Abstract
Intraoperative image-guided surgery (IGS) has attracted extensive research interests in determination of tumor margins from surrounding normal tissues. Introduction of near infrared (NIR) fluorophores into IGS could significantly improve the in vivo imaging quality thus benefit IGS. Among the reported NIR fluorophores, rare-earth nanoparticles exhibit unparalleled advantages in disease theranostics by taking advantages such as large Stokes shift, sharp emission spectra, and high chemical/photochemical stability. The recent advances in elements doping and morphologies controlling endow the rare-earth nanoparticles with intriguing optical properties, including emission span to NIR-II region and long life-time photoluminescence. Particularly, NIR emissive rare earth nanoparticles hold advantages in reduction of light scattering, photon absorption and autofluorescence, largely improve the performance of nanoparticles in biological and pre-clinical applications. In this review, we systematically compared the benefits of RE nanoparticles with other NIR probes, and summarized the recent advances of NIR emissive RE nanoparticles in bioimaging, photodynamic therapy, drug delivery and NIR fluorescent IGS. The future challenges and promises of NIR emissive RE nanoparticles for IGS were also discussed.
Keywords: near infrared fluorescence, rare earth nanoparticle, bioimaging, image guided surgery
1. Introduction
Surgical operation is one of the most frequently used therapy to cancer treatment for centuries 1,2. In common cancer surgeries, intraoperative evaluation of margins of tumor is essential to determine the final curative result 3. However, it is mainly dependent on the visual senses and subjective palpation to decide excision 4 during the surgical operation. Inevitably, it is very difficult for the surgeons to discriminate the tumor margins from surrounding normal tissues 5,6. It has been reported that tumor recurrence happens as high as 20-30% after surgical therapy, and subsequent cancer metastasis largely increases the complexity 7,8. It is highly demanded to maximize tumor removal, minimize damage to the normal tissues and shorten surgical time 9. Thus, intraoperative image-guided surgery (IGS) 5,10,11 is introduced to provide real-time tumor visualization to oncological surgeons to do them a favor in cancer margin recognition12.
Among various optical imaging techniques 13, near-infrared (NIR) fluorescence imaging 14,15 is one of the latest trends in IGS applications16, for use in both fundamental medical research and clinical practice17,18. Due to advantages in reduction of light scattering, photon absorption and autofluorescence via broadening to the 700-1,700 nm NIR window 19, NIR fluorescence-based imaging technique provides high spatial resolution along with increased tissue penetration depths. Very recently, NIR phosphors that extended to the entire NIR window, including small molecules 20-22, inorganic nanoparticles 23,24, organic macromolecules 25,26 and quantum dots (QDs) 27,28 with tunable emission wavelength were developed 29. Besides the benefits of efficient detection of NIR photons, recently developed NIR fluorophores have enabled biomedical imaging 30 of specific biomarkers 31 and anatomical structures with better signal-to-noise ratio, application for preclinical animal studies 32,33, clinical diagnostics 34 and translational medicine 35.
Compared with the visible spectrum widely employed for fluorescence imaging, the studies over the broadly defined NIR window are still in their infancy 36. In the past decade, researches in NIR fluorescence imaging have focused on the conventional NIR window (NIR-I, 700-900 nm)37, and have recently extended their efforts to the second NIR window (NIR-II, 1,000-1,700 nm)38,39. The NIR-I window is typically named as the 'biological transparent window' because in this range there is low tissue absorption and fluorescence background in vivo (compared with the visible range)40. The studies of molecular imaging to the novel NIR-II window has been achieved by the development of biocompatible NIR fluorophores with increasingly longer wavelengths throughout the field of chemistry, materials science and nanotechnology 41. Also, we shall thank to the development of more efficient photon detectors with high NIR-II sensitivity as well as the drop of the price. It is more and more widely accepted that in vivo NIR-II fluorescence technology is superior to traditional NIR-I one due to the further reduced scattering, absorption and tissue autofluorescence 42.
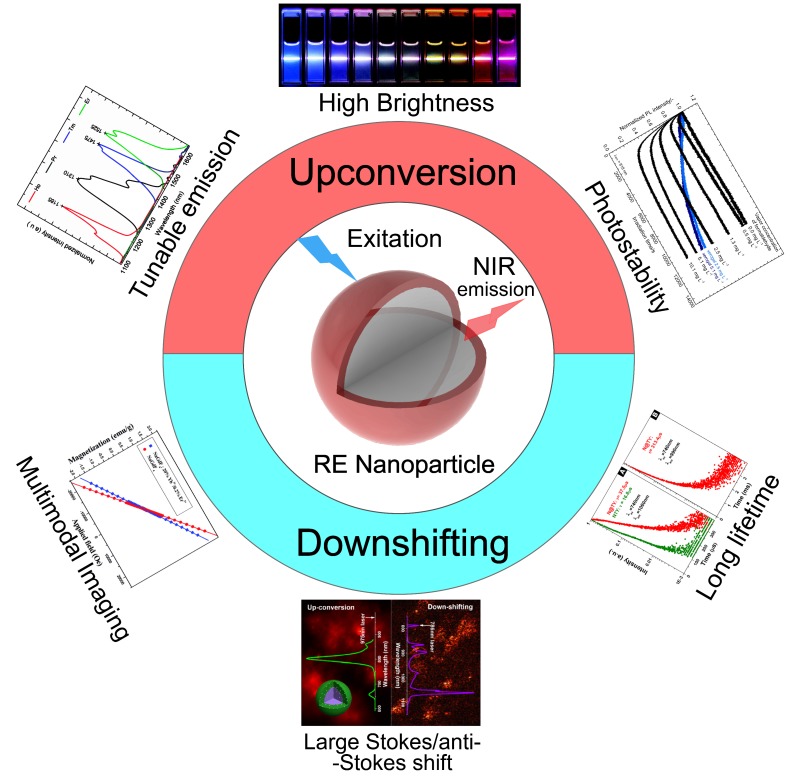
Among the existed NIR materials, Rare earth (RE) nanoparticles 43,44 can afford good stability, ease to fabricate 45,46, high emissive efficiency 47 and long luminescence lifetime to microseconds48. Compared with lanthanide chelates 49, QDs 50,51, polymers 52, and organic dyes 53,54, lanthanide-doped inorganic RE nanoparticles hold all the advantages, including tunable emission55, large Stokes shift 56, sharp emission peaks 57, and high chemical/photochemical stability 58 (Figure 1). Moreover, facile to multiple choices of doping 59, RE-doped inorganic materials can provide efficient emission from the ultraviolet (UV), passing through the whole visible range, to the mid-infrared region upon excitation 55,60,61. All the advantages mentioned above have enabled the promising potential of NIR emissive RE nanoparticles in bioimaging 62, theranostics 63,64, photothermal therapy 65,66, drug delivery 67, and also the clinical IGS 20,68.
Figure 1.
Characteristic properties of NIR emissive RE nanoparticles. Inset figures were adapted from Ref 63,69-72. Copyright 2014, Royal Society of Chemistry; Copyright 2008, 2011, 2013, 2016, American Chemical Society.
In this review, we provided a comprehensive introduction to the RE based NIR emissive nanoparticles. We systematically compared the benefits and of RE nanoparticles with other NIR probes, and summarized the recent advances of NIR emissive RE nanoparticles in bioimaging, photodynamic therapy, drug delivery and NIR fluorescence enabled IGS. The future challenges and promises of RE nanoparticles with NIR emission were also discussed.
2. NIR emissive rare earth nanoparticles
2.1. Emission mechanism of RE nanoparticle
RE nanoparticles are important fluorescent materials, due to their ability to enable intriguing emission properties, including tunable fluorescence color (Figure 1) 70,73, long life-time photoluminescence 74, highly efficient upconversity 75, long persistent phenomenon 76,77. Generally speaking, RE elements are composed of 15 lanthanides (from lanthanum to lutetium), and usually plus scandium and yttrium. With abundant f shell orbitals, trivalent lanthanide (Ln) ions can exhibit sharp fluorescent emissions through intra-4f or 4f-5d transitions and thus are widely used as emitting centers in many fluorophores 78. There are multiple methods to endow RE nanoparticles with NIR emission, using either upconversion 79 or down-shifting mechanisms (Figure 1) 69, and even long lifetime fluorophore and “after-glow” persistent luminescence 80-82. Various luminescence features have been achieved in a wide spectrum of matrix materials, such as RE oxides, fluorides, and other matrices.
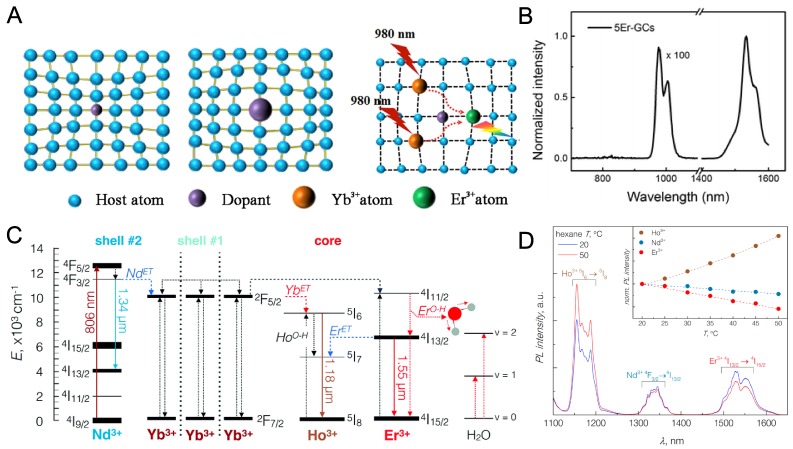
Due to the involvement of multiple steps in one single luminescence process, including electron transition and the transition probability (Figure 2a), the excitation selection, multiphonon relaxation and energy transfer, Stokes shift and line broadening,86 the study of RE nanoparticle emission is highly confusing where lots of details remains unclear. For example, at least 6 states were involved in an Yb, Er, Nd-co-doped triple-layered core-shell NIR fluorophore 84. During the absorption process of 800 nm light, the 4I9/2→4F5/2 transition of Nd3+ is firstly involved. After that, the energy is fast transferred to the inner layer by a 2F7/2→2F5/2 process between Nd3+ and Yb3+. Consequent energy transfer happens by the co‐doped Yb3+ and then sensitize Er3+ (Yb3+→Er3+, 4I15/2→4I11/2). After all, the relaxation from the excited state of Er3+ finally releases a 1525 nm (4I13/2→4I15/2) photon via phonon vibration process (Figure 2b). Similarly, a complicated phonon-assisted Yb3+ (2F5/2) → Ho3+ (5I6) and Nd3+ (4F3/2) → Yb3+ (2F5/2) energy transfer mechanism was proposed in Yb3+, Ho3+, Nd3+ doped core/shell NaGdF4 nanoparticles and it was designed as a nanothermometer due to a temperature-dependent promotion of the electronic-to-vibrational energy transfer (Figure 2c,d). Steady/transient state fluorescence spectroscopy, fluorescence polarization spectroscopy, and femto-second laser pulse luminescence etc. were widely used to study the emission mechanisms of RE fluorophores 87,88. The better understanding of the luminescence mechanism will help to design better NIR probes as well as further broadened applications.
Figure 2.
Luminescence mechanism of RE nanoparticles. (A) Scheme of emission mechanism of Yb, Er doped RE nanoparticles exited by 980 nm NIR light. Adapted with permission from 83, Copyright 2018, Nature Springer. (B) NIR-spectra of 5% Er3+ doped glass ceramics upon 400 nm irradiation. Adapted with permission from 84, Copyright 2015, Royal Society of Chemistry. (C) Principal operation scheme for the NIR-to-NIR emission of RE doped nanoparticles. (D) NIR emission bands of Ho3+ (1.18 µm), Nd3+ (1.34 µm), and Er3+ (1.55 µm) ions excited with 806 nm irradiation. Adapted with permission from 85, Copyright 2017, Royal Society of Chemistry.
2.2. Material Subclasses of NIR emissive RE nanoparticles
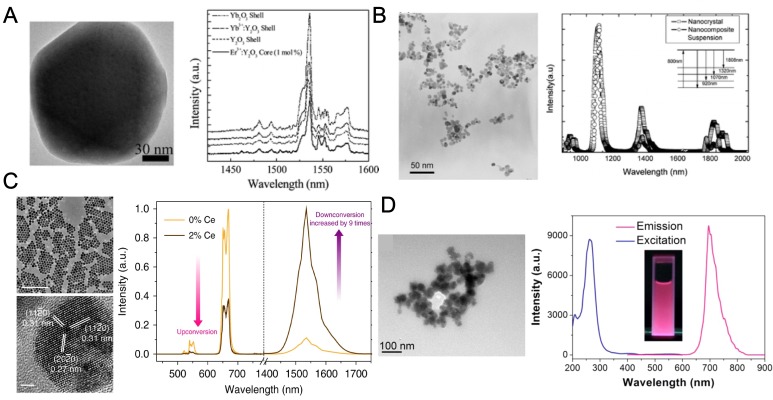
RE oxides 89,90, in most cases, Y2O3, (Figure 3a) is the first generation NIR emissive lanthanide material 91,92. In a typical synthesis of RE oxides, the nanoparticles were synthesized through homogeneous precipitation and then high temperature calcination is required to increase the emission efficiency if necessary. In 2003, Vetrone and his coworkers investigated the upconversion emission of nanocrystalline and bulk Y2O3:Er3+ and the influence of the erbium concentration to the luminescence 93. They reported that by adjusting the doping concentration, a transition in emission from visible to NIR region was observed. Soga group further developed liposome encapsulated, Er-doped Y2O3 nanoparticles with various surface modifications as a fluorescent probe for NIR bioimaging 90. The authors introduced PEG on the liposome surface to avoid nonspecific interaction with proteins. Both microscopic and macroscopic NIR imaging systems were applied to image the organs of a mouse injected with the NIR-encapsulated liposomes as a demonstration of successful NIR bioimaging. But limited by the relatively low NIR emission efficiency of lanthanide oxides, their applications stop at organ bioimaging and no more clinical approaches were conducted.
Figure 3.
Subclasses of RE nanoparticles. TEM images (left) and NIR fluorescent spectra of (A) Yb2O3 nanoparticles, adapted with permission from 91, Copyright 2012, American Chemical Society. (B) YF3 nanoparticles, adapted with permission from 94, Copyright 2007, American Chemical Society. (C) NaYF4 nanoparticles, adapted with permission from 101, Copyright 2017, Nature Springer and (D) Zn2.94Ga1.96Ge2O10 nanoparticles, adapted with permission from 102, Copyright 2014, American Chemical Society.
RE fluorides 94,95, referring to YF3 and LaF3, 96 and maybe more frequently, NaYF4 and NaGdF4 73,87, were the most widely used doping matrices (Figure 3c) for lanthanide phosphor 79. This is largely because NaLnF4 exhibits the lowest non-radiative energy loss and endow the highest quantum yield for a major of lanthanides doping. The NaLnF4 is still the most widely used matrix 97 for NIR emission through either upconversion or downshifting luminescence by different rare earth doping, largely on account to the high emission efficiency and extraordinary chemical stability. In addition, NaGdF4 also has excellent magnetic properties and it was widely employed as contrast agents in MRI 98,99. The classic synthesis of NaYF4 or NaGdF4 nanoparticles was conducted in oleic acid and 1‐octadecene through a solvothermal method, using lanthanide nitric salts or lanthanides acetates, reacting with NaF or NH4F. The versatile luminescent properties as well their intriguing magnetic and electronic properties of RE fluorides open an avenue to multi-mode molecular imaging and dual signal guided surgery 100. For example, Riman's group prepared highly NIR emissive fluoride nanopowders (LaF3: Nd and CaF2: Er) with solvothermal methods (Figure 3b). The quantum efficiencies were as high as 95% for LaF3: Nd and 51% for CaF2: Er, which are much higher than RE oxides 94. In 2013, Zhou et al. employed Tm and Nd doped NaGdF4 nanoparticles to efficient NIR-to-NIR upconversion and down-shifting emission, providing a dual mode platform for NIR fluorescence bioimaging and promisingly even magnetic resonance imaging (MRI) probes 69.
Recently, a series of new matrices were reported for NIR emission of lanthanides 80,103,104, where solid state high-temperature synthesis were usually employed. The new matrices bring new properties to NIR based bioimaging, such as long persistence emissive phenomenon and degradability in physiological fluids. For example, Scherman et al. reported to successfully prepare lanthanide doped Ca0.2Zn0.9Mg0.9Si2O6 nanoparticles with NIR persistent luminescence 82. The NIR persistent nanoparticles can be excited before injection to mouse, and the biodistribution of the nanoparticle can be monitored in real-time for more than 1 h without any external illumination source. The nanoparticles were modified with targeting ligands, would guide the nanoparticles specifically to lung, liver or to long-lasting blood circulation. This system can be employed to evaluate tumor mass and showed great clinical potential. Another similar work was reported by Yan's lab that they synthesized NIR emitting Zn2.94Ga1.96Ge2O10 nanoparticles co-doped with Cr3+, Pr3+ for long persistent luminescence (Figure 3d). The nanoprobe was further functionalized with gadolinium complexes and enabled a multimodal in vivo MRI and NIR luminescence imaging 102.
3. RE nanoparticles for NIR bioimaging
Fluorescence based bioimaging in the NIR window features deep tissue penetration, reduced tissue scattering, and decreased tissue autofluorescence. These advantages would largely improve the performance of nanoparticles in biological and pre-clinical applications. Hence, NIR fluorescent probes, especially RE nanoparticles, are constructed into platforms for NIR bioimaging 105, biosensing 106, drug delivery 107, photodynamic therapy and NIR based IGS 108. The application in bioimaging is the first step for successive preclinical studies and practices. The good performance in NIR bioimaging of RE nanoparticles plays as cornerstones for the follow-up photodynamic therapy, drug delivery and surgical navigation.
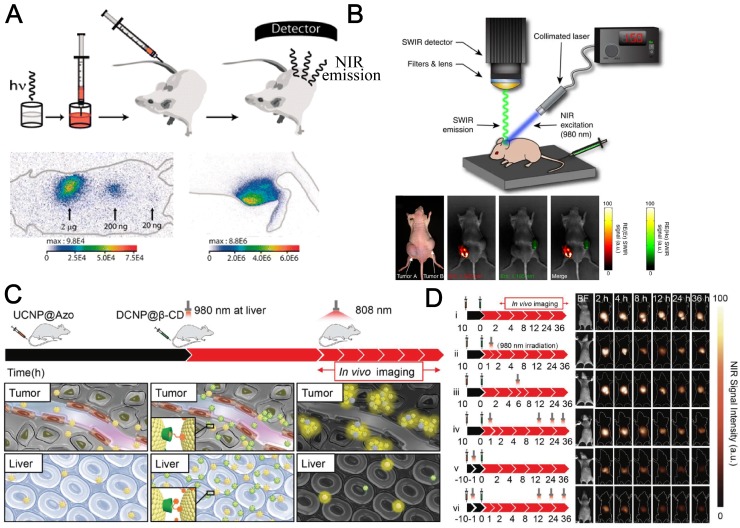
Various groups have successfully reported NIR emissive RE nanoparticles for bioimaging 109 (Figure 4a). Hammond's group constructed LbL-modified NIR-II nanoparticels from RE doped NaYF4 fluorescent materials to perform a side-by-side investigation and comparison for the biodistribution, pharmacokinetics, and toxicities of these probes 110. Moghe et al. reported a multispectral, real-time short-wavelength infrared imaging offering anatomical resolution using brightly emitting RE nanomaterials and demonstrate their practicability as a disease-targeted imaging method (Figure 4b) 111. RE nanomaterials modified with human serum albumin (HSA) endowed systemic study of biodistribution of the RE nanoparticles. It was reported by the authors that accumulation and retention in tumor tissue was improved after protein conjugation, which was visualized by the localized enhancement of NIR signal intensity (Figure 4b). The involvement of HSA was drawn as experiences by a lot of other studies and was verified to improve the biocapability and retention time in organs and tumors 112. Liu's group found another route of NIR emitting nanomaterials for theranostic applications on how RE moieties were involved. They fabricated a nanocomplex where Gd3+ chelate were functionalized onto HSA, conjugated with a NIR dye IR825 113. The albumin-based probe was capable of multimodal imaging and photothermal therapy (PTT). The authors also validated the practicability of an NIR 'photothermal ablation assisted surgery' strategy using the theranostic nanoassay, which is promising for future clinical cancer treatment.
Figure 4.
RE nanoprobes for NIR bioimaging in vivo. (A). Principal scheme for in vivo experiments and NIR bioimaging. NIR images of mice at different localizations with different nanoparticle amounts. Adapted with permission from 82, Copyright 2007, National Academy of Sciences. (B). Schematic of the portable short-wave infrared (SWIR) imaging prototype using 980 nm NIR excitation and the bioimaging for injected tumor on nude mouse. Adapted with permission from 114, Copyright 2013, Nature Springer. (C) Scheme illustration of assembly and NIR laser‐regulated disassembly of nanoprobes for stable and accurate NIR‐II bioimaging. (D) Schematic depiction of experimental timeline for the in vivo assembly and 980 nm laser‐triggered in vivo disassembly and NIR‐II fluorescence bioimaging results for the abdomen (1000 nm long‐pass filter) of the nude mice with murine epidermal tumor by two‐staged in‐sequence injection of RE nanoparticles (interval between two injections is 10 h) under 808 nm excitation. Adapted with permission from 115, Copyright 2018, John Wiley & Sons, Inc.
In 2019, a surge of RE nanoparticles for NIR bioimaging have been reported by different research groups. Zhang's group reported that in vivo assembly and disassembly of supramolecularly engineered NIR‐II emissive RE nanoparticles (Figure 4 c,d) can greatly improve the quality of bioimaging 115. In another work by the same team, they succeeded in precise in vivo inflammation imaging technique using in situ responsive cross‐linking of glutathione‐modified NIR‐II lanthanide nanoparticles. NIR‐II signals in the inflamed area were observed within 10 min and lasted as long as 8 h. The signal-noise ratio of inflammatory bioimaging was enhanced 2.9‐fold compared with reference groups at the same time. Their ROS‐responsive in vivo crosslinking strategy provides a safe and easy route for the fast location of and long‐term imaging of inflamed areas 116. Li et al. proposed the poly(acrylic acid) (PAA)-modified NaLnF4:40Gd/20Yb/2Er nanorods (Ln = Y, Yb, Lu, PAA-Ln-NRs) with enhanced downshifting NIR-IIb emission for improved quality of bioimaging 117. The downshifting emission beyond 1500 nm is doubled by suppressing the upconversion path through Ce3+ doping. The explored bright NIR-IIb emitted PAA-Lu-NRs were used for a series of applications, including high sensitivity small tumor (∼4 mm) imaging, metastatic tiny tumor detection (∼3 mm), high spatial resolution (41 μm) tumor vessel visualization, and brain vessel imaging. Their findings opened the opportunity of utilizing the RE based NIR-IIb probe for in vivo tumor vessel/metastasis and noninvasive brain vascular imaging. It should be drawn more interests that Gu et al. reported an important progress of NIR bioimaging using RE nanoparticles 118. In their work, a time-domain (τ) based light transducer was applied instead of conventional spectra-domain signaling, serving as a new weapon for in vivo NIR imaging. The ytterbium-based transducer can convert the pulsed NIR irradiation into long-decaying luminescence with an efficiency approaching 100%. This technique can largely improve the signal-to-noise ratio and bioimaging quality in mice models.
DNA nanotechnology 119 also plays an important role in bioimaging using RE nanoparticles. DNA structures, including G‐quadruplexes 120, aptamers 121, molecular switches 122, framework nucleic acids 123 (FNAs, eg. DNA tetrahedrons 124,125), and origamis 126, were widely involved in design of probes for RE nanoparticles based bioimaging systems 127 or theranostic devices 128,129. In comparison of other materials such as inorganic gold nanoparticles 130,131, DNA nanostructures 132,133 showed extraordinary biocompatibility, degradability, low size dispersibility 134 and programmability 135. The reversible Watson-Crick pairing of DNA also provide a versatile platform to construct dynamic, programmable, precisely controlled devices 136 for sensing 137 and imaging in combination of RE nanoparticles. For example, Lu and his group introduced DNA modifications to RE nanoparticles and successfully obtained controllable assemblies of gold nanoparticles onto RE upconversion nanoparticles for improved drug delivery and bioimaging 138. Kuang et al. reported the self-assemblies of RE nanoparticles with DNA tetrahedrons and applied them as a chiral sensing platform for cell imaging and direct observation of autophagy 139.
4. RE nanoparticles for NIR photodynamic therapy (PDT) and drug delivery
The NIR bioimaging systems were widely studied in various biological applications and clinical attempts, such as cell and tissue imaging, tumour diagnosis and therapy, and surgical navigation. However, limited by the difficulties of clinical practices, most of the researches of NIR bioimaging did not reach the surgical guidance level. Considering this, we also concluded the recent progresses in photodynamic therapy (PDT) and drug delivery using rare earth nanoparticles since the requirements of the probes and the NIR imaging equipment are similar with IGS but practically much easier to achieve to a lot of research groups in this field. The highly related fields will share a view in material design, safety estimation, animal models and so on 140. For bioimaging and IGS applications, the performance is largely determined by the signal-to-background ratio and targeting affinity. It requires higher fluorescence efficiency, lower tissue photo-absorption and stabilized functionalization. Down-shifting RE nanoparticles with NIR-II emission excited by NIR-I laser is commonly used to achieve good in vivo bioimaging quality. For PDT and drug delivery design, higher photon energy is demanded to trigger the ROS generation or release of cargos. And upconversion nanoparticles that will give rise to the photon energies are preferred.
Photodynamic therapy (PDT) is a non-invasive treatment modality for a variety of diseases including cancer 141,142. A recent popular strategy to conduct PDT is based on a subclass of RE nanoparticles, upconversion nanoparticles (UCNPs). Upon NIR excitation, UCNPs emit visible light with anti-Stokes shifts, which can be applied to activate modified photosensitizers to produce reactive oxygen species (such as 1O2) and damage cancer cells through oxidative stress and activated metabolic autophagy 143. NIR-excited UCNPs can be utilized to activate photosensitizers in deep tissues and exhibit wider coverage of therapies and better efficiency than traditional PDT under visible or UV light illumination. Similarly, RE nanoparticles could also be used for NIR light-triggered drug release 144 through photothermal process or photochemical cascade reactions 145,146.
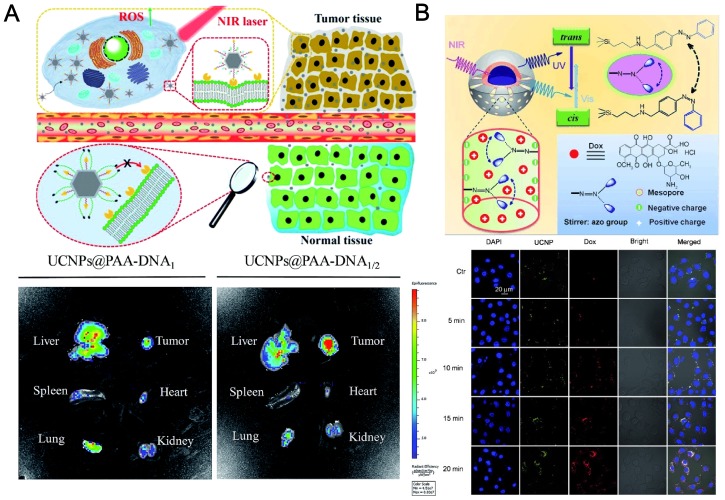
The first in vivo UCNP-based PDT study on animals was demonstrated by Liu's team. 48 They applied non-covalently incorporated Ce6 onto PEGylated amphiphilic polymer-coated upconversion nanoparticles (UCNPs). The obtained UCNP-Ce6 complex could enter cancer cells and induce 4T1 cell death after being exposed to the 980-nm NIR light. The survival of mice after UCNP-Ce6 injection and PDT treatment was dramatically pro-longed compared to the control group. They also found that the injected UCNPs could be gradually cleared out after 2 months, determined by ex vivo ICP-AES measurement, without noticeable toxicity to the treated mice. It is valuable that the authors also compared the tissue penetration abilities for the same NIR probes induced by 980-nm NIR light and 660 nm visible light. It is observed that more singlet oxygens were generated under 660-nm illumination, in comparison to UCNP-Ce6 sample under the 980 nm excitation. But under 8 mm tissue (pork) blocking, 660 nm visible light will lose its power in singlet oxygen production but 980 nm NIR illumination remains high efficiency. Very recently, Yu et al. developed a pre-protective strategy using a switchable folic acid modified UCNPs conjugated with two types of DNA in different lengths. In normal tissues, folic acid is protected by longer DNA. The platform can be triggered in tumor site to exposed folic acids for tumor targeting and NIR PDT (Figure 5a) 147.
Figure 5.
(A) Precise tumor targeting and specific PDT for cancer of UCNPs@PAA-DNA. In vivo imaging of five major organs harvested from a mouse at 8 h postinjection with UCNPs@PAA-DNA1(Ce6) (left) or UCNPs@PAA-DNA1/2 (right). Adapted with permission from 147, Copyright 2018, Royal Society of Chemistry. (B) Upper: NIR light‐triggered Dox release by making use of the upconversion property of UCNPs and trans-cis photoisomerization of azo molecules grafted in the mesopore network of a mesoporous silica layer. Down: CLSM observations of the photocontrolled Dox release in HeLa cells. Adapted with permission from 145, Copyright 2013, John Wiley & Sons, Inc.
Besides the application of UCNPs in PDT, photo-responsive drug release systems using NIR triggering, have received remarkable emphasis in recent years, due to their promising potential in noninvasive theranostics at the site of nidus (e.g. tumors) 148. For example, Shi et al. fabricated mesoporous silica coated UCNPs modified by azobenzene molecules. 145 The anticancer drug doxorubicin (DOX) were controllably released from the outer layer of the mesoporous silica under NIR laser irradiation (Figure 5b). Qu et al. reported a NIR upconversion responsive system carrying two cargos (clioquinol and curcumin) to stepwise sequential release 149. When the UCNP platform is irradiated at low intensity of the NIR laser, clioquinol is first released for chelating with free metal ions such as Cu2+, which hinders the efficacy of curcumin. Subsequently, under higher intensity of NIR illumination, curcumin is subsequently released. The stepwise-release strategy can greatly improve the activity of curcumin for the inhibition of amyloid aggregation. Excess Cu2+ ions and superfluous ROS can be cleaned up by the NIR-triggered drug delivery platform.
5. Surgery guide using NIR emissive RE nanoparticles
Inspired by the success of bioimaging and PDT therapies using NIR emissive nanoparticles, researchers urged to put forwards the employment of NIR probes into clinical practices. Tian et al. used ZnGa2O4Cr0.004 (ZGC) nanoparticles for guided surgery during operation to accurate delineation of hepatocellular carcinoma (HCC) 23. ZGC showed excellent long-lasting NIR afterglow properties that lasted for hours, which can improve real-time guided surgical quality. Though the ZGC nanoparticles employed in this work were not consisting of any RE elements, the ZGC probes with NIR emission is surely a continuum of its prototype counterpart-- Zn2.94Ga1.96Ge2O10:Cr3+,Pr3+ nanoparticles, where RE element Pr plays as emitters 80.
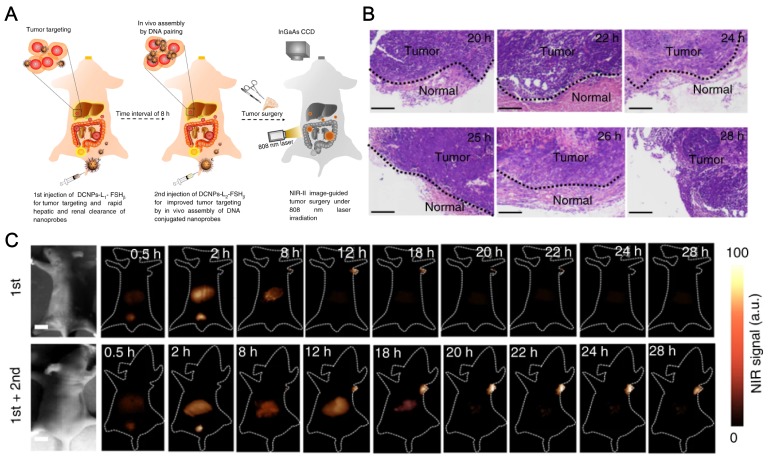
Very recently, Zhang's lab at Fudan University also reported in vivo assembly of the lanthanides doped NaGdF4 based NIR-II emitting nanoparticles to improve the IGS for metastatic ovarian cancer (Figure 6) 150. The NIR-II probes were modified with DNA and targeting peptides while the imaging quality is largely improved with good photostability and deep tissue penetration over 8 mm, in comparison to that of conventional organic dye, indocyanine green (ICG). The authors observed in vivo assembly of the nanoprobes, which increases the tumor retention period to 6 h, enabled precise tumor resection. Also, better tumor-to-normal tissue ratio is successfully achieved to facilitate the abdominal ovarian metastases surgical operation. The preclinical practice proved that metastases smaller than 1 mm can be completely excised under Zhang's NIR-II bioimaging guidance. This work is a milestone of the applications of RE based NIR emissive nanoparticles and greatly encourages researchers to bring NIR fluorescence IGS to clinical surgery.
Figure 6.
NaGdF4 based NIR-II nanoprobes in-vivo assembly to improve IGS for metastatic ovarian cancer. (A) Schematic illustration of NIR-II nanoprobes fabrication for ovarian metastasis surgery under NIR-II bioimaging guidance. (B) Hematoxylin and eosin (H&E) staining results of the tumors resected in 20-28 h PI under NIR-II fluorescence bioimaging guidance. (C) NIR-II fluorescence bioimaging (1000 nm long-pass filter) of the nude mice with murine epidermal tumor by single caudal vein first injection and two-staged in sequence injection (first + second) (interval between two injection is 8 h) under 808 nm excitation (fluence rate = 40 mW cm-2). The concentration of DCNPs in single injection is same to the sum of that for two-staged injection. All scale bars: 1 cm. Representative images are for n = 5 per group. Adapted with permission from 150, Copyright 2017, Nature Springer.
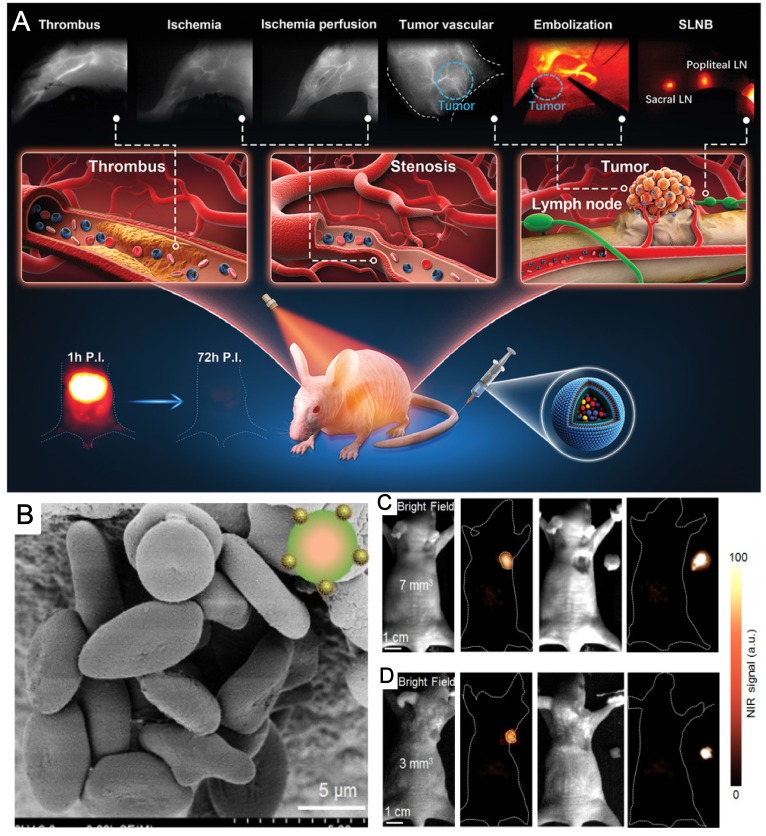
There is an increase of reports of NIR-II based IGS using RE nanoparticles since last year. Liu and his collaborators fabricated functionalized red blood cells with RE UCNPs as a multimodal probe for NIR-II luminescence guiding precise tumor resection under an 808-nm laser irradiation and meanwhile laser activated O2 release to help PDT therapy for popliteal lymph node metastasis 152. In their work, it is clearly shown that NIR-II fluorescence imaging largely improves the penetration of light and exhibits lower signal-noise ratio. The penetration depth of the NIR-II fluorescence of their probe doubled in comparison of that for NIR-I fluorescence. The red blood cell and RE nanoparticles based NIR-II probe enabled the successfully NIR-II guided surgical removal of small tumor with a size of 7 mm3 and 3 mm3 (Figure 7 b,c).
Figure 7.
RE nanoparticles for surgical guidance with NIR imaging. (A) Schematic illustration of excretable RE nanoparticle for multifunctional biomedical imaging and ICG in the NIR-II window. (B) SEM images of multimodal probes enabled by red blood cell coated with NIR-II emissive lanthanide nanoparticles. Adapted with permission from 151, Copyright 2019, John Wiley & Sons, Inc. (C, D) NIR II fluorescence bioimaging results (12 h PI) of epidermal tumors with sizes of 7 mm3 (C) and 3 mm3 (D) and NIR II fluorescence bioimaging results after the surgical resection of tumors. Adapted with permission from 152, Copyright 2019, Ivyspring International Publisher.
All the above-mentioned reports of RE based NIR-bioimaging guided surgery concentrated on direct targeting to the tumor or immunological recognition of cancer tissues. However, Li et al. provided us another choice for NIR imaging based IGS with RE nanoparticles, without any targeting strategies to tumors 151. The RE nanoparticles was used for NIR-II visualization of circulatory systems instead of the tumors. Due to the moderate half-time of blood circulation, their probes are capable of monitoring vascular disorders including artery thrombosis, ischemia, and tumor angiogenesis. The cancer therapy was constructed through a blood vessel embolization surgery conducted with NIR-II navigation of femur orthotopic osteosarcoma on nude mice. In addition, the NIR-II probe is also applicable for sentinel lymph nodes imaging and sequential biopsy by tail injection.
6. Conclusion and Perspective
Rare earth nanoparticles have many advantages, such as high NIR luminescence efficiency, low toxicity, and good biocompatibility. They hold great promise in a wide range of applications in cancer diagnosis and treatment, and surgical navigation. However, there are only limited reports on the application of RE nanoparticles in surgical navigation at clinical level. NIR small molecular dyes and quantum dots are still the mainstream of probes for NIR fluorescence ICG. This is mainly because of the following two reasons: 1) Concerns about the safety of RE nanoparticles, including their refractoriness and toxicity of possibly released rare earth ions; 2) In order to achieve higher sensitivity and spatiotemporal resolution in IGS, smaller RE nanoparticles are required, however, the luminescence efficiency of RE nanoparticles decreases rapidly within smaller size nanoparticles 153. Whereas the nanoparticles smaller than 10 nm has no advantage against competing semiconductor quantum dots in terms of luminescence efficiency.
On the other hand, the current reports of NIR surgical navigations using lanthanide nanoparticles are mostly focused on simple animal models such as ovarian tumor metastases and unilateral thrombus on nude mice. Larger animals such as rabbits 154 and dogs 155 have not yet been employed in NIR emissive RE nanoparticles based IGS. Considering that the major advantage of using NIR emissive RE nanoparticles is to boost the penetration depth of the excitation light, it is important to verify it in larger animals with thicker tissues. Thus, it is of great urgency to develop new disease models to larger mammals which can be better mimics for human body. However, the penetration depth of NIR fluorescence of current reports are mostly no larger than 10 mm, which is obviously impractical for clinical surgery of human body. From this aspect, we shall prospect that there is still great space for the improvement of the fluorescence intensity, quantum yield, noise-to-background ratio and eventually penetration depth for the RE nanoparticles of NIR emission.
Therefore, the future development trends of RE nanoparticles in the field of NIR fluorescent IGS are proposed as follows: a) Develop degradable and metabolizable rare earth nanoparticles, where the metabolites of the nanoparticles are required to be non-toxic too; b) Further improve the luminescence efficiency of NIR, especially for small size nanoparticles, it is necessary to surpass inorganic semiconductor quantum dots (such as Ag2S) 156 and also improve the penetration depth of NIR fluorescence. c) Expand the unique luminescent properties such as long afterglow and time-resolved luminescence, and utilize the magnetism of rare earth elements such as gadolinium to develop multi-mode molecular imaging technology including MRI and multiple optical imaging techniques.
Acknowledgments
This work was supported by the National Science Foundation of China (21834007, 21904087), National Key R&D Program of China (2016YFA0400900), Science and Technology Commission of Shanghai Municipality (19ZR1474600), China Postdoctoral Science Foundation (2018M641995), the Key Research Program of Frontier Sciences (QYZDJ-SSW-SLH031), the Open Large Infrastructure Research of CAS, Chinese Academy of Sciences, LU Jiaxi International Team of the Chinese Academy of Sciences, K. C. Wong Foundation at Shanghai Jiao Tong University, and Innovative research team of high-level local universities in Shanghai.
References
- 1.Demicheli R, Retsky MW, Hrushesky WJM, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–8. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 2.de Boer E, Harlaar NJ, Taruttis A. et al. Optical innovations in surgery. Brit J Surg. 2015;102:e56–72. doi: 10.1002/bjs.9713. [DOI] [PubMed] [Google Scholar]
- 3.Cabioglu N, Hunt KK, Sahin AA. et al. Role for Intraoperative Margin Assessment in Patients Undergoing Breast-Conserving Surgery. Ann Surg Oncol. 2007;14:1458–71. doi: 10.1245/s10434-006-9236-0. [DOI] [PubMed] [Google Scholar]
- 4.Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs. histologic measurement of breast cancer following chemotherapy: Comparison with x-ray mammography and palpation. J Magn Reson Imaging. 2001;13:868–75. doi: 10.1002/jmri.1124. [DOI] [PubMed] [Google Scholar]
- 5.Debie P, Hernot S. Emerging Fluorescent Molecular Tracers to Guide Intra-Operative Surgical Decision-Making. Front Pharmacol. 2019;10:510–20. doi: 10.3389/fphar.2019.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Sajja HK, Cao Z. et al. uPAR-targeted optical imaging contrasts as theranostic agents for tumor margin detection. Theranostics. 2013;4:106–18. doi: 10.7150/thno.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagao T, Inoue S, Yoshimi F. et al. Postoperative recurrence of hepatocellular carcinoma. Ann Surg. 1990;211:28–33. doi: 10.1097/00000658-199001000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L, Wang H, He B. et al. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics. 2016;6:762–72. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homann M, Sansjofre P, Van Zuilen M. et al. Microbial life and biogeochemical cycling on land 3,220 million years ago. Nat Geosci. 2018;11:665–71. [Google Scholar]
- 10.Sun Y, Ding F, Zhou Z. et al. Rhomboidal Pt(II) metallacycle-based NIR-II theranostic nanoprobe for tumor diagnosis and image-guided therapy. Proc Natl Acad Sci. 2019;116:1968–1973. doi: 10.1073/pnas.1817021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi C, Du Y, Ye J. et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;4:1072–84. doi: 10.7150/thno.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu K, Xu Y, Li H. et al. Real-Time Tracking and In Vivo Visualization of β-Galactosidase Activity in Colorectal Tumor with a Ratiometric Near-Infrared Fluorescent Probe. J Am Chem Soc. 2016;138:5334–40. doi: 10.1021/jacs.6b01705. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Wang D, Zhang Y. et al. A Phosphorus Phthalocyanine Formulation with Intense Absorbance at 1000 nm for Deep Optical Imaging. Theranostics. 2016;6:688–97. doi: 10.7150/thno.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Sun X, Guo Z. et al. In Vivo and in Situ Tracking Cancer Chemotherapy by Highly Photostable NIR Fluorescent Theranostic Prodrug. J Am Chem Soc. 2014;136:3579–88. doi: 10.1021/ja412380j. [DOI] [PubMed] [Google Scholar]
- 15.Lv G, Guo W, Zhang W. et al. Near-Infrared Emission CuInS/ZnS Quantum Dots: All-in-One Theranostic Nanomedicines with Intrinsic Fluorescence/Photoacoustic Imaging for Tumor Phototherapy. ACS Nano. 2016;10:9637–45. doi: 10.1021/acsnano.6b05419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Fan W, Zhang Z, Wen Y, Xiong L, Chen X. Advanced Nanotechnology Leading the Way to Multimodal Imaging-Guided Precision Surgical Therapy. Adv Mater. 2019;0:1904329.. doi: 10.1002/adma.201904329. in press. doi: 10.1002/adma.201904329. [DOI] [PubMed] [Google Scholar]
- 17.Vahrmeijer AL, Hutteman M, Van Der Vorst JR, Van De Velde CJH, Frangioni J V. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–18. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low PS, Singhal S, Srinivasarao M. Fluorescence-guided surgery of cancer: applications, tools and perspectives. Curr Opin Chem Biol. 2018;45:64–72. doi: 10.1016/j.cbpa.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Tian F, Zhang Y, Zhang Y, Li C, Wang Q. Tracking of Transplanted Human Mesenchymal Stem Cells in Living Mice using Near-Infrared Ag2S Quantum Dots. Adv Funct Mater. 2014;24:2481–8. [Google Scholar]
- 20.Kelderhouse LE, Chelvam V, Wayua C. et al. Development of tumor-targeted near infrared probes for fluorescence guided surgery. Bioconjug Chem. 2013;24:1075–80. doi: 10.1021/bc400131a. [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam SM, Chu H, Liu X, Leamon CP, Low PS. Carbonic Anhydrase IX-Targeted Near-Infrared Dye for Fluorescence Imaging of Hypoxic Tumors. Bioconjug Chem. 2018;29:3320–31. doi: 10.1021/acs.bioconjchem.8b00509. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Li X, Tse BW-C. et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics. 2018;8:1227–42. doi: 10.7150/thno.22872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ai T, Shang W, Yan H. et al. Near infrared-emitting persistent luminescent nanoparticles for Hepatocellular Carcinoma imaging and luminescence-guided surgery. Biomaterials. 2018;167:216–25. doi: 10.1016/j.biomaterials.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Deng H, Zhong Y, Du M. et al. Theranostic self-assembly structure of gold nanoparticles for NIR photothermal therapy and X-Ray computed tomography imaging. Theranostics. 2014;4:904–18. doi: 10.7150/thno.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal K, Sharma V, Sahoo D, Kapuria N, Koner AL. Large Stokes-shifted NIR-emission from nanospace-induced aggregation of perylenemonoimide-doped polymer nanoparticles: imaging of folate receptor expression. Chem Commun. 2018;54:523–6. doi: 10.1039/c7cc08404h. [DOI] [PubMed] [Google Scholar]
- 26.Palner M, Pu K, Shao S, Rao J. Semiconducting Polymer Nanoparticles with Persistent Near-Infrared Luminescence for in Vivo Optical Imaging. Angew Chem Int Ed. 2015;54:11477–80. doi: 10.1002/anie.201502736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Yue J, Cui R. et al. Bright quantum dots emitting at ∼1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc Natl Acad Sci. 2018;115:6590–6595. doi: 10.1073/pnas.1806153115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Lim YT, Soltesz EG. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S, Yung BC, Chandra S, Niu G, Antaris AL, Chen X. Near-Infrared-II (NIR-II) Bioimaging via Off-Peak NIR-I Fluorescence Emission. Theranostics. 2018;8:4141–51. doi: 10.7150/thno.27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivero-Escoto JL, Huxford-Phillips RC, Lin W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem Soc Rev. 2012;41:2673–85. doi: 10.1039/c2cs15229k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinho O, Silva-Oliveira R, Cury FP. et al. HER Family Receptors are Important Theranostic Biomarkers for Cervical Cancer: Blocking Glucose Metabolism Enhances the Therapeutic Effect of HER Inhibitors. Theranostics. 2017;7:717–32. doi: 10.7150/thno.17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiler M, Dixon JB. Differential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front Physiol. 2013;4:215–10. doi: 10.3389/fphys.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp DW, Adams LG, DeGrand AM. et al. Sentinel Lymph Node Mapping of Invasive Urinary Bladder Cancer in Animal Models Using Invisible Light. Eur Urol. 2007;52:1700–9. doi: 10.1016/j.eururo.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergholt MS, Zheng W, Lin K. et al. Combining near-infrared-excited autofluorescence and Raman spectroscopy improves in vivo diagnosis of gastric cancer. Biosens Bioelectron. 2011;26:4104–10. [Google Scholar]
- 35.Keereweer S, Hutteman M, D.F. Kerrebijn J, J.H. van de Velde C, L. Vahrmeijer A, W.G.M. Lowik C. Translational Optical Imaging in Diagnosis and Treatment of Cancer. Current Pharmaceutical Biotech. 2012;13:498–503. doi: 10.2174/138920112799436294. [DOI] [PubMed] [Google Scholar]
- 36.Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng. 2017;1:10–22. [Google Scholar]
- 37.Choi HS, Gibbs SL, Lee JH. et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148–53. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Lu L, Zhao M, Lei Z, Zhang F. An Efficient 1064 nm NIR-II Excitation Fluorescent Molecular Dye for Deep-Tissue High-Resolution Dynamic Bioimaging. Angew Chem Int Ed. 2018;57:7483–7. doi: 10.1002/anie.201801226. [DOI] [PubMed] [Google Scholar]
- 39.He S, Song J, Qu J, Cheng Z. Crucial breakthrough of second near-infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem Soc Rev. 2018;47:4258–78. doi: 10.1039/c8cs00234g. [DOI] [PubMed] [Google Scholar]
- 40.Kam NWS, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci. 2005;102:11600–5. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Q, Ma Z, Wang H. et al. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv Mater. 2017;29:1605497–9. doi: 10.1002/adma.201605497. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Bai G, Zeng S, Hao J. Theranostic Carbon Dots with Innovative NIR-II Emission for in Vivo Renal-Excreted Optical Imaging and Photothermal Therapy. ACS Appl Mater Interfaces. 2019;11:4737–44. doi: 10.1021/acsami.8b14877. [DOI] [PubMed] [Google Scholar]
- 43.Bouzigues C, Gacoin T, Alexandrou A. Biological applications of rare-earth based nanoparticles. ACS Nano. 2011;5:8488–505. doi: 10.1021/nn202378b. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Li Z, Hu D, Lin CT, Li J, Lin Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J Am Chem Soc. 2010;132:9274–6. doi: 10.1021/ja103169v. [DOI] [PubMed] [Google Scholar]
- 45.Tian G, Gu Z, Liu X. et al. Facile Fabrication of Rare-Earth-Doped Gd2O3 Hollow Spheres with Upconversion Luminescence, Magnetic Resonance, and Drug Delivery Properties. J Phys Chem C. 2011;115:23790–6. [Google Scholar]
- 46.Gai S, Li C, Yang P, Lin J. Recent Progress in Rare Earth Micro/Nanocrystals: Soft Chemical Synthesis, Luminescent Properties, and Biomedical Applications. Chem Rev. 2014;114:2343–89. doi: 10.1021/cr4001594. [DOI] [PubMed] [Google Scholar]
- 47.Kömpe K, Borchert H, Storz J. et al. Green-Emitting CePO4:Tb/LaPO4 Core-Shell Nanoparticles with 70 % Photoluminescence Quantum Yield. Angew Chem Int Ed. 2003;42:5513–6. doi: 10.1002/anie.200351943. [DOI] [PubMed] [Google Scholar]
- 48.Sun W, Yu J, Deng R. et al. Semiconducting Polymer Dots Doped with Europium Complexes Showing Ultranarrow Emission and Long Luminescence Lifetime for Time-Gated Cellular Imaging. Angew Chem Int Ed. 2013;52:11294–7. doi: 10.1002/anie.201304822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonnet CS, Buron F, Caillé F. et al. Pyridine-Based Lanthanide Complexes Combining MRI and NIR Luminescence Activities. Chem A Eur J. 2012;18:1419–31. doi: 10.1002/chem.201102310. [DOI] [PubMed] [Google Scholar]
- 50.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni J V. Tissue- and Organ-Selective Biodistribution of NIR Fluorescent Quantum Dots. Nano Lett. 2009;9:2354–9. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz D, del Rosal B, Acebrón M. et al. Ag/Ag2S Nanocrystals for High Sensitivity Near-Infrared Luminescence Nanothermometry. Adv Funct Mater. 2017;27:1604629–9. [Google Scholar]
- 52.Shou K, Tang Y, Chen H. et al. Diketopyrrolopyrrole-based semiconducting polymer nanoparticles for in vivo second near-infrared window imaging and image-guided tumor surgery. Chem Sci. 2018;9:3105–10. doi: 10.1039/c8sc00206a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–38. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 54.Kanduluru AK, Srinivasarao M, Low PS. Design, Synthesis, and Evaluation of a Neurokinin-1 Receptor-Targeted Near-IR Dye for Fluorescence-Guided Surgery of Neuroendocrine Cancers. Bioconjug Chem. 2016;27:2157–65. doi: 10.1021/acs.bioconjchem.6b00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L-D, Wang Y-F, Yan C-H. Paradigms and Challenges for Bioapplication of Rare Earth Upconversion Luminescent Nanoparticles: Small Size and Tunable Emission/Excitation Spectra. Acc Chem Res. 2014;47:1001–9. doi: 10.1021/ar400218t. [DOI] [PubMed] [Google Scholar]
- 56.Chen H-Q, Fu J, Wang L. et al. Ultrasensitive mercury(II) ion detection by europium(III)-doped cadmium sulfide composite nanoparticles. Talanta. 2010;83:139–44. doi: 10.1016/j.talanta.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 57.Wolska E, Kaszewski J, Kiełbik P, Grzyb J, Godlewski MM, Godlewski M. Rare earth activated ZnO nanoparticles as biomarkers. Opt Mater. 2014;36:1655–9. [Google Scholar]
- 58.Wang Y, Qin W, Zhang J. et al. Synthesis, photoluminescence and bioconjugation of rare-earth (Eu) complexes-embedded silica nanoparticles. Solid State Commun. 2007;142:689–93. [Google Scholar]
- 59.Rafique R, Kailasa SK, Park TJ. Recent advances of upconversion nanoparticles in theranostics and bioimaging applications. TrAC Trends Anal Chem. 2019;120:115646–19. [Google Scholar]
- 60.Skripka A, Karabanovas V, Jarockyte G. et al. Decoupling Theranostics with Rare Earth Doped Nanoparticles. Adv Funct Mater. 2019;29:1807105–12. [Google Scholar]
- 61.Zhao X, He S, Tan MC. Design of infrared-emitting rare earth doped nanoparticles and nanostructured composites. J Mater Chem C. 2016;4:8349–72. [Google Scholar]
- 62.Hemmer E, Venkatachalam N, Hyodo H. et al. Upconverting and NIR emitting rare earth based nanostructures for NIR-bioimaging. Nanoscale. 2013;5:11339–61. doi: 10.1039/c3nr02286b. [DOI] [PubMed] [Google Scholar]
- 63.Naczynski DJ, Tan MC, Riman RE, Moghe P V. Rare earth nanoprobes for functional biomolecular imaging and theranostics. J Mater Chem B. 2014;2:2958–73. doi: 10.1039/C4TB00094C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan MH, Liu RS. Advanced sensing, imaging, and therapy nanoplatforms based on Nd3+-doped nanoparticle composites exhibiting upconversion induced by 808 nm near-infrared light. Nanoscale. 2017;9:18153–68. doi: 10.1039/c7nr06693g. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Wang H, Liu D, Song S, Wang X, Zhang H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials. 2013;34:7715–24. doi: 10.1016/j.biomaterials.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Jiang M, Zeng S, Liu H. Polydopamine coated multifunctional lanthanide theranostic agent for vascular malformation and tumor vessel imaging beyond 1500 nm and imaging-guided photothermal therapy. Theranostics. 2019;9:3866–78. doi: 10.7150/thno.31864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin M, Ju E, Chen Z, Li Z, Ren J, Qu X. Upconverting Nanoparticles with a Mesoporous TiO2 Shell for Near-Infrared-Triggered Drug Delivery and Synergistic Targeted Cancer Therapy. Chem A Eur J. 2014;20:14012–7. doi: 10.1002/chem.201403733. [DOI] [PubMed] [Google Scholar]
- 68.Mangeolle T, Yakavets I, Marchal S. et al. Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis. Nanomaterials. 2018;8:572–20. doi: 10.3390/nano8080572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J, Shirahata N, Sun HT. et al. Efficient dual-modal NIR-to-NIR emission of rare earth ions co-doped nanocrystals for biological fluorescence imaging. J Phys Chem Lett. 2013;4:402–8. doi: 10.1021/jz302122a. [DOI] [PubMed] [Google Scholar]
- 70.Wang F, Liu X. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J Am Chem Soc. 2008;130:5642–3. doi: 10.1021/ja800868a. [DOI] [PubMed] [Google Scholar]
- 71.Takeshita S, Takebayashi Y, Nakamura H, Yoda S. Gas-Responsive Photoluminescence of YVO4:Eu3+ Nanoparticles Dispersed in an Ultralight, Three-Dimensional Nanofiber Network. Chem Mater. 2016;28:8466–9. [Google Scholar]
- 72.Ren G, Zeng S, Hao J. Tunable Multicolor Upconversion Emissions and Paramagnetic Property of Monodispersed Bifunctional Lanthanide-Doped NaGdF4 Nanorods. J Phys Chem C. 2011;115:20141–7. [Google Scholar]
- 73.Mi C-C, Tian Z, Han B, Mao C, Xu S. Microwave-assisted one-pot synthesis of water-soluble rare-earth doped fluoride luminescent nanoparticles with tunable colors. J Alloys Compd. 2012;525:154–8. doi: 10.1016/j.jallcom.2012.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu S-M, Liu F-Q, Wang Z-G. Relaxation of carriers in terbium-doped ZnO nanoparticles. Chem Phys Lett. 2001;343:489–92. [Google Scholar]
- 75.Wang Y-F, Sun L-D, Xiao J-W. et al. Rare-Earth Nanoparticles with Enhanced Upconversion Emission and Suppressed Rare-Earth-Ion Leakage. Chem A Eur J. 2012;18:5558–64. doi: 10.1002/chem.201103485. [DOI] [PubMed] [Google Scholar]
- 76.Qiu Z, Zhou Y, Lü M, Zhang A, Ma Q. Combustion synthesis of long-persistent luminescent MAl2O4: Eu2+, R3+ (M=Sr, Ba, Ca, R=Dy, Nd and La) nanoparticles and luminescence mechanism research. Acta Mater. 2007;55:2615–20. [Google Scholar]
- 77.Lécuyer T, Teston E, Ramirez-Garcia G. et al. Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics. 2016;6:2488–524. doi: 10.7150/thno.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buono-core GE, Li H, Marciniak B. Quenching of excited states by lanthanide ions and chelates in solution. Coord Chem Rev. 1990;99:55–87. [Google Scholar]
- 79.Yi G, Peng Y, Gao Z. Strong red-emitting near-infrared-to-visible upconversion fluorescent nanoparticles. Chem Mater. 2011;23:2729–34. [Google Scholar]
- 80.Abdukayum A, Chen J-T, Zhao Q, Yan X-P. Functional Near Infrared-Emitting Cr3+/Pr3+ Co-Doped Zinc Gallogermanate Persistent Luminescent Nanoparticles with Superlong Afterglow for in Vivo Targeted Bioimaging. J Am Chem Soc. 2013;135:14125–33. doi: 10.1021/ja404243v. [DOI] [PubMed] [Google Scholar]
- 81.Ni X, Zhang X, Duan X, Zheng HL, Xue XS, Ding D. Near-Infrared Afterglow Luminescent Aggregation-Induced Emission Dots with Ultrahigh Tumor-to-Liver Signal Ratio for Promoted Image-Guided Cancer Surgery. Nano Lett. 2019;19:318–30. doi: 10.1021/acs.nanolett.8b03936. [DOI] [PubMed] [Google Scholar]
- 82.le Masne de Chermont Q, Chaneac C, Seguin J. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci. 2007;104:9266–71. doi: 10.1073/pnas.0702427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyu L, Cheong H, Ai X. et al. Near-infrared light-mediated rare-earth nanocrystals: recent advances in improving photon conversion and alleviating the thermal effect. NPG Asia Mater. 2018;10:685–702. [Google Scholar]
- 84.Shang X, Chen P, Jia T. et al. Upconversion luminescence mechanisms of Er3+ ions under excitation of an 800 nm laser. Phys Chem Chem Phys. 2015;17:11481–9. doi: 10.1039/c5cp00057b. [DOI] [PubMed] [Google Scholar]
- 85.Skripka A, Benayas A, Marin R, Canton P, Hemmer E, Vetrone F. Double rare-earth nanothermometer in aqueous media: opening the third optical transparency window to temperature sensing. Nanoscale. 2017;9:3079–85. doi: 10.1039/c6nr08472a. [DOI] [PubMed] [Google Scholar]
- 86.Kar A, Patra A. Impacts of core-shell structures on properties of lanthanide-based nanocrystals: crystal phase, lattice strain, downconversion, upconversion and energy transfer. Nanoscale. 2012;4:3608–19. doi: 10.1039/c2nr30389b. [DOI] [PubMed] [Google Scholar]
- 87.Ma D, Xu X, Hu M. et al. Rare-Earth-Based Nanoparticles with Simultaneously Enhanced Near-Infrared (NIR)-Visible (Vis) and NIR-NIR Dual-Conversion Luminescence for Multimodal Imaging. Chem An Asian J. 2016;11:1050–8. doi: 10.1002/asia.201501456. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Li B, Wang B. et al. Magnetic nanoparticles modified with DTPA-AMC-rare earth for fluorescent and magnetic resonance dual mode imaging. Dalt Trans. 2012;41:8723–8. doi: 10.1039/c2dt30125c. [DOI] [PubMed] [Google Scholar]
- 89.Kodaira CA, Loureno AVS, Felinto MCFC. et al. Biolabeling with nanoparticles based on Y2O3: Nd 3+ and luminescence detection in the near-infrared. J Lumin. 2011;131:727–31. [Google Scholar]
- 90.Soga K, Tokuzen K, Tsuji K, Yamano T, Hyodo H, Kishimoto H. NIR Bioimaging: Development of Liposome-Encapsulated, Rare-Earth-Doped Y2O3 Nanoparticles as Fluorescent Probes. Eur J Inorg Chem. 2010;2010:2673–7. [Google Scholar]
- 91.Dorman JA, Choi JH, Kuzmanich G, Chang JP. Elucidating the effects of a rare-earth oxide shell on the luminescence dynamics of Er3+ :Y2O3 nanoparticles. J Phys Chem C. 2012;116:10333–40. [Google Scholar]
- 92.Min Y-L, Wan Y, Yu S-H. Au@Y2O3:Eu3+ rare earth oxide hollow sub-microspheres with encapsulated gold nanoparticles and their optical properties. Solid State Sci. 2009;11:96–101. [Google Scholar]
- 93.Vetrone F, Boyer J-C, Capobianco JA, Speghini A, Bettinelli M. Concentration-Dependent Near-Infrared to Visible Upconversion in Nanocrystalline and Bulk Y2O3:Er3+ Chem Mater. 2003;15:2737–43. [Google Scholar]
- 94.Kumar GA, Chen CW, Ballato J, Riman RE. Optical characterization of infrared emitting rare-earth-doped fluoride nanocrystals and their transparent nanocomposites. Chem Mater. 2007;19:1523–8. [Google Scholar]
- 95.Wong H-T, Chan HLW, Hao J. Towards pure near-infrared to near-infrared upconversion of multifunctional GdF3:Yb3+,Tm3+ nanoparticles. Opt Express. 2010;18:6123–30. doi: 10.1364/OE.18.006123. [DOI] [PubMed] [Google Scholar]
- 96.Nyk M, Kumar R, Ohulchanskyy TY, Bergey EJ, Prasad PN. Photoluminescence Bioimaging Using Near Infrared to Near Infrared Up-Conversion in Tm3+ and Yb3+ Doped Fluoride Nanophosphors. Assay Drug Dev Technol; 2008. pp. 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan Q, He S, Qian J, Cheng H, Cai F. Optimization of optical excitation of upconversion nanoparticles for rapid microscopy and deeper tissue imaging with higher quantum yield. Theranostics. 2013;3:306. doi: 10.7150/thno.6007. —316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leary J, Key J. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int J Nanomedicine. 2014;9:711–725. doi: 10.2147/IJN.S53717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdukayum A, Yang C-X, Zhao Q, Chen J-T, Dong L-X, Yan X-P. Gadolinium Complexes Functionalized Persistent Luminescent Nanoparticles as a Multimodal Probe for Near-Infrared Luminescence and Magnetic Resonance Imaging in Vivo. Anal Chem. 2014;86:4096–101. doi: 10.1021/ac500644x. [DOI] [PubMed] [Google Scholar]
- 100.Fan W, Yung B, Huang P, Chen X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem Rev. 2017;117:13566–638. doi: 10.1021/acs.chemrev.7b00258. [DOI] [PubMed] [Google Scholar]
- 101.Zhong Y, Ma Z, Zhu S. et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat Commun. 2017;8:737–7. doi: 10.1038/s41467-017-00917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdukayum A, Yang CX, Zhao Q, Chen JT, Dong LX, Yan XP. Gadolinium complexes functionalized persistent luminescent nanoparticles as a multimodal probe for near-infrared luminescence and magnetic resonance imaging in vivo. Anal Chem. 2014;86:4096–101. doi: 10.1021/ac500644x. [DOI] [PubMed] [Google Scholar]
- 103.Bunton J, Calvez L, Kadan V, Blonskyi I, Shpotyuk O, Golovchak R. Near-IR emission of Er3+ ions in CsCl-Ga-Ge-S glasses excited by visible light. Opt Mater. 2017;72:195–200. [Google Scholar]
- 104.Zhang M, Zheng W, Liu Y. et al. A New Class of Blue-LED-Excitable NIR-II Luminescent Nanoprobes Based on Lanthanide-Doped CaS Nanoparticles. Angew Chem Int Ed. 2019;58:9556–60. doi: 10.1002/anie.201905040. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Jia Q, Zhou J. Recent Advance in Near-Infrared (NIR) Imaging Probes for Cancer Theranostics. Adv Ther. 2018;1:1800055–21. [Google Scholar]
- 106.Liu L, Wang S, Zhao B. et al. Er3+ Sensitized 1530 nm to 1180 nm Second Near-Infrared Window Upconversion Nanocrystals for In Vivo Biosensing. Angew Chem Int Ed. 2018;57:7518–22. doi: 10.1002/anie.201802889. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Gong CS, Dai Y. et al. In situ polymerization on nanoscale metal-organic frameworks for enhanced physiological stability and stimulus-responsive intracellular drug delivery. Biomaterials. 2019;218:62–9. doi: 10.1016/j.biomaterials.2019.119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wenk CHF, Ponce F, Guillermet S. et al. Near-infrared optical guided surgery of highly infiltrative fibrosarcomas in cats using an anti-αvß3 integrin molecular probe. Cancer Lett. 2013;334:188–95. doi: 10.1016/j.canlet.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 109.Miao Q, Xie C, Zhen X. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat Biotechnol. 2017;35:1102–10. doi: 10.1038/nbt.3987. [DOI] [PubMed] [Google Scholar]
- 110.Dang X, Gu L, Qi J. et al. Layer-by-layer assembled fluorescent probes in the second near-infrared window for systemic delivery and detection of ovarian cancer. Proc Natl Acad Sci. 2016;113:5179–84. doi: 10.1073/pnas.1521175113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naczynski DJ, Tan MC, Zevon M. et al. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat Commun. 2013;4:2199–10. doi: 10.1038/ncomms3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang L, Wang J, Yang S, Lu Q, Li P, Li N. Rod-shape MSN@MoS2 Nanoplatform for FL/MSOT/CT Imaging-Guided Photothermal and Photodynamic Therapy. Theranostics. 2019;9:3992–4005. doi: 10.7150/thno.32715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Q, Liang C, Wang X, He J, Li Y, Liu Z. An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials. 2014;35:9355–62. doi: 10.1016/j.biomaterials.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 114.Thimsen E, Sadtler B, Berezin MY. Shortwave-infrared (SWIR) emitters for biological imaging: a review of challenges and opportunities. Nanophotonics. 2017;6:1043–54. [Google Scholar]
- 115.Zhao M, Li B, Wang P. et al. Supramolecularly Engineered NIR-II and Upconversion Nanoparticles In Vivo Assembly and Disassembly to Improve Bioimaging. Adv Mater. 2018;30:1804982–8. doi: 10.1002/adma.201804982. [DOI] [PubMed] [Google Scholar]
- 116.Zhao M, Wang R, Li B. et al. Precise In Vivo Inflammation Imaging Using In Situ Responsive Cross-linking of Glutathione-Modified Ultra-Small NIR-II Lanthanide Nanoparticles. Angew Chem. 2019;131:2072–6. doi: 10.1002/anie.201812878. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Zeng S, Hao J. Non-Invasive Optical Guided Tumor Metastasis/Vessel Imaging by Using Lanthanide Nanoprobe with Enhanced Down-Shifting Emission beyond 1500 nm. ACS Nano. 2019;13:248–59. doi: 10.1021/acsnano.8b05431. [DOI] [PubMed] [Google Scholar]
- 118.Gu Y, Guo Z, Yuan W. et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat Photonics. 2019;13:525–31. [Google Scholar]
- 119.Chao J, Zhu D, Zhang Y, Wang L, Fan C. DNA nanotechnology-enabled biosensors. Biosens Bioelectron. 2016;76:68–79. doi: 10.1016/j.bios.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 120.Yuan Q, Wu Y, Wang J. et al. Targeted Bioimaging and Photodynamic Therapy Nanoplatform Using an Aptamer-Guided G-Quadruplex DNA Carrier and Near-Infrared Light. Angew Chem Int Ed. 2013;52:13965–9. doi: 10.1002/anie.201305707. [DOI] [PubMed] [Google Scholar]
- 121.Tang Y, Hu H, Zhang MG. et al. An aptamer-targeting photoresponsive drug delivery system using “off-on” graphene oxide wrapped mesoporous silica nanoparticles. Nanoscale. 2015;7:6304–10. doi: 10.1039/c4nr07493a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Y, Liu G, Liu H, Li D, Fan C, Liu D. An Electrochemically Actuated Reversible DNA Switch. Nano Lett. 2010;10:1393–7. doi: 10.1021/nl100169p. [DOI] [PubMed] [Google Scholar]
- 123.Liu Q, Ge Z, Mao X. et al. Valency-Controlled Framework Nucleic Acid Signal Amplifiers. Angew Chem Int Ed. 2018;57:7131–5. doi: 10.1002/anie.201802701. [DOI] [PubMed] [Google Scholar]
- 124.Liang L, Li J, Li Q. et al. Single-Particle Tracking and Modulation of Cell Entry Pathways of a Tetrahedral DNA Nanostructure in Live Cells. Angew Chem Int Ed. 2014;53:7745–50. doi: 10.1002/anie.201403236. [DOI] [PubMed] [Google Scholar]
- 125.Qu X, Yang F, Chen H. et al. Bubble-Mediated Ultrasensitive Multiplex Detection of Metal Ions in Three-Dimensional DNA Nanostructure-Encoded Microchannels. ACS Appl Mater Interfaces. 2017;9:16026–34. doi: 10.1021/acsami.7b03645. [DOI] [PubMed] [Google Scholar]
- 126.Fu Y, Zeng D, Chao J. et al. Single-Step Rapid Assembly of DNA Origami Nanostructures for Addressable Nanoscale Bioreactors. J Am Chem Soc. 2013;135:696–702. doi: 10.1021/ja3076692. [DOI] [PubMed] [Google Scholar]
- 127.Qu A, Sun M, Xu L. et al. Quantitative zeptomolar imaging of miRNA cancer markers with nanoparticle assemblies. Proc Natl Acad Sci. 2019;116:3391–400. doi: 10.1073/pnas.1810764116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He L, Brasino M, Mao C. et al. DNA-Assembled Core-Satellite Upconverting-Metal-Organic Framework Nanoparticle Superstructures for Efficient Photodynamic Therapy. Small. 2017;13:1700504–7. doi: 10.1002/smll.201700504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He L, Dragavon J, Cho S. et al. Self-assembled gold nanostar-NaYF4:Yb/Er clusters for multimodal imaging, photothermal and photodynamic therapy. J Mater Chem B. 2016;4:4455–61. doi: 10.1039/c6tb00914j. [DOI] [PubMed] [Google Scholar]
- 130.Chen N, Wei M, Sun Y. et al. Self-Assembly of Poly-Adenine-Tailed CpG Oligonucleotide-Gold Nanoparticle Nanoconjugates with Immunostimulatory Activity. Small. 2014;10:368–75. doi: 10.1002/smll.201300903. [DOI] [PubMed] [Google Scholar]
- 131.Huang Y, Li M, Huang D. et al. Depth-Resolved Enhanced Spectral-Domain OCT Imaging of Live Mammalian Embryos Using Gold Nanoparticles as Contrast Agent. Small. 2019;15:1902346–13. doi: 10.1002/smll.201902346. [DOI] [PubMed] [Google Scholar]
- 132.Rinker S, Ke Y, Liu Y, Chhabra R, Yan H. Self-assembled DNA nanostructures for distance-dependent multivalent ligand-protein binding. Nat Nanotechnol. 2008;3:418–22. doi: 10.1038/nnano.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilner OI, Willner I. Functionalized DNA Nanostructures. Chem Rev. 2012;112:2528–56. doi: 10.1021/cr200104q. [DOI] [PubMed] [Google Scholar]
- 134.Edwardson TGW, Lau KL, Bousmail D, Serpell CJ, Sleiman HF. Transfer of molecular recognition information from DNA nanostructures to gold nanoparticles. Nat Chem. 2016;8:162. doi: 10.1038/nchem.2420. [DOI] [PubMed] [Google Scholar]
- 135.Langecker M, Arnaut V, Martin TG. et al. Synthetic Lipid Membrane Channels Formed by Designed DNA Nanostructures. Science. 2012;338:932–6. doi: 10.1126/science.1225624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goodman RP, Heilemann M, Doose S, Erben CM, Kapanidis AN, Turberfield AJ. Reconfigurable, braced, three-dimensional DNA nanostructures. Nat Nanotechnol. 2008;3:93–6. doi: 10.1038/nnano.2008.3. [DOI] [PubMed] [Google Scholar]
- 137.Ye D, Li L, Li Z. et al. Molecular Threading-Dependent Mass Transport in Paper Origami for Single-Step Electrochemical DNA Sensors. Nano Lett. 2019;19:369–74. doi: 10.1021/acs.nanolett.8b04051. [DOI] [PubMed] [Google Scholar]
- 138.Li L-L, Wu P, Hwang K, Lu Y. An Exceptionally Simple Strategy for DNA-Functionalized Up-Conversion Nanoparticles as Biocompatible Agents for Nanoassembly, DNA Delivery, and Imaging. J Am Chem Soc. 2013;135:2411–4. doi: 10.1021/ja310432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun M, Hao T, Li X. et al. Direct observation of selective autophagy induction in cells and tissues by self-assembled chiral nanodevice. Nat Commun. 2018;9:4494–10. doi: 10.1038/s41467-018-06946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McDannold N, Zhang Y, Supko JG. et al. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics. 2019;9:6284–99. doi: 10.7150/thno.35892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang Z, Fan W, Zou J. et al. Precision Cancer Theranostic Platform by In Situ Polymerization in Perylene Diimide-Hybridized Hollow Mesoporous Organosilica Nanoparticles. J Am Chem Soc. 2019;141:14687–98. doi: 10.1021/jacs.9b06086. [DOI] [PubMed] [Google Scholar]
- 142.Sun W, Luo L, Feng Y, Aggregation-Induced Emission Gold Clustoluminogens for Enhanced Low-Dose X-ray-Induced Photodynamic Therapy. Angew Chem Int Ed; 2019. in press. doi:10.1002/anie.201908712. [DOI] [PubMed] [Google Scholar]
- 143.Azad MB, Chen Y, Gibson SB. Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxid Redox Signal. 2008;11:777–90. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 144.Wang C, Cheng L, Liu Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics. 2013;3:317–30. doi: 10.7150/thno.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu J, Bu W, Pan L, Shi J. NIR-Triggered Anticancer Drug Delivery by Upconverting Nanoparticles with Integrated Azobenzene-Modified Mesoporous Silica. Angew Chem Int Ed. 2013;52:4375–9. doi: 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- 146.Yan B, Boyer J-C, Branda NR, Zhao Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J Am Chem Soc. 2011;133:19714–7. doi: 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- 147.Yu Z, Ge Y, Sun Q. et al. A pre-protective strategy for precise tumor targeting and efficient photodynamic therapy with a switchable DNA/upconversion nanocomposite. Chem Sci. 2018;9:3563–9. doi: 10.1039/c8sc00098k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Staninec M, Douglas SM, Darling CL. et al. Non-destructive clinical assessment of occlusal caries lesions using near-IR imaging methods. Lasers Surg Med. 2011;43:951–9. doi: 10.1002/lsm.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma M, Gao N, Sun Y, Ren J, Qu X. A Near-Infrared Responsive Drug Sequential Release System for Better Eradicating Amyloid Aggregates. Small. 2017;13:1701817. doi: 10.1002/smll.201701817. [DOI] [PubMed] [Google Scholar]
- 150.Wang P, Fan Y, Lu L. et al. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat Commun. 2018;9:2898–10. doi: 10.1038/s41467-018-05113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li D, He S, Wu Y. et al. Excretable Lanthanide Nanoparticle for Biomedical Imaging and Surgical Navigation in the Second Near-Infrared Window. Adv Sci. 2019;0:1902042.. doi: 10.1002/advs.201902042. in press. doi:10.1002/advs.201902042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang P, Wang X, Luo Q. et al. Fabrication of Red Blood Cell-Based Multimodal Theranostic Probes for Second Near-Infrared Window Fluorescence Imaging-Guided Tumor Surgery and Photodynamic Therapy. Theranostics. 2019;9:369–380. doi: 10.7150/thno.29817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gargas DJ, Chan EM, Ostrowski AD. et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat Nanotechnol. 2014;9:300–5. doi: 10.1038/nnano.2014.29. [DOI] [PubMed] [Google Scholar]
- 154.Feng Y, Chen F, Ma Z. et al. Towards stratifying ischemic components by cardiac MRI and multifunctional stainings in a rabbit model of myocardial infarction. Theranostics. 2013;4:24–35. doi: 10.7150/thno.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dewitte H, Vanderperren K, Haers H. et al. Theranostic mRNA-loaded microbubbles in the lymphatics of dogs: implications for drug delivery. Theranostics. 2015;5:97–109. doi: 10.7150/thno.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hong G, Robinson JT, Zhang Y. et al. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew Chem Int Ed. 2012;51:9818–21. doi: 10.1002/anie.201206059. [DOI] [PubMed] [Google Scholar]