Abstract
Study Objectives:
Although previous studies suggested an increased prevalence of obstructive sleep apnea (OSA) among patients with rheumatoid arthritis (RA), no existing large prospective study has addressed this association using objective measures. This study aims to assess the prevalence of OSA using polysomnography (PSG) in patients with RA and its relationship with RA activity.
Methods:
Patients with RA who presented at the rheumatology clinic at a university hospital from 2017 to 2018 were eligible. In the first stage, data from the Disease Activity Score 28, Berlin questionnaire, and Epworth Sleepiness Scale were obtained, along with personal data and a comprehensive medical history. The second stage involved a case-control study confirming OSA with PSG. OSA was defined as an apnea-hypopnea index (AHI) ≥ 5 events/h, whereas patients with an AHI ≥ 15 events/h were categorized as having moderate-severe OSA.
Results:
In total, 199 patients with RA were recruited, 110 patients (55%) underwent PSG, and 5 were excluded. The mean age was 48.93 ± 12.7 years, and the mean body mass index was 31.70 ± 9.74 kg/m2; 94% were female. In total, 67 participants (33.2%) were at high risk for OSA (36 [55.4%] underwent PSG), whereas 132 (66.8%) were at low risk (69 [51.5%] underwent PSG). The estimated prevalence of OSA (AHI ≥ 5 events/h) in the whole population was 58.1%, whereas the prevalence of moderate-to-severe OSA (AHI ≥ 15) was 22.9%.
Conclusions:
This prospective PSG-based study demonstrated that OSA is more common in patients with RA than in the general population, but there appears to be no relationship with disease activity.
Citation:
Wali S, Mustafa M, Manzar D, et al. Prevalence of obstructive sleep apnea in patients with rheumatoid arthritis. J Clin Sleep Med. 2020;16(2):259–265.
Keywords: disease activity, obstructive sleep apnea, polysomnography, prevalence, rheumatoid arthritis
BRIEF SUMMARY
Current Knowledge/Study Rationale: The current literature suggests an increased prevalence of obstructive sleep apnea (OSA) among patients with rheumatoid arthritis (RA). However, a large prospective polysomnography-based study with a gold-standard confirmatory diagnosis of OSA is lacking. The current study is the first to assess the prevalence of OSA among patients with RA.
Study Impact: This study shows a higher prevalence of OSA among patients with RA than among the general population, which may worsen disease morbidity.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition characterized by recurrent upper airway occlusion during sleep.1 The estimated prevalence of OSA worldwide is 3% to 7% in men and 2% to 5% in women.2–4 Several studies have shown that OSA is associated with rheumatoid arthritis (RA).1,5 Patients with RA have the same risk factors for OSA that affect the general population, such as advanced age, obesity, and neck circumference.6 Although obesity is present in most patients with OSA and is considered a major risk factor for its development, OSA has been reported in patients with low body mass index (BMI) and gross anatomic abnormalities causing upper airway narrowing.5,7 Indeed, the presence of OSA has been observed in some patients with RA irrespective of BMI. Acquired retrognathia secondary to temporomandibular joint (TMJ) destruction in RA has been proposed as a rare risk factor for OSA and has been reported as a contributing factor to the development of OSA in patients with RA.8
Although earlier studies linking OSA to RA were limited to case reports, subsequent observational studies and population-based studies have suggested that OSA is more likely to develop in patients with RA than are members of the general population. Reading et al7 used the Berlin questionnaire to assess OSA and found the risk of OSA in 164 patients with RA and 328 control patients without RA to be 50% and 31%, respectively. Conversely, other studies using the Berlin questionnaire to assess OSA showed no significant difference in OSA prevalence between patients with RA and the general population.9 However, studies using more objective methods, such as polysomnography (PSG), have suggested that there is an elevated risk of OSA among patients with RA.8,10 However, most of these studies had limited numbers of patients, apart from one large retrospective study in Taiwan. In that large retrospective cohort study, Shen et al10 compared 33,418 patients with newly diagnosed RA who underwent PSG with a similar number of individuals without RA and found that the overall incidence rates of OSA in the RA and non-RA cohorts were 3.04 and 1.73/10,000 person-years, respectively. Nevertheless, no large prospective PSG-based study has systematically examined the association between OSA and RA. Hence, our aim in the current study was to prospectively assess the prevalence of OSA among patients with RA using a standard objective measure (PSG). Furthermore, the relationship between OSA and RA disease activity was explored.
METHODS
Our target population consisted of all patients with RA aged 18 to 80 years who presented at the rheumatology clinic at King Abdulaziz University Hospital, Jeddah, in the period from June 2017 to September 2018. Ethical approval was obtained from the ethics committee of King Abdulaziz University Hospital, Jeddah. All participating patients signed an informed consent form. The study was performed in two stages: a screening stage and a confirmatory stage.
The first (screening) stage
In this stage, all patients older than 18 years in whom RA was diagnosed (based on the diagnostic criteria of the American Rheumatology Association (ARA)11 presented to the King Abdulaziz University Hospital Rheumatology Clinic to be interviewed by trained physicians in a cross-sectional study. The purpose was to collect personal data and a brief medical history and to administer the Disease Activity Score 28 (DAS28) to assess the activity of RA, the Berlin questionnaire to assess the risk of OSA, and the Epworth Sleepiness Scale (ESS) to assess daytime sleepiness. In addition, all participants had their anthropometric measures recorded, including height, weight, and neck circumference.
Instruments
Berlin Questionnaire
The widely used Berlin questionnaire9,12 is composed of 11 self-reported questions used to reliably identify individuals at high risk of OSA. These questions focus on three main apnea signs and symptoms: snoring, daytime sleepiness, and obesity/high blood pressure. The questionnaire was originally designed as a means for clinicians to quickly establish OSA risk factors and has been validated in patients 18 years or older. The scoring process includes evaluating “yes or no” responses as well as multiple-choice questions. It also includes space for calculating the BMI based on the measurements of the respondent. For “yes or no” questions, one point is given for each positive answer. In the case of the multiple-choice questions, the two answers that correspond to the highest severity of apnea each receive one point. Responses in the first and second categories indicate high risk if the individual receives two or more points. The respondent is considered at high risk based on category three questions when his/her blood pressure is high or his/her BMI is greater than 30 kg/m2.
Epworth Sleepiness Scale
The ESS is a validated questionnaire that was first published in 1991 and is used to assess level of daytime sleepiness.13 The advantage of the ESS is that it is a reliable instrument for classifying humans as healthy or chronically somnolent during the daytime.13 The ESS asks people to use a three-point scale to rate their usual chances of dozing off in eight situations/activities that most people encounter in their daily life.13 The scores for all eight items are then aggregated to give a score ranging from 0 to 24, which is used as a measurement of the respondent’s average sleep propensity in those eight situations. A score < 10 is considered normal, with a score ≥ 10 being indicative of excessive daytime sleepiness.13
DAS28 Scoring System
The assessment of the disease activity of RA was based on the DAS28 score, which provides a global summative and continuous score for disease activity assessment. Ranges of DAS28 scores that correspond to high, moderate and low disease activity and remission have been proposed. A DAS28 score > 5.1 indicates high disease activity; moderate disease activity is indicated by a DAS28 score range of 3.2 to 5.1; low disease activity is indicated when the score is in the range of 2.6 to 3.2; and remission is represented by a score less than 2.6.14 The components of the assessment include a count of the number of joints with swelling and tenderness, the measurement of C-reactive protein levels, and the patient’s global assessment of disease activity using a score from 0 to 100 (higher numbers indicate more active disease).15
The second (confirmatory) stage
The second stage of this study was a case-control study that confirmed the presence of OSA with PSG and assessed the roles of different risk factors. After analyzing the stage 1 data, the respondents were classified based on their risk for OSA using the Berlin questionnaire. Once classified into their respective groups, 50% of each of the high-risk and low-risk groups were selected to undergo PSG. PSG (SOMNO Medics Plus; SOMNO Medics, Randersacker, Germany) consisted of continuous recordings from the following: surface leads for electroencephalography (EEG), electrooculography, electromyography (submental and bilateral anterior tibialis muscles), electrocardiography, nasal pressure, nasal and oral airflow (thermocouple), chest and abdominal impedance belts for respiratory muscle effort, pulse oximetry for oxygen saturation, pulse rate, a tracheal microphone for snoring, and body position sensors for sleep position. PSG records were then scored manually according to the criteria published by the American Academy of Sleep Medicine.16 Registered polysomnographic technologists were assigned to manually score the data from the PSG studies. Full PSG was conducted in two different locations, either in the participants’ homes or at the Sleep Medicine and Research Center at King Abdulaziz University Hospital; the same device was used in both locations to increase convenience and participant cooperation.
Abnormal obstructive breathing events during monitored sleep were described according to the latest recommendation of the AASM.16 Such events included a decrease in airflow by at least 90% from the baseline value for at least 10 seconds (apnea) and a discernible reduction in airflow of at least 30% of the pre-event baseline value according to the nasal pressure that was associated with a reduction in oxygen saturation of at least 3% and/or followed by an EEG arousal (hypopnea), despite persistent chest and abdominal muscle efforts to overcome the obstruction.16 EEG arousal was defined according to the recommendation by the AASM.16 The average number of these apnea and hypopnea events/h of sleep (apnea-hypopnea index [AHI]), was then calculated. The primary outcome obtained was the prevalence of OSA, as assessed by the AHI. An AHI score of ≥ 5 events/h was the minimal threshold for an OSA diagnosis, whereas patients with AHI scores of ≥ 15 events/h were categorized as having moderate-to-severe OSA as per AASM recommendation.17
Calculation of prevalence
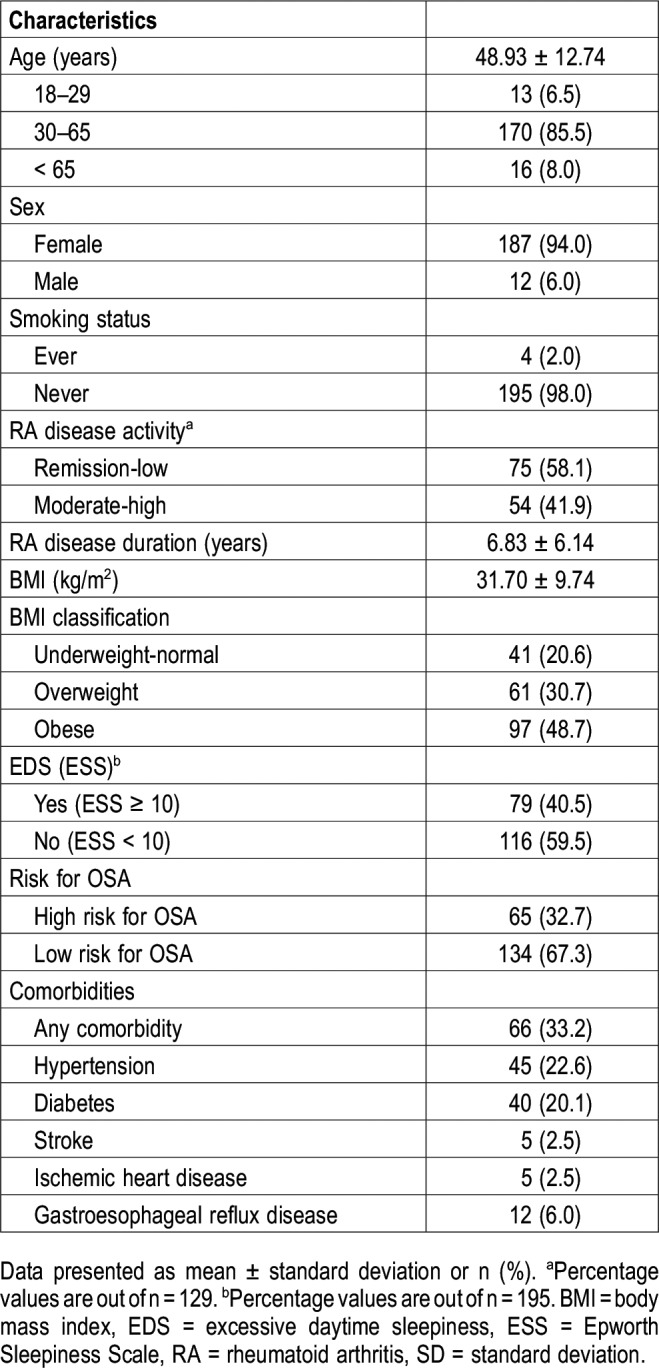
The mean age and BMI of patients who underwent PSG (PSG group) were compared with those who did not undergo PSG (non-PSG group). The lack of significant differences between the two groups implies that the prevalence of OSA in the PSG group is representative of that in the entire screened population.18 However, any significant differences detected between the PSG and non-PSG groups would have required a conservative approach, with the assumption that patients with OSA in the PSG group should be considered the only patients with OSA in the entire screened population.18,19
Statistical analysis
We used the SPSS 23.0 software package (IBM Corp, Armonk, New York, United States) to perform the statistical analysis. Mean values with standard deviations, frequencies, and percentages are used for the descriptive presentation of the variables. The chi-square test was used to assess differences in the distributions of categorical variables. t tests, Mann-Whitney U tests, and Kruskal-Wallis tests were used to assess differences in continuous variables according to their distributions and sample sizes. Binary logistic regression was used to assess the presence of associations between OSA and its predictors. Correlation tests were performed to evaluate bivariate associations between OSA and the target independent variables. Those target independent variables with P < .25 for a correlation with OSA were used as prospective predictive variables in binary logistic regression.20 Age, BMI group (less than or equal to/greater than 30 kg/m2), the Berlin score, the presence of diabetes mellitus, the presence of hypertension, and the presence of any other comorbidity were chosen as covariates because their P values were all less than .25 for their correlations with OSA.20 Sex, smoking and duration of RA were not used as covariates because their P values were greater than .25.20 Furthermore, to manage the zero-cell effect because of the modest sample size, categorical covariates, that is, diabetes (model 1), hypertension (model 2) and the presence of any comorbidity (model 3), were run in separate models.
RESULTS
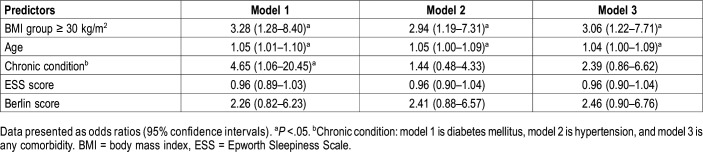
Participants’ characteristics
Most of the 199 participants (85.5%) were in the 30- to 65-year age group (Table 1). Females accounted for most (94%) of the study participants. More than one-fourth of the participants (27.1%) showed moderate-to-high levels of RA activity. More than two-thirds of the study population (79.4%) was either overweight or obese. Nearly 40% of the participants reported an ESS score of 10 or greater. Approximately one-third of the participants were classified as being at high risk of the development of OSA according to the Berlin questionnaire. Hypertension and diabetes mellitus were the most prevalent comorbidities in the study population (Table 1). Of the 199 participants, 110 (55%) underwent PSG; however, 5 were excluded because of inaccurate PSG data and the participants’ refusal to repeat the study. Successful PSG was performed for 55.4% of high-risk patients (36/65) and 51.5% of low-risk patients (69/134).
Table 1.
Characteristics of the study population (n = 199).
Demographic comparison between the PSG group and the non-PSG group
There were no significant differences between patients who underwent PSG and those who did not in terms of age, BMI, and sex distribution (Table 2).
Table 2.
Demographic comparison between the PSG group and non-PSG group.
Prevalence of OSA in patients with RA (n = 105)
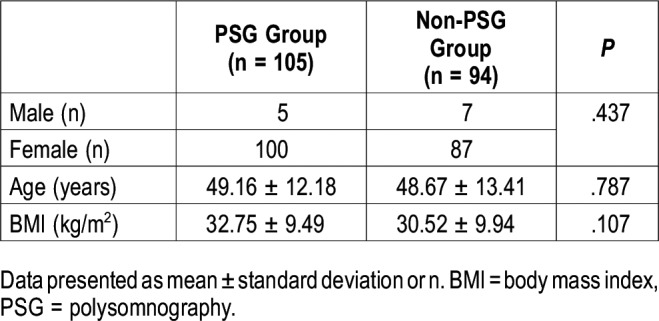
The prevalence of OSA (AHI ≥ 5 events/h) among the 105 patients with RA who underwent PSG was 58.1% (61/105) (Table 3). Of these patients, 19 had excessive daytime sleepiness (19/61; 31.1%). However, when a more stringent definition was used, that is, AHI ≥ 15 events/h, 22.9% (24/105) of the patients had OSA.
Table 3.
Prevalence of obstructive sleep apnea in patients with rheumatoid arthritis (n = 105).
Prevalence of OSA in the cohort of patients with RA (n = 199)
Because there were no differences between the PSG and non-PSG groups in terms of sex, BMI, and age (Table 2), the estimated prevalence of OSA (AHI ≥ 5 events/h) in the whole cohort of 199 patients with RA was 58.1%, and the prevalence of moderate-severe OSA was estimated to be 22.9% in the entire screened population.
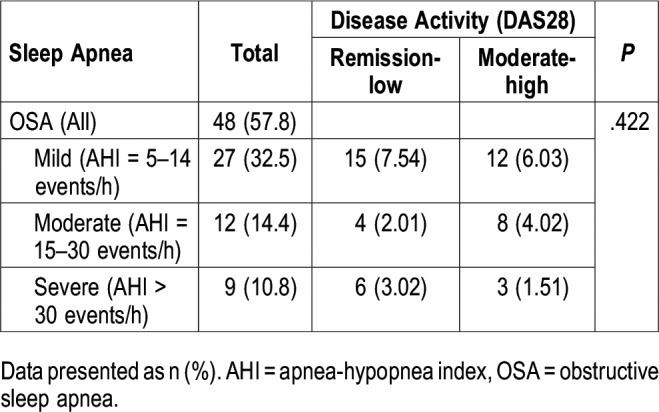
OSA and its correlation with RA disease activity
No relationships were found between OSA and RA disease activity levels (remission-low or moderate-high), as determined by the DAS28 scores (Table 4). The percentages presented are out of a total of 83 participants (those with an OSA diagnosis and DAS28 data).
Table 4.
Obstructive sleep apnea and its correlation with rheumatoid arthritis disease activity (n = 83)
Predictors of OSA in patients with RA
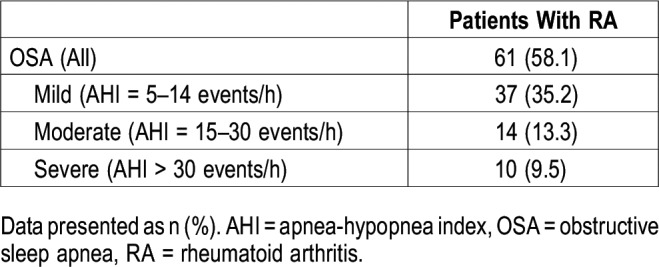
Table 5 shows the relationship between the potential predictors and OSA. Binary logistic regression models investigated the relationships between the covariates (BMI ≥ 30 kg/m2, age, diabetes, hypertension, other comorbidities, ESS score, and Berlin score) and OSA. Model 1 was statistically significant (χ26 = 24.00, P < .001) and explained 28.4% (Nagelkerke R2) of the variance in the status of OSA, with a classification accuracy of 70.3%. Advanced age, BMI ≥ 30 kg/m2, and the presence of diabetes were associated with an increased likelihood of the development of OSA.
Table 5.
Predictors of obstructive sleep apnea status identified by binary logistic regression analysis.
Model 2 was also statistically significant (χ26 = 19.55, P < .01) and explained 23.6% (Nagelkerke R2) of the variance in the status of OSA, with a classification accuracy of 71.3%. Model 3 was additionally seen to be statistically significant (χ26 = 22.07, P < .001). The model explained 26.4% (Nagelkerke R2) of the variance in the status of OSA, with a classification accuracy of 70.3%. For the last two models, advanced age and BMI ≥ 30 kg/m2 were associated only with an increased likelihood of the development of OSA.
DISCUSSION
This study demonstrated an OSA (AHI ≥ 5 events/h) prevalence of 58.1%, and moderate-to-severe OSA (AHI ≥ 15 events/h) was seen in 22.9% of patients with RA, a much higher prevalence than that observed among the general population. Previous studies on the prevalence of OSA in the general Saudi population have reported prevalence rates of 8.8% (diagnosed OSA) and 4.3% (moderate to severe OSA),4 which were much lower than those found in patients with RA in the current study. Worldwide, the prevalence of OSA in the overall adult population ranges from 6% to 17%,21 and notably, OSA has been reported to be equally common among both the developing and developed worlds.22 Furthermore, the prevalence reported in Asia is comparable to that in the rest of the world, despite the lower obesity rates among the Asian populations. One possible reason is the craniofacial structure prevalent in Asian populations.19,23,24 Similarly, large studies using the Berlin questionnaire, such as those conducted by the National Sleep Foundation in America, demonstrated that 26% of the general population is at high risk of the development of sleep apnea, which again, is a lower rate than that reported among patients with RA in the current study (32.3%).25
To the best of our knowledge, this is the first prospective study with a relatively large number of participants to examine the prevalence of OSA among patients with RA using PSG as the standard diagnostic test. Earlier studies linking OSA to RA were limited to case reports and small observational and population-based studies. Most of these studies did not use PSG as an objective test to verify the diagnosis of OSA but depended on validated questionnaires. Alamoudi,8 however, reported 10 patients with RA who underwent PSG, nine of whom were found to have OSA. Furthermore, Gjevre et al26 reported a high prevalence of OSA (68%), defined as an AHI of ≥ 5 events/h in 25 patients with RA, and found that ESS had a high predictive value for OSA. Only one large study evaluated the prevalence of OSA among patients with RA using PSG as an objective test. However, the study was a retrospective study conducted in Taiwan.10 In that study, the authors reviewed data from the Longitudinal Health Insurance Database, which covers more than 99% of Taiwan’s population.10 They compared 33,418 patients with newly diagnosed RA with a similar number of individuals without RA and found that the overall incidence rate of OSA based on PSG in RA patients was 3.04/10,000 person-years, which was almost double the incidence observed in the non-RA group (1.73/10,000 person-years).10 The actual figures from the Taiwan study cannot be compared with our figures, as they were measuring the incidence, whereas our focus was on prevalence. Nevertheless, both studies suggested an elevated risk of developing OSA among patients with RA.
Patients with RA have the same risk factors for OSA that are usually found in the general population, such as advanced age, obesity, and neck circumference.4,6 Obesity remains the main risk factor for OSA in the general population. In fact, a meta-analysis that systematically assessed the association between BMI and RA found that an increased BMI is associated with a greater risk of the development of RA.27 Furthermore, our study has demonstrated that increased age and obesity are associated with an increased likelihood of the development of OSA among patients with RA (Table 5). Moreover, patients with RA may be at higher risk of the development of OSA than the general population due to the presence of features specifically related to RA, in addition to conventional risk factors. Such features include micrognathia and retrognathia, resulting from destruction of the TMJ, as well as occipitocervical lesions. Davies and Iber in 1983 were the first to report the presence of OSA caused by micrognathia in a patient with RA.28 This report was followed by several case reports linking OSA with RA due to micrognathia. Subsequently, Redlund-Johnell studied 400 patients with RA and found that 70% of those with severe arthritic TMJ destruction had episodes of upper airway obstruction.29 This report was followed by a study by Sugahara et al30 who reported that RA is an indirect risk factor for OSA caused by TMJ damage. Shoda et al31 examined the prevalence of OSA in patients with RA and occipitocervical lesions using a portable monitoring device and found that 23 patients (79%) had OSA. Unfortunately, we did not evaluate the contributions of these risk factors to the development of OSA among patients with RA in the current study. However, as observed in the general population, our study demonstrated that patients with RA and OSA are at risk of having concurrent comorbidities, such as diabetes.3 This finding corresponds with a recent retrospective study revealing that OSA is again associated with risk factors such as obesity, hypertension, and diabetes in both RA groups and the general population.32
The coexistence of OSA in patients with RA has been suggested to clinically influence the severity of the disease, with more reported symptoms of pain and fatigue, as well as having a biologic influence through possible effects on the levels of circulating inflammatory markers and mediators.33,34 Cytokines are known to play roles in physiological sleep regulation. Special interest has been given to the link between tumor necrosis factor alpha (TNF-α) and sleep disorders. Increased levels of TNF-α have been reported in patients with OSA, and the initiation of anti-TNF-α therapy (etanercept) led to a noticeable decrease in sleepiness and AHI.35 However, in a large population study, Wolfe and Michaud failed to demonstrate a significant change in sleep scores in patients with RA reated with anti-TNF therapies.36 Therefore, the effect of OSA on RA activity remains unknown. Although our study suggested an increased prevalence of OSA among individuals with RA, no significant differences in the prevalence of OSA were observed among patients with different disease activities of RA (Table 4). The results of the study by Mutoh et al37 concur with our findings, as they reported sleep apnea in 20% of inpatients with RA, although they found no correlation between the severity of sleep apnea and RA activity.
The main limitation of the current work is the restricted cohort of patients included in the study. The study examined patients who presented at the rheumatology clinic at a specific single-center hospital. However, the study was comprehensive and involved the assessment of many clinical parameters in addition to PSG, which might not be available in many centers in the Kingdom of Saudi Arabia. Another limitation of this study is that we did not address the roles played by individual features specifically related to RA, such as micrognathia and cervical myelopathy, on the elevated risk of OSA in such patients. Nevertheless, the primary objective of the study was to determine the prevalence of sleep apnea among patients with RA, irrespective of these contributing factors.
In summary, to the best of our knowledge, this was the first prospective study with a relatively large sample size to use PSG as an objective test to address the prevalence of sleep apnea among patients with RA. The study demonstrated a higher prevalence of this sleep disorder among patients with RA than among the general population. Furthermore, the study did not find a correlation between the existence of OSA and the level of disease activity of RA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. RG-01-140-38. The authors, therefore, gratefully acknowledge the DSR for its technical and financial support. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff of the Sleep Medicine and Research Center of King Abdulaziz University Hospital for scoring all the sleep studies, Mrs. Haneen Mansour, Mrs. Dalyah AlQaidi, Mrs. Raina Abdullah, Ms. Rozanith Damasco, Mrs. Loida Asi, and Mrs. Rose Anne Credo. Special thanks to Mrs. Walaa Abuzahra, Research Coordinator at the Sleep Medicine and Research Center, for her time and effort in coordinating all of the procedures.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- BMI
body mass index
- DAS28
Disease Activity Score
- EEG
electroencephalography
- ESS
Epworth Sleepiness Scale
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RA
rheumatoid arthritis
- TMJ
temporomandibular joint
REFERENCES
- 1.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wali SO, Abalkhail B, Krayem A. Prevalence and risk factors of obstructive sleep apnea syndrome in a Saudi Arabian population. Ann Thorac Med. 2017;12(2):88–94. doi: 10.4103/1817-1737.203746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westhovens R, van der Elst K, Matthys A, Tran M, Gilloteau I. Sleep problems in patients with rheumatoid arthritis. J Rheumatol. 2014;41(1):31–40. doi: 10.3899/jrheum.130430. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Gjevre RM, Nair BV, Gjevre JA. Obstructive sleep apnoea in relation to rheumatic disease. Rheumatology. 2012;52(1):15–21. doi: 10.1093/rheumatology/kes210. [DOI] [PubMed] [Google Scholar]
- 7.Reading SR, Crowson CS, Rodeheffer RJ, Fitz-Gibbon PD, Maradit-Kremers H, Gabriel SE. Do rheumatoid arthritis patients have a higher risk for sleep apnea? J Rheumatol. 2009;36(9):1869–1872. doi: 10.3899/jrheum.081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alamoudi OS. Sleep-disordered breathing in patients with acquired retrognathia secondary to rheumatoid arthritis. Med Sci Monit. 2006;12(12):Cr530–Cr534. [PubMed] [Google Scholar]
- 9.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 10.Shen TC, Hang LW, Liang SJ, et al. Risk of obstructive sleep apnoea in patients with rheumatoid arthritis: a nationwide population-based retrospective cohort study. BMJ Open. 2016;6(11):e013151. doi: 10.1136/bmjopen-2016-013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi N, Chung SA, Gibbs A, Shapiro CM. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12(1):39–45. doi: 10.1007/s11325-007-0125-y. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 14.Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625–2636. doi: 10.1002/art.21235. [DOI] [PubMed] [Google Scholar]
- 15.Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 16.Berry R, Brooks R, Gamaldo C, et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.4. Darien, IL: American Academy of Sleep Medicine; 2017. [Google Scholar]
- 17.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 18.Keenan SP, Ferguson KA, Chan-Yeung M, Fleetham JA. Prevalence of sleep disordered breathing in a population of Canadian grainworkers. Can Respir J. 1998;5(3):184–190. doi: 10.1155/1998/403649. [DOI] [PubMed] [Google Scholar]
- 19.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125(1):127–134. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Bendel RB, Afifi AA. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72(357):46–53. [Google Scholar]
- 21.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care. 2010;55(9):1155–1167. [PubMed] [Google Scholar]
- 23.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170(10):1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 25.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130(3):780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 26.Gjevre JA, Taylor-Gjevre RM, Nair BV, Lim HJ. Do sleepy rheumatoid arthritis patients have a sleep disorder? Musculoskelet Care. 2012;10(4):187–195. doi: 10.1002/msc.1016. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Sun M. A meta-analysis of the relationship between body mass index and risk of rheumatoid arthritis. EXCLI J. 2018;17:1079–1089. doi: 10.17179/excli2018-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies SF, Iber C. Obstructive sleep apnea associated with adult-acquired micrognathia from rheumatoid arthritis. Am Rev Respir Dis. 1983;127(2):245–247. doi: 10.1164/arrd.1983.127.2.245. [DOI] [PubMed] [Google Scholar]
- 29.Redlund-Johnell I. Upper airway obstruction in patients with rheumatoid arthritis and temporomandibular joint destruction. Scand J Rheumatol. 1988;17(4):273–279. doi: 10.3109/03009748809098796. [DOI] [PubMed] [Google Scholar]
- 30.Sugahara T, Mori Y, Kawamoto T, Sakuda M. Obstructive sleep apnea associated with temporomandibular joint destruction by rheumatoid arthritis: report of case. J Oral Maxillofac Surg. 1994;52(8):876–880. doi: 10.1016/0278-2391(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 31.Shoda N, Seichi A, Takeshita K, et al. Sleep apnea in rheumatoid arthritis patients with occipitocervical lesions: the prevalence and associated radiographic features. Eur Spine J. 2009;18(6):905–910. doi: 10.1007/s00586-009-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilton KM, Matteson EL, Crowson CS. Risk of obstructive sleep apnea and its association with cardiovascular and noncardiac vascular risk in patients with rheumatoid arthritis: a population-based study. J Rheumatol. 2018;45(1):45–52. doi: 10.3899/jrheum.170460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135(4):950–956. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 34.Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12(3):211–228. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89(9):4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe F, Michaud K. Fatigue, rheumatoid arthritis, and anti-tumor necrosis factor therapy: an investigation in 24,831 patients. J Rheumatol. 2004;31(11):2115–2120. [PubMed] [Google Scholar]
- 37.Mutoh T, Okuda Y, Mokuda S, et al. Study on the frequency and risk factors of moderate-to-severe sleep apnea syndrome in rheumatoid arthritis. Mod Rheumatol. 2016;26(5):681–684. doi: 10.3109/14397595.2015.1131352. [DOI] [PubMed] [Google Scholar]