Abstract
Study Objectives:
Sleep restriction therapy (SRT) has been shown to be comparably effective relative to cognitive behavioral therapy for insomnia (CBT-I), but with lower requirements for patient contact. As such, SRT appears to be a viable alternate treatment for those who cannot complete a full course of CBT-I. However, it is unclear whether SRT—a treatment solely focusing on restricting time in bed—increases risk for sleepiness comparably to CBT-I. The current study tested objective sleepiness as an outcome in a randomized controlled trial comparing SRT, CBT-I, and attention control in a sample of postmenopausal women in whom insomnia was diagnosed according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Methods:
Single-site, randomized controlled trial. A total of 150 postmenopausal women (56.44 ± 5.64 years) with perimenopausal or postmenopausal onset of Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition insomnia disorder were randomized to 3 treatment conditions: sleep education control (6 sessions); SRT (2 sessions with interim phone contact); and CBT-I (6 sessions). Blinded assessments were performed at pretreatment and posttreatment. Risk of excessive sleepiness was evaluated using a symmetry analysis of sleepiness measured through the Multiple Sleep Latency Test (MSLT).
Results:
The odds ratios (ORs) of being excessively sleepy versus nonsleepy were not different than 1.0 for both SRT (OR = 0.94, 95% confidence interval [0.13–6.96]) and CBT-I (OR = 0.62, 95% confidence interval [0.09–4.46]), indicating that the odds of becoming excessively sleepy following treatment was not different from the odds of being nonsleepy. This suggests that excessive sleepiness is not of unique concern following SRT relative to CBT-I or sleep education.
Conclusions:
SRT appears to have a comparable risk profile for excessive sleepiness as CBT-I, and thus may be considered a safe alternative to CBT-I. Future research should characterize objective measures of excessive sleepiness immediately following sleep restriction.
Clinical Trail Registration:
Registry: ClinicalTrials.gov; Name: Behavioral Treatment of Menopausal Insomnia; Sleep and Daytime Outcomes; Identifier: NCT01933295
Citation:
Cheng P, Kalmbach D, Fellman-Couture C, Arnedt JT, Cuamatzi-Castelan A, Drake CL. Risk of excessive sleepiness in sleep restriction therapy and cognitive behavioral therapy for insomnia: a randomized controlled trial. J Clin Sleep Med. 2020;16(2):193–198.
Keywords: CBT-I, excessive sleepiness, MSLT, sleep restriction therapy
BRIEF SUMMARY
Current Knowledge/Study Rationale: As a single-component therapy, sleep restriction therapy (SRT) has been advocated as an alternate treatment to cognitive behavioral therapy for insomnia (CBT-I). SRT may be more feasible for those who cannot engage in a full course of CBT-I because it is conducted in fewer sessions; however, the relative safety of SRT versus CBT-I has not been established via a head-to-head comparison.
Study Impact: This randomized controlled trial used the gold-standard objective measurement of sleepiness and found near-identical risk of excessive sleepiness between SRT and CBT-I. This result provides evidence that SRT is a safe alternative to a full course of CBT-I.
INTRODUCTION
Restriction of time in bed (TIB) with the aim of consolidating sleep (ie, sleep restriction) is a core component of cognitive behavioral therapy for insomnia (CBT-I), and has efficacy as a single-component therapy.1 As a stand-alone treatment, sleep restriction therapy (SRT) putatively leverages the homeostatic sleep drive by restricting TIB and then titrating back to the desired TIB based on improvement in sleep efficiency.2 SRT typically requires less contact time (∼2 face-to-face sessions) and thus is an ideal alternative for those who are unable to commit to a full course of CBT-I (6 or more face-to-face sessions).
In comparison to SRT as a stand-alone treatment (∼2 sessions), sleep restriction implemented as part of CBT-I (∼6 sessions) provides more time and opportunity to titrate time-in-bed up toward the recommended duration for adults (7 to 9 hours) by the end of therapy. Although the titration rate in stand-alone SRT can be accelerated (eg, titrating every few days instead of weekly as is typical in CBT-I), full correction of this disparity is rarely achieved, particularly when SRT is chosen as a briefer alternative treatment to CBT-I. As such, excessive sleepiness as an adverse event is a common concern associated with SRT, especially after treatment when patients are no longer monitored by a care provider.
While prior studies have examined sleepiness as a consequence of SRT, the literature consists primarily of studies with small samples that use self-report instruments, and findings have been inconsistent. For example, a small study (n = 18) using qualitative methods to document adverse events following SRT found that reports of sleepiness and fatigue were highly prevalent.3 Another study (n = 16) in insomnia with short sleep (sleep duration ≤ 6 hours) also found that SRT increased reported sleepiness and impaired attention, both of which recovered by 8 weeks after treatment.4 However, a more recent study did not find differences on the Epworth Sleepiness Scale (n = 16) or performance deficits associated with sleepiness following SRT.5 Another study in older adults (55 years and older) compared SRT, SRT with an afternoon nap, and sleep hygiene, but found no differences in pretreatment and posttreatment Multiple Sleep Latency Test (MSLT) scores or self-reported sleepiness (n = 16).6 Furthermore, no well-controlled studies have conducted head-to-head comparisons of SRT and CBT-I to control. Additionally, prior studies have not adequately examined excessive sleepiness (ie, sleepiness that exceeds a clinically significant threshold). This is critical because a clinically significant change in sleepiness may be more strongly associated with safety concerns (eg, drowsy driving), and should be a central consideration for the recommendation of SRT as an alternative to CBT-I.
To determine whether the risk of excessive sleepiness as an adverse event is higher in SRT than CBT-I, this study examined objective sleepiness via the gold standard MSLT as an outcome in a randomized controlled trial comparing SRT, CBT-I, and a sleep education control. Participants were postmenopausal women with insomnia. We hypothesized that the risk of excessive sleepiness would not differ between SRT and CBT-I groups relative to the sleep education control.
METHODS
Participants and procedure
This study was conducted in a large six-hospital health system in the state of Michigan. Participants were recruited from the health system in primary care and the sleep clinic, as well as from the community via newspaper advertisements and from a database of prior sleep center studies. Participants were postmenopausal women meeting Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for insomnia disorder. Additionally, all participants showed objective sleep disturbance via polysomnography at baseline as defined by wake after sleep onset ≥ 45 minutes.7 Exclusionary criteria also included prior or current Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition major depression per diagnostic interview, sleep-wake disorders other than insomnia (examined on polysomnography [PSG] adaptation night and per patient report), and medications influencing sleep.
A final sample of 150 participants were randomized into three treatment conditions: (1) sleep education (n = 50), (2) SRT (n = 50), and (3) CBT-I (n = 50). Six individuals at pretreatment and nine individuals at posttreatment had technological errors or difficulties that precluded the valid and reliable scoring of the MSLT. These data were excluded from analyses. Although double-blind conditions could not be achieved given the nature of the behavioral interventions, participants were not informed which treatments were considered control versus active, or of the specific hypotheses. All procedures were approved by the Henry Ford Health System Institutional Review Board.
Treatment conditions
Sleep education
Women in the sleep education condition received six weekly psychoeducation emails that also included sleep hygiene. Sleep hygiene is neither the primary cause nor a sufficient therapeutic target in insomnia disorder and therefore served as an ideal control condition and real-world comparator.
Cognitive behavioral therapy for insomnia
Women randomized to CBT-I completed 6 face-to-face sleep therapy sessions8 with a registered nurse specialized in behavioral sleep medicine. Weekly sessions covered behavioral (sleep restriction and stimulus control) and cognitive components (eg, cognitive restructuring), as well as relaxation strategies (eg, progressive muscle relaxation and autogenic training) and sleep hygiene education. Sleep restriction and stimulus control were introduced during the first and second sessions and reviewed as necessary throughout the treatment.
Sleep restriction therapy
SRT was delivered via 2 face-to-face sessions and three intervening brief phone contacts (< 15 minutes) with a registered nurse specialized in behavioral sleep medicine. The first and second sessions were separated by 2 weeks. The prescribed TIB was based on average total sleep time from 2 weeks of baseline sleep diaries, with a minimum of 5 hours for safety as is standard.1 Interim phone contacts were conducted between the first and second sessions to titrate the TIB prescription based on the average sleep efficiency over at least 4 days. TIB was titrated according to the following rules: increase 30 minutes if sleep efficiency was 90% or higher; increase 15 minutes if sleep efficiency was 85% to 89%; no change if sleep efficiency was 80% to 84%; reduce 15 minutes if sleep efficiency < 80%. The second and final session introduced relapse prevention and also reviewed principles for implementing TIB titration for continued use if the desired TIB had not been reached, or for future episodes of insomnia.
Dependent variable
The primary outcome was objective sleepiness measured via the standard research MSLT.9 Self-reported sleepiness from the sleep diary was also included (0–10 scale, 10 = highest). The pretreatment MSLT was conducted within 10 days prior to the first treatment session, and the posttreatment MSLT was conducted within 10 days following the last treatment session. MSLTs were conducted following full-night PSG with 8 hours in bed starting at habitual bedtime based on self-report. A total of four sleep latency tests were conducted at 2-hour intervals starting 1.5 hours after the scheduled rise time following the nocturnal PSG. Tests were terminated after 4 consecutive epochs of sleep (2 minutes) or 20 minutes after lights out if sleep onset did not occur. Sleep onset latency (SOL) was scored for each sleep latency test, and a mean score was calculated for each set of MSLT conducted per individual.
To evaluate the probability of excessive sleepiness following SRT—rather than simply comparing changes in mean SOL—we tested the risk for a level of sleepiness that would be sufficiently large to imply potential risk of functional impairments. We selected a cutoff point of a mean SOL below 8 minutes on the MSLT for “excessive sleepiness” that is consistent with the threshold used in the International Classification of Sleep Disorders to identify excessive sleepiness associated with narcolepsy and idiopathic hypersomnia.10 A variance of ± 2 minutes around 10 minutes was determined as significant change based on prior evidence of a 2-minute margin of error above the 8-minute threshold in capturing hypersomnolence.11,12 As such, a threshold of greater than 12 minutes on the MSLT was categorized as “nonsleepy,” and an MSLT score between 8 and 12 minutes was categorized as “subclinical sleepiness.”
Analysis plan
A multinomial generalized linear mixed-effects approach was used to evaluate the risk of sleepiness (excessive sleepiness, subclinical sleepiness, nonsleepiness) following CBT-I and SRT relative to the control condition. Independent variables in the model included condition (sleep education [reference category], CBT-I, SRT), time (pretreatment [reference category], posttreatment) and the interaction between condition × time, with level of sleepiness (excessive sleepiness, subclinical sleepiness, nonsleepiness) as the dependent variable. A random intercept was included in order to account for individual differences in baseline sleepiness. The final model included two covariates. The first accounted for differences in TIB at the final treatment session and the 8-hour TIB for the posttreatment PSG. The second accounted for the number of days between the final treatment session and the posttreatment PSG.
To test for significant changes in odds of excessive sleepiness, we specifically examined the symmetry of odds of excessive sleepiness versus nonsleepiness following each respective treatment relative to the control condition. This approach is appropriate for safety evaluations because pre–post differences that may be statistically significant but not clinically significant should not strongly affect inferences regarding safety, as such changes may not correspond to differences in safety risk.13,14 In the multinomial generalized linear mixed-effects model, symmetrical risk would be represented if the change in odds of excessive sleepiness versus nonsleepiness associated with receiving the treatments of interest (SRT or CBT-I) were comparable to that of the control condition. Symmetry would indicate that SRT or CBT-I are not likely to confer additional risks of excessive sleepiness over sleep education control. The analyses were set up so that a significant condition × time interaction would indicate nonsymmetrical odds for being excessively sleepy versus nonsleepy following treatment (CBT-I or SRT) compared to the sleep education control. An odds ratio (OR) significantly higher than 1.0 would suggest increased risk for excessive sleepiness. Finally, probabilities for each outcome level by condition at posttreatment were also calculated and used for interpretation.
A secondary analysis was also conducted using MSLT values to determine whether smaller differences in objective sleepiness were present between the three treatment groups. This was conducted using a linear mixed-effects model with MSLT scores as the dependent variable and condition (sleep education [reference category], CBT-I, SRT), time (pretreatment [reference category], posttreatment) and the interaction between condition × time as predictor variables. A random intercept was included in order to account for individual differences in baseline sleepiness. A significant condition × time interaction would indicate that MSLT scores were differentially affected by the treatment conditions. In the context of symmetrical odds from the primary analysis, a positive finding from this secondary analysis would indicate that smaller differences in objective sleepiness may still exist between the treatment groups.
RESULTS
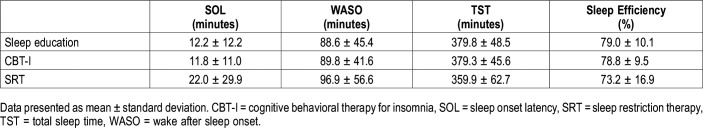
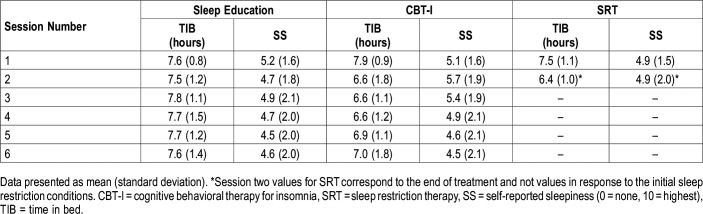
Our sample was largely composed of non-Hispanic white (52.0%) and non-Hispanic black women (39.3%). Participants exhibited more difficulties with sleep maintenance: 75.2% of the sample reported greater than 30 minutes of wake time after sleep onset from baseline sleep diaries, compared to just 42.3% who reported a SOL greater than 30 minutes.7 Table 1 includes sleep characteristics from pretreatment and posttreatment PSG. Average TIB from sleep diaries confirm that sleep restriction was implemented in CBT-I and SRT conditions, but not the sleep education control (Table 2).
Table 1.
Baseline characteristics of sleep from pretreatment polysomnography.
Table 2.
Mean self-reported time in bed and self-reported sleepiness by session number.
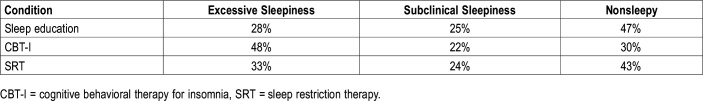
Results from the multinomial generalized linear mixed-effects model indicated that the odds of excessive sleepiness relative to nonsleepiness was not significantly different among groups at baseline. Following treatment, the sleep education control condition did not engender increased odds of excessive sleepiness versus nonsleepiness, marginal effect of time (reference = sleep education), OR (95% confidence interval [CI] = 2.00 [0.48–8.42]). Relative to the control condition, those in the SRT condition showed symmetrical odds, condition (SRT) × time, OR (95% CI) = 0.94 (0.13–6.96), suggesting that SRT did not result in a higher risk for excessive sleepiness. Similarly, those in the CBT-I condition also showed symmetrical odds of sleepiness compared to the control group, condition (CBT-I) × time, OR (95% CI) = 0.62 (0.09–4.46). The probability of excessive sleepiness at posttreatment was 33% in the SRT condition, 48% in the CBT-I condition, and 28% in the control condition. Neither covariates were significant, indicating that sleepiness levels were not associated with differences in TIB between the final treatment session and the posttreatment PSG (OR [95% CI] = 1.00 [0.99–1.01]), or with the number of days between the final treatment session and the posttreatment PSG (OR [95% CI] = 1.03 [0.90–1.20]). Mean MSLT scores are presented in Table 3, and probabilities of sleepiness outcomes are presented in Table 4.
Table 3.
Mean sleep onset latency from the Multiple Sleep Latency Test at pretreatment and posttreatment between experimental conditions.
Table 4.
Probabilities of sleepiness an outcome following each treatment condition.
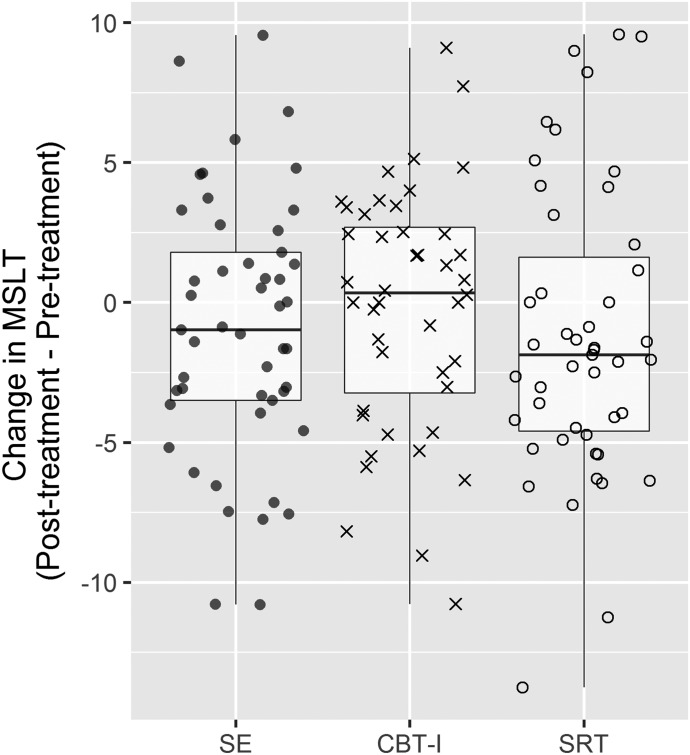
Secondary analysis was also conducted using linear mixed-effects approach with MSLT scores as a continuous dependent variable. Results indicated that the condition × time interaction was not significant F2,137 = 0.77, P = .46 (Table 3 shows mean MSLT scores, and Figure 1 shows boxplots of change in MSLT scores).
Figure 1. Boxplot with individual change scores in MSLT from pretreatment to posttreatment for SRT and CBT-I relative to the control condition (sleep education).
Change scores greater than zero represent increased MSLT score (ie, increases in alertness) following treatment. CBT-I = cognitive behavioral therapy for insomnia, MSLT = Multiple Sleep Latency Test, SE = sleep education, SRT = sleep restriction therapy.
DISCUSSION
This study examined the risk of excessive sleepiness following SRT and CBT-I relative to a sleep education control in a randomized controlled trial of 150 postmenopausal women with chronic insomnia. We found that SRT and CBT-I did not increase the risk of excessive sleepiness at the conclusion of treatment. Moreover, SRT did not differ from CBT-I with regard to risk for excessive sleepiness. These findings add to extant support for SRT as a safe and efficacious alternative to CBT-I, particularly for patients unable to engage in full multimodal CBT-I.
This is the first study to conduct a head-to-head comparison of excessive sleepiness between SRT and CBT-I. The primary strength of this study is the utilization of the MSLT as the gold standard assessment of objective sleepiness. This is important because perception of sleepiness may not be as sensitive compared to objective measures.15 As such, objective measures of sleepiness may also be stronger predictors of sleepiness related-performance deficits such as driving impairment.16 One limitation may be the implementation of the standard 8-hour sleep period prior to the final MSLT, which could have dissipated homeostatic pressure associated with sleep restriction; however, the results did not suggest that final MSLT results varied significantly by TST on the posttreatment PSG.
Notably, these results also support distinguishing SRT conceptually from chronic partial sleep deprivation. When conducted in accordance with clinical guidelines,2 the goal of SRT is not to deprive sleep, but rather to consolidate total sleep time. This is evidenced by the practice of only restricting TIB equal to pretreatment total sleep time—as opposed to sleep deprivation, which shrinks total sleep time—and an enforced minimum TIB. Although, in practice, the initiation of SRT usually does increase sleep drive, this drive dissipates with the consolidation of sleep bouts and with the upward titration of TIB. In contrast, chronic partial sleep deprivation from a consistent shrinkage of total sleep time below sleep need leads to a continual buildup of sleep drive. Although sleepiness could increase temporarily at the initiation of SRT, this pressure should dissipate throughout treatment. Consequentially, the risk of excessive sleepiness should not differ greatly from that of CBT-I, as observed in this study. This would also explain why differences in time in bed between the final treatment session and the posttreatment PSG did not predict levels of excessive sleepiness.
Although results of this study support the general safety of SRT relative to CBT-I, they do not detract from the importance of safety assessments with individual patients throughout both SRT and CBT-I. These results do not reflect individuals’ responses shortly following the initiation of sleep restriction. Instead, the results indicate that, in aggregate, SRT does not confer additional risk of excessive sleepiness compared to CBT-I; however, there are likely interindividual differences that may make certain individuals more vulnerable to excessive sleepiness than others in response to sleep restriction. For example, prior studies have shown that genetic differences may moderate individual differences in sleepiness and performance impairments associated with sleep loss.17–20 Importantly, some of the candidate genes also influence circadian rhythms (eg, chronotype),21–24 which has implications for sleep propensity at various times in a 24-hour period. Age may be another factor. While excessive sleepiness is not normative of healthy aging,25,26 some studies have suggested that older adults may actually be less impaired following sleep restriction.27–30 Future research should further examine these and other patient characteristics that may moderate individual risk of excessive sleepiness in response to SRT. Additionally, studies should also examine whether the safety profile of SRT generalizes to other domains such as driving and cognitive performance (ie, reaction time, decision making) in high-stakes contexts.
Another important consideration is that the analyses occurred in the context of a larger study examining the effectiveness of behavioral sleep interventions in postmenopausal women. Thus, the generalizability of these findings is limited to perimenopausal and postmenopausal insomnia. Additional differences of this sample include age and/or sex of the participants. Future studies should replicate these findings in larger samples that are more representative of the general population with insomnia as well as other specific insomnia populations (eg, chronic pain, mild sleep-disordered breathing). For example, phenotypic differences in insomnia may be important, particularly as SRT in insomnia with short sleep has been previously associated with increased self-reported sleepiness and attentional impairments lasting beyond treatment conclusion4; however, the safety implications of these results have yet to be tested.
CONCLUSIONS
Results from this study indicated the risk of excessive sleepiness was not higher in stand-alone SRT relative to CBT-I in a sample of postmenopausal women with insomnia. This adds to a growing body of evidence supporting the safety of SRT as an alternate treatment for those who are unable to engage in a full course of CBT-I.
DISCLOSURE STATEMENT
All authors have seen and approved the submission of this manuscript. This work was performed at the Henry Ford Health System. The authors report no conflicts of interest.
ABBREVIATIONS
- CBT-I
cognitive behavioral therapy for insomnia
- CI
confidence interval
- MSLT
Multiple Sleep Latency Test
- SOL
sleep onset latency
- SRT
sleep restriction therapy
- TIB
time in bed
REFERENCES
- 1.Miller CB, Espie CA, Epstein DR, et al. The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep Med Rev. 2014;18(5):415–424. doi: 10.1016/j.smrv.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- 3.Kyle SD, Morgan K, Spiegelhalder K, Espie CA. No pain, no gain: an exploratory within-subjects mixed-methods evaluation of the patient experience of sleep restriction therapy (SRT) for insomnia. Sleep Med. 2011;12(8):735–747. doi: 10.1016/j.sleep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Kyle SD, Miller CB, Rogers Z, Siriwardena AN, MacMahon KM, Espie CA. Sleep restriction therapy for insomnia is associated with reduced objective total sleep time, increased daytime somnolence, and objectively impaired vigilance: implications for the clinical management of insomnia disorder. Sleep. 2014;37(2):229–237. doi: 10.5665/sleep.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittall H, Pillion M, Gradisar M. Daytime sleepiness, driving performance, reaction time and inhibitory control during sleep restriction therapy for Chronic Insomnia Disorder. Sleep Med. 2018;45:44–48. doi: 10.1016/j.sleep.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13(1):17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 7.Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2) doi: 10.1093/sleep/zsy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. Vol. 1. New York, NY: Springer Science & Business Media; 2006. [Google Scholar]
- 9.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 11.Guilleminault C, Partinen M, Maria Antonia Q-S, Hayes B, Dement WC, Nino-Murcia G. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94(1):32–37. doi: 10.1378/chest.94.1.32. [DOI] [PubMed] [Google Scholar]
- 12.van den Hoed J, Kraemer H, Guilleminault C, et al. Disorders of excessive daytime somnolence: polygraphic and clinical data for 100 patients. Sleep. 1981;4(1):23–37. doi: 10.1093/sleep/4.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Arnaud M, Bégaud B, Thurin N, Moore N, Pariente A, Salvo F. Methods for safety signal detection in healthcare databases: a literature review. Expert Opin Drug Saf. 2017;16(6):721–732. doi: 10.1080/14740338.2017.1325463. [DOI] [PubMed] [Google Scholar]
- 14.Vermeeren A, Vuurman EFPM, Leufkens TRM, et al. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. Sleep. 2014;37(3):489–496. doi: 10.5665/sleep.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. Sleep loss and sleepiness: current issues. Chest. 2008;134(3):653–660. doi: 10.1378/chest.08-1064. [DOI] [PubMed] [Google Scholar]
- 16.Ftouni S, Sletten TL, Howard M, et al. Objective and subjective measures of sleepiness, and their associations with on-road driving events in shift workers. J Sleep Res. 2013;22(1):58–69. doi: 10.1111/j.1365-2869.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14(3):151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Rupp TL, Wesensten NJ, Newman R, Balkin TJ. PER3 and ADORA2A polymorphisms impact neurobehavioral performance during sleep restriction. J Sleep Res. 2013;22(2):160–165. doi: 10.1111/j.1365-2869.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Holst SC, Müller T, Valomon A, Seebauer B, Berger W, Landolt H-P. Functional polymorphisms in dopaminergic genes modulate neurobehavioral and neurophysiological consequences of sleep deprivation. Sci Rep. 2017;7(1):45982. doi: 10.1038/srep45982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tkachenko O, Dinges DF. Interindividual variability in neurobehavioral response to sleep loss: a comprehensive review. Neurosci Biobehav Rev. 2018;89:29–48. doi: 10.1016/j.neubiorev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Archer SN, Schmidt C, Vandewalle G, Dijk D-J. Phenotyping of PER3 variants reveals widespread effects on circadian preference, sleep regulation, and health. Sleep Med Rev. 2018;40:109–126. doi: 10.1016/j.smrv.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk D-J. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31(5):608–617. doi: 10.1093/sleep/31.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira DS, Tufik S, Louzada FM, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005;28(1):29–32. [PubMed] [Google Scholar]
- 24.Kalmbach DA, Schneider LD, Cheung J, et al. Genetic basis of chronotype in humans: insights from three landmark GWAS. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 26.Hoch CC, Reynolds CF, Jennings JR, et al. Daytime sleepiness and performance among healthy 80 and 20 year olds. Neurobiol Aging. 1992;13(2):353–356. doi: 10.1016/0197-4580(92)90049-4. [DOI] [PubMed] [Google Scholar]
- 27.Stenuit P, Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005;28(10):1283–1288. doi: 10.1093/sleep/28.10.1283. [DOI] [PubMed] [Google Scholar]
- 28.Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57(7):1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep. 2005;28(1):123–144. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]