39)2]13–,12 [LnW30O110]12–13) and polyoxomolybdates ([Ln(β-Mo8O26)2]5–,14 [Ln{Mo5O13(OMe)4NNC6H4-p-NO2}2]3–14). Although a growing number of first-row d-block single-ion magnets (SIMs) has been reported,15 d-block SIMs based on POMs are particularly rare.16,17 Even though there are a few examples of heteropolynuclear SMMs containing chromium(III) centers,18 there are no reported chromium(III) SIMs.
Herein, we report the first CrIII-centered Keggin-type anion [α-CrW12O40]5– (CrW12), representing a new member of the Keggin ([XM12O40]n−, better represented as [XO4M12O36]n−, X: heteroatom; M: Mo, W, V, or Nb) POM family. CrW12 was characterized by elemental analysis, single-crystal and powder X-ray diffraction analysis, IR spectroscopy, thermogravimetric analysis, electrospray ionization mass spectrometry, cyclic voltammetry, X-ray absorption and photoelectron spectroscopy, UV–vis−NIR electronic spectroscopy, high-frequency and -field electron paramagnetic resonance, magnetic susceptibility measurements, and DFT calculations (see the SI, Figures S1–S14, Tables S1–S5). The {CrIIIO4} unit is stabilized inside the Keggin cavity, which causes a slow relaxation of magnetization. This type of system, where a POM cavity isolates the SIM unit from the environment, is an interesting approach to acquire direct and explicit information about the mechanisms that govern the SIM behavior.
CrW12 was isolated as the double salt Na2.4(TMA)3[α-CrW12O40]0.6[α-H2W12O40]0.4·37H2O (TMA: tetramethylammonium, (CH3)4N+) from the system CrIII–WO42– (c = 0.04 M)–H+–Tris-NH2 with a molar ratio of 1 CrIII:10 WVI:12 H+:5 Tris-NH2 (Tris-NH2: tris(hydroxymethyl)aminomethane, H2NC(CH2OH)3) at the final pH of 7.5. Interestingly, when the pH was adjusted to 7.5 with NaOH instead of Tris-NH2 or with a phosphate buffer, CrW12 could not be isolated (Scheme S1). The use of phosphate buffer most likely leads to the formation of lacunary forms of [α-PW12O40]3–. The failure to crystallize the target product with NaOH indicates the importance of buffering during the CrW12 synthesis due to the complex hydrolysis equilibria in POT solution. The formation of monolacunary anions in the unbuffered system was also shown by ESI-MS (Figure S2).
CrW12 possesses a typical disordered α-Keggin structure (CCDC 1913668, Figure 1). The central cation CrIII is surrounded by eight half-occupied oxygen atoms (Figure 1A), thus reducing its effective coordination number to four. This disorder of the oxygen atoms in the Keggin ion was first mentioned in 198419 and reported for many Keggin structures with different heteroatoms, e.g., Si4+20 or P5+.21 The cubic environment around the central heteroatom X can be formed from the superimposition of one tetrahedrally coordinated X in one unit cell with another X tetrahedron (just oppositely oriented) in the neighboring unit cell.
Figure 1.
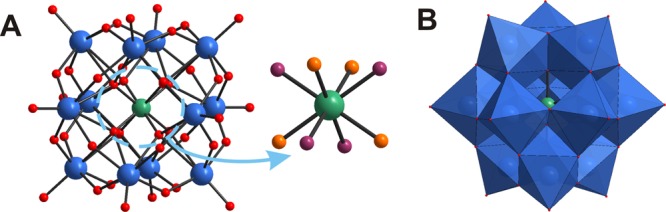
CrW12 anion. (A) Ball-and-stick representation including the crystallographic disorder in {CrO4}. (B) Polyhedral representation. Color code: W, blue; Cr, green; O, red, orange, and purple.
The CrIII site occupancy amounts up to 60% based on the X-ray data, which indicates that an alternative population of the central Keggin tetrahedron with CrIII or two protons is most likely. The synthesized compound can be described as a double salt of CrW12 and the metatungstate anion [α-H2W12O40]6– (H2W12). The alternating occupancy with two hetero ions in a Keggin anion was previously described for CuII and two protons in [α-Cu0.4(H2)0.6W12O40]6–.22
The direct current (dc) magnetic behavior of CrW12 is shown in Figure 2 as a plot of χMT against T, where χMT is the molar magnetic susceptibility, and of the magnetization (M) versus H/T at different applied dc fields. At room temperature, χMT of CrW12 amounts to 0.65 cm3·mol–1·K and remains constant by cooling down to 50 K. This value is distinctly lower than the spin-only value (1.874 cm3·mol–1·K) for a high-spin d3 ion supporting the partial occupancy of CrIII site in CrW12.
Figure 2.
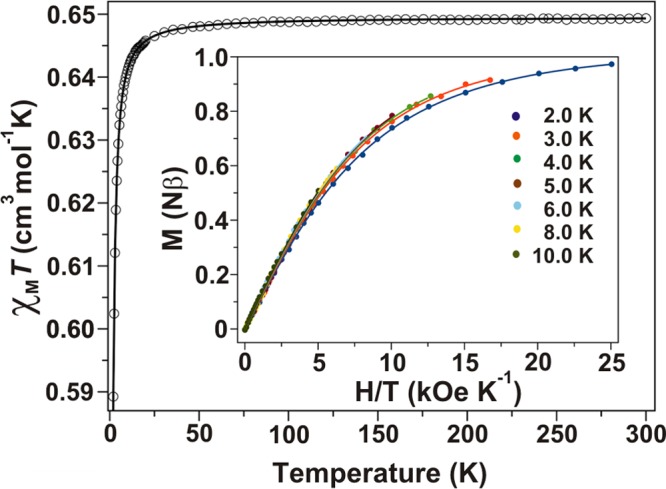
Magnetometry of CrW12. Temperature dependence of χMT and the magnetization curves (inset) for CrW12: (○, ●) experimental; (−) best-fit curves to the experimental data using the parameters provided in the text. The χMT value supports a partial (36%) occupancy of CrIII.
Since the paramagnetic CrIII in CrW12 is kept isolated from other neighbors inside the polyoxotungstate, the decrease of χMT below 12 K and the non-superposition of the curves of the reduced magnetization can be attributed only to zero-field splitting (zfs). The simultaneous analysis of all data through the spin Hamiltonian, Ĥ = D(Ŝz2 – S(S+1)/3) + E(Ŝx2 – Ŝy2) + gβHŜ, provided a good agreement between experimental and simulated curves with the best-fit parameters: |D| = +0.98 cm–1, E/D = 0.095, and g = 2.005. Although there is no doubt about the presence of a zfs in CrW12, the sign of the axial component (D) is uncertain.
In order to more accurately determine zfs in CrW12, we used high-frequency and -field electron paramagnetic resonance (HFEPR). Although the spectral quality was moderate at best, a low-temperature, multifrequency experiment in the V-band range (Figure 3) delivered a direct measure of zfs by observing a near-zero field absorption between the ±1/2 and ±3/2 Kramers doublets at 65 ± 1 GHz = 2.16(3) cm–1. This value represents the parameter Δ = 2[(D2 + 3E2)1/2] for an S = 3/2 spin state. In order to deconvolute Δ into D and E we performed standard X-band EPR (Figure S6), which resulted in spectra originating from the ±1/2 Kramers doublet only, and typical for the case |D| > hν where h is Planck’s constant and ν is the EPR operating frequency (9.47 GHz = ∼ 0.3 cm–1). The spectra could be satisfactorily simulated using |D| = 1.07 and |E| = 0.07 cm–1 (rhombicity factor E/D = 0.07). Both zfs parameters are in excellent agreement with the magnetometric result. These parameters were then used in plotting the field vs frequency (or energy) dependence of the EPR turning points, confirming their accuracy (Figure S7). Given the modest zfs magnitude and the relatively low EPR frequencies employed, it was not possible to unequivocally determine the sign of D. A perfect cancellation of large contributions to zfs in an ideal tetrahedral high-spin d3 system is broken due to small distortions and a partial charge transfer in the ground state from the highly charged oxo-groups bound to CrIII ion, which can accept electrons, with an unstable geometry that facilitates this electronic drain (see SI text and Table S4). The UV–vis−NIR solid reflectance spectrum of CrW12 also supports a moderate charge-transfer from the oxo groups to the CrIII ion and the presence of a high-spin d3 CrIII ion in a tetrahedral coordination environment (see SI text and Figure S14). Alternating current (ac) magnetic susceptibility measurements below 10 K did not show out-of-phase magnetic susceptibility χM″ signals at frequencies up to 10 000 Hz in the absence of an applied dc field. However, the suppression of fast tunneling of the magnetization (QTM) by the application of an external dc field (Hdc, 1000 and 2500 G) resulted in a set of frequency-dependent signals in plots of χM″ vs T (Figure S8). This slow relaxation of the magnetization in mononuclear complexes is characteristic for SIMs. The relaxation times (τ) at each temperature were extracted from χM″ vs frequencies (ν) plots, in which χM″ reaches its highest value (Figure S9). The obtained data in the form of {T, v} pairs was used to build Arrhenius plots (Figure S10), showing a linear dependence that can only be described by a unique relaxation process following an Arrhenius law, τ–1 = τ0–1 exp(−Ea/kT), where k is the Boltzmann constant and Ea is the energy barrier governing the spin reversal. The Ea and τ0 values calculated for CrW12 (Table S3) are in the range of those found in octahedral and tetrahedral complexes with first-row transition metal ions. In both magnetic fields, the Ea values are about 3.0 cm–1, which is the energy barrier calculated from |D| = 1.5 cm–1, a value close to those derived from the EPR study and magnetometry. However, an energy barrier for the spin reversal governed by an Orbach mechanism is only possible for a negative value of D, which could not be experimentally confirmed. SIMs with positive D values or even with an S = 1/2 ground state are increasingly common; however, the relaxation mechanism governing their spin dynamics is still under discussion.15,23−25
Figure 3.
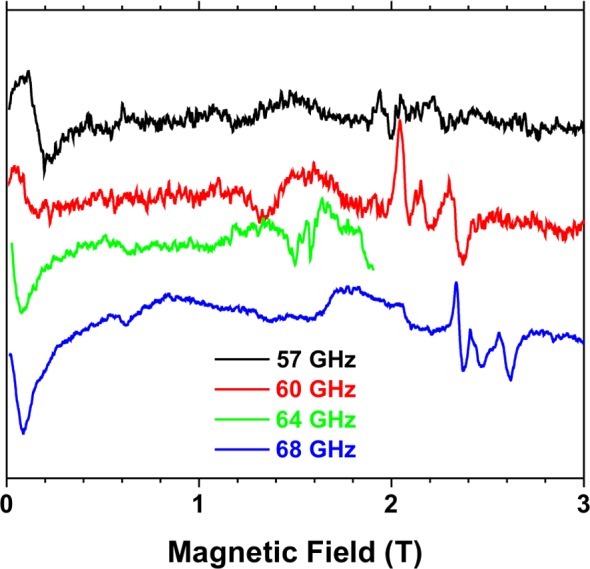
HFEPR spectra of CrW12 at 4.5 K and indicated frequencies in the vicinity of the zero-field transition between the ±1/2 and ±3/2 Kramers doublets. The resonances appearing between 2 and 3 T depending on frequency (g ∼ 1.95) belong to a different spin species, which has a much lower anisotropy (about 0.1 cm–1) and thus represents a small fraction of total absorption despite its amplitude, signifying low abundance.
Only two cases of POM-based d-block (FeIII and CoII) SIMs have been described in literature.16,17 The single-ion magnet behavior is unusual in high-spin FeIII complexes and rarely encountered for the systems based on POMs; however, this effect is well-known for FeIII porphyrin complexes.26 In contrast, in CrW12, the zfs is induced through a unique tetrahedral geometry for a d3 ion. Compared with other SIMs based on third-row transition metal ions (Table S5), the energy barrier for CrW12 is small, but more importantly, the magnetic blocking temperature is above 10 K, which places it in the same range or better than other analogs. The chemical surrounding can hamper, randomly modify or even abolish the slow relaxation of magnetization. In our case, the use of POMs as shielding ligands is a strategy to avoid these unwanted interactions. Moreover, tetrahedral high-spin CrIII complexes with a small zfs, as in CrW12, feature both allowed and forbidden transitions and can therefore be applied as qubits.27
The existence of CrW12 demonstrates that CrIII can adopt a tetrahedral coordination in the Keggin cavity and opens a new perspective for Cr-centered lacunary POMs. The Keggin cavity is capable of stabilizing an a priori unstable tetrahedral geometry for a high-spin d3 ion, which results in a single-ion magnet behavior. These novel properties of POMs enhance the relevance of these inorganic compounds in molecular magnetism because a variety of a new series of 3d-metal SIMs can be foreseen by a suitable choice of POM ligands.
Acknowledgments
This research was funded by the Austrian Science Fund (FWF): M2203 (N.G.), P27534 (A.R.), the University of Vienna and the Ministerio Español de Ciencia e Innovación (Projects CTQ2016-75068P and CTQ2016-75671P, and Unidad de Excelencia María de Maetzu MDM-2015-0538). A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by NSF Cooperative Agreement DMR-1644779 and the State of Florida. We acknowledge Diamond Light Source for time on B18 under proposal SP24909 and G. Cibin for his support with XAS measurements at beamline B18 at Diamond Light Source, UK; A. Fabisikova for support with ESI-MS measurements; P. Unfried for support with TGA measurement; M. Bujdoš for support with ICP-MS and AAS; Global Tungsten & Powders Corp. for providing ammonium metatungstate providing; V. Arion, J. Breibeck, E. Al-Sayed, and E. Tanuhadi for valuable discussions concerning this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b12797.
The authors declare no competing financial interest.
Notes
CCDC 1913668 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
Supplementary Material
References
- Waroquiers D.; Gonze X.; Rignanese G.-M.; Welker-Nieuwoudt C.; Rosowski F.; Göbel M.; Schenk S.; Degelmann P.; Andre R.; Glaum R.; Hautier G. Statistical analysis of coordination environments in oxides. Chem. Mater. 2017, 29, 8346–8360. 10.1021/acs.chemmater.7b02766. [DOI] [Google Scholar]
- Beale A. M.; Grandjean D.; Kornatowski J.; Glatzel P.; de Groot F. M. F.; Weckhuysen B. M. Unusual coordination behavior of Cr3+ in microporous aluminophosphates. J. Phys. Chem. B 2006, 110, 716–722. 10.1021/jp0531006. [DOI] [PubMed] [Google Scholar]
- Lunk H.-J. Discovery, properties and applications of chromium and its compounds. ChemTexts 2015, 1, 6. 10.1007/s40828-015-0007-z. [DOI] [Google Scholar]
- a Liu W.; Christian J. H.; Al-Oweini R.; Bassil B. S.; van Tol J.; Atanasov M.; Neese F.; Dalal N. S.; Kortz U. Synthesis, detailed characterization, and theoretical understanding of mononuclear chromium(III)-containing polyoxotungstates [CrIII(HXVW7O28)2]13– (X = P, As) with exceptionally large magnetic anisotropy. Inorg. Chem. 2014, 53, 9274–9283. 10.1021/ic501385r. [DOI] [PubMed] [Google Scholar]; b Liu W.; Al-Oweini R.; Meadows K.; Bassil B. S.; Lin Z.; Christian J. H.; Dalal N. S.; Bossoh A. M.; Mbomekallé I. M.; de Oliveira P.; Iqbal J.; Kortz U. CrIII-substituted heteropoly-16-tungstates [CrIII2(B-β-XIVW8O31)2]14– (X = Si, Ge): magnetic, biological, and electrochemical studies. Inorg. Chem. 2016, 55, 10936–10946. 10.1021/acs.inorgchem.6b01458. [DOI] [PubMed] [Google Scholar]
- a Perloff A. Crystal structure of sodium hexamolybdochromate(III) octahydrate, Na3(CrMo6O24H6).8H2O. Inorg. Chem. 1970, 9, 2228–2239. 10.1021/ic50092a006. [DOI] [Google Scholar]; b Liu W.; Lin Z.; Bassil B. S.; Al-Oweini R.; Kortz U. Synthesis and Structure of Hexatungstochromate(III), [H3CrIIIW6O24]6–. Chimia 2015, 69, 537–540. 10.2533/chimia.2015.537. [DOI] [PubMed] [Google Scholar]; c Gumerova N. I.; Caldera Fraile T.; Roller A.; Giester G.; Pascual-Borràs M.; Ohlin C. A.; Rompel A. Direct single- and double-side triol-functionalization of the mixed type Anderson polyoxotungstate [Cr(OH)3W6O21]6–. Inorg. Chem. 2019, 58, 106–113. 10.1021/acs.inorgchem.8b01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. H. The preparation, properties, structure, and spectra of 12-tungstochromic(III) acid. J. Chem. Soc. 1962, 0, 3322–3324. 10.1039/jr9620003322. [DOI] [Google Scholar]
- Nomiya K.; Miwa M. Undecatungstomanganate and undecatungstochromate. Polyhedron 1984, 3, 1161–1163. 10.1016/S0277-5387(00)88075-4. [DOI] [Google Scholar]
- Clemente-Juan J. M.; Coronado E.; Gaita-Ariño A. Magnetic polyoxometalates: from molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. 10.1039/c2cs35205b. [DOI] [PubMed] [Google Scholar]
- Ritchie C.; Ferguson A.; Nojiri H.; Miras H. N.; Song Y.-F.; Long D.-L.; Burkholder E.; Murrie M.; Kögerler P.; Brechin E. K.; Cronin L. Polyoxometalate-mediated self-assembly of single-molecule magnets: {[XW9O34]2[Mn(III)4Mn(II)2O4(H2O)4]}12-. Angew. Chem., Int. Ed. 2008, 47, 5609–5612. 10.1002/anie.200801281. [DOI] [PubMed] [Google Scholar]
- Lehmann J.; Gaita-Ariño A.; Coronado E.; Loss D. Spin qubits with electrically gated polyoxometalate molecules. Nat. Nanotechnol. 2007, 2, 312–317. 10.1038/nnano.2007.110. [DOI] [PubMed] [Google Scholar]
- AlDamen M. A.; Clemente-Juan J. M.; Coronado E.; Martí-Gastaldo C.; Gaita-Ariño A. Mononuclear lanthanide single-molecule magnets based on polyoxometalates. J. Am. Chem. Soc. 2008, 130, 8874–8875. 10.1021/ja801659m. [DOI] [PubMed] [Google Scholar]
- AlDamen M. A.; Cardona-Serra S.; Clemente-Juan J. M.; Coronado E.; Gaita-Ariño A.; Martí-Gastaldo C.; Luis F.; Montero O. Mononuclear lanthanide single molecule magnets based on the polyoxometalates [Ln(W5O18)2]9– and [Ln(β2-SiW11O39)2]13– (LnIII = Tb, Dy, Ho, Er, Tm, and Yb). Inorg. Chem. 2009, 48, 3467–3479. 10.1021/ic801630z. [DOI] [PubMed] [Google Scholar]
- Cardona-Serra S.; Clemente-Juan J. M.; Coronado E.; Gaita-Ariño A.; Camón A.; Evangelisti M.; Luis F.; Martínez-Pérez M. J.; Sesé J. Lanthanoid single-ion magnets based on polyoxometalates with a 5-fold symmetry: the series [LnP5W30O110]12– (Ln3+ = Tb, Dy, Ho, Er, Tm, and Yb). J. Am. Chem. Soc. 2012, 134, 14982–14990. 10.1021/ja305163t. [DOI] [PubMed] [Google Scholar]
- Baldoví J. J.; Duan Y.; Bustos C.; Cardona-Serra S.; Gouzerh P.; Villanneau R.; Gontard G.; Clemente-Juan J. M.; Gaita-Ariño A.; Giménez-Saiz C.; Proust A.; Coronado E. Single ion magnets based on lanthanoid polyoxomolybdate complexes. Dalton Trans. 2016, 45, 16653–16660. 10.1039/C6DT02258H. [DOI] [PubMed] [Google Scholar]
- Frost J. M.; Harriman K. L. M.; Murugesu M. The rise of 3-d single-ion magnets in molecular magnetism: towards materials from molecules?. Chem. Sci. 2016, 7, 2470–2491. 10.1039/C5SC03224E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R.; Suzuki K.; Minato T.; Shinoe M.; Yamaguchi K.; Mizuno N. Field-induced slow magnetic relaxation of octahedrally coordinated mononuclear Fe(III)-, Co(II)-, and Mn(III)-containing polyoxometalates. Chem. Commun. 2015, 51, 4081–4084. 10.1039/C4CC09435B. [DOI] [PubMed] [Google Scholar]
- Minato T.; Aravena D.; Ruiz E.; Yamaguchi K.; Mizuno N.; Suzuki K. Effect of heteroatoms on field-induced slow magnetic relaxation of mononuclear FeIII (S = 5/2) ions within polyoxometalates. Inorg. Chem. 2018, 57, 6957–6964. 10.1021/acs.inorgchem.8b00644. [DOI] [PubMed] [Google Scholar]
- Sokol J. J.; Hee A. G.; Long J. R. A cyano-bridged single-molecule magnet: slow magnetic relaxation in a trigonal prismatic MnMo6(CN)18 cluster. J. Am. Chem. Soc. 2002, 124, 7656–7657. 10.1021/ja0263846. [DOI] [PubMed] [Google Scholar]
- Evans T.; Pope M. T. Reinterpretation of five recent crystal structures of heteropoly and isopoly complexes: divanadodecamolybdophosphate, trivanadoenneamolybdophosphate, ″gamma.-dodecatungstophosphate″, the dodecamolybdate-dodecamolybdomolybdate blue complex, and dihydrogen decavanadate. Inorg. Chem. 1984, 23, 501–504. 10.1021/ic00172a024. [DOI] [Google Scholar]
- Roy S.; Sarkar S.; Pan J.; Waghmare U. V.; Dhanya R.; Narayana C.; Peter S. C. Crystal structure and band gap engineering in polyoxometalate-based inorganic–organic hybrids. Inorg. Chem. 2016, 55, 3364–3377. 10.1021/acs.inorgchem.5b02718. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Z.; Lu C. Z. Hydrothermal synthesis and structures of two new molybdenum phosphate compounds based on Keggin cluster units. Z. Anorg. Allg. Chem. 2006, 632, 330–334. 10.1002/zaac.200500357. [DOI] [Google Scholar]
- Lunk H. J.; Giese S.; Fuchs J.; Stösser R. Das erste KEGGIN-Anion mit tetraedrischer Kupfer(II)-Sauerstoff-Koordination: [α-Cu0,4(H2)0,6O4W12O36]6–. Z. Anorg. Allg. Chem. 1993, 619, 961–968. 10.1002/zaac.19936190526. [DOI] [Google Scholar]
- Marinho M. V.; Reis D. O.; Oliveira W. X. C.; Marques L. F.; Stumpf H. O.; Déniz M.; Pasán J.; Ruiz-Pérez C.; Cano J.; Lloret F.; Julve M. Photoluminescent and slow magnetic relaxation studies on lanthanide(III)-2,5-pyrazinedicarboxylate frameworks. Inorg. Chem. 2017, 56, 2108–2123. 10.1021/acs.inorgchem.6b02774. [DOI] [PubMed] [Google Scholar]
- Maeda M.; Hino S.; Yamashita K.; Kataoka Y.; Nakano M.; Yamamura T.; Kajiwara T. Correlation between slow magnetic relaxation and the coordination structures of a family of linear trinuclear Zn(II)–Ln(III)–Zn(II) complexes (Ln = Tb, Dy, Ho, Er, Tm and Yb). Dalton Trans. 2012, 41, 13640–13648. 10.1039/c2dt31399e. [DOI] [PubMed] [Google Scholar]
- Boča R.; Rajnák C.; Titiš J.; Valigura D. Field supported slow magnetic relaxation in a mononuclear Cu(II) complex. Inorg. Chem. 2017, 56, 1478–1482. 10.1021/acs.inorgchem.6b02535. [DOI] [PubMed] [Google Scholar]
- Stavretis S. E.; Atanasov M.; Podlesnyak A. A.; Hunter S. C.; Neese F.; Xue Z. L. Magnetic transitions in iron porphyrin halides by inelastic neutron scattering and ab initio studies of zero-field splittings. Inorg. Chem. 2015, 54, 9790–9801. 10.1021/acs.inorgchem.5b01505. [DOI] [PubMed] [Google Scholar]
- Fataftah M. S.; Zadrozny J. M.; Coste S. C.; Graham M. J.; Rogers D. M.; Freedman D. E. Employing forbidden transitions as qubits in a nuclear spin-free chromium complex. J. Am. Chem. Soc. 2016, 138, 1344–1348. 10.1021/jacs.5b11802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


