Abstract
Study Objectives:
Hypoglossal nerve stimulation (HNS) is an effective surgical alternative for patients with obstructive sleep apnea (OSA). HNS therapy relies on the stimulation of the hypoglossal nerve to open the upper airways. This stimulation could lead to alterations in tongue strength and fatigability, which could alter treatment outcome over time. The aim of the study was to investigate whether HNS alters tongue strength and fatigability.
Methods:
Tongue protrusion strength (peak pressure in kPa) and fatigability (time to task failure during 50% of peak pressure contraction) were measured with a pressure transducer at least 2 months after HNS implantation (n = 30). These results were compared to a group of patients with OSA (n = 38) and a non-OSA control group (n = 35).
Results:
Median tongue protrusion strength was lower (54.7 [43.8, 63.0] versus 60.7 [53.7, 66.0] kPa, P = .013) and fatigue occurred more quickly (21.3 [17.4, 26.3] versus 26.0 [19.3, 31.3] seconds, P = .017) in the patients with OSA compared to the non-OSA control group. In multiple regression analysis, age was a significant factor for tongue strength and diagnosis of OSA for tongue fatigability. Tongue strength and fatigability did not differ between patients with OSA with conservative therapy or observation versus after HNS implantation (51.8 [41.3, 63.4] versus 56.3 [45.0, 62.3] kPa, P = .502; 20.8 [16.3, 26.2] versus 21.8 [18.3, 26.8] seconds, P = .418).
Conclusions:
Tongue strength decreases with age. Tongue fatigability is more pronounced in people with OSA. However, approximately 1.5 years of HNS therapy on average does not alter tongue strength or fatigability compared to an OSA control group.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Change in Tongue Strength and Fatigue After Upper Airway Stimulation Therapy; Identifier: NCT03980158
Citation:
Wirth M, Unterhuber D, von Meyer F, et al. Hypoglossal nerve stimulation therapy does not alter tongue protrusion strength and fatigability in obstructive sleep apnea. J Clin Sleep Med. 2020;16(2):285–292.
Keywords: CPAP, genioglossus, selective upper airway stimulation, sleep-disordered breathing, upper airway physiology
BRIEF SUMMARY
Current Knowledge/Study Rationale: Hypoglossal nerve stimulation (HNS), an effective therapeutic alternative for patients with OSA, is based on activation of the main pharyngeal airway dilatory muscle and tongue protruder (genioglossus) through selective stimulation of the hypoglossal nerve during sleep. Currently no study has investigated whether this activation of the genioglossus muscle influences tongue strength and fatigability over time, which could alter the treatment outcome.
Study Impact: This is the first study comparing tongue strength and fatigue of patients with OSA after implantation of HNS therapy with patients with OSA undergoing conservative or no treatment and a test group. The findings of the study demonstrate that, after an average of approximately 1.5 years of HNS therapy, tongue strength and fatigue in patients with OSA undergoing HNS therapy was unaltered compared to patients with OSA undergoing conservative or no treatment.
INTRODUCTION
In a recent population-based study 23% of women and 50% of men experienced from moderate to severe sleep-disordered breathing.1,2 Common symptoms include excessive daytime sleepiness and cognitive dysfunction.3 Additionally, untreated OSA is associated with increased risk of the development of secondary diseases, especially cardiovascular disease.4 OSA has a high societal burden; approximately 20% of car accidents are related to OSA or sleep deprivation.5 Long-term adherence rates (defined as daily usage for > 4 hours in 5 nights per week) for the standard treatment, continuous positive airway pressure (CPAP), range between 40% to 70%.6–10 For patients with poor CPAP adherence, alternatives such as various surgical interventions, mandibular advancement devices, and hypoglossal nerve stimulation (HNS) have been developed.7,11,12 HNS is based on activation of the main pharyngeal airway dilatory muscle and tongue protruder (genioglossus) through stimulation of the hypoglossal nerve to open the airways during sleep.13 Different stimulation strategies have been developed that are either breathing dependent (closed loop) or independent of respiratory phase (open loop).14 Systems also vary by activation of distal nerve fibers or specific sectors of the proximal nerve.14 To date, most patients have been implanted with breathing triggered stimulation to the distal branches of the hypoglossal nerve supplying tongue protrudors.15–17 This technique is termed selective hypoglossal nerve stimulation (HNS). HNS resulted in a treatment response according to Sher in 75% of patients after 5 years.18 It is generally well tolerated19 and has a high adherence of 6.6 hours usage per night after 12 months treatment, which has been published recently.20 HNS also improved sleep architecture in patients with OSA and also is effective in older patients.21,22 Long-term HNS could alter tongue muscle strength and fatigability via changes in muscle architecture. Increased propensity for muscle fatigue could negatively affect long-term treatment success. However, this has not been investigated for selective HNS.
Alternatively, decreased muscle fatigue and/or increased muscle strength can be beneficial in the treatment of other disorders in the head and neck area. For example, external neuromuscular electrical stimulation has been evaluated in swallowing therapy with promising results.23–25 Furthermore, repetitive transcranial magnetic stimulation, for example, for vagal nerve stimulation is being actively investigated.26 Direct neural stimulation in dysphagia could offer advantages such as more selective stimulation of muscles without the need to apply external electrodes. Knowledge of the effect of direct stimulation of hypoglossal nerve on tongue muscle parameters could therefore also be relevant for the development of dysphagia therapies. Indeed, many people with OSA also have impaired swallowing function27–31 such as a diminished gag reflex.32 Swallowing dysfunction in OSA may be reversible, at least in part, as CPAP therapy improved dysphagia in a recent small pilot study.33 HNS has been assessed in rats in the context of swallowing function34,35 but there are no data in humans.
The aim of this study was to determine whether HNS has an effect on tongue strength and fatigability. Another aim of the study was to compare tongue strength and fatigability in patients with OSA and control patients without OSA.
METHODS
Patient selection
A total of 68 patients with OSA (10 female and 58 male; mean age 55.8 ± 12.3 years, ranging from 29 to 82 years) and a control group without OSA (n = 35; 6 female and 29 male; mean age 41.1 ± 10.1 years, ranging from 27 to 61 years) were enrolled in the study. Patients presenting to the sleep laboratory for consultation (department of otolaryngology, Technical University of Munich) were enrolled if they had a diagnosis of OSA, did not meet the exclusion criteria stated in the next paragraphs, and gave informed written consent. The non-OSA control group was recruited at a local volunteer fire department to reflect the normal population. Participants in the control group were enrolled if they met the following criteria: no indications for OSA in the anamnesis and an Epworth Sleepiness Scale (ESS) score below 11. Thirty of the 68 patients with OSA had been implanted with selective HNS (Inspire II/IV Upper Airway Stimulation System, Inspire Medical Systems, Golden Valley, Minnesota, USA) system at least 2 months prior as described in the next paragraphs. In addition, in four of the patients with OSA implanted with HNS, tongue strength and fatigability was also measured 4 weeks after implantation (prestimulation) and again 1 month poststimulation. The remaining 38 patients with OSA (n = 34) were either being treated conservatively for OSA (CPAP, mandibular advancement device, or positional therapy) or were not receiving any therapy (n = 4). The diagnosis of OSA was confirmed by 18-channel inpatient overnight polysomnography (PSG) in the sleep laboratory. Anyone with a history of a neuromuscular disorder or tongue surgery were also excluded from the study.
All participants provided informed consent prior to any testing. The study was approved by the local ethics committee of the Technical University of Munich (project number 425/17S) and registered at clinicaltrials.gov (NCT03980158).
Selective hypoglossal nerve stimulation
Patients were selected for HNS as described previously.22,36 Patients meeting the criteria were implanted with the HNS system between 2015 and 2018. Implantation was performed as described in recent studies.15–17,37 Patients were implanted under general anesthesia on the right hypoglossal nerve.15 The electrode cuff was placed around the hypoglossal nerve fibers responsible for tongue protrusion, and verification of the nerve fibers to be included was performed by nerve integrity monitoring.37 The pulse generator was implanted in a pocket inferior to the clavicle and the sensing lead for breathing detection on the right chest wall between internal and external intercostal muscles. System integrity including tongue motion was checked intraoperatively.
Activation and titration of selective HNS and tongue motion direction
The stimulation system was activated 1 month after implantation. After instruction in the system and programming the initial amplitude, stimulation was gradually increased by patients as previously described.38,39 The second month after the implantation, stimulation was titrated during overnight PSG. An additional control PSG was performed 3 months postimplantation. Stimulation frequency was set at 33 Hz in all patients. Stimulation amplitude (V) and electrode configurations were individually titrated.
Different tongue motions occur after activation of the stimulation system. The direction of the tongue motion with turned on stimulation was classified as bilateral (bilateral elongation and anterior displacement of the tongue), right protrusion (ipsilateral extension of the tongue with deviation to the left side), or mixed activation (includes every other type of tongue motion such as shortening, retracting, or curling of the tongue).40
Tongue protrusion strength and fatigability measurements
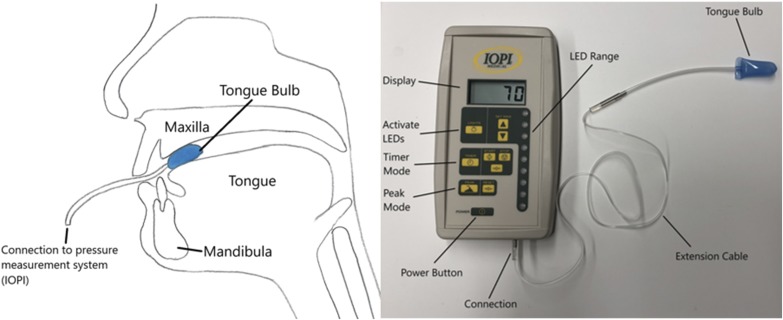
Tongue protrusion strength and fatigability measurements were performed using the Iowa Oral Performance Instrument (IOPI; Medical LLC, Woodinville, Washington, USA) as described in detail previously.41,42 Briefly, IOPI is an instrument designed to assess tongue and lip strength. Measurements are based on individuals pressing against an air-filled bulb placed against the hard palate behind the alveolar ridge (Figure 1). The bulb is connected to a pressure transducer that is connected to an amplifier, signal conditioning conduit, and digital voltmeter.41
Figure 1. Iowa Oral Performance Instrument.
(left) Schematic depiction of intraoral position of tongue bulb for measurements. (right) Picture of Iowa Oral Performance Instrument (IOPI) connected to tongue bulb.
Tongue strength is measured in kilopascal (kPa). The light display indicates the relative pressure exerted with light emitting diodes (LED) and provides visual feedback for the test individuals for the endurance testing.41 Tongue strength was assessed as the maximal isometric pressure generated by the participants (Pmax).41 For Pmax, participants were asked to: “Press up on the bulb with your tongue and squeeze the bulb against the roof of your mouth.”41 Tongue strength measurements were performed three times with a 30-second break between each measurement.
The mean value for Pmax was used to calculate the target pressure for the tongue fatigability measurements in each individual. Specifically, tongue fatigability was determined as the time in seconds that participants could generate at least 50% of Pmax.41,42 The upper LED of IOPI’s light pressure indicator was set at 50% of Pmax. When at least 50% of Pmax was reached, the upper LED was illuminated in green and the rest of the LEDs in red. Patients were instructed to sustain at least 50% of Pmax as long as possible after a short training period.41 Patients were verbally encouraged during each trial. Patients were allowed to briefly (1 second) decrease tongue strength by one LED light. Three trials of fatigability testing were executed with 30 seconds between tests. The duration of each trial was timed and the average endurance was calculated.
Polysomnography
During PSG the following parameters were recorded: electroencephalography, electrooculography, electromyography, air flow (by thermistor and nasal flow), oxygen saturation, abdominal and thoracic respiratory movement, and position changing and leg movement. Respiratory events were scored according to American Academy of Sleep Medicine criteria.43
Respiratory events were classified into apneas (reduction of air flow ≥ 90%) and hypopneas (reduction of air flow ≥ 30% and drop of oxygen saturation ≥ 3%). Both had to last for ≥ 10 seconds.
Statistical analysis
All statistical tests were two-sided and significance was determined at an alpha level of 0.05. Statistical calculations were executed with the SPSS version 25 (IBM, Ehningen, Germany) and case-control matching was performed with MedCalc Statistical Software version 19.0.7 (MedCalc Software bvba, Ostend, Belgium). For normality testing, the Shapiro-Wilk test was applied. In normally distributed groups, comparison of distribution was performed with t test or ANOVA. Otherwise, a Mann-Whitney U or Kruskal-Wallis test was used. Correlation testing was performed with a Pearson test in normally distributed groups or otherwise with a Spearman test. Multiple linear regression (forward) was performed to investigate potential associations between key independent variables (tongue strength, tongue fatigue, age, body mass index (BMI), diagnosis of OSA, preoperative AHI, stimulation amplitude).
RESULTS
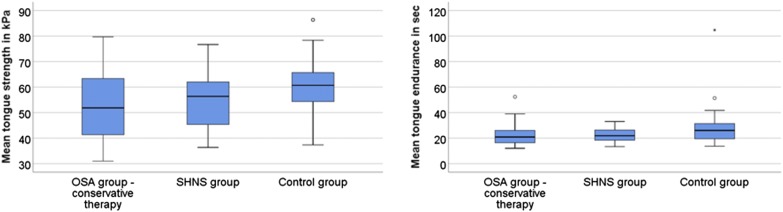
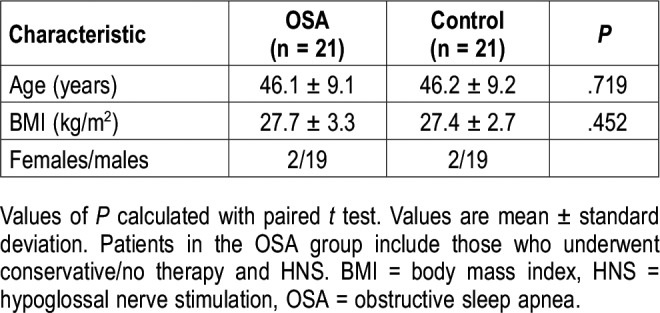
The characteristics of patients and the control group are depicted in Table 1 and mean tongue strength and endurance measurements in the three groups (OSA group with conservative therapy and after HNS implantation and control group) in Figure 2. AHI of patients ranged from 5.2–115.1 events/h. Tongue strength and fatigue were measured on average 17 ± 13 months (range 2 to 53 months) after HNS stimulation. Because the control group was significantly younger and had a lower BMI (Table 1), patients with OSA (CPAP/conservative therapy + HNS groups combined) and control group were matched 1:1 for sex, age (maximum allowable difference 3 years) and BMI (maximum allowable difference 3 kg/m2) creating two subgroups (n = 21 each). The characteristics of these subgroups are shown in Table 2.
Table 1.
Participant characteristics.
Figure 2. Tongue strength and endurance.
Depiction of tongue strength (left) and tongue endurance (right) in the obstructive sleep apnea (OSA) group with conservative therapy, after HNS implantation and control group. Tongue strength and endurance tended to be higher in the control group (P = .054 and P = .044, Kruskal-Wallis test). Patients in the control group were significantly younger and had lower body mass index than both OSA cohorts.
Table 2.
Characteristics of matched subgroups.
Tongue strength depending on age
Median tongue strength in the patients with OSA (CPAP/conservative therapy + HNS groups combined) was 54.7 kPa [43.8, 63.0] compared to 60.7 kPa [53.7, 66.0] in the younger, thinner control group (P = 0.013). In the OSA subgroup (n = 21), median tongue strength was 57.0 kPa [50.7, 63.3] in comparison with 61.0 kPa [52.3, 71.5] in the matched control group (n = 21) (P = .139). Tongue strength did not differ between the OSA group with CPAP/conservative therapy and the HNS group (51.8 [41.3, 63.4] versus 56.3 [45.0, 62.3] kPa, P = .502). There was a significant correlation between tongue strength and age (Spearman correlation −.258, P = .008) but not AHI (P = .193). In a multiple linear regression (forward) with tongue strength as dependent variable and age, BMI and OSA versus control group as independent variables, only age was a significant explanatory variable (R square = 0.084; unstandardized regression coefficient B = −0.265; 95% confidence interval for B −0.438 to −0.092; P = .003).
No difference in tongue endurance between OSA groups
Median tongue endurance in the patients with OSA (CPAP/conservative therapy + HNS groups combined) was 21.3 seconds [17.4, 26.3] compared to 26.0 seconds [19.3, 31.3] in the control group (P = .017, Mann-Whitney-U test). In the OSA subgroup (n = 21), median tongue endurance was 19.3 seconds [16.3, 23.7] in comparison to 25.3 seconds [19.2, 29.3] in the matched control group (n = 21) (P = .014). Tongue endurance (in seconds) did not differ between the OSA groups with conservative therapy versus after HNS implantation (20.8 [16.3, 26.2] versus 21.8 [18.3, 26.8], P = .418, Mann-Whitney U test). There was no significant correlation between tongue endurance and age or AHI (P = .058 and P = .228). In the linear regression with tongue endurance as dependent variable and age, BMI and OSA versus control group as independent variables only diagnosis of OSA had a significant influence on tongue endurance (R square = 0.062; regression coefficient B = 5.72; 95% confidence interval for B = 1.33 to 10.11; P = .011).
Influence of length of implantation on tongue parameters
Patients with HNS therapy were divided into two groups according to duration of HNS therapy (lowest quartile, stimulation < 7 months, n = 8 and > 7 months, n = 22). Tongue strength did not differ between both groups (53.3 ± 7.6 versus 55.3 ± 11.6 kPa, P = .658, t test). Similarly, tongue fatigability was not different between groups (24.9 ± 5.7 versus 21.6 ± 5.2 seconds, P = .148, t test). In 4 patients tongue parameters were measured 4 weeks postimplantation (prestimulation) and 4 weeks after stimulation (8 weeks poststimulation). Tongue strength was unaltered (54.8 ± 11.8 versus 54.9 ± 8.5 kPa, P = .981) whereas tongue fatigability significantly improved after stimulation (16.1 ± 1.5 versus 21.3 ± 3.9 seconds, P = .046).
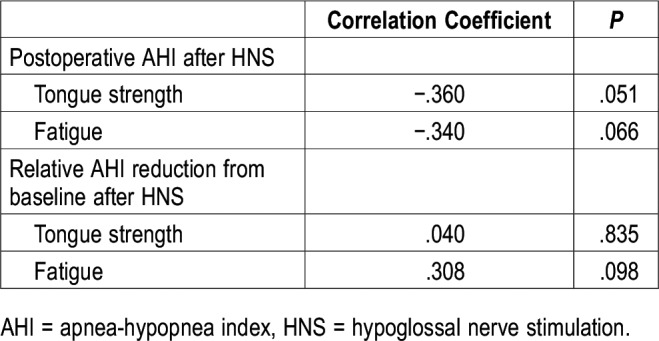
Association between postoperative AHI and tongue parameters
There were no significant associations between postoperative AHI after HNS and measures of tongue strength and fatigue (Table 3).
Table 3.
Associations between postoperative AHI after HNS and measures of tongue strength and fatigue.
Correlation of stimulation amplitude and tongue parameters
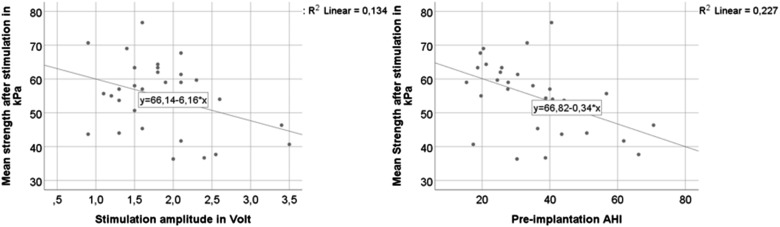
Therapeutic stimulation amplitude 2 months postimplantation ranged from 0.6 to 3.5 V. Stimulation electrode was configured bipolar (+ – +) in 25 patients and unipolar (off – off) in 5 patients. In general, considerably lower stimulation amplitudes are required to result in the same therapeutic effect in unipolar compared to bipolar stimulation-configurations. Bipolar stimulation amplitudes are about 50% higher compared to unipolar stimulation. For better comparison, amplitudes of unipolar stimulated patients were corrected by multiplying by a factor of 1.5. Stimulation amplitude was negatively correlated with tongue strength after stimulation (Pearson correlation −.366, P = .047, association also shown in Figure 3) but not with tongue endurance (Pearson correlation .048, P = .802). The tongue protruded bilateral in 23 and right in 7 patients after stimulation was turned on. Tongue strength was slightly higher in patients with bilateral protrusion (56.1 ± 11.2 versus 50.5 ± 7.7 kPa, P = .231, t test). However, there was no difference in tongue endurance (22.6 ± 5.2 versus 22.4 ± 6.5 seconds, P = .939, t test). Multiple linear regression revealed that postoperative tongue strength was significantly negatively associated with preoperative AHI (correlation also shown in Figure 3) but not with stimulation amplitude or age (R square = 0.227; unstandardized coefficient = −0.335; 95% confidence interval for B = −0.575 to −0.096; P = 0.008).
Figure 3. Tongue protrusion strength.
Relationship of tongue protrusion strength and stimulation amplitude (left) and preimplantation apnea-hypopnea index (AHI) (right).
DISCUSSION
In this study, tongue strength and fatigability were analyzed in patients with OSA after HNS and results compared with those of patients treated with CPAP/conservative treatment. In addition, tongue parameters were measured in a non-OSA control group.
AHI was not significantly correlated with tongue parameters in the OSA group. The control group had a significantly higher tongue strength and fatigability compared to OSA patients. Because patients with OSA were significantly older and had a higher BMI than patients in the control group, case-control matching and multiple linear regression analysis was performed to determine relevant influencing factors. In the matched-group comparison, tongue endurance was significantly higher in the control group compared to the OSA group. In accordance with the finding in the matched subgroups, regression analysis showed age as a significant influencing factor for tongue strength and diagnosis of OSA for tongue endurance. These findings are consistent with decreased tongue protrusion strength with age reported by Mortimore et al in a healthy cohort.44 A subsequent study by the same group detected no difference in tongue protrusion strength and fatigability between a cohort of patients with OSA and nonsnoring individuals and only a weak correlation of tongue protrusion strength and AHI.45 Increased genioglossus fatigue was reported in a small cohort of patients with OSA (n = 9) in comparison with control patients in another study (n = 9).46 However, in this investigation muscle fatigue was measured as the rate of decline of muscle fiber conduction velocity during an isometric fatiguing contraction at 30% Tpmax.46 In another study with 12 patients with untreated OSA versus 13 control patients, maximal tongue protrusion force was greater in patients with OSA compared to control patients and tongue fatigue occurred in approximately half the time during a repetitive, intermittent (5 seconds on, 5 seconds off) isometric contraction task at 70% max in the patients with OSA.47 The potential influence of age dependency on tongue parameters in these studies is difficult to discern due to the smaller sample sizes. Overall, data in the literature are in favor of increased muscle fatigue in patients with OSA, which is in accordance with our findings. The influence of OSA on tongue strength remains less clear.
The BMI was not associated with tongue parameters in our examination. In a study published by Carrera et al, in vitro genioglossus endurance was higher in nonobese patients with OSA but not in obese patients with OSA compared to control patients.48 No difference in genioglossus endurance was detected in CPAP-treated nonobese patients with OSA.48 These results are consistent with our findings because most patients with OSA were either treated with CPAP or HNS in our cohort. The comparability to our tongue measurements, however, is limited because fatigue was tested in vitro after a genioglossus biopsy. In another study, lower tongue total muscle work was detected in nonobese compared to untreated obese patients with moderate to severe OSA.49 In this study patients with OSA were also not treated, which could explain the differing results compared to our cohort. In summary, BMI seems to affect tongue fatigue in untreated nonobese patients with OSA but not in treated nonobese patients with OSA.
Subsequently, tongue parameters were compared between the patients with OSA who received CPAP/conservative therapy or were not treated, and those who received HNS implantation. Patients who received HNS were measured at least 2 months (average ∼1.5 years) after implantation and 1 month after stimulation. No significant difference in tongue protrusion strength or tongue fatigability was detected between the two groups. To further rule out that long-term stimulation influences tongue parameters, the HNS group was divided into patients implanted less than 7 months and longer than 7 months ago. No effect on tongue strength or fatigue through HNS was seen between groups. In addition, there was no change in muscle force in four patients who were measured 1 month after implantation/before any stimulation and again after the first month of stimulation. Rather, tongue fatigue decreased after stimulation. However, in addition to the small sample size, a limitation to this finding is that we did not measure the preoperative value for the comparison to the postoperative finding. This requires prospective investigation. Thus, it is possible that tongue endurance was recovering to normal levels after surgery at the time of 1-month testing rather than reflecting a direct stimulation therapy effect. Indeed, there was no difference in the larger between OSA group comparisons. Nonetheless, reduced tongue fatigue after HNS is consistent with reductions in OSA severity after 12 months of tonic HNS.50
To date, tongue strength and fatigability have not been tested in patients with HNS to our knowledge. Limited data exist on the effect of hypoglossal nerve stimulation on tongue contraction parameters in animal models. Hypoglossal nerve stimulation resulted in increased muscle strength and endurance in aged rats.34,35 Connor et al used 40 Hz as stimulation frequency, which is similar to the 33 Hz applied in patients in our cohort. However, Connor et al provided stimulation with supramaximal current in rats.34 In human patients, stimulation is performed with the functional amplitude tolerated. In theory, altered tongue strength and fatigability after HNS could also impact swallowing. However, there is a paucity of data on the effect of HNS on swallowing. Bowen tested the Eating Assessment Tool-10 score in 14 patients before and after HNS implantation and did not detect a clinically significant difference after the stimulation.51 This is in accordance with our finding of unaltered tongue strength and fatigability after HNS.
Furthermore, there were no significant associations between postoperative AHI and tongue parameters in the current study. These potential associations require further investigation in a bigger patient cohort with preoperative and postoperative measurements. It remains speculative whether decreased tongue fatigability affects AHI reduction or if patients with a lower tongue fatigability respond better to the stimulation therapy at first. To further test this, stimulation strategies to increase tongue endurance would need to be developed. In rats, stimulation of the hypoglossal nerve with 100 Hz produced a more pronounced reduction in tongue fatigue and a significantly longer contraction time compared with a frequency of 10 Hz.35 A trial of increased stimulation frequency in poor responders to HNS could help to further understand this mechanism.
Last, stimulation amplitude was negatively correlated with tongue strength and multiple linear regression revealed that preoperative AHI had a significant negative influence on postoperative tongue strength. Reducing stimulation amplitude would be clinically meaningful because this could reduce adverse effects and prolong battery life. A possible cause for the association between preoperative AHI and postoperative tongue strength could be that patients with higher preoperative AHI need higher stimulation amplitudes for airway patency, which could negatively affect postoperative tongue strength. Other potential determinants could be patient factors such as lower preoperative tongue strength or higher nerve vulnerability in patients with higher preoperative AHI. Surgical technique could play a role as well. The change of tongue motion seen in some patients after implantation from bilateral protrusion to right protrusion could be a sign of mild postoperative neurapraxia.52 “Soft” surgery with the aim of preserving the integrity of the hypoglossal nerve with measures such as not ligating the vena comitans of the hypoglossal nerve, reduction of nerve manipulation to a minimum, or using atraumatic instruments could therefore result in lower stimulation amplitude needed.
There are several limitations to our study. Most importantly the lack of preoperative and postoperative measurement in the HNS group confine the delineation of the causes of measured changes in tongue parameters. The small sample size restricts the detection of relevant changes especially in smaller subgroup analyses. The significant age and BMI difference in the healthy control group impedes the comparison to the OSA group. However, this was controlled for with the multiple regression analysis. In subsequent projects, these variables should be matched in the control group to verify our findings. Also, comparison of AHI and tongue parameters are only possible in the OSA group because AHI was not measured in the control group. Furthermore, despite no signs of OSA based on a historical screen and ESS within the normal range, it is possible that some of the participants in the control group could have had sleep disordered breathing. In future studies, the STOP BANG questionnaire could be used in addition to the ESS or formal PSG testing to more definitively rule out OSA in the control group. Finally, we cannot rule out the possibility that a difference in sleepiness between the patients with OSA and the control group could have influenced the tongue response findings due to differences in volitional efforts.
CONCLUSIONS
In summary, consistent with previous findings in healthy individuals, tongue strength is associated with age and tongue fatigability in people with OSA. An average of approximately 1.5 years of HNS therapy does not alter tongue strength and fatigability compared to conventional therapy. Postoperative tongue strength after HNS is inversely correlated with preoperative AHI.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. MW, BH, and CH received financial research support and surgical training support from Inspire Medical Systems Inc. CH is a consultant for Inspire Medical Systems Inc. DJE is funded by a National Health and Medical Research Council of Australia (NHMRC) Senior Research Fellowship (1116942). Outside the current study, DJE has received research grants from Bayer and Apnimed and has a Cooperative Research Centre (CRC)-P grant, a collaboration between the Australian Government, Academia and Industry (Industry partner Oventus Medical). The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Katharina Eckbauer for her excellent support as study nurse during this trial.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- HNS
hypoglossal nerve stimulation
- IOPI
Iowa Oral Performance Instrument
- LED
light-emitting diode
- OSA
obstructive sleep apnea
- Tpmax
maximum voluntary tongue protrusion force
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuckenbrock JK, Freuschle A, Nakajima I, Stuck BA. The influence of pharyngeal and esophageal pressure measurements on the parameters of polysomnography. Eur Arch Otorhinolaryngol. 2014;271(5):1299–1304. doi: 10.1007/s00405-013-2771-y. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb DJ, Ellenbogen JM, Bianchi MT, Czeisler CA. Sleep deficiency and motor vehicle crash risk in the general population: a prospective cohort study. BMC Med. 2018;16(1):44. doi: 10.1186/s12916-018-1025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C, Won T-B, Cha W, Yoon I, Chung S, Kim J-W. Obstructive site localization using multisensor manometry versus the Friedman staging system in obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2008;265(2):171–177. doi: 10.1007/s00405-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 7.Rotenberg BW, Vicini C, Pang EB, Pang KP. Reconsidering first-line treatment for obstructive sleep apnea: a systematic review of the literature. J Otolaryngol Head Neck Surg. 2016;45(1):23. doi: 10.1186/s40463-016-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson MK, Carter R, Nicol A, Paton R, Banham SW. Long-term continuous positive airway pressure (CPAP) outcomes from a sleep service using limited sleep studies and daycase CPAP titration in the management of obstructive sleep apnoea/hypopnoea syndrome. Chron Respir Dis. 2004;1(2):83–88. doi: 10.1191/1479972304cd019oa. [DOI] [PubMed] [Google Scholar]
- 9.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res. 2010;131:245–258. [PMC free article] [PubMed] [Google Scholar]
- 10.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 13.Heiser C, Edenharter G, Bas M, Wirth M, Hofauer B. Palatoglossus coupling in selective upper airway stimulation. Laryngoscope. 2017;127(10):E378–e383. doi: 10.1002/lary.26487. [DOI] [PubMed] [Google Scholar]
- 14.Fleury Curado T, Oliven A, Sennes LU, Polotsky VY, Eisele D, Schwartz AR. Neurostimulation Treatment of OSA. Chest. 2018;154(6):1435–1447. doi: 10.1016/j.chest.2018.08.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heiser C, Thaler E, Boon M, Soose RJ, Woodson BT. Updates of operative techniques for upper airway stimulation. Laryngoscope. 2016;126(Suppl 7):S12–S16. doi: 10.1002/lary.26158. [DOI] [PubMed] [Google Scholar]
- 16.Heiser C, Thaler E, Soose RJ, Woodson BT, Boon M. Technical tips during implantation of selective upper airway stimulation. Laryngoscope. 2018;128(3):756–762. doi: 10.1002/lary.26724. [DOI] [PubMed] [Google Scholar]
- 17.Heiser C, Knopf A, Hofauer B. Surgical anatomy of the hypoglossal nerve: A new classification system for selective upper airway stimulation. Head Neck. 2017;39(12):2371–2380. doi: 10.1002/hed.24864. [DOI] [PubMed] [Google Scholar]
- 18.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 19.Hofauer B, Steffen A, Knopf A, Hasselbacher K, Heiser C. Patient experience with upper airway stimulation in the treatment of obstructive sleep apnea. Sleep Breath. 2019;23(1):235–241. doi: 10.1007/s11325-018-1689-4. [DOI] [PubMed] [Google Scholar]
- 20.Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727–1734. doi: 10.1007/s00405-016-4297-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Hofauer B, Wirth M, et al. Selective upper airway stimulation in older patients. Respir Med. 2018;140:77–81. doi: 10.1016/j.rmed.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Hofauer B, Philip P, Wirth M, Knopf A, Heiser C. Effects of upper-airway stimulation on sleep architecture in patients with obstructive sleep apnea. Sleep Breath. 2017;21(4):901–908. doi: 10.1007/s11325-017-1519-0. [DOI] [PubMed] [Google Scholar]
- 23.Lin PH, Hsiao TY, Chang YC, et al. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19(1):91–99. doi: 10.1007/s00520-009-0792-2. [DOI] [PubMed] [Google Scholar]
- 24.Frost J, Robinson HF, Hibberd J. A comparison of neuromuscular electrical stimulation and traditional therapy, versus traditional therapy in patients with longstanding dysphagia. Curr Opin Otolaryngol Head Neck Surg. 2018;26(3):167–173. doi: 10.1097/MOO.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 25.Park JS, Oh DH, Hwang NK, Lee JH. Effects of neuromuscular electrical stimulation combined with effortful swallowing on post-stroke oropharyngeal dysphagia: a randomised controlled trial. J Oral Rehabil. 2016;43(6):426–434. doi: 10.1111/joor.12390. [DOI] [PubMed] [Google Scholar]
- 26.Lin WS, Chou CL, Chang MH, Chung YM, Lin FG, Tsai PY. Vagus nerve magnetic modulation facilitates dysphagia recovery in patients with stroke involving the brainstem - a proof of concept study. Brain Stimul. 2018;11(2):264–270. doi: 10.1016/j.brs.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Saboisky JP, Butler JE, Gandevia SC, Eckert DJ. Functional role of neural injury in obstructive sleep apnea. Front Neurol. 2012;3:95. doi: 10.3389/fneur.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zohar Y, Grusko I, Sulkes J, Melloul MM. Oropharyngeal scintigraphy: a computerized analysis of swallowing in patients with obstructive sleep apnea. Laryngoscope. 1998;108(1 Pt 1):37–41. doi: 10.1097/00005537-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Teramoto S, Sudo E, Matsuse T, et al. Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest. 1999;116(1):17–21. doi: 10.1378/chest.116.1.17. [DOI] [PubMed] [Google Scholar]
- 30.Jaghagen EL, Berggren D, Isberg A. Swallowing dysfunction related to snoring: a videoradiographic study. Acta Otolaryngol. 2000;120(3):438–443. doi: 10.1080/000164800750000702. [DOI] [PubMed] [Google Scholar]
- 31.Levring Jaghagen E, Franklin KA, Isberg A. Snoring, sleep apnoea and swallowing dysfunction: a videoradiographic study. Dentomaxillofac Radiol. 2003;32(5):311–316. doi: 10.1259/dmfr/29209140. [DOI] [PubMed] [Google Scholar]
- 32.Valbuza JS, de Oliveira MM, Zancanella E, et al. Swallowing dysfunction related to obstructive sleep apnea: a nasal fibroscopy pilot study. Sleep Breath. 2011;15(2):209–213. doi: 10.1007/s11325-010-0474-9. [DOI] [PubMed] [Google Scholar]
- 33.Caparroz FA, de Almeida Torres Campanholo M, Sguillar DA, et al. A pilot study on the efficacy of continuous positive airway pressure on the manifestations of dysphagia in patients with obstructive sleep apnea. Dysphagia. 2019;34(3):333–340.. doi: 10.1007/s00455-018-9944-1. [DOI] [PubMed] [Google Scholar]
- 34.Connor NP, Russell JA, Jackson MA, et al. Tongue muscle plasticity following hypoglossal nerve stimulation in aged rats. Muscle Nerve. 2013;47(2):230–240. doi: 10.1002/mus.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kletzien H, Russell JA, Leverson G, Connor NP. Effect of neuromuscular electrical stimulation frequency on muscles of the tongue. Muscle Nerve. 2018;58(3):441–448. doi: 10.1002/mus.26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heiser C, Steffen A, Boon M, et al. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01405-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiser C, Hofauer B, Lozier L, Woodson BT, Stark T. Nerve monitoring–guided selective hypoglossal nerve stimulation in obstructive sleep apnea patients. Laryngoscope. 2016;126(12):2852–2858. doi: 10.1002/lary.26026. [DOI] [PubMed] [Google Scholar]
- 38.Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378–384. doi: 10.1177/0194599816683378. [DOI] [PubMed] [Google Scholar]
- 39.Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509–515. doi: 10.1002/lary.26688. [DOI] [PubMed] [Google Scholar]
- 40.Heiser C, Maurer JT, Steffen A. Functional outcome of tongue motions with selective hypoglossal nerve stimulation in patients with obstructive sleep apnea. Sleep Breath. 2016;20(2):553–560. doi: 10.1007/s11325-015-1237-4. [DOI] [PubMed] [Google Scholar]
- 41.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 42.Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10(2):183–193. doi: 10.5664/jcsm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring Manual Updates for 2017 (Version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatiguability in male and female subjects. Eur Respir J. 1999;14(1):191–195. doi: 10.1034/j.1399-3003.1999.14a32.x. [DOI] [PubMed] [Google Scholar]
- 45.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res. 2000;9(4):389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 46.McSharry D, O’Connor C, McNicholas T, et al. Genioglossus fatigue in obstructive sleep apnea. Respir Physiol Neurobiol. 2012;183(2):59–66. doi: 10.1016/j.resp.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 47.Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A. Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol. 2011;111(6):1644–1653. doi: 10.1152/japplphysiol.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrera M, Barbe F, Sauleda J, et al. Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. Eur Respir J. 2004;23(3):425–429. doi: 10.1183/09031936.04.00099404. [DOI] [PubMed] [Google Scholar]
- 49.Li WY, Gakwaya S, Saey D, Series F. Assessment of tongue mechanical properties using different contraction tasks. J Appl Physiol. 2017;123(1):116–125. doi: 10.1152/japplphysiol.00934.2016. [DOI] [PubMed] [Google Scholar]
- 50.Rodenstein D, Rombaux P, Lengele B, Dury M, Mwenge GB. Residual effect of THN hypoglossal stimulation in obstructive sleep apnea: a disease-modifying therapy. Am J Respir Crit Care Med. 2013;187(11):1276–1278. doi: 10.1164/rccm.201211-2129LE. [DOI] [PubMed] [Google Scholar]
- 51.Bowen AJ, Nowacki AS, Kominsky AH, Trask DK, Benninger MS, Bryson PC. Voice and swallowing outcomes following hypoglossal nerve stimulation for obstructive sleep apnea. Am J Otolaryngol. 2018;39(2):122–126. doi: 10.1016/j.amjoto.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Steffen A, Kilic A, König IR, Suurna MV, Hofauer B, Heiser C. Tongue motion variability with changes of upper airway stimulation electrode configuration and effects on treatment outcomes. Laryngoscope. 2018;128(8):1970–1976. doi: 10.1002/lary.27064. [DOI] [PubMed] [Google Scholar]