Abstract
Study Objectives:
Limb movements during sleep (LMS) and periodic limb movements during sleep (PLMS) have been shown to vary by age in children. In the current study, we examined this relationship in more detail in a large clinically referred sample adjusting for iron status and sex.
Methods:
Retrospective data analysis was done on a large pediatric population who underwent an overnight sleep study and had ferritin levels measured within 30 days of sleep study between May 2013 and October 2017 at pediatric sleep center. Patients with obstructive or central sleep apneas were excluded.
Results:
A total of 1,070 patients were included in the study, with 60% males. Younger age and male sex were associated with increased PLMS and LMS. In addition, there was an increase in PLMS and LMS during adolescence that subsided at a later age, independent of sex. These associations remained significant in models controlling for ferritin level. Ferritin level, in contrast, was not a significant predictor of PLMS or LMS when controlling for sex and age.
Conclusions:
Age and sex may need to be considered when interpreting limb movement indices in pediatric sleep patients regardless of ferritin level.
Citation:
Al-Shawwa B, Ehsan Z, Perry GV, Ingram DG. Limb movements during sleep in children: effects of age, sex, and iron status in more than 1,000 patients referred to a pediatric sleep center. J Clin Sleep Med. 2020;16(1):49–54.
Keywords: age, children ferritin, LMS, periodic limb movements
BRIEF SUMMARY
Current Knowledge/Study Rationale: Age appears to be a contributing factor for limb movements in pediatric sleep. This study was done to assess the relationship between age and PLMS and LMS in pediatric patients when controlling for ferritin levels.
Study Impact: The study shows that both age and sex are important factors in pediatric limb movements during sleep, independent of ferritin levels. These findings may inform the interpretation of limb movements on sleep studies in children.
INTRODUCTION
Periodic limb movements during sleep (PLMS) is diagnosed based on polysomnogram findings of periodic episodes of repetitive stereotypical limb movements.1–3 Clinical presentation of PLMS in children could be nonspecific such as growing pains, restless sleep, insomnia, and daytime sleepiness and frequently goes unnoticed by the family.4–8 The 2016 report by the joint task force from the International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) reviewed and updated the standards for recording and scoring limb movements (LM) in polysomnography (PSG).1 It also highlighted the differences between pediatric PLMS compared to that in adults, which included less periodicity and higher limb movements in the younger age group. Age appears to be a strong factor in periodic limb movements (PLM) and periodicity index as shown by Ferri and colleagues when patients between the ages 7.5 years to 83.2 years were studied. Both of these measures increased with age.9 However, in the pediatric population, PLM appears to increase with decreasing age. The study by Scholle and Scholle conducted on 52 children between the ages of 1 to 18 years demonstrated that limb movements during sleep (LMS) and PLMS increase with decreasing age.10 Results from these studies and others suggest that younger children may have different normative values of PLMS and LMS.9–11 However, these studies did not present measures of iron status in their populations, which could be a strong contributing factor and may have affected the results. There is a strong correlation between PLMS and restless legs syndrome (RLS), and both may be prevalent in patients with iron deficiency.12–14 In addition, iron therapy has been shown to reduce RLS symptoms and PLMS in patients who had ferritin levels less than 50 mcg/L.12,15–17 It has been hypothesized that iron may play a crucial role in the pathogenesis of RLS and PLMS. Therefore, controlling for iron deficiency would be prudent when studying PLM or RLS, especially in growing children.
In this study, we examined the associations between age, sex, and iron status and PLMS and LMS in a large sample of pediatric patients who presented to the sleep laboratory.
METHODS
The study was approved by the institutional review board where it was conducted. The data on all patients who had serum ferritin testing within 30 days of PSG during the period between May 2013 and October 2017 were pulled electronically and reviewed retrospectively. The sample represented children referred for sleep study by their treating specialists for clinical reasons, which include otherwise healthy children, as well as many children with an array of complex medical and neurodevelopmental disorders. The sample would be typical of a pediatric sleep clinic in a tertiary referral academic children’s hospital. With the goal to limit the interference of sleep-disordered breathing with the results, patients were excluded if obstructive apnea-hypopnea index was more than 2 events/h or central apnea-hypopnea index more than 5 events/h. PSG was performed in an American Academy of Sleep Medicine (AASM) accredited laboratory and was interpreted by a board-certified sleep specialist. Overnight sleep studies were included if there were at least 6 hours of total recording time. The following parameters were measured during PSG: chest and abdominal wall movements by inductance plethysmography, heart rate by electrocardiography, air flow by an oronasal thermistor and nasal end tidal carbon dioxide monitoring (which was also used to assess the ventilation), arterial pulse oxygen saturation (SpO2) by pulse oximetry, bilateral electro-oculogram, six channels of electroencephalogram (two frontal, two occipital, and two central leads), chin electromyogram, bilateral leg electromyogram, and a digital time-synchronized video recording. All measures were digitized using a commercially available system (Polysmith acquisition software version 11.0, Nihon Kohden, Tokyo, Japan). The sleep technician followed patient behavior and confirmed sleep position using the infrared camera inside the room. Sleep studies were scored by a registered polysomnographic technologist based on AASM criteria. Patients were organized into 6 groups based on their age: group 1 (0 to 2 years), group 2 (3 to 4 years), group 3 (5 to 10 years), group 4 (11 to 13 years), group 5 (14 to 15 years), group 6 (16 to 17 years). These categories were based on the study by Scholle and Scholle.10
Statistical analysis
Categorical variables were analyzed using contingency tables where chi-square tests or Fisher exact tests were performed. Bivariate correlations were examined via Pearson correlation. Statistical comparisons for the means were done by t tests appropriately corrected for nonequal variance where needed. Values of P < .05 were considered statistically significant. Summary data are presented as mean ± standard deviation except otherwise noted. General linear models were used to examine the association between age group, sex, ferritin, and LM indices. All analyses were performed using SPSS software (version 24, IBM Corp, Armonk, New York, United States).
RESULTS
A total of 1,070 patients were included in the study, and 645 (60.2%) were male. The age range was 0 to 17 years, with an average age of 5.9 years (± 3.7 years). Overall, 287 children (26%) had PLMS index ≥ 5 events/h.
Correlates of age
Table 1 shows the descriptive statistics for the study population by different age groups. Results from analysis of variance demonstrated that age group was significantly associated with PLMS (F5,1064 = 3.2, P = .007), LMS (F5,1064 = 7.2, P < .001), and LM arousal index (F5,1064 = 4.4, P = .001) (Table 2). Interestingly, PLMS and LMS decreased with age until the early teen years (group 4, 11 to 13 years) where there was a spike in LM that subsided gradually at older ages (Figure 1). Similarly, the proportion of children with PLMS index > 5 events/h varied significantly by age group (χ25 = 14.8, P = .011) as follows: 35% in group 1, 24% in group 2, 23% in group 3, 32% in group 4, 32% in group 5, and 14% in group 6. To further assess this relationship, we used cutoff values for PLMS previously reported by age,10 and the results continued to demonstrate an age effect (χ25 = 38.3, P < .001) with particularly high prevalence in early teenage groups: as follows: 13% in group 1, 6% in group 2, 7% in group 3, 24% in group 4, 29% in group 5, and 14% in group 6.
Table 1.
Study population descriptive data.
Table 2.
Analysis of variance summary table.

Figure 1. Total leg movements during sleep and PLMS according to age group.

Group 1 (0–2 years), group 2 (3–4 years), group 3 (5–10 years), group 4 (11–13 years), group 5 (14–15 years), group 6 (16–17 years). Bars represent 95% confidence intervals. PLMS = periodic limb movements during sleep.
Correlates of sex
Male patients had significantly higher PLMS (4.7 ± 6.5 versus 3.4 ± 4.2, P < .001), LMS (12.6 ± 7.8 versus 10.8 ± 6.3, P < .001), and LM arousal index (5.4 ± 2.7 versus 5.0 ± 2.5, P = .041) compared to female patients. In contrast, neither ferritin levels (28.4 ± 19.5 versus 28.4 ± 19.1, P = .986) nor age (5.8 ± 3.6 versus 6.0 ± 4.0, P = .503) differed by sex.
Correlates of ferritin
Ferritin level was weakly and negatively associated with LMS (r = −.073, P = .017) and LM arousal index (r = −.066, P = .031), but not PLMS (r = −.041, P = .177). In the total population, mean ferritin levels in mcg/L were not significantly different between those children with PLMS index above or below 5 events/h (27.3 ± 18.9 versus 28.8 ± 19.5 respectively, P = .275). Ferritin levels did not differ between patients who were > 90th percentile according to Scholle and Scholle10) for PLMS (26.7 ± 17.7 versus 28.7 ± 19.5, P = .205) or LMS (27.2 ± 21.4 versus 28.5 ± 19.2, P = .566). As noted previously, ferritin levels did not differ by sex but were strongly associated with age group.
Age, sex, ferritin level, and LM
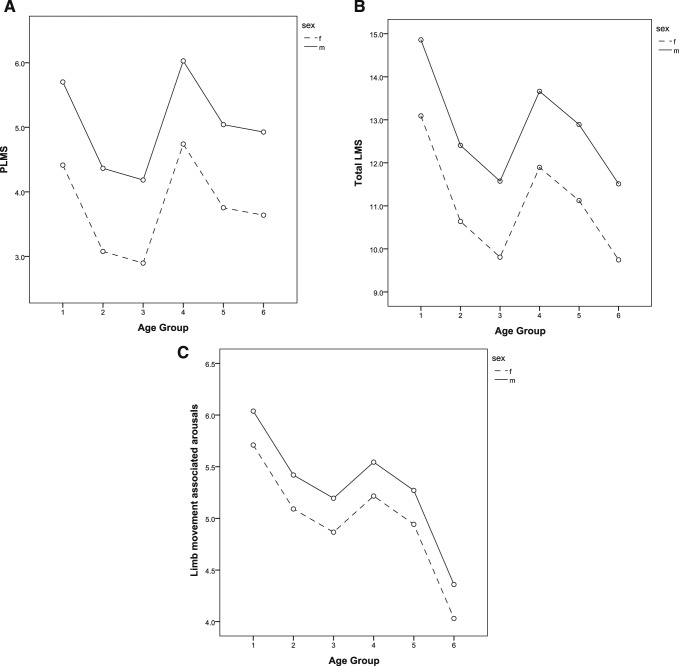
For each LM index, separate general linear models were built with age group, sex, and ferritin levels included. Figure 2 demonstrates the main independent effects of age group and sex on limb indices. Age group was significantly associated with PLMS (F5,1062 = 3.2, P = .007), LMS (F5,1062 = 6.7, P < .001), and LM arousal index (F5,1062 = 3.8, P = .002). Likewise, there was a statistically significant main effect of sex for PLMS (F1,1062 = 12.9, P < .001), LMS (F1,1062 = 15.4, P < .001), and LM arousal index (F1,1062 = 3.9, P = .046). Ferritin level was not significantly associated with PLMS, LMS, or LM arousal index in any model controlling for age and sex.
Figure 2. Relationship between age, sex, limb movements and associated arousals.
Relationship between age, sex and (A) PLMS, (B) LMS, and (C) limb movement associated arousal index. Age groups as follows: group 1 (0–2 years), group 2 (3–4 years), group 3 (5–10 years), group 4 (11–13 years), group 5 (14–15 years), group 6 (16–17 years). Estimated marginal means from linear models with age, sex, and ferritin levels included. LMS = limb movements during sleep, PLMS = periodic limb movements during sleep.
DISCUSSION
Evaluation of LMS is commonplace in pediatric sleep clinics. Despite this, normative data and correlates of PLMS and LMS are limited. In the current study, which includes more than 1,000 children referred to pediatric sleep center, we found that age and sex are major contributing factors to LMS, even when controlling for ferritin levels. Overall, males had higher LMs. The association with age was complex, with limb movements decreasing with age with the exception of the early teenage years. Surprisingly, we found very little association between ferritin levels and LM indices.
Our results support previous findings by Scholle and Scholle where LMS or PLMS were higher in younger children, and the authors proposed having different normal values for younger patients.10 They showed patients younger than 10 years have median PLMS index ranging from 4.4 to 9.6 events/h and with 90th percentile just over 10 events/h. Our data suggested the same distribution but with higher prevalence of patients with periodic limb movement in sleep index > 5 events/h (26% in the total population in this study). This difference is likely the result of our sample consisting of children referred to the sleep center rather than those without any clinical sleep problems. These findings are similar to those in a study by Martinez and Guilleminault who found that 23% of their patients who were referred with sleep complaints had periodic limb movement disorder.7 Most of the studies looking into normative data for PLMS in children were done in samples lacking children younger than 5 years or failed to stratify PLMS based on age.9,18,19
Our data also demonstrate a strong association of LMs with sex. Males had higher indices of PLMS and LMS that persisted in all age categories. These findings are congruent with a study by Marcus et al where they found 10.5% males compared to 3.7% of female having PLMS index > 5 events/h, but failed to reach a statistical significance (P = .078) likely because of their relatively small sample size.18
Interestingly, we found that all indices of limb movements increased in the early teen years. Similarly, the study by Marcus et al showed higher percentages of patients with PLM index > 5 events/h in adolescents (10.5%) compared to school-age children (5%), but this did not reach statistically significance.18 The physiology underlying the spike in limb movements in early teenagers remains a mystery. One possibility is that as adolescents undergo rapid growth, iron deficiency resulting in increased limb movements may develop. However, this spike remained in our data while controlling for serum ferritin levels. Another possibility is that hormonal changes associated with puberty could contribute to differences in LMs; supporting this notion are studies demonstrating increased PLMS and/or RLS with hormonal changes associated with menopausal transition or pregnancy.20,21 Another possibility is that it could be due to medication effect because adolescents are at increased risk of the development of mood disorders and therefore could be placed on psychotropic medications which can increase PLMS; in the current study we did not have medication data readily available to examine this hypothesis.
Surprisingly, and of great clinical interest, we found minimal to no relationship between LMs and ferritin levels. The association of ferritin levels with RLS is well established in the literature, but this association is more tenuous with respect to PLMS.12,22 One possibility is that serum ferritin levels are a poor marker of brain iron stores in children. It also could be due to the fact that childhood ferritin level varies with age. In addition, ferritin in children may not reflect iron stores due to lower iron reserves. This hypothesis is supported by prior studies demonstrating no differences in serum ferritin levels between adult patients with or without RLS, but significant differences in cerebrospinal fluid ferritin levels, suggesting that RLS is due to a dysfunction of iron transportation from serum to the central nervous system.23 Furthermore, a study by Simakajornboon and colleagues found that PLMS were associated with low serum iron but not ferritin levels.12 Similar findings have been demonstrated in adults, where iron stores are more predictable and age is less of a factor on ferritin concentration. For example, a study in adults by Sun et al showed ferritin < 50 mcg/L correlated significantly with RLS symptoms but not with PLMS; they related this finding to the inclusion of a couple of outlier patients.22 In contrast to examining the correlation of ferritin with PLMS, there is stronger evidence for a therapeutic response of iron supplementation on RLS symptoms and/or PLMS.12,16,17,24,25 We speculate that the aforementioned findings of a lack of baseline association of serum ferritin with PLMS but seemingly strong clinical response to iron supplementation reflects the poor ability of serum measures of iron status to provide information regarding central nervous system iron stores. Alternatively, given the lack of any placebo-controlled trial for iron supplementation for RLS/PLMD in children, placebo effect remains an alternative explanation; it should be noted that placebo-controlled trials in adults demonstrate substantial placebo response in RLS.26
Although this study has many strengths, we would like to highlight some of the limitations. The study was retrospective in nature, and we lacked information on potential confounders such as medications use. In addition, the study population was referred for PSG based on clinical symptoms and was not a normal population. That said, we attempted to decrease bias by excluding patients with sleep-disordered breathing based on apnea-hypopnea index. Although we cannot make a direct comparison of our results to prior studies on healthy children without any clinical sleep problems, it is interesting that the results of our large clinical sample are generally in agreement with previously published normative data from smaller studies.9,10,18 Another limitation is the lack of periodicity index for LM.10,27 However, pediatric patients typically have less periodicity compared to adults and therefore we included total LMs in the results.1,9 In conclusion, these data demonstrate significant associations of sex and age with LMS and should be considered when interpreting PSG data. We found little to no association between serum ferritin levels and any LM parameters, which we speculate reflects its poor ability to characterize brain iron stores. Further studies are needed to identify superior markers of brain iron stores as well as to elucidate the physiology underlying the observed age and sex effects.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. Dr. Ingram has served as a consultant for Jazz pharmaceuticals and received research support from WakeUpNarcolepsy. The other authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- LM
limb movement
- LMS
limb movements during sleep
- PLMS
periodic limb movements during sleep
- PSG
polysomnography
REFERENCES
- 1.Ferri R, Fulda S, Allen RP, et al. World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) Sleep Med. 2016;26:86–95. doi: 10.1016/j.sleep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Simakajornboon N, Kheirandish-Gozal L, Gozal D. Diagnosis and management of restless legs syndrome in children. Sleep Med Rev. 2009;13(2):149–156. doi: 10.1016/j.smrv.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri R, Rundo F, Zucconi M, et al. Diagnostic accuracy of the standard and alternative periodic leg movement during sleep indices for restless legs syndrome. Sleep Med. 2016;22:97–99. doi: 10.1016/j.sleep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Wong MW, Williamson BD, Qiu W, Champion D, Teng A. Growing pains and periodic limb movements of sleep in children. J Paediatr Child Health. 2014;50(6):455–460. doi: 10.1111/jpc.12493. [DOI] [PubMed] [Google Scholar]
- 5.Picchietti DL, Walters AS. Moderate to severe periodic limb movement disorder in childhood and adolescence. Sleep. 1999;22(3):297–300. doi: 10.1093/sleep/22.3.297. [DOI] [PubMed] [Google Scholar]
- 6.Walters AS, Picchietti DL, Ehrenberg BL, Wagner ML. Restless legs syndrome in childhood and adolescence. Pediatr Neurol. 1994;11(3):241–245. doi: 10.1016/0887-8994(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 7.Martinez S, Guilleminault C. Periodic leg movements in prepubertal children with sleep disturbance. Dev Med Child Neurol. 2004;46(11):765–770. doi: 10.1017/s0012162204001318. [DOI] [PubMed] [Google Scholar]
- 8.Picchietti DL, Stevens HE. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Med. 2008;9(7):770–781. doi: 10.1016/j.sleep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Ferri R, Manconi M, Lanuzza B, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med. 2008;9(7):790–798. doi: 10.1016/j.sleep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Scholle S, Scholle HC. Leg movements and periodic leg movements during sleep in the development across childhood and adolescence from 1 to 18 years. Sleep Med. 2014;15(9):1068–1074. doi: 10.1016/j.sleep.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Pennestri MH, Whittom S, Adam B, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006;29(9):1183–1187. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 12.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26(6):735–738. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 13.Muhle H, Neumann A, Lohmann-Hedrich K, et al. Childhood-onset restless legs syndrome: clinical and genetic features of 22 families. Mov Disord. 2008;23(8):1113–1121. doi: 10.1002/mds.22016. quiz 1203. [DOI] [PubMed] [Google Scholar]
- 14.Kotagal S, Silber MH. Childhood-onset restless legs syndrome. Ann Neurol. 2004;56(6):803–807. doi: 10.1002/ana.20292. [DOI] [PubMed] [Google Scholar]
- 15.Amos LB, Grekowicz ML, Kuhn EM, et al. Treatment of pediatric restless legs syndrome. Clin Pediatr (Phila) 2014;53(4):331–336. doi: 10.1177/0009922813507997. [DOI] [PubMed] [Google Scholar]
- 16.Tilma J, Tilma K, Norregaard O, Ostergaard JR. Early childhood-onset restless legs syndrome: symptoms and effect of oral iron treatment. Acta Paediatr. 2013;102(5):e221–e226. doi: 10.1111/apa.12173. [DOI] [PubMed] [Google Scholar]
- 17.Grim K, Lee B, Sung AY, Kotagal S. Treatment of childhood-onset restless legs syndrome and periodic limb movement disorder using intravenous iron sucrose. Sleep Med. 2013;14(11):1100–1104. doi: 10.1016/j.sleep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Traylor J, Gallagher PR, et al. Prevalence of periodic limb movements during sleep in normal children. Sleep. 2014;37(8):1349–1352. doi: 10.5665/sleep.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40(1):22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 20.Wesstrom J, Ulfberg J, Sundstrom-Poromaa I, Lindberg E. Periodic limb movements are associated with vasomotor symptoms. J Clin Sleep Med. 2014;10(1):15–20. doi: 10.5664/jcsm.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzaja A, Wehrle R, Lancel M, Pollmacher T. Elevated estradiol plasma levels in women with restless legs during pregnancy. Sleep. 2009;32(2):169–174. doi: 10.1093/sleep/32.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep. 1998;21(4):371–377. [PubMed] [Google Scholar]
- 23.Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, O’Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10(9):973–975. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Kryger MH, Otake K, Foerster J. Low body stores of iron and restless legs syndrome: a correctable cause of insomnia in adolescents and teenagers. Sleep Med. 2002;3(2):127–132. doi: 10.1016/s1389-9457(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 26.Silva MA, Duarte GS, Camara R, et al. Placebo and nocebo responses in restless legs syndrome: a systematic review and meta-analysis. Neurology. 2017;88(23):2216–2224. doi: 10.1212/WNL.0000000000004004. [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Rundo F, Zucconi M, et al. Putting the periodicity back into the periodic leg movement index: an alternative data-driven algorithm for the computation of this index during sleep and wakefulness. Sleep Med. 2015;16(10):1229–1235. doi: 10.1016/j.sleep.2015.05.019. [DOI] [PubMed] [Google Scholar]