Abstract
Night-to-night variability (NNV) of the degree of obstructive sleep apnea (OSA) over the long term is not well investigated. In our case, we investigated the NNV of the apnea-hypopnea index (AHI) with regard to sleep structure. Unattended polysomnography (PSG) at home was used to determine the AHI in the course of 4 weeks in a single patient with a mild-to-moderate OSA, by using the Somnocheck R&K system. The mean sleep period was 6.7 ± 1.1 hours and the mean AHI was 14.1 ± 5.7 events/h (range: 5.1–28.3 events/h; coefficient variability [CV] 40.4%). Independent of non-rapid eye movement and rapid eye movement (REM) sleep, the AHI in supine position (43.6 ± 16.9 events/h; CV 38.8%) was greater than during lateral-recumbent sleep (4.8 ± 4.1 events/h; CV 85.4%, P < .0001). A negative correlation was found for both: the AHI in supine position with the duration of supine position sleep (r = .59, P < .001), as well as the AHI in REM with the duration of REM sleep (r = −.37, P < .025). The AHI shows no rhythmicity neither from day to day nor from week to week. We found a high long-term NNV of the AHI, which was typically not influenced by the particular day of the week. Supine AHI is evidently dependent on the duration spent in that position throughout the night. We found it advisable to consider the existence of NNV in association with the degree of OSA, especially for patients with questionable therapeutic indication.
Citation:
Fietze I, Glos M, Zimmermann S, Penzel T. Long- term variability of the apnea-hypopnea index in a patient with mild to moderate obstructive sleep apnea. J Clin Sleep Med. 2020;16(2):319–323.
Keywords: apnea-hypopnea index, AHI variability, obstructive sleep apnea, polysomnography, sleep- disordered breathing
INTRODUCTION
In addition to the clinical symptom complex, the severity of obstructive sleep apnea (OSA) is substantially determined by the number of episodes with absent or reduced airflow.
The question addressed here is: how great is the validity and the reliability of data gathered on sleep-disordered breathing? Studies conducted until now on the variability and the reproducibility of the apnea-hypopnea index (AHI) differ in methodology with respect to the type of evaluation, the use of either home sleep apnea testing (HSAT) or polysomnography (PSG) for measurement, the duration of study (from night to night, up to 6 months), the age of the patients, the severity of the sleep-related respiratory disorder,1,2 and to limitation to using merely the oxygen desaturation index as denominator.3
In routine clinical work with use of the same measured variables and the same analysis definitions, interrater variability should be slight, especially for hypopnea. Other factors such as sleeping position,4,5 sleep quality, consumption of alcohol, use of medication, extent of activity during the daytime, and the day of the week fluctuate from day to day and are assumed to influence the AHI—which has until now been investigated to only a limited degree.
It has been assumed that night-to-night variability of the AHI is around 10 events/h, regardless of whether measurement is performed by HSAT or PSG.6 As a result, one PSG night suffices for diagnosis in many cases. However, all authors mentioned previously point out that variability can in some cases play a great and individually differing role, especially in mild forms of OSA.
In order to investigate intraindividual variability, we initiated a case report, covering a period of 4 weeks, of one patient with mild to moderate OSA.
REPORT OF CASE
Among the outpatients of the Interdisciplinary Center for Sleep Medicine of the Charité University Medical Center in Berlin, we searched for one patient with the following inclusion criteria: mild to moderate OSA diagnosed on the basis of six-channel HSAT (Embletta pds, Natus Neuro, Middleton, Wisconsin, United States), willingness to undergo 4 weeks of consecutive unattended PSG and to fill out a sleep diary during that time, age older than 18 years, and a regular weekly routine regarding social and professional activities.
Exclusion criteria were previous therapy of OSA, an additional acute illness that would impair sleep, comorbidities, shift work, or jet lag. Unattended PSG was performed using the Somnocheck R&K system (Weinmann, Hamburg, Germany). Body position was recorded by the device using a built-in three-axis position sensor with a sensitivity of 45° ± 15°. Recording took place on 28 consecutive days in a home environment. Each evening, the same trained technician attached the system and removed it again on the following morning. The patient was instructed to carry on his daily routines as usual, to maintain his usual sleeping pattern, and to keep a sleep log.
After all recordings had been made, sleep and respiration were scored manually by one single trained expert (IR) who was blinded with regard to the date and the number of the recording.
The study was approved by the local ethics committee of the Charité University Medical Center, and the participant gave his written consent for participation.
For statistical analysis of the data, we used SPSS version 22.0 (IBM Corp, Armonk, New York, United States). Mean ± standard deviation (SD) is given for the individual measured values, with statistical analysis by analysis of variance with measurement repetitions, nonparametric test procedures, and correlation analysis. Autocorrelation function (ACF) analysis was performed on 28 days’ time series of AHI, amount of supine body position, rapid eye movement (REM) sleep, and slow wave sleep (SWS) for testing of self-similarity covering the range of one up to 7 days. For AHI, coefficient of variation (CV) values were given by dividing the SD by the mean value. Individual standardized AHI values for mean body position distribution were given by the sum of (individual AHI in supine position multiplied by 28 days’ mean percentage of supine position duration) and of (individual AHI in lateral position multiplied by 28 days’ mean percentage of lateral position duration). Values of P < .05 were considered significant.
RESULTS
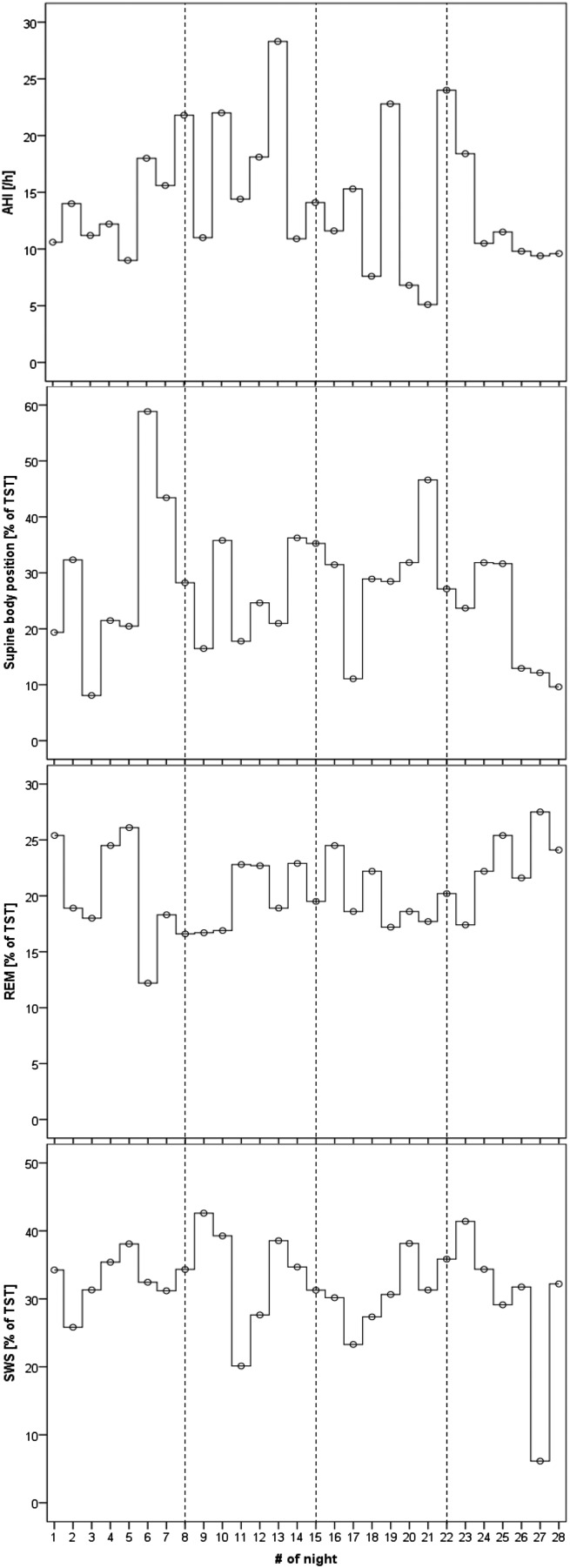
The mean total sleep time (TST) over the recording period of 28 days was 6.7 ± 1.1 hours, which was somewhat greater than the self-reported sleeping time of 6.4 ± 0.5 hours. The participant did not take any medication during that recording period. The mean AHI was 14.1 ± 5.7 events/h (range 5.1–28.3 events/h; CV 40.4%) and the standardized AHI was 15.1 ± 7.5 events/h (range 3.0–29.4 events/h; CV 49.7%). In 15 nights, the standardized AHI was higher than the original AHI (range 0.4 to 10.0 events/h), and in 13 nights it was lower (range 0.8–8.4 events/h). Severity class based on standardized AHI would be higher than by original AHI by one category for 5 nights and would be lower by one category for 2 nights. The mean apnea index was 8.4 ± 4.3 events/h (range 2.8–19.4 events/h), and the mean hypopnea index was 5.7 ± 2.8 events/h (range 1.6–12.4 events/h). The variability for recorded apnea events was greater than for hypopnea. Explorative analyses of 28 nights’ time series show that AHI and supine body position varies the most whereas amount of REM sleep and of SWS sleep tend to vary the least (Figure 1). ACF analysis for testing self-similarity for time lags of 1 day up to 7 days revealed for AHI absolute values up to 0.13, for amount of supine body position values up to 0.33, for REM sleep values up to 0.37, and for SWS values up to 0.22. None of these r values are significant.
Figure 1. Synchronized time series of 28 nights.
Synchronized time series of 28 nights’ values for the apnea-hypopnea index (AHI), percentage of supine body position (Supine) based on total sleep time (TST), percentage of rapid eye movement (REM) sleep based on TST, and percentage of slow wave sleep (SWS) based on TST are presented in four panels. Dashed lines indicate each week of recording.
The AHI in REM sleep (mean 12.0 ± 11.5 events/h; range 0–53.7 events/h; CV 95.8%) did not differ significantly from the AHI in non-rapid eye movement (NREM) sleep (mean 14.7 ± 5.1 events/h; range 6–24 events/h; CV 34.7%).
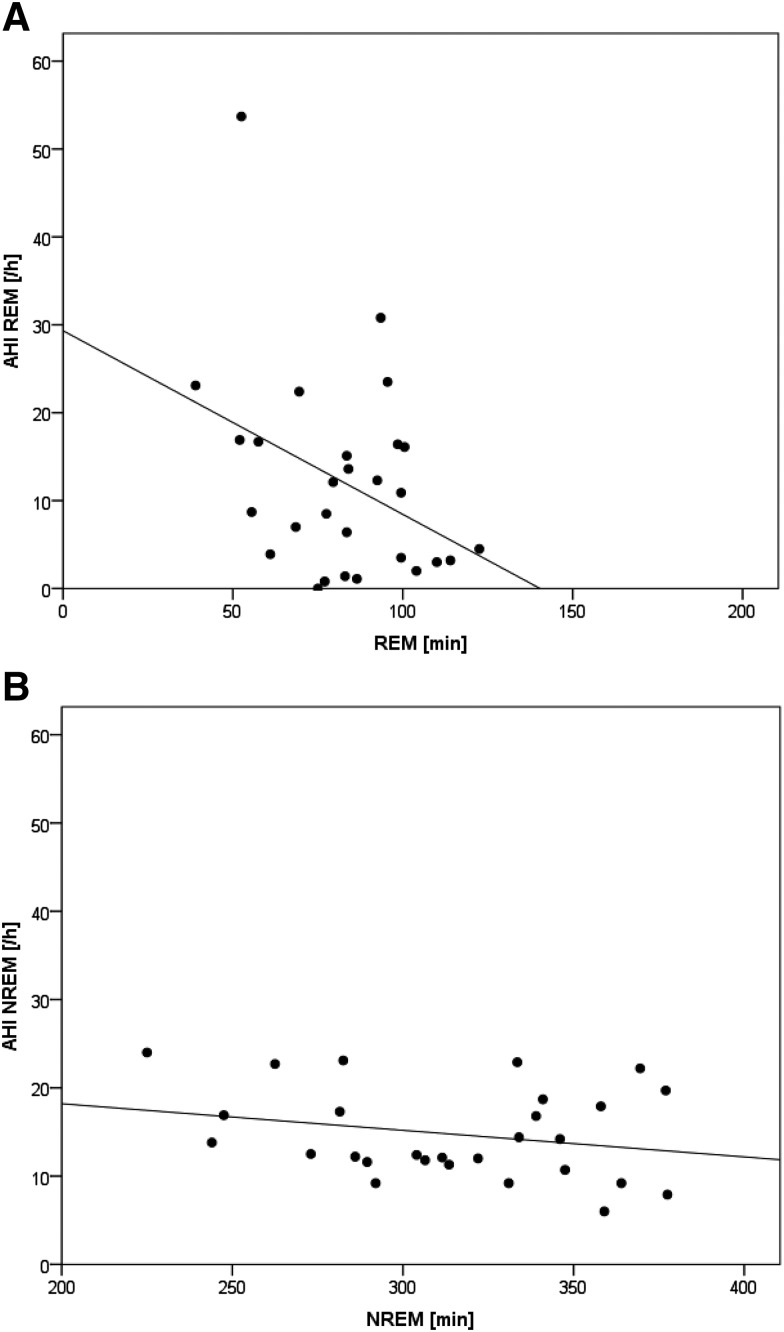
AHI in REM sleep was negatively correlated with summarized REM sleep duration throughout the night (r = −.37, P < .025) (Figure 2A). AHI in NREM sleep revealed no relationship with NREM sleep duration (r = .25) (Figure 2B).
Figure 2. AHI in REM and NREM sleep.
Scatterplots and linear regression lines for the 28 days of recording of (A) the apnea-hypopnea index (AHI) of rapid eye movement (REM) sleep (AHI REM) versus amount of REM sleep in the night and (B) the AHI of non-rapid eye movement (NREM) sleep (AHI NREM) versus amount of NREM sleep in the night.
During REM sleep the mean AHI in the supine position (44.6 ± 31.5 events/h; CV 70.6%) was greater than in the lateral-recumbent positions (right position = 3.7 ± 5.6 events/h; CV 151.3%; P < .001 and left position = 5.9 ± 12.7 events/h; CV 215.3%; P < .001), and the AHI during NREM sleep in the supine position (38.7 ± 17.5 events/h; CV 45.2%) was greater than the AHI in the NREM lateral-recumbent positions (right position = 3.4 ± 5.2 events/h; CV 152.9%; P < .001 and left position = 9.5 ± 14.9 events/h; CV 156.8%; P < .001).
In the supine body position (mean 26.3 ± 11.6% of TST; range 8.1–58.8% of TST), the AHI (mean 43.6 ± 16.9 events/h; range 10.6–72.6 events/h; CV 38.8%) was greater than in the lateral body position (mean 4.8 ± 4.1 events/h; range 0–19.5 events/h; CV 85.4%; P < .0001).
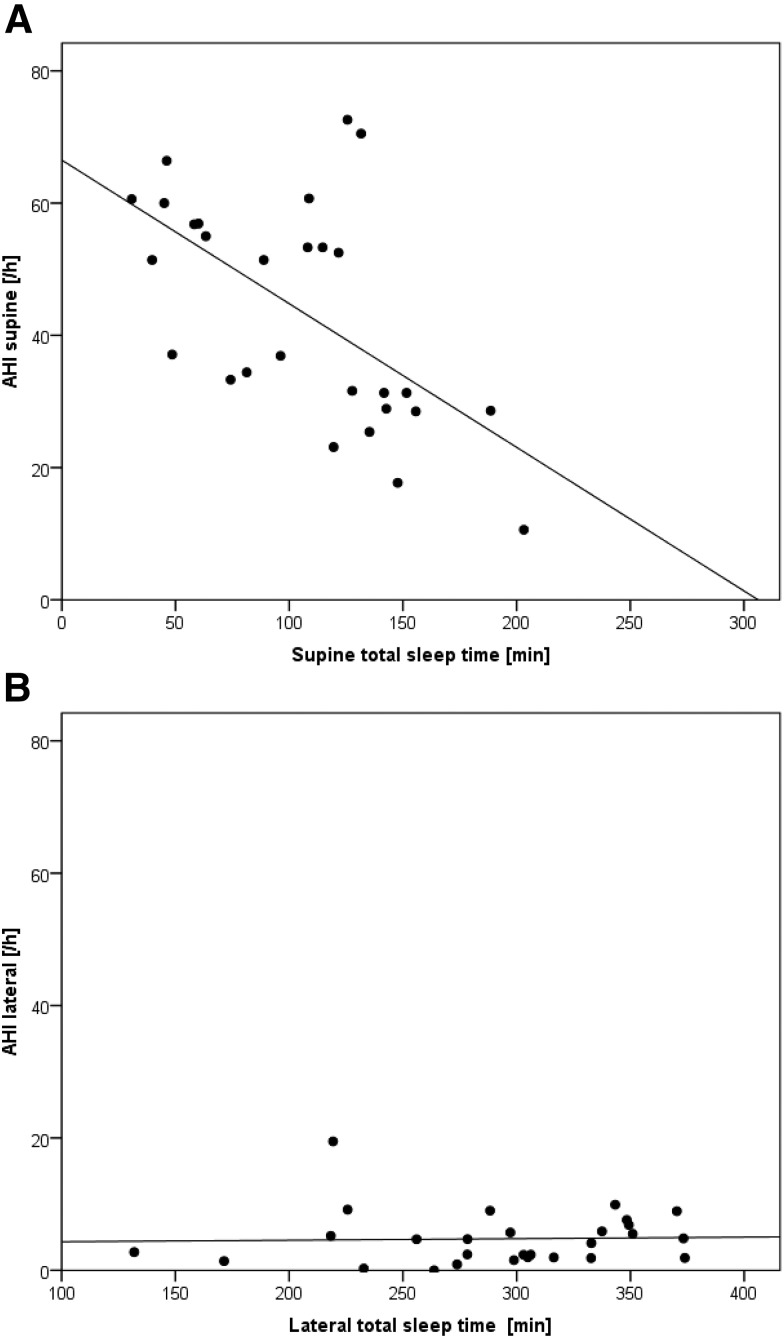
The AHI was not correlated (r = .1) with the percentage of supine sleep of the TST. A negative relationship was found for correlation between the AHI in supine position and the summarized time spent in supine position throughout the night (r = −.59, P < .001) (Figure 3A). In contrast, the AHI in the lateral body position was not correlated with the time spent in lateral body position (r = .035) (Figure 3B).
Figure 3. AHI in supine and lateral positions.
Scatterplots and linear regression lines for the 28 days of recording of (A) the apnea-hypopnea index (AHI) of supine body position sleep (AHI supine) versus amount of sleep in supine body position in the night and (B) the AHI of lateral body position sleep (AHI lateral) versus amount of sleep in lateral body position in the night.
The number of self-reported awakenings, (mean 3.8 ± 2.0; range 1–8), objective awakenings (mean 11.6 ± 7.3; range 1–29), and changes in sleeping position (mean 28.8 ± 8.4; range 10–43), as well as the wake time after sleep onset (mean 15.9 ± 14.4 minutes; range 1.5–51.5 minutes), revealed no correlation with the AHI.
DISCUSSION
Individual variability of OSA severity can be very great, which appreciably influences diagnosis and therapy decisions. This is the first study that investigates the variability of AHI based on home PSG recording over the long term in a patient with mild to moderate OSA. Continuous 4-week night-by-night unattended measurement revealed a high variability ranging from the diagnosis of mild sleep apnea (AHI = 5.3 events/h) not requiring therapy if there is no sleepiness or OSA-correlated comorbidity to an AHI of 28.3 events/h that does necessitate treatment.
Night-to-night variability of AHI is known and has been estimated at approximately 10 events/h.6 However, the prevalence of variability is unknown and possible influencing factors are under discussion. Studies by Stepnowski et al2 and Cartwright7 determined a prevalence of 10% and more than 50%, respectively, among patients with OSA. In a more recent study, Skiba et al found that, in 125 patients for whom PSG was negative for OSA, the second night revealed OSA in 63% of recordings.8 In other studies it was shown that 25% to 45% of patients undergoing 2 nights of PSG have AHI variability > 5–20 events/h. The increase in AHI is more relevant in those with lower initial AHI values. As a result, it is often advisable in cases of low AHI to seriously consider 2 diagnosis nights. Other predictors, except REM sleep and supine position, have not yet become known or have been variously interpreted. For example, it has not been established until now whether variability depends on age or sex, or whether influences result from comorbid sleep disorders such as restless legs syndrome and insomnia.
The influence on AHI by the supine position4,7 and by the REM sleep phase5 has now been established. For our patient, the mean AHI in REM sleep was only slightly greater than in NREM sleep. However, the CV analysis showed that the variability of AHI in REM sleep over 28 days is almost twice as high as that in NREM sleep, concluding that it is difficult to interpret the AHI in REM from a single-night measurement. Significant differences of mean values occurred by imposition of the additional condition of supine sleep. The main determinant of AHI variability was therefore body position, such as reported by Yalciner et al9 and Oksenberg et al.5 However, one case report is not comparable to the study of 100 patients by Oksenberg et al5 during a single night. The site of the study also plays an important role for results. Levendowski et al1 described variability for repeated studies under domestic conditions (unattended PSG) that was lower than that determined by studies conducted in a sleep laboratory. In contrast, the great intraindividual variability determined by the current case report shows that, in a single case, even 2 nights do not necessarily suffice to determine great test-retest correlation.
Therefore, sleeping position can play a key role in diagnosis and therapy decisions, especially for patients with mild OSA.4 It has been established that positional sleep apnea is the form of apnea in which a 50% reduction in AHI can result by changing from the supine to the nonsupine position.7 Consideration of these findings could lead to the conclusion that our patient experienced positional sleep apnea and that he is among those 50% to 60% of patients with OSA with this form of apnea.7 Permut et al10 defined positional sleep apnea as apnea for which the AHI in the lateral recumbent position is not more than 5 events/h and for which reduction of at least 50% in AHI takes place upon change from the supine to the lateral position. In accordance with this definition, our patient—as based on his mean measured values—does not have positional sleep apnea. Consideration, however, of the individual nights would justify a diagnosis of positional apnea in 9 of the 28 nights studied here (30%). By considering body position we were able to calculate a standardized AHI. Analysis revealed that in 54% of nights OSA was underestimated up to 10 events/h. It can be concluded that after standardization, in 5 nights OSA severity was counted as “moderate” rather than “mild.” Some authors concluded that positional apnea occurs frequently in cases of mild sleep apnea, and that a split night cannot serve as a diagnostic tool. Our analysis of variability complements the statement of these authors to the extent that, for greater effectiveness, 2 or more diagnostic nights are necessary to enable valid diagnosis for these patients. Zheng et al11 likewise emphasize the great variability of sleep parameters from the first to the second and the third PSG night.
Bittencourt et al6 conducted a PSG study of 20 patients for 4 consecutive nights and were not able to report major influence of position or the stage of sleep on AHI variability. In the present case report as well, the phase of sleep—and especially WASO and related objective and self-reported parameters such as waking frequency and position change—was not related to changes in AHI.
Under consideration of the combined influence of sleep position and REM, Oksenberg et al5 have described a relevant relationship. Lying in the supine position in REM sleep results in the most instances of sleep-disordered breathing. An interesting finding is the negative correlation of AHI in a supine position with the duration of sleep in a supine position and the negative correlation of AHI in REM sleep with the REM duration of sleep. In both situations, frequent apneas and hypopneas initially occur during the first half of the night that do not increase in severity with ongoing duration of REM sleep or supine position. The influence of supine position on AHI also plays an important role in NREM sleep. In contrast to REM sleep, the AHI in NREM sleep was not correlated to the amount of NREM sleep during the night, but higher AHI values were present in the supine than in the lateral body position.
Oksenberg et al5 showed that even in the course of a single REM sleep phase the frequency and duration of apneas change. The longer the REM sleep phase, the lower the AHI, because the respiratory disorders diminish in the process.
This might be an explanation for our results of elevated CV values for AHI during REM sleep compared to NREM sleep. These relationships are extremely important because they illustrate that interpretation of OSA severity could be skewed if it is based on a single-night measurement without recording of NREM-REM sleep pattern in relation to body position. Medication intake, musculoskeletal diseases, and other comorbidities could influence REM sleep duration and body position as well. Current HSAT devices typically record body position. Most devices are not able to record NREM-REM sleep pattern. The increased use of HSAT to diagnose OSA12 may result in a need for repeated measurements. This may increase the number of subsequent PSG tests. This may be specifically important in patients in whom clinical symptoms do not match AHI results from HSAT. This may indicate patients with comorbidities influencing body position.
Other influences can affect AHI variability, such as sleep deficits, increased alcohol consumption, and weight change. None of these influences prevailed for our patient or did not change during the study. On 4 nights with only moderate alcohol consumption (< 30 g/d), no significant increase in AHI took place.
Our technical solution entailed the conduct of unattended PSG at home. From our standpoint, this was advantageous for patient adherence. Previously conducted studies are not necessarily comparable to our work. These other studies included analysis of only oxygen desaturation index,3 HSAT studies,2 and PSG,1,5,6 as well as studies at home1,2 or in a sleep laboratory.1,5,6 The message is nevertheless comparable. A considerable number of patients need a second diagnostic night. It is therefore necessary to obtain predictors from the patient history, comorbidity, or from the initial PSG to enable a decision as to which patients require 2 or more diagnostic nights.
Interrater variability did not play a role in our case, because only one PSG scorer evaluated all the nights.
Our case study determined neither a predictor for AHI variability nor any special influence of a day of the week. ACF analysis revealed no significant rhythmicity of data. Nevertheless, our data substantiate the requirement that variability in the severity of OSA must be considered and advanced analysis methods could be used to explore them. This is important when the AHI is low or moderate and when clinical symptoms and the AHI do not match—or when the clinical symptoms are not indicative for OSA, as was the case with our patient.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Charité-Universitätsmedizin Berlin. Thomas Penzel has received research grants from Heinen & Löwenstein, Itamar, Philips / Respironics, Resmed, and Somnodent. He received speaker fees and travel support from Bayer, Itamar, Inspire, Somnodent, UCB, Weinmann. He is a shareholder of Advanced Sleep Research GmbH, The Siestagroup GmbH, Somnico GmbH. He was supported by the project no. LQ1605 from the National Program of Sustainability II (MEYS CR) and by the project FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 (OP VaVpI). Ingo Fietze has received research grants from Actelion, Eisai, Heinen & Löwenstein, Jazz Pharmaceuticals, Philips / Respironics, Resmed, Somnodent, UCB, Vanda. He is a shareholder of Somnico GmbH. Martin Glos has received a research grant from Phasya. The other authors report no conflicts of interest.
ABBREVIATIONS
- ACF
autocorrelation function
- AHI
apnea-hypopnea index
- CV
coefficient of variation
- HSAT
home sleep apnea testing
- NNV
night-to-night variability
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PSG
polysomnography
- REM
rapid eye movement
- TST
total sleep time
REFERENCES
- 1.Levendowski D, Steward D, Woodson BT, Olmstead R, Popovic D, Westbrook P. The impact of obstructive sleep apnea variability measured in-lab versus in-home on sample size calculations. Int Arch Med. 2009;2(1):2. doi: 10.1186/1755-7682-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stepnowsky CJ, Jr, Orr WC, Davidson TM. Nightly variability of sleep-disordered breathing measured over 3 nights. Otolaryngol Head Neck Surg. 2004;131(6):837–843. doi: 10.1016/j.otohns.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Stöberl AS, Schwarz EI, Haile SR, et al. Night-to-night variability of obstructive sleep apnea. J Sleep Res. 2017;26(6):782–788. doi: 10.1111/jsr.12558. [DOI] [PubMed] [Google Scholar]
- 4.Mador MJ, Choi Y, Bhat A, et al. Are the adverse effects of body position in patients with obstructive sleep apnea dependent on sleep stage? Sleep Breath. 2010;14(1):13–17. doi: 10.1007/s11325-009-0269-z. [DOI] [PubMed] [Google Scholar]
- 5.Oksenberg A, Arons E, Nasser K, Vander T, Radwan H. REM-related obstructive sleep apnea: the effect of body position. J Clin Sleep Med. 2010;6(4):343–348. [PMC free article] [PubMed] [Google Scholar]
- 6.Bittencourt LR, Suchecki D, Tufik S, et al. The variability of the apnoea-hypopnoea index. J Sleep Res. 2001;10(3):245–251. doi: 10.1046/j.1365-2869.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 8.Skiba V, Goldstein C, Schotland H. Night-to-night variability in sleep disordered breathing and the utility of esophageal pressure monitoring in suspected obstructive sleep apnea. J Clin Sleep Med. 2015;11(6):597–602. doi: 10.5664/jcsm.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yalciner G, Babademez MA, Gul F. Association of sleep time in supine position with apnea-hypopnea index as evidenced by successive polysomnography. Sleep Breath. 2017;21(2):289–294. doi: 10.1007/s11325-016-1401-5. [DOI] [PubMed] [Google Scholar]
- 10.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng H, Sowers M, Buysse DJ, et al. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN Sleep Study. J Clin Sleep Med. 2012;8(1):87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]