Abstract
Study Objectives:
The aim of this study was to obtain preliminary data on the efficacy, credibility, and acceptability of Exposure, relaxation, and rescripting therapy for military service members and veterans (ERRT-M) in active duty military personnel with trauma-related nightmares.
Methods:
Forty participants were randomized to either 5 sessions of ERRT-M or 5 weeks of minimal contact control (MCC) followed by ERRT-M. Assessments were completed at baseline, posttreatment/postcontrol, and 1-month follow-up.
Results:
Differences between ERRT-M and control were generally medium in size for nightmare frequency (Cohen d = −0.53), nights with nightmares (d = −0.38), nightmare severity (d = −0.60), fear of sleep (d = −0.44), and symptoms of insomnia (d = −0.52), and depression (d = −0.51). In the 38 participants who received ERRT-M, there were statistically significant, medium-sized decreases in nightmare frequency (d = −0.52), nights with nightmares (d = −0.50), nightmare severity (d = −0.55), fear of sleep (d = −0.48), and symptoms of insomnia (d = −0.59), posttraumatic stress disorder (PTSD) (d = −0.58) and depression (d = −0.59) from baseline to 1-month follow-up. Participants generally endorsed medium to high ratings of treatment credibility and expectancy. The treatment dropout rate (17.5%) was comparable to rates observed for similar treatments in civilians.
Conclusions:
ERRT-M produced medium effect-size reductions in nightmares and several secondary outcomes including PTSD, depression, and insomnia. Participants considered ERRT-M to be credible. An adequately powered randomized clinical trial is needed to confirm findings and to compare ERRT-M to an active treatment control.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: A Pilot Randomized Controlled Trial of Treatment for Trauma-Related Nightmares In Active Duty Military Personnel; Identifier: NCT02506595; URL: https://clinicaltrials.gov/ct2/show/NCT02506595
Citation:
Pruiksma KE, Taylor DJ, Mintz J, et al; on behalf of the STRONG STAR Consortium. A pilot randomized controlled trial of cognitive behavioral treatment for trauma-related nightmares in active duty military personnel. J Clin Sleep Med. 2020;16(1):29–40.
Keywords: nightmares, trauma, rescripting, imagery rehearsal, exposure
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research among civilians and veterans supports the efficacy of cognitive behavioral treatments for trauma-related nightmares. This pilot randomized controlled trial was conducted to obtain preliminary data of treatment effect in active duty service members.
Study Impact: This pilot study demonstrates that a cognitive behavioral treatment for nightmares in an active duty military sample is acceptable and feasible, and it suggests a positive effect on nightmares and secondary outcomes, as the therapy also led to reductions in symptoms of PTSD, depression, and insomnia.
INTRODUCTION
Chronic nightmares are frequently experienced following traumatic experiences1 and are associated with the prevalence and severity of posttraumatic stress disorder (PTSD),2–4 alcohol abuse,3 and health problems.5 Nightmares are also associated with suicide risk after controlling for the effects of depression and PTSD.6,7 Little research has examined the prevalence, impact, or treatment of nightmares in active duty military populations who, by the nature of their responsibilities in combat operations, are at increased risk for trauma exposure and subsequent difficulties with nightmares. Active duty service members face a myriad of unique circumstances that negatively impact sleep and may exacerbate nightmares and poor sleep. Those circumstances include overnight shifts, frequent stressful transitions between units and station assignments, and early morning start times. In terms of the prevalence of nightmares in active duty service members, one study of 4,119 active duty service members scheduled for deployment found that 40% reported experiencing nightmares.8 A military sleep clinic record review of 500 treatment-seeking patients serving in the Army (46%), Air Force (45%), or Navy and Marines (9%) found that 19% reported trauma-related nightmares at least weekly.9 Prevalence of nightmares tends to be much higher among individuals who meet full criteria for PTSD, which supports the idea that disturbed sleep is a hallmark symptom that maintains and exacerbates PTSD.10 Approximately 10% to 18% of active duty service members who have deployed in support of overseas military combat operations meet criteria for PTSD,11–14 and 69% of treatment-seeking service members with PTSD report nightmares.15 Nightmares may not fully remit following evidence–based, trauma-focused therapy for PTSD. One study found that nightmares persisted in 52% of active duty service members who were treated for PTSD and in 13% who no longer met criteria for PTSD after treatment.15
Research in civilian and veteran samples largely supports the efficacy of cognitive behavioral treatments for trauma-related nightmares.16–18 The American Academy of Sleep Medicine position paper for the treatment of nightmare disorder in adults19 states that a cognitive behavioral therapy called imagery rehearsal therapy (IRT) “is recommended for the treatment of PTSD-associated nightmares” and is similar to a cognitive behavioral approach, exposure, relaxation, and rescripting therapy (ERRT), which is included as a treatment that “may be used for the treatment of PTSD-associated nightmares.” Although several IRT protocols exist,20 IRT involves identifying a target nightmare (with or without specific discussion of, or exposure to the nightmare content), writing a different storyline for the nightmare during wakefulness (ie, rescripting), and repeatedly imagining the new dream before sleep. ERRT is a variant of IRT that incorporates techniques consistent with trauma-focused therapies including targeting one of the worst or most frequent nightmares, written and verbal exercises with exposure to nightmare content during session, and guidelines for rescripting dreams based on trauma-related themes (eg, safety, power/control, esteem, intimacy, trust). ERRT (and some IRT protocols) also include relaxation exercises and sleep habit modification (similar to cognitive behavioral therapy for insomnia), which appear to be important elements of nightmare treatment.21,22
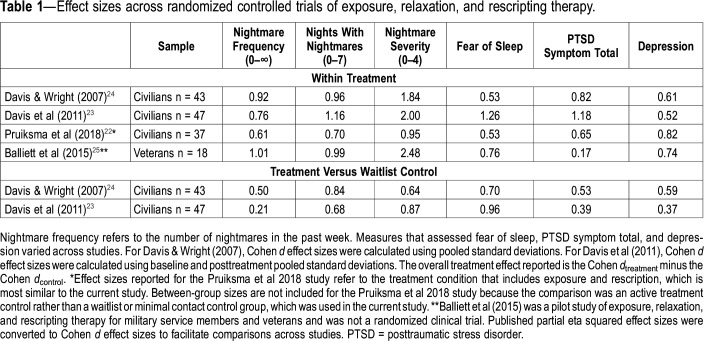
ERRT has been examined in 3 randomized controlled trials (RCTs) in civilian samples22–24 and in 1 nonrandomized pilot study in veterans.25 Effect sizes found in these studies are shown in Table 1. In the RCTs in civilian samples, the protocol consisted of three 90-minute sessions delivered in individual and group formats and included psychoeducation about trauma, nightmares, and sleep; modification of sleep habits; relaxation training; written exposure exercises to nightmare content; and writing new dream content based on themes identified in the nightmare (ie, rescription). The majority of participants in these studies were women, and the most frequently reported types of traumatic events were unwanted sexual contact, serious accidents, and physical assaults. Two of these RCTs compared treatment to a waitlist control (n = 43 and n = 47) and found medium to large within-group and between-group effect sizes for improved nightmare frequency, nightmare severity, fear of sleep, PTSD symptoms, and depression symptoms.23,24 One of these RCTs also found reductions in physiological reactions to nightmare imagery.26 The third RCT compared the full treatment protocol to an active treatment control protocol that included all of the same components except that, in the control, the written nightmare exposure and rescription exercises were replaced with additional relaxation training. This study found that treatment for both groups resulted in medium to large within-group effect sizes for the same variables noted above with no significant differences between the two groups.22 The 3-session protocol was subsequently adapted for use in veteran and military samples (ERRT-M) and examined in a nonrandomized pilot study of veterans (n = 19). This study found pre- to posttreatment improvements in nightmare frequency, nightmare severity, sleep quality, depression, and insomnia that were maintained at a 2-month follow-up (Table 1).25,27
Table 1.
Effect sizes across randomized controlled trials of exposure, relaxation, and rescripting therapy.
To date, one small case series using IRT has shown that it is possible to treat nightmares effectively in active duty service members. That study, reported in a letter to the editor, describes the treatment of 11 U.S. Army combat soldiers experiencing acute nightmares shortly after a trauma while deployed to Iraq.28 One provider treated all 11 individuals in theater, reporting that 7 soldiers experienced decreases in nightmares along with improvements in PTSD and insomnia symptoms. Active duty service members differ significantly from civilians and veterans in terms of sleep schedules and types of stressful life experiences (eg, deployments, combat, head injuries, etc). There have been no studies of nightmare treatments in active duty service members while in garrison or in their nondeployed, home station setting. Research examining nightmare treatments in active duty service members is needed to inform clinical treatment guidelines and dissemination efforts to address sleep concerns that are highly prevalent in active duty populations. However, it is known that treatments first validated in civilian populations tend to have smaller effects in military populations.29–31 Therefore, pilot studies are needed to help design interventions, plan procedures, and establish feasibility (eg, establish collaborations, estimate recruitment potential, examine credibility and acceptability) before investing substantial fiscal and human resources into a larger randomized controlled trial for military populations. The purpose of the current pilot study was to obtain preliminary data of the efficacy, credibility, and acceptability of ERRT-M for trauma-related nightmares among active duty U.S. military personnel. We hypothesized the following: (1) comparisons after 5 sessions of ERRT-M delivered over 5 weeks and a 5-week minimal contact control (MCC) group would reveal at least a medium effect of treatment; (2) ERRT-M treatment would result in significant improvements in nightmare frequency, nightmare severity, insomnia, PTSD, depression, and fear of sleep, and that gains would persist through 1-month follow-up; (3) participants would rate treatment as credible after Session 1 and at posttreatment; and (4) treatment dropout rates would be similar to civilian studies of similar treatment protocols (ie, 13% to 26%18,22–24).
METHODS
Participants
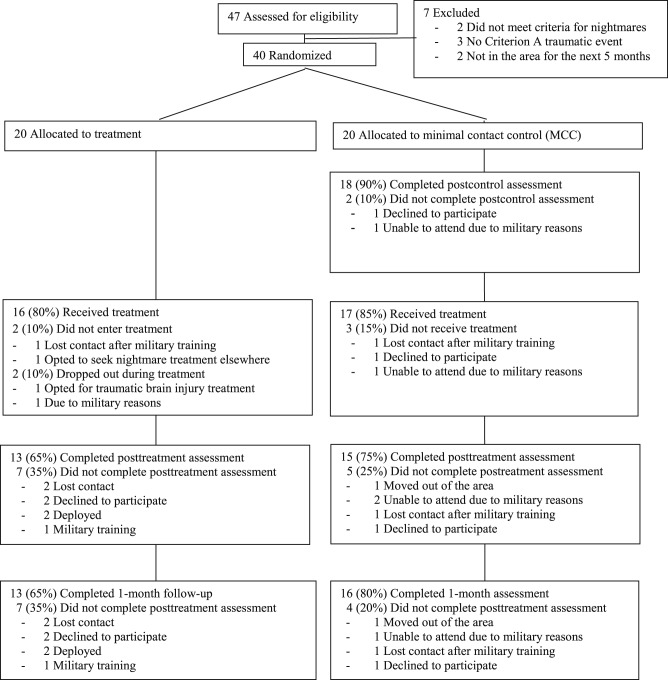
Participants were 40 active duty U.S. Army soldiers stationed at Fort Hood, Texas. Participant enrollment and attrition are presented in Figure 1. Participants were eligible for the study if they met the following criteria: (1) aged 18–50, (2) able to speak and read English, (3) history of experiencing at least one Criterion A traumatic event defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),32 (4) experienced at least 1 nightmare that caused an awakening per week for at least 1 month, (5) stable on psychotropic and/or hypnotic medications and/or interventions for sleep (eg, continuous positive airway pressure for sleep apnea) for at least one month, (6) willing to refrain from beginning new behavioral health or medication treatment for issues pertaining to sleep, PTSD, or nightmares during participation in the study, and (7) planned to be in the area for the 5 months following the baseline assessment.
Figure 1. CONSORT chart showing participant flow through the randomized clinical trial.
Exclusion criteria included the following: (1) current suicide or homicide risk meriting immediate crisis intervention, (2) severe brain damage resulting in the inability to comprehend baseline questionnaires, (3) pregnancy at baseline assessed by self-report and review of medical record (because the impact of pregnancy on nightmares and sleep outcomes is unknown), (4) serious mental health diagnosis such as bipolar disorder or psychosis, (5) currently engaged in evidence-based treatment for PTSD (ie, prolonged exposure or cognitive processing therapy) or insomnia (ie, cognitive behavioral therapy for insomnia), (6) taking propranolol (a medication known to cause nightmares).
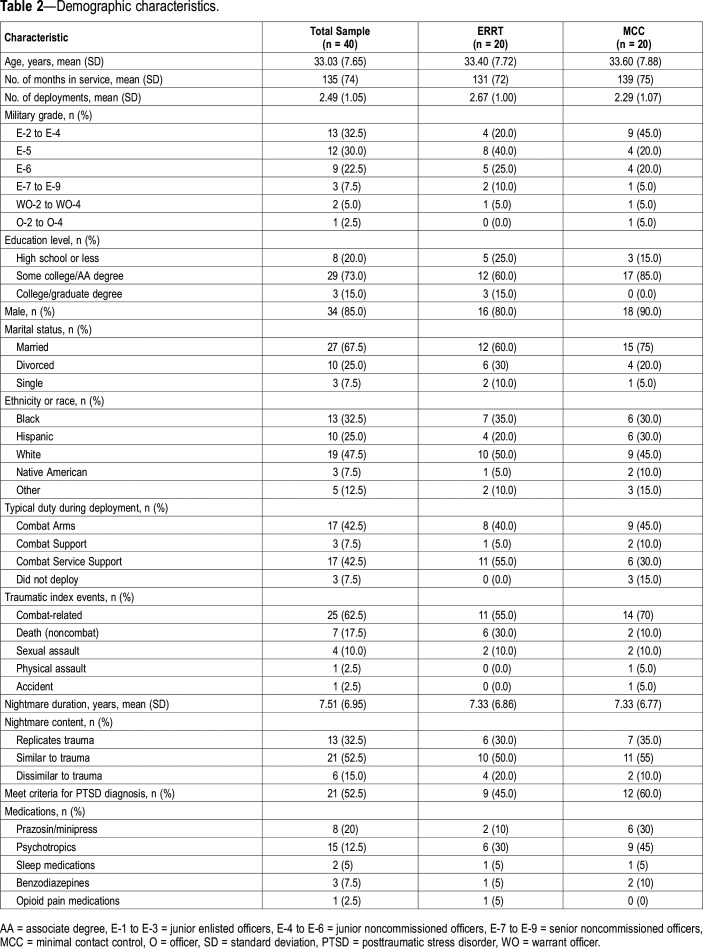
Participant demographics, trauma and nightmare characteristics, and medication use are shown in Table 2. The study was approved by The University of Texas Health Science Center at San Antonio (UT Health San Antonio) Institutional Review Board (IRB). The Brooke Army Medical Center IRB, the reviewing IRB for the Carl R. Darnall Army Medical Center at Fort Hood, deferred its review to the UT Health San Antonio IRB. The U.S. Army Medical Research and Materiel Command Human Research Protection Office also reviewed and approved the study.
Table 2.
Demographic characteristics.
Procedure
The study was affiliated with the UT Health San Antonio-led South Texas Research Organizational Network Guiding Studies on Trauma and Resilience (STRONG STAR Consortium), a multi-institutional and multidisciplinary research consortium of investigators focused on the diagnosis, treatment, and epidemiology of combat-related PTSD and comorbid conditions. The affiliation with STRONG STAR enabled the study to employ trained research staff and use standardized procedures for the recruitment, screening, assessment, and monitoring of research participants. The STRONG STAR Data Safety and Monitoring Board monitored the conduct of the study, which was conducted from February 2016 to August 2017. Individuals were recruited at Fort Hood, Texas, via flyers, clinician referrals, and self-referrals in response to briefings conducted at sleep clinic orientation classes at the Carl R. Darnall Army Medical Center Sleep Disorders Clinic. Interested individuals completed a brief phone screen and, if eligible, were scheduled for informed consent and an initial evaluation. Eligible participants were randomly assigned to ERRT-M or the 5-week MCC group using a random number generator provided by the biostatistician via prefilled ordered envelopes that only the project coordinator could access. After completion of the post MCC assessment (ie, postcontrol), participants were invited to receive the 5-week treatment. Assessments were conducted at baseline, at 1-week posttreatment/postcontrol, and at the 1-month follow-up by trained clinicians who participated in weekly assessment supervision and completed coratings on a regular basis throughout the study to prevent assessment drift.
Treatment
Exposure, relaxation, and rescripting therapy for military service members and veterans
ERRT-M was composed of 5 individual 90-minute sessions. The original ERRT manual used in civilian samples included 3 sessions of 90 minutes each.33 Balliett et al25 adapted the manual for military service members and veterans (ERRT-M) and included additional session time to allow for psychoeducation and more opportunities to engage in exposure and rescription exercises. The ERRT and ERRT-M developer and workgroup now recommend that 4 to 5 sessions be utilized and, based on these recommendations, a 5-session protocol was selected for this pilot study.
Session 1 included an overview of treatment, psychoeducation about helpful sleep habits, implementation of stimulus control therapy and sleep hygiene habits collaboratively chosen by the patient and therapist, and introduction to progressive muscle relaxation (PMR). Homework consisted of nightly PMR practice, modification of at least 1 sleep habit, and completion of a sleep and nightmare log. Session 2 consisted of homework review, psychoeducation about psychological trauma and the development and maintenance of nightmares, and another PMR exercise. Homework consisted of reviewing the session content, nightly PMR practice, and modification of another sleep habit. Session 3 included homework review followed by a nightmare exposure exercise. Patients were instructed to target a nightmare that was either the most frequent or most vivid, to write a detailed description of the nightmare, and then to read the account aloud. The therapist and patient then identified trauma-related themes in the nightmare (ie, safety, power/control, trust, intimacy, and esteem), and the patient was encouraged to rescript the nightmare according to the themes identified. Patients then read the rescription aloud. The session concluded with the patient learning a diaphragmatic breathing relaxation procedure. For homework, patients were instructed to modify another sleep habit, to read and visualize their rescripted dream nightly, and to practice PMR and/or diaphragmatic breathing before going to sleep. Session 4 included homework review with a focus on the patient’s experience with imagery rehearsal. If indicated, a second rescription of the dream was developed in-session to bolster the effects of the imagery rehearsal, or the exposure and rescription of another nightmare was implemented. The session concluded with the patient learning a mindfulness technique. For homework, patients were instructed to modify another sleep habit, to read and visualize their rescripted dream nightly, and to practice PMR and/or diaphragmatic breathing before going to sleep. Session 5 began with homework review followed by a discussion about treatment focusing on identifying the skills and techniques most useful to the patient. A plan for continued improvement was also discussed.
Minimal contact control
Those assigned to the MCC group were contacted for a brief (5-minute) check-in safety call every week for 5 weeks. At the end of 5 weeks, participants completed the postcontrol assessment and were invited to receive ERRT-M.
Therapists and fidelity
Treatment was conducted by 3 licensed clinical psychologists and 1 licensed clinical social worker. All therapists were trained and supervised by the primary investigator (KEP). One independent PhD-level clinician trained in the treatment reviewed a random selection of 10% of sessions using an adherence measure adapted from trials of cognitive processing therapy for PTSD34 and used in previous studies of ERRT22 to rate elements unique and specific to each session (eg, “Had client write out nightmare”), essential but not unique elements of each session (eg, “Therapist established good rapport with the client”), and proscribed elements for each session (eg, conducted any treatment procedures that are outside of the protocol). Over 94% of unique and essential elements and 100% of essential but not unique elements were included in all sessions. There were no significant deviations from the protocol. Overall, 87% of the unique and essential elements and 97% of essential but not unique elements were rated as “good” or “excellent” competence (from the following options: poor, mediocre, satisfactory, good, or excellent). Throughout all sessions, 89% of therapists' overall skills were rated as “excellent.”
Instruments
Instruments for this study were selected in accordance with the common data elements developed for the Consortium to Alleviate PTSD35 and to assess nightmares in detail. Psychometric data are included here for those measures that were not included in the common data elements.35
Demographics questionnaire
This questionnaire is a 24-item self-report measure used to gather the participants’ demographics (eg, race/ethnicity, sex, marital status, education) and military status (eg, number of deployments, highest rank held, typical duties).
Life Events Checklist
The Life Events Checklist is a 17-item self-report instrument used to screen for Criterion A events by asking if participants have learned about, witnessed, or experienced various difficult and/or stressful index events.36 This measure was used for the current study in combination with the Clinician-Administered PTSD Scale for DSM-5 in order to evaluate for a PTSD diagnosis and to screen for inclusion to the study.
Clinician-Administered PTSD Scale for DSM-5
The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) is a semistructured, clinician-administered interview to assess response to a traumatic event and is used to determine if a participant meets DSM-5 diagnostic criteria for PTSD.37 Additionally, the CAPS-5 assesses the frequency and severity of any posttraumatic symptoms that are endorsed by the participant.
Structured Clinical Interview for DSM-5 Sleep Disorders
The Structured Clinical Interview for DSM-5 Sleep Disorders (SCISD) is a semistructured diagnostic interview designed to screen for sleep disorders according to DSM-5 criteria.38 For the current study, it was used to determine if participants met inclusion criteria for nightmare disorder and to assess for comorbid sleep disorders including insomnia disorder, hypersomnolence disorder, circadian rhythm sleep-wake disorders, sleep walking, sleep terrors, and restless legs syndrome. The SCISD also provided an initial screening of rapid eye movement (REM) sleep behavior disorder, narcolepsy, and obstructive sleep apnea. A psychometric study conducted in an active duty military population indicated the SCISD has excellent interrater reliability for insomnia (κ = 1.0) and restless legs syndrome (κ = 0.83); very good reliability for nightmare disorder (κ = 0.78) and obstructive sleep apnea (κ = 0.73); and good reliability for hypersomnolence (κ = 0.50) and circadian rhythm sleep-wake disorders (κ = 0.50).38
Health questionnaire
This questionnaire is a 6-item, self-report measure used to gather information about the participants’ overall health and current medication regimens.
Trauma-Related Nightmare Survey
The Trauma-Related Nightmare Survey is a 14-item measure that uses dichotomous and Likert-scale items to assess nightmare frequency, severity, and characteristics (eg, similarity between nightmares and traumatic events, duration of chronic nightmares, onset of chronic nightmares), as well as cognitions, emotions, and behaviors associated with nightmares.39 The items have good test-retest reliabilities (r = 0.60–0.88 with an average r = 0.73) and good convergent validities (range r = 0.44–0.78) with other commonly used measures for sleep and mood symptoms.39
Insomnia Severity Index
The Insomnia Severity Index is a 7-item measure that assesses difficulties falling asleep, staying asleep, and waking too early, as well as distress related to sleep difficulties.40 Items are summed to derive a total score ranging from 0 to 28, with higher scores indicating greater insomnia severity.
Fear of Sleep Inventory-Short Form
The Fear of Sleep Inventory-Short Form is a 13-item measure that assesses trauma-related thoughts and activities associated with sleep and the occurrence of traumas associated with the bedroom or sleep.41 Items are summed to derive a total score from 0 to 52, with higher scores indicating greater fear of sleep. The items have high internal consistency (α = 0.76–0.94), good convergenet validities with measures of PTSD (r = 0.39–0.61) and insomnia (r = 0.39–0.48) and good discriminant validity with a measure of sleep hygiene (r = 0.19–0.27).41
PTSD Checklist for DSM-5
The PTSD Checklist for DSM-5 is a 20-item measure of PTSD symptoms, with total scores ranging from 0–80, where higher scores indicate greater PTSD symptoms.42 It has been found to be a psychometrically sound measure in a sample of treatment-seeking military service members that is very similar to that of the current study.43
Patient Health Questionnaire
The Patient Health Questionnaire is a self-report scale that assesses the frequency of each of the 9 symptoms of major depressive disorder as defined by the DSM-5 during the past 2 weeks.44 Scores range from 0–9.
Depressive Symptom Index-Suicide Subscale
The Depressive Symptom Index-Suicide Subscale is a 4-item self-report measure that assesses the participants’ depressive symptoms and suicidal ideation over the previous week.45 This measure was used in the current study to determine the need for crisis intervention.
Credibility and Expectancy Questionnaire
The Credibility and Expectancy Questionnaire is a 6-item self-report measure used to assess the participants’ beliefs and thoughts about the effectiveness of the treatment being offered.46 Three items assess treatment credibility and were rated on a scale from 1 (“not at all”) to 9 (“very”) related to the perceived level of credibility. Three items assessed treatment expectancy, including 1 item rated on a scale from 1 (“not at all”) to 9 (“very”) and 2 items rated on a scale from 0 to 100%. This questionnaire was completed by the participants following their first session of ERRT-M as well as after their final session.
Training and supervision of independent evaluators
The diagnostic clinical interviews (ie, the CAPS-537 and the SCISD)38 were conducted by masters- or doctoral-level independent evaluators who were unaware of the treatment condition of the participants. The independent evaluators participated in extensive training prior to conducting interviews with study participants and in regularly scheduled calibration exercises throughout the study to ensure adherence to assessment procedures.
Data analysis
This pilot study was designed to examine the credibility and acceptability of ERRT-M and to determine the effect size associated with ERRT-M when used to treat a sample of active duty service members experiencing nightmares. A total of 40 participants were randomized to treatment or MCC. A medium effect (Cohen d = 0.50) was expected, based on medium to large effect sizes on nightmare frequency in civilian populations.16 To examine the effects of the intervention, intent-to-treat analyses were conducted using Statistical Analysis Software (SAS) mixed effects regression analyses with group (immediate treatment versus MCC) entered as a nominal between-subjects variable and time (pretreatment versus posttreatment) entered as a nominal within-subjects variable. For follow-up analyses, the treatment group was combined with participants from the control group who completed treatment after the postcontrol assessment. Outcomes were examined with multilevel models with time (baseline [or postcontrol for the control group], posttreatment, 1-month follow-up) entered as a nominal within-subjects variable. The magnitude of before-to-after treatment effects were calculated from the between group t tests using the conversion formula 2 × (t / √df) for between-group tests, and t / √df for within-group tests. Cohen d effect sizes are generally interpreted as small (0.2), medium (0.5) and large (0.8). Consistent with previous studies in veterans,25,47 participants were considered to achieve a clinically significant treatment response if they reported experiencing ≤ 1 nightmare in the week prior to the 1-month assessment. To examine treatment credibility and expectancy, the proportion of participants endorsing items on the Credibility and Expectancy Questionnaire in the low (1–3), medium (4–6) and high (7–9) are reported after Session 1 and at posttreatment.
RESULTS
Intent-to-treat analyses comparing treatment to minimal contact control
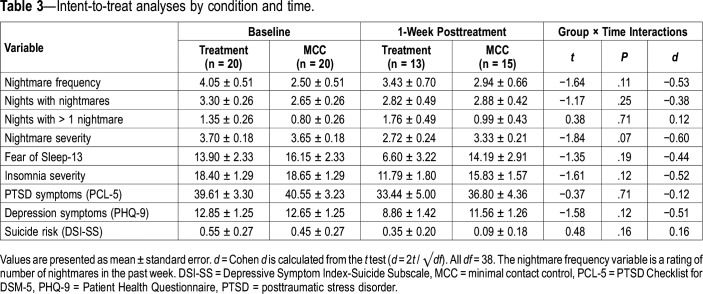
Comparisons of the treatment group and the MCC showed medium interaction effect sizes for nightmare frequency (d = −0.53), nights with nightmares (d = −0.38), nightmare severity (d = −0.60), fear of sleep (d = −0.44), and symptoms of insomnia (d = −0.52) and depression (d = −0.51). Very small effects were seen on nights with more than 1 nightmare (d = 0.12), PTSD (d = −0.12) and on suicide risk (d = 0.16). However, none of the differences between the ERRT-M and MCC groups were statistically significant at the 1-week posttreatment/postcontrol assessment on any outcome variables (Table 3). Given that this was a pilot RCT, it was not powered to find significant results between ERRT-M and control.
Table 3.
Intent-to-treat analyses by condition and time.
ERRT-M treatment effects through follow-up
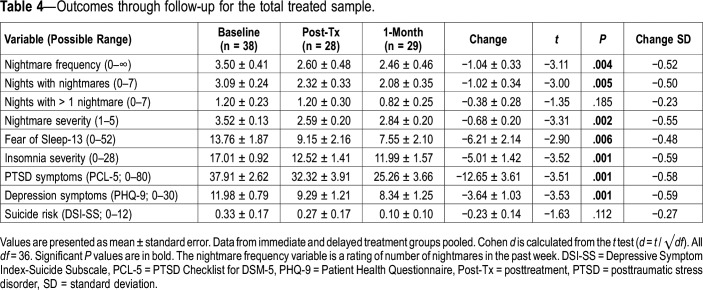
We next investigated the effect of ERRT-M in the 38 participants who had a baseline assessment and were randomized to the treatment or who had a postcontrol “baseline” assessment and then opted to participate in treatment. As can be seen in Table 4, analyses examining changes through the posttreatment and 1-month follow-up revealed significant decreases with medium within-subjects effect sizes for nightmare frequency in the past week (d = −0.52), nights with nightmares in the past week (d = −0.50), nightmare severity (d = −0.55), fear of sleep (d = −0.58), and symptoms of insomnia (d = −0.59), PTSD (d = −0.58) and depression (d = −0.59). No differences over time were seen in number of nights with greater than 1 nightmare (d = −0.23) or with suicide risk (d = −0.27).
Table 4.
Outcomes through follow-up for the total treated sample.
Clinically significant treatment response
Of the 29 participants who completed ERRT-M and the 1-month follow-up assessment, 48% met the treatment response criteria of experiencing ≤ 1 nightmare in the past week,47 with half of those reporting no nightmares in the past week.
Credibility and acceptability
As shown in Figure 2 and Figure 3, participants generally endorsed medium to high ratings indicating that they perceived the treatment to be credible and that they expected to experience benefits as a result of treatment. This was the case at the end of Session 1 after receiving an overview and rationale of the treatment (credibility scale: mean = 6.8, standard error [SE] = 0.3; expectancy scale: mean = 5.9, SE = 0.2), and also after completing all 5 sessions of treatment (credibility scale: mean = 7.1, SE = 0.3; expectancy scale mean = 5.9, SE = 0.4). There were no significant changes over time.
Figure 2. Treatment credibility ratings.
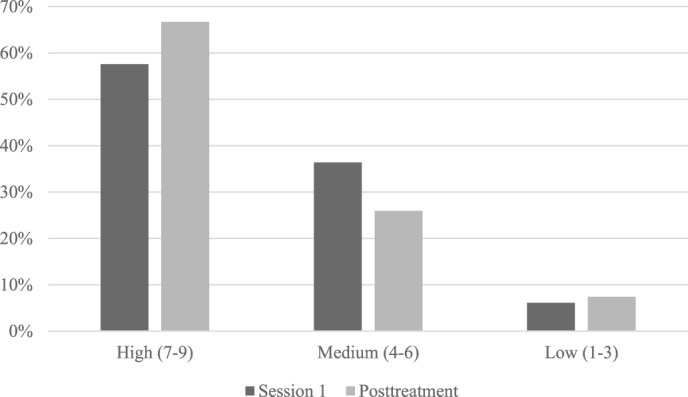
Percent of participants endorsing low, medium, and high treatment credibility after session 1 and at 1-week posttreatment. Participants rated items on a scale from 1 “not at all credible” to 9 “very credible” following the first session of treatment and at 1-week posttreatment. Ratings are grouped into High (7–9), Medium (4–6), and Low (1–3) treatment credibility ratings.
Figure 3. Treatment expectancy ratings.
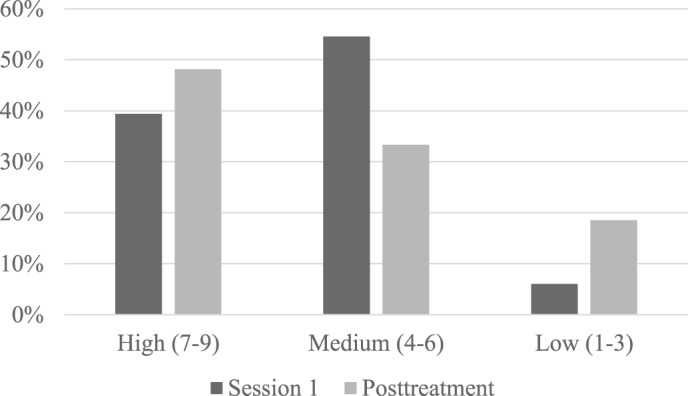
Percent of participants endorsing low, medium, and high treatment expectancy after session 1 and at 1-week posttreatment.
Treatment dropout and attrition
As shown in the CONSORT chart (Figure 1), the overall treatment dropout rate was 17.5% (n = 7; defined as the number of participants who did not complete all treatment sessions either in the immediate treatment group or during treatment following MCC [n = 5] and the number who dropped out of the study during the 5-week MCC [n = 2] and who, therefore, did not enter treatment). In the immediate treatment group, 10% (n = 2) did not enter treatment, 5% (n = 1) dropped out during treatment, and 5% (n = 1) were unable to attend treatment due to military reasons (eg, leaving the area for training, deployments, etc.). In the control group, 90% (n = 18) completed the postcontrol assessment. Similar to the immediate treatment group, 10% (n = 2) dropped during treatment and 5% (n = 1) were unable to complete treatment due to military reasons. Dropout from the study was higher at the 1-month follow-up with 35% and 20% of the immediate treatment group and control group, respectively, failing to complete the 1-month follow-up assessment.
Adverse events
This study used the STRONG STAR Consortium standardized adverse event monitoring48 at each treatment session and each week during the control condition. Therapists asked participants, “Have you had any problems since we last spoke?” All reported events that were a change from baseline were recorded as adverse events and were adjudicated during regular intervals across the study. A total of 65 adverse events were reported by 29 study participants; 11 participants did not report any adverse events throughout their participation in the study. Most adverse events were related to general medical problems the participants were having, and they were determined to be unrelated to the study. However, 6 adverse events were at least possibly related to the study; 3 involved increased frequency of nightmares, 1 involved increased PTSD symptoms, 1 involved a rash due to wearing a wrist-worn actigraphy device, and 1 involved increased stress related to moving, work, and attending appointments. Each event was monitored by the participant’s therapist. Of note, only 3 out of 38 participants (7.9%) reported an increase in nightmares, indicating that an increase in nightmares as a result of ERRT-M was a rare occurrence in this sample.
DISCUSSION
The present study is the first RCT of nightmare treatment to be conducted in an active duty military population. It is the fourth RCT of ERRT (although it used a 5-session variant of the 3-session version that was included in previous RCTs). Consistent with hypotheses that treatment would result in significant improvements in within-group analyses of the participants who received treatment, statistically significant reductions were found in past week nightmare frequency, nights with nightmares, nightmare severity, fear of sleep, and symptoms of insomnia, PTSD, and depression with medium effect sizes of ERRT-M for most measures. Between-group differences were also medium in size for most measures but were not statistically significant in this pilot study.
The findings from this study were similar to, or slightly smaller than, those found in previous trials of ERRT tested in civilan samples, although two additional sessions were provided with this study as compared with previous trials of 3-session ERRT22–24 (Table 1). The treatment response rate of 48% reporting ≤ 1 nightmare the week prior to the 1-month assessment is also promising. This rate is lower than the 72% found in the open trial of ERRT-M in veterans25 but higher than the 23% found in an RCT of IRT in veterans.47
Overall, active duty military personnel reported the treatment to be credible and had positive expectations after learning about the treatment at Session 1. These positive expectations persisted throughout treatment when assessed 1 week following the final session, which is important because behavioral treatments depend upon patients being willing to engage in the treatment components. Active duty service members in this study also were able to engage effectively in nightmare exposure and rescription activities. As hypothesized, the treatment dropout rate of 17.5% for this study was similar to rates found in civilian studies of similar treatment protocols (ie, 12.8% to 26%18,22–24). Treatment initiation rates were slightly higher than in civilian studies.26 This is particularly notable since active duty service members are highly mobile with frequent transfers between duty stations and, in accordance with the Department of Defense Instruction 3216.02, participants were not paid for study participation. These data suggest that active duty service members in the U.S. Army find this treatment approach to be credible and that implementing the treatment is feasible in this population.
In contrast to many clinicians’ and patients’ concerns that talking about nightmares or trauma may increase nightmares or other PTSD symptoms, our study found this was a rare occurrence (ie, only 7.9% reported any such symptoms). Previous studies have provided limited information about adverse events resulting from treatment or have not monitored them in a similar way. We encouraged standardized reporting of adverse events in behavioral therapy clinical trials to advance our understanding of treatment and to be able to fully inform patients of potential adverse events of treatment.
Limitations and future directions
While this pilot study provides important data for the investigation of nightmare treatment among active duty service members, this study was limited by a number of factors. First, caution should be used when examining effect sizes from small samples.49 Second, this study did not include detailed assessment of potential barriers to the treatment, such as detailed homework compliance assessment or tracking of adherence to treatment for comorbid sleep disorders such as sleep apnea. Third, the sample for this study included primarily male, lower-ranking enlisted personnel, and all were members of the U.S. Army stationed at a single military installation. Results may not generalize to active duty women, higher-ranking personnel, or to members of other branches of the military or those serving at different locations.
Future directions include studies to examine homework compliance and strategies for increasing adherence to assigned practice, such as motivational interviewing. Future studies are also needed to examine the impact of comorbid sleep disorders on nightmare treatment, such as sleep apnea. There is some indication in the civilian literature that nightmare treatment is effective regardless of risk for sleep apnea.50 However, previous studies were limited by use of self-report measures only, whereas objective overnight polysomnography is required for assessing sleep apnea. Also, sleep apnea may be more prevalent in a primarily male population. Studies are also needed to clarify the optimal approaches for addressing nightmares, comorbid sleep disorders (eg, insomnia, sleep apnea), and PTSD. Nightmare treatment may be more effective if sleep apnea and/or PTSD have already been treated; however, nightmare treatment may also help patients be more responsive to treatment of these comorbid conditions. While nightmares appear to be more responsive to effective PTSD treatment, they are resistant to treatment in some cases.15
In conclusion, this study demonstrated that ERRT-M resulted in significant reductions in various measures of nightmares in active duty military personnel. Participants considered ERRT-M both credible and feasible. An adequately powered randomized clinical trial is needed to confirm findings and to compare ERRT-M to an active treatment control.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This research was conducted at Carl R. Darnall Army Medical Center, Fort Hood, TX. This was an investigator-initiated study funded by an American Academy of Sleep Medicine Foundation Focused Program Award grant awarded to Dr. Pruiksma. The authors report no conflicts of interest. The study did not involve off-label or investigational use of any pharmaceutical or medical treatment. The views expressed herein are solely those of the authors and do not reflect an endorsement by or the official policy or position of the U.S. Army, the Department of Defense, the Department of Veterans Affairs, or the U.S. Government.
ACKNOWLEDGMENTS
The authors thank Julie Collins and Joel Williams, who provided editorial support for this manuscript, Antoinette Brundige and Bryce Williams who provided project management support, Ray Aguilar who provided database development support, and the following individuals who supported this study as assessors and therapists (listed alphabetically): Lucas Brilliott, Brooke Fina, Brittany Hall-Clark, Vanessa Jacoby, and Dana Larson. We also thank Tara Casady for serving as the treatment fidelity rater.
ABBREVIATIONS
- CAPS-5
Clinician-Administered PTSD Scale for DSM-5
- ERRT
exposure, relaxation, and rescripting therapy
- ERRT-M
exposure, relaxation, and rescripting therapy for military service members and veterans
- ES
effect size
- IRB
institutional review board
- IRT
imagery rehearsal therapy
- MCC
minimal contact control
- PMR
progressive muscle relaxation
- PTSD
posttraumatic stress disorder
- RCT
randomized controlled trial
- REM
rapid eye movement
- SAS
Statistical Analysis Software
- SCISD
Structured Clinical Interview for DSM-5 Sleep Disorders
- STRONG STAR
South Texas Research Organizational Network Guiding Studies on Trauma and Resilience
REFERENCES
- 1.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 2.Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126–132. doi: 10.1097/NMD.0b013e3181961d8e. [DOI] [PubMed] [Google Scholar]
- 3.Gellis LA, Gehrman PR, Mavandadi S, Oslin DW. Predictors of sleep disturbances in Operation Iraqi Freedom/Operation Enduring Freedom veterans reporting a trauma. Mil Med. 2010;175(8):567–573. doi: 10.7205/milmed-d-09-00123. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41(6):469–478. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- 5.Mohr D, Vedantham K, Neylan T, Metzler TJ, Best S, Marmar CR. The mediating effects of sleep in the relationship between traumatic stress and health symptoms in urban police officers. Psychosom Med. 2003;65(3):485–489. doi: 10.1097/01.psy.0000041404.96597.38. [DOI] [PubMed] [Google Scholar]
- 6.Sjöström N, Hetta J, Waern M. Persistent nightmares are associated with repeat suicide attempt: a prospective study. Psychiatry Res. 2009;170(2-3):208–211. doi: 10.1016/j.psychres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Nadorff MR, Nazem S, Fiske A. Insomnia symptoms, nightmares, and suicide risk: Duration of sleep disturbance matters. Suicide Life Threat. Behav. 2013;43(2):139–149. doi: 10.1111/sltb.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruiksma KE, Slavish D, Wardle S, et al. Prevalence and correlates of nightmares in active duty service members. Sleep. 2019;42(suppl_1):A267. [Google Scholar]
- 9.Creamer JL, Brock MS, Matsangas P, Motamedi V, Mysliwiec V. Nightmares in United States military personnel with sleep disturbances. J Clin Sleep Med. 2018;14(03):419–426. doi: 10.5664/jcsm.6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 12.Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq War veterans. Am J Psychiatry. 2007;164(1):150–153. doi: 10.1176/ajp.2007.164.1.150. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JK, Levin-Rector A, Van Dorn RA, et al. Trends in mental health outcomes and combat exposure among US marines returning from Iraq, Afghanistan or other deployments, 2004–13. J Public Health (Oxf) 2019;41(2):313–320. doi: 10.1093/pubmed/fdy078. [DOI] [PubMed] [Google Scholar]
- 14.Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA. 2006;296(5):519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- 15.Pruiksma KE, Taylor DJ, Wachen JS, et al. on behalf of the STRONG STAR Consortium Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol Trauma. 2016;8(6):697–701. doi: 10.1037/tra0000150. [DOI] [PubMed] [Google Scholar]
- 16.Casement MD, Swanson LM. A meta-analysis of imagery rehearsal for post-trauma nightmares: Effects on nightmare frequency, sleep quality, and posttraumatic stress. Clin Psychol Rev. 2012;32(6):566–574. doi: 10.1016/j.cpr.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augedal AW, Hansen KS, Kronhaug CR, Harvey AG, Pallesen S. Randomized controlled trials of psychological and pharmacological treatments for nightmares: a meta-analysis. Sleep Med Rev. 2013;17(2):143–152. doi: 10.1016/j.smrv.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Ho FY-Y, Chan CS, Tang KN-S. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90–102. doi: 10.1016/j.cpr.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Morgenthaler TI, Auerbach S, Casey KR, et al. Position paper for the treatment of nightmare disorder in adults: an American Academy of Sleep Medicine position paper. J Clin Sleep Med. 2018;14(6):1041–1055. doi: 10.5664/jcsm.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harb GC, Phelps AJ, Forbes D, Ross RJ, Gehrman PR, Cook JM. A critical review of the evidence base of imagery rehearsal for posttraumatic nightmares: pointing the way for future research. J Trauma Stress. 2013;26(5):570–579. doi: 10.1002/jts.21854. [DOI] [PubMed] [Google Scholar]
- 21.Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757–767. doi: 10.5664/jcsm.7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruiksma KE, Cranston CC, Rhudy JL, Micol RL, Davis JL. Randomized controlled trial to dismantle exposure, relaxation, and rescripting therapy (ERRT) for trauma-related nightmares. Psychol Trauma. 2018;10(1):67–75. doi: 10.1037/tra0000238. [DOI] [PubMed] [Google Scholar]
- 23.Davis JL, Rhudy JL, Pruiksma KE, et al. Physiological predictors of response to exposure, relaxation, and rescripting therapy for chronic nightmares in a randomized clinical trial. J Clin Sleep Med. 2011;7(6):622–631. doi: 10.5664/jcsm.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis JL, Wright DC. Randomized clinical trial for treatment of chronic nightmares in trauma-exposed adults. J Trauma Stress. 2007;20(2):123–133. doi: 10.1002/jts.20199. [DOI] [PubMed] [Google Scholar]
- 25.Balliett NE, Davis JL, Miller KE. Efficacy of a brief treatment for nightmares and sleep disturbances for veterans. Psychol Trauma. 2015;7(6):507–515. doi: 10.1037/tra0000055. [DOI] [PubMed] [Google Scholar]
- 26.Rhudy JL, Davis JL, Williams AE, et al. Cognitive-behavioral treatment for chronic nightmares in trauma-exposed persons: Assessing physiological reactions to nightmare-related fear. J Clin Psychol. 2010;66(4):365–382. doi: 10.1002/jclp.20656. [DOI] [PubMed] [Google Scholar]
- 27.Davis JL, Balliett NE, Friedlander JN, Pruiksma KE, Miller KE. Exposure, Relaxation, and Rescripting Therapy for Military and Veterans (ERRT-M) for Chronic Trauma-Related Nightmares and Sleep Disturbances: A Treatment Workbook. Tulsa, OK: University of Tulsa; 2016; [Google Scholar]
- 28.Moore B, Krakow B. Imagery rehearsal therapy for acute posttraumatic nightmares among combat soldiers in Iraq. Am J Psychiatry. 2007;164(4):683–684. doi: 10.1176/ajp.2007.164.4.683. [DOI] [PubMed] [Google Scholar]
- 29.Foa EB, McLean CP, Zang Y, et al. for the STRONG STAR Consortium Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354–364. doi: 10.1001/jama.2017.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resick PA, Wachen JS, Mintz J, et al. on behalf of the STRONG STAR Consortium A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058–1068. doi: 10.1037/ccp0000016. [DOI] [PubMed] [Google Scholar]
- 31.Resick PA, Wachen JS, Dondanville KA, et al. and the STRONG STAR Consortium Effect of group vs individual cognitive processing therapy in active-duty military seeking treatment for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(1):28–36. doi: 10.1001/jamapsychiatry.2016.2729. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013; [Google Scholar]
- 33.Davis JL. Treating Post-trauma Nightmares: A Cognitive Behavioral Approach. New York, NY: Springer Publishing Co; 2009; [Google Scholar]
- 34.Resick PA, Galovski TE, Uhlmansiek MOB, Scher CD, Clum GA, Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J Consult Clin Psychol. 2008;76(2):243–258. doi: 10.1037/0022-006X.76.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes BJ, Presseau C, Jordan AH, et al. and the Consortium to Alleviate PTSD Common data elements in the assessment of military-related PTSD research applied in the consortium to alleviate PTSD. Mil Med. 2019;184(5-6):e218–e226. doi: 10.1093/milmed/usy226. [DOI] [PubMed] [Google Scholar]
- 36.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the Life Events Checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 37.Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor DJ, Wilkerson AK, Pruiksma KE, et al. on behalf of the STRONG STAR Consortium Reliability of the Structured Clinical Interview for DSM-5 Sleep Disorders Module. J Clin Sleep Med. 2018;14(3):459–464. doi: 10.5664/jcsm.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cranston CC, Miller KE, Davis JL, Rhudy JL. Preliminary validation of a brief measure of the frequency and severity of nightmares: The Trauma-Related Nightmare Survey. J Trauma Dissociation. 2017;18(1):88–99. doi: 10.1080/15299732.2016.1191578. [DOI] [PubMed] [Google Scholar]
- 40.Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 41.Pruiksma KE, Taylor D, Ruggero C, et al. A psychometric study of the Fear of Sleep Inventory-Short Form (FoSI-SF) J Clin Sleep Med. 2014;10(5):551–558. doi: 10.5664/jcsm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weathers F, Litz B, Keane T, Palmieri P, Marx B, Schnurr P. PTSD Checklist for DSM–5 (PCL-5). https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp. Published 2013. Accessed May 2, 2019.
- 43.Wortmann JH, Jordan AH, Weathers FW, et al. Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychol. Assess. 2016;28(11):1392–1403. doi: 10.1037/pas0000260. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metalsky GI, Joiner TE., Jr The Hopelessness Depression Symptom Questionnaire. Cognit Ther Res. 1997;21(3):359–384. [Google Scholar]
- 46.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 47.Nappi CM, Drummond SPA, Thorp SR, McQuaid JR. Effectiveness of imagery rehearsal therapy for the treatment of combat-related nightmares in veterans. Behav Ther. 2010;41(2):237–244. doi: 10.1016/j.beth.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Peterson AL, Roache JD, Raj J, Young-McCaughan S. for the STRONG STAR Consortium The need for expanded monitoring of adverse events in behavioral health clinical trials. Contemp Clin Trials. 2013;34(1):152–154. doi: 10.1016/j.cct.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller KE, Cranston CC, Simonet DV, Pruiksma KE, Davis JL. The impact of suspected sleep apnea on exposure, relaxation, and rescripting therapy (ERRT): a preliminary examination. J Sleep Disord Med Care. 2018;1(1) [Google Scholar]